16 Non-Neuroendocrine Carcinomas (Excluding “Sarcomatoid” Carcinoma) and Salivary Gland Analog Carcinomas in the Lung
Carcinoma of the lung is a growing public health problem of worrisome proportions, not only in the United States but also internationally.1–5 The American Cancer Society has estimated that more than 170,000 new cases of lung cancer will occur each year in the United States in the foreseeable future. This unfortunate reality reflects the continuing use of cigarettes, cigars, and pipes by a significant fraction of the world population and the undeniable causal relationship between inhaled tobacco smoke and pulmonary carcinoma.1–7 To make matters worse, it has been shown that persons who are in constant and close proximity to heavy smokers, either at home or in the workplace, inhale sufficient “sidestream” smoke to place them at definite risk for lung cancer even if they have never smoked themselves.6,8,9 The potential pathogenetic influence of other proposed etiologic agents (e.g., human papillomavirus, radon, pneumoconiosis-related minerals, hereditary syndromes) on pulmonary carcinogenesis also continues to undergo analysis.3,10–21 Women now have an incidence of lung cancer on a rough numerical parity with men, and the mortality rate in both sexes is so high that lung cancer represents the leading worldwide cause of death related to malignancy.2,5,6
Nosology of Lung Cancer and Diagnostic Effects of Sampling Methods
Seventy-five years ago, the first attempt at morphologic classification of lung cancer was undertaken by Marchesani.10 This scheme, outlining the now-classic categories of squamous cell carcinoma (SCC), adenocarcinoma (ACA), and small cell “undifferentiated” and large cell “undifferentiated” carcinomas, is still widely recognized and has gone through several iterations over the ensuing decades. Recently, a pragmatic, clinically attuned movement has been enjoined wherein a more simplified system was embraced, dividing malignant epithelial tumors of the lung into “small cell” and “non–small cell” carcinomas. At the same time, research by pathologists has resulted in ever-greater refinement of morphologic categorization, and the interface between the practical needs of the operating suite and data generated in the clinical laboratory has become problematic. In light of this situation, a prognostically oriented nosologic scheme for lung cancer was devised by the Veterans Administration Lung Group in 1991.22 It has since been modified by other organizations, most notably, the World Health Organization.23
Whether a pathologist elects to use one or another of these systems is a decision that should be made after consultation with clinical colleagues. In any event, nonstandard designations for pulmonary carcinomas (i.e., those that are not sanctioned by the World Health Organization)23 should be well defined in surgical pathology reports, with pertinent references provided in cases that are particularly uncommon or conceptually contentious.
A particular problem that must be recognized by everyone involved in treating lung cancer is the common heterogeneity that it may demonstrate at a light microscopic level. The literature is now well supplied with reports of admixtures of virtually all of the lung carcinoma histotypes in the same tumor mass.3,12,24–26 An analysis by Roggli and colleagues27 demonstrated such heterogeneity in fully 66% of a consecutive series of lung cancers, and others have reported similar findings. This biologic diversity may attain prognostic importance in the future, particularly in light of recent work that appears to affirm the effect of histologic features on tumor evolution.28 Neoplastic heterogeneity is a practical diagnostic problem for surgeons and medical oncologists, because small biopsies often do not represent “divergent” tumor elements as a consequence of sampling bias. At a time when ever more limited methods of tissue procurement are being advanced, it is clear that substantial discrepancies will be observed between biopsy results and findings of resection specimens in the surgical pathology laboratory.29,30 That is not to imply that such techniques as fine-needle aspiration biopsy should not be used, because they are extremely helpful in planning therapy in many cases. Nonetheless, the potential limitations of all sampling procedures must be weighed carefully against their benefits.
Clinicopathologic Features of Non-Neuroendocrine Pulmonary Carcinomas, Excluding “Sarcomatoid” Carcinoma
Squamous Cell Carcinoma
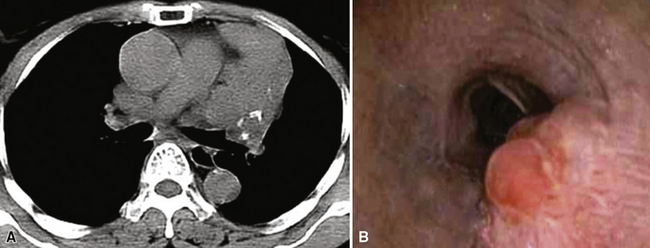
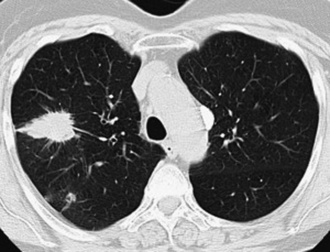
