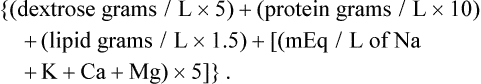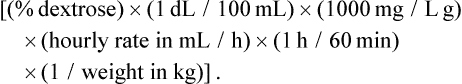Nutritional Support for The Pediatric Patient
Despite advances in the field of nutritional support, the prevalence of malnutrition among hospitalized patients, especially those with a protracted clinical course, has remained largely unchanged over the last two decades.1,2 The provision of optimal nutritional therapy requires a careful assessment of energy needs and the provision of macronutrients and micronutrients via the most suitable feeding route. The profound and stereotypic metabolic response to injury places unique demands on the hospitalized child. Standard equations available for estimating energy needs have proven to be unreliable in this population.3,4 In addition, children with critical illness have a marked net protein catabolism and often lack adequate nutritional support.5 Ultimately, an individualized nutritional regimen should be tailored for each child and reviewed regularly during the course of illness. An understanding of the metabolic events that accompany illness and surgery in a child is the first step in implementing appropriate nutritional support.
The Metabolic Response to Stress
The metabolic response to illness due to stressors such as trauma, surgery, or inflammation has been well described. Cuthbertson was the first investigator to realize the primary role that whole-body protein catabolism plays in the systemic response to injury.6 Based on his work, the metabolic stress response has been conceptually divided into two phases. The initial, brief ‘ebb phase’ is characterized by decreased enzymatic activity, reduced oxygen consumption, low cardiac output, and a core temperature that may be subnormal. This is followed by the hypermetabolic ‘flow phase’ characterized by increased cardiac output, oxygen consumption, and glucose production. During this phase, fat and protein mobilization is manifested by increased urinary nitrogen excretion and weight loss. This catabolic phase is mediated by a surge in cytokines and the characteristic endocrine response to trauma or operation that results in an increased availability of substrates essential for healing and glucose production.
Neonates and children share similar qualitative metabolic responses to illness as adults, albeit with significant quantitative differences. The metabolic stress response is beneficial in the short term, but the consequences of sustained catabolism are significant as the child has limited tissue stores and substantial nutrient requirements for growth. Thus the prompt institution of nutritional support is a priority in sick neonates and children. The goal of nutrition in this setting is to augment the short-term benefits of the metabolic response to injury while minimizing long-term consequences. In general, the metabolic stress response is characterized by an increase in net muscle protein degradation and the enhanced movement of free amino acids through the circulation (Fig. 2-1). These amino acids serve as the building blocks for the rapid synthesis of proteins that act as mediators for the inflammatory response and structural components for tissue repair. The remaining amino acids not used in this way are channeled through the liver where their carbon skeletons are utilized to create glucose through gluconeogenesis. The provision of additional dietary protein may slow the rate of net protein loss, but does not eliminate the overall negative protein balance associated with injury.7
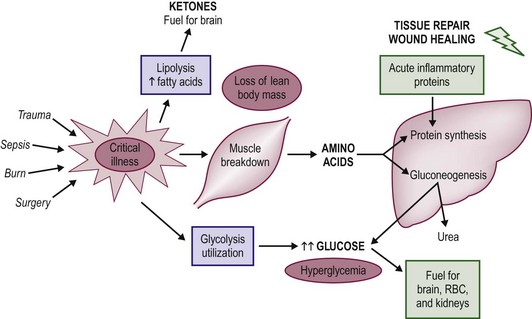
FIGURE 2-1 The metabolic changes associated with the pediatric stress response to critical illness and injury. In general, net protein catabolism predominates and amino acids are transported from muscle stores to the liver, where they are converted to inflammatory proteins and glucose through the process of gluconeogenesis.
Carbohydrate and lipid turnover are also increased several fold during the metabolic response. Although these metabolic alterations would be expected to increase overall energy requirements, data show that such an increase is quantitatively variable, modest, and evanescent. Overall, the energy needs of the critically ill or injured child are governed by the severity and persistence of the underlying illness or injury. Accurate assessment of energy requirements in individual patients allows optimal caloric supplementation and avoids the deleterious effects of both under- and overfeeding. Children with critical illness demonstrate a unique hormonal and cytokine profile characterized by an elevation in serum levels of insulin, the catabolic hormones (glucagons, cortisol, catecholamines), and specific cytokines known to interact with the inflammatory process.8 Novel ways to manipulate these hormonal and cytokine alterations with an aim to minimize the deleterious consequences induced by the stress response are a focus of research.
Body Composition and Nutrient Reserves
The body composition of the young child contrasts with that of the adult in several ways that significantly affect nutritional requirements. Table 2-1 lists the macronutrient stores of the neonate, child, and adult as a percentage of total body weight.9,10 Carbohydrate stores are limited in all age groups and provide only a short-term supply of glucose. Despite this fact, neonates have a high demand for glucose and have shown elevated rates of glucose turnover when compared with those of the adult.11 This is thought to be related to the neonate’s increased brain-to-body mass ratio because glucose is the primary energy source for the central nervous system. Neonatal glycogen stores are even more limited in the early postpartum period, especially in the preterm infant.12 Short periods of fasting can predispose the newborn to hypoglycemia. Thus when infants are burdened with illness or injury, they must rapidly turn to the breakdown of protein stores to generate glucose through the process of gluconeogenesis.
TABLE 2-1
The Body Composition of Neonates, Children, and Adults as a Percentage of Total Body Weight
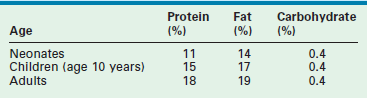
Lipid reserves are low in the neonate, gradually increasing with age. Premature infants have the lowest proportion of lipid stores as the majority of polyunsaturated fatty acids accumulate in the third trimester.13 This renders lipid less useful as a potential fuel source in the young child.14 The most dramatic difference between adult and pediatric patients is in the relative quantity of stored protein. The protein reserve of the adult is nearly twofold that of the neonate. Thus infants cannot afford to lose significant amounts of protein during the course of a protracted illness or injury. An important feature of the metabolic stress response, unlike in starvation, is that the provision of dietary glucose does not halt gluconeogenesis. Consequently, the catabolism of muscle protein to produce glucose continues unabated.15 Neonates and children also share much higher baseline energy requirements. Studies have demonstrated that the resting energy expenditure for neonates is two to three times that of adults when standardized for body weight.14,16 Clearly, the child’s need for rapid growth and development is a large component of this increase in energy requirement. Moreover, the relatively large body surface area of the young child may increase heat loss and further contributes to elevations in energy expenditure.
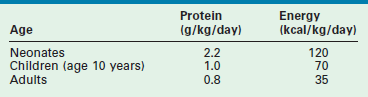
The basic requirements for protein and energy in the healthy neonate, child, and adult, based on recent recommendations by the National Academy of Sciences, are listed in Table 2-2.17 As illustrated, the recommended protein provision for the neonate is almost three times that of the adult. In premature infants, a minimum protein allotment of 2.8 g/kg/day is required to maintain in utero growth rates.18 The increased metabolic demand and limited nutrient reserves of the infant mandates early nutritional support in times of injury and critical illness to avoid negative nutritional consequences.
Accurate assessment of body composition is necessary for planning nutritional intake, monitoring dynamic changes in the body compartments (such as the loss of lean body mass), and assessing the adequacy of nutritional supportive regimens during critical illness. Ongoing loss of lean body mass is an indicator of inadequate dietary supplementation and may have clinical implications in the hospitalized child. However, current methods of body composition analysis (such as anthropometry, weight and biochemical parameters) are either impractical for clinical use or inaccurate in a subgroup of hospitalized children with critical illness. One of the principal problems in critically ill children is the presence of capillary leak, manifesting as edema and large fluid shifts. These make anthropometric measurements invalid and other bedside techniques have not been validated.
Energy Expenditure during Illness
REE can be measured using direct or indirect methods. The direct calorimetric method measures the heat released by a subject at rest and is based on the principle that all energy is eventually converted to heat. In practice, the patient is placed in a thermally isolated chamber, and the heat dissipated is measured for a given period of time.19 This method is the true gold standard for measured energy expenditure. Direct calorimetry is not practical for most hospitalized children and REE is often estimated using standard equations. Unfortunately, REE estimates using standardized World Health Organization (WHO) predictive equations are unreliable, particularly in underweight subjects.19–21
REE estimation is difficult in critically ill or postoperative children. Their energy requirements show individual variation and are dependent upon severity of injury. For instance, an infant with respiratory distress on pressure support is likely to have high energy requirement due to increased work of breathing. The same patient, when started on mechanical ventilation with muscle relaxants, is unlikely to have sustained high energy requirements. Infants with congenital diaphragmatic hernia on extracorporeal membrane oxygenation (ECMO) support have been shown to have energy expenditures of approximately 90 kcal/kg/day. Following extubation, the same patients may have energy requirements as high as 140 kcal/kg/day. Although stress factors ranging from 1.0 to 2.7 have been applied to correct for these variations, calculated standardized energy expenditure equations have not been satisfactorily validated in critically ill children.22–25
Indirect calorimetry measures VO2 (the volume of oxygen consumed) and VCO2 (the volume of CO2 produced), and uses a correlation factor based on urinary nitrogen excretion to calculate the overall rate of energy production.26 The measurement of energy needs is ‘indirect’ because it does not use direct temperature changes to determine energy needs. Indirect calorimetry provides a measurement of the overall respiratory quotient (RQ), defined as the ratio of CO2 produced to O2 consumed (VCO2/ VO2), for a given patient. Oxidation of carbohydrate yields an RQ of 1.0, whereas fatty acid oxidation gives an RQ of 0.7. However, the role of the RQ as a marker of substrate use and as an indicator of underfeeding or overfeeding is limited. The body’s ability to metabolize substrate may be impaired during illness, making assumptions invalid about RQ values and substrate oxidation.
