67 Nosocomial Pneumonia
 Definitions
Definitions
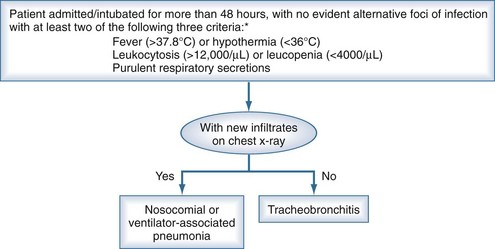
Nosocomial pneumonia is an infection of the pulmonary parenchyma caused by pathogens predominantly present in hospital settings.1 Nosocomial pneumonia develops in patients admitted to the hospital for more than 48 hours, and usually the incubation period of this infection is no longer than 2 days. Nosocomial pneumonias include ventilator-associated pneumonia (VAP), which commonly develops in intensive care unit (ICU) patients who have been tracheally intubated and mechanically ventilated for at least 48 hours. Ventilator-associated tracheobronchitis (VAT) has not been as extensively studied as VAP in patients undergoing mechanical ventilation, but this disorder is characterized by signs of respiratory infection such as an increase in the volume and purulence of respiratory secretions, fever, and leukocytosis. However, in contrast to VAP, radiologic infiltrates suggestive of consolidation on chest x-ray are not observed.1 Healthcare-associated pneumonia (HCAP) is a new clinical entity that has recently been defined in the latest guidelines of the American Thoracic Society1 for the diagnosis and treatment of nosocomial pneumonia. Patients who develop HCAP are not hospitalized, but they present several risks for being colonized by pathogens present in hospital settings, including multiresistant microorganisms. Risk factors for developing HCAP are hospitalization for 2 days or more within the preceding 90 days, residence in a nursing home or extended care facility, home infusion therapy (including antibiotics), chronic dialysis within 30 days, home wound care, and a family member with multidrug-resistant (MDR) pathogen colonization or infection. Interestingly, there is still controversy about the etiology and definition of HCAP. Indeed, several North American studies have reported that HCAP is mostly caused by multiresistant microorganisms; conversely, European data show larger similarities between etiology of HCAP and community-acquired pneumonia.
Nosocomial pneumonia can also be classified based on the presence of microorganisms isolated through respiratory surveillance cultures and includes the following categories2:
The time of onset for nosocomial pneumonia also has important implications for possible etiology, empirical antimicrobial treatment, and outcomes. Langer et al.3 first differentiated between nosocomial pneumonia at early onset (developing within the first 4 days after hospital admission) and late onset (5 days or more). However, there are no well-designed trials supporting these time cutoffs. An interesting trial performed by Trouillet et al.4 found that three variables were significant for predicting infection with MDR VAP: duration of mechanical ventilation (MV) ≥ 7 days (odds ratio [OR] = 6.0), prior antibiotic use (OR = 13.5), and prior use of broad-spectrum drugs (third-generation cephalosporin, fluoroquinolone, and/or imipenem) (OR = 4.1).
 Epidemiology
Epidemiology
Incidence
Nosocomial pneumonia is the second most common nosocomial infection and the leading cause of death from nosocomial infections for critically ill patients. The incidence of nosocomial pneumonia is age dependent, with 5 of every 1000 cases affecting hospitalized patients younger than 35 years of age and up to 15 of 1000 in hospitalized patients older than 65.5 In earlier reports, nosocomial pneumonia increased hospital stay by 7 to 9 days per patient, accounting for up to 25% of all ICU infections and for more than 50% of the antibiotics prescribed.6 A Spanish report by Sopena et al.7 in 2005 studied the epidemiology of nosocomial pneumonia in 186 non-ICU patients from 12 hospitals and found that nosocomial pneumonia was observed mostly in elderly patients with underlying diseases and was primarily caused by S. pneumoniae, Legionella pneumophila, Aspergillus spp., and P. aeruginosa. The mortality rate was 26%, with an attributable mortality of 13%. Cook et al.8 estimated that the risk of VAP is 1% per day on mechanical ventilation8; they also demonstrated that the risk changes over time, being 3% the first 5 days on mechanical ventilation, 2% from the 5th to 10th day, and 1% for the remaining days. Considering that most invasively ventilated patients are intubated for less than a week, nearly half of VAP cases occur during the first days of mechanical ventilation.
Mortality
The crude mortality from nosocomial pneumonia may be as high as 30% to 70%, although several cofactors influence mortality during critical illness and make it extremely difficult to determine attributable mortality.9 Mortality caused by VAP has been defined as the percentage of deaths that would not have occurred in the absence of the infection. Several case-matching studies have estimated that one third to one half of all VAP-related deaths are the direct result of the infection, with a higher mortality rate in cases caused by P. aeruginosa or Acinetobacter spp. and associated with bacteremia.10 The development of VAP is accompanied by a 1.8- to 4-fold increase in the risk of death. A multicenter French study9 evaluated the attributable mortality and risk factors for death for late-onset pneumonia. They evaluated 764 patients admitted to the ICU for more than 96 hours and found a 47% mortality in patients with VAP versus 22% in the total population. Moreover, mortality was inversely related to adequacy of the initial empirical therapy. Similarly, Luna et al.11 assessed the appropriateness and delay of antibiotic therapy in 76 mechanically ventilated patients with bacteriologically confirmed VAP and found an overall mortality of 52.6%. Based on current evidence, nosocomial pneumonia is associated with a high mortality rate, but precise estimates of mortality attributable to this condition are not possible, owing to heterogeneity between patient populations, microbial patterns, antibiotic treatment, and diagnostic methods.12
 Pathogenesis
Pathogenesis
Role of Tracheal Tube in the Pathogenesis of ventilator-associated pneumonia
Pulmonary aspiration of colonized oropharyngeal secretions across the tracheal tube cuff is the main pathogenic mechanism for development of VAP. The endotracheal tube (ETT), commonly used in the ICU for long-term mechanically ventilated patients, includes a high-volume, low-pressure (HVLP) cuff. HVLP cuffs were originally designed to control pressure exerted against the tracheal wall and prevent tracheal injury.13–15 However, the potential diameter of the HVLP cuff is two to three times larger than the tracheal diameter, so when the tracheal cuff is inflated within the trachea, folds invariably form along the cuff surface, causing consistent micro- and macroaspiration of oropharyngeal secretions.16
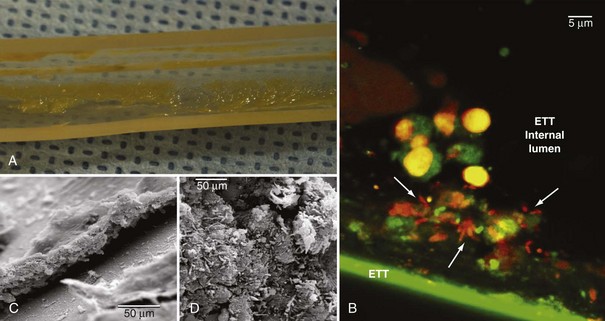
Pathogens may also grow on the internal surface of the ETT and ultimately translocate into the lungs. The ETT is commonly made of polyvinyl chloride (PVC), and bacteria easily adhere to its internal surface to form a complex structure called biofilm17,18 (Figure 67-1). Biofilm is composed of sessile bacteria embedded within a self-produced exopolysaccharide matrix.19 Biofilm on the internal surface of an ETT can be identified early following tracheal intubation.20,21 Sessile bacteria undergo phenotypic differentiation from their planktonic counterparts, and most of such differentiation constitutes a survival advantage. Indeed, bacteria within the biofilm are difficult to eradicate, and antibacterial efficacy of the host’s immune response and antibiotics are largely reduced. During mechanical ventilation, biofilm particles may dislodge into the airways as a result of inspiratory airflow17 and invasive medical interventions such as tracheal aspiration22 and bronchoscopy. Several studies have assessed the role of bacterial biofilm on pathogenesis of VAP and confirmed that the ETT biofilm is difficult to eradicate and constitutes a persistent source of colonization. Adair et al.23 studied 40 tracheal tubes obtained from critically ill patients with and without VAP and compared the genotype of bacteria retrieved from the lower airways and the ETT. In 70% of the samples obtained from patients with VAP, the authors found the same genotype in bacteria from ETT biofilm and the patients’ airways. Moreover, they confirmed that antibiotic susceptibility was lower in pathogens isolated from within the biofilm.
Sources of Colonization
Tracheally intubated patients in the ICU can be colonized either exogenously or endogenously. Patients are colonized exogenously by contaminated respiratory equipment, the ICU environment, and the hands of the ICU staff. Several reports have described ICU outbreaks due to colonized bronchoscopes,24,25 water supply,26,27 respiratory equipment,28,29 humidifiers,30 ventilator temperature sensors,31,32 respiratory nebulizers,33,34 and contaminated environment.35 Several factors play a significant role in reducing risks for cross-transmission of pathogens. Indeed, in every ICU an adequate ratio of single rooms to open beds should be provided; healthcare personnel should be adequately trained on infection control and preventive strategies; strict sterilization protocols and hand washing with alcohol-based solutions should be implemented, and finally, lower patient-to-nurse ratios are advantageous.
Endogenous colonization is believed to be the primary pathogenic mechanism for VAP development. In the critically ill patient, the oral flora shifts early to a predominance of aerobic gram-negative pathogens,36,37 Pseudomonas aeruginosa, and methicillin-resistant Staphylococcus aureus (MRSA). Therefore, pulmonary aspiration of oropharyngeal contents increases the risk for airway colonization and infection. Following aspiration and colonization of the airways, the occurrence of VAP primarily depends on the size of the inoculum, functional status, and the competency of host defenses. There is still controversy regarding the exact sequence of colonization and sources of infection in the pathogenesis of VAP. Early studies by Feldman et al.38 found that in patients undergoing mechanical ventilation, the oropharynx is the first site to be colonized by pathogens (36 hours), followed by the stomach (36-60 hours), the lower respiratory tract (60-84 hours), and thereafter the tracheal tube (60-96 hours).
Dental Plaque
In the healthy human, oral colonization with pathogens is prevented by the physical-chemical properties of the oral mucosa surface, the salivary enzymatic content, and specific proteases and immunoglobulins. ICU patients are at higher risk for dental plaque colonization due to difficulties in oral hygiene, changes in salivary properties during critical illness, and change of oral flora by antibiotic therapy. An early study by Scannapieco et al.39 showed that ICU patients are often colonized by aerobic pathogens on admission. More recently, Fourrier and collaborators40 found that prolonged ICU stay increases risks for colonization of dental plaque by aerobic pathogens. Moreover, the authors found that colonization of dental plaque was highly predictive of concurrent or subsequent nosocomial infection. Azarpazhooh et al.41 found evidence of an association of pneumonia with oral health (OR = 1.2 to 9.6, depending on oral health indicators). The authors reported that improved oral hygiene and frequent professional oral health care reduces the progression or occurrence of respiratory infection among high-risk elderly adults living in nursing homes and especially those in ICUs (number needed to treat [NNT] = 2-16; relative risk reduction [RRR] = 34%-83%). During critical illness and extensive antibiotic therapy, the oral flora may rapidly change; unfortunately, standard culture-based microbiological assays determine neither the dominant bacterial species nor the range of bacterial diversity within the community. In a study by Heo,42 18 ICU patients who developed VAP were studied, comparing genetic features of strains obtained from oral, tracheal, and bronchoalveolar lavage (BAL) samples. The authors found that oral respiratory pathogens were often genetically identical to pathogens recovered from the lower airways, and rapid changes of bacterial species in both oral and pulmonary sites appeared to occur.
Sinuses
The association between sinusitis and VAP has long been debated. Several studies have confirmed that orotracheal as compared to nasotracheal intubation is associated with a decreased incidence of sinusitis,43–45 and that incidence of VAP is lower in patients who do not develop sinusitis.46 A study by Holzapfel et al.47 evaluated the incidence of nosocomial maxillary sinusitis and pneumonia in patients who underwent either nasotracheal or orotracheal intubation. The authors found that sinusitis increased the risk of nosocomial pneumonia by a factor of 3.8.
Stomach
According to the gastropulmonary hypothesis of colonization, the stomach of ICU patients is often colonized by pathogens as a consequence of alkalinization of gastric contents by enteral nutrition and drugs. Continuous gastroesophageal reflux facilitates translocation of microbes into the oropharynx, which are then aspirated across the ETT cuff. Several trials have investigated the benefits of selective gut decontamination and stress ulcer prophylaxis and confirmed that preventing gastric alkalinization and reducing the bacterial burden of the stomach is associated with a lower incidence of nosocomial respiratory infections.48 Early studies have shown that in tracheally intubated patients, gastric pH higher than 4 is consistently associated with gastric colonization with pathogens.49,50 However, the association between gastric colonization and VAP found in early studies51–53 has been challenged by more recent studies.54–57 Overall, this area remains highly controversial, and several studies38,54,58–63 have not found a relationship with bacteria causing VAP as first originating in the stomach.
Impairment of Respiratory Defense During Critical Illness and Tracheal Intubation
In healthy subjects, the physiologic adduction of the true and false vocal folds provides full closure of the airways, and efficiently prevents aspiration of pathogen-laden oropharyngeal contents. The airways are additionally protected by the epiglottis, which moves over the top of the larynx to divert any fluid or solids from passing into the airways. Following intubation, the tracheal tube completely bypasses these anatomic barriers and creates a direct conduit for bacteria to be aspirated and reach lower airways. Cough is one of the most efficient mechanisms to prevent further translocation of pathogens that may have gained access into airways. In the healthy human, cough begins with an inspiratory effort followed by a forced expiratory effort against a closed glottis and ultimately, opening of the glottis to generate rapid expiratory airflow. Tracheal intubation prevents closure of the glottis, hence it fully hinders cough; moreover, intubated patients are often sedated and unable to generate high expiratory flows. Mucociliary clearance is the primary innate airway defense mechanism to clear pathogens. In young, healthy nonsmokers, the mucociliary velocity ranges between 10 and 15 mm/min. Studies in animals have consistently shown that inflation of the tracheal tube cuff within the trachea lowers mucociliary velocity by 37% within an hour, and 52% after 2 hours.64 Clinical studies65 in critically ill, tracheally intubated patients have confirmed those results and found that mucociliary velocity is very low (0.8-1.4 mm/min); lower mucociliary clearance has been associated with higher risks for pulmonary complications.
Although many ICU patients develop tracheal bacterial colonization during the course of mechanical ventilation, only a small proportion subsequently develop VAP. As previously mentioned, the daily hazard rate for developing VAP is higher during the first days of mechanical ventilation.8 Investigators have found that a temporary immunoparalysis can be found early in the course of the critical illness and admission to the ICU.66 In particular, researchers have focused on assessing human leukocyte antigen DR (HLA-DR) expression on peripheral monocytes as a marker of immune function.67–69 According to the rationale that impaired immune function may predispose to the development of VAP, low levels of HLA-DR expression have been found in patients who subsequently developed nosocomial pneumonia.70
Etiologic Agents
Nosocomial pneumonia may be caused by a variety of pathogens and, in many patients, more than one pathogen may be isolated. Microorganisms responsible for nosocomial pneumonia differ according to the ICU population, the duration of hospital and ICU stays, and the specific diagnostic method(s) used. VAP is commonly caused by aerobic gram-negative bacilli such as P. aeruginosa, Escherichia coli, Klebsiella pneumoniae, or Acinetobacter species, while S. aureus is the predominant isolated gram-positive pathogen.1,71–74 Data from 7087 infected patients (63.5% with respiratory tract infection) from the Extended Prevalence of Infection in Intensive Care (EPIC II) study75 have confirmed that Pseudomonas spp. and S. aureus are the most common isolated pathogens in intensive care units.
Few studies have assessed whether the pathogens that cause pneumonia in ventilated patients differ from those in patients who are not mechanically ventilated. Weber et al.76 evaluated 158,519 patients admitted to a single center over a 4-year period and identified 327 episodes of VAP and 261 episodes of nosocomial pneumonia. The infecting flora in ventilated patients mostly included MRSA (17.75%) and gram-negative bacilli such as P. aeruginosa (59.0%), Stenotrophomonas maltophilia (17.50%), and Acinetobacter species (6.75%). Similarly, in 20.37% of the patients not requiring mechanical ventilation, MRSA was identified, while a lower incidence of nosocomial pneumonia due to P. aeruginosa, Acinetobacter spp., and S. maltophilia was found. Nevertheless, the overall frequency of infection with these gram-negative pathogens was sufficiently high to warrant the use of empirical therapy likely to be active against them.
The high rate of polymicrobial infection in VAP has been shown repeatedly. Combes and colleagues77 studied 124 ICU patients, of whom 65 (52%) had monomicrobial VAP and 59 had (48%) polymicrobial VAP. In most patients, two different bacteria were isolated (42 patients, 34%); however, up to four different bacteria coexisted in 7 patients (6%). Interestingly, no differences were detected in mortality rate at 30 days between patients with polymicrobial or monomicrobial infection. A study by Teixeira et al.78 investigated risk factors for inadequate empirical antimicrobial therapy in 151 ICU patients and found that 69 (45.7%) patients with a clinical diagnosis of VAP received inadequate empirical antimicrobial treatment. Multiple logistic regression analysis revealed that inadequate antimicrobial treatment was associated with polymicrobial VAP (OR, 3.67; 95% confidence interval [CI], 1.21-11.12; P = 0.02), and importantly, inadequate antimicrobial treatment was associated with higher mortality for patients with VAP.
