CHAPTER 6 Obesity
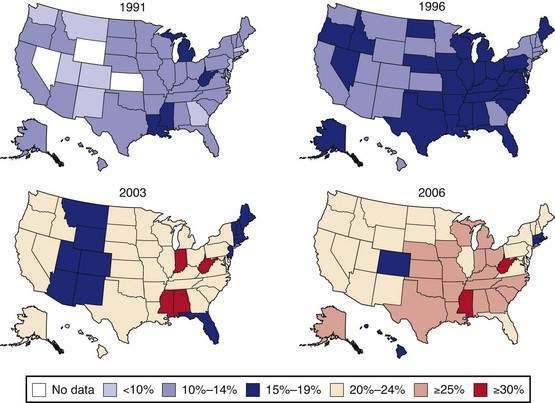
Obesity is a chronic disease that is increasing in prevalence. Both the World Health Organization1 and the National Heart, Lung, and Blood Institute2 of the U.S. National Institutes of Health have labeled obesity an epidemic. More than 30% of adult Americans are now obese3 and the prevalence of obesity in children and adults has increased more than 50% in the past decade. The progress of this epidemic is shown in maps indicating obesity rates in all states and regions of the United States. In 1991, 18% of the states (9/50) had obesity rates exceeding 15%, and by 1998 this rate had increased to 78% (39/50; Fig. 6-1). This epidemic is a time bomb for the future development of diabetes and its many complications.4
Data of the Behavioral Risk Factor Surveillance System. (From the Centers for Disease Control and Prevention. U.S. obesity trends 1985-2007. Available at http://www.cdc.gov/nccdphp/dnpa/obesity/trend/maps/index.htm.)
As a disease, obesity has its pathology rooted in the enlargement of fat cells, the secretory products of which produce most of the pathogenic changes that result in the complications associated with obesity. The remaining changes are a consequence of the fat mass per se.4 Physicians and the health care system have two strategies to deal with this problem. First, we can prevent the development of obesity, or treat it before complications develop. Alternatively, we can wait until complications develop and then treat these. Many physicians would prefer to wait until the comorbidities arise, because there are more treatment options for diabetes, hypertension, and heart disease than for obesity. In one long-term trial, the incidence of new cases of diabetes was reduced to zero over two years in patients who lost weight and maintained a weight loss of 12% or more, compared with an incidence of 8.5% for new cases of diabetes in those who did not lose weight.5
Obesity is a stigmatized disease.4 A common view shared by the public and by health professionals alike is that obese people are lazy and weak-willed. It is not unusual to hear statements such as, “If fat people just had will power, they would push themselves away from the table and not be so fat.” This view is supported by the perception in advertising that thin women are more attractive than full-figured women; the declining relative weights of center-fold models in Playboy and other publications and of women who are winners of the Miss America contest substantiate this view.
Another issue that aggravates the problem of treating obesity is the negative perception that surrounds the use of appetite suppressants.4 Amphetamine, the first widely used weight loss drug, is addictive. This concern about addiction has been transferred to other drugs, whether warranted or not.
With all treatments for obesity, weight loss slows and then stops. This so-called plateau effect arises when homeostatic mechanisms in the body come into play and stabilize weight, although at a lower level than the starting level. A similar phenomenon also is observed in the treatment of hypertension; when an antihypertensive drug produces a drop in blood pressure, there is a plateau at a new, lower level.3 The antihypertensive drug has not lost its effect when the plateau occurs, but its effect is being counteracted by physiologic mechanisms. In the treatment of obesity, this new plateau in body weight often is viewed as a therapeutic failure for the weight loss drug or other treatment. Cessation of weight loss often prompts patients to think that they are “cured” and they stop treatment only to regain weight.
Finally, many treatments for obesity have provided unwanted side effects.4 A recent example is what befell many patients who took the combination of fenfluramine and phentermine. The aortic regurgitant lesions that occurred in up to 25% of the patients treated with this combination of drugs led many physicians to say “I told you so” and “I’m certainly glad I didn’t use those drugs.” Much of this suspicion will subside with time, but there will remain residual concerns about such potential side effects of antiobesity agents among physicians, government regulators, and the public.
DEFINITION
BODY MASS INDEX
Throughout the past 50 years, there has been a steady rightward, upward shift in the distribution curve for body weight. This trend can be traced most effectively using the body mass index (BMI), defined as the weight in kilograms divided by the height (in meters) squared [W/(H)2], which provides a useful operating definition of overweight. A normal BMI is between 18.5 and 25 kg/m2. A BMI between 25 and 29.9 kg/m2 is operationally defined as overweight, and individuals with a BMI higher than 30 kg/m2 are obese, with special consideration for muscle builders and other resistance-trained athletes. Above a BMI of 30 kg/m2, BMI is specific, but not very sensitive when compared with body fat for determining obesity.6 Values below a BMI of 30 kg/m2 are less specific, but still represents a good starting point in evaluating overweight people. BMI also provides a good risk measure for obesity.1,2,4
CENTRAL ADIPOSITY
The waist circumference is a practical measure of central adiposity that is a surrogate for more precise measures of visceral fat, such as computed tomography (CT) or magnetic resonance imaging (MRI) scans of the abdomen. When BMI and waist circumference were used to predict the risk of hypertension, dyslipidemia, and the metabolic syndrome, the waist circumference was shown to be a better predictor than the BMI.7
PREVALENCE AND COSTS
Using the BMI, it is clear that obesity is increasing in prevalence. This increase began in the 1980s and continues at present, although recent data suggest that it may be abating somewhat.3 Obesity affects children as well as adults. We are now seeing a rise in the prevalence of type 2 diabetes in adolescents that is directly related to the rising prevalence of obesity. Obesity has a higher prevalence in Hispanic and African American populations.8,9 Both mean height and weight increased in adults ages 20 to 74 years between 1960 and 2002. Men increased in height from a mean of 68 inches (172.7 cm) to 69.5 inches (176.5 cm) and women from a mean of 63 to 64 inches (160 cm to 162.6 cm) during this period. For men, weight rose from a mean of 166.3 to 191 pounds (75.4 to 86.8 kg) and for women from a mean 140.2 to 164.3 pounds (63.7 to 74.6 kg), for an average increase of BMI from 25.2 to 28 kg/m2 for men and from 24.8 to 28.2 kg/m2 for women during this 42-year period. The increase in weight was greater in older men than in younger men, but the reverse was true for women, with older women gaining less weight than younger women. Similar effects are seen in children, with the weight of 10-year-old boys rising from a mean of 74.2 pounds (33.7 kg) in 1963 to 85 pounds (38.6 kg) in 2002, and of 10-year-old girls rising from a mean of 77.4 pounds (35.2 kg) to 88 pounds (40 kg) in this same interval. These increases in weight were associated with increases in BMI for boys and girls. For 7-year-old boys, BMI increased from a mean of 15.8 to 17.0 kg/m2 between 1963 and 2002 and for 7-year-old girls it rose from a mean of 15.8 to 16.6 kg/m2. For 16-year-old boys, it rose from 21.3 to 24.1 kg/m2 and for girls, from 21.9 to 24.0 kg/m2 in this same interval.
Obesity is expensive, representing between 3% and 8% of health care budgets in various countries.4,10 Hospital costs and use of medication also escalate with increasing BMI. In a large health maintenance organization, mean annual costs were 25% higher in participants with a BMI between 30 and 35 kg/m2 and 44% higher in those with a BMI greater than 35 kg/m2 than in those with a BMI between 20 and 25 kg/m2.11 Costs for lifetime treatment of hypertension, hypercholesterolemia, type 2 diabetes, heart disease, and stroke in men and women with a BMI of 37.5 kg/m2 was $10,000 higher than for men and women with a BMI of 22.5 kg/m2, according to data from the National Center for Health Statistics and the Framingham Heart Study.12
CAUSES
ENERGY IMBALANCE: EPIDEMIOLOGIC MODEL
We become obese because over an extended period of time, we ingest more carbon- and nitrogen-containing compounds as food than we need for daily energy expenditure. We and other animals thus obey the First Law of Thermodynamics, which describes this energy imbalance. Unfortunately, however, this important law of nature fails to inform us about such important issues as how food intake is regulated, where fat is stored, why men store fat differently than women, and how genes control these processes.4,13
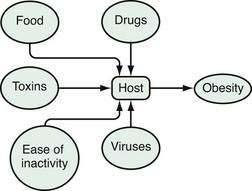
An epidemiologic model is a good way to look at energy balance and to conceptualize obesity as a disease.14 In an epidemiologic model, environmental agents act on a host to produce a disease (Fig. 6-2). Disease is a function of the virulence of the agent and susceptibility of the host. For obesity, the environmental agents include drugs, food, toxins, physical inactivity, viruses, and other people.4,13,15 Among the important drugs that cause weight gains are some antipsychotic drugs (e.g., clozapine, olanzapine), some antidepressants (e.g., amitriptyline), some antidiabetic drugs (insulin, sulfonylureas, and thiazolidinediones), and glucocorticoids (Table 6-1). Although neurotropic viruses can produce obesity in experimental animals, the most intriguing human example is the presence of antibodies to adenovirus 36 in some obese people, and the fact that this virus can produce obesity in marmosets, a nonhuman primate. Exposure to estrogen-like compounds in utero may enhance the risk of obesity later in life. In affluent Western societies, foods, particularly foods high in fat, are abundant. In addition, portion sizes have increased, providing more energy to people with each portion. Toxins are an interesting potential group of agents for which more research is needed. Viruses are known to produce obesity and their potential role in obesity needs to be studied further. Physical activity may have gradually decreased, thereby reducing energy expenditure. Some have described the current environment as a virulent or toxic environment that has heightened the risk for obesity for people who are genetically susceptible to becoming obese.15 For the genetically susceptible host, this excess of food energy, environmental toxins, and viruses, along with a reduced level of physical activity, may lead to an accumulation of fat in fat cells.
Table 6-1 Drugs That Produce Weight Gain and Potential Alternatives
| CATEGORY | DRUGS THAT CAUSE WEIGHT GAIN | POTENTIAL ALTERNATIVES |
|---|---|---|
| Neuroleptics | Thioridazine, olanzepine, quetiapine, resperidone, clozapine | Molindone, haloperidol, ziprasodone |
| Antidepressants | ||
| Tricyclics | Amitriptyline, nortriptyline | Protriptyline |
| Monoamine oxidase inhibitors | Imipramine, mitrazapine | Bupropion, nefazadone |
| Selective serotonin-reuptake inhibitors | Paroxetine | Fluoxetine, sertraline |
| Anticonvulsants | Valproate, carbamazepine, gabapentin | Topiramate, lamotrigine, zonisamide |
| Antidiabetic drugs | Insulin, sulfonylureas, thiazolidinediones | Acarbose, miglitol, metformin, pramlintide exenatide |
| Antiserotonin agents | Pizotifen | — |
| Antihistamines | Cyproheptadine | Inhalers, decongestants |
ENVIRONMENTAL AGENTS
Intrauterine Factors
Several environmental intrauterine events influence postnatal weight and lifetime weight gain and fatness.4 These include maternal diabetes, maternal smoking,16 and intrauterine nutrition, all of which increase an individual’s risk for increased body weight and diabetes later in life. Offspring of mothers who smoke during pregnancy are at increased risk of weight gain in their first decades of life,16 as are infants of diabetic mothers and small-for-gestational-age infants.
Neonatal Environment
Infants who are breast-fed for more than three months may have a reduced risk of future obesity.17 Children who get more sleep tend to weigh less when they enter school than those who sleep less.4
Adiposity Rebound
Adiposity rebound occurs in childhood at the age that the BMI stops falling and begins to rise. Early adiposity rebound predicts future obesity.18
Drug-Induced Weight Gain
In our current practice of medicating much of society, it would not be surprising to find drugs that produce weight gain. Table 6-1 is a list of medications that produce weight gain when used to treat other diseases, such as psychosis, depression, allergies, and diabetes. Also listed in the table are possible alternatives that can be used to avoid such weight gain. In most cases, there are alternative strategies that can be used to treat a patient when weight gain is closely associated with the initiation of a new medication for one of these conditions. When the binding of drugs listed in Table 6-1 to receptors in the brain was examined, several—including the histamine (H1), α1A-adrenergic, and serotonin (5-HT2C and 5-HT6) receptors—could explain much of the differences in weight gain associated with the atypical antipsychotic drugs shown in Table 6-1.19
Diet
Portion size, fat intake, and consumption of beverages sweetened with sucrose (table sugar) or high-fructose corn syrup have all been implicated in the current obesity epidemic.4,20 Consumption of soft drinks predicted future weight gain in children and adults, and also may be related to the risk for the metabolic syndrome and gout.21
Physical Inactivity
Low levels of physical activity, such as watching television, correlate with weight gain. In a 10-year study of individuals 20 to 74 years of age in the National Health and Examination Survey (NHANES I), those with low levels of recreational activity gained more weight than those with higher levels of activity.22 Similarly, low levels of baseline energy expenditure predicted weight gain in the Pima Indians,23 and exercise capacity and body composition predict mortality among men with diabetes.24 Time spent watching television correlates with percentage of overweight children4 and the more television watched, the greater the risk of overweight and obesity.
