CHAPTER 88 Noninvasive Imaging of Atherosclerosis
Atherosclerosis is a disease process in which fatty infiltration and inflammation of the wall of medium to large arteries lead to plaque formation and multiple adverse sequelae including rupture, obstruction, and embolism. It is a major source of morbidity and mortality, with 25 million people in the United States demonstrating at least one clinical manifestation of atherosclerosis.1 It has been the number one cause of death in the United States since 1900 except for 1918, the year of the influenza epidemic.2 Atherosclerosis also leads to significant losses in functional capacity and quality of life.
ATHEROSCLEROSIS
Definition
Atherosclerosis is a form of arteriosclerosis characterized by the deposition of plaques containing cholesterol and lipids on the innermost layer of the walls of large and medium-sized arteries.3 The atheroma is a complex of lipids and fibrous tissue with surrounding hypertrophied smooth muscle and inflammatory cells that leads to progressive vessel luminal narrowing and obstruction of blood flow, directly causing or indirectly mediating the many clinical manifestations of atherosclerotic disease.
Prevalence and Epidemiology
The exact overall prevalence of atherosclerosis is not known because of the numerous arterial beds it encompasses and because it is often silent and found only through screening, which is not universally performed. Moreover, atherosclerotic disease often coexists in multiple vascular systems, making the individual disease prevalence not additive. Atherosclerosis does account for almost three quarters of all deaths from cardiovascular disease.2 The majority of these are from coronary atherosclerosis. Although the mortality from coronary heart disease is decreasing, that from noncoronary atherosclerotic disease is increasing, partly owing to the overall aging of the population. Information on the prevalence of noncoronary atherosclerotic disease and the resultant complications is available for the individual arterial systems affected.
Cerebrovascular Atherosclerotic Disease
Cerebrovascular atherosclerosis has the highest impact of noncoronary disease processes. Stroke is the third leading cause of death in the United States, with an annual incidence of 700,000 events. It is associated with high morbidity and mortality, with a 50% 5-year survival and 15% to 30% risk of permanent disability. Sixty percent of new or recurrent strokes are the result of atherothrombotic disease. One third of these are due to carotid atherosclerosis. At the age of 75 years, 53% of women and 63% of men have carotid stenoses of more than 10%.4 The presence of carotid bruits approximately doubles the expected stroke risk, but the disease is often in a different cerebral vascular territory and not related to the initial lesion auscultated. Despite the high morbidity and mortality from stroke, individuals with carotid artery disease are more likely to die of cardiovascular causes than of cerebrovascular disease, underscoring the close relationship between noncoronary atherosclerosis and cardiovascular risk.
Peripheral Atherosclerotic Disease
Defining peripheral arterial disease (PAD) as a noninvasive ankle-brachial index (ABI) below 0.90, its prevalence in the lower extremities is 2.5% for those younger than 60 years and increases markedly with age to 18.8% for those 70 years of age or older.5 Claudication is associated with significant disability and is present in as many as 6% to 7% of the general population 65 years of age and older. The prevalence also varies significantly by risk factor profile. The PAD Awareness, Risk, and Treatment: New Resources for Survival (PARTNERS) study examined PAD prevalence in the American primary care setting and found that the prevalence was as high as 29% in older Americans or those with significant risk factors (especially tobacco use and diabetes). The disease has high morbidity from both peripheral and cardiovascular events. The annual mortality for those with PAD is 4% to 6% and is as high as 25% for the 1% to 2% of patients with critical limb ischemia.5 Individuals with PAD have a 60% to 80% prevalence of significant coronary artery disease (CAD), with a twofold to sixfold increase in cardiovascular death and a 40% increase in the risk of stroke. PAD is often undetected without careful exercise tolerance questioning and judicious screening, going undiagnosed in as many as 55% of affected individuals.
Aortic Atherosclerotic Disease
Atherosclerosis of the aorta rarely leads to occlusion but causes abdominal aortic aneurysms (AAAs), peripheral embolization of aortic atheromatous material, penetrating aortic ulcers, and intramural hematomas. The prevalences of these entities are difficult to discern as aneurysms have wide variations in definition and the other aortic manifestations are under-reported. The Veterans Affairs Aneurysm Detection and Management (ADAM) study of 125,000 veterans revealed a prevalence of 4.3% and 1.0% for AAAs of 3.0 cm or larger in men and women, respectively, aged 50 to 79 years but only 1.3% and 0.1%, respectively, for AAAs of 4.0 cm or larger.4 The prevalence increases markedly with age; one Scandinavian study showed a peak of 5.9% for men aged 80 to 85 years and 4.5% for women older than 90 years. This disease is almost always asymptomatic because the clinical manifestations are typically catastrophic. The risk of AAA rupture is amplified with increasing diameter; AAAs larger than 6 cm have a 25% yearly risk of rupture.4 Despite the focus on rupture, approximately 60% of patients with AAAs die of other cardiovascular complications.
Renal Atherosclerotic Disease
Renal atherosclerotic disease causes 90% of the cases of renal artery stenosis, an uncommon but highly morbid cause of secondary refractory hypertension and renal failure. The prevalence of renal artery stenosis in the population older than 65 years is 6.8%, but it is much higher in high-risk populations. Patients undergoing cardiac catheterization have a 30% prevalence, whereas renal artery stenosis is present in 22% to 59% of those with PAD.5
Renal arterial disease has a high rate of progression; occlusion occurs in up to 39% of cases by 5 years. Significant renal impairment in individuals with two functional kidneys typically occurs only with bilateral renal artery stenosis, but 44% of patients have bilateral disease. Almost half of individuals with renal artery stenosis have increased creatinine concentration, and 29% have a 25% to 50% decline in glomerular filtration rate. Despite these factors, patients with renal artery stenosis have a 2-year dialysis-free survival of 97.3% and 82.4% for unilateral and bilateral disease, respectively. The risk of cardiovascular events in this population (more than fourfold increase) far exceeds that of significant renal impairment.6 Nevertheless, the mortality rate is high with renal artery stenosis and end-stage renal disease, with a mean life expectancy of only 2.7 years.5
Etiology and Pathophysiology
Response to Injury Hypothesis
The primary step in the formation of atherosclerosis is the development of vessel wall endothelial dysfunction, which occurs as a response to injury from risk factors such as elevations and alterations in low-density lipoprotein (LDL), genetic abnormalities, and toxic free radicals stemming from tobacco use.7
Oxidation Hypothesis
Oxidation of LDL is required for its uptake by macrophages and accumulation within the vessel wall.8 Arterial LDL is progressively oxidized by oxygen free radicals and internalized by macrophages, forming lipoperoxides, a reactive species that triggers further LDL oxidation and plasma membrane destruction. Oxidized LDL is also a potent chemoattractant for macrophages, inducing the expression of vascular cell adhesion molecules and inhibiting macrophage mobility, thereby furthering macrophage and lipid accumulation within the vessel wall. These lipid-laden macrophages are known as foam cells because of their histologic appearance.7 As these foam cells accumulate, they undergo apoptosis and necrosis from increased proteolytic activity, forming a necrotic lipid core.
Progression to Clinical Significance
During the initial stages of atherosclerosis, the blood vessel dilates to maintain lumen size, a process known as the Glagov phenomenon (Fig. 88-1). However, the repeated cycles of inflammation, smooth muscle cell and fibrous tissue proliferation, and expansion of the lipid core eventually overwhelm the compensatory response, leading to progressive luminal obstruction. Decreased luminal blood flow from the increasing vessel blockage will eventually lead to insufficient supply to meet oxygen demand, and ischemia will ensue.
