Chapter 16 Nuclear medicine
 The radiation dose from any diagnostic procedure should be as low as reasonably practicable, consistent with diagnostic results.1
The radiation dose from any diagnostic procedure should be as low as reasonably practicable, consistent with diagnostic results.1 This is governed in nuclear medicine by the administration of strictly controlled amounts of radioactivity under a licensing arrangement and the use of optimum imaging conditions.2
This is governed in nuclear medicine by the administration of strictly controlled amounts of radioactivity under a licensing arrangement and the use of optimum imaging conditions.2 Any person working within the nuclear medicine environment should have an awareness of the practical radiation protection considerations.
Any person working within the nuclear medicine environment should have an awareness of the practical radiation protection considerations. Nuclear medicine studies require the administration of less than a microgram of the radioactive substance under test. This has the advantage that it does not interfere with the physiological pathways but demonstrates their function.
Nuclear medicine studies require the administration of less than a microgram of the radioactive substance under test. This has the advantage that it does not interfere with the physiological pathways but demonstrates their function. The radioactive substances may be given in one of two forms: in their radionuclide form or attached to a drug and known as a radiopharmaceutical.
The radioactive substances may be given in one of two forms: in their radionuclide form or attached to a drug and known as a radiopharmaceutical. The radiopharmaceutical or radionuclide determines which system or organ the radioactive substance targets.
The radiopharmaceutical or radionuclide determines which system or organ the radioactive substance targets. Most radioactive substances used in diagnostic imaging emit gamma radiation, which can subsequently be detected externally to the patient, and an image is produced of the uptake in the specific area.
Most radioactive substances used in diagnostic imaging emit gamma radiation, which can subsequently be detected externally to the patient, and an image is produced of the uptake in the specific area. Nuclear medicine studies can often detect certain disorders earlier than other diagnostic imaging procedures because they rely on functional rather than structural changes.
Nuclear medicine studies can often detect certain disorders earlier than other diagnostic imaging procedures because they rely on functional rather than structural changes.INTRODUCTION
Nuclear medicine is concerned with providing diagnostic information about patients following the administration of a radioactive product. The patient is imaged using a gamma camera. Images are produced of the distribution of the radioactive substance within different organs and systems. This can be compared with normal distribution to diagnose if a medical condition is present and assess its extent or severity.
Key terms
RADIONUCLIDES USED IN MEDICAL IMAGING
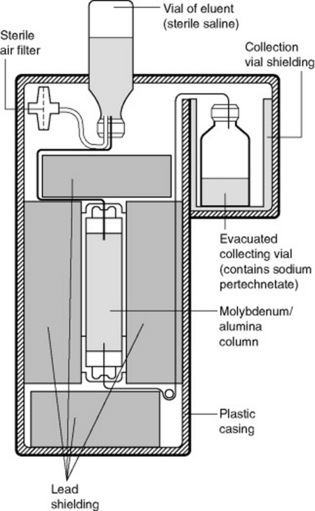
TECHNETIUM
OTHER RADIONUCLIDES USED IN DIAGNOSTIC IMAGING
81mKrypton
Krypton is used for scanning the lungs as it can show the ventilation. It is metastable like technetium and also a pure gamma emitter. It has an energy level of 190 keV, but has a half-life of 13 seconds. This means that the radionuclide has to be produced and then administered directly to the patient. In this case the patient inhales the radionuclide. Air is passed over a column of rubidium, which results in krypton being produced.
EQUIPMENT
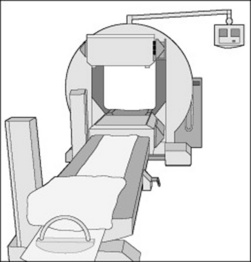
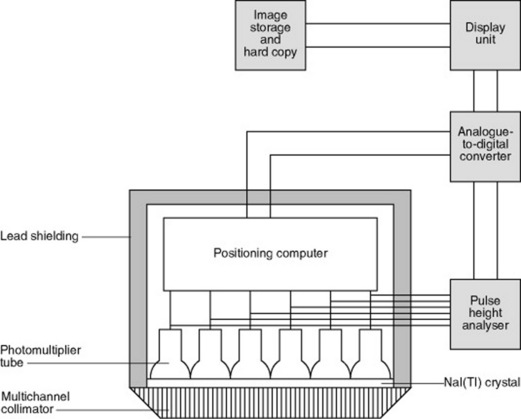
The basic design of a gamma camera has not significantly changed for over 40 years and the use of devices such as sodium iodine crystals and photomultiplier tubes are the main reasons why nuclear medicine images have such low resolution in comparison to CT. However, technology advancements may see the development and production of solid-state gamma cameras in the future. The modern gamma camera consists of a large detector (or two detectors in dual head systems, Fig. 16.4), which is positioned as close to the patient as possible during examinations.