Probably because of changes in the smoking habits of the public, with a greater preference for filtered cigarettes in the last 35 years,7 SCC is no longer the most common type of lung cancer and has been eclipsed by ACA in recent years.25,31 However, SCC has retained its classic clinicopathologic attributes. These include a tendency for multifocal but clonal in situ disease in the bronchial mucosa, often preceding the appearance of a discrete mass by several years, and a propensity to arise in large central airways proximal to the subsegmental bronchi. Because of the common presence of an endobronchial tumor mass, obstructive pneumonia is a relatively common accompaniment of this neoplasm, but it is not pathognomonic. “Pure” SCC may originate in the peripheral pulmonary parenchyma as well, and rare examples are even subpleural.3
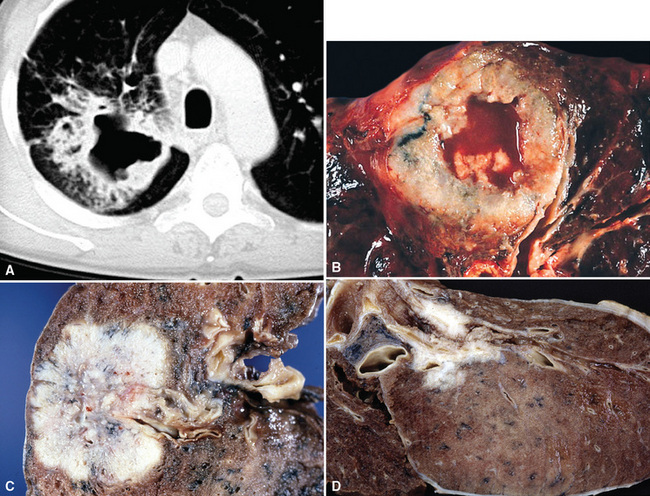
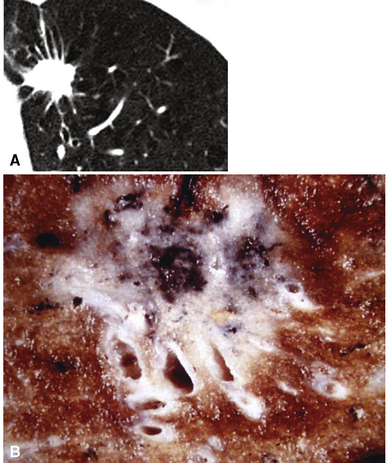
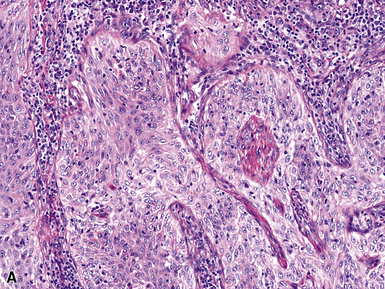
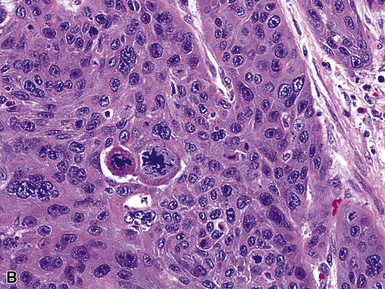
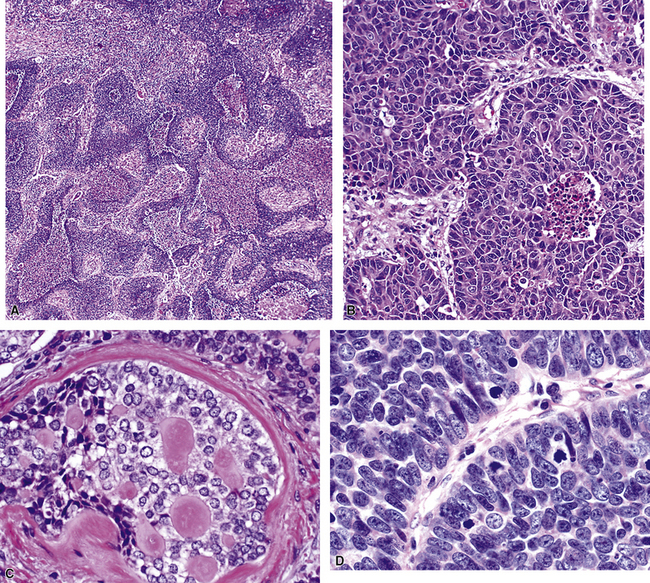
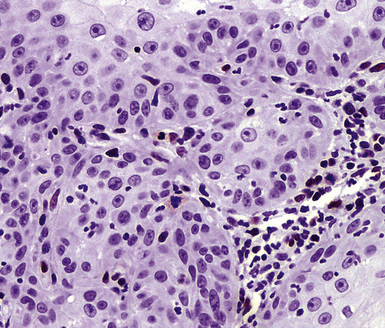
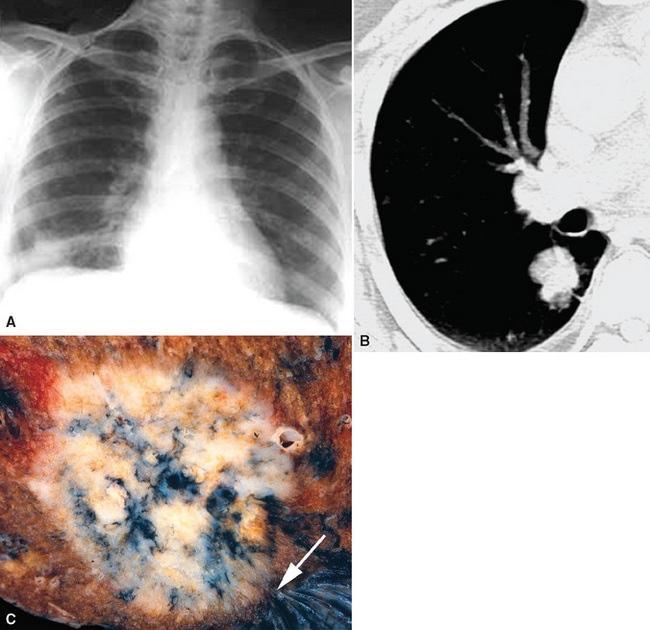
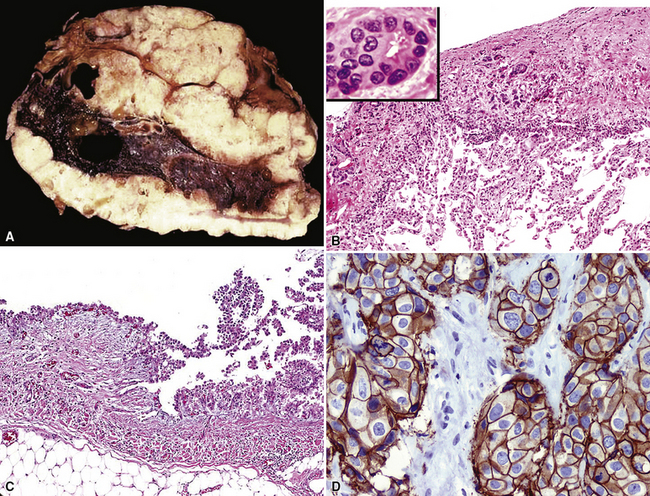
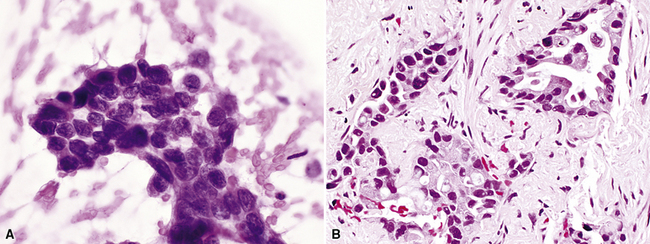
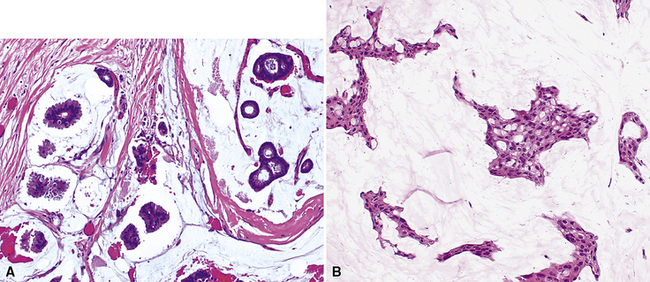
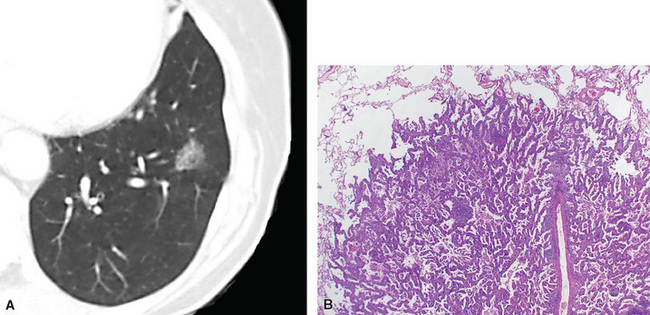
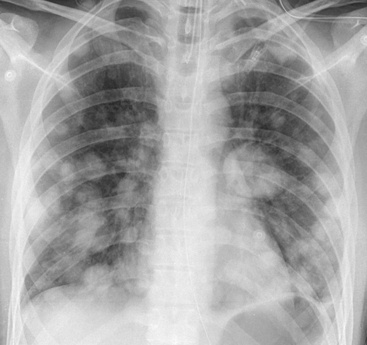
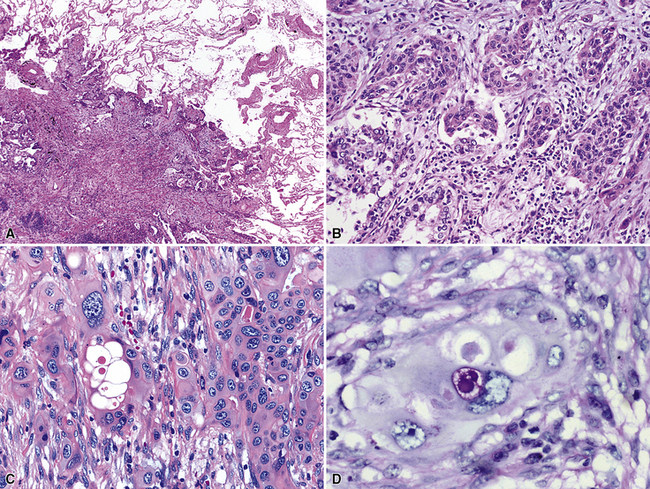
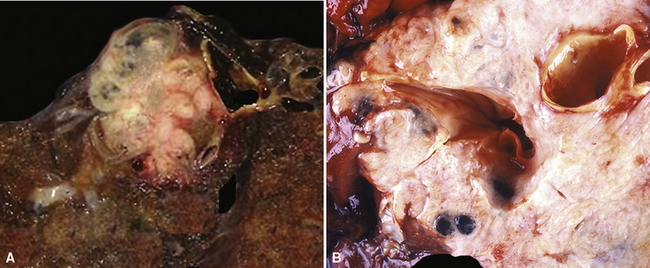
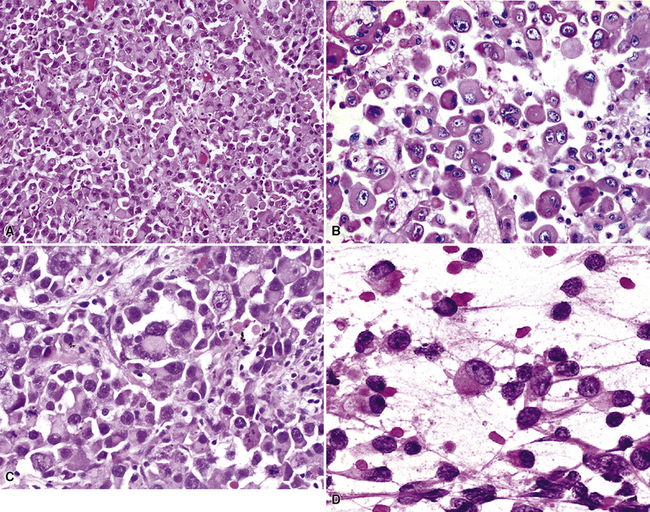
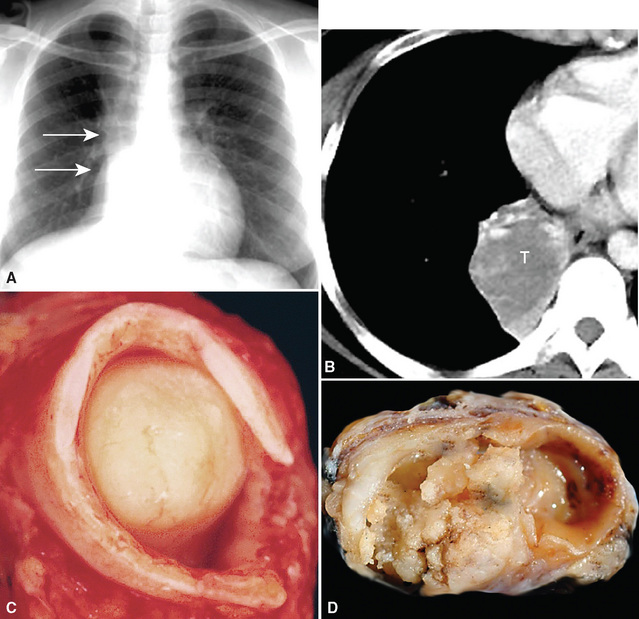
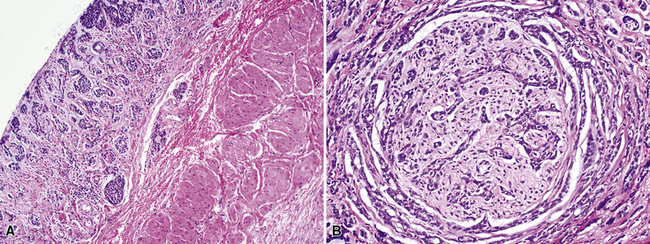
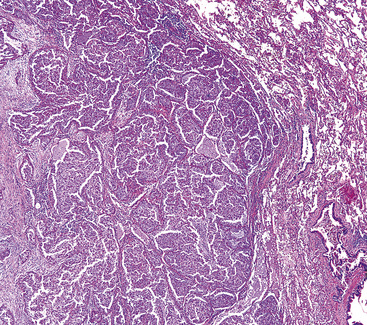
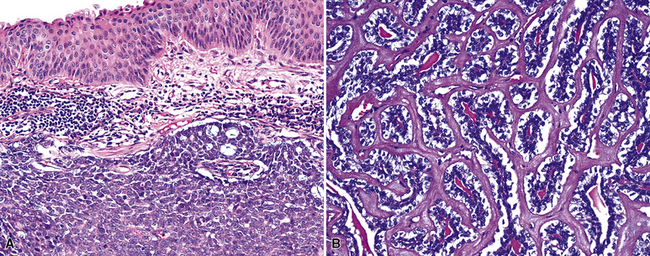
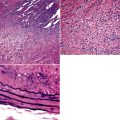
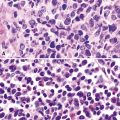
Squamous cell cancer of the lung has an irregular, often friable, gray-white cut surface, commonly showing a large area of central necrosis, with or without cavitation (Fig. 16-1). The surrounding pulmonary parenchyma is frequently tethered to the mass, giving it a “spiculated” appearance that may be well seen on radiographic images (Fig. 16-2). Microscopically, SCC is defined by its resemblance to stratified squamous epithelium of the upper airway, but with disordered architectural and cytologic maturation (Figs. 16-3 to 16-6). Anucleate keratin and squamous pearls are observed in better differentiated lesions, which account for a minority of pulmonary carcinomas. Poorly differentiated SCC may be extremely difficult for the pathologist to distinguish from high-grade ACAs, small cell carcinomas, or large cell undifferentiated carcinomas (LCCs), because they commonly take the form of rather nondescript proliferations of primitive epithelioid cells of varying size, arranged in nests and sheets, with no other distinguishing features. In the absence of special studies, one may have to use the default terminology of “poorly differentiated carcinoma, not further specified,” in the frozen section laboratory and in small biopsies of such lesions. Recognized and distinct subtypes of poorly differentiated SCC include spindle cell and pleomorphic (sarcomatoid) forms32–35 (see Chapter 14); adenoid-acantholytic (pseudoglandular) variants (Fig. 16-7)36; a pseudovascular (angiosarcoma-like) subtype (Fig. 16-8)36,37; “lymphoepithelioma-like” carcinoma (Fig. 16-9)38; and a basaloid form (Fig. 16-10) (“small cell squamous carcinoma” or “basaloid squamous cell carcinoma”)39 that is analogous to primary poorly differentiated SCC of the anorectum, hypopharynx, thymus, and other anatomic sites. Other than presenting special problems in microscopic differential diagnosis, these tumor variants have no singular clinical significance in comparison with other high-grade carcinomas.
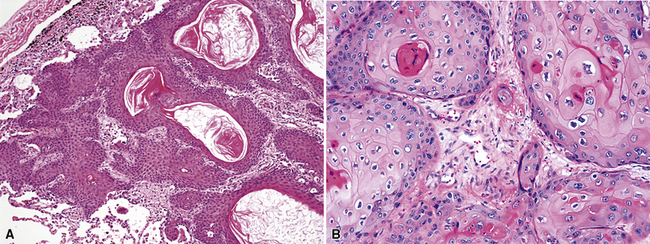
Figure 16-3 A and B, Photomicrographs of well-differentiated squamous carcinoma showing obvious keratinization.
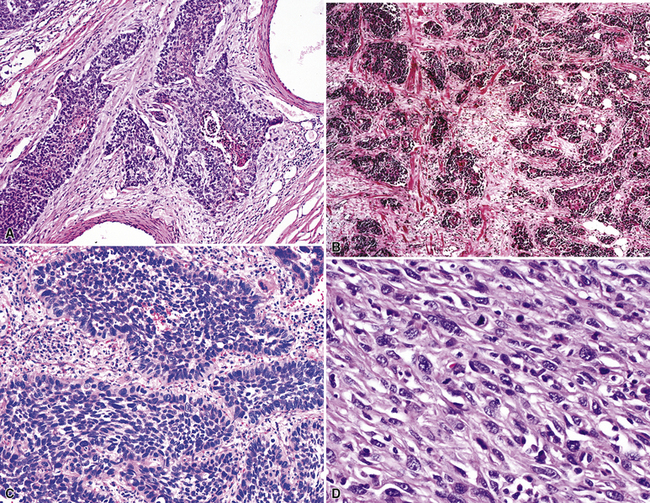
Figure 16-5 A, Poorly differentiated squamous cell carcinoma (PDSCC) showing only focal keratinization and predominant composition by primitive epithelial cells. B, PDSCC comprising groups of relatively primitive polygonal cells invading the soft tissue of the chest wall. C, Only very focal keratinization is apparent in this PDSCC. D, PDSCC with sarcomatoid features, presenting difficulty in recognition of the lesion as epithelial (see Chapter 14).
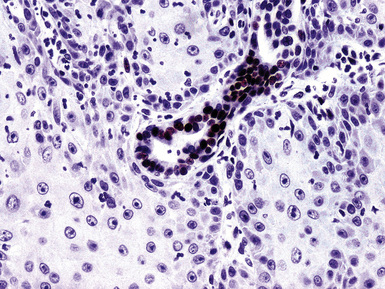
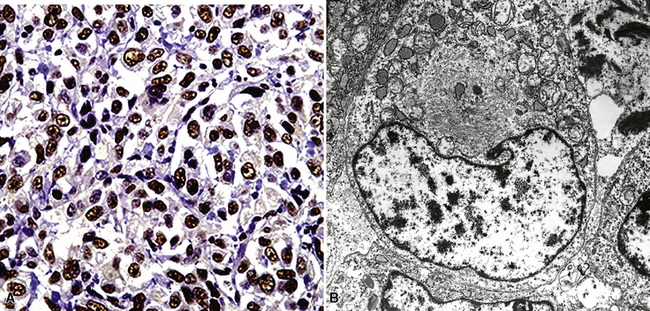
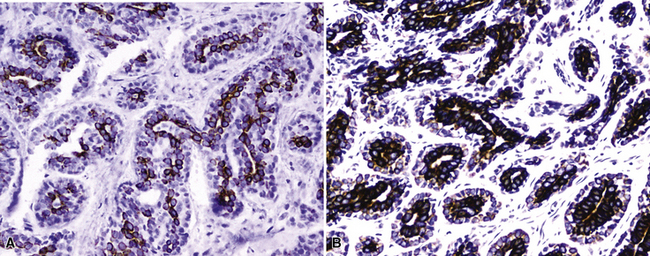
For unknown reasons, SCC has a greater association with selected hereditary syndromes than do other forms of lung carcinoma.11–21 These conditions are extremely rare causes of pulmonary neoplasia, but they include such disorders as Muir-Torre syndrome, von Hippel-Lindau disease, and dysplastic nevus (“atypical mole”) syndrome. In one of them—Muir-Torre syndrome—immunohistochemical analyses can be done for the absence of nucleic acid mismatch repair proteins in the tumor cells. The three most commonly assessed gene products are MLH1, MSH2, and MSH6 (Figs. 16-11 and 16-12).