More rapid vessel occlusion can also occur, leading to ischemia and potentially infarction, depending on the vascular bed. The activated T lymphocytes present can secrete matrix metalloproteinases and other lytic molecules that can degrade the fibrous cap, leading to cap rupture and the uncovering of the prothrombotic elements underneath. This exposure, along with other procoagulant factors released by activated inflammatory cells, can induce platelet aggregation and ultimately thrombosis and rapid vessel occlusion (Fig. 88-2).
Risk Factors
The risk factors for atherosclerosis are similar across the multiple arterial beds affected, regardless of the end-organ perfused. They fall into two categories: those that are modifiable and those beyond our control. Modifiable risk factors can be further broken down into those that are predominantly a result of lifestyle indiscretions and those that are primarily manifestations of clinical disease that can be treated (Table 88-1).
TABLE 88-1 Risk Factors for the Development of Atherosclerosis
| Modifiable Risk Factors |
| Lifestyle indiscretions |
| Obesity |
| Tobacco use |
| Physical inactivity |
| Clinical comorbid conditions |
| Lipid abnormalities* |
| Elevated low-density lipoprotein or total cholesterol level |
| Low high-density lipoprotein level |
| Elevated triglyceride levels |
| Diabetes mellitus |
| Metabolic syndrome, insulin resistance |
| Hypertension (both systolic and diastolic are independently associated) |
| Risk Factors (Not Modifiable) |
| Advanced age |
| Male gender |
| Race† |
| Genetic predisposition (positive family history) |
| Prothrombotic and proinflammatory comorbid conditions (such as systemic lupus erythematosus, rheumatoid arthritis) |
| New/Under Investigation |
| Increased lipoprotein(a) |
| High-sensitivity C-reactive protein elevation |
| Homocysteine elevation |
| Increased fibrinogen |
* Each of these lipid abnormalities provides independent incremental risk.
† The black population has a higher rate of atherosclerosis than the white population does.
Risk Factors (Not Modifiable)
Increasing age is the most powerful risk factor for noncoronary atherosclerotic vascular disease (AVD). The atherosclerotic process occurs in a stepwise fashion over time, and those with advanced age are more likely to have a higher burden and greater complexity of disease. Data from the Framingham study show that 7% to 9% of individuals 75 years of age or older have carotid stenoses of 50% or more. In contrast, less than 1% had that degree of obstruction at 50 years of age.4
Gender also plays a significant role in the prevalence of atherosclerosis. However, with the increasing number of female smokers and disproportionate prevalence and rate of increase in obesity, these gender differences are narrowing.2 Race also has a significant impact on the likelihood of atherosclerotic disease. For instance, black populations have a 38% higher incidence than do white populations of ischemic stroke and stroke mortality adjusted for risk factors.4
Known genes that promote lipid abnormalities include apolipoprotein E (APOE) and cholesteryl ester transfer protein (CETP). Mutations in the LDL receptor gene are particularly damaging, leading to familial hypercholesterolemia in its homozygous form and significant lipid abnormalities even when heterozygous, which occurs in approximately 1 in 500 persons.8 Contributors to the inflammatory process include peroxisome proliferator–activated receptor γ, vascular cell adhesion molecule, and tumor necrosis factor α.9 Significant research is necessary to identify new genes and to determine the full impact of known genetic abnormalities, their response to environmental conditions, and the subsequent therapeutic implications.
Modifiable Risk Factors
Many of the known modifiable risk factors have well-established interactions with the pathophysiologic processes of noncoronary atherosclerosis. For example, hypertension causes increased levels of angiotensin II, which stimulates smooth muscle growth and lipoxygenase activity, a contributor to LDL oxidation and inflammation. Lipoxygenase also increases free radical production and subsequently reduces nitric oxide formation. Homocysteine decreases nitric oxide availability in addition to its direct toxicity to the endothelium and its prothrombotic effects.7
Tobacco use and diabetes mellitus appear to confer the greatest risk of noncoronary AVD (Fig. 88-3).9 Tobacco use doubles the risk of ischemic stroke. Smoking also increases the risk of PAD by twofold to sixfold, and more than 80% of those with PAD have smoked or continue to do so. This effect occurs in a dose-dependent manner.5 In the Edinburgh Artery Study, the odds ratio (OR) for PAD with tobacco use (OR, 1.8-5.6) was approximately twofold to threefold higher than for CAD (OR, 1.1-1.6).
The proposed pathophysiologic mechanisms for the increased risk of disease in PAD versus CAD are (1) increased endothelial dysfunction (measured through von Willebrand and tissue plasminogen activator antigens), (2) reduced circulating antioxidants, (3) increased plasma fibrinogen levels, and (4) altered lipoprotein profiles. The Edinburgh Artery Study specifically addressed the differential odds ratios by measuring risk factors and analyzing the prevalence of these two conditions in 1592 subjects both with and without a history of tobacco use.10 This study confirmed increased levels of von Willebrand and tissue plasminogen activator antigens (markers of endothelial disruption), reduced antioxidant levels, and increased fibrinogen levels. However, correction for these variables decreased the PAD odds ratio to only 2.7 from 3.9, with little change in the CAD odds ratio. Although the differential effect of tobacco use was partly mitigated by adjusting for these potential contributors, it is clear that other unknown mechanisms still predominate.10
Diabetes mellitus is the other of the two most significant modifiable risk factors, increasing the risk of PAD by 2- to 4-fold and ischemic stroke by 1.8- to 6-fold.5,11 The risks of critical limb ischemia and major amputation are also higher with diabetes. The pathophysiologic mechanism underlying this increased risk is multifactorial. Increased levels of C-reactive protein promote apoptosis and stimulate procoagulant tissue factors, leukocyte adhesion molecules, and inhibitors of fibrinolysis. The hyperglycemia, insulin resistance, and fatty acid production associated with diabetes reduce the bioavailability of nitric oxide, decreasing vasodilation and allowing increased smooth muscle cell proliferation and platelet activation. Finally, diabetes increases procoagulant tissue factor and fibrinogen production, leading to a hypercoagulable state.12 Unlike tobacco use, diabetes does not appear to increase the risk of noncoronary AVD disproportionately to the risk of CAD.
Unlike with coronary vascular disease, the lipid abnormality most strongly associated with noncoronary AVD is the combination of high triglyceride and low high-density lipoprotein (HDL) levels, which are also highly linked with diabetes. These lipid abnormalities are closely involved in the noncoronary atherosclerotic process along with increased LDL. Triglyceride-rich lipoproteins stimulate smooth muscle cell proliferation and extracellular matrix deposition. Low levels of HDL increase atherosclerotic risk through a relative decrease in its beneficial processes, including reverse cholesterol transport for its excretion, endothelial protection, and anti-inflammatory effects.8
Novel Risk Factors
Greater understanding of the most common risk factors associated with noncoronary AVD have led to the development of risk scores for stroke and claudication based on the Framingham data. However, novel contributors of risk, especially those estimating inflammation, such as high-sensitivity C-reactive protein, lipoprotein(a), and homocysteine, are challenging these existing paradigms.9 Lipoprotein(a), for instance, self-aggregates and increases inflammation by impairing fibrinolysis through regulation of fibrinogen activator inhibitor 1 and by inducing smooth muscle cell proliferation.
