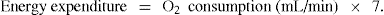Chapter 45 NUTRITION IN PATIENTS WITH LIVER DISEASE
NUTRITIONAL ASSESSMENT IN CIRRHOSIS
Protein–energy malnutrition
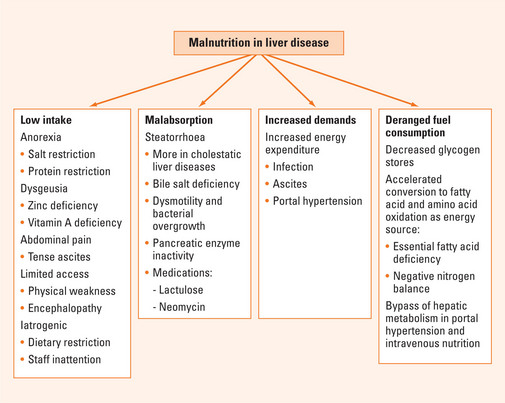
Multiple factors may be involved in the development of PEM in advanced liver disease (Figure 45.1). Inadequate dietary intake has been described in up to 87% of patients. This may be caused by anorexia and dysgeusia, which may be related to zinc and vitamin A deficiencies. Commonly prescribed salt and protein dietary restrictions may also affect appetite. In addition, patients with large ascites may experience early satiety and abdominal pain, limiting food intake, and generalised weakness and encephalopathy may limit physical access to adequate intake. In the hospital, deficient intake is commonly iatrogenic. Malabsorption may be another factor contributing to PEM. Steatorrhoea has been reported in both cholestatic and non-cholestatic liver disease and may be caused by decreased intestinal bile salt pool, pancreatic insufficiency and bacterial overgrowth associated with impaired intestinal motility. The use of lactulose and neomycin in encephalopathy may exacerbate malabsorption. It has been suggested that increased nutritional requirement may exacerbate malnutrition in cirrhosis. However, studies comparing measured energy expenditure using indirect calorimetry and that calculated by Harris Benedict equations (Table 45.1) suggest the presence of large variability among patients with cirrhosis, with both hypermetabolism and hypometabolism equally observed in about one-third of patients (more than 20% discrepancy). Of significance, patients with increased energy expenditure experienced increased rates of morbidity and mortality. The mechanism of hypermetabolism in some cirrhotic patients may be related to infection, ascites and portal hypertension. Both the removal of ascites and the reduction of portal hypertension by transjugular intrahepatic portosystemic shunt (TIPS) have been associated with reduction of energy expenditure.
| Basal energy expenditure for women = 665 + (9.6 × W) + (1.8 × H) + (4.7 × A) |
| Basal energy expenditure for men = 66 + (13.7 × W) + (5 × H) + (6.8 × A) |
W = weight in kg; H = height in cm; A = age in years.
Malnutrition in cirrhotic patients is associated with increased risk of complications including variceal bleeding, ascites, encephalopathy and infections. Malnutrition was also found to be an independent predictor of mortality in cirrhosis. In addition, some studies indicate that malnutrition prior to transplant is associated with increased risk of post-transplant infections, variceal bleeding, prolonged hospital stay and longer ventilatory support time.
Energy and protein requirements
Harris Benedict equations (Table 45.1) can be utilised to estimate basal energy expenditure (BEE) and most cirrhotic patients require 120% of the BEE. It is recommended that the ideal rather than the actual body weight be used in the calculations to avoid overfeeding. In general, most patients require 25–35 kcal/kg/day. Indirect calorimetry may be helpful in determining energy requirements of patients with possible hypermetabolism failing to respond to regular nutritional recommendations. This involves measurement of oxygen consumption and CO2 output at the mouth using metabolic cart. Another simpler estimate of energy expenditure is based on O2 consumption as follows:
TREATMENT
Can nutrition intervention improve nutritional status in patients with advanced liver disease and improve clinical outcome? In patients with alcoholic cirrhosis, oral nutrition supplement providing 1000 calories and 34 grams of protein improved nutritional parameters including mid-arm circumference, albumin level and handgrip strength along with fewer episodes of hospitalisations. Both late-evening meals and nocturnal feeding have been shown to improve nitrogen balance. However, oral nutritional supplement may not increase total energy and protein intake, probably reflecting compensatory decrease in voluntary food intake. Aggressive nutritional support with enteral tube feeding was associated with faster clinical improvement, decreased bilirubin and hepatic encephalopathy and decreased in-hospital mortality in patients with alcoholic liver disease. Early enteral feeding has been successfully attempted post transplant and was associated with improved nitrogen balance and decreased viral infections and is as effective as total parenteral nutrition in maintaining nutritional status post transplant with lower cost and complications.
