CHAPTER 85 Nuclear Medicine
Extrathoracic Vascular Imaging
Compared with catheter angiography, CT angiography, and MR angiography, nuclear medicine is a small component of extrathoracic vascular imaging. This component is crucial, however; the specific questions answered safely and efficiently by nuclear medicine cannot be answered by any other modality. Historically, radioisotope techniques have been used widely to answer research and clinical vascular questions. Some early nuclear angiographic procedures contributed greatly to our knowledge of cardiac and vascular physiology and diagnosis of various peripheral vascular disorders.1 These techniques capitalize on the ability of noninvasive radioisotope imaging to depict existing physiologic parameters accurately without changing the physiology being interrogated.
Bleeding studies benefit from dynamic cine sequences, which show in rapid succession multiple images acquired at short intervals (Fig. 85-1). This sequence of images gives the interpreting physician greater confidence in localizing a bleeding site or visualizing the progressive accumulation of activity in a Meckel diverticulum (Fig. 85-2). Brain SPECT studies have benefited from advances in software allowing digital image fusion of the radioisotope study with CT or MRI anatomic sectional images, further computer comparison with probabilistic brain atlases, and three-dimensional volume rendering.
Nuclear medicine continues to refine its preexisting role in extrathoracic cardiac imaging even as it expands to assess atherosclerosis with positron emission tomography with 18FDG. This study is still in its experimental phase, but shows great promise as an imaging adjunct.2
GASTROINTESTINAL BLEED LOCALIZATION SCAN
Technique Description
No specific patient preparation is required for a Tc 99m–radiolabeled erythrocyte scan. This is an advantage because studies for gastrointestinal bleeding are usually requested emergently. Various labeling methods and kits are available to label the red blood cell, including an in vitro method, an in vivo method, and a hybrid method. The in vitro method using widely available kits typically provides very high labeling efficiency (e.g., Ultratag kit [Mallinckrodt, Inc., St. Louis]) provides a labeling efficiency of 98.5%).3 What the methods have in common is the use of stannous ion as a reducing agent and the Tc 99m label.
The patient is positioned supine with the camera located anteriorly. On initial intravenous injection of the radiolabeled erythrocytes, initial flow images are obtained at 1 s/frame for 60 frames. Subsequent dynamic images are obtained at 1 min/frame for 60 additional frames. A 128 × 128 image matrix is normally used for both acquisitions.4 Additional imaging may be repeated at intervals up to 24 hours, but all acquisitions should be in a dynamic series to enable detection of subtle bleeding sites and peristaltic transit through the bowel.
Pitfalls and Solutions
The most common technical pitfall of the Tc 99m–radiolabeled erythrocyte scan relates to an imperfect tagging of the blood cells, leaving free Tc 99m pertechnetate in the bloodstream. As described earlier, tagging efficiency is typically very high, especially with the in vitro technique. Common drugs such as heparin, penicillin, and iodinated contrast media interfere with the entry of the reducing agent (Sn++) through the red blood cell membrane, however, causing an increased amount of free pertechnetate in the blood.5 Also, alternative in vivo labeling techniques may allow a certain amount of free pertechnetate to circulate.
Free pertechnetate appears in the stomach where it is physiologically secreted by the gastric parietal cells (see Fig. 85-2). This activity has an intraluminal configuration and moves antegrade over time. This movement can simulate an upper gastrointestinal bleed. In some instances, an upper gastrointestinal bleed may have been ruled out from a recent endoscopy, or the clinical presentation may not be consistent with one. A static image of the neck also reveals physiologic uptake of pertechnetate in the thyroid gland. After confirmation of the presence of free pertechnetate, the best solution is to allow for it in the interpretation. A brisk lower gastrointestinal bleed should be differentiated easily on dynamic imaging.