Smoking
Smokers have lower body weights than nonsmokers, and cessation of smoking generally is associated with weight gain.4,25 Two explanations have been offered for the effect of smoking on body weight. First, smoking is thermogenic; that is, the metabolic rate during the act of smoking is higher than when the subject is not smoking. Second, smoking reduces hunger and changes taste perceptions; smokers tend to eat less.
Viruses
One laboratory has reported that obese humans have higher antibody levels to one strain of adenovirus (AM-36).26 As noted, this virus can produce obesity when given to nonhuman primates and this viral antibody, as a marker of viral infection, is found in the circulation of many obese people.
HOST AGENTS
Genetic Causes
Several genes have a strong relation to the development of obesity.27 The melanocortin-4 receptor gene, leptin gene, pro-opiomelanocortin (POMC) gene, and agouti gene all have significant effects on body fat and fat stores. There are five melanocortin receptors, MC4 and MC being primarily in areas of the brain that affect feeding. Genetic abnormalities in this receptor may account for up to 6% of cases in early-onset, severely obese children.28 Absence of leptin or an ineffective leptin receptor is associated with massive obesity in human beings and animals. Leptin has the dual effect of reducing food intake and increasing energy expenditure, both of which favor loss of body fat. Treatment of leptin-deficient children with leptin decreased their body weight and hunger, indicating the importance of leptin in normal subjects (see later, “Neurophysiologic Factors”). Heterozygotes for leptin deficiency have low but detectable serum leptin levels and have increased adiposity, indicating that low levels of leptin are associated with increased hunger and gain in body fat. Leptin also can increase energy expenditure. Thus, when leptin is given and caloric intake simultaneously is reduced, the leptin attenuates the decreases in thyroid hormones and 24-hour energy expenditure that result, because leptin normally falls with reduced caloric intake.29
There are several rare clinical syndromes of obesity with a genetic basis.30 The Prader-Willi syndrome is the most common.31 This disease is transmitted as an abnormality on chromosome 15 and is characterized by a floppy baby who has difficulty feeding. These children are mentally slow, short in stature, have hypotonia and hypogonadism, and are obese. The Bardet-Biedl syndrome can result from changes at many different genetic loci. These children have visual impairment caused by abnormalities in the retina, are mentally slow, and have increased numbers of digits (polydactylism). In at least one pedigree, the genetic defect was caused by a fault in the chaperonin-like gene, which produces a product involved in folding proteins in the reticular endoplasm.32
The epidemic of obesity is occurring on a genetic background that does not change as fast as the epidemic has been exploding. Nonetheless, it is clear that genetic factors play an important role in the development of obesity, for which more than 100 genes have so far been implicated.30
Neurophysiologic Factors
A number of peptides play an important role in the development of obesity.33 The discovery of leptin in 1994 opened a new window for understanding control of food intake and body weight. The response of leptin-deficient children to treatment with leptin revealed the critical role that this peptide plays in the control of energy balance.27 Leptin enters brain tissue, probably by transport across the blood-brain barrier, where it acts on receptors in the arcuate nucleus to regulate, in a conjugate fashion, the production and release of at least four peptides.34 Leptin inhibits the production of neuropeptide Y (NPY) and agouti-related peptide (AGRP), both of which increase food intake; it also enhances production of POMC, the source of α-melanocyte stimulating hormone (α-MSH), which reduces food intake.
Three other brain peptide systems also have been linked to the control of feeding. Melanin-concentrating hormone (MCH) is found in the lateral hypothalamus and decreases food intake when injected into the ventricular system of the brain.35 Orexin (also called hypocretin) was identified in a search for G protein-linked peptides that affect food intake34; it increases food intake and plays a role in sleep. Endocannabinoids (anandamide and 2-arachidonoyl glycerol) also increase food intake by acting on cannabinoid-1 (CB1) receptors. There are two cannabinoid receptors that originally were identified when tetrahydrocannabinol, the active ingredient of marijuana, was used to identify its endogenous receptor. These receptors are located in the brain and in many peripheral tissues. An antagonist to the CB1 receptor has served as the basis for a new antiobesity drug.36
Intestinal peptides, including cholecystokinin, pancreatic polypeptide, and polypeptide YY, reduce food intake,4 whereas ghrelin, a small peptide produced in the stomach, stimulates food intake.33
Metabolism of fatty acids in the brain may be another important control point. An experimental chemical that blocks fatty acid synthase in the brain was noted to result in significant weight loss and accumulation of malonyl coenzyme A. In pursuing this system, 5′-adenosine monophosphate kinase (AMPK), an enzyme that is activated or inhibited in relation to the ratio of adenosine monophosphate (AMP) to adenosine triphosphate (ATP), was thought perhaps to be the underlying central point in this control system.37
PATHOLOGY AND PATHOPHYSIOLOGY
The pathology and pathophysiology of obesity lie in the changes in the fat cells that store fat. Enlarged fat cells are the hallmark of this process. These enlarged cells produce effects through their increased mass, which increases the wear and tear on joints and makes overweight individuals obvious candidates for stigmatization. In addition, enlarged fat cells produce many adipokine products that affect distant cells. In addition to enlarged fat cells, some individuals also have an increased number of fat cells.14,38
FAT CELL AS AN ENDOCRINE CELL
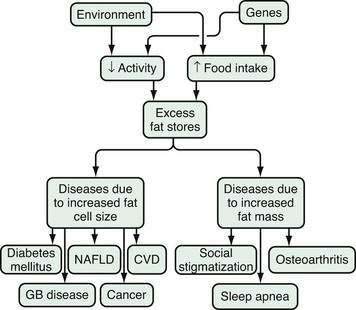
Two mechanisms are integral to understand the pathophysiology of health problems associated with obesity. The first is the increased fat mass, which explains the stigmatization of physically obvious obesity and accompanying osteoarthritis and sleep apnea (Fig. 6-3).14 The second is the consequences of the increased number of peptides produced by the enlarged fat cells that act on distant organs. The discovery of leptin catapulted the fat cell into the arena of endocrine cells. In addition to leptin, fat cells secrete, for example, cytokines, angiotensinogen, adipsin (complement D), and metabolites, such as free fatty acids and lactate. Angiotensinogen is an enzyme involved in the control of blood pressure. Adipsin was one of the first substances secreted by fat cells to be identified, and turned out to be a component of the coagulation system. In contrast to these fat cell products, the release of adiponectin, the most abundant peptide produced by fat cells, is decreased in obesity.39 High levels of adiponectin are associated with insulin sensitivity and low levels with insulin resistance. These products of the fat cell in turn modify the metabolic processes of other organs in the host. For the susceptible host, these metabolic changes lead in turn to hyperinsulinemia, atherosclerosis, hypertension, and physical stress on bones and joints.
VISCERAL FAT
A considerable body of data suggests that visceral fat has a stronger relationship with the complications associated with obesity than does total body fat.7 Moreover, central adiposity is one of the key components of the metabolic syndrome, the diagnostic criteria of which are based on the recommendations of the National Cholesterol Education Program Adult Treatment Panel III (Table 6-2).40,41
Table 6-2 National Cholesterol Education Program Adult Treatment Panel (ATP) III Modified Criteria for Metabolic Syndrome*
| PARAMETER | ATP III MODIFIED CRITERION |
|---|---|
| Waist circumference | |
| Female | >35 inches (>88 cm) |
| Male | >40 inches (>102 cm) |
| HDL cholesterol | |
| Female | <50 mg/dL (<1.29 mmol/L) |
| Male | <40 mg/dL (<1.03 mmol/L) |
| Fasting glucose | ≥110 mg/dL (≥6.2 mmol/L) |
| Triglycerides | ≥150 mg/dL |
| Blood pressure | ≥130/85 mm Hg |
HDL, high-density lipoprotein.
* The metabolic syndrome is present when any three of the five criteria listed in the table are abnormal.
Adapted from Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA 2001; 285:2486-97; and Park YW, Zhu S, Palaniappan L, et al. The metabolic syndrome: Prevalence and associated risk factor findings in the U.S. population from the Third National Health and Nutrition Examination Survey, 1988-1994. Arch Intern Med 2003; 163:427-36.
COMPLICATIONS AND CONSEQUENCES
DEATH
Mortality and BMI have a J-shaped relationship in essentially all studies.4 Among the more than 90,000 women in the Women’s Health Initiative, there was a graded increase in the risk of death as BMI increased from normal to a BMI > 40 kg/m2.42 In the more than 527,000 men and women in the NIH-AARP American cohort of individuals ages 50 to 71 years, the risk of death was increased both in those who were overweight and in those who were obese.43 In an even larger Korean study, both overweight and obesity in men and women were related to higher death rates compared with normal-weight subjects.44 Another American cohort of more than 80,000 men and women was monitored for over 14.7 years and over 1.23 million person-years of follow-up. Excluding the first five years of death in men and women, those younger than 55 years showed a risk of death that was directly related to BMI, beginning at a BMI of 21.0 kg/m2 in women and 23.0 kg/m2 in men; in those older than 55 years, the increase in mortality occurred at a higher BMI (25 kg/m2 in women and 30 kg/m2 in men).45
DISEASES AND DISORDERS
Disorders Related to Enlarged and Visceral Fat Cells
Excess body fat, particularly visceral fat, increases the risk for a number of diseases as a metabolic consequence of the enlarged fat cells or as a result of the increased mass of fat.4,14,46
Diabetes
The risk of diabetes rises as BMI increases and is particularly steep when the BMI is more than 30 kg/m2. Weight gain in middle age, independent of attained weight, increases the risk of impaired glucose tolerance45 and increases the risk of heart disease. Blood pressure increases linearly with BMI and hypertension is present in approximately half of very obese subjects at initial evaluation.47 In the Nurses Health Study, the BMI values at age 18 years and at midlife were positively associated with the occurrence of hypertension.4
Lipid Derangements
Dyslipidemia, characterized by a low high-density lipoprotein (HDL) cholesterol and high triglyceride level, is more common in obesity, particularly with central adiposity48 and, when accompanied by hypertension and an elevated serum glucose level, meet the National Cholesterol Education Program criteria for the so-called metabolic syndrome. A meta-analysis of 21 cohort studies has suggested that the adverse effects of obesity on blood pressure and lipids account for approximately half of the excess risk of coronary heart disease.49
Cardiovascular Diseases
Because coronary heart disease accounts for nearly half of all deaths in our society, its relationship to obesity is particularly important.50 In one study, an increase in BMI of 1.1 kg/m2 increased the risk for major cardiovascular disease by 6%.51 Obesity also increases the risk of congestive heart failure52 and atrial fibrillation.53 Much of this increased risk of heart disease is associated with central adiposity.7 The INTERHEART study of patients from 52 countries showed that abdominal adiposity accounts for 20% of the population’s attributable risk for a first myocardial infarction.54
Hypertension
Blood pressure often is increased in overweight individuals.55 In the Swedish Obese Subjects Study,48 hypertension was present at baseline in 44% to 51% of subjects. For each decline of 1 mm Hg in diastolic blood pressure, the risk of myocardial infarction decreased an estimated 2% to 3%. Obesity and hypertension interact with cardiac function. In overweight individuals, ventricular eccentric dilation occurs, whereas hypertension in normal-weight people produces concentric hypertrophy of the heart, with uniform thickening of ventricular walls. Increased preload and stroke work are associated with hypertension. The combination of overweight and hypertension leads to thickening of the ventricular wall and larger heart volume, and thus to a greater likelihood of cardiac failure.
Kidney Disease
Obesity may affect the kidney in several ways. First, an obesity-related glomerulopathy characterized as focal segmental glomerulosclerosis has increased significantly, from 0.2% of biopsies collected between 1986 and 1990 to 2.0% of specimens taken between 1996 and 2000.56 Second, overweight patients also are at increased risk for kidney stones.57 Finally, BMI is related to the risk of end-stage renal disease. In a study from the Kaiser Permanente Group of Northern California, Hsu and colleagues58 found that a higher BMI is a progressively greater risk factor for end-stage renal disease that persists even after correcting for multiple potential confounding factors, including baseline blood pressure or diabetes mellitus.
Gallbladder Disease
Cholelithiasis is the primary hepatobiliary pathology associated with overweight,59 part of the explanation for which is increased cholesterol turnover related to total body fat.60 Cholesterol production is linearly related to body fat; approximately 20 mg of additional cholesterol is synthesized for each kilogram of extra body fat. Thus, a 10-kg increase in body fat leads to the daily synthesis of as much cholesterol as contained in the yolk of one egg. The increased cholesterol is in turn excreted in the bile, where high cholesterol concentrations relative to bile acids and phospholipids increase the likelihood of precipitation of cholesterol gallstones in the gallbladder (see Chapter 65). Additional factors, however, such as nidation conditions, also are involved in determining whether gallstones form.60 During weight loss, the likelihood of gallstone formation increases because the flux of cholesterol mobilized from fat is increased through the biliary system. Diets with moderate levels of fat that trigger gallbladder contraction and thus empty its cholesterol content may reduce this risk. Similarly, the use of bile acids, such as ursodeoxycholic acid, may be advisable if the risk of gallstone formation is thought to be increased.