Underlying diseases may predispose patients to infection with specific organisms, Patients with chronic obstructive pulmonary disease (COPD), for example, are at increased risk for Haemophilus influenzae, Moraxella catarrhalis, P. aeruginosa, or Streptococcus pneumoniae infections.79,80 Patients with acute respiratory distress syndrome (ARDS) are at higher risk for developing VAP caused by S. aureus, P. aeruginosa, and Acinetobacter baumannii, and often in these patients, VAP is caused by multiple pathogens.81,82 Finally, trauma and neurologic patients are at increased risk for S. aureus, Haemophilus, and S. pneumoniae infections.83–85
It is important to identify MDR pathogens in order to guide appropriate antibiotic treatment. Causative pathogens of VAP that are potentially multiresistant are P. aeruginosa, MRSA, Acinetobacter spp., S. maltophilia, Burkholderia cepacia, and extended-spectrum β-lactamase (ESBL+) Klebsiella pneumonia. Conversely, S. pneumoniae, H. influenzae, methicillin-sensitive S. aureus (MSSA), and antibiotic-sensitive Enterobacteriaceae are not considered MDR pathogens. Patients at risk of being colonized by MDR pathogens are extremely heterogeneous, often present several comorbidities, and many receive antibiotics prior to and during the course of their hospitalization. Therefore, it is extremely challenging to accurately define risk factors for carrying MDR pathogens. Langer et al.3 tried to better classify patients who develop VAP, in order to provide data for guiding empirical antibiotic treatment. They compared early-onset and late-onset pneumonia and found that early-onset pneumonia is rarely caused by MDR pathogens, is less severe, and is associated with better outcome. However, recent reports are challenging such conclusions and demonstrate no association between MDR pathogens and time of onset of pneumonia.86,87 Those data suggest the need for additional studies to accurately identify risk factors for harboring MDR pathogens, rather than risk stratification based on nonspecific factors such as severity of pneumonia and time of onset. The incidence of MDR pathogens is also closely linked to local factors and varies widely from one institution to another. Consequently, each ICU has to continuously collect accurate epidemiologic data. Rello et al.88 analyzed variations of VAP etiology among three Spanish ICUs and compared them with data collected in Paris. The authors concluded that VAP pathogens varied widely among the four clinical centers, with marked differences in the microorganisms isolated from VAP episodes in Spanish centers as compared with the French site. Therefore, clinicians must be aware of the common microorganisms associated with both early-onset and late-onset VAP in their own hospitals to avoid the administration of inadequate initial antimicrobial therapy.
Legionella pneumophila as a cause of nosocomial pneumonia should be considered, particularly in immunocompromised patients.89 Often the source of legionellosis outbreaks within the hospital is a water system that has become colonized by the microorganism.90
The role of anaerobes in the pathogenesis of VAP requires further assessment, since the primary mechanism for VAP development is through aspiration of oropharyngeal contents, and the oropharynx is highly colonized by anaerobes. Robert et al.91 studied 26 mechanically ventilated patients and found that 15 patients became colonized with 28 different anaerobic strains. Similarly Dore et al.92 found anaerobic bacteria in 30 (23%) of 130 patients diagnosed with VAP, but always in association with aerobic pathogens. Importantly, empirical antibiotic therapy active against anaerobic bacteria appears to improve short-term outcomes in patients with VAP.93 Nevertheless, several authors94,95 were unable to reproduce those data, and the role of anaerobes in VAP is still considered controversial. In particular, Marik et al.95 studied microbiology of 185 episodes of suspected VAP through blind protected specimen brush sampling and mini-BAL and were unable to identify anaerobes as the causative pathogens of VAP.
Rarely, the causative organism of VAP is a fungus. Candida spp. and Aspergillus fumigatus are the most common isolated fungi, predominately in immunocompromised patients. In mechanically ventilated patients, the clinical significance of respiratory tract colonization by Candida is controversial. In a retrospective analysis of 639 patients from a Canadian study or VAP, 114 patients had Candida colonization of the respiratory tract.96 Interestingly, patients with Candida colonization had a significant increase in hospital mortality (34% versus 21% in patients without Candida colonization, P = 0.003). However, it is still unclear whether Candida colonization is associated with or responsible for worse outcomes. Moreover, a recent report showed that in ICU patients, isolation of Candida species in respiratory samples demonstrates only colonization rather than Candida pneumonia.97
It is commonly reported that VAP is infrequently due to viruses; however, it should also be acknowledged that patients with clinical suspicion of VAP are rarely screened for viruses. Daubin et al.98 studied 139 patients mechanically ventilated for more than 48 hours, of which 39 (28%) developed VAP. Although P. aeruginosa and MRSA still accounted for most of the VAP cases, herpes simplex virus type 1 was found in 12 cases of VAP and cytomegalovirus (CMV) in 1 case. Several studies have reported a high incidence of active CMV infection in mechanically ventilated patients.99–101 Recently, Chiche et al.102 studied 242 immunocompetent ICU patients and found active CMV infection in 39 (16%). At 28 days, only 15% of the patients with active CMV infection were weaned and alive, in comparison to 52% of patients free of CMV infection (P < 0.001).
 Prevention
Prevention
Nosocomial pneumonia is associated with high morbidity and mortality and constitutes an important burden for the healthcare system1,103; therefore, preventive strategies should be implemented to reduce overall incidence of the disease (Box 67-1). Strategies have focused on reducing cross-transmission, the likelihood of aspiration across the ETT cuff, and the bacterial load in the oropharynx. The Institute for Healthcare Improvement recommends that approaches with proven efficacy for reduction of morbidity and mortality related to infection control should be grouped and implemented together as a bundle, because together they are expected to result in a better outcome than when implemented individually. Designing a preventive bundle is just the first step and must be followed by continuous assessment of healthcare personnel compliance and improvements to implement interventions. Several reports104–106 have found drastic reductions in the incidence of VAP following implementation of VAP preventive bundles.
Box 67-1
Preventive Strategies for Nosocomial Pneumonia
General Prophylactic Measures
Maintaining high levels of education among ICU personnel relating to VAP pathophysiology and preventive strategies can be effective in reducing incidence of this problem.107–110 Respiratory care practitioners and nurses should be the primary recipients of education programs, and frequent performance feedback and compliance assessment should be undertaken.111 Interestingly, Needleman et al.112 studied administrative data from 799 hospitals in 11 states (covering 5,075,969 discharges of medical patients and 1,104,659 discharges of surgical patients) and found that a higher proportion of hours of care per day provided by registered nurses, compared to licensed practical nurses and nurses’ aides, were associated with lower incidence of pneumonia.
Adherence to simple infection-control measures such as alcohol-based hand disinfection effectively reduces cross-transmission of pathogens and incidence of VAP.113 The World Health Organization has endorsed hand hygiene as the single most important element of strategies to prevent healthcare-associated infections.114 Overall, most studies conducted in ICUs have shown consistent results and temporal association between implementation of alcohol-based hand hygiene and reduction of nosocomial infections.115–117
Kollef et al.118 demonstrated that patient transport outside the ICU was associated with increased risks for VAP. Clinicians and nursing staff should carefully carry out transport of intubated patients. In particular, the internal pressure of the ETT cuff should be always kept within the recommended range, particularly when the patient is expected to be maintained supine during diagnostic or therapeutic procedures; ventilator circuits should be carefully manipulated in order to prevent aspiration of colonized fluids from within the circuit.
Daily interruption or lightening of sedation to avoid constant impairment of respiratory defenses, as well as avoidance of paralytic agents, is highly recommended. It is well acknowledged that prolonged tracheal intubation is associated with VAP.8 A report by Kress et al.119 was recently confirmed by Schweickert et al.,120 who studied 128 mechanically ventilated patients randomized to continuous infusions of sedation with or without daily interruption. The authors demonstrated reduction of duration of mechanical ventilation and length of stay in the ICU when patients were allowed to wake up daily. Moreover, a trial by Schweickert et al.121 has shown that early physical and occupational therapy during critical illness is associated with more ventilator-free days. In a study by Strøm et al.,122 140 critically ill adult patients expected to be intubated for more than 24 hours were randomized to receive no sedation or sedation with daily interruption until awake. Both groups were treated with bolus doses of morphine. In that study, patients receiving no sedation had significantly more days without ventilation and shorter stay in the ICU. Results from these clinical trials are challenging standard sedation protocols for intubated patients and hold promise for reducing length of stay on mechanical ventilation and, ultimately, risks for VAP.
There is evidence of shorter length of mechanical ventilation, reduced rate of failed extubation, and decreased incidence of VAP when protocol-driven weaning from the ventilator is implemented.123,124 Marelich et al.123 randomized 385 patients to receive either a protocol-driven weaning procedure or standard care and found that duration of mechanical ventilation was decreased from a median of 124 hours for the control group to 68 hours in the protocol-driven weaning group (P = 0.0001). Moreover, a trend toward less VAP was found in the treatment group (P = 0.061).
Noninvasive Ventilation
Tracheal intubation and mechanical ventilation account for the main risk for nosocomial pneumonia and therefore should be avoided whenever possible. Noninvasive ventilation (NIV) is an attractive alternative for patients with acute exacerbations of COPD or acute hypoxemic respiratory failure and for some immunocompromised patients with pulmonary infiltrates and respiratory failure.125–128 NIV can also be safely used to facilitate early extubation and avoid continued invasive weaning. A meta-analysis129 evaluated 12 trials enrolling 530 participants, mostly with chronic obstructive pulmonary disease, and confirmed that noninvasive weaning is significantly associated with reduced mortality, VAP, and length of stay in the ICU and hospital. Another report130 emphasized the role of NIV in preventing reintubation in recently extubated patients at risk for relapse and respiratory failure. Kohlenberg et al.131 pooled data of 400 ICUs in Germany and found mean pneumonia incidence of 1.58 and 5.44 cases per 1000 ventilator days for NIV and invasive mechanical ventilation, respectively. Therefore, when indicated, NIV should be attempted to avoid tracheal intubation and reduce overall duration of tracheal intubation.
Tracheal Tube Cuff
Several strategies have been applied to improve the design of tracheal tubes and reduce the likelihood of aspiration of pathogen-laden secretions across the cuff. Novel ETT cuffs made of new materials such as polyurethane,16 silicone,132 and latex133,134 have been developed and tested in laboratory and clinical trials. Particularly, the polyurethane cuff has a thickness of 5 to 10 µm in comparison to 50 µm of PVC cuffs; hence, upon inflation, smaller folds form, and aspiration of secretions above the cuff can be prevented or reduced. Lorente et al.135 compared a standard ETT to an ETT incorporating an ultrathin polyurethane cuff and intermittent aspiration of subglottic secretions and found a reduction of incidence of VAP from 22.1% to 7.9% between the standard and new tubes, respectively (P = 0.001). A single-center study by Miller et al.136 tested the use of a polyurethane-cuff ETT versus a standard PVC cuff ETT, with a before and after design, and found that VAP rates decreased from 5.3 per 1000 ventilator days before the use of the polyurethane-cuffed ETT to 2.8 per 1000 ventilator days during the intervention year (P = 0.0138). The polyurethane-cuffed ETT has also shown benefits in reducing early postoperative pneumonia in cardiac surgical patients. Poelaert et al.137 studied 134 cardiothoracic surgery patients and demonstrated that the incidence of early postoperative pneumonia was significantly lower in the polyurethane group than in the polyvinyl chloride group (23% versus 42%, P < 0.03). Silicone132,138 and latex133,134,139 cuffs are low-volume, low-pressure cuffs and are promising alternatives to PVC cuffs. Upon inflation, folds are never formed, yet compliance of those materials is extremely high; thus they allow reliable control of the pressure exerted against the trachea. In a clinical trial on patients undergoing anesthesia or admitted to the ICU, Young et al.132 demonstrated high effectiveness of a silicone cuff in reducing pulmonary aspiration.
The shape of the cuff plays an important role in prevention of aspiration.133,140 In comparison to standard cuffs with cylindrical shapes, cuffs designed with a smooth, tapering shape allow elimination of folds for a full circumference of the trachea/cuff contact zone, irrespective of the cuff material.
It is important to maintain the internal pressure of ETT cuff pressure between 25 and 30 cm H2O, particularly when no positive end-expiratory pressure (PEEP) is applied; this serves to prevent either macroleakage of contaminated secretions into the lower airways or tracheal injury. A recent study141 demonstrated that frequently the ETT cuff was deflated or hyperinflated using standard management.
Ventilatory settings may play a role in pathogenesis of VAP. In particular, PEEP may decrease the incidence of VAP by counteracting hydrostatic pressure exerted by oropharyngeal secretions above the ETT cuff, hence reducing pulmonary aspiration.142 A recent report143 assessed effects of 5 to 8 cm H2O in normoxemic ventilated patients and showed reduction of the rate of VAP (PEEP group 9.4%, control patients 25.4%, relative risk, 0.37; 95% CI = 0.15-0.84; P = 0.017).
Tracheal Tubes Coated with Antimicrobial Agents
Coating the ETT with antimicrobial agents such as silver is a promising strategy to prevent biofilm formation within its internal surface and VAP. Olson et al.144 examined a silver-coated tracheal tube in comparison to standard tube in intubated dogs. The dogs were challenged with P. aeruginosa into the oropharynx. Using the new tube, the investigators were able to postpone colonization of the ETT inner surface (3.2 ± 0.8 versus 1.8 ± 0.4 days; P = 0.02) and reduce bacterial burden in the lung parenchyma (4.8 ± 0.8 versus 5.4 ± 9 log colony-forming unit [CFU]/g lung tissue; P = 0.01). Similarly, Berra et al.20 randomized 16 sheep to be intubated with a standard ETT or a silver sulfadiazine/chlorhexidine–coated ETT. After 24 hours of mechanical ventilation, all eight ETTs and ventilatory circuits in the control group were heavily colonized, and biofilm was found within the ETT. Pathogenic bacteria colonized the trachea and the lungs in five of eight sheep (up to 109 CFU/g). In the study group, seven of eight ETTs and ventilator circuits showed no growth and no biofilm; moreover, there was no bacterial growth in the lungs and bronchi, except for one bronchus in one sheep. Interestingly, the efficacy of silver-based coatings seems to decrease over time. Indeed, animal studies consistently reported no colonization and biofilm formation after 24 hours of mechanical ventilation. However, heavy ETT colonization was reported when studies were prolonged after 72 hours. To date, only one laboratory study145 has reported the absence of ETT colonization and biofilm formation following up to 168 hours of mechanical ventilation. In that study, the authors used ETTs internally coated with silver-sulfadiazine that were regularly cleaned with a novel concentric inflatable silicone device, the Mucus Shaver,146 devised to keep the ETT lumen free of mucus. The North American Silver-Coated Endotracheal Tube (NASCENT) randomized trial72 studied 1509 patients expected to require mechanical ventilation for more than 24 hours and randomized to be intubated with either a silver-coated or a conventional tube. The silver-coated ETT was associated with a lower incidence of microbiologically confirmed VAP (37/766 (4.8%) versus 56/743 (7.5%); P = 0.03), for a relative risk reduction of 35.9%. More importantly, the silver-coated tube had its greatest impact during the first 10 days of tracheal intubation. A retrospective cohort analysis by Afessa et al.,147 based on the NASCENT study, showed that the silver-coated ETT was associated with reduced mortality in patients with VAP (silver ETT versus control, 5/37 [14%] versus 20/56 [36%], P = 0.03), but mortality was higher in those without VAP (silver versus control, 228/729 [31%] versus 178/687 [26%], P = 0.03). In conclusion, there is promising evidence that ETTs coated with antimicrobial agents could reduce incidence of VAP. Nevertheless, clinicians should carefully consider benefits and limitations of these new ETTs and properly direct the use of silver-coated tubes to patients expected to be ventilated for longer periods of time and with higher associated risks for nosocomial pneumonia.
Shorr et al.148 analyzed cost-effectiveness of the silver-coated ETT as a preventive tool for VAP. Based on the NASCENT trial, the authors assumed a reduction in the relative risk of VAP from 35.9% to 24%. Assuming marginal VAP costs of $16,620 and costs of $90.00 for coated and $2.00 for uncoated ETTs, the authors found that savings per case of VAP prevented were $12,840.
Aspiration of Subglottic Secretions
Aspiration of colonized subglottic secretions through dedicated ETTs reduces hydrostatic pressure exerted above the cuff and potentially prevents macroleakage across the cuff. A meta-analysis149 comprising data from five studies and 896 patients has shown that subglottic secretion drainage reduced the incidence of VAP by nearly half (risk ratio [RR] = 0.51; 95% CI, 0.37-0.71), primarily by reducing early-onset pneumonia. Likewise, in the trial by Bouza et al.150 of 690 patients undergoing major cardiac surgery and on mechanical ventilation for more than 48 hours, the use of ETT tubes with aspiration of subglottic secretions was able to reduce incidence of VAP, median length of ICU stay, and antibiotic use and led to overall cost savings. In the multicenter trial by Lacherade et al.,151 333 patients were randomized to be intubated with either an ETT that allowed drainage of subglottic secretions or a standard ETT. Microbiologically confirmed VAP occurred in 14.8% of the patients in the treatment group, compared to 25.6% of the patients intubated with standard tube (P = 0.02). Importantly, this was the first trial reporting efficacy of subglottic secretions aspiration in reducing both early- and late-onset VAP in comparison to earlier studies (late onset VAP in 18.6% of patients in treatment group versus 33.0% of the patients in control group, P = 0.01). In more recent studies, aspiration of subglottic secretions was performed intermittently.135,151 The benefits of intermittent subglottic suction were similar to studies in which continuous aspiration of subglottic secretions was used. Hence, based on current evidence, intermittent aspiration (every 4-6 hours) is advisable to avoid potential risks for tracheal injury using continuous aspiration.152
Tracheostomy
Tracheostomized patients present the same risks for aspiration of pathogen-laden secretions pooled above the cuff153,154 as do orotracheally intubated patients. An observational study by Ibrahim et al.155 on 880 mechanically ventilated patients demonstrated an association between tracheostomy and higher incidence of VAP (adjusted OR, 6.71; 95% CI, 3.91-11.50; P < 0.001). Conversely, a case-control study by Nseir et al.156 on 354 patients mechanically ventilated for more than 7 days showed a lower rate of nosocomial pneumonia associated with tracheostomy (4.8 versus 9.2 episodes per 1000 ventilator days in patients with or without tracheostomy, respectively). Meta-analyses have assessed outcomes of early versus late tracheostomy.157–159 Unfortunately, all included studies were highly heterogeneous owing to differences in studied populations and no clear classification of “early” versus “late” tracheostomy. Nevertheless, the meta-analyses failed to demonstrate benefits of early tracheostomy on reduction of VAP incidence. To date, the latest multicenter randomized trial160 enrolled 419 mechanically ventilated patients to undergo either early or late tracheostomy. Patients in the early tracheostomy group underwent tracheostomy after a mean of 7 days, whereas patients in the late tracheostomy group underwent tracheostomy after a mean of 14 days. Although the authors found shorter length of mechanical ventilation and ICU stay with early tracheostomy, they were able to demonstrate only a trend toward a lower incidence of pneumonia and no difference in survival. Clinicians should consider that early tracheostomy may offer several benefits for mechanically ventilated patients: improved patient comfort, ability to communicate, capability for oral feeding, less need for sedation and analgesia, and reduced airway resistance in comparison to standard ETTs, which could be extremely important during the weaning period to shorten the duration of tracheal intubation.
