Chapter 12 Nonvariceal Upper Gastrointestinal Bleeding
Introduction
Upper gastrointestinal (GI) bleeding is a common GI emergency. Frequency of occurrence is 40 to 150 cases per 100,000 in the Western population, and it accounts for a total expenditure of $2.5 billion annually in the United States.1,2 Significant advances have been made not only in decreasing the overall incidence, but also in treatment (medical treatment and endoscopic hemostasis), prevention of complications, and recurrence of bleeding.3 These advances have resulted in an overall decrease in the rate of hospitalizations and mortality associated with upper GI hemorrhage in the Western world.4 However, more recent data suggest that although hospital admission rates may have decreased, patients being admitted are older and have more comorbid illnesses. These patients are known to carry a worse prognosis.3 Acute nonvariceal upper GI bleeding is still associated with significant morbidity and mortality despite significant development in the understanding of the pathophysiology of the disease and the armamentarium available to the endoscopist for endoscopic therapy to treat these lesions. This chapter discusses the approach, assessment, and management strategies in patients presenting with nonvariceal upper GI bleeding.
Clinical Presentation
The presentation of GI bleeding depends on the volume and site of bleeding. Hematemesis is vomiting of blood and is the most common presentation of an upper GI bleed. The source is almost always proximal to the ligament of Treitz. Blood may be bright red, indicating a recent bleed, or resemble “coffee grounds,” representing older blood reduced by acid in the stomach. Melena is black, tarry, and sticky stool with a specific foul odor caused by degradation of blood in the intestines and colon. This presentation most commonly indicates an upper GI source; however, blood from the right colon may also manifest as melena. A massive upper GI bleed can manifest as hematochezia (bright red blood per rectum) in 15% of cases and carries a worse prognosis.5
Initial Evaluation
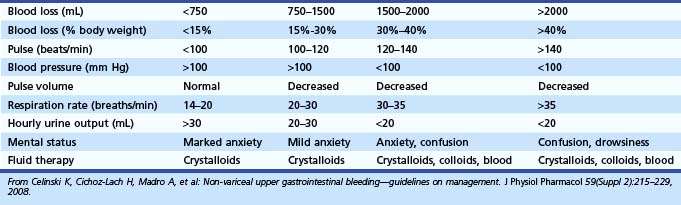
The initial assessment of a patient suspected to have an acute GI bleed must focus on hemodynamic stability so that resuscitative measures can be started without delay. Good clinical assessment determines the fluid requirement for hemodynamic stabilization.6 A focused history and physical examination provides vital information on the severity of bleeding and other confounding medical problems (e.g., coronary disease, chronic obstructive pulmonary disease, malignancy) that may affect medical management and therapeutic intervention. The initial evaluation should focus on vital signs and orthostatic changes because postural hypotension represents a significant volume loss (>15%) and is predictive of a poor outcome (Table 12.1).7,8
Nasogastric Aspirate
Nasogastric tubes can be helpful in the localization of bleeding because a positive nasogastric aspirate (coffee ground or bright red blood) confirms the source to be from the upper GI tract. However, the localization of bleeding can be determined by a careful history and physical examination. A reliable history of hematemesis confirms the source to be proximal to the ligament of Treitz. Use of a nasogastric tube to stratify patients with high-risk lesions is controversial.9 In the national survey of the American Society of Gastrointestinal Endoscopy (ASGE), about 16% of patients with clear nasogastric aspirates were found to have active bleeding at the time of endoscopy.10 The bleeding sources in these patients also included esophagitis (10.7%) and varices (5.1%).
Aljebreen and colleagues11 performed a retrospective analysis on 1869 patients with upper GI bleeding. A bloody nasogastric aspirate was significantly associated with high-risk lesions (active bleeding, nonbleeding visible vessel, adherent clot). A bloody aspirate had a specificity of 75.8% and negative predictive value of 77.9% for high-risk lesions. Although nasogastric lavage provides no information about the etiology of bleeding, it can be valuable in localizing the source in a hemodynamically unstable patient because it is a quick and easy test that is performed at bedside. A negative or bilious aspirate does not rule out upper GI bleeding. Nasogastric suction often is not needed with the availability of large-channel therapeutic endoscopes, with forward water jets built into the endoscope and the ability to suction directly from the endoscopic channel port, bypassing the internal suction channeling that passes through the handle of the endoscope and through the umbilicord, which can contain a stepdown in channel size.
Laboratory Data
Laboratory tests appropriate at initial presentation include hemoglobin level, hematocrit, platelet count, prothrombin time, and partial thromboplastin time. Initial hemoglobin level may not depict the degree of bleeding, and initial decision making must be based on clinical grounds. An increased blood urea nitrogen (BUN)-to-creatinine ratio (>36) has been suggestive of an upper GI source of bleeding with a sensitivity of 90% and specificity of 27%.12 BUN levels can weakly predict severity of bleeding but are not helpful in predicting high-risk lesions.13 BUN values can be helpful in the diagnosis; however, the clinical picture is often complicated by other medical illnesses (e.g., renal insufficiency, congestive heart failure) and polypharmacy. Other important laboratory data include liver function tests and cardiac enzyme analysis.
Risk Stratification
Most GI bleeds stop spontaneously without any recurrence. Approximately 20% of bleeds can continue or recur leading to patient morbidity and mortality.14 Numerous clinical factors have been identified that predict a high rate of recurrent bleeding or poor outcome. Old age (>65 years), shock, comorbid illnesses, low hemoglobin on evaluation, melena, multiple transfusions (more than five), hematochezia, fresh blood emesis or nasogastric aspirate, and need for emergency surgery are clinical predictors of increased risk of rebleeding.15,16 A history of alcoholism, a history of cancer, and an Acute Physiology, Age, and Chronic Health Evaluation (APACHE) II score of 11 or greater have also been associated with poor outcomes.17,18
Chiu and colleagues19 prospectively studied 3220 patients with bleeding peptic ulcers from 1993 to 2003 and identified risk factors for mortality. Among patients with bleeding peptic ulcers after endoscopic hemostasis, advanced age, multiple comorbidities, hypovolemic shock, in-hospital bleeding, rebleeding, and need for surgery were significant factors predicting in-hospital mortality (Table 12.2). Based on these individual clinical criteria and endoscopic findings, numerous scoring systems have been formulated to try to stratify high-risk patients for appropriate intervention.
Table 12.2 Statistically Significant Predictors of Persistent or Recurrent Bleeding
| Risk Factor | Odds Ratio for Increased Risk |
|---|---|
| Age | |
| >65 yr | 1.3 |
| >70 yr | 2.3 |
| Shock (systolic blood pressure <100 mm Hg) | 1.2–3.65 |
| Comorbid illness | 1.6–7.63 |
| Transfusion requirements | NA |
| Initial hemoglobin <10 g/dL | 0.8–2.99 |
| Coagulopathy (prolonged prothrombin time) | 1.96 (1.46–2.64) |
| Melena | 1.6 |
| Blood in nasogastric tube or stomach | 1.1–11.5 |
| Hematemesis | 1.2–5.7 |
| Continued bleeding | 3.14 (2.4–4.12) |
| Need for emergency surgery | NA |
NA, Not available.
Modified from Barkun A, Bardou M, Marshall JK; Nonvariceal Upper GI Bleeding Consensus Conference Group: Consensus recommendations for managing patients with non-variceal upper gastrointestinal bleeding. Ann Intern Med 139:843–859, 2003.
Blatchford and associates20 devised a scoring system based on admission hemoglobin, BUN, pulse, systolic blood pressure, presentation with syncope and melena, and evidence of hepatic disease and cardiac failure. Their criteria could identify 20% of patients with very low risk of needing treatment to control bleeding, who could be offered outpatient treatment. The Blatchford scoring system has been validated by other investigators.21 In 1974, Forrest and colleagues23 first classified stigmata of active or recent bleeding based on visualization at the time of endoscopy (Table 12.3). Visible vessel is the term used for an elevated area within the ulcer base. It is thought to be a coagulum over a small-caliber arterial vessel in the ulcer base.24 The color of the lesion can also be predictive of rebleeding: Nonpigmented lesions (white-pale) have a higher risk (71%) than pigmented lesions (38%).25 The frequency with which a visible vessel can be found also depends on the timing of endoscopy and how aggressively the clot is washed to expose the ulcer base. Chung and coworkers26 reported disappearance of visible vessels in 62 patients who underwent endoscopy for 3 consecutive days.
Table 12.3 Modified Forrest Criteria
| Forrest Class | Type of Lesion |
|---|---|
| IA | Arterial spurting |
| IB | Active oozing |
| IIA | Ulcer with nonbleeding visible vessel |
| IIB | Ulcer with adherent clot on surface |
| IIC | Ulcer with red or dark blue flat spot |
| III | Ulcer with clean base |
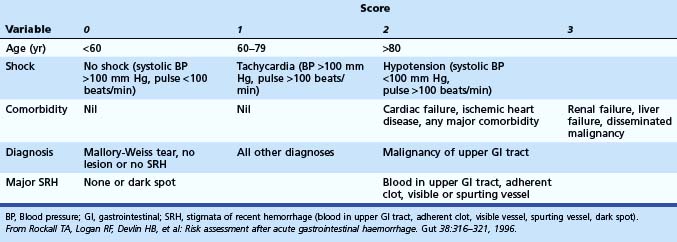
Rockall and colleagues27 developed a scoring system involving clinical and endoscopic criteria. The main aim is to predict risk of rebleeding and mortality. An initial calculation is based on clinical parameters, and further categorization of the bleeding lesion and the stigmata of hemorrhage at the time of endoscopy enables the complete score to be calculated. A total score of less than 3 was associated with excellent prognosis, whereas a score greater than 8 carried a high mortality. For cases with a score less than 3, rebleeding occurred in less than 5%, and mortality was 0%. Multiple studies have been performed to validate the Rockall scoring system.28,29 It has been validated in other studies to be predictive and cost-effective (Table 12.4).30
Table 12.4 Rockall Scoring System for Risk of Rebleeding and Death after Admission to the Hospital for Acute Gastrointestinal Bleeding
Hay and coworkers31 derived a scoring system by using hemodynamics, time from bleeding, comorbidity, and endoscopy findings. Use of the practice guidelines reduced hospital stay in low-risk patients from 4.6 to 2.9 days (P < .001). Saeed and associates32 proposed another scoring system based on endoscopy findings. In a retrospective analysis, Chuan and colleagues compared the Blatchford score with the Rockall score in 354 patients. The Blatchford score identified 92.1% of patients, whereas the Rockall score identified 70.1%. Blatchford scoring based on clinical and laboratory parameters was concluded to have a higher sensitivity to identify high-risk patients (P < .0001).33,34
Kim and associates35 conducted a prospective study to compare the clinical utility of five scoring systems for the prediction of rebleeding and death in patients with nonvariceal upper GI bleeding. Using five scoring systems (Forrest classification, Rockall scoring system, Cedars-Sinai Medical Center Predict Index, Blatchford scoring system, and Baylor College scoring system), 239 consecutive patients were investigated. All patients underwent endoscopy for nonvariceal bleed. There were 35 patients (14.6%) who experienced rebleeding and 20 patients (8.4%) who died. Forrest classification was superior to the others in predicting rebleeding and death. The Cedars-Sinai Medical Center Predict Index and the Rockall scoring system showed high positive predictive values for predicting rebleeding (Cedars-Sinai Medical Center Predict Index) and death (Rockall scoring system). Forrest classification was thought to be the most useful scoring system for the prediction of rebleeding and death in patients with nonvariceal upper GI bleeding. The development of clinical scoring systems is very encouraging because these can be used by emergency department physicians, general practitioners, and junior house staff to triage patients effectively. These scoring systems have been validated in large cohorts in diverse populations and have been shown to be effective in risk stratification and cost-effectiveness.
Timing of Endoscopy
Endoscopy primarily is used to determine the cause of bleeding, prognosticate, and administer appropriate endoscopic therapy to control bleeding and prevent rebleeding.36,37 The timing of endoscopy has been a subject of debate, especially in patients who are clinically stable with no evidence of further bleeding. It is now evident that early endoscopy provides essential data affecting patient triage.
Lee and associates38 showed that early “endoscopy triage” leads to early discharge of patients with low risk of rebleeding, without increasing morbidity and mortality. Median cost savings were $2068. Cipolletta and coworkers39 used endoscopic and clinical criteria to identify patients at low risk for recurrent bleeding. Patients were randomly assigned to outpatient care versus hospital admission. No patients underwent surgery or died. Rates of recurrent bleeding were comparable (2.1% and 2.2%) in both groups. Median costs were $340 for outpatients and $3940 for inpatients. Based on multiple studies confirming the beneficial effects of endoscopic therapy, urgent endoscopy has been recommended by the National Institutes of Health40 and ASGE41 for patients who have active bleeding or who are at high risk for rebleeding. The definition of urgent endoscopy ranges in various studies from 2 to 24 hours after presentation to the hospital. It has been determined that 76% to 78% of patients with acute GI bleeding undergo endoscopy within the first 24 hours.42,43 Even if discharge is not always the best option, triage results might help choose the correct level of care within the hospital.