There is some variation in risk factors based on the anatomic localization of disease. For instance, in aortic disease, tobacco use continues to play a significant role (partly because of elastin degradation). It and male sex confer the highest risk for AAAs, whereas those of Asian descent rarely develop this disorder. A family history of AAA is especially important. In PAD, however, diabetes plays a larger role, especially for the female gender. PAD appears to have a higher incidence in African-American and Hispanic subgroups. Other than these examples, few data are available on gender- and ethnicity-based risk differences.9
Manifestations of Disease
Clinical Presentation
History
The presenting symptoms of noncoronary AVD in the different arterial beds can be found in Table 88-2, which can also be used as a comprehensive vascular review of systems.
TABLE 88-2 Common Presenting Clinical Findings and Vascular Review of Systems
| Condition | Clinical Symptoms and Review of Systems |
|---|---|
| Cerebrovascular disease |
AAA, abdominal aortic aneurysm; ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; ESR, erythrocyte sedimentation rate.
AAAs are almost always asymptomatic, although they can be picked up on a careful physical examination. An essential part of a general review of systems is a history of aortic aneurysmal disease in a first-degree relative. Up to 28% of patients with an AAA have a first-degree relative with disease, and the relative risk for male relatives of affected men is as high as 18.5 Individuals with a family history may have onset of disease at a younger age, although the rate of progression and location do not appear to differ. Inflammatory AAA is one subset that does often have symptoms with no significant differences in risk factor makeup.
Physical Examination
The key aspects of the peripheral vascular examination include the following5:
Given the limited sensitivity and specificity of the history and physical examination for noncoronary AVD, any concerning findings should be evaluated further through noninvasive vascular testing.5
Imaging Indications and Algorithm
Carotid intimal-medial thickness (CIMT) measurement by ultrasonography, on the other hand, is primarily used to evaluate cardiovascular risk and is typically performed in asymptomatic individuals. It is best used in patients with an intermediate risk of cardiovascular disease or with a strong family history, an especially severe risk factor, or other reason for which the optimal aggressiveness of medical therapy in an individual is unknown. More than 1000 asymptomatic patients in nine studies have shown a strong association between an abnormal CIMT and increased risk of cardiovascular death, nonfatal myocardial infarction (MI), stroke, or a combination of these. The presence of carotid plaque or a CIMT greater than the 75th age- and gender-matched percentile indicates the need for more aggressive medical therapy.13
Noninvasive evaluation of PAD almost always starts with ABI ascertainment as a high-risk asymptomatic screening method or to evaluate symptoms in the absence of arterial insufficiency ulcers or critical limb ischemia (Fig. 88-4). ABIs correlate highly with the site and severity of peripheral arterial obstruction as well as with overall cardiovascular risk. Subsequent further studies typically involve high-resolution MRA, computed tomographic angiography (CTA), or digital subtraction angiography.
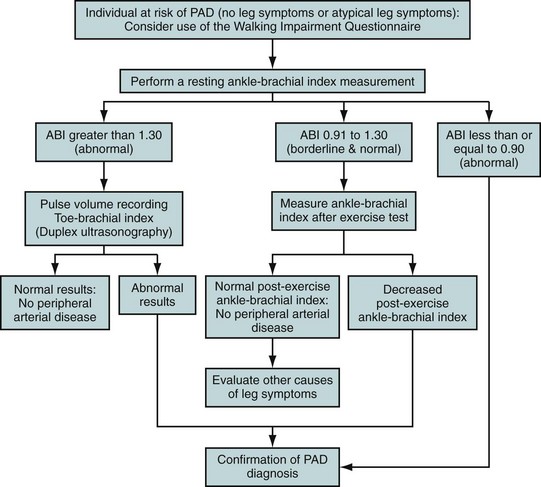
 FIGURE 88-4 Diagnostic algorithm for peripheral arterial disease (PAD).
FIGURE 88-4 Diagnostic algorithm for peripheral arterial disease (PAD).
(Modified from Hirsch AT, Haskal ZJ, Hertzer NR, et al. ACC/AHA 2005 guidelines for the management of patients with peripheral arterial disease [lower extremity, renal, mesenteric, and abdominal aortic]: executive summary a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines [Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease] endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. J Am Coll Cardiol 2006; 47:1239.)
Imaging Techniques and Findings
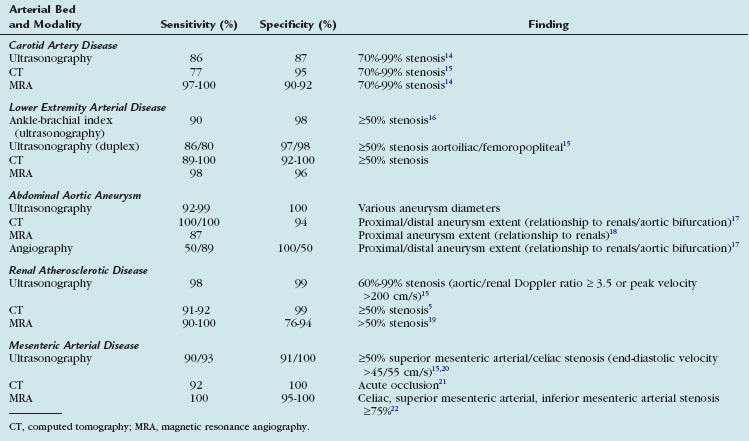
The sensitivities and specificities of the multiple imaging modalities that are used to assess noncoronary atherosclerotic disease are presented in Table 88-3.
TABLE 88-3 Sensitivities and Specificities of Noninvasive, Noncoronary Atherosclerosis Assessment Techniques
Ultrasonography
Ultrasonography has demonstrated utility in all of the noncoronary arterial beds. In the cerebrovascular arterial system, carotid ultrasound examination is performed in patients with symptoms of cerebrovascular ischemia or an asymptomatic bruit to assess for focal atherosclerotic plaques quantified through Doppler analysis. An increase in velocity typically does not occur until a stenosis of 50% or more is present. The plaque thickness can be assessed (with discrete plaques defined as a 50% increase in wall thickness), but this typically correlates poorly with the overall plaque size and volume because it is one two-dimensional measurement.23 Despite these limitations, this technique remains a primary tool to investigate for significant cerebrovascular atherosclerotic disease.
An assessment of the overall atherosclerotic burden can also be obtained for epidemiologic or risk stratification purposes through measurement of the CIMT (Fig. 88-5).23 An absolute cutoff is difficult to ascertain because age has a large effect on the CIMT. Moreover, because of the frequent inability to separate the intima from the media, diseases such as hypertension that cause medial hypertrophy increase the CIMT in the absence of atherosclerosis. However, increasing CIMT has been shown to correlate well with cardiovascular morbidity, with an age- and sex-adjusted 15% and 18% increase in risk of MI and stroke, respectively, with each 0.1-mm increase in CIMT.24 Interobserver variability is good, averaging approximately 0.4 mm with a 3.1% coefficient of variation for experienced readers. Although it has significant value as a predictor of adverse outcome in large populations, CIMT has extensive variability not directly related to atherosclerosis that limits its ability to provide sufficient prognostic information at the level of the individual patient at this time. A consensus statement of the American Society of Echocardiography recommends against CIMT in patients with established atherosclerosis or use in serial fashion to assess progression.13
In the peripheral arterial system, ultrasonography has utility in the measurement of ABIs, in the evaluation of brachial artery reactivity, and for arterial duplex scanning. ABI assessment uses hand-held Doppler ultrasound, although this is not an imaging study. The ratio of the ankle to the higher of the brachial systolic blood pressures is obtained; a ratio below 0.90 indicates moderate to significant upstream peripheral arterial obstruction. This technique has fair test-retest reliability (±10% to 16%) and a sensitivity and specificity of 90% and 98%, respectively, for a peripheral arterial stenosis of 50% or more. An ABI below 0.40 is consistent with severe ischemia. Symptomatic patients with normal ABIs can often have dysfunction unmasked with exercise (treadmill or active, repeated pedal plantar flexion). Abnormal ABIs correlate with claudication and functional status (walking distance, overall physical activity) and indicate a higher risk of overall mortality and likelihood of CAD (relative risks at 4 years of 3.1 and 3.7, respectively) and stroke.16
Brachial artery reactivity testing assesses the endothelial dysfunction that typically precedes the clinical manifestations of atherosclerosis, making it potentially effective for screening the early stages of disease. Forearm ischemia is created with blood pressure cuff inflation for more than 5 minutes, and the percentage increase in brachial artery diameter is compared with baseline. Functional endothelium releases nitric oxide and should induce reactive hyperemia, increasing the vessel diameter. Flow-mediated dilation of less than 10% was associated in one study with an increased risk of MI and revascularization.25 The major limitation of this technique is the large variation between patients in the vasodilator response to forearm ischemia, and its clinical use is minimal.
