CHAPTER 56 Nuclear Medicine Imaging of Ventricular Function
Left ventricular (LV) function is an important, well-established indicator of patient prognosis. Radionuclide imaging techniques are established as the most highly reproducible methods to assess LV ejection fraction noninvasively. Comprehensive textbooks have summarized the results of studies over several decades showing the high reproducibility and accuracy of gated equilibrium blood pool scintigraphy and radionuclide angiography in determining LV and right ventricular (RV) ejection fraction measurements.1–4 Cardiac mortality is strongly associated with impaired ventricular function as assessed by decreased LV ejection fraction. Additionally, identifying dysfunctional segments in viable myocardium is crucial in clinical management. Numerous studies have shown an almost threefold higher incidence of cardiac mortality in medically treated patients with viable compared with nonviable myocardium.5 The identification of focal dysfunctional segments is highly relevant to clinical management.
The introduction of Tc-99m radiotracers has made gated myocardial perfusion imaging (MPI) readily available, easily feasible, and now a standard technique requiring little additional effort or cost.6 Because MPI is the most commonly performed procedure in nuclear cardiology, evaluation of LV function in nuclear cardiology is most often performed in conjunction with MPI. Similarly, assessment of ventricular function by gated MPI can be performed with positron emission tomography (PET).7
For quantitative, functional analysis of the right ventricle by RV ejection fraction, first-pass angiocardiography currently remains the method of choice because planar equilibrium blood pool imaging cannot separate underlying or adjacent blood pool structures sufficiently for an accurate determination of RV ejection fraction. Newer techniques, such as tomographic ECG gated blood pool (GBP) imaging, are becoming more widespread as quantitative software programs are being developed and validated. These newer techniques have the potential to evaluate simultaneously and quantitatively RV and LV size and function, with high spatial and temporal resolution. Elegant first-pass techniques have been developed to evaluate intraventricular shunts quantitatively; the need for these is relatively uncommon, and they are described elsewhere.1
LEFT VENTRICULAR FUNCTIONAL ASSESSMENT FROM GATED MYOCARDIAL PERFUSION IMAGING
ECG gating to assess ventricular function is now a standard, integral part of MPI. The quantitative evaluation of LV ejection fraction has exhibited high reproducibility and accuracy. Compared with CT, LV ejection fraction assessed by SPECT is not significantly different, although absolute values for end-diastolic volume and end-systolic volume are different with SPECT.8 As expected, LV functional impairment quantified by SPECT is associated with a poor prognosis, including cardiac death. LV ejection fraction less than 45% and LV end-systolic volume greater than 70 mL were associated with approximately 8% to 9%/yr cardiac death rate compared with approximately 1%/yr for LV ejection fraction 45% or greater and end-systolic volume 70 mL or less.9 Although perfusion defect reversibility is most predictive of future myocardial infarction, LV ejection fraction is the strongest predictor of mortality.10
Several studies have shown the clinical utility with improved specificity by identifying normal wall motion and wall thickening in areas of decreased activity because of tissue attenuation. This improvement in specificity may be particularly important in women because of breast attenuation artifacts. The gating information also assists in improving the degree of certainty when interpreting studies as definitely normal or definitely abnormal.6,11 In a prospective study of 115 women, gated Tc 99m sestamibi significantly improved specificity (92%) compared with the same study without gating (84%; P = .0004) or with thallium 201 without gating (59%).12
In addition, gating improves sensitivity for detection of coronary artery disease (CAD) in the subset of patients with multivessel disease (“balanced disease”) who have transient, stress-induced wall motion abnormalities that are not detected by MPI alone.13 Additional wall motion information significantly increased the sensitivity for detection of multivessel CAD without adversely affecting specificity.14 In this study, the sensitivity for multivessel disease was significantly increased from 46% to 60%, and sensitivity for three-vessel disease significantly increased from 10% to 25%.14 Principles of gated MPI with SPECT are similar to gating with PET radiotracers such as ammonia N 13 or rubidium 82, providing regional and global wall motion information.15,16 The more recent development of combined PET/CT and SPECT/CT instrumentation may also permit additional, independent, high spatial resolution CT assessment of regional myocardial function.17
Electrocardiogram Gated Acquisition
