CHAPTER 2 Nonoperative Treatment of Distal Radius Fractures
Indications
The fractures of the distal radius best suited for nonoperative treatment are (1) undisplaced and minimally displaced stable fractures, (2) bending fractures in good bone, and (3) intra-articular fractures where there is almost no step between the intra-articular fragments.
Undisplaced and Minimally Displaced Fractures
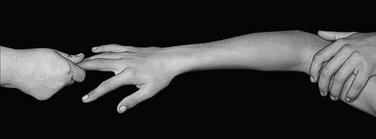
The cast is made up of an 8-inch plaster of Paris slab extending from the distal palmar crease around the radial aspect of the wrist and proximally up to the proximal third of the forearm. A hole is cut out for the thumb. The pronated forearm is held by two assistants, one holding the arm just above the elbow and the other holding the extended fingers. These assistants must give slight traction along the line of the forearm (Fig. 2-1). The hand and forearm are covered firmly with synthetic wool, and the plaster slab is applied to extend around the radial side of the forearm and wrist and ulnarward both dorsally and on the palmar side. This slab is then firmly bandaged in place with a crepe bandage. As the plaster sets it is molded with three points of compression to counter the dorsal bending deformity of the distal radius fracture. The palmar point of compression overlies the site of the fracture and forms the fulcrum over which the dorsal two compression points stretch the broken bone, thereby countering the dorsal bending tendency of such a fracture (Figs. 2-2 and 2-3).
Do Undisplaced Fractures Need Immobilization?
Undisplaced or minimally displaced fractures can be safely treated in a plaster of Paris slab. Stable fractures can also be safely treated without immobilization. In a prospective randomized study of 97 fractures, Dias and associates1 demonstrated that the treatment of such fractures without a cast did not result in greater displacement or more discomfort than those treated in a cast. Moreover, more patients treated without immobilization recovered grip and movement within 8 weeks compared to those immobilized in a cast.
Displaced Fractures
Extra-articular Fractures
Extra-articular displaced fractures need to be carefully manipulated back into place. The technique described by Charnley2 needs to be followed to disimpact the fracture by giving traction. Traction may be easily given using manual distraction or alternatively may be provided by finger traps and weights as described by Earnshaw and coworkers.3 Once the fracture has been disimpacted, the wrist can be manipulated in pronation to counter the tendency of the distal fragment to supinate. Although flexion of the wrist has been suggested to counter the dorsal angulation of the distal radius, the fracture can be gently manipulated to correct the dorsal tilt by molding. The physician stands facing the patient’s head, on the thumb side of the pronated forearm. The physician’s hand nearest the patient supports the palmar side of the radius just proximal to the fracture. The physician’s hand away from the patient is used to push down on the dorsum of the distal fragment (see Fig. 2-2). The fracture is then assessed under fluoroscopy. If the position is satisfactory, especially with the traction released, a plaster slab is applied as described above.
Intra-articular Fractures
Severely displaced or comminuted fractures cannot be treated satisfactorily using a cast. Casting can only be considered in these cases if the physiologic state of the patient does not permit surgical intervention. The physician must anticipate a high rate of loss of position, with remanipulation not offering much hope of improved position.4
Aftercare
Based on these observations, the patient is taught wrist exercises, concentrating on wrist circumduction and forearm rotation. Formal physiotherapy may need to be organized if the wrist and forearm range is less than half of the opposite, uninjured side. The patient is advised to resume activity but to avoid heavy work and any activity or sport where there is a real risk of falling. This is because bone healing is still progressing.
Advantages, Disadvantages, and Limitations of Nonoperative Treatment
Loss of Position and Malunion
The biggest risk with nonoperative treatment of distal radius fractures is loss of position. Mackenney and colleagues5 carefully investigated factors that could help predict loss of position after manipulation and established that the patient’s age, initial metaphyseal comminution, and shortening promoted such a loss. The rate of malunion, defined as shortening of 3 mm and a dorsal tilt of 10 degrees, has been calculated as 27% for minimally displaced fractures and 60% for initially displaced fractures. This rate has also been established by other studies. Dias and associates6 demonstrated that osteoporosis caused greater impaction at injury, a greater early loss of position, and a greater progression of deformity during the subsequent healing phase after the cast was removed because osteoporotic bone was less able to resist the deformation of bone caused by the loads borne by the wrist during everyday activity.