Ventilator Circuit Management
Decreased frequency of ventilator circuit changes, replacement of heated humidifiers by heat and moisture exchangers, decreased frequency of heat and moisture exchanger changes, and closed suctioning systems have been tested as measures for preventing VAP. Results from clinical trials in adults161–167 and meta-analyses168,169 yield consistent evidence that routine change of the ventilator circuit does not decrease risks for VAP and costs. Therefore, circuits should not be changed unless the circuit is soiled or damaged. Importantly, inadvertent flushing of the contaminated condensate into the lower airways or nebulizers should be always avoided by careful emptying of ventilator circuits and water traps.170–172
Two meta-analyses assessed the effects of heated humidifiers (HH) and heat and moisture exchangers (HME) on prevention of nosocomial pneumonia. Kola et al.173 pooled data from nine clinical trials on 1378 patients and found that the use of HME decreased the rate of VAP (relative risk = 0.7; 95% CI = 0.50-0.94). Conversely, a meta-analysis by Siempos et al.174 including 13 studies on 2580 patients found no difference between HME and HH patients in the prevention of VAP and secondary outcomes such as ICU mortality, length ICU stay, duration of mechanical ventilation, or episodes of airway occlusion. Hence, to date there are no consistent data showing reduction in the incidence of VAP and better outcome using either HME or HH. Based on the ongoing controversy, neither humidification strategy can be recommended as a pneumonia prevention tool. However, it is rational to deliver inspiratory gases at body temperature or slightly below and at the highest relative humidity in order to prevent loss of heat and moisture from the airways and, more importantly, change in rheologic properties of secretions and impairment of mucociliary clearance.175 Therefore, the use of HH is indicated particularly in patients with hypothermia, prolonged mechanical ventilation, thick secretions, and chronic respiratory disorders. Finally, studies that have evaluated the effect of less frequent changes of heat and moisture exchangers on the development of VAP have found no increased risks.176–180 However, it is important to emphasize that when HMEs are used for prolonged periods of time, the technical performance of the devices should be periodically checked.
Closed tracheal suctioning systems have been introduced in clinical settings to avoid adverse events associated with ventilator disconnection during open tracheal suctioning and exogenous contamination of suction catheters entering the ETT. Three meta-analyses181–183 have compared the closed tracheal suction system to the open tracheal suction system in mechanically ventilated patients and found no benefits on VAP prevention. One meta-analysis182 evaluated nine randomized trials comprising 1292 patients and found no difference in the incidence of VAP between patients suctioned with closed or open systems (OR = 0.96, 95% CI 0.72-1.28). Moreover, data pooled from four of these nine studies showed a higher incidence of respiratory tract colonization in the group managed with a closed system (OR = 2.88, 95% CI 1.50-5.52).
The use of a saline solution instilled into the tracheal tube before tracheal suctioning remains controversial. Caruso et al.184 published a report on 262 patients randomized to receive either isotonic saline instillation before tracheal suctioning or no treatment. The authors found a lower incidence of microbiologically proven VAP (saline instillation versus no treatment: 23.5% versus 10.8%; P = 0.008), and no significant differences were found in secondary outcomes such as the incidence of ETT obstruction, pulmonary and lobar atelectasis, mortality, and duration of mechanical ventilation and ICU stay. Theoretically, in sedated patients in the semirecumbent position and with the internal surface of the ETT highly colonized, saline instillation may increase risks for translocation of pathogens into the airways, so current limited evidence suggests that routine saline instillation should not be recommended.
Body Position
Early studies clearly demonstrated that intubated patients are at higher risk for gastropulmonary aspiration when placed in the supine position (0 degrees) as compared with a semirecumbent position (45 degrees).185,186 One randomized trial187 demonstrated a reduction in the incidence of VAP in patients positioned in the semirecumbent position compared with patients treated completely supine. Moreover, the trial confirmed increased risk for VAP in patients enterally fed. A later randomized trial188 studied the feasibility of maintaining the head of the bed oriented 45 degrees during mechanical ventilation. This study found that patients were positioned on average only 28 degrees above horizontal, and no difference on VAP incidence was found. Thus, as strongly suggested by the American1 and European189 guidelines, intubated patients should be preferentially kept in the semirecumbent position (30-45 degrees) rather than supine (0 degrees) to prevent aspiration, especially when receiving enteral feeding.
Laboratory reports190,191 challenge the role of the semirecumbent position in patients with oropharyngeal colonization due to tracheal intubation or during the course of mechanical ventilation. Theoretically, in such patients a tracheal orientation above horizontal, as in the semirecumbent position, might facilitate aspiration across the tracheal tube cuff. Laboratory studies in animals190,191 consistently found that tracheal orientation and body position to avoid aspiration across the ETT cuff enhance mucus drainage and decrease risks for VAP; however, such results need to be confirmed in humans.
Rotating Bed
Normal healthy people change body position, even during sleep, every few minutes. Conversely, when critically ill patients are tracheally intubated and on mechanical ventilation, they are maintained in the supine, semirecumbent position for days with few or no changes in body position. Several ICU beds allow rotation of patients in the longitudinal axis from one lateral position to the other and seem to reduce extravascular lung water, improve ventilation-perfusion ratio, and enhance mobilization of airway secretions.192 Several studies have evaluated the effects of rotational therapy on VAP; however, most of these studies present limitations. For example, most studies used a clinical diagnosis of pneumonia, lack of standardization of VAP preventive measures, and included heterogeneity on the duration and type of rotation therapy. Three meta-analyses193–195 showed significant reduction in the incidence of VAP in patients undergoing rotation therapy, but they consistently failed to show beneficial effects on secondary outcomes such as duration of mechanical ventilation, length of stay, and mortality. An article by Staudinger et al.196 studied the effects of continuous lateral rotation therapy on the incidence of microbiologically confirmed VAP in 3 medical ICUs and found an incidence of 11% in the rotation group and 23% in the control group (P = 0.048), respectively. The authors also found that the duration of ventilation (8 ± 5 versus 14 ± 23 days, P = 0.02) and length of stay (25 ± 22 days versus 39 ± 45 days, P = 0.01) were significantly shorter in the rotation group. In conclusion, in patients at higher risk for prolonged immobilization and respiratory infection, continuous lateral rotation therapy should be considered as a feasible method exerting additive effects to other preventive measures for VAP.
Stress Ulcer Prophylaxis and Enteral Feeding
There is clear evidence that in intubated and mechanically ventilated patients, the stomach is often colonized by pathogens. Early studies showed that in tracheally intubated patients, gastric pH higher than 4 was consistently associated with gastric colonization.49,50 Alkalinization of gastric contents due to drugs for stress ulcer prophylaxis and continuous enteral nutrition were the main risk factors for gastric colonization.
In the ICU, stress ulcer prophylaxis is usually achieved with either sucralfate, histamine type 2 blockers (H2 blockers), or proton pump inhibitors (PPI). Sucralfate is the only treatment that potentially prevents stress GI ulceration without raising gastric pH. Several randomized studies have compared the effects of drugs that alkalinize gastric contents to sucralfate.52–57,197,198 Two studies compared either H2 blockers51 or sucralfate199 to placebo. Nine studies assessed gastric pH and colonization and consistently found lower pH and gastric colonization with regular use of sucralfate. Conversely, studies conducted by Bonten et al.54 and Thomason et al.56 found gastric luminal alkalinization and colonization, irrespective of the use of sucralfate. Finally, Eddleston et al.199 found no differences in gastric colonization when sucralfate was compared to placebo.
Randomized trials reported inconsistent results regarding stress ulcer prophylaxis and the incidence of VAP. In particular, early studies found a higher incidence of pneumonia in patients with alkalinized gastric contents,51–53 while more recent studies have not found such an association.54,55,124 Cook et al.55 studied 1200 patients randomized to receive either H2 blockers or sucralfate for stress ulcer prophylaxis. The authors found a higher risk for GI bleeding using sucralfate and no significant difference in VAP incidence, 19.1% and 16.2% in patients treated with H2 blockers or sucralfate, respectively. A recent meta-analysis200 on the efficacy and safety of PPIs in comparison with H2 blockers pooled data by seven randomized controlled trials on 936 patients and found no difference between PPI and H2-blocker therapy on the risk for pneumonia and ICU mortality. In conclusion, GI bleeding is a serious complication in critically ill patients at high risk for stress ulcers (i.e., patients with coagulopathy, need for prolonged mechanical ventilation, and history of GI ulceration or bleeding). The actual risk for VAP is unknown when accurate methods of enteral feeding (i.e., avoiding large gastric residual volumes) or other preventive measures are used in combination with stress ulcer prophylaxis. Therefore, clinicians must weigh the potential benefit of sucralfate (with potentially less VAP and more GI bleeding) versus H2 blockers/PPI (with potentially more VAP and less GI bleeding) and probably limit stress ulcer prophylaxis to high-risk patients.
Enteral nutrition has been considered a risk factor for the development of nosocomial pneumonia, mainly because of increased risks for alkalinization of gastric contents, gastro-esophageal reflux, and gastropulmonary aspiration. However, its alternative, parenteral nutrition, is associated with higher risks for catheter-related infections, complications of line insertions, higher costs, and loss of intestinal villous architecture, which may facilitate enteral microbial translocation. A large meta-analysis201 of 15 studies comprising 753 patients admitted to the ICU for trauma, head injury, burns, and abdominal surgery found a significantly lower incidence of infections and a reduced length of hospital stay associated with early enteral feeding. Conversely, studies in medical ICU patients have proven higher risk for VAP with early enteral feeding.202,203 Nonetheless, in a study by Artinian et al.202 the increased risk of VAP associated with early enteral feeding did not translate into an increased risk of death. Therefore, in medical ICU patients, the benefits of early nutrition should be balanced with associated increased risks for VAP.
A large number of studies have evaluated the risks for ICU-acquired pneumonia in patients randomized to either gastric or postpyloric feeding. Theoretically, many ICU patients present impaired gastric emptying; hence placement of the feeding tube beyond the pylorus has the potential to achieve nutrition goals without increased risks for gastropulmonary aspiration. A meta-analysis by Heyland et al.204 found that small-bowel feedings were associated with a lower incidence of pneumonia (RR, 0.77; 95% CI, 0.60-1.0); conversely, a meta-analysis by Ho et al.205 found no significant benefit on the risk of diarrhea, length of ICU stay, mortality, or risk of pneumonia. Therefore, ICU physicians should preferentially indicate postpyloric feeding in critically ill patients who have impaired gastric emptying.
Modulation of Oropharyngeal and Gastrointestinal Colonization
One of the most important factors in the pathogenesis of nosocomial pneumonia is the early shift of oral flora following tracheal intubation into a predominance of aerobic gram-negative pathogens. Therefore, extensive efforts have been devoted to modulating oropharyngeal flora of ICU patients and reducing the risks for aspiration of pathogens. Several antiseptics for oropharyngeal decontamination have been evaluated: chlorhexidine gluconate, iseganan, or povidone iodine; chlorhexidine has been the focus of most research. Chlorhexidine is a cationic chlorophenyl bis-biguanide antiseptic that has long been used as an inhibitor of dental plaque formation and gingivitis. Meta-analyses206–208 of studies assessing the benefits of chlorhexidine on reduction of VAP have shown good results, particularly in cardiothoracic ICU patients. Results in noncardiac ICU populations are more uncertain. Most of the aforementioned studies used chlorhexidine concentrations of 0.12% and 0.2%. However, recent studies in general ICU patients have demonstrated significant reductions in VAP rates when chlorhexidine concentration was increased to 2%.209,210 Therefore, oral decontamination with chlorhexidine should be routinely used, particularly in cardiothoracic patients. The usefulness of chlorhexidine as VAP preventive strategy in other ICU populations still requires more evidence before being put into general practice, but the use of higher chlorhexidine concentrations has showed promising results.
Since the original studies published by Stoutenbeek and coworkers,211,212 selective decontamination of the digestive tract (SDD) has been used as a preventive strategy for nosocomial pneumonia for almost 3 decades. SDD comprises a combination of nonabsorbable antibiotics against gram-negative pathogens (i.e., tobramycin, polymyxin E) plus either amphotericin B or nystatin administered into the GI tract in order to prevent oropharyngeal and gastric colonization with aerobic gram-negative bacilli and Candida spp., while preserving the anaerobic flora. Some regimens include a short course of systemic antibiotics (most commonly cefotaxime) in addition to nonabsorbable GI antibiotics. Randomized clinical trials213–215 and meta-analyses216–218 confirm results of earlier studies and suggest that SDD confers protection against pneumonia. Interestingly, SDD is the only preventive strategy for VAP that has shown reduction of mortality rates. A clinical trial by de Smet et al.214 evaluated the effectiveness of SDD and selective oropharyngeal decontamination in a crossover study using cluster randomization in 13 ICUs and applied SDD, oropharyngeal decontamination, or standard care in random order for 6 months. SDD consisted of 4 days of intravenous cefotaxime and topical application of tobramycin, colistin, and amphotericin B in the oropharynx and stomach. Oropharyngeal decontamination consisted of oropharyngeal application only of the same antibiotics. The authors enrolled a total of 5939 patients. Post hoc analysis in a random-effects logistic-regression model found that the odds ratio for death at day 28 in the oropharyngeal decontamination and SDD groups, as compared with the standard-care group, were 0.86 (95% CI, 0.74-0.99, P = 0.045) and 0.83 (95% CI, 0.72-0.97, P = 0.02), respectively.
It is important to acknowledge that prophylactic use of antibiotics to modulate GI flora may potentially increase risks for antibiotic resistance, and results from randomized clinical trials still remain controversial; moreover, standard SDD is aimed at preventing overgrowth of aerobic gram-negative bacteria, yet it could increase colonization by gram-positive bacteria such as MRSA and Enterococcus spp. A large Dutch randomized controlled trial213 demonstrated that carriage of multiresistant gram-negative bacteria was actually reduced in patients who had SDD, compared with controls (RR, 0.61; 95% CI, 0.46-0.81). Overall, emergence of resistance of gram-negative bacteria was consistently a rare event with SDD. Recently, Bonten’s group reported data219 on bacterial ecology in 13 ICUs that participated in their previous study of SDD.214 Rectal and respiratory samples were analyzed once monthly in all ICU patients and showed that during SDD, average proportions of patients with intestinal colonization with GNB resistant to either ceftazidime, tobramycin, or ciprofloxacin increased from 5%, 7%, and 7%, respectively to 15%, 13%, and 13% post intervention (P <0.05). During SDD/SOD, resistance levels in the respiratory tract were not more than 6% for all three antibiotics but increased gradually (for ceftazidime, P <0.05 for trend) during intervention and to levels of 10% or more for all three antibiotics post intervention (P <0.05).
Early attempts at VAP prophylaxis using parenteral antibiotics were unsuccessful.222 Only one study223 showed that a short course of cefuroxime upon emergent intubation and during 48 hours following intubation in patients with structural coma or severe burns was an effective prophylactic strategy to decrease the VAP rate. However, routine use of parenteral antibiotics is not recommended until more data become available.
Several clinical trials have attempted to modify GI and oropharyngeal growth of pathogens through the use of probiotics. Probiotics are microorganisms that can be administered either as individual strains or in various combinations. These microorganisms are often administered with nondigestible food ingredients that facilitate bacterial growth and/or activity (prebiotics); products containing both probiotics and prebiotics are called synbiotics. A meta-analysis224 on the effects of probiotics, pooling data from five randomized controlled trials in 689 patients, showed a lower incidence of VAP. The use of probiotics is a promising strategy for VAP; however, additional evidence is required before recommending its use in all mechanically ventilated patients, particularly owing to the heterogeneity of previous studies.
 Diagnosis
Diagnosis
The diagnosis of VAP is a controversial issue.225,226 Clinical signs suggestive of pneumonia in non-ICU patients, such as fever, tachycardia, and leukocytosis, are too nonspecific to be of diagnostic value for ventilated patients.103,227,228 Moreover, the chest radiograph is often difficult to interpret in intubated, critically ill patients. Indeed, when the chest radiograph is normal, pneumonia may not be completely ruled out because of the limited technical quality. Also, chest radiographs may not reveal subtle lung infiltrates that may be detected with computed tomography (CT) scans, particularly in patients with COPD.229 When infiltrates are evident, it is often difficult to differentiate among cardiogenic and noncardiogenic pulmonary edema, pulmonary contusion, atelectasis, and pneumonia.