Analogous to traditional methods of acquiring planar gated studies, the ECG is used to bin counts from the various phases of the cardiac cycle temporally during data acquisition. Because of count limitations, most studies currently use eight time frames per cardiac cycle. In patients with irregular rhythms, such as atrial fibrillation, ventricular function may be better assessed with methods that do not rely on data from multiple, averaged heartbeats (e.g., echocardiography). If gated MPI is used, the averaged data may be subject to inaccuracies because of misplacement of counts within the cardiac cycle, and careful inspection of the ECG and gating data is necessary to ensure that appropriate beats with similar RR intervals are included for analysis.18,19
Data Processing, Reconstruction, and Analysis
Well-developed programs to evaluate LV wall motion have been commercially available for several years.20–22 Generally, these use boundary detection algorithms, count densities, or a combination of variables to define the endocardial and epicardial borders for LV volume calculations. Another important parameter is LV wall thickening. Detected activity within the myocardium depends on radioactive concentration and volume (or thickness) of the myocardium. When small objects (LV myocardial wall thickness) are below twice the full-width half-maximum of the system resolution, detected activity within the ventricular wall appears lower than the true activity because of partial volume effects.23 Because myocardial wall thickness is susceptible to the partial volume effect, a higher wall thickness at end-systole corresponds to higher detected activity by SPECT. The degree of regional wall thickening is related to the change in detected regional myocardial wall activity. Detected activity at end-diastole typically is lower than that at end-systole because of differences in ventricular wall thickness. Some algorithms assume there is an exact linear relationship between myocardial wall thickening and percent systolic count increases.
Cardiac volume and myocardial thickening can be appreciated visually for cine display formats of individual tomographic slices and computer-generated derived surface renderings. In addition, quantitative, physiologic indices are derived from tomographic volumes and from counts within the myocardium. Fourier analysis is applied to the time-activity curve of each data element in the volume (voxel) to generate parametric images of contraction. Fourier analysis forms the basis of phase and amplitude analysis by which the pattern of myocardial contraction and regional wall motion is assessed quantitatively. More recently, high correlation of LV ejection fraction measurements was reported between techniques in hybrid instrumentation combining high-resolution 64-slice CT with SPECT; mean values were not significantly different.8
PITFALLS AND SOLUTIONS
Potential limitations of this technique should also be mentioned. In patients with prior infarction, boundaries in regions of low or absent tracer uptake may be difficult to define accurately. Although edge detection algorithms are in most cases beneficial, in some cases the extremely low activity renders myocardial boundary definition very difficult and susceptible to computer boundary definition errors. In a canine experimental model, boundary detection and reproducibility were adversely affected by prior infarction, low tracer dose administration, and high background activity.24 Additionally, in patients with small LV cavities (e.g., small women), the endocardial borders may be difficult to identify, and an underestimation of LV cavity size at end systole may lead to artifactually high LV ejection fraction values.6
As with all ECG gated techniques, arrhythmias may also interfere with appropriate data collection, and ECG data should be inspected carefully. Differences in boundary detection algorithms exist in different software packages, and volumes and LV ejection fraction values may differ from one algorithm to the next because of variability in data analysis methods. Comparing ejection fraction or volume calculations produced by more than one nuclear cardiology software package, or comparing against values obtained from another imaging modality such as MRI, should be performed carefully and should take into account methodologic differences, including the normal limits specific to each algorithm.25
TECHNIQUES
Equilibrium Gated Blood Pool Imaging
Indications
This technique has been well established as the clinical method of choice to assess objectively oncology patients at risk of developing cardiotoxicity from chemotherapy.26 Although echocardiography is rapid and results in little patient discomfort, its low quantitative reproducibility obviates its use in standard chemotherapeutic monitoring. Radionuclide gated equilibrium blood pool imaging similarly provides rapid assessment with little discomfort, but the high quantitative reproducibility, technical simplicity, and widespread availability, combined with relatively low cost, make this the method of choice for quantitative assessment of LV function in this population.