Nonbronchioloalveolar Adenocarcinomas
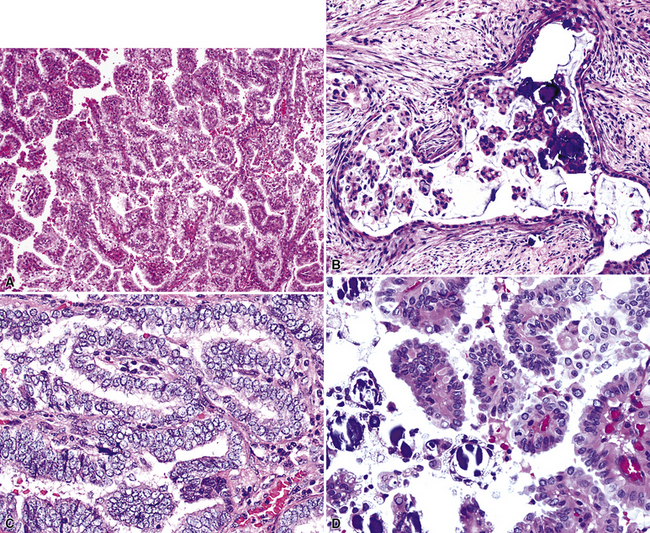
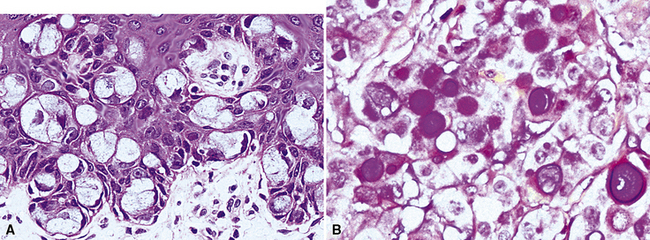
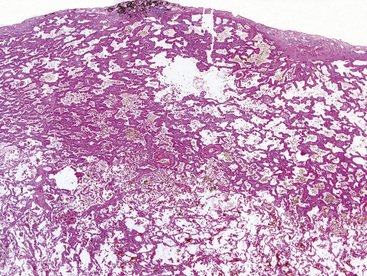
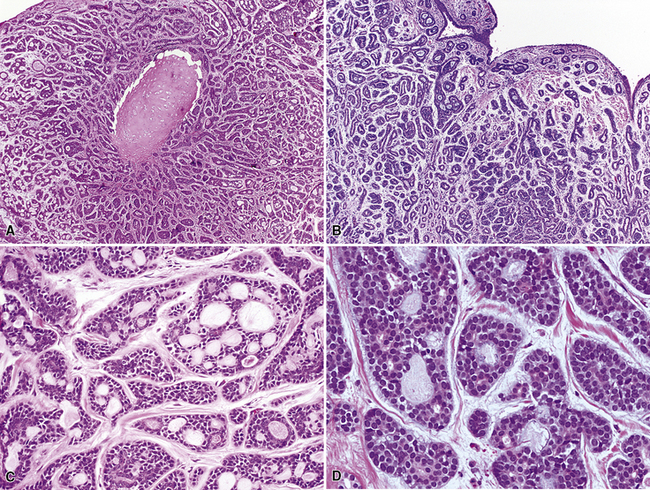
As stated earlier, ACA has now successfully eclipsed SCC as the most common form of monodifferentiated lung cancer. In North America and Europe, most ACAs are predominantly peripheral parenchymal masses; however, interestingly, histologically identical lesions in India and Asia are as likely to be central as peripheral in location. Grossly, ACA typically has an irregularly lobulated configuration, with a gray-white cut surface (Fig. 16-13). Anthracotic pigment is commonly entrapped in the tumor mass as well, but foci of necrosis and hemorrhage are seen only in large (>5 cm) lesions. A relationship to tubular airways is only rarely obvious. Close inspection may also demonstrate the presence of “satellite” nodules around the main tumor mass; however, this phenomenon may be a reflection of the tendency for pulmonary ACA to be synchronously or metachronously multifocal, either in one lobe of the lung or in both lungs. The latter statement applies particularly to a special subtype of ACA, bronchioloalveolar carcinoma (BAC; discussed later). One peculiar and uncommon gross presentation of pulmonary ACA that merits special mention is its “pseudomesotheliomatous” form, wherein the pleurotropic growth of extremely peripheral intrapulmonary tumors creates a “rind” of tumor tissue surrounding the lung (Fig. 16-14). This virtually perfectly simulates the appearance of mesothelioma, both intraoperatively and radiographically.40–42
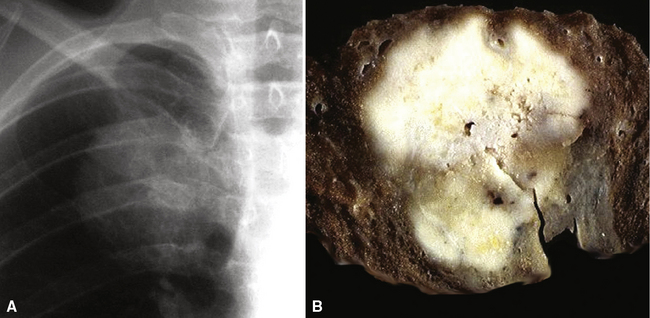
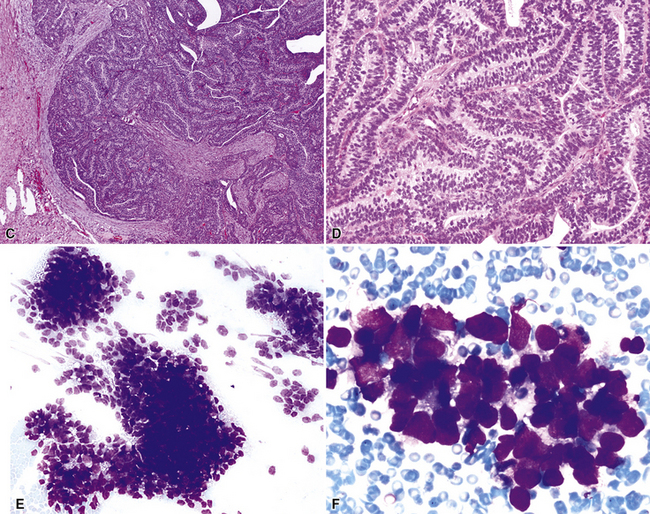
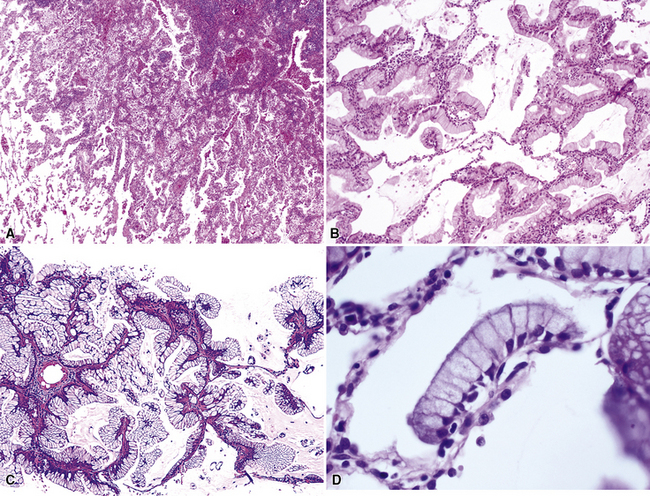
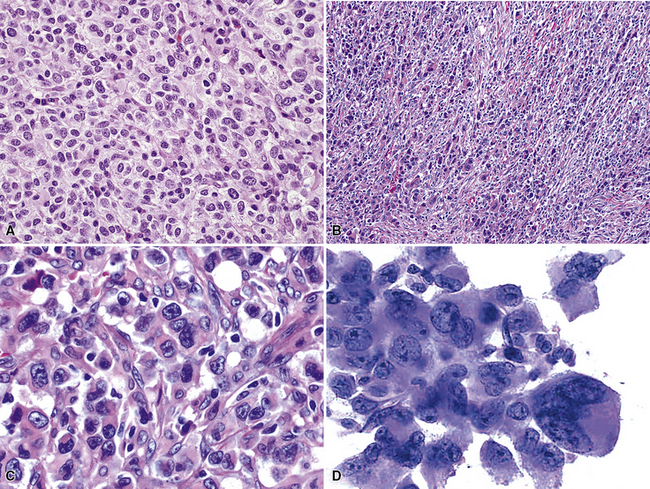
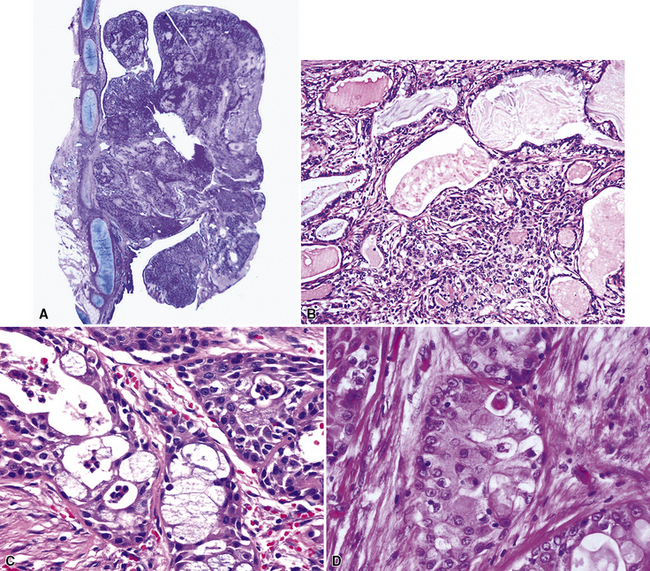
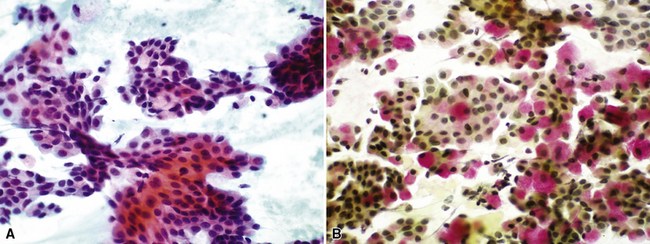
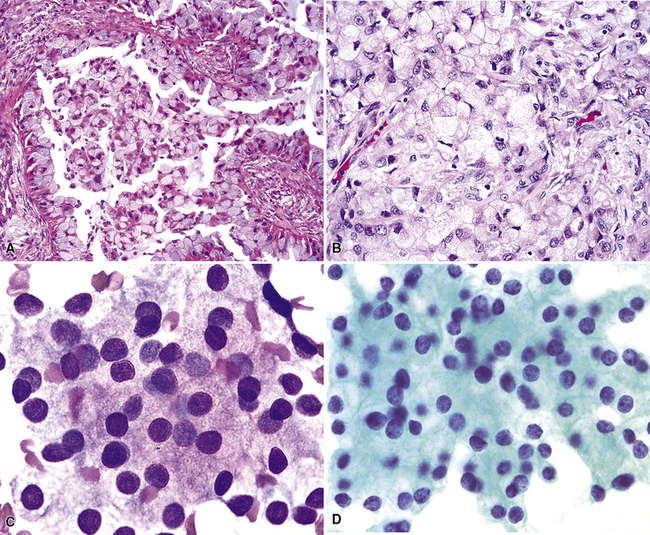
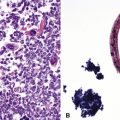
Figure 16-18 A, “Fetal” adenocarcinoma of the lung is considered to be in the same general family of tumors as pulmonary “blastoma,” and is shown here in an adult patient as a peripheral nodule in the right upper lung field on a plain-film radiograph. B, The resected tumor is a solid, relatively well-demarcated, and homogeneous mass. C and D, Microscopically, fetal adenocarcinoma comprises closely apposed glandular arrays made up of compact columnar cells, resembling those seen in embryonic lungs. E and F, Fine-needle aspiration specimens of fetal adenocarcinoma show small cells with high nucleocytoplasmic ratios, dispersed chromatin, and a tendency to surround small central spaces. Cytologic differential diagnosis includes neuroendocrine and neuroectodermal lesions (see Chapter 13).
(A and B, Courtesy of Dr. Samuel A. Yousem, Pittsburgh, PA. E and F, Courtesy of Dr. Kim Geisinger, Winston-Salem, NC).
However, more variants exist, such as clear cell and enteric (intestinal-like; Fig. 16-22).47–52 In some cases, because of the overlap between these histologic groups and the appearance of metastatic ACAs in the lung, it may be extremely difficult for the pathologist to separate primary from secondary lesions. This is particularly true of enteric and signet ring cell tumors, which can closely imitate the attributes of gastric or colorectal carcinomas (see Chapter 17). Psammoma bodies can also be seen in primary papillary ACAs of the lung,43,53 and these structures therefore raise the question of whether one is instead viewing a solitary metastasis from an occult tumor of the thyroid, ovary, or another location wherein psammomatous carcinomas are potentially found. Needless to say, in cases featuring multifocality of ACA in more than one lobe, this problem is even more striking. Special studies, including electron microscopy and immunohistology, are helpful in resolving this differential diagnosis. Ultimately, however, diagnostic reliance is also placed on such banal characteristics as peritumoral fibrosis and inflammation, which are more common in primary pulmonary lesions than in metastases.
Pulmonary ACA may rarely involve the bronchial epithelium diffusely in a pagetoid fashion (Fig. 16-23).54 Whether this represents intraepithelial spread of the tumor or multifocal synchronous growth is an open question, but pagetoid lesions are excruciating management problems because of the difficulty in obtaining tumor-free bronchial margins.
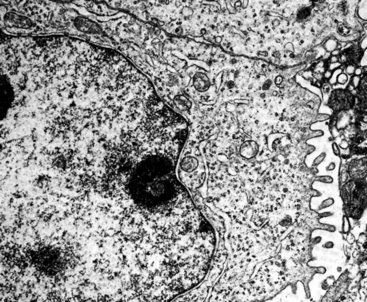
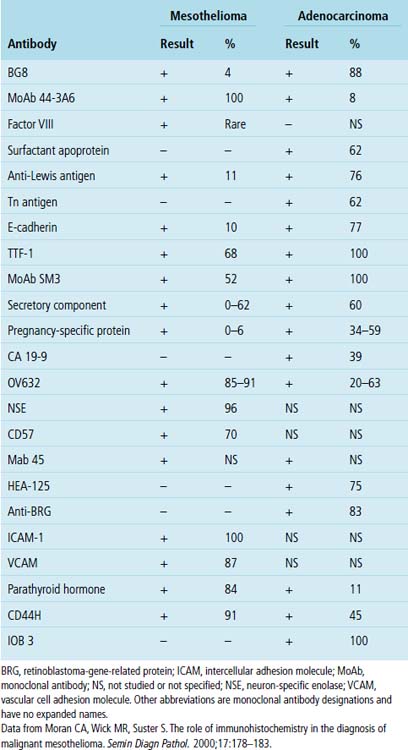
“Pseudomesotheliomatous” (pleurotropic) ACAs are indeed separable from true mesotheliomas using adjuvant pathologic techniques, particularly immunophenotyping and electron microscopy (Figs. 16-24 and 16-25 and Table 16-1).55 Nonetheless, whether this exercise has any more than academic significance is an open question. Available therapies for both of these tumor types are suboptimal, and the only real significance of the differential diagnosis may be a medicolegal one. In the absence of asbestosis, pleurotropic ACA has no proven causal relationship to asbestos exposure.
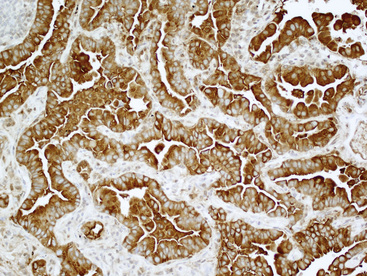
Figure 16-24 The tumor depicted in Figure 16-14 shows diffuse immunoreactivity for carcinoembryonic antigen, as expected in most adenocarcinomas of the lung but not in mesotheliomas.
Bronchioloalveolar Carcinoma
Bronchioloalveolar carcinoma of the lung was initially described in the 1800s, but was most fully characterized as a distinct entity by Liebow in 1960.56 Since that time, BAC has been the subject of intense interest and controversy. In particular, the pathologic criteria that apply to its diagnosis and how its histologic attributes relate to prognosis represent perhaps the two most contentious issues.57–65
There are no particular distinguishing demographic features associated with BAC vis-à-vis other ACAs of the lung. They tend to occur in elderly individuals, with an essentially equal distribution by sex.58,65 Contrary to reports in the 1970s63—before such phenomena as “passive” tobacco exposure were recognized—there is a definite relationship between cigarette smoking and the genesis of this tumor, as is true of virtually all pulmonary carcinomas. Nonetheless, BAC is indeed over-represented (with regard to other histotypes of lung cancer) in patients who have never smoked and who have never lived with smokers. This point has raised the issue of whether other etiologic factors—in particular, infection with human papillomavirus—might account for the genesis of some BACs.
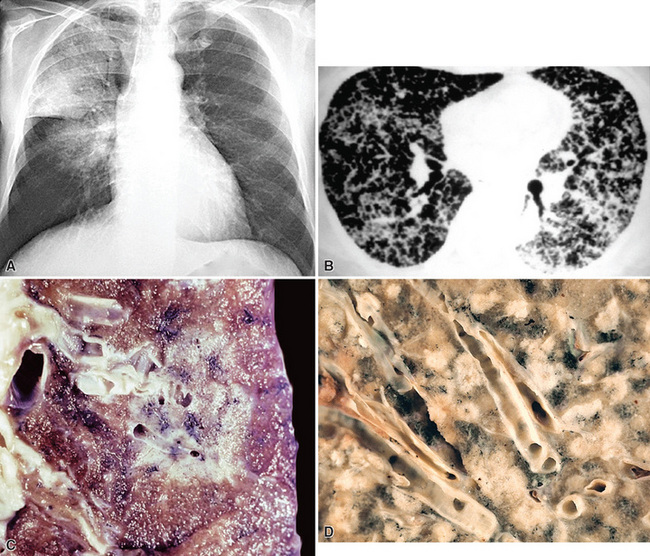
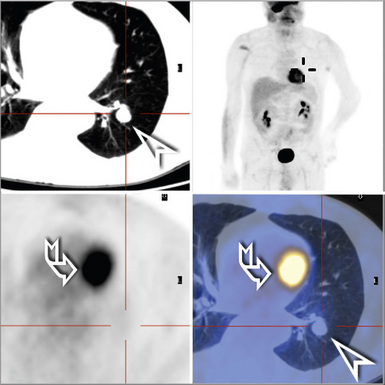
Radiologically and clinically, three discrete subsets of patients with BAC are recognized.65,66 These include individuals who have chest radiographic findings and clinical symptoms suggesting pneumonia (fever, productive cough, and lobar or segmental consolidation; Fig. 16-26) as well as some patients with a solitary peripheral mass lesion (Fig. 16-27) and others with multiple rounded densities throughout one or both lung fields (Fig. 16-28). On computed tomograms, the latter lesions may assume a “Cheerio” shape, in that they commonly demonstrate small central areas of cavitation. Hence, BAC may simulate an infectious disease, represent a nondescript “coin” lesion of the lung, or imitate the pattern of metastases to the lung from an occult visceral neoplasm.
