Chapter 15 Obscure Gastrointestinal Bleeding
Introduction
Obscure gastrointestinal (GI) bleeding accounts for approximately 5% of all GI bleeding and is defined as bleeding from an unknown source that persists or recurs after negative endoscopic diagnostic evaluation.1 A negative diagnostic evaluation is commonly agreed on as consisting of negative upper endoscopy (esophagogastroduodenoscopy [EGD]) and colonoscopy with careful evaluation of the terminal ileum. Obscure GI bleeding is subcategorized as overt or occult. Obscure-overt GI bleeding is persistent or recurrent visible evidence of bleeding (hematemesis, hematochezia, or melena), whereas obscure-occult GI bleeding is persistent or recurrent positive fecal occult blood, iron deficiency anemia, or both, when there is no evidence of visible blood loss to the patient or health care provider.2
Etiology of Obscure Gastrointestinal Bleeding
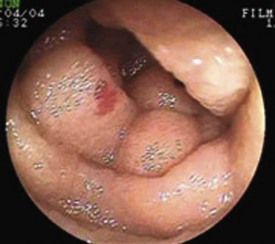
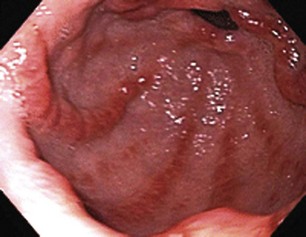
Obscure GI bleeding has numerous possible causes and may originate from the upper GI tract (proximal to the ligament of Treitz), the mid-GI tract (ligament of Treitz to the terminal ileum), or the colon. Most causes (approximately 75%) of obscure GI bleeding are found between the ligament of Treitz and the ileocecal valve—in other words, in the small bowel.3 Arteriovenous malformations (AVMs) of the small bowel account for 30% to 40% of obscure GI bleeding and are the most common source of obscure GI bleeding in older patients (Fig. 15.1).4 In persons 30 to 50 years of age, tumors such as GI stromal tumors, leiomyomas, leiomyosarcomas, schwannomas, carcinoids, lymphomas, and adenocarcinomas predominate (Fig. 15.2). Younger individuals most commonly have obscure bleeding from Crohn’s disease or Meckel’s diverticula–associated ulceration (Fig. 15.3).5 Nonsteroidal antiinflammatory drug (NSAID) enteropathy has been associated with erosions, ulcers, and strictures of the small bowel and can also be a potential cause of obscure GI bleeding.6 Less common causes of obscure GI bleeding include Dieulafoy’s lesions, hemosuccus pancreaticus, Strongyloides stercoralis infection, radiation-induced enteritis, and pseudoxanthoma elasticum (Table 15.1).7–11
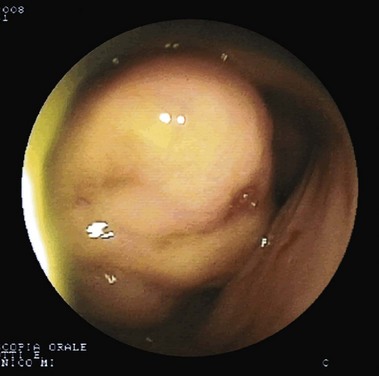
Fig. 15.2 Gastrointestinal stromal tumor as seen by double-balloon enteroscopy.
(Courtesy of Professor Roberto De Franchis.)
Table 15.1 Etiology of Obscure Gastrointestinal (GI) Bleeding
| UPPER GI LESIONS |
NSAID, Nonsteroidal antiinflammatory drug.
Investigating Obscure Gastrointestinal Bleeding
A review of medications (including over-the-counter medications) may reveal inadvertent use of NSAIDs or products containing acetylsalicylic acid (ASA) and NSAIDs. A family history of cancer occurring at an early age, particularly colorectal or endometrial, may suggest the presence of hereditary nonpolyposis colorectal cancer. Skin, nail, and oral mucosal changes may suggest the presence of several disorders associated with obscure GI bleeding or iron deficiency anemia, including telangiectasias, which may reflect hereditary hemorrhagic telangiectasia (Osler-Weber-Rendu syndrome); dermatitis herpetiformis, which may reflect celiac disease; or other conditions with cutaneous and GI manifestations (e.g., Kaposi’s sarcoma, Peutz-Jeghers syndrome, tylosis, pseudoxanthoma elasticum, Ehlers-Danlos syndrome, blue rubber bleb nevus syndrome, Henoch-Schönlein purpura, neurofibromatosis, malignant atrophic papulosis, and Klippel-Trenaunay-Weber syndrome).2
Diagnostic Investigations
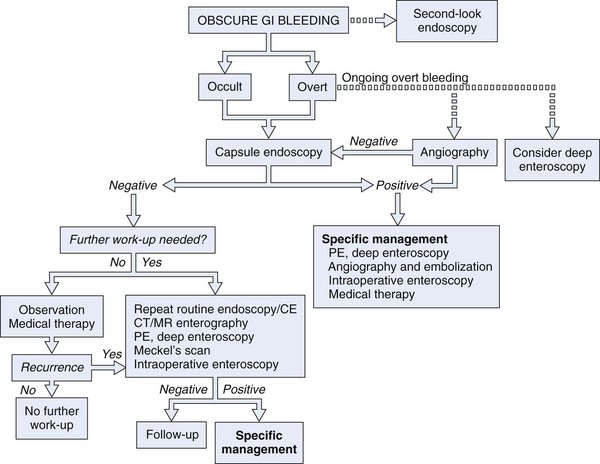
Numerous diagnostic investigations may be performed to identify the etiology of obscure GI bleeding. Endoscopic investigations include repeat EGD and colonoscopy, push enteroscopy, capsule endoscopy (CE), deep enteroscopy using balloon-assisted or non–balloon-assisted techniques, and intraoperative enteroscopy (IOE). Radiologic testing includes barium studies such as small bowel follow-through and enteroclysis, nuclear medicine studies such as tagged red blood cell (RBC) scan and Meckel’s scan, computed tomography (CT) and magnetic resonance (MR) imaging in combination with enteroclysis (CT and MR enterography), and angiography (Fig. 15.4).