Technique Description
for background-corrected end-diastolic and end-systolic counts, where LVED is LV end-diastolic volume counts and LVES is LV end-systolic volume counts after background correction.
Background counts typically are determined using an extraventricular region defined from the lateral to inferolateral LV wall. The normal LV ejection fraction is defined as 50% or greater. Reproducibility data show that greater than 5% difference in LV ejection fraction constitutes a statistically significant difference. Curve fitting with Fourier analysis is performed for each voxel in the best septal separation view (typically LAO 45); this provides parametric phase and amplitude images representing the onset and magnitude of contraction. These aid in image processing by defining ventricular boundaries and valve planes; they also aid in image interpretation by providing an objective method to assess the pattern of contraction and regional asynchrony (Figs. 56-1 and 56-2).
RV wall motion can be assessed visually (Fig. 56-3); however, planar images do not permit accurate or reproducible RV ejection fraction calculations because of the adjacent structures that overlap the right ventricle. This issue can be addressed by calculating RV ejection fraction from a gated first-pass list mode radionuclide angiogram or by tomographic methods, as described later. Specific pathologic entities (e.g., atrial septal defect, ventricular septal defect) can also affect ventricular size and function as seen on GBP imaging and have been described elsewhere in detail.1
Tomographic blood pool imaging has shown high correlation of RV ejection fraction with planar first-pass techniques.27 More recently, automated analysis software for tomographic GBP imaging has confirmed high accuracy and reproducibility in the assessment of RV function as confirmed by MRI and echocardiography.21,28 Because of the increasing incidence of heart failure, accurate biventricular functional assessment may become increasingly important in clinical practice and investigations.
Advantages of tomography include higher contrast, three-dimensional localization, potential for higher accuracy with respect to ventricular volume and wall motion measurements, and simultaneous assessment of the right and left ventricles. These advantages are particularly useful in assessing the right ventricle because of its irregular shape, which is difficult to model using simple assumptions (Fig. 56-4). It has been shown more recently that LV and RV measurements of function are highly reproducible by tomographic blood pool techniques.29 Consequently, interest has re-emerged in the use of GBP SPECT to identify candidates with ventricular asynchrony who may benefit from cardiac resynchronization therapy. GBP SPECT incorporates analysis of RV and LV contraction phase information, in contrast to gated MPI phase analysis, which currently measures only contraction phases for the left ventricle, but not for the right ventricle.30
First-Pass Radionuclide Angiography
Technical Aspects
Data Acquisition
The technical details of acquisition are extensive and have been reviewed more recently.5 To perform planar gated first-pass imaging, an ECG gated list mode acquisition is used in conjunction with Tc-99m pertechnetate injection of 740 to 1110 MBq (20 to 30 mCi). Because this is the same radionuclide activity used for GBP imaging, this study can be combined with the aforementioned GBP study to obtain RV ejection fraction and LV ejection fraction measurements. For the first-pass acquisition, the gamma camera is placed in the shallow right anterior oblique (20 to 30 degrees) position to maximize the likely separation of the right atrium and RV outflow tract. The radiotracer is injected over several seconds to allow more heartbeats for RV analysis. A large peripheral vein (antecubital or external jugular) is used, and radiotracer administration is followed by a saline flush.
First-pass images for RV evaluation typically are acquired as high temporal resolution list mode data. The bolus is viewed in cine mode to select the appropriate frames for analysis. RV analysis requires isolation of the specific heartbeats (typically three to six beats) during which the bolus first traveled through the right ventricle. For this imaging application, the very low background activity produces high image contrast and reduces the effect of boundary variability on the RV ejection fraction calculation. The selected heartbeat data are binned with respect to the phase of the ECG cycle, and background-corrected RV end-diastolic and end-systolic counts are used to determine the RV ejection fraction. Spatially smoothed data are displayed in cine format for visual and quantitative analysis (Fig. 56-5).