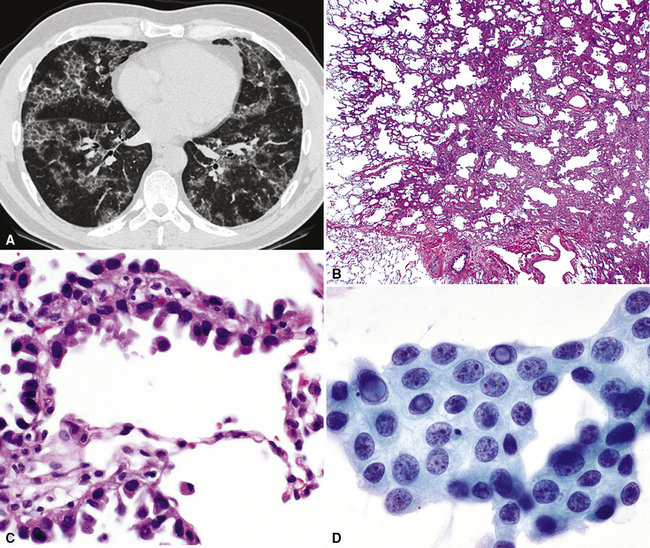
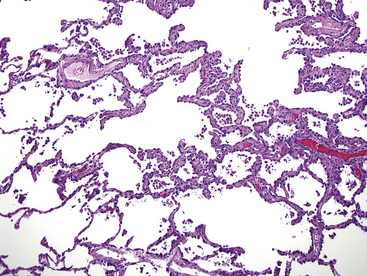
Histologically, two distinct cytologic subtypes of BAC are recognized: mucinous and nonmucinous.57,58,62 These are of importance because of their clinical associations. Mucinous tumors (Fig. 16-29) are those that tend to assume a pseudopneumonic or multifocal/multinodular clinical appearance, whereas nonmucinous BAC (Fig. 16-30) is more commonly a solitary lesion. Furthermore, stage for stage, nonmucinous variants may show a more favorable clinical evolution.62,67 The criteria for the distinction of BAC from “ordinary” pulmonary ACAs continue to be debated. In accord with current convention, we restrict the use of this diagnosis to neoplasms that demonstrate a mantling of pre-existing air spaces (“lepidic” or noninvasive growth) by single layers or limited strata and micropapillae of only modestly atypical cuboidal or columnar epithelial cells, with or without intracellular or extracellular mucin production. Intranuclear inclusions of cytoplasm containing surfactant proteins are also common.68 Save for mucin production, such characteristics are shared by both forms of BAC, mucinous and nonmucinous (serous). Septal widening and sclerosis may be seen, especially with nonmucinous BAC, but there must be no desmoplasia or inflammation within or around the lesion if it is to be considered a bona fide BAC. Caution should be exercised in diagnosing BACs with central sclerosis because this is often a site of subtle early invasion. This demanding definition differs from that of some other observers, who have accepted the existence of a “sclerosing” BAC subtype.69 Justification for more narrow requirements is gained from biologic data, which show worse behavior of “sclerosing” or “invasive” BAC than of nonfibrotic tumors.57,70,71 Indeed, Clayton,58 who devoted much attention to BAC, clearly stated that bronchioloalveolar carcinomas with sclerosis should be classified with other peripheral ACAs.
Another traditional point of discussion pertaining to the microscopic features of BAC (particularly its mucinous form) is that this neoplasm is said to disseminate within the lung by “aerogenous” means. That is to say, tumor cells are believed to detach from a “mother lesion” and spread to other foci in the pulmonary parenchyma by the process of inhalation and exhalation. Some molecular analyses, however, have cast doubt on that premise and instead suggest that the lesions are multiclonal.72
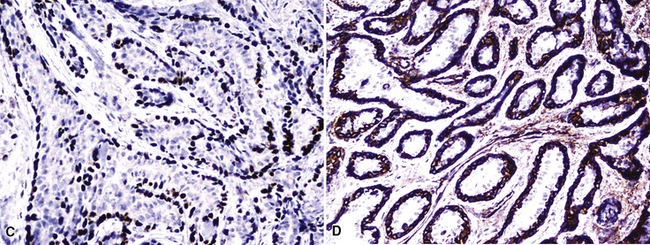
Other lesions that may be confused pathologically with BAC include foci of florid type II pneumocytic hyperplasia surrounding areas of diffuse alveolar damage73 and interstitial fibrosing pneumonitides or organizing pulmonary infarcts74; the proliferation known as “atypical adenomatous alveolar hyperplasia” (discussed later)75; and the unicentric neoplasms known as “papillary alveolar adenoma” and “sclerosing hemangioma.”76 The latter two entities are bland cytologically and show a sharp interface with the surrounding pulmonary parenchyma, unlike BAC. Another point that was often raised in the older literature on BAC concerned the great difficulty with which metastases to the lung could be distinguished from the former neoplasm. Selected immunohistologic markers—especially nuclear labeling for thyroid transcription factor-1 (TTF-1; Fig. 16-31) and cytoplasmic staining for napsin-A—are valuable in distinguishing BAC from metastases of extrapulmonary ACAs,77–80 but that separation must ultimately rest on careful analysis of conventional clinicopathologic data.
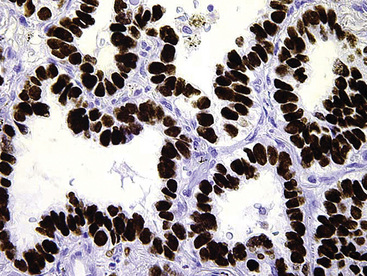
Figure 16-31 Intense nuclear immunoreactivity for thyroid transcription factor-1 is present in this bronchioloalveolar carcinoma.
Prognostically, patients with multifocal or pseudopneumonic BACs have an outlook that is worse than that of patients with unicentric tumors of this type because many of the former lesions are inoperable. Furthermore, mucinous tumors tend to behave more aggressively—with a higher incidence of extrapulmonary metastasis—than nonmucinous BAC when they are matched by size and stage.57,58 Overall, nonmucinous tumors measuring less than 3 cm in maximum dimension have a good prognosis, approximating 90% at 5 years. Indeed, the current surgical approach to such lesions is limited resection, principally by wedge excision (Fig. 16-32).81–85
The natural history of BAC is more protracted than that of more conventional pulmonary ACAs, and tumor-related fatalities will continue to accrue even at 10 years after diagnosis.57,63 Nevertheless, in an often-cited study by Manning and colleagues,62 the 5-year survival rate for nonmucinous BAC was 72%, whereas only 26% of patients with mucinous tumors survived at that point.
Atypical Adenomatous Hyperplasia and Its Relationship to Bronchioloalveolar Carcinoma
Kitamura and colleagues86 addressed the conceptual mechanistic relationship between topographically small atypical glandular proliferations of the lung—“atypical adenomatous hyperplasia” (AAH)—and BAC. Their model appeared to demonstrate a stepwise progression from AAH to BAC, with further evolution to invasive growth. That concept also had been advanced before, especially by Miller and colleagues,87 who postulated that such a stepwise process existed in the lung in analogy to the adenoma-carcinoma sequence in the colon.
Atypical adenomatous hyperplasia must be differentiated from reactive or regenerative pneumocytic lesions, at one end of the spectrum, and from small bona fide ACAs, at the other. With regard to separation of AAH from various reactive lesions, Kitamura and co-workers86 have emphasized the tendency of reactive lesions to include multiple cell types, including type II pneumocytes, ciliated cells, and mucinous cells, and they have described the relatively more conspicuous interstitial inflammation and edema in localized interstitial pneumonitis. Additional criteria that are found to be helpful in this context involve assessment of the lesional borders and patterns of fibrosis. There is a tendency for lesions of AAH to be more sharply circumscribed and for the mild interstitial fibrosis and inflammation to stop at the same boundary as the atypical alveolar cells. Conversely, reactive lesions tend to be less well defined, and the interstitial scarring extends beyond the areas of alveolar cell atypia. The individual cells in AAH, although less homogeneous than those in BAC, also tend to be more uniformly atypical than those of reactive hyperplasias (Figs. 16-33 and 16-34).
Gupta and colleagues88 identified factors that favored the diagnosis of BAC over reactive changes in an evidence-based construct: multiple growth patterns; anisocytosis; nuclear atypia in 75% or more of the lesional cells; macronucleoli; and atypical mitoses. Conflicting information has emerged on the use of p63 immunostains to make this diagnostic distinction. Sheikh and colleagues89 suggested that p63 positivity was restricted to reactive processes and absent in nonmucinous BAC, whereas Saad and coworkers90 found p63 reactivity in 89% of cases of BAC.
A variety of chemotherapeutic agents can induce striking degrees of cytologic atypia, and this phenomenon is a well-documented pitfall in exfoliative cytology.74,91 We have also seen several examples of pneumocytes with intranuclear cytoplasmic inclusions in clear-cut cases of organizing phase diffuse alveolar damage; hence, their presence should not be viewed as pathognomonic of neoplasia.
It is our view that the distinction between AAH and small nonmucinous BAC is conceptually arbitrary. Standard criteria that have been cited as useful in this distinction include uniformly atypical nuclei, large lesional size (>5 mm), and complex growth, with budding or tufting of tumor cells in the alveolar spaces in BAC but not AAH.75,92 Kitamura and coworkers86 suggested that a nuclear area of less than 40 μm2 and a lesional diameter of less than 5 mm could effectively identify AAH as opposed to small BAC. Miller93 also proposed a limit of 5 mm to separate “bronchioloalveolar cell adenoma” from BAC.
Nevertheless, morphometric and immunohistologic analyses have shown a synonymity rather than a disparity between AAH and nonmucinous BAC.94,95 Similarly, molecular studies, which show some differences in statistical groups, have demonstrated many more shared features than differences.96–100
In light of the current availability of biologic agents that can be used to treat lung cancer, it is appropriate to mention the genotypes of nonmucinous and mucinous BAC. In general, nonmucinous lesions demonstrate mutations in the epidermal growth factor receptor (EGFR) molecule, to which tyrosine kinase inhibitor (TKI) therapeutic agents are directed.101,102 On the other hand, mucinous BAC manifests preferential aberrations in the K-ras gene and accordingly does not show a response to TKIs.101 Mutant EGFR protein overexpression, as indicated indirectly by strong cell membrane immunoreactivity in tumor cells, correlates to some degree, but very imperfectly, with in situ hybridization studies or polymerase chain reaction-based assays.103
“Micropapillary” ACA of the lung was formerly considered by many observers to be a subtype of nonmucinous BAC. That nosologic approach has now changed,43 occasioned by the demonstrably worse prognosis of micropapillary carcinoma.104,105 Accordingly, the latter neoplasm is now regarded as a pathologic entity unto itself. In analogy to the diagnostic approach to BAC, a conclusive interpretation of micropapillary carcinoma should be avoided in cytologic specimens. Rudomina and colleagues106 have shown that papillary profiles in the latter samples do not correlate well with a final diagnosis of micropapillary ACA.
Adenocarcinomas Associated with Scars
In the relatively recent past, it was taught that fibrous scarring in the lung—seen as a consequence of pneumonia, interstitial fibroproliferative diseases, or pneumoconioses—predisposed to ACA and had a directly causative role in the genesis of that tumor type.107 Nevertheless, several investigators have concluded that the central fibrosis seen in “scar adenocarcinomas” (Fig. 16-35) is formed after initiation of the carcinoma and that it is the product of the tumor cells themselves.108–110 At a practical level, there is no reason to suspect that localized fibrosing conditions in the lung are, in and of themselves, preneoplastic. However, it appears that an increased frequency of lung carcinoma may exist in patients with diffuse interstitial fibrosis—as seen in late-stage “usual interstitial pneumonia,” for example—over and above that associated with smoking alone.111 Nevertheless, the relative weights of those potentially causal elements are still unclear.
With particular reference to asbestosis and silicosis, special forms of pulmonary interstitial fibrosis, our review of the aggregated literature leads to the conclusion that the risk of pulmonary carcinoma may (debatably) be increased by those conditions of the lung, although such an association does not prove causation. It is clear that cigarette smoking is, by far, the most significant carcinogenic factor in this specific context.112
Adenosquamous Carcinoma
Adenosquamous carcinoma (ASC) is a “composite” tumor, exhibiting simultaneous squamous and glandular differentiation in the same mass (Fig. 16-36). It accounts for no more than 5% of all lung cancers in most surgical series.113–115 The clinical, radiographic, and gross pathologic attributes of ASC are most similar to those of “pure” ACAs of the lung. A point of contention with regard to this lesion is whether it is synonymous with high-grade mucoepidermoid carcinoma of the salivary glandular type. Our opinion is that those two neoplasms are typically separable. Salivary gland analog tumors in the lung, as considered subsequently, tend to arise in the large central airways, in contrast to the propensity for ASC to be peripheral. In addition, foci of lower-grade mucoepidermoid carcinoma are often present in the former, but not the latter, of these tumor types. The prognosis of pulmonary ASC is said to be adverse. In studies reported by Ishida and colleagues,113 Takamori and colleagues,115 and Cakir and co-workers,116 the survival of patients with adenosquamous tumors was statistically worse than that of individuals with “pure” ACAs or SCCs.