Preparation and Place for Endoscopy
Endoscopy is best performed in a fully equipped endoscopy suite where staff members are trained to take care of the patient and in the use and maintenance of endoscopes and their accessories (Fig. 12.1). A general recommendation is that patients with mild to moderate bleeding can have endoscopy the next day in the endoscopy unit. Patients whose presentation suggests a major or severe bleeding event (e.g., hemodynamic compromise) can undergo endoscopy at the bedside in the intensive care unit after initial resuscitation. Endotracheal intubation to protect the airway and prevent aspiration should be considered in high-risk patients who are experiencing active hematemesis, marked agitation, lethargy, and persistent shock. In the United States, there is increasing use of propofol for routine and even emergency endoscopy; use of propofol not administered by an anesthetist has also been studied but is not widely employed in current practice.44
In patients with active or recent hemorrhage, residual blood in the stomach, especially in the fundus, can limit the quality of the examination, and residual clots must be removed or flushed away. Poor visualization at the time of endoscopy has been associated with worse outcomes.45 Multiple methods have been proposed to overcome this problem. Vigorous gastric lavage using a large-bore nasogastric tube at the time of endoscopy and instillation of 3% hydrogen peroxide46 to dissolve small clots have been proposed. Use of an endoscope with a large device channel may offer a convenient and effective method to remove clots and blood from the stomach.47,48 Connecting a suction line directly to the endoscope device channel, bypassing the internal system, provides rapid and impressive clearing of blood and clot. Randomized controlled trials have looked into using erythromycin as a promotility agent to improve quality and yield of endoscopy.49–52 Patients with acute GI bleeding were randomly assigned to receive erythromycin (3 mg/kg intravenously) and were compared with a no treatment arm. The treatment group (erythromycin 3 mg/kg intravenously 20 to 60 minutes or 120 minutes before endoscopy) had an improved quality of endoscopic examination and reduced need for second-look endoscopy, and erythromycin was deemed a cost-effective strategy.
Etiology of Nonvariceal Upper Gastrointestinal Bleeding
Causes of nonvariceal upper GI bleeding are presented in Table 12.5.
Table 12.5 American Society of Gastrointestinal Endoscopy Bleeding Survey: Endoscopic Diagnosis for Upper Gastrointestinal Bleeding in 2225 Patients
| Diagnosis | Frequency (%) |
|---|---|
| Duodenal ulcer | 24.3 |
| Gastric erosions | 23.4 |
| Gastric ulcer | 21.3 |
| Varices | 10.3 |
| Mallory-Weiss tear | 7.2 |
| Esophagitis | 6.3 |
| Erosive duodenitis | 5.8 |
| Neoplasm | 2.9 |
| Stomal ulcer | 1.8 |
| Esophageal ulcer | 1.7 |
| Miscellaneous | 6.8 |
Gastric and Duodenal Ulcers
Gastroduodenal ulcer disease is the most common cause of acute GI bleeding in the Western world despite an overall decline in the disease incidence in the last 3 decades.53–56 Ulcer disease is responsible for 50% of patients presenting with upper GI bleeding.57 With better understanding of the risk factors and better treatment protocols, overall prevalence and hospital admissions for peptic ulcer disease have decreased. The hospitalization rate for ulcer-related GI bleeding has not changed significantly, however.56 More elderly patients (>65 years old) are being hospitalized with bleeding ulcers.54,58–60 Mortality associated with peptic ulcer disease is still about 14% and is known to increase progressively with age.14,61 Previously, all peptic ulcers were considered idiopathic; in the 1980s and 1990s, most ulcer disease was attributed to Helicobacter pylori and nonsteroidal antiinflammatory drugs (NSAIDs). H. pylori was associated with 90% of duodenal ulcers and 70% of gastric ulcers.62 With awareness and aggressive therapy, there has been a decline in the prevalence of H. pylori in the Western world. The epidemiology of peptic ulcer disease has changed with a much higher percentage of H. pylori–negative ulcer disease now being reported.63,64
Mamdani and colleagues65 reported an increase in admission rate from upper GI bleeding in patients older than 65 years with greater use of cyclooxygenase-2 (COX-2) and NSAIDs. H. pylori infection and NSAID use both increase risk of peptic ulcer disease and upper GI bleeding.14,66–68 In a meta-analysis by Huang and colleagues,66 NSAID use was significantly greater in patients with peptic ulcer disease than in matched controls. H. pylori infection marginally increased the risk of ulcer bleeding. Risk of bleeding was much greater when both risk factors coexisted, having an additive effect. COX-2 inhibitors increase the risk modestly—less than NSAID use but higher than no use at all.69 Cardiovascular risks associated with some COX-2 inhibitors must also be taken into consideration. Patients seen with GI bleeding with ulcers with high-risk features (active bleeding, nonbleeding visible vessel) commonly have continued or recurrent bleeding, and up to 35% require urgent surgery.70 Over the last 15 to 20 years, endoscopic therapy has been shown to benefit this population of patients. Endoscopic therapy has been associated with reduction in the rates of rebleeding, blood transfusion, length of hospital stay, need for other therapeutic interventions, costs, and mortality.71–73
Indications for Endoscopic Therapy for Gastroduodenal Ulcers
After initial resuscitation of patients with severe upper GI bleeding and initiation of medical therapy for patients with suspected ulcer hemorrhage, urgent endoscopy is integral to the initial care, permitting effective triage of patients into low-risk and high-risk categories. Endoscopic treatment directed at major stigmata for ulcer hemorrhage should be intentionally planned because it has shown to improve outcome. A multicenter U.S. trial of 4090 patients hospitalized for nonvariceal GI bleeding showed 10.3% had active bleeding (arterial or oozing), 12.2% had nonbleeding visible vessel, 8.3% had adherent clot, 9.9% had flat spot, and 58.4% had an ulcer with clean base.74 Endoscopic treatment is not recommended for patients with low-risk endoscopic stigmata, including an ulcer with a clean base or a dark nonprotuberant pigmented spot in the ulcer base. Patients who have active bleeding, spurting or oozing from the ulcer, or a nonbleeding visible vessel in the ulcer base should receive endoscopic therapy.75,76 In a meta-analysis by Bardou and colleagues,77 endoscopic treatment was associated with statistically significant absolute decrease in rates of rebleeding, surgery, and mortality.
Nonbleeding Adherent Clot
Endoscopic therapy for ulcers with an adherent clot is also recommended. A high rate of rebleeding has been documented in these patients (33% to 40%) caused by an underlying visible vessel. Earlier studies did not show any benefit of endoscopic monotherapy in ulcers with adherent clots.78,79 Severe bleeding may be induced from removal of the clot.76 This observation has often been a deterrent to intervention and has led to confusion regarding whether or not to intervene. Later studies have shown combination therapy to be superior to medical therapy.
In a multicenter study organized by the Mayo Clinic GI Bleeding Team, Bleau and colleagues80 randomly assigned patients with adherent clots to receive medical therapy or endoscopic therapy. In the treatment arm, 1 : 10,000 epinephrine was injected in four quadrants around the ulcer before removing the overlying clot aggressively (cold snare, suction, manipulation with biopsy forceps and tip of the endoscope). Underlying stigmata were treated with heater probe coagulation. Rates of rebleeding were 34.3% in the medical treatment arm and 4.8% in the endoscopic treatment arm. Jensen and coworkers81 randomly assigned 32 patients with severe bleeding and adherent clots to combination endoscopic therapy or medical management. In their study, after epinephrine injection in four quadrants, cold guillotining was used to shave off the clot to 3 to 4 mm above the ulcer base. The residual clot was treated with coaptive coagulation. Rebleeding rate in the medical treatment group was 35.3% and in the endoscopic therapy group was 0%.
A meta-analysis by Kahi and associates82 of six studies comprising 240 patients showed significant reduction in rebleeding rates (relative risk 0.35, 95% confidence interval [CI] 0.14 to 0.83) in patients who received endoscopic treatment versus medical treatment alone (8.2% vs. 24.7%). Other outcomes, such as length of hospital stay, need for surgery, transfusion requirements, and surgery, were not significant. The underlying mechanism of rebleeding in the case of an adherent clot is thought to be stigmata underlying the clot, which are the major determinants of rebleeding. Evaluation of the ulcer base is vital. Injection of 1 : 10,000 epinephrine seems to reduce the risk of bleeding and allows for the safe removal of the overlying clot.
Although these studies are promising, aggressive removal of clot and endoscopic therapy have their own risks. Adherent clot removal is advisable in the appropriate clinical setting (high-risk patients) in the hands of expert endoscopists. It is important for the endoscopist to maintain the entire clinical picture in focus, including the appearance and location of the ulcer, before removing a clot. Large ulcers (>2 cm) and especially deep ulcers may contain exposed serosally based arteries that are expectedly large. Ulcers with these features in the posterior duodenal bulb and along the lesser curvature of the stomach may be particularly vulnerable to containing large compromised arteries—the gastroduodenal in the posterior duodenal bulb and the left gastric along the lesser curve. The caliber of these arteries may exceed the capabilities of standard endoscopic therapies. If there is question regarding the size of a potential artery underlying a dense adherent clot, endoscopic ultrasound (EUS) may be performed to screen the lesion. Preliminary data suggest that EUS-guided therapy may have an important role in delivering more effective treatment.83
Available Modalities for Endoscopic Therapy
Injection Therapy
Injection therapy is used as an adjunct with other modalities for ulcer hemostasis. Epinephrine is the most established injection agent used for peptic ulcer injection therapy. Epinephrine diluted to 1 : 10,000 or 1 : 20,000 in normal saline is found to be most effective and safest.84,85 The mechanism of action is thought to be vasoconstriction, platelet activation, and stimulation of the coagulation cascade.86 In addition, a tamponade effect resulting from the volume of fluid injected into the ulcer base is thought to be therapeutic.87
Technique
A disposable injection needle with a retractable tip is used. Injection is undertaken in 0.5- to 1.0-mL increments in all four quadrants around the nonbleeding stigmata because the path of the exposed artery is unknown. A total volume of 20 to 45 mL may be injected to achieve the desired results.88,89 No cardiac side effects have been reported as a result of larger volume of epinephrine being injected. In the setting of active bleeding, if the initial injection results in noticeable slowing and, more so, cessation of bleeding, a single injection site may suffice. Additional volume may be injected via a single site as long as any lifting effect does not impair access to the bleeding vessel for second-line therapy with coaptive coagulation or mechanical closure.
Thermal Coaptive Therapy
Thermal methods formerly included the neodymium:yttrium-aluminum-garnet (Nd:YAG) laser and presently include heater probe and electrocoagulation (Fig. 12.2). Laser therapy and monopolar coagulation are no longer popular because of the lack of portability to bedside, high cost, and perforation risks. Multipolar electrocoagulation and heater probe are the most widely used thermal modalities. The advantages of these devices are their excellent efficacy; safety; portability; and ability to combine irrigation, tamponade, and coagulation.
In coaptive coagulation, the probe is used to compress physically and tamponade the bleeding vessel, followed by thermal energy sealing the walls of the vessel. In an animal model, arteries with a diameter of 2.5 mm can be coagulated with a heater probe using this technique.93
Technique
Bipolar Electrocautery (Multipolar Probe)
The technique for applying bipolar electrocoagulating energy has not been standardized and varies among reported clinical trials. In canine models, a large (3.2-mm) multipolar probe produced better hemostasis than a smaller (2.3-mm) probe.94,95 Laine96 recommends forcefully applying a large (3.2-mm) bipolar circumactive probe (BICAP) on a power setting of 3 to 5 for a prolonged duration, such as 14 seconds, or seven pulses of 2 seconds each. Jensen and Hirabayashi94 used a setting of 3 to 4 with 10-second pulses on a BICAP II generator. The Gold Probe (Boston Scientific, Microvasive Endoscopy, Natick, MA), which contains a gold foil–wrapped tip, has been shown in clinical trials to be effective at low power settings with longer pulse duration.97
Heater Probe
The present technique for bleeding peptic ulcers involves using the larger heater probe, firm tamponade directly on the bleeding point or visible vessel, and coagulation with at least 120 J (four pulses of 30 J each) before moving the probe.98 An additional application of energy when using the heater probe or multipolar (bipolar) probes as the probe is removed from the treatment site can reduce unwanted tissue adhesion and the inadvertent tearing of the coagulated sealant with resultant rebleeding. In a prospective study by Bianco and colleagues,99 combined therapy with epinephrine injection with bipolar probe showed better results than epinephrine alone.
Mechanical Devices
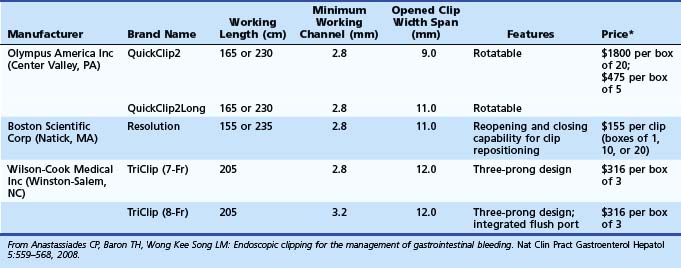
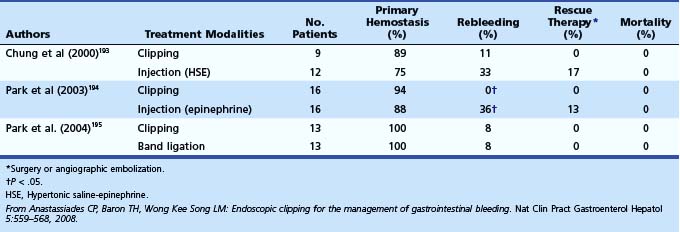
Numerous mechanical devices, including hemoclip, endoloop, and rubber band, are now available for endoscopic treatment of GI bleeding. Although endoloop and rubber band ligation have little role in treating peptic ulcer bleeding, use of hemoclips has become very popular for emergency nonvariceal hemostasis. The underlying mechanism of action is mechanical clamping of the bleeding vessel. Various clips are available (Table 12.6). Multiple randomized trials have compared use of clips alone or in combination with other therapies. Most studies have been performed on first-generation clips. Jensen and associates100 studied hemostasis capability of three presently available clips in a canine model; initial hemostasis was 100% for all types with longer retention times reported for the largest of the available clips, which can also be opened and closed on demand before deployment (see Table 12.6).
