9 OA-PICA Bypass
Introduction
Yasargil et al.1 demonstrated the feasibility of EC-IC bypass surgery in 1967 after performing the first successful superficial temporal artery to middle cerebral artery anastomosis. Ausman and colleagues2 applied these techniques for vertebral artery (VA) occlusive disease and reported the first intracranial posterior circulation revascularization procedure in 1976 after performing an occipital artery (OA) to posterior inferior cerebellar artery (PICA) anastomosis. Posterior circulation bypass surgery has since been used to treat vertebrobasilar insufficiency, skull base tumors involving the VA and its branches, and complex or giant posterior circulation aneurysms. Current advances in endovascular techniques and the availability of intracranial stents have largely limited the need for posterior circulation bypass surgery for vertebrobasilar insufficiency. Occipital artery–PICA bypass surgery, however, remains a valuable tool in the management of fusiform aneurysms of the VA encompassing the PICA origin as well as giant and complex VA/PICA and PICA aneurysms that cannot be reconstructed with surgical clipping or endovascular coiling and require parent artery occlusion or trapping. In the context of VB insufficiency, the bypass may still have a role in the rare settings of poor endovascular access due to tortuosity or occlusion. This chapter reviews the relevant anatomy, surgical technique, perioperative care, and complications associated with OA-PICA revascularization.
Anatomy
Occipital Artery
The OA is an ideal choice for PICA revascularization as it can be encountered during the suboccipital approach and closely approximates the size of the PICA. The course of the OA can be divided into three segments.3
The first segment, also known as the digastric segment, extends from the origin of the OA from the external carotid artery to the point of emergence from the occipital groove of the mastoid process. The OA originates from the posterior or lateral wall of the external carotid artery at the level of the angle of the mandible. The artery ascends medial to the external carotid artery and lateral to the internal jugular vein to a point posterior and medial to the styloid process. The OA then courses posteriorly and laterally superficial to the rectus capitus lateralis muscle first and then superior oblique muscle.4 The artery is covered by the posterior belly of the digastric muscle laterally, hence known as the digastric segment. The artery then runs in the occipital groove or occasionally a true bony canal3 medial to the mastoid notch, in which the posterior belly of the digastric muscle arises.4
The second segment, also known as the suboccipital or horizontal segment, extends from the emergence of the OA from the occipital groove of the mastoid process to the superior nuchal line. The OA exits the occipital groove between the superior oblique muscle and posterior belly of the digastric and is covered by the splenium capitis and sternocleidomastoid. The artery courses medially in a horizontal plane either superficial or deep to the longissimus capitus muscle, depending on whether the occipital groove is absent or present. The OA then continues superficial to the semispinalis capitis muscle just below the superior nuchal line in the upper part of the posterior triangle. The artery then changes course and runs vertically upward, piercing the fascia connecting the cranial attachment of the trapezius and sternocleidomastoid muscles to the superior nuchal line.5 The suboccipital segment gives rise to ascending and descending muscular branches as well as transosseous branches to the dura of the posterior fossa. The diameter of the suboccipital segment ranges from 1.6 to 2.2 mm (mean 1.9 mm) and the length ranges from 75 to 85 mm (range 79.3 mm).6
The third segment, also known as the occipital or subgaleal segment, begins at the superior nuchal line after the OA pierces the fascial attachment of the trapezius and sternocleidomastoid muscles. In a cadaveric study, the OA was found to cross the superior nuchal line approximately 35 mm (±0.5 mm) lateral to the inion.3 The artery continues underneath the galea and above the occipitalis muscle before dividing into its terminal branches. The diameter of the OA at the superior nuchal line is approximately 1.4 mm (±0.3 mm).3
PICA Artery
Segments of the PICA
It is very convenient and appropriate to subscribe to the concept advanced by Lister et al.,7 that the PICA can be divided into five segments based on its relationship to the medulla and the cerebellum: anterior medullary, lateral medullary, tonsillomedullary, telovelotonsillar, and cortical.