Large Cell Carcinoma
Large cell undifferentiated carcinomas account for approximately 15% of all lung cancers.117 As mentioned earlier, diagnostic use of the term “non–small cell carcinoma” has produced some confusion among poorly differentiated SCC, poorly differentiated ACA, poorly differentiated ASC, and true LCC. Accordingly, it has been suggested that the designation of “large cell carcinoma” should be employed restrictively as a synonym for LCC. An even more extreme point of view is that current pathologic tools for cellular analysis have made this diagnosis completely obsolete.118,119
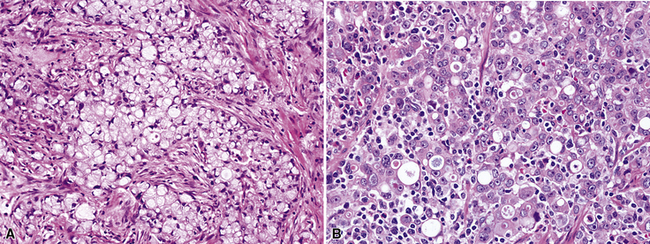
Large cell carcinomas are typically larger than 5 cm in maximum dimension, have a white-gray cut surface (which may be lobulated and resemble “fish flesh,” thus potentially simulating the appearance of a sarcoma or a hematolymphoid lesion; Fig. 16-37), and are rarely multicentric. Internal necrosis is a relatively common feature. Approximately 50% demonstrate a connection to a large tubular airway.
Histologically, LCCs show a composition of large polygonal cells with vesicular chromatin, prominent nucleoli, discernible cytoplasmic borders, and by definition, a lack of glandular differentiation or keratinization (Fig. 16-38). They are typically arranged in sheets or large clusters, potentially exhibiting foci of central necrosis. Two distinctive subtypes also exist—giant cell carcinoma and clear cell carcinoma.
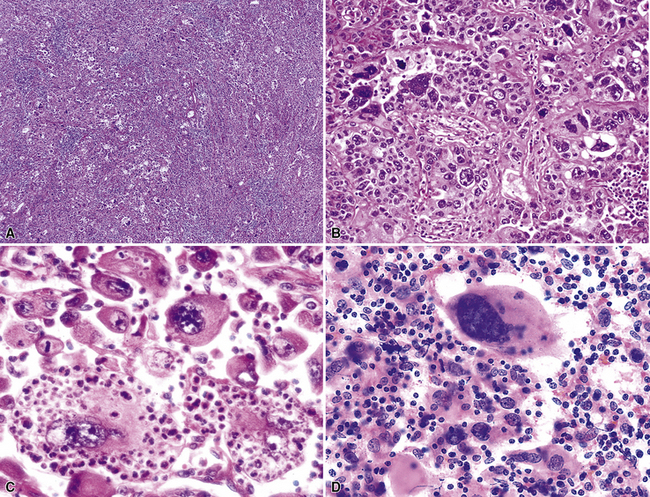
Giant cell carcinoma was initially believed to be a separate clinicopathologic entity,120,121 but that philosophy is no longer considered valid.122 Microscopically, this variant of LCC is composed of extremely pleomorphic large tumor cells, which are often multinucleated (Fig. 16-39). There is a regular admixture of polymorphonuclear leukocytes with the neoplastic elements, even in the absence of necrosis, suggesting tumoral synthesis of leukocyte cytokines, such as granulocyte colony–stimulating factor.123 Parenthetically, this phenomenon can also be associated with systemic neutrophilia in association with the giant cell subtype of LCC. Neoplastic “cannibalism” may also be observed, wherein the giant tumor cells appear to engulf one another. One subtype of pulmonary giant cell carcinoma bears a close resemblance to choriocarcinoma of the gonads, complete with tumor synthesis of beta-human chorionic gonadotropin.124
Primary clear cell carcinoma of the lung (CCCL) is a diagnosis of exclusion, and it is likely that tumors with both squamous and glandular differentiation are included in that group. A number of other clear cell neoplasms of the lung, including some “carcinoids,” the benign “sugar tumor,” metastatic renal cell carcinoma, and metastatic “balloon cell” melanoma, must also be considered before making the diagnosis of CCCL.50,125–129 This can be accomplished by a combination of radiographic, electron microscopic, and immunohistologic evaluations. The overall clinicopathologic attributes of CCCL are comparable to those of LCC, not otherwise specified.
In the last two decades, it has been suggested that a subset of pulmonary large cell carcinomas that show occult neuroendocrine differentiation—as detected only by electron microscopy or immunohistology—should be nosologically separated from truly undifferentiated large cell tumors.130–133 Hence, the terms “exocrine large cell carcinoma” (another synonym for LCC) and “endocrine large cell carcinoma” have entered use.132 In our opinion, “endocrine large cell carcinomas” are best specified as either high-grade “pure” neuroendocrine carcinomas, large cell type, or LCCs with occult neuroendocrine differentiation; those two entities are considered in more detail in another chapter on neuroendocrine neoplasms of the lung (see Chapter 13).
Selected large cell lung cancers have a “rhabdoid” phenotype134 with eosinophilic hyaline inclusions in the cytoplasm and large vesicular nuclei with prominent nucleoli (Fig. 16-40). It is likely that this image represents a final common pathway of clonal evolution (“dedifferentiation”) in carcinomas of the lung, as in several other neoplasms demonstrating a rhabdoid configuration.135 That conclusion is supported by the fact that other forms of carcinoma may be admixed with the rhabdoid elements (“composite” extrarenal rhabdoid tumors).135 Moreover, nuclear expression of the INI1 gene—a tumor suppressor—is retained in “composite” extrarenal rhabdoid tumors (Fig. 16-41), but is consistently deleted in prototypical rhabdoid tumor of the kidney in children.136–138
Returning to the issue of how a pathologic diagnosis of pulmonary LCC is made, we—and others139–141—believe that conventional light microscopic morphologic analysis is still the method of both convenience and choice. At this point, no credible data have shown significant differences in survival among cases that are categorized as LCC in prospective and rigorously constructed clinical trials employing “modern” modalities of pathologic evaluation as well as treatment, compared with the outcomes of poorly differentiated “solid” ACAs or high-grade SCCs.
Pathologic Classification of Lung Cancer and Selection of Nonsurgical Therapies
As mentioned earlier, attention to pathologic detail has been strong in the last several years on the part of clinicians, with the aim of selecting nonsurgical therapy for patients with advanced carcinomas of the lung. The ever-increasing availability of new biologic and chemical agents has driven that scrutiny. In a generic sense, the construct that has been applied by oncologists pairs the preferred management of ACAs with TKIs and that of nonglandular and neuroendocrine carcinomas with “conventional” chemotherapeutic drugs.100,140,142,143 Although initial responses to TKIs appeared to be promising in patients with lung cancer, enthusiasm recently has been tempered.144–146
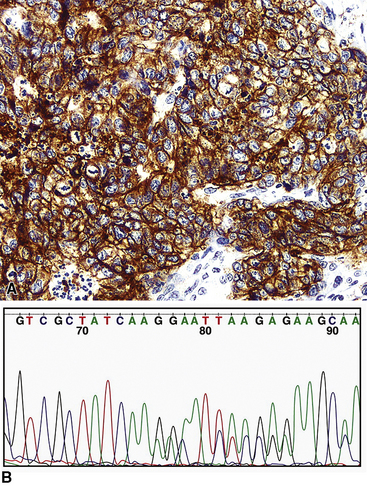
Some of this disappointment has likely been caused, at least in part, by pathologists. For example, it is common to equate strong immunoreactivity for EGFR (Fig. 16-42) with pathologic alterations in the EGFR gene; in reality, the former finding is only a crude substitute for results of molecular analyses, such as in situ hybridization or polymerase chain reaction–based methods.103,147 Moreover, the diagnostic boundaries of ACA have been “stretched” by some pathologist observers to qualify patients for entrance into TKI trials. That is particularly true in laboratories that routinely “type” lung cancers with immunohistochemical panels. For example, even though a poorly differentiated neoplasm may demonstrate an “adenocarcinoma profile” by such assessment, prospective and evidence-based clinicopathologic studies are lacking to demonstrate that this finding translates into a differential benefit of one treatment as opposed to another. Markers that are currently applied in this context include high–molecular-weight (“squamous”) keratins, CD141, and p63 for squamous carcinomas (Fig. 16-43); cytokeratin-7, napsin-A (Fig. 16-44), and TTF-1 for ACAs; mixtures of reactivities for those determinants in ASCs; and an absence of them all in LCCs.35,118,148
The problems with the approach of linking immunophenotypes with therapeutic choices are threefold. First, we reiterate the fact that lung carcinomas often demonstrate mixed patterns of differentiation at light microscopic and ultrastructural levels of analysis.3,12,24,26 Accordingly, immunoprofiles and genotypes also vary considerably, even within the same morphologic tumor groups.149–151 Secondly, large groups of high-stage, immunohistochemically “defined” lung carcinoma types have not been treated using systematic protocols after careful matching of comorbid variables in construction of the studies. Third, several studies have shown that the responses to EGFR- and K-ras–directed treatments may differ between metastatic pulmonary cancers and their primary tumors.152–154
Thus, for now, we urge caution in the reflexive immunohistochemical or immunogenetic assessment of all lung carcinomas, which is not yet a “standard of practice.” Pathologists should attempt to comply with the requirements of various developmental therapeutic trials, vis-à-vis methods for nosologic categorization, but that is different from the diagnosis of lung cancers in the current clinical environment. Sartori and associates155 have proposed the use of a scoring system that integrates clinical, morphologic, and molecular-genetic data to yield a probability score that indicates the likelihood of tumor response to EGFR inhibitors in clinical trials.
So that this commentary is not nihilistic, it can be said that some reproducible correlative trends have emerged from adjunctive studies on pulmonary carcinomas. Nonmucinous BACs, micropapillary ACAs, and ACAs comprising “hobnail” cells generally show some response to TKIs.98,102,143 In contrast, “solid” poorly differentiated ACAs often do not.143 The latter lesions more often exhibit mutations in the K-ras gene rather than in EGFR, as is true of most mucinous BACs (discussed earlier).155 Finally, some TKI-responsive ACAs have also manifested integration of nucleic acid from the human papillomavirus, at least in Asian patients.156
Salivary Gland–Type Carcinomas of the Lung
Primary salivary gland–type tumors of the lung (SGTTLs) are unusual, comprising no more than 1% of all pulmonary neoplasms.157 Moreover, their diagnosis may pose a problem not only because of their rarity but also because in small biopsies it is relatively easy to consign them to the broad category of “non–small cell carcinoma.” That would be unfortunate because the clinical behavior of SGTTLs can be quite different from that of conventional lung cancers. This family of tumors generally has an immunohistochemical profile similar to that of ordinary pulmonary carcinomas, a fact that tends to lessen the value of immunophenotyping in the differential diagnosis.
Mucoepidermoid Carcinoma
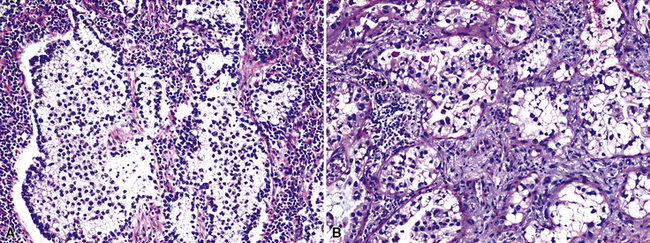
Mucoepidermoid carcinoma (MEC) is the most common of the SGTTLs and may be encountered in any age group; however, most cases have been seen in adults.158–168 In a series of cases reported by Yousem and Hochholzer,166 58 patients ranged from 9 to 78 years of age, with a male-to-female distribution of almost 1.5:1. When the tumors were separated into low- and high-grade lesions, no preference for any particular age group was noted for either group. Nevertheless, the great majority of these tumors belong in the low-grade category. Clinical symptoms in patients with MECs are dependent on the size and location of the neoplasms. Large central tumors cause symptoms of obstruction, with pneumonia, dyspnea, or chest pain.168 More peripheral lesions may be asymptomatic and are discovered on routine chest radiography.
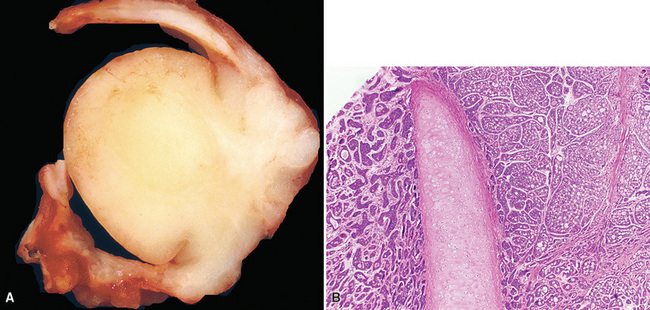
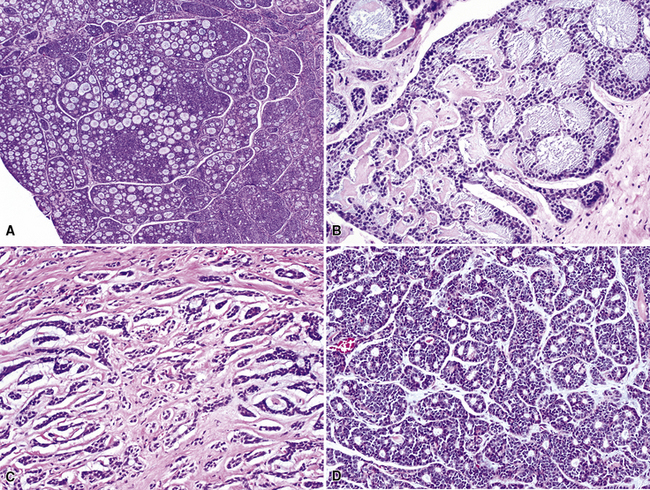
Mucoepidermoid carcinomas classically present as exophytic endobronchial tumors and are potentially greater than 5 cm in greatest diameter. They are usually well circumscribed, with smooth overlying mucosal surfaces (Fig. 16-45). On cut section, these tumors are tan-gray or yellow. They are solid or cystic, or both, and may show overtly mucoid features. There is no topographic predilection for any particular pulmonary lobe or segment. Obstruction of the bronchial lumen by tumor is often associated with postobstructive “lipoid” pneumonia (Fig. 16-46).
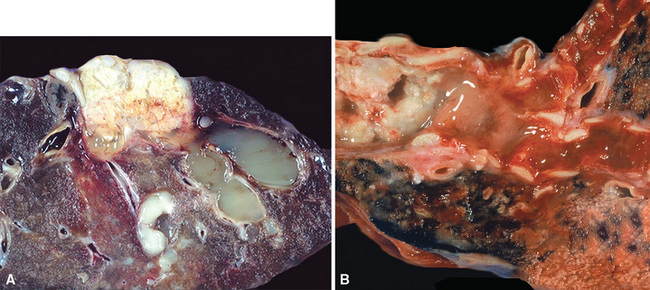
Figure 16-46 A and B, Central endobronchial mucoepidermoid carcinomas often produce postobstructive mucoid and lipoid pneumonia.
