CHAPTER 37 Nonosseous Spinal Tumors
INTRODUCTION
Only 15% of primary central nervous system (CNS) tumors are intraspinal.1 Nonosseous spinal tumors represent a collection of pathologic entities that are difficult to differentiate from each other based on clinical findings, and more importantly are sometimes difficult to distinguish from the much more common degenerative spinal disorders. Obviously, it is important to make this distinction when evaluating patients with spinal disorders, but the ready availability of high-resolution imaging has removed the majority of suspense in making these distinctions. However, a basic understanding of the pathophysiology of spinal tumors and the key elements in the history and physical examination that help differentiate them from degenerative spinal pathologies are essential to avoid delayed or erroneous diagnosis. A suspicion of a spinal tumor may lead to earlier spinal imaging, as well as more comprehensive imaging to include portions of the spine that would otherwise not be imaged. We cannot overemphasize the importance of evaluating the history and physical examination for inconsistencies and ‘red flags’ such as a history of systemic malignancy, family history of syndromic tumors (neurofibromatosis, von Hippel – Lindau), nocturnal or recumbent pain, unusual radicular or medullary (burning, dysesthetic, nonradicular, as with a syrinx) symptoms, spasticity or other pathologic reflexes, bowel/bladder/sexual involvement. One additional difficulty in differentiating spinal tumor types from each other is that because the majority of symptoms are from compression, symptomatology in general is more related to the level of the lesion that the identity of the lesion itself. However, as will be discussed in subsequent sections, there is a regional predilection for each tumor and some lesions have distinct clinical findings secondary to associated pathologic changes (i.e. a syrinx associated with an intramedullary tumor). Our intention with this chapter is to give a concise and organized summary of nonosseous spinal tumors in addition to suggesting a rational approach to differentiating these lesions from more common spinal pathologies.
Nonosseous spinal tumors can be generally separated in three general categories: (1) extradural (55–60%), arising in the epidural space or in vertebral bodies; (2) intradural/extramedullary (30–40%); and (3) intramedullary (5–10%).2 These distinct categories are somewhat oversimplified as some tumors can span more than one compartment. Additionally, while metastasis can be found in all groups, the vast majority are extradural.
EXTRADURAL TUMORS
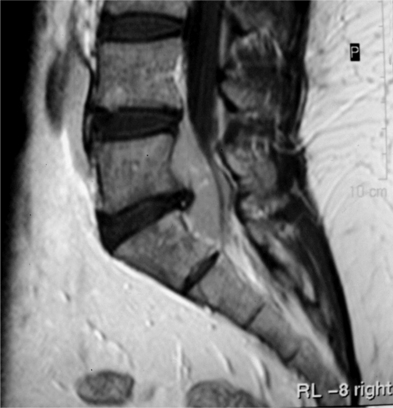
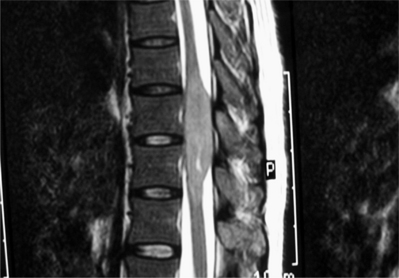
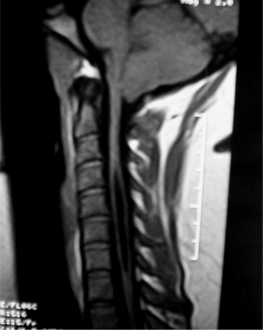
Tumors arising in and from bone will be discussed in other sections of this book. The majority of extradural tumors, with or without bone involvement, are metastatic in nature. Lymphomas are somewhat unique in that they can present with only epidural involvement and little or no bone involvement (Fig. 37.1).3 Schwannomas and meningiomas can be wholly or partially extradural but these tumors will be covered in the section on intradural/extramedullary tumors. Other more unusual causes of extradural spinal cord compressions include benign angiolipomas.4 Epidural lipomatosis is an infrequent cause of epidural spinal cord compression associated with chronic steroid use.5 Finally, epidural abscess can present as a mass lesion with or without associated discitis and osteomyelitis (Fig. 37.2).
