CHAPTER 5 Noninvasive Examination of the Patient Before Sclerotherapy
Confirmatory diagnostic testing is performed after the history is taken and the physical examination performed. The handheld continuous-wave Doppler historically was a routine part of the physical examination but is slowly being replaced by compact, portable duplex ultrasound visualization. At present, the vascular laboratory (level 2) provides a reliable tool for acquiring anatomic and functional information that not only confirms the diagnosis but also formulates treatment. Elements of the diagnostic vascular laboratory are slowly becoming part of a routine physical examination.1 A duplex examination of the venous system is becoming a more important diagnostic tool. This approach has been confirmed in the guidelines of the American Venous Forum.2
Medical History
CEAP classification
An international ad hoc committee of the American Venous Forum developed the CEAP classification for chronic venous disease in 1994 (Table 5.1; see also Chapter 2) with the goal of stratifying clinical levels of venous insufficiency. The four categories and descriptors selected for classification were clinical state (C), etiology (E), anatomy (A), and pathophysiology (P). The CEAP classification has been endorsed worldwide, despite its acknowledged deficiencies. It has been adopted as a standard in many clinics in Europe, Asia, South America, and the United States it is considered the only modern method for reporting data. It’s weakness is the inability to distinguish between levels of smaller superficial veins. The CEAP classification was revised in 2004,3 and is now referred to as ‘advanced’ CEAP.4 It includes:
| CLINICAL CLASSIFICATION | |
| C0 | No visible or palpable signs of venous disease |
| C1 | Telangiectasias or reticular veins |
| C2 | Varicose veins – separated from reticular veins by a diameter of 3 mm as the upper limit of size of a reticular vein |
| C3 | Edema |
| C4 | Changes in the skin and subcutaneous tissue secondary to chronic venous disease are divided into two subclasses to better define the differing severity of venous disease: |
| C4a | Pigmentation and eczema |
| C4b | Lipodermatosclerosis and atrophie blanche |
| C5 | Healed venous ulcer |
| C6 | Active venous ulcer |
| Each clinical class is further characterized by a subscript for the presence of symptoms (S, symptomatic) or their absence (A, asymptomatic). Symptoms include aching, pain, tightness, skin irritation, heaviness, and muscle cramps, as well as other complaints attributable to venous dysfunction. | |
| ETIOLOGIC CLASSIFICATION | |
| Ec | Congenital |
| Ep | Primary |
| Es | Secondary (postthrombotic) |
| En | No venous etiology identified |
| ANATOMIC CLASSIFICATION | |
| As | Superficial veins |
| Ap | Perforator veins |
| Ad | Deep veins |
| An | No venous location identified |
| PATHOPHYSIOLOGIC CLASSIFICATION | |
| Basic CEAP | |
| Pr | Reflux |
| Po | Obstruction |
| Pro | Reflux and obstruction |
| Pn | No venous pathophysiology identifiable |
| Advanced CEAP | |
| Same as Basic with the addition that any of 18 named venous segments (below) can be utilized as locators for venous pathology. | |
| All items listed in C should be repeated. | |
| VENOUS SEGMENTS | |
| Superficial veins | |
| 1 | Telangiectasias/reticular veins |
| 2 | GSV above knee |
| 3 | GSV below knee |
| 4 | SSV |
| 5 | Nonsaphenous veins |
| Deep veins | |
| 6 | IVC |
| 7 | Common iliac vein |
| 8 | Internal iliac vein |
| 9 | External iliac vein |
| 10 | Pelvic: gonadal, broad ligament veins, other |
| 11 | Common femoral vein |
| 12 | Deep femoral vein |
| 13 | Femoral vein |
| 14 | Popliteal vein |
| 15 | Crural: anterior tibial, posterior tibial, fibular veins (all paired) |
| 16 | Muscular: gastrocnemial, soleal veins, other |
| Perforating veins | |
| 17 | Thigh |
| 18 | Calf |
The descriptor (a) is added for asymptomatic patients and (s) in case of symptoms.
Diagnostic approach
The first step in evaluating a patient with venous disease is to establish his or her clinical class, which rapidly progresses from cosmetic to chronic venous insufficiency. The next is to correlate the symptoms, which place the limb being examined into one of the classes shown in Table 5.1. The patient’s clinical class will dictate the need for further evaluation. In patients with telangiectasias (class 1 or 2), the evaluation can be limited to a physical examination and evaluation of the superficial venous system with a handheld continuous-wave Doppler. Imaging of 83 limbs with clinical evidence of only telangiectatic vessels demonstrated that nearly 25% had insufficiency of the great saphenous vein (GSV) or small saphenous veins (SSV), which was not apparent on physical examination.5 Patients with symptomatic class 2 varicosities and class 4, 5, and 6 skin changes require a duplex venous reflux examination because surgical intervention is indicated.6–10 Recalcitrant cases may require more extensive imaging studies to detect venous occlusive disease. Physiologic testing can be relegated to documentation rather than to diagnosis, and phlebography should be performed only when venous reconstruction is contemplated.
Prior treatment
The physician should discuss prior treatment for venous disease. However, he or she must realize that although proper ligation, with or without stripping of the main saphenous trunks, implies that reflux through the saphenofemoral junction (SFJ) and saphenopopliteal junction (SPJ) has been prevented, this is not always the case. Some have proposed that in up to 27% of patients there is a duplication of the GSV;11–13 thus, the removal of the GSV may be followed by the development of varicosity in the remaining GSV, although this is not universally accepted.
In 20% to 40% the SSV has a variable termination14–17 that is not in the popliteal vein at or above the popliteal fossa. Therefore, the actual SPJ must be correctly diagnosed otherwise it will lead to an apparent rapid recurrence with varicose changes occurring in the remaining segment of the SSV and its tributaries. Finally, in a number of patients, a recurrence of varicose veins in the upper thigh may be the result of incomplete ligation and division of the other tributaries arising at the level of the SFJ or failure to accomplish the ligation flush with the femoral vein. In fact, in a review of 341 extremities that underwent repeat operations for varicose veins, Lofgren et al17 found that 61% had inadequate ligation. These facts make it imperative that, even in the patient with a history of ligation, division, and stripping, an examination for reflux through the SFJ and SPJ be performed.
Symptoms
It is not well known that presence and severity of symptoms has no correlation with the size or severity of varicose veins present. Symptoms usually attributable to varicose veins include feelings of heaviness, tiredness, aching, burning, throbbing, itching, and cramping in the legs (Box 5.1). These symptoms are generally worse with prolonged sitting or standing and are improved with leg elevation or walking. A premenstrual exacerbation of symptoms is also common. Patients typically find relief with the use of compression in the form of either support hose or an elastic bandage if they are compliant. Compliance can be a challenge. Weight loss or the commencement of a regular program of lower extremity exercise may also lead to a diminution in the severity of varicose vein symptoms. Clearly, these symptoms are not specific, as they may also be indicative of a variety of rheumatologic or orthopedic problems. However, their relationship to lower extremity movement and compression is usually helpful in establishing a venous origin for the symptoms. Significant symptoms suggestive of chronic venous disease should prompt further evaluation for valvular insufficiency and calf muscle pump dysfunction. If a venous etiology is suspected but all examinations are negative, repeat examination during a symptomatic period is warranted and often fruitful.
The recent development of an extremely painful area on the lower leg associated with an overlying area of erythema and warmth may be indicative of lipodermatosclerosis, which may be associated with insufficiency of underlying perforator veins or reflux from a proximal point. Examination for underlying perforator vein reflux should be performed. Lipodermatosclerosis may precede ulceration and has been shown to be improved by stiff compression and certain pharmacologic interventions.18
Rarely, patients with a history of iliofemoral thrombophlebitis who describe ‘bursting’ pain with walking may be suffering from ‘venous claudication’. In these patients, an evaluation for persistent hemodynamically significant obstruction, possibly treatable with venous bypass surgery, is appropriate.19
Complications of varicose vein disease
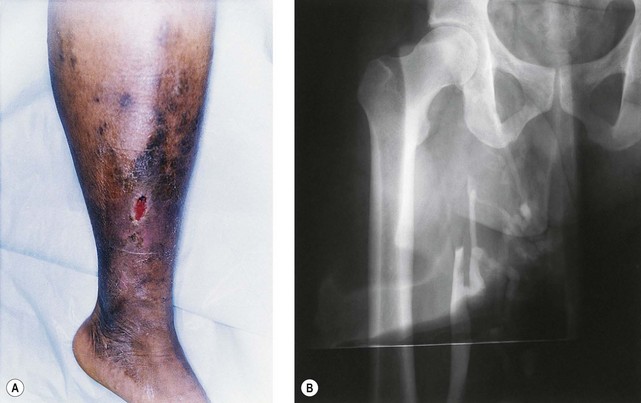
Complications such as ulceration and hemorrhage should be discussed with the patient because this provides additional insight into both the severity and the probable locations of abnormality within the venous system. A history of ulceration of the medial aspect of the lower leg should prompt further examination of the GSV trunk,6 whereas involvement of the lateral aspect of the lower leg suggests an abnormality in the SSV, in addition to the deep and perforating vein systems. A history of hemorrhage from telangiectasias in a particular area suggests further examination for underlying incompetent perforators and is an indication to treat all suspicious telangiectasias.20
Physical Examination
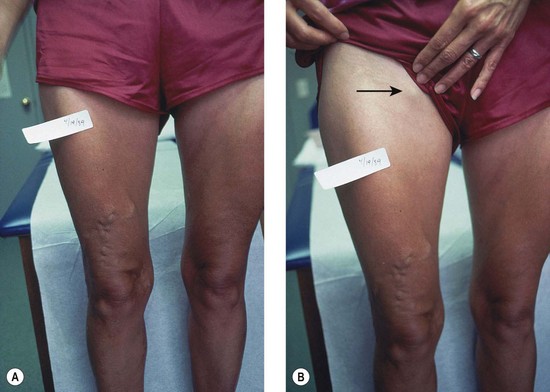
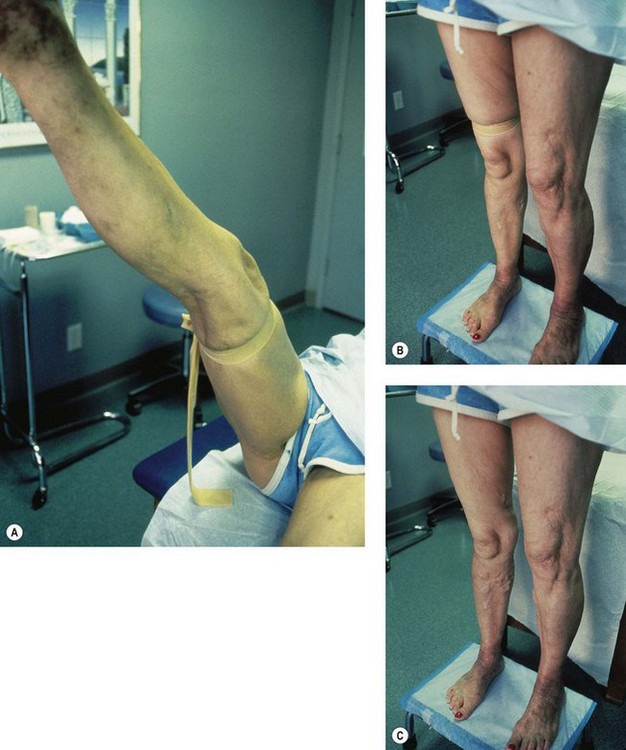
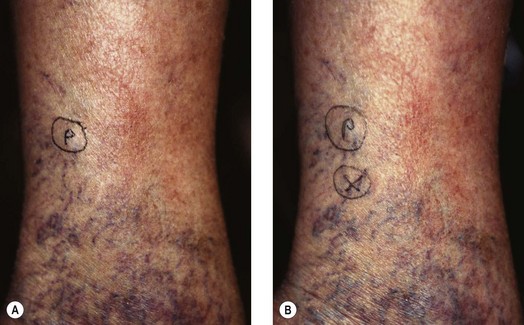
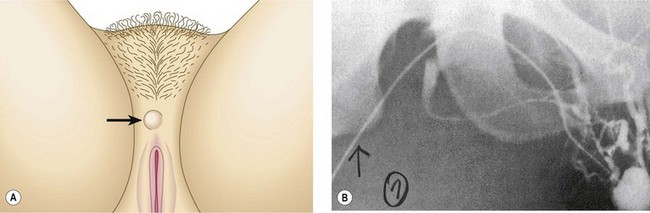
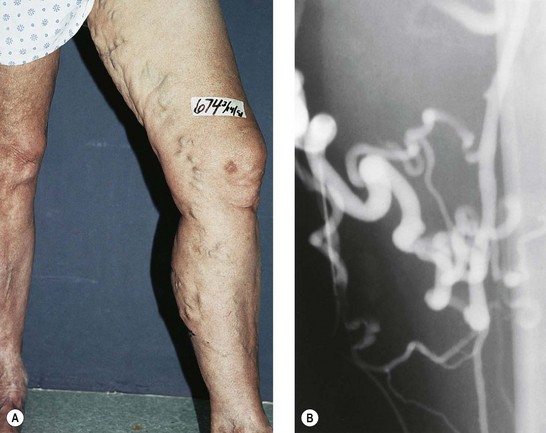
The screening physical examination consists of careful observation of the legs. Any patient with the following conditions should be examined more fully: large varicose veins; bulges in the thigh, calf, or the inguinal region representative of incompetent perforating veins (IPVs) or a saphena varix;21 signs of superficial venous hypertension, such as an accumulation of telangiectasias in the ankle region (corona phlebectatica); or any finding suggestive of venous dermatitis (pigmentation, induration, eczema). This includes patients with obvious cutaneous signs of venous disease, such as venous ulceration, atrophie blanche, or lipodermatosclerosis. An obvious but often forgotten point is the necessity of observing the entire leg and not confining the examination simply to the area that the patient feels is abnormal. The importance of this is demonstrated in Figure 5.1. This patient came for treatment of an obviously dilated anterior thigh vein, but further inspection revealed a saphena varix, with incompetence at the level of the SFJ; thus defining the first step in her treatment. Similarly, patients often seek treatment of specific clusters of telangiectasia and do not notice the underlying reticular veins that should be treated before or at the same time (see Chapter 12).
Finally, because the veins of the leg empty into the pelvic and abdominal veins, inspection of the abdomen is very important, since dilation of veins on the abdominal wall or across the pubic region suggests an old iliofemoral thrombus22 or, rarely, a developmental anomaly of the venous system.23 Dilated veins along the medial or posterior aspect of the proximal thigh or buttocks most often arise from varicosities involving the pudendal or other pelvic vessels. These can be associated with vulvar varices that may remain symptomatic after the completion of the pregnancy during which they formed. The enlarged veins in the thigh or buttocks may also be quite symptomatic and respond well to treatment.
Clinical testing
Trendelenburg test
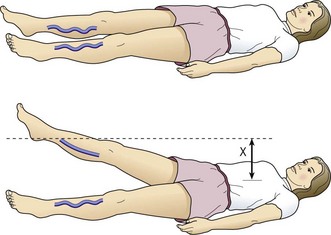
When compared with the contralateral leg, the method just described may demonstrate a degree of venous obstructive disease. Another approach is to elevate the leg while the patient is supine and to observe the height of the heel in relation to the level of the heart that is required for the prominent veins to collapse (Fig. 5.2). Unfortunately, neither procedure is sufficiently sensitive or accurate, or able to differentiate acute from chronic obstruction, which means neither of them is much assistance in current medical practice. This emphasizes the important role of duplex ultrasound in modern evaluation of the superficial venous system. One study found that pneumatic tourniquets only occluded 27% of saphenous trunks.24 Several other physical examination maneuvers, described below, can provide information on the competence of the venous valves.