Liver Disease
Nonalcoholic fatty liver disease (NAFLD) is the term given to describe a constellation of liver abnormalities associated with overweight, including hepatomegaly, elevated liver biochemical test results, and abnormal liver histology, including steatosis, steatohepatitis, fibrosis, and cirrhosis (see Chapter 85).61 NAFLD may reflect increased very low-density lipoprotein (VLDL) production associated with hyperinsulinemia. A study using a cross-sectional analysis of liver biopsies has suggested that in overweight patients, the prevalences of steatosis, steatohepatitis, and cirrhosis are approximately 75%, 20%, and 2% respectively.62 Data from the Homeostasis Assessment Model (HOMA), a means used to develop mathematical models to describe glucose regulation, have shown that the more marked the insulin resistance, the higher the prevalence of severe steatosis.63 Using ultrasound for diagnosing increased liver fat, Hamaguchi and colleagues64 found that in a Japanese population there was a 10% incidence of new cases of NAFLD after a mean follow-up of 414 days, and that this was predicted by the presence of the metabolic syndrome. If increased fat in the liver is suspected, an ultrasound of the liver can provide a quantitative estimate that is much better than serum liver biochemical test results.
Gastroesophageal Reflux Disease
Overweight also may be a contributing factor in gastroesophageal reflux disease (GERD) (see Chapter 43). A total of nine studies examined the association of GERD with BMI,65 six of which showed a statistically significant association. Erosive esophagitis and esophageal adenocarcinoma also were more common in obesity. The odds ratio for GERD was 1.43 in the overweight group (BMI, 25 to 29.9 kg/m2) compared with the normal-weight group and rose to 1.94 when the BMI was higher than 30 kg/m2.66
Cancer
Certain forms of cancer are significantly increased in obesity.67–69 Obese men face increased risk for neoplasms of the colon, rectum, and prostate, whereas in women, cancers of the reproductive system and gallbladder are more common than in nonobese women. One explanation for the increased risk of endometrial cancer in overweight women is the increased production of estrogens by stromal cells in adipose tissue. This increased production is related to the degree of excess body fat and accounts for a major source of estrogen production in postmenopausal women. Breast cancer is not only related to total body fat, but also may have a more important relationship to central body fat,70 which may help explain why breast cancer risk is increased at age 75 in women in the highest versus the lowest quartile of BMI.71 Increased visceral fat as measured by CT shows an important relationship to the risk of breast cancer.
The Nurses Health Study has added significant insight to the relationship of body weight and breast cancer. Women who gained 25 kg or more after age 18 were at increased risk of breast cancer (relative risk [RR], 1.45; P < 0.001), and women who gained 10 kg or more after menopause were at increased risk for breast cancer compared with women whose weight remained stable. Women who achieved and maintained a 10-kg or more weight loss and who did not use postmenopausal hormones were at lower risk than those who maintained a stable weight.72 Finally, a pooling project with data from 13 cohort studies found that the relative risk of renal cell carcinoma was increased to 2.10 in those with a BMI > 30 kg/m2 compared with those with a BMI < 23 kg/m2.73
Endocrine Effects
A variety of endocrine changes are associated with obesity, including the polycystic ovary syndrome (PCOS), which is characterized by hirsutism, oligomenorrhea, and marked insulin resistance, hyperactivity of the adrenal glands (Cushing’s syndrome), and reduced fertility in men and women. In the Nurses Health Study, as BMI increased, the relative risk of infertility rose. Compared with the reference group, which had a BMI of 20 to 21.9 kg/m2, the relative risk of infertility was 1.7 for a BMI of 26 to 27.9 kg/m2 and 2.7 for a BMI above 30 kg/m2.74
Obesity influences the outcome of pregnancy. Increasing prepregnancy body weight was associated with a significant and weight-related increase in the likelihood of cesarean delivery. Infant pre-term birth weight was higher in smaller women. Heavier women had heavier babies, but no increased risk of low birth weight infants. Low birth weight infants were less likely in heavier women and also in those who gain more weight during their pregnancy.75 Weight gain of more than18.6 kg (41 pounds) also increases the risk of cesarean delivery. The risk of postpartum urinary tract infection also appears to be increased in overweight women, based on an observational study of 60,167 women.76
In a large retrospective study from Scotland, nulliparous women, compared with multiparous women, had increased elective preterm delivery, neonatal death, and infant weights less than 1000 g, and these effects were greatest in women with a BMI > 35 kg/m2.77
Pneumonia
Community-acquired pneumonia may be an additional risk related to being obese. In the Health Professionals Follow-up Study and the Nurses Health Study II, the risk of pneumonia increased as BMI increased.78 Significant weight gain in women after age 18 years also increased the risk of pneumonia.
Disorders Associated with Increased Fat Mass
Obstructive Sleep Apnea
The chief disturbance of pulmonary function in obese individuals results from a decrease in residual lung volume because of increased abdominal pressure on the diaphragm,79 although fat distribution, independent of total fat, also affects ventilatory capacity. In contrast to the relatively benign effects of excess weight on respiratory function, overweight often is associated with sleep apnea, which can be severe and associated with significant reduction in nocturnal oxygen saturation.79,80 Sleep apnea is considerably more common in men than in women. People with sleep apnea have an increased snoring index and increased maximal nocturnal sound intensity. An interesting hypothesis is that the increased neck circumference and fat deposits in the pharyngeal area may lead to the obstructive sleep apnea of overweight.
Diseases of the Bones, Joints, Muscles, Connective Tissue, and Skin
Osteoarthritis is significantly increased in overweight individuals. The osteoarthritis that develops in the knees and ankles may be directly related to the trauma associated with the degree of excess body weight,81 but the increased osteoarthritis in other non–weight-bearing joints suggests that some components of the overweight syndrome alter cartilage and bone metabolism, independent of weight bearing. Increased osteoarthritis accounts for a significant component of the health cost of being overweight. Increased body weight also produces disability from joint disease. Using the Behavioral Risk Factor Surveillance System telephone survey data on individuals older than 45 years, Okoro and colleagues82 found that class 3 overweight (BMI > 40 kg/m2) was associated with disability in individuals who reported arthritis and those who did not report arthritis. Even lighter weight obese individuals had an increased likelihood of disability compared with normal-weight respondents.83,84
Rheumatoid arthritis and BMI have a paradoxical relationship. In a study of rheumatoid arthritis that accrued 123 deaths in 3460 patient-years of observation, the BMI was found to be inversely related to mortality, although the study period was relatively short and the number of subjects relatively small.85
Several skin changes are associated with excess weight.86 Stretch marks, or striae, are common and reflect the pressure on the skin from expanding lobular deposits of fat. Acanthosis nigricans refers to a deepening of the pigmentation in the folds of the neck, knuckles, and extensor surfaces that occurs in many overweight individuals. In normal-weight individuals, this can be a sign of increased risk of malignancy or insulin resistance but, when associated with obesity, such risks are unusual. Hirsutism in obese women may reflect altered increased androgen production, which can impair menstrual cycles and ovulation.4
Psychosocial Dysfunction
Overweight is stigmatized in both children and adults87–89; that is, overweight individuals are exposed to the consequences of public disapproval of their fatness. The disapproval of obesity has, if anything, worsened over the past 40 years.90 Overweight children have a negative self-image and also a significant decrease in physical and social functioning compared with normal-weight children. A study using the Medical Outcomes Study Short-form Health Survey (SF-36) found that obese people had profound abnormalities in health-related quality of life.91 Overweight women appear to be at greater risk of psychological dysfunction, compared with overweight men, possibly because of increased societal pressures on women to be thin.92 A systematic review has shown that in four of eight studies that met criteria for inclusion in the investigation, obese subjects had an increased risk of dementia.93
BENEFITS OF WEIGHT LOSS
Weight loss improves a person’s health outlook in many ways.4 Weight loss reduces the risk of death in obese patients treated with bariatric surgery. In one study from Sweden94 with more than 4000 patients, half of whom received one of three bariatric operations (see later), there was a reduction in mortality of 24% after 10.9 years. Another study from Utah95 compared over 7000 patients who received a gastric bypass (see later) with those matched for weight and age and showed a reduction in mortality of 56% after 7.1 years.95
Weight reduction also reduces the risk from diseases that result from obesity. The Diabetes Prevention Program (DPP) is a clear example of a reduction in the risk of developing diabetes with weight loss. After an average of 2.8 years of follow-up in 3234 individuals with impaired glucose tolerance, those who were randomized to the intensive lifestyle treatment lost 7% of their body weight and showed a 58% reduction in the risk of developing diabetes.96
Blood pressure also benefits from weight loss. In the Framingham study, a modest weight loss of at least 6.8 kg or more led to a 28% reduction in the risk of hypertension in middle-aged adults and a 37% reduction in older adults.97 In a clinical trial using lifestyle interventions to lower blood pressure (TOHP II), the risk of being hypertensive was reduced 42% at 6 months and 22% at 18 months. In those who maintained a weight loss of 4.5 kg for 30 months, the risk of hypertension was reduced 65%.98
Apneic episodes also are influenced by changes in weight. Relative to stable weight, a 10% weight loss predicted a 26% reduction in the apnea-hyperpnea index.99 In a systematic review of long-term weight loss studies in obese adults, dietary and lifestyle approaches and pharmacologic interventions improved markers of cardiovascular disease, particularly in patients with cardiovascular risk factors at the beginning of the study.100 Quality of life also improves following weight loss.101
EVALUATION
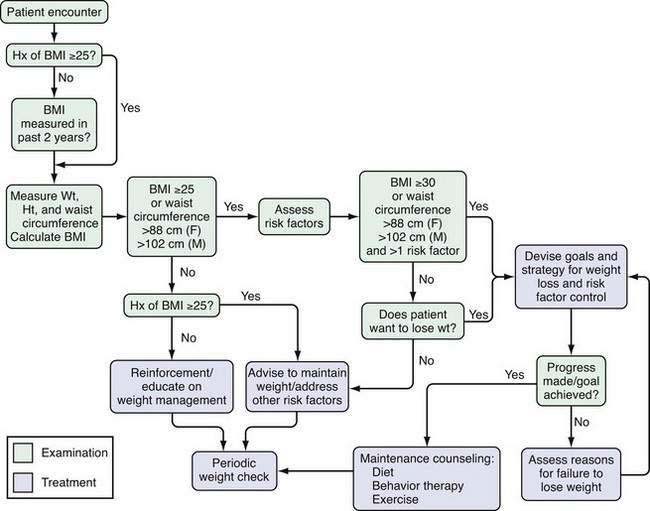
The hazards of excess weight and the benefits of weight loss point to the need for careful evaluation of the overweight patient. The National Heart, Lung, and Blood Institute has provided an algorithm for evaluating the overweight patient. It is a useful framework for viewing the information that is collected during the evaluation of individual patients (Fig. 6-4).
The basic components in the evaluation of any overweight or obese patient are a record of the historical events associated with the patient’s weight problem, a physical examination, and an appropriate laboratory assessment. Here, I have used the criteria recommended by the U.S. Preventive Services Task Force102 and also taken into account reports from the National Heart, Lung, and Blood Institute2 and the World Health Organization.1 The importance of evaluating overweight individuals has increased as the epidemic of overweight has worsened, and the number of patients potentially needing treatment has increased.
CLINICAL HISTORY
It is important to determine whether the patient comes from a family in which overweight is common, the usual setting, or whether she or he has become overweight in a family in which few people are overweight. The latter setting suggests a need to search for environmental factors that may be contributing to weight gain. Studies have shown that alteration in the melanocortin-4 receptor occurs in 2.5% to 5.5% of children and adolescents with a BMI > 30 kg/m2.28 This genetic defect is among the most common of those associated with any chronic disease, and evaluation for this defect may become important in the treatment of overweight people.
PHYSICAL EXAMINATION
The first step in the clinical examination of the overweight patient is to determine vital signs, which include BMI and waist circumference, as well as pulse and blood pressure.103 Accurate measurement of height and weight is the initial step in the clinical assessment,104 because these are needed to determine the BMI (see earlier). The BMI has a reasonable correlation with body fat and a curvilinear relationship to risk. Risk arbitrarily has been subdivided by cut points derived from data collected on whites. It is now clear, however, that different ethnic groups have different percentages of body fat for the same BMI105 and BMI thus needs to be interpreted in an ethnically specific context. An Asian Conference selected lower levels of BMI to define overweight (BMI < 23 kg/m2) and obesity (BMI > 25 kg/m2); the same BMI presumably carries a different level of risk in various populations. These differences need to be taken into consideration when making clinical judgments about the degree of risk for the individual patient. During weight loss, body weight is more useful than the BMI, because height doesn’t change during this period, and the need to use the height squared makes it more difficult for the physician and patient to calculate.