Endoscopic Investigations
Repeat Upper Endoscopy (Esophagogastroduodenoscopy) and Colonoscopy
Second-look upper endoscopy may be helpful in identifying lesions potentially overlooked or unrecognized at the time of the initial endoscopic evaluation.12 Data suggest that 20% of lesions leading to obscure GI bleeding may be overlooked and are within reach of a standard upper endoscope.13 Commonly overlooked lesions in the upper GI tract include Cameron’s erosions or ulcerations in large hiatal hernias, isolated gastric fundal varices, peptic ulcers, AVMs including gastric antral vascular ectasia (watermelon stomach), and Dieulafoy’s lesion (Fig. 15.5).14–16 An aortoenteric fistula should be considered in patients with prior abdominal aortic aneurysm repair. Although the reported yield of repeat colonoscopy is low (3% to 6%), it may be helpful in selected patients when the original colonoscopy examination was documented to have had a mediocre or poor preparation, the extent of the examination was not to the cecum, or the terminal ileum was not evaluated.17 Lesions missed or unrecognized during colonoscopy may include AVMs, polyps, solitary rectal ulcers, rectal varices, and neoplasms.
Push Enteroscopy
Push enteroscopy permits evaluation of the proximal small intestine to a distance that is approximately 50 to 100 cm beyond the ligament of Treitz. Dedicated videoenteroscopes (160 to 250 cm in length) are commercially available, but if these instruments are not available at the endoscopy site, a pediatric or standard adult colonoscope can be used instead.18 The use of an overtube, back-loaded onto the endoscope insertion tube, may help limit looping of the enteroscope within the stomach and facilitate deeper small bowel intubation.19 The diagnostic yield of push enteroscopy is reported to increase with greater depth of scope insertion. When using a pediatric or adult colonoscope, the reported diagnostic yield in the evaluation of obscure GI bleeding ranges from 13% to 38%. With the use of a dedicated videoenteroscope, reported diagnostic yield rates increase to 26% to 80%.13,20,21
With the development of endoscopic devices for dedicated videoenteroscopes, such as biopsy forceps, snares, thermal probes (contact and noncontact), and injection needles, push enteroscopy is preferred over radiologic diagnostic modalities because of the ability to obtain tissue, perform polypectomy or hemostasis if necessary, and mark lesion sites with India ink tattoo.13 However, push enteroscopy does not allow for the visualization of the entire small bowel, and complications, including perforation and mucosal laceration, have been reported with the use of an overtube. With the advent of CE and “deep” enteroscopy, diagnostic push enteroscopy is less commonly used.
Capsule Endoscopy
CE is an endoscopic technology that is capable of obtaining endoscopic images from the entire small bowel.22,23 CE is safe, easy, minimally invasive, and patient-friendly and has become a first-line tool in imaging small bowel pathologies. With this realization, there has been rapid uptake and wide acceptance of this revolutionary endoscopic technology for detecting small bowel abnormalities.24
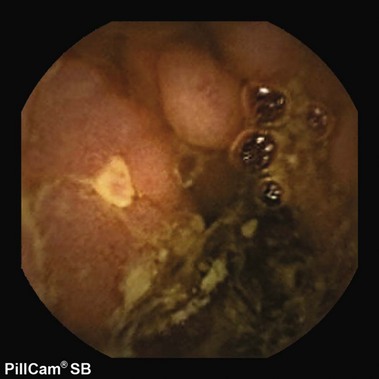
The Pillcam SB video capsule endoscope (Given Imaging Ltd, Yoqneam, Israel) is a wireless capsule (11 mm × 26 mm) composed of a light source, lens, complementary metal oxide semiconductor imager, battery, and wireless transmitter. The Pillcam SB has a battery life of approximately 7 to 8 hours in which time the capsule captures two images per second (approximately 60,000 total images per examination) in a 140-degree field of view and 8 : 1 magnification.25 The smooth outer coating of the capsule allows easy ingestion and prevents adhesion of intestinal contents, whereas the capsule moves via natural peristalsis from the mouth to the anus. Endoscopic images are transmitted via sensor arrays to a recording device worn as a belt by the patient. The recorded images are downloaded into a Reporting and Processing of Images and Data (RAPID) computer workstation and reviewed as a continuous video by the physician. The PillCam SB 2 video capsule offers advanced optics and a wider field of view for imaging the small bowel. It has the same dimensions as the PillCam SB and captures nearly twice the mucosal area per image compared with the PillCam SB. The PillCam SB 2 also provides Automatic Light Control for optimal illumination of each image.