INTRADURAL/EXTRAMEDULLARY TUMORS
The clinical features of intradural/extramedullary tumors generally reflect the slow-growing nature of these tumors and are more dependent on location within the spinal axis rather than specific pathology. Upper cervical and foramen magnum lesions often arise ventrally and can present with long tract signs as well as headaches and a syndrome of distal arm weakness, hand clumsiness, and intrinsic hand muscle wasting.6,7 Local/regional pain is a common initial symptom in both nerve sheath tumors and meningiomas. Because of the origin of nerve sheath tumors, they are more likely to also have radicular symptoms.8,9 These regional/radicular symptoms generally precede compressive cord symptoms. Hand and arm weakness as well as local and/or radicular sensory disturbances, and long tract signs, are common in mid and lower cervical lesions. Lateralizing asymmetry including Brown-Séquard type of findings (ipsilateral motor and proprioceptive and contralateral sensory derangement) can also be seen.10 Other less common presenting syndromes include hydrocephalus (thought to be related to increased cerebrospinal fluid [CSF] protein which alters absorption)11 and subarachnoid hemorrhage from schwannomas.12
Lesions in the cauda equina present very commonly with back and radiating leg pain except for lipomas. Early sphincter disturbances are more common in lesions at the conus or cauda equine.13
Nerve sheath tumors
Nerve sheath tumors consist of neurofibromas and schwannomas which have a common cell of origin, the myelin-producing Schwann cell. The distinction is ultimately histopathological, as neurofibromas expand the involved nerve in a fusiform fashion with nerve fibers intermingled in the substance of the tumor. Schwannomas arise in a more eccentric, globoid fashion with a more discrete attachment to the nerve. Nerve sheath tumors account for approximately 40% of intradural/extramedullary tumors in adults, with an annual incidence of 0.3–0.4 per 100 000.9 Men and women are affected equally, and generally within the fourth to sixth decades of life. Most solitary schwannomas are nonsyndromic and are distributed proportionally throughout the spine. Multiple nerve sheath tumors are seen in patients with neurofibromatosis, with neurofibromas predominating in NF1 and schwannomas predominating in NF2. Multiple schwannomas can occur in patients without a recognized genetic predisposition. Approximately 2.5% of nerve sheath tumors show malignant degeneration,9 with more than half associated with neurofibromatosis. Malignant nerve sheath tumors have a very poor prognosis.
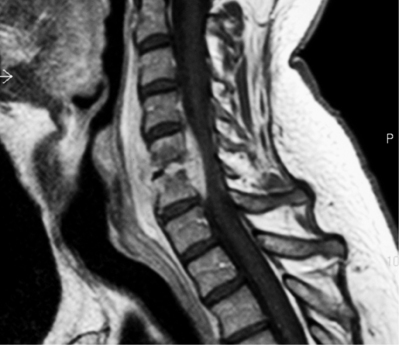
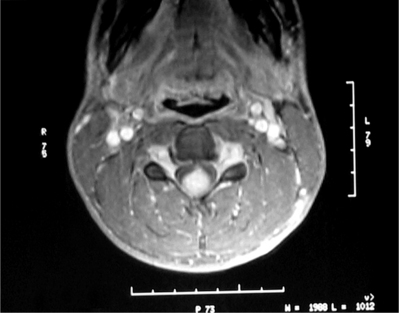
Schwanommas with extradural extension (dumbbell schwannomas) often show a smooth expansion of the bony foramen (Fig. 37.3). Extramedullary tumors by definition displace the cord by magnetic resonance imaging (MRI) and myelogram. MRI has become the imaging modality of choice for spinal lesions. The MRI appearance of schwannomas can help differentiate them from meningiomas in that schwannomas are somewhat hypointense to spinal cord on T1 and hyperintense on T2 (Fig. 37.4).2 Enhancement tends to be heterogeneous and a predominantly peripheral pattern of enhancement is not uncommon. This may be due to cystic, hemorrhagic, or necrotic changes. Nerve sheath tumors that are predominantly intradural are usually amenable to complete resection and have a low recurrence rate with complete resection.10 The nerve fibers of origin of the tumor often have to be sectioned to allow complete resection. This rarely results in significant neurologic deficit even at the cervical or lumbar level, as adjacent roots may have assumed some of the neurologic function.8,14–16 However, intraoperative nerve monitoring/stimulation helps guide intraoperative decision-making.