After BMI, waist circumference is the second vital sign in the evaluation of the overweight individual. Waist circumference is the easiest measurement to evaluate central adiposity. It is determined using a metal or nonstretchable plastic tape. Measurements at the level of the umbilicus or at the midpoint between the lower rib and suprailiac crest are the two most common locations. Waist circumference is a good strategy for following the clinical progress of weight loss and is particularly valuable if patients become more physically active. Physical activity may slow loss of muscle mass and thus slow weight loss, whereas fat continues to be mobilized. Waist circumference can help in making these distinctions. The relationship of central fat to risk factors for health varies among populations as well as within them. Japanese Americans and Indians from South Asia have relatively more visceral fat and are thus at higher risk for a given BMI or total body fat than whites. Even though the BMI is below 25 kg/m2, central fat may be increased, particularly in Asian populations, and may increase the risk of disease.106 Central adiposity is important, particularly with a BMI between 22 and 29 kg/m2.
Blood pressure should be measured carefully. Hypertension is amenable to improvement with diet107 and is an important criterion for diagnosis of the metabolic syndrome. The patient should sit quietly for 5 minutes before measuring the blood pressure with a calibrated instrument to increase the accuracy of measurement. The blood pressure criteria from the Seventh Joint National Commission recommendations should be followed. A normal blood pressure is less than 120/80 mm Hg. Prehypertension was defined by this group as a systolic blood pressure (SBP) of 120 to 139 mm Hg and diastolic BP (DBP) of 80 to 89 mm Hg. Hypertension is then a SBP/DBP of 140/90 mm Hg and clearly needs treatment if such blood pressure values are confirmed. Individuals with prehypertension need to be carefully observed.
LABORATORY STUDIES
Serum lipids, glucose, C-reactive protein (now measured as high-sensitivity CRP [hs-CRP]), and other values indicated from the history and physical examination should be measured. An increased fasting glucose, low HDL cholesterol, and high triacylglycerol levels are atherogenic components of the metabolic syndrome. Along with elevated blood pressure, it is possible to categorize the patient as having the metabolic syndrome by using criteria proposed by the National Cholesterol Education Program (see Table 6-2). An individual has the metabolic syndrome if three of the five criteria are abnormal. Measurement of LDL cholesterol also is important because it may need treatment independently of obesity or central adiposity. A positive hs-CRP assay along with an elevated serum LDL cholesterol level is a clear risk factor for heart disease.
PREVENTION
Studies designed to prevent obesity have been conducted in children and in adults.4 For children and adolescents, many school-based programs have been tried and, although there have been some promising results, the long-term impact of such programs has been small. In one successful study in children, a reduction in television watching slowed their gain in BMI.108 Another study has shown that decreasing children’s consumption of carbonated beverages, primarily soft drinks, was associated with slower weight gain than that of children who were not given this advice.109 In studies involving adults, however, there are few successful preventive programs.
TREATMENT
Realism is one important aspect of treatment for obesity. For most forms of treatment, including behavior therapy, diet, and exercise, weight loss levels off at less than 10% below baseline. For many patients this is a frustrating experience, because their dream weight would require a loss of almost 30% of their body weight.110 A weight loss of less than 17% would be a disappointment with participation in a weight loss program. Yet, other than surgery, a weight loss of 10% is the expected outcome. It is important for the patient and physician to realize that an initial weight loss of 10% of body weight should be considered a success and that this amount of weight loss lessens the health risks of obesity.100 Because obesity left to itself will lead to a number of associated diseases, there are two therapeutic strategies: (1) wait until associated diseases develop (e.g., diabetes, hypertension, or dyslipidemia) and treat them individually; or (2) treat the obesity itself, thus reducing the risk of developing diabetes, hypertension, and other associated diseases. The second approach is preferable.
DIETS
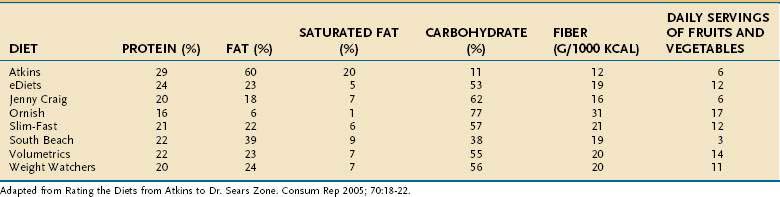
To lose weight, a person must consume fewer calories (eat less food) than the body needs for daily activities. Many diet plans are available for overweight individuals (Table 6-3).111 These can be categorized as those that are low in fat (Ornish, Jenny Craig), those that are low in carbohydrate (Atkins, South Beach), those that restrict most nutrients, the so-called balanced deficit diets (Weight Watchers, Volumetrics, Slim Fast, eDiets), those that highlight one type of food or another (e.g., the low glycemic index diet), or diets that highlight special foods (e.g., the grapefruit diet).
The efficacy of dietary counseling versus control therapy has been examined in a meta-analysis.112 A random effects model of 46 studies of dietary counseling showed a maximum net treatment effect of −1.9 BMI units (95% confidence interval [CI]: −2.3, −1.5) or approximately a 6% weight loss over 12 months. There was a loss of about 0.1 BMI unit/month for the 12 months of active treatment and a regain of about 0.02 to 0.03 BMI unit/month during subsequent phases of the program. There were many different strategies used in the studies reviewed in this meta-analysis, but there was no clear basis for selecting one dietary approach over another.
Popular Diets
Low-Fat Diets
Low-fat diets are a standard strategy to help patients lose weight. One benefit from a very low level of fat intake is the slowing or reversal of coronary artery disease.113 A meta-analysis of five randomized controlled trials of low-fat diets, however, has shown that these diets produce significant weight loss, but no more so than the control diets.114 Subsequently, 48,835 women were randomly assigned in a large clinical trial to low-fat or control diets.115 Weight loss was 2.2 kg below baseline at year one and 0.6 kg at an average of 7.5 years of follow-up. At both time points, weight loss was significantly less in the women on the low-fat diet compared with those on the normal-fat diet, and there was a clear relationship between the decrease in percentage of fat ingested and weight loss (P < 0.001 for trend). A meta-analysis of weight loss studies has found that over the first six months, low-fat diets produce weight loss and that heavier individuals lose more weight than lighter weight individuals.116
Low Energy Density Diets
The theory behind the use of low energy density diets117 is that filling the stomach with low-fat, high-fiber foods (low energy density) reduces hunger and produces satiety. Conversely, in experimental settings, people eat more food when it is more energy dense—that is, has more calories per unit weight.
Low-Carbohydrate Diets
The most popular diets are the low-carbohydrate, high-protein, high-fat diets. Daily carbohydrate intake in some of these diets is as low as 13 g; when carbohydrate intake is less than 50 g/day, ketosis develops. In short-term metabolic ward studies, patients did not increase the intake of other foods to compensate for the lower calories in a very low carbohydrate diet.118 Several randomized clinical trials have reported greater weight loss in the low-carbohydrate diet group up to six months, but not at one year.119
Four clinical trials have compared the effect of macronutrient composition on weight loss in one-year studies120,121 and two-year studies.122,123 The two one-year studies compared the Atkins, Zone, and Ornish diets, with the Weight Watchers diet in one study120 or the LEARN manual in another study.121 One two-year study compared the Atkins diet, a Mediterranean-style diet, and the American Heart Association low-fat diet122 and the other compared four diets: 20% or 40% fat with 15% or 25% protein.123 In the first one-year study,120 160 participants were randomly assigned to each diet. After one year, there was no significant difference in the weight loss of patients using any of the four diets. The weight loss was as follows: −3.9 ± 6 kg (−8.58 ± 13.2 pounds) with the Atkins diet; −4.9 ± 6.9 kg with the Zone diet (CI, 10.78 ± 15.18 pounds); −4.6 ± 5.4 kg (−10.12 + 11.88 pounds) with the Weight Watchers diet; and −6.6 ± 9.3 kg (−14.52 ± 20.46 pounds) with the Ornish diet—thus, no differences among the diets. The principal determinant of weight loss was the degree of adherence to the diet, not the diet itself. In the second one-year trial, 311 premenopausal women were randomly assigned to one of four diets.121 In this study, the Atkins diet produced more weight loss at 12 months (−0.7 kg) compared with the other three diets (Zone, −1.6 kg; LEARN, −2.6 kg; Ornish, −2.2 kg), a difference that was not statistically different. Also in this study, adherence to the diet was the principal determinant of success.
The first two-year study compared a Mediterranean diet, Atkins diet, and low-fat diet in a group composed of 90% men living in a town in Israel. At the end of two years, the weight loss was −4.7 kg for the low-carbohydrate group, −2.9 kg for the low-fat group, and −4.4 kg for the Mediterranean diet group. After reaching a plateau at six to seven months, the group on the Mediterranean diet had another drop in weight equal to that of the low-carbohydrate group.122
The second two-year study was the most complex, using a 2 × 2 design that had four carbohydrate levels (35%, 45%, 55%, and 65%) resulting from the two fat levels (20% or 40%) and the two protein levels (15% or 25%).123 In this study, 811 individuals were randomized to one of the four diets. The weight loss at one year averaged 7% across diets, with no significant differences. Thereafter, there was a small weight regain. Attendance at support, education, and diet groups strongly predicted success. The authors concluded that “the content of dietary fat, carbohydrate, and protein had little influence on body weight loss over two years in obese people.”
Very Low-Calorie Diets
Very low-calorie diets (i.e., diets with an energy level below 800 kcal/day), can be used for rapid weight loss prior to major surgery. In other settings, the weight rebound that usually occurs at the end of a program with very low-calorie diets may not make them worth the effort for some people, and may deter them from using similar diets in the future. A systematic review of 29 studies of weight loss programs using a very low-calorie diet that lasted more than two years124 found that participants in the very low-calorie diet program lost significantly more weight than those eating hypoenergetic balanced diets. The very low-calorie diets, however, have been replaced largely by portion-controlled diets, in which the calories from beverages, bars, or frozen meals provided at breakfast or lunch are fixed by the manufacturer. In a four-year study, this approach resulted in early initial weight loss, which then was maintained.125
Commercial Programs
A number of commercial and self-help programs, including Overeaters Anonymous, Take Off Pounds Sensibly (TOPS), Weight Watchers, Jenny Craig, Herbalife, OPTIFAST, LA Health, and eDiets, are available to the consumer. Tsai and Wadden126 have examined the effectiveness of a number of these programs. The Weight Watchers program is done in groups, in contrast to Jenny Craig and LA Weight Loss, in which clients are seen individually. Jenny Craig uses prepackaged food and Weight Watchers and LA Weight loss use diet plans. All three programs encourage physical activity. In one trial lasting two years and including 423 subjects, the participants in the intervention group attended the Weight Watchers meetings and experienced a mean weight loss of 5.3% at one year and 3.2% at two years, compared with 1.5% at one year and 0% weight loss for the control group that received the self-help intervention with two visits to a dietitian.127
LIFESTYLE MODIFICATION
A basic strategy in helping obese patients lose weight is through lifestyle changes. The first step in this process is to determine whether the individual is really ready to make lifestyle changes. Patients often have a dream weight that involves a weight loss of nearly 30% of their initial body weight.110 An initial loss of 5% to 10% of body weight is a more realistic goal, because it will significantly reduce many of the health hazards described above, if they are present.100
Behavioral strategies include helping patients learn to monitor their eating behavior by recording what is eaten, the setting in which it is eaten, and the situations that trigger eating. With this information, the health care provider can help a patient change his or her eating habits. Patients should be encouraged to use a defined eating plan. People who are successful in losing weight and maintaining weight loss tend to monitor their behavior, eat low-fat diets, increase their physical activity, and practice positive self-thinking and techniques for stress reduction, as documented by the National Weight Loss Registry.128 Use of the Internet is a promising new tool.129
EXERCISE
Exercise is one strategy for balancing energy intake and expenditure, whether as a primary treatment for weight loss or for prevention of weight regain. Walking expends approximately 100 kcal/mile. A deficit of 3500 kcal (500 kcal/day) maintained for one week should result in a loss of 0.45 kg (1 pound). To obtain this effect from exercise alone, an individual would need to walk five miles/day, seven days/week. For this reason, exercise alone has not been very effective as a primary weight loss technique.4 Moderate to vigorous exercise for 60 min/day, six days/week, produced more weight loss (−1.4 kg in women; −1.8 kg in men) than that of a nonexercise group in over 12 months.130 A meta-analysis of weight loss trials lasting at least one year has found that exercise-alone groups had minimal weight loss. Use of resistance training, as opposed to aerobic exercise, may help retain lean body mass and reduce the associated fall in resting energy expenditure.131
For individuals wanting to monitor their exercise, inexpensive pedometers can be worn on the belt. A mile is about 2000 steps, and increasing the number of monitored steps walked each day is a good way to encourage walking.132
PHARMACOTHERAPY
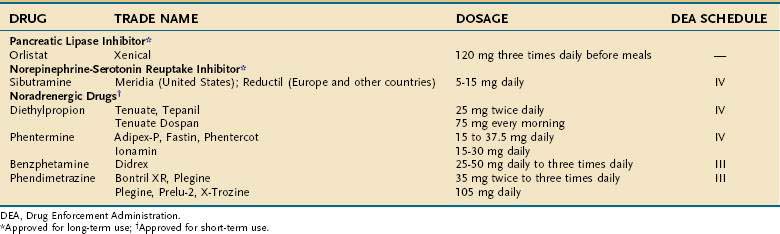
Because all medications inherently have more risks than diet and exercise, medications should only be used when the benefits justify the risk. Current medications for the treatment of obesity can be divided into two broad categories: (1) those that act primarily on the central nervous system to reduce food intake; and (2) those that act primarily outside the brain. Wherever the primary site of action may be, however, the net effect must be a reduction in food intake, an increase in energy expenditure, or both. There currently are several drugs available in the United States to treat obesity133–135 (Table 6-4).