The anterior medullary segment begins at the origin of the PICA from the VA anterior to the medulla oblongata. Congenital anomalies of the PICA include double origin, fenestration, or duplicated PICAs, a common anterior inferior cerebellar artery (AICA)–PICA configuration, a VA termination at the PICA, extradural origins at C1 and C2 levels (5% to 20%), origins at the hypoglossal, proatlantal, or posterior meningeal arteries, and at all points along the intradural VA.8–15 From its origin, the artery runs posteriorly around the medulla past the exit of the hypoglossal nerve rootlets from the anterior border of the inferior olivary complex to the boundary between the anterior and lateral surfaces of the medulla, which is marked by a rostrocaudal line through the most prominent part of the inferior olive. The lateral medullary segment begins at the point where the PICA passes the most prominent part of the inferior olive and extends posteriorly around the lateral aspect of the medulla to the origin of the glossopharyngeal, vagus, and accessory rootlets from the posterior border of the inferior olivary complex. The PICA continues medially as the tonsillomedullary segment between the lower half of the cerebellar tonsil and the posterior aspect of the medulla oblongata. The artery makes a caudally convex curve as it passes around the lower pole of the cerebellar tonsil known as the caudal or infratonsillar loop. The caudal loop is frequently used as a recipient during OA-PICA bypasses and its diameter ranges from 0.9 to 1.4 mm (mean 1.2 mm).6 After forming the caudal loop, the PICA ascends to the midlevel of the medial surface of the tonsil where it becomes the telovelotonsillar segment. The artery continues along the medial surface of the tonsil toward the roof of the fourth ventricle. The PICA then forms a rostrally convex curve referred to as the cranial or supratonsillar loop. This loop consists of proximal ascending and distal descending limbs and an apex that lies caudal to the fastigium at the center of the inferior medullary velum. The ascending limb runs posterior to the tela choroidea and inferior medullary velum toward the fastigium of the fourth ventricle. The descending limb runs posteriorly in the fissure between the vermis medially and the superomedial surface of the tonsil and cerebellar hemisphere laterally. The PICA emerges from the fissure and continues as the cortical segment. The artery divides into a smaller medial and a larger lateral trunk and subsequently gives rise to hemispheric, vermian, and tonsillar branches.
Branches of the PICA
The PICA gives rise to three types of branches:7
Preoperative evaluation
Physiological imaging modalities such as positron emission tomography, xenon computed tomography, single-photon emission computed tomography, computed tomography perfusion, and magnetic resonance perfusion commonly used to detect hemodynamic compromise in anterior circulation occlusive disease are less effective in assessing the posterior circulation as a result of their limited regional resolution.16 Furthermore, the validity of these imaging modalities in detecting posterior circulation hypoperfusion remains uncertain. Phase-contrast quantitative magnetic resonance angiography (QMRA) has become available in recent years and is capable of directly measuring volumetric blood flow (milliliters per minute) through the major vessels of the posterior (as well as anterior) circulation. The technique is now implemented and enhanced in commercially available software called the NOVA (Noninvasive Optimal Vessel Analysis) system (VasSol, Inc., Chicago, IL). Table 9–1 shows the mean blood flow values and ranges for posterior circulation vessels in 50 healthy patients.17 In a retrospective study of 47 patients with symptomatic vertebrobasilar disease, patients with >20% reduction of blood flow in the basilar artery (<120 cc/min) and PCAs (<40 cc/min) had a higher risk of stroke as compared to patients with preserved blood flow.17 The VERiTAS (Vertebrobasilar Flow Evaluation and Risk of Transient Ischemic Attack and Stroke) is an ongoing prospective multicenter observational study funded by the National Institutes of Health and aimed at determining the utility of QMRA in assessing patients with symptomatic vertebrobasilar occlusive disease of ≥50%. If predictive, QMRA evaluation could help identify high-risk patients who would benefit most from either surgical revascularization or endovascular angioplasty and stenting.
Table 9–1 The Mean Value and Ranges of Blood Flow for Posterior Circulation Vessels in 50 Healthy Volunteers.
| Vessel | Mean Flow (cc/min) | Range* (cc/min) |
|---|---|---|
| BA | 190 | 150–230 |
| LPCA | 72 | 50–94 |
| RPCA | 68 | 50–86 |
| LVA | 126 | 94–158 |
| RVA | 110 | 81–139 |
BA, indicates basilar artery; PCA, posterior cerebral artery; VA, vertebral artery; L, left; R, right.