Meningiomas
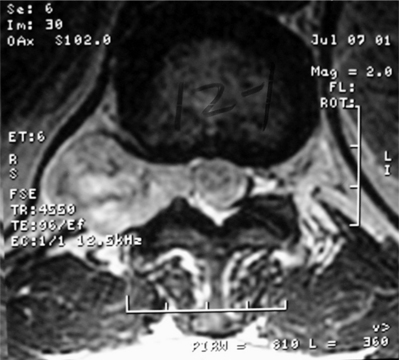
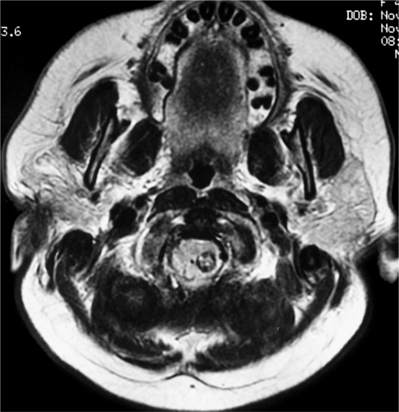
Meningiomas and nerve sheath tumors occur at approximately the same incidence in adults. However, there is an extreme gender bias, as approximately 80% of spinal meningiomas occur in women in the fifth to seventh decades of life.2 Almost 80% occur in the thoracic spine but they are also found in the upper cervical spine and foramen magnum. Almost all meningiomas are completely intradural; however, as many as 10% have an extradural component. The MRI appearance of meningiomas as compared to schwannomas tends to be isointense to spinal cord on T1 and T2 sequences with a tendency towards more homogeneous enhancement (Fig. 37.5). An enhancing dural tail by MRI is also suggestive of a meningioma. Calcification is also a common finding. In general, spinal meningiomas can be completely removed in 90% of cases. Recurrence rates range 3–23%.17,18 One potential recurrence risk is the extent to which involved dura can be removed. In dura that cannot easily be removed, extensive coagulation is also used. It is unclear if there is a significant difference in recurrence rates between these two methods.18 Often, resection of involved dura aids in removal in accessible areas (dorsal). Dural involvement more ventral is difficult to remove safely and effectively and is probably better coagulated.
Other neoplastic process
As stated previously, metastatic lesions predominate in the extradural space. However, metastasis can be intradural (4%) and should be considered especially in the patient with a known history of systemic malignancy.19 Paragangliomas are rare benign tumors that are histologically related to other nonadrenal paraganglion tumors such as carotid body and glomus jugulare tumors. They tend to be very vascular, well-circumscribed tumors arising from the filum terminale or cauda equine.20 Radiographically, they are difficult to distinguish from filum ependymomas. The histologic determination of neurosecretory granules betrays the neural crest origin of these tumors.