Although RQ is not a sensitive marker for adequacy of feeding in individual cases, RQ values greater than 1.0 are generally associated with lipogenesis secondary to overfeeding.27,28 A recent study has suggested the utility of extremes of RQ in monitoring feeding adequacy, where an RQ higher than 0.85 reliably indicates the absence of underfeeding and an RQ higher than 1.0 reliably indicates the presence of overfeeding.29 However, numerous factors, related and unrelated to feeding, can alter the value of a measured RQ in critically ill patients, e.g., hyperventilation, acidosis, effects of cardiotonic agents and neuromuscular blocking, and an individual response to a given substrate load, injury, or disease. Furthermore, in the setting of wide diurnal and day-to-day variability of REE in critically ill individuals, the extrapolation of short-term calorimetric REE measurements to 24-hour REE may introduce errors. The use of steady-state measurements may decrease these errors. Steady state is defined by change in VO2 and VCO2 of <10% over a period of five consecutive minutes. The values for the mean REE from this steady-state period may be used as an accurate representation of the 24-hour TEE in patients with low levels of physical activity.30 In a patient who fails to achieve steady state and is metabolically unstable, prolonged testing is required (minimum of 60 minutes), and 24-hour indirect calorimetry should be considered. With the advent of newer technology, the application of indirect calorimetry at the bedside for continuous monitoring shows promise.
Indirect calorimetry is not accurate in the setting of air leaks around the endotracheal tube, ventilator circuit or through a chest tube, or in subjects on ECMO. High inspired oxygen fraction (FiO2 >0.6) will also affect indirect calorimetry. Indirect calorimetry is difficult to use in babies on ECMO because a large proportion of the patient’s oxygenation and ventilation is performed through the membrane oxygenator. The use of indirect calorimetry for assessment and monitoring of nutrition intake requires attention to its limitations and expertise in the interpretation. Nonetheless, its application in children at high risk for underfeeding and overfeeding may be helpful.31,32
Nonradioactive stable isotope techniques have been used to measure REE in the pediatric patient. Stable isotope technology has been available for many years and was first applied for energy expenditure measurement in humans in 1982.33,34 Both 13C-labeled bicarbonate and doubly labeled water (2H218O) have been used to measure TEE in pediatric surgical patients, and have been shown to correlate well with indirect calorimetry.31,34,35 The 13C-labeled bicarbonate method allows the calculation of REE on the basis of infusion rate and the ratio of labeled to unlabeled CO2 in expired breath samples.35 Orally administered stable isotopes of water (2H2O and H218O) mix with the body water and the 18O is lost from the body as both water and CO2, while the 2H is lost from the body only as water. The difference in the rates of loss of the isotopes 18O and 2H from the body reflects the rate of CO2 production, which can be used to calculate the TEE.36–38 However, the doubly labeled water method has its limitations in children with active capillary leak, decreased urine output, fluid overload, and diuretic use.36
In general, any increase in energy expenditure during illness or after an operation is variable, and studies suggest that the increase is far less than originally hypothesized. In children with severe burns, the initial REE during the flow phase of injury is increased by 50% but then returns to normal during convalescence.39 In neonates with bronchopulmonary dysplasia, in which the illness increases the patient’s work of breathing, a 25% elevation in energy requirement is evident.40 Newborns undergoing major surgery have only a transient 20% increase in energy expenditure that returns to baseline values within 12 hours postoperatively, provided no major complications develop.41,42 Stable extubated neonates, five days after operation, have been shown to have REE comparable to normal infants.43 Effective anesthetic and analgesic management may play a significant role in muting the stress response of the neonate. Studies have demonstrated no discernible increase in REE in neonates undergoing patent ductus arteriosus ligation who received intraoperative fentanyl anesthesia and postoperative intravenous analgesic regimens.42 A retrospective stratification of surgical infants into low- and high-stress cohorts based on the severity of underlying illness found that high-stress infants undergo moderate short-term elevations in energy expenditure after operation, whereas low-stress infants do not manifest any increase in energy expenditures during the course of illness.44 Finally, by using stable isotopic methods, it has been found that the mean energy expenditures of critically ill neonates on ECMO are nearly identical to age- and diet-matched nonstressed controls.45
All these studies suggest that critically ill neonates have only a small and usually short-term increase in energy expenditure. Although children have increased energy requirements from increased metabolic turnover during illness, their caloric needs may be lower than previously considered due to possible halted or slow growth,46 and the use of sedation and muscle paralysis.47 This could result in overfeeding when energy intake is based on presumed or estimated energy expenditure with stress factors. On the other hand, unrecognized hypermetabolism in select individuals result in underfeeding with negative nutritional consequences.31 The variability in energy requirements may result in cumulative energy imbalances in the intensive care unit (ICU) over a period of time.32 A direct relationship has been reported between cumulative caloric imbalance and the mortality rate in critically ill surgical patients.48
For practical purposes, the recommended dietary caloric intake for healthy children may represent a reasonable starting point for the upper limit of caloric allotment in the hospitalized child.17 However, as discussed earlier, energy requirement estimates in select groups of patients remain variable and possibly overestimated, mandating an accurate estimation using measured energy expenditure where available. Regular anthropometric measurements plotted on a growth chart to assess the adequacy of caloric provision will allow relatively prompt detection of underfeeding or overfeeding in most cases. However, some critically ill children may be too sick for regular weights or have changes in body water that make anthropometric measurements unreliable.
Macronutrient Intake
Protein Metabolism and Requirement During Illness
Amino acids are the key building blocks required for growth and tissue repair. The vast majority (98%) are found in existing proteins, and the remainder reside in the free amino acid pool. Proteins are continually degraded into their constituent amino acids and resynthesized through the process of protein turnover. The reutilization of amino acids released by protein breakdown is extensive. Synthesis of proteins from the recycling of amino acids is more than two times greater than from dietary protein intake. An advantage of high protein turnover is that a continuous flow of amino acids is available for the synthesis of new proteins. This allows the body tremendous flexibility in meeting ever-changing physiologic needs. However, the process of protein turnover requires the input of energy to power both protein degradation and synthesis. At baseline, infants are known to have higher rates of protein turnover than adults. Healthy newborns have a protein-turnover rate of 6–12 g/kg/day compared with 3.5 g/kg/day in adults.49 Even greater rates of protein turnover have been measured in premature and low birth weight infants.50 For example, it has been demonstrated that extremely low birth weight infants receiving no dietary protein can lose in excess of 1.2 g/kg/day of endogenous protein.51 At the same time, infants must maintain a positive protein balance to attain normal growth and development, whereas the healthy adult can subsist with a neutral protein balance.
In the metabolically stressed patient, such as the child with severe burn injury or cardiorespiratory failure requiring ECMO, protein turnover is doubled when compared with normal subjects.34,49 A study of critically ill infants and children found an 80% increase in protein turnover, which correlated with the duration of the critical illness.52 This process redistributes amino acids from skeletal muscle to the liver, wound, and tissues taking part in the inflammatory response. The factors required for the inflammatory response (acutely needed enzymes, serum proteins, and glucose) are thereby synthesized from degraded body protein stores. The well-established increase in hepatically derived acute phase proteins (including C-reactive protein, fibrinogen, transferrin, and α-1-acid glycoprotein), along with the concomitant decrease in transport proteins (albumin and retinol-binding protein), is evidence of this protein redistribution. As substrate turnover is increased during the stress response, rates of both whole-body protein degradation and whole-body protein synthesis are accelerated. However, protein breakdown predominates, thereby leading to a hypercatabolic state with an ensuing net negative protein and nitrogen balance.27
Protein loss is evident in elevated levels of excreted urinary nitrogen during critical illness. For example, infants with sepsis demonstrate a severalfold increase in the loss of urinary nitrogen that directly correlates with the degree of illness.53 Clinically, severe protein loss can be manifested by skeletal muscle wasting, weight loss, delayed wound healing, and immune dysfunction.54 In addition to the reprioritization of protein for tissue repair, healing and inflammation, the body appears to have an increased need for glucose production during times of metabolic stress.55 The accelerated rate of gluconeogenesis during illness and injury is seen in both children and adults, and this process appears to be accentuated in infants with low body weight.13,54 The increased production of glucose in times of illness is necessary as glucose represents a versatile energy source for tissues taking part in the inflammatory response. For example, it has been shown that glucose utilization by leukocytes is significantly increased during the inflammatory response.56 Unfortunately, the provision of additional dietary glucose does not suppress the body’s need for increased glucose production. Therefore, net protein breakdown continues to predominate.14,57,58
Specific amino acids are transported from muscle to the liver to facilitate hepatic glucose production. The initial step of amino acid catabolism involves removal of the toxic amino group (NH3). Through transamination, the amino group is transferred to α-ketoglutarate, thereby producing glutamate. The addition of another amino group converts glutamate to glutamine, which is subsequently transported to the liver. Here, the amino groups are removed from glutamine and detoxified to urea through the urea cycle. The amino acid carbon skeleton can then enter the gluconeogenesis pathway. Alternatively, in skeletal muscle, the amino group can be transferred to pyruvate, thereby forming alanine. When alanine is transported to the liver and detoxified, pyruvate is reformed and can be converted to glucose through gluconeogenesis. The transport of alanine and pyruvate between peripheral muscle tissue and the liver is termed the glucose-alanine cycle.59 Hence the transport amino acid systems involving glutamine and alanine provide carbon backbones for gluconeogenesis, while facilitating the hepatic detoxification of ammonia by the urea cycle.