Olympus (Olympus Corporation, Tokyo, Japan) has introduced a wireless capsule endoscope (EndoCapsule) with similar features. The Pillcam SB, Pillcam SB 2, and EndoCapsule have been approved by the U.S. Food and Drug Administration (FDA) (Fig. 15.6). Other small bowel capsule endoscopes available in the marketplace but not yet approved by the FDA are the OMOM pill (Jinshan Science & Technology, Chongqing, China) and the MiroCam (Intromedic, Seoul, Korea).
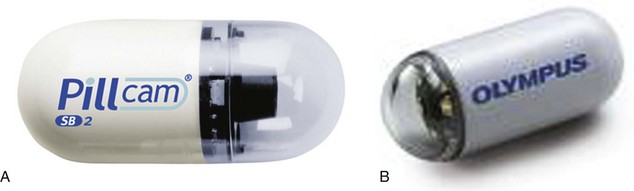
Fig. 15.6 A, Capsule endoscope (Pillcam SB 2). B, Capsule endoscope (Olympus EndoCapsule).
(A, Courtesy of Given Imaging Ltd, Yoqneam, Israel; B, courtesy of Olympus Corporation, Tokyo, Japan.)
Compared with other diagnostic modalities for evaluating obscure GI bleeding (e.g., small bowel series and enteroclysis, push enteroscopy, IOE), CE has been shown to have very good test characteristics and diagnostic yields in the range of 63% to 74%.2 Patient selection is important and seems to influence the diagnostic yield of CE. CE performed close to the time of the bleeding event, hemoglobin less than 10 g/dL, recurrent episodes of bleeding, or persistent bleeding (>6 months) may increase CE diagnostic yield.2 Limitations of CE include that at the present time biopsy, therapy, and endoscopic marking (e.g., India ink tattooing) are impossible. In addition, not all CE examinations reach the cecum (complete small bowel examination occurs approximately 80% to 85% of the time), and in some patients, luminal debris and bubbles interfere with viewing. Some physicians use polyethylene glycol–based preparations, prokinetic agents, simethicone, or a combination of these products before capsule ingestion.26 Other limitations are that the imaging cannot be controlled or localized, and images are not viewed in real time.
Some patients may be unsuitable candidates for CE, including patients with cardiac pacemakers, patients with defibrillators, and patients with suspected small bowel obstruction. However, small case series suggest that CE, when performed with careful patient monitoring, is safe in patients with pacemakers and cardiac defibrillators.2 Patients with swallowing disorders may have the capsule placed endoscopically into the duodenum using a capsule delivery device. Capsule retention is the major, and for all practical purposes, the only complication of CE. The reported incidence of capsule retention ranges from 0% in healthy volunteers, to 1.4% in patients with obscure GI hemorrhage, to 21% in patients with suspected small bowel obstruction.27
Double-Balloon Enteroscopy
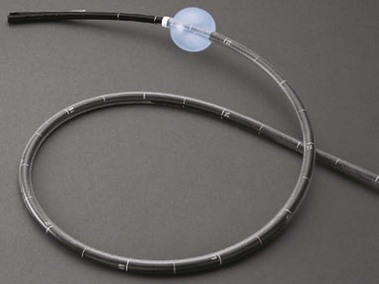
Double-balloon enteroscopy (DBE), initially described and reported by Yamamoto and colleagues,28 is a novel endoscopic insertion technique that attempts to improve on currently available endoscopic insertion methods to evaluate the entire length of the small bowel. The DBE system (Fujinon Inc, Saitama, Japan), uses a high-resolution, dedicated videoendoscope that has a working length of 200 cm and two soft, latex balloons: One balloon is attached to the tip of the endoscope, and the other is attached to the distal end of a soft, flexible overtube (Fig. 15.7). The balloons can be inflated and deflated using an air pump that is controlled by the endoscopist while monitoring air pressure.29 The balloons grip the wall of the bowel, allowing the endoscope to be advanced without looping.