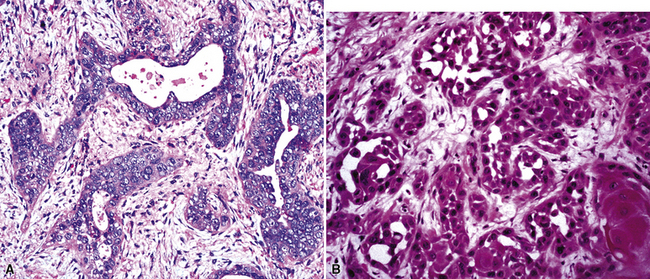
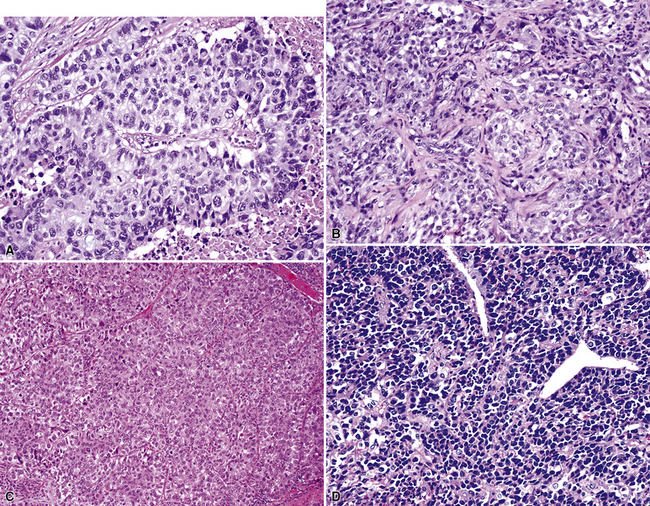
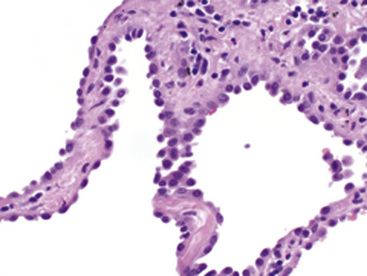
Mucoepidermoid carcinomas of the lung are classified into low-grade and high-grade tumors morphologically, principally based on their cytologic features. In low-grade lesions, the panoramic appearance is one of a neoplasm, with cystic and solid areas in close association with a tubular airway (Fig. 16-47). On closer inspection of the solid areas, it is possible to identify clear cells, squamoid cells, or transitional (intermediate) polygonal cells. These elements are interspersed with areas in which there are mucus-secreting glandular cells. Most examples of MEC do not contain large foci of keratinization. However, areas of papillary growth may be seen as well as others with spindle cell proliferation. When the last of those components is extensive, such tumors are designated as “sclerosing” MECs.
Low-grade tumors characteristically lack necrosis and hemorrhage. As mentioned earlier, the cytologic features of these tumors are bland and mitotic activity is minimal or absent (Fig. 16-48). High-grade tumors share some of the architectural features seen in low-grade lesions but manifest a much higher degree of cytologic atypia and mitotic activity (Fig. 16-49). In some cases, necrosis and hemorrhage are also present.
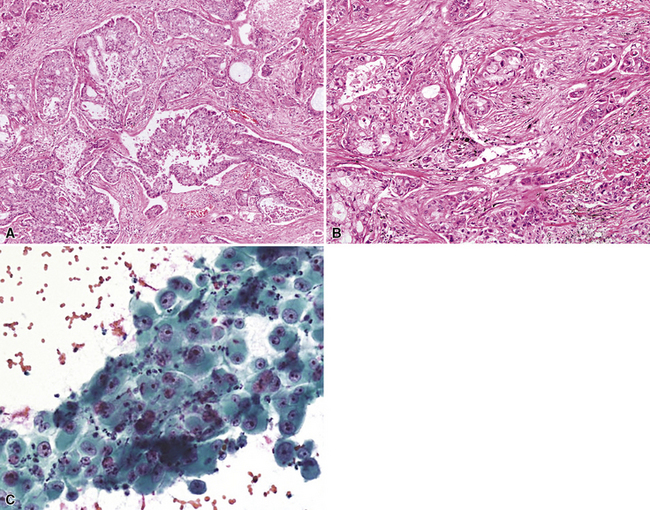
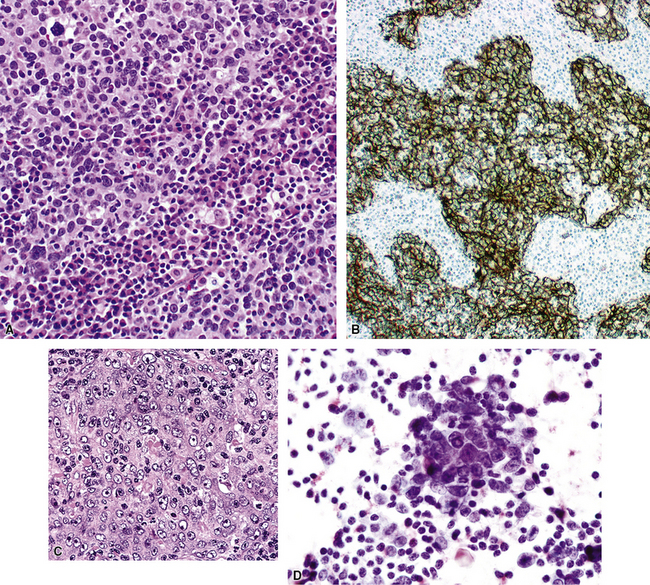
Figure 16-49 A and B, High-grade mucoepidermoid carcinoma (HGMEC) demonstrates the presence of lower-grade tumor as well as foci with a greater degree of cytologic anaplasia in both its squamoid and glandular elements (compare with Fig. 16-47). C, Fine-needle aspiration biopsy specimen of HGMEC demonstrates a relatively uniform squamoid cell population with little or no mucus in the background.
Shilo and colleagues169 described a series of low-grade tubulocystic MECs in which a prominent lymphoplasmacytic infiltrate permeated the tumors (Fig. 16-50). Russell bodies were often associated with the plasma cellular foci. The clinical presentations and behaviors of such lesions were comparable to those of ordinary MECs, but their lymphoid components raised pathologic concern over the possibility of concomitant low-grade lymphoma. Nonetheless, they were polytypic and analogous to lymphoid infiltrates that can be seen in salivary glands, with or without associated epithelial neoplasms.
The most important tumor to be distinguished from low-grade MECs, especially in small biopsies, is mucous gland adenoma (MGA). Unfortunately, a distinction between those two entities may not be possible until a complete resection of the tumor is performed. MGAs are generally confined to the internal aspect of bronchi in which they arise (luminal to the bronchial cartilage). In contrast, mucoepidermoid carcinomas commonly invade through the entirety of the bronchial wall. One possible discriminant between MEC and MGA is immunoreactivity for TTF-1; that marker appears to be present only in MGA.169 Another pertinent differential diagnostic problem is that of well-differentiated SCC; in the context under discussion, the presence of marked keratinization and the lack of demonstrable mucin in the tumor cells would favor a diagnosis of a pure squamous tumor over MEC.
The behavior of these neoplasms is related to their stage and grade. Tumors in the low-grade MEC category can be managed by resection alone, and an indolent course is expected, with rare exceptions.167,168 However, in high-grade tumors, surgical resection is usually followed by adjuvant radiation or chemotherapy, and their behavior approximates that of more ordinary forms of lung cancer.
As discussed earlier in reference to another tumor type, MECs commonly demonstrate immunolabeling for EGFR but do not show abnormal copy numbers or mutations in the EGFR gene on more detailed analysis.170,171 Hence, they are unlikely to respond to therapy with TKI agents. Several reproducible chromosomal translocations have been found in MEC, including t(1;11)(p22;q13), t(11;19)(q14-21;p12), and t(11;19)(q21;p13).167 In some instances, the cyclin-D1 gene may be up-regulated.
Adenoid Cystic Carcinoma
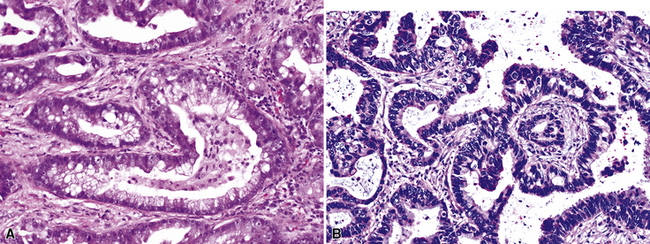
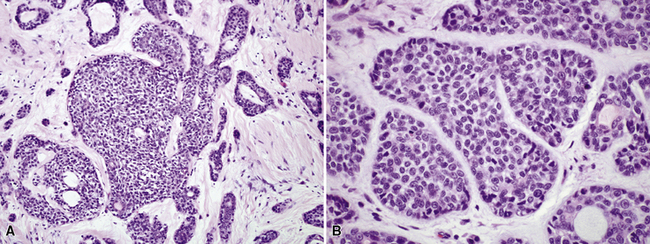
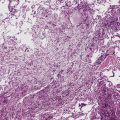
Adenoid cystic carcinoma (ACC) is the second most common SGTTL168,172–179 and typically occurs in adults. In one large series,172 patients ranged from 29 to 79 years of age (mean, 54 years) with a male-to-female ratio of 2:1. Clinically, because of their characteristic central location, pulmonary ACCs present with symptoms and signs of bronchial erosion or obstruction, including pneumonia, dyspnea, cough, wheezing, and hemoptysis. However, tumors arising in the peripheral lung have been reported as well, and these are usually asymptomatic.180 The average size of a pulmonary ACC is 4 cm, and the lesions are deceptively circumscribed on gross examination, with a soft yellow-white cut surface. Despite that attribute, surgical bronchial margins are positive for tumor more often in ACC than in other forms of lung cancer.181 This probably reflects the notorious ability of this tumor to track along neurovascular bundles and cartilaginous plates.168
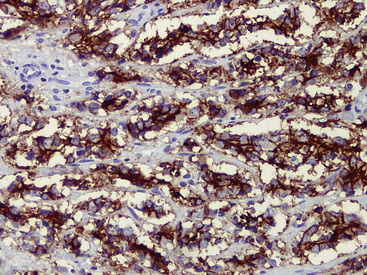
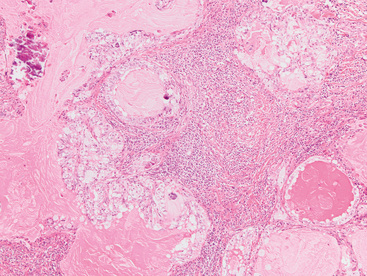
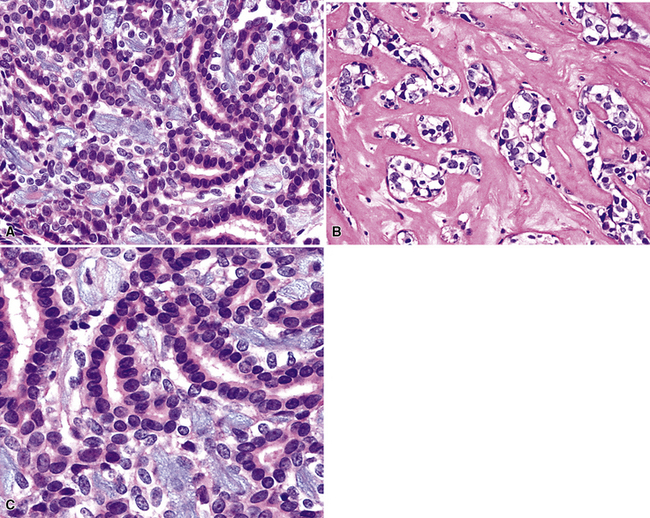
Adenoid cystic carcinoma is a prototypical tumor from a morphologic perspective, composed of monotonous arrays of compact polyhedral cells with uniformly round and hyperchromatic nucleoli and amphophilic cytoplasm. Indeed, were it not for the obviously infiltrative growth that the neoplasms exhibit—with permeation of bronchial walls, blood vessels, and perineurial sheaths (Figs. 16-51 and 16-52)—the cytologic features of ACC would not lead one to immediately interpret it as a malignant lesion.
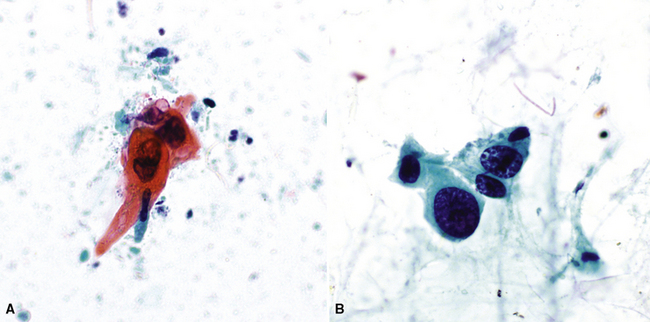
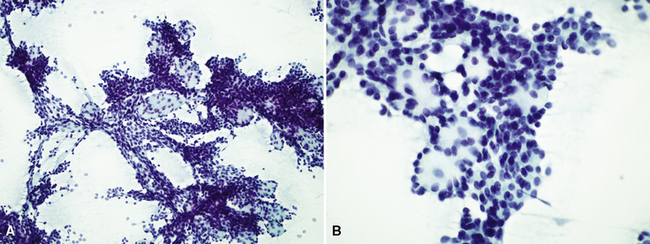
Fine-needle aspiration is effective in demonstrating the cytologic homogeneity of the tumor in most cases,182 and it also shows interspersed cylinders or spheres of eosinophilic matrical material contained within the epithelial cell groups (Fig. 16-53). Nevertheless, exceptions to these statements have been reported. Daneshbod and associates183 found that myxochondroid material—as also seen in pulmonary chondromas—could be a confounding finding in cytologic preparations from ACCs. These authors also observed nuclear molding in some lesions, similar to the image of neuroendocrine carcinoma. Ozkara and Turan184 studied an example of “solid” ACC (discussed later) cytologically; the eosinophilic stromal matrix was sparse in that tumor type. Chuah and colleagues185 suggested that bronchial brushing cytology may not be productive in ACC because the tumors are often covered by intact bronchial mucosa.
The most common histologic growth pattern is the “cylindromatous” one, reflected by islands and cords of tumor cells that are arranged in a characteristic “jigsaw puzzle piece” pattern (Figs. 16-54 and 16-55). Many cell groups encompass luminal or pseudoluminal spaces that may contain mucinous material—potentially yielding a cribriform image—and the nests are separated by bands of fibroconnective tissue with variable thickness. Cystic foci in ACC are lined by at least two layers of cells. Mitotic figures, nuclear pleomorphism, necrosis, and hemorrhage are almost always absent.