Increased muscle protein catabolism is a successful short-term adaptation during critical illness, but it is limited and ultimately harmful to the child with reduced protein stores and elevated protein demands. Unless the inciting stress is eliminated, the progressive breakdown of diaphragmatic, cardiac, and skeletal muscle can lead to respiratory compromise, fatal arrhythmia, and loss of lean body mass. Moreover, a prolonged negative protein balance may have a significant impact on the child’s growth and development. Healthy, nonstressed neonates require a positive protein balance of nearly 2 g/kg/day.48,60 In contrast, critically ill, premature neonates requiring mechanical ventilation have a negative protein balance of −1 g/kg/day.61,62 Critically ill neonates who require ECMO have exceedingly high rates of protein loss, with a net negative protein balance of –2.3 g/kg/day.63 It has been well established that the extent of protein catabolism correlates with morbidity and mortality in surgical patients.
Fortunately, amino acid supplementation tends to promote increased nitrogen retention and positive protein balance in critically ill patients.59,64 The mechanism appears to be an increase in protein synthesis while rates of protein degradation remain constant.60,61 Therefore the provision of dietary protein sufficient to optimize protein synthesis, facilitate wound healing and the inflammatory process, and preserve skeletal muscle mass is the single most important nutritional intervention in critically ill children. The quantity of protein needed to enhance protein accrual is greater in hospitalized sick children than in healthy children. Table 2-3 lists recommended quantities of dietary protein for hospitalized children. Extreme cases of physiologic stress, including the child with extensive burns or the neonate on ECMO, may necessitate additional protein supplementation to meet metabolic demands.
TABLE 2-3
Recommended Protein Requirements for Hospitalized Infants and Children
| Age (years) | Estimated Protein Requirement (g/kg/day) |
| Extremely low birth weight infants | up to 3.5 |
| Very low birth weight | up to 3.0 |
| 0–2 | 2.0–3.0 |
| 2–13 | 1.5–2.0 |
| 13–18 | 1.0–1.5 |
The influence of macronutrient intake on protein balance has been explored in a limited number of studies. A systematic review of all such studies in mechanically ventilated children showed that a minimum of 1.5 g/kg/day protein and 57 kcal/kg/day energy intake was needed to achieve a positive protein balance in this group.62 However, it should be noted that toxicity from excessive protein administration can occur, particularly in children with impaired renal and hepatic function. The provision of protein at levels greater than 3 g/kg/day is rarely indicated and is often associated with azotemia. In premature neonates, the possible beneficial effects of protein allotments of 3–3.5 g/kg/day are being actively investigated in an effort to replicate intrauterine growth rates. Studies using protein provisions of 6 g/kg/day in children have demonstrated significant morbidity, including azotemia, pyrexia, strabismus, and lower IQ scores.64,65
Protein Quality
In addition to the sufficient quantity of dietary protein, an increased focus has been placed on the protein quality of nutritional provisions. The specific amino acid formulation to best increase whole-body protein balance has yet to be fully determined, although numerous clinical and basic science research projects are actively focusing on this topic. It is known that infants have an increased requirement per kilogram for the essential amino acids compared to the adult.66 In particular, neonates have immature biosynthetic pathways that may temporarily alter their ability to synthesize specific amino acids. One example is the amino acid histidine, which has been shown to be a conditionally essential amino acid in infants up to age 6 months. Data suggest that cysteine, taurine, and proline also may be limited in the premature neonate.67–70 Interest has also been expressed in the use of arginine as an ‘immunonutrient’ to enhance the function of the immune system in critically ill patients. Although preliminary studies show that arginine supplementation may reduce the risk of infectious complications, its safety and efficacy in infants and children has yet to be established.71
The restricted availability of the amino acid cysteine may have clinical relevance in the critically ill child. Cysteine is a required substrate for the production of glutathione, the body’s major antioxidant. In critically ill children, cysteine turnover is increased significantly, whereas rates of glutathione synthesis are decreased by 60%. In this way, cysteine may become a conditionally essential amino acid in the sick child. Recent experiments have demonstrated that the enteral feeding of cysteine in small quantities to rats dependent on total parenteral nutrition (TPN) significantly increases the hepatic concentration of glutathione.72 The enteral supplementation of cysteine in a pediatric nutritional regimen warrants further basic science and clinical investigation.
Glutamine is another amino acid that has been studied extensively in both children and adults in the ICU. Glutamine is an important amino acid source for gluconeogenesis, intestinal energy production, and ammonia detoxification. In healthy subjects, glutamine is a nonessential amino acid, although it has been hypothesized that glutamine may become conditionally essential in critically ill patients. Because it is difficult to keep glutamine soluble in solution, standard TPN formulas do not include glutamine in the amino acid mixture. Although preliminary data on glutamine supplementation in the clinical setting are encouraging, numerous problems with study methodology have been noted.73 Additional prospective, randomized trials are needed to define its utility fully in both the adult and pediatric population.
Modulating Protein Metabolism
The dramatic increase in protein breakdown during critical illness, coupled with the known association between protein loss and patient mortality and morbidity, has stimulated a wide array of research efforts. The measurement of whole-body nitrogen balance through urine and stool was once the only way to investigate changes in protein metabolism, but new and validated stable isotope tracer techniques now allow precise measurement of protein turnover, breakdown, and synthesis.74 However, the modulation of protein metabolism in critically ill patients has been difficult. Dietary supplementation of amino acids increases protein synthesis, but appears to have no effect on rates of protein breakdown. Thus investigators have recently focused on the use of alternative anabolic agents to decrease protein catabolism. Studies have used various pharmacologic tools to achieve this goal, including growth hormone, insulin-derived growth factor I (IGF-I), and testosterone, with varying degrees of success.75–77 One of the more promising agents, however, may be the anabolic hormone insulin. Multiple studies have used insulin to reduce protein breakdown in healthy volunteers and adult burn patients.50,78 In children with extensive burns, intravenous insulin has been shown to increase lean body mass and mitigate peripheral muscle catabolism.79 A recent prospective, randomized trial of more than 1500 adult postoperative patients in the ICU demonstrated significant reductions in mortality and morbidity with the use of intravenous insulin.80 Preliminary stable isotopic studies demonstrate that an intravenous insulin infusion may reduce protein breakdown by over 30% in critically ill neonates on ECMO.81 The use of intensive insulin therapy for critically ill children and adults continues to be another active and interesting area of clinical investigation. Some recent studies examining the role of insulin for tight glycemic control in critically ill patients have been less encouraging and are discussed in the next section.
Carbohydrate Metabolism and Requirement During Illness
Glucose production and availability are a priority in the pediatric metabolic stress response. Glucose is the primary energy source for the brain, erythrocyte, and renal medulla, and is used extensively in the inflammatory response. Injured and septic adults demonstrate a threefold increase in glucose turnover, glucose oxidation, and gluconeogenesis.16 This increase is of particular concern in neonates who have an elevated glucose turnover at baseline.10 Moreover, glycogen stores provide only a limited endogenous supply of glucose in adults and an even smaller reserve in the neonate. Thus the critically ill neonate has a greater glucose demand and reduced glucose stores. During illness, the administration of exogenous glucose does not halt the elevated rates of gluconeogenesis, and thus net protein catabolism continues unabated.14 It is clear, however, that a combination of dietary glucose and amino acids can effectively improve protein balance during critical illness, primarily through augmentation of protein synthesis.
In the past, nutritional support regimens for critically ill patients used large amounts of glucose in an attempt to reduce endogenous glucose production. Unfortunately, excess glucose increases CO2 production, engenders fatty liver, and does not result in a reduction in endogenous glucose turnover.82 Thus a surplus of carbohydrate may increase the ventilatory burden on the critically ill patient. In one study, adults in the ICU fed with high-glucose TPN demonstrate a 30% increase in oxygen consumption, a 57% increase in CO2 production, and a 71% elevation in minute ventilation.83 In critically ill infants, the conversion of excess glucose to fat has also been correlated with increased CO2 production and higher respiratory rates.84 In addition, excessive carbohydrate may play a role in the genesis of TPN-associated cholestatic liver injury. Finally, some data in critically ill neonates have shown that excess caloric allotments of carbohydrate are paradoxically associated with an increased rate of net protein breakdown.85
When designing a nutritional regimen for the critically ill child, excessive carbohydrate calories should be avoided. A mixed fuel system, with both glucose and lipid substrates, should be used to meet the child’s caloric requirements. When the postoperative neonate is fed a high-glucose diet, the corresponding RQ is approximately 1.0, and may be higher than 1.0 in selected patients, signifying increased lipogenesis.86 A mixed dietary regimen of glucose and lipid (at 2–4 g/kg/day) lowers the effective RQ in neonates to 0.83.87 This approach provides the infant with full nutritional supplementation while alleviating an increased ventilatory burden and difficulties with hyperglycemia.