The procedure can be performed via an oral or anal approach with or without fluoroscopic guidance. Choice of oral or transanal approach may be dictated by suspicion for the location of a possible lesion as determined by preceding small bowel CE or other nonendoscopic small bowel imaging technique.30,31 In a peroral approach, when the two balloons reach the duodenum, the overtube balloon is inflated to fix the overtube to the small bowel wall. The overtube is held in place as the endoscope is inserted further. Once the tip of the endoscope is maximally inserted, the balloon on the tip of the endoscope is inflated, the balloon on the overtube is deflated, and the overtube is advanced over the shaft of the endoscope. When the distal end of the overtube reaches the tip of the endoscope, the overtube balloon is reinflated, again fixing the overtube to a second point on the small bowel wall. This sequence is repeated until the entire small bowel is evaluated or further advancement of the endoscope is difficult.32
Two types of double-balloon enteroscopes are available: one for general diagnostic use (EN-450P5) and one for therapeutic use (EN-450T5). The EN-450P5 is a thinner endoscope with an external diameter of 8.5 mm and maximum working channel diameter of 2.2 mm. The EN450T5 has an external diameter of 9.4 mm and working channel of 2.8 mm.29 DBE has been shown to be able to visualize the entire length of the small bowel and allows for biopsy, marking of lesions for subsequent surgical resection (e.g., India ink tattoo), and therapeutics.
Published studies evaluating DBE for obscure GI bleeding have reported diagnostic yields ranging from 40% to 80% and diagnostic or treatment success in 43% to 76% of cases.2 Limitations of DBE include concerns about the endoscopic learning curve, common need for endoscopy on two separate days (peroral followed by transanal approach), limitations in visualization of the entire small bowel, miss rates for submucosal lesions owing to insufflation issues, time-consuming procedure that also requires a high level of ancillary staffing, increased sedation requirements (including possible anesthesiology support), and patient tolerance and preferences. Although initial reports of complete evaluation of the small bowel using DBE were encouraging, subsequent studies have failed to show similar results.2 In addition, although uncommon, the reported incidence of severe complications associated with DBE has ranged from 0% to 2.5% and has included pancreatitis, perforations, bleeding, abdominal pain, and fever.29
Single-Balloon Enteroscopy
A novel balloon enteroscope system has been developed more recently using only a single balloon (single-balloon enteroscopy [SBE]) (Fig. 15.8). The endoscopist needs to manipulate only one balloon, and time and complexity for preparation of the system and for the examination itself may be reduced.33 Data for the SBE system to compare its performance and safety profile with DBE are sparse.34 In the limited literature available, SBE seems to provide similar diagnostic and therapeutic yield compared with DBE.35,36 SBE is performed using the SBE endoscope system (SIF-Q180, Olympus Optical Co, Ltd, Tokyo, Japan). The SBE endoscope consists of a 200-cm long videoendoscope with an outer diameter of 9.2 mm and a flexible overtube with a length of 140 cm and an outer diameter of 13.2 mm. One single silicone balloon is attached to the tip of the overtube. The insertion process follows the method used for DBE, but instead of inflation of the endoscope balloon, the tip of the endoscope may be optionally angulated during the pullback or straightening of the enteroscope and balloon-inflated overtube.37 Similar to DBE, SBE can be performed via an oral or transanal approach, according to suspicion for the location of a possible small bowel lesion.
Spiral Enteroscopy
Spiral enteroscopy is an alternative technique for accomplishing deep small bowel intubation that uses a special overtube (Endo-Ease, Discovery Small Bowel (DSB), Spirus Medical) to pleat the small bowel onto itself (Fig. 15.9).38 The DSB overtube can be paired with existing enteroscopes or colonoscopes. Rotation of the DSB beyond the ligament of Treitz allows the manually rotating overtube with an outer fixed spiral coil to pleat the small bowel onto the overtube. Preliminary data suggest that use of the Endo-Ease DSB overtube for enteroscopy is a safe and effective technique for visualization of the small bowel.39 Studies of spiral enteroscopy have shown diagnostic yield, total time of procedure, and depth of small bowel insertion to compare favorably with DBE and SBE.40 The strengths of spiral enteroscopy are rapid advancement in the small bowel and controlled, stable withdrawal that facilitates endoscopic therapy.
Intraoperative Enteroscopy
Exploratory laparotomy with IOE has been used since the 1980s and is a diagnostic and potentially therapeutic endoscopic modality in suspected small bowel disease.41,42 IOE is considered to be the ultimate endoscopic evaluation of the small bowel. In addition to being able to diagnose small bowel sources of obscure bleeding, IOE allows for identification of lesions for definitive surgical resection. Using an orally passed colonoscope or, more optimally, a dedicated small bowel videoenteroscope, the endoscope is either advanced beyond the ligament of Treitz into the proximal jejunum or advanced into a mid–small bowel enterotomy. The latter technique expedites the examination, especially of the distal small bowel, and minimizes the potential for iatrogenic trauma to the small bowel arising from excessive manipulation that can arise from peroral passage. At that point, the surgeon gently telescopes the small bowel over the shaft of the endoscope, allowing for the careful inspection of the mucosa.