Anesthetic technique and neuroprotection
The surgery is performed under general anesthesia. Hypotension must be avoided during the initial part of the procedure particularly in patients with marginal cerebral perfusion. Hyperventilation and alpha-adrenergic agents are not recommended because of their vasoconstrictive effects. We routinely use modest hypothermia (33°C) throughout the procedure as well as induced blood pressure elevation to 20% to 30% of baseline during the period of temporary PICA cross-clamping to augment collateral flow. Electrophysiologic monitoring using somatosensory and motor-evoked potentials and brainstem auditory-evoked potentials allows early detection of ischemia or excessive retraction and manipulation. Barbiturates have been used during bypass surgery to increase tolerance to cerebral hypoperfusion. The mechanisms of barbiturate neuroprotection are multifactorial and incompletely understood. It is believed that a reversible, dose-dependent depression of cerebral blood flow occurs, with subsequent reduction in cerebral metabolic rate and intracranial pressure.18–21 Furthermore, vasoconstriction in normal areas of the brain may result in an inverse steal phenomenon with redistribution of cerebral blood flow to ischemic tissue.22 At a cellular level, barbiturates have been demonstrated to reduce ischemia-induced glutamate release,23 enhance GABA-ergic transmission,24,25 and reduce ischemia-induced intracellular calcium through inhibition of both voltage-gated calcium channels and NMDA receptors.26 In addition to the fore mentioned neuroprotective properties, barbiturates may also act as a scavenger of membrane-damaging free radicals. Despite these potential benefits, we do not routinely use barbiturate neuroprotection during OA-PICA bypass surgery due to problems associated with circulatory and respiratory depression as well as delayed postoperative wake-up. However, if technical difficulties during the anastomosis result in excessively prolonged temporary occlusion, we may consider using barbiturates.
Surgical technique
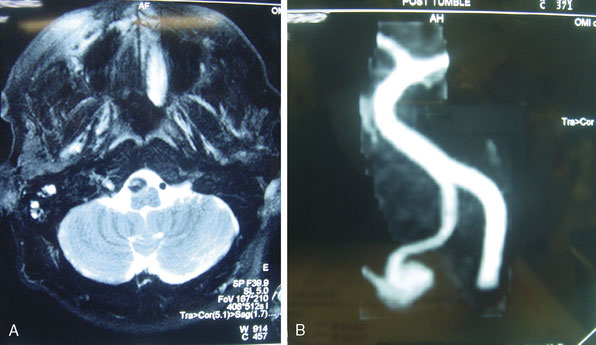
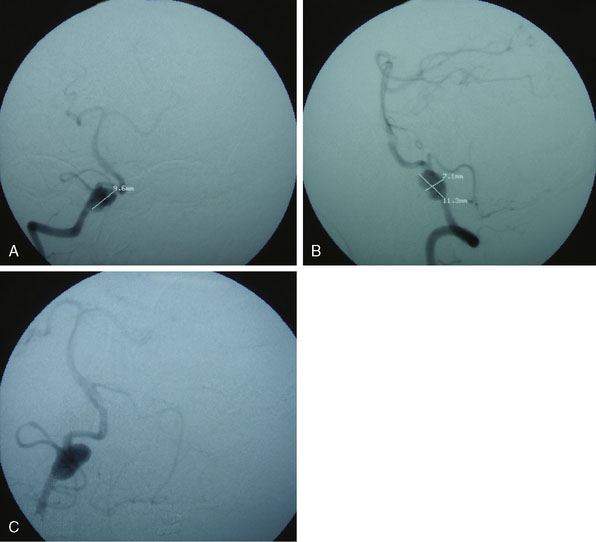
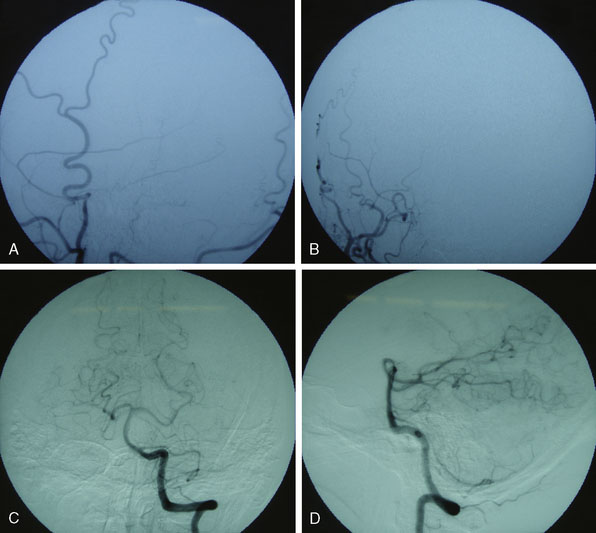
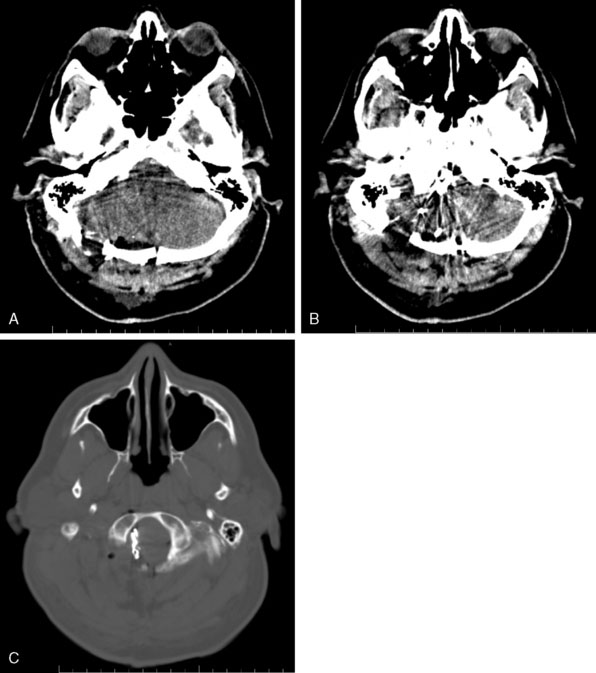
Figures 9-1 through 9-3 show preoperative imaging of a patient diagnosed with fusiform right VA-PICA origin large aneurysm.
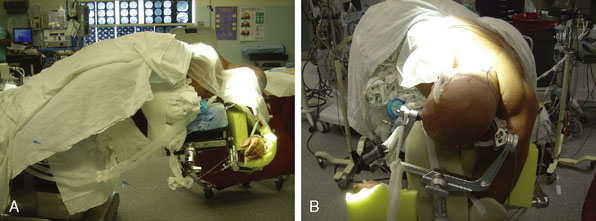
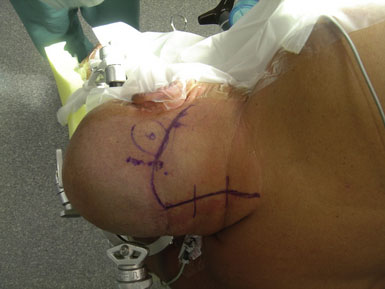
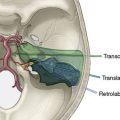
The patient is either placed prone (more likely for a pure ischemia case), or more commonly in a three-quarter prone position (more likely for an aneurysm or tumor case) for a far lateral approach if access to the PICA origin is necessary. For the prone position, the head is placed in moderate flexion and secured in a three-pin head holder. Proper Mayfield pin placement is crucial, as it may hinder the procedure if placed improperly. The pins are placed so that the single pin is 2 cm superior and anterior to the pinna ipsilateral to the donor OA. The paired pins are positioned so that the posterior pin is 2 cm above the contralateral ear pinna. The head is positioned above the level of the heart to reduce cerebral venous congestion. In the three-quarter prone position, the head is positioned with four movements (Figure 9–4):