Mechanisms of Drug Action
The brain plays a central role in regulating food intake by receiving and processing information from the environment and internal milieu.34 A number of neurotransmitter systems, including monoamines, amino acids, and neuropeptides, are involved in modulating food intake. The monoamines include norepinephrine, serotonin, dopamine, and histamine, as well as certain amino acids. The serotonin system has been one of the most extensively studied of the monoamine pathways. Its receptors modulate both the quantity of food eaten and macronutrient selection. Stimulation of the serotonin receptors in the paraventricular nucleus reduces fat intake, with little or no effect on the intake of protein or carbohydrate. This reduction in fat intake is probably mediated through 5-HT2C receptors, because its effect is attenuated in mice that cannot express the 5-HT2C receptor. Sibutramine blocks reuptake of serotonin and norepinephrine. Lorcaserin is a drug in clinical trials that acts directly on serotonin receptors in the brain.
Stimulation of α1-adrenergic receptors also reduces food intake,134 as shown by the α1 agonist phenylpropanolamine. Some of the α1 receptor antagonists used to treat hypertension produce weight gain, further indicating a role for this receptor in weight control. In contrast, stimulation of α2-adrenergic receptors increases food intake in experimental animals, and a polymorphism in the α2a adrenoceptor has been associated with reduced metabolic rate in humans. Activation of β2 receptors in the brain reduces food intake. These receptors can be activated by β agonists, which release norepinephrine in the vicinity of these receptors or block the reuptake of norepinephrine. Sibutramine is both a serotonin and norepinephrine reuptake inhibitor and also uses this mechanism.
Histamine receptors also can modulate feeding. Stimulation of the H1 receptor in the central nervous system reduces feeding. Experimentally, this has been addressed by modulating the H3 autoreceptor, which controls histamine release. When the autoreceptor is stimulated, histamine secretion is reduced and food intake increases. Blockade of this H3 autoreceptor decreases food intake. The histamine system is important in control of feeding because some psychoactive drugs bind to histamine receptors and produce weight gain.19
The endocannabinoid system is the most recent addition to the central controllers of feeding.36 Tetrahydrocannabinol, isolated from the marijuana plant, stimulates food intake. Isolation of the cannabinoid receptor was followed by the identification of two fatty acids, anandamide and 2-arachidonoylglycerol, which are endogenous ligands for this receptor; infusion of either ligand into the brain stimulates food intake. The CB1 receptor is a preganglionic receptor, meaning that its activation inhibits synaptic transmission. Antagonists to this receptor have been shown to reduce food intake and lead to weight loss.36
In addition to the drugs that act on the central nervous system, there are also drugs that act peripherally.33 Thus, for example, blockade of intestinal lipase by orlistat will produce weight loss. A second drug in this class, cetilistat, is in clinical trials.
FDA-Approved Medications
The FDA has approved several drugs for the treatment of obesity, shown in Table 6-4. Two of them, sibutramine and orlistat, are approved for long-term use (12 months); the others are approved for up to a few weeks, which is usually interpreted as 12 weeks.
Sibutramine
Sibutramine (Meridia in the United States and Reductil in Europe and other countries) has been marketed in the United States since March 1998. It is a selective reuptake inhibitor of serotonin and norepinephrine into neurons, but does not act on any known receptors. Sibutramine promotes satiety, but may also increase energy expenditure by blocking the reduction in metabolic rate that accompanies weight loss. In a randomized, placebo-controlled, six-month dose-ranging study of 1047 patients, there was a clear dose-response effect in dosages of 1 to 30 mg/day. In this study, 67% of subjects treated with sibutramine achieved a 5% weight loss from baseline, and 35% lost 10% or more.136 In a one-year trial of 456 patients who received sibutramine (10 or 15 mg/day) or placebo, 56% of those who stayed in the trial for 12 months lost at least 5% of their initial body weight, and 30% of the patients lost 10% of their initial body weight while taking the 10-mg dose.137 In a trial of patients who initially lost weight eating a very low-calorie diet before being randomized to sibutramine (10 mg/day) or placebo, sibutramine produced additional weight loss, whereas the placebo-treated patients regained weight.134,138
Sibutramine also is effective for weight maintenance. The Sibutramine Trial of Obesity Reduction and Maintenance (STORM) Trial began with a six-month open-label phase to induce weight loss using 10 mg/day of sibutramine. Patients who lost more than 8 kg then were randomized to sibutramine or placebo.139 During the 18-month double-blind phase of this trial, the placebo-treated patients steadily regained weight, maintaining only 20% of their weight loss at the end of the trial. In contrast, the subjects treated with sibutramine maintained 80% of their weight loss after two years.139 The blood pressure levels of the sibutramine-treated patients were still higher than in patients treated with placebo (see later). Clinical trials with sibutramine have shown that about 75% of patients treated with 15 mg/day of sibutramine achieved more than 5% weight loss, and 80% of those maintained that loss for 2 years if they stayed on the drug. In a meta-analysis of clinical trials with sibutramine,135 the drug produced a weighted mean weight loss of 6.35 ± 6.47 kg (−13.9 pounds) compared with 2.18 ± 5.23 kg (−4.8 pounds) for the placebo group, giving a net effect, or what is often called the placebo-subtracted weight loss, of −4.16 kg (95% CI: −4.73 to −3.59).
Orlistat
Orlistat (Xenical) is available by prescription in a dosage of 120 mg three times daily and Alli is offered over-the-counter (OTC) at a lower dosage, 60 mg three times daily; both drugs should be taken before meals. Orlistat inhibits the enzymatic action of pancreatic lipase. In a two-year trial with orlistat,140 patients received a hypocaloric diet that was 500 kcal/day less than their calculated requirements for the first year, and a diet that was calculated to maintain body weight in the second year. By the end of the first year, the placebo-treated patients lost 6.1% of their initial body weight and the drug-treated patients lost 10.2%. At the end of the second year, the patients who were switched from orlistat to placebo after one year gained weight, from 10% to 6% below baseline, a gain of 4%. Patients switched from placebo to orlistat lost weight, from 6% to 8.1% below baseline (a loss of 2.1%), an amount essentially identical to the 7.9% weight loss in the patients treated with orlistat for the full 2 years.
In a three-year study,141 a very low-energy diet was used for eight weeks, and subjects who lost a minimum of 5% of their body weight were randomized to lifestyle or lifestyle plus orlistat. Weight loss continued to decline for three months and remained below randomization levels at 12 months in the orlistat group, but had risen above randomization level by six months in the lifestyle controls. At the end of three years, those on orlistat were still 2.4 kg lighter than the controls. Clinical trials show that ∼70% of patients will achieve more than 5% weight loss and at 2 years, 70% of them will have maintained that loss. There are clinical trials documenting orlistat use for up to four years.142 One advantage to orlistat’s use is its beneficial effect on LDL cholesterol. Because orlistat blocks fat absorption, the LDL reduction is about twice that seen with weight loss alone. A meta-analysis has shown that the orlistat-treated patients had a weighted mean weight loss of −5.70 ± 7.28 kg (−12.6 pounds) compared with −2.40 ± 6.99 kg (−5.3 pounds), giving a net, or placebo-subtracted weighted mean, weight loss of −2.87 (95% CI −3.21 to −2.53) (−6.4 pounds).135
Sympathomimetic Amines
Four sympathomimetic drugs have been approved by the FDA.134 Two (phentermine, diethylpropion) are schedule IV drugs and the other two (benzphetamine and phendimetrazine) are schedule III drugs (see Table 6-4). These drugs are only approved for a few weeks of use, which usually is interpreted as up to 12 weeks. Phentermine is not available in Europe. Obtaining written informed consent if phentermine is prescribed for longer than 12 weeks is good medical practice, because of the paucity of published reports on the long-term use of phentermine.
Non–FDA-Approved Medications
Fluoxetine
Fluoxetine is a selective serotonin reuptake inhibitor (SSRI) that blocks serotonin transporters, thus prolonging the action of serotonin. Fluoxetine is approved by the FDA for the treatment of depression. Fluoxetine at a dosage of 60 mg/day (three times the usual dose for treatment of depression) was effective in reducing food intake4 and body weight in overweight patients. A meta-analysis of six studies using fluoxetine has shown a wide range of results with a mean weight loss in one study of 14.5 kg and a weight gain of 0.40 kg in another.133 In the meta-analysis by Avenell and colleagues,143 the weight loss at 12 months was 0.33 kg (95% CI, −1.49 to 0.82 kg). Goldstein and colleagues reviewed the trials with fluoxetine that included one 36-week trial in type II diabetic subjects, one 52-week trial in subjects with uncomplicated overweight, and two 60-week trials in subjects with dyslipidemia, diabetes, or both.144 A total of 719 subjects were randomized to fluoxetine and 722 to placebo. Six months of treatment were completed by 522 subjects on fluoxetine and 504 subjects on placebo. Weight losses in the placebo and fluoxetine groups at six months were 2.2 and 4.8 kg and at one year were 1.8 and 2.4 kg, respectively. The regain of 50% of the lost weight during the second six months of treatment with fluoxetine makes this drug inappropriate for the long-term treatment of obesity. Fluoxetine, however, although not a good drug for long-term treatment of obesity, may be preferred for the treatment of depressed obese patients over some of the tricyclic antidepressants, which are associated with significant weight gain.
Bupropion
Bupropion is a norepinephrine and dopamine reuptake inhibitor approved for the treatment of depression and for help in smoking cessation. Two multicenter clinical trials, one in obese subjects with depressive symptoms and one in uncomplicated overweight patients, have tested this drug. In the study of overweight patients with depressive symptom ratings of 10 to 30 on a Beck Depression Inventory, 213 patients were randomized to 400 mg/day of bupropion and 209 subjects were assigned to placebo over a 24-week period. In the bupropion group, 121 subjects completed the trial and lost 6.0% ± 0.5% of initial body weight; the 108 subjects in the placebo group who completed the trial lost 2.8% ± 0.5% (P < 0.0001).145 A study in uncomplicated overweight subjects randomized 327 subjects to bupropion, 300 mg/day, bupropion 400 mg/day, or placebo in equal proportions.146 At 24 weeks, 69% of those randomized remained in the study and the percent losses of initial body weight were 5% ± 1%, 7.2% ± 1%, and 10.1% ± 1% for the placebo, bupropion 300-mg, and bupropion 400-mg groups, respectively (P < 0.0001). The placebo group was randomized to the 300- or 400-mg group at 24 weeks and the trial was extended to week 48. By the end of the trial, the dropout rate was 41%, and the weight losses in the bupropion 300- and 400-mg groups were 6.2% ± 1.25% and 7.2% ± 1.5% of initial body weight, respectively.146 Thus, it appears that nondepressed subjects may respond to bupropion with more weight loss than those with depressive symptoms.
Topiramate
Topiramate is an antiepileptic drug that was discovered to be associated with weight loss in its clinical trials for epilepsy.147 Weight losses of 3.9% of initial weight were seen at three months and losses of 7.3% of initial weight were seen at one year. Bray and colleagues reported a six-month, placebo-controlled, dose-ranging study of topiramate in which 385 obese subjects were randomized to placebo or topiramate at 64, 96, 192, or 384 mg/day.148 These doses were gradually reached by a tapering increase and were reduced in a similar manner at the end of the trial. Weight loss from baseline to 24 weeks was 2.6%, 5%, 4.8%, 6.3%, and 6.3% in the placebo, 64-, 96-, 192-, and 384-mg groups, respectively. The most frequent adverse events were paresthesias, somnolence, and difficulty with concentration, memory, and attention. This trial was followed by two other multicenter trials.149,150 The first trial randomized 1289 obese subjects to placebo or topiramate 89, 192, or 256 mg/day. This trial was terminated early because of the sponsor’s decision to pursue a time-release form of the drug. The 854 subjects who completed one year of the trial before it was terminated lost 1.7%, 7%, 9.1%, and 9.7% of their initial body weight in the placebo, 89-, 192-, and 256-mg groups, respectively. Subjects in the topiramate groups also had significant improvement in blood pressure and glucose tolerance.149 The second trial enrolled 701 subjects who were treated with a very low-calorie diet to induce an 8% loss of initial body weight.150 The 560 subjects who achieved an 8% weight loss were randomized to topiramate 96 or 192 mg/day, or placebo. This study also was terminated early. At the time of termination, 293 subjects had completed 44 weeks of the trial. The topiramate groups lost 15.4% and 16.5% of their baseline weight and the placebo group lost 8.9%.150 Topiramate also is effective in producing weight loss in diabetic patients.151 Although topiramate still is available as an antiepileptic drug, the development program to obtain an indication for overweight was terminated by the sponsor because of the associated adverse events.