Administration of high caloric (glucose load) diets in the early phase of critical illness may exacerbate hyperglycemia, increase carbon dioxide generation with an increased load on the respiratory system, promote hyperlipidemia resulting from increased lipogenesis, and result in a hyperosmolar state. Several reports have linked hyperglycemia with increased mortality and established the role of insulin-assisted tight glycemic control in improving outcomes in critically ill adults.80,88,89 A remarkable 43% reduction in mortality was reported in postcardiac surgery patients in an adult ICU by implementing strict glycemic control (arterial blood glucose levels below 110 mg/dL) and using insulin infusion in the treatment group compared with the control group (average blood glucose level of 150–160 mg/dL).80 The precise mechanism(s) responsible for this beneficial effect of tight glycemic control with an insulin protocol remains unanswered. A recent meta-analysis of studies examining the role of tight glycemic control in adult ICUs has shown a high incidence of hypoglycemia in the treatment group and less impressive benefit.90 Although the incidence of hyperglycemia in children is high and may be associated with increased mortality and length of stay,91 there are no data for similar benefits of tight glycemic control in this patient population. Studies examining the role of a tight glycemic control strategy in infants and children are currently underway.
Lipid Metabolism and Requirements During Illness
Similar to protein and carbohydrate metabolism, the turnover of lipid is generally increased by critical illness, major surgery, and trauma in the pediatric patient.92 During the early ebb phase, triglyceride levels may initially increase as the rate of lipid metabolism decreases. However, this process reverses itself in the predominant flow phase, and during this time, critically ill adults have demonstrated two- to fourfold increases in lipid turnover.93 Also, it has been shown that critically ill children on mechanical ventilation have increased rates of fatty acid oxidation.94 The increased lipid metabolism is thought to be proportional to the overall degree of illness. The process of lipid turnover involves the conversion of free fatty acids and their glycerol backbone into triglycerides. Approximately 30–40% of free fatty acids are oxidized for energy. RQ values may decline during illness, reflecting an increased utilization of fat as an energy source.95 This suggests that fatty acids are a prime source of energy in metabolically stressed pediatric patients. In addition to the rich energy supply from lipid substrate, the glycerol moiety released from triglycerides may be converted to pyruvate and used to manufacture glucose. As seen with the other catabolic changes associated with illness and trauma, the provision of dietary glucose does not decrease fatty acid turnover in times of illness. The increased demand for lipid utilization in critical illness coupled with the limited lipid stores in the neonate puts the metabolically stressed infant/child at high risk for the development of essential fatty acid deficiency.96,97 Preterm infants have been shown to develop biochemical evidence of essential fatty acid deficiency two days after the initiation of a fat-free nutritional regimen.98
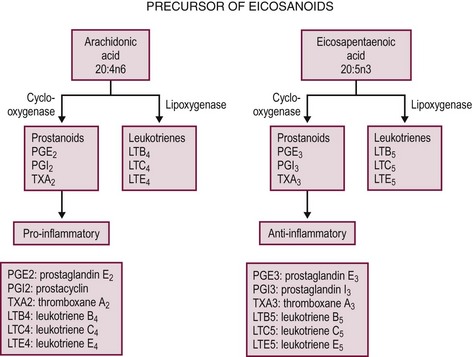
In the human, the polyunsaturated fatty acids linoleic and linolenic acid are considered essential fatty acids because the body cannot manufacture them by desaturating other fatty acids. Linoleic acid is used by the body to synthesize arachidonic acid, an important intermediary in prostaglandin synthesis. The prostaglandin family includes the leukotrienes and thromboxanes, all of which serve as mediators in wide-ranging processes such as vascular permeability, smooth muscle reactivity, and platelet aggregation. If an individual lacks dietary linoleic acid, the formation of arachidonic acid (a tetraene) cannot occur, and eicosatrienoic acid (a triene) accumulates. Clinically, a fatty acid profile can be performed on human serum, and an elevated triene-to-tetraene ratio greater than 0.4 is characteristic of biochemical essential fatty acid deficiency, though this value is somewhat variable and dependent upon the specific laboratory assay utilized. Signs of fatty acid deficiencies include dermatitis, alopecia, thrombocytopenia, increased susceptibility to infection, and overall failure to thrive. To avoid essential fatty acid deficiency in neonates, the allotment of linoleic and linolenic acid is recommended at concentrations of 4.5% and 0.5% of total calories, respectively. In addition, some evidence exists that the long-chain fatty acid docosahexaenoic acid (DHA), a derivative of linolenic acid, also may be deficient in preterm and formula-fed infants. At present, clinical trials are actively seeking to determine whether supplementation with long-chain polyunsaturated fatty acids will be of clinical benefit in this population.
Parenterally delivered lipid solutions also limit the need for excessive glucose intake. These lipid emulsions provide a higher quantity of energy per gram than does glucose (9 kcal/g vs 4 kcal/g). This reduces the overall rate of CO2 production, the RQ value, and the incidence of hepatic steatosis.99 There are risks when starting a patient on intravenous lipid administration. These include hypertriglyceridemia, a possible increased risk of infection, and decreased alveolar oxygen-diffusion capacity.100–102 Most institutions, therefore, initiate lipid provisions in children at 0.5–1.0 g/kg/day and advance over a period of days to 2–4 g/kg/day. During this time, triglyceride levels are monitored closely. Lipid administration is generally restricted to 30–40% of total caloric intake in ill children in an effort to obviate immune dysfunction, although this practice has not been validated in a formal clinical trial.
In settings of prolonged fasting or uncontrolled diabetes mellitus, the accelerated production of glucose depletes the hepatocyte of needed intermediaries in the citric acid cycle. When this occurs, the acetyl-coenzyme A (CoA) generated from the breakdown of fatty acids cannot enter the citric acid cycle and instead forms ketone bodies, acetoacetate, and b-hydroxybutyrate. These ketone bodies are released by the liver to extrahepatic tissues, particularly, skeletal muscle and the brain, where they can be used for energy production instead of glucose. During surgical illness, however, ketone body formation is relatively inhibited secondary to elevated serum insulin levels.103 Thus in surgical patients, ketone bodies do not significantly supplant the need for glucose and do not play a major role in the metabolic management of the pediatric stress response.
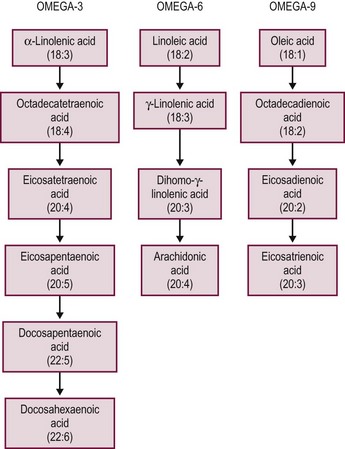
In addition to their nutritional role, fatty acids profoundly influence inflammatory and immune events by changing lipid mediators and inflammatory protein and coagulation protein expression. After ingestion, n-6 and n-3 fats are metabolized by an alternating series of desaturase and elongase enzymes, transforming them into the membrane associated lipids: arachidonic acid, eicosapentaenoic acid (EPA), DHA, respectively (Figs 2-2 and 2-3).104 Substitution of the intralipid component of parenteral nutrition (PN) (rich in proinflammatory omega-6 fatty acids) with fish oil (a source of omega-3 fatty acids) may alleviate some of the toxic hepatic effects of long-term PN.105 The beneficial effects of omega-3 fatty acids have been shown in animal and human models and this is an area of great interest. Omega-3 fatty acids have an anti-inflammatory effect, with decreased cytokine production in some models.104 More recently, a commercially available omega-3 fatty acid for parenteral administration has been administered in children with exciting results. In a cohort of PN dependent children with PN associated hyperbilirubinemia, Omegaven®, at a low lipid allocation of 1 g/kg/day, was associated with a normalization of bilirubin levels.106 A clinical trial, in surgical neonates, to test the relative benefits of Omegaven® vs reduced omega-6 lipid allotments alone is currently underway.