Cough test
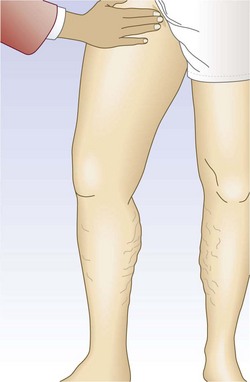
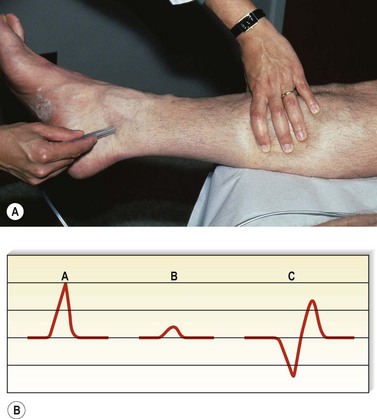
One hand is placed gently over the GSV or SFJ and the patient is asked to cough or perform a Valsalva maneuver (Fig. 5.3). Simply palpating an impulse over the vein being examined may be indicative of insufficiency of the valve at the SFJ and below to the level of the palpating hand. This test, however, is not applicable to the examination of the SSV and SPJ (see following section).14 Palpation of a thrill during this maneuver is generally more diagnostic. One study found a low sensitivity of 0.59 and low specificity of 0.67 with this test.25
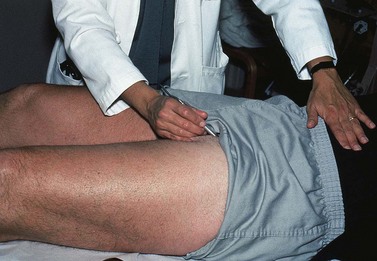
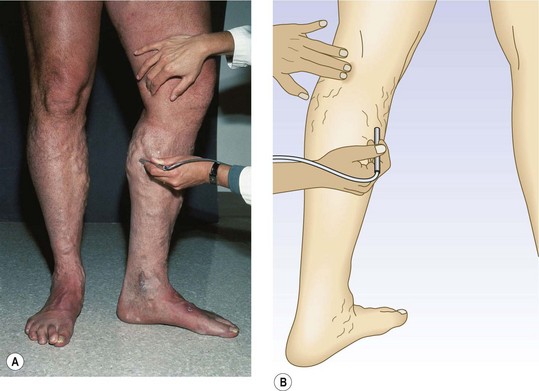
Percussion/Schwartz test
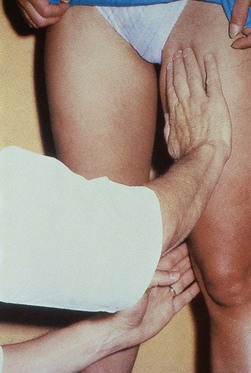
One hand is placed over the SFJ or SPJ while the other hand is used to tap very lightly on a distal segment of the GSV or SSV (Fig. 5.4). The production of an impulse in this manner implies insufficiency of the valves in the segment between the two hands. Confirmation of valvular insufficiency can be achieved by tapping proximally while palpating distally. This test can also be used to detect whether an enlarged tributary is in direct connection with the GSV or SSV by palpating over the main trunk and tapping lightly on the dilated tributary, or vice versa. The presence of a direct connection results in a palpable impulse being transmitted from the percussing to the palpating hand. As might be expected, these tests are far from infallible. In a study of 105 limbs, Chan et al26 found that these clinical examination techniques correctly identified SFJ incompetence in only 82% of limbs. False negatives were believed to be caused primarily by previous groin surgery with resultant scarring and by obesity. However, false positives were the result of variations in venous anatomy, such as a dilated tributary emptying into the common femoral vein (CFV) adjacent to the GSV or the absence of valves in an otherwise normal CFV and external iliac vein (seen in 5% to 30% of patients).27,28 Another study showed a low sensitivity of 0.59 with a high specificity of 0.92.25 A further source of error with the cough and/or percussion test is simply a misinterpretation of the muscle contraction that occurs with coughing as a reflux impulse.
Brodie-Trendelenburg test
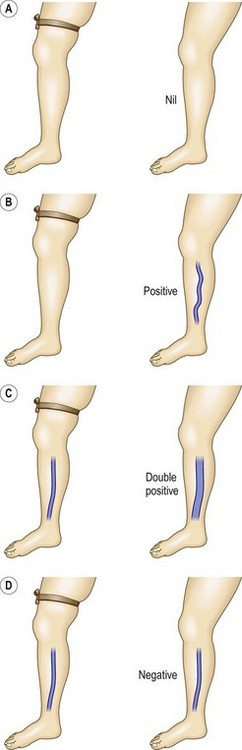
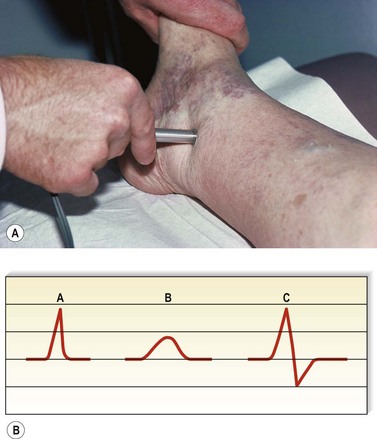
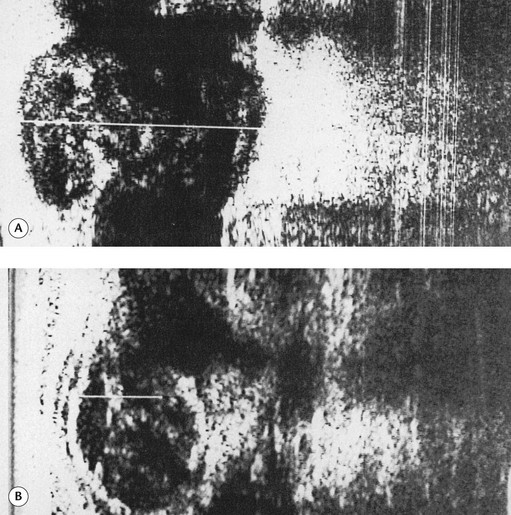
The Brodie-Trendelenburg test traditionally involves the manual obstruction of the proximal end of the GSV (or SSV) while the patient lies supine with the leg elevated, after stroking the vein in a cephalad direction to empty it of blood.22,29–32 The patient then assumes the standing position, and the leg is observed for 30 seconds (Fig. 5.5). In a ‘nil’ test there is slow filling of the veins from below, and the release of the compression does not result in rapid filling from above, indicating competence of valves in deep and perforating veins and at the SFJ (Fig. 5.6A). Rapid filling of the GSV or more distal tributaries that occurs only after release of the compression constitutes a ‘positive’ test, indicating the presence of an insufficient valve at the SFJ (Fig. 5.6B). In the ‘double-positive’ test, some distension of the veins occurs within the initial 30 seconds while the compression is maintained, as well as additional filling once the compression is released (Fig. 5.6C). This is taken as evidence of incompetent deep and perforating veins as well as reflux through the SFJ. A ‘negative’ test occurs when the veins fill within the initial 30 seconds with no increased filling after the compression is released, implying only deep and perforating valvular insufficiency (Fig. 5.6D). The reverse may not be true; that is, filling in longer than 30 seconds does not imply competence of perforating veins. In a study of 901 extremities, Sherman33 found that 95% had a nil Trendelenburg test, but surgical exploration later showed incompetent perforators in 90% of these patients. Another study showed a high sensitivity of 0.91 with a low specificity of 0.15.25
The Brodie-Trendelenburg test thus can be an important method of localizing the most proximal site of reflux in most dilated superficial veins by obstructing the GSV, SSV, or whichever vein is suspected of refluxing into a more distal vein. The physician can also place the examining finger over palpable fascial defects in the leg while the patient is supine and then release the obstructions one by one after the patient is standing. This allows the sites of insufficient perforators, or ‘points of control’ (considered so crucial in Fegan’s technique of sclerotherapy), to be defined, because the superficial veins distal to the insufficient perforator fill rapidly once the obstructing fingers are removed (see Chapter 9).34,35
With this technique, described well in many papers,21,34–37 the practitioner first marks on the leg the sites of all dilated varicosities. The patient then assumes the supine position with the leg elevated to approximately 60 degrees to empty the veins. After at least 20 seconds, or when the distended veins are flattened, the leg is gently and rapidly palpated to detect any defects in the fascia. With experience, these can be detected easily as places that allow the entrance of the examining finger without the use of any pressure. Fascial defects can be caused by many abnormalities other than perforating veins, thus the practitioner continues the examination by compressing the individual fascial defects with his or her fingers and then having the patient stand. The fingers are then released one by one, starting with the most distal defect, and rapid filling of more distal varicosities is noted (Fig. 5.7). Those defects that cause distal filling when released are assumed to correspond to sites of IPVs. In the presence of a dilated GSV or SSV, these points of reflux first must be controlled with either digital compression or a tourniquet to evaluate the lower volume reflux through the perforators. For the evaluation to be helpful, compression of the defects must first cause sustained flattening of the varicosities when the patient initially stands. If the veins fill before any of the fingers are released, the test must be restarted and other sites compressed until the sites responsible for the reflux are located. This examination is associated with a 50% to 70% accuracy36–39 compared with findings at surgical exploration. Repeated examination at different times and improvement of edema allows the detection of increased numbers of perforators.
Bracey Variation
A clever variation of the Brodie-Trendelenburg technique was proposed by Bracey40 in 1958 (Fig. 5.8). He used a flat, 3.8-cm-wide, rubber tourniquet and two rubber rings covered with latex, with inside diameters of 7 cm and 8.2 cm. The smaller ring is used between the ankle and knee and may also be used for the thigh if the patient is thin. If not, the larger ring is used for the thigh. With the patient standing, the small ring is rolled over the foot to just above the ankle, and the rubber tourniquet is then placed below the ring to obstruct any upward flow of blood through the superficial veins. The small ring is then slowly rolled upward, emptying the superficial veins as it moves. As soon as it passes an IPV, the blood enters the superficial vein that connects with it, causing a dilation of the vein. The exit site of the perforating vein may then be marked. This reflux of blood can be accentuated by asking the patient to repetitively dorsiflex the foot. When the ring reaches the knee, the tourniquet is moved up to the knee, just below the ring. Either the smaller or larger ring is then used similarly to examine the thigh.
Perthes’ test
The Perthes’ test22,32,41 has several uses, including distinguishing between venous valvular insufficiency in the deep, perforator, and superficial systems and screening for DVT (Table 5.2). To localize the site of valvular disease, the physician places a tourniquet around the proximal thigh with the patient standing. When the patient ambulates, a decrease in the distension of varicose veins suggests a primary process without underlying deep venous disease because the calf muscle pump effectively removes blood from the leg and empties the varicose veins. Secondary varicose veins do not change caliber (if there is patency of the deep venous system) because of the inability to empty blood out of the veins as a result of impairment of the calf muscle pump. In the setting of a concurrent DVT, they may increase in size. If there is significant chronic or acute obstructive disease in the iliofemoral segment, the patient may note pain (venous claudication)42–44 as a result of the obstruction to outflow through both the deep and superficial systems. Information regarding the presence of deep venous valvular insufficiency and thrombosis is important to note in patient selection. This avoids causing catastrophic complications, such as pulmonary embolism resulting from an undiagnosed and worsened DVT or venous claudication caused by further impairment of venous return. Indeed, these two complications are serious enough to warrant the use of a much more sensitive and accurate method; therefore the Perthes’ test is now of more historical than actual clinical importance.
| Finding | Interpretation |
|---|---|
| Decreased diameter of varicose veins | Primary varicose veins |
| No change in diameter of varicose veins | Secondary varicose veins |
| Deep venous patency | Impairment of calf muscle pump |
| Increased diameter of varicose veins | Deep venous obstruction |
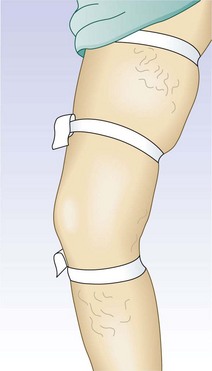
To test for perforator valvular defects, the physician may embellish the traditional Perthes’ test by placing a tourniquet around the calf just below the popliteal fossa.34 If the dilated superficial veins in the calf and ankle become less prominent as the patient ambulates, this implies that the blood is being drawn into the deep system through competent perforating veins. However, if the veins become increasingly dilated, the perforating veins must be incompetent. A more involved test, the Mahorner-Ochsner comparative tourniquet test, similarly localizes the site(s) of reflux by observing the leg while the patient walks with the tourniquet placed at various levels on the leg (upper, middle, and lower thigh) (Fig. 5.9).32
Noninvasive Diagnostic Techniques
The preceding three decades have been very fruitful and have provided a wealth of noninvasive technology that has revolutionized vascular diagnosis. A thorough description of all these techniques is certainly beyond the scope of this book, but those not presented here may be found in several excellent texts.5,13,45 Some of the new technologies have real use in the everyday performance of sclerotherapy, and the following discussion attempts to acquaint the reader with their uses and limitations.
Doppler ultrasound
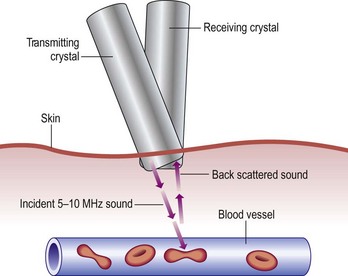
Although rapidly being replaced by duplex ultrasound, the most practical instrument for evaluating patients with venous disease is Doppler ultrasound. Its first vascular application came in 1960 when Satomura and Kaneko46 described a method of studying changes in blood flow in peripheral arteries using an ultrasonic blood rheograph. Its use in the field of venous disease was promoted by many groups, including Sumner et al,47 Strandness et al,48 Felix and Sigel,49, Sigel et al50–54 and Pourcelot et al.55 The instrument is based on the principle of the Doppler effect and consists of an emitting crystal and a receiving crystal. Sound waves are directed into the limb and reflected off the blood cells traveling through the vessel being examined (Fig. 5.10). The input picked up by the receiving crystal may be connected to a variety of audio or graphic recording systems. Dopplers come with either continuous or pulsed-wave ultrasound beams; the continuous-wave Doppler is adequate for venous examination, even though the signal represents a composite of the flow in all vessels in the path of the ultrasound beam. Thus, selective examination of one particular vessel may not always be possible. Pulsed Dopplers are used in sonar systems and in medical ultrasound imaging and are required when the intent is to focus the beam at a particular depth. Dopplers are also available in either directional or nondirectional forms. The directional type is capable of determining the direction of blood flow and depicts the direction on the tracing as either a positive (toward the probe) or negative (away from the probe) deflection (Fig. 5.11). Although the directionality greatly simplifies the interpretation of the tracing, experience with a nondirectional Doppler allows the examiner to make this determination easily, based on certain augmentation maneuvers.
The transmission frequency of the ultrasound beam may range from 2 to 10 MHz; the depth of penetration varies inversely with the frequency. Therefore, a frequency of 4 MHz produces a broad beam with deep penetration, which is especially useful for examining the deep veins in the pelvis and abdomen. A frequency of 8 MHz is much better suited for the examination of more superficial veins, including superficial segments of the deep veins of the legs, since it produces a narrower beam with relatively less penetration. Dopplers used for evaluation of the venous system generally permit detection of flow rates as low as 6 cm/second.51
Characteristics of Doppler waveform
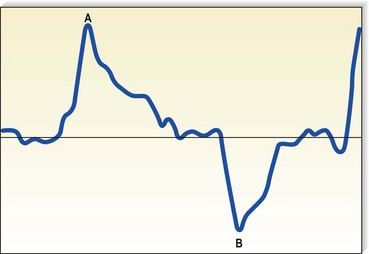
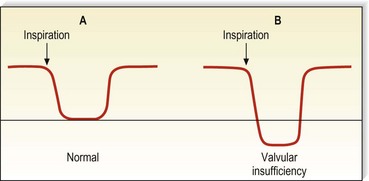
Venous Doppler signals display five characteristics (Box 5.2). In a normal patient, there should be a spontaneous signal over any vessel not otherwise vasoconstricted, and the flow should be only unidirectional. This signal diminishes in intensity with inspiration as descent of the diaphragm causes a rise in intra-abdominal pressure, thus decreasing venous outflow from the leg. It will be augmented similarly with exhalation. This waxing and waning of the intensity of the signal with the respiratory cycle is a phenomenon known as phasicity. Venous signals are continuous except for their respiratory variation and are not pulsatile, except in the setting of elevated right heart pressure such as congestive heart failure or tricuspid insufficiency56 or in the normal CFV.57 Finally, and most important to their usefulness in the evaluation of patients with varicose veins, venous signals may be augmented with certain compression maneuvers. It is the response to these maneuvers that provides information regarding the sites of valvular insufficiency and obstruction of the venous system.