Reported complications associated with IOE include mucosal lacerations, perforations, prolonged ileus, abdominal abscess, and bowel ischemia.41,42 Because of its invasive nature and potential for complications, the decision to perform IOE must not be made lightly. All risks and benefits need to be fully considered and explained well to the patient; only experienced endoscopists and surgeons should perform this procedure. Currently, this technique is reserved as a last option if deep enteroscopy using DBE, SBE, or spiral enteroscopy cannot be performed successfully because of the presence of abdominal adhesions, obesity, or other technical factors.
Small Bowel Contrast Radiography
A small bowel series (upper GI series with small bowel follow-through) involves the oral ingestion of a dilute barium solution with serial abdominal images obtained of the small bowel. Enteroclysis is a double-contrast study performed by passing a tube into the proximal small bowel and injecting barium, methylcellulose, and air.43 This technique is considered to be superior to standard small bowel follow-through.44 An upper GI series with small bowel follow-through is sometimes used to evaluate obscure GI bleeding either before performing push enteroscopy or after a negative push enteroscopy examination.
The role of small bowel series and enteroclysis in the evaluation of obscure GI bleeding has declined substantially because of low diagnostic yield (0% to 6% for small bowel follow-through and 10% to 25% for enteroclysis) and the advent of improved endoscopic diagnostic techniques for the small bowel.45,46 In addition to limited diagnostic sensitivity in obscure GI bleeding, enteroclysis can be uncomfortable for the patient and involves more radiation exposure than small bowel follow-through. Generally, the role of enteroclysis in the evaluation of obscure GI bleeding is limited to settings in which CE and enteroscopy are unavailable or contraindicated, such as clinical situations that suggest small bowel obstruction secondary to malignancy, Crohn’s disease, or prior significant NSAID use. Otherwise, there is little or no role today for either small bowel series or enteroclysis in the evaluation of obscure GI bleeding.
Cross-Sectional Imaging
Novel cross-sectional imaging techniques for evaluation of the small bowel include helical CT enteroclysis, helical CT angiography, and MR enteroclysis.2 Helical CT enteroclysis combines standard enteroclysis to distend the small bowel followed by helical CT.47 More recent reports suggest the utility of helical CT in identifying small and large intestinal bleeding angiectasias.48,49 Further research and clinical experience should define the precise role of CT enteroclysis in the investigation of small bowel disease.
CT angiography involves catheterization of the abdominal aorta followed by helical CT angiography before and after intraarterial injections of a contrast medium. The site of hemorrhage is recognized as a hyperdense area owing to the extravasation of contrast medium into the intestinal lumen. In a prospective study of 18 patients with bleeding colonic angiectasis, the sensitivity, specificity, and positive predictive value of helical CT angiography were 70%, 100%, and 100% compared with a “gold standard” of colonoscopy and mesenteric angiography.50 In a report of 22 patients with obscure GI bleeding, CT enteroclysis was found to be inferior to CE in the detection of potential bleeding lesions such as angiectasis in the small bowel.51
Nuclear Medicine Scans
Tagged RBC scans are noninvasive, safe, and readily available. Most commonly performed with technetium-labeled RBCs, a radionuclide bleeding scan detects bleeding that is occurring at a rate of 0.1 to 0.5 mL/min. It is of little or no value in patients with obscure-occult bleeding who appear to have a low rate of blood loss because the rate of bleeding is too slow to be detected. Bleeding scans may be more sensitive than angiography but less specific than either a positive endoscopic or angiographic examination.52,53 Early scans (up to 4 hours after initial injection) may be helpful in gross localization of bleeding when the rate of blood loss exceeds 0.1 to 0.5 mL/min. Tagged RBC scans can identify only a general area of bleeding, however, and assessment with other diagnostic modalities such as angiography is often necessary after nuclear scans. The role of nuclear scans continues to be limited in patients with obscure GI bleeding, and the ability to localize the source of bleeding accurately is poor.54
Angiography
The data on the clinical utility of angiography in the setting of obscure GI bleeding are very limited.55 Compared with tagged RBC scans, mesenteric angiography is more likely to document the specific site of bleeding, yet the rate of bleeding must be greater than 0.5 mL/min. Angiography can also identify lesions that are not actively bleeding because of demonstration of typical vascular features seen in vascular ectasia (e.g., slow-emptying veins, vascular tuft, and early-filling veins) and tumors. It is possible to administer embolization therapy if an amenable lesion is detected.
Provocation angiography using anticoagulation, vasodilators, or thrombolytic agents may increase the likelihood that a source of bleeding can be identified and has been advocated by some investigators.56,57 However, the risk of inducing uncontrolled bleeding limits the use of this technique. As noted with tagged RBC scans, the utility of mesenteric angiography in the evaluation of patients with obscure GI bleeding is limited, and it is not commonly recommended.