Many nuclear medicine departments will utilise one gamma camera to undertake a range of examinations. Some larger departments may employ a dual and a single head gamma camera to perform clinical examinations. Dual head gamma camera systems allow the operator to perform certain examinations (e.g. whole body bone scans) quicker than single head units, which is particularly useful for patients who may be in considerable discomfort.
The basic components of a modern gamma camera detector unit are:
Collimator
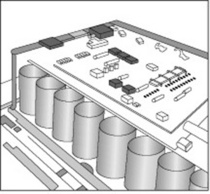
The majority of investigations performed within a nuclear medicine department involve the use of technetium-99m based radiopharmaceutical agents. Technetium-99m has a photopeak energy of 140 keV and low energy collimators are used to absorb any gamma events that are not perpendicular to the crystal. For dynamic renal examinations a ‘low energy all purpose’ (LEAP) collimator may be employed and the holes are of a particular design to allow more gamma events to interact with the crystal than a ‘low energy high resolution’ (LEHR) collimator. The collection of counts on a dynamic examination is crucial if image processing is to take place afterwards. Figure 16.5 depicts a set of collimators being exchanged on a dual head gamma camera system. This process is mainly automated and requires minimal practitioner involvement. Caution should, however, be exercised when collimators are being exchanged, as the detector crystal is exposed and the collimators themselves are very heavy.
Photomultiplier (PM) tubes
The fundamental purpose of a PM tube is to convert a light pulse into an electrical signal. A number of PM tubes are positioned behind the crystal and protected with black plastic covers (Fig. 16.6).
CREATION OF AN IMAGE
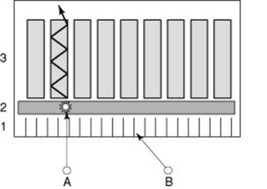
Gamma events leaving the patient’s body may be travelling at various angles – some may be perpendicular to the detector of the gamma camera (gamma event A in Fig. 16.8). Gamma events that are perpendicular to the crystal within the detector unit have to pass through the collimator; those that are not perpendicular to the crystal are absorbed by the collimator (gamma event B in Fig. 16.8). The collimator is the first tool used to ensure the accurate representation of physiological tracer uptake within the patient and without it there would not be any recognisable image on the monitor.
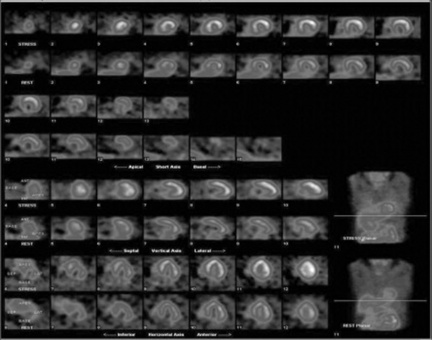
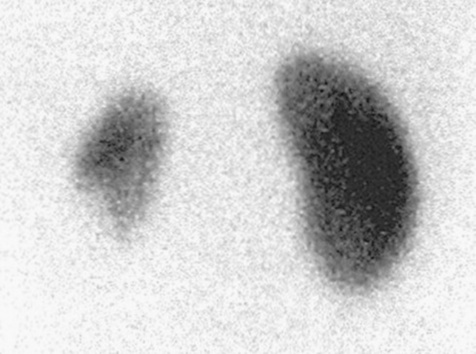
The second tool is the PHA, which as previously mentioned, discriminates against scatter or background radiation. Like in most clinical examinations, the nuclear medicine practitioner will have access to a number of preset protocols, which are stored on an operator’s computer console. Modern computer consoles use software driven platforms and graphical user interfaces (GUI). These permit quick access to a range of common clinical protocols and radioisotopes. The photopeak energy value per second of these isotopes is stored within the gamma camera’s computer and, as a result, determines the energy ‘window’ for that particular isotope. The practitioner has the ability to adjust the energy window if circumstances require this action. Nuclear medicine images may also be presented in different colour scales. Grey scale is normally utilised for skeletal and respiratory images and colour may be used for renal and cardiac examinations. Colour intensity scales are also presented with the images to assess the degree of tracer uptake within a particular part of the area under examination (Figs 16.9 and 16.10).