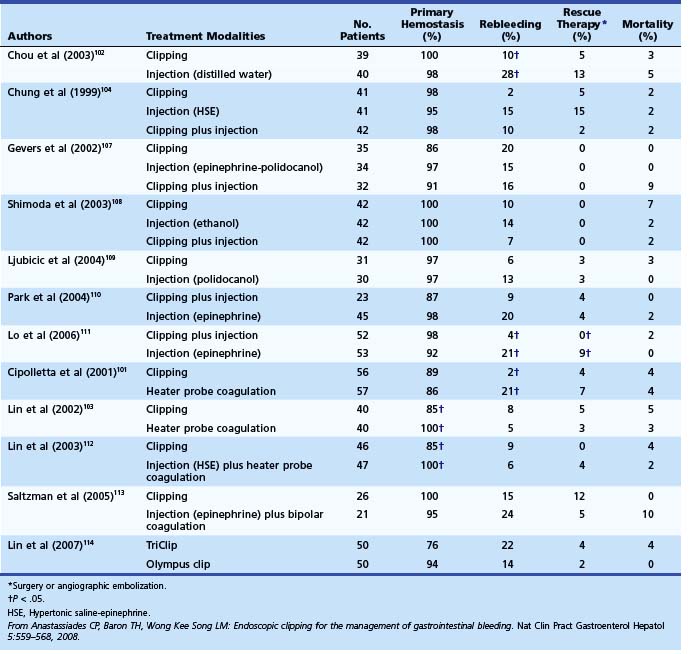
Among trials comparing hemoclip with other endoscopic therapy, Cipolletta and colleagues101 randomly assigned 112 patients with major stigmata to heater probe or hemoclip therapy (Fig. 12-3). Rates of recurrent bleeding (21% vs. 1.8%) and need for surgery (7% vs. 3.6%) were significantly lower in the hemoclip group. The investigators concluded that hemoclip therapy was safe and superior to heater probe therapy. In a randomized study by Chou and coworkers,102 hemoclip therapy was found to be superior to distilled water injection therapy in patients with major stigmata (active bleeding vessel, nonbleeding visible vessel). In their study comparing hemoclip versus heater probe, Lin and associates103 reported 85% initial hemostasis in the hemoclip group compared with 100% in the heater probe group. Rebleeding rate was 8.8% in the hemoclip group and 5% in the heater probe group. In trials comparing combination therapy, Chung and colleagues104 prospectively assigned 124 patients to hemoclip, hypertonic saline–epinephrine (HSE), and combined treatment groups. Initial hemostasis was comparable in all groups. Rates of recurrent bleed in hemoclip, HSE, and combination therapy groups were 2.4%, 14.6%, and 9.5%. Buffoli and colleagues105 did not find an additional advantage of hemoclip therapy when used in combination with epinephrine injection therapy. A favored trend toward reducing surgery was seen in the combination therapy group (0% vs. 7.4%).
Several randomized controlled trials investigating the use of endoscopic clips alone or in combination with other endoscopic modalities have reported variable success (Table 12.7).106 The most important factor seems to be difficulty in appropriate placement of the clip for effective hemostasis, especially for ulcers in difficult-to-approach sites such as high on the lesser curvature and posterior duodenal wall.103 Improvements in endoscopic clip devices have included single-use, rotatable, and reopening features. Present data suggest clips to be as effective as thermal therapy. The choice between the two modalities is at the discretion of the endoscopist depending on his or her comfort level with the device and the location of the lesion (see Table 12.7).106
Combination Therapy
There is a theoretical advantage of combining therapies because hemostasis is achieved by different mechanisms. The most popular combination therapy used is injection therapy and thermal coagulation (Fig. 12.4); epinephrine causes vasoconstriction, reduced blood flow, and platelet activation, which potentiates the coaptive coagulation by thermal energy. Another advantage may be improved visualization of the target area for thermal coagulation after initial injection therapy in an actively bleeding ulcer. Combination therapy with injection and coaptive therapy has consistently shown superiority over medical therapy.115
In a randomized controlled trial, Chung and colleagues116 compared epinephrine injection with epinephrine injection plus heater probe in actively bleeding ulcers. Patients with major, active arterial bleeding had a better outcome regarding need for surgery (29.6% vs. 6.5%) and length of hospital stay (6 days vs. 4 days) after combination therapy. Combination therapy did not improve outcomes of patients with lesions with oozing only. Lin and coworkers117 compared epinephrine injection alone, bipolar electrocoagulation alone, and combined treatment. Combination therapy was associated with reduced rate of rebleeding, reduced treatment failure, and reduced transfusion requirements. In contrast, Marmo and associates118 showed single endoscopic therapy with thermal probes or clips is as effective as combination therapy and may be safer than combination therapy including injection therapy in high-risk ulcers.
A meta-analysis performed by Calvet and colleagues119 looked at trials comparing epinephrine injection alone versus epinephrine plus a second method (thermal, mechanical, sclerosant) for patients with upper GI hemorrhage from peptic ulcer disease. The analysis included 16 studies with 1673 patients; only studies with major stigmata of bleeding defined by Forrest criteria IA, IIA, and IIB were included. Addition of a second endoscopic method reduced rate of rebleeding (18.4% vs. 10.6%), need for surgery (11.3% vs. 7.6%), and mortality (5.1% vs. 2.6%) compared with epinephrine injection alone. Risk of further bleeding decreased regardless of the type of second method used. Risk of significant complications, including massive bleeding, gastric wall necrosis, and perforation, was the same in both groups (1.1%).
A meta-analysis by Yuan and associates120 comparing clips with other modalities showed that initial hemostasis was 92% for clips versus 96% for other modalities (CI 0.19 to 1.75); however, rate of rebleeding was 8.5% for clips versus 15.5% for other modalities (CI 0.30 to 1.05). Both values did not reach statistical significance. Hemoclips are equivalent to other endoscopic modalities in terms of initial hemostasis, rebleeding rates, emergency surgery, and mortality rates for treatment of peptic ulcer bleed. Sung and colleagues121 reached a similar conclusion that hemoclips improved hemostasis compared with injection alone but were comparable to thermal coagulation. In a Cochrane review meta-analysis in 2007 that included 1763 patients, adding a second procedure reduced further bleeding rate from 18.8% to 10.4% (odds ratio [OR] 0.51), emergency surgery from 10.8% to 7.1% (OR 0.63), and mortality from 5% to 2.5% (OR 0.50). Subanalysis showed that risk decreased regardless of which modality was used as second therapy. Risk was reduced in all groups.122
Role of Argon Plasma Coagulator
Argon plasma coagulation (APC) is a noncoaptive method that allows controlled noncontact electrocoagulation via high-frequency monopolar energy delivered to the tissue through ionized gas (argon plasma). It has been used instead of standard electrocoagulation, and preliminary data show some promise. In a prospective trial of 41 patients, Cipolletta and colleagues123 compared APC with heater probe therapy; rates of initial hemostasis, recurrent bleeding, emergency surgery, and 30-day mortality were comparable in both groups. Chau and coworkers124 reported similar results in a large (185 cases) randomized trial comparing epinephrine plus heater probe with epinephrine plus APC. A Cochrane review concluded that APC was similar to other endoscopic therapies for nonvariceal upper GI bleeding.125 These data suggest that APC therapy is safe and effective for treatment of ulcer bleeding.
Other Options
Various agents have been used for injection therapy with variable results, including agents causing tamponade (hypertonic saline, distilled water),87 sclerosing agents (ethanolamine, cyanoacrylate, polidocanol),126–128 tissue-fixating (desiccating) agent (alcohol),129 and agents stimulating clot formation (thrombin, fibrin).88,130 There is little evidence that addition of other agents significantly reduces the rate of rebleeding. Some of these agents (e.g., alcohol) have been associated with tissue necrosis of injected areas, clinical perforation, and death. Current data do not show superiority of these agents to currently available therapeutic options, including the settings of rebleeding alternative therapy and therapy in patients with coagulopathies.
Role of Medical Therapy
Medical management targets reduction in morbidity, risk of rebleeding, need for transfusions, hospitalization duration, need for endoscopic or surgical intervention, and mortality. Testing and eradication for H. pylori is important in all patients with peptic ulcer bleeds. Several randomized controlled trials have shown that eradication of H. pylori in patients with bleeding peptic ulcers significantly decreases recurrent bleeding compared with nontreated patients.131,132 In addition to endoscopic therapy, acid suppression therapy has been shown to benefit patients with bleeding peptic ulcers.133 The role of gastric acid inhibition in stopping bleeding or preventing recurrent bleeding is related to sealant clot stability, which is favored at a higher gastric pH.134,135 A pH greater than 6 is required for platelet aggregation, whereas clot lysis occurs at pH less than 6. IV and oral H2 antagonists have previously been used to prevent recurrent bleeding in these patients. Two meta-analyses showed that H2-receptor inhibitors provide no additional benefit in patients with bleeding duodenal ulcers, but statistically significant absolute risk reductions in rebleeding, surgery, and death were seen in patients with bleeding gastric ulcers.133,136 Proton pump inhibitors (PPIs) have been found to be more effective than H2-receptor antagonists in decreasing the rate of recurrent bleeding.137,138
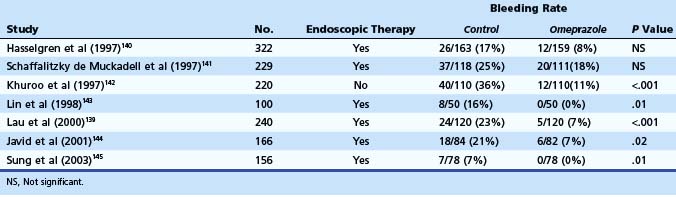
Lau and colleagues139 reported results of a large double-blind randomized trial in which omeprazole was compared with placebo in patients receiving endoscopic therapy for bleeding ulcers (Table 12.8). After receiving endoscopic therapy (active bleeding, nonbleeding visible vessel, stigmata under adherent clot), patients were randomly assigned to IV omeprazole (8 mg/hr continuous infusion) versus placebo for 72 hours. Recurrent bleeding at 30 days, the primary endpoint of the study, occurred in 6.7% of the patients in the omeprazole group compared with 22.5% of the patients in the placebo group (relative risk reduction 70%, P < .001). Most cases of rebleeding occurred within 72 hours of endoscopic therapy. A Cochrane meta-analysis by Leontiadis and colleagues146 on the role of PPI therapy in peptic ulcer bleeding included 24 trials with 4373 patients. Reductions in rebleeding, the need of surgery, and repeat endoscopic therapy were statistically significant with PPI use. Other meta-analyses reached similar conclusions.147–150 Post hoc analysis of the Cochrane data by Leontiadis and colleagues150 showed important differences between Asian and non-Asian patients. The effects of PPI therapy on rebleeding and need for surgery were more marked in the Asian trials, and PPI therapy was associated with significant reduction in 30-day mortality in Asian patients.
Lau and coworkers151 prospectively showed that IV bolus followed by infusion of PPI started before endoscopy decreased the need for endoscopic therapy, number of actively bleeding ulcers, and duration of hospitalization. A retrospective study from Keyvani and colleagues152 showed that PPI (oral and IV) started before endoscopy significantly reduced rebleeding, surgery, duration of hospital stay, and mortality in patients presenting with ulcer hemorrhage. In a meta-analysis, Laine and McQuaid153 studied the role of PPI therapy as an adjunct to endoscopic therapy. Three groups were analyzed: (1) IV PPI (bolus followed by infusion) versus placebo, (2) IV PPI (bolus followed by infusion) versus H2 blockers, and (3) oral or intermittent IV PPI versus placebo. Their conclusions were that PPI therapy after endoscopic therapy reduced rebleeding, need for surgery, need for urgent intervention, and mortality. The results were most consistent for bolus followed by continuous IV PPI for 72 hours.
Based on randomized clinical trials, the recommended dose of PPI is equivalent to omeprazole 80 mg IV bolus followed by 8 mg/hr infusion for 72 hours. The U.S. Food and Drug Administration (FDA) has not approved PPIs for the indication of upper GI bleed. In view of their known benefits and very good side-effect profile, starting PPI therapy seems to be a reasonable approach. Increasing data indicate that perhaps it is safe to switch the patient to oral PPI early in the course of management.154,155 Somatostatin, octreotide, and tranexamic acid may have a theoretical advantage by their different mechanism of action of helping hemostasis, but there is no firm evidence to recommend them over PPI therapy for nonvariceal upper GI bleeding.
Second-Look Endoscopy
Routine repeat (second-look) endoscopy is done 24 hours after the initial procedure with the intent of repeat endoscopic treatment of residual high-risk lesions and has been advocated to be beneficial in some clinical situations. Few randomized trials have addressed the issue, and these trials show conflicting results. Messmann and colleagues156 reported no improvements in outcomes when scheduled repeat endoscopy was compared with second-look endoscopy performed at recurrent bleeding. Villanueva and associates157 noted a nonsignificant trend toward better outcomes in the group that received routine second-look endoscopy within 24 hours. Saeed and colleagues158 adopted an approach of scheduled retreatment in high-risk patients based on a composite clinical and endoscopic score and showed significant benefit in the prevention of rebleeding.
Pooled data on second-look endoscopy showed that routine second-look endoscopy with retreatment significantly reduced the risk of recurrent bleeding compared with expectant management (absolute risk reduction 6.2%, P < .01, number needed to treat 16).159 The risk of surgery and death were not significantly influenced by second-look endoscopy. Cost-effectiveness of repeat endoscopy has been verified in a hypothetical model,160 but no prospective studies are available to date. Routine second-look endoscopy in all patients is not recommended. It may be justified in selected high-risk patients. Second-look endoscopy may be needed in patients with an incomplete initial endoscopy because of poor visualization or other technical difficulties.