Arterial duplex ultrasonography is performed in stepwise fashion along the entire vessel in the extremity of interest. Color Doppler study is used to identify stenoses, which are then quantified by pulse- and continuous-wave velocities. This technique was evaluated in a meta-analysis of 14 studies that showed sensitivities and specificities of 86% and 97% for aortoiliac disease (≥50% stenosis) and 80% and 98% for femoropopliteal disease.15 In many instances, ultrasonographic evaluation can avoid invasive diagnostic angiography for patients before intervention, with a 97% accuracy compared with arteriography. It can also be used for serial surveillance of grafts and native vessels after stent placement.
Arterial duplex ultrasonography is also very useful in the assessment of renal and mesenteric atherosclerotic disease. An aortic–renal artery Doppler ratio of 3.5 or higher or a peak systolic velocity above 200 cm/sec corresponds to a stenosis of 60% to 99% with a sensitivity of 98%, specificity of 99%, positive predictive value of 99%, and negative predictive value of 97%.15 This technique may also predict blood pressure and renal function improvement with revascularization through the resistive index; a value above 80 is associated with a small chance of improvement. Limitations include the need for deep penetration, which is difficult in obese patients, and a relatively poor sensitivity (approximately 60%) for identifying accessory renal arteries.
Mesenteric duplex ultrasonography is contraindicated in the evaluation of acute intestinal ischemia because of the deep location, lack of fasting and optimal timing in the early morning to avoid excessive bowel gas, increased time required for ultrasound examination, and abdominal distention and fluid often present with this condition.5 Ultrasonography is a good screening modality for chronic mesenteric arterial obstruction, on the other hand, with a sensitivity exceeding 90% for 50% celiac or superior mesenteric arterial stenoses and a 99% negative predictive value. Thus, a normal study should induce work-up of nonatherosclerotic causes of abdominal pain. Mesenteric evaluation is also limited by large body habitus, examiner experience, gas pattern, and prior abdominal surgery.
Computed Tomography
Recent improvements in CTA have markedly shortened image acquisition times that enabled broader anatomic coverage such that a full lower extremity arterial study can now be performed. Moreover, the improved speed allows more detailed visualization of smaller vessels with thinner sections, greater detail, and more uniform vascular enhancement, often with lower doses of contrast agents.5,15 A single breath-hold (if necessary) image is obtained with multiple contiguous or overlapping axial cross sections of the region of interest. These images have the highest diagnostic utility and less chance for artifacts related to postprocessing. However, the more typical angiographic appearance is created with digital smoothing to reduce stair-step artifacts and multiple techniques, such as multiplanar reformation, maximum intensity projection, and volumetric rendering.
CTA provides a high-resolution image of the carotid artery lumen, and lumen diameter can be determined with high accuracy from the three-dimensional reconstructed images (Fig. 88-6). A meta-analysis comparing CTA with invasive cerebral angiography has shown CTA to identify 70% to 99% stenoses with good sensitivity (77%) and excellent specificity (95%). It is especially important to differentiate near from total occlusions, as a benefit has not been shown for revascularization in patients with total occlusions. CTA is 97% to 100% sensitive and 99% to 100% specific for this differentiation.15 Like MRA and ultrasonography, CTA has a significantly lower sensitivity and specificity for the identification of 50% to 69% stenoses.
The renal and mesenteric arteries can be imaged with high resolution by CTA. With use of multidetector CT to assess renal atherosclerosis, the sensitivity is 91% to 92%, with specificity of 99% compared with MRA.5 The interobserver and intermodality agreements between CTA and MRA are excellent (κ, 0.88-0.90). The need for 100 to 150 mL of contrast material limits the use of CTA in renal insufficiency, but this amount is expected to decrease with further technologic advancement.
Magnetic Resonance
An exciting new frontier in MR assessment of atherosclerosis is vessel wall imaging with plaque assessment and resolution of individual plaque components.26 Other imaging techniques primarily focus on the arterial lumen. However, because of positive arterial remodeling (the Glagov effect; see Fig. 88-1), luminal obstruction does not occur until 40% of the intima is occupied by plaque. This phenomenon allows significant underestimation of the burden of disease by currently used imaging modalities. Moreover, the majority of clinical events are initiated at sites with nonsignificant obstruction. Vessel wall imaging with MR uses special sequences that can provide an estimate of fibrous cap thickness and lipid core volume, which are critical determinants of plaque stability or lack thereof.26 Vessel wall imaging can assess earlier atherosclerotic changes than are visible with traditional techniques. It is not widely used in clinical practice, and further research is necessary before its routine adoption. It remains an exciting, ongoing new direction.
Contrast-enhanced MRA remains a method of choice to evaluate the carotid arteries (ACC/AHA class I recommendation).5 This technique was formerly thought to overestimate plaque burden. However, it is more likely that digital subtraction angiography underestimated asymmetric stenoses because of the limited number of carotid artery projections available. It remains highly sensitive and specific for the diagnosis of high-grade carotid stenoses. In a 2006 meta-analysis, the sensitivity and specificity for diagnosis of a 70% to 99% carotid stenosis were 94% and 93%, respectively, compared with invasive angiography. It is critical to separate high-grade stenoses from complete occlusions, and the sensitivity and specificity of MRA compared with invasive angiography for this difference ranges from 97% to 98% and 99% to 100%, respectively.
MRA of the peripheral arterial circulation can be achieved through multiple techniques, such as time-resolved three-dimensional MRA and bolus chase three-dimensional MRA. It performs well in determining the location and degree of atherosclerotic stenosis and has rendered invasive diagnostic angiography almost unnecessary. With use of intraoperative angiography as the gold standard, MRA has accuracy similar to that of invasive catheter angiography; both have accuracies of 91% to 99% for a stenosis of 50% or more and close agreement (91% to 97%), which makes MRA a good technique for preoperative evaluation.5 Compared with ultrasonography, the sensitivity is improved with MRA (98% versus 88%), with similar specificity (96% and 95%, respectively). It can overestimate stenoses because of turbulent flow.
MRA had previously been used extensively in patients with significant renal dysfunction to evaluate for renal artery stenosis and is increasingly regarded as the first-line study (Fig. 88-7). Unfortunately, more caution must be taken given the recently discovered risk of nephrogenic systemic fibrosis. Compared with catheter-based contrast angiography, MRA can identify renal artery stenosis secondary to atherosclerotic disease with sensitivities ranging from 90% to 100%, specificities of 76% to 100%, and accuracy approaching 100%. The interobserver agreement was high, as was that between modalities (κ, 0.88-0.90).5 MRA can also evaluate the surrounding vasculature and the renal parenchyma, and it may even assess renal function. It has a more limited role in the evaluation of patients with renal stents because of artifact that limits intra-stent analysis of stenosis.