ACCESSORY EQUIPMENT FEATURES
A typical nuclear medicine department will utilise a range of ancillary items to position patients and ensure the production of optimal quality images. Paediatric patients require considerable preparation for examinations within nuclear medicine and the practitioner may employ the use of special imaging pallets, sandbags and immobilisation devices during the scan. It is crucial that patients do not move during examinations, as image unsharpness will occur. If patient movement occurs during a dynamic renal examination, this could potentially lead to an incorrect assessment of renal function. Some departments also use DVD and audio equipment to distract paediatric patients during examinations. The use of such equipment also reduces the close contact between the practitioner and patients and therefore helps to reduce the radiation dose received.
SPECIALISED EQUIPMENT
As previously mentioned, the majority of nuclear medicine departments also perform SPECT imaging. Common SPECT procedures undertaken within clinical practice include cardiac and skeletal examinations. However, some departments may also use SPECT techniques to perform neurological and oncology related procedures. SPECT imaging involves the collection of a number of views around the patient using the detector head/s. Most gamma cameras employed in clinical practice are dual head and can be configured to perform different SPECT examinations. As the detector head/s move around the patient (normally in a step fashion) each view collects a preset number of gamma photon events. Figure 16.11 demonstrates the set-up for a cardiac examination, with the detector heads presented in an inverted ‘V’ fashion. Performing SPECT examinations permits the presentation of data in three image planes: transaxial, sagittal and coronal. Powerful computers process the collected data and the practitioner may manipulate the generated data to provide the final images.
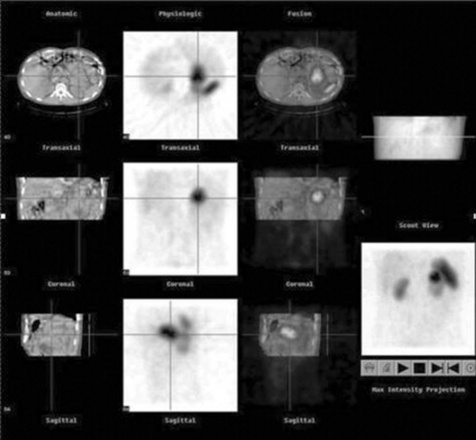
Recently the use of ‘hybrid’ gamma cameras has been introduced into clinical practice. Inherently, nuclear medicine images possess inferior spatial resolution compared to computed tomography. This is mainly due to the aforementioned inefficiencies of current gamma camera technology. The introduction of a low power X-ray tube and detector bank on the same gantry as the gamma camera heads is allowing practitioners to ‘fuse’ anatomical and functional data from the same imaging environment. Such technology is beginning to redefine the physical layout of nuclear medicine departments (given the use of an X-ray source) and is assisting in the localisation of certain physiological tracers. Figure 16.12 demonstrates an example of hybrid imaging.
HANDLING AND SAFETY
SAFETY OF THE GAMMA CAMERA
The gamma camera interlocks should be checked every day and every time the collimators are changed.
RADIATION SAFETY
Radiopharmaceuticals can be shielded before they are injected into the patient. Tungsten syringe shields and lead and tungsten pots can be used. Radiopharmaceuticals are unsealed sources and therefore have the potential to contaminate through spillage. Pots and shields may be contaminated with radioactivity and therefore should not be touched. Treat all pots and shields as if they are contaminated and wear gloves and use tongs or other devices to keep a distance from them.
Gloves should be worn for handling radioactive materials, contaminated objects and if a suspected spillage has occurred. It is important to check for contamination: hands should be monitored after contact with radioactive products and departments should be monitored regularly (Fig. 16.13). A radioactive trefoil sign indicates areas containing radioactive materials or patients.
A spill kit should be available in case some unsealed radioactivity has been spilt (Fig. 16.14). The nuclear medicine department should have a contingency plan in case of a spill.1 If a spill has occurred, the radiation may have been spread and door handles and the bottom of shoes should be monitored as well as any area that might have been contaminated. The area of any spillage should be demarked. After donning protective clothing the spill should be wiped up, from the outside in, to avoid further spread. If the radiopharmaceutical is short lived it might be easier to close the area until decay has occurred. Records should be kept of the incident.
EXAMINATION OVERVIEWS
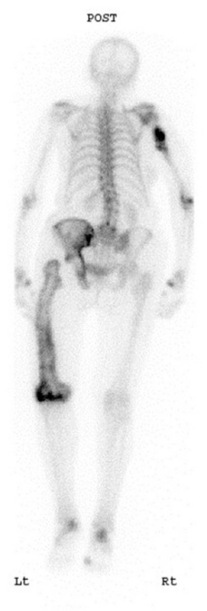
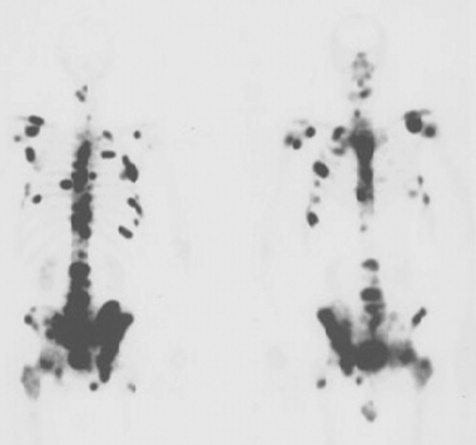
BONE SCANS
Main indications
Metastases, Paget’s disease, infection, loose prosthesis, primary bone tumours, osteomyelitis, fractures, avascular necrosis, osteomalacia and hyperparathyroidism.
