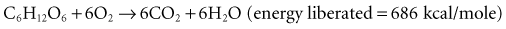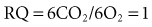95 Nutrition Issues in Critically Ill Children
Recent reviews of the literature on nutritional support for critically ill children details the lack of definitive or reliable studies to guide our practice based upon scientific evidence.1,2 Therefore, many of the recommendations made rest upon “good practice” principles which rely upon expert consensus and avoidance of known harm whenever possible. The American Society of Parenteral and Enteral Nutrition (A.S.P.E.N.) has promulgated a set of expert guidelines for supporting critically ill children that represent a reasonable standard of care for children in the pediatric intensive care unit (PICU).3
 Impact of Physiologic Stress on Children
Impact of Physiologic Stress on Children
Alterations in protein and energy metabolism are hallmarks of critical illness and have been studied for many decades.4 This work has demonstrated a great difference between short-term starvation states in otherwise healthy individuals and the dramatic “autocannibalism” seen in critically ill patients who are not receiving appropriate nutritional support as summarized in Table 95-1.
TABLE 95-1 Comparison of Nutrient Metabolism in Starvation Versus Sepsis/Trauma
| Starvation | Sepsis/Trauma | |
|---|---|---|
| Protein breakdown | ++ | +++ |
| Hepatic protein synthesis | ++ | ++++ |
| Ureagenesis | ++ | ++++ |
| Gluconeogenesis | ++ | ++++ |
| Energy expenditure | Reduced | Increased |
| Mediator activity | Low | High |
| Hormone counterregulatory capacity | Preserved | Poor |
| Use of ketones | +++ | + |
| Loss of body stores | Gradual | Rapid |
| Primary fuels | Fat | Amino acids, glucose, triglycerides |
Adapted from Barton R, Cerra FB. The hypermetabolism-multiple organ failure syndrome. Chest 1989;96:1153-60.
The events that lead to ICU admission are extremely varied, yet the body’s response to acute physiologic stress tends to be similar whether the inciting event is sepsis, ischemia-reperfusion, trauma, burns, or other inflammatory conditions. Beyond low levels of stress, such as minor elective surgery, life-threatening illness, burns, organ transplantation, or major surgical procedures elicit dramatic systemic inflammatory responses due to activation of the immune system, clotting mechanisms, and the endothelium. The patient’s ability to withstand the metabolic responses to such stresses and ultimately to reverse the process is central to recovery. A complete discussion of the metabolic response to stress is beyond the scope of this chapter; the reader is referred to other sources.5,6
The initial response to injury is to activate endothelial cells and to prime inflammatory cells such as neutrophils, macrophages, and lymphocytes through proinflammatory mediators including tumor necrosis factor, interleukin 2, histamine, eicosanoids, heat-shock proteins, free radicals, platelet-activating factor, and tryptases.7 These same signals that produce activation of the endothelium lead to permeability changes, activation of clotting mechanisms, and changes in hepatic and peripheral protein metabolism.8 If recovery is to occur, this process must be extinguished by a decrease in the inflammatory state and an increase in tissue repair.9 Although it may seem that simply shutting off the proinflammatory signals should lead to resolution, the process of resolving inflammation appears more complex.10 Studies show the importance of many of the proinflammatory stimuli in regeneration and repair, and the timing of interventions is important.11 In response to injury, a wide range of neurohumoral reactions occur, forming the classic “stress response,” which includes elevation of growth hormone, endogenous catecholamines, glucagon, and cortisol. Recognition of the role of insulin-like growth factor-1 along with growth hormone in promoting protein synthesis and counter-regulating inflammatory states suggests important potential treatment options that have been best studied in burns. Despite these studies showing benefit from growth hormone supplementation, evidence of increased mortality rate after growth hormone supplementation also has been reported.12 Clinicians must balance the relative benefit of hormonal manipulation with potential risks.
In the inflammatory state, unremitting gluconeogenesis occurs through the release of glycerol and gluconeogenic amino acids from the periphery with their conversion to glucose in the liver and kidney. Hyperglycemia frequently is associated with this state and may induce glycosuria and an osmotic diuresis. Insulin activity becomes impaired at the tissue level, leading to so-called insulin resistance in the face of the powerful gluconeogenesis driven by the stress hormones. It seems that the impairment of insulin results from decreased phosphorylation of the insulin receptor and second messengers.11 In the last decade, evidence from adult ICU experience has suggested a benefit from the use of insulin infusions to maintain tight control over serum glucose level.13 Although much of the preceding information derives from adult studies, it has found its way into contemporary pediatric practice in many centers in children of various ages. This question is receiving intense scrutiny in critically ill children through multicenter trials which are currently underway. The use of insulin infusions to control hyperglycemia in premature infants continues to be standard practice; however, the potential to produce marked hepatic steatosis under the influence of insulin should be born in mind when choosing the amount of carbohydrate to provide.