Use of MRA in mesenteric ischemia is less well established. In acute mesenteric ischemia, there are few data evaluating the use of MRA. It is unclear if MRA can assess the distal vasculature adequately for microthromboemboli and areas of nonocclusive ischemia. Angiography can evaluate for these entities and is still the test of choice. In addition, vascular access in these patients who are often severely ill is limited. CT is generally preferred in this setting as it allows improved access to the patient, is lower in cost, and has high sensitivity for detection of mesenteric venous thrombosis.27
MRA has been used increasingly in the preoperative assessment before AAA repair. One study of 28 preoperative patients found MRA to correctly predict the proximal extent of the aneurysm and thereby appropriate proximal cross-clamp sites in 87% of patients. Proximal anastomotic sites were identified with similar high accuracy in both MRA and catheter angiography (95% to 97%). Moreover, MRA was able to assess for iliac or femoral aneurysms, which complicate bypass, with sensitivity of 79% and specificity of 86%.18
Nuclear Medicine/Positron Emission Tomography
Radionuclide techniques to evaluate noncoronary atherosclerotic disease currently have very limited clinical value. Positron emission tomography and single photon emission computed tomography can image both perfusion and metabolic abnormalities and provide a functional assessment of their disease in quantifiable fashion. Current research focuses on anatomic correlation with symptoms and outcomes. Muscle perfusion can be difficult to differentiate from skin perfusion.28
MRA, ultrasonography, and CTA can provide an assessment of localization and extent of disease with far more precision. They also have more background research to validate their findings and are often less expensive and faster. There are currently no clinical applications of metabolic imaging in noncoronary atherosclerosis, and promising agents, such as radiolabeled platelets and lipoproteins, have ongoing technical limitations. Ongoing research and technical advancement may identify relevant metabolic tracers and increase the role of these imaging modalities in the future.28
Angiography
Invasive catheter angiography has been widely available for a long time. It has been considered the gold standard for defining vascular anatomy and pathology. Digital subtraction angiography has intrinsic high resolution, and individual vessels can be selectively evaluated. Moreover, hemodynamic information can be captured to evaluate physiology, whereas it can be estimated only indirectly with noninvasive techniques. Bolus chasing, rapid acquisition of images, three-dimensional reconstruction, and smaller catheters have further improved the utility of digital subtraction angiography, which decreases the dose of contrast material, improves visualization of the vascular tree, and speeds acquisition time compared with conventional angiography.15
Likewise, in PAD, invasive angiography is typically reserved for those with conflicting or inconclusive noninvasive results, although it retains an ACC/AHA class I indication, especially in the setting of planned revascularization. Smaller catheters, improved access site choice (such as radial access), and closure device use have improved the safety of this procedure. It is important that suspected vessels be selectively imaged; this improves the overall accuracy and reduces the burden of contrast material. A complete study should include assessment of the major bifurcations in profile without vessel overlap (iliac, femoral, and tibial), and indeterminate lesions should have translesional pressure gradients measured (Fig. 88-8).5 Although digital subtraction angiography has been traditionally used for preoperative evaluation, it has several important limitations, such as poor identification of distal vessels in the setting of critical limb ischemia and underestimation of eccentric lesions.
Invasive angiography is controversial in the setting of acute intestinal ischemia because it takes extra time that may be critical in determining the patient’s outcome. In many instances, proceeding directly to exploratory laparotomy is the appropriate course. However, angiography detects arterial occlusion with high sensitivity (>90%) and can assist in differentiating occlusive from nonocclusive disease and in presurgical planning.29 Moreover, therapy in the form of intra-arterial vasodilators or thrombolytic therapy can be given, or thrombectomy devices can be deployed. This approach is best for patients who present long after symptom onset, with an unclear diagnosis, or with a high likelihood of nonocclusive disease. Angiography should not be used in those with concurrent hypotension or in the setting of vasopressors because these can falsely mimic nonocclusive disease. Moreover, beneficial vasodilators cannot be given in this circumstance.29
In chronic mesenteric ischemia, catheter angiography can display arterial obstruction in a fashion similar to that in the other arterial beds. An important caveat is that the majority of chronic mesenteric disease occurs at the origin of the major mesenteric arteries, such as the superior and inferior mesenteric arteries. Angiography may miss these lesions if the catheter is selectively engaged into the partially obstructed vessel. Lateral aortography can help display the origin of disease. In complete superior mesenteric artery origin occlusion, the entire vessel may not be seen, the “naked aorta” sign. A prominent meandering artery can represent an enlarged marginal artery of Drummond rather than chronic atherosclerotic disease.29 As with invasive angiography in other arterial distributions, the limited projections of the mesenteric arteries obtained can lead to underestimation of eccentric vessels.
Differential Diagnosis
Clinical Presentation
The differential diagnosis for noncoronary atherosclerosis is broad and specific to the arterial bed affected (Table 88-4). Consideration of any possible alternative diagnoses is essential before further invasive testing or therapy is undertaken.
TABLE 88-4 Differential Diagnosis of Noncoronary Atherosclerotic Disease
| Affected Arterial Bed | Differential Diagnosis* |
|---|---|
| Cerebrovascular arterial disease |
* This list represents the most common alternative diagnoses. It is not exhaustive.
AAAs and mesenteric ischemia both cause acute abdominal pain (during AAA rupture and acute mesenteric arterial occlusion) and chronic pain (AAA leakage and chronic mesenteric ischemia) that can have multiple potential causes, as listed in Table 88-2. Noninvasive imaging is essential to help narrow the differential and should be considered in those with a high risk of noncoronary atherosclerotic disease, as in those with multiple cardiovascular risk factors, especially advanced age, male gender, and ongoing tobacco use.
Synopsis of Treatment Options
Medical
Peripheral Atherosclerotic Disease
PAD is considered a cardiovascular disease risk equivalent, and treatment targets the same medication classes as for other noncoronary atherosclerotic disease: lipid-lowering, antihypertensive, and antiplatelet medications. Patients with PAD should meet the current Joint National Commission hypertension guidelines, which suggest a goal of below 140/90 mm Hg for all patients and below 130/80 mm Hg for those with diabetes or advanced renal disease. Certain classes have been shown to have beneficial effects in addition to their blood pressure–lowering effect. β Blockers reduce MI and death for patients with CAD and prior MI and should be used because they do not reduce walking capacity as previously thought. Angiotensin-converting enzyme inhibitors are also recommended (class IIa); ramipril was studied in the HOPE trial in 4000 patients with PAD and reduced cardiovascular death, nonfatal MI, and stroke by 22%.5,30 Additional antihypertensives can be added as necessary to achieve goal blood pressure.
Lipid-lowering therapy is another essential component to retard the progression of atherosclerosis. Statins are the definitive first-line therapy and should be used in all patients with PAD unless an allergy or other contraindication exists. The Heart Protection Study included patients with atherosclerosis, many of whom had PAD, and showed a 25% reduction in cardiac events and overall mortality with titration of simvastatin to achieve the National Cholesterol Eduction Program goal LDL of less than 100 mg/dL.30 Use of statins to attain this goal has an ACC/AHA class I indication; a goal of less than 70 mg/dL is appropriate, especially in patients with multiple or poorly controlled risk factors (class IIa).5 Patients who require additional LDL lowering despite maximum-tolerated statin use can benefit from niacin therapy, although flushing and sweats can limit the tolerability of this medication. Niacin also assists in raising the HDL level, low values of which are a known independent risk factor for cardiac events. Finally, fibric acid derivatives are beneficial for those with high triglyceride levels, especially in the setting of low HDL level. One study of patients with low HDL levels and CAD showed a 22% reduction in cardiovascular death and nonfatal MI.