Proposed Diagnostic Strategy
In a patient with obscure GI bleeding, defined as careful, quality endoscopic examinations (EGD plus ileocolonoscopy) being negative, the small bowel should be assumed to be the source of blood loss. Unless contraindicated, CE should be the next method of small bowel evaluation (see Fig. 15.4).2,58 In patients with obscure overt bleeding, data support the role of expedited CE.12,59 CE performed closer to the episode of obscure overt bleeding has a higher likelihood of finding the site of small bowel bleeding.12,59 If CE defines a small bowel source of obscure bleeding, appropriate specific therapy may be instituted (e.g., endoscopic, surgical, or pharmacologic). If no small bowel source is identified, a decision must be made as to whether additional diagnostic evaluations are immediately indicated (e.g., repeat EGD with or without ileocolonoscopy, repeat CE, Meckel’s scan in pediatric and young adult patients) or a period of “watchful waiting” may be warranted.
Pharmacologic Therapy
Pharmacotherapy should be considered whenever endoscopic therapy, surgical intervention, or angiographic therapy is impractical or ineffective, such as in patients in whom the source and the etiology of bleeding remains undefined or the underlying bleeding pathology is too diffuse to be amenable to ablative therapies. The role of hormonal therapy continues to be most debated.60,61 Somatostatin and its analogue octreotide have been anecdotally reported to be beneficial in patients with obscure GI bleeding from angiectasis and blue rubber bleb nevus syndrome, possibly secondary to their inhibitory effect on angiogenesis and splanchnic blood flow.62–64 A beneficial effect of thalidomide and its antiangiogenic effects in patients with obscure GI bleeding secondary to angiectasis and hereditary hemorrhagic telangiectasia has also been reported.65,66 In a case report, erythropoietin was believed to stop chronic diffuse hemorrhage from the GI mucosa. The exact mechanism of erythropoietin is unknown but may be related to the complex effect of erythropoietin on the platelet-subendothelial interactions and on protein C, protein S, and anti–thrombin III levels.67
1 Leighton JA, Goldstein J, Hirota W, et al. Obscure gastrointestinal bleeding. Gastrointest Endosc. 2003;58:650-655.
2 Raju GS, Gerson L, Das A, et al. American Gastroenterological Association (AGA) Institute technical review on obscure gastrointestinal bleeding. Gastroenterology. 2007;133:1697-1717.
3 Katz LB. The role of surgery in occult gastrointestinal bleeding. Semin Gastrointest Dis. 1999;10:78-81.
4 Lewis BS, Kornbluth A, Waye JD. Small bowel tumours: yield of enteroscopy. Gut. 1991;32:763-765.
5 Bartram CI, Amess JA. The diagnosis of Meckel’s diverticulum by small bowel enema in the investigation of obscure intestinal bleeding. Br J Surg. 1980;67:417-418.
6 Kwo PY, Tremaine WJ. Nonsteroidal anti-inflammatory drug-induced enteropathy: Case discussion and review of the literature. Mayo Clin Proc. 1995;70:55-61.
7 Risti B, Marincek B, Jost R, et al. Hemosuccus pancreaticus as a source of obscure upper gastrointestinal bleeding: Three cases and literature review. Am J Gastroenterol. 1995;90:1878-1880.
8 Bhatt BD, Cappell MS, Smilow PC, et al. Recurrent massive upper gastrointestinal hemorrhage due to Strongyloides stercoralis infection. Am J Gastroenterol. 1990;85:1034-1036.
9 Taverner D, Talbot IC, Carr-Locke DL, et al. Massive bleeding from the ileum: A late complication of pelvic radiotherapy. Am J Gastroenterol. 1982;77:29-31.
10 Morgan AA. Recurrent gastrointestinal hemorrhage: An unusual cause. Am J Gastroenterol. 1982;77:925-928.
11 Blecker D, Bansal M, Zimmerman RL, et al. Dieulafoy’s lesion of the small bowel causing massive gastrointestinal bleeding: Two case reports and literature review. Am J Gastroenterol. 2001;96:902-905.
12 Pennazio M, Eisen G, Goldfarb N. ICCE consensus for obscure gastrointestinal bleeding. Endoscopy. 2005;37:1046-1050.
13 Chak A, Koehler MK, Sundaram SN, et al. Diagnostic and therapeutic impact of push enteroscopy: Analysis of factors associated with positive findings. Gastrointest Endosc. 1998;47:18-22.
14 Zaman A, Katon RM. Push enteroscopy for obscure gastrointestinal bleeding yields a high incidence of proximal lesions within reach of a standard endoscope. Gastrointest Endosc. 1998;47:372-376.
15 Mullan FJ, McKelvey ST. Pancreatic carcinoma presenting as bleeding from segmental gastric varices: Pitfalls in diagnosis. Postgrad Med J. 1990;66:401-403.
16 Lee YT, Walmsley RS, Leong RW, et al. Dieulafoy’s lesion. Gastrointest Endosc. 2003;58:236-243.
17 Leaper M, Johnston MJ, Barclay M, et al. Reasons for failure to diagnose colorectal carcinoma at colonoscopy. Endoscopy. 2004;36:499-503.