Few studies have examined the accuracy of portable chest radiographs in the diagnosis of pneumonia in the ICU.228,230–232 In mechanically ventilated patients with autopsy-proven pneumonia, no single radiographic sign had a diagnostic accuracy greater than 68%.231 The presence of air bronchograms or alveolar opacities in patients without ARDS correlated with pneumonia; however, no such correlation was found for patients with ARDS. Many causes other than pneumonia can explain asymmetrical consolidation in patients with ARDS, and marked heterogeneity of radiographic abnormalities has also been reported in patients with uncomplicated ARDS.233 A clinical study showed the presence of lung infection in only 42% of the patients with clinically suspected VAP, with frequent occurrence of multiple infectious and noninfectious processes,234 indicating a poor correlation between clinical signs and bacteriologic demonstration of VAP.
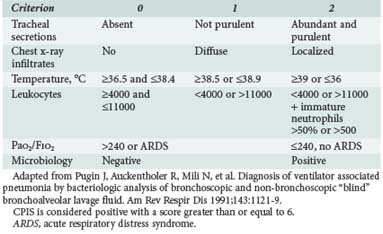
The Clinical Pulmonary Infection Score (CPIS) is based on six clinical assessments—temperature, blood leukocyte count, volume and purulence of tracheal secretions, oxygenation, pulmonary radiographic findings, and semiquantitative culture of tracheal aspirate—each worth between 0 and 2 points (Table 67-1).235 The CPIS showed a good correlation (r = 0.84, P < 0.0001) with quantitative bacteriology of BAL samples. Moreover, a value ≥ 6 was the threshold to accurately identify patients with pneumonia. Yet the value of CPIS remains to be validated in a large prospective study, especially in patients with bilateral pulmonary infiltrates.
The presence of bacteria in the lower airways of intubated patients is not sufficient to diagnose true lung infection. The tracheobronchial tree and the oropharynx of mechanically ventilated patients are frequently colonized by enteric gram-negative bacilli.1,37,61 Cultures of endotracheal aspirate from patients with respiratory failure and histologically documented pneumonia, simultaneously obtained from the trachea and lung tissue, agreed in only 40% of cases, with a 82% sensitivity and 27% specificity.236 Similarly, another study demonstrated that only 23% of colonized patients subsequently developed nosocomial pneumonia.37
Qualitative cultures of endotracheal aspirates have a high percentage of false-positive results due to frequent bacterial colonization of the proximal airways in ICU patients. Conversely, quantitative culture techniques of endotracheal aspirates may have an acceptable overall diagnostic accuracy. When patients develop pneumonia, pathogens are present in the lower respiratory tract secretions at concentrations of at least 105 to 106 CFU/mL,237–240 and contaminants are generally present at less than 104 CFU/mL. The current diagnostic threshold proposed for tracheal aspirates is 106 CFU/mL. Similarly, PSB collects between 0.001 and 0.01 mL of secretions; therefore the presence of more than 103 bacteria in the originally diluted sample (1 mL) actually represents 105 to 106 CFU/mL in pulmonary secretions. Finally, 104 CFU/mL is considered the cutoff for BAL, which collects 1 mL of secretions in 10 to 100 mL of effluent.
Results of quantitative endotracheal aspirate cultures cannot always be used to accurately predict which microorganisms found in the proximal trachea are actually present in the lungs. In one study,240 only 40% of the microorganisms cultured in endotracheal aspirate samples coincided with those obtained from PSB specimens. Also, when quantitative cultures of different lower respiratory tract specimens were compared with postmortem quantitative lung biopsy cultures, all techniques for detecting VAP were of limited value.241
A major problem in the management of patients with suspicion of VAP is the use of antibiotics. The indiscriminate administration of antimicrobial agents for patients in the ICU may contribute to the emergence of multiresistant pathogens and increase the risk of severe superinfections with increased morbidity and mortality, as well as expose the patient to antibiotic-related adverse effects and higher costs.242,243 On the other hand, correct and prompt treatment of pneumonia results in better patient survival.103,244,245 Inappropriate therapy is strongly associated with worse survival.56,246 Inadequate empirical antibiotic treatment initiated before obtaining the results of cultures from respiratory secretions was associated with greater hospital mortality rate compared with an antibiotic regimen that provided adequate antimicrobial coverage based on microbiologic culture results.247–251 However, the choice of the initial antibiotic treatment is often difficult due to several factors: (1) high frequency of resistant organisms in ICU patients previously treated with antibiotics,252 (2) high risks for MDR pathogens in late-onset pneumonia occurring more than 7 days after initiation of mechanical ventilation,4 and (3) frequent isolation of multiple organisms from pulmonary secretions when the sampling technique is not specific enough to differentiate colonizing from infecting pathogens.253–255
The importance of a microbiological diagnosis of VAP is aimed not only at determining whether a patient has pneumonia but also in optimizing antimicrobial treatment.189 To allow narrowing or de-escalation of the initial empirical treatment, antimicrobial susceptibility data should be available as soon as possible. Recently, several alternative techniques to microbial cultures have been developed to achieve a more rapid and accurate diagnosis of nosocomial pneumonia. Among the most recent improvements, the direct antibiogram using E-test strips applied directly to respiratory tract samples have proved to be both reliable and effective and can anticipate the availability of antimicrobial susceptibility data by more than 48 hours.256,257 Other advances include clinical application of quantitative polymerase chain reaction (qPCR) for direct measurement of microbial genetic material in patient specimens.258 The mecA gene that confers resistance to methicillin in S. aureus can be detected using qPCR; qPCR of mecA in mini-BAL samples was able to rapidly and accurately diagnose MRSA pneumonia.259
 Diagnostic Strategies for Hospital-Acquired Pneumonia
Diagnostic Strategies for Hospital-Acquired Pneumonia
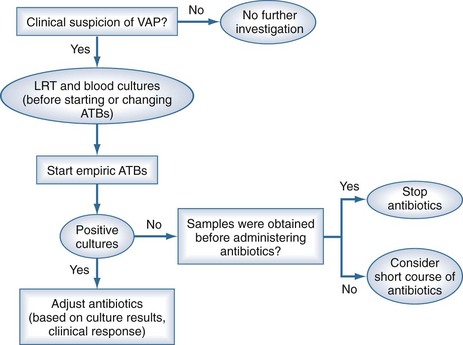
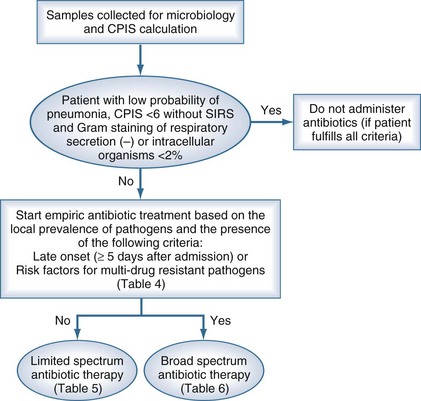
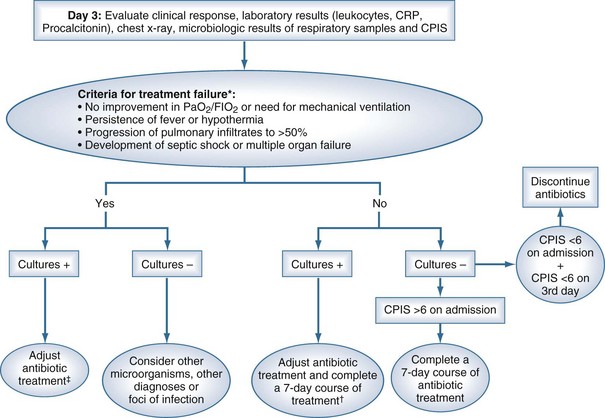
Two diagnostic algorithms can be used following clinical suspicion of nosocomial pneumonia. The clinical approach recommends treating every patient with suspicion of having a pulmonary infection with new antibiotics even when the likelihood of infection is low (Figure 67-2). However, samples of respiratory secretions such as endotracheal aspirate or sputum should be obtained before the initiation of antibiotic treatment. In this strategy, the selection of appropriate empirical therapy is based on risk factors and local resistance patterns. The etiology of pneumonia is defined by semiquantitative cultures of endotracheal aspirates or sputum, with initial microscopic examination of the Gram stain. Antimicrobial therapy is adjusted according to culture results or clinical response. Semiquantitative culture of tracheal aspirates has the advantage that no specialized microbiologic techniques are required, and the sensitivity is high. This clinical strategy provides antimicrobial treatment to the majority of the patients with suspicion of HAP and yields a low rate of false negatives. Still, if the tracheal aspirate culture does not demonstrate pathogens, and the patient has not received new antibiotics within the previous 72 hours, the diagnosis of pneumonia is unlikely.251 This strategy is useful in centers where bronchoscopic methods are not always available for sampling the lower respiratory tract. The main drawback of this strategy is that the high sensitivity of semiquantitative cultures of tracheal aspirates leads to overestimation of the incidence of nosocomial pneumonia, hence antibiotic treatments can be administered to patients without pneumonia.
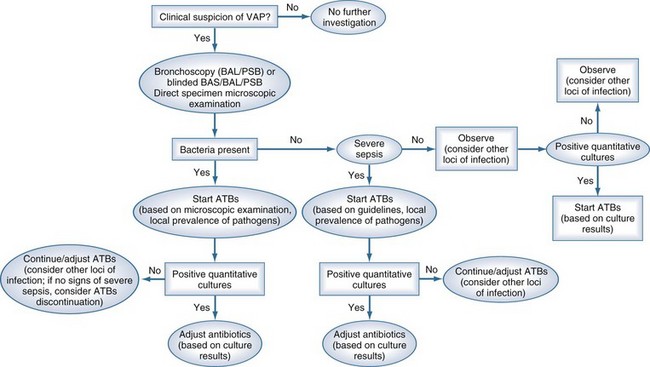
The bacteriologic strategy is based on the results of quantitative cultures of lower respiratory secretions (Figure 67-3). The procedure used to collect the samples (endotracheal aspirate, BAL, or PSB) may be invasive (bronchoscopic) or noninvasive (blind procedures). Specific threshold cutoffs for each test to discriminate between colonizing microorganisms and those producing infection are used in this strategy. The cutoff point used for endotracheal aspirates is 106 CFU/mL, 104 CFU/mL for BAL, and 103 CFU/mL for PSB. The bacteriologic strategy attempts to accurately identify patients with true nosocomial pneumonia so that only infected patients are treated and clinical outcomes are improved.84,250,255,260 Such a strategy reduces risks for overuse of antibiotics, since quantitative cultures yield fewer microorganisms above the threshold in comparison to semiquantitative cultures. Among the disadvantages of the bacteriologic strategy is the possibility of obtaining false-negative results that lead to delayed antibiotic treatment in a patient with pneumonia. Moreover, results using the microbiology strategy may lack of reproducibility, and often no microbiological information is available at the time of initiation of empirical antibiotic therapy.
Evaluation of Diagnostic Strategies
Four randomized controlled trials250,261–263 have assessed the impact of diagnostic strategies on antibiotic use and outcome in patients with clinical suspicion of nosocomial pneumonia. In three small studies,250,261,262 invasive diagnostic techniques resulted in a greater number of antibiotic changes than noninvasive techniques; however, no differences in mortality and morbidity were found when either invasive (PSB and/or BAL) or noninvasive (quantitative endotracheal aspirate cultures) techniques were used. By contrast, a larger trial263 showed a reduction in mortality, better Sequential Organ Failure Assessment (SOFA) score at follow-up, reduced use of antibiotics, and increased number of antibiotic-free days using invasive diagnostic techniques. This study was limited, however, by the use of qualitative cultures of tracheal aspirates, thereby limiting comparison with other clinical trials. A meta-analysis by Shorr et al.264 pooled data from these randomized studies on 628 patients and found that overall, an invasive approach did not alter mortality (OR, 0.89; 95% CI, 0.56-1.41). Invasive testing, though, affected antibiotic utilization (OR for change in antibiotic management after invasive sampling, 2.85; 95% CI, 1.45-5.59). Importantly, it should be realized that diagnostic cultures of pulmonary secretions after initiation of new antibiotic therapy in patients with suspicion of HAP can lead to a high number of false-negative results, irrespective of the sampling technique. In this clinical setting, a lower threshold should be used to define a positive quantitative result.265,266 Nevertheless, it is strongly recommended that diagnostic sampling of the respiratory tract be obtained before starting any new antibiotic or changing previous antimicrobial therapy.
A clinical trial267 compared quantitative culture of BAL fluid and culture of endotracheal aspirate in critically ill patients with suspected VAP. This study was part of a larger 2-by-2 factorial design also comparing empirical antimicrobial monotherapy (a carbapenem) and combination therapy (a carbapenem plus a fluoroquinolone). A total of 740 patients in 28 ICUs in Canada and the United States were enrolled, and the authors found no difference in the 28-day mortality rate between the BAL group and the endotracheal aspiration group (18.9% and 18.4%, respectively; P = 0.94). The BAL group and the endotracheal aspiration group also had similar rates of targeted therapy (74.2% and 74.6%, respectively; P = 0.90), days alive without antibiotics (10.4 ± 7.5 and 10.6 ± 7.9; P = 0.86), and maximum organ-dysfunction scores (mean [±SD], 8.3 ± 3.6 and 8.6 ± 4.0; P = 0.26). The two groups did not differ significantly in the length of stay in the ICU or hospital. Unfortunately, at least 40% of the screened patients were excluded because they were at risk for colonization with Pseudomonas spp. or MRSA or were immunosuppressed. Therefore, translation of these findings into clinical practice is a major concern, because many ICU patients evaluated for suspected VAP fall into these categories.
Practical Implementation of a Diagnostic Strategy in Suspected Ventilator-Associated Pneumonia
In practice, the development of local clinical guidelines can combine both clinical and bacteriologic strategies (Table 67-2). The diagnostic protocol begins with clinical suspicion of nosocomial respiratory infection (Figure 67-4). In mechanically ventilated patients, the presence of an infiltrate on chest radiograph differentiates between the possible presence of pneumonia and tracheobronchitis. The next step is to sample the lower respiratory tract (see Table 67-2) to identify the causative microorganism. Sampling should be performed before initiation or change of antibiotic treatment, even though it should not delay the administration of antibiotic therapy, particularly for septic patients. Respiratory tract specimens can be obtained through expectoration, bronchial aspirate, BAL, or PSB. The latter two techniques can be performed with bronchoscopy or blindly. Several other samples should also be collected, as noted in Table 67-2. With clinical suspicion of pneumonia, CPIS235 should be calculated to improve objective assessment of the clinical parameters (see Table 67-1).
TABLE 67-2 Diagnostic Protocol to Combine Clinical and Bacteriologic Strategies for the Diagnosis of Ventilator-Associated Pneumonia
* Samples should be sent to the microbiology department, or if not available, maintained in refrigerator at 4°C (only respiratory samples) for a maximum of 1 hour for Gram staining, intracellular organism counting (only in BAL and mini-BAL), and quantitative cultures. The collection of lower respiratory secretion samples should not delay the initiation of empirical treatment in patients with severe sepsis.
** These techniques may be performed by bronchoscopy or blind procedures. Quantitative cultures are performed with the respiratory secretions obtained by BAS, BAL, or PBS. The cutoff count to diagnose pneumonia is the following: BAS 106 CFU/mL; BAL 104 CFU/mL, and PSB 103 CFU/mL.
 Treatment
Treatment
Likely Etiologic Microorganisms
The microorganisms most frequently isolated from the bronchial secretions of patients with VAP are S. aureus and P. aeruginosa, comprising around 50% of the isolates. These are followed, in order of frequency, by Enterobacteriaceae (E. coli, Klebsiella spp., Enterobacter spp., Citrobacter spp., Serratia spp., and Proteus spp.) representing 15%, nonfermentative gram-negative bacilli other than P. aeruginosa (Acinetobacter spp., Stenotrophomonas spp., and Burkholderia spp.) in 10%, and H. influenzae and S. pneumoniae (among others) in the remaining cases.103
The microorganisms causing VAP generally come from the oropharyngeal flora of the patient. Underlying chronic diseases,268 specific risk factors, acute inflammatory processes, and factors specific to each hospital or ICU can facilitate abnormal bacterial colonization of the oropharynx and may predispose patients to infection with specific organisms.1,269 Therefore, the selection of initial antimicrobial therapy must be tailored to the local prevalence of pathogens and antimicrobial patterns of resistance of each institution.88,270 Healthy subjects rarely have significant colonization with gram-negative bacilli in the oropharynx, even after prolonged exposure to the hospital or ICU environment. Conversely, elderly individuals and patients with comorbidities and/or previous exposure to antibiotics may be at increased risk for abnormal oropharyngeal colonization.271 The dynamics of change of oropharyngeal flora during hospital stay can be described as follows (Figure 67-5):
Choice of Empirical Antimicrobials Likely to be Active Against Causative Microorganisms
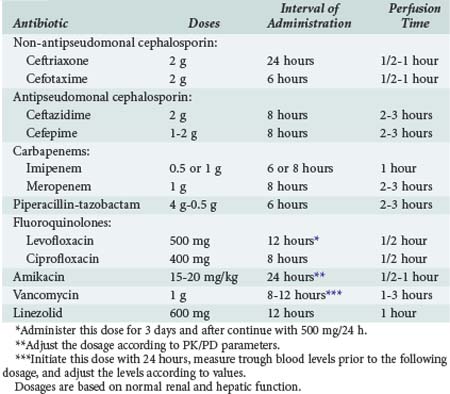
The latest guidelines of the American Thoracic Society and Infectious Diseases Society of America (ATS/IDSA) for the management of adult patients with nosocomial pneumonia1 recommend that the selection of empirical antibiotic therapy for each patient should be based on the timing of onset and presence of risk factors for MDR pathogens. Risk factors for MDR pathogens defined by the ATS/IDSA guidelines are summarized in Box 67-2. An algorithm for the initial management of patients with nosocomial respiratory infection and selection of appropriate antimicrobials is shown in Figure 67-6. The antibiotics recommended by the current ATS/IDSA guidelines are shown in Tables 67-3 and 67-4. Adequate dosing of antibiotics for empirical therapy is summarized in Table 67-5. Broad-spectrum empirical antibiotic therapy should be rapidly deescalated as soon as microbiological data become available in order to limit the emergence of resistance in the hospital. In brief, initial empiric therapy should be based on patient’s risk of colonization by MDR organisms and managed as follows:
Box 67-2
Risk Factors for Multidrug-Resistant Pathogens Causing Nosocomial Pneumonia
Adapted from American Thoracic Society. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med 2005;171:388-416.