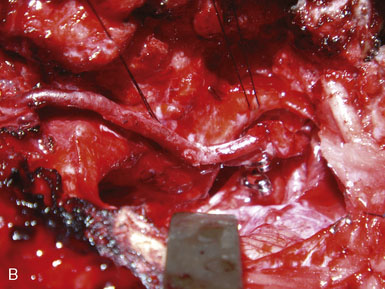
A portable transcutaneous Doppler probe is used to identify the course of the OA over the scalp from the mastoid to approximately 4 cm above the superior nuchal line (Figure 9–5). Scalp infiltration solutions containing vasoconstrictive agents should not be used. A hockey-stick incision is then made. The incision starts approximately at the level of the spinous process of C3 and extends superiorly in the avascular midline plane to approximately 2 cm above the superior nuchal line. The incision is then turned laterally parallel to the superior nuchal line. As this limb of the incision approaches the point at which the distal OA crosses the incision, a curved hemostat is used to dissect over and protect the OA. The incision is then continued over the OA to a point immediately superior to the mastoid process. Finally, the incision is curved inferiorly to end just inferior to the mastoid tip. The distal OA is then identified and dissected proximally in its muscular plane. Although this may be performed under the operative microscope, in our opinion loupe magnification is sufficient and more efficient. Dissection of the OA is generally the most difficult part of the procedure due to the tortuosity and adherence of the artery to the surrounding tissue. The OA is typically surrounded by a venous plexus and runs with the occipital nerve in a fascial sheath. A generous cuff of periadventitial tissue is left around the artery. Small side branches are carefully coagulated using low current bipolar forceps, so as not to cause thermal injury to the parent artery. The side branches are then sectioned at a distance from their origin from the OA trunk. It is important to dissect the artery as far proximally as the occipital groove to ensure adequate length of the graft. The OA is wrapped in a papaverine-soaked cottonoid to relieve spasms related to vessel manipulation, and is left in continuity until just before it is required for the anastomosis (Figure 9–6). The suboccipital musculature is swept laterally in a subperiosteal fashion to expose the occiput as far laterally as the mastoid process as well as the arch of C1. The skin and muscle flap are retracted inferolaterally and held in position by fish hooks. It is important to leave a muscle cuff attached to the superior nuchal line. This facilitates tight muscle closure at the end of the procedure, as a water-tight dural closure is not possible because of the necessity of creating an opening for passage of the OA.
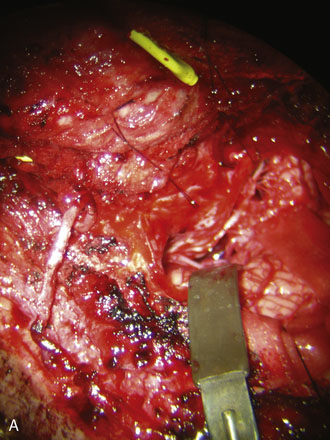
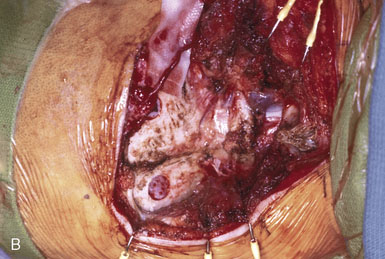
An ipsilateral suboccipital craniotomy extending just across the midline and a C1 hemi-laminectomy are performed. The craniotomy may be extended to a far lateral transcondylar approach by exposure of the sigmoid sinus and resection of the posterior medial third of the occipital condyle if access to the PICA origin is required. Any opened mastoid air cells must be thoroughly sealed with bone wax to avoid postoperative cerebrospinal fluid leaks. The dura is opened in the midline at the level of C1 and extended in a curvilinear fashion to the superolateral extent of the exposure. An additional incision is made from the caudal end of the dural incision toward the C1/C2 joint, caudal to the vertebral artery ring. The dural flap is then sutured to the surrounding tissues with 4-0 neurolon traction sutures (Figure 9–7).