Recurrent Bleeding
The size and the depth of ulcer influence the bleeding rate. Increased ulcer size (>1 cm) is associated with an increased risk of rebleeding and mortality. The failure rate of therapeutic endoscopy is much greater in ulcers larger than 2 cm.161 Ulcers located high on the lesser curvature of the stomach and on the posteroinferior wall of the duodenum also seem to have a higher rate of rebleeding.24 Primary hemostasis is achieved in greater than 95% of patients with bleeding peptic ulcers and ulcers with a nonbleeding visible vessel. Rebleeding can occur in 10% to 20% of these patients with mortality of 4% to 10%. In a prospective study, Manguso and colleagues162 recruited patients admitted for nonvariceal upper GI bleeding with Forrest I lesions (active bleeding) at time of endoscopy. All patients received standard endoscopic therapy and IV PPI. The investigators reported a rebleeding rate of 12%, 2.8% of patients required surgery, and there was an overall mortality of 5.6% in a 24-day period.
In a retrospective trial of patients with nonvariceal upper GI bleeding, rate of recurrent bleed was 20% after endoscopic therapy. The authors developed a score to predict rate of rebleeding in patients after endoscopic therapy. The score was based on six factors: failure to use a PPI after the procedure, endoscopically shown active bleeding, treatment with epinephrine monotherapy, peptic ulcer as a source of bleeding, IV heparin or low-molecular-weight heparin after procedure, and moderate or severe cirrhosis.163
In a prospective randomized trial, Lau and coworkers164 randomly assigned 92 patients with recurrent bleeding after endoscopic hemostasis; 48 patients were assigned to repeat endoscopic therapy, and 44 patients were assigned to emergency ulcer surgery. Of patients in the repeat endoscopic therapy group, 73% (35 of 48) had long-term control of bleeding by repeat endoscopic therapy; 27% (13 of 48) required surgery because of 11 endoscopic failures and 2 perforations secondary to thermal coagulation. Overall, a lower complication rate (14.6% vs. 36.4%) was seen in the repeat endoscopy group. This study suggests that repeat endoscopy is effective in reducing the need for surgical intervention and its associated complications.
During endoscopic retreatment with coagulation therapy, a large thermal probe (3.2-mm) is desirable. Repeat thermal therapy at the same location increases the risk of clinical perforation and should be used with caution in therapy of acute lesions, such as a Mallory-Weiss tear, stress ulcer, and Dieulafoy’s lesion in the gastric fundus, and in severely ill immunocompromised patients with poor tissue healing capability.164 Injection therapies may be ineffective because of increased fibrosis, especially of chronic and penetrating ulcers. Injection therapy is reasonable to attempt during active spurting. If there is no immediate response, alternative therapy is needed. Refractory rebleeding in patients who are not candidates for surgical intervention or who have also failed angiographic intervention may be candidates for EUS-guided therapy by direct vascular injection of embolic material or glue. Fine needle application of tissue glues and even alcohol can be performed into specific types of lesions (e.g., gastrointestinal stromal tumors, pseudoaneurysms).165
Complications of Endoscopic Therapy
Perforations are typically small (probe-sized), and efforts should be directed at closing the perforation with hemostatic clips. For larger perforations in the stomach, except posteriorly, and in the anterior duodenum, omental fat can be grasped and pulled into the defect, then clipped into place (omental patch). Laine and Peterson14 reported induction of uncontrollable bleeding requiring urgent surgery in 0.3% of cases and perforation in 0.5% of patients who underwent endoscopic therapy for peptic ulcer hemorrhage. In a meta-analysis by Cook and associates,71 endoscopic hemostasis induced bleeding requiring surgery in 0.39% and 0.4% of cases for contact thermal devices and injection therapy, and perforation rates of 0.7% and 0% were reported.
Failure of Endoscopic Therapy
Surgery for bleeding peptic ulcer has declined in the past 2 decades secondary to improvements in endoscopic hemostasis techniques and acid suppression therapy. However, active nonvariceal GI hemorrhage that cannot be controlled by endoscopic intervention and especially hemorrhage that overwhelms the ability to visualize any bleeding site requires an urgent operation. It is imperative to obtain surgical consultation in all patients presenting with the aforementioned clinical situation. Accurate prediction of patients likely to do poorly after endoscopic therapy is desirable. Wong and colleagues166 analyzed risk factors associated with treatment failure with combined injection therapy and heater probe thermal coagulation. Hypotension, hemoglobin less than 10 g/dL, fresh blood in the stomach, ulcer with active bleeding, and ulcers larger than 2 cm were independent risk factors predicting poor outcome. In a prospective study by Chung and coworkers,167 presence of spurting and ulcers larger than 2 cm were significantly related to failure of endoscopic therapy. Few data support elective surgery in these patients after their first endoscopic intervention, although it sounds appealing in theory because elective surgery carries a much lower mortality than emergency surgery. Maximal acid suppression therapy, close monitoring for signs of rebleeding, and surgical team on standby may be the best treatment strategy.
Therapeutic Angiography
Therapeutic angiography is an alternative option for patients who have failed endoscopic therapy and have a high surgical risk. Therapeutic options include selective intraarterial vasopressin infusion or embolization with microcoils, gelatin, or polyvinyl alcohol particles. Embolization has been shown to stop bleeding in massive gastroduodenal ulcers. Detection of bleeding site at the time of endoscopy provides vital information to the interventional radiologist to select the target area for catheterization. Technical success rates have been reported to be 50% to 90%.168–170 Known complications of embolization are bowel ischemia, necrosis with perforation, abscess formation, and hepatic infarction especially in patients with poor hepatic reserve.
Prevention of Recurrent Bleed
Prevention of recurrent ulcer disease and bleeding is very important in patients with ulcer hemorrhage. Convincing data indicate that treatment with PPIs prevents ulcer rebleeding. Follow-up endoscopy is warranted to exclude malignancy in certain patients with gastric ulcer. The data linking persistent H. pylori with recurrent ulcer hemorrhage are compelling, making eradication of infection the best approach for these patients.171–174 Most tests of active infection may exhibit increased false-negative rates in the context of acute bleeding.175–178 The optimal diagnostic approach may include testing for H. pylori by antral biopsy at the time of endoscopy, with reconfirmation of negative results by repeat testing outside the acute setting of bleeding. Oral therapy can be started immediately or during follow-up in patients found to have H. pylori. Patients with peptic ulcer bleeding should be encouraged to discontinue use of NSAIDs. If stopping NSAID usage is impossible, therapy with misoprostol (200 µg four times daily) or omeprazole seems to be effective in prevention of gastroduodenal ulcers and erosions.171,179 Switching to COX-2 inhibitors would still require concurrent prophylaxis therapy.
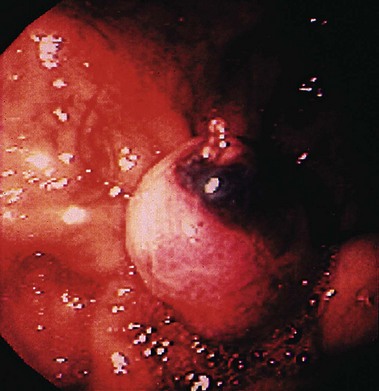
Dieulafoy’s Lesion
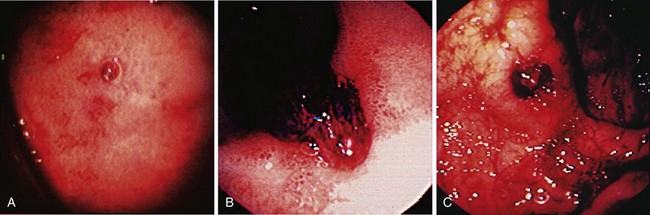
Dieulafoy’s lesion is an uncommon cause of acute nonvariceal GI bleeding, but it can be associated with massive, life-threatening hemorrhage. Originally described by Gallard in 1884 and designated “exulceratio simplex” by the French surgeon Dieulafoy 14 years later, Dieulafoy’s lesion consists of an abnormal, submucosal “caliber persistent artery” (1 to 3 mm diameter) that retains the large caliber and typically protrudes through a small mucosal defect without any surrounding ulceration.180 The underlying mechanism is poorly understood but thought to be due to mechanical compression of overlying mucosa by the large artery resulting in small erosion with rupture of the vessel into the lumen.181 Dieulafoy’s lesion accounts for 1.2% to 1.9% of cases of acute nonvariceal upper GI bleeding,182,183 but an incidence of 5.8% has been reported.184 Although reported in all age groups, Dieulafoy’s lesion predominantly manifests in old men with multiple comorbid conditions.181,184 Typically, a Dieulafoy lesion is located in the stomach usually within 6 cm of the gastroesophageal junction, the classic site, in 60% to 64% of cases and in the duodenum in another 14% to 18%.184,185
Diagnosis
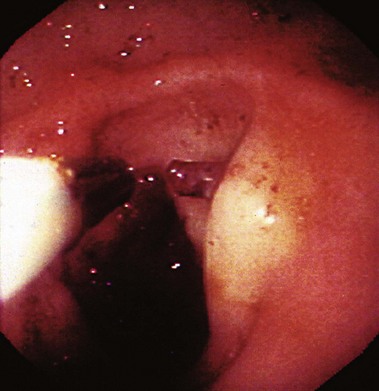
The endoscopic criteria for the diagnosis of Dieulafoy’s lesion are (1) active arterial spurting or micropulsatile streaming; (2) visualization of a protruding vessel with or without active bleeding; or (3) fresh, densely adherent clot with a narrow point of attachment, from a minute (<3 mm) mucosal defect or through normal surrounding mucosa (Fig. 12.5).186 Diagnosis at the time of endoscopy can be difficult because there is no identifying lesion (ulcer) to indicate the source. The diagnosis rates for initial endoscopy range from 49% to 63%, and repeat endoscopy is often required.182,187 In view of the intermittent nature of bleeding from Dieulafoy’s lesion, which can span years, the sensitivity of endoscopic diagnosis is increased through early endoscopy in patients with acute GI bleeding.
Endoscopic Therapy
Hemostasis and long-term ablation of the caliber persistent artery of Dieulafoy’s lesion can be achieved by endoscopic treatment in more than 90% of patients.183,184 Endoscopic therapy can be effective with most methods—injection or thermal probe monotherapy, mechanical devices, or combination treatment. Most data are from case series because randomized trials are difficult to perform secondary to the rarity of the condition. Therapy is directed at controlling bleeding and preventing rebleeding over the long-term. To accomplish this goal, the underlying caliber persistent artery must have its path altered. Although mechanical devices (clips) have been successful for controlling bleeding from acute events, it is uncertain that they would provide long-term benefit because this form of therapy is very superficial (mucosal).
Injection and Thermal Monotherapy
Multiple agents, including epinephrine, sclerosant, alcohol, hypertonic glucose, and cyanoacrylate glue, have been reported for successful injection therapy. Although there is a high initial success rate, there seems to be a high rate of rebleeding with injection monotherapy. Baettig and colleagues184 treated 19 patients using injection therapy with epinephrine or polidocanol and had a rebleeding rate of 21%, whereas Kasapidis and colleagues188 in their retrospective analysis reported a rebleeding rate of 55% in patients with Dieulafoy lesions treated with injection therapy using epinephrine, ethanolamine oleate (5%), or combination of the two.
Thermal probe monotherapy has been found to be effective, but variable success rates have been reported. Lin and coworkers189 had 100% success in their six patients. Parra-Blanco and associates190 had two of six patients with recurrent bleed after treatment with heater probe.
Combination Therapy
Stark and colleagues191 presented the Mayo Clinic GI Bleeding Team data of 10 years on management of Dieulafoy’s lesion. Dieulafoy’s lesion was found in 19 of 1124 consecutive patients with upper GI bleeding. Patients (18 of 19) treated with combination therapy with epinephrine injection and thermal therapy (17 heater probe, 1 BICAP) had a 100% success rate of initial hemostasis, and only 1 patient had rebleeding in the follow-up period. In a review of 6 years of experience at a tertiary care center, 40 cases of Dieulafoy’s lesion were identified with a 90% success rate of hemostasis with endoscopic therapy. Combination therapy with epinephrine injection and heater probe was used in most (28 of 40) patients. Rates of rebleeding were not specified, but only two patients required surgical intervention in the combined treatment group.180 Kasapidis and colleagues188 retrospectively compared injection therapy (epinephrine, ethanolamine) with thermal therapy alone or in combination with epinephrine injection. Initial hemostasis was achieved in all 18 patients. Five of nine patients had recurrent bleeding, whereas none of the patients in the other group (eight of nine had combined therapy; one of nine had heater probe only) had rebleeding.
Mechanical Devices
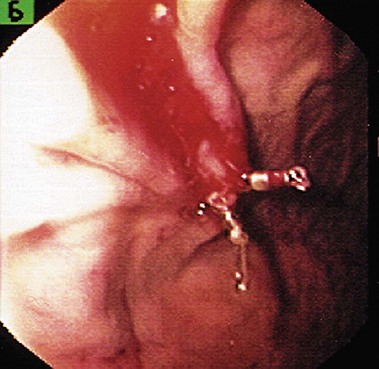
Hemoclip Application
Hemoclip application hemostasis has been used for Dieulafoy’s lesion (Fig. 12.6) similar to other lesions. Yamaguchi and coworkers192 used hemoclip application as first-choice hemostatic treatment for bleeding from Dieulafoy’s lesion. Initial hemostasis was achieved in 94.1% of patients requiring 3.1 (mean, range 1 to 6) clips per patient. Rate of recurrent bleeding was 9.4%, which was successfully treated with repeat endoscopic therapy. None of the patients required surgery. Parra-Blanco and associates190 used hemoclip application to control bleeding from Dieulafoy’s lesion in 69% of their patients. Initial success rate was 97% (17 of 18) with a mean of 2.7 clips per patient.