TABLE 67-3 Initial Empirical Antibiotic Treatment in Nosocomial and Ventilator-Associated Pneumonia of Early Onset in Patients Without Risk Factors for Infection by Multidrug-Resistant Pathogens
| Probable Microorganism | Recommended Antibiotic |
|---|---|
| Streptococcus pneumoniae Haemophilus influenzae Methicillin-sensitive Staphylococcus aureus Enteric gram-negative bacilli Escherichia coli Klebsiella pneumoniae Enterobacter spp. Proteus spp. Serratia marcescens |
Ceftriaxone or Levofloxacin, moxifloxacin or Ampicillin/sulbactam or Ertapenem |
Adapted from American Thoracic Society. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med 2005;171:388-416.
TABLE 67-4 Initial Empirical Antibiotic Treatment for Nosocomial and Ventilator-Associated Pneumonia of Late Onset or in Patients with Risk Factors for Infection by Multidrug-Resistant Pathogens and Any Degree of Severity
| Probable Microorganism | Combined Antibiotic Treatment |
|---|---|
| Microorganisms from Table 67-3 plus: Pseudomonas aeruginosa Klebsiella pneumoniae (ESBL+)† Acinetobacter spp.† Other nonfermenting gram-negative bacilli Methicillin-resistant Staphylococcus aureus (MRSA) Legionella pneumophila‡ |
Antipseudomonal cephalosporin (ceftazidime or cefepime)* or Carbapenem (imipenem, meropenem)* or β-lactam/β-lactamase inhibitor (piperacillin-tazobactam)* + Antipseudomonal fluoroquinolone (ciprofloxacin, levofloxacin)** or Aminoglycoside** (amikacin) ± Linezolid or vancomycin*** |
ESBL, extended-spectrum β-lactamase; GNB, gram-negative bacilli.
* The choice of β-lactam is made as follows: patients who have not received any antipseudomonal β-lactam within the last 30 days should be administered piperacillin-tazobactam or an antipseudomonal cephalosporin. Patients who have received these drugs should be given empirical therapy with a carbapenem. Patients with infection by ESBL-producing microorganisms should be treated with carbapenem regardless of the results of the antibiogram.
** For combined empirical therapy for multidrug-resistant GNB, an antipseudomonal fluoroquinolone should be used in cases of renal failure or concomitant use of vancomycin. In other settings, combined empirical therapy is initiated with amikacin and maintained for a 5-day period.
*** Empirical therapy aimed against MRSA is initiated in patients with proven colonization (ψ), previous infection by this microorganism, or implementation of MV for more than 6 days. The antibiotic of choice is either vancomycin (except in patients allergic to this medication, with creatinine values ≥ 1.6 mg/dL, or in patients presenting signs of empirical treatment failure after 48 hours of antibiotic therapy) or linezolid. (Ψ) For epidemiologic surveillance, nasal and perineal cultures should be performed on admission and at 1-week intervals thereafter while remaining in the ICU.
† If an ESBL+ strain such as K. pneumoniae or Acinetobacter spp. is suspected, a carbapenem is the first choice.
‡ If L. pneumophila is suspected, the combination antibiotic regimen should include a macrolide (e.g., azithromycin), or a fluoroquinolone (e.g., ciprofloxacin, levofloxacin) should be used rather than an aminoglycoside.
Antimicrobial Therapy in Special Situations
The addition of antibiotics with activity against MRSA depends on the local prevalence of MRSA, the presence of risk factors for MRSA, and the severity of infection. In geographic areas with documented presence of community-acquired MRSA, severe pneumonia with radiologic images of cavitation and presence of gram-positive cocci in respiratory secretions, empirical treatment with linezolid or vancomycin may be appropriate. Recently an outbreak of MRSA and linezolid-resistant S. aureus (LRSA) was reported in an intensive care department of a 1000-bed tertiary care university teaching hospital in Madrid, Spain, and was associated with nosocomial transmission and extensive usage of linezolid.272 In that report, 12 patients with LRSA were identified, and a mortality of 50% was reported. CFR-mediated linezolid resistance was demonstrated in all isolates. Tigecycline may be a useful alternative in this setting, although clinical experience is scanty.
Modifications of Therapy and Duration of Treatment
A suggested flowchart for follow-up of patients with nosocomial pneumonia is shown in Figure 67-7. After 72 hours, treatment should be adjusted based on microbiological results. The initial β-lactam should be continued if the microorganism is susceptible to the empirical β-lactam originally prescribed. If not, another β-lactam, possibly a carbapenem, may be introduced. The empirical antibiotic against MRSA should be discontinued if the presence of this pathogen is not confirmed by cultures. Discontinuation of the fluoroquinolone and especially the aminoglycoside should be considered after 3 to 5 days of treatment. The bactericidal activity of aminoglycosides and fluoroquinolones leads to a rapid reduction in the bacterial load during the first days of treatment. After this time, monotherapy may be sufficient. This approach would decrease emergence of resistant mutants and minimize nephrotoxicity caused by aminoglycosides.
The majority of infections can be effectively treated with regimens lasting up to 8 days. Four situations may justify prolonged treatment: (1) infection by microorganisms that may multiply in the cellular cytoplasm, such as Legionella spp.; (2) the presence of biofilms or prosthetic devices; (3) the development of tissue necrosis, the formation of abscesses, or infection within a closed cavity, such as empyema; and (4) persistence of the original infection (such as perforation or endocarditis). If the clinical course from the pneumonia is favorable—as defined by defervescence, improvement in PaO2/FIO2, and reduction in C-reactive protein (CRP) levels within the first 3 to 5 days of antimicrobial therapy—treatment may be withdrawn after the completion of 7 days. If the causative microorganism is a nonfermenting gram-negative bacillus, the treatment can be extended beyond 14 days. A large prospective, multicenter, randomized trial study comparing the efficacy of 8-day and 15-day antibiotic regimens for treating VAP suggested that an 8-day regimen reduces antibiotic use and decreases the emergence of multiresistant bacteria in the lung, without modification of the prognosis.273 However, this study observed that in cases of pneumonia produced by nonfermenting gram-negative bacilli, eradication of these microorganisms from bronchial secretions was lower with the shorter regimen.273 On the other hand, the 14-day treatment regimen was associated with a greater trend to colonization by multiresistant flora and a greater frequency of reinfection.274
In patients with clinical suspicion of ICU-acquired pneumonia who have a CPIS lower than 6 on the third day of treatment, the treatment may be withdrawn. In this setting, the patient probably does not have pneumonia, or the pneumonia is sufficiently mild such that prolonged antibiotic treatment is not required.235
 Implementation of Guidelines
Implementation of Guidelines
There is evidence that appropriate and timely antibiotic treatment can improve outcome in patients with VAP. Guidelines can play a significant role in accomplishing this aim. However, to significantly reduce morbidity and mortality, guidelines should (1) be implemented in specific clinical settings and (2) guide appropriate antibiotic treatment, tailored on specific risk factors for acquiring MDR pathogens. Although guideline implementation is difficult to achieve and requires effort, there is evidence demonstrating that management and outcome of patients is improved. Our report275 on the validation of the current 2005 ATS/IDSA guidelines demonstrates worse microbial prediction, in comparison to previous guidelines, in patients considered to be at low risk for acquiring MDR pathogens, and similar low prediction for fungi. Those data suggest the need for further research to improve the accuracy of future guidelines and, ultimately, the outcome of patients with VAP.
Although guideline-recommended strategies may provide significant benefits for patients, implementation is often hard to achieve.276,277 Guidelines can be translated into clinical practice via education and behavioral changes of healthcare personnel, design and distribution of dedicated protocols, and frequent audit and feedback. Few studies have assessed the effects of the implementation of guidelines on outcomes. Soo-Hoo et al.277 developed hospital guidelines to manage patients with severe hospital-acquired pneumonia based on the 1996 ATS guidelines.269 Recommendations designed by a multidisciplinary taskforce focused particularly on empirical antibiotic treatment and specimen collection. A strict campaign to educate healthcare personnel was undertaken, and progress in guidelines implementation was frequently reviewed. After the guidelines were introduced into clinical settings, the authors found that adequate antibiotic therapy was administered in more than 81% of the patients with pneumonia, compared with 46% before implementation (P < 0.01). Moreover, a lower mortality at 14 days was found after guidelines implementation (P = 0.03). Similarly, Ibrahim and co-workers developed a protocol to provide appropriate initial antibiotic treatment for patients with VAP and encouraged a 7-day course of treatment.270 The authors adapted the 1996 guidelines to the microbial patterns of their institution. Patients more often received adequate antimicrobial treatment after guidelines implementation (94%, in comparison to 48% before implementation; P < 0.001). Length of treatment was reduced by 6 days, and a second episode of VAP was less likely to occur after implementation.
Focus of Guidelines on Prevention and Management of VAP to Improve Outcome
Preventive approaches to VAP have focused on reducing cross-transmission, pulmonary aspiration across the ETT cuff, and reducing bacterial load in the oropharynx. Several strategies with proven efficacy in reducing morbidity and mortality related to mechanical ventilation have been grouped by the Institute for Healthcare Improvement as the “Ventilator Bundle.” Although the bundle was not designed to specifically prevent VAP, interventions such as semirecumbent position187 and sedation vacation119,124 have proven to significantly reduce VAP rates. The bundle was later modified to specifically address VAP by adding two strategies: daily oral use of chlorhexidine and subglottic secretion drainage. A 2-year multifaceted program to prevent VAP with eight targeted measures based on well-recognized published guidelines showed that increasing compliance with these measures was followed by an important reduction in the incidence of VAP.111
Key Points
American Thoracic Society. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171:388-416.
Valles J, Pobo A, Garcia-Esquirol O, Mariscal D, Real J, Fernandez R. Excess ICU mortality attributable to ventilator-associated pneumonia: the role of early vs. late onset. Intensive Care Med. 2007;33:1363-1368.
Kollef MH, Afessa B, Anzueto A, et al. Silver-coated endotracheal tubes and incidence of ventilator-associated pneumonia: the NASCENT randomized trial. JAMA. 2008;300:805-813.
Giantsou E, Liratzopoulos N, Efraimidou E, et al. Both early-onset and late-onset ventilator-associated pneumonia are caused mainly by potentially multiresistant bacteria. Intensive Care Med. 2005;31:1488-1494.
Schweickert WD, Gehlbach BK, Pohlman AS, Hall JB, Kress JP. Daily interruption of sedative infusions and complications of critical illness in mechanically ventilated patients. Crit Care Med. 2004;32:1272-1276.
Lorente L, Lecuona M, Alejandro J, Maria M, Antonio S. Influence of an endotracheal tube with polyurethane cuff and subglottic drainage on pneumonia. Am J Respir Crit Care Med. 2007;176:1979-1983.
Lacherade JC, De JB, Guezennec P, et al. Intermittent subglottic secretion drainage and ventilator-associated pneumonia: a multicenter trial. Am J Respir Crit Care Med. 2010;182:910-917.
Liberati Liberati A, D’Amico R, Pifferi S, Torri V, Brazzi L. Antibiotic prophylaxis to reduce respiratory tract infections and mortality in adults receiving intensive care. Cochrane Database Syst Rev 2009;4:CD000022.
Canadian Critical Care Trials Group. A randomized trial of diagnostic techniques for ventilator-associated pneumonia. N Engl J Med. 2006;355:2619-2630.
Ferrer M, Liapikou A, Valencia M, et al. Validation of the American Thoracic Society–Infectious Diseases Society of America Guidelines for hospital-acquired pneumonia in the intensive care unit. Clin Infect Dis. 2010;50:945-952.
1 American Thoracic Society. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated Pneumonia. Am J Respir Crit Care Med. 2005;171:388-416.
2 Murray AE, Chambers JJ, van Saene HKF. Infections in patients requiring ventilation in intensive care: application of a new classification. Clin Microbiol Infect. 1998;4(2):94-102.
3 Langer M, Cigada M, Mandelli M, et al. Early-onset pneumonia: a multicenter study in intensive care units. Intensive Care Med. 1987;13:342-346.
4 Trouillet JL, Chastre J, Vuagnat A, et al. Ventilator-associated pneumonia caused by potentially drug-resistant bacteria. Am J Respir Crit Care Med. 1998;157:531-539.
5 Torres A, Rello J. Update in community-acquired and nosocomial pneumonia 2009. Am J Respir Crit Care Med. 2010;181(8):782-787.
6 Alcon A, Fabregas N, Torres A. Pathophysiology of pneumonia. Clin Chest Med. 2005;26(1):39-46.
7 Sopena N, Sabria M. Multicenter study of hospital-acquired pneumonia in non-ICU patients. Chest. 2005;127:213-219.
8 Cook DJ, Walter SD, Cook RJ, et al. Incidence of and risk factors for ventilator-associated pneumonia in critically ill patients. Ann Intern Med. 1998;129:433-440.
9 Valles J, Pobo A, Garcia-Esquirol O, Mariscal D, Real J, Fernandez R. Excess ICU mortality attributable to ventilator-associated pneumonia: the role of early vs late onset. Intensive Care Med. 2007;33(8):1363-1368.
10 Bonten MJ, Kollef MH, Hall JB. Risk factors for ventilator-associated pneumonia: from epidemiology to patient management. Clin Infect Dis. 2004;38:1141-1149.
11 Luna CM, Aruj P, Niederman MS, et al. Appropriateness and delay to initiate therapy in ventilator-associated pneumonia. Eur Respir J. 2006;27(1):158-164.
12 Craven DE, De Rosa FG, Thornton D. Nosocomial pneumonia: emerging concepts in diagnosis, management, and prophylaxis. Curr Opin Crit Care. 2002;8(5):421-429.
13 Belson TP. Cuff induced tracheal injury in dogs following prolonged intubation. Laryngoscope. 1983;93(5):549-555.
14 Homi J, Notcutt W, Jones JJ, Sparke BR. A method for comparing endotracheal cuffs. A controlled study of tracheal trauma in dogs. Br J Anaesth. 1978;50(5):435-444.
15 Nordin U. The trachea and cuff-induced tracheal injury. An experimental study on causative factors and prevention. Acta Otolaryngol Suppl. 1977;345:1-71.
16 Dullenkopf A, Gerber A, Weiss M. Fluid leakage past tracheal tube cuffs: evaluation of the new Microcuff endotracheal tube. Intensive Care Med. 2003;29:1849-1853.
17 Inglis TJ, Millar MR, Jones JG, Robinson DA. Tracheal tube biofilm as a source of bacterial colonization of the lung. J Clin Microbiol. 1989;27:2014-2018.
18 Inglis TJ, Lim TM, Ng ML, Tang EK, Hui KP. Structural features of tracheal tube biofilm formed during prolonged mechanical ventilation. Chest. 1995;108(4):1049-1052.
19 Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science. 1999;21:1318-1322.
20 Berra L, De ML, Yu ZX, Laquerriere P, Baccarelli A, Kolobow T. Endotracheal tubes coated with antiseptics decrease bacterial colonization of the ventilator circuits, lungs, and endotracheal tube. Anesthesiology. 2004;100(6):1446-1456.
21 Berra L, Kolobow T, Laquerriere P, et al. Internally coated endotracheal tubes with silver sulfadiazine in polyurethane to prevent bacterial colonization: a clinical trial. Intensive Care Med. 2008;34:1030-1037.
22 Ng KS, Kumarasinghe G, Inglis TJ. Dissemination of respiratory secretions during tracheal tube suctioning in an intensive care unit. Ann Acad Med Singapore. 1999;28(2):178-182.
23 Adair CG, Gorman SP, Feron BM, et al. Implications of endotracheal tube biofilm for ventilator-associated pneumonia. Intensive Care Med. 1999;25:1072-1076.
24 Bou R, Aguilar A, Perpinan J, et al. Nosocomial outbreak of Pseudomonas aeruginosa infections related to a flexible bronchoscope. J Hosp Infect. 2006;64(2):129-135.
25 Srinivasan A, Wolfenden LL, Song X, et al. An outbreak of Pseudomonas aeruginosa infections associated with flexible bronchoscopes. N Engl J Med. 2003;348(3):221-227.
26 Crane LR, Tagle LC, Palutke WA. Outbreak of Pseudomonas paucimobilis in an intensive care facility. JAMA. 1981;246(9):985-987.