There are two additional growth patterns in pulmonary ACC that may suggest alternative diagnoses. The “tubular” pattern characteristically shows arrangement of the neoplastic cells in small gland-like spaces or elongated cylinders (Fig. 16-56). Cytologically, the tumor cells in that variant are similar to those seen in cylindromatous ACC. The “solid” variant of ACC exhibits a medullary or insular proliferation of tumor cells, with few if any intercellular spaces and only scant stromal matrix (Fig. 16-57). Mitotic activity is more brisk in this subtype. The diagnosis is furthered if abortive areas of cylindromatous differentiation can be identified; otherwise, several other tumor types with a basaloid cellular constituency enter diagnostic consideration. In particular, we have seen several cases in which a distinction between solid ACC and “basal cell adenocarcinoma” of the salivary gland type—an uncommon lesion usually seen in minor salivary glands186–188—was virtually impossible. Purely tubular or solid ACCs are rare, with most cases demonstrating mixed histologic patterns from field to field in the lesion.
Adenoid cystic carcinoma of the lung may show ultrastructural or immunohistologic evidence of partial myoepithelial differentiation. Hence, potential reactivity for keratin, vimentin, actin, and S-100 protein is observed. Immunolabeling for CD117 (c-kit protein) has been described as well (Fig. 16-58), but it is not accompanied by activating mutations in the c-kit gene.189–191 The same relationship applies to the paradoxical immunoreactivity for EGFR but a lack of demonstrable gene aberrations in ACC.170 Immunohistologic studies are usually not necessary for diagnosis, with the exception of some cases of “solid” ACC; in those lesions, stains for collagen type IV or laminin may be useful in highlighting small cylindrical stromal accumulations of basement membrane material. Tubular ACC may be confused with conventional well-differentiated ACA of the lung; however, the latter tumor type typically contains larger cells with more discernible nucleoli.
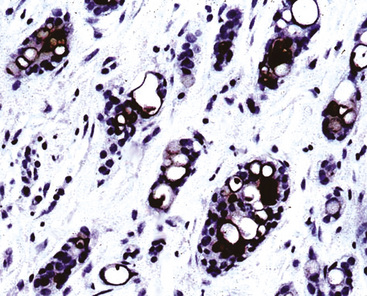
Figure 16-58 Immunoreactivity is seen for CD117 (c-kit protein) in this bronchial adenoid cystic carcinoma.
Adenoid cystic carcinomas are generally considered to be slowly growing tumors of low-grade malignancy. However, that view is deceptive in many cases. The lesions often pursue a tenaciously persistent course, with recalcitrant intrathoracic recurrences over several years, potentially culminating with distant metastasis. Tumor stage at initial diagnosis is important in determining the clinical outcome, and complete surgical excision offers the best chance of cure.168,192
Acinic Cell Carcinoma of the Lung (Fechner Tumor)
Intrapulmonary acinic cell carcinoma has been designated as the “Fechner tumor” (FT) to honor the first description of this neoplasm in 1972 by Robert Fechner and colleagues.193 It is an uncommon primary lesion in the lung but may be seen either as an endobronchial mass or a peripheral neoplasm that abuts a tubular airway.194–196 Most descriptions of FTs have been as isolated case reports. In the series that exist on acinic cell carcinomas of the lung,194 such tumors occur predominantly in adults, with an equal sex predilection. Occasional examples have been reported in children.197 Because the majority of FTs are peripherally located, most patients are asymptomatic and their tumors are discovered on screening chest radiographs. When FTs assume a central location, the patient is more likely to report dyspnea, cough, or hemoptysis.
These lesions are usually well circumscribed but not encapsulated, and they vary in size from 1 to 5 cm in greatest dimension. Their cut surfaces are tan-gray and homogeneous. Scanning microscopy shows a circumscribed lesion with internal effacement of the normal lung parenchyma. Solid growth is typical, with a composition of round-to-polygonal cells with prominently granular eosinophilic cytoplasm, round nuclei, and inconspicuous nucleoli. Mitotic activity and nuclear atypia are minimal, and areas of hemorrhage or necrosis are lacking (Figs. 16-59 and 16-60). Occasionally, FTs may comprise a proliferation of clear cells with granular cytoplasm and nuclei that are displaced toward the periphery of the cells, superficially simulating “signet ring” cells. The neoplastic cells also may be arranged in ill-defined nests separated by delicate fibroconnective tissue, with interspersed lymphocytes and plasma cells, as discussed earlier in connection with MEC. Finally, as in the salivary glands, intrapulmonary acinic cell carcinoma may show acinar, oncocytic, cystic, and papillocystic growth patterns. One unusual case has been documented in which an FT was juxtaposed with a low-grade neuroendocrine carcinoma of the lung within the same mass.198
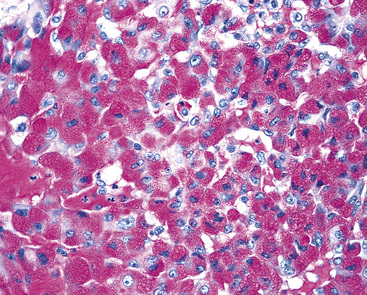
One of the most useful histochemical stains in the evaluation of acinic cell carcinoma is the periodic acid/Schiff (PAS) method, used to demonstrate glycogen in the tumor cells (Fig. 16-61). Mucicarmine stains may show foci of intracellular mucin production as well. Immunostains may demonstrate positivity for such lysosomal proteins as alpha-1-antitrypsin and amylase in the tumor cell granules.
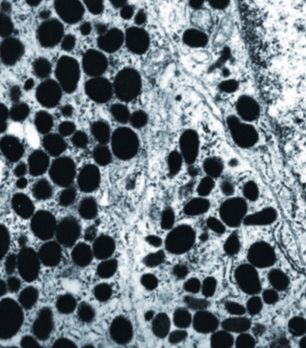
Electron microscopy is still helpful in the diagnosis of acinic cell carcinoma. The finding of large dark electron-dense or electron-lucent (immature) zymogen granules in the cytoplasm of the neoplastic cells is characteristic (Fig. 16-62).
Fechner tumors are low-grade malignancies, and they have limited but definite potential to metastasize. Examples have been reported that involved regional lymph nodes in the thorax,199,200 and one case featured a pleural recurrence.201 However, the overall clinical evolution is usually favorable.
Epithelial-Myoepithelial Carcinoma
Epithelial-myoepithelial carcinoma (EMC) is one of the most unusual primary tumors of the lung, and it also belongs to the SGTTL group. Only a few cases of this lesion have been reported202–211; they have involved adults with endobronchial masses, and because of their central location, the neoplasms were associated with obstructive symptoms and signs. EMC is considered a low-grade malignancy. It is reported to be well circumscribed but not encapsulated and may measure up to 4 cm in greatest dimension.
The scanning image of EMC is that of a predominantly glandular proliferation that is transected by thin fibroconnective stromal septa. It is centered in a large tubular airway but extends into and obliterates the adjacent lung parenchyma. On closer inspection, the glandular components of EMC show a characteristically biphasic cellular population, with inner ductal epithelial cells surrounded by an outer layer of myoepithelium (Figs. 16-63 and 16-64).207 This neoplasm does not have a high mitotic rate or notable nuclear atypia; similarly, necrosis and hemorrhage are absent. Hence, the diagnosis must be made on the basis of infiltrative architecture, together with the noted cytologic attributes. Immunohistochemically, the inner cell layer in tumoral glands shows strong reactivity for pankeratin and keratin-7, whereas the outer layer demonstrates positivity for alpha-isoform actin, p63 protein, keratin 5/6, and S-100 protein (Fig. 16-65).206,207 Because of its prominently glandular appearance, EMC can be easily confused with a well-differentiated ACA. Detailed morphologic examination and demonstration of a myoepithelial immunophenotype are keys to the correct diagnosis. Mixed tumors (pleomorphic adenomas) also manifest a similar immunohistologic profile but differ substantially on morphologic grounds from EMC (see Chapter 19).
In light of the rarity of this tumor in the lungs, it is difficult to draw overarching conclusions regarding the behavior of pulmonary EMC. Pelosi and colleagues206 reviewed the pertinent literature and concluded that the term “pulmonary epithelial-myoepithelial tumor of unproven malignant potential” was preferable to EMC. On the other hand, based on their experience, Nguyen and colleagues207 stated unequivocally that “these tumors, when in the lung, clearly have the capacity to infiltrate and metastasize, and therefore should be designated as epithelial-myoepithelial carcinoma.” Complete excision is recommended.
Histopathologic and Oncogenetic Factors with Putative Prognostic Significance for Carcinomas of the Lung
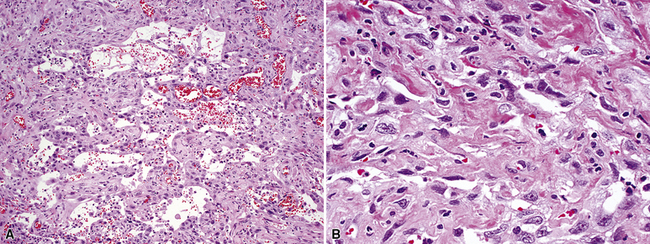
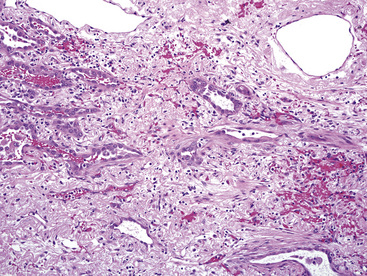
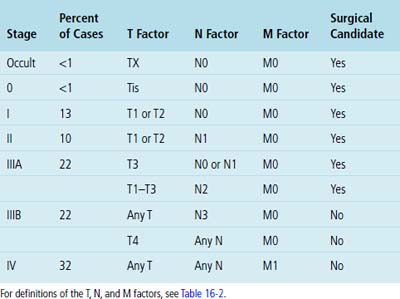
Several histologic factors that can be observed in conventionally stained microscopic sections have putative prognostic significance. Principal among these are, quite simply, the histologic type of the tumor, its level of differentiation, and the status of the surgical margins and resected lymph nodes (if any) (Tables 16-2 and 16-3).212–214 In addition, invasion of the visceral pleura by lung cancers increases their T substages and worsens survival, all other variables being equal.215 Several authors have shown that application of the Verhoeff–van Gieson elastic stain to paraffin sections enhances the pathologist’s ability to recognize this feature.216–220 A semiquantitative estimate of the degree of tumor necrosis was also reported to have predictive value by Elson and coworkers,221 in a study of non-neuroendocrine carcinomas of the lung. Moreover, documentation of angiolymphatic vascular invasion by tumor (Fig. 16-66) has similar importance,222–224 and because it is more frequently observed in ACA,222 it may explain the worsened survival rate associated with that tumor type compared with SCC of the lung.
| T—Tumor Size or Extent of Involvement | |
| TX | Tumor proven by the presence of malignant cells in bronchopulmonary secretions but not visualized roentgenographically or bronchoscopically, or any tumor that cannot be assessed as in treatment staging |
| T0 | No evidence of primary tumor |
| Tis | Carcinoma in situ |
| T1 | A tumor that is 3 cm or less in greatest dimension, surrounded by lung or visceral pleura, and without evidence of invasion proximal to a lobar bronchus at bronchoscopy. (Note: The uncommon superficial tumor of any size with its invasive component limited to the bronchial wall, which may extend proximal to the main stem bronchus, is also classified as T1.) |
| T2 | A tumor more than 3 cm in greatest dimension or a tumor of any size that either invades the visceral pleura or has associated atelectasis or obstructive pneumonitis extending to the hilar region. At bronchoscopy, the proximal extent of demonstrable tumor must be within a lobar bronchus or at least 2 cm distal to the carcinoma. Any associated atelectasis or obstructive pneumonitis must involve less than an entire lung. |
| T3 | A tumor of any size with direct extension into the chest wall (including superior sulcus tumors), diaphragm, or the mediastinal pleura or pericardium without involving the heart, great vessels, trachea, esophagus, or vertebral body, or a tumor in the main stem bronchus within 2 cm of the carina without involving the carina |
| T4 | A tumor of any size with invasion of the mediastinum or involving the heart, great vessels, trachea, esophagus, vertebral body, or carina, or the presence of malignant pleural effusion. (Note: Most pleural effusions associated with lung cancer are due to tumor. There are, however, a few patients in whom the cytopathologic findings of pleural fluid—on more than one specimen—are negative for tumor and the fluid is nonbloody and is not an exudate. In such cases, where these elements and clinical judgment dictate that the effusion is not related to the tumor, the patient should be staged TI, T2, or T3, excluding effusion as a staging element.) |
| N—Nodal Status | |
| NX | Regional lymph nodes cannot be assessed |
| N0 | No demonstrable metastasis to the regional lymph nodes |
| N1 | Metastasis to the lymph nodes in the peribronchial or ipsilateral hilar region, or both, including direct extension |
| N2 | Metastasis to the ipsilateral mediastinal lymph nodes or subcarinal lymph nodes |
| N3 | Metastasis to the contralateral mediastinal lymph nodes, contralateral hilar nodes, ipsilateral or contralateral scalene nodes, or supraclavicular lymph nodes |
| M—Distant Metastases | |
| MX | Presence of distant metastases cannot be assessed |
| M0 | No (known) distant metastasis |
| M1 | Distant metastasis present—specify site(s) |
In other anatomic sites, particularly the head and neck and axillae, extranodal extension by tumor metastases of carcinomas has been associated with worsened prognosis. Data from a study by Lee and associates225 suggest that this association also holds for lung cancers.
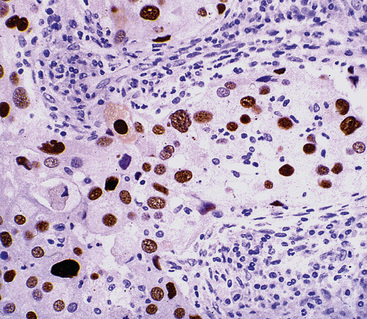
A number of investigators have examined the use of adjunctive techniques for the evaluation of proliferative activity in lung cancer. One may apply flow cytometry to measure the S-phase fraction of the tumor cell population212 or immunohistology to assess the expression of S-phase–related nuclear proteins, such as Ki-67/MIB-1/PCNA (Fig. 16-67).226 Published work on such markers has not found them to offer a great deal more information than simple counting of mitotic figures. Similarly, measurement of DNA ploidy in pulmonary carcinomas does not appear to provide prognostically valuable data.212
Over the last decade, a great deal of research has addressed the possible role of genetic alterations in predicting the clinical outcome of patients with lung cancer. In particular, aberrations of the p53, c-erbB-2, K-ras, RB-1, myc, and bcl-2 genes have been evaluated in the greatest detail, but with conflicting and inconclusive results.227–237 Therefore, we do not recommend the routine use of such assessments for prognosis. Despite the work of Fontanini and coworkers,238 purportedly demonstrating the predictive utility of microvessel counts in lung cancers, a similar comment applies to that adjunctive area of analysis.232 A meta-analysis of immunohistologic “prognosticators” by Zhu and colleagues239 has supported this position. These authors also suggested that cyclin-E, vascular endothelial growth factor-A, p16 (Cink4a), p27 (kip1), and beta-catenin were promising analytes in this context, but they should still be regarded as investigational markers.