In a randomized prospective trial, Chung and colleagues193 compared mechanical methods (hemoclip and band ligation) with injection therapy with HSE (Table 12.9). Of 24 patients, 12 were assigned to mechanical therapy (9 hemoclip, 3 rubber band ligation), and 12 were assigned to the injection therapy group. Mechanical therapy was more effective than injection therapy in terms of initial hemostasis (91.7% vs. 75%), recurrent bleeding (8.3% vs. 33.3%, P < .05), and need for surgery (0% vs. 17%).
Endoscopic Rubber Band Ligation
Matsui and coworkers196 compared endoscopic rubber band ligation with bipolar electrocoagulation in patients with acute GI hemorrhage exclusive of chronic gastroduodenal disease (Fig. 12.7). Endoscopic therapy was provided to 27 patients with Dieulafoy’s lesion. Success rate of hemostasis was 100% (13 of 13) for endoscopic band ligation versus 85.7% (12 of 14) for bipolar electrocoagulation. In a retrospective Veterans Affairs study, Mumtaz and colleagues197 presented data of 23 patients with Dieulafoy’s lesion treated with endoscopic band ligation (14 of 23) and injection with or without thermal therapy. Initial hemostasis was achieved in all patients in both groups, and only one patient had early rebleeding (within 72 hours) in both groups. Nikolaidis and associates198 retrospectively reviewed the results of endoscopic band ligation in 23 patients with Dieulafoy’s lesion. Initial hemostasis success rate was 96% (22 of 23). One patient with jejunal Dieulafoy’s lesion required surgery for rebleeding.
Recurrent Bleeding
There is little evidence to support acid suppression therapy to prevent rebleeding from Dieulafoy’s lesions. Because the underlying pathophysiology that causes the aberrantly located artery to bleed is unknown, long-term use cannot be justified. It is often administered either as empiric therapy for acute GI bleeding or to treat concurrent GI pathology. Short-term recurrent bleeding is common, occurring in 9% to 22% of cases.182,184,186,187,189,199 In case of rebleeding, repeat endoscopic therapy is recommended to destroy the aberrant artery effectively because it is successful in most patients. The long-term rate of recurrent bleeding is low once Dieulafoy’s lesion is completely treated. Studies have reported no recurrent bleeding with follow-up of up to 3 years after treatment188,190; however, repeat bleeding from the same site has been known to occur 6 years after the initial episode.186
Salvage Therapy
Despite a high success rate of endoscopic therapy, surgery may be required as salvage therapy in 3% to 16% of patients. Endoscopy is extremely important for localizing the lesion. Surgical oversewing of the vessel is associated with a higher risk of recurrent bleeding for reasons mentioned earlier. Wedge resection is more intuitive and is a better surgical procedure for these patients with refractory bleeding.185,187 Angiography can be used not only to localize but also to embolize the bleeding vessel selectively. Variable success has been reported, and angiography should be reserved for patients who have failed endoscopic therapy and are poor candidates for surgical intervention. After the vessel site has been identified and bleeding has become refractory, EUS-guided ablative therapy may become the next and more ideal choice over angiography and surgery.
Mallory-Weiss Tear
Mallory-Weiss tears are a common cause of nonvariceal upper GI bleeding and account for 3% to 10% of cases.200,201 A Mallory-Weiss tear was found by endoscopy in 7% of patients in the ASGE survey.10 Mallory-Weiss tear is a mucosal laceration at the gastroesophageal junction or gastric cardia usually caused by retching or forceful vomiting. The underlying mechanism is thought to be rapid propulsion of gastric cardia into the thoracic cavity through the hiatus from retching or vomiting. If this is forceful enough, a longitudinal laceration can follow. Most patients with Mallory-Weiss tears are in their third to fifth decade, and there is a male preponderance in most case series.200,201 Alcohol use is a common factor and is found in 40% to 70% of patients with Mallory-Weiss tear.202–204 Many other factors have been associated with the development of Mallory-Weiss tears, including severe coughing, pregnancy, heavy lifting, straining, and colonic lavage using polyethylene glycol. Mallory-Weiss tears are well known, although uncommon complications of upper endoscopy.205
Risk Assessment
Few prospective data exist about clinical or endoscopic stigmata of recent hemorrhage and benefits of endoscopic hemostasis for bleeding Mallory-Weiss tears. Bharucha and colleagues200 did a retrospective review of 56 patients presenting with acute GI bleeding to the Mayo Clinic GI Bleeding Team over a 4-year period. Clinical features and endoscopic findings were compared to determine prognostic factors for rebleeding and outcomes. Rebleeding, although unusual (7%), was significantly greater in patients with bleeding diathesis (P = .02); only active bleeding (not other stigmata) was associated with a higher transfusion requirement. The investigators concluded that GI bleed from a Mallory-Weiss tear without (1) clinical features of severe bleeding (hematochezia, hemodynamic instability), (2) coagulopathy or other major systemic disease, or (3) active bleeding at endoscopy can be managed with a brief period of observation.
Kortas and colleagues202 did a retrospective analysis of 73 cases of Mallory-Weiss tear. The most common risk factor was alcohol use (40%). A complicated course was present in 17 (23%) patients. Among the factors studied, a complicated course was associated with a low hematocrit (P = .009) and active bleeding on initial endoscopy (P = .013).
Endoscopic Stigmata
Present data suggest that endoscopic stigmata that predict a high risk of rebleeding in peptic ulcer disease do not apply to Mallory-Weiss tears. Jensen and colleagues showed that less than 20% of patients with Mallory-Weiss tears with nonbleeding visible vessels or adherent clots had rebleeding with medical management. In the study by Chung and associates,206 no recurrent bleed (0 of 40) was reported with medical management in patients with Mallory-Weiss bleed with a protruding vessel (23 patients) and adherent clot (17 patients). Bataller and coworkers207 reported similar results where none (0 of 18) of the patients with an adherent clot had rebleeding in the medical management group. In view of current data, endoscopic therapy is recommended for active spurting and active oozing. Data do not show any benefit of endoscopic treatment of a visible vessel or an adherent clot in patients with Mallory-Weiss tear with an acute GI hemorrhage. Despite the above-described general experience, a Mallory-Weiss tear, if sufficiently deep, can compromise an esophageal branch from the left gastric artery, which would result in massive life-threatening bleeding. This is a very infrequent occurrence for which awareness is appropriate.
Endoscopic Therapy
Similar to other etiologies of GI bleeding, different endoscopic methods have been used to achieve hemostasis, including injection. Laine208 showed that, compared with medical treatment, multipolar electrocoagulation is beneficial in achieving hemostasis, reducing emergency interventions and transfusion requirements in patients with major GI bleeding from a Mallory-Weiss tear.
Peng and colleagues209 retrospectively compared data of 76 patients with Mallory-Weiss bleeding. Patients with visible vessel or fresh blood coating the tear were included. Isotonic saline–epinephrine injection (15 of 36) was compared with conservative management (21 of 36). The initial hemostasis rates (93% vs. 95%) and rebleeding rates (7% vs. 5%) were similar in the two groups. Llach and colleagues210 showed injection sclerotherapy (epinephrine and polidocanol) to be superior to conservative management (rebleeding; 25.8% vs. 6.2%, P < .05) in patients with active bleeding or nonbleeding visible vessel with Mallory-Weiss tear.
Chung and associates206 studied 76 patients with Mallory-Weiss tear in whom there was active bleeding, who contained a protruding vessel, or who had an adherent clot. The first 30 patients were randomly assigned to injection therapy with HSE (n = 14), hemoclipping, or band ligation. In the remaining 46 patients, lesions were irrigated with a 1 : 100,000 epinephrine solution until hemostasis occurred (medical management group). All patients received IV H2 blockers. Rebleeding occurred in 1 of 6 patients with oozing in the medical management group and 3 of 15 patients in the endoscopic therapy group. All of these patients received injection therapy; no bleeding occurred with mechanical hemostasis (hemoclip, band ligation). The authors concluded that endoscopic therapy for Mallory-Weiss tear is unnecessary in patients without active bleeding, and mechanical hemostatic method is more effective than HSE injection.
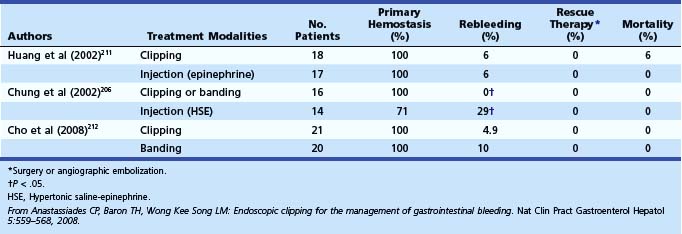
Huang and colleagues211 prospectively compared epinephrine injection therapy (n = 17 of 35) with endoscopic hemoclip application (n = 18 of 35) in patients with spurting vessels or active oozing from Mallory-Weiss tears (Table 12.10).106 In each group, there was one case of recurrent bleeding that was successfully controlled by repeat endoscopic therapy. There were no procedure-related complications, and none of the patients required surgery. Cho and colleagues212 found hemoclip placement and band ligation to be equally effective and safe for the management of active bleeding even in patients with shock or comorbid diseases.
Future Directions for Endoscopic Therapy
Doppler ultrasound may provide objective findings in patients with ulcer hemorrhage. Doppler signal correlates with rebleeding suggesting that it may be useful to continue endoscopic treatment until underlying signal of blood flow stops.213,214 Interobserver disagreement in interpretation and its availability preclude wide use of Doppler ultrasound at present. EUS-guided therapy has been used in sporadic cases to control refractory bleeding.165 EUS-guided therapy has the advantage because therapy is delivered under ultrasound guidance, and direct visualization may not be needed. Hemoclips are the only mechanical device available for widespread clinical use, but it is expected that development of natural orifice transendoscopic surgery will offer additional endoscopic suturing devices that eventually will be applicable to endoscopic therapy of nonvariceal bleeding.215
1 Barkun A, Sabbah S, Enns R, et al. The Canadian Registry on Nonvariceal Upper Gastrointestinal Bleeding and Endoscopy (RUGBE): Endoscopic hemostasis and proton pump inhibition are associated with improved outcomes in a real-life setting. Am J Gastroenterol. 2004;99:1238-1246.
2 Gleeson F, Clarke E, Lennon J, et al. Outcome of accident and emergency room triaged patients with low risk non-variceal upper gastrointestinal haemorrhage. Ir Med J. 2006;99:114-117.
3 van Leerdam ME. Epidemiology of acute upper gastrointestinal bleeding. Best Pract Res Clin Gastroenterol. 2008;22:209-224.
4 Barkun AN, Bardou M, Kuipers EJ, et al. International consensus recommendations on the management of patients with nonvariceal upper gastrointestinal bleeding. Ann Intern Med. 2010;152:101-113.
5 Wilcox CM, Alexander LN, Cotsonis G. A prospective characterization of upper gastrointestinal hemorrhage presenting with hematochezia. Am J Gastroenterol. 1997;92:231-235.
6 Baradarian R, Ramdhaney S, Chapalamadugu R, et al. Early intensive resuscitation of patients with upper gastrointestinal bleeding decreases mortality. Am J Gastroenterol. 2004;99:619-622.
7 Silverstein FE, Gilbert DA, Tedesco FJ, et al. The national ASGE survey on upper gastrointestinal bleeding. II. Clinical prognostic factors. Gastrointest Endosc. 1981;27:80-93.
8 Celinski K, Cichoz-Lach H, Madro A, et al. Non-variceal upper gastrointestinal bleeding—guidelines on management. J Physiol Pharmacol. 2008;59(Suppl 2):215-229.
9 Leung FW. The venerable nasogastric tube. Gastrointest Endosc. 2004;59:255-260.
10 Gilbert DA, Silverstein FE, Tedesco FJ, et al. The national ASGE survey on upper gastrointestinal bleeding. III. Endoscopy in upper gastrointestinal bleeding. Gastrointest Endosc. 1981;27:94-102.
11 Aljebreen AM, Fallone CA, Barkun AN. Nasogastric aspirate predicts high-risk endoscopic lesions in patients with acute upper-GI bleeding. Gastrointest Endosc. 2004;59:172-178.
12 Ernst AA, Haynes ML, Nick TG, et al. Usefulness of the blood urea nitrogen/creatinine ratio in gastrointestinal bleeding. Am J Emerg Med. 1999;17:70-72.
13 Al-Naamani K, Alzadjali N, Barkun AN, et al. Does blood urea nitrogen level predict severity and high-risk endoscopic lesions in patients with nonvariceal upper gastrointestinal bleeding? Can J Gastroenterol. 2008;22:399-403.
14 Laine L, Peterson WL. Bleeding peptic ulcer. N Engl J Med. 1994;331:717-727.
15 Barkun A, Bardou M, Marshall JK, Nonvariceal Upper GI Bleeding Consensus Conference Group. Consensus recommendations for managing patients with nonvariceal upper gastrointestinal bleeding. Ann Intern Med. 2003;139:843-857.
16 Huang CS, Lichtenstein DR. Nonvariceal upper gastrointestinal bleeding. Gastroenterol Clin North Am. 2003;32:1053-1078.
17 Imperiale TF, Dominitz JA, Provenzale DT, et al. Predicting poor outcome from acute upper gastrointestinal hemorrhage. Arch Intern Med. 2007;167:1291-1296.
18 Almela P, Benages A, Peiro S, et al. A risk score system for identification of patients with upper-GI bleeding suitable for outpatient management. Gastrointest Endosc. 2004;59:772-781.
19 Chiu PWY, Ng EKW, Cheung FKY, et al. Predicting mortality in patients with bleeding peptic ulcers after therapeutic endoscopy. Clin Gastroenterol Hepatol. 2009;7:311-316.
20 Blatchford O, Murray WR, Blatchford M. A risk score to predict need for treatment for upper-gastrointestinal haemorrhage. Lancet. 2000;356:1318-1321.
21 Masaoka T, Suzuki H, Hori S, et al. Blatchford scoring system is a useful scoring system for detecting patients with upper gastrointestinal bleeding who do not need endoscopic intervention. J Gastroenterol Hepatol. 2007;22:1404-1408.