The breakdown of protein is a central theme in the body’s response to stress, which has wide-ranging significance beyond simple protein losses. The conversion of certain amino acids to glucose and the oxidation of others in peripheral tissues lead to the liberation of large quantities of amino-nitrogen, which would become toxic if not for the efficient conversion to urea. A dramatic increase in the rate of urea production is seen in critically ill patients. Concomitantly, other non-urea nitrogen is liberated in the form of uric acid and creatine and accounts for the dramatic increase in nitrogen wasting seen during stress states. Total urinary nitrogen losses in critically ill children may be 0.3 g/kg/d, which represents the loss of approximately 1.8 g/kg/d of whole protein catabolized. In parallel with the increased turnover of proteins, the metabolic rate for oxidation of energy substrates may increase following acute critical illness during the recovery phase (see subsequent section on energy expenditure).
The body’s response to withholding feeding (i.e., starvation) in healthy individuals is qualitatively and quantitatively different than that seen when nutrient intake is absent during critical illness. These differences are fundamental to understanding nutritional support in the ICU and are summarized in Table 95-1. In simple starvation, the body’s regulatory mechanisms for sparing lean tissue and using triglycerides as the primary energy source are intact, whereas under the influence of the stress response, rapid depletion of lean tissues occurs with oxidation of amino acids, carbohydrate, and fat as energy substrates.
One of the major consequences of life-threatening physiologic stress is the net depletion of body protein representing the somatic protein pool (e.g., skeletal muscle mass) and functional (e.g., plasma proteins, enzyme systems, antibodies) tissues contained in the visceral protein pool. With protein catabolism rates increased up to twofold, synthesis does not keep pace, and a state of negative nitrogen balance ensues when patients are not given adequate calories and protein.14 These changes produce depressed function of T and B lymphocytes, monocytes, and neutrophils as cumulative protein loss increases. The synthesis of antibodies, chemotaxis, phagocytosis, and bacterial killing is impaired in the face of advanced protein-calorie malnutrition.15 A decrease in total lymphocyte count may be seen in many patients, but a total lymphocyte count less than 1200/mm3 should raise concern for the presence of possible immune dysfunction. These alterations lead to impairment of host defense mechanisms. As noted earlier, for resolution of the inflammatory response, the patient’s immune system plays a central role in recovery of wound healing and recovery of immune competence.16 It is likely that the syndrome of multiple organ dysfunction seen in critically ill patients is due in part to the inability of the immune system to down-regulate the inflammatory response to injury in specific organs, as well as acquired mitochondrial dysfunction leading to ineffective cellular energy production.17 Nutritional support of a critically ill patient is thought to be essential to achieving recovery and minimizing the subsequent period of convalescence.
Considerable attention currently is focused on the use of modified nutritional support regimens in critically ill adults to modify the inflammatory response and reduce secondary organ system dysfunction.18 A wide range of substances have been studied in an attempt to improve outcome or minimize nitrogen loss during critical illness in specific populations of patients. Glutamine supplementation appears to benefit critically ill adults, particularly those with burns.18 Omega-3 fatty acids appear to also be beneficial in patients with sepsis and systemic inflammatory response syndrome (SIRS). The results in adults suggest that formulas supplemented with these products improve oxygenation and reduce the alveolar inflammatory response during acute respiratory distress syndrome (ARDS). While trials of these agents are underway in critically ill children, there is still not a strong enough consensus among pediatric specialists to consider their use as standard therapy.3
 Nutrition Assessment
Nutrition Assessment
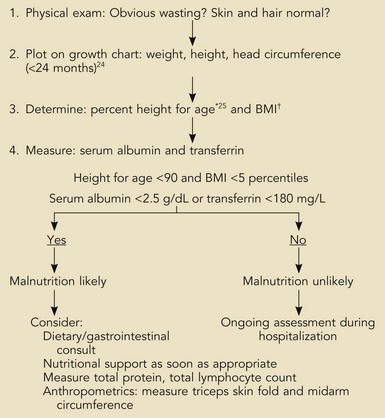
The nutrition assessment of hospitalized children is a central and critical part of the initial examination and evaluation of all patients. The existence of chronic malnutrition as well as the development of acute malnutrition during critical illness has been recognized in pediatric critical care for many years19–21 and appears to be an unmet need even today.22 Therefore, clinicians must assess newly admitted patients for the presence of malnutrition that may complicate the response to therapies or impair recovery (Box 95-1). The presence of previous severe malnutrition may complicate critical care management through the presence of marasmic cardiomyopathy, severe intracellular energy deficiency, and the development of refeeding disequilibrium when nutrients are provided in the ICU.23
Box 95-1
Assessment of Nutrition Status on Admission
*Actual height (cm) × 100/expected height at 50th percentile for age.
†BMI (kg/m2) = body mass index: actual weight (kg)/[actual weight × height (m)] 2
From Statistics NCfH. CDC growth charts, United States, 2000. Available at: http://www.cdc.gov/growthcharts; and from Waterlow J. Classification and definition of protein-calorie malunutrition. Br J Med 1972;3:566-9.