Routes of Nutritional Provision
Enteral Nutrition
Following the estimation of energy expenditure and macronutrient requirement in the hospitalized child, the next challenge is to facilitate the provision of this nutritional support. In most pediatric patients with a functioning gastrointestinal tract, the enteral route of nutrient administration is preferable to PN. Enteral nutrition (EN) is physiologic and has been shown to be more cost effective without the added risk of nosocomial infection inherent in PN. Early EN has been shown to decrease infectious episodes and decrease length of hospitalization in critically ill patients.107 Based on level 1 and level 2 evidence in the adult critical care literature, The Canadian Clinical Practice Guidelines for Nutrition Support have strongly recommended the use of early enteral feeds (within 24–48 hourrs after ICU admission).108 The optimal route of nutrient delivery has not been systematically studied in children. In the absence of a randomized-controlled trial comparing the effects of EN vs PN, many centers have adopted institutional guidelines. Current practice includes the initiation of gastric or post-pyloric enteral feeding within 48 hrs to 72 hrs after admission. PN is being used to supplement or replace EN in those patients where EN alone is unable to meet the nutritional goal.
In children on EN, there are insufficient data to make recommendations regarding the site of enteral feeding (gastric vs postpyloric). Both enteral routes have been successfully used for nutritional support of the critically ill child.109–111 In a study examining the role of small bowel feeding in 74 critically ill children randomized to receive either gastric or postpyloric feeds, no significant difference was observed in microaspiration, tube displacement and feeding intolerance between the two groups.112 The study was not powered to detect differences in mortality and enteral feeds were interrupted in a large number of subjects in this study. Also, caloric goals were met in only a small percentage of the population studied. A higher percentage of subjects in the small bowel group achieved their daily caloric goal when compared to the gastric fed group. Critically ill children receiving early (less than 24 hours after ICU admission) postpyloric feeds have been shown to have better feeding tolerance (decreased incidence of abdominal distension) compared to those where post-pyloric feeding was initiated late.113
EN in critically ill children is often interrupted for a variety of reasons, some of which may be avoidable.114 Children with frequent interruptions have a higher reliance on PN. Intolerance to enteral feeds may be a limiting factor and supplementation with PN in this group of patients allows for improved nutritional intake. Enteral feeds are held for a period of time before procedures such as elective endotracheal intubation, general anesthesia, procedural sedation, extubation, and other such interventions to lower the corresponding risk of aspiration. Most centers do not to use enteral feeds with patients who are on multiple vasopressor drugs for hypotension or who have evidence of bowel ischemia so as to limit the risk of small bowel necrosis associated with rapid enteral feeding.115 In a subgroup of critically ill patients, TPN may be required for a period before initiation of enteral feeds.
Prospective cohort studies and retrospective chart reviews have reported the inability to achieve the daily caloric goal in many critically ill children.4,116 In a recent study of mechanically ventilated children from 30 centers, energy and protein intake were found to be grossly inadequate.117 By the end of the first week in the ICU just over 50% of the prescribed energy and protein were delivered. Patients with energy intake less than 66% of the prescribed goal had higher mortality rates. The most common reasons for suboptimal enteral nutrient delivery in these studies were fluid restriction, procedures interrupting feeds, and feeding intolerance. In a study examining the endocrine and metabolic response of children with meningococcal sepsis, goal nutrition was achieved in only 25%.7 Similar observations have been made in a group of 95 children in a pediatric ICU (PICU) where patients received a median of 58.8% (range 0–277%) of their estimated energy requirements.116 In this review, enteral feeding was interrupted on 264 occasions to allow clinical procedures. In another review of nutritional intake in 42 patients in a tertiary-level PICU over 458 days, actual energy intake was compared with estimated energy requirement.4 Only 50% of patients were reported to have received full estimated energy requirements after a median of seven days. Prolonged fluid resuscitation was a major factor hindering the achievement of estimated energy requirements despite maximizing the energy content of the feedings. Protocols for use of transpyloric feeding tubes and changing from bolus to continuous feeds during brief periods of intolerance are strategies that may help achieve estimated energy goals in this population. Consistently underachieved EN goals are thought to be one of the reasons for the absence of a beneficial effect in multiple studies and meta-analyses of the efficacy of immunonutrition in preventing infection.118 Addressing preventable interruptions in enteral feeding in critically ill children is essential to attaining goal feeds. At this time, there is not enough evidence to recommend the use of prokinetic medications, motility agents (for feeding intolerance or to facilitate enteral tube placement), prebiotics, probiotics, or synbiotics in critically ill children. Randomized studies comparing enteral feeds administered by bolus or continuously are also lacking.
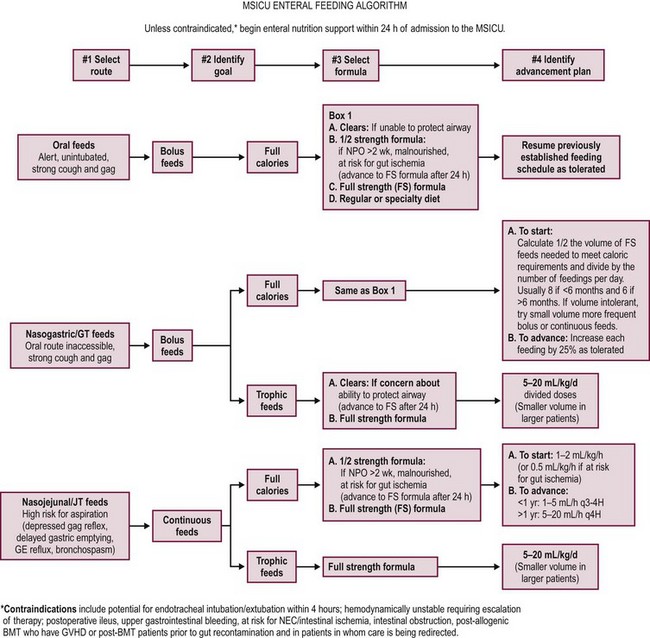
In summary, EN must be initiated early in hospitalized children with bowel activity. Postpyloric EN may be utilized in children with a high risk of aspiration or when gastric feeding is either contraindicated or has failed. Enterally administered feeds meet nutritional requirements in critically ill children with a functional gastrointestinal system and have the advantages of low cost, manageability, safety, and preservation of hepatic and other gastrointestinal function. Early introduction of enteral feeds in critically ill patients helps to achieve positive protein and energy balance and restores nitrogen balance during the acute state of illness. It maintains gut integrity and elicits release of growth factors and hormones that maintain gut integrity and function.119 Despite its perceived benefits, current practice in ICUs shows that a significant proportion of eligible patients do not receive EN.120 Fig. 2-4 offers an algorithm for initiating and advancing EN in children admitted to the multidisciplinary ICU at Children’s Hospital, Boston.
Parenteral Nutrition
Fluid and electrolytes status will guide the initial PN prescription. The patient’s hydration, size, age, and underlying disease will dictate the amount of the fluid to be administered. Fluid requirements in the pediatric age group are routinely estimated based on the Holliday–Segar method (Table 2-4). PN should not routinely be used to replace ongoing losses. Fluid shifts, increased insensible losses, drainage of bodily secretions, and renal failure can complicate electrolyte management in these patients. PN should be prescribed daily after reviewing the electrolytes (Na+, K+, Cl−, HCO3−, Ca2+) and glucose to allow adjustments in the macro- and micronutrient composition. In sick patients with significant gastrointestinal fluid loss (gastric, pancreatic, small intestinal, or bile), the measurement of electrolytes from the drained fluid is recommended. However, urgent changes in serum electrolytes should not be managed by changes in PN infusion rate or composition because these represent imprecise methods to treat a potentially serious electrolyte abnormality. In addition, careful attention to phosphate and magnesium levels is important. Hypophosphatemia may lead to hemolytic anemia, respiratory muscle dysfunction, and cardiac failure. A significant decrease in serum phosphate also may be seen with the re-feeding syndrome. In contrast, renal failure can result in the retention of phosphate and potassium, and nutritional allotments must be reduced accordingly. Magnesium deficiency can cause fatal cardiac arrhythmia in children and adults alike. Abnormalities of acid-base physiology also can influence the nutritional regimen of the hospitalized child. If a metabolic alkalosis from active diuresis or gastric suction occurs, chloride administration is used to correct the alkalosis. Severe, untreated alkalemia may inhibit the patient’s respiratory drive, shift potassium intracellularly, decrease ionized calcium concentrations by increasing the affinity of albumin for calcium, and promote refractory cardiac arrhythmias. Metabolic acidosis is often seen in critically ill children and may be associated with hypotension, ischemia, or renal failure. In such patients, the use of acetate instead of chloride in the PN regimen may be helpful.121
TABLE 2-4
Daily Fluid Requirement for Infants and Children
| Body Weight (kg) | Maintenance Daily Fluid Requirement |
| 0–10 | 100 mL/kg |
| 10–20 | 1000 mL + 50 mL/kg >10 kg |
| >20 | 1500 mL + 20 mL/kg >20 kg |
The three main macronutrients in PN are carbohydrate, lipid, and protein. Protein is administered in the form of crystalline amino acids starting at 0.5 g/kg/day in preterm neonates and 1 g/kg/day in others. The protein intake is advanced daily in increments of 1 g/kg/day until the goal intake is achieved. Table 2-3 lists recommended quantities of dietary protein needs for hospitalized children. Dextrose provides the main source of energy in PN and is initiated at a rate of 5 mg/kg/min using 5–10% concentration. The glucose infusion rate in mg/kg/min can be calculated with the help of the equation:
Infusion rates higher than 12 mg/kg/min are infrequently required and overfeeding with carbohydrate is associated with lipogenesis (RQ >1.0), hepatic steatosis, hyperglycemia, and osmotic diuresis. Three to 5% of the energy needs must be met using intravenous lipids, which are usually initiated at a rate of 1 g/kg/day, and advanced in increments to reach a maximum of 3 g/kg/day or 50% of total energy intake. Intravenous lipids prevent essential fatty acid deficiency and are a concentrated and isotonic source of energy. Triglyceride levels should be monitored and intralipid infusion rate is lowered when hypertriglyceridemia is noted. As noted previously, available evidence suggests that limiting lipids to 1g/kg/day may be indicated in patients with intestinal failure-associated PN cholestasis or in those patients who are likely to require a protracted course of PN.122
The vitamin and micronutrient (trace element) needs of healthy children and neonates are relatively well defined in the literature.17 In the neonate and child, required vitamins include the fat-soluble vitamins (A, D, E, and K) as well as the water-soluble vitamins: ascorbic acid, thiamine, riboflavin, pyridoxine, niacin, pantothenate, biotin, folate, and vitamin B12. Because vitamins are not consumed stoichiometrically in biochemical reactions but instead act as catalysts, the administration of large vitamin supplements in metabolically stressed states is not logical from a nutritional standpoint. The trace elements required for normal growth and development include zinc, iron, copper, selenium, manganese, iodide, molybdenum, and chromium. Trace elements are usually used in the synthesis of the active sites of a ubiquitous and extraordinarily important class of enzymes called metalloenzymes. More than 200 zinc metalloenzymes alone exist, and both DNA and RNA polymerase are included in this group. As with vitamins, these metalloenzymes act as catalytic agents. Unless specific mineral losses occur, such as enhanced zinc loss with severe diarrhea, large nutritional requirements would not be anticipated during critical illness. Selenium and carnitine may be added after 30 days of exclusive PN administration. The addition of copper and manganese in the PN of children with cholestasis is controversial and usually the dose is halved in view of their biliary excretion. The pharmacologic use of vitamins and trace minerals in pediatric illness has not been adequately studied. Reviews of both vitamin and trace mineral toxicity clearly demonstrate that excessive intake is a health risk.123,124
Immune-Enhancing Diets
Immunomodulation is thought to play a significant role in the response to an infectious insult and impacts outcome in children with sepsis. In 1996, Bone and colleagues outlined the role of the compensatory anti-inflammatory response that follows the initial proinflammatory response engendered by trauma or infection.125 Therapies aimed at modulating or stimulating the immune response have been a focus of many recent nutritional studies.