Radiopharmaceutical and rationale
99mTc-MDP (methylene diphosphonate) or 99mTc-HDP (hydoxydiphosphonate):
Procedure
STATIC RENAL SCANS
Procedure
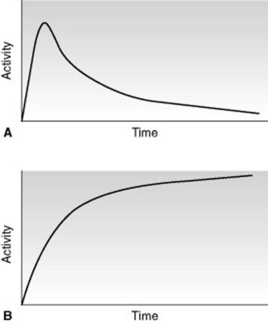
DYNAMIC RENAL SCANS (RENOGRAM)
INDIRECT MICTURATING CYSTOGRAM
If a child is potty trained and can cooperate well then the acquisition of an indirect cystogram can occur. This demonstrates reflux, which can cause scarring to the kidneys. The child micturates into a bedpan whilst sitting against the gamma camera and time/activity curves can be drawn. If urine is refluxing back to the kidneys it will show on the curves. This examination avoids the need for catheterisation of the child. Adults can also have a cystogram in this way, although requests for adult patients are not a common occurrence.
PATIENT CARE AND ADVICE
PATIENT CARE PRIOR TO THE SCAN
Patients are normally sent information leaflets in advance of their scan that explain the procedure and precautions to be taken afterwards. All patients who receive radiation should be given clear written advice which sets out the risks associated with ionising radiation and specifies how doses resulting from their exposure during the scan can be restricted as far as reasonably possible so as to protect persons in contact with them (IR(ME)R 2000, see p. 14). Most patients have to avoid prolonged close contact with pregnant women and children for the rest of the day. These precautions may be longer for scans involving higher radiation doses or a radionuclide other than 99mTc.
PATIENT CARE DURING THE SCAN
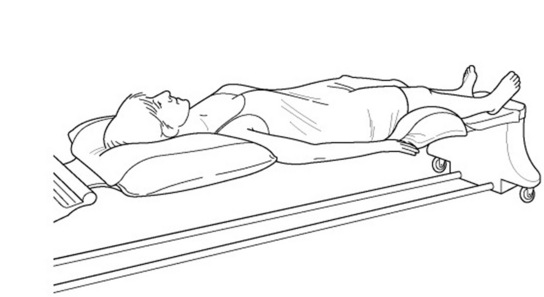
Patients are required to lie flat on the scanning table, with no more than one pillow under their head, in order to enable the gamma camera to be positioned as close as possible to their body. The scanning tables are very narrow and the armrests are normally hard Perspex (Fig. 16.18). The scan must start on an empty bladder, so patients are instructed to visit the toilet before the scan commences and then should be warned about the length of time they will have to spend on the scanning table. Metal objects such as coins and keys, which could cause artefacts, have to be removed from the patients before they get on the table. Patients usually keep their clothes on and do not change into gowns. The gamma camera rooms are air-conditioned and can feel cold to the patients, so blankets should be available to keep them warm. A pillow or knee support should be placed under the knees to increase comfort.
PATIENT PATHWAYS
These pathways can be seen in Appendix 2.5,6 These are simple generic pathways, which may differ from patient to patient depending on the imaging modalities available and the patient’s condition. Nuclear medicine involvement in the pathway is in bold type.
1 The Ionising Radiations Regulations. (SI 1999/3232). London: HMSO. 1999.
2 Administration of Radioactive Substances Advisory Committee. Notes for guidance on the clinical administration of radiopharmaceuticals and use of sealed radioactive sources. Oxford: Health Protection Agency, 2006.
3 Sampson C. Textbook of radiopharmacy; theory and practice, 3rd edn. Amsterdam: Gordon and Breach, 1999.
4 Sharp PF, Gemmell HG, Smith FW, editors. Practical nuclear medicine. Oxford: Oxford University Press, 1998.
5 Eisenberg R, Johnson N. Comprehensive radiographic pathology. St. Louis: Mosby, 2003.
6 Underwood J., editor. General and systematic pathology. London: Churchill Livingstone, 2004.