The initial nutrition evaluation consists of assessing the patient’s weight, height, historical evidence for recent weight loss, and anthropometric measurements including midarm circumference and skinfold determination (when edema is not present). Nutrition history must include the presence and duration of nausea, vomiting, diarrhea, fever, frequent infections, fatigue, food aversion, abdominal discomfort, or feeding intolerance. For growth standards, norms exist reflecting age and gender.24 Ethnic background and considerations such as the presence of certain syndromes (e.g., Down syndrome) or the child’s birth status (e.g., premature, growth restricted, etc.) may affect the child’s growth status.
In particular, determination of body mass index (BMI, previously known as weight-for-height) for children older than 2 years of age provides important information regarding the previous nutritional status (see Box 95-1). In children younger than 2 years, the weight-for-age in light of the previous growth status is most useful. These straightforward measurements have withstood the test of time and were used by Pollack and coworkers to estimate the risk of malnutrition in critically ill children admitted to a multidisciplinary PICU.19,20,25 Their findings demonstrated higher rates of preexisting malnutrition than had been previously thought. In addition, there was an unexpected deterioration in nutrition indices following admission, suggesting the powerful effects of life-threatening illness on nutritional stores and status even with excellent clinical care. Clinicians caring for children who will experience more than a few days of hospitalization must therefore be especially aware of the potential for acquired nutritional depletion. Potential sources of error exist in interpreting anthropometric measurements that are primarily related to changes in body water associated with many acute critical illnesses in children (i.e., conditions producing capillary leak syndrome or defects in renal water clearance). Such conditions may invalidate the measurement of skinfold or midarm circumference; however, their longitudinal use in patients can be very useful in estimating the accretion of fat and lean tissue stores. It is standard practice to measure these parameters in patients at risk for malnutrition, such as those with cystic fibrosis, short bowel syndrome, and other conditions in which malabsorption or chronically elevated metabolic demands exist (e.g., congenital heart failure, bronchopulmonary dysplasia, and similar chronic conditions).
The triceps and scapular skin folds measure the subcutaneous tissue compartment (consisting primarily of adipose tissue) but also tissue edema in patients with anasarca from any cause. Triceps skin fold is measured by standardized skin caliper and is subject to considerable error if not performed in a consistent manner midway between the acromion and olecranon. The midarm circumference should be measured at the same point with a nonstretchable tape measure. The two indices taken together permit a reliable estimate of muscle mass. In general, good correlation exists between skinfold and arm circumference and weight-for-height percentile.26 During critical illness, anasarca may obscure the loss of lean tissue, which may only be apparent following resolution of edema when successful diuresis has occurred. A very reliable indicator of global loss of lean body mass can be seen in the wasting of the interosseous and thenar muscles of the hand, which becomes apparent 2 or 3 weeks after hospitalization with resolution of edema.
Shorter half-life serum proteins such as prealbumin [T1/2 = 2 days] and transferrin [T1/2 = 7 days] also reflect nutrition status and respond more quickly to changes in anabolic state.27 As noted earlier, the pool of proteins in the plasma, interstitial space, and some intracellular proteins represent a relatively labile pool of protein referred to as the visceral protein pool. Visceral proteins are rapidly turned over relative to structural proteins that comprise the somatic protein pool. In critical illness, the synthesis of specific proteins such as C-reactive protein, ceruloplasmin, and α2-macroglobulin is increased, whereas the synthesis of other proteins such as albumin [T1/2 = ~20 days] is decreased.28 These changes may be seen within 6 hours of the onset of severe physiologic stress. This response to physiologic stress is under the regulation of complex neurohumoral control and is referred to as the acute phase response. It is largely responsible for the increase in erythrocyte sedimentation rate associated with acute inflammatory conditions.29 When followed longitudinally, the return of previously depressed levels of certain visceral proteins such as albumin, transferrin, retinol-binding protein, or prealbumin represents the abatement of physiologic stress or improvement in nutrition when levels are low due to protein-calorie malnutrition. Such positive changes herald the impending return to a state of growth and tissue accretion, barring reentry into a new inflammatory state.
 Energy Expenditure
Energy Expenditure
All cellular processes require energy, generally in the form of ATP which is produced through oxidation of metabolic fuels, with heat and water as byproducts. The production of ATP is closely coupled to cellular metabolism and must be maintained to prevent cell death. As ATP levels fall, ionic gradients cannot be maintained, excitatory cells cannot depolarize, the synthesis of new cells and repair of damaged cell constituents cannot occur, and mechanical work such as cardiac pump function and respiratory activity cease. Thus, the body has numerous mechanisms for efficiently producing energy from a wide variety of substrates including protein, fat, and carbohydrates. Following the adaptation to decreased nutrient intake, an otherwise healthy individual will rely upon ketone bodies derived from the breakdown of fat stores to provide critical intracellular energy. Protein stores are relatively spared as the decrease in insulin output allows the metabolism to shift to a ketone-based state. As indicated in Table 95-1, critical illness prevents the body’s conservational mechanisms in response to decreased intake, leading to relatively rapid depletion of carbohydrate and available protein stores.