Unfortunately investigations examining the role of immune-enhancing diets in critically ill patients are marred by heterogeneous clinical populations, methodological flaws, and the use of nutritional formulations that often contain multiple potentially active components. Thus, studies and meta-analyses offer conflicting conclusions.69,114,121–123 Furthermore, there are no published studies specifically evaluating the role of immunonutrition in critically ill children. Potentially promising, but unproven, additives include arginine, glutamine, cysteine, nucleic acids, and omega-3 fatty acids.
Conclusion
The nutritional status of children influences outcome in surgical patients. Malnutrition is associated with physiological instability and a longer ICU stay accompanied by increased utilization of resources.126 The first step in implementing appropriate nutritional support is an understanding of the metabolic events that accompany critical illness and surgery. Individualized, quantitative assessments of protein, carbohydrate, lipid, electrolyte, vitamin and micronutrient requirements are made, and the appropriate route of nutrient delivery is determined. This nutrition regimen needs to be reviewed and amended regularly during the course of illness. The goal of nutrition therapy in sick pediatric surgical patients is to augment the short-term benefits of the metabolic stress response while minimizing any long-term consequences.
References
1. Pollack, MM, Wiley, JS, Kanter, R, et al. Malnutrition in critically ill infants and children. J Parenter Enteral Nutr. 1982; 6:20–24.
2. Hults, J, Joosten, K, Zimmermann, L, et al. Malnutriion in critically il children: From admision to 6 months after discharge. Clin Nutr. 2004; 23:223–232.
3. Alexander, E, Susla, GM, Burstein, AH, et al. Retrospective evaluation of commonly used equations to predict energy expenditure in mechanically ventilated, critically ill patients. Pharmacotherapy. 2004; 24:1659–1667.
4. Chwals, WJ, Bistrian, BR. Predicted energy expenditure in critically ill children: Problems associated with increased variability. Crit Care Med. 2000; 28:2655–2656.
5. Rogers, EJ, Gilbertson, HR, Heine, RG, et al. Barriers to adequate nutrition in critically ill children. Nutrition. 2003; 19:865–868.
6. Cuthbertson, D. Intensive-care-metabolic response to injury. Br J Surg. 1970; 57:718–721.
7. Munro, HN. Nutrition and muscle protein metabolism: Introduction. Fed Proc. 1978; 37:2281–2282.
8. de Groof, F, Joosten, KF, Janssen, JA, et al. Acute stress response in children with meningococcal sepsis: Important differences in the growth hormone/insulin-like growth factor I axis between nonsurvivors and survivors. J Clin Endocrinol Metab. 2002; 87:3118–3124.
9. Fomon, SJ, Haschke, F, Ziegler, EE, et al. Body composition of reference children from birth to age 10 years. Am J Clin Nutr. 1982; 35:1169–1175.
10. Forbes, GB, Bruining, GJ. Urinary creatinine excretion and lean body mass. Am J Clin Nutr. 1976; 29:1359–1366.
11. Long, CL, Spencer, JL, Kinney, JM, et al. Carbohydrate metabolism in man: Effect of elective operations and major injury. J Appl Physiol. 1971; 31:110–116.
12. Ogata, ES. Carbohydrate metabolism in the fetus and neonate and altered neonatal glucoregulation. Pediatr Clin N Am. 1986; 33:25–45.
13. Herrera, E, Amusquivar, E. Lipid metabolism in the fetus and the newborn. Diabetes Metab Res Rev. 2000; 16:202–210.
14. Schulze, KF, Stefanski, M, Masterson, J, et al. Energy expenditure, energy balance, and composition of weight gain in low birth weight infants fed diets of different protein and energy content. J Pediatr. 1987; 110:753–759.
15. Long, CL, Kinney, JM, Geiger, JW. Nonsuppressability of gluconeogenesis by glucose in septic patients. Metabolism. 1976; 25:193–201.
16. Whyte, RK, Haslam, R, Vlainic, C, et al. Energy balance and nitrogen balance in growing low birthweight infants fed human milk or formula. Pediatr Res. 1983; 17:891–898.
17. National Academy of Sciences. Recommended Dietary Allowances, 10th ed. Washington, DC: National Academy Press; 1989.
18. Kashyap, S, Schulze, KF, Forsyth, M, et al. Growth, nutrient retention, and metabolic response in low birth weight infants fed varying intakes of protein and energy. J Pediatr. 1988; 113:713–721.
19. Seale, JL, Rumpler, WV. Comparison of energy expenditure measurements by diet records, energy intake balance, doubly labeled water and room calorimetry. Eur J Clin Nutr. 1997; 51:856–863.
20. Daly, JM, Heymsfield, SB, Head, CA, et al. Human energy requirements: Overestimation by widely used prediction equation. Am J Clin Nutr. 1985; 42:1170–1174.
21. Schofield, WN. Predicting basal metabolic rate, new standards and review of previous work. Hum Nutr Clin Nutr. 1985; 39(Suppl 1):5–41.
22. Hunter, DC, Jaksic, T, Lewis, D, et al. Resting energy expenditure in the critically ill: Estimations versus measurement. Br J Surg. 1988; 75:875–878.
23. Muller, MJ, Bosy-Westphal, A, Klaus, S, et al. World Health Organization equations have shortcomings for predicting resting energy expenditure in persons from a modern, affluent population: Generation of a new reference standard from a retrospective analysis of a German database of resting energy expenditure. Am J Clin Nutr. 2004; 80:1379–1390.
24. Elwyn, DH, Kinney, JM, Askanazi, J. Energy expenditure in surgical patients. Surg Clin North Am. 1981; 61:545–556.
25. Tilden, SJ, Watkins, S, Tong, TK, et al. Measured energy expenditure in pediatric intensive care patients. Am J Dis Child. 1989; 143:490–492.
26. Ferrannini, E. The theoretical bases of indirect calorimetry: A review. Metabolism. 1988; 37:287–301.
27. Joosten, KF, Verhoeven, JJ, Hazelzet, JA. Energy expenditure and substrate utilization in mechanically ventilated children. Nutrition. 1999; 15:444–448.
28. Chwals, WJ. Overfeeding the critically ill child: Fact or fantasy? New Horiz. 1994; 2:147–155.
29. Hulst, JM, van Goudoever, JB, Zimmermann, LJ, et al. Adequate feeding and the usefulness of the respiratory quotient in critically ill children. Nutrition. 2004; 21:192–198.
30. McClave, SA, Spain, DA, Skolnick, JL, et al. Achievement of steady state optimizes results when performing indirect calorimetry. J Parenter Enteral Nutr. 2003; 27:16–20.