27 Eckmanns T, Oppert M, Martin M, et al. An outbreak of hospital-acquired Pseudomonas aeruginosa infection caused by contaminated bottled water in intensive care units. Clin Microbiol Infect. 2008;14(5):454-458.
28 Lee JK. Two outbreaks of Burkholderia cepacia nosocomial infection in a neonatal intensive care unit. J Paediatr Child Health. 2008;44(1-2):62-66.
29 Kikuchi T, Nagashima G, Taguchi K, et al. Contaminated oral intubation equipment associated with an outbreak of carbapenem-resistant Pseudomonas in an intensive care unit. J Hosp Infect. 2007;65(1):54-57.
30 Bou R, Ramos P. Outbreak of nosocomial legionnaires’ disease caused by a contaminated oxygen humidifier. J Hosp Infect. 2009;71(4):381-383.
31 Berthelot P, Grattard F, Mahul P, et al. Ventilator temperature sensors: an unusual source of Pseudomonas cepacia in nosocomial infection. J Hosp Infect. 1993;25(1):33-43.
32 Rogues AM, Maugein J, Allery A, et al. Electronic ventilator temperature sensors as a potential source of respiratory tract colonization with Stenotrophomonas maltophilia. J Hosp Infect. 2001;49(4):289-292.
33 Pegues CF, Pegues DA, Ford DS, et al. Burkholderia cepacia respiratory tract acquisition: epidemiology and molecular characterization of a large nosocomial outbreak. Epidemiol Infect. 1996;116(3):309-317.
34 Cobben NA, Drent M, Jonkers M, et al. Outbreak of severe Pseudomonas aeruginosa respiratory infections due to contaminated nebulizers. J Hosp Infect. 1996;33(1):63-70.
35 Enoch DA, Summers C, Brown NM, et al. Investigation and management of an outbreak of multidrug-carbapenem-resistant Acinetobacter baumannii in Cambridge, UK. J Hosp Infect. 2008;70(2):109-118.
36 Johanson WG, Pierce AK, Sanford JP. Changing pharyngeal bacterial flora of hospitalized patients: emergence of gram-negative bacilli. N Engl J Med. 1969;281:1137-1140.
37 Johanson WG, Pierce AK, Sanford JP, et al. Nosocomial respiratory infection with gram-negative bacilli: the significance of colonization of the respiratory tract. Ann Intern Med. 1972;77:701-706.
38 Feldman C, Kassel M, Cantrell J, et al. The presence and sequence of endotracheal tube colonization in patients undergoing mechanical ventilation. Eur Respir J. 1999;13:546-551.
39 Scannapieco FA, Stewart EM, Mylotte JM. Colonization of dental plaque by respiratory pathogens in medical intensive care patients. Crit Care Med. 1992;20(6):740-745.
40 Fourrier F, Duvivier B, Boutigny H, et al. Colonization of dental plaque: a source of nosocomial infections in intensive care unit patients. Crit Care Med. 1998;26(2):301-308.
41 Azarpazhooh A, Leake JL. Systematic review of the association between respiratory diseases and oral health. J Periodontol. 2006;77(9):1465-1482.
42 Heo SM, Haase EM, Lesse AJ, et al. Genetic relationships between respiratory pathogens isolated from dental plaque and bronchoalveolar lavage fluid from patients in the intensive care unit undergoing mechanical ventilation. Clin Infect Dis. 2008;47(12):1562-1570.
43 Bach A, Boehrer H, Schmidt H, Geiss HK. Nosocomial sinusitis in ventilated patients. Nasotracheal versus orotracheal intubation. Anaesthesia. 1992;47(4):335-339.
44 Michelson A, Kamp HD, Schuster B. [Sinusitis in long-term intubated, intensive care patients: nasal versus oral intubation]. Anaesthesist. 1991;40(2):100-104.
45 Salord F, Gaussorgues P, Marti-Flich J, et al. Nosocomial maxillary sinusitis during mechanical ventilation: a prospective comparison of orotracheal versus the nasotracheal route for intubation. Intensive Care Med. 1990;16(6):390-393.
46 Rouby JJ, Laurent P, Gosnach M, et al. Risk factors and clinical relevance of nosocomial maxillary sinusitis in the critically ill. Am J Respir Crit Care Med. 1994;150:776-783.
47 Holzapfel L, Chevret S, Madinier G, et al. Influence of long-term oro- or nasotracheal intubation on nosocomial maxillary sinusitis and pneumonia: results of a prospective, randomized, clinical trial. Crit Care Med. 1993;21(8):1132-1138.
48 Torres A, El Ebiary M, Soler N, et al. Stomach as a source of colonization of the respiratory tract during mechanical ventilation: association with ventilator-associated pneumonia. Eur Respir J. 1996;9(8):1729-1735.
49 Donowitz LG, Page MC, Mileur BL, et al. Alteration of normal gastric flora in critical care patients receiving antacid and cimetidine therapy. Infect Control. 1986;7(1):23-26.
50 Hillman KM. Colonisation of the gastric contents in critically ill patients. Acta Anaesthesiol Belg. 1983;34(3):191-192.
51 Apte NM, Karnad DR, Medhekar TP, Tilve GH, Morye S, Bhave GG. Gastric colonization and pneumonia in intubated critically ill patients receiving stress ulcer prophylaxis: a randomized, controlled trial. Crit Care Med. 1992;80:590-593.
52 Driks MR, Craven DE, Celli BR, et al. Nosocomial pneumonia in intubated patients given sucralfate as compared with antacids or histamine type 2 blockers. The role of gastric colonization. N Engl J Med. 1987;317:1376-1382.
53 Prodhom G, Leuenberger P, Koerfer J, et al. Nosocomial pneumonia in mechanically ventilated patients receiving antacid, ranitidine, or sucralfate as prophylaxis for stress ulcer. Ann Intern Med. 1994;120:653-662.
54 Bonten MJ, Gaillard CA, van der Geest S, et al. The role of intragastric acidity and stress ulcer prophylaxis on colonization and infection in mechanically ventilated ICU patients. A stratified, randomized, double-blind study of sucralfate versus antacids. Am J Respir Crit Care Med. 1995;152:1825-1834.
55 Cook D, Guyatt G, Marshall J, et al. A comparison of sucralfate and ranitidine for the prevention of upper gastrointestinal bleeding in patients requiring mechanical ventilation. Canadian Critical Care Trials Group. N Engl J Med. 1998;338:791-797.
56 Thomason MH, Payseur ES, Hakenewerth AM, et al. Nosocomial pneumonia in ventilated trauma patients during stress ulcer prophylaxis with sucralfate, antacid, and ranitidine. J Trauma. 1996;41(3):503-508.
57 Kantorova I, Svoboda P, Scheer P, et al. Stress ulcer prophylaxis in critically ill patients: a randomized controlled trial. Hepatogastroenterology. 2004;51(57):757-761.
58 Bonten MJ, Gaillard CA, van Thiel FH, Smeets HG, van der Geest S, Stobberingh EE. The stomach is not a source for colonization of the upper respiratory tract and pneumonia in ICU patients. Chest. 1994;105:878-884.
59 Cardeñosa Cendrero JA, Sole-Violan J, Bordes Benitez A, et al. Role of different routes of tracheal colonization in the development of pneumonia in patients receiving mechanical ventilation. Chest. 1999;116:462-470.
60 de Latorre FJ, Pont T, Ferrer A, et al. Pattern of tracheal colonization during mechanical ventilation. Am J Respir Crit Care Med. 1995;152:1028-1033.
61 Garrouste-Orgeas M, Chevret S, Arlet G, et al. Oropharyngeal or gastric colonization and nosocomial pneumonia in adult intensive care unit patients. A prospective study based on genomic DNA analysis. Am J Respir Crit Care Med. 1997;156:1647-1655.
62 Palmer LB, Donelan SV, Fox G, et al. Gastric flora in chronically mechanically ventilated patients. Relationship to upper and lower airway colonization. Am J Respir Crit Care Med. 1995;151(4):1063-1067.
63 Valles J, Mariscal D, Cortes P, et al. Patterns of colonization by Pseudomonas aeruginosa in intubated patients: a 3-year prospective study of 1,607 isolates using pulsed-field gel electrophoresis with implications for prevention of ventilator-associated pneumonia. Intensive Care Med. 2004;30(9):1768-1775.
64 Sackner MA, Hirsch J, Epstein S. Effect of cuffed endotracheal tubes on tracheal mucous velocity. Chest. 1975;68(6):774-777.
65 Konrad F, Schreiber T, Brecht-Kraus D, et al. Mucociliary transport in ICU patients. Chest. 1994;105(1):237-241.
66 Wunderink RG. Nosocomial pneumonia, including ventilator-associated pneumonia. Proc Am Thorac Soc. 2005;2(5):440-444.
67 Fumeaux T, Pugin J. Role of interleukin-10 in the intracellular sequestration of human leukocyte antigen-DR in monocytes during septic shock. Am J Respir Crit Care Med. 2002;166(11):1475-1482.
68 Hynninen M, Pettila V, Takkunen O, et al. Predictive value of monocyte histocompatibility leukocyte antigen-DR expression and plasma interleukin-4 and -10 levels in critically ill patients with sepsis. Shock. 2003;20(1):1-4.
69 Lekkou A, Karakantza M, Mouzaki A, et al. Cytokine production and monocyte HLA-DR expression as predictors of outcome for patients with community-acquired severe infections. Clin Diagn Lab Immunol. 2004;11(1):161-167.
70 Muehlstedt SG, Lyte M, Rodriguez JL. Increased IL-10 production and HLA-DR suppression in the lungs of injured patients precede the development of nosocomial pneumonia. Shock. 2002;17:443-450.
71 Gastmeier P, Sohr D, Geffers C, et al. Early- and late-onset pneumonia: is this still a useful classification? Antimicrob Agents Chemother. 2009;53(7):2714-2718.
72 Kollef MH, Afessa B, Anzueto A, et al. Silver-coated endotracheal tubes and incidence of ventilator-associated pneumonia: the NASCENT randomized trial. JAMA. 2008;300:805-813.
73 Kollef MH, Morrow LE, Niederman MS, et al. Clinical characteristics and treatment patterns among patients with ventilator-associated pneumonia. Chest. 2006;129(5):1210-1218.
74 Rello J, Ollendorf DA, Oster G, et al. Epidemiology and outcomes of ventilator-associated pneumonia in a large US database. Chest. 2002;122(6):2115-2121.
75 Vincent JL, Rello J, Marshall J, et al. International study of the prevalence and outcomes of infection in intensive care units. JAMA. 2009;302(21):2323-2329.
76 Weber DJ, Rutala WA, Sickbert-Bennett EE, et al. Microbiology of ventilator-associated pneumonia compared with that of hospital-acquired pneumonia. Infect Control Hosp Epidemiol. 2007;28:825-831.
77 Combes A, Figliolini C, Trouillet JL, et al. Incidence and outcome of polymicrobial ventilator-associated pneumonia. Chest. 2002;121(5):1618-1623.
78 Teixeira PJ, Seligman R, Hertz FT, et al. Inadequate treatment of ventilator-associated pneumonia: risk factors and impact on outcomes. J Hosp Infect. 2007;65(4):361-367.
79 Soler N, Torres A, Ewig S, et al. Bronchial microbial patterns in severe exacerbations of chronic obstructive pulmonary disease (COPD) requiring mechanical ventilation. Am J Respir Crit Care Med. 1998;157:1498-1505.
80 Ferrer M, Ioanas M, Arancibia F, et al. Microbial airway colonization is associated with noninvasive ventilation failure in exacerbation of chronic obstructive pulmonary disease. Crit Care Med. 2005;33:2003-2009.
81 Fagon JY, Chastre J. Diagnosis and treatment of nosocomial pneumonia in ALI/ARDS patients. Eur Respir J Suppl. 2003;42:77s-83s.
82 Delclaux C, Roupie E, Blot F, et al. Lower respiratory tract colonization and infection during severe acute respiratory distress syndrome. Am J Respir Crit Care Med. 1997;156:1092-1098.
83 Bronchard R, Albaladejo P, Brezac G, et al. Early onset pneumonia: risk factors and consequences in head trauma patients. Anesthesiology. 2004;100(2):234-239.
84 Baker AM, Meredith JW, Haponik EF. Pneumonia in intubated trauma patients. Microbiology and outcomes. Am J Respir Crit Care Med. 1996;153:343-349.
85 Sirvent JM, Torres A, Vidaur L, et al. Tracheal colonisation within 24 h of intubation in patients with head trauma: risk factor for developing early-onset ventilator-associated pneumonia. Intensive Care Med. 2000;26:1369-1372.
86 Gastmeier P, Sohr D, Geffers C, et al. Early- and late-onset pneumonia: is this still a useful classification? Antimicrob Agents Chemother. 2009;53(7):2714-2718.
87 Giantsou E, Liratzopoulos N, Efraimidou E, et al. Both early-onset and late-onset ventilator-associated pneumonia are caused mainly by potentially multiresistant bacteria. Intensive Care Med. 2005;31(11):1488-1494.
88 Rello J, Sa-Borges M, Correa H, et al. Variations in etiology of ventilator-associated pneumonia across four treatment sites: implications for antimicrobial prescribing practices. Am J Respir Crit Care Med. 1999;160:608-613.
89 Carratala J, Garcia-Vidal C. An update on Legionella. Curr Opin Infect Dis. 2010;23(2):152-157.
90 Garbe PL, Davis BJ, Weisfeld JS, et al. Nosocomial legionnaires’ disease. Epidemiologic demonstration of cooling towers as a source. JAMA. 1985;254(4):521-524.
91 Robert R, Grollier G, Frat JP, et al. Colonization of lower respiratory tract with anaerobic bacteria in mechanically ventilated patients. Intensive Care Med. 2003;29(7):1062-1068.
92 Dore P, Robert R, Grollier G, et al. Incidence of anaerobes in ventilator-associated pneumonia with use of a protected specimen brush. Am J Respir Crit Care Med. 1996;153:1292-1298.
93 Robert R, Grollier G, Dore P, et al. Nosocomial pneumonia with isolation of anaerobic bacteria in ICU patients: therapeutic considerations and outcome. J Crit Care. 1999;14(3):114-119.
94 Mier L, Dreyfuss D, Darchy B, et al. Is penicillin G an adequate initial treatment for aspiration pneumonia? Intensive Care Med. 1993;19:279-284.
95 Marik PE, Careau P. The role of anaerobes in patients with ventilator-associated pneumonia and aspiration pneumonia: a prospective study. Chest. 1999;115:178-183.
96 Delisle MS, Williamson DR, Perreault MM, et al. The clinical significance of Candida colonization of respiratory tract secretions in critically ill patients. J Crit Care. 2008;23(1):11-17.
97 Meersseman W, Lagrou K, Spriet I, et al. Significance of the isolation of Candida species from airway samples in critically ill patients: a prospective, autopsy study. Intensive Care Med. 2009;35(9):1526-1531.
98 Daubin C, Vincent S, Vabret A, et al. Nosocomial viral ventilator-associated pneumonia in the intensive care unit: a prospective cohort study. Intensive Care Med. 2005;31(8):1116-1122.
99 Heininger A, Jahn G, Engel C, et al. Human cytomegalovirus infections in nonimmunosuppressed critically ill patients. Crit Care Med. 2001;29(3):541-547.
100 Jaber S, Chanques G, Borry J, et al. Cytomegalovirus infection in critically ill patients: associated factors and consequences. Chest. 2005;127(1):233-241.
101 Papazian L, Fraisse A, Garbe L, et al. Cytomegalovirus. An unexpected cause of ventilator-associated pneumonia. Anesthesiology. 1996;84(2):280-287.
102 Chiche L, Forel JM, Roch A, et al. Active cytomegalovirus infection is common in mechanically ventilated medical intensive care unit patients. Crit Care Med. 2009;37(6):1850-1857.
103 Chastre J, Fagon JY. Ventilator-associated pneumonia. Am J Respir Crit Care Med. 2002;165:867-903.
104 Al-Tawfiq JA, Abed MS. Decreasing ventilator-associated pneumonia in adult intensive care units using the Institute for Healthcare Improvement bundle. Am J Infect Control. 2010.
105 Bird D, Zambuto A, O’Donnell C, et al. Adherence to ventilator-associated pneumonia bundle and incidence of ventilator-associated pneumonia in the surgical intensive care unit. Arch Surg. 2010;145(5):465-470.
106 Marra AR, Cal RG, Silva CV, et al. Successful prevention of ventilator-associated pneumonia in an intensive care setting. Am J Infect Control. 2009;37(8):619-625.
107 Apisarnthanarak A, Pinitchai U, Thongphubeth K, et al. Effectiveness of an educational program to reduce ventilator-associated pneumonia in a tertiary care center in Thailand: a 4-year study. Clin Infect Dis. 2007;45(6):704-711.
108 Babcock HM, Zack JE, Garrison T, et al. An educational intervention to reduce ventilator-associated pneumonia in an integrated health system: a comparison of effects. Chest. 2004;125(6):2224-2231.
109 Zack JE, Garrison T, Trovillion E, et al. Effect of an education program aimed at reducing the occurrence of ventilator-associated pneumonia. Crit Care Med. 2002;30(11):2407-2412.
110 Salahuddin N, Zafar A, Sukhyani L, et al. Reducing ventilator-associated pneumonia rates through a staff education programme. J Hosp Infect. 2004;57(3):223-227.
111 Bouadma L, Mourvillier B, Deiler V, et al. A multifaceted program to prevent ventilator-associated pneumonia: impact on compliance with preventive measures. Crit Care Med. 2010;38(3):789-796.
112 Needleman J, Buerhaus P, Mattke S, Stewart M, Zelevinsky K. Nurse-staffing levels and the quality of care in hospitals. N Engl J Med. 2002;346(22):1715-1722.