Non-alcoholic fatty liver disease
Non-alcoholic fatty liver disease (NAFLD) represents a spectrum of liver diseases, characterised mainly by macrovesicular steatosis in the absence of significant alcohol ingestion. It is now known to lead to progressive fibrosis and cirrhosis. Major risk factors include overweight (specifically central obesity), sedentary lifestyles and insulin resistance. Lipid peroxidation and high levels of reactive oxygen substrates are thought to be key mediators in the progression from simple steatosis to NASH providing the rationale for using antioxidants such as vitamin E in non-alcoholic steatohepatitis (NASH). Diets low in processed carbohydrates and saturated fats with a goal to achieve a 500–1000 cal/day deficit improve insulin sensitivity, reduce serum aminotransferases and decrease hepatic steatosis. As an antioxidant and inhibitor of fibrosing cytokine and transforming growth factor, vitamin E has been tested in several clinical trials. Some trials showed improvement in transaminases with vitamin E supplement of 400–1200 IU/day, particularly among children. Current recommendations limit vitamin E supplement to no more that 400 IU/day, as recent studies suggest a possible increase in the rate of all-cause mortality and cardiac complications with vitamin E supplements >400 IU/day.
Parenteral nutrition-associated liver disease
The risk of developing parenteral nutrition-associated liver disease (PNALD) increases with longer parenteral nutrition (PN) dependence. Steatosis and progression to steatohepatitis with fibrosis is the predominant histological picture observed in adults and older children, while cholestasis is more predominant in infants. Abnormal liver tests are observed in 55%, 64% and 72% of patients and cirrhosis with complications is observed in 26%, 39% and 50% after 2, 4 and 6 years of PN dependence, respectively. PNALD is more predominant in infants with reported prevalence up to 85% after 6 weeks of PN dependence. In addition, the risk of PNALD is higher among patients with shorter remnant intestine, those with diseased remnant intestine and those totally dependent on PN with no enteral intake. Several mechanisms have been postulated for the pathogenesis of PNALD, including essential fatty acid and carnitine deficiency, although the former is unlikely unless lipids are totally omitted from PN. In addition, carnitine supplementation has not been associated with improvement in PNALD. Intravenous nutrition bypasses the liver where most of transsulfuration of amino acids occurs. In support of this, taurine and choline deficiencies have been observed in PN dependence, and taurine supplementation in infants reduced the rate of PNALD, although their roles in adults are not clear. Overfeeding results in higher insulin:glucagon ratio, promotes free fatty acid mobilisation and the development of steatosis. Increased oxidative stress and lipid peroxidation may be involved. Lack of enteral nutrition has been associated with intestinal mucosal atrophy, which may be associated with bacterial translocation and the induction of systemic inflammation. In addition, higher risk of PNALD has been reported in association with higher rate of lipid perfusion above 1 g/kg/day. Therefore, recommendations to decrease the risk of PNALD include encouraging enteral nutrition as much as clinically possible, avoiding overfeeding, limiting lipid infusion to less than 1 g/kg/day and cycling of PN (over 12–16 hours versus continuous). However, PNALD remains one of the more serious complications of PN in patients with short bowel syndrome and is a major indication for small bowel transplant in these patients.
Liver transplant
As previously described, malnutrition prior to transplant is a risk factor for increased post-transplant complications. However, randomised studies of the effects of nutritional support on transplant outcome are lacking. In a prospective study enteral nutrition supplement providing 750 cal and 20 g protein to transplant candidates with less than the 25th percentile of midarm circumference had no impact on outcome although there was a tendency towards decreased mortality. In a pilot study, 15 liver transplant candidates given an immunomodulatory-enteral formula orally had a lower rate of infection (33% versus 71%, respectively) and improved total body protein, compared with standard nutrition. In post-transplant patients, energy requirements are about 20% above resting energy expenditure. Protein catabolism with nitrogen loss is observed in the immediate post-transplant period when protein intake should be 1.5–2 g/day. Water and electrolyte imbalances are also common in the post-transplant patients and may be exacerbated by gastric and biliary drainage. In addition, cyclosporin and tacrolimus increase magnesium loss. In the first few days after transplant, oral intake may be limited by ileus. Therefore, PN is utilised in many centres. PN was shown to improve nitrogen balance and shorten intensive care unit stay. BCAA-enriched solution was not superior to the standard solution. Significantly, enteral nutrition using intraoperative jejunostomy tube or nasojejunal tube has been used successfully post transplant. In a prospective study of 50 liver transplantation patients, enteral nutrition resulted in greater calorie and protein intakes, better nitrogen balance, quicker recovery in grip strength and fewer infections compared with control patients.
Buchman AL, Lyer K, Fryer J. Parenteral nutrition-associated liver disease and the role for isolated intestine and intestine/liver transplantation. Hepatology. 2006;43:9-19.
Cabre E, Gassull MA. Nutrition in liver disease. Curr Opin Clin Nutr Metab Care. 2005;8:545-551.
Campos AC, Matias JE, Coelho JC. Nutritional aspects of liver transplantation. Curr Opin Clin Nutr Metab Care. 2002;5:297-307.
Chang CY, Argo CK, Al-Osaimi AM, et al. Therapy of NAFLD: antioxidants and cytoprotective agents. J Clin Gastroenterol. 2006;40:S51-S60.
DiCecco SR, Francisco-Ziller N. Nutrition in alcoholic liver disease. Nutr Clin Pract. 2006;21:245-254.
Marchesini G, Marzocchi R, Noia M., et al. Branched-chain amino acid supplementation in patients with liver diseases. J Nutr. 2005;135:1596S-1601S.