By compressing the limb distal to the Doppler probe (Fig. 5.12), the examiner increases the flow through the vein; an immediate increase in the signal intensity should be heard if there is no proximal obstruction. In the presence of a hemodynamically significant DVT, the augmented response is weaker and delayed compared with the contralateral side. With the patient in the upright position, release of distal compression should be followed by silence as the valves close in response to the downward pressure of the blood being pulled by gravity. With the patient in the supine position, release of the compression should normally be followed by the return of the lower intensity spontaneous signal or by silence in the smaller veins. In the setting of valvular insufficiency at the level of the Doppler probe, a loud reflux flow signal can be heard on release of distal compression as blood is pulled in a caudal direction by gravity. To quantitate this reflux flow, the compression used may be standardized by using a pneumatic cuff inflated to a standard pressure (e.g. 80–120 mmHg), and the amplitude and duration of reflux may be read from the tracing obtained. To be considered true reflux and not merely delayed valve closure, the duration of reflux must be at least 0.5 seconds.8,58 Although many now believe that over 1 second is the appropriate duration above which to consider it abnormal.
The other method of augmentation is proximal compression and release (Fig. 5.13). Proximal compression produces a transient obstruction to outflow and thus causes an accumulation of blood distally, with an associated interruption of the Doppler signal. On its release, the large bolus of blood flowing past the Doppler probe creates a loud signal. This has also been found to be the more sensitive maneuver in diagnosing DVT, even that limited to calf veins, with a diminished or delayed signal indicative of a significant thrombosis.59,60 Valvular insufficiency is discovered easily, because proximal compression yields a loud reflux flow instead of silence.
In early descriptions of the use of Doppler ultrasound for detection of venous disease, Sigel et al51 named the various sounds ‘S’ for spontaneous and ‘A’ for augmented. They further specified ‘A’ sounds as distal (if the compression was distal to the probe) or proximal (if the compression was proximal to the probe), and positive if the ‘A’ sound was heard directly with compression or negative if heard on release of the compression. This notation thus makes it possible for four ‘A’ sounds to be generated at each site being examined. Table 5.3 summarizes these sounds and their significance. This schema provides a useful method of categorizing these sounds; however, the ‘S’ and ‘A’ nomenclature has not found generalized acceptance. Instead, sounds are referred to as manifesting flux or reflux, antegrade or retrograde flow, patency or incompetence, etc.
| Type of ‘A’ Sound | Condition If Present | Condition If Absent |
|---|---|---|
| Distal positive | Normal | Venous obstruction |
| Distal negative | Valvular insufficiency | Normal |
| Proximal positive | Valvular insufficiency | Normal |
| Proximal negative | Normal | Venous obstruction or marked valvular insufficiency |
Augmentation of the most proximal portion of the GSV and of the more proximal deep veins is accomplished either by compressing the abdomen or by using a variation of the proximal compression and release, and the Valsalva maneuver (Fig. 5.14). The rise in intra-abdominal pressure caused by descent of the diaphragm is accentuated by contraction of the intercostal muscles. In the normal patient, an abrupt closure of the valves results in silence. However, more than 38% of normal persons have a brief period of reflux at the commencement of the Valsalva.61 Also, with a weak effort by the patient, a slow retrograde flow may pass through the valve and produce a Doppler flow signal because sufficient force to cause valve closure has not been generated. Visualization of the valves using ultrasound demonstrates that these valves do close eventually, and that they are not actually insufficient.62 Therefore, the continuation of reflux through at least half of the period of compression, or at least 0.5 seconds (usually 1–4 seconds), is important in diagnosing pathologic valvular incompetence.8,58 A less sensitive, but perhaps more specific, response may be elicited simply with deep breathing. With valvular insufficiency, instead of hearing the cessation of flow as the patient takes a deep breath, flow is reversed and a continuous signal heard, which shows a reverse deflection on a directional Doppler tracing (Fig. 5.15). The Valsalva maneuver is sometimes hard to explain to patients, and difficult to standardize; to facilitate, several tricks have been proposed, for example blowing into a surgical latex glove.63 When using the Valsalva maneuver to produce reflux while listening over more distal veins, the examiner must realize that the path of the reflux may be either straight down the superficial vein or through the deep vein to the perforating vein and into the superficial vein (Fig. 5.16).64 Therefore, additional testing is necessary to further delineate the exact site of abnormality. This is easily accomplished by manually obstructing the superficial vein; if reflux is still heard, the retrograde flow is assumed to be traveling through the deep and perforating systems.
Doppler examination technique
Femoral Vein
With the patient supine and the hips slightly flexed and externally rotated, the physician first locates the pulsatile signal of the femoral artery in the groin. If desired, the examination can also be performed with the patient standing, which may provide a more physiologic evaluation because most symptoms occur when the patient is upright and reflux is more easily elicited. The Doppler probe is then gradually angled medially until the spontaneous, continuous sound of the femoral vein, suggestive of a windstorm, is heard. Clear phasicity with respiration should be detected easily. Patency can be further tested by manually compressing the thigh or calf and listening for a strongly augmented signal. Valvular competence may be assessed by listening first for the phasic waxing and waning of the signal that, in severe cases of insufficiency, shows a decrease in intensity of the signal followed by a reversal of flow direction as inspiration progresses, rather than the expected silence. The patient is then asked to perform a Valsalva maneuver; alternatively, the physician can press on the abdomen. These latter maneuvers should cause an abrupt closure of the valve and silence, followed by a more intense antegrade flow on release if the valves are competent. A loud reflux flow heard through the Valsalva maneuver is pathognomonic of valvular insufficiency, which may be present in 5% to 30% of normal patients and, in one study, was found in 100% of patients with bilateral GSV varicosities.65 The effort invested in the Valsalva maneuver may be standardized to ensure the proper force and reproducibility by asking the patient to blow into a tube connected to a mercury manometer until the mercury column rises to 30 mm.
Differentiation of femoral from saphenous veins
Because the SFJ is located close to the femoral artery pulsation, valvular incompetence at the junction can sometimes be mistaken for CFV insufficiency. Several techniques can be used to aid in making this important differentiation. The saphenous vein is much easier to compress than the femoral vein, so manual compression using the Doppler probe may occlude the saphenous vein and allow the physician to listen selectively to the femoral vein. A separate occlusive device, such as the physician’s other hand or a tourniquet, may be used to compress the GSV distal to the Doppler probe and thus prevent reflux through it. Any reflux still heard is assumed to be through the femoral vein. Finally, moving or angling the Doppler probe in a cephalad direction may enable the physician to direct the ultrasound beam away from the saphenous vein to a more proximal segment of the femoral vein. Still, there are a small number of patients in whom differentiation of femoral from junctional signals may be impossible to determine by use of only the continuous-wave Doppler; an imaging procedure such as duplex scanning, which uses a pulsed ultrasound beam, may be necessary in such cases.66,67
To achieve uniform testing of venous reflux between institutions, comparable methods of testing by duplex and Doppler ultrasound scanning are desirable. In one study, the Valsalva maneuver was compared with rapid cuff deflation performed in the 15-degree reverse Trendelenburg position and in patients’ standing. Duplex technology allowed estimation of duration of retrograde flow and peak velocity. The general conclusions of the study was that the Valsalva method is best performed in the reverse Trendelenburg position as opposed to standing, but the cuff technique is more effective in the standing position.68
Popliteal Vein
For the next site of examination, the popliteal vein, the patient may be in the supine, prone, or standing position. The most physiologic position is standing, and it is advisable to perform all presclerotherapy Doppler examinations in this position. It is important to have the knee slightly flexed, however, since full extension of the knee joint may cause a functional obstruction of the popliteal vein. Also, if the examination is performed while the patient is standing, the weight should be borne on the opposite foot (Fig. 5.17). The pulsatile arterial signal is located, generally, in the popliteal crease just lateral to the midline; the Doppler probe may be angled medially to find a softer, although spontaneous, venous signal, or it may be left over the popliteal artery. Augmentation with either calf compression or thigh compression and release, as described previously, discloses both obstruction and valvular insufficiency. The Valsalva maneuver discloses reflux only if the more proximal deep veins (CFV) are also incompetent. As with reflux heard at the femoral level, reflux at the popliteal level may actually be caused by reflux through the SPJ. Therefore, in any patient who appears to have reflux through the popliteal vein, the test should be repeated while firm manual compression is applied to the SSV. Obliteration of the reflux in this manner localizes the site of reflux to the SPJ and not to the popliteal vein itself. Another method consists of slightly compressing an uninvolved portion of the calf with one finger, which causes flow through the popliteal vein and not the SSV.64 Popliteal vein reflux can be detected in this way with a sensitivity of 100% and a specificity of 92%, with most false positives being the result of variations in the anatomy of the SSV (see Chapter 1).8,15,69 The presence of popliteal valvular insufficiency is an important finding because it is associated with diminished calf muscle pump function and may be the most important prognostic factor in the development of venous ulceration.8,70,71 This relationship is not absolute, however; one study showed that popliteal incompetence was found in only 20% of patients with ulceration and 31.2% of postphlebitic legs.61
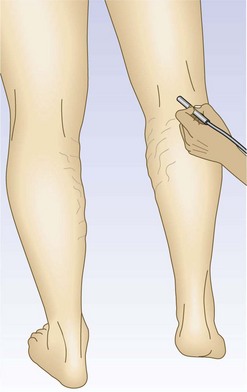
Figure 5.17 The popliteal vein is examined with the knee flexed and the weight borne on the opposite foot.
Although the continuous-wave Doppler is adequate for testing GSV incompetence, all reflux detected in the popliteal fossa should be checked by duplex examination. The continuous-wave Doppler examination has a sensitivity of 95% and a specificity of 100% for SFJ examinations, and a sensitivity of 90% and a specificity of 93% at the SPJ.72
Posterior Tibial Vein
The final deep vein that should be examined is the posterior tibial vein, located just posterior to the medial malleolus and beside the posterior tibial artery, which has an easily locatable pulsatile signal. This vein is frequently vasoconstricted, except if the patient is examined in a warm room, in which a spontaneous signal may be noticeable. Augmentation maneuvers are the same as described for the other deep veins. Again, although the Doppler is generally not felt to be sufficiently sensitive in the diagnosis of DVT below the knee, the response of posterior tibial venous flow to the release of calf compression has been found to allow an 87% accuracy in this diagnosis.59,60
Scanning veins below the knees by ultrasound presents unique difficulties because of the small size of the veins and their deep position. The addition of color to the Doppler examination has improved this situation immeasurably, and the rates for detection of the posterior tibial, anterior tibial, and peroneal veins has been raised to 98%, 96%, and 96%, respectively.73
Superficial Veins
After the deep veins mentioned previously have been examined, attention is turned to the superficial and perforating systems. The major saphenous trunks and their junctions with the deep veins should be examined with the patient in the standing position. Because of the lower flow rate in these vessels, a spontaneous signal may only rarely be audible. The presence of a saphena varix, or a visible bulge over the SFJ, is nearly pathognomonic of valvular incompetence. The junction is easily located with the Doppler approximately two fingerbreadths in the femoral triangle below the inguinal ligament. Alternatively, the physician may first locate the SSV in the thigh and then gradually move the Doppler probe superiorly and laterally while repetitively compressing the GSV until its location is reached. A positive cough or percussion test may also help to localize the site of the SFJ. The presence of reflux on release of more distal compression is indicative of SFJ insufficiency. Again, the magnitude of the compression can be standardized for serial comparisons by using a pneumatic cuff inflated to a specific level. Also, the Valsalva maneuver may be used to elicit reflux, although manual compression of the SFJ by the inguinal ligament may occur during a forceful Valsalva, and more proximal competent valves may impede the retrograde flow,74 thus creating the false impression of a competent valve.
If no reflux is heard over the SFJ, the physician should not assume that the entire GSV is competent.75–77 The perforator(s) in Hunter’s canal may frequently be the first abnormality to develop, leading to dilation and incompetence beginning just below the level of the middle thigh (see Chapters 1 and 3).33,78 Reflux frequently originates in branches of the GSV79; the ‘atypical refluxes’ described by Schultz-Ehrenburg and Hubner64 may be the most common. Incompetence of the GSV that is limited to the calf suggests insufficiency of the geniculate or lower leg perforators. Therefore, it is important to test the GSV for reflux in the groin, at the level of the knee, and in the lower leg and not to assume that it is normal until all sites fail to demonstrate reflux. In addition, there is a growing consensus that dilation may occur because of biochemical abnormalities in the muscle of the varicose vein wall. Thus, valvular insufficiency may not necessarily be a descending process, as was once assumed. This underscores the need to evaluate the entire length of the GSV in determining which portions of the vein to treat.75
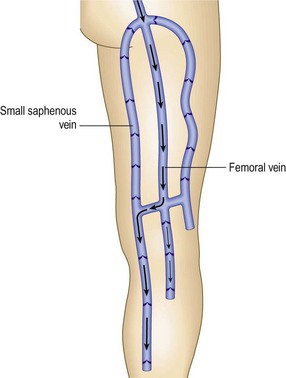
Examination of the SSV and SPJ is best carried out with the patient standing and the knee slightly flexed, as previously described. The SSV is felt more easily with the knee flexed and the popliteal fossa relaxed.14 When enlarged, the SSV generally is still not visible, but it is easily palpable as a spongy tubular structure leading inferiorly from the popliteal crease. By listening over the popliteal vein and tapping the leg very gently 5 to 10 cm below the probe, the examiner selectively compresses and thus listens to the SSV and not the popliteal vein, which requires a much stronger force. Since the termination of the SSV is variable, the exact location of the probe cannot be known for certain; therefore it is difficult to determine if any reflux heard is originating from the SPJ or is simply within a dilated SSV. The Valsalva maneuver or compression of the thigh aids in this differentiation because it results in reflux only if the SPJ is incompetent. Distinction between flow through the SPJ and popliteal vein can also be difficult but is facilitated by manually compressing the SSV below the probe while pressing on the calf, as described previously. Abolition of the reflux is evidence that the source is the SPJ.15 Another method is to listen over a more distal segment of the SSV, along the posterolateral calf, and to compress and release the SSV at the popliteal crease. Reflux or only augmentation after release is detected easily.
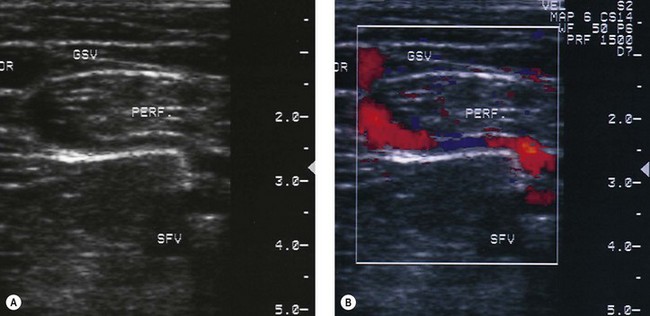
Perforating Veins
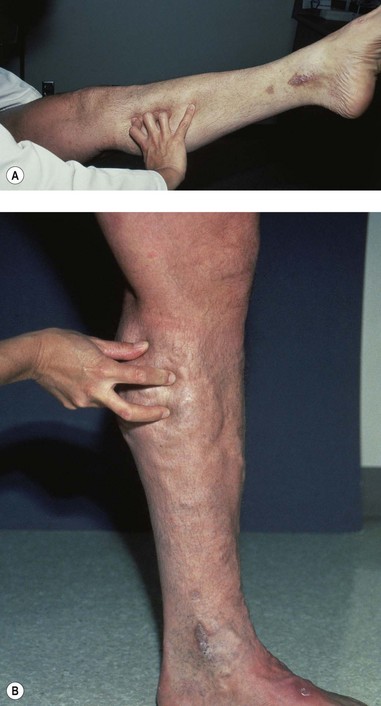
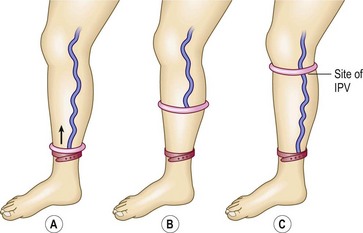
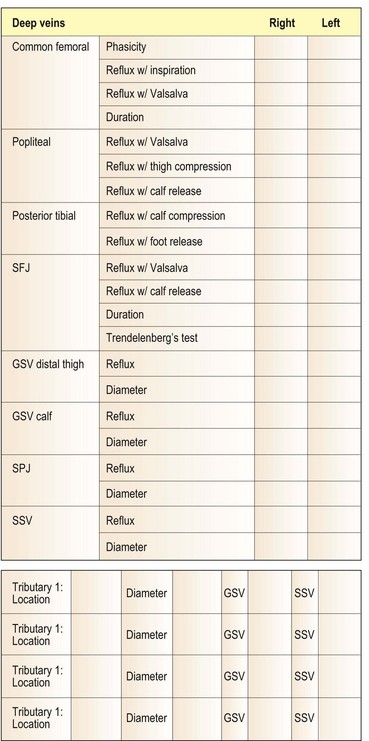
The examination of perforating veins is, at best, only 80% accurate using the Doppler.80–82 Many believe that physical examination – that is, palpation of fascial defects in which the incompetent perforator meets a dilated superficial vein at the depth of the superficial fascia – is perhaps even more helpful.83 In fact, published studies document that palpation is accurate only 51%37 to 69%39 of the time (Fig. 5.18). This technique, which is discussed more fully in Chapter 9, yields a large number of false-positive results because a fascial defect may result merely from dilation of a superficial varicosity or even from a separate pathologic process, such as a muscle hernia. In these situations, the Doppler affords increased reliability. In fact, Doppler examination for IPVs is advised after preliminary clinical localization of suspected sites (by listening for the characteristic to-and-fro movement of blood over sites of palpable defects in the fascia). Some authors have advocated the placement of tourniquets at 10 cm (4 inch) increments along the course of the lower leg before listening for flux and reflux at the sites of fascial weakness while the calf or thigh is repetitively compressed.80–82 A simpler approach is to place one tourniquet just below the level of the fascial defect and another just above it. While listening with the Doppler over each marked fascial defect, the physician compresses the foot (Fig. 5.19). Any audible signal thus represents flow proximally through the deep system and outward through an IPV. This provides greater specificity because it interrupts the flow through the superficial veins, thus allowing selective examination of the perforating veins. Figure 5.20 provides a rational method of recording the venous Doppler examination findings.
Post-treatment evaluation
Follow-up examinations of injected veins using the Doppler contribute more precise information regarding the response to treatment than physical examination does, because a vein that has been sclerosed loses both spontaneous and augmented flow signals. However, the Doppler detects flow through any vessel passing within the sound-wave beam and thus does not allow the examiner to be certain that the signal is from a particular vessel. Also, the Doppler does not differentiate thrombus from fibrosis, because both lead to an absence of a flow signal. These limitations illustrate the advantages of the duplex scanner, another technologic advance that is revolutionizing the practice of phlebology (Table 5.4).84–87
Table 5.4 Comparison of Doppler ultrasound and duplex scanning in the presclerotherapy evaluation
| Doppler | Duplex | |
|---|---|---|
| Portability | Portable | Portable |
| Ease of use | Requires short period of training and experience | Requires longer period of training |
| Information obtained | Patency, competence of venous valves | Patency, competence of venous valves |
| DVT in thigh (?calf) | DVT with greater accuracy | |
| Velocity of reflux | ||
| Anatomy and anomalies of venous system | ||
| Termination of SSV | ||
| Thrombosis vs sclerosis | ||
| Reliability | Less reliable because of blind, nonpulsed sound beam | More reliable because of actual visualization of vein being examined |
DVT, deep venous thrombosis; SSV, small saphenous vein.
Duplex ultrasound scanning
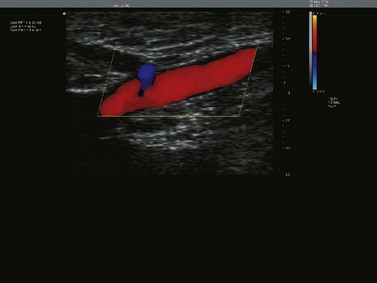
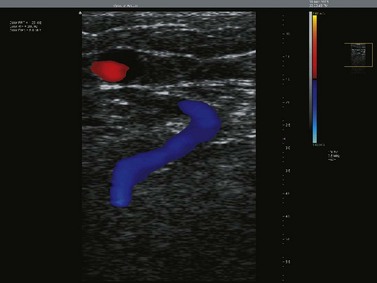
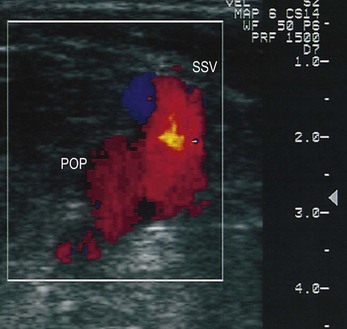
Duplex scanners are ultrasound machines that generally use a 7.5–12 MHz imaging probe along with a 3–5 MHz pulsed Doppler to enable visualization of the superficial venous system and to determine the direction of blood flow within the examined veins. Anatomy, flow within the veins, and the movement of the valves may also be studied (Fig. 5.21). Current scanners (sometimes termed triplex if displaying real-time color imaging and pulsed Doppler at the same time) use a computer-generated color system in which antegrade and retrograde flow may be coded to appear as different colors (red or blue) with varying intensities (brighter with lower velocities, paler with higher velocities), thus allowing immediate integration of this information by the examiner (Figs 5.22–5.24). Many new features have been introduced to enhance picture detail and contrast (B flow, Power Doppler, etc.), which can be helpful in specific cases. Visual ultrasound images have one of their greatest uses within the field of venous disease in the diagnosis of DVT and now have all but replaced venography in centers where the instrumentation is available (Fig. 5.25).88–93 Since the 1990s, alterations in the frequency range of the probes (higher frequencies: 10–20 MHz) have enabled clear resolution of superficial and deep veins, thus introducing an entirely new era in the diagnosis of varicose veins and their treatment by sclerotherapy.
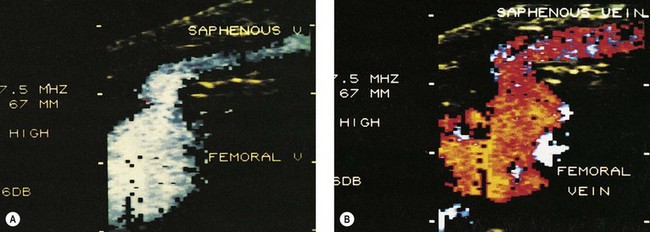
Figure 5.22 Color scanners display flow: in the normal direction in blue (A), and reflux flow in red (B).
While studies have demonstrated that the examination is best performed with the patient standing,94 it is often difficult to perform this practically. Most examinations are not performed on a tilt table with patients at least 30 degrees in reverse Trendelenburg position. The cut-off value for reflux in the veins is greater than 500 ms, except for the femoropopliteal vein, where it is 1 second (Fig. 5.26).
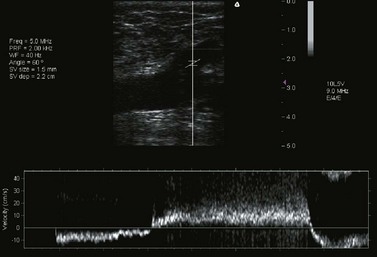
Figure 5.26 Duplex image showing reflux by pulsed-wave Doppler, lasting more than 3 seconds.
(Taken with Terason 2000 system.)
Aid to sclerotherapy
If the Doppler used to act as the ‘ears’ of the phlebologist, the duplex scanner must be considered both the ears and eyes as it allows the examiner to ‘see’ much more than is ascertainable otherwise. The duplex scanner allows for determination of the exact anatomy, including the important SFJs and SPJs. The anatomy of the SFJ is generally believed to be similar in all persons; however, there is actually significant variation. Although not generally accepted as fact, duplication of the GSV has been reported to be found in up to 27% of persons11–13 and is easily demonstrable with this technology (Fig. 5.27). Because the termination of the SSV is so variable, the exact location of the SPJ or the termination of the SSV in the GSV or its tributaries (the superficial or common femoral veins) or in tributaries of the internal iliac veins93 can be seen on the duplex scan (Fig. 5.28). In the past, selective venography was advised to determine the exact site of termination of the SSV before the SSV was operated on.95–97 Duplex ultrasound provides this piece of information noninvasively.98,99 Injections into the SFJ or SPJ, if performed under ultrasonic guidance,100,101 confer an added degree of accuracy and potentially safety to this procedure (Fig. 5.29). Injections into IPVs, particularly in areas of ulceration or lipodermatosclerosis, can be facilitated greatly by performing them under ultrasonic guidance.102 These areas may be particularly difficult to examine clinically or with a Doppler alone, and injections administered blindly into these areas can be quite risky because of the proximity of the posterior tibial vein and artery. Finally, the anatomic basis for proximal recurrences following GSV or SSV ligation may be found through duplex scanning. Because recurrent varicose veins occur in 20% to 80% of patients who have had varicose vein surgery, duplex scanning has allowed classification of the recurrences so that future studies can be conducted in a rational and well-planned fashion.103
In addition, as McMullin and Appleberg104 have found, duplex measurement of antegrade flow rates through the CFV, superficial femoral vein, popliteal vein, and GSV may be used to determine the degree of resistance within the deep veins and thus the preferential flow up the superficial veins in patients with chronic venous insufficiency. If it is found that the flow rate upward through an incompetent GSV is quite high in a given patient with disease in more than one segment of his or her venous system, removal or closure of this vein might be contraindicated. The amount of flow generated by active dorsiflexion of the foot may also provide information on the efficacy of the musculovenous pump.
Duplex scanning has allowed definition of the saphenous vein and its relationship to the superficial fascia and the deep or muscular fascia (Fig. 5.30). Throughout its length, duplex scanning has shown the GSV to lie on the muscular fascia. It is covered in its full length by the superficial fascia or membranous fascia, a connective tissue lamina that descends from the inguinal ligament to the ankle. This lamina is formed by the interlacing of connective tissue sheets. After the superficial fascia arches over the GSV, it fuses with the muscular fascia to create a saphenous compartment. In duplex scanning, this compartment has been called the ‘Egyptian eye’.105 This identification is crucial for correct duplex scanning and separating varicose tributaries of the saphenous vein from the saphenous vein itself.
Post-treatment evaluation
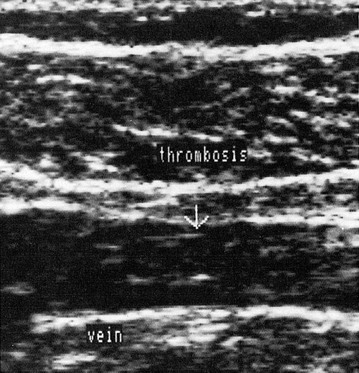
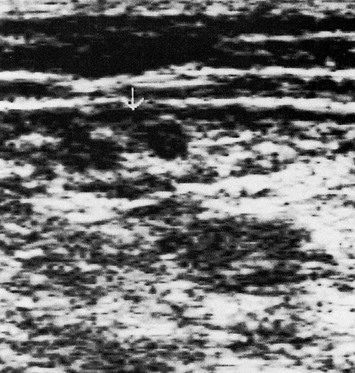
Another major use of duplex ultrasound with sclerotherapy is for follow-up of treatment. As mentioned previously, the Doppler does not allow differentiation between thrombus and fibrosis, both of which yield abolition of flow through the involved vein segment. The duplex scanner can differentiate these two situations very clearly. Depending on its age, thrombus may appear as a variably echogenic space associated with soft tissue swelling and inflammation, whereas fibrosis appears more often as a dense line with no associated inflammatory reaction (Fig. 5.31). Because patient response to treatment is so variable, physicians now can more accurately determine if the treatment rendered has been completely effective, thus producing fibrosis, or if the vessel is occluded by thrombus, thereby necessitating additional treatment. Many apparent treatment failures with early recurrence are likely to be found to be the result of inadequate treatment and not inadequate response.
Another important advantage of duplex ultrasound over the Doppler is its ability to quantitate venous reflux. This parameter has been found to have some prognostic potential. The flow in milliliters per second at peak reflux was measured in 47 limbs of patients who had chronic venous problems. It was found that dermatitis or ulceration did not develop if the sum of the peak refluxes in the GSV, SSV, and popliteal vein was less than 10 mL/second. A sum of greater than 15 mL/second was associated with a high incidence of these sequelae.8,106 In addition, superficial venous reflux alone may cause ulceration if the peak flow is greater than 7 mL/second.107
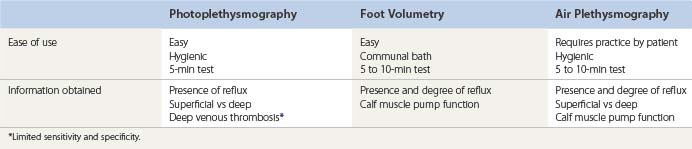
Photoplethysmography
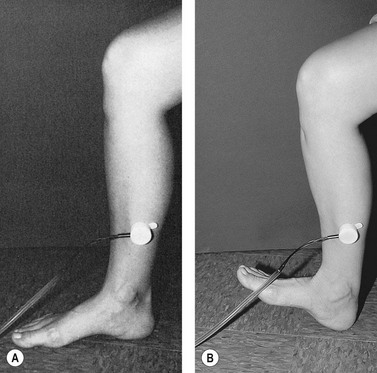
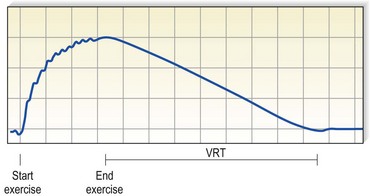
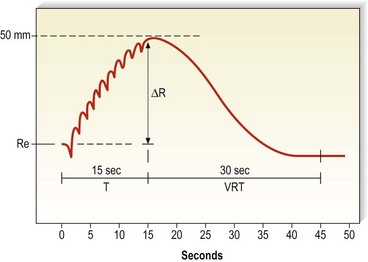
The most widely used functional evaluation for presclerotherapy purposes is photoplethysmography (PPG).108–111 Various forms of plethysmography have been used to evaluate venous function since 1956,112 and they have been shown to correlate well with venographic findings113 and ambulatory venous pressure (AVP) measurements. In one study of 338 paired measurements of PPG and AVP, the correlation co-efficient was 0.9.108 The principle of PPG is quite simple, and the test is easy and quick to perform. An infrared light source and sensor are attached with adhesive to the medial aspect of the lower leg, approximately 10 cm proximal to the medial malleolus. The infrared light is transmitted into the leg to a depth of approximately 0.5 to 1.5 mm, within the subdermal venous plexus, where it is absorbed by hemoglobin in red blood cells. Of the light that is not absorbed, a certain amount returns to the sensor. Therefore, the amount of infrared light reflected is inversely proportional to the volume of blood in the skin. Once a baseline level is reached, the patient is asked to actively dorsiflex the foot 5 to 10 times, which activates the calf muscle pump and produces venous outflow (Fig. 5.32). With a reduced volume of blood in the calf and the subdermal plexus, more light is reflected and the tracing shows a gradual deflection (the direction of the deflection depends on the electronics of the particular instrument). At the conclusion of exercise, the patient is asked to relax and the tracing then either returns to the original baseline value or levels off at a new value of light transmission. The time required for this value to be reached, the venous refilling time (VRT), provides information on the presence and degree of reflux of blood through either superficial or deep veins (Fig. 5.33).
The need for the awareness just mentioned is especially important in obese patients, but it is also necessary in patients of normal weight. By using a duplex scanner to visualize the flow through the GSV, McMullin et al114 found that the pressure within a 2.5-cm-wide tourniquet required to prevent reflux through the vein varied between 40 and 300 mmHg in the 40 patients studied. Therefore, manual compression applied directly to the vein being considered is the preferred method because it allows more reliable interruption of the flow and gives reproducibly accurate results.
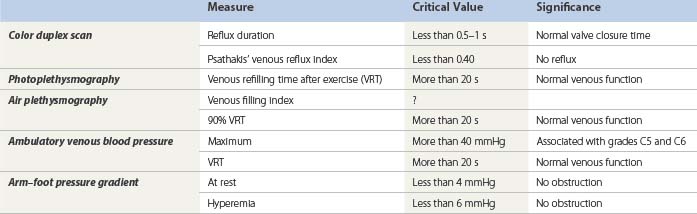
VRT has been found to correlate well with AVP measurements, which have long been considered the gold standard in the functional evaluation of venous hemodynamics. VRT may vary between 20 and 65 seconds when AVP is below 40 mmHg, but a VRT of less than 15 seconds is found only if the AVP is higher than 40 mmHg.115 AVP measurements show a linear relationship between their value and the incidence of venous ulceration.8,116
Photoplethysmography may be used to quantify the blood changes within the subdermal plexus, thus quantifying the degree of reflux. This involves performing an in vivo calibration maneuver that allows the examiner to assign a numeric value to the deflections on the tracing.117,118 The transducer is placed on the leg in its usual location while the patient rests in the supine position, and the tracing on the recorder is set to a zero baseline. The patient then stands, bearing weight on the opposite leg, and after the tracing levels off, the gain is adjusted so that the deflection reflects the calculated hydrostatic pressure in the superficial veins, measured by the distance from the right atrium to the site of the transducer on the leg. This maneuver is repeated until the zero baseline and standing levels of subdermal plexus blood content reproducibly reflect the hydrostatic pressures. The decrement in the tracing is then proportional to the degree of fall in AVP, as measured by invasive venous pressure recordings.
Plethysmography has been vigorously defended and advocated by some.119 However, others have questioned the use of this tool because of its lack of correlation with duplex scanning.120 The authors of this study stated, ‘These results do not warrant the continued use of photoplethysmography for surgical decision-making in patients with suspected venous insufficiency.’
Recent studies have demonstrated the efficacy of PPG in evaluating venous hemodynamics.121,122 A PPG system can be calibrated to quantify the blood volume displacement with leg elevation and/or exercise. In a study of patients with isolated superficial venous disease, digital PPG was found to give reproducable results.123 A determination of venomuscular pump efficiency has been demonstrated to quickly gauge the severity of venous disease. The use of PPG may also allow the practitioner to assess the effectiveness of superficial vein treatment.
Light reflection rheography
Light reflection rheography (LRR), which is basically a form of PPG, was intended to improve on the original PPG system.124,125 On account of its incorporating three light sources, the infrared light beam can be focused at a standardized depth of penetration (0.3–2.3 mm) to cover the subcutaneous venous plexus. Dermal pigment, such as that commonly found in patients with chronic venous insufficiency, is concentrated in the more superficial layers of the skin and interferes with light transmission, yielding inaccurate and variable values. It was hoped that by focusing its light beam on the deeper tissues, the LRR would not be as affected by the tissues containing the majority of the pigment. This did not prove to be the case, however, and it was felt that calibration of the system might neutralize the effect of variables such as skin thickness, skin pigment, and local blood volume on light absorption. This improvement is now available as digital PPG (D-PPG) or calibratable PPG (C-PPG).115
The D-PPG contains a computer that permits changes in light intensity from the infrared light source according to the optical properties of the skin. The machine emits a standard light intensity and awaits reflection of the unabsorbed light. If it is below a certain level, the intensity of the emitted light is automatically increased until the intensity of reflected light reaches a level at which the machine can function accurately. This was demonstrated nicely by Kerner et al,126,127 who recorded essentially the same response to dorsiflexion even after the leg was covered with a dark paint.
The C-PPG is essentially the same as the D-PPG, except that the changes in light intensity are adjusted manually. This device was tested on normal subjects and on patients with venous disease. By comparing the time with 90% refilling, or by combining the results obtained during postural changes and dorsiflexion in order to obtain exercise drainage volume, it was possible to significantly differentiate patients with venous ulcers from those with varicose veins and from the normal controls.115 Other parameters that can be calculated include venous filling volume and pump efficiency.
The usefulness of PPG in the assessment of venous valvular insufficiency is undisputed; however, claims that it is accurate in diagnosing DVT are controversial. The general statement that a ‘picket fence’ pattern (Fig. 5.34) produced by the 10 dorsiflexions with essentially no vertical movement off of the baseline is diagnostic of DVT is certainly incorrect, because there are many false-positives using this criterion. In a study of 30 limbs, the correlation coefficient between venous emptying and AVP was only 0.73.124 However, in a study performed at the University of Miami,128 the slope of the deflection correlated well with the presence of acute DVT as documented by venography. As shown in Figure 5.35, the finding of a slope (R/T) of less than 0.31 mm/s predicts the presence of DVT with a 96% sensitivity. Still, LRR alone currently is not considered sufficient to make the diagnosis of DVT, and at least one other noninvasive test is required to confirm the diagnosis.
Air plethysmography
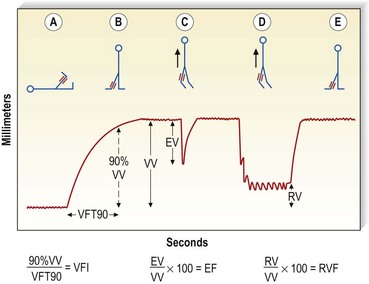
Air plethysmography (APG) is one technology that is just as simple to use and potentially supplies a great deal of additional information compared with the conventional PPG.129 This device consists of a 14-inch-long, tubular, polyvinyl-chloride air chamber that surrounds the leg from knee to ankle. This is inflated to 6 mmHg and connected to a pressure transducer, an amplifier, and a recorder. A smaller bag placed between the air chamber and the leg is used for calibration by injecting a certain volume of air or water and measuring the change in the recording that is associated with that volume (Fig. 5.36). Parameters assessed include: (1) functional venous volume (VV), or the volume in the leg while the patient stands; (2) venous filling time 90 (VFT90), or the time required to achieve 90% of the VV; (3) venous filling index (VFI), or 90% VV/VFT90; (4) ejection volume (EV), or the volume expelled from the leg with one tiptoe motion; (5) residual volume (RV), or the volume at the end of 10 tiptoe motions; and (6) residual volume fraction (RVF), or RV/VV100 (Fig. 5.37).
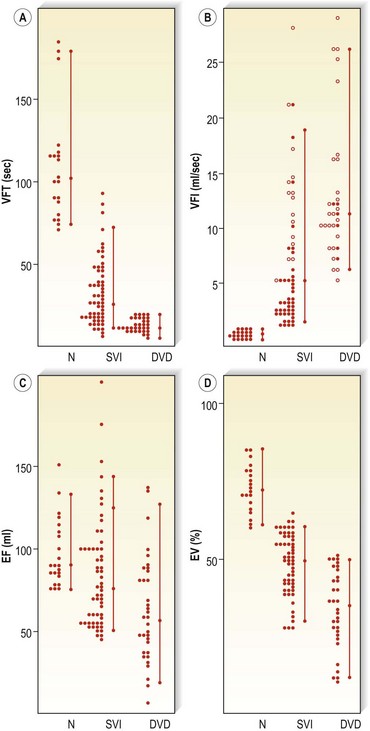
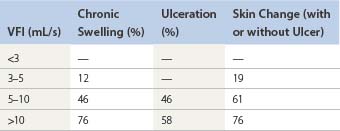
In a study of 22 patients with superficial venous insufficiency and nine patients with deep venous disease, VV was found to be elevated in 80% of patients.130 VFT90 was greater than 70 seconds in normal limbs, 8 to 82 seconds in limbs with superficial venous insufficiency, and 9 to 19 seconds in limbs with deep venous disease. In normal limbs the VFI was less than 1.7 mL/second, in limbs with superficial venous insufficiency it was 2 to 30 mL/second, and in limbs with deep venous disease the value was 7 to 28 mL/second. Ejection fraction (EF) appeared to show better discrimination than EV. The RVF was 20% in normal legs, 45% in legs with superficial venous insufficiency, and 60% in legs with deep venous disease (Fig. 5.38). A linear correlation with r = 0.83 was present between RVF and AVP. In another study of 104 patients,107,131 VFI was found to correlate with the incidence of sequelae of venous disease such as chronic swelling, skin changes, and ulceration (Table 5.5). In a third study of 205 limbs,132 the same authors found an increasing incidence of ulceration in patients with diminished EF and elevated VFI (Table 5.6) and found that the RVF showed a good correlation (r = 0.81) with the incidence of ulceration and AVP measurements.
Table 5.6 Effect of venous filling index (VFI) and ejection fraction (EF) on incidence of venous ulceration
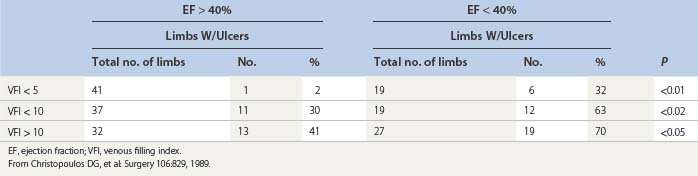
The real advantages of this method are its ability to quantitate reflux with the VFI and thus determine prognosis, and its ability to measure calf muscle pump function through the determination of the EF.107,130–133 Still, APG measurements should not be used in a vacuum. They should be combined with Doppler or duplex findings and clinical evaluation, since a great deal of overlap in values between normal and abnormal occur, decreasing the predictive value of abnormal APG measurements.134 Neglen and Raju135 demonstrated quite well in their evaluation of 118 limbs that VFI alone had a positive predictive value of 66% in separating clinical severity class 0 or class 1 from class 2 or class 3. VFI combined with information gleaned from duplex scanning had a positive predictive value of 83%.
Validation of use of APG in clinical practice has been achieved by some.136,137 However, in clinical practice, the use of APG appears to be limited. Part of the problem is the difficulty in testing a large number of patients in a busy clinical setting. Another is the fact that the skin changes of severe chronic venous insufficiency are caused by many factors, and the data obtained by APG can represent only the hemodynamic factor and not the effects of leukocyte infiltration, activation, and leukocyte–endothelial interactions that produce the inflammatory response.138
Perhaps the most carefully performed evaluation of the use of APG was accomplished under David Sumner’s direction in Springfield, Illinois.139 In his report, he stated that, ‘We conclude that plethysmographic measurements of functional venous parameters do not discriminate well between limbs with uncomplicated varicose veins and limbs with ulcers or stasis dermatitis and that the venous filling index correlates poorly with the presence of incompetent veins and their diameters.’ Both duplex scanning and plethysmography seem to be necessary for a complete evaluation of limbs with chronic venous insufficiency.
Foot volumetry
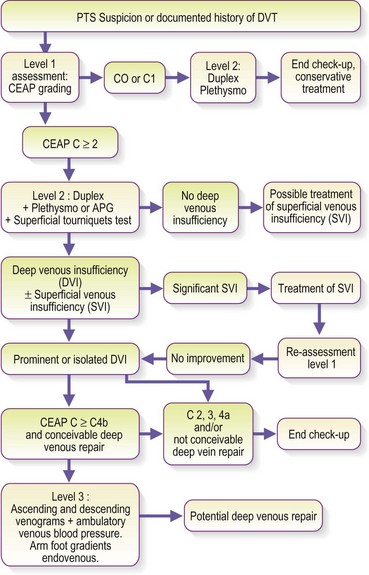
Yet another method for evaluation of the functional state of the venous system is foot volumetry.140–145 Introduced in the early 1970s, this technique has not earned a prominent place in phlebology, probably because of certain logistics of performing the test. However, it is necessary to have an accurate way to measure leg swelling either for evaluation of chronic venous disorders (day-to-day edema measurement) or calf pump function assessment.146 The patient stands with their feet in an open water-filled plethysmograph (Fig. 5.39). The water level is monitored by a photoelectric sensor, and changes in foot volume are continuously measured, first while the patient is standing still, then during the performance of 20 knee bends, and again while standing still. The parameters measured include the volume of blood expelled from the foot during exercise, the flow rate after exercise, and the time required for half and then full refilling to occur. Norgren et al143 have shown good correlation between foot volumetry and invasive venous pressure measurements in control subjects (r = 0.662) and in patients with varicose veins (r = 0.760) but poor correlation in patients with deep venous valvular insufficiency (r = 0.410). In their study, venous pressure measurements differed significantly in patients with varicose veins and controls but were similar in patients with primary varicose veins and those with deep venous valvular insufficiency. However, with the use of foot volumetry, there were significant differences between all three groups. Thus, although it is possible that foot volumetry is inaccurate in this important categorization, it is likely that this technique is more sensitive in distinguishing these groups than are venous pressure measurements.144 It has been shown that both the volume expelled with exercise and the refilling time increase after treatment of varicose veins.145 Therefore, this test could be used to evaluate the success of a particular treatment, to follow the effect of different stages in treatment, and to monitor the severity of chronic venous insufficiency. It does not, however, allow localization of a particular site of reflux, thus limiting its usefulness for presclerotherapy evaluation when compared with other methods (Table 5.7–5.10, Fig. 5.40).
Table 5.8 The instrumental evaluation of post-thrombotic syndrome – relevance of investigations according to considered abnormalities
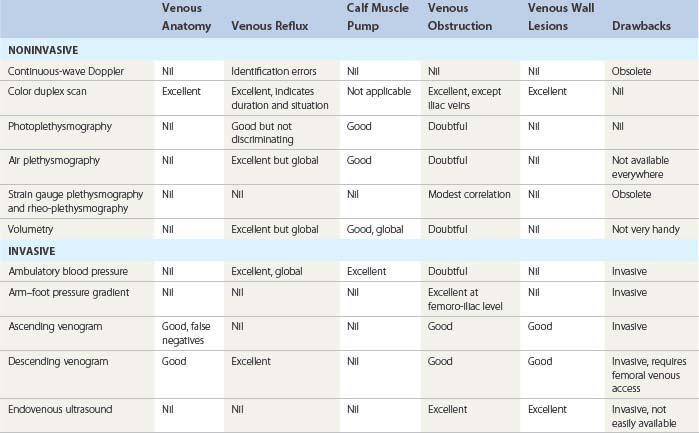
Table 5.9 Relevance of investigations according to considered abnormalities – associated investigations
| Basics | Optionals | |
|---|---|---|
| Level 1 | Interrogation, medical history Physical examination, CEAP grading |
Continuous-wave Doppler |
| Level 2 | Color duplex scan Photoplethysmography or air plethysmography |
Superficial tourniquets test |
| Level 3 | Ascending venogram Descending venogram Ambulatory venous blood pressure Arm–foot pressure gradient |
Endovenous ultrasound |
Use of Noninvasive Techniques
Each of the previously described techniques has advantages, limitations, and uses in specific situations. Prohibitive cost or limited access may preclude the use of the most sensitive and accurate method. The following section discusses a reasonable use of various noninvasive techniques for a variety of situations commonly encountered in the everyday practice of sclerotherapy (Table 5.11).
Many practitioners have noted changes in the findings of flow and reflux depending on timing within the menstrual cycle, time of day, recent use of compression hosiery, and psychologic stress of the patient. Clearly, there are enough experimental data to support a physiologic cause for these fluctuations.147 Therefore, an effort to examine patients in the most physiologic circumstances (e.g. premenstrually, late in the day) may be rewarded by a more revealing study.
Examination of deep veins
Duplex ultrasound is the standard for examination of deep veins of the leg. A reflux duration of at least 0.5 seconds after the release of calf compression identifies valvular insufficiency.58 Descending venography provides accurate information on the state of the deep venous valves. Although its invasiveness, associated risks, and pain make it a much less attractive option for routine use, occasionally it provides information unobtainable with other studies (Fig. 5.41). Photoplethysmography detects the presence of valvular insufficiency, and compression of the superficial veins (either manually or with a tourniquet) allows differentiation between superficial and deep venous reflux; however, PPG cannot localize the reflux to the level within the deep system (femoral versus popliteal, etc.). When both superficial and deep venous reflux are present, PPG will allow the determination of the relative importance of each segment. Although ascending venography was once considered the gold standard for the diagnosis of acute or chronic deep venous obstructive disease, most institutions now use B-mode ultrasound or color duplex scanning in everyday clinical practice. Magnetic resonance venography may find a place in the diagnostic armamentarium as well, since it has been found to be as accurate as duplex scanning in the diagnosis of DVT.148
Descending venography detects deep venous valvular reflux but, again, the duplex scanner offers the additional advantage of quantifying the reflux by determining flow velocities. The finding of deep venous valvular insufficiency is worrisome because it may be associated with chronic venous obstructive disease, which may give rise to venous claudication,42–44 since there are a small number of patients who rely on their dilated superficial channels for venous return. This has been determined using strain gauge plethysmography149 and most likely may be assessed with PPG as well. Impairment of VRT with the tourniquet might caution the examiner to avoid treatment. Alternatively, the simplest and most practical test is to place a 30- to 40-mmHg compression stocking on the patient for 24 hours. The development of pain while walking contraindicates sclerotherapy and suggests the need for a venous bypass procedure. Using APG, Spence et al150 found that compression therapy in patients with venous claudication caused a deterioration in the EF and/or AVP as measured by RVF. Noninvasive tests do not provide sufficient sensitivity if there is genuine concern about venous claudication. The examiner must proceed with invasive pressure measurements, such as the arm–foot vein pressure differential or the foot vein pressure elevation after reactive hyperemia, as described by Shami et al.10 Deep venous valvular insufficiency has also been found to reduce the likelihood of successful long-term sclerosis of the main saphenous trunk.64
Examination of saphenous vein trunks
Duplex ultrasound is now the standard for examination of saphenous vein trunks. Historically, Thomas and Bowles151 found that Doppler ultrasound grossly overdiagnosed incompetence when compared with venography, and they recommended that all GSV be examined with venography before ligation and stripping. One reason for this is the fact that Doppler examination is ‘blind’; thus, dilated tributaries of the saphenous vein or pelvic varicosities may be mistaken easily for the GSV. This was addressed in two early studies that compared the results of continuous-wave Doppler examination with those obtained with duplex scanning.66,67 The researchers found that in the examination of the GSV, Doppler was no better than 77% sensitive and 83% specific compared with the duplex scan. In a more recent study using duplex scanners with even greater sensitivities, DePalma et al152 found a sensitivity of 48%, specificity of 83%, positive predictive value of 83%, and negative predictive value of 44% in the determination of GSV reflux. By obtaining duplex scans preoperatively, 10 limbs out of 80 were spared GSV stripping.
The Doppler examination of the junction of the saphenous trunks with the deep veins (SFJ and SPJ), and the distinction between reflux through these junctions versus reflux through the deep veins themselves, is often difficult and a common source of error. In fact, studies using Doppler ultrasound have quoted an incidence of deep venous valvular reflux from 5% to 30%,27,28,64 a range that is most likely partially determined by the difficulty of the examination and not solely by differences between the populations examined. The distinction between junctional and actual deep venous reflux is important for several reasons. First, Schultz-Ehrenburg28,64 found that patients with deep venous valvular incompetence fared far better with a surgical approach to their disease than with treatment that was limited to sclerotherapy. Thus, the accuracy of this portion of the examination has a direct application to the treatment plan. In addition, patients with deep venous valvular insufficiency should be questioned regarding a history of iliofemoral thrombosis and possible chronic venous obstructive disease, which might contraindicate treatment of their GSV. Finally, it is possible to have only deep venous valvular insufficiency with a normally functioning saphenous vein. Thus, without this differentiation, a patient may be sent for treatment of a normal superficial vein. The technique of examination is quite simple. The Doppler probe may be placed over the site of the SFJ or over the femoral vein with the patient standing or supine, and the patient is asked to perform the Valsalva maneuver. The procedure is then repeated with the GSV firmly compressed below the Doppler probe. If reflux still can be heard after compression is applied, the examiner assumes that the reflux is in the femoral vein. In contrast, if the reflux is obliterated with this maneuver, the retrograde flow is only through the SFJ and not through the femoral vein itself.
Examination of tributaries of the saphenous trunks
As mentioned previously, although the Trendelenburg test is reasonably accurate, the cough and percussion tests are now considered confirmatory because the Doppler provides a more accurate answer to these questions. Placement of the Doppler probe over the tributary while intermittently compressing or percussing either the GSV or SSV allows determination of the origin of the tributary (Fig. 5.42). Listening for reflux while the patient coughs or performs the Valsalva maneuver uncovers connections to the deep system (since there should be no reflux unless there is a pathway directly to the deep vein that is unobstructed by incompetent valves). The applicability of the first piece of information is obvious. Careful evaluation must then be directed to that particular incompetent saphenous trunk to achieve sclerosis of the tributary as well. However, the connection of the tributary to the deep system must be explored further because the exact route that the blood has taken from the deep to the superficial system must be defined. If the connection is simply through the SFJ or SPJ, again the treatment directive is apparent. On the other hand, if the connection is actually through a perforating vein or another tributary,64 treatment must be aimed at that particular vein and, perhaps, treatment of the saphenous trunk may be unnecessary. Manual occlusion of the involved saphenous trunk at its proximal end, followed by repeat examination, offers this important differentiation. If this occlusion causes the obliteration of reflux with the cough or Valsalva maneuver then the blood must have flowed through the SFJ or SPJ. If, on the other hand, this maneuver does not change the result of the test then the saphenous trunk is an important conduit and the perforating vein must be the important route.
The importance of duplex scanning in patients with varicose disease has been verified by studies in which clinical examination, duplex ultrasound, and plethysmography have been compared.153 These studies have revealed that quantitative plethysmography was not particularly helpful because of its nonspecificity. The duplex scan, however, was able to identify patients without SFJ reflux and could ascribe varicosities to tributary incompetence. Such incompetence would be the target for sclerotherapy or isolated ambulatory phlebectomy.
Examination of perforating veins
The perforator segment of the venous system is probably the most mysterious because of its variability, the difficulty of locating perforators even under direct visualization in the operating room, and the overwhelming importance ascribed to perforating veins in the development of varicose veins and the skin changes associated with chronic venous insufficiency.154 It is no wonder, therefore, that no consensus exists about what constitutes the best method for examination of perforating veins and their valvular competence. Nearly every technique, including venography, Doppler, duplex, thermography, fluorescein injection, and physical examination of fascial defects, has been used with varying degrees of success. Complicating the evaluation of each method is the fact that all are compared with later surgical findings, which most likely also miss many IPVs and which are impossible to standardize. Underscoring the current difficulties in this aspect of venous diagnosis are data that show that the number of IPVs detected per limb, in studies of the various diagnostic methods ranges from 1 to 4, whereas anatomic studies have shown a range of 1 to 14, with an average of 7.32 Thus it is still impossible to test the exact sensitivity and accuracy of each method. At best, the examiner may miss a great deal of important pathology. In spite of the pitfalls and limitations of current diagnostic methods, examination for IPVs is crucial and usually productive. As mentioned previously, in a study of 901 limbs with varicose veins, 90% were found to have incompetent perforators.32 Of interest, only 9% of the perforators were found in the thigh. Thigh perforators may be either single or multiple and may occur anywhere from just proximal to the patella to just below the SFJ, with most being single and located in the middle third of the thigh.155 In evaluating patients with IPVs in the lower leg, Dodd156 found that 45% were associated with incompetence of the GSV, 15% with incompetence of the SSV, and 2% with an IPV in Hunter’s canal. Therefore, any patient with significant truncal varicosities, as well as those with signs of chronic venous insufficiency, should undergo evaluation for perforator valvular insufficiency.
Historically, the most important and probably the most commonly used technique for detection of outward flow through perforating veins was that of clinical examination. With its ability to detect 50% to 70% of IPVs, clinical examination should be the first step in the evaluation. Other techniques have been studied extensively, such as thermography,36,39,157,158 fluorescein injection,159,160 and ascending36,37,159 and intraosseus39 venography, generally demonstrating accuracies of between 60% and 90%. Unfortunately, the required instrumentation makes these techniques impractical for most practitioners. The techniques can be used, however, when perforator disease is strongly suspected but has escaped localization by other methods.
Ultrasound technology can be helpful and is associated with greater ease and lower risks than the other methods. Probably the most effective method of locating perforator veins is to inject a low concentration of foamed detergent sclerosant and follow its flow into perforating veins. Doppler evaluation of perforator incompetence provides a diagnostic accuracy of 60% to 90%37,80,82,160 and definitely improves with experience. In one study of 39 legs,37 its accuracy improved from 60% to 87% when it was combined with clinical examination, thus making this combination of techniques well suited for routine clinical practice. Duplex scans are extremely useful in visualizing the site(s) of IPVs (Fig. 5.43) and may be considered if the examiner is otherwise unable to localize a vein in a suspected area and has the necessary equipment.
The clinical significance of outward flow through perforating veins has long been debated, and the physiologic to-and-fro movement of blood through perforating veins in normal feet is well accepted. Thus, the finding by Sarin et al161 that the direction of blood flow within medial calf perforators can be either inward or outward in legs without venous disease is quite interesting. They observed outward blood flow in 21% of medial calf perforators in normal limbs during compression of the foot with a cuff inflated to 60 mmHg. However, during the relaxation phase (distal cuff deflation), flow occurred in 33% to 44% of perforators in limbs with venous disease but in none of the perforators in limbs without venous disease. Thus, this criterion may allow the first true differentiation of pathologic flow within perforating veins.
Evaluation of the origin of recurrences after ligation and stripping
Duplex ultrasound is considered to be the best method to evaluate recurrences after ligation and stripping. There are several noninvasive or minimally invasive methods that provide information helpful to understanding the remaining connections and therefore the necessary sites of treatment. Lofgren,17 in his study of 510 operations performed on patients with recurrence after ligation and stripping, found most of the recurrences to be the result of inadequate surgery. Either treatment of a dilated tributary with the actual saphenous trunk left untouched or ligation distal to the SFJ/SPJ are the usual findings.
The approach to the kind of patient just mentioned begins in the same way as the approach to any patient with involvement of the saphenous trunk. The palpation of the GSV or SSV is attempted, followed by the Doppler examination for reflux. It is always possible that the dilated vein noticed by the patient may simply be, in fact, a collateral that is functioning normally and does not need to be treated. Once reflux through the vein has been documented, a Valsalva maneuver will demonstrate whether this vein is still in connection with the deep venous system. If not, sclerotherapy generally may be used successfully. If reflux is heard with a Valsalva maneuver, the patient may then be approached as if he or she was presenting de novo with SFJ or SPJ reflux. Historically, venography has been used in this situation and provides a good image of the exact connection of the recurrent varix. However, duplex scanning provides excellent images without the associated risks of the contrast media and radiation, and it has been recognized as the gold standard for evaluation of recurrences.162
Evaluation of vulvar varices
Vulvar varices generally arise from the pudendal or iliac veins and require treatment only of the varices themselves. In some cases, however, they connect directly with the long saphenous system. Therefore, before treatment, the presence of an incompetent SFJ should be sought, since long-term control of these varices requires control of the SFJ if blood is refluxing through it into the vulvar veins. The technique for this determination has been described previously. The exact connection may also be determined by varicography (Fig. 5.44). Since the association between pelvic varices (often complicated by pelvic congestion syndrome) and inguinal varices is common, a retrograde selective catheterism and venogram of pelvic veins will frequently help to solve both problems at the same time by means of specific endovenous treatment (association of coils and foam sclerotherapy).163–165
Invasive Diagnostic Techniques
Venography
The most serious disadvantages of venography are its invasive method, the frequent development of superficial phlebitis as a result of the procedure, and a 5% to 10% incidence of allergy to the contrast medium. The latter complications have been significantly reduced through the introduction of newer nonionic contrast media,13 and venography continues to find some use in the diagnosis and treatment planning of venous disease. It also has great use in the evaluation of groin and pelvic recurrences, including vulvar varicosities.166 Four techniques have been described: ascending venography, descending venography, intraosseous venography, and varicography.
A variation of venography involving the injection of fluorescein into a vein on the dorsum of the foot has been used for the localization of incompetent ankle perforating veins.167 This test is performed by placing a 2.5-cm cuff, inflated to 80 mmHg, just above the malleoli. The leg is elevated to 90 degrees, and the patient is asked to plantarflex the foot 10 times. A second 13-cm cuff is then inflated to 120 mmHg just above the knee, and the leg is lowered. Five mL of aqueous fluorescein is then injected into the distal portion of the foot, and an ultraviolet light is directed toward the leg in the darkened room. A second set of 10 plantar flexions is performed, drawing the solution into the deep veins and outward through any incompetent perforators. This is reflected on the skin surface as a circle of yellow–green fluorescence, 1 to 2 cm in diameter, within 30 seconds to 2 minutes. In 37 legs studied, this method had a 96% accuracy, identifying 50 of the 52 perforating veins later found to be incompetent at surgery, with two false-negatives and four false-positives.
Ascending venography
Ascending venography13 is performed by injecting the contrast medium into a superficial vein on the dorsum of the foot after a 2.5-cm tourniquet has been placed around the ankle to prevent flow through the superficial venous system. This forces the contrast to enter only the deep veins and allows clearer visualization of the deep system. Fluoroscopic imaging with the patient in various positions allows the deep veins, from the foot to the lower segment of the inferior vena cava, to be examined for the presence of thrombi (Fig. 5.45A). Passage of the contrast into the perforating veins is abnormal and diagnostic of valvular insufficiency (Fig. 5.45B).38 The addition of a Valsalva maneuver shows competent venous valves and defines bicuspid structures with a concentration of contrast media in their sinuses, and it may obviate the need for descending venography if this procedure were to be considered later. Ascending functional venography requires the patient to plantarflex the foot, thus forcing the contrast into the superficial veins through any IPVs during muscle relaxation.7
Descending venography
Descending venography involves injection of the contrast into the femoral or the popliteal vein with the patient lying supine or tilted head-up and performing a Valsalva maneuver. Valvular insufficiency is readily demonstrated because the contrast flows rapidly in a retrograde direction. Five grades of reflux have been described (Fig. 5.46).61,168 One of the disadvantages of this technique is that the demonstration of reflux at a given level relies on the presence of reflux at the higher levels. Therefore, using descending venography alone, the examiner might miss isolated incompetence of tibial veins or gastrocnemius veins, both of which have been shown to be responsible for the production of significant symptoms (see Chapters 3 and 4).169,170
Dynamic popliteal phlebography171 had been the latest, and successful attempt to improve phlebography, especially for analyzing deep venous function and structure. It has, however, been rendered obsolete by ultrasound duplex scanning.
Intraosseus venography
Intraosseus venography is simply a variation of ascending venography in which the contrast is injected into bone rather than directly into a vein, with rapid distribution throughout the deep venous system. This technique may be significantly easier to perform in patients with edema172 and may be especially helpful in diagnosing IPVs,38 yet it has not been found to have significant use recently.
Varicography
Varicography (Fig. 5.47) is a very helpful technique in which the contrast agent is injected directly into the dilated varix, showing the extent of the varicosities and their connection with other superficial, perforating, and deep veins.13,166,173,174 The direction of blood flow, and therefore the competence of valves, is not discernible, although other criteria have been found to correlate with valvular insufficiency. For instance, if a perforator is greater than 3 mm in diameter and is seen to be tortuous, it is assumed to be incompetent.13 When other methods, such as duplex scanning, fail to demonstrate the proximal connections of a varicosity (because of obesity of the patient, masses of varicosities in the area, etc.), varicography provides a relatively simple way of showing this anatomy clearly (Fig. 5.48).
Thermography
Infrared thermography is based on the fact that infrared waves radiate from the skin surface in proportion to the temperature of that surface.175,176 It is possible to scan the surface with an infrared detector and create an image in which gradations of white and black or color reflect degrees of heat. Two identical symmetric skin areas of the body should be at the same temperature unless certain factors are present. These factors include structural abnormalities of vessels (dilation and incompetence), abnormalities of vascular control, local effects on vessels, changes in thermal conductivity of the tissues, and increased heat production of the tissues. When veins are dilated, such as with varicose veins or with an underlying arteriovenous communication, the overlying skin is warmer because of the accumulation of the extra volume of blood in the dilated vein. Similarly, when venous valves are incompetent, the reflux of blood from the more central CFV down the GSV (when the limb is lowered), or outward from the warmer deep veins through a perforating vein when the calf muscle is activated, produces a rise in skin temperature; this may be visualized on the thermograph as a white, or ‘hot’, spot (Fig. 5.49). In fact, this technique may be used to localize the site of an IPV (see Chapter 3).39 After the general distribution of thermal patterns is recorded in the standing position, the leg is elevated for 1 minute to drain the veins, and the leg temperature is lowered with a cold, wet towel or a fan for 5 minutes. A tourniquet is placed around the proximal thigh to occlude the superficial veins, and the patient is asked to stand. Areas of rapid rewarming suggest possible sites of IPVs. These areas are re-examined after tourniquets are placed above and below each site. The segment in question is again cooled, and the appearance of a hot area within 60 seconds of standing (in the case of a thigh perforator) or of calf muscle action (in the case of a lower leg perforator) identifies the site of an incompetent perforator. Of 84 IPVs later found at operation, thermography correctly identified 79 (94%). The five that were missed by thermography were found at surgery to be very small in diameter or in close proximity to a larger incompetent perforator that was correctly identified. Of the 12 false positives, 4 were caused by inadequate surgical exploration of the area, and the others were the result of heat changes from communicating sites of superficial veins or from the penetration of the GSV into the deep fascia of the thigh. The relationship of arteriovenous fistulas to varicose veins remains controversial, and thermography has been validated as a technique for identifying arteriovenous fistulas when they are present.177
Future Evaluation Techniques
Studies are underway for objective noninvasive quantification of superficial varicose veins utilizing infrared photography. Photographs taken through a 700-nm lens filter allow visualization of blood-containing vessels 2.5 mm below the skin.178 When analyzed in a computer grid, this technique may allow for a reproducible evaluation of veins before and after treatment, as well as validate C1 and C2 classification.
Infrared imaging of subcutaneous veins as an aid in performing surgical or injection therapy is also being investigated. An infrared device comprising a head-mounted infrared LED array (880 nm) has been developed.179 This device provides good contrast of subcutaneous veins 0.5 to 2 mm in diameter at a depth of 1–3 mm.
Near infrared imaging
Infrared imaging can be used to visualize superficial reticular veins. Bustos et al showed that this could be performed either with the use of a red light source close to 700 nm or an infrared night-day vision camera system (Vision Viewer Gen 3 (Night Vision Experts, Buffalo N.Y.). Both of these systems allow the operator to visualize veins 2 to 3 mm in depth.180 A more elaborate infrared imaging device, the Luminetx VeinViewer (Luminetx Corp. Memphis, Tenn.), was used by Miyake et al and found to be useful in treating reticular veins.181 A study of 23 subjects with varicose veins and telangiectasia demonstrated that 100% had feeder veins which could be detected by the VeinViewer. These visualization techniques may be useful in allowing more efficient treatment of superficial veins. Near-infrared fluorescence venography using indocyanine green has also been demonstrated to be helpful in visualizing superficial veins prior to ambulatory phlebectomy.182
1 Thibault P, Bray A, Wlodarczyk J, Lewis J. Cosmetic leg veins: evaluation using duplex venous imaging. J Dermatol Surg Oncol. 1990;16:612.
2 Mclafferty SRB, Lambert AD. Diagnostic algorithm for telangiectasias, varicose veins, and venous ulcers: current guidelines. In Gloviczki P, editor: Handbook of chronic venous disorders, 3rd ed, London: Hodder Arnold, 2009.
3 Eklof B, Rutherford RB, Bergan JJ, et al. Revision of the CEAP classification. J Vasc Surg. 2004;40:1248.
4 Kistner RL, Eklof B. Classification and etiology of chronic venous diseases. In Gloviczki P, editor: Handbook of chronic venous disorders, 3rd ed, London: Hodder Arnold, 2009.
5 AbuRahma AF, Bergan JJ, editors. Noninvasive vascular diagnosis. New York: Springer-Verlag, 2000.
6 Hoare MC, Nicolaides AN, Miles CR, et al. The role of primary varicose veins in venous ulceration. Surgery. 1982;92:450.
7 Sethia KK, Darke SG. Long saphenous incompetence as a cause of venous ulceration. Br J Surg. 1984;71:754.
8 Nicolaides A, Christopoulous D, Vasdekis S. Progress in the investigation of chronic venous insufficiency. Ann Vasc Surg. 1989;3:278.
9 Hanrahan LM, Araki CT, Rodriguez AA. Distribution of valvular incompetence in patients with venous stasis ulceration. J Vasc Surg. 1991;13:805.
10 Shami SK, Sarin S, Cheatle TR, et al. Venous ulcers and the superficial venous system. J Vasc Surg. 1993;17:487.
11 Haeger D. The anatomy of the veins of the leg. In: Hobbs JT, editor. The treatment of venous disorders. Philadelphia: Lancaster MTP, 1977.
12 May R, Nissl R. Surgery of the veins of the leg and pelvis. In: May R, editor. Anatomy. Stuttgart: Thieme, 1979.
13 Browse NL, Burnand KG, Lea TM. Diseases of the veins: pathology, diagnosis, and treatment. London: Arnold; 1988.
14 Dodd H. The varicose tributaries of the popliteal vein. Br J Surg. 1965;52:350.
15 Hoare MC, Royle JP. Doppler ultrasound detection of saphenofemoral and saphenopopliteal incompetence and operative venography to ensure precise saphenopopliteal ligation. Aust N Z J Surg. 1984;54:49.
16 Sherman RS. Varicose veins: anatomy, reevaluation of Trendelenburg tests, and an operative procedure. Surg Clin North Am. 1964;44:1369.
17 Lofgren KA, Myers TT, Webb WD. Recurrent varicose veins. Surg Gynecol Obstet. 1956;102:729.
18 The Alexander House Group. Consensus paper on venous leg ulcer. J Dermatol Surg Oncol. 1992;18:592.
19 Neglen P, Raju S. Detection of outflow obstruction in chronic venous insufficiency. J Vasc Surg. 1993;17:583.
20 Tretbar LL. Bleeding from varicose veins: treatment with injection sclerotherapy. Abstract 3G 15. Presented at the 10th World Congress of Phlebology, Strasbourg, September 25–9, 1989.
21 Nabatoff RA. Simple palpation to detect valvular incompetence in patients with varicose veins. JAMA. 1955;159:27.
22 Dodd H, Cockett FB. The pathology and surgery of the veins of the lower limb, 2nd ed. London: Churchill Livingstone; 1986.
23 Knudtzen J, Gudmundsen TE, Svane S. Congenital absence of the entire inferior vena cava. Acta Chir Scand. 1986;152:541.
24 Scriven JM, Bianchi V, Hartshorne T, London NJM. Tourniquets cannot identify superficial venous reflux nor predict the haemodynamic outcome of saphenous vein surgery. Phlebology. 2002;16:154.
25 Kim J, Richards S, Kent PJ. Clinical examination of varicose veins – a validation study. Ann R Coll Surg Engl. 2000;82:171.
26 Chan A, Chisholm I, Royle JP. The use of directional ultrasound in the assessment of saphenofemoral incompetence. Aust N Z J Surg. 1983;53:399.
27 Schultz-Ehrenburg U. Functional sclerotherapy. Presented at the Second Annual Congress of the North American Society of Phlebology, New Orleans, February 25–6, 1989.
28 Schadeck M. Personal communication. September 1989.
29 Brodie B. Lecture illustrative of various subjects in pathology and surgery. London: Longman; 1846.
30 Trendelenburg F. Über die Unterbindung der Vena Saphena magna bei Unterschendelvaricen. Beitr Z Klin Chir. 1891;7:195.
31 Steiner CA, Palmer LH. A simplification of the diagnosis of varicose veins. Ann Surg. 1948;127:362.
32 Mahorner HR, Ochsner A. The modern treatment of varicose veins as indicated by the comparative tourniquet test. Ann Surg. 1938;7:927.
33 Sherman RS. Varicose veins: further findings based on anatomic and surgical dissections. Ann Surg. 1949;130:218.
34 Fegan WG. Compression sclerotherapy. Ann R Coll Surg Engl. 1967;41:364.
35 Fegan G. Varicose veins: compression sclerotherapy. London: Heinemann; 1967.
36 Beesley WH, Fegan WG. An investigation into the localization of incompetent perforating veins. Br J Surg. 1970;57:30.
37 O’Donnell TFJr, Burnand KG, Clemenson G, et al. Doppler examination vs clinical and phlebographic detection of the location of incompetent perforating veins. Arch Surg. 1977;112:31.
38 Townsend J, Jones H, Williams JE. Detection of incompetent perforating veins by venography at operation. Br Med J. 1967;3:583.
39 Patil KD, Williams JR, Williams KL. Thermographic localization of incompetent perforating veins in the leg. Br Med J. 1970;1:195.
40 Bracey DW. Simple device for location of perforating veins. Br Med J. 1958;2:101.
41 Perthes G. Über die Operation der Unterschenkelvaricen nach Trendelenburg. Deutsche Med Wehrschr. 1895;21:253.
42 Killewich LA, Martin R, Cramer M, et al. Pathophysiology of venous claudication. J Vasc Surg. 1984;1:507.
43 Anastasios JT. The physiology of venous claudication. Am J Surg. 1980;139:447. (abstract)
44 Tripolitis AJ, Milligan EB, Bodily KC, Strandness DEJr. The physiology of venous claudication. Am J Surg. 1980;139:447.
45 Fronek A. Noninvasive diagnostics in vascular disease. New York: McGraw-Hill; 1989.
46 Satomura S, Kaneko Z. Study of the flow patterns in peripheral arteries by ultrasonics. J Acoust Soc Japan. 1959;15:151.
47 Sumner DS, Baker DW, Strandness DEJr. The ultrasonic velocity detector in a clinical study of venous disease. Arch Surg. 1968;97:75.
48 Strandness DEJr, Schultz RD, Sumner DS, Rushmer RF. Ultrasonic flow detection: a useful technique in the evaluation of peripheral vascular disease. Am J Surg. 1967;113:311.
49 Felix WR, Sigel B. Doppler ultrasound diagnosis in vascular disease. Penn Med. 1972;75:67.
50 Sigel B, Popky GL, Wagner DK, et al. A Doppler ultrasound method for diagnosing lower extremity venous disease. Surg Gynecol Obstet. 1968;127:339.
51 Sigel B, Popky GL, Boland JP, et al. Augmentation flow sounds in the ultrasonic detection of venous abnormalities: a preliminary report. Invest Radiol. 1967;2:256.
52 Sigel B, Popky GL, Boland JP, et al. Diagnosis of venous disease by ultrasonic flow detection. Surg Forum. 1967;18:185.
53 Sigel B, Popky GL, Wagner DK, et al. Comparison of clinical and Doppler ultrasound evaluation of confirmed lower extremity venous disease. Surgery. 1968;64:332.
54 Sigel B, Popky GL, Mapp EM, et al. Evaluation of Doppler ultrasound examination: its use in diagnosis of lower extremity venous disease. Arch Surg. 1970;100:535.
55 George P, Pourcelot L, Fourcade C, et al. The Doppler effect and measurement of the blood flow [in French]. C R Acad Sci Hebd Seances Acad Sci D. 1965;261:253.
56 Krahenbuhl B, Restellini A, Frangos A. Peripheral venous pulsatility detected by Doppler method for diagnosis of right heart failure. Cardiology. 1984;71:173.
57 Folse R. The influence of femoral vein dynamics on the development of varicose veins. Surgery. 1970;68:974.
58 Araki CT, Back TL, Padberg FTJr, et al. Refinements in the ultrasonic detection of popliteal vein reflux. J Vasc Surg. 1993;18:742.
59 Barnes RW, Russell HE, Wu KK, et al. Accuracy of Doppler ultrasound in clinically suspected venous thrombosis of the calf. Surg Gynecol Obstet. 1976;143:425.
60 Sumner DS, Lambeth A. Reliability of Doppler ultrasound in the diagnosis of acute venous thrombosis both above and below the knee. Am J Surg. 1979;130:218.
61 Ackroyd JS, Lea Thomas M, Browse NL. Deep venous reflux: an assessment by descending venography. Br J Surg. 1986;73:31.
62 Bishop C. Personal communication. September 1989.
63 Guex J-J, Hiltbrand B, Bayon J-M, et al. Anatomical patterns in varicose vein disease: a duplex scanning study. Phlebology. 1995;10:94.
64 Schultz-Ehrenburg U, Hubner HJ. Reflux diagnosis with Doppler ultrasound. In: Findings in angiology and phlebology. New York: FK Schattauer Verlag; 1989.
65 Barnes RW, Russell HE, Wilson MR. Doppler ultrasonic evaluation of venous disease – a programmed audiovisual instruction, 2nd ed. Iowa City: University of Iowa; 1975.
66 Stonebridge PA, Ruckley CV, Harper DR, et al. Comparison of Doppler and duplex scanning for the evaluation of valvular reflux within the veins of the lower limb. Abstract 4S 09:20. Presented at the 10th World Congress of Phlebology, Strasbourg, September 25–9, 1989.
67 Bishop C, Fronek HS, Dilley RB, Bernstein EF. Real-time color duplex scanning after sclerotherapy of the greater saphenous vein. J Vasc Surg. 1991;14:505.
68 Masuda EM, Kistner KL, Eklof B. Prospective study of duplex scanning for venous reflux: comparison of Valsalva and pneumatic cuff techniques in the reverse Trendelenburg and standing position. J Vasc Surg. 1994;20:711.
69 Nicolaides A, Christopoulos D, Vasdekis S, et al. A comparison of duplex scanning, Doppler ultrasound, and perioperative venography in assessing the termination of the short saphenous vein. Abstract 25 ii2. Presented at the Fourth European-American Symposium on Venous Diseases, Washington DC, March 31–April 2, 1987.
70 Partsch H. Investigations on the pathogenesis of venous leg ulcers. Acta Chir Scand Suppl. 1988;544:25.
71 Shull KC, Nicolaides AN, Fernandes E, Fernandes J, et al. Significance of popliteal reflux in relation to ambulatory venous pressure and ulceration. Arch Surg. 1979;114:1304.
72 Darke SG, Vetrievel S, Foy DMA. A comparison of duplex scanning and continuous-wave Doppler in the assessment of primary and uncomplicated varicose veins. Eur J Vasc Endovasc Surg. 1997;14:457.
73 Ziegenbein W, Myers KA, Matthews PG, Zeng GH. Duplex ultrasound scanning for chronic venous disease. I. Techniques for examination of the crural veins. Phlebology. 1994;9:104.
74 Van Bemmelen PS, Bedford G, Beach K, Strandness DE. Quantitative segmental evaluation of venous valvular reflux with duplex ultrasound scanning. J Vasc Surg. 1989;10:425.
75 Rose SR, Ahmed A. Some thoughts on the aetiology of varicose veins. J Cardiovasc Surg. 1986;27:534.
76 Goren G, Yellin AE. Primary varicose veins: topographic and hemodynamic correlations. J Cardiovasc Surg. 1990;31:672.
77 Abu-Own A, Scurr JH, Coleridge-Smith PD. Saphenous vein reflux without saphenofemoral or saphenopopliteal junction incompetence. Presented at the Seventh Annual Congress of the North American Society of Phlebology, Maui, February 21–3, 1994.
78 Rettori R. Recurrence of varicose veins due to the incompetence of the perforating veins at the medial aspect of the thigh. Abstract W51–18. Presented at the Ninth World Congress of Phlebology, Kyoto, 1986.
79 Labropoulos N, Giannoukas AD, Delis K, et al. Where does venous reflux start? J Vasc Surg. 1997;26:736.
80 Folse A, Alexander RH. Directional flow detection for localizing venous valvular incompetency. Surgery. 1970;67:114.
81 Miller SS, Foote AV. The ultrasonic detection of incompetent perforating veins. Br J Surg. 1974;61:653.
82 Foote AV, Miller SS. Ultrasonic flow probe detection of incompetent perforating veins. Scott Med J. 1969;14:96.
83 O’Donnell TFJr, Burnand KG, Clemenson G, et al. Doppler examination vs clinical and phlebographic detection of the location of incompetent perforating veins: a prospective study. Arch Surg. 1977;112:31.
84 Zweibel WJ, editor. Introduction to vascular ultrasonography, 2nd ed, Philadelphia: Saunders, 1986.
85 Knight RM, Zygmunt JA. Ultrasonic detection of the efficacy of injection sclerotherapy of the saphenofemoral junction. Abstract 3G 15. Presented at the 10th World Congress of Phlebology, Strasbourg, September 25–9, 1989.
86 Schadeck M. Sclerotherapy of the long saphenous veins: methodology and results controlled by echo-Doppler on 400 patients. Abstract 2S 15:30. Presented at the 10th World Congress of Phlebology, Strasbourg, September 25–9, 1989.
87 Raymond-Martimbeau P. Duplex ultrasonography, color flow Doppler and magnetic resonance imaging in phlebology. Abstract 1T 15:50. Presented at the 10th World Congress of Phlebology, Strasbourg, September 25–9, 1989.
88 Day TK, Rish PJ, Kakkar VV. Detection of deep vein thrombosis by Doppler angiography. Br Med J. 1976;1:618.
89 Sullivan ED, Peter DJ, Cranley JJ. Real-time B-mode venous ultrasound. J Vasc Surg. 1984;1:465.
90 Raghavendra BN, Horii SC, Hilton S, et al. Deep venous thrombosis: detection by probe compression of veins. J Ultrasound Med. 1986;5:80.
91 Talbot SR. Use of real time imaging in identifying deep venous obstruction: a preliminary report. Bruit. 1982;1:41.
92 Hannan LJ, Stedje KJ, Skorcz MJ, et al. Venous imaging of the extremities: our first 2500 cases. Bruit. 1986;10:29.
93 Flanagan LD, Sullivan ED, Cranley JJ. Venous imaging of the extremities using real-time B-mode ultrasound. In: Bergan JJ, Yao JST, editors. Surgery of the veins. Orlando: Grune & Stratton, 1984.
94 Labropoulos N, Tiongson J, Pryor L, et al. Definition of venous reflux in lower-extremity veins. J Vasc Surg. 2003;38:793.
95 Hobbs JT, et al. Comparison of clinical examination, Doppler ultrasound, and color duplex scanning with perioperative venography in the assessment of the short saphenous vein termination. Presented at the Second Annual Congress of the North American Society of Phlebology, New Orleans, February 25–6, 1989.
96 Hobbs JT. Perioperative venography to ensure accurate sapheno-popliteal vein ligation. Br Med J. 1980;280:1578.
97 Corecos L, Romeo V, Fiori C, et al. Intra-operative phlebography of the short saphenous vein. Phlebology. 1987;2:241.
98 Vasdekis SN, Clarke GH, Hobbs JT, et al. Evaluation of noninvasive and invasive methods in the assessment of short saphenous vein termination. Br J Surg. 1989;76:929.
99 Somjen GM, Royle JP, Fell G, et al. Venous reflux patterns in the popliteal fossa. J Cardiovasc Surg. 1992;33:85.
100 Knight RM, Vin F, Zygmunt JA. Ultrasonic guidance of injections into the superficial venous system. Abstract 4S 14:50. Presented at the 10th World Congress of Phlebology, Strasbourg, September 25–9, 1989.
101 Kanter A Grondin L, Soriano J. Echosclerotherapy: a Canadian Study. In: Raymond-Martinbeau P, Prescott R, Zumme M, editors. Phlébologie ’92. Paris: John Libbey Eurotext, 1992.
102 Thibault PK, Lewis WA. Recurrent varicose veins: injection of incompetent perforating veins using ultrasound guidance. J Dermatol Surg Oncol. 1992;18:895.
103 Perrin M. Venous leg perforating veins [in French]. J Mal Vasc. 1999;24:19.
104 McMullin G, Appleberg M. Measurement of venous flow rates by duplex Doppler ultrasound. Presented at the Seventh Annual Congress of the North American Society of Phlebology, Maui, February 1994.
105 Caggiati A. Fascial relationships of the long saphenous vein. Circulation. 1999;100:2547.
106 Vasdekis SN, Clarke GH, Nicolaides AN. Quantification of venous reflux by means of duplex scanning. J Vasc Surg. 1989;10:670.
107 Christopoulos D, Nicolaides AN, Szendro G. Venous reflux: quantitation and correlation with the clinical severity of chronic venous disease. Br J Surg. 1988;75:352.
108 Abramowitz HB, Queral LA, Finn WR, et al. The use of photoplethysmography in the assessment of venous insufficiency: a comparison to venous pressure measurements. Surgery. 1979;86:434.
109 Pearce WH, Ricco JB, Queral LA, et al. Hemodynamic assessment of venous problems. Surgery. 1983;93:715.
110 Barnes RW, Garrett WV, Humel B, et al. Photoplethysmographic assessment of altered cutaneous circulation in the postphlebitic syndrome. Proceedings of the 13th Annual Meeting of AAMI, Washington DC, March 28–April 1, 1978.
111 Killewich LA, Martin R, Cramer M, et al. An objective assessment of the physiologic changes in the postthrombotic syndrome. Arch Surg. 1985;120:424.
112 Dohn K. Plethysmography during functional states for investigation of the peripheral circulation. Proceedings of the Second International Congress of Physical Medicine, Copenhagen, 1957.
113 Bygdeman S, Aschberg S, Hindmarsh T. Venous plethysmography in the diagnosis of chronic venous insufficiency. Acta Chir Scand. 1971;137:423.
114 McMullin G, Coleridge-Smith P, Scurr J. An assessment of pneumatic tourniquets. Personal communication, 1990.
115 Fronek A. Recent developments in venous photoplethysmography. In Bernstein EF, editor: Vascular diagnosis, 4th ed, St Louis: Mosby, 1993.
116 Nicolaides AN, Hussein MK, Szendro G, et al. The relation of venous ulceration with ambulatory venous pressure measurements. J Vasc Surg. 1993;17:414.
117 Barnes RW, Yao JST. Photoplethysmography in chronic venous insufficiency. In Bernstein EF, editor: Noninvasive diagnostic techniques in vascular disease, 2nd ed, St Louis: CV Mosby, 1982.
118 Norris CS, Beyrau A, Barnes RW. Quantitative photoplethysmography in chronic venous insufficiency: a new method of noninvasive estimation of ambulatory venous pressure. Surgery. 1983;94:758.
119 Fronek A. Photoplethysmography in the diagnosis of venous disease. Dermatol Surg. 1995;21:64.
120 Van Bemmelen PS, van Ramshorst B, Eikelboom BC. Photoplethysmography reexamined: lack of correlation with duplex scanning. Surgery. 1992;112:544.
121 Fronek A, Minn C, Kim R. Venous outflow and inflow resistance in health and venous disease. J Vasc Surg. 2000;31:472.
122 Fronek A, Vanderweijer I. Noninvasive determination of venomuscular efficiency. J Vasc Surg. 2003;37:839.
123 Sam RC, Darvall KAL, Adam DJ, et al. Digital venous photoplethysmography in the seated position is a reproducable noninvasive measure of lower limb venous function in patients with isolated superficial venous reflux. J Vasc Surg. 2006;43:335.
124 Shepard AD, Machey WL, O’Donnell TF, Heggerich PA. Light reflection rheography (LRR): a new non-invasive test of venous function. Bruit. 1984;8:266.
125 Hubner K. Is the light reflection rheography (LRR) suitable as a diagnostic method for the phlebology practice? Phlebol Proktol. 1986;15:209.
126 Kerner J, Schultz-Ehrenburg U, Blazek V. Digitale photoplethysmograph (D-PPG). Phlebol Proktol. 1989;18:98.
127 Kerner J, Schultz-Ehrenburg U, Blazek V. First clinical experiences on two new plethysmographic measuring systems: digital-PPG and computer aided gravimetric plethysmography (CGP). Abstract 5T 08:40. Presented at the 10th World Congress of Phlebology, Strasbourg, September 25–9, 1989.
128 Hemodynamics Inc: Guidelines for measuring venous emptying with LRR as validated by the University of Miami. Personal communication, 1989.
129 Christopoulos D, Nicolaides AN. Air plethysmography in the assessment of the calf muscle pump in man. J Phys. 1986;374:11.
130 Christopoulos DG, Nicolaides AN, Szendro G, et al. Air plethysmography and effect of elastic compression on venous hemodynamics of the leg. J Vasc Surg. 1987;5:148.
131 Nicolaides AN, Christopoulos D. Diagnosis and quantitation of venous reflux. In: Baccolon H, editor. Angiologie. Paris: John Libbey Eurotext, 1988.
132 Christopoulos D, Nicolaides AN, Cook A, et al. Pathogenesis of venous ulceration in relation to calf muscle pump function. Surgery. 1989;106:829.
133 Christopoulos DG, Nicolaides AN. Noninvasive diagnosis and quantitation of popliteal reflux in the swollen and ulcerated leg. J Cardiovasc Surg. 1988;29:535.
134 Van Bemmelen PS, Mattos MA, Hodgson KJ, et al. Does air plethysmography correlate with Duplex scanning in patients with chronic venous insufficiency? J Vasc Surg. 1993;18:796.
135 Neglen P, Raju S. A rational approach to detection of significant reflux with duplex Doppler scanning and air plethysmography. J Vasc Surg. 1993;17:590.
136 Welkie JF, Kerr RP, Katz ML, Comerota AJ. Can noninvasive venous volume determinations accurately predict ambulatory venous pressure? J Vasc Technol. 1991;15:198.
137 Bundens WP. Use of air plethysmography in the evaluation and treatment of patients with venous stasis disease. Dermatol Surg. 1995;21:67.
138 Bays RA, Healy DA, Atnip RG, et al. Validation of air plethysmography, photoplethysmography, and duplex ultrasonography in the evaluation of severe venous stasis. J Vasc Surg. 1994;20:721.
139 Van Bemmelen PS, Mattos MA, Hodgson KJ, et al. Does air plethysmography correlate with duplex scanning in patients with venous insufficiency? J Vasc Surg. 1993;18:796.
140 Norgren L. Functional evaluation of chronic venous insufficiency by foot volumetry. Acta Chir Scand Suppl. 1974;444:1.
141 Kakkar VV, Lawrence DA. Venous pressure measurement and foot volumetry in venous disease. Int Angiol. 1982;1:87.
142 Thulesius O, Norgren L, Gjores JE. Foot-volumetry, a new method for objective assessment of edema and venous function. Vasa. 1973;2:325.
143 Norgren L, Thulesius O, Gjores JE, Soderlundh S. Foot-volumetry and simultaneous venous pressure measurements for evaluation of venous insufficiency. Vasa. 1974;3:140.
144 Lawrence D, Kakkar VV. Post-phlebitic syndrome – a functional assessment. Br J Surg. 1980;67:686.
145 Norgren L. Foot-volumetry before and after surgical treatment of patients with varicose veins. Acta Chir Scand. 1975;141:129.
146 Perrin M, Guex J-J. Edema and leg volume: methods of assessment. Angiology. 2000;51:9.
147 Camerota AJ, Datz ML, Derr RP. Variability of venous valve function with daily activity. J Vasc Surg. 1993;17:440.
148 Carpenter JP, Holland GA, Baum RA, et al. Magnetic resonance venography for the detection of deep venous thrombosis: comparison with contrast venography and duplex Doppler ultrasonography. J Vasc Surg. 1993;18:734.
149 Barnes RW, Ross EA, Strandness DEJr. Differentiation of primary from secondary varicose veins by Doppler ultrasound and strain gauge plethysmography. Surg Gynecol Obstet. 1975;141:207.
150 Spence RK, Cahall EJ, Alexander JB, et al. Compression therapy fails to relieve venous claudication: a plethysmographic analysis of treatment failure and guidelines for surgical treatment. J Vasc Surg. 1993;17:432.
151 Thomas ML, Bowles JN. Descending phlebography in the assessment of long saphenous vein incompetence. Am J Radiol. 1985;145:1255.
152 DePalma RG, Hart MT, Zanin L, Massarin EH. Physical examination, Doppler ultrasound, and colour flow duplex scanning: guides to therapy for primary varicose veins. Phlebology. 1993;8:7.
153 Iafrati MD, O’Donnell TFJr, Kunkemueller A, et al. Clinical examination, duplex ultrasound, and plethysmography for varicose veins. Phlebology. 1994;9:114.
154 Burnand KG, O’Donnell TFJr, Thomas ML, Browse NL. The relative importance of incompetent communicating veins in the production of varicose veins and venous ulcers. Surgery. 1977;82:9.
155 Papadakis K, Christodoulou C, Christopoulos D, et al. Number and anatomical distribution of incompetent thigh perforating veins. Br J Surg. 1989;76:581.
156 Dodd H. The diagnosis and ligation of incompetent ankle perforating veins. Ann R Coll Surg Engl. 1964;34:186.
157 Noble J, Gunn AA. Varicose veins: comparative study of methods of detecting incompetent perforators. Lancet. 1972;299:1253.
158 Elem B, Shorey BA, Williams KL. Comparison between thermography and fluorescein test in the detection of incompetent perforating veins. Br Med J. 1971;4:651.
159 Thomas ML, McAllister V, Rose DH, Tonge K. A simplified technique of phlebography for the localization of incompetent perforating veins of the legs. Clin Radiol. 1972;23:486.
160 Miller SS, Crossman JA, Foote AV. The ultrasound detection of incompetent perforating veins. Br J Surg. 1971;58:872.
161 Sarin S, Scurr JH, Coleridge-Smith PD. Medial calf perforators in venous disease: the significance of outward flow. J Vasc Surg. 1992;16:40.
162 Perrin MR, Guex J-J, Ruckley CV, et al. Recurrent varices after surgery, a consensus document. and the REVAS group]. Cardiovasc Surg. 2000;8:233.
163 Perrin M, Gillet JL. Management of recurrent varices at the popliteal fossa after surgical treatment. Phlebology. 2008;23:64.
164 Liddle AD, Davies AH. Pelvic congestion syndrome: chronic pelvic pain caused by ovarian and internal iliac varices. Phlebology. 2007;22:100.
165 Greiner M, Gilling-Smith GL. Leg varices originating from the pelvis: diagnosis and treatment. Vascular. 2007;15:70.
166 Lea Thomas M, Mahraj RPM. A comparison of varicography and descending phlebography in clinically suspected recurrent groin and upper thigh varicose veins. Phlebology. 1988;3:155.
167 Chilvers AS, Thomas MH. A method for the localization of incompetent ankle perforating veins. Br Med J. 1970;2:577.
168 Herman RJ, Neiman HL, Yao JST. Descending venography: a method of evaluating lower extremity valvular function. Radiology. 1980;137:63.
169 Moore DF, Himmel PD, Sumner DS. Distribution of venous valvular incompetence in patients with postphlebitic syndrome. J Vasc Surg. 1986;3:49.
170 Thiery L. Varicose veins as a result of gastrocnemial vein pathology: a 20-year survey. Presented at the Second Annual Congress of the North American Society of Phlebology, New Orleans, February 25–6, 1989.
171 Perrin M, Bolot JE, Genevois A, Hiltband B. Dynamic popliteal phlebography [in French]. Phlebologie. 1988;41:429.
172 Begg AC. Intraosseus venography of the lower limb and pelvis. Br J Radiol. 1954;27:318.
173 Lea Thomas M, Posniak HV. Varicography. Int Angiol. 1985;4:475.
174 Lea Thomas M, Keeling FP. Varicography in the management of recurrent varicose veins. Angiology. 1986;37:570.
175 Rosenberg N, Stefanides A. Thermography in the management of varicose veins and venous insufficiency. Ann NY Acad Sci. 1964;122:113.
176 Williams KL. Infrared thermography as a tool in medical research. Ann NY Acad Sci. 1964;121:99.
177 Bergqvist B, Halböök T, Lindhagen A. Thermography: non-invasive diagnostic method for the detection of arterio-venous anastomoses in varicosis. Vasa. 1979;8:63.
178 De Zeeuw R, Noordmans HJ, Verdaasdonk RM, Wittens CHA. Objective non-invasive technique for quantification of superficial varicose veins. Phlebology. 2005;20:60.
179 Zharov VP, Ferguson S, Eidt JF, et al. Infrared imaging of subcutaneous veins. Lasers Surg Med. 2004;34:56.
180 Bustos LL, Fronek A, Lopez-Kapke L, Henriquez JA. Total reticular vision, a non-invasive method that exposes panoramically non visible insufficient subcutaneous reticular venous plexus. Eur J Vasc Endovas Surg. 2010. (in press)
181 Miyake RK, Zeman HD, Duarte FH, et al. Vein imaging: a new method of near infrared imaging, where a processed image is projected onto the skin for the enhancement of vein treatment. Dermatol Surg. 2006;32:1031.
182 Kikuchi M, Hosokawa K. Near-infrared fluorescence venography: a navigation system for varicose surgery. Dermatol Surg. 2009;35:1495.