18 Foutch PG, Sawyer R, Sanowski RA. Push-enteroscopy for diagnosis of patients with gastrointestinal bleeding of obscure origin. Gastrointest Endosc. 1990;36:337-341.
19 Taylor AC, Chen RY, Desmond PV. Use of an overtube for enteroscopy—does it increase depth of insertion? A prospective study of enteroscopy with and without an overtube. Endoscopy. 2001;33:227-230.
20 Gralnek IM. Obscure-overt gastrointestinal bleeding. Gastroenterology. 2005;128:1424-1430.
21 Eisen GM, Dominitz JA, Faigel DO, et al. Enteroscopy. Gastrointest Endosc. 2001;53:871-873.
22 Lewis BS, Swain P. Capsule endoscopy in the evaluation of patients with suspected small intestinal bleeding: Results of a pilot study. Gastrointest Endosc. 2002;56:349-353.
23 Lewis B, Goldfarb N. The advent of capsule endoscopy—a not-so-futuristic approach to obscure gastrointestinal bleeding. Aliment Pharmacol Ther. 2003;17:1085-1096.
24 Mishkin DS, Chuttani R, Croffie J, et al. ASGE Technology Status Evaluation Report: Wireless capsule endoscopy. Gastrointest Endosc. 2006;63:539-545.
25 Eliakim R. Video capsule endoscopy of the small bowel. Curr Opin Gastroenterol. 2008;24:159-163.
26 Niv Y. Efficiency of bowel preparation for capsule endoscopy examination: A meta-analysis. World J Gastroenterol. 2008;14:1313-1317.
27 Eliakim AR. Video capsule endoscopy of the small bowel (PillCam SB). Curr Opin Gastroenterol. 2006;22:124-127.
28 Yamamoto H, Sekine Y, Sato Y, et al. Total enteroscopy with a nonsurgical steerable double-balloon method. Gastrointest Endosc. 2001;53:216-220.
29 Akahoshi K, Kubokawa M, Matsumoto M, et al. Double-balloon endoscopy in the diagnosis and management of GI tract diseases: Methodology, indications, safety, and clinical impact. World J Gastroenterol. 2006;12:7654-7659.
30 Manabe N, Tanaka S, Fukumoto A, et al. Double-balloon enteroscopy in patients with GI bleeding of obscure origin. Gastrointest Endosc. 2006;64:135-140.
31 Gerson LB. Double-balloon enteroscopy: The new gold standard for small-bowel imaging? Gastrointest Endosc. 2005;62:71-75.
32 Hsu CM, Chiu CT, Su MY, et al. The outcome assessment of double-balloon enteroscopy for diagnosing and managing patients with obscure gastrointestinal bleeding. Dig Dis Sci. 2007;52:162-166.
33 Tsujikawa T, Saitoh Y, Andoh A, et al. Novel single-balloon enteroscopy for diagnosis and treatment of the small intestine: preliminary experiences. Endoscopy. 2008;40:11-15.
34 Tominaga K, Iida T, Nakamura Y, et al. Small intestinal perforation of endoscopically unrecognized lesions during peroral single-balloon enteroscopy. Endoscopy. 2008;40(Suppl 2):E213-E214.
35 Upchurch BR, Vargo JJ. Single-balloon enteroscopy. Gastrointest Endosc Clin N Am. 2009;19:335-347.
36 Ramchandani M, Reddy DN, Gupta R, et al. Diagnostic yield and therapeutic impact of single-balloon enteroscopy: Series of 106 cases. J Gastroenterol Hepatol. 2009;24:1631-1638.
37 Hartmann D, Eickhoff A, Tamm R, et al. Balloon-assisted enteroscopy using a single-balloon technique. Endoscopy. 2007;39(Suppl 1):E276.
38 Akerman PA, Agrawal D, Cantero D, et al. Spiral enteroscopy with the new DSB overtube: A novel technique for deep peroral small-bowel intubation. Endoscopy. 2008;40:974-978.
39 Akerman PA, Agrawal D, Chen W, et al. Spiral enteroscopy: A novel method of enteroscopy by using the Endo-Ease Discovery SB overtube and a pediatric colonoscope. Gastrointest Endosc. 2009;69:327-332.
40 Buscaglia JM, Dunbar KB, Okolo PI3rd, et al. The spiral enteroscopy training initiative: results of a prospective study evaluating the Discovery SB overtube device during small bowel enteroscopy (with video). Endoscopy. 2009;41:194-199.
41 Zaman A, Sheppard B, Katon RM. Total peroral intraoperative enteroscopy for obscure GI bleeding using a dedicated push enteroscope: Diagnostic yield and patient outcome. Gastrointest Endosc. 1999;50:506-510.