Epidermoids and dermoids are generally developmentally retained ectodermal implants. However, epidermoid tumors were also documented to occur when nonstyleted lumbar puncture needles were used.21 Both have linear growth rates like skin as opposed to the exponential growth rate of neoplasm. Epidermoids are lined by stratified squamous epithelium and the contents include keratin, cellular debris, and cholesterol. They tend to be isointense to CSF by MRI but show diffusion restriction in comparison to arachnoid cysts.21 Dermoids are lined by squamous epithelium but also include dermal appendage organs (hair follicles and sebaceous glands). Their contents are similar to epidermoids but also contain hair and sebum. Their appearance by MRI is variable but classically show patterns similar to fat signal.22
Dorsal arachnoid cysts in the thoracic spine can present as extramedullary mass lesions.23 A neurenteric is a cyst formed by failure of separation of the gastrointestinal tract from the primitive neural crest because of the neural connection with the spinal canal, which may be via a sinus tract or a fibrous band. Neurenteric cysts have a high incidence of associated vertebral anomalies or anterior vertebral defects.24,25 Inflammatory or infectious processes such as sarcoid, tuberculoma, or subdural empyema can present as intradural lesions.26–28
INTRADURAL/INTRAMEDULLARY TUMORS
Intradural/intramedullary (IM-ID) spinal cord lesions only represent 5–10% of spinal tumors. The majority are either astrocytomas or ependymomas which together make up 95% or ID-IM tumors.29,30 In adults, regional pain is the most common initial presentation, which may precede diagnosis by several months to years.31 The location of the tumor dictates the distribution and progression of symptoms. Upper extremity symptoms occur in cervical lesions, while thoracic lesions produce spasticity and sensory disturbances. Numbness in the lower extremities often progresses from distal to proximal. Conus lesions often present with back pain, leg pain, and early sphincter disturbances.2,10
Astrocytomas
Astrocytomas of the spinal cord can occur at any age but predominate in the first three decades of life. They account for 90% of spinal cord tumors in patients less than 10 years old, and 60% in adolescents. The majority of these tumors (60%) are found in the cervical and thoracic regions. There is an associated syrinx in approximately 20% of cases. Spinal cord astrocytomas have a higher incidence in neurofibromatosis. Histologically, most adult spinal astrocytomas are fibrillary in nature. Pilocytic astrocytomas are more common in pediatric cases. Approximately 25% of adult spinal astrocytomas are malignant.31–33 Astrocytomas are hypo- to isointense on T1 and hyperintense on T2. They appear as focal enlargements of the spinal cord and even the lower-grade lesions can have significant enhancement (Fig. 37.6).2
Ependymoma
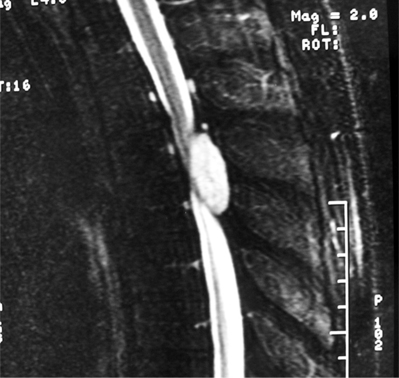
Ependymomas are the most common intramedullary tumors in adults. They occur predominantly in the third through sixth decades of life with a slight male predominance. They commonly occur in the conus and cauda equina where myxopapillary histology predominates.2 These tend to be low grade but can, however, be more aggressive in younger patients and may be refractory to resection if they heavily encase the nerve roots. Symptoms are present 1 year before diagnosis in 82% of cases.10 Intramedullary ependymomas are more commonly found in the cervical cord and frequently (65%) have an associated syrinx.34 There is an association between NF2 and spinal ependymomas. Contrast enhancement is generally intense by MRI with variable homogeneity (Fig. 37.7).
Hemangioblastoma
Hemangioblastomas are histologically benign tumors of vascular origin. The peak incidence is in the fourth decade of life. Approximately 25% are associated with von Hippel–Lindau syndrome.35 This is an autosomal dominant condition associated with brain/spine hemangioblastomas, retinal angiomas, and renal cell carcinoma. By MRI, they often show an intensely enhancing nodule associated with a cyst or syrinx. By angiography, they show a robust vascular blush.
