5 Non-permanent fillers
Belotero®/Esthélis® and Teosyal®
Summary and Key Features
• Belotero®, Esthelis®, and Teosyal® are three hyaluronic acid families produced in Switzerland
• Good clinical data are available only for Belotero® Basic and Teosyal® Deep Lines
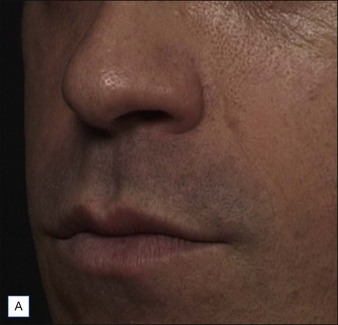
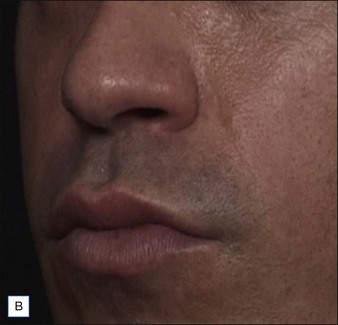
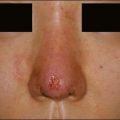
• At 6 months Belotero® Basic and Teosyal® Deep Lines still show at least a one-grade difference after treatment of the nasolabial folds
• Belotero® Basic and Teosyal® Deep Lines have a good safety profile
Hyaluronic acid dermal fillers
How to differentiate the different HA fillers? There are certain possibilities:
Belotero®
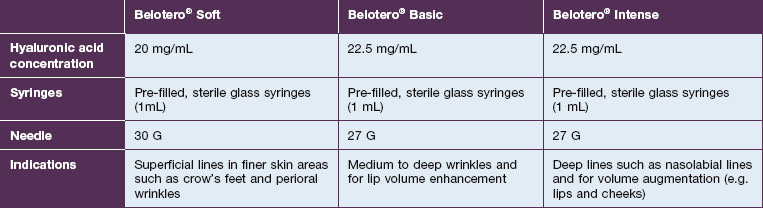
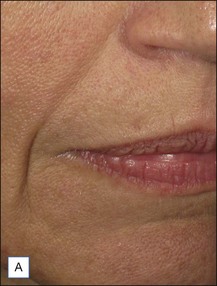
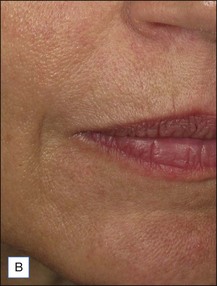
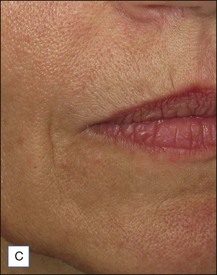
Belotero® is produced by the Swiss company Anteis SA and distributed by Merz Pharmaceuticals. The stabilizer is BDDE and according to the manufacturer two cross-linking processes are used leading to a cohesive and polydensified matrix, which is supposed to ease injection while maintaining long-lasting results. Belotero® offers three different formulations: Belotero® Soft, Belotero® Basic and Belotero® Intense (Table 5.1, Figs 5.1 and 5.2).
Esthélis®
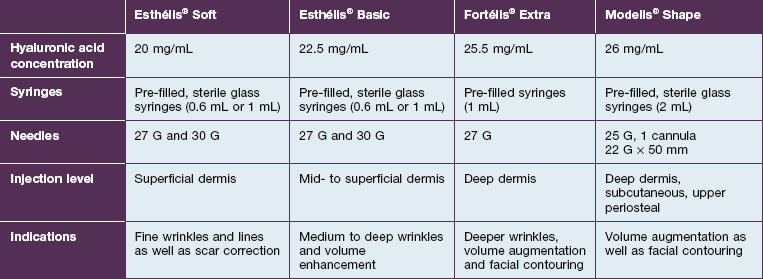
The Esthélis brand is also produced by Anteis SA. The Esthélis® range provides three different main products: Fortélis® Extra, Esthélis® Soft, and Esthélis® Basic (Table 5.2). In addition, as a filler for volume augmentation, Modelis® has been added to the Esthélis® range. No good clinical data – not even a case series – are available for this family of fillers. It might be probably safe to assume that they are similar to the Belotero® group of fillers.
Teosyal®
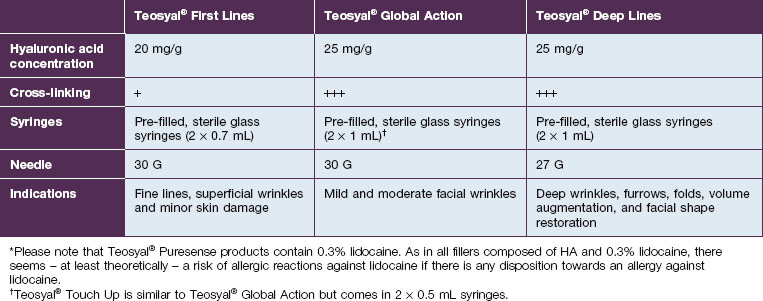
Teoxane is the manufacturer of the HA filler family Teosyal® (Tables 5.3 and 5.4, Figs 5.1 and 5.2). Like Belotero® only one good clinical trial has been published, by Nast and co-workers. No large case series on this product family could be found.
| Teosyal® Ultimate | Teosyal® Kiss | |
|---|---|---|
| Hyaluronic acid concentration | 22 mg/g | 25 mg/g |
| Cross-linking | ++++ | +++ |
| Syringes | Pre-filled, sterile glass syringes (1 × 3 mL) | Pre-filled, sterile glass syringes (2 × 1 mL) |
| Needle | 23 G | 27 G |
| Indications | Volume augmentation and remodeling of facial contours | Lip contour and volume |
* Please note that Teosyal® Puresense products contain 0.3% lidocaine.
Dirting K, Lampe H, Wolters M, et al. [Hyaluronic acid filler for correction of nasolabial grooves – results of a clinical study]. Journal der Deutschen Dermatologischen Gesellschaft [Journal of the German Society of Dermatology]. 2008;6(suppl 2):S10–S14.
Narins RS, Coleman W, Donofrio L, et al. Nonanimal sourced hyaluronic acid-based dermal filler using a cohesive polydensified matrix technology is superior to bovine collagen in the correction of moderate to severe nasolabial folds: results from a 6-month, randomized, blinded, controlled, multicenter study. Dermatologic Surgery. 2010;36(suppl 1):730–740.
Narins RS, Coleman WP, 3rd., Donofrio LM, et al. Improvement in nasolabial folds with a hyaluronic acid filler using a cohesive polydensified matrix technology: results from an 18-month open-label extension trial. Dermatologic Surgery. 2010;36(suppl 3):1800–1808.
Nast A, Reytan N, Hartmann V, et al. Efficacy and durability of two hyaluronic acid-based fillers in the correction of nasolabial folds: results of a prospective, randomized, double-blind, actively controlled clinical pilot study. Dermatologic Surgery. 2011;37:768–775.
Pavicic T. Efficacy and tolerability of a new monophasic, double-crosslinked hyaluronic acid filler for correction of deep lines and wrinkles. Journal of Drugs in Dermatology. 2011;10:134–139.