Image Interpretation
The technical aspects of the study, including data acquisition, image processing, and variability in patient physiology, can have a significant impact on final accuracy and interpretation. The first step in interpretation is review of raw data. Assessment is performed for adequacy of the bolus, tracer transit, counting statistics, ECG rhythm, and number and quality of beats selected for processing. For data analysis, the regions of interest, background region, and parametric phase and amplitude images are inspected. Images displayed in cine format are assessed for chamber size, wall motion, and ejection fraction. RV ejection fraction of 45% or greater is considered normal.1
CONCLUSION
KEY POINTS
 ECG gating, in conjunction with MPI SPECT, provides accurate and reproducible assessment of LV function; these benefits are without additional cost in acquisition time and without additional radiation exposure.
ECG gating, in conjunction with MPI SPECT, provides accurate and reproducible assessment of LV function; these benefits are without additional cost in acquisition time and without additional radiation exposure. Planar ECG gated equilibrium blood pool imaging is established as the most accurate and reproducible method to evaluate LV function.
Planar ECG gated equilibrium blood pool imaging is established as the most accurate and reproducible method to evaluate LV function.Gerson MC. Cardiac Nuclear Medicine, 3rd ed. New York: McGraw-Hill; 1997.
Iskandrian AE, Garcia EV. Nuclear Cardiac Imaging: Principles and Applications, 3rd ed. New York: Oxford University Press; 2003.
Lima RSL, Watson DD, Goode AR, et al. Incremental value of combined perfusion and function over perfusion alone by gated SPECT myocardial perfusion imaging for detection of severe three-vessel coronary artery disease. J Am Coll Cardiol. 2003;42:64-70.
Nakajima K, Higuchi T, Taki J, et al. Accuracy of ventricular volume and ejection fraction measured by gated myocardial SPECT: comparison of 4 software programs. J Nucl Med. 2001;42:1571-1578.
Nichols KJ, Van Tosh A, Wang Y, et al. Validation of gated blood-pool SPECT regional left ventricular function measurements. J Nucl Med. 2009;50:53-60.
Phelps ME. PET: Molecular Imaging and Its Biological Applications. New York: Springer; 2004.
Sharir T, Germano G, Kavanagh PB, et al. Incremental prognostic value of post-stress left ventricular ejection fraction and volume by gated myocardial perfusion single photon emission computed tomography. Circulation. 1999;100:1035-1042.
1 Gerson MC. Cardiac Nuclear Medicine, 3rd ed. New York: McGraw-Hill; 1997.
2 Iskandrian AE, Garcia EV. Nuclear Cardiac Imaging: Principles and Applications, 3rd ed. New York: Oxford University Press; 2003.
3 Zaret BL, Beller G. Clinical Nuclear Cardiology: State of the Art and Future Directions. St Louis: Mosby; 1993.
4 Heller GV, Hendel R. Nuclear Cardiology: Practical Applications. New York: McGraw Hill; 2004.
5 Friedman JD, Berman DS, Borges-Neto S, et al. First-pass radionuclide angiography (abstract). J Nucl Cardiol. 2006;13:e42-e55.
6 Go V, Bhatt MR, Hendel RC. The diagnostic and prognostic value of ECG-gated SPECT myocardial perfusion imaging. J Nucl Med. 2004;45:912-921.
7 Phelps ME. PET: Molecular Imaging and Its Biological Applications. New York: Springer; 2004.
8 Schepis T, Gaemperli O, Koepfli P, et al. Comparison of 64-slice CT with gated SPECT for evaluation of left ventricular function. J Nucl Med. 2006;47:1288-1294.
9 Sharir T, Germano G, Kavanagh PB, et al. Incremental prognostic value of post-stress left ventricular ejection fraction and volume by gated myocardial perfusion single photon emission computed tomography. Circulation. 1999;100:1035-1042.