The close coupling between oxidative metabolism and substrate utilization is reflected in the amount of oxygen consumed (VO2) and carbon dioxide produced (VCO2) through the pathways of intermediary metabolism, which include the glycolytic pathway and the tricarboxylic acid cycle. Specific substrates such as fat, protein, and various carbohydrates have a characteristic relationship between VO2 and VCO2 based upon the stoichiometry of their unique oxidation. This relationship is referred to as the respiratory quotient (RQ = VCO2/VO2) and may be measured through the quantification of respiratory gas exchange through the patient’s lung. The overall metabolic rate is most easily determined in the clinical setting through the process of indirect calorimetry, a process that estimates the resting energy expenditure (REE) based upon VO2 and VCO2.30 Indirect calorimetry is well established in clinical nutrition but has been elusively difficult to perform with consistent results and easily applied technology in children. The respiratory quotient for fats is around 0.707 and for proteins around 0.80 and, in conjunction with urinary nitrogen determination, forms the basis for determining the specific substrates being utilized.30 This concept is demonstrated for the aerobic metabolism of glucose:
The availability of equipment to reliably perform indirect calorimetry in children has been a major obstacle to its widespread application. Several factors limit the reliability with which indirect calorimetry can be performed in young children, including non–steady state due to patient movement and nursing interventions, use of uncuffed endotracheal tubes producing loss of respiratory gases, high bias flows on infant ventilators, use of elevated inspired oxygen in nonintubated infants, as well as the small tidal volumes seen in the smallest patients. When indirect calorimetry is not feasible, VO2 can be calculated in many patients via the Fick equation (A × VdO2 × cardiac output) when a reliable measure of cardiac output is available. Based upon a conversion factor of approximately 5 kcal of energy per liter of oxygen consumed, one can closely estimate metabolic rate30 if the oxygen consumption is known and the RQ is assumed to be in a normal range.
Through indirect calorimetry it has become clear that patients with similar clinical appearances may have widely differing metabolic rates when adjusted for age and weight.3,31–34 The differences may be as great as 300%, suggesting the potential for severe over- or undernutrition depending upon the values assumed.35 Thus, clinicians generally must rely upon information provided in controlled studies to guide the delivery of calories, since most will not have a means of easily determining the REE. A wide range of predictive equations have been devised which attempt to predict energy requirements of critically ill children, but it is clear that no single method of estimating caloric expenditures will be successful for all critically ill children.3,4,36
In very young infants, the effects of environmental cold stress is recognized as a source of unnecessary morbidity.37 The thermal neutral zone in infants up to 1 year of age tends to be several degrees higher than that for burned adults or older children. Heat lost to the environment produces rapid drops in core temperature in young children, with concomitant increase in metabolic demands. Maintaining the environment in a range of 30°C to 34°C with servo-controlled heaters or other means can significantly reduce energetic requirements in critically ill infants.
 Nutritional Support for the Critically Ill Child
Nutritional Support for the Critically Ill Child
Nutritional support for critically ill children is fundamentally different than conventional nutrition of healthy children because of the alterations in metabolism outlined previously. During periods of critical illness, utilization of nutrients for growth is markedly inhibited by hormonal response to stress and circulating inflammatory mediators. Utilization of calories for activity is much lower than under normal conditions. In addition, diet-induced thermogenesis is also affected in hospitalized patients by the different routes and formulations of nutrients provided. Estimates of increased caloric and protein requirements during acute illness and recovery indicate that compared to critically ill adults, children have greater requirements for both on a body weight basis. Therefore, one of the most important points for clinicians prescribing nutritional support is to provide calories in a thoughtful manner based upon the guidelines that follow and to avoid excess caloric intake during the acute phase of illness. During acute critical illness in children, many investigators have found REE to be less elevated than previously expected, with significant risk for overfeeding.33,35
Maintenance Fluids
Maintenance fluids for most patients can be estimated based upon body weight as indicated in Table 95-2. Children have generally increased requirements in relation to body weight for fluid, energy, protein, and many of the micronutrients. Water metabolism is closely coupled to metabolic activity because of the central role water plays in intermediary metabolism. For the term newborn, these amounts should be reduced during the first few days of life, owing to their increased intrinsic total body water. Premature infants have other considerations (e.g., high insensible losses), and consultation with a pediatrician or neonatologist is critical to provide appropriate and adequate fluid. Volumes must be increased for fever or persistent tachypnea to compensate for increased insensible fluid losses. Additional fluids must be provided to cover abnormal losses due to diarrhea, nasogastric drainage, or wound loss in burns or from other sites. Composition of the replacement fluid is based upon the content of sodium, potassium, bicarbonate, and chloride lost and conforms to conventional surgical and medical guidelines for fluid replacement. Typical maintenance fluids should provide sodium (3-5 mEq/kg/d) and potassium (2-3 mEq/kg/d) salts as well as a modest amount of glucose (5% or 10% if younger than 6 months of age). Recent trends in providing electrolytes has favored a balanced electrolyte solution that contains acetate salts of 1 to 2 mEq/kg/d to minimize development of hyperchloremic metabolic academia in the young child. Provision of glucose in maintenance fluids is intended to spare lean tissue through the elicitation of insulin release, which exerts an anticatabolic effect in minimally stressed patients.