22 Cameron EA, Pratap JN, Sims TJ, et al. Three-year prospective validation of a pre-endoscopic risk stratification in patients with acute upper-gastrointestinal haemorrhage. Eur J Gastroenterol Hepatol. 2002;14:497-501.
23 Forrest JA, Finlayson ND, Shearman DJ. Endoscopy in gastrointestinal bleeding. Lancet. 1974;2:394-397.
24 Swain CP, Storey DW, Bown SG, et al. Nature of the bleeding vessel in recurrently bleeding gastric ulcers. Gastroenterology. 1986;90:595-608.
25 Freeman ML, Cass OW, Peine CJ, et al. The non-bleeding visible vessel versus the sentinel clot: Natural history and risk of rebleeding. Gastrointest Endosc. 1993;39:359-366.
26 Chung SCS, Leung JUC, Lo KK, et al. 1990 Natural history of the sentinel clot: an endoscopic study [abstract]. Gastroenterology. 1990;98:31.
27 Rockall TA, Logan RF, Devlin HB, et al. Risk assessment after acute upper gastrointestinal haemorrhage. Gut. 1996;38:316-321.
28 Sanders DS, Carter MJ, Goodchap RJ, et al. Prospective validation of the Rockall risk scoring system for upper GI hemorrhage in subgroups of patients with varices and peptic ulcers. Am J Gastroenterol. 2002;97:630-635.
29 Vreeburg EM, Terwee CB, Snel P, et al. Validation of the Rockall risk scoring system in upper gastrointestinal bleeding. Gut. 1999;44:331-335.
30 Dulai GS, Gralnek IM, Oei TT, et al. Utilization of health care resources for low-risk patients with acute, nonvariceal upper GI hemorrhage: an historical cohort study. Gastrointest Endosc. 2002;55:321-327.
31 Hay JA, Lyubashevsky E, Elashoff J, et al. Upper gastrointestinal hemorrhage—clinical guideline determining the optimal hospital length of stay. Am J Med. 1996;100:313-322.
32 Saeed ZA, Ramirez FC, Hepps KS, et al. Prospective validation of the Baylor bleeding score for predicting the likelihood of rebleeding after endoscopic hemostasis of peptic ulcers. Gastrointest Endosc. 1995;41:561-565.
33 Stanley AJ, Ashley D, Dalton HR, et al. Outpatient management of patients with low-risk upper-gastrointestinal haemorrhage: Multicentre validation and prospective evaluation. Lancet. 2009;373:42-47.
34 Chen I, Hung M, Chiu T, et al. Risk scoring systems to predict need for clinical intervention for patients with nonvariceal upper gastrointestinal tract bleeding. Am J Emerg Med. 2007;25:774-779.
35 Kim B, Park M, Kim S, et al. Comparison of scoring systems for the prediction of outcomes in patients with nonvariceal upper gastrointestinal bleeding: A prospective study. Dig Dis Sci. 2009;54:2523-2529.
36 Kovacs TOG, Jensen DM. Endoscopic treatment of ulcer bleeding. Curr Treat Options Gastroenterol. 2007;10:143-148.
37 Gralnek IM, Barkun AN, Bardou M. Management of acute bleeding from a peptic ulcer. N Engl J Med. 2008;359:928-937.
38 Lee JG, Turnipseed S, Romano PS, et al. Endoscopy-based triage significantly reduces hospitalization rates and costs of treating upper GI bleeding: A randomized controlled trial. Gastrointest Endosc. 1999;50:755-761.
39 Cipolletta L, Bianco MA, Rotondano G, et al. Outpatient management for low-risk nonvariceal upper GI bleeding: A randomized controlled trial. Gastrointest Endosc. 2002;55:1-5.
40 Consensus conference: Therapeutic endoscopy and bleeding ulcers. JAMA. 1989;262:1369-1372.
41 American Society for Gastrointestinal Endoscopy Standard of Practice Committee. The role of endoscopy in the management of non-variceal acute upper gastrointestinal bleeding: Guidelines for clinical application. Gastrointest Endosc. 1992;38:760-764.
42 Barzun AN, Chiba N, Enns R, et al. Use of national endoscopic database to determine the adoption of emerging pharmacological and endoscopic technologies in the everyday care of patients with upper GI bleeding: The RUGBE initiative [abstract]. Am J Gastroenterol. 2001;96:S261.
43 Vreeburg EM, Snel P, de Bruijne JW, et al. Acute upper gastrointestinal bleeding in the Amsterdam area: Incidence, diagnosis, and clinical outcome. Am J Gastroenterol. 1997;92:236-243.
44 Lichtenstein DR, Jagannath S, Baron TH, et al. Sedation and anesthesia in GI endoscopy. Gastrointest Endosc. 2008;68:815-826.
45 Stollman NH, Putcha RV, Neustater BR, et al. The uncleared fundal pool in acute upper gastrointestinal bleeding: Implications and outcomes. Gastrointest Endosc. 1997;46:324-327.
46 Kalloo AN, Canto MI, Wadwa KS, et al. Clinical usefulness of 3% hydrogen peroxide in acute upper GI bleeding: A pilot study. Gastrointest Endosc. 1999;49:518-521.
47 Kodali VP, Petersen BT, Miller CA, et al. A new jumbo-channel therapeutic gastroscope for acute upper gastrointestinal bleeding. Gastrointest Endosc. 1997;45:409-411.
48 Hintze RE, Binmoeller KF, Adler A, et al. Improved endoscopic management of severe upper gastrointestinal hemorrhage using a new wide-channel endoscope. Endoscopy. 1994;26:613-616.
49 Coffin B, Pocard M, Panis Y, et al. Erythromycin improves the quality of EGD in patients with acute upper GI bleeding: A randomized controlled study. Gastrointest Endosc. 2002;56:174-179.
50 Frossard JL, Spahr L, Queneau PE, et al. Erythromycin intravenous bolus infusion in acute upper gastrointestinal bleeding: A randomized, controlled, double-blind trial. Gastroenterology. 2002;123:17-23.
51 Carbonell N, Pauwels A, Serfaty L, et al. Erythromycin infusion prior to endoscopy for acute upper gastrointestinal bleeding: A randomized, controlled, double-blind trial. Am J Gastroenterol. 2006;101:1211-1215.
52 Winstead NS, Wilcox CM. Erythromycin prior to endoscopy for acute upper gastrointestinal haemorrhage: A cost-effectiveness analysis. Aliment Pharmacol Ther. 2007;26:1371-1377.
53 Brown RC, Langman MJ. Proceedings: Hospital admission for peptic ulcer and acute gastrointestinal bleeding in the United Kingdom 1958–1970. Gut. 1974;15:335.
54 Primatesta P, Goldacre MJ, Seagroatt V. Changing patterns in the epidemiology and hospital care of peptic ulcer. Int J Epidemiol. 1994;23:1206-1217.
55 Kang J, Tinto A, Higham J, et al. Peptic ulceration in general practice in England and Wales 1994–98: Period prevalence and drug management. Aliment Pharmacol Ther. 2002;16:1067-1074.
56 Czernichow P, Hochain P, Nousbaum JB, et al. Epidemiology and course of acute upper gastrointestinal haemorrhage in four French geographical areas. Eur J Gastroenterol Hepatol. 2000;12:175-181.
57 Skok P. The epidemiology of hemorrhage from the upper gastrointestinal tract in the mid-nineties—has anything changed? Hepatogastroenterology. 1998;45:2228-2233.
58 Elashoff JD, Grossman MI. Trends in hospital admissions and death rates for peptic ulcer in the United States from 1970 to 1978. Gastroenterology. 1980;78:280-285.
59 Bardhan KD, Cust G, Hinchliffe RF, et al. Changing pattern of admissions and operations for duodenal ulcer. Br J Surg. 1989;76:230-236.
60 Sonnenberg A. Time trends of ulcer mortality in Europe. Gastroenterology. 2007;132:2320-2327.
61 van Leerdam ME, Vreeburg EM, Rauws EAJ, et al. Acute upper GI bleeding: Did anything change? Time trend analysis of incidence and outcome of acute upper GI bleeding between 1993/1994 and 2000. Am J Gastroenterol. 2003;98:1494-1499.
62 Marshall BJ, McGechie DB, Rogers PA, et al. Pyloric Campylobacter infection and gastroduodenal disease. Med J Aust. 1985;142:439-444.
63 Ciociola AA, McSorley DJ, Turner K, et al. Helicobacter pylori infection rates in duodenal ulcer patients in the United States may be lower than previously estimated. Am J Gastroenterol. 1999;94:1834-1840.
64 Meucci G, Di Battista R, Abbiati C, et al. Prevalence and risk factors of Helicobacter pylori-negative peptic ulcer: A multicenter study. J Clin Gastroenterol. 2000;31:42-47.
65 Mamdani M, Warren L, Kopp A, et al. Changes in rates of upper gastrointestinal hemorrhage after the introduction of cyclooxygenase-2 inhibitors in British Columbia and Ontario. Can Med Assoc J. 2006;175:1535-1538.
66 Huang JQ, Sridhar S, Hunt RH. Role of Helicobacter pylori infection and non-steroidal anti-inflammatory drugs in peptic-ulcer disease: A meta-analysis. Lancet. 2002;359:14-22.
67 van Leerdam ME, Tytgat GNJ. Helicobacter pylori infection in peptic ulcer haemorrhage. Aliment Pharmacol Ther. 2002;16(Suppl 1):66-78.
68 Chan FKL, To KF, Wu JCY, et al. Eradication of Helicobacter pylori and risk of peptic ulcers in patients starting long-term treatment with non-steroidal anti-inflammatory drugs: A randomised trial. Lancet. 2002;359:9-13.
69 Garcia Rodriguez LA, Barreales Tolosa L. Risk of upper gastrointestinal complications among users of traditional NSAIDs and COXIBs in the general population. Gastroenterology. 2007;132:498-506.
70 Perng CL, Lin HJ, Chen CJ, et al. Characteristics of patients with bleeding peptic ulcer requiring emergency endoscopy and aggressive treatment. Am J Gastroenterol. 1994;89:1811-1814.
71 Cook DJ, Guyatt GH, Salena BJ, et al. Endoscopic therapy for acute nonvariceal upper gastrointestinal hemorrhage: A meta-analysis. Gastroenterology. 1992;102:139-148.
72 Sacks HS, Chalmers TC, Blum AL, et al. Endoscopic hemostasis: An effective therapy for bleeding peptic ulcers. JAMA. 1990;264:494-499.
73 Spiegel BM, Vakil NB, Ofman JJ. Endoscopy for acute nonvariceal upper gastrointestinal tract hemorrhage: Is sooner better? A systematic review. Arch Intern Med. 2001;161:1393-1404.
74 Jensen DM, Savides T, Sitaer M, et al. Demographics, risk factors and outcomes of peptic ulcer hemorrhage in the United States: Results of a large multi-center study [abstract]. Gastroenterology. 2003;124:A17.
75 Consensus statement on therapeutic endoscopy and bleeding ulcers. Consensus Development Panel. Gastrointest Endosc. 1990;36:S62-S65.
76 British Society of Gastroenterology Endoscopy. Non-variceal upper gastrointestinal haemorrhage: Guidelines. Gut. 2002;51(Suppl 4):iv1-iv6.
77 Bardou M, Youssef M, Toubouti Y, et al. Newer endoscopic therapies decrease both re-bleeding and mortality in high risk patients with acute peptic ulcer bleeding: A series of meta-analyses [abstract]. Gastroenterology. 2003;123:A239.
78 Matthewson K, Swain CP, Bland M, et al. Randomized comparison of Nd YAG laser, heater probe, and no endoscopic therapy for bleeding peptic ulcers. Gastroenterology. 1990;98:1239-1244.
79 Jensen DM, Kovacs TOG, Jutabha R, et al. Final results and cost assessment of endoscopic vs medical therapies for prevention of recurrent ulcer hemorrhage from adherent clots in a randomized controlled trial [abstract]. Gastrointest Endosc. 1995;41:279.
80 Bleau BL, Gostout CJ, Sherman KE, et al. Recurrent bleeding from peptic ulcer associated with adherent clot: A randomized study comparing endoscopic treatment with medical therapy. Gastrointest Endosc. 2002;56:1-6.
81 Jensen DM, Kovacs TOG, Jutabha R, et al. Randomized trial of medical or endoscopic therapy to prevent recurrent ulcer hemorrhage in patients with adherent clots. Gastroenterology. 2002;123:407-413.
82 Kahi CJ, Jensen DM, Sung JJY, et al. Endoscopic therapy versus medical therapy for bleeding peptic ulcer with adherent clot: A meta-analysis. Gastroenterology. 2005;129:855-862.
83 Levy MJ, Chak A. EUS 2008 Working Group document: Evaluation of EUS-guided vascular therapy. Gastrointest Endosc. 2009;69:S37-S42.
84 Chung SC, Leung JW, Steele RJ, et al. Endoscopic injection of adrenaline for actively bleeding ulcers: A randomised trial. Br Med J (Clin Res Educ). 1988;296:1631-1633.
85 Randall GM, Jensen DM, Hirabayashi K, et al. Controlled study of different sclerosing agents for coagulation of canine gut arteries. Gastroenterology. 1989;96:1274-1281.
86 O’Brien JR. Some effects of adrenaline and anti-adrenaline compounds on platelets in vitro and in vivo. Nature. 1963;200:763-764.
87 Lai KH, Peng SN, Guo WS, et al. Endoscopic injection for the treatment of bleeding ulcers: Local tamponade or drug effect? Endoscopy. 1994;26:338-341.
88 Lin H, Hsieh Y, Tseng G, et al. A prospective, randomized trial of large- versus small-volume endoscopic injection of epinephrine for peptic ulcer bleeding. Gastrointest Endosc. 2002;55:615-619.
