Chapter 28 Nuclear Imaging in Revascularized Patients with Coronary Artery Disease
BACKGROUND
Single-photon emission tomography (SPECT) continues to be the mainstay of scintigraphy. Many technical improvements have been made over last decade, allowing shortening of acquisition protocols and attenuation correction. Hybrid SPECT/CT and positron emission tomography PET/CT systems have grown throughout the United States, opening an opportunity for more accurate noninvasive myocardial perfusion imaging1 PET unlike standard SPECT allows assessment of true coronary flow reserve.1 However, vasodilator stress nitrogen (N)-13-ammonia or oxygen (O)-15-water requires on-site isotope production and additional capital investment for a cyclotron that may be prohibitively expensive for most PET centers. Rubidium-82 PET imaging is a more practical and financially feasible alternative to SPECT imaging, with advantages of higher image quality and shorter patient time commitment.2
EVALUATION AFTER CORONARY ARTERY BYPASS GRAFT
Abnormalities observed on stress nuclear myocardial perfusion imaging (MPI) in patients who have undergone CABG will reflect a complex constellation of underlying pathophysiology that involves variable time courses. Early after CABG, abnormal perfusion may be related to early graft closure, which occurs in 12% to 20% within the first year.3 However, it may also reflect myocardium supplied by diseased vessels that were technically unable to be grafted due to anatomic limitations. Alternatively, early MPI ischemia may be the result of coronary lesions proximal to patent grafts that compromise retrograde flow. When this involves the left anterior descending (LAD), a pattern of basal ischemia involving the anterior and lateral walls may be seen. Therefore, analysis and interpretation of MPI in revascularized patients should be done with knowledge of the coronary anatomy and operative results on hand.
Late after CABG, MPI abnormalities can reflect development of intercurrent venous graft closure, which is 2% to 4% per graft per year for years 1 through 5 and reaches 50% by 5 years.3 Late ischemia on MPI can also reflect progression of native CAD.
Detection of Graft Disease
Several studies have evaluated the ability of nuclear MPI to detect graft disease, although their value is limited by relativity small sample size and older planar technology.4,5 Combining these studies, the sensitivity for detecting occluded grafts was 19/22 (86%) grafts, with a specificity of 56/64 (88%). MPI SPECT imaging has shown similar results. In a series of 109 patients undergoing adenosine SPECT MPI nearly 7 years after CABG, the sensitivity of detecting graft disease was 96%. The specificity was only 61%, but the large majority of “false positives” were explained by significant lesions in nonrevascularized native CAD or prior myocardial infarction with fixed defects.6 Sensitivity of 80% to 84% with specificity of 80% to 90% has been reported in several smaller studies.7,8 Localization of graft-site occlusion using stress nuclear MPI is quite accurate, with individual coronary territory sensitivities of 92%, 82%, and 75%, respectively, for the LAD, right coronary (RCA), and left circumflex (LCX).8 The corresponding specificities were 91%, 90%, and 75%. The lower accuracy for the LCX territory is consistent with MPI data for native CAD and probably reflects the smaller myocardial territory and overlapping distributions with both RCA and LAD.
Anatomy Versus Physiology Considerations
Because stress nuclear MPI will reflect the hemodynamic significance of a coronary or graft lesion that may not correspond to conventional paradigms of angiographically significant lesions, descriptions of sensitivity and specificity for stress MPI based on such anatomic standards is limited. For example, Salm and colleagues found that 50% of grafts with a greater than 50% diameter stenosis had normal perfusion.9 However, when graft lesions were assessed hemodynamically by Doppler coronary flow reserve, all of the grafts with depressed flow reserve had abnormal stress MPI, and 79% of grafts with normal flow reserve had normal MPI. Similar findings were reported by the same group using coronary flow reserve measured by cardiac MRI.10 Zafrir and colleagues identified that in patients with left internal mammary artery (LIMA) to LAD anastomosis, myocardial ischemia by MPI can occur without angiographic luminal stenosis, concluding that flow-limiting factors like mismatch between LAD and LIMA diameters at the anastomosis take place.11
Assessment Early After CABG (2 to 3 Years)
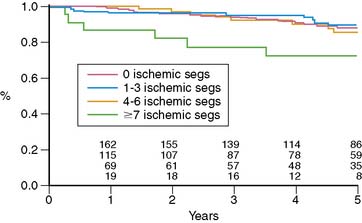
Miller and colleagues evaluated the prognostic value of stress thallium-201 MPI SPECT performed within 2 years of CABG (mean, 11 months) and followed for 5.8 years.12 They found that the only significant multivariate predictors of cardiac death or MI were the exercise angina score and the number of abnormal segments on stress MPI, which reflects the extent of ischemia plus scar. Five-year event-free survival was 93% without angina and either normal MPI or a small postexercise perfusion defect (less than 3 abnormal segments of 14 total segments), compared to 71% for patients with angina and a moderate to large defect and 83% for patients with either of these adverse prognostic factors. Looking at the prognostic impact of reversible defects, indicating jeopardized viable myocardium, there was a threshold effect where patients with more than 7 ischemic segments had a worse outcome (72% 5-year death/MI event-free rate) compared to patients with 0 to 6 reversible segments (85% to 89% 5-year event-free survival) (Fig. 28-1). Importantly, patients with normal MPI had a 5-year death/MI free rate of 92%, yielding an annual event rate of 1.6% per year. An interesting finding in this study was that ischemia proximal to bypass anastomosis was not associated with adverse outcome.