Zonisamide
Zonisamide is an antiepileptic drug that has serotonergic and dopaminergic activity in addition to inhibiting sodium and calcium channels. Weight loss was noted in the clinical trials for the treatment of epilepsy, again suggesting the drug as a potential agent for weight loss. Gadde and colleagues tested this possibility by performing a 16-week randomized controlled trial in 60 obese subjects.152 Subjects were placed on a calorie-restricted diet and randomized to zonisamide or placebo. Zonisamide was started at 100 mg/day and increased to 400 mg/day. At 12 weeks, the dosage in those subjects who had not lost 5% of initial body weight was increased to 600 mg/day. The zonisamide group lost 6.6% of initial body weight at 16 weeks compared with 1% in the placebo group. Thirty-seven subjects completing the 16-week trial elected to continue the trial for 32 weeks, 20 in the zonisamide group and 17 in the placebo group. At the end of 32 weeks, the 19 subjects in the zonisamide group lost 9.6% of their initial body weight compared with 1.6% for the 17 subjects in the placebo group.152
Metformin
Metformin is a biguanide approved for the treatment of diabetes mellitus that reduces hepatic glucose production, decreases glucose absorption from the gastrointestinal tract, and enhances insulin sensitivity. In clinical trials in which metformin was compared with sulfonylureas, it produced weight loss.134 In one French trial, called BIGPRO, metformin was compared with placebo in a one-year multicenter study of 324 middle-aged subjects with upper body adiposity and the metabolic syndrome (insulin resistance syndrome). The subjects on metformin lost significantly more weight (1 to 2 kg) than those in the placebo group, and the study concluded that metformin may have a role in the primary prevention of type 2 diabetes.153 In a meta-analysis of three of these studies, Avenell and colleagues143 reported a weighted mean weight loss at 12 months of 1.09 kg (95% CI, −2.29 to 0.11 kg).
The best trial of metformin for obesity, however, is the Diabetes Prevention Program (DPP) study of individuals with impaired glucose tolerance. This study included a double-blind comparison of metformin 850 mg twice daily with placebo. During the 2.8 years of this trial, the 1073 patients treated with metformin lost 2.5% of their body weight (P < 0.001) compared with the 1082 patients treated with placebo, and the conversion from impaired glucose tolerance to diabetes was reduced by 31% compared with placebo. In the DPP trial, metformin was more effective in reducing the development of diabetes in persons in the subgroup who were most overweight and in the younger members of the cohort.96 Although metformin does not produce enough weight loss to qualify as a weight loss drug (FDA criteria require 5% weight loss), it would appear to be a very useful choice for overweight individuals who have diabetes or are at high risk for diabetes. One area in which metformin has found use is in treating overweight women with the polycystic ovary syndrome, in whom the modest weight loss may contribute to increased fertility and reduced insulin resistance.154
Pramlintide
Amylin is a peptide found in the beta cells of the pancreas that is cosecreted along with insulin to circulate in the blood. Amylin and insulin are deficient in type 1 diabetics, in whom beta cells are destroyed immunologically. Pramlintide, a synthetic amylin analog, has a prolonged biological half-life.155 Pramlintide is approved by the FDA for the treatment of diabetes. Unlike insulin and many other diabetic medications, pramlintide use is associated with weight loss. In a study in which 651 subjects with type 1 diabetes were randomized to placebo or subcutaneous pramlintide 60 µg three or four times daily, along with an insulin injection, the hemoglobin A1c level decreased 0.29% to 0.34% and weight decreased −1.2 kg relative to placebo.156 Maggs and colleagues analyzed the data from two one-year studies in insulin-treated type 2 diabetic subjects randomized to pramlintide 120 µg twice daily or 150 µg three times daily.157 Weight decreased by 2.6 kg and hemoglobin A1c decreased 0.5%. When weight loss was then analyzed by ethnic group, African Americans lost 4 kg, whites lost 2.4 kg, and Hispanics lost 2.3 kg; the improvement in diabetes correlated with the weight loss, suggesting that pramlintide is effective in ethnic groups with the greatest burden from overweight. The most common adverse event was nausea, which usually was mild and confined to the first four weeks of therapy.
Exenatide
GLP-1 is derived from the processing of the preproglucagon peptide, which is secreted by L cells in the terminal ileum in response to a meal. Increased GLP-1 inhibits glucagon secretion, stimulates insulin secretion, stimulates gluconeogenesis, and delays gastric emptying.155 It has been postulated to be responsible for the superior weight loss and improvement in diabetes seen after gastric bypass surgery for overweight.158 GLP-1 is rapidly degraded by dipeptidyl peptidase-4 (DPP-4), an enzyme that is elevated in obese individuals. Bypass operations for overweight increase GLP-1, but do not change the levels of DPP-4.155
In humans, exenatide reduces fasting and postprandial glucose levels, slows gastric emptying, and decreases food intake by 19%.155 The side effects of exenatide in humans are headache, nausea, and vomiting that are lessened by gradual dose escalation. Several clinical trials of 30 weeks’ duration have been reported using exenatide at a daily dosage of 5 µg twice daily subcutaneously.159–161 In one trial with 377 type 2 diabetic subjects who were failing maximal sulfonylurea therapy, exenatide produced a fall of 0.74% more in hemoglobin A1c than placebo. Fasting glucose levels also decreased and there was a progressive weight loss of 1.6 kg.161 The interesting feature of this weight loss is that it occurred without change in lifestyle, diet, or exercise. In a 26-week randomized controlled trial, exenatide produced a 2.3-kg weight loss compared with a gain of 1.8 kg in the group receiving insulin glargine.162 The FDA has issued a notice about potential risks of pancreatitis.
Drug Combinations
The first important clinical trial combining drugs that acted by separate mechanisms used phentermine and fenfluramine.163 This trial showed that by using combination therapy, a highly significant weight loss was achieved of almost 15% below baseline and with fewer side effects than those seen with the individual agents. This combination became very popular, but because of reports of aortic valvular regurgitation associated with its use, fenfluramine was withdrawn from the market worldwide on September 15, 1997.164
SURGERY
Surgical intervention for obesity has become ever more popular (see Chapter 7).165 The Swedish Obese Subjects Study has offered surgical intervention for obese Swedish patients aimed at reducing their obesity through a gastrointestinal operation. The control group included obese Swedish patients who did not get surgical treatment but were treated with the best alternatives in the Swedish health care system. The effect of weight change on dyslipidemia, blood pressure, and serum insulin levels in the surgically treated group two and 10 years after surgery was compared with these parameters in the control group.166 There was a graded effect of weight change on HDL cholesterol, triglycerides, systolic and diastolic blood pressure, insulin, and glucose. This surgery has now been shown to reduce mortality.94,95 A comparison of surgically and nonsurgically treated patients has shown that weight loss improves long-term health outcomes, but at a cost of significant short-term health problems.167
Avenell A, Brown TJ, McGee MA, et al. What interventions should we add to weight reducing diets in adults with obesity? A systematic review of randomized controlled trials of adding drug therapy, exercise, behaviour therapy or combinations of these interventions. J Hum Nutr Diet. 2004;7:293-316. (Ref 143.)
Bray GA. The metabolic syndrome and obesity. Totowa, NJ: Humana Press; 2007. (Ref 4.)
Bray G, Greenway F. Pharmacological treatment of the overweight patient. Pharmacol Rev. 2007;59:151-84. (Ref 134.)
Christakis NA, Fowler JH. The spread of obesity in a large social network over 32 years. N Engl J Med. 2007;357:370-9. (Ref 15.)
Dansinger ML, Tatsioni A, Wong JB, et al. Meta-analysis: The effect of dietary counseling for weight loss. Ann Intern Med. 2007;147:41-50. (Ref 112.)
Farooqi IS, O’Rahilly S. Genetic factors in human obesity. Obes Rev. 2007;8(Suppl 1):37-40. (Ref 27.)
Freedman DM, Ron E, Ballard-Barbash R, et al. Body mass index and all-cause mortality in a nationwide US cohort. Int J Obes (Lond). 2006;30:822-9. (Ref 45.)
Klein S, Burke LE, Bray GA, et al. American Heart Association Council on Nutrition, Physical Activity, and Metabolism. Clinical implications of obesity with specific focus on cardiovascular disease: A statement for professionals from the American Heart Association Council on Nutrition, Physical Activity, and Metabolism: Endorsed by the American College of Cardiology Foundation. Circulation. 2004;110:2952-67. (Ref 46.
Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393-403. (Ref 96.)
National Institutes of Health. Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults—The Evidence Report. National Institutes of Health. Obes Res. 1998;6(Suppl 2):51S-209S. (Ref 2.)
Poirier P, Giles TD, Bray GA, et al. American Heart Association; Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss: An update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2006;113:898-918. (Ref 50.)
Rucker D, Padwal R, Li SK, et al. Long term pharmacotherapy for obesity and overweight: updated meta-analysis. BMJ. 2007;335:1194-9. (Ref 135.)
Sjöström L, Narbro K, Sjöström CD, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007;357:741-52. (Ref 94.)
1. Obesity: Preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. 2000;894:i-xii. 1-253
2. National Institutes of Health. Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults—The Evidence Report. Obes Res. 1998;6(Suppl 2):51S-209S.
3. Ogden CL, Yanovski SZ, Carroll MD, et al. The epidemiology of obesity. Gastroenterology. 2007;132:2087-102.
4. Bray GA. The metabolic syndrome and obesity. Totowa, NJ: Humana Press; 2007.
5. Sjöström L. Surgical treatment of obesity. In: Bray GA, Bouchard C, editors. Handbook of obesity: Clinical applications. 2nd ed. New York: Marcel Dekker; 2004:359.
6. Romero-Corral A, Somers VK, Sierra-Johnson J, et al. Accuracy of body mass index in diagnosing obesity in the adult general population. Int J Obes. 2008;32:959-66.
7. Lee CM, Huxley RR, Wildman RP, Woodward M. Indices of abdominal obesity are better discriminators of cardiovascular risk factors than BMI: A meta-analysis. J Clin Epidemiol. 2008;61:646-53.
8. Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among U.S. adults, 1999-2000. JAMA. 2002;288:1723-7.
9. Ogden CL, Flegal KM, Carroll MD, Johnson CL. Prevalence and trends in overweight among U.S. children and adolescents, 1999-2000. JAMA. 2002;288:1728-32.
10. Finkelstein EA, Fiebelkorn IC, Wang G. State-level estimates of annual medical expenditures attributable to obesity. Obes Res. 2004;12:18-24.
11. Quesenberry CPJr, Caan B, Jacobson A. Obesity, health services use, and health care costs among members of a health maintenance organization. Arch Intern Med. 1998;158:466-72.
12. Thompson D, Edelsberg J, Colditz GA, et al. Lifetime health and economic consequences of obesity. Arch Intern Med. 1999;159:2177-83.
13. Bray GA, Champagne CM. Beyond energy balance: There is more to obesity than kilocalories. J Am Diet Assoc. 2005;105:S17-23.
14. Bray GA. Medical consequences of obesity. J Clin Endocrinol Metab. 2004;89:2583-9.
15. Christakis NA, Fowler JH. The spread of obesity in a large social network over 32 years. N Engl J Med. 2007;357:370-9.
16. Toschke AM, Ehlin AG, von Kries R, et al. Maternal smoking during pregnancy and appetite control in offspring. J Perinat Med. 2003;31:251-6.
17. Harder T, Bergmann R, Kallischnigg G, Plagemann AL. Duration of breastfeeding and risk of overweight: A meta-analysis. Am J Epidemiol. 2005;162:397-403.
18. Taylor RW, Grant AM, Goulding A, Williams SM. Early adiposity rebound: Review of papers linking this to subsequent obesity in children and adults. Curr Opin Clin Nutr Metab Care. 2005;8:607-12.
19. Kroeze WK, Hufeisen SJ, Popadak BA, et al. H1-histamine receptor affinity predicts short-term weight gain for typical and atypical antipsychotic drugs. Neuropsychopharmacology. 2003;28:519-26.
20. Vartanian LR, Schwartz MB, Brownell KD. Effects of soft drink consumption on nutrition and health: A systematic review and meta-analysis. Am J Public Health. 2007;97:667-75.
21. Dhingra R, Sullivan L, Jacques RF, et al. Soft drink consumption and risk of developing cardiometabolic risk factors and the metabolic syndrome in middle-aged adults in the community. Circulation. 2007;116:480-8.
22. Williamson DF, Madans J, Anda RF, et al. Recreational physical activity and ten-year weight change in a U.S. national cohort. Int J Obes Relat Metab Disord. 1993;17:279-86.
23. Ravussin E, Lillioja S, Knowler WC, et al. Reduced rate of energy expenditure as a risk factor for body-weight gain. N Engl J Med. 1988;318:467-72.
24. Church TS, Cheng YJ, Earnest CP, et al. Exercise capacity and body composition as predictors of mortality among men with diabetes. Diabetes Care. 2004;27:83-8.