10 Sharir T, Germano G, Kang XP, et al. Prediction of myocardial infarction versus cardiac death by gated myocardial perfusion SPECT: risk stratification by the amount of stress-induced ischemia and the poststress ejection fraction. J Nucl Med. 2001;42:831-837.
11 DePuey EG, Rozanski A. Using gated technetium-99m-sestamibi SPECT to characterize fixed myocardial defects as infarct or artifact. J Nucl Med. 1995;36:952-955.
12 Taillefer R, DePuey EG, Udelson JE, et al. Comparative diagnostic accuracy of Tl-201 and Tc-99m sestamibi SPECT imaging (perfusion and ECG-gated SPECT) in detecting coronary artery disease in women. J Am Coll Cardiol. 1997;29:69-77.
13 Shirai N, Yamagishi H, Yoshiyama M, et al. Incremental value of assessment of regional wall motion for detection of multivessel coronary artery disease in exercise Tl-201 gated myocardial perfusion imaging. J Nucl Med. 2002;43:443-450.
14 Lima RSL, Watson DD, Goode AR, et al. Incremental value of combined perfusion and function over perfusion alone by gated SPECT myocardial perfusion imaging for detection of severe three-vessel coronary artery disease. J Am Coll Cardiol. 2003;42:64-70.
15 Kanayama S, Matsunari I, Kajinami K. Comparison of gated N-13 ammonia PET and gated Tc-99m sestamibi SPECT for quantitative analysis of global and regional left ventricular function. J Nucl Cardiol. 2007;14:680-687.
16 Khorsand A, Graf S, Eidherr H, et al. Gated cardiac N-13-NH3 PET for assessment of left ventricular volumes, mass, and ejection fraction: Comparison with electrocardiography-gated F-18-FDG PET. J Nucl Med. 2005;46:2009-2013.
17 Di Carli MF, Dorbala S, Meserve J, et al. Clinical myocardial perfusion PET/CT. J Nucl Med. 2007;48:783-793.
18 Nichols K, Dorbala S, DePuey EG, et al. Influence of arrhythmias on gated SPECT myocardial perfusion and function quantification. J Nucl Med. 1999;40:924-934.
19 Nichols K, Yao SS, Kamran M, et al. Clinical impact of arrhythmias on gated SPECT cardiac myocardial perfusion and function assessment. J Nucl Cardiol. 2001;8:19-30.
20 Faber TL, Cooke CD, Folks RD, et al. Left ventricular function and perfusion from gated SPECT perfusion images: an integrated method. J Nucl Med. 1999;40:650-659.
21 Nichols K, Lefkowitz D, Faber T, et al. Echocardiographic validation of gated SPECT ventricular function measurements. J Nucl Med. 2000;41:1308-1314.
22 Germano G, Kiat H, Kavanagh PB, et al. Automatic quantification of ejection fraction from gated myocardial perfusion SPECT. J Nucl Med. 1995;36:2138-2147.
23 Galt JR, Garcia EV, Robbins WL. Effects of myocardial wall thickness on SPECT quantification. IEEE Trans Med Imaging. 1990;9:144-150.
24 Vallejo E, Dione DP, Bruni WL, et al. Reproducibility and accuracy of gated SPECT for determination of left ventricular volumes and ejection fraction: experimental validation using MRI. J Nucl Med. 2000;41:874-882.
25 Nakajima K, Higuchi T, Taki J, et al. Accuracy of ventricular volume and ejection fraction measured by gated myocardial SPECT: comparison of 4 software programs. J Nucl Med. 2001;42:1571-1578.
26 Gurusher SP, Diwakar J. Monitoring chemotherapy-induced cardiotoxicity: role of cardiac nuclear imaging (abstract). J Nucl Cardiol. 2006;13:415-426.
27 Chin BB, Bloomgarden DC, Xia WS, et al. Right and left ventricular volume and ejection fraction by tomographic gated blood-pool scintigraphy. J Nucl Med. 1997;38:942-948.