Lifestyle changes have an essential role in PAD therapy, the most important of which is tobacco cessation. Physician counseling is effective, leading to a 50-fold increase in 1-year cessation rates (increase from 0.1% to 5%). The addition of nicotine replacement therapy increases the 1-year success rate to 16% and of bupropion to 30%.5 Weight reduction, especially waist circumference, and regular exercise are also critical.
Cilostazol is a phosphodiesterase type 3 inhibitor that improves treadmill time and quality of life. A meta-analysis of six trials showed an improved pain-free walking distance of 30% to 60%. Cilostazol is contraindicated in heart failure. Pentoxifylline (a methylxanthine derivative), l-arginine, propionyl-l-carnitine, and gingko biloba are less effective and not used as frequently. Chelation therapy, vitamin E, and prostaglandins such as iloprost have class III indications.5 Critical limb ischemia is a surgical condition. There is no clear benefit to the parenteral administration of pentoxifylline or prostaglandins.
Aortic Atherosclerotic Disease
The greatest risk factors for rupture include tobacco use and uncontrolled hypertension. Ongoing tobacco use increases the aneurysm growth rate by 20% to 25% and significantly increases the risk of rupture. Cessation advice and assistance with psychotropic medication, nicotine replacement, and adjunctive pharmacotherapy are given a class I indication in this setting per the ACC/AHA 2005 guidelines.5
Renal Atherosclerotic Disease
Resistant, severe hypertension and progressive renal function decline are the two primary complications of renal atherosclerotic disease and stem from renal artery stenosis. Angiotensin-converting enzyme inhibitors, angiotension receptor blockers, calcium channel blockers, and β blockers have all been shown to have some effect on renal artery stenosis–associated hypertension. Both angiotensin-converting enzyme inhibitors and angiotension receptor blockers have been shown to slow the decline in renal function.5
Surgical/Interventional
Cerebrovascular Atherosclerotic Disease
Carotid endarterectomy (CEA) is the primary surgical technique for carotid stenoses. It is currently the primary option for revascularization but remains imperfect, with a 3% to 7.4% surgical complication rate. Individuals with symptoms and carotid stenoses of 70% to 99% were found to have a 2.9 relative risk reduction in ipsilateral stroke with CEA compared with medical therapy at 2 years in the North American Symptomatic Carotid Endarterectomy Trial (NASCET). A Department of Veterans Affairs Cooperative Studies trial likewise showed a 3.4 relative risk reduction.31
Because of the high risk of CEA, percutaneous revascularization options are undergoing intensive investigation, but their use remains in a state of evolution. Stenting has supplanted angioplasty alone because this technique is not as effective as CEA. The multiple trials examining percutaneous stenting versus CEA have conflicting noninferiority data, and there is no evidence of long-term efficacy with stenting. Small studies with short-term follow-up have shown benefit comparable to that with CEA, with a marginally higher restenosis rate (3% to 4% versus 1%, respectively). Whereas percutaneous revascularization techniques and outcomes data are improved, the American Heart Association/American Stroke Association 2006 guidelines recommend this approach only in patients at high operative risk for CEA, especially those with early post-CEA restenosis or radiation-induced stenosis.32 Aggressive antiplatelet therapy is especially important in those receiving carotid stents.
Peripheral Atherosclerotic Disease
Revascularization in patients without symptoms to prevent critical limb ischemia and intervention on lesions without significant pressure gradients are both contraindicated (class III in the ACC/AHA guidelines).5 Claudication does not often progress to critical limb ischemia. There are limited randomized trial data comparing revascularization to medical therapy.
The location of obstructive atherosclerotic disease and lesion characteristics dictate the form of revascularization. The type of revascularization determines the operative mortality and patency rates (Table 88-5). Focal aortic disease may be treated with surgery or PTA with or without stenting; surgery is recommended for aortic disease that extends into the iliac arteries. In the iliac system, surgery is the preferred treatment of long or irregular stenoses or occlusions. PTA has an ACC/AHA class I indication for shorter stenoses or focal occlusions. The patency is 60% to 80% at 4 to 5 years. Patency rates are slightly higher with surgery, but the increased cost and perioperative risk of surgery make PTA safer and less expensive even if repeated revascularization is required. PTA has 1- to 3-year patency rates of 60% for single venous bypass graft stenoses, but this drops to 6% with multigraft involvement.31 There are limited data suggesting that stenting may be better in the iliac system, and stenting is preferred for lesions of 2.0 cm or larger. The use of drug-eluting stents is not well studied. Stents are recommended in all vessel distributions for salvage therapy.
TABLE 88-5 Vascular Surgical and Percutaneous Transluminal Angioplasty Revascularization Procedure Success and Mortality Rates
| Surgical Procedure | Operative Mortality Rate (%) | Expected Patency Rate (%) |
|---|---|---|
| Aortobifemoral bypass | 3.3 | 87.5 (5 years) |
| Aortoiliac or aortofemoral bypass | 1-2 | 85-90 (5 years) |
| Iliac endarterectomy | 0 | 79-90 (5 years) |
| Femorofemoral bypass | 6 | 71 (5 years) |
| Axillofemoral bypass | 6 | 49-80 (3 years) |
| Axillofemoral-femoral bypass | 4.9 | 63-67.7 (5 years) |
| Percutaneous transluminal angioplasty or stenting | ||
| Iliac focal stenosis or occlusion | 1 | 60-80 (4-5 years) |
| With stent placed | — | 74 (4 years) |
| Venous bypass graft stenosis | — | 60 (1-3 years) |
| Femoral-popliteal stenosis | <1 | 70 (4-5 years) |
| With stent placed | — | 43 (3 years) |
Modified from Kandarpa K, Becker GJ, Hunink MG, et al. Transcatheter interventions for the treatment of peripheral atherosclerotic lesions: part I. J Vasc Interv Radiol 2001; 12:683.
In the femoral-popliteal area, surgery has good outcomes data. The use of PTA is controversial, but patency rates are as high as 70% at 4 to 5 years. Stents are likely to worsen prognosis, especially in patients with poor distal runoff, probably because of distal embolization, and are contraindicated under the current ACC/AHA guidelines.5
Aortic Atherosclerotic Disease
Aortic aneurysms can lead to thromboembolic ischemic events and impinge on neighboring structures, but the primary concern is the risk for rupture. Properly timed, elective intervention is critical for AAAs; repair in this setting has an improving mortality rate of approximately 5% compared with up to 50% during aortic leakage or rupture. Given this dramatic increase in mortality, all symptomatic patients should undergo repair immediately, regardless of aneurysm diameter. For asymptomatic patients, surgical decision-making is based on the risk/benefit ratio of procedural morbidity versus likelihood of rupture, which is directly related to the maximal diameter, rate of expansion, and gender of the patient.5
Two major trials show no benefit to surgery for aneurysms with a diameter of less than 5.5 cm. Thus, the current recommendation is to repair those with a diameter of 5.5 cm or more (class I indication).5 AAAs located above the renal arteries have a slightly higher rate of complications, such as acute renal failure and death, and may either be repaired or watched between 5.5 and 6.0 cm. Growth rates above 7 to 8 mm yearly or a diameter two times the size of the largest normal segment should prompt consideration of repair. Given the higher rate of rupture in women (up to four times greater), the American Association for Vascular Surgery recommends consideration of elective repair for aneurysms with diameters as small as 4.5 cm in women.33
Renal Atherosclerotic Disease
Revascularization of renal artery stenosis secondary to atherosclerotic disease can be attained with both percutaneous and surgical approaches. Revascularization is not currently recommended for asymptomatic renal artery stenosis with no evidence of end-organ dysfunction. The ACC/AHA 2005 guidelines give revascularization a class I indication for recurrent unexplained congestive heart failure or flash pulmonary edema.5 The goal of intervention in this instance is to reverse the persistent activation of the renin-angiotensin system and progressive renal functional deterioration that lead to volume expansion and intolerance to angiotensin-converting enzyme inhibitors and angiotension receptor blockers.