Recovery of Movement
It is usual to recover almost a full range of wrist and forearm rotation after distal radius fractures treated nonoperatively within 1 year.6–8
The outcome in the long term also remains satisfactory, with no patient treated nonoperatively changing occupation over a lifetime.9
Osteoarthritis After Nonoperative Treatment
Gartland and Werley10 reviewed 60 distal radius fractures around 18 months after injury and found no arthritis if the fracture was undisplaced and mild arthritis in 11% of 27 undisplaced intra-articular fractures and 40% of 26 displaced intra-articular fractures.
Smaill8 reported on the results of 41 fractures 5 years after injury and found that 10 (24%) had some narrowing of joint space, but only 3 had minor aching symptoms. This rate of between 21% and 33% is unchanged for minimally displaced fractures over the long term of between 32 and 38 years.9,11
Knirk and Jupiter12 related the presence of radiologic arthritis to the step in the intra-articular fracture and reported an 11% arthritis rate if the joint was congruous but a 95% arthritis rate if the joint was not congruous. Their data, however, had 21 cases of intra-articular fractures treated nonoperatively and 8 (38%) had slight decrease of joint space at 6.7 years after injury. This is similar to the 40.5% slight decrease in joint space reported by Kopylov and coworkers9 in 47 intra-articular fractures seen 32 years after injury. Ford and associates11 reported that 24 of 40 (60%) intra-articular fractured wrists had radiologic arthritis at least one grade worse than the other wrist 38 years after injury. They calculated that the odds of developing arthritis in the fractured wrist doubled (2.4 times) with 2-mm shortening and tripled (3.2 times) in intra-articular fractures.
The possibility of remodeling of the step also needs to be borne in mind. This has been demonstrated by Ford and colleagues11 in a prospective study following patients with fractures over 9 years. Arthritis may not be completely avoided if there is significant damage to the articular cartilage at the time of injury.
The observation that a step of greater than 2 mm results in osteoarthritis may be less true for nonoperatively treated fractures because the step may decrease with loading of the hand as the carpus pushes the articular step displacement into better alignment. This may explain why the rate of osteoarthritis is greater in fractures that are partly fixed with wires and other metal12 where one column is better fixed than the other, resulting in inadequate correction or loss of correction during healing. This promotes the formation of a step within the articular surface, which may result in early onset of arthritis. The fixation device prevents the leveling of the intra-articular displacement and, by maintaining the step, promotes degenerative change.
All alternative methods of intervention must be able to, at the very least, deliver the rate and severity of osteoarthritis following nonoperative treatment or improve on it. The information available at present does not suggest that internal fixation methods have delivered an improved rate,12,13 even if one were to discount the occasional serious and irreversible complications of fixation, such as screws cutting out into the radiocarpal joint and destroying it.
Tendon Rupture
The extensor pollicis tendon can rupture even after nonoperative treatment of distal radius fractures. The rate of rupture is very low, between 0.3% (14 of 4000),14 1.2% (7 of 565),15 and 2% (4 of 207).16 Up to 50% of ruptures of this tendon are due to a distal radius fracture.17 It usually occurs soon after the cast is removed, at between 4 and 12 weeks after injury, and is often seen after minimally displaced fractures.
The tendon may fray over a sharp edge at the fracture site or as it is contained in an osseoligamentous third extensor compartment pulley. Its circulation could be compromised, causing an ischemic rupture. Heidemann and associates18 were able to demonstrate encroachment of the third extensor compartment by the edge of the fracture at Lister’s tubercle using CT scans.
The usual treatment, if needed, is a transfer of the extensor indicis proprius tendon to the extensor pollicis distal tendon with satisfactory but incomplete restoration of thumb extension.17 Although rare, isolated cases of rupture of flexor tendons have been reported, and there are also a very few cases of rupture of more than one tendon.
Nerve Problems
Nerve symptoms occur in between 6% and 17% of patients.7,15,16 Ulnar nerve and radial nerve symptoms are less common.
Stewart and coworkers7 recorded that symptoms of median nerve dysfunction after nonoperatively treated distal radius fracture, with altered feeling in the thumb, index, middle, and ring fingers, occurred in 17% of 209 patients with distal radius fractures, and if treated nonoperatively, resolution of symptoms occurred in 55%.