Interpretive pitfalls consist of confounding patterns of activity. The only solution for these interpretive pitfalls is for the interpreter to be aware of their appearance. Physiologic penile blood flow may be mistaken for rectal bleeding, or mesenteric vascular activity may overlie the expected location of the bowel. What these confounding patterns have in common is their lack of intraluminal configuration and the absence of antegrade or retrograde movement over time. Table 85-1 presents examples of these patterns.
MECKEL DIVERTICULUM SCAN
Indications and Contraindications
The Tc 99m–pertechnetate scan is useful in detecting a Meckel diverticulum, which contains ectopic gastric mucosa whose acid production ulcerates the small bowel mucosa. These congenital abnormalities are almost always located in the right lower quadrant of the abdomen and manifest with symptomatic gastrointestinal bleeding (see Fig. 85-2). In 50% of cases, diverticula manifest before 2 years of age. The pertechnetate administered crosses the placenta,6 and women of reproductive age should be questioned regarding the possibility of pregnancy.
Technique Description
After appropriate positioning, 5 to 10 mCi of Tc 99m pertechnetate is injected intravenously. The dose may be administered by weight for pediatric patients. A standard protocol includes flow images, obtained at 1 s/frame for 60 frames. Dynamic images are obtained at 30 s/frame for 60 additional frames. A 128 × 128 matrix is used.4
BRAIN PERFUSION TOMOGRAPHY
Indications and Contraindications
Acetazolamide-augmented brain perfusion SPECT scans are obtained in patients with compromised carotid circulation who must be evaluated for external-to-internal carotid arterial bypass. If there is appropriate augmentation of cerebral blood flow from baseline after administration of the vasodilator, the cerebrovascular reserve is intact. If there is a failure of perfusion augmentation, this implies a critically stenotic vessel with compromised ability to increase regional blood flow to the brain parenchyma it supplies (Fig. 85-3).
The main contraindication to this study is a sulfa drug allergy. As with any drug allergy, a full investigation into the nature and severity of a past reaction should be obtained. Isolated reports of “steal phenomenon” leading to ischemia exist in the literature,7 but there are no data to suggest a significant risk of stroke after administration of this carbonic anhydrase inhibitor. Patients should be advised regarding side effects such as nausea, numbness around the mouth or fingers, lightheadedness, or facial flushing.
Technique Description
The patient should be placed in a dark, quiet room for 20 minutes before injection of radiotracer. The patient should not read or speak. Approximately 20 minutes after intravenous injection of 10 mCi of a Tc 99m–radiolabeled lipophilic amine compound—Tc99m HMPAO (Ceretec) or Tc 99m ECD (Neurolite)—a baseline SPECT scan should be acquired. Subsequently, 1 g of acetazolamide is infused intravenously over 5 minutes. Ten minutes after this, a higher dose (30 mCi) of the same Tc 99m radiotracer is administered. Another 20-minute delay precedes the second SPECT image acquisition.8
Pitfalls and Solutions
Postprocessing errors can confound the interpretation as well. Many software programs compare the acquired data with a probabilistic brain atlas.9 Any results obtained through these means should be verified with direct visual inspection and used at best as an adjunct corroborating the interpreter’s own impression.
Image Interpretation
Reporting
Areas of absent or severely limited cerebrovascular reserve appear as areas of decreased perfusion compared with baseline after vasodilator administration (Fig. 85-4). The report should list these areas, ideally taking advantage of digital image fusion or coregistration with correlative anatomic studies, such as MRI or CT.
KEY POINTS
 Tc 99m–radiolabeled erythrocyte scans guide selective angiographic intervention in the setting of acute lower gastrointestinal tract bleeding.
Tc 99m–radiolabeled erythrocyte scans guide selective angiographic intervention in the setting of acute lower gastrointestinal tract bleeding. Tc 99m pertechnetate scanning detects the ectopic gastric mucosa that is present in symptomatic Meckel diverticula.