Mesenteric Atherosclerotic Disease
The therapy for mesenteric ischemia depends on the acuity of the presentation. Acute mesenteric ischemia frequently leads to sepsis, bowel infarction, and death, making early diagnosis and treatment imperative. Patients with suspected perforation or gangrene should go directly to surgery. Surgical arterial reconstruction involves bypass grafting, local or transaortic endarterectomy, and resection of nonviable bowel segments. These approaches have a 79% 5-year symptom-free survival but very high surgical mortality rate (approximately 70%).5,34 Repeated laparotomy at 24 to 48 hours can be beneficial to ensure that no infarcted bowel remains.
Given the high surgical mortality, percutaneous approaches can be considered (class IIb in the 2005 ACC/AHA guidelines).5 However, most patients still require laparotomy because of infarcted bowel, and percutaneous approaches are difficult because the most common site of involvement is at the origin of the major splanchnic vessels and often involves more than one artery. Moreover, in patients with a high degree of damage, restoration of flow can release dangerous endotoxins that can be controlled with surgery but not with percutaneous therapy. However, a percutaneous approach may reduce the magnitude of a dangerous surgery and should thus be considered.
Angioplasty has an 80% technical success rate, with an 80% rate of clinical remission at 2 to 3 years. Although there is a high rate of restenosis (27% to 46% during 1 to 3 years), recurrent obstruction can generally be resolved with additional percutaneous therapy. Although there has been little formal evaluation of stenting, use of stents appears to be at least as effective as primary angioplasty and may reduce the high recurrence rate.5
Surgical approaches include bypass grafting (most commonly used), endarterectomy, and reimplantation. The choice of procedure should be guided by the expertise of the individual surgeon as trials have not shown clear superiority of any technique. Revascularization is successful in 98% to 100% of patients, with lower 1- to 3-year recurrence rates of 19% to 24% compared with percutaneous therapy.5 Regardless of the choice of revascularization technique, close follow-up is essential.
Reporting: Information for the Referring Physician
KEY POINTS
 Noncoronary atherosclerotic disease carries a high morbidity and mortality, both from the arterial bed affected and from comorbid coronary artery disease.
Noncoronary atherosclerotic disease carries a high morbidity and mortality, both from the arterial bed affected and from comorbid coronary artery disease. A careful, detailed history and physical examination and a high degree of suspicion are the only ways to adequately assess these often silent or misclassified disease processes.
A careful, detailed history and physical examination and a high degree of suspicion are the only ways to adequately assess these often silent or misclassified disease processes. Noninvasive modalities have become sufficient for initial and often definitive evaluation of noncoronary atherosclerotic disease without the need for invasive testing.
Noninvasive modalities have become sufficient for initial and often definitive evaluation of noncoronary atherosclerotic disease without the need for invasive testing.Faxon DP, Creager MA, Smith SCJr, et al. Atherosclerotic Vascular Disease Conference: Executive summary: Atherosclerotic Vascular Disease Conference proceeding for healthcare professionals from a special writing group of the American Heart Association. Circulation. 2004;109:2595.
Kim AY, Ha HK. Evaluation of suspected mesenteric ischemia: efficacy of radiologic studies. Radiol Clin North Am. 2003;41:327.
Kramer CM, Anderson JD. MRI of atherosclerosis: diagnosis and monitoring therapy. Expert Rev Cardiovasc Ther. 2007;5:69.
Redberg RF, Vogel RA, Criqui MH, et al. 34th Bethesda Conference: Task force #3—What is the spectrum of current and emerging techniques for the noninvasive measurement of atherosclerosis? J Am Coll Cardiol. 2003;41:1886.
Ross R. Atherosclerosis—an inflammatory disease. N Engl J Med. 1999;340:115.
Stein JH, Korcarz CE, Hurst RT, et al. Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: a consensus statement from the American Society of Echocardiography Carotid Intima-Media Thickness Task Force. Endorsed by the Society for Vascular Medicine. J Am Soc Echocardiogr. 2008;21:93.
1 Faxon DP, Creager MA, Smith SCJr, et al. Atherosclerotic Vascular Disease Conference: Executive summary: Atherosclerotic Vascular Disease Conference proceeding for healthcare professionals from a special writing group of the American Heart Association. Circulation. 2004;109:2595.
2 Rosamond W, Flegal K, Friday G, et al. Heart disease and stroke statistics—2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007;115:e69.
3 Atherosclerosis. The American Heritage Dictionary of the English Language, 4th ed. Boston: Houghton Mifflin; 2004.
4 Pasternak RC, Criqui MH, Benjamin EJ, et al. Atherosclerotic Vascular Disease Conference: Writing Group I: epidemiology. Circulation. 2004;109:2605.
5 Hirsch AT, Haskal ZJ, Hertzer NR, et al. ACC/AHA 2005 guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): executive summary a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease) endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. J Am Coll Cardiol. 2006;47:1239.
6 Kalra PA, Guo H, Kausz AT, et al. Atherosclerotic renovascular disease in United States patients aged 67 years or older: risk factors, revascularization, and prognosis. Kidney Int. 2005;68:293.
7 Ross R. Atherosclerosis—an inflammatory disease. N Engl J Med. 1999;340:115.
8 Mallika V, Goswami B, Rajappa M. Atherosclerosis pathophysiology and the role of novel risk factors: a clinicobiochemical perspective. Angiology. 2007;58:513.
9 Smith SCJr, Milani RV, Arnett DK, et al. Atherosclerotic Vascular Disease Conference: Writing Group II: risk factors. Circulation. 2004;109:2613.
10 Price JF, Mowbray PI, Lee AJ, et al. Relationship between smoking and cardiovascular risk factors in the development of peripheral arterial disease and coronary artery disease: Edinburgh Artery Study. Eur Heart J. 1999;20:344.
11 Goldstein LB, Adams R, Alberts MJ, et al. Primary prevention of ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council: cosponsored by the Atherosclerotic Peripheral Vascular Disease Interdisciplinary Working Group; Cardiovascular Nursing Council; Clinical Cardiology Council; Nutrition, Physical Activity, and Metabolism Council; and the Quality of Care and Outcomes Research Interdisciplinary Working Group. Circulation. 2006;113:e873.
12 Peripheral arterial disease in people with diabetes. Diabetes Care. 2003;26:3333.
13 Stein JH, Korcarz CE, Hurst RT, et al. Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: a consensus statement from the American Society of Echocardiography Carotid Intima-Media Thickness Task Force. Endorsed by the Society for Vascular Medicine. J Am Soc Echocardiogr. 2008;21:93.