In a series of 75 patients undergoing stress MPI a mean of 38 months after CABG, the best predictor of cardiac events was the summed reversibility score (SRS), reflecting the extent of jeopardized viable myocardium.13 This variable added significant incremental prognostic value to clinical and exercise data and nearly doubled the chi-square of the predictive model. A meta-analysis of functional testing for graft stenosis or progression of native disease showed superior sensitivity for stress MPI compared to exercise treadmill testing (ETT) (68% versus 45%), with similar specificity (84% versus 82%, respectively).14
Assessment Late After CABG (Beyond 5 Years)
Several studies have consistently shown that stress MPI performed late after CABG predicts future cardiac death and MI.15–20 Palmas and colleagues evaluated 294 patients undergoing stress 201Tl MPI at least 5 years after CABG.16 They found that the SRS reflecting jeopardized viable myocardium was significantly related to future death or MI and had significant incremental predictive value to clinical and exercise data, doubling the predictive model chi-square. Stress nuclear MPI data in patients 5 years after CABG have also been shown to add significant prognostic value to angiographic data, doubling the predictive model chi-square for cardiac death/MI when added to clinical, exercise, and angiographic data.17 Significant multivariate predictors of cardiac death or MI included the extent of the perfusion defect, multivessel perfusion defects, and increased 201Tl lung uptake. Zellweger and colleagues also found that the extent of reversible defects in patients undergoing stress MPI more than 5 years after CABG was a significant multivariate predictor of cardiac death.18
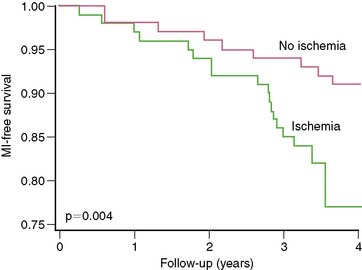
Even in asymptomatic patients presenting 6 to 7 years out from their CABG, stress nuclear MPI is a powerful predictor of death or MI.19 Reversible perfusion defects and exercise capacity were multivariate predictors of death or MI when clinical factors were adjusted. The presence of any reversible defect was associated with a 13% death/MI rate over 3 years, compared to 7% without reversible defect (P = 0.004) (Fig. 28-2), with separation in risk appearing to grow over additional time.
EVALUATION AFTER PERCUTANEOUS CORONARY INTERVENTION
Early After PCI
There is little role for performing screening MPI before 6 weeks, because generally the restenosis phenomenon does not begin to occur before this time. Nevertheless, in patients presenting with chest pain, stress MPI may be helpful in ruling out ischemia as a basis even in this time frame, although interpretation of images requires an understanding of the impact of coronary intervention on coronary responsiveness to hyperemic stimuli. In the days of balloon angioplasty without stent placement, the initial vascular response to reduction of diameter narrowing with mechanical plaque rupture was variable. Several studies showed that perfusion defects on stress MPI early after angioplasty predicted development of restenosis.20–23 Furthermore, even in arteries that do not show restenosis early, perfusion defect on stress MPI can be seen at a mean 9 days after angioplasty and progressively resolves on serial imaging at 3 and 7 months after angioplasty.24 Similar findings have been described for coronary stenting, despite generally more satisfactory initial angiographic results.25–28 The initial perfusion defect may reflect residual vessel obstruction, regional microvascular distal dysfunction in the territory of the dilated artery, angiographically unapparent dissections, or new accumulations of focally extruded plaque. The initial perfusion defect and diminished coronary flow reserve may also reflect endothelial dysfunction and medial injury without coronary stenosis that resolves with time.29–31 These factors contribute to decreased specificity of MPI early after PCI, which substantially impairs the clinical utility of stress MPI in this time frame.