Miscellaneous ID-IM lesions
Other primary neoplasms found much less commonly in the spinal cord include gangliogliomas,36 subependymomas,37 and oligodendrogliomas.38 Intramedullary metastasis is extremely rare but, again, must be considered in patients with a history or suspicion of a systemic malignancy.39
Lipomas occur often in conjunction with spinal dysraphism. Of those not associated with dysraphism, they occur with no sex predominance in the second to fifth decades of life. They often present with ascending mono- or paraparesis. At the conus level they present with early sphincter disturbances and less frequently with pain. They often have intramedullary and extramedullary components.40 By MRI, they show a classic fat pattern. Vascular lesions such as cavernous malformations (Fig. 37.8) or arteriovenous malformations (AVMs) can present with acute onset of symptoms, especially with recurrent hemorrhage.41
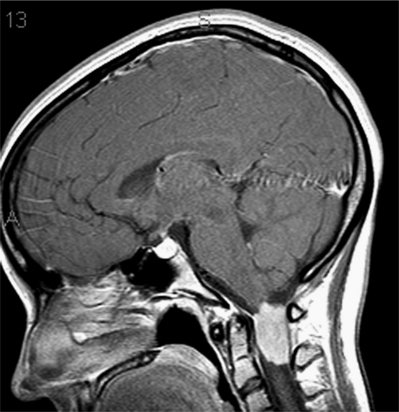
A syrinx not associated with tumor can be seen post-traumatically and in association with a Chiari malformation type 1 (Fig. 37.9). Inflammatory processes such as multiple sclerosis and transverse myelitis can often have presentations similar to mass lesions in the spine.42 In addition to presenting as extramedullary lesions, granulomatous conditions such as sarcoid and tuberculoma can present as intramedullary lesions.43,44
1 Kopelson G, et al. Management of intramedullary spinal cord tumors. Radiology. 1980;135(2):473-479.
2 Van Goethem JW, et al. Spinal tumors. Eur J Radiol. 2004;50(2):159-176.
3 Chamberlain MC, Kormanik PA. Epidural spinal cord compression: a single institution’s retrospective experience. Neurooncol. 1999;1(2):120-123.
4 Samdani AF, et al. Spinal angiolipoma: case report and review of the literature. Acta Neurochir (Wien). 2004;146(3):299-302. discussion 302
5 Clancey JK. Spinal epidural lipomatosis: a case study. J Neurosci Nurs. 2004;36(4):208-209. 213
6 Goodridge AE, et al. Hand wasting due to mid-cervical spinal cord compression. Can J Neurol Sci. 1987;14(3):309-311.
7 Hirano H, et al. Foramen magnum and upper cervical cord tumors. Diagnostic problems. Clin Orthop. 1983;176:171-177.
8 McCormick PC. Anatomic principles of intradural spinal surgery. Clin Neurosurg. 1994;41:204-223.
9 Conti P, et al. Spinal neurinomas: retrospective analysis and long-term outcome of 179 consecutively operated cases and review of the literature. Surg Neurol. 2004;61(1):34-43. discussion 44
10 Schwartz TH, McCormick PC. Spinal cord tumors in adults. In: Winn HR, editor. Youman’s textbook of neurosurgery. Philadelphia: WB Saunders; 2001:4817-4833.
11 Prasad VS, et al. Intraspinal tumour presenting as hydrocephalus in childhood. Childs Nerv Syst. 1994;10(3):156-157.
12 Parmar H, et al. Spinal schwannoma with acute subarachnoid hemorrhage: a diagnostic challenge. Am J Neuroradiol. 2004;25(5):846-850.
13 Bagley CA, Gokaslan ZL. Cauda equina syndrome caused by primary and metastatic neoplasms. Neurosurg Focus. 2004;16(6):e3.
14 Kim P, et al. Surgery of spinal nerve schwannomas. Risk of neurologic deficit after resection of involved root. J Neurosurg. 1989;71(6):810-814.
15 Celli P. Treatment of relative nerve roots involved in nerve sheath tumors: removal or preservation? Neurosurg. 2002;51(3):684-692.
16 Miura T, et al. Resection of cervical spinal neuroma including affected nerve root: recovery of neurologic deficits in 15 patients. Acta Orthop Scand. 1998;69(3):280-282.
17 Solero CL, et al. Spinal meningiomas: review of 174 operated cases. Neurosurgery. 1989;25(2):153-160.
18 King AT, et al. Spinal meningiomas: a 20-year review. Br J Neurosurg. 1998;12(6):521-526.
19 Perrin RG, Livingston KE, Aarabi B. Intradural extramedullary spinal metastasis. A report of 10 cases. J. Neurosurg. 1982;56(6):835-837.