89 Park C, Lee S, Park J, et al. Optimal injection volume of epinephrine for endoscopic prevention of recurrent peptic ulcer bleeding. Gastrointest Endosc. 2004;60:875-880.
90 Liou T, Chang W, Wang H, et al. Large-volume endoscopic injection of epinephrine plus normal saline for peptic ulcer bleeding. J Gastroenterol Hepatol. 2007;22:996-1002.
91 Park WG, Yeh RW, Triadafilopoulos G. Injection therapies for nonvariceal bleeding disorders of the GI tract. Gastrointest Endosc. 2007;66:343-354.
92 Laine L, Estrada R. Randomized trial of normal saline solution injection versus bipolar electrocoagulation for treatment of patients with high-risk bleeding ulcers: Is local tamponade enough? Gastrointest Endosc. 2002;55:6-10.
93 Johnston JH, Jensen DM, Auth D. Experimental comparison of endoscopic yttrium-aluminum-garnet laser, electrosurgery, and heater probe for canine gut arterial coagulation: Importance of compression and avoidance of erosion. Gastroenterology. 1987;92:1101-1108.
94 Jensen D, Hirabayashi K, CURE Hemostasis Research Group. A study of coagulation depths with BICAP and heater probe to improve endoscopic hemostasis of bleeding peptic ulcers [abstract]. Gastrointest Endosc. 1989;35:181.
95 Morris DL, Brearley S, Thompson H, et al. A comparison of the efficacy and depth of gastric wall injury with 3.2- and 2.3-mm bipolar probes in canine arterial hemorrhage. Gastrointest Endosc. 1985;31:361-363.
96 Laine L. Determination of the optimal technique for bipolar electrocoagulation treatment: An experimental evaluation of the BICAP and Gold probes. Gastroenterology. 1991;100:107-112.
97 Jensen DM, Kovacs TOG, Freeman M, et al. A multicenter randomized prospective study of Gold probe for hemostasis of very severe ulcer or Mallory-Weiss bleeding [abstract]. Gastroenterology. 1991;100(Suppl A):92.
98 Jensen DM. Heat probe for hemostasis of bleeding peptic ulcers: Technique and results of randomized controlled trials. Gastrointest Endosc. 1990;36:S42-S49.
99 Bianco MA, Rotondano G, Marmo R, et al. Combined epinephrine and bipolar probe coagulation vs. bipolar probe coagulation alone for bleeding peptic ulcer: a randomized, controlled trial. Gastrointest Endosc. 2004;60:910-915.
100 Jensen DM, Machicado GA, Hirabayashi K. Randomized controlled study of 3 different types of hemoclips for hemostasis of bleeding canine acute gastric ulcers. Gastrointest Endosc. 2006;64:768-773.
101 Cipolletta L, Bianco MA, Marmo R, et al. Endoclips versus heater probe in preventing early recurrent bleeding from peptic ulcer: A prospective and randomized trial. Gastrointest Endosc. 2001;53:147-151.
102 Chou Y, Hsu P, Lai K, et al. A prospective, randomized trial of endoscopic hemoclip placement and distilled water injection for treatment of high-risk bleeding ulcers. Gastrointest Endosc. 2003;57:324-328.
103 Lin H, Hsieh Y, Tseng G, et al. A prospective, randomized trial of endoscopic hemoclip versus heater probe thermocoagulation for peptic ulcer bleeding. Am J Gastroenterol. 2002;97:2250-2254.
104 Chung IK, Ham JS, Kim HS, et al. Comparison of the hemostatic efficacy of the endoscopic hemoclip method with hypertonic saline-epinephrine injection and a combination of the two for the management of bleeding peptic ulcers. Gastrointest Endosc. 1999;49:13-18.
105 Buffoli F, Graffeo M, Nicosia F, et al. Peptic ulcer bleeding: Comparison of two hemostatic procedures. Am J Gastroenterol. 2001;96:89-94.
106 Anastassiades CP, Baron TH, Wong Kee Song LM. Endoscopic clipping for the management of gastrointestinal bleeding. Nat Clin Pract Gastroenterol Hepatol. 2008;5:559-568.
107 Gevers A, De Goede E, Simoens M, et al. A randomized trial comparing injection therapy with hemoclip and with injection combined with hemoclip for bleeding ulcers. Gastrointest Endosc. 2002;55:466-469.
108 Shimoda R, Iwakiri R, Sakata H, et al. Evaluation of endoscopic hemostasis with metallic hemoclips for bleeding gastric ulcer: Comparison with endoscopic injection of absolute ethanol in a prospective, randomized study. Am J Gastroenterol. 2003;98:2198-2202.
109 Ljubicic N, Supanc V, Vrsalovic M. Efficacy of endoscopic clipping for actively bleeding peptic ulcer: Comparison with polidocanol injection therapy. Hepatogastroenterology. 2004;51:408-412.
110 Park C, Joo Y, Kim H, et al. A prospective, randomized trial comparing mechanical methods of hemostasis plus epinephrine injection to epinephrine injection alone for bleeding peptic ulcer. Gastrointest Endosc. 2004;60:173-179.
111 Lo C, Hsu P, Lo G, et al. Comparison of hemostatic efficacy for epinephrine injection alone and injection combined with hemoclip therapy in treating high-risk bleeding ulcers. Gastrointest Endosc. 2006;63:767-773.
112 Lin HJ, Perng CL, Sun IC, et al. Endoscopic haemoclip versus heater probe thermocoagulation plus hypertonic saline-epinephrine injection for peptic ulcer bleeding. Dig Liver Dis. 2003;35:898-902.
113 Saltzman JR, Strate LL, Di Sena V, et al. Prospective trial of endoscopic clips versus combination therapy in upper GI bleeding (PROTECCT-UGI bleeding). Am J Gastroenterol. 2005;100:1503-1508.
114 Lin H, Lo W, Cheng Y, et al. Endoscopic hemoclip versus triclip placement in patients with high-risk peptic ulcer bleeding. Am J Gastroenterol. 2007;102:539-543.
115 Tekant Y, Goh P, Alexander DJ, et al. Combination therapy using adrenaline and heater probe to reduce rebleeding in patients with peptic ulcer haemorrhage: A prospective randomized trial. Br J Surg. 1995;82:223-226.
116 Chung SS, Lau JY, Sung JJ, et al. Randomised comparison between adrenaline injection alone and adrenaline injection plus heat probe treatment for actively bleeding ulcers. BMJ. 1997;314:1307-1311.
117 Lin HJ, Tseng GY, Perng CL, et al. Comparison of adrenaline injection and bipolar electrocoagulation for the arrest of peptic ulcer bleeding. Gut. 1999;44:715-719.
118 Marmo R, Rotondano G, Piscopo R, et al. Dual therapy versus monotherapy in the endoscopic treatment of high-risk bleeding ulcers: A meta-analysis of controlled trials. Am J Gastroenterol. 2007;102:279-289.
119 Calvet X, Vergara M, Brullet E, et al. Addition of a second endoscopic treatment following epinephrine injection improves outcome in high-risk bleeding ulcers. Gastroenterology. 2004;126:441-450.
120 Yuan Y, Wang C, Hunt RH. Endoscopic clipping for acute nonvariceal upper-GI bleeding: A meta-analysis and critical appraisal of randomized controlled trials. Gastrointest Endosc. 2008;68:339-351.
121 Sung JJY, Tsoi KKF, Lai LH, et al. Endoscopic clipping versus injection and thermo-coagulation in the treatment of non-variceal upper gastrointestinal bleeding: A meta-analysis. Gut. 2007;56:1364-1373.
122 Vergara M, Calvet X, Gisbert JP: Epinephrine injection versus epinephrine injection and a second endoscopic method in high risk bleeding ulcers. Cochrane Database Syst Rev CD005584, 2007.
123 Cipolletta L, Bianco MA, Rotondano G, et al. Prospective comparison of argon plasma coagulator and heater probe in the endoscopic treatment of major peptic ulcer bleeding. Gastrointest Endosc. 1998;48:191-195.
124 Chau CH, Siu WT, Law BKB, et al. Randomized controlled trial comparing epinephrine injection plus heat probe coagulation versus epinephrine injection plus argon plasma coagulation for bleeding peptic ulcers. Gastrointest Endosc. 2003;57:455-461.
125 Havanond C, Havanond P: Argon plasma coagulation therapy for acute non-variceal upper gastrointestinal bleeding. Cochrane Database Syst Rev CD003791, 2005.
126 Choudari CP, Palmer KR. Endoscopic injection therapy for bleeding peptic ulcer: A comparison of adrenaline alone with adrenaline plus ethanolamine oleate. Gut. 1994;35:608-610.
127 Lee KJ, Kim JH, Hahm KB, et al. Randomized trial of N-butyl-2-cyanoacrylate compared with injection of hypertonic saline-epinephrine in the endoscopic treatment of bleeding peptic ulcers. Endoscopy. 2000;32:505-511.
128 Villanueva C, Balanzo J, Espinos JC, et al. Endoscopic injection therapy of bleeding ulcer: A prospective and randomized comparison of adrenaline alone or with polidocanol. J Clin Gastroenterol. 1993;17:195-200.
129 Chung SC, Leung JW, Leong HT, et al. Adding a sclerosant to endoscopic epinephrine injection in actively bleeding ulcers: A randomized trial. Gastrointest Endosc. 1993;39:611-615.
130 Kubba AK, Murphy W, Palmer KR. Endoscopic injection for bleeding peptic ulcer: A comparison of adrenaline alone with adrenaline plus human thrombin. Gastroenterology. 1996;111:623-628.
131 Graham DY, Hepps KS, Ramirez FC, et al. Treatment of Helicobacter pylori reduces the rate of rebleeding in peptic ulcer disease. Scand J Gastroenterol. 1993;28:939-942.
132 Lai KC, Hui WM, Wong WM, et al. Treatment of Helicobacter pylori in patients with duodenal ulcer hemorrhage—a long-term randomized, controlled study. Am J Gastroenterol. 2000;95:2225-2232.
133 Collins R, Langman M. Treatment with histamine H2 antagonists in acute upper gastrointestinal hemorrhage: Implications of randomized trials. N Engl J Med. 1985;313:660-666.
134 Peterson WL, Cook DJ. Antisecretory therapy for bleeding peptic ulcer. JAMA. 1998;280:877-878.
135 Saltzman JR, Zawacki JK. Therapy for bleeding peptic ulcers. N Engl J Med. 1997;336:1091-1093.
136 Levine JE, Leontiadis GI, Sharma VK, et al. Meta-analysis: The efficacy of intravenous H2-receptor antagonists in bleeding peptic ulcer. Aliment Pharmacol Ther. 2002;16:1137-1142.
137 Brunner G, Chang J. Intravenous therapy with high doses of ranitidine and omeprazole in critically ill patients with bleeding peptic ulcerations of the upper intestinal tract: An open randomized controlled trial. Digestion. 1990;45:217-225.
138 Gisbert JP, Gonzalez L, Calvet X, et al. Proton pump inhibitors versus H2-antagonists: A meta-analysis of their efficacy in treating bleeding peptic ulcer. Aliment Pharmacol Ther. 2001;15:917-926.
139 Lau JY, Sung JJ, Lee KK, et al. Effect of intravenous omeprazole on recurrent bleeding after endoscopic treatment of bleeding peptic ulcers. N Engl J Med. 2000;343:310-316.
140 Hasselgren G, Lind T, Lundell L, et al. Continuous intravenous infusion of omeprazole in elderly patients with peptic ulcer bleeding: Results of a placebo-controlled multicenter study. Scand J Gastroenterol. 1997;32:328-333.
141 Schaffalitzky de Muckadell OB, Havelund T, Harling H, et al. Effect of omeprazole on the outcome of endoscopically treated bleeding peptic ulcers: Randomized double-blind placebo-controlled multicentre study. Scand J Gastroenterol. 1997;32:320-327.
142 Khuroo MS, Yattoo GN, Javid G, et al. A comparison of omeprazole and placebo for bleeding peptic ulcer. N Engl J Med. 1997;336:1054-1058.
143 Lin HJ, Lo WC, Lee FY, et al. A prospective randomized comparative trial showing that omeprazole prevents rebleeding in patients with bleeding peptic ulcer after successful endoscopic therapy. Arch Intern Med. 1998;158:54-58.
144 Javid G, Masoodi I, Zargar SA, et al. Omeprazole as adjuvant therapy to endoscopic combination injection sclerotherapy for treating bleeding peptic ulcer. Am J Med. 2001;111:280-284.
145 Sung JJY, Chan FKL, Lau JYW, et al. The effect of endoscopic therapy in patients receiving omeprazole for bleeding ulcers with nonbleeding visible vessels or adherent clots: A randomized comparison. Ann Intern Med. 2003;139:237-243.
146 Leontiadis GI, Sharma VK, Howden CW. Proton pump inhibitor therapy for peptic ulcer bleeding: Cochrane collaboration meta-analysis of randomized controlled trials. Mayo Clin Proc. 2007;82:286-296.
147 Andriulli A, Annese V, Caruso N, et al. Proton-pump inhibitors and outcome of endoscopic hemostasis in bleeding peptic ulcers: A series of meta-analyses. Am J Gastroenterol. 2005;100:207-219.
148 Bardou M, Toubouti Y, Benhaberou-Brun D, et al. Meta-analysis: Proton-pump inhibition in high-risk patients with acute peptic ulcer bleeding. Aliment Pharmacol Ther. 2005;21:677-686.
149 Leontiadis GI, Sharma VK, Howden CW: Proton pump inhibitor treatment for acute peptic ulcer bleeding. Cochrane Database Syst Rev CD002094, 2006.