3 to 6 Months After PCI
This time frame represents the general period during which the restenosis phenomenon becomes completed. A large body of literature has evaluated the role of stress nuclear MPI in this clinical setting. It supports the use of stress MPI for detecting the presence of silent or symptomatic restenosis and documents its incremental prognostic value compared to treadmill testing and angiography, its cost-effectiveness, and its overall ability to predict cardiac events and guide management decisions.32–41
In a series of 116 patients 6 months out from angioplasty, stress nuclear MPI and treadmill testing were compared for predicting restenosis.42 SPECT MPI had superior sensitivity (93% versus 52%), specificity (77% versus 64%), and accuracy (86% versus 57%) compared to treadmill testing. In addition, MPI was able to correctly identify the culprit vessel with 86% accuracy. Importantly, many patients with angiographic restenosis are asymptomatic, and the accuracy of stress MPI is not affected by manifestation of the symptoms. Hecht and colleagues reported that in their series of 116 patients, angiographic restenosis occurred in 61% of symptomatic and 59% of asymptomatic patients.43 The ability of stress MPI to detect restenosis was similar: 96% and 91% sensitivity for asymptomatic and symptomatic patients, respectively, with 75% and 77% specificity, respectively. The sensitivity of stress EKG was substantially lower, 40% and 59%, respectively for silent and symptomatic patients. A series by Marie et al. confirmed that asymptomatic restenosis is common but well detected by stress MPI. Silent and symptomatic restenosis occurred at equal rates, and stress nuclear MPI detected 100% of the lesions, whereas stress ECG detected only 25%.44 Numerous other studies, including a meta-analysis of publications between 1975 and 2000, have showed sensitivity of 80% to 90% for detecting restenosis, compared to 40% to 50% for treadmill exercise testing.45–47
Late After PCI
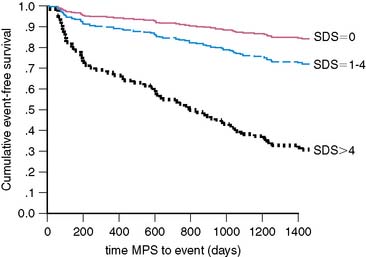
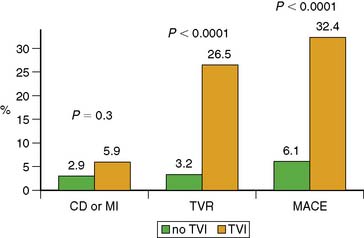
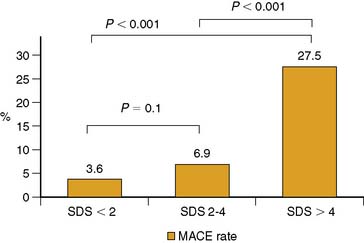
During the late time period after PCI, progression of disease in untreated vessel segments is more prevalent than restenosis.48–52 Beyond simply detecting the presence or absence of restenosis after PCI, stress nuclear MPI performed outside the window of restenosis has the ability to predict major cardiac events, including death or MI. In a series of 211 patients undergoing stress 201Tl MPI 1 to 3 years after balloon angioplasties, future cardiac death or MI was best predicted by the summed stress score, which will reflect the extent of ischemia plus scar.53 The Duke Treadmill Score was not a significant predictor of outcome. In a series of 206 patients undergoing stress nuclear MPI 12 to 18 months after PCI (139 with stenting), the summed difference score (SDS) reflecting ischemia was the best predictor of death or MI.54 Death or MI occurred in 36% patients with reversible defects compared to only 5% without reversible defects. Importantly, of the 124 asymptomatic patients, 24 (19%) had ischemia on MPI, of whom 8/24 (33%) died or developed MI.54 In a larger series of 356 patients who had stress nuclear MPI 6 months after PCI stenting, 23% of patients had evocable target vessel ischemia by MPI that was clinically silent in 62% of cases.55 Risk of cardiac events was directly proportionally related to the extent of reversible defects manifested by the SDS: As SDS increased from 0, 1 to 4, and greater than 4, the cardiac event rates were 17%, 29%, and 69%, respectively (Fig. 28-3). Thus, absence of symptoms in this cohort was not a marker of low risk, and stress MPI was able to identify the high-risk subgroup. Similarly, in another study of 318 post-PCI patients, multivariate analysis showed the summed stress score to be the best independent predictor for hard cardiac events and the SDS the strongest independent predictor of soft cardiac events.56 In the cohort, outcome was also not related to symptomatic status.56 Target vessel ischemia appears to be less common with drug-eluting stents, but late cardiac events are still predicted by stress nuclear MPI. In a series of 476 patients who underwent stress nuclear MPI at 6 months after PCI (66% drug-eluting stents [DES], 34% bare metal stents [BMS]), target vessel ischemia was seen in 10% of BMS compared to 5% of DES (P = 0.045).57 Most patients (68%) with target vessel ischemia were asymptomatic. Target vessel ischemia was associated with a greater then fivefold increased risk of major adverse cardiac events (MACE) (Fig. 28-4). Furthermore, the risk of cardiac events was directly related to extent of jeopardized viable myocardium reflected in the SDS (Fig. 28-5). The increased risk of MACE with target vessel ischemia persisted even if events occurring within 2 months of the MPI (which might have included revascularization events induced by the positive MPI results) were excluded (28% MACE with target vessel ischemia compared to only 5% without; P < 0.0001). Importantly, target vessel ischemia did not predict events related to late stent thrombosis, suggesting that such events are related to a different pathophysiology than intima hyperplasia.