14 Auerbach EG, Martin ET. Magnetic resonance imaging of the peripheral vasculature. Am Heart J. 2004;148:755.
15 Olin JW, Kaufman JA, Bluemke DA, et al. Atherosclerotic Vascular Disease Conference: Writing Group IV: imaging. Circulation. 2004;109:2626.
16 Vogt MT, Cauley JA, Newman AB, et al. Decreased ankle/arm blood pressure index and mortality in elderly women. JAMA. 1993;270:465.
17 Errington ML, Ferguson JM, Gillespie IN, et al. Complete pre-operative imaging assessment of abdominal aortic aneurysm with spiral CT angiography. Clin Radiol. 1997;52:369.
18 Petersen MJ, Cambria RP, Kaufman JA, et al. Magnetic resonance angiography in the preoperative evaluation of abdominal aortic aneurysms. J Vasc Surg. 1995;21:891.
19 Tan KT, van Beek EJ, Brown PW, et al. Magnetic resonance angiography for the diagnosis of renal artery stenosis: a meta-analysis. Clin Radiol. 2002;57:617.
20 Zwolak RM, Fillinger MF, Walsh DB, et al. Mesenteric and celiac duplex scanning: a validation study. J Vasc Surg. 1998;27:1078.
21 Zandrino F, Musante F, Gallesio I, et al. Assessment of patients with acute mesenteric ischemia: multislice computed tomography signs and clinical performance in a group of patients with surgical correlation. Minerva Gastroenterol Dietol. 2006;52:317.
22 Meaney JF, Prince MR, Nostrant TT, et al. Gadolinium-enhanced MR angiography of visceral arteries in patients with suspected chronic mesenteric ischemia. J Magn Reson Imaging. 1997;7:171.
23 Redberg RF, Vogel RA, Criqui MH, et al. 34th Bethesda Conference: Task force #3—What is the spectrum of current and emerging techniques for the noninvasive measurement of atherosclerosis? J Am Coll Cardiol. 2003;41:1886.
24 Lorenz MW, Markus HS, Bots ML, et al. Prediction of clinical cardiovascular events with carotid intima-media thickness: a systematic review and meta-analysis. Circulation. 2007;115:459.
25 Neunteufl T, Heher S, Katzenschlager R, et al. Late prognostic value of flow-mediated dilation in the brachial artery of patients with chest pain. Am J Cardiol. 2000;86:207.
26 Kramer CM, Anderson JD. MRI of atherosclerosis: diagnosis and monitoring therapy. Expert Rev Cardiovasc Ther. 2007;5:69.
27 Kim AY, Ha HK. Evaluation of suspected mesenteric ischemia: efficacy of radiologic studies. Radiol Clin North Am. 2003;41:327.
28 Wolfram RM, Budinsky AC, Sinzinger H. Assessment of peripheral arterial vascular disease with radionuclide techniques. Semin Nucl Med. 2001;31:129.
29 Martinez JP, Hogan GJ. Mesenteric ischemia. Emerg Med Clin North Am. 2004;22:909.
30 Creager MA, Jones DW, Easton JD, et al. Atherosclerotic Vascular Disease Conference: Writing Group V: medical decision making and therapy. Circulation. 2004;109:2634.
31 Bettmann MA, Dake MD, Hopkins LN, et al. Atherosclerotic Vascular Disease Conference: Writing Group VI: revascularization. Circulation. 2004;109:2643.
32 Sacco RL, Adams R, Albers G, et al. Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack: a statement for healthcare professionals from the American Heart Association/American Stroke Association Council on Stroke: co-sponsored by the Council on Cardiovascular Radiology and Intervention: the American Academy of Neurology affirms the value of this guideline. Stroke. 2006;37:577.
33 Brewster DC, Cronenwett JL, Hallett JWJr, et al. Guidelines for the treatment of abdominal aortic aneurysms. Report of a subcommittee of the Joint Council of the American Association for Vascular Surgery and Society for Vascular Surgery. J Vasc Surg. 2003;37:1106.
34 Cho JS, Carr JA, Jacobsen G, et al. Long-term outcome after mesenteric artery reconstruction: a 37-year experience. J Vasc Surg. 2002;35:453.

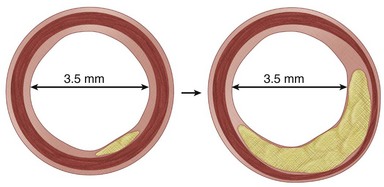
 FIGURE 88-1
FIGURE 88-1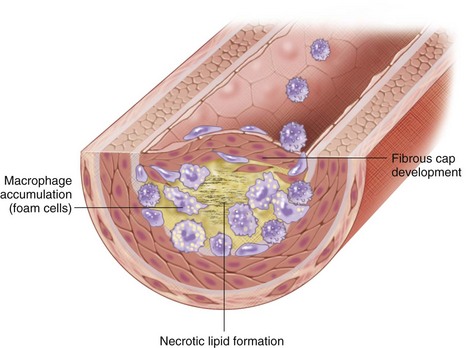
 FIGURE 88-2
FIGURE 88-2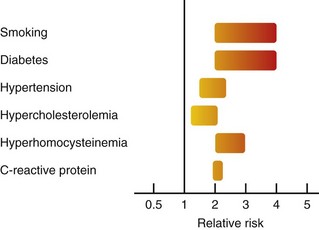
 FIGURE 88-3
FIGURE 88-3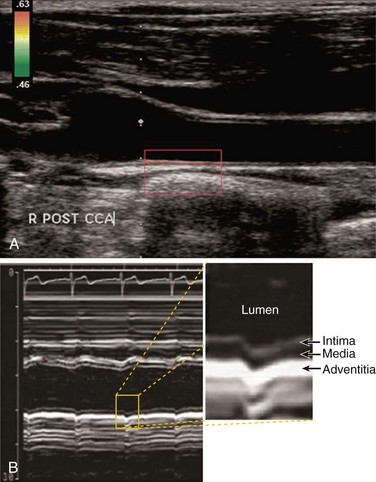
 FIGURE 88-5
FIGURE 88-5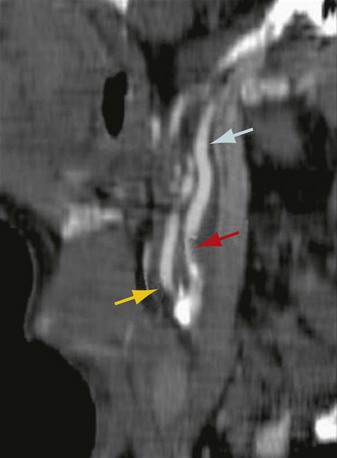
 FIGURE 88-6
FIGURE 88-6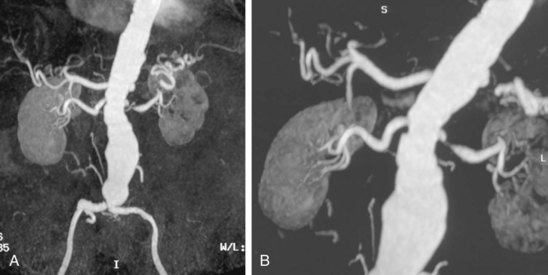
 FIGURE 88-7
FIGURE 88-7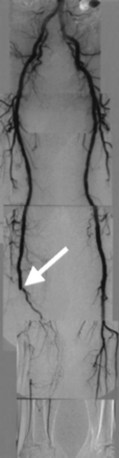
 FIGURE 88-8
FIGURE 88-8