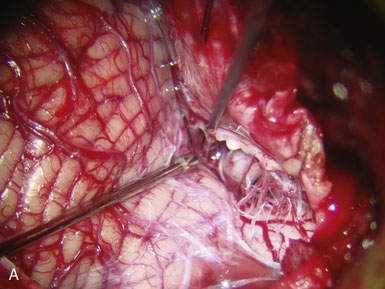
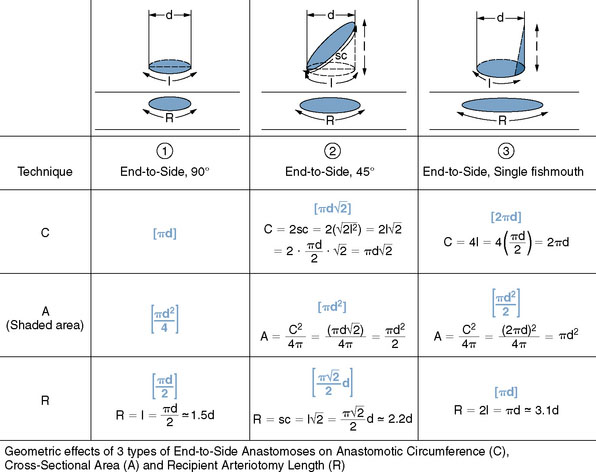
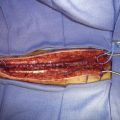
The OA is then prepared for the anastomosis. A temporary clip is first applied to the proximal end of the artery, which is then cut distally at an appropriate length for the bypass. The occipital artery is notoriously tortuous and amenable to lengthening by “undoing” the various turns and loops. It is, however, critical to let the artery find its own natural contour after dissecting it, to avoid forcing an unnatural kink that might result in graft occlusion. The mean length of OA required for a PICA bypass is 58 mm (range 54 to 60 mm).6 The distal 1 cm of the artery is then stripped of its periadventitial layer. Although this step may be easier to perform while the OA is still in continuity, because of the benefit of countertraction, we prefer to first section the artery to the required length for the bypass to avoid having to repeat the periadventitial stripping if the artery proves too long. There are several ways that the distal OA—or any donor vessel, for that matter—can be prepared, but the three most common techniques are depicted in Figure 9–8, and are as follows:
The latter technique is our favored technique because it increases the cross-sectional area available for the anastomosis and provides redundancy of donor artery wall, thereby minimizing the possibility of stenosis at the site of anastomosis. We firmly believe that end-to-side anastomoses in general fare better if an “elephant foot design” is achieved, with the redundant edges of the donor allowing a flaring of the completed anastomosis and a reduced risk of stenosis or occlusion. Furthermore, the obliquity of the resulting construct allows flow to be preferentially directed toward the proximal PICA and its medullary branches. Using simple high school mathematics (Pythagorean theorem, circumference, and area formulas of circles), we can easily show in Figure 9–8 that the three different techniques result in three different anastomosis circumferences and areas at the suture line. As can be seen from the calculations, compared to the unmodified simple 90-degree, end-to-side technique (Technique 1), the 45-degree cut (Technique 2) “doubles” the cross-sectional area at the suture line, but—better yet—the simple one-sided fishmouthing (Technique 3) “quadruples” it! This, of course, comes at the cost of lengthening the needed arteriotomy in the recipient vessel by a factor of 2 (Technique 3 versus Technique 1). Surgeons often wonder what the arteriotomy length in the recipient vessel needs to be, and Figure 9–8 shows clearly that, for Technique 3, it needs to be twice the “flattened” diameter of the donor, which is about three times its “true” diameter. In summary, when focusing on the geometry of the anastomotic line, Technique 3, compared to Technique 1, results in four times the cross-sectional area and two times the circumference, at the cost of two times the recipient arteriotomy. These geometrical concepts have been clearly simplified