25. O’Hara P, Connett JE, Lee WW, et al. Early and late weight gain following smoking cessation in the Lung Health Study. Am J Epidemiol. 1998;148:821-30.
26. Dhurandhar NV, Kulkarni PR, Ajinkya SM, et al. Association of adenovirus infection with human obesity. Obes Res. 1997;5:464-9.
27. Farooqi IS, O’Rahilly S. Genetic factors in human obesity. Obes Rev. 2007;8(Suppl 1):37-40.
28. Farooqi IS, Keogh JM, Yeo GS, et al. Clinical spectrum of obesity and mutations in the melanocortin 4 receptor gene. N Engl J Med. 2003;348:1085-95.
29. Rosenbaum M, Goldsmith R, Bloomfield D, et al. Low-dose leptin reverses skeletal muscle, autonomic, and neuroendocrine adaptations to maintenance of reduced weight. J Clin Invest. 2005;115:3579-86.
30. Rankinen T, Zuberi A, Chagnon YC, et al. The human obesity gene map: The 2005 update. Obesity (Silver Spring). 2006;14:529-644.
31. Goldstone AP, Holland AJ, Hauffa BP, et al. Speakers Contributors at the Second Expert Meeting of the Comprehensive Care of Patients with PWS. Recommendations for the diagnosis and management of Prader-Willi syndrome. J Clin Endocrinol Metab. 2008;93:4183-97.
32. Katsanis N, Beales PL, Woods MO, et al. Mutations in MKKS cause obesity, retinal dystrophy and renal malformations associated with Bardet-Biedl syndrome. Nat Genet. 2000;26:67-70.
33. Cummings DE, Schwartz MW. Genetics and pathophysiology of human obesity. Annu Rev Med. 2003;54:453-71.
34. Berthoud HR, Morrison C. The brain, appetite, and obesity. Annu Rev Psychol. 2008;59:55-92.
35. Pissios P, Maratos-Flier E. Melanin-concentrating hormone: From fish skin to skinny mammals. Trends Endocrinol Metab. 2003;14:243-8.
36. Bellocchio L, Cervino C, Pasquali R, Pagotto U. The endocannabinoid system and energy metabolism. J Neuroendocrinol. 2008;20:850-7.
37. Xue B, Kahn BB. AMPK integrates nutrient and hormonal signals to regulate food intake and energy balance through effects in the hypothalamus and peripheral tissues. J. Physiol. 2006;574(Pt 1):73-83.
38. Spalding KL, Arner E, Westermark PO, et al. Dynamics of fat cell turnover in humans. Nature. 2008;453:783-7.
39. Wang ZV, Scherer PE. Adiponectin, cardiovascular function, and hypertension. Hypertension. 2008;51:8-14.
40. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult treatment Panel III). JAMA. 2001;285:2486-97.
41. Park YW, Zhu S, Palaniappan L, et al. The metabolic syndrome: Prevalence and associated risk factor findings in the U.S. population from the Third National Health and Nutrition Examination Survey, 1988-1994. Arch Intern Med. 2003;163:427-36.
42. McTigue K, Larson JC, Valoski A, et al. Mortality and cardiac and vascular outcomes in extremely obese women. JAMA. 2006;296:79-86.
43. Adams KF, Schatzkin A, Harris TB, et al. Overweight, obesity, and mortality in a large prospective cohort of persons 50 to 71 years old. N Engl J Med. 2006;355:763-78.
44. Jee SH, Sull JW, Park J, et al. Body-mass index and mortality in Korean men and women. N Engl J Med. 2006;355:779-87.
45. Freedman DM, Ron E, Ballard-Barbash R, et al. Body mass index and all-cause mortality in a nationwide US cohort. Int J Obes (Lond). 2006;30:822-9.
46. Klein S, Burke LE, Bray GA, et al. American Heart Association Council on Nutrition, Physical Activity, and Metabolism. Clinical implications of obesity with specific focus on cardiovascular disease: A statement for professionals from the American Heart Association Council on Nutrition, Physical Activity, and Metabolism: Endorsed by the American College of Cardiology Foundation. Circulation. 2004;110:2952-67.
47. Black E, Holst C, Astrup A, et al. Long-term influences of body-weight changes, independent of the attained weight, on risk of impaired glucose tolerance and type 2 diabetes. Diabet Med. 2005;22:1199-205.
48. Sjöström L, Lindroos AK, Peltonen M, et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004;351:2683-93.
49. Bogers RP, Bemelmans WJ, Hoogenveen RT, et al. for the BMI-CHD Collaboration Investigators. Association of overweight with increased risk of coronary heart disease partly independent of blood pressure and cholesterol levels: A meta-analysis of 21 cohort studies including more than 300 000 persons. Arch Intern Med. 2007;167:1720-8.
50. Poirier P, Giles TD, Bray GA, et al. American Heart Association; Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Obesity and cardiovascular disease: Pathophysiology, evaluation, and effect of weight loss: An update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2006;113:898-918.
51. Emberson JR, Whincup PH, Morris RW, et al. Lifestyle and cardiovascular disease in middle-aged British men: The effect of adjusting for within-person variation. Eur Heart J. 2005;26:1774-82.
52. Ingelsson E, Sundström J, Arnlöv J, et al. Insulin resistance and risk of congestive heart failure. JAMA. 2005;294:334-41.
53. Wang TJ, Parise H, Levy D, et al. Obesity and the risk of new-onset atrial fibrillation. JAMA. 2004;292:2471-7.
54. Yusuf S, Hawken S, Ounpuu S, et al. INTERHEART Study Investigators. Obesity and the risk of myocardial infarction in 27,000 participants from 52 countries: A case-control study. Lancet. 2005;366:1640-9.
55. Rocchini AP. Obesity and blood pressure regulation. In: Bray G, Bouchard C, editors. Handbook of obesity: Clinical applications. 2nd ed. New York: Marcel Dekker; 2002:873.
56. Kambham N, Markowitz GS, Valeri AM, et al. Obesity-related glomerulopathy: An emerging epidemic. Kidney Int. 2001;59:1498-509.
57. Taylor EN, Stampfer MJ, Curhan GC. Obesity, weight gain, and the risk of kidney stones. JAMA. 2005;293:455-62.
58. Hsu CY, McCulloch CE, Iribarren C, et al. Body mass index and risk for end-stage renal disease. Ann Intern Med. 2006;144:21-8.
59. Ko CW, Lee SP. Obesity and gallbladder disease. In: Bray GA, Bouchard C, editors. Handbook of obesity: Etiology and pathophysiology. New York: Marcel Dekker; 2003:919.
60. Caroli-Bosc FX, Pugliese P, Peten EP, et al. Gallbladder volume in adults and its relationship to age, sex, body mass index, body surface area and gallstones. An epidemiologic study in a nonselected population in France. Digestion. 1999;60:344-8.
61. Matteoni CA, Younossi ZM, Gramlich T, et al. Nonalcoholic fatty liver disease: A spectrum of clinical and pathological severity. Gastroenterology. 1999;116:1413-19.
62. Bellentani S, Saccoccio G, Masutti F, et al. Prevalence of and risk factors for hepatic steatosis in Northern Italy. Ann Intern Med. 2000;132:112-17.
63. Angelico F, Del Ben M, Conti R, et al. Insulin resistance, the metabolic syndrome, and nonalcoholic fatty liver disease. J Clin Endocrinol Metab. 2005;90:1578-82.
64. Hamaguchi M, Kojima T, Takeda N, et al. The metabolic syndrome as a predictor of nonalcoholic fatty liver disease. Ann Intern Med. 2005;143:722-8.
65. Hampel H, Abraham NS, El-Serag HB. Meta-analysis: obesity and the risk for gastroesophageal reflux disease and its complications. Ann Intern Med. 2005;143:199-211.
66. Utzschneider KM, Kahn SE. Review: The role of insulin resistance in nonalcoholic fatty liver disease. J Clin Endocrinol Metab. 2006;91:4753-61.
67. Manson JE, Willett WC, Stampfer MJ, et al. Body weight and mortality among women. N Engl J Med. 1995;333:677-85.
68. Adams KF, Leitzmann MF, Albanes D, et al. Body mass and colorectal cancer risk in the NIH-AARP cohort. Am J Epidemiol. 2007;166:36-45.
69. Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625-38.
70. Schapira DV, Clark RA, Wolff PA, et al. Visceral obesity and breast cancer risk. Cancer. 1994;74:632-9.
71. Sweeney C, Blair CK, Anderson KE, et al. Risk factors for breast cancer in elderly women. Am J Epidemiol. 2004;160:868-75.
72. Eliassen AH, Colditz GA, Rosner B, et al. Adult weight change and risk of postmenopausal breast cancer. JAMA. 2006;296:193-201.
73. Mannisto S, Smith-Warner S. Body mass index, height and risk of renal cell cancer in a pooled analysis of 13 cohort studies. Obes Rev. 2006;7(Suppl 2):106-7.
74. Rich-Edwards JW, Goldman MB, Willett WC, et al. Adolescent body mass index and infertility caused by ovulatory disorder. Am J Obstet Gynecol. 1994;171:171-7.
75. Rosenberg TJ, Garbers S, Lipkind H, Chiasson MA. Maternal obesity and diabetes as risk factors for adverse pregnancy outcomes: Differences among 4 racial/ethnic groups. Am J Public Health. 2005;95:1545-51.
76. Usha Kiran TS, Hemmadi S, Bethel J, Evans J. Outcome of pregnancy in a woman with an increased body mass index. BJOG. 2005;112:768-72.
77. Smith GC, Shah I, Pell JP, et al. Maternal obesity in early pregnancy and risk of spontaneous and elective preterm deliveries: A retrospective cohort study. Am J Public Health. 2007;97:157-62.
78. Baik I, Curhan GC, Rimm EB, et al. A prospective study of age and lifestyle factors in relation to community-acquired pneumonia in U.S. men and women. Arch Intern Med. 2000;160:3082-8.
79. Subramanian S, Strohl KP. Obesity and pulmonary function. In: Bray GA, Bouchard C, editors. Handbook of obesity: Evaluation and treatment. New York: Marcel Dekker; 2004:935.
80. Young T, Peppard PE, Taheri S. Excess weight and sleep-disordered breathing. J Appl Physiol. 2005;99:1592-9.
81. Felson DT, Anderson JJ, Naimark A, et al. Obesity and knee osteoarthritis. The Framingham Study. Ann Intern Med. 1988;109:18-24.
82. Okoro CA, Hootman JM, Strine TW, et al. Disability, arthritis, and body weight among adults 45 years and older. Obes Res. 2004;12:854-61.
83. Messier SP, Gutekunst DJ, Davis C, DeVita P. Weight loss reduces knee-joint loads in overweight and obese older adults with knee osteoarthritis. Arthritis Rheum. 2005;52:2026-32.
84. Griffin FM, Scuderi GR, Insall JN, Colizza W. Total knee arthroplasty in patients who were obese with 10 years’ follow-up. Clin Orthop Relat Res. 1998;356:28-33.
85. Escalante A, Haas RW, del Rincón I. Paradoxical effect of body mass index on survival in rheumatoid arthritis: Role of comorbidity and systemic inflammation. Arch Intern Med. 2005;165:1624-9.
86. Garcia Hidalgo L. Dermatological complications of obesity. Am J Clin Dermatol. 2002;3:497-506.
87. Gortmaker SL, Must A, Perrin JM, et al. Social and economic consequences of overweight in adolescence and young adulthood. N Engl J Med. 1993;329:1008-12.
88. Williams J, Wake M, Hesketh K, et al. Health-related quality of life of overweight and obese children. JAMA. 2005;293:70-6.
89. Strauss RS, Pollack HA. Social marginalization of overweight children. Arch Pediatr Adolesc Med. 2003;157:746-52.
90. Latner JD, Stunkard AJ. Getting worse: The stigmatization of obese children. Obes Res. 2003;11:452-6.
91. Fontaine KR, Cheskin LJ, Barofsky I. Health-related quality of life in obese persons seeking treatment. J Fam Pract. 1996;43:265-70.
92. Carpenter KM, Hasin DS, Allison DB, Faith MS. Relationships between obesity and DSM-IV major depressive disorder, suicide ideation, and suicide attempts: Results from a general population study. Am J Public Health. 2000;90:251-7.
93. Gorospe EC, Dave JK. The risk of dementia with increased body mass index. Age. Aging. 2007;36:23-9.
94. Sjöström L, Narbro K, Sjöström CD, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007;357:741-52.
95. Adams TD, Gress RE, Smith SC, et al. Long-term mortality after gastric bypass surgery. N Engl J Med. 2007;357:753-61.
96. Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393-403.
97. Moore LL, Visioni AJ, Qureshi MM, et al. Weight loss in overweight adults and the long-term risk of hypertension: the Framingham study. Arch Intern Med. 2005;165:1298-303.
98. Stevens VJ, Obarzanek E, Cook NR, et al. Trials for the Hypertension Prevention Research Group. Long-term weight loss and changes in blood pressure: Results of the Trials of Hypertension Prevention, phase II. Ann Intern Med. 2001;134:1-11.