Time Dependency of Risk
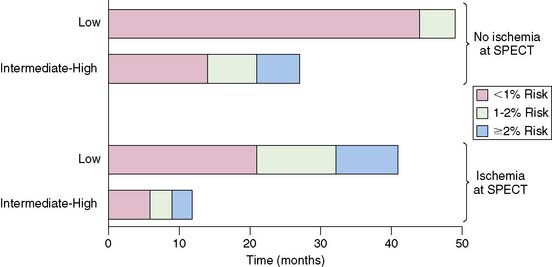
Although normal stress MPI performed late after PCI generally predicts a low cardiac event rate, the event rate is time dependent and also influenced by clinical risk factors. Prior studies have showed that in patients with CAD but normal MPI results, the risk of hard cardiac events increases with time.58 In a series of 396 patients undergoing stress MPI 12 to 18 months after PCI, Acampa and colleagues found, over a 31-month follow-up, that clinical likelihood of ischemia and MPI SPECT ischemia were independent significant predictors of cardiac death or MI.59 Importantly, they found that cardiac risk accelerated with time for both patients with and without MPI ischemia, and this risk was also influenced by clinical likelihood of ischemia (Fig. 28-6). Patients with MPI ischemia and a high clinical likelihood of ischemia exceeded a calculated 2% annual death/MI rate by 10 months after imaging, while it took patients 30 months to reach that threshold if they had a low clinical likelihood of ischemia. Conversely, in patients without MPI ischemia and low clinical likelihood of ischemia, the annual risk of death/MI remained less than 2% throughout the follow-up. Patients without MPI ischemia but an intermediate-high clinical likelihood of ischemia exceeded 2% annual risk of death/MI by 20 months.
NOVEL REVASCULARIZATION METHODS AND RESEARCH TRENDS
Transmyocardial laser revascularization (TMLR) is a surgical technique performed through a left thoracotomy or thoracoscopically, that uses a laser to bore transmural channels from the epicardial to the endocardial surfaces through the left ventricular myocardium in an attempt to improve local perfusion to ischemic myocardial territories not being reached by diseased arteries. TMLR has demonstrated improvement of symptoms in patients with refractory angina.60,61 The basis of the beneficial effect of the procedure is unclear. Although it has been proposed that the clinical benefit could be related to creation of new intramuscular oxygen delivery channels, several studies have shown modest to no myocardial perfusion improvement by SPECT or quantitative PET.62–65 Others have attributed the decrease in anginal symptoms to a decrease in sympathetic innervation after TMLR rather than improvement in myocardial perfusion.66 Interestingly, myocardial sympathetic denervation has been documented by I-123 metaiodobenzylguanidine (MIBG) imaging in patients undergoing TMLR, and the duration of denervation corresponds to the duration of reduced anginal symptoms after TMLR.67 Further data are required to determine whether MIBG SPECT has a role for following patients after TMLR treatment.
Percutaneous transmyocardial laser revascularization (PTMR) is a catheter-based technique of laser revascularization for refractory angina in no-option patients.72 However, similar to TMLR, a reduction in anginal score does not correspond to an improvement in myocardial perfusion,72 suggesting that any beneficial effect of PTMR is also not based on increased myocardial perfusion through the bored channels. Furthermore, initial promising results of PTMR68 could not be reproduced in a subsequent randomized trial.69
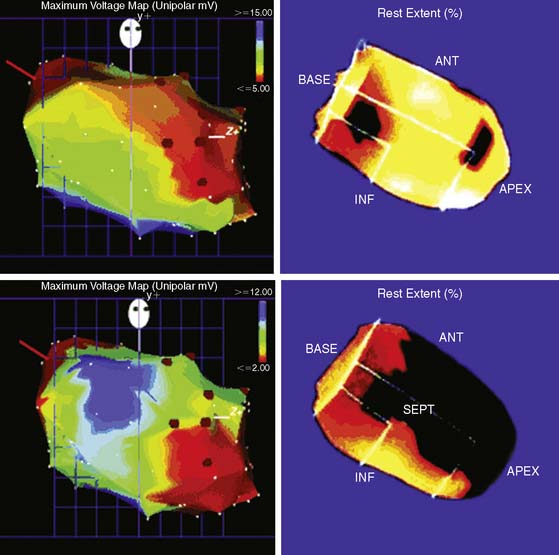
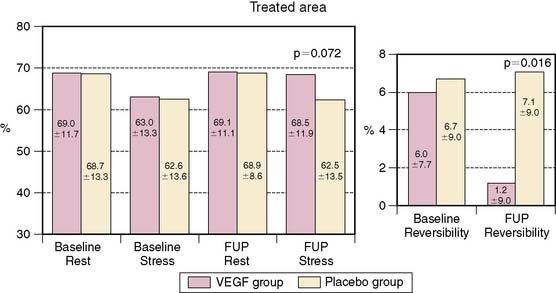
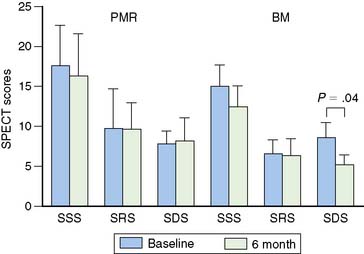
Medical induction of angiogenesis: Over a decade of research in coronary angiogenesis70 has led to developments of exciting methods in nonsurgical coronary revascularization, allowing more effective and targeted induction of angiogenesis. MPI has been shown to be useful as a surrogate of treatment efficacy in this cohort. Intracoronary administration of adenoviral particles containing gene-encoding fibroblast growth factor Ad5FGF-4 resulted in reduction of ischemic defect size by SPECT adenosine MPI.71 Vascular endothelial growth factor (VEGF) gene transfer using adenoviral vector administered after PTCA and stenting showed no measurable benefit in restenosis rate at 6 months but showed improvement in myocardial perfusion by exercise MPI.72 Intracoronary administration of these vectors may be intrinsically limited in reaching the target myocardium in no-option patients with severely diseased or occluded vessels. Therefore, transendocardial stereotactic injection systems have been developed to deliver angiogenesis factors directly to ischemic viable myocardium.73 Stress nuclear MPI has been used then to determine objective response to treatment. Tio and colleagues compared treatment of end-stage CAD with transendocardial-delivered VEGF (using electrical and mechanical data to define ischemic viable