150 Leontiadis GI, Sharma VK, Howden CW. Systematic review and meta-analysis: Enhanced efficacy of proton-pump inhibitor therapy for peptic ulcer bleeding in Asia—a post hoc analysis from the Cochrane Collaboration. Aliment Pharmacol Ther. 2005;21:1055-1061.
151 Lau JY, Leung WK, Wu JCY, et al. Omeprazole before endoscopy in patients with gastrointestinal bleeding. N Engl J Med. 2007;356:1631-1640.
152 Keyvani L, Murthy S, Leeson S, et al. Pre-endoscopic proton pump inhibitor therapy reduces recurrent adverse gastrointestinal outcomes in patients with acute non-variceal upper gastrointestinal bleeding. Aliment Pharmacol Ther. 2006;24:1247-1255.
153 Laine L, McQuaid KR. Endoscopic therapy for bleeding ulcers: An evidence-based approach based on meta-analyses of randomized controlled trials. Clin Gastroenterol Hepatol. 2009;7:33-47.
154 Murthy S, Keyvani L, Leeson S, et al. Intravenous versus high-dose oral proton pump inhibitor therapy after endoscopic hemostasis of high-risk lesions in patients with acute nonvariceal upper gastrointestinal bleeding. Dig Dis Sci. 2007;52:1685-1690.
155 Yilmaz S, Bayan K, Tuzun Y, et al. A head to head comparison of oral vs intravenous omeprazole for patients with bleeding peptic ulcers with a clean base, flat spots and adherent clots. World J Gastroenterol. 2006;12:7837-7843.
156 Messmann H, Schaller P, Andus T, et al. Effect of programmed endoscopic follow-up examinations on the rebleeding rate of gastric or duodenal peptic ulcers treated by injection therapy: A prospective, randomized controlled trial. Endoscopy. 1998;30:583-589.
157 Villanueva C, Balanzo J, Torras X, et al. Value of second-look endoscopy after injection therapy for bleeding peptic ulcer: A prospective and randomized trial. Gastrointest Endosc. 1994;40:34-39.
158 Saeed ZA, Cole RA, Ramirez FC, et al. Endoscopic retreatment after successful initial hemostasis prevents ulcer rebleeding: A prospective randomized trial. Endoscopy. 1996;28:288-294.
159 Marmo R, Rotondano G, Bianco MA, et al. Outcome of endoscopic treatment for peptic ulcer bleeding: Is a second look necessary? A meta-analysis. Gastrointest Endosc. 2003;57:62-67.
160 Spiegel BMR, Ofman JJ, Woods K, et al. Minimizing recurrent peptic ulcer hemorrhage after endoscopic hemostasis: The cost-effectiveness of competing strategies. Am J Gastroenterol. 2003;98:86-97.
161 Branicki FJ, Coleman SY, Fok PJ, et al. Bleeding peptic ulcer: A prospective evaluation of risk factors for rebleeding and mortality. World J Surg. 1990;14:262-269.
162 Manguso F, Riccio E, Bennato R, et al. In-hospital mortality in non-variceal upper gastrointestinal bleeding Forrest 1 patients. Scand J Gastroenterol. 2008;43:1432-1441.
163 Travis AC, Wasan SK, Saltzman JR. Model to predict rebleeding following endoscopic therapy for non-variceal upper gastrointestinal hemorrhage. J Gastroenterol Hepatol. 2008;23:1505-1510.
164 Lau JY, Sung JJ, Lam YH, et al. Endoscopic retreatment compared with surgery in patients with recurrent bleeding after initial endoscopic control of bleeding ulcers. N Engl J Med. 1999;340:751-756.
165 Levy MJ, Wong Kee Song LM, Farnell MB, et al. Endoscopic ultrasound (EUS)-guided angiotherapy of refractory gastrointestinal bleeding. Am J Gastroenterol. 2008;103:352-359.
166 Wong SKH, Yu L, Lau JYW, et al. Prediction of therapeutic failure after adrenaline injection plus heater probe treatment in patients with bleeding peptic ulcer. Gut. 2002;50:322-325.
167 Chung IK, Kim EJ, Lee MS, et al. Endoscopic factors predisposing to rebleeding following endoscopic hemostasis in bleeding peptic ulcers. Endoscopy. 2001;33:969-975.
168 Defreyne L, Vanlangenhove P, De Vos M, et al. Embolization as a first approach with endoscopically unmanageable acute nonvariceal gastrointestinal hemorrhage. Radiology. 2001;218:739-748.
169 Lang EK. Transcatheter embolization in management of hemorrhage from duodenal ulcer: Long-term results and complications. Radiology. 1992;182:703-707.
170 Aina R, Oliva VL, Therasse E, et al. Arterial embolotherapy for upper gastrointestinal hemorrhage: Outcome assessment. J Vasc Interv Radiol. 2001;12:195-200.
171 Graham DY, White RH, Moreland LW, et al. Duodenal and gastric ulcer prevention with misoprostol in arthritis patients taking NSAIDs. Misoprostol Study Group. Ann Intern Med. 1993;119:257-262.
172 Labenz J, Borsch G. Role of Helicobacter pylori eradication in the prevention of peptic ulcer bleeding relapse. Digestion. 1994;55:19-23.
173 Rokkas T, Karameris A, Mavrogeorgis A, et al. Eradication of Helicobacter pylori reduces the possibility of rebleeding in peptic ulcer disease. Gastrointest Endosc. 1995;41:1-4.
174 Jaspersen D, Koerner T, Schorr W, et al. Helicobacter pylori eradication reduces the rate of rebleeding in ulcer hemorrhage. Gastrointest Endosc. 1995;41:5-7.
175 Grino P, Pascual S, Such J, et al. Comparison of diagnostic methods for Helicobacter pylori infection in patients with upper gastrointestinal bleeding. Scand J Gastroenterol. 2001;36:1254-1258.
176 Lee JM, Breslin NP, Fallon C, et al. Rapid urease tests lack sensitivity in Helicobacter pylori diagnosis when peptic ulcer disease presents with bleeding. Am J Gastroenterol. 2000;95:1166-1170.
177 Colin R, Czernichow P, Baty V, et al. Low sensitivity of invasive tests for the detection of Helicobacter pylori infection in patients with bleeding ulcer. Gastroenterol Clin Biol. 2000;24:31-35.
178 Houghton J, Ramamoorthy R, Pandya H, et al. Human plasma is directly bacteriocidal against Helicobacter pylori in vitro, potentially explaining the decreased detection of Helicobacter pylori during acute upper GI bleeding. Gastrointest Endosc. 2002;55:11-16.
179 Hawkey CJ, Karrasch JA, Szczepanski L, et al. Omeprazole compared with misoprostol for ulcers associated with nonsteroidal antiinflammatory drugs. Omeprazole versus Misoprostol for NSAID-Induced Ulcer Management (OMNIUM) Study Group. N Engl J Med. 1998;338:727-734.
180 Miko TL, Thomazy VA. The caliber persistent artery of the stomach: A unifying approach to gastric aneurysm, Dieulafoy’s lesion, and submucosal arterial malformation. Hum Pathol. 1988;19:914-921.
181 Goldman RL. Submucosal arterial malformation (“aneurysm”) of the stomach with fatal hemorrhage. Gastroenterology. 1964;46:589-594.
182 Norton ID, Petersen BT, Sorbi D, et al. Management and long-term prognosis of Dieulafoy lesion. Gastrointest Endosc. 1999;50:762-767.
183 Schmulewitz N, Baillie J. Dieulafoy lesions: A review of 6 years of experience at a tertiary referral center. Am J Gastroenterol. 2001;96:1688-1694.
184 Baettig B, Haecki W, Lammer F, et al. Dieulafoy’s disease: Endoscopic treatment and follow up. Gut. 1993;34:1418-1421.
185 Veldhuyzen van Zanten SJ, Bartelsman JF, Schipper ME, et al. Recurrent massive haematemesis from Dieulafoy vascular malformations—a review of 101 cases. Gut. 1986;27:213-222.
186 Dy NM, Gostout CJ, Balm RK. Bleeding from the endoscopically-identified Dieulafoy lesion of the proximal small intestine and colon. Am J Gastroenterol. 1995;90:108-111.
187 Reilly HF, al-Kawas FH. Dieulafoy’s lesion: Diagnosis and management. Dig Dis Sci. 1991;36:1702-1707.
188 Kasapidis P, Georgopoulos P, Delis V, et al. Endoscopic management and long-term follow-up of Dieulafoy’s lesions in the upper GI tract. Gastrointest Endosc. 2002;55:527-531.
189 Lin HJ, Lee FY, Tsai YT, et al. Therapeutic endoscopy for Dieulafoy’s disease. J Clin Gastroenterol. 1989;11:507-510.
190 Parra-Blanco A, Takahashi H, Mendez Jerez PV, et al. Endoscopic management of Dieulafoy lesions of the stomach: A case study of 26 patients. Endoscopy. 1997;29:834-839.
191 Stark ME, Gostout CJ, Balm RK. Clinical features and endoscopic management of Dieulafoy’s disease. Gastrointest Endosc. 1992;38:545-550.
192 Yamaguchi Y, Yamato T, Katsumi N, et al. Short-term and long-term benefits of endoscopic hemoclip application for Dieulafoy’s lesion in the upper GI tract. Gastrointest Endosc. 2003;57:653-656.
193 Chung IK, Kim EJ, Lee MS, et al. Bleeding Dieulafoy’s lesions and the choice of endoscopic method: Comparing the hemostatic efficacy of mechanical and injection methods. Gastrointest Endosc. 2000;52:721-724.
194 Park CH, Sohn YH, Lee WS, et al. The usefulness of endoscopic hemoclipping for bleeding Dieulafoy lesions. Endoscopy. 2003;35:388-392.
195 Park CH, Joo YE, Kim HS, et al. A prospective, randomized trial of endoscopic band ligation versus endoscopic hemoclip placement for bleeding gastric Dieulafoy’s lesions. Endoscopy. 2004;36:677-681.
196 Matsui S, Kamisako T, Kudo M, et al. Endoscopic band ligation for control of nonvariceal upper GI hemorrhage: Comparison with bipolar electrocoagulation. Gastrointest Endosc. 2002;55:214-218.
197 Mumtaz R, Shaukat M, Ramirez FC. Outcomes of endoscopic treatment of gastroduodenal Dieulafoy’s lesion with rubber band ligation and thermal/injection therapy. J Clin Gastroenterol. 2003;36:310-314.
198 Nikolaidis N, Zezos P, Giouleme O, et al. Endoscopic band ligation of Dieulafoy-like lesions in the upper gastrointestinal tract. Endoscopy. 2001;33:754-760.
199 Pointner R, Schwab G, Konigsrainer A, et al. Endoscopic treatment of Dieulafoy’s disease. Gastroenterology. 1988;94:563-566.
200 Bharucha AE, Gostout CJ, Balm RK. Clinical and endoscopic risk factors in the Mallory-Weiss syndrome. Am J Gastroenterol. 1997;92:805-808.
201 Sugawa C, Benishek D, Walt AJ. Mallory-Weiss syndrome: A study of 224 patients. Am J Surg. 1983;145:30-33.
202 Kortas DY, Haas LS, Simpson WG, et al. Mallory-Weiss tear: Predisposing factors and predictors of a complicated course. Am J Gastroenterol. 2001;96:2863-2865.
203 Knauer CM. Mallory-Weiss syndrome: Characterization of 75 Mallory-Weiss lacerations in 528 patients with upper gastrointestinal hemorrhage. Gastroenterology. 1976;71:5-8.
204 Dagradi AE, Broderick JT, Juler G, et al. The Mallory-Weiss syndrome and lesion: A study of 30 cases. Am J Dig Dis. 1966;11:710-721.
205 Penston JG, Boyd EJ, Wormsley KG. Mallory-Weiss tears occurring during endoscopy: A report of seven cases. Endoscopy. 1992;24:262-265.
206 Chung IK, Kim EJ, Hwang KY, et al. Evaluation of endoscopic hemostasis in upper gastrointestinal bleeding related to Mallory-Weiss syndrome. Endoscopy. 2002;34:474-479.
207 Bataller R, Llach J, Salmeron JM, et al. Endoscopic sclerotherapy in upper gastrointestinal bleeding due to the Mallory-Weiss syndrome. Am J Gastroenterol. 1994;89:2147-2150.
208 Laine L. Multipolar electrocoagulation in the treatment of active upper gastrointestinal tract hemorrhage: A prospective controlled trial. N Engl J Med. 1987;316:1613-1617.
209 Peng YC, Tung CF, Chow WK, et al. Efficacy of endoscopic isotonic saline-epinephrine injection for the management of active Mallory-Weiss tears. J Clin Gastroenterol. 2001;32:119-122.
210 Llach J, Elizalde JI, Guevara MC, et al. Endoscopic injection therapy in bleeding Mallory-Weiss syndrome: A randomized controlled trial. Gastrointest Endosc. 2001;54:679-681.
211 Huang S, Wang H, Lee Y, et al. Endoscopic hemoclip placement and epinephrine injection for Mallory-Weiss syndrome with active bleeding. Gastrointest Endosc. 2002;55:842-846.
212 Cho Y, Chae H, Kim H, et al. Endoscopic band ligation and endoscopic hemoclip placement for patients with Mallory-Weiss syndrome and active bleeding. World J Gastroenterol. 2008;14:2080-2084.
213 Wong RCK. Endoscopic Doppler US probe for acute peptic ulcer hemorrhage. Gastrointest Endosc. 2004;60:804-812.
214 Chen VK, Wong RCK. Endoscopic Doppler ultrasound versus endoscopic stigmata-directed management of acute peptic ulcer hemorrhage: A multimodel cost analysis. Dig Dis Sci. 2007;52:149-160.
215 Moran EA, Gostout CJ, Bingener J. Preliminary performance of a flexible cap and catheter-based endoscopic suturing system. Gastrointest Endosc. 2009;69:1375-1383.