42 Kovacs TO, Jensen DM. Recent advances in the endoscopic diagnosis and therapy of upper gastrointestinal, small intestinal, and colonic bleeding. Med Clin North Am. 2002;86:1319-1356.
43 Aliperti G, Zuckerman GR, Willis JR, et al. Enteroscopy with enteroclysis. Gastrointest Endosc Clin N Am. 1996;6:803-810.
44 Vallance R. An evaluation of the small bowel enema based on an analysis of 350 consecutive examinations. Clin Radiol. 1980;31:227-232.
45 Fried AM, Poulos A, Hatfield DR. The effectiveness of the incidental small-bowel series. Radiology. 1981;140:45-46.
46 Rabe FE, Becker GJ, Besozzi MJ, et al. Efficacy study of the small-bowel examination. Radiology. 1981;140:47-50.
47 Rajesh A, Maglinte DD. Multislice CT enteroclysis: Technique and clinical applications. Clin Radiol. 2006;61:31-39.
48 Schmidt S, Lepori D, Meuwly JY, et al. Prospective comparison of MR enteroclysis with multidetector spiral-CT enteroclysis: interobserver agreement and sensitivity by means of “sign-by-sign” correlation. Eur Radiol. 2003;13:1303-1311.
49 Grassi R, di Mizio R, Romano S, et al. Multiple jejunal angiodysplasia detected by enema-helical CT. Clin Imaging. 2000;24:61-63.
50 Junquera F, Quiroga S, Saperas E, et al. Accuracy of helical computed tomographic angiography for the diagnosis of colonic angiodysplasia. Gastroenterology. 2000;119:293-299.
51 Voderholzer WA, Ortner M, Rogalla P, et al. Diagnostic yield of wireless capsule enteroscopy in comparison with computed tomography enteroclysis. Endoscopy. 2003;35:1009-1014.
52 Dusold R, Burke K, Carpentier W, et al. The accuracy of technetium-99m-labeled red cell scintigraphy in localizing gastrointestinal bleeding. Am J Gastroenterol. 1994;89:345-348.
53 Brunnler T, Klebl F, Mundorff S, et al. Significance of scintigraphy for the localisation of obscure gastrointestinal bleedings. World J Gastroenterol. 2008;14:5015-5019.
54 Howarth DM, Tang K, Lees W. The clinical utility of nuclear medicine imaging for the detection of occult gastrointestinal haemorrhage. Nucl Med Commun. 2002;23:591-594.
55 Rollins ES, Picus D, Hicks ME, et al. Angiography is useful in detecting the source of chronic gastrointestinal bleeding of obscure origin. AJR Am J Roentgenol. 1991;156:385-388.
56 Bloomfeld RS, Smith TP, Schneider AM, et al. Provocative angiography in patients with gastrointestinal hemorrhage of obscure origin. Am J Gastroenterol. 2000;95:2807-2812.
57 Berkelhammer C, Radvany A, Lin A, et al. Heparin provocation for endoscopic localization of recurrent obscure GI hemorrhage. Gastrointest Endosc. 2000;52:555-556.
58 Sidhu R, Sanders DS, Morris AJ, et al. Guidelines on small bowel enteroscopy and capsule endoscopy in adults. Gut. 2008;57:125-136.
59 Apostolopoulos P, Liatsos C, Gralnek IM, et al. Evaluation of capsule endoscopy in active, mild-to-moderate, overt, obscure GI bleeding. Gastrointest Endosc. 2007;66:1174-1181.
60 Grant EC. Avoid hormones in gastrointestinal angiodysplasia. Lancet. 2002;360:1254.
61 Hodgson H. Hormonal therapy for gastrointestinal angiodysplasia. Lancet. 2002;359:1630-1631.
62 Blich M, Fruchter O, Edelstein S, et al. Somatostatin therapy ameliorates chronic and refractory gastrointestinal bleeding caused by diffuse angiodysplasia in a patient on anticoagulation therapy. Scand J Gastroenterol. 2003;38:801-803.
63 Bowers M, McNulty O, Mayne E. Octreotide in the treatment of gastrointestinal bleeding caused by angiodysplasia in two patients with von Willebrand’s disease. Br J Haematol. 2000;108:524-527.
64 Gonzalez D, Elizondo BJ, Haslag S, et al. Chronic subcutaneous octreotide decreases gastrointestinal blood loss in blue rubber-bleb nevus syndrome. J Pediatr Gastroenterol Nutr. 2001;33:183-188.
65 Perez-Encinas M, Rabunal Martinez MJ, Bello Lopez JL. Is thalidomide effective for the treatment of gastrointestinal bleeding in hereditary hemorrhagic telangiectasia? Haematologica. 2002;87:ELT34.
66 Shurafa M, Kamboj G. Thalidomide for the treatment of bleeding angiodysplasias. Am J Gastroenterol. 2003;98:221-222.
67 Zaharia-Czeizler V. Erythropoietin stops chronic diffuse transfusion-dependent gastrointestinal bleeding. Ann Intern Med. 2001;135:933.