113 Allegranzi B, Pittet D. Role of hand hygiene in healthcare-associated infection prevention. J Hosp Infect. 2009;73(4):305-315.
114 World Health Organization. WHO guidelines for hand hygiene in health care. Geneva, Switzerland: World Health Organization; 2009.
115 Pittet D, Hugonnet S, Harbarth S, et al. Effectiveness of a hospital-wide programme to improve compliance with hand hygiene. Infection Control Programme. Lancet. 2000;356(9238):1307-1312.
116 Won SP, Chou HC, Hsieh WS, et al. Handwashing program for the prevention of nosocomial infections in a neonatal intensive care unit. Infect Control Hosp Epidemiol. 2004;25(9):742-746.
117 Capretti MG, Sandri F, Tridapalli E, et al. Impact of a standardized hand hygiene program on the incidence of nosocomial infection in very low birth weight infants. Am J Infect Control. 2008;36(6):430-435.
118 Kollef MH, Von HB, Prentice D, et al. Patient transport from intensive care increases the risk of developing ventilator-associated pneumonia. Chest. 1997;112(3):765-773.
119 Kress JP, Pohlman AS, O’Connor MF, et al. Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation. N Engl J Med. 2000;342:1471-1477.
120 Schweickert WD, Gehlbach BK, Pohlman AS, et al. Daily interruption of sedative infusions and complications of critical illness in mechanically ventilated patients. Crit Care Med. 2004;32(6):1272-1276.
121 Schweickert WD, Pohlman MC, Pohlman AS, et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet. 2009;373(9678):1874-1882.
122 Strom T, Martinussen T, Toft P. A protocol of no sedation for critically ill patients receiving mechanical ventilation: a randomised trial. Lancet. 2010;375(9713):475-480.
123 Marelich GP, Murin S, Battistella F, et al. Protocol weaning of mechanical ventilation in medical and surgical patients by respiratory care practitioners and nurses: effect on weaning time and incidence of ventilator-associated pneumonia. Chest. 2000;118:459-467.
124 Girard TD, Kress JP, Fuchs BD, et al. Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (Awakening and Breathing Controlled trial): a randomised controlled trial. Lancet. 2008;371(9607):126-134.
125 Brochard L, Mancebo J, Wysocki M, et al. Noninvasive ventilation for acute exacerbations of chronic obstructive pulmonary disease. N Engl J Med. 1995;333:817-822.
126 Hilbert G, Gruson D, Vargas F, et al. Noninvasive ventilation in immunosuppressed patients with pulmonary infiltrates, fever, and acute respiratory failure. N Engl J Med. 2001;344:481-487.
127 Keenan SP. Noninvasive positive pressure ventilation in acute respiratory failure. JAMA. 2000;284:2376-2378.
128 Nourdine K, Combes P, Carton MJ, et al. Does noninvasive ventilation reduce the ICU nosocomial infection risk? A prospective clinical survey. Intensive Care Med. 1999;25:567-573.
129 Burns KE, Adhikari NK, Keenan SP, et al. Use of non-invasive ventilation to wean critically ill adults off invasive ventilation: meta-analysis and systematic review. BMJ. 2009;338:b1574.
130 Ferrer M, Sellares J, Valencia M, et al. Non-invasive ventilation after extubation in hypercapnic patients with chronic respiratory disorders: randomised controlled trial. Lancet. 2009;374:1082-1088.
131 Kohlenberg A, Schwab F, Behnke M, Geffers C, Gastmeier P. Pneumonia associated with invasive and noninvasive ventilation: an analysis of the German nosocomial infection surveillance system database. Intensive Care Med. 2010;36(6):971-978.
132 Young PJ, Pakeerathan S, Blunt MC, Subramanya S. A low-volume, low-pressure tracheal tube cuff reduces pulmonary aspiration. Crit Care Med. 2006;34(3):632-639.
133 Young PJ, Blunt MC. Improving the shape and compliance characteristics of a high-volume, low-pressure cuff improves tracheal seal. Br J Anaesth. 1999;83(6):887-889.
134 Zanella A, Li Bassi G, Kolobow T. Evaluation of fluid leakage around 3 different endotracheal tube cuffs. Proceedings American Thoracic Society. 2006;3(abstracts issue):A742.
135 Lorente L, Lecuona M, Alejandro J, Maria M, Antonio S. Influence of an Endotracheal Tube with Polyurethane Cuff and Subglottic Drainage on Pneumonia. Am J Respir Crit Care Med. 2007;176:1979-1983.
136 Miller MA, Arndt JL, Konkle MA, et al. A polyurethane cuffed endotracheal tube is associated with decreased rates of ventilator-associated pneumonia. J Crit Care. 2010.
137 Poelaert J, Depuydt P, De WA, Van d V, Herck I, Blot S. Polyurethane cuffed endotracheal tubes to prevent early postoperative pneumonia after cardiac surgery: a pilot study. J Thorac Cardiovasc Surg. 2008;135(4):771-776.
138 Young PJ, Burchett K, Harvey I, Blunt MC. The prevention of pulmonary aspiration with control of tracheal wall pressure using a silicone cuff. Anaesth Intensive Care. 2000;28(6):660-665.
139 Young PJ, Ridley SA, Downward G. Evaluation of a new design of tracheal tube cuff to prevent leakage of fluid to the lungs. Br J Anaesth. 1998;80(6):796-799.
140 Dave MH, Frotzler A, Spielmann N, et al. Effect of tracheal tube cuff shape on fluid leakage across the cuff: an in vitro study. Br J Anaesth. 2010.
141 Valencia M, Ferrer M, Farre R, et al. Automatic control of tracheal tube cuff pressure in ventilated patients in semirecumbent position: a randomized trial. Crit Care Med. 2007;35:1543-1549.
142 Lucangelo U, Zin WA, Antonaglia V, et al. Effect of positive expiratory pressure and type of tracheal cuff on the incidence of aspiration in mechanically ventilated patients in an intensive care unit. Crit Care Med. 2008;36(2):409-413.
143 Manzano F, Fernandez-Mondejar E, Colmenero M, et al. Positive-end expiratory pressure reduces incidence of ventilator-associated pneumonia in nonhypoxemic patients. Crit Care Med. 2008;36(8):2225-2231.
144 Olson ME, Harmon BG, Kollef MH. Silver-coated endotracheal tubes associated with reduced bacterial burden in the lungs of mechanically ventilated dogs. Chest. 2002;121(3):863-870.
145 Berra L, Curto F, Li BG, et al. Antibacterial-coated tracheal tubes cleaned with the Mucus Shaver: a novel method to retain long-term bactericidal activity of coated tracheal tubes. Intensive Care Med. 2006;32:888-893.
146 Kolobow T, Berra L, Li BG, Curto F. Novel system for complete removal of secretions within the endotracheal tube: the Mucus Shaver. Anesthesiology. 2005;102(5):1063-1065.
147 Afessa B, Shorr AF, Anzueto AR, et al. Association between a silver-coated endotracheal tube and reduced mortality in patients with ventilator-associated pneumonia. Chest. 2010;137(5):1015-1021.
148 Shorr AF, Zilberberg MD, Kollef M. Cost-effectiveness analysis of a silver-coated endotracheal tube to reduce the incidence of ventilator-associated pneumonia. Infect Control Hosp Epidemiol. 2009;30(8):759-763.
149 Dezfulian C, Shojania K, Collard HR, et al. Subglottic secretion drainage for preventing ventilator-associated pneumonia: a meta-analysis. Am J Med. 2005;118(1):11-18.
150 Bouza E, Perez MJ, Munoz P, et al. Continuous aspiration of subglottic secretions in the prevention of ventilator-associated pneumonia in the postoperative period of major heart surgery. Chest. 2008;134(5):938-946.
151 Lacherade JC, De JB, Guezennec P, et al. Intermittent Subglottic Secretion Drainage and Ventilator-associated Pneumonia: A Multicenter Trial. Am J Respir Crit Care Med. 2010.
152 Berra L, De Marchi L, Panigada M, Yu ZX, Baccarelli A, Kolobow T. Evaluation of continuous aspiration of subglottic secretion in an in vivo study. Crit Care Med. 2004;32:2071-2078.
153 Elpern EH, Scott MG, Petro L, Ries MH. Pulmonary aspiration in mechanically ventilated patients with tracheostomies. Chest. 1994;105(2):563-566.
154 Leder SB. Incidence and type of aspiration in acute care patients requiring mechanical ventilation via a new tracheotomy. Chest. 2002;122(5):1721-1726.
155 Ibrahim EH, Tracy L, Hill C, Fraser VJ, Kollef MH. The occurrence of ventilator-associated pneumonia in a community hospital: risk factors and clinical outcomes. Chest. 2001;120(2):555-561.
156 Nseir S, Pompeo CD, Jozefowicz E, et al. Relationship between tracheotomy and ventilator-associated pneumonia: a case-control study. Eur Respir J. 2006;30:314-320.
157 Griffiths J, Barber VS, Morgan L, Young JD. Systematic review and meta-analysis of studies of the timing of tracheostomy in adult patients undergoing artificial ventilation. BMJ. 2005;330(7502):1243.
158 Dunham CM, Ransom KJ. Assessment of early tracheostomy in trauma patients: a systematic review and meta-analysis. Am Surg. 2006;72(3):276-281.
159 Durbin CGJr, Perkins MP, Moores LK. Should tracheostomy be performed as early as 72 hours in patients requiring prolonged mechanical ventilation? Respir Care. 2010;55(1):76-87.
160 Terragni PP, Antonelli M, Fumagalli R, et al. Early vs late tracheotomy for prevention of pneumonia in mechanically ventilated adult ICU patients: a randomized controlled trial. JAMA. 2010;303(15):1483-1489.
161 Dreyfuss D, Djedaini K, Weber P, et al. Prospective study of nosocomial pneumonia and of patient and circuit colonization during mechanical ventilation with circuit changes every 48 hours versus no change. Am Rev Respir Dis. 1991;143:738-743.
162 Fink JB, Krause SA, Barrett L, et al. Extending ventilator circuit change interval beyond 2 days reduces the likelihood of ventilator-associated pneumonia. Chest. 1998;113(2):405-411.
163 Han JN, Liu YP, Ma S, et al. Effects of decreasing the frequency of ventilator circuit changes to every 7 days on the rate of ventilator-associated pneumonia in a Beijing hospital. Respir Care. 2001;46(9):891-896.
164 Hess D, Burns E, Romagnoli D, Kacmarek RM. Weekly ventilator circuit changes. A strategy to reduce costs without affecting pneumonia rates. Anesthesiology. 1995;82(4):903-911.
165 Kollef MH, Prentice D, Shapiro SD, et al. Mechanical ventilation with or without daily changes of in-line suction catheters. Am J Respir Crit Care Med. 1997;156(2 Pt 1):466-472.
166 Kotilainen HR, Keroack MA. Cost analysis and clinical impact of weekly ventilator circuit changes in patients in intensive care unit. Am J Infect Control. 1997;25(2):117-120.
167 Long MN, Wickstrom G, Grimes A, et al. Prospective, randomized study of ventilator-associated pneumonia in patients with one versus three ventilator circuit changes per week. Infect Control Hosp Epidemiol. 1996;17(1):14-19.
168 Stamm AM. Ventilator-associated pneumonia and frequency of circuit changes. Am J Infect Control. 1998;26(1):71-73.
169 Han J, Liu Y. Effect of ventilator circuit changes on ventilator-associated pneumonia: a systematic review and meta-analysis. Respir Care. 2010;55(4):467-474.
170 Cook D, De Jonghe B, Brochard L, Brun-Buisson C. Influence of airway management on ventilator-associated pneumonia: evidence from randomized trials. JAMA. 1998;279:781-787.
171 Craven DE, Goularte TA, Make BJ. Contaminated condensate in mechanical ventilator circuits. A risk factor for nosocomial pneumonia? Am Rev Respir Dis. 1984;129:625-628.
172 Craven DE, Lichtenberg DA, Goularte TA, Make BJ, McCabe WR. Contaminated medication nebulizers in mechanical ventilator circuits. Am J Med. 1984;77:834-838.
173 Kola A, Eckmanns T, Gastmeier P. Efficacy of heat and moisture exchangers in preventing ventilator-associated pneumonia: meta-analysis of randomized controlled trials. Intensive Care Med. 2005;31(1):5-11.
174 Siempos II, Vardakas KZ, Kopterides P, Falagas ME. Impact of passive humidification on clinical outcomes of mechanically ventilated patients: A meta-analysis of randomized controlled trials. Crit Care Med. 2007;35:2843-2851.
175 Hirsch JA, Tokayer JL, Robinson MJ, Sackner MA. Effects of dry air and subsequent humidification on tracheal mucous velocity in dogs. J Appl Physiol. 1975;39(2):242-246.
176 Ricard JD, Le ME, Markowicz P, et al. Efficiency and safety of mechanical ventilation with a heat and moisture exchanger changed only once a week. Am J Respir Crit Care Med. 2000;161(1):104-109.
177 Boisson C, Viviand X, Arnaud S, et al. Changing a hydrophobic heat and moisture exchanger after 48 hours rather than 24 hours: a clinical and microbiological evaluation. Intensive Care Med. 1999;25(11):1237-1243.
178 Davis KJr, Evans SL, Campbell RS, et al. Prolonged use of heat and moisture exchangers does not affect device efficiency or frequency rate of nosocomial pneumonia. Crit Care Med. 2000;28(5):1412-1418.
179 Thomachot L, Boisson C, Arnaud S, et al. Changing heat and moisture exchangers after 96 hours rather than after 24 hours: a clinical and microbiological evaluation. Crit Care Med. 2000;28(3):714-720.
180 Djedaini K, Billiard M, Mier L, et al. Changing heat and moisture exchangers every 48 hours rather than 24 hours does not affect their efficacy and the incidence of nosocomial pneumonia. Am J Respir Crit Care Med. 1995;152(5 Pt 1):1562-1569.
181 Jongerden IP, Rovers MM, Grypdonck MH, Bonten MJ. Open and closed endotracheal suction systems in mechanically ventilated intensive care patients: a meta-analysis. Crit Care Med. 2007;35(1):260-270.
182 Siempos II, Vardakas KZ, Falagas ME. Closed tracheal suction systems for prevention of ventilator-associated pneumonia. Br J Anaesth. 2008;100(3):299-306.
183 Subirana M, Sola I, Benito S. Closed tracheal suction systems versus open tracheal suction systems for mechanically ventilated adult patients. Cochrane Database Syst Rev 2007;4:CD004581.
184 Caruso P, Denari S, Ruiz SA, Demarzo SE, Deheinzelin D. Saline instillation before tracheal suctioning decreases the incidence of ventilator-associated pneumonia. Crit Care Med. 2009;37(1):32-38.
185 Torres A, Serra-Batlles J, Ros E, et al. Pulmonary aspiration of gastric contents in patients receiving mechanical ventilation: the effect of body position. Ann Intern Med. 1992;116:540-543.
186 Orozco-Levi M, Torres A, Ferrer M, et al. Semirecumbent position protects from pulmonary aspiration but not completely from gastroesophageal reflux in mechanically ventilated patients. Am J Respir Crit Care Med. 1995;152:1387-1390.
187 Drakulovic MB, Torres A, Bauer TT, et al. Supine body position as a risk factor for nosocomial pneumonia in mechanically ventilated patients: a randomised trial. Lancet. 1999;354:1851-1858.
188 van Nieuwenhoven CA, Vandenbroucke-Grauls C, van Tiel FH, et al. Feasibility and effects of the semirecumbent position to prevent ventilator-associated pneumonia: A randomized study. Crit Care Med. 2006;34:396-402.
189 Torres A, Ewig S, Lode H, Carlet J. Defining, treating and preventing hospital acquired pneumonia: European perspective. Intensive Care Med. 2009;35:9-29.
190 Bassi GL, Zanella A, Cressoni M, Stylianou M, Kolobow T. Following tracheal intubation, mucus flow is reversed in the semirecumbent position: possible role in the pathogenesis of ventilator-associated pneumonia. Crit Care Med. 2008;36(2):518-525.
191 Panigada M, Berra L, Greco G, Stylianou M, Kolobow T. Bacterial colonization of the respiratory tract following tracheal intubation-effect of gravity: an experimental study. Crit Care Med. 2003;31(3):729-737.
192 Raoof S, Chowdhrey N, Raoof S, et al. Effect of combined kinetic therapy and percussion therapy on the resolution of atelectasis in critically ill patients. Chest. 1999;115(6):1658-1666.
193 Hess DR. Patient positioning and ventilator-associated pneumonia. Respir Care. 2005;50(7):892-898.
194 Goldhill DR, Imhoff M, McLean B, Waldmann C. Rotational bed therapy to prevent and treat respiratory complications: a review and meta-analysis. Am J Crit Care. 2007;16(1):50-61.
195 Delaney A, Gray H, Laupland KB, Zuege DJ. Kinetic bed therapy to prevent nosocomial pneumonia in mechanically ventilated patients: a systematic review and meta-analysis. Crit Care. 2006;10(3):R70.
196 Staudinger T, Bojic A, Holzinger U, et al. Continuous lateral rotation therapy to prevent ventilator-associated pneumonia. Crit Care Med. 2010;38(2):486-490.
197 Ephgrave KS, Kleiman-Wexler R, Pfaller M, et al. Postoperative pneumonia: a prospective study of risk factors and morbidity. Surgery. 1993;114(4):815-819.
198 Mahul PH, Auboyer C, Jospe R, et al. Prevention of nosocomial pneumonia in intubated patients: Respective role of mechanical sugglottic secretions drainage and stress ulcer prophylaxis. Intensive Care Med. 1992;18:20-25.