Algodystrophy
Algodystrophy needs early identification and rigorous management to control pain, decrease swelling, and maintain and improve movement if the devastating and essentially permanent dysfunction of the wrist and hand is to be avoided. Up to 25% of patients with distal radius fracture will have some features of algodystrophy,19 which can be identified early and are usually exacerbated by tight circumferential casts. If inadequately treated, the consequences are lasting.
1. Dias JJ, Wray CC, Jones JM, Gregg PJ. The value of early mobilisation in the treatment of Colles’ fractures. J Bone Joint Surg [Br]. 1987;69:463-467.
2. Charnley J. The Colles Fracture. In The Closed Treatment of Common Fractures, 3rd ed., Edinburgh: E and S Livingstone; 1961:128-142.
3. Earnshaw SA, Aladin A, Surendran S, Moran CG. Closed reduction of Colles fractures: comparison of manual manipulation and finger-trap traction. A prospective, randomised study. J Bone Joint Surg [Br]. 2002;84(3):354-358.
4. McQueen MM, Hajducka C, Court-Brown CM. Redisplaced unstable fractures of the distal radius: a prospective randomised comparison of four methods of treatment. J Bone Joint Surg [Br]. 1996;78(3):404-409.
5. Mackenney PJ, McQueen MM, Elton R. Prediction of instability in distal radial fractures. J Bone Joint Surg [Am]. 2006;88(9):1944-1951.
6. Dias JJ, Wray CC, Jones JM. Osteoporosis and Colles’ fractures in the elderly. J Hand Surg [Br]. 1987;12(1):57-59.
7. Stewart HD, Innes AR, Burke FD. Functional cast-bracing for Colles’ fractures. A comparison between cast-bracing and conventional plaster casts. J Bone Joint Surg [Br]. 1984;66(5):749-753.
8. Smaill GB. Long-term follow-up of Colles’ fracture. J Bone Joint Surg [Br]. 1965;47:80-85.
9. Kopylov P, Johnell O, Redlund-Johnell I, Bengner U. Fractures of the distal end of the radius in young adults: a 30-year follow-up. J Hand Surg [Br]. 1993;18(1):45-49.
10. Gartland JJJr, Werley CW. Evaluation of healed Colles’ fractures. J Bone Joint Surg [Am]. 1951;33(4):895-907.
11. Ford DR, Sithole J, Davis TRC. The 38-year outcome of distal radial fractures in young adults. J Hand Surg [Br]. 2006;31(Suppl 1):6-7.
12. Knirk JL, Jupiter JB. Intra-articular fractures of the distal end of the radius in young adults. J Bone Joint Surg [Am]. 1986;68(5):647-659.
13. Catalano LW3rd, Cole RJ, Gelberman RH, et al. Displaced intra-articular fractures of the distal aspect of the radius. Long-term results in young adults after open reduction and internal fixation. J Bone Joint Surg [Am]. 1997;79:1290-1302.
14. Hove LM. Delayed rupture of the thumb extensor tendon. A 5-year study of 18 consecutive cases. Acta Orthop Scand. 1994;65(2):199-203.
15. Cooney WP3rd, Dobyns JH, Linscheid RL. Complications of Colles’ fractures. J Bone Joint Surg [Am]. 1980;62:613-619.
16. McKay SD, MacDermid JC, Roth JH, Richards RS. Assessment of complications of distal radius fractures and development of a complication checklist. J Hand Surg [Am]. 2001;26(5):916-922.
17. Loos A, Kalb K, Van Schoonhoven J, et al. Rekonstruktion der Extensor pollicis longus-Sehne mittels Extensor indicis-Transposition. Handchir Mikrochir Plast Chir. 2003;35(6):368-372.
18. Heidemann J, Gausepohl T, Pennig D. Einengung des dritten Strecksehnenfaches bei gering dislozierter distaler Radiusfraktur mit Gefahr einer Extensor pollicis longus-Sehnenruptur. Handchir Mikrochir Plast Chir. 2002;35(5):324-327.
19. Atkins RM, Duckworth T, Kanis JA. Algodystrophy following Colles’ fracture. J Hand Surg [Br]. 1989;14(2):161-164.