and idealized for the purpose of approximation and illustration, and rely on the assumption that the sutured anastomosis will assume a close-to-circular configuration under flow conditions and that the vessel wall edges do not stretch significantly, both very reasonable assumptions. In addition, as a slight departure from Technique 3 as described, we often “round” (small tissue excision) very slightly the sharp corners of the 90-degree fishmouth angles to facilitate running the suture.
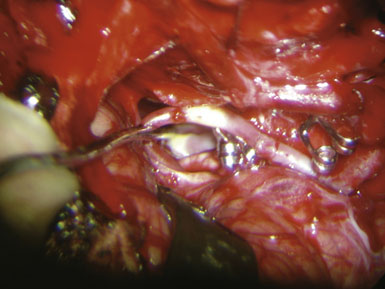
We routinely make detailed intraoperative measurements of blood flow in the donor and recipient arteries during bypass surgeries, using a microvascular ultrasonic flow probe (Charbel Micro-Flow Probe, Transonic Systems, Inc.). The “cut flow,” or the maximal flow carrying capacity of the donor vessel in the absence of downstream resistance, is first measured. The baseline flow in the recipient artery is also measured and gives an indication of the amount of flow that is desirable to replace or augment. Once the anastomosis has been completed, flow through the OA is measured and is known as the “bypass flow” (Figure 9–9). This measurement represents flow through the bypass and provides immediate verification of bypass patency. The ratio of the bypass flow measurement to the initial cut-flow measurement is known as the “cut-flow index” (CFI). Values greater than 0.5 have been found to be a sensitive predictor for acute and long-term postoperative bypass patency, at least in STA-MCA bypasses, primarily done for flow augmentation in ischemic cases.27
The anastomosis may be carried out using interrupted stitches or a continuous suture. Although interrupted stitches have a theoretical advantage of allowing future enlargement and maturation of the bypass, we do not believe this to be a real concern unless the vessels are particularly small (<0.8 mm), and we thus generally favor the running suture for its expediency. Furthermore, we suture the back and front wall of the anastomosis separately and thereby allow some enlargement of the bypass over time, if needed. Third, since we utilize Technique 3 in most cases, we believe we are already providing a four-fold increase in cross-sectional area (see Figure 9–8), negating any concerns about stenotic complications. The initial anchoring suture is passed through the wall of the donor OA at the “heel,” from outside the lumen to inside the lumen of the donor (colloquially called the “out-in” stitch), and then the needle is passed through the wall of the recipient PICA at the apex from inside the lumen to outside the lumen (colloquially called the “in-out” stitch) so that the knot ends up lying outside the lumen, naturally. The anastomosis proceeds by first suturing the more difficult (i.e., less accessible) back wall. Care must be taken to avoid grasping the luminal surface of the vessel wall with either the forceps or the needle holder, regardless of the delicacy of the instruments, as the intima may be injured. The forceps should be used to gently anchor the artery as the needle is passed through its wall. The sharp tip of the needle itself is a very useful “hook” that should be used to precisely manipulate, position, and pierce wall edges. The suture is kept loose