31. Mehta, NM, Bechard, LJ, Leavitt, K, et al. Severe weight loss and hypermetabolic paroxysmal dysautonomia following hypoxic ischemic brain injury: The role of indirect calorimetry in the intensive care unit. J Parenter Enteral Nutr. 2008; 32:281–284.
32. Mehta, NM, Bechard, LJ, Leavitt, K, et al. Cumulative energy imbalance in the pediatric intensive care unit: Role of targeted indirect calorimetry. J Parenter Enteral Nutr. 2009; 33:336–344.
33. Schoenheimer, R, Rittenberg, D. Deuterium as an indicator in the study of intermediary metabolism. Science. 1935; 82:156–157.
34. Schoeller, DA, van Santen, E. Measurement of energy expenditure in humans by doubly labeled water method. J Appl Physiol. 1982; 53:955–959.
35. Shew, SB, Beckett, PR, Keshen, TH, et al. Validation of a [13C]bicarbonate tracer technique to measure neonatal energy expenditure. Pediatr Res. 2000; 47:787–791.
36. Schoeller, DA, Hnilicka, JM. Reliability of the doubly labeled water method for the measurement of total daily energy expenditure in free-living subjects. J Nutr. 1996; 126:348S–354S.
37. Schoeller, DA, Kushner, RF, Jones, PJ. Validation of doubly labeled water for measuring energy expenditure during parenteral nutrition. Am J Clin Nutr. 1986; 44:291–298.
38. Jones, PJ, Winthrop, AL, Schoeller, DA, et al. Validation of doubly labeled water for assessing energy expenditure in infants. Pediatr Res. 1987; 21:242–246.
39. Jahoor, F, Desai, M, Herndon, DN, et al. Dynamics of the protein metabolic response to burn injury. Metabolism. 1988; 37:330–337.
40. Weinstein, MR, Oh, W. Oxygen consumption in infants with bronchopulmonary dysplasia. J Pediatr. 1981; 99:958–961.
41. Jones, MO, Pierro, A, Hammond, P, et al. The metabolic response to operative stress in infants. J Pediatr Surg. 1993; 28:1258–1262.
42. Shew, SB, Keshen, TH, Glass, NL, et al. Ligation of a patent ductus arteriosus under fentanyl anesthesia improves protein metabolism in premature neonates. J Pediatr Surg. 2000; 35:1277–1281.
43. Pierro, A, Carnielli, V, Filler, RM, et al. Partition of energy metabolism in the surgical newborn. J Pediatr Surg. 1991; 26:581–586.
44. Chwals, WJ, Letton, RW, Jamie, A, et al. Stratification of injury severity using energy expenditure response in surgical infants. J Pediatr Surg. 1995; 30:1161–1164.
45. Jaksic, T, Shew, SB, Keshen, TH, et al. Do critically ill surgical neonates have increased energy expenditure? J Pediatr Surg. 2001; 36:63–67.
46. Briassoulis, G, Venkataraman, S, Thompson, AE. Energy expenditure in critically ill children. Crit Care Med. 2000; 28:1166–1172.
47. Goran, MI, Kaskoun, M, Johnson, R. Determinants of resting energy expenditure in young children. J Pediatr. 1994; 125:362–367.
48. Bartlett, RH, Dechert, RE, Mault, JR, et al. Measurement of metabolism in multiple organ failure. Surgery. 1982; 92:771–779.
49. Beaufrere, B. Protein turnover in low-birth-weight (LBW) infants. Acta Paediatr Suppl. 1994; 405:86–92.
50. Denne, SC, Karn, CA, Ahlrichs, JA, et al. Proteolysis and phenylalanine hydroxylation in response to parenteral nutrition in extremely premature and normal newborns. J Clin Invest. 1996; 97:746–754.
51. Hay, WW, Jr., Lucas, A, Heird, WC, et al. Workshop summary: Nutrition of the extremely low birth weight infant. Pediatrics. 1999; 104:1360–1368.
52. Cogo, PE, Carnielli, VP, Rosso, F, et al. Protein turnover, lipolysis, and endogenous hormonal secretion in critically ill children. Crit Care Med. 2002; 30:65–70.
53. Mrozek, JD, Georgieff, MK, Blazar, BR, et al. Effect of sepsis syndrome on neonatal protein and energy metabolism. J Perinatol. 2000; 20:96–100.
54. Williamson, DH, Farrell, R, Kerr, A, et al. Muscle-protein catabolism after injury in man, as measured by urinary excretion of 3-methylhistidine. Clin Sci Mol Med. 1977; 52:527–533.
55. Pierro, A. Metabolism and nutritional support in the surgical neonate. J Pediatr Surg. 2002; 37:811–822.
56. Meszaros, K, Bojta, J, Bautista, AP, et al. Glucose utilization by Kupffer cells, endothelial cells, and granulocytes in endotoxemic rat liver. Am J Physiol. 1991; 260:G7–12.
57. Denne, SC, Karn, CA, Wang, J, et al. Effect of intravenous glucose and lipid on proteolysis and glucose production in normal newborns. Am J Physiol. 1995; 269:E361–E367.
58. Mitton, SG, Garlick, PJ. Changes in protein turnover after the introduction of parenteral nutrition in premature infants: Comparison of breast milk and egg protein-based amino acid solutions. Pediatr Res. 1992; 32:447–454.
59. Felig, P. The glucose-alanine cycle. Metabolism. 1973; 22:179–207.
60. Duffy, B, Pencharz, P. The effects of surgery on the nitrogen metabolism of parenterally fed human neonates. Pediatr Res. 1986; 20:32–35.
61. Poindexter, BB, Karn, CA, Leitch, CA, et al. Amino acids do not suppress proteolysis in premature neonates. Am J Physiol. 2001; 281:E472–E478.
62. Bechard, LJ, Parrott, JS, Mehta, NM. Systematic review of the influence of energy and protein intake on protein balance in critically ill children. J Pediatr. 2012; 161:333–339.
63. Keshen, TH, Miller, RG, Jahoor, F, et al. Stable isotopic quantitation of protein metabolism and energy expenditure in neonates on- and post-extracorporeal life support. J Pediatr Surg. 1997; 32:958–962.
64. Goldman, HI, Freudenthal, R, Holland, B, et al. Clinical effects of two different levels of protein intake on low-birth-weight infants. J Pediatr. 1969; 74:881–889.
65. Goldman, HI, Liebman, OB, Freudenthal, R, et al. Effects of early dietary protein intake on low-birth-weight infants: Evaluation at 3 years of age. J Pediatr. 1971; 78:126–129.
66. Imura, K, Okada, A. Amino acid metabolism in pediatric patients. Nutrition (Burbank, Los Angeles County, Calif. 1998; 14:143–148.
67. Miller, RG, Jahoor, F, Jaksic, T. Decreased cysteine and proline synthesis in parenterally fed, premature infants. J Pediatr Surg. 1995; 30:953–957.
68. Miller, RG, Keshen, TH, Jahoor, F, et al. Compartmentation of endogenously synthesized amino acids in neonates. J Surg Res. 1996; 63:199–203.
69. Reeds, PJ, Berthold, HK, Boza, JJ, et al. Integration of amino acid and carbon intermediary metabolism: Studies with uniformly labeled tracers and mass isotopomer analysis. Eur J Pediatr. 1997; 156(Suppl 1):S50–S58.
70. Zlotkin, SH, Anderson, GH. The development of cystathionase activity during the first year of life. Pediatr Res. 1982; 16:65–68.
71. Heyland, DK, Novak, F, Drover, JW, et al. Should immunonutrition become routine in critically ill patients? A systematic review of the evidence. JAMA. 2001; 286:944–953.
72. Dzakovic, A, Kaviani, A, Eshach-Adiv, O, et al. Trophic enteral nutrition increases hepatic glutathione and protects against peroxidative damage after exposure to endotoxin. J Pediatr Surg. 2003; 3(8):844–847.
73. Duggan, C, Gannon, J, Walker, WA. Protective nutrients and functional foods for the gastrointestinal tract. Am J Clin Nutr. 2002; 75:789–808.
74. Liu, Z, Barrett, EJ. Human protein metabolism: Its measurement and regulation. Am J Physiol. 2002; 283:E1105–E1112.
75. Demling, RH, Orgill, DP. The anticatabolic and wound healing effects of the testosterone analog oxandrolone after severe burn injury. J Crit Care. 2000; 15:12–17.
76. Takala, J, Ruokonen, E, Webster, NR, et al. Increased mortality associated with growth hormone treatment in critically ill adults. N Engl J Med. 1999; 341:785–792.
77. Yarwood, GD, Ross, RJ, Medbak, S, et al. Administration of human recombinant insulin-like growth factor-I in critically ill patients. Crit Care Med. 1997; 25:1352–1361.
78. Sakurai, Y, Aarsland, A, Herndon, DN, et al. Stimulation of muscle protein synthesis by long-term insulin infusion in severely burned patients. Ann Surg. 1995; 222:283–294.
79. Thomas, SJ, Morimoto, K, Herndon, DN, et al. The effect of prolonged euglycemic hyperinsulinemia on lean body mass after severe burn. Surgery. 2002; 132:341–347.