TABLE 95-2 Approximate Parenteral Maintenance Fluid Requirements
| Body Weight | Fluid Volume (Parenterally) |
|---|---|
| First 10 kg | 100 mL/kg/d |
| Second 10 kg | 50 mL/kg/d |
| Additional kg | 20 mL/kg/d |
Prescribing Nutritional Support
The decision to provide nutrition via a parenteral or enteral route takes many factors into consideration, including anticipated time to resumption of normal dietary intake, available routes of nutrient administration, underlying metabolic or endocrine conditions, and the existence of organ dysfunction. When patients will not receive conventional nutrition for a prolonged period of time, it is appropriate to consider support via the gut or intravenously (IV). There is general agreement that the enteral route is superior to TPN when a patient is able to tolerate it. Advantages of the enteral route include better maintenance of gut structure and function, reduced bacterial translocation, fewer metabolic complications, decreased intrahepatic cholestasis, greater ease and safety of administration, better outcomes, and reduced cost.3
Nutrition is frequently started as soon as the patient is metabolically stable; however, reliable data regarding the necessity or benefits of nutritional support in the first 5 days of critical illness have not be convincing.3 For a critically ill patient, sufficient metabolic stability has been achieved when aggressive correction of electrolyte derangements has been achieved and the acid-base status no longer requires aggressive correction.
Carbohydrate
Carbohydrate serves predominantly as an energy source. The carbon backbone of sugars also provides the basis for synthesis of many nonessential nutrients in the body. Carbohydrate is provided as sugars or starches in enteral formulas and as dextrose in parenteral nutrition. The caloric density of common dietary carbohydrate is generally 4 kcal/g, except for dextrose solutions, which provide 3.4 kcal/g because of energy lost through the process of hydration in solution. As the primary energy source, the rate of infusion should be adjusted to achieve the goals outlined in Table 95-3.
TABLE 95-3 Target Goals for Nonprotein Calories When Resting Energy Expenditure Determination Not Available
| Acute Phase (First 3-5 Days; kcal/kg/d) |
Convalescent Phase (After 5 Days; kcal/kg/d) |
|
|---|---|---|
| Young children (<10 kg) | 50-80 | 80-120 |
| Children (1-7 years) | 45-65 | 75-90 |
| Children (>7 years) | 30-50 | 30-75 |
In general, the cellular energy requirements of most critically ill children and adults can be met and euglycemia can be maintained through the infusion of 5 to 8 mg/kg/min of dextrose. This range represents about 25 to 40 kcal/kg/d of carbohydrate calories and is a close first approximation of basal energy expenditure seen in many hospitalized children. In healthy nonstressed individuals, ketosis ensues when glucose entry into the circulation falls below 1.5 to 2 mg/kg/min. As an additional point of reference, infusion of over 10 to 12 mg/kg/min of glucose results in net lipogenesis and excess carbon dioxide production in most hospitalized patients. When hyperglycemia develops in the face of appropriate rates of glucose infusion, it has become routine to administer insulin as a continuous infusion. Recent reports suggest that maintaining serum glucose in a narrow euglycemic range in critically ill adults may be associated with greater morbidity due to hypoglycemia and offers limited actual benefit.38 This practice has become commonplace in pediatric critical care, with several multicenter trials of this approach currently underway. Clinicians can expect the rate of insulin infusion required to control the serum glucose to be as much as 2 to 3 times higher than is routinely used in the treatment of diabetes as a result of the insulin resistance seen during critical illness.
Protein
Protein requirements are met through the provision of conventional enteral formulas or formulas containing hydrolysates of complex proteins that provide oligopeptides. Enteral formulas containing primary amino acids tend to be hypertonic with limited absorptive advantages, owing to the presence of mucosal transporter mechanisms that absorb di- and tripeptides more efficiently. The high rate of protein turnover during critical illness is associated with an increase in ureagenesis and urinary nitrogen losses that may amount to as much as 1 to 2 g/kg/d of protein equivalent. The supraphysiologic ureagenesis may represent additional metabolic stress on the liver and kidneys. To minimize nitrogen loss and assure that no amino acid falls to a level that would limit protein synthesis, high-quality nutritional protein must be given through the acute and convalescent phase of illness. Conceptually, proteins must be administered in amounts sufficient to replace losses, with additional protein to synthesize new tissue. Table 95-4 provides guidelines for the administration of protein to children in the ICU. It is important to recognize that nitrogen balance in response to nutritional support represents a continuum. In one recent study, the authors found that nitrogen balance was obtained at an intake of 2.8 g/kg/d.39 Positive nitrogen balance was only achieved with amino acid infusion rates at the upper end of those typically used by clinicians. Furthermore, calories must be provided in sufficient quantity to ensure that protein can be used for synthesis rather than as an energy substrate.