between stitches, like the spiral binding of a book, until the entire back wall of the anastomosis has been sutured. There is a happy medium for how loose the loops should be: too loose, and they overlap on each other and interfere with each other and the passing of the next bite; too tight, and they are too close to the vessel wall and become harder to grasp later for the tightening phase. At the end of the run, the loops should ideally be equally spaced and lying parallel to each other, without interweaving. The suture is then tightened one loop at a time using two forceps starting from the initial anchoring suture. One forceps tightens the suture while the other handles the next loop, in a sequential manner, ensuring that steady tension is maintained constantly on the previously tightened suture. These forceps have to be relatively new or at least well maintained and preferably diamond-dusted, in order to provide a generous grasping surface area with less risk of slippage or of weakening the suture. The final loop is then tied to itself. Once the back wall has been sutured, the OA is rotated to expose the front wall. A new anchoring suture is placed and the front wall is then sutured in a similar manner. The lumen of the PICA is filled with heparinized saline prior to completion of the final stitch. The anastomosis is carried out using a 10-0 monofilament suture. We prefer a BV 75-3 (Ethicon, Johnson & Johnson, Somerville, NJ) tapered point needle. The needle forms three-eighths of a circle, and therefore minimizes the degree of wrist rotation required during suturing as compared to needles forming one-half of a circle. We do not use a silicon splint in the recipient, as some surgeons do.
Postoperative care
Patients are monitored in an intensive care unit for 1 to 2 days (Figure 9–10). Neurological examinations and assessment of bypass graft patency with bedside Doppler are performed every hour. Patients are kept normotensive. Postoperative hypertension should be avoided particularly in patients with vertebrobasilar ischemia, as it may predispose to parenchymal hemorrhage or cerebral edema. Computed tomography angiography (CTA) or preferably a digital subtraction angiography (DSA) is routinely performed 1 to 2 days after surgery. Patients are generally placed on daily aspirin (325 mg) for life.
Common alternatives to oa-pica in posterior fossa revascularization
1 Yaşargil M.G. Anastomosis between Superficial Temporal Artery and a Branch of the Middle Cerebral Artery. Stuttgart: Georg Thieme Verlag, 1969.
2 Ausman J.I., Lee M.C., Klassen A.C., et al. Stroke: what’s new? Cerebral revascularization. Minn Med. 1976;59(4):223-227.
3 Alvernia J.E., Fraser K., Lanzino G. The occipital artery: a microanatomical study. Neurosurgery. 2006;58(Suppl 1):ONS114-ONS122.
4 Rhoton A.L.Jr. The far-lateral approach and its transcondylar, supracondylar, and paracondylar extensions. Neurosurgery. 2000;47(Suppl 3):S195-S209.
5 Kawashima M., Rhoton A.L.Jr, Tanriover N., et al. Microsurgical anatomy of cerebral revascularization. Part II: posterior circulation. J Neurosurg. 2005;102(1):132-147.
6 Ateş O., Ahmed A.S., Niemann D., et al. The occipital artery for posterior circulation bypass: microsurgical anatomy. Neurosurg Focus. 2008;24(2):E9.