199 Eddleston JM, Pearson RC, Holland J, et al. Prospective endoscopic study of stress erosions and ulcers in critically ill adult patients treated with either sucralfate or placebo. Crit Care Med. 1994;22(12):1949-1954.
200 Lin PC, Chang CH, Hsu PI, et al. The efficacy and safety of proton pump inhibitors vs histamine-2 receptor antagonists for stress ulcer bleeding prophylaxis among critical care patients: a meta-analysis. Crit Care Med. 2010;38(4):1197-1205.
201 Marik PE, Zaloga GP. Early enteral nutrition in acutely ill patients: a systematic review. Crit Care Med. 2001;29(12):2264-2270.
202 Artinian V, Krayem H, DiGiovine B. Effects of early enteral feeding on the outcome of critically ill mechanically ventilated medical patients. Chest. 2006;129(4):960-967.
203 Ibrahim EH, Mehringer L, Prentice D, et al. Early versus late enteral feeding of mechanically ventilated patients: results of a clinical trial. JPEN J Parenter Enteral Nutr. 2002;26(3):174-181.
204 Heyland DK, Dhaliwal R, Drover JW, Gramlich L, Dodek P. Canadian clinical practice guidelines for nutrition support in mechanically ventilated, critically ill adult patients. JPEN J Parenter Enteral Nutr. 2003;27(5):355-373.
205 Ho KM, Dobb GJ, Webb SA. A comparison of early gastric and post-pyloric feeding in critically ill patients: a meta-analysis. Intensive Care Med. 2006;32(5):639-649.
206 Chlebicki MP, Safdar N. Topical chlorhexidine for prevention of ventilator-associated pneumonia: a meta-analysis. Crit Care Med. 2007;35(2):595-602.
207 Pineda LA, Saliba RG, El Solh AA. Effect of oral decontamination with chlorhexidine on the incidence of nosocomial pneumonia: a meta-analysis. Crit Care. 2006;10(1):R35.
208 Chan EY, Ruest A, Meade MO, Cook DJ. Oral decontamination for prevention of pneumonia in mechanically ventilated adults: systematic review and meta-analysis. BMJ. 2007;334(7599):889.
209 Tantipong H, Morkchareonpong C, Jaiyindee S, Thamlikitkul V. Randomized controlled trial and meta-analysis of oral decontamination with 2% chlorhexidine solution for the prevention of ventilator-associated pneumonia. Infect Control Hosp Epidemiol. 2008;29(2):131-136.
210 Koeman M, van d V, Hak E, et al. Oral decontamination with chlorhexidine reduces the incidence of ventilator-associated pneumonia. Am J Respir Crit Care Med. 2006;173(12):1348-1355.
211 Stoutenbeek CP, van Saene HK, Miranda DR, Zandstra DF, Binnendijk B. The prevention of superinfection in multiple trauma patients. J Antimicrob Chemother. 1984;14(Suppl. B):203-211.
212 Stoutenbeek CP, van Saene HK, Miranda DR, Zandstra DF. The effect of selective decontamination of the digestive tract on colonisation and infection rate in multiple trauma patients. Intensive Care Med. 1984;10(4):185-192.
213 de Jonge E, Schultz MJ, Spanjaard L, et al. Effects of selective decontamination of digestive tract on mortality and acquisition of resistant bacteria in intensive care: a randomised controlled trial. Lancet. 2003;362:1011-1016.
214 de Smet AMGA, Kluytmans JAJW, Cooper BS, et al. Decontamination of the Digestive Tract and Oropharynx in ICU Patients. N Engl J Med. 2009;360(1):20-31.
215 Krueger WA, Lenhart FP, Neeser G, et al. Influence of Combined Intravenous and Topical Antibiotic Prophylaxis on the Incidence of Infections, Organ Dysfunctions, and Mortality in Critically Ill Surgical Patients: A Prospective, Stratified, Randomized, Double-Blind, Placebo-controlled Clinical Trial. Am J Respir Crit Care Med. 2002;166:1029-1037.
216 Liberati A, D’Amico R, Pifferi S, Torri V, Brazzi L, Parmelli E. Antibiotic prophylaxis to reduce respiratory tract infections and mortality in adults receiving intensive care. Cochrane Database Syst Rev 2009;4:CD000022.
217 Liberati A, D’Amico R, Pifferi S, Torri V, Brazzi L. Antibiotic prophylaxis to reduce respiratory tract infections and mortality in adults receiving intensive care. Cochrane Database Syst Rev 2004;1:CD000022.
218 D’Amico R, Pifferi S, Leonetti C, Torri V, Tinazzi A, Liberati A. Effectiveness of antibiotic prophylaxis in critically ill adult patients: systematic review of randomised controlled trials. BMJ. 1998;316(7140):1275-1285.
219 Oostdijk EA, de Smet AM, Blok HE, et al. Ecological effects of selective decontamination on resistant gram-negative bacterial colonization. Am J Respir Crit Care Med. 2010;181(5):452-457.
220 Li Bassi G, Saucedo LM. The use of prophylactic vancomycin to prevent MRSA colonization: does this double-edged sword promote future vancomycin resistance or is it a safe preventative strategy that should be used in all patients in the context of MRSA endemicity? Minerva Anestesiol. 2010;76(3):175-177.
221 de la Cal MA, Cerda E, van Saene HK, et al. Effectiveness and safety of enteral vancomycin to control endemicity of methicillin-resistant Staphylococcus aureus in a medical/surgical intensive care unit. J Hosp Infect. 2004;56(3):175-183.
222 Mandelli M, Mosconi P, Langer M, Cigada M. Prevention of pneumonia in an intensive care unit: a randomized multicenter clinical trial. Intensive Care Unit Group of Infection Control. Crit Care Med. 1989;17(6):501-505.
223 Sirvent JM, Torres A, El-Ebiary M, et al. Protective effect of intravenously administered cefuroxime against nosocomial pneumonia in patients with structural coma. Am J Respir Crit Care Med. 1997;155:1729-1734.
224 Siempos II, Ntaidou TK, Falagas ME. Impact of the administration of probiotics on the incidence of ventilator-associated pneumonia: a meta-analysis of randomized controlled trials. Crit Care Med. 2010;38(3):954-962.
225 Niederman MS, Torres A, Summer W. Invasive diagnostic testing is not needed routinely to manage suspected ventilator-associated pneumonia. Am J Respir Crit Care Med. 1994;150:565-569.
226 Chastre J, Fagon JY. Invasive diagnostic testing should be routinely used to manage ventilated patients with suspected pneumonia. Am J Respir Crit Care Med. 1994;150:570-574.
227 Fagon JY, Chastre J, Hance AJ, et al. Detection of nosocomial lung infection in ventilated patients. Use of a protected specimen brush and quantitative culture techniques in 147 patients. Am Rev Respir Dis. 1988;138:110-116.
228 Andrews CP, Coalson JJ, Smith JD, Johanson WG. Diagnosis of nosocomial bacterial pneumonia in acute, diffuse lung injury. Chest. 1981;80:254-258.
229 Patel IS, Vlahos I, Wilkinson TMA, et al. Bronchiectasis, Exacerbation Indices, and Inflammation in Chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med. 2004;170:400-407.
230 Wunderink RG. Radiologic diagnosis of ventilator-associated pneumonia. Chest. 2000;117(4 Suppl. 2):188S-190S.
231 Wunderink RG, Woldenberg LS, Zeiss J, et al. The radiologic diagnosis of autopsy-proven ventilator associated-pneumonia. Chest. 1992;101:458-463.
232 Lefcoe MS, Fox GA, Leasa DJ, et al. Accuracy of portable chest radiography in the critical care setting. Diagnosis of pneumonia based on quantitative cultures obtained from protected brush catheter. Chest. 1994;105(3):885-887.
233 Meduri GU, Belenchia JM, Estes RJ, et al. Proliferative phase of ARDS. Clinical findings and effects of corticosteroids. Chest. 1991;100:943-952.
234 Meduri GU, Mauldin GL, Wunderink RG, et al. Causes of fever and pulmonary densities in patients with clinical manifestations of ventilator-associated pneumonia. Chest. 1994;106:221-235.
235 Pugin J, Auckenthaler R, Mili N, et al. Diagnosis of ventilator-associated pneumonia by bacteriologic analysis of bronchoscopic and non-bronchoscopic “blind” bronchoalveolar lavage fluid. Am Rev Respir Dis. 1991;143:1121-1129.
236 Hill JD, Ratliff JL, Parrott JC, et al. Pulmonary pathology in acute respiratory insufficiency: lung biopsy as a diagnostic tool. J Thorac Cardiovasc Surg. 1976;71(1):64-71.
237 Marquette CH, Georges H, Wallet F, et al. Diagnostic efficiency of endotracheal aspirate with quantitative bacterial cultures in intubated patients with suspected pneumonia period. Comparison with the protected specimen brush. Am Rev Respir Dis. 1993;148:138-144.
238 El-Ebiary M, Torres A, González J, et al. Quantitative cultures of endotracheal aspirates for the diagnosis of ventilator-associated pneumonia. Am Rev Respir Dis. 1993;147:1552-1557.
239 Torres A, Martos A, Puig dlB, et al. Specificity of endotracheal aspiration, protected specimen brush, and bronchoalveolar lavage in mechanically ventilated patients. Am Rev Respir Dis. 1993;147(4):952-957.
240 Jourdain B, Novara A, Joly-Guillou ML, et al. Role of quantitative cultures of endotracheal aspirates in the diagnosis of nosocomial pneumonia. Am J Respir Crit Care Med. 1995;152(1):241-246.
241 Torres A, Fabregas N, Ewig S, et al. Sampling methods for ventilator-associated pneumonia: validation using different histologic and microbiological references. Crit Care Med. 2000;28:2799-2804.
242 Kollef MH. Ventilator-associated pneumonia: A multivariate analysis. JAMA. 1993;270:1965-1970.
243 McGowan JEJr. Antimicrobial resistance in hospital organisms and its relation to antibiotic use. Rev Infect Dis. 1983;5(6):1033-1048.
244 Kollef MH, Sherman G, Ward S, Fraser VJ. Inadequate antimicrobial treatment of infections: a risk factor for hospital mortality among critically ill patients. Chest. 1999;115:462-474.
245 Iregui M, Ward S, Sherman G, Fraser VJ, Kollef MH. Clinical importance of delays in the initiation of appropriate antibiotic treatment for ventilator-associated pneumonia. Chest. 2002;122(1):262-268.
246 Celis R, Torres A, Gatell JM, et al. Nosocomial pneumonia: A multivariate analysis of risk and prognosis. Chest. 1988;93:318-324.
247 Alvarez-Lerma F. Modification of empiric antibiotic treatment in patients with pneumonia acquired in the intensive care unit. ICU-acquired Pneumonia Study Group. Intensive Care Med. 1996;22:387-394.
248 Rello J, Gallego M, Mariscal D, Sonora R, Valles J. The value of routine microbiologic investigation in the diagnosis of ventilator-associated pneumonia. Am J Respir Crit Care Med. 1997;156:196-200.
249 Kollef MH, Bock KR, Richards RD, Hearns ML. The safety and diagnosis accuracy of minibronchoalveolar lavage in patients with suspected ventilator associated pneumonia. Ann Intern Med. 1995;122:743-748.
250 Sanchez-Nieto JM, Torres A, Garcia-Cordoba F, et al. Impact of invasive and noninvasive quantitative culture sampling on outcome of ventilator-associated pneumonia: a pilot study. Am J Respir Crit Care Med. 1998;157(2):371-376.
251 Dupont H, Mentec H, Sollet JP, Bleichner G. Impact of appropriateness of initial antibiotic therapy on the outcome of ventilator-associated pneumonia. Intensive Care Med. 2001;27(2):355-362.
252 Hanberger H, Garcia-Rodriguez JA, Gobernado M, et al. Antibiotic susceptibility among aerobic gram-negative bacilli in intensive care units in 5 European countries. French and Portuguese ICU Study Groups. JAMA. 1999;281(1):67-71.
253 Fagon JY, Chastre J, Domart Y, et al. Nosocomial pneumonia in patients receiving continuous mechanical ventilation. Prospective analysis of 52 episodes with use of a protected specimen brush and quantitative culture techniques. Am Rev Respir Dis. 1989;139:877-884.
254 Torres A, Aznar R, Gatell JM, et al. Incidence, risk, and prognosis factors of nosocomial pneumonia in mechanically ventilated patients. Am Rev Respir Dis. 1990;142:523-528.
255 Rello J, Ausina V, Ricart M, Castella J, Prats G. Impact of previous antimicrobial therapy on the etiology and outcome of ventilator-associated pneumonia. Chest. 1993;104:1230-1235.
256 Bouza E, Torres MV, Radice C, et al. Direct E-test (AB Biodisk) of respiratory samples improves antimicrobial use in ventilator-associated pneumonia. Clin Infect Dis. 2007;44:382-387.
257 Cercenado E, Cercenado S, Marin M, et al. Evaluation of direct E-test on lower respiratory tract samples: a rapid and accurate procedure for antimicrobial susceptibility testing. Diagn Microbiol Infect Dis. 2007;58(2):211-216.
258 Reygaert W. Methicillin-resistant Staphylococcus aureus (MRSA): identification and susceptibility testing techniques. Clin Lab Sci. 2009;22(2):120-124.
259 Ost DE, Poch D, Fadel A, et al. Mini-bronchoalveolar lavage quantitative polymerase chain reaction for diagnosis of methicillin-resistant Staphylococcus aureus pneumonia. Crit Care Med. 2010;38(7):1536-1541.
260 Heyland DK, Cook DJ, Griffith L, et al. The attributable morbidity and mortality of ventilator-associated pneumonia in the critically ill patient. The Canadian Critical Trials Group. Am J Respir Crit Care Med. 1999;159:1249-1256.
261 Sole-Violan J, Fernandez JA, Benitez AB, et al. Impact of quantitative invasive diagnostic techniques in the management of outcome of mechanically ventilated patients with suspected pneumonia. Crit Care Med. 2000;28:2737-2741.
262 Ruiz M, Torres A, Ewig S, et al. Noninvasive versus invasive microbial investigation in ventilator-associated pneumonia: evaluation of outcome. Am J Respir Crit Care Med. 2000;162(1):119-125.
263 Fagon JY, Chastre J, Wolff M, et al. Invasive and noninvasive strategies for management of suspected ventilator-associated pneumonia. A randomized trial. Ann Intern Med. 2000;132:621-630.
264 Shorr AF, Sherner JH, Jackson WL, Kollef MH. Invasive approaches to the diagnosis of ventilator-associated pneumonia: a meta-analysis. Crit Care Med. 2005;33(1):46-53.
265 Montravers P, Fagon JY, Chastre J, et al. Follow-up protected specimen brushes to assess treatment in nosocomial pneumonia. Am Rev Respir Dis. 1993;147:38-44.
266 Souweine B, Veber B, Bedos JP, et al. Diagnostic accuracy of protected specimen brush and bronchoalveolar lavage in nosocomial pneumonia: impact of previous antimicrobial treatments. Crit Care Med. 1998;26(2):236-244.
267 Pugin J, Auckenthaler R, Mili N, et al. Diagnosis of ventilator associated pneumonia by bacteriologic analysis of bronchoscopic and non-bronchoscopic “blind” bronchoalveolar lavage fluid. Am Rev Respir Dis. 1991;143:1121-1129.
268 Mackowiak PA, Martin RM, Jones SR, Smith JW. Pharyngeal colonization by gram-negative bacilli in aspiration-prone persons. Arch Intern Med. 1978;138:1224-1227.
269 American Thoracic Society. Hospital-acquired pneumonia in adults: Diagnosis, assessment of severity, initial antimicrobial therapy, and preventative strategies. A consensus statement. Am J Respir Crit Care Med. 1996;153:1711-1725.
270 Ibrahim EH, Ward S, Sherman G, et al. Experience with a clinical guideline for the treatment of ventilator-associated pneumonia. Crit Care Med. 2001;29:1109-1115.
271 Valenti WM, Trudell RG, Bentley DW. Factors predisposing to oropharyngeal colonization with gram-negative bacilli in the aged. N Engl J Med. 1978;298:1108-1111.
272 Chastre J, Wolff M, Fagon JY, et al. Comparison of 8 vs 15 days of antibiotic therapy for ventilator-associated pneumonia in adults: a randomized trial. JAMA. 2003;290(19):2588-2598.
273 Chastre J, Wolff M, Fagon JY, et al. Comparison of 8 vs 15 days of antibiotic therapy for ventilator-associated pneumonia in adults: a randomized trial. JAMA. 2003;290(19):2588-2598.
274 Chastre J, Luyt CE, Combes A, Trouillet JL. Use of quantitative cultures and reduced duration of antibiotic regimens for patients with ventilator-associated pneumonia to decrease resistance in the intensive care unit. Clin Infect Dis. 2006;43(Suppl. 2):S75-S81.
275 Ferrer M, Liapikou A, Valencia M, et al. Validation of the American Thoracic Society–Infectious Diseases Society of America Guidelines for Hospital-Acquired Pneumonia in the Intensive Care Unit. Clin Infect Dis. 2010;50:945-952.
276 Sinuff T, Muscedere J, Cook D, Dodek P, Heyland D. Ventilator-associated pneumonia: Improving outcomes through guideline implementation. J Crit Care. 2008;23(1):118-125.
277 Soo Hoo GW, Wen YE, Nguyen TV, Goetz MB. Impact of clinical guidelines in the management of severe hospital-acquired pneumonia. Chest. 2005;128(4):2778-2787.