| Acute Phase (First 3-5 Days; g/kg/d) | Convalescent Phase (After 5 Days; g/kg/d) | |
|---|---|---|
| Infants/Children < 7 years | 1.5-2.5 | 2.0-3.0 |
| Children > 7 years | 1.5-2.0 | 1.5-2.0 |
Special Considerations
Patients with renal insufficiency should receive nutrition optimized to achieve wound healing, without excessive concern for the increase in blood urea seen. In general, the increased nitrogen load is handled through dialysis, so optimal nutrition can be provided to promote recovery. Patients receiving continuous renal replacement therapies are at risk for both amino acid and micronutrient loss across the dialysis membrane and should have their nutritional support adjusted correspondingly.40
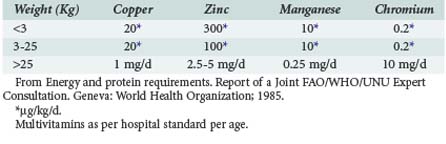
Micronutrients
Multivitamin preparations are provided either as unit doses by the pharmacy in parenteral nutrition or as MVI in standard formulas. Occasionally, additional vitamins or trace elements will be required for specific deficiency states or diseases, but fine tuning of micronutrients other than minerals and electrolytes has been difficult to achieve clinically. Current recommendations are given in Table 95-5.41
Route of Administration
Nutrition should be provided via the gastrointestinal tract whenever possible, supplementing with peripheral or central parenteral nutrition when adequate enteral intake cannot be achieved.3 In patients with significant burns, an enteral feeding tube should be placed within the first hours of hospitalization. Continuous drip feedings should begin within hours to minimize bowel dysmotility and feeding intolerance often seen if feeding is delayed in such patients. In other patients, initiating feedings on the second hospital day is feasible in most cases and should be provided initially as a continuous infusion at a minimal rate of about 1 mL/kg/h and advanced as tolerated. The provision of trophic feedings is thought to provide a number of benefits even though significant nutritional intake cannot be achieved. These benefits include maintenance of gut motility, improved mesenteric blood flow, the release of trophic factors from the gut and pancreas, which maintain enterocyte mass and hepatocyte function.42 In addition, enterocytes derive a significant portion of their nutrient and energetic requirements from the luminal contents during digestion, making enteral nutrition ideal when tolerated.
During acute critical illness, continuous drip feedings are often better tolerated than bolus feedings, especially in patients with respiratory distress. Transpyloric feeding when possible via weighted Silastic feeding tubes should be used to minimize the risk of gastroesophageal reflux and aspiration. It has been used with excellent results in critically ill children.43 Placement of transpyloric feeding tubes can be done blindly by some experienced clinicians44 or may be done by a radiologist under fluoroscopic guidance. Occasionally, metoclopramide or erythromycin may facilitate passage of a transpyloric tube. Even when a transpyloric feeding tube cannot be placed, continuous enteral feeding via a nasogastric tube may confer most of the benefits, although the risk of gastroesophageal reflux is somewhat greater. For young infants, the availability of breast milk is the optimal nutrient source and can be easily delivered by feeding tube when the infant cannot nurse. In older patients, the initial enteral nutrition formula for most critically ill children should be lactose free, have some of the fat provided as medium-chain triglycerides, and contain easily absorbed proteins such as di- and tripeptides (see earlier discussion). Most of the currently available formulas developed for children between the ages of 1 and 10 years of age conform to these recommendations. A wide variety of formulas exist; availability may vary from region to region. The hospital dietitian is best prepared to help select appropriate formulas and knows which products are available locally.
While beyond the scope of the current discussion, special considerations for premature infants and newborns include the use of formulas supplemented with docosahexaenoic acid (DHA) and arachidonic acid (ARA).45 DHA and ARA are long-chain polyunsaturated fatty acids found in breast milk and recently added to infant formulas. Their importance in infant nutrition was recognized by the rapid accretion of these fatty acids in the brain during the first postnatal year. Subsequent reports of enhanced intellectual development in breastfed children and recognition of the physiologic importance of DHA in visual and neural systems from studies in animal models has led to formulas being developed that contain them.46 It is becoming routine in the neonatal population to supplement DHA and ARA when providing enteral feedings.
Infants younger than 6 months of age should receive isotonic or hypotonic feedings initially until tolerance has been demonstrated. Young children between 1 and 5 years of age should receive an age-appropriate formula or an adult formula with appropriate supplements of protein, vitamins, and trace elements. Critically ill children older than 10 generally tolerate enteral formulas developed for adult patients, with supplementation of vitamins and micronutrients as needed for age. Enteral formulas should be initially iso- or hypotonic in order to minimize the possibility of diarrhea from excess osmotic load to the gut and to facilitate absorption. Infusion rates are begun conservatively at around 1 mL/kg/h, with a stepwise increase every 4 to 6 hours as tolerated up to the desired final rate. Once an acceptable rate is achieved, caloric density may be increased as tolerated. The clinician must maintain vigilance for evidence of feeding intolerance. In patients with poor tissue perfusion, enteral feedings are feasible; however, the risk of necrotizing enterocolitis is increased slightly when using the gut for nutrition. Thus, any signs of pronounced abdominal distension, profuse diarrhea, severe gastroesophageal reflux, or development of a new metabolic acidemia should lead to a hold on feedings and assessment of the abdomen prior to reinstituting feedings. Common manifestations of enteral feeding intolerance are outlined in Table 95-6.