7 Lister J.R., Rhoton A.L.Jr, Matsushima T., et al. Microsurgical anatomy of the posterior inferior cerebellar artery. Neurosurgery. 1982;10(2):170-199.
8 Lesley W.S., Rajab M.H., Case R.S. Double origin of the posterior inferior cerebellar artery: association with intracranial aneurysm on catheter angiography. AJR Am J Roentgenol. 2007;189(4):893-897.
9 Fine A.D., Cardoso A., Rhoton A.L.Jr. Microsurgical anatomy of the extracranial-extradural origin of the posterior inferior cerebellar artery. J Neurosurg. 1999;91:645-652.
10 Ahuja A., Graves V.B., Crosby D.L., et al. Anomalous origin of the posterior inferior cerebellar artery from the internal carotid artery. AJNR Am J Neuroradiol. 1992;13:1625-1626.
11 Lasjaunias P., Vallee B., Person H., et al. The lateral spinal artery of the upper cervical spinal cord. Anatomy, normal variations, and angiographic aspects. J Neurosurg. 1985;63:235-241.
12 Manabe H., Oda N., Ishii M., et al. The posterior inferior cerebellar artery originating from the internal carotid artery, associated with multiple aneurysms. Neuroradiology. 1991;33:513-515.
13 Margolis M.T., Newton T.H. The posterior inferior cerebellar artery. Newton T.H., Potts D.G., editors. Radiology of the Skull and Brain, vol. 2. St. Louis: Mosby, 1974;1719-1775. bk. 2
14 Ogawa T., Fujita H., Inugami A., et al. Anomalous origin of the posterior inferior cerebellar artery from the posterior meningeal artery. AJNR Am J Neuroradiol. 1991;12:186.
15 Tanaka A., Kimura M., Yoshinaga S., et al. Extracranial aneurysm of the posterior inferior cerebellar artery: case report. Neurosurgery. 1993;33:742-744.
16 Haase J., Magnussen I.B., Ogilvy C.S., et al. Evaluating patients with vertebrobasilar transient ischemic attacks. Surg Neurol. 1999;52(4):386-392.
17 Amin-Hanjani S., Du X., Zhao M., et al. Use of quantitative magnetic resonance angiography to stratify stroke risk in symptomatic vertebrobasilar disease. Stroke. 2005;36(6):1140-1145.
18 Howe J.R., Kindt G.W. Cerebral protection during carotid endarterectomy. Stroke. 1974;5:340-343.
19 Michenfelder J.D., Milde J.H., Sundt T.M.Jr. Cerebral protection by barbiturate anesthesia. Use after middle cerebral artery occlusion in Java monkeys. Arch Neurol. 1976;33:345-350.
20 Gross C.E., Adams H.P.Jr, Sokoll M.D., et al. Use of anticoagulants, electroencephalographic monitoring, and barbiturate cerebral protection in carotid endarterectomy. Neurosurgery. 1981;9:1-5.
21 Imparato A.M., Ramirez A., Riles T., et al. Cerebral protection in carotid surgery. Arch Surg. 1982;117:1073-1078.
22 Feustel P.J., Ingvar M.C., Severinghaus J.W. Cerebral oxygen availability and blood flow during middle cerebral artery occlusion: effects of pentobarbital. Stroke. 1981;12:858-863.
23 Amakawa K., Adachi N., Liu K., et al. Effects of pre- and postischemic administration of thiopental on transmitter amino acid release and histologic outcome in gerbils. Anesthesiology. 1996;85:1422-1430.
24 Buggy D.J., Nicol B., Rowbotham D.J., et al. Effects of intravenous anesthetic agents on glutamate release: a role for GABAA receptor-mediated inhibition. Anesthesiology. 2000;92:1067-1073.
25 Bieda M.C., MacIver M.B. Major role for tonic GABAA conductances in anesthetic suppression of intrinsic neuronal excitability. J Neurophysiol. 2004;92:1658-1667.
26 Zhan R.Z., Fujiwara N., Endoh H., et al. Thiopentone inhibits increases in [Ca2+] induced by membrane depolarization, NMDA receptor activation and ischemia in rat hippocampal and cortical slices. Anesthesiology. 1998;89:456-466.
27 Ashley W.W., Amin-Hanjani S., Alaraj A., et al. Flow-assisted surgical cerebral revascularization. Neurosurg Focus. 2008;24(2):E20.