28 Nichols KJ, Saouaf R, Ababneh A, et al. Validation of SPECT equilibrium radionuclide angiographic right ventricular parameters by cardiac magnetic resonance imaging (abstract). J Nucl Cardiol. 2002;9:153-160.
29 Nichols K, Van Tosh A, De Bondt P, et al. Normal limits of gated blood pool SPECT count-based regional cardiac function parameters. Int J Cardiovasc Imaging. 2008;24:717-725.
30 Chen J, Henneman MM, Trimble MA, et al. Assessment of left ventricular mechanical dyssynchrony by phase analysis of ECG-gated SPECT myocardial perfusion imaging. J Nucl Cardiol. 2008;15:127-136.

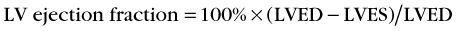
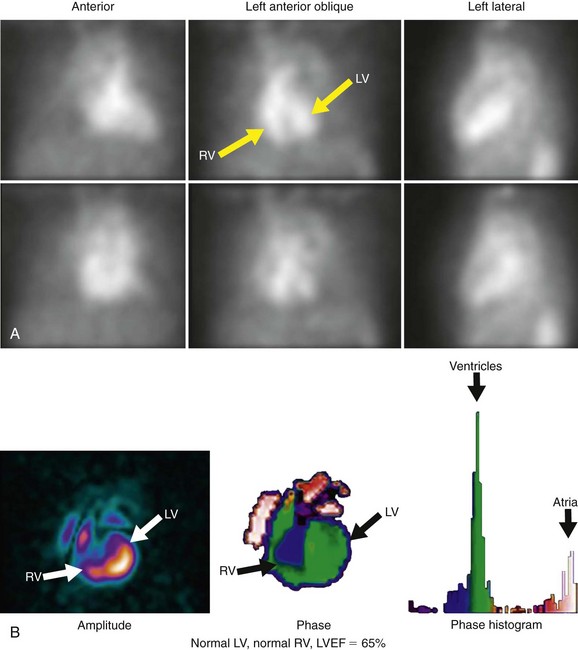
 FIGURE 56-1
FIGURE 56-1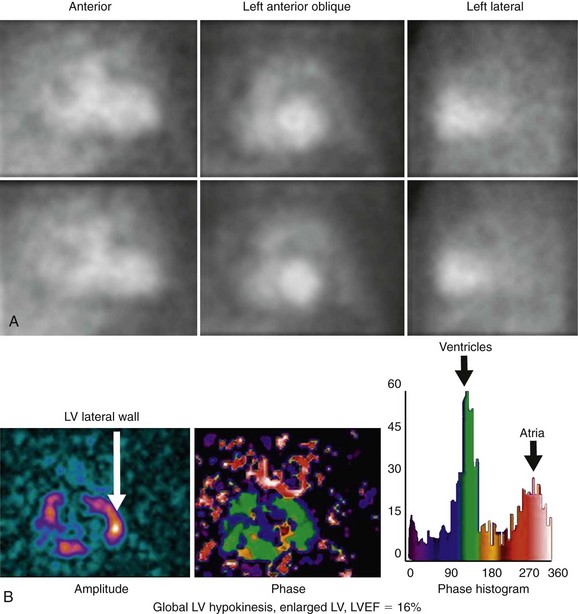
 FIGURE 56-2
FIGURE 56-2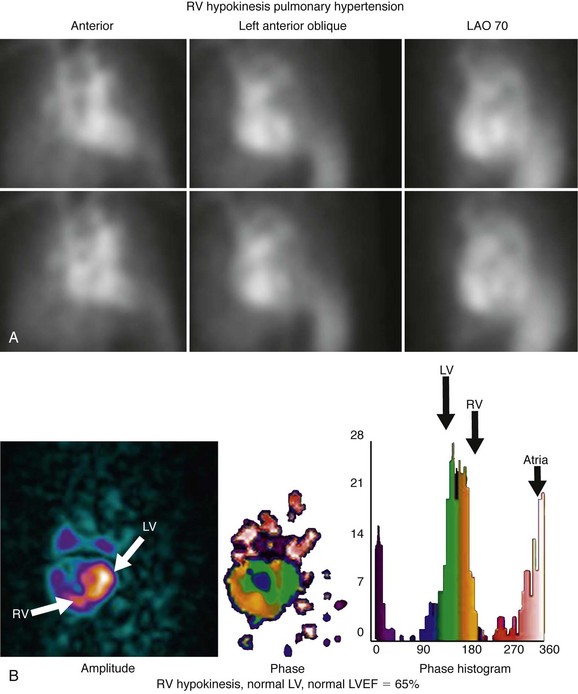
 FIGURE 56-3
FIGURE 56-3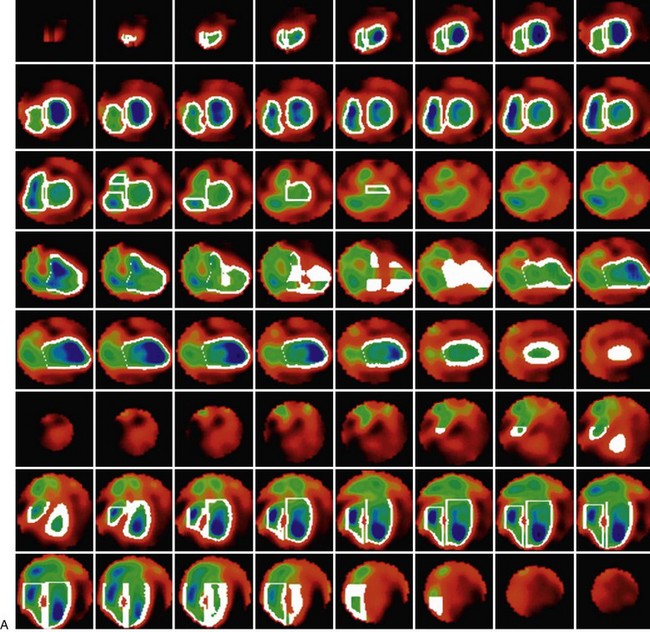
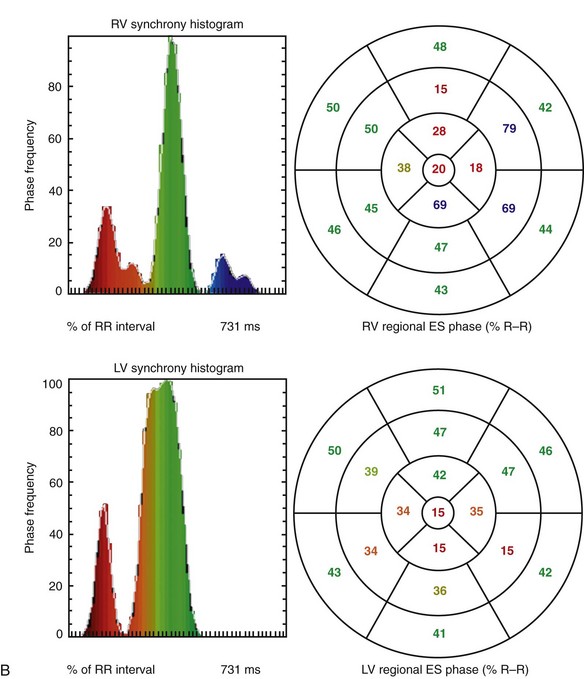
 FIGURE 56-4
FIGURE 56-4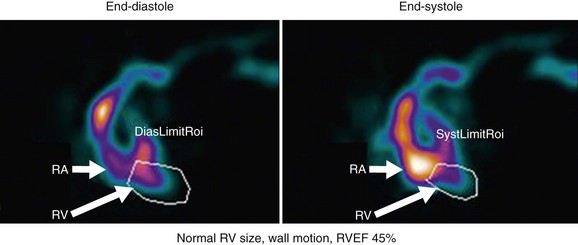
 FIGURE 56-5
FIGURE 56-5