Molecular Analysis of Gene Sets in Non-Neuroendocrine Lung Carcinomas
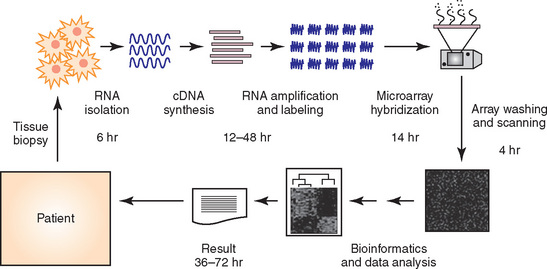
A rapidly growing area of clinical investigation on the behavior and prognosis of lung cancers is represented by “molecular” analyses of those neoplasms. As reviewed by Petty and coworkers,240 this can be accomplished with several laboratory techniques, such as “global” gene profiling, complementary deoxyribonucleic acid (cDNA) and oligonucleotide microarrays, or single-nucleotide polymorphism (SNP) microarrays. The goals of these assessments are basically twofold—to separate metastatically capable from incapable low-stage pulmonary carcinomas and to predict the likely visceral sites of metastasis for tumors that are capable of extrapulmonary growth. The genes comprising study sets in this setting are diverse, governing proteins that have roles in immune response, transcriptional modulation, cell cycle regulation, apoptosis, intracellular signaling, extracellular matrix synthesis or degradation, and angiogenesis.240,241 As suggested by Potti and colleagues,242 gene assessments should employ a “multi-metagene” model to identify reproducible biologic gene profiles because a single gene or even a small group of genes will not determine ultimate tumor behavior. In that context, assessments that have compared primary tumors with their metastases are particularly interesting. Hoang and associates243 have shown that secondary carcinomatous deposits are genetically still very similar to their primary “parents,” but differences are consistently represented by mutations in an aggregation of selected and biologically important genes in the metastatic lesions.
To date, several well-constructed and well-conducted studies have shown definite promise for this form of pathologic analysis. For example, Chen and coworkers244 identified a five-gene “signature” of non-neuroendocrine lung cancers that effectively separated survivors from nonsurvivors. Similarly, Xi and colleagues245 used gene expression profiles and “prediction analysis of microarrays” to predict the behavior of pulmonary ACAs. Patients with high-risk prediction analysis of microarrays ultimately had a worse prognosis overall, even if their morphologic tumor substage was T1–T2/pN0. The latter finding implies that gene profiling is capable of identifying patients with distant metastasis but uninvolved regional lymph nodes—in other words, patients with stage I tumors that ultimately will not respond to treatment. It also runs counter to the often-held premise that carcinomatous metastasis invariably proceeds in a linear fashion through lymph node groups before spreading to visceral organs.246 Other studies have shown comparable predictive abilities and reached similar conclusions.247–253
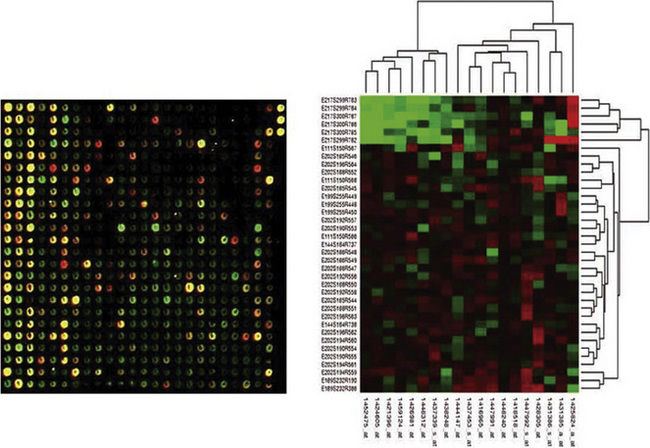
We believe that gene profiling will eventually become an integrated part of the pathologic evaluation of lung cancers. When and how that occurs must await future developments. Nonetheless, genetic analysis—using methods such as “heat mapping” of gene expression (Fig. 16-68)—can be done in a relatively rapid fashion, potentially providing clinicians with important data for treatment planning (Fig. 16-69).254 In an optimal scenario, that information could hypothetically be used as depicted in Figure 16-70.
Problems with Current Staging Protocols for Lung Carcinomas
Pathologic staging systems for lung cancer have undergone several alterations through the last 15 years, under the aegis of such organizations as the American Joint Committee on Cancer (AJCC), the International Union for Cancer Control, and the International Association for the Study of Lung Cancer Staging Committee. Pertinent changes and remaining shortcomings of existing staging schemes have been well summarized by Flieder.255 These are summarized as follows:
Intraoperative Consultations in Lung Carcinoma Cases
Intraoperative consultations (IOPs) are commonly requested of pathologists by thoracic surgeons in the course of procedures for presumed or proven pulmonary carcinomas.258 Confirmation of malignant diagnoses, determination of the status of bronchial or soft tissue margins, and examination of regional lymph nodes for possible tumor involvement are the principal reasons for such interactions.258–262 The pathologist may elect to perform any or all of a variety of examinations, including “naked-eye” perusal, frozen (cryostat) sections, touch-imprint slide preparations, or ex vivo fine-needle aspiration.263 Several studies have affirmed the value and the efficacy of these procedures; however, there is an irreducible margin of error (approximately 1% to 2% of cases overall), of which clinicians should be apprised.264 Frozen sections are not equivalent to permanent sections of paraffin-embedded tissues with regard to histologic detail and the scope of tissue sampling.
Several particularly difficult, if not impossible, scenarios can be encountered in this context. The first concerns requests for a primary tissue diagnosis because none has been obtained previously through biopsy. In some circumstances—alluded to earlier—even that process can be complicated. For example, distinguishing BAC from florid reactive pneumocytic proliferations is a potential problem,88 as are the definitive recognition of “carcinoid tumors” and the diagnostic separation of solitary metastasis of a previous nonpulmonary malignancy from a primary lung cancer.265,266 Useful guidelines have been advanced for morphologic findings that are useful in these IOPs,88,265–267 but those recommendations are not foolproof.181,260,261,264,267,268
Selected publications have suggested that “ultra-rapid” immunohistochemical studies could be done during an IOP to facilitate histopathologic interpretations.269,270 However, most surgical pathology laboratories are not equipped to undertake these procedures, lacking the necessary staffing and funding. We would argue that they are not necessary in a pragmatic sense. If the questions are “primary tumor or solitary metastasis” and “small peripheral carcinoma or localized benign pseudotumor,” the surgeon can be advised to perform limited but complete resection of the mass, optimally with additional sampling of regional lymph nodes. Such an approach typically does not compromise the overall prognosis if the lesion proves to be a primary lung cancer; furthermore, it is not associated with significant morbidity if the mass is determined to be either metastastic or benign.
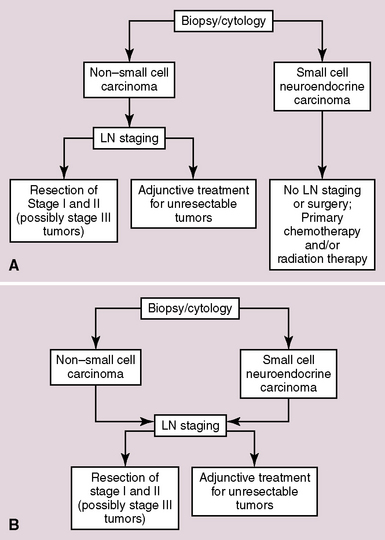
A related topic is the traditional onus for pathologists to separate “small cell” from “non–small cell” carcinomas in primary diagnoses made during IOPs.271 Based on information that has also been presented in Chapter 13, we believe that the latter paradigm is outmoded. The more appropriate question pertaining to surgery for any given lung cancer should be “resectable or nonresectable,” regardless of cell type and tumor grade. The answer to that query, in turn, principally hinges on the stage of the neoplasm as determined by results of radiographic imaging and intraoperative tissue sampling. Recent publications confirm the contention that patients with limited-stage small cell carcinoma do benefit from surgical intervention.272,273
These “standard” and “revisionist” approaches to treatment, in which IOP often plays a role, are summarized in Figure 16-73.
Stylized Surgical Pathology Reports on Carcinoma of the Lung
Obviously, not all pulmonary carcinomas are resectable. Colby and Deschamps274 have nicely summarized the clinicopathologic features of these tumors, including that subset that is surgically approachable (Table 16-4). For lesions that can be completely excised, pathology organizations such as the College of American Pathologists and the Association of Directors of Anatomic & Surgical Pathology (ADASP) have published guidelines for the current reporting of morphologic findings.275,276 In our opinion, the most tenable is that provided by the latter of those two organizations, which is reproduced in modified form here.276
Suggested ADASP Reporting Format for Resected Lung Carcinomas
1 Wingo P.A., Tong T., Boldens S. Cancer statistics, 1995. Ca. 1995;45:8-30.
2 Andre F., Jacot W., Pujol J.L., Grunenwald D., LeChevalier T. Epidemiology, prognostic factors, staging, and treatment of non-small cell lung cancer. Bull Cancer. 1999;3(suppl):17-41.
3 Fraire A.E. Pathology of lung cancer. In: Aisner J., Arriagada R., Green M.R., Martini N., Perry M.C., editors. Comprehensive Textbook of Thoracic Oncology. Baltimore: Williams & Wilkins; 1996:245-275.
4 Davila D.G., Williams D.E. The etiology of lung cancer. Mayo Clin Proc. 1993;68:170-182.
5 Franceschi S., Bidoli E. The epidemiology of lung cancer. Ann Oncol. 1999;10(suppl):S3-S6.
6 Emmons K.M. Smoking cessation and tobacco control: an overview. Chest. 1999;116(suppl3):490S-492S.
7 Kubina M., Hedelin G., Charloux A., et al. Do patients with squamous cell carcinoma or adenocarcinoma of the lung have different smoking histories? Rev Mal Respir. 1999;16:539-549.
8 Boffetta P., Nyberg F., Agudo A., et al. Risk of lung cancer from exposure to environmental tobacco smoke from cigars, cigarillos, and pipes. Int J Cancer. 1999;83:805-806.
9 Lubin J.H. Estimating lung cancer risk with exposure to environmental tobacco smoke. Environ Health Perspect. 1999;107(suppl 6):879-883.
10 The World Health Organization histological typing of lung tumors, 2nd edn. Am J Clin Pathol. 1982;77:123-136.
11 Nolan L., Eccles D., Cross E., et al. First case report of Muir-Torre syndrome associated with non-small cell lung cancer. Fam Cancer. 2009;8:359-362.
12 Hassan M.M., Phan A., Li D., Dagohoy C.G., Leary C., Yao J.C. Family history of cancer and associated risk of developing neuroendocrine tumors: a case-control study. Cancer Epidemiol Biomarkers Prev. 2008;17:959-965.
13 Weinstein A., Nouri K., Bassiri-Tehrani S., Flores F., Jimenez G. Muir-Torre syndrome: a case of this uncommon entity. Int J Dermatol. 2006;45:311-313.
14 Lynch H.T., Katz D.A., Bogard P., Lynch J.F. Cancer genes, multiple primary cancers, and von Hippel-Lindau disease. Cancer Genet Cytogenet. 1985;16:13-19.
15 Lynch H.T., Fain P.R., Albano W.A., et al. Genetic-epidemiological findings in a study of smoking-associated tumors. Cancer Genet Cytogenet. 1982;6:163-169.
16 Lynch H.T., Fusaro R.M., Pester J., et al. Tumor spectrum in the FAMMM syndrome. Br J Cancer. 1981;44:553-560.
17 Lissowska J., Foretove L., Dabek J., et al. Family history and lung cancer risk: international multicenter case-control study in Eastern and Central Europe and meta- analyses. Cancer Causes Control. 2010. March 21 (E-pub before print)
18 Landi M.T., Chatterjee N., Yu K., et al. A genome-wide association study of lung cancer identifies a region of chromosome 5p15 associated with risk for adenocarcinoma. Am J Hum Genet. 2009;85:679-691.
19 Carney A., Sheahan K., Keegan D., Tolan M., Hyland J., Green A. Synchronous lung tumors in a patient with metachronous colorectal carcinoma and a germline MSH2 mutation. J Clin Pathol. 2009;62:471-473.
20 Nitadori J., Inoue M., Iwasaki M., et al. Association between lung cancer incidence and family history of lung cancer: data from a large-scale population-based cohort study, the JPHC study. Chest. 2006;130:968-975.
21 Li X., Hemminki K. Familial and second lung cancers: a nation-wide epidemiologic study from Sweden. Lung Cancer. 2003;39:255-263.
22 Yesner R., Seydel G., Asbell S.O., et al. Biopsies of non-small cell lung cancer: central review in cooperative studies of the radiation therapy oncology group. Mod Pathol. 1991;4:432-440.
23 Travis W.D., Brambilla E., Muller-Hermelink H.K., Harris C.C., editors. Pathology & Genetics of Tumors of the Lung, Pleura, Thymus, & Heart. Geneva: World Health Organization, 2004.
24 Olcott C.T. Cell types and histologic patterns in carcinoma of the lung: observations on the significance of tumors containing more than one type of cell. Am J Pathol. 1955;31:975-995.
25 Fraire A.E., Cooper S.P., Greenberg S.D., Buffler P.A. Carcinoma of the lung: changing cell distribution and histopathologic cell types. Prog Surg Pathol. 1992;12:129-149.
26 Motoi N., Szoke J., Riely G.J., et al. Lung adenocarcinoma: modification of the 2004 WHO mixed subtype to include the major histologic subtype suggests correlations between papillary and micropapillary adenocarcinoma subtypes, EGFR mutations and gene expression analysis. Am J Surg Pathol. 2008;32:810-827.
27 Roggli V.L., Vollmer R.T., Greenberg S.D., et al. Lung cancer heterogeneity: a blinded and randomized study of 100 consecutive cases. Hum Pathol. 1985;16:569-579.
28 Fraire A.E., Roggli V.L., Vollmer R.T., et al. Lung cancer heterogeneity: prognostic implications. Cancer. 1987;60:370-379.
29 Selvaggi G., Scagliotti G.V. Histologic subtype in NSCLC: does it matter? Oncology (Williston Park). 2009;23:1133-1140.
30 Butnor K.J. Avoiding underdiagnosis, overdiagnosis, and misdiagnosis of lung carcinoma. Arch Pathol Lab Med. 2008;132:1118-1132.
31 Vincent R.G., Pickren J.W., Lane N.V.W., et al. The changing histopathology of lung cancer: a review of 1682 cases. Cancer. 1977;39:1617-1655.
32 Fishback N.F., Travis W.D., Moran C.A., et al. Pleomorphic (spindle/giant-cell) carcinