Non-Ocular Sources for Cell-Based Ocular Surface Reconstruction
Development of Cultivated Oral Mucosal Epithelial Transplantation (COMET, Preclinical Trail)
The Successful Culture of Rabbit Oral Mucosal Epithelial Cells
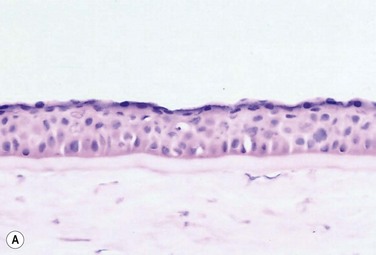
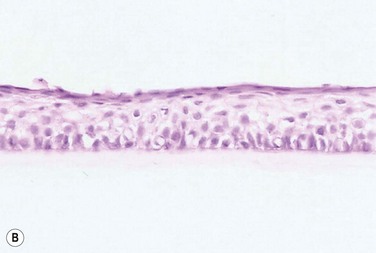
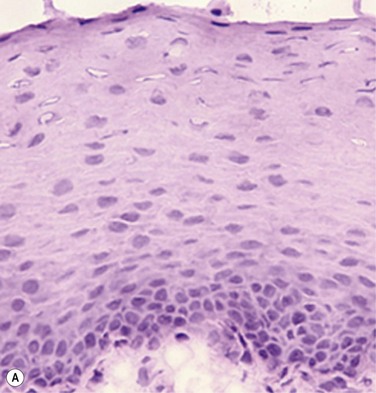
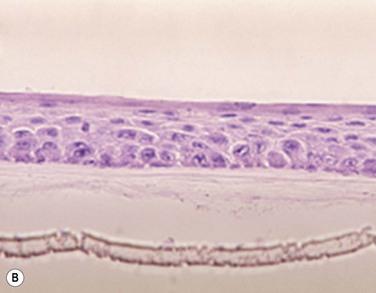
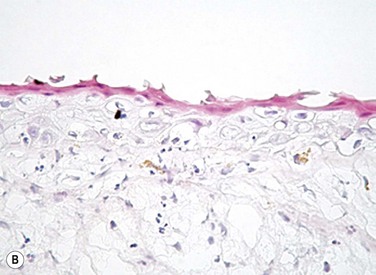
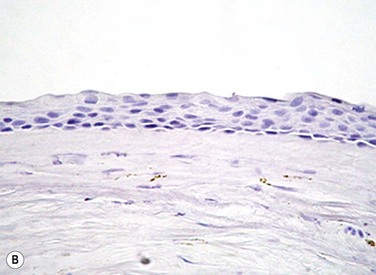
The problem of allograft rejection is the main reason that we decided to develop a new method of autologous oral mucosal epithelium transplantation for OSR.1 In our laboratory experiment, epithelial cells from the oral mucosa began to form colonies on the denuded AM within 3 days. At 3 weeks, the cultivated oral epithelial cells showed 4–5 layers of stratification, were well differentiated (Fig. 45.1A), and appeared very similar to in vivo normal corneal epithelium (Fig. 45.1B).1
Cell Biological Characteristics of the Rabbit Cultivated Oral Mucosal Epithelial Sheet
Cytokeratins play an important structural and protective role in maintaining the integrity of the epithelium of the anterior segment of the eye. Defined subsets of individual cytokeratin pairs are characteristically expressed, depending on the type of epithelial cell tissue and level of differentiation. In this study, we used immunohistochemistry to demonstrate that the keratin 1/10 pair, which is involved in the physiological keratinization process in the epidermis, is not expressed in any layers of the cultivated oral epithelial sheet. We also found that the keratin 4/13 pair, which is observed in nonkeratinized, stratified epithelia, is expressed in the superficial and intermediate layers of the cultivated oral epithelial cells. These results led us to believe that the oral epithelial cells cultivated on AM have the characteristics of nonkeratinized mucosa, not keratinized mucosa. Immunohistochemical examination revealed no cornea-specific keratin 12 expression in any layers of the cultivated oral epithelial sheets (Fig. 45.2A), whereas cornea-specific keratin 3 was expressed in all epithelial layers of the cultivated oral epithelial sheet (Fig. 45.2B). Even though the cells of the cultivated oral epithelial sheet are not able to become corneal epithelial cells, we suggest that they might have the potential ability to become cornea-like epithelial cells under proper in vitro culture conditions.
Morphological Characteristics of the Rabbit Cultivated Oral Mucosal Epithelial Sheet
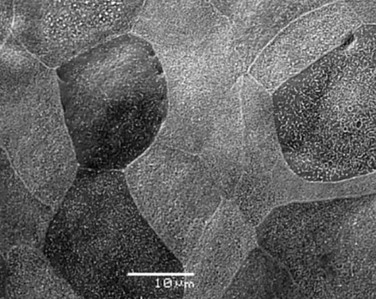
Electron microscopic results of the cultivated oral mucosal epithelial sheet are of particular interest. Examination by use of a scanning electron microscope (SEM) revealed that rabbit oral epithelial cells appeared healthy and well formed with tightly opposed cell junctions (Fig. 45.3). The cultivated oral cells were similar in size and appearance to rabbit corneal epithelial cells. Transmission electron microscopy (TEM) confirmed that the cultivated oral epithelial sheet was very similar in appearance to that of corneal epithelium, and very different from conjunctiva and oral mucosa. Like corneal epithelium, it had 4–5 layers of stratified cells which were differentiated into columnar, wing, and squamous cells (Fig. 45.4). Both our SEM and TEM results show clearly that our oral mucosal cells, cultivated on AM, resemble normal corneal epithelial cells more closely than any other cell type.
Successful Transplantation of the Rabbit Cultivated Oral Mucosal Epithelial Sheet
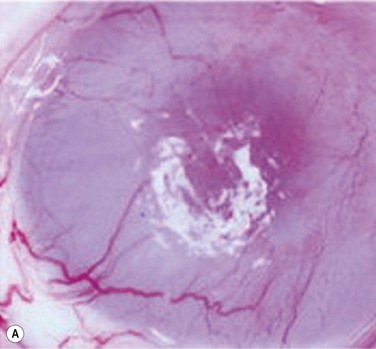
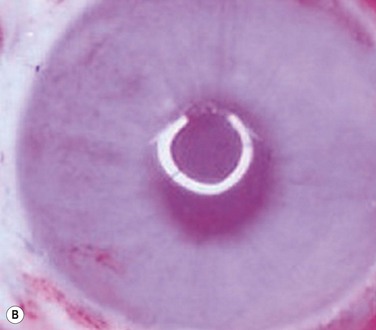
After the successful culture of rabbit oral mucosal epithelial cells on AM, we tried to reconstruct the damaged corneal surfaces by transplantation of autologous cultivated oral mucosal epithelial cells in order to test the viability of using these cells as a substitute for cultivated corneal epithelial cells. At 48 hours after surgery, most of the area of the transplanted cultivated oral mucosal epithelial sheet possessed intact epithelium. At 10 days after transplantation, the ocular surface covered by the transplanted epithelium was intact and without defects, thus suggesting that the autologous transplantation of cultivated oral mucosal epithelia is a viable procedure for ocular surface reconstruction (Fig. 45.5). Histological examination of the transplanted sheets at 10 days after surgery revealed that the sheets were well adhered to the host corneal stroma, with no evidence of subepithelial cell infiltration or stromal edema. Superficial cells of the transplanted sheets had nuclei, indicating that they were indeed nonkeratinized mucosal epithelial cells.
The Successful Culture of Human Oral Mucosal Epithelial Cells
We next focused our attention on cultivating human oral mucosal epithelial cells using our previously reported culture methods for rabbit oral mucosal epithelial cells, yet with several modifications. It should be noted that it was quite difficult to cultivate human oral mucosal epithelial cells using the previously reported culture technique for rabbit oral epithelial cells, and therefore, the culture process did require some modification. In the end, and as a result of the modification, we were able to successfully generate a well-stratified and differentiated human cultivated oral mucosal epithelial sheet (Fig. 45.6). Light microscopy revealed that the human cultivated oral mucosal epithelial cells were very similar in appearance to normal corneal epithelial cells. Moreover, immunohistochemistry confirmed the presence of keratins 4/13 and 3 in the human cultivated oral mucosal epithelial cells, similar to those found in the rabbit model.
Transplantation of Cultivated Oral Mucosal Epithelial Cells in Patients with Severe OSD (Clinical Trial)
In the past, several researcher groups investigated the possibility of using oral mucosa for OSR. Ballen reported that oral mucosal grafts which included both epithelium and subepithelial tissues, heavily vascularized with early fibrosis.2 In addition, Gipson et al. reported that in vivo oral epithelium, freed of underlying connective tissue, was not maintained in the central avascular corneal regions.3
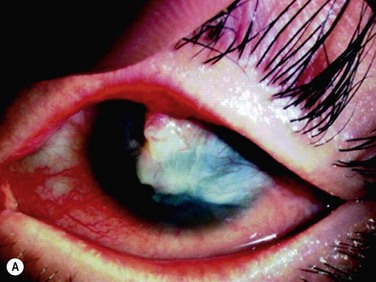
We investigated the possibility of reconstructing the human ocular surface using autologous mucosal epithelium of non-ocular-surface origin. Using rabbits, we have already established a surgical method for transplanting cultivated autologous oral mucosal epithelial cells.1 We next applied this method in six eyes of four patients with severe OSD.4
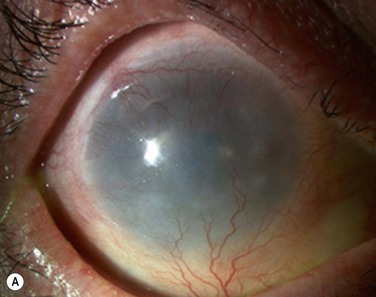
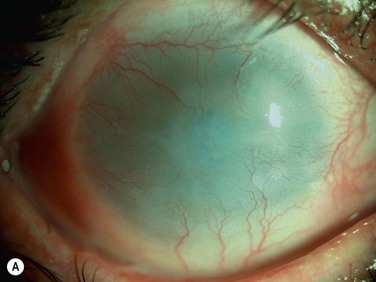
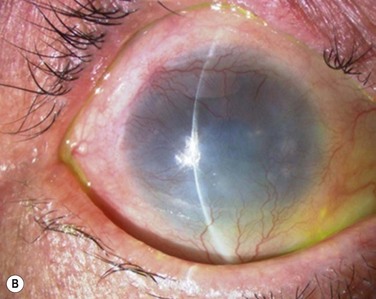
At 48 hours post transplant, the entire corneal surface of all six eyes was free of epithelial defects, indicating complete survival of the transplanted cultivated oral mucosal epithelium. Visual acuity was improved in all eyes. During follow-up (13.8 ± 2.9 months), the ocular surface remained stable, although all eyes showed mild peripheral neovascularization. Therefore, autologous cultivated oral mucosal epithelial sheets can be transplanted to treat severe OSD (Fig. 45.7). This initial clinical study represents a first step toward assessing the feasibility of transplanting autologous cultivated epithelial transplants of non-ocular-surface origin.
Mid-Term Clinical Results
We next present the mid-term clinical data on 15 eyes of 12 patients transplanted with cultivated autologous oral mucosal epithelial sheets.5 We also assessed their clinical outcomes, with special reference to postoperative neovascularization.
Phenotypic Investigation of the Transplanted Cultivated Oral Mucosal Epithelial Sheets
Our initial and mid-term clinical assessments of COMET yielded favorable results from the perspective of corneal surface stabilization;4,5 longevity and phenotypic analyses of the cultivated oral mucosal epithelial sheets to the corneal surface needs to be performed. Since it had not been confirmed what happens to failed and successful grafts on the corneal surface, we compared our clinical observations with the results of the cellular phenotype analysis of autologous COMET.6
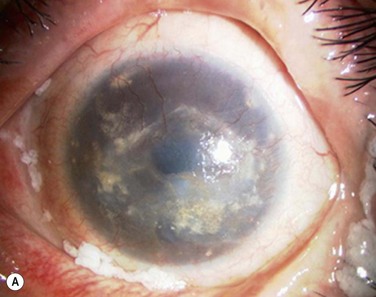
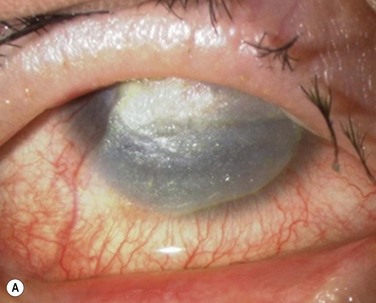
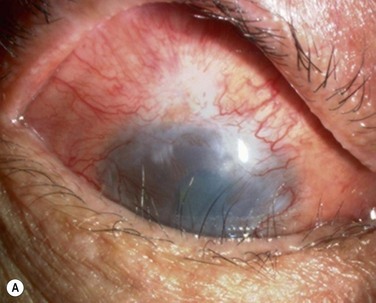
In the clinically failed transplants, electron microscopy (EM) and immunohistochemical analysis showed only small areas where the original cultivated oral mucosal epithelial sheets persisted. Surrounding conjunctival epithelial cells had apparently invaded a large portion of the corneal surface (oral mucosal epithelial marker keratin 3(−), goblet cell marker Muc5ac (+) ). Our clinical, ultrastructural, and cell biological findings revealed that the process of graft failure was responsible for the loss of cultivated oral mucosal epithelial sheets, due to postoperative bacterial infections and that this event was followed by neighboring conjunctival invasion onto the corneal surface (Fig. 45.8).
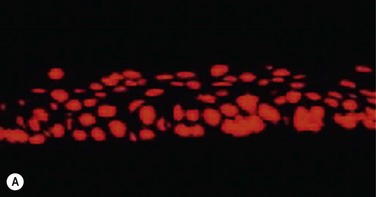
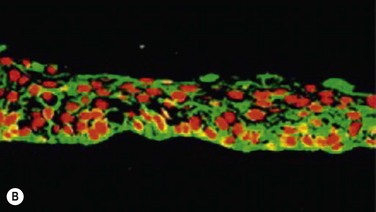
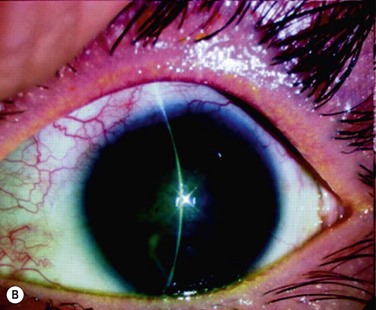
In the clinically successful transplants, the cultivated oral mucosal epithelial sheets survived and had adapted well to the host corneal tissues (oral mucosal epithelial marker keratin 3 (+), goblet cell marker Muc5ac (−) ). In the course of postoperative follow-up, their distinctive fluorescein staining patterns made it easy to distinguish transplanted cultivated oral mucosal epithelial sheets from surrounding conjunctival epithelium. The staining pattern of cultivated oral mucosal epithelial cells is more like that of superficial punctate keratopathy than of conjunctival epithelium. This set of findings confirmed that transplanted cultivated oral mucosal epithelial cells can survive on the ocular surface and maintain ocular surface integrity (Fig. 45.9).
Long-Term Clinical Results in the Scar Phase of Severe OSDs
More recently, our experimental and serial clinical studies demonstrated the efficacy and usefulness of COMET for the treatment of severe OSD. Even though initial and mid-term clinical results of COMET have been reported from several groups worldwide,7,8 the long-term clinical assessments of COMET are entirely unknown and the feasibility of this technique still requires detailed investigation.
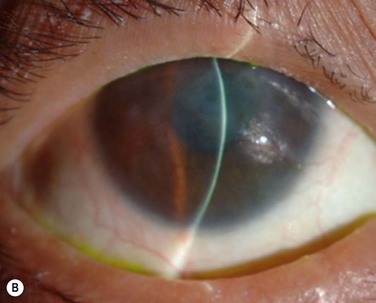
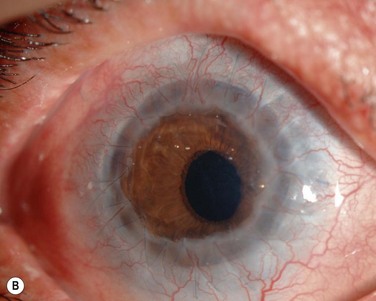
Finally, we present the long-term clinical data on 19 eyes that received COMET, for which the mean follow-up period was 55 months; the longest follow-up period being 90 months.9 The study included 19 eyes of 17 patients in the scar phase of severe OSD who underwent OSR with COMET and who could be followed up for more than 36 months. In this study, to precisely examine the long-term clinical results of COMET for OSR, we excluded the patients who received penetrating keratoplasty (PKP) after the initial COMET, as well as patients who received COMET for conjunctival fornix reconstruction. Clinical efficacy was evaluated in terms of the patients’ postoperative visual acuity. The clinical results were evaluated and graded on a scale from 0 to 3 according to their severity. Clinical safety was evaluated in terms of persistent epithelial defects, ocular hypertension, and infections. During the postoperative follow-up, best-corrected visual acuity was improved by more than two lines in 15 eyes, and visual acuity at the postoperative thirty-sixth month was improved in eight eyes. During the long-term follow-up period, postoperative clinical conjunctivalization was significantly inhibited (Fig. 45.10). Moreover, corneal opacification tended to improve during the long-term follow-up period. All eyes manifested various degrees of superficial corneal vascularization, but it gradually abated and its activity was comparatively stable from 6 months after transplantation. During the long-term follow-up period, postoperative symblepharon formation was also significantly inhibited; seven of the 19 eyes manifested persistent epithelial defects at least once during the follow-up. Postoperative ocular hypertension was observed in a total of three eyes during the follow-up periods. The occasional increase in intraocular pressure was mainly managed by the administration of carbonic anhydrase inhibitor. Corneal infection was mainly observed within 6 months after transplantation, and methicillin-resistant Staphylococcus aureus was found to be the only cause the infection.
Development of the Next Generation of COMET
The Use of Autologous Serum in the Development of Cultivated Epithelial Sheets
The currently preferred method of cultivating epithelial sheets requires the use of xenobiotic materials in the culture system, such as fetal bovine serum (FBS) and mouse-derived 3T3 feeder cells. However, the use of FBS in the culture system is a major concern, as bovine spongiform encephalopathy cannot be detected by any known in vitro assay. Various serum-free culture systems, developed to delete the FBS from the culture system, have mainly been used to study the roles of various growth factors.10 The clinical use of these serum-free culture systems has been limited because of their lower efficacy for cell proliferation, compared to FBS-supplemented medium. In the development of a cultivated oral mucosal epithelial sheet for clinical application, the ideal culture system is one that is free from disease transmission, as well as being able to support cell proliferation and differentiation. Therefore, the use of autologous serum (AS) as an alternative to FBS is significantly advantageous, as it eliminates the need for bovine material in the culture process. We wanted to determine if AS from patients with severe OSD was similarly useful in supporting the cell proliferation and differentiation of cultivated oral mucosal epithelial sheets, compared to conventional FBS-supplemented culture methods. Here, we demonstrate that the AS-supplemented culture system derived from severe OSD patients was able to support epithelial cell proliferation, as well as the development of transplants showing morphological and ultrastructural characteristics that are similar to normal tissues.11
AS-Derived COMET
We previously demonstrated the use of COMET for treating severe OSD using the FBS culture system, which is particularly useful in bilateral severe OSDs.4,5 However, the use of FBS may be associated with the risk of transmission of zoonotic infection and other unknown pathogens. As bovine spongiform encephalitis cannot be detected by any known in vitro assay, the use of bovine-derived products is a major health concern in many parts of the world. We previously showed that human AS was able to support epithelial cell proliferation,11 which raises the possibility of using the patient’s own serum as an alternative to FBS in the culture system. The use of AS is advantageous, as it eliminates the need for bovine material and reduces the risk of disease transmission. In this clinical study, we compared the efficacy of AS supplementation with conventional FBS supplementation in developing cultivated oral mucosal epithelial sheets, and evaluated the usefulness of AS-derived COMET for the treatment of severe OSD.12
Potential Diversity of COMET
Combination of COMET and Penetrating Keratoplasty
Severe OSD is sometimes accompanied by severe corneal stromal scarring and opacity and/or corneal endothelial dysfunction. Therefore, most patients require PKP for visual recovery. To improve the clinical outcome and long-term prognosis of these patients, their reconstructed cornea must be provided with a more stable epithelial supply, such as cultivated epithelial transplantation. Therefore, a two-step surgical strategy that utilizes a combination of COMET and PKP has been developed.13
The initial COMET for OSR may be performed as described in previous reports (Fig. 45.11).4,5 PKP is then performed 5–6 months after the initial COMET. PKP with cataract surgery was also performed according to the usual clinical procedures. In brief, a 7-mm diameter trephination was performed on the host cornea, followed by continuous circular capsulorrhexis using micro-forceps. The lens was removed through the cornea using the regular phacoemulsification and aspiration technique. After inserting the intraocular lens, a fresh donor cornea was transplanted and then fixed with interrupted and continuous sutures. Finally, the ocular surface was covered with a medical-use soft contact lens.
Combination of COMET and Automated Lamellar Therapeutic Keratoplasty
Combined surgical modalities, such as lamellar keratoplasty and cataract surgery are commonly required at the time of COMET. However, these procedures are not easy, compared with the single surgical procedure for a non-ocular-surface disease. In particular, corneal opacity prevents the corneal transparency that is essential for intraocular surgery. Therefore, special surgical modifications that increase visibility and reduce reflected light are necessary for safe cataract surgery. Lamellar keratoplasty and deep lamellar keratoplasty are effective procedures to remove scarred corneal opacity. Automated lamellar therapeutic keratoplasty (ALTK) is a newly developed surgical procedure based on microkeratome-assisted keratoplasty that provides a smooth and sharp corneal stromal excision. This surgical procedure is also very useful to improve intraoperative visibility at the time of cataract surgery in patients with a hazy cornea. Once the cornea was excised by microkeratome following removal of the conjunctivalized tissue, corneal clarity improved well enough for cataract surgery to be performed. Staining the anterior lens capsule with indocyanine green and surgical slit lamp illumination are effective and useful procedures when performing cataract surgery in conditions of poor visibility. A corneal graft of the same size was then created using the Moria ALTK manual microkeratome system and sutured with 10-0 nylon. The corneal surface was then covered with cultivated oral mucosal epithelium, resulting in improvement of visual acuity. Not only the new cultivated epithelial transplantation, but also the combined surgery using new instruments, has dramatically improved clinical results and postoperative visual quality.14
Combination of COMET and Eyelid Surgery
In severe OSD, the corneal epithelial stem cells in the corneal limbus are destroyed and coverage of the corneal surface by invading neighboring conjunctival epithelium results in the ingrowth of fibrous tissue, chronic inflammation, neovascularization, and stromal scarring. In specific, various degrees of pathologic symblepharon formation and entropion frequently occur in patients with severe OSD and disturb the integrity of the ocular surface. Furthermore, an abnormal eyelid can often make a severe OSD worse, due to the fact that the eyelid margin rotation or structural abnormalities disturb tear spreading and corneal wetting. Malfunction of the conjunctival fornix by symblepharon can cause severe OSDs, such as dry eye and an inflamed ocular surface resulting from cicatricial entropion and restriction of ocular motility. Therefore, it was recently reported that the selection of the proper surgical procedure depends upon the severity of pathogenic symblepharon formation. Moreover, it was also recommended that the eyelid and fornix abnormalities be reconstructed prior to performing OSR. Therefore, it is necessary to reconstruct not only the ocular surface but also the formation of the eyelid in patients with severe OSD. (Fig. 45.12).15
Future Challenges of OSR: a Novel Cell Origin for OSR
Mesenchymal Stem Cells
Mesenchymal stem cells (MSCs) have been used in an attempt to treat a variety of diseases based on the theory that MSCs could become functional cells in host tissues. Using an animal model, Ma et al. investigated whether the transplantation of MSCs could successfully reconstruct the corneal surface, and also whether transplanted MSCs could transdifferentiate into corneal epithelial cells.16 In that study, after growth and expansion on AM, MSCs were transplanted onto a rat corneal surface 7 days after chemical injury. The results of that study showed that the transplantation of MSCs, like corneal limbal epithelial stem cells, successfully reconstructed the damaged corneal surface. Interestingly, the therapeutic effect of MSC transplantation may be associated with the inhibition of postoperative inflammation and angiogenesis rather than with corneal epithelial differentiation from the MSCs. Thus, that study provided the novel findings that MSCs can be used for OSR in the treatment of severe OSD.
Epidermal Stem Cells
It is well known that the epidermal and corneal keratinocytes are derived from ectoderm during embryogenesis. The keratinocyte stem cells of these two biological systems share several important attributes, such as expression of the same markers and transdifferentiation from corneal to epidermal. Yang et al. recently reported a new surgical strategy for OSR using an autologous transplantation of epidermal adult stem cells (EpiASCs) in a goat model.17 The goats in that report were treated by the transplantation of tissue-engineered cell sheets composed of EpiASCs, leading to the restoration of corneal transparency and improvement of postoperative visual acuity in 80% of the experimental eyes. The authors also showed that the reconstructed corneal epithelium expressed keratins 3/12, which are both thought to be cornea-specific markers, and that it had the function of secreting glycocalyx-like material. These findings not only indicate that EpiASCs can reconstruct the damaged corneal surface of goats with severe OSD, but also that EpiASCs can transdifferentiate into corneal epithelial cell types in vivo. However, further studies are needed to elucidate whether these experimental results hold true for humans.
Immature Dental-Pulp Stem Cells
Searching for a source of epithelial stem cells that have characteristics similar to those of corneal limbal epithelial stem cells, Monteiro et al. recently reported that human immature dental-pulp stem cells (hIDPSCs) share similar characteristics with corneal limbal epithelial stem cells and might be used as a potential alternative cell source for OSR.18 Interestingly, the authors showed that hIDPSCs display all of the unique characteristics of multipotent adult stem cells, expressing human embryonic stem cell markers (OCT4, SSEA-3/4, and NANOG) and several MSCs (SH2/3/4). Interestingly, hIDPSCs were also shown to express markers that are in common with corneal limbal epithelial stem cells, such as p63, ABCG2, and keratins 3/12. In that study, transplantation of a tissue-engineered hIDPSC sheet using a temperature-responsive culture dish was shown to be successful for the OSR in an animal model of severe OSD.
Nasal Mucosa
Kim et al. reported that transplantation of autologous nasal mucosa is a useful surgical procedure for achieving OSR in cases of severe OSD.19 In that report, they showed that nasal mucosal epithelium expressed an abundance of p63 and keratin 3. Moreover, goblet cells and MUC5AC expression were also observed in the nasal mucosal epithelium. In all patients, ocular surface integrity recovered with no major postoperative complications and an increase of goblet cells was apparently observed on the ocular surface. In view of those clinical findings, they concluded that transplantation of nasal mucosa may be an ideal surgical method for the treatment of severe OSD.
Hair Follicle Bulge-Derived Stem Cells
It has been reported that hair follicle bulge-derived stem cells (HFSCs) have a high degree of tissue plasticity and can cross cell lineage boundaries and differentiate into a different cell phenotype. Most recently, Meyer-Blazejewska et al. reported the therapeutic potential of murine vibrissae HFSCs as an autologous stem cell source for OSR.20 That study is an expansion of their previously studies showing transdifferentiation of HFSCs into a corneal epithelial cell phenotype, and their findings showed that the HFSC transplant successfully reconstructed the ocular surface in 80% of the transplanted eyes. These experimental data, including the use of a mouse model of severe OSD, highlight the therapeutic potential of using HFSCs to reconstruct the ocular surface.
References
1. Nakamura, T, Endo, K, Cooper, LJ, et al. The successful culture and autologous transplantation of rabbit oral mucosal epithelial cells on amniotic membrane. Invest Ophthalmol Vis Sci. 2003;44:106–116.
2. Ballen, PH. Mucous membrane grafts in chemical (lye) burns. Am J Ophthalmol. 1963;55:302–312.
3. Gipson, IK, Geggel, HS, Spurr-Michaud, SJ. Transplant of oral mucosal epithelium to rabbit ocular surface wounds in vivo. Arch. Ophthalmol. 1986;104:1529–1533.
4. Nakamura, T, Inatomi, T, Sotozono, C, et al. Transplantation of cultivated autologous oral mucosal epithelial cells in patients with severe ocular surface disorders. Br J Ophthalmol. 2004;88:1280–1284.
5. Inatomi, T, Nakamura, T, Koizumi, N, et al. Mid-term results on ocular surface reconstruction using cultivated autologous oral mucosal epithelial transplantation. Am J Ophthalmol. 2006;141:267–275.
6. Nakamura, T, Inatomi, T, Cooper, LJ, et al. Phenotypic investigation of human eyes with transplanted autologous cultivated oral mucosal epithelial sheets for severe ocular surface diseases. Ophthalmology. 2007;114:1080–1088.
7. Nishida, K, Yamato, M, Hayashida, Y, et al. Corneal reconstruction with tissue-engineered cell sheets composed of autologous oral mucosal epithelium. N Engl J Med. 2004;351:1187–1196.
8. Satake, Y, Dogru, M, Yamane, GY, et al. Barrier function and cytologic features of the ocular surface epithelium after autologous cultivated oral mucosal epithelial transplantation. Arch Ophthalmol. 2008;126:23–28.
9. Nakamura, T, Takeda, K, Inatomi, T, et al. Long-term results of autologous cultivated oral mucosal epithelial transplantation in the scar phase of severe ocular surface disorders. Br J Ophthalmol. 2011;95:942–946.
10. Kruse, FE, Tseng, SCG. Growth factors modulate clonal growth and differentiation of cultured rabbit limbal and corneal epithelium. Invest Ophthalmol Vis Sci. 1993;34:1963–1976.
11. Nakamura, T, Ang, LPK, Rigby, H, et al. The use of autologous serum in the development of corneal and oral epithelial equivalents in patients with Stevens-Johnson syndrome. Invest Ophthalmol Vis Sci. 2006;47:909–916.
12. Ang, LPK, Nakamura, T, Inatomi, T, et al. Autologous serum-derived cultivated oral epithelial transplants for severe ocular surface disease. Arch Ophthalmol. 2006;124:1543–1551.
13. Inatomi, T, Nakamura, T, Kojyo, M, et al. Ocular surface reconstruction with combination of cultivated autologous oral mucosal epithelial transplantation and penetrating keratoplasty. Am J Ophthalmol. 2006;142:757–764.
14. Inatomi, T, Nakamura, T, Koizumi, N, et al. Current concepts and challenges in ocular surface reconstruction using cultivated mucosal epithelial transplantation. Cornea. 2005;24(8 Suppl):S32–S38.
15. Takeda, K, Nakamura, T, Inatomi, T, et al. Ocular surface reconstruction using the combination of autologous cultivated oral mucosal epithelial transplantation and eyelid surgery for severe ocular surface disease. Am J Ophthalmol. 2011;152:195–201.
16. Ma, Y, Xu, Y, Xiao, Z, et al. Reconstruction of chemically burned rat corneal surface by bone marrow-derived human mesenchymal stem cells. Stem Cells. 2006;24:315–321.
17. Yang, X, Moldovan, NI, Zhao, Q, et al. Reconstruction of damaged cornea by autologous transplantation of epidermal adult stem cells. Mol Vis. 2008;14:1064–1070.
18. Monteiro, BG, Serafim, RC, Melo, GB, et al. Human immature dental pulp stem cells share key characteristic features with limbal stem cells. Cell Prolif. 2009;42:587–594.
19. Kim, JH, Chun, YS, Lee, SH, et al. Ocular surface reconstruction with autologous nasal mucosa in cicatricial ocular surface disease. Am J Ophthalmol. 2010;149:45–53.
20. Meyer-Blazejewska, EA, Call, MK, Yamanaka, O, et al. From hair to cornea: toward the therapeutic use of hair follicle-derived stem cells in the treatment of limbal stem cell deficiency. Stem Cells. 2011;29:57–66.