99. Peppard PE, Young T, Palta M, et al. Longitudinal study of moderate weight change and sleep-disordered breathing. JAMA. 2000;284:3015-21.
100. Douketis JD, Macie C, Thabane L, et al. Systematic review of long-term weight loss studies in obese adults: Clinical significance and applicability to clinical practice. Int J Obes (Lond). 2005;29:1153-67.
101. Coakley EH, Kawachi I, Manson JE, et al. Lower levels of physical functioning are associated with higher body weight among middle-aged and older women. Int J Obes Relat Metab Disord. 1998;22:958-65.
102. U.S. Preventive Services Task Force. Screening and interventions to prevent obesity in adults. http://www.ahrq.gov/clinic/uspstf/uspsobes.htm, 2003. Available at
103. Bray GA. Classification and evaluation of the overweight patient. In: Bray GA, Bouchard C, editors. Handbook of obesity: Clinical applications. 3rd ed. New York: Informa Healthcare; 2008:1.
104. U.S. Department of Health and Human Services. Body measurements. In: Clinician’s handbook of preventive services: Put prevention into family practice. Washington, DC: U.S. Government Printing Office; 1994:141.
105. Gallagher D, Heymsfield SB, Heo M, et al. Healthy percentage body fat ranges: An approach for developing guidelines based on body mass index. Am J Clin Nutr. 2000;72:694-701.
106. Hozawa A, Okamura T, Oki I, et al. NIPPON DATA80 Study Group. Relationship between BMI and all-cause mortality in Japan: NIPPON DATA80. Obesity (Silver Spring). 2008;16:1714-17.
107. Appel LJ, Moore TJ, Obarzanek E, et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N Engl J Med. 1997;336:1117-24.
108. Robinson TN. Reducing children’s television viewing to prevent obesity: A randomized controlled trial. JAMA. 1999;282:1561-7.
109. James J, Thomas P, Cavan D, Kerr D. Preventing childhood obesity by reducing consumption of carbonated drinks: Cluster randomised controlled trial. BMJ. 2004;328:1237.
110. Foster GD, Wadden TA, Vogt RA, Brewer G. What is a reasonable weight loss? Patients’ expectations and evaluations of obesity treatment outcomes. J Consult Clin Psychol. 1997;65:79-85.
111. Rating the diets from Atkins to Dr. Sears Zone. Consum Rep. 2005;70:18-22.
112. Dansinger ML, Tatsioni A, Wong JB, et al. Meta-analysis: The effect of dietary counseling for weight loss. Ann Intern Med. 2007;147:41-50.
113. Ornish D, Scherwitz LW, Billings JH, et al. Intensive lifestyle changes for reversal of coronary heart disease. JAMA. 1998;280:2001-7.
114. Pirozzo S, Summerbell C, Cameron C, et al. Should we recommend low-fat diets for obesity? Obes Rev. 2003;4:83-90.
115. Howard BV, Manson JE, Stefanick ML, et al. Low-fat dietary pattern and weight change over 7 years: The Women’s Health Initiative Dietary Modification Trial. JAMA. 2006;295:39-49.
116. Astrup A, Grunwald GK, Melanson EL, et al. The role of low-fat diets in body weight control: A meta-analysis of ad libitum dietary intervention studies. Int J Obes Relat Metab Disord. 2000;24:1545-52.
117. Rolls BJ, Barnett RA. Volumetrics: Feel full on fewer calories. New York: Harper Collins; 2000.
118. Boden G, Sargrad K, Homko C, et al. Effect of a low-carbohydrate diet on appetite, blood glucose levels, and insulin resistance in obese patients with type 2 diabetes. Ann Intern Med. 2005;142:403-11.
119. Nordmann AJ, Nordmann A, Briel M, et al. Effects of low-carbohydrate vs low-fat diets on weight loss and cardiovascular risk factors: A meta-analysis of randomized controlled trials. Arch Intern Med. 2006;166:285-93.
120. Dansinger ML, Gleason JA, Griffith JL, et al. Comparison of the Atkins, Ornish, Weight Watchers, and Zone diets for weight loss and heart disease risk reduction: A randomized trial. JAMA. 2005;293:43-53.
121. Gardner CD, Kiazand A, Alhassan S, et al. Comparison of the Atkins, Zone, Ornish, and LEARN diets for change in weight and related risk factors among overweight premenopausal women: The A TO Z Weight Loss Study: A randomized trial. JAMA. 2007;297:969-77.
122. Shai I, Schwarzfuchs D, Henkin Y, et al. Dietary Intervention Randomized Controlled Trial (DIRECT) Group. Weight loss with a low-carbohydrate, Mediterranean, or low-fat diet. N Engl J Med. 2008;359:229-41.
123. Sacks FM, Bray GA, Carey VJ, et al. Comparison of weight loss diets with different compositions of fat, protein, and carbohydrates. N Engl J Med. 2009;360:859-73.
124. Anderson JW, Konz EC, Frederich RC, Wood CL. Long-term weight loss maintenance: A meta-analysis of US studies. Am J Clin Nutr. 2001;74:579-84.
125. Flechtner-Mors M, Ditschuneit HH, Johnson TD, et al. Metabolic and weight loss effects of long-term dietary intervention in obese patients: Four-year results. Obes Res. 2000;8:399-402.
126. Tsai AG, Wadden TA. Systematic review: An evaluation of major commercial weight loss programs in the United States. Ann Intern Med. 2005;142:56-66.
127. Heshka S, Anderson JW, Atkinson RL, et al. Weight loss with self-help compared with a structured commercial program: A randomized trial. JAMA. 2003;289:1792-8.
128. Wing RR, Phelan S. Long-term weight loss maintenance. Am J Clin Nutr. 2005;82(Suppl):222S-5S.
129. Tate DF, Jackvony EH, Wing RR. A randomized trial comparing human e-mail counseling, computer-automated tailored counseling, and no counseling in an Internet weight loss program. Arch Intern Med. 2006;166:1620-5.
130. McTiernan A, Sorensen B, Irwin ML, et al. Exercise effect on weight and body fat in men and women. Obesity (Silver Spring). 2007;15:1496-512.
131. Jakicic JM, Marcus BH, Lang W, Janney C. Effect of exercise on 24-month weight loss maintenance in overweight women. Arch Intern Med. 2008;168:1550-9.
132. Bohannon RW. Number of pedometer-assessed steps taken per day by adults: A descriptive meta-analysis. Phys Ther. 2007;87:1642-50.
133. Li Z, Maglione M, Tu W, et al. Meta-analysis: Pharmacologic treatment of obesity. Ann Intern Med. 2005;142:532-46.
134. Bray G, Greenway F. Pharmacological treatment of the overweight patient. Pharmacol Rev. 2007;59:151-84.
135. Rucker D, Padwal R, Li SK, et al. Long-term pharmacotherapy for obesity and overweight: updated meta-analysis. BMJ. 2007;335:1194-9.
136. Bray GA, Blackburn GL, Ferguson JM, et al. Sibutramine: A dose-ranging and long-term efficacy study in the treatment of obesity. Obes Res. 1999;7:189-98.
137. Smith IG, Goulder MA. Sibutramine Clinical Study 1047 Team. Randomized placebo-controlled trial of long-term treatment with sibutramine in mild to moderate obesity. J Fam Pract. 2001;50:505-12.
138. Apfelbaum M, Vague P, Ziegler O, et al. Long-term maintenance of weight loss after a very-low-calorie diet: A randomized blinded trial of the efficacy and tolerability of sibutramine. Am J Med. 1999;106:179-84.
139. James WP, Astrup A, Finer N, et al. Effect of sibutramine on weight maintenance after weight loss: A randomized trial. STORM Study Group. Sibutramine Trial of Obesity Reduction and Maintenance. Lancet. 2000;356:2119-25.
140. Sjöström L, Rissanen A, Andersen T, et al. Randomised placebo-controlled trial of orlistat for weight loss and prevention of weight regain in obese patients. European Multicentre Orlistat Study Group. Lancet. 1998;352:167-72.
141. Richelsen B, Tonstad S, Rössner S, et al. Effect of orlistat on weight regain and cardiovascular risk factors following a very-low-energy diet in abdominally obese patients: A 3-year randomized, placebo-controlled study. Diabetes Care. 2007;30:27-32.
142. Torgerson JS, Hauptman J, Boldrin MN, Sjöström L. XENical in the prevention of diabetes in obese subjects (XENDOS) study: A randomized study of orlistat as an adjunct to lifestyle changes for the prevention of type 2 diabetes in obese patients. Diabetes Care. 2004;27:155-61.
143. Avenell A, Brown TJ, McGee MA, et al. What interventions should we add to weight reducing diets in adults with obesity? A systematic review of randomized controlled trials of adding drug therapy, exercise, behaviour therapy or combinations of these interventions. J Hum Nutr Diet. 2004;7:293-316.
144. Goldstein DJ, Rampey AHJr, Roback PJ, et al. Efficacy and safety of long-term fluoxetine treatment of obesity—maximizing success. Obes Res. 1995;3(Suppl 4):481S-490S.
145. Jain AK, Kaplan RA, Gadde KM, et al. Bupropion SR vs. placebo for weight loss in obese patients with depressive symptoms. Obes Res. 2002;10:1049-56.
146. Anderson JW, Greenway FL, Fujioka K, et al. Bupropion SR enhances weight loss: A 48-week double-blind, placebo-controlled trial. Obes Res. 2002;10:633-41.
147. Astrup A, Toubro S. Topiramate: A new potential pharmacological treatment for obesity. Obes Res. 2004;12(Suppl):167S-173S.
148. Bray GA, Hollander P, Klein S, et al. A 6-month randomized, placebo-controlled, dose-ranging trial of topiramate for weight loss in obesity. Obes Res. 2003;11:722-33.
149. Wilding J, Van Gaal L, Rissanen A, et al. A randomized double-blind placebo-controlled study of the long-term efficacy and safety of topiramate in the treatment of obese subjects. Int J Obes Relat Metab Disord. 2004;28:1399-410.
150. Astrup A, Caterson I, Zelissen P, et al. Topiramate: Long-term maintenance of weight loss induced by a low-calorie diet in obese subjects. Obes Res. 2004;12:1658-69.
151. Rosenstock J, Hollander P, Gadde KM, et al. OBD-202 Study Group. A randomized, double-blind, placebo-controlled, multicenter study to assess the efficacy and safety of topiramate controlled release in the treatment of obese type 2 diabetic patients. Diabetes Care. 2007;30:1480-6.
152. Gadde KM, Franciscy DM, Wagner HR2nd, et al. Zonisamide for weight loss in obese adults: A randomized controlled trial. JAMA. 2003;289:1820-5.
153. Fontbonne A, Charles MA, Juhan-Vague I, et al. The effect of metformin on the metabolic abnormalities associated with upper-body fat distribution. BIGPRO Study Group. Diabetes Care. 1996;19:920-6.
154. Ortega-Gonzalez C, Luna S, Hernandez L, et al. Responses of serum androgen and insulin resistance to metformin and pioglitazone in obese, insulin-resistant women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2005;90:1360-5.
155. Riddle MC, Drucker DJ. Emerging therapies mimicking the effects of amylin and glucagon-like peptide 1. Diabetes Care. 2006;29:435-49.
156. Ratner RE, Dickey R, Fineman M, et al. Amylin replacement with pramlintide as an adjunct to insulin therapy improves long-term glycaemic and weight control in Type 1 diabetes mellitus: A 1-year, randomized controlled trial. Diabet Med. 2004;21:1204-12.
157. Maggs D, Shen L, Strobel S, et al. Effect of pramlintide on A1C and body weight in insulin-treated African Americans and Hispanics with type 2 diabetes: A pooled post hoc analysis. Metabolism. 2003;52:1638-42.
158. Small CJ, Bloom SR. Gut hormones as peripheral anti obesity targets. Curr Drug Targets CNS Neurol Disord. 2004;3:379-88.
159. DeFronzo RA, Ratner RE, Han J, et al. Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes Care. 2005;28:1092-100.
160. Kendall DM, Riddle MC, Rosenstock J, et al. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in patients with type 2 diabetes treated with metformin and a sulfonylurea. Diabetes Care. 2005;28:1083-91.
161. Buse JB, Henry RR, Han J, et al. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in sulfonylurea-treated patients with type 2 diabetes. Diabetes Care. 2004;27:2628-35.
162. Heine RJ, Van Gaal LF, Johns D, et al. Exenatide versus insulin glargine in patients with suboptimally controlled type 2 diabetes: A randomized trial. Ann Intern Med. 2005;143:559-69.
163. Weintraub M. Long-term weight control: the National Heart, Lung, and Blood Institute funded multimodal intervention study. Clin Pharmacol Ther. 1992;51:581-5.
164. Connolly HM, Crary JL, McGoon MD, et al. Valvular heart disease associated with fenfluramine-phentermine. N Engl J Med. 1997;337:581-8.
165. Steinbrook R. Surgery for severe obesity. N Engl J Med. 2004;350:1075-9.
166. Sjöström L, Lindroos AK, Peltonen M, et al. Swedish Obese Subjects Study Scientific Group. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004;351:2683-93.
167. Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292:1724-37.