segments) to PTLR74 in a randomized trial.73 PET imaging showed an improvement in extent of ischemia only in the VEGF-treated group. Gyongyosi and colleagues took a different approach and instead used the results of stress nuclear MPI SPECT as a navigational map to define segments of ischemic viable myocardium, based on the location or reversible defects, that were suitable for transendocardial injections of VEGF (Fig. 28-7).75 Patients were randomized to active agent or placebo injections. The target area of SPECT reversibility was substantially and significantly reduced in the VEGF group compared to placebo (P < 0.01) (Fig. 28-8). Direct endocardial implantation of bone marrow cells has also been used to attempt to improve myocardial perfusion in end-stage CAD. Tse and colleagues compared perfusion and clinical symptom response to endocardial injections of bone marrow cells versus PTMR.76 Selection of sites for endocardial injections was guided by SPECT MPI and electromechanical mapping, whereas PTMR was guided by electromechanical mapping alone. Although there was significant improvement in quality of life scores in both groups, only the bone marrow injection group showed improvement in extent of jeopardized viable myocardium reflected in reversibility score (Fig. 28-9) at 6 months. Ongoing research in this area will further define the long-term benefit of these novel approaches to revascularization as well as the utility of stress MPI for mapping vulnerable areas and determining the success of revascularization.
Gene transfection is another promising technology of medical myocardial revascularization.77 Ongoing investigations are addressing the appropriate choice of the therapeutic gene type, its structure and form, vector for delivery, method of selective delivery to target tissue, and preferable controllable gene expression.74,78 Molecular imaging probes may be helpful in monitoring the gene expression at the cellular level. Combining traditional scintigraphic technologies with precise anatomic information from CT in hybrid technologies like SPECT/CT and PET/CT may result in a potent imaging tool for translational research,79 but much more research will be required to define the role of radionuclide imaging in this setting.
1. Raymondo T., et al. A prospective comparison of rubidium-82 and thallium-201 SPECT myocardial perfusion imaging utilizing a single dipyridamole stress in the diagnosis of coronary artery disease. J Nucl Med. 1990;21:1899-1905.
2. Katsuya Y., et al. Coronary flow and flow reserve by PET simplified for clinical applications using rubidium-82 or nitrogen-13-ammonia. J Nucl Med. 1996;37:1701-1712.
3. Grondin C.N., et al. Coronary artery bypass grafting with saphenous vein. Circulation. 1989;79(Suppl I):24.
4. Pfisterer M., Emmenegger H., Schmitt H.E., Muller-Brand J., Hasse J., Gradel E., Laver M.B., Burckhardt D., Burkart F. Accuracy of serial myocardial perfusion scintigraphy with thallium-201 for prediction of graft patency early and late after coronary artery bypass surgery. A controlled prospective study. Circulation. 1982;66(5):1017-1024.
5. Ritchie J.L., Narahara K.A., Trobaugh G.B., Williams D.L., Hamilton G.W. Thallium-201 myocardial imaging before and after coronary revascularization. Assessment of regional myocardial blood flow and graft patency. Circulation. 1977;56:830-836.
6. Khoury A.F., Rivera J.M., Mahmarian J.J., Verani M.S. Adenosine thallium-201 tomography in evaluation of graft patency late after coronary artery bypass graft surgery. J Am Coll Cardiol. 1997;29:1290-1295.
7. Lakkis N.M., Mahmarian J.J., Verani M.S. Exercise thallium-201 single photon emission computed tomography for evaluation of coronary artery bypass graft patency. Am J Cardiol. 1995;76:107-111.
8. Elhendy A., et al. Dobutamine-atropine stress myocardial perfusion SPECT imaging in the diagnosis of graft stenosis after coronary bypass grafting. J Nucl Cardiol. 1998;5:491-497.
9. Salm L.P., Bax J.J., Jukema J.W., Langerak S.E., Vliegen H.W., Steendijk P., Lamb H.J., de Roos A., van der Wall E.E. J Nucl Cardiol. 2005;12:545-552.
10. Salm L.P., Bax J.J., Vliegen H.W., Langerak S.E., Dibbets P., Jukema J.W., Lamb H.J., Pauwels E.K., de roos A., van der Wall E.E. Functional significance of stenoses in coronary artery bypass grafts. Evaluation by single-photon emission tomography perfusion imaging, cardiovascular magnetic resonance, and angiography. J Am Coll Cardiol. 2004;44:1877-1882.
11. Zafrir N., et al. Discrepancy between myocardial ischemia and luminal stenosis in patients with left internal mammary artery grafting to left anterior descending coronary. J Nucl Cardiol. 2003;10:663-668.
12. Miller T.D., Christian T.F., Hodge D.O., Mullan B.P., Gibbons R.J. Prognostic value of exercise thallium-201 imaging performed within 2 years of coronary artery bypass graft surgery. J Am Coll Cardiol. 1988;31:848-854.
13. Desideri A., et al. Exercise technetium-99m sestamibi single-photon emission tomography late after coronary artery bypass surgery: Long term follow-up. Clin Cardiol. 1997;20:779.
14. Chin A.S., et al. Functional testing after coronary artery bypass graft surgery: a meta analysis. Can J Cardiol. 2003;19(7):802-808.
15. Sales dos Santos M.M., et al. Prognostic value of technetium-99m labeled single photon emission computerized tomography in the follow-up of patients after their first myocardial revascularization surgery. Arq Bras Cardiol. 2003;80:25-30.
16. Palmas W., Bingham S., Diamond G.A., Denton T.A., Kiat H., Friedman J.D., Scarlata D., Maddahi J., Cohen I., Berman D. Incremental prognostic value of exercise thallium-201 myocardial single-photon emission computed tomography late after coronary artery bypass surgery. J Am Coll Cardiol. 1995;25:403-409.
17. Nallamothu N., Johnson J.H., Bagheri B., Heo J., Iskandrian A.E. Utility of stress single-photon emission computed tomography (SPECT) perfusion imaging in predicting outcome after coronary artery bypass grafting. Am J Cardiol. 1997;80:1517-1521.
18. Zellweger M.J., Lewin H.C., Lai S., Dubois E.A., Friedman J.D., Germano G., Kang X., Sharir T., Berman D.S. When to stress patients after coronary artery bypass surgery. J Am Coll Cardiol. 2001;37:144-152.
19. Lauer M.S., Lytle B., Pashkow F., Snader C.E., Marwick T.H. Prediction of death and myocardial infarction by screening with exercise-thallium testing after coronary-artery-bypass grafting. Lancet. 1998;351:615-622.
20. Cubukcu A.A., Sivananthan U.M., Thorley P.J., Verma S.P., Sheard K., Williams G.J., Rees M.R. Value of thallium-201 studies to assess coronary stent patency and to detect restenosis. Am J Noninvas Cardiol. 1994;8:225-229.
21. Wijns W., Serruys P.W., Simoons M.L., van den Brand M., De Feijter P.J., Reiber J.H., Hugenholtz P.G. Predictive value of early maximal exercise test and thallium scintigraphy after successful percutaneous transluminal coronary angioplasty. Br Heart J. 1985;53:194-200.
22. Breisblatt W.M., Weiland F., Spaccavento L.J. Stress thallium-201 imaging after coronary angioplasty predicts restenosis and recurrent symptoms. J Am Coll Cardiol. 1988;12:1199-12204.
23. Wijns W., Surruys P.W., Reiber J.H.C., de Feyter P.J., den Brand M., Simoons M.L., Hugenholtz P.G., Tijssen J.G.P. Early detection of restenosis after successful percutaneous transluminal coronary angioplasty by exercise-redistribution thallium scintigraphy. Am J Cardiol. 1985;55:357-361.
24. Manyari D.E., Knudtson M., Kloiber R., Roth D. Sequential thallium-201 myocardial perfusion studies after successful percutaneous transluminal coronary artery angioplasty: delayed resolution of exercise-induced scintigraphic abnormalities. Circulation. 1988;77:86-95.
25. Rodes-Cabau J., et al. Frequency of Clinical significance of myocardial ischemia detected early after coronary stent implantation. J Nucl Med. 2001;42:1768-1772.
26. Nagaoka H., et al. Redistribution in thalium-201 imaging soon after successful coronary stenting: tomographic evaluation during coronary hyperemia induced by adenosine. Jpn Circ J. 1998;62:160-166.
27. Bachmann R., et al. Dipyridamole scintigraphy and intravascular ultrasound after successful coronary intervention. J Nucl Med. 1997;38:553-558.
28. Versafil F., et al. Differences of regional coronary flow reserve by adenosine thallium-201 scintigraphy early and six months after successful percutaneous transluminal coronary angioplasty or stent implantation. Am J Cardiol. 1996;78:1097-11102.
29. DePuey E.G. Myocardial perfusion imaging with thallium-201 to evaluate patients before and after percutaneous transluminal coronary angioplasty. Circulation. 1991;84:I59-I65.
30. Wilson R.F., et al. The effect of coronary angioplasty on coronary flow reserve. Circulation. 1988;77:873-885.
31. Uren N.G., et al. Delayed recovery of resistive vessel function after coronary angioplasty. J Am Coll Cardiol. 1993;21:612-621.
32. Mishra J., Iskandrian A.E. Stress myocardial perfusion imaging after coronary angioplasty. Am J Cardiol. 1998;81:766.
33. Alzaraki P., Krawczynska E.G. Thallium imaging in management of post-revascularization patients. Q J Nucl Med. 1996;40:85.
34. Miller D.D., Verani M.S. Current status of myocardial perfusion imaging after percutaneous transluminal coronary angioplasty. J Am Coll Cardiol. 1994;24:260.
35. Miller D.D., Liu P., Strauss H.W., et al. Prognostic value of computer-quantitated exercise thallium imaging early after percutaneous transluminal coronary angioplasty. J Am Coll Cardiol. 1987;10:275.
36. Rosin D.R., Van Raden M.J., Mincemoyer R.M., et al. Exercise electrocardiographic and functional responses after percutaneous transluminal coronary angioplasty. Am J Cardiol. 1984;53:36C.
37. Herzel H.O., Nuesch K., Gruentzig A.R., et al. Short and long-term changes in myocardial perfusion after percutaneous transluminal coronary angioplasty assessed with thallium-201 exercise scintigraphy. Circulation. 1981;63:1001.
38. Scoll J.M., Chaitman B.R., David P.R., et al. Exercise electrocardiography and myocardial scintigraphy in the serial evaluation of the results of percutaneous transluminal coronary angioplasty. Circulation. 1982;66:380.
39. Cottin Y., Rezaizadeh K., Touzery C., et al. Long-term prognostic value of 201Tl single-photon emission computed tomographic myocardial perfusion imaging after coronary stenting. Am Heart J. 2001;141:999-1006.
40. Kaminek M., et al. Prognostic value of myocardial perfusion tomographic imaging in patients after percutaneous transluminal coronary angioplasty. Clin Nucl Med. 2000;25:775-778.
41. Zhang X.L., et al. 99mTc-MIBI stress-rest SPECT imaging for evaluation of outcome after percutaneous transluminal coronary angioplasty. Chin J Nucl Med. 2000;20:97-100.
42. Hecht H.S., Shaw R., Buce R.T., et al. Usefulness of tomographic thallium-201 imaging for detection of restenosis after percutaneous transluminal coronary angioplasty. Am J Cardiol. 1990;6:1314.
43. Hecht H.S., Shaw R.E., Chin H.L., et al. Silent ischemia after coronary angioplasty: Evaluation of restenosis and extent of ischemia in asymptomatic patients by tomographic thallium-201 exercise imaging and comparison with symptomatic patients. J Am Coll Cardiol. 1991;17:670.
44. Marie P.Y., Danchin N., Karcher G., et al. Usefulness of exercise SPECT thallium to detect asymptomatic restenosis in patients who had angina before coronary angioplasty. Am Heart J. 1993;126:571.
45. Georoulias P., Demakopoulos N., Kontos A., et al. Tc-99m tetrofosmin myocardial perfusion imaging before and six months after percutaneous transluminal coronary angioplasty. Clin Nucl Med. 1998;23:678.
46. Milan E., Zoccarto O., Terzi A., et al. Technetium-99m Sestamibi SPECT to detect restenosis after successful percutaneous coronary angioplasty. J Nucl Med. 1996;37:1300.
47. Garzon P.P., Eisnberg M.J. Functional testing for the detection of restenosis after percutaneous transluminal coronary angioplasty: a meta-analysis. Can J Cardiol. 2001;17:41.
48. Suresh C.G., et al. Late symptom recurrence after successful coronary angioplasty: angiographic outcome. Int J Cardiol. 1993;42:257-262.
49. Fleisch M., et al. Management and outcome of stents in 1998. Cardiol Rev. 1999;7:215-218.
50. Kober G., et al. Results of repeat angiography up to eight years following percutaneous transluminal angioplasty. Eur Hear J. 1989;10(Suppl G):49-53.
51. Kober G., et al. Results of repeat angiography up to eight years following percutaneous transluminal angioplasty. Eur Heart J. 1989;10(Suppl G):49-53.
52. Serruys P.W., Luijten H.E., Beatt K.J., et al. Incidence of restenosis after successful coronary angioplasty: A time-related phenomenon. A quantitative angiographic study in 342 consecutive patients at 1, 2, 3, and 4 months. Circulation. 1988;77:361-371.
53. Ho K.T., Miller T.D., Holmes D.R., et al. Long-term prognostic of Duke Treadmill Score and exercise thallium-201 imaging performed one to three years after percutaneous transluminal coronary angioplasty. Am J Cardiol. 1999;84:1323.
54. Acampa W., Petretta M., Florimonte L., et al. Prognostic value of exercise cardiac tomography performed late after percutaneous coronary intervention in symptomatic and symptom free patient. Am J Cardiol. 2003;91:259-263.
55. Zellweger M.J., Weinbacher M., Zutter A.W., et al. Long-term outcome of patients with silent versus symptomatic ischemia six months after percutaneous coronary intervention and stenting. J Am Coll Cardiol. 2003;42:33-40.
56. Zhang X., et al. Long-term prognostic value of exercise 99mTc-MIBI SPET myocardial perfusion imaging in patients after percutaneous coronary intervention. Eur J Nucl Med Mol Imaging. 2004;31:655-662.
57. Zellweger M.J., Kaiser C., Brunner-LaRocca H.P., Buser P.T., Osswald S., Weiss P., Mueller-Brand J., Pfisterer M.E. Value and limitations of target-vessel ischemia in predicting late clinical events after drug-eluting stent implantation. J Nucl Med. 2008;49:550-556.
58. Hachamovitch R., et al. Determinants of risk and its temporal variation in patients with normal stress myocardial perfusion scans: what is the warranty period of a normal scan? J Am Coll Cardiol. 2003;41:1329-1340.
59. Acampa W., et al. Usefulness of stress cardiac single-photon emission computed tomographic imaging late after percutaneous coronary intervention for assessing cardiac events and time of such events. Am J Cardiol. 2007;100:436-441.
60. Spertus J.A., Jones P.G., Coen M., et al. Transmyocardial CO2 laser revascularization improves symptoms, function and quality of life: 12 month results from a randomized controlled trial. Am J Med. 2001;111:341.
61. Horvath K.A., Aranki S.F., Chn L.H., et al. Sustained angina relief 5 years after transmyocardial laser revascularization with a CO2 laser. Circulation. 2001;104:181.
62. Cooley D.A., Frazier O.H., Kadipasaoglu K.A., et al. Transmyocardial laser revascularization: clinical experience with twelve months follow-up. J Thorac Cardiovasc Surg. 1996;111:791-799.
63. Rimoldi O., Burns S.M., Rosen S.D., et al. Measurements of myocardial blood flow with positron emission tomography before and after transmyocardial laser revascularization. Circulation. 1999;100:II 134.
64. Horvath K.A., Cohn L.H., Cooley D.A., et al. Transmyocardial laser revascularization: results of a multicenter trial with transmyocardial laser revascularization used as sole therapy for end-stage coronary artery disease. J Thorac Cardiovasc Surg. 1997;113:645-653.
65. Holmstrom M., et al. Wall motion and perfusion analysis of transmyocardial laser revascularization. Scand Cardiovasc J. 2003;37(2):91-97.
66. Al-Sheihk T., Allen K.B., Straka S.P., et al. Cardiac sympathetic denervation after transmyocardial laser revascularization. Circulation. 1999;100:135.
67. Teresinska, et al. Changes in cardiac adrenergic nervous system after transmyocardial laser revascularization assessed by I-123 MIBG SPECT. Kardiol Pol. 2004;60:15-20.
68. Oesterle S.N., Sanborn T.A., Ali N., et al. Percutaneous transmyocardial laser revascularization for severe angina: The PACIFIC randomized trial. Potential Class Improvement From Intramyocardial Channels. Lancet. 2000;356(9243):1705-1710.
69. Liao L., Sarria-Santamera A., Matchar D.B., et al. Meta-analysis of survival and relief of angina pectoris after transmyocardial revascularization. Am J Cardiol. 2005;95(10):1243-1245.
70. Lazarous D.F., Shou M., Scheinowitch M., et al. Comparative effects of basic fibroblast growth factor and vascular endothelial growth factor on coronary collateral development and the arterial response to injury. Circulation. 1996;94:1074-1082.
71. Grines C., Watkins M., Mahmarian J., et al. A randomized, double-blind, placebo-controlled trial of Ad5FGF-4 gene therapy and its effect on myocardial perfusion in patients with stable angina. J Am Coll Cardiol. 2003;42:1339-1347.
72. Hedman M., Hartikainen J., Syvanne M., et al. Safety and feasibility of catheter-based local intracoronary vascular endothelial growth factor gene transfer in the prevention of postangioplasty and in-stent restenosis and in the treatment of chronic myocardial ischemia. Circulation. 2003:107. 2677
73. Tio R.A., et al. PET for evaluation of differential myocardial perfusion dynamics after VEGF gene therapy and laser therapy in end-stage coronary artery disease. J Nucl Med. 2004;45(9):1437-1443.
74. Gaffney M.M., Hynes S.O., Barry F., O’Brien T. Cardiovascular gene therapy: current status and therapeutic potential. Br J Pharmacol. 2007;152:175-188.
75. Gyongyosi M., Khorsand A., Zamini S., et al. NOGA-guided analysis of regional myocardial perfusion abnormalities treated with intramyocardial injections of plasmid encoding vascular endothelial growth factor A-165 in patients with chronic myocardial ischemia: Subanalysis of the EUROINJECT-ONE Multicenter Double-Blind Randomized Study. Circulation. 2005;112:I-157–I-165.
76. Tse H.F., et al. Comparative evaluation of long-term clinical efficacy with catheter-based percutaneous intramyocardial autologous bone marrow cell implantation versus laser myocardial revascularization in patients with severe coronary artery disease. Am Heart J. 2007;14(982e):1-1982.e6.
77. Hedman M., Turunen M.P., Ylä-Herttuala S. New technologies in cardiovascular research: Gene therapy. In: Pasterkamp G., de Kleijn D., editors. Cardiovascular Research: New Technologies, Methods, and Applications. New York: Springer; 2005:89-98.
78. Rissanen T.T., Ylä-Herttuala S. Current status of cardiovascular gene therapy. Mol Ther. 2007;15:1233-1247.
79. Jaffer F.A. Seeing within. Molecular imaging of the cardiovascular system. Circ Res. 2004;94:433-445.