Tc 99m pertechnetate scanning detects the ectopic gastric mucosa that is present in symptomatic Meckel diverticula.Grant K, Umphrey H, Liu HG, et al. Clinical use of Diamox brain SPECT: comparison of protocols, evaluation of interpretive findings, and use correlative imaging. J Nucl Med. 2007;48(Suppl 2):214P.
Gutierrez C, et al. The use of technetium-labeled erythrocyte scintigraphy in the evaluation and treatment of lower gastrointestinal hemorrhage. Am Surg. 1998;64:989-992.
Leonidas JC, Germann DR. Technetium-99m pertechnetate imaging in diagnosis of Meckel’s diverticulum. Arch Dis Child. 1974;49:21-26.
Ozgur HT, et al. Correlation of cerebrovascular reserve as measured by acetazolamide-challenged SPECT with angiographic flow patterns and intra- or extracranial arterial stenosis. AJNR Am J Neuroradiol. 2001;22:928-936.
Parkman HP, Maurer AH. Update on gastrointestinal scintigraphy. Semin Nucl Med Volume. 2006;36:110.
1 MacIntyre WJ, Storaasli JP, Krieger H, et al. I-131-labeled serum albumin: its use in the study of cardiac output and peripheral vascular flow. Radiology. 1952;59:849-857.
2 Rudd JH, Myers KS, Bansilal S, et al. Atherosclerosis inflammation imaging with 18F-FDG PET: carotid, iliac, and femoral uptake reproducibility, quantification methods, and recommendations. J Nucl Med. 2008;49:871-878.
3 Patrick ST, Glowniak JV, Turner FE, et al. Comparison of in vitro RBC labeling with the UltraTag RBC kit versus in vivo labeling. J Nucl Med. 1991;32:242-244.
4 Klingensmith W, Eshima D, Goddard J. Nuclear Medicine Procedure Manual, 2000-2002 edition. Engelwood, CO: Wick Publishing; 2002.
5 Saha GB. Characteristics of specific radiopharmaceuticals. In: Fundamentals of Nuclear Pharmacy. Berlin: Springer-Verlag; 1992:117.
6 Husak V, Wiedermann M. Radiation absorbed dose estimates to the embryo from some nuclear medicine procedures. Eur J Nucl Med. 1980;5:205-207.
7 Komiyama M, Nishikawa M, Yasui T, et al. Reversible pontine ischemia caused by acetazolamide challenge. AJNR Am J Neuroradiol. 1997;18:1782-1784.
8 Juni JE, Waxman AD, Devous MD, et al. Society of Nuclear medicine procedure guideline for brain perfusion single photon emission computed tomography (SPECT) using Tc-99m radiopharmaceuticals. Version 2.0 (approved February 7, 1999).
9 Lee HY, Paeng JC, Lee DS, et al. Efficacy assessment of cerebral arterial bypass surgery using statistical parametric mapping and probabilistic brain atlas on basal/acetazolamide brain perfusion SPECT. J Nucl Med. 2004;45:202-206.

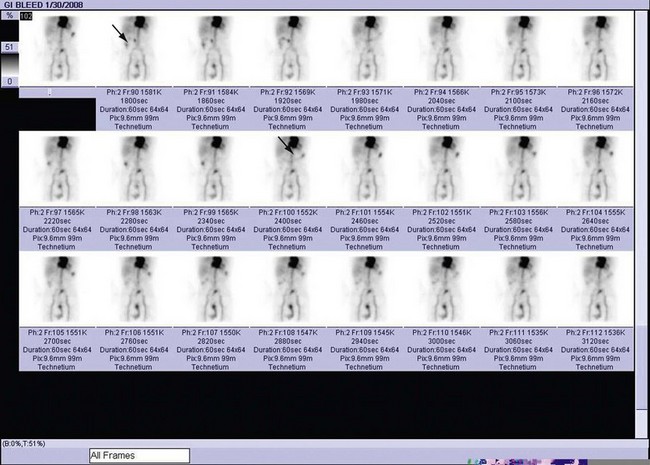
 FIGURE 85-1
FIGURE 85-1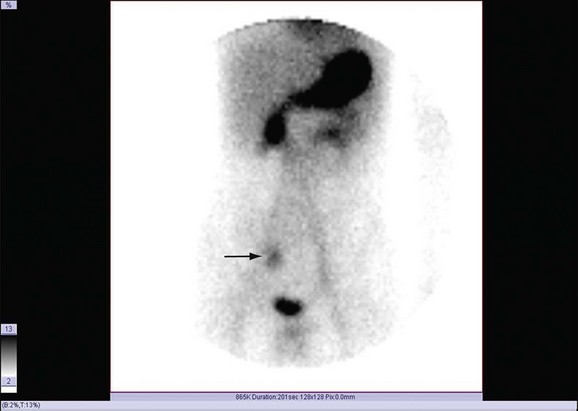
 FIGURE 85-2
FIGURE 85-2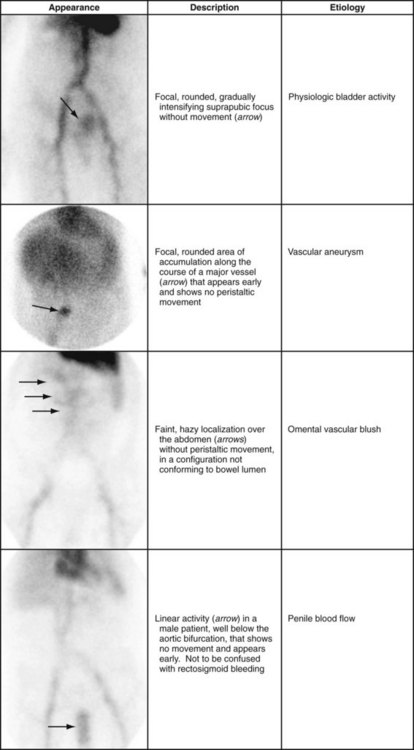
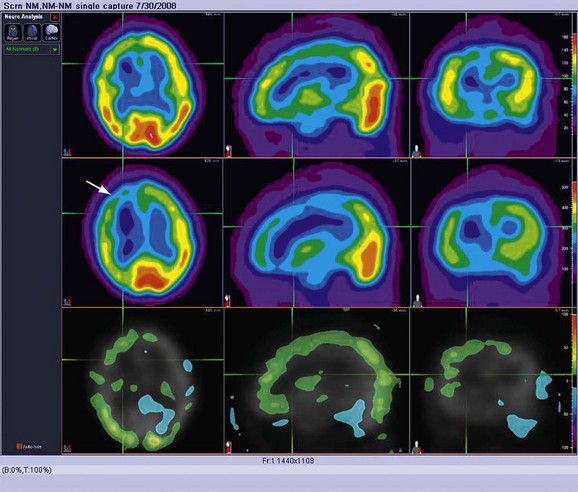
 FIGURE 85-3
FIGURE 85-3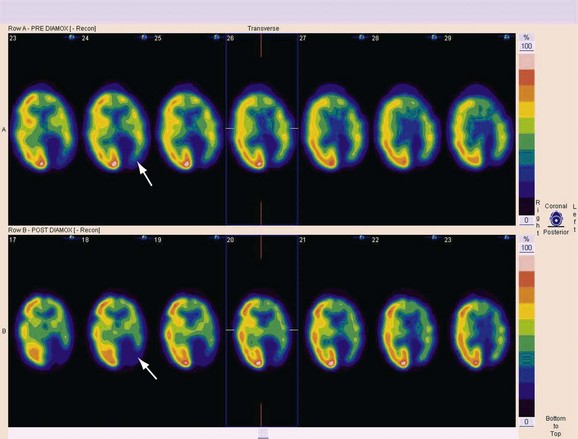
 FIGURE 85-4
FIGURE 85-4