80. van den Berghe, G, Wouters, P, Weekers, F, et al. Intensive insulin therapy in the critically ill patients. N Engl J Med. 2001; 345:1359–1367.
81. Agus, MS, Javid, PJ, Ryan, DP, et al. Intravenous insulin decreases protein breakdown in infants on extracorporeal membrane oxygenation. J Pediatr Surg. 2004; 39:839–844.
82. Tappy, L, Schwarz, JM, Schneiter, P, et al. Effects of isoenergetic glucose-based or lipid-based parenteral nutrition on glucose metabolism, de novo lipogenesis, and respiratory gas exchanges in critically ill patients. Crit Care Med. 1998; 26:860–867.
83. Askanazi, J, Rosenbaum, SH, Hyman, AI, et al. Respiratory changes induced by the large glucose loads of total parenteral nutrition. JAMA. 1980; 243:1444–1447.
84. Jones, MO, Pierro, A, Hammond, P, et al. Glucose utilization in the surgical newborn infant receiving total parenteral nutrition. J Pediatr Surg. 1993; 28:1121–1125.
85. Shew, SB, Keshen, TH, Jahoor, F, et al. The determinants of protein catabolism in neonates on extracorporeal membrane oxygenation. J Pediatr Surg. 1999; 34:1086–1090.
86. Forsyth, JS, Murdock, N, Crighton, A. Low birthweight infants and total parenteral nutrition immediately after birth. III. Randomised study of energy substrate utilisation, nitrogen balance, and carbon dioxide production. Arch Dis Child. 1995; 73:F13–F16.
87. Jones, MO, Pierro, A, Garlick, PJ, et al. Protein metabolism kinetics in neonates: Effect of intravenous carbohydrate and fat. J Pediatr Surg. 1995; 30:458–462.
88. Krinsley, JS. Association between hyperglycemia and increased hospital mortality in a heterogeneous population of critically ill patients. Mayo Clin Proc. 2003; 78:1471–1478.
89. Laird, AM, Miller, PR, Kilgo, PD, et al. Relationship of early hyperglycemia to mortality in trauma patients. J Trauma. 2004; 56:1058–1062.
90. Wiener, RS, Wiener, DC, Larson, RJ. Benefits and risks of tight glucose control in critically ill adults: A meta-analysis. JAMA. 2008; 300:933–944.
91. Srinivasan, V, Spinella, PC, Drott, HR, et al. Association of timing, duration, and intensity of hyperglycemia with intensive care unit mortality in critically ill children. Pediatr Crit Care Med. 2004; 5:329–336.
92. Jeevanandam, M, Young, DH, Schiller, WR. Nutritional impact on the energy cost of fat fuel mobilization in polytrauma victims. J Trauma. 1990; 30:147–154.
93. Nordenstrom, J, Carpentier, YA, Askanazi, J, et al. Metabolic utilization of intravenous fat emulsion during total parenteral nutrition. Ann Surg. 1982; 196:221–231.
94. Coss-Bu, JA, Klish, WJ, Walding, D, et al. Energy metabolism, nitrogen balance, and substrate utilization in critically ill children. Am J Clin Nutr. 2001; 74:664–669.
95. Powis, MR, Smith, K, Rennie, M, et al. Effect of major abdominal operations on energy and protein metabolism in infants and children. J Pediatr Surg. 1998; 33:49–53.
96. Friedman, Z, Danon, A, Stahlman, MT, et al. Rapid onset of essential fatty acid deficiency in the newborn. Pediatrics. 1976; 58:640–649.
97. Paulsrud, JR, Pensler, L, Whitten, CF, et al. Essential fatty acid deficiency in infants induced by fat-free intravenous feeding. Am J Clin Nutr. 1972; 25:897–904.
98. Giovannini, M, Riva, E, Agostoni, C. Fatty acids in pediatric nutrition. Pediatr Clin North Am. 1995; 42:861–877.
99. Van Aerde, JE, Sauer, PJ, Pencharz, PB, et al. Metabolic consequences of increasing energy intake by adding lipid to parenteral nutrition in full-term infants. Am J Clin Nutr. 1994; 59:659–662.
100. Cleary, TG, Pickering, LK. Mechanisms of intralipid effect on polymorphonuclear leukocytes. J Clin Lab Immunol. 1983; 11:21–26.
101. Freeman, J, Goldmann, DA, Smith, NE, et al. Association of intravenous lipid emulsion and coagulase-negative staphylococcal bacteremia in neonatal intensive care units. N Engl J Med. 1990; 323:301–308.
102. Periera, GR, Fox, WW, Stanley, CA, et al. Decreased oxygenation and hyperlipemia during intravenous fat infusions in premature infants. Pediatrics. 1980; 66:26–30.
103. Birkhahn, RH, Long, CL, Fitkin, DL, et al. A comparison of the effects of skeletal trauma and surgery on the ketosis of starvation in man. J Trauma. 1981; 21:513–519.
104. Lee, S, Gura, KM, Kim, S, et al. Current clinical applications of omega-6 and omega-3 fatty acids. Nutr Clin Pract. 2006; 21:323–341.
105. Lee, S, Gura, KM, Puder, M. Omega-3 fatty acids and liver disease. Hepatology. 2007; 45:841–845.
106. Gura, KM, Duggan, CP, Collier, SB, et al. Reversal of parenteral nutrition-associated liver disease in two infants with short bowel syndrome using parenteral fish oil: Implications for future management. Pediatrics. 2006; 118:e197–e201.
107. Zaloga, GP. Early enteral nutritional support improves outcome: Hypothesis or fact? Crit Care Med. 1999; 27:259–261.
108. Heyland, DK, Dhaliwal, R, Drover, JW, et al. Canadian clinical practice guidelines for nutrition support in mechanically ventilated, critically ill adult patients. JPEN. 2003; 27:355–373.
109. Briassoulis, G, Zavras, N, Hatzis, T. Malnutrition, nutritional indices, and early enteral feeding in critically ill children. Nutrition. 2001; 17:548–557.
110. Briassoulis, GC, Zavras, NJ, Hatzis, MT. Effectiveness and safety of a protocol for promotion of early intragastric feeding in critically ill children. Pediatr Crit Care Med. 2001; 12:113–121.
111. Chellis, MJ, Sanders, SV, Webster, H, et al. Early enteral feeding in the pediatric intensive care unit. J Parenter Enteral Nutr. 1996; 20:71–73.
112. Meert, KL, Daphtary, KM, Metheny, NA. Gastric vs. small bowel feeding in critically ill children receiving mechanical ventilation: A randomized controlled trial. Chest. 2004; 126:872–878.
113. Sanchez, C, Lopez-Herce, J, Carrillo, A, et al. Early transpyloric enteral nutrition in critically ill children. Nutrition. 2007; 23:16–22.
114. Mehta, NM, McAleer, D, Hamilton, S, et al. Challenges to optimal enteral nutrition in a multidisciplinary pediatric intensive care unit. J Parenter Enteral Nutr. 2010; 34:38–45.
115. Munshi, IA, Steingrub, JS, Wolpert, L. Small bowel necrosis associated with early postoperative jejunal tube feeding in a trauma patient. J Trauma. 2000; 49:163–165.
116. Taylor, RM, Preedy, VR, Baker, AJ, et al. Nutritional support in critically ill children. Clin Nutr. 2003; 22:365–369.
117. Mehta, NM, Bechard, LJ, Cahill, N, et al. Nutritional practices and their relationship to clinical outcomes in critically ill children–an international multicenter cohort study. Crit Care Med. 2012; 40:2204–2211.
118. Atkinson, S, Sieffert, E, Bihari, D. A prospective, randomized, double-blind, controlled clinical trial of enteral immunonutrition in the critically ill. Guy’s Hospital Intensive Care Group. Crit Care Med. 1998; 26:1164–1172.
119. Briassoulis, G, Tsorva, A, Zavras, N, et al. Influence of an aggressive early enteral nutrition protocol on nitrogen balance in critically ill children. J Nutr Biochem. 2002; 13:560.
120. Heyland, DK, Cook, DJ, Guyatt, GH. Enteral nutrition in the critically ill patient: A critical review of the evidence. Intensive Care Med. 1993; 19:435–442.
121. Peters, O, Ryan, S, Matthew, L, et al. Randomised controlled trial of acetate in preterm neonates receiving parenteral nutrition. Arch Dis Child. 1997; 77:F12–F15.
122. Garza, JJ, Shew, SB, Keshen, TH, et al. Energy expenditure in ill premature neonates. J Pediatr Surg. 2002; 37:289–293.
123. Flodin, NW. Micronutrient supplements: Toxicity and drug interactions. Prog Food Nutr Sci. 1990; 14:277–331.
124. Marks, J. The safety of the vitamins: An overview. Int J Vitam Nutr Res Suppl. 1989; 30:12–20.
125. Bone, RC, Grodzin, CJ, Balk, RA. Sepsis: A new hypothesis for pathogenesis of the disease process. Chest. 1997; 112:235–243.
126. Pollack, MM, Ruttimann, UE, Wiley, JS. Nutritional depletions in critically ill children: Associations with physiologic instability and increased quantity of care. J Parenter Enteral Nutr. 1985; 9:309–313.