20 Sundgren P, et al. Paragangliomas of the spinal canal. Neuroradiology. 1999;41(10):788-794.
21 Lai SW, et al. MRI of epidermoid cyst of the conus medullaris. Spinal Cord. 2005;43(5):320-323.
22 Krishna KK, et al. Dermoid of the conus medullaris. J Clin Neurosci. 2004;11(7):796-797.
23 Wang MY, Levi AD, Green BA. Intradural spinal arachnoid cysts in adults. Surg Neurol. 2003;60(1):55-56.
24 Lippman CR, et al. Intramedullary neurenteric cysts of the spine. Case report and review of the literature. J Neurosurg Spine. 2001;94(2):305-309.
25 Singhal BS, et al. Intramedullary neurenteric cyst in midthoracic spine in an adult: a case report. Neurol India. 2001;49(3):302-304.
26 Bernaerts A, et al. Tuberculosis of the central nervous system: overview of neuroradiological findings. Eur Radiol. 2003;13(8):1876-1890.
27 Bose B. Extramedullary sarcoid lesion mimicking intraspinal tumor. Spine J. 2002;2(5):381-385.
28 Greenlee JE. Subdural empyema. Curr Treat Options Neurol. 2003;5(1):13-22.
29 Bowers DC, Weprin BE. Intramedullary spinal cord tumors. Curr Treat Options Neurol. 2003;5(3):207-212.
30 Kane PJ, et al. Spinal intradural tumours: Part II – Intramedullary. Br J Neurosurg. 1999;13(6):558-563.
31 Houten JK, Cooper PR. Spinal cord astrocytomas: presentation, management and outcome. J Neurooncol. 2000;47(3):219-224.
32 Banczerowski P, et al. Primary intramedullary glioblastoma multiforme of the spinal cord: report of eight cases. Ideggyogy Sz. 2003;56(1–2):28-32.
33 Santi M, et al. Spinal cord malignant astrocytomas. Clinicopathologic features in 36 cases. Cancer. 2003;98(3):554-561.
34 Koyanagi II, et al. Diagnosis of spinal cord ependymoma and astrocytic tumours with magnetic resonance imaging. J Clin Neurosci. 1999;6(2):128-132.
35 Huang JS, Chang CJ, Jeng CM. Surgical management of hemangioblastomas of the spinal cord. J Formos Med Assoc. 2003;102(12):868-875.
36 Jallo GI, Freed D, Epstein FJ. Spinal cord gangliogliomas: a review of 56 patients. J Neurooncol. 2004;68(1):71-77.
37 Shimada S, et al. Subependymoma of the spinal cord and review of the literature. Pathol Int. 2003;53(3):169-173.
38 Aman RA, et al. Intramedullary oligodendroglioma: a case report. Gan To Kagaku Ryoho. 2000;27(Suppl 2):571-573.
39 Kaya RA, et al. Intramedullary spinal cord metastasis: a rare and devastating complication of cancer – two case reports. Neurol Med Chir (Tokyo). 2003;43(12):612-615.
40 Kulkarni AV, Pierre-Kahn A, Zerah M. Conservative management of asymptomatic spinal lipomas of the conus. Neurosurgery. 2004;54(4):868-873. discussion 873–875
41 Sandalcioglu IE, et al. Intramedullary spinal cord cavernous malformations: clinical features and risk of hemorrhage. Neurosurg Rev. 2003;26(4):253-256.
42 Krishnan C, et al. Transverse myelitis: pathogenesis, diagnosis and treatment. Front Biosci. 2004;9:1483-1499.
43 Prelog K, Blome S, Dennis C. Neurosarcoidosis of the conus medullaris and cauda equina. Australas Radiol. 2003;47(3):295-297.
44 Torii H, et al. Intramedullary spinal tuberculoma – case report. Neurol Med Chir (Tokyo). 2004;44(5):266-268.