| Problem | Possible Reason | Possible Remedy |
|---|---|---|
| Diarrhea, malabsorption | Delivery too fast | Decrease delivery rate |
| High osmotic load | Reduce osmolarity or volume | |
| Mucosal injury | Start TPN, continuous slow enteral feeding to allow bowel recovery | |
| Substrate intolerance | Use elemental formula, especially disaccharide-free with MCT | |
| Gastric retention/gastroesophageal reflux | Hypertonic formula | Decrease osmolarity, dilute. |
| High long-chain fat content | Change to MCT containing formula | |
| Hypodynamic gut | Positioning right-side down, consider prokinetic agent (e.g., Reglan, opiate antagonist) | |
| Abdominal distension | Ileus, constipation | R/O surgical abdomen, R/O constipation |
| Add bulking agent or stool softener |
MCT, medium-chain triglycerides; R/O, rule out; TPN, total parenteral nutrition.
Parenteral Nutrition
Amino acid solutions developed for neonates (e.g., TrophAmine, which contains taurine, tyrosine, cysteine, and histidine) provide an advantage for select newborns and young infants with biliary disease, sepsis, or under high physiologic stress. This effect derives from increased branched-chain amino acids, the presence of amino acids which are conditionally “essential-for-age” in infants, and a reduction in nonessential amino acids. In premature infants or those on prolonged TPN, IV carnitine supplementation has been advocated to aid in triglyceride clearance through enhanced beta-oxidation of fatty acids.47 In older children, conventional amino acid solutions provide adequate dietary nitrogen.
Key Points
Pollack MM, Ruttiman UE, Wiley JS. Nutritional depletions in critically ill children: associations with physiologic instability and increased quantity of care. JPEN J Parenter Enteral Nutr. 1985;9:309-313.
Joffe Joffe A, Anton N, Lequier L, Vandermeer B, Tjosvold L, Larsen B et al. Nutritional support for critically ill children. Cochrane Database Syst Rev 2009;2:CD005144.
Mehta NM, Compher C, A.S.P.E.N. Board of Directors. Clinical Guidelines: nutrition support of the critically ill child. JPEN J Parenter Enteral Nutr. 2009;33:260-276.
Le HD, Fallon EM, de Meijer VE, Malkan AD, Puder M, Gura KM. Innovative parenteral and enteral nutrition therapy for intestinal failure. Semin Pediatr Surg. 2010;19:27-34.
Diamond IR, Pencharz PB, Wales PW. What is the current role for parenteral lipid emulsions containing omega-3 fatty acids in infants with short bowel syndrome? Minerva Pediatr. 2009;61:263-272.
1 Skillman H, Wischmeyer P. Nutrition therapy in critically ill infants and children. JPEN J Parenter Enteral Nutr. 2008;32:520-534.
2 Joffe A, et al. Nutritional support for critically ill children. Cochrane Database Syst Rev. 2009;2:1-10.
3 Mehta N, Compher C, et alA.S.P.E.N. Clinical guidelines: nutrition support of the critically ill child. JPEN J Parenter Enteral Nutr. 2009;33:260-276.
4 Mehta N, Jaksic T. The critically ill child, in Nutrition in Pediatrics. Duggan C. Watkins J. Walker W. Hamilton, ON: BC Decker; 2008:663-673.
5 Anand KJS. The stress response to surgical trauma: From physiological basis to therapeutic implications. Prog Food Nutr Sci. 1986;10:67-132.
6 Wesley J. Nutrient metabolism in relation to the systemic stress response. In: Fuhrman B, Zimmerman J, editors. Pediatric Critical Care. St. Louis: Mosby; 1998:799-819.
7 Hill A. Initiators and propogators of the metabolic response to injury. World J Surg. 2000;24:624-629.
8 Nathan C. Points of control in inflammation. Nature. 2002;420:846-852.
9 Lin L, Hopf H. Paradigm of the injury-repair continuum during critical illness. Crit Care Med. 2003;31S:S493-S495.
10 Ward N, Casserly B, Ayala A. The compensatory anti-inflammatory response syndrome (CARS) in critically ill patients. Clin Chest Med. 2008;29:617-625.
11 Shipman J, Guy J, Abumrad N. Repair of metabolic processes. Crit Care Med. 2003;31S:S512-S517.
12 Takala J, et al. Increased mortality associated with growth hormone treatment in critically ill adults. N Engl J Med. 1999;341:785-792.
13 van den Berghe G, et al. Intensive insulin therapy in the surgical intensive care unit. N Engl J Med. 2001;345:1417-1418.
14 Shew S, Jaksic T. The metablic needs of critically ill children and neonates. Semin Pediatr Surg. 1999;8:131-139.
15 Calvano S. Hormonal mediation of immune dysfunction following thermal and traumatic injury. In: Gallin J, Fauci A, editors. Advances in host defense mechanisms. New York: Raen Press, 1986.
16 Ayala A, et al. Mechanisms of immune resolution. Crit Care Med. 2003;31S:S558-S571.
17 Dare A, et al. A systematic review of experimental treatments for mitochondrial dysfunction in sepsis and multiple organ dysfunction syndrome. Free Radical Bio Med. 2009;47:1517-1525.
18 Marik P, Zaloga G. Immunonutrition in critically ill patients: a systematic review and analysis of the literature. Intensive Care Med. 2008;34:1980-1990.
19 Pollack M, et al. Malnutrition in critically-ill infants and children. JPEN J Parenter Enteral Nutr. 1982;6:20-24.
20 Pollack M, Wiley J, Holbrook P. Early nutritional depletion in critically ill children. Crit Care Med. 1981;9:580-583.
21 Pollack M, Ruttiman U, Wiley J. Nutritional depletions in critically ill children: associations with physiologic instability and increased quantity of care. JPEN J Parenter Enteral Nutr. 1985;9:309-313.
22 Mehta N, Duggan C. Nutritional deficiencies during critical illness. Pediatr Clin N Am. 2009;556:1143-1160.
23 Fuentebella J, Kerner J. Refeeding syndrome. Pediatr Clin N Am. 2009;56:1201-1210.
24 Statistics NCfH. CDC growth charts. United States, 2000. Available from. http://www.cdc.gov/growthcharts/.
25 Waterlow J. Classification and definition of protein-calorie malunutrition. Br J Med. 1972;3:566-569.
26 Carter P, et al. Nutritional parameters in children with cancer. J Am Diet Assoc. 1983;82:616-622.
27 Fletcher J, Little J, Guest P. A comparison of serum transferrin and serum prealbumin as nutritional parameters. JPEN J Parenter Enteral Nutr. 1987;11:144-147.
28 Fleck A, Colley C, Myers M. Liver export proteins and trauma. Br Med Bull. 1985;41:265-273.
29 Pannen B, Robotham J. The acute phase response. New Horizons. 1995;3:183-197.
30 Bursztein S, et al. Energy metabolism, indirect calorimetry, and nutrition. Baltimore: Williams & Wilkins; 1989.
31 Chwals W, et al. Measured energy expenditure in critically ill infants and young children. J Surg Res. 1988;44:467-472.
32 Verhoeven J, et al. Comparison of measured and predicted energy expenditure in mechanically ventilated children. Intensive Care Med. 1998;24:464-468.
33 Briassoulis G, Venkataraman S, Thompson A. Energy expenditure in critically ill children. Crit Care Med. 2000;28:1166-1172.
34 Turi R, et al. Energy metabolism of infants and children with systemic inflammatory resonse syndrome and sepsis. Ann Surg. 2001;233:581-587.
35 Chwals W. Overfeeding the critically ill child: fact or fantasy? New Horizons. 1994;2:147-155.
36 Coss-Bu J, et al. Resting energy expenditure in children in a pediatric intensive care unit: comparison of Harrisedict and Talbot predications with indirect calorimetry values. Am J Clin Nutr. 1998;67:74-80.
37 Soll R. Heat loss prevention in neonates. J Perinatol. 2008;28:S57-S59.
38 Griesdale D, et al. Intensive insulin therapy and mortality among critically ill patients: a meta-analysis including NICE-SUGAR study data. CMAJ Can Med Assoc J. 2009;180:821-827.
39 Coss-Bu J, et al. Resting energy expenditure and nitrogen balance in critically ill pediatric patients on mechanical ventilation. Nutrition. 1998;14:649-652.
40 Zappitelli M, et al. Continuous renal replacement therapy amino acid, trace metal and folate clearance in critically ill children. Intensive Care Med. 2009;35:698-706.
41 Energy and protein requirements. Report of a Joint FAO/WHO/UNU Expert Consultation. Geneva: World Health Organization; 1985.
42 Schmidt H, Martindale R. The gastrointestinal tract in critical illness: nutritional implications. Curr Opin Clin Nutr Metab Care. 2003;6:587-591.
43 Mehta N. Approach to enteral feeding in the PICU. Nutr Clin Practice. 2009;24:377-387.
44 Joffe A, et al. Validation of a blind transpyloric feeding tube placement technique in pediatric intensive care: rapid, simple, and highly successful. Pediatr Crit Care Med. 2000;1:151-155.
45 Auestad N, et al. Visual, cognitive, and language assessments at 39 months: a follow-up study of children fed formulas containing long-chain polyunsaturated fatty acids to 1 year of age. Pediatrics. 2003;112:e177-e183.
46 Birch E, et al. The DIAMOND (DHA Intake and Measurement of Neural Development) Study: a double-masked, randomized controlled clinical trial of the maturation of infant visual acuity as a function of the dietary level of docosahexaenoic acid. Am J Clin Nutr. 2010;91:848-859.
47 Cairns P, Stalker D. Carnitine supplementation of parenterally fed neonates. Cochrane Database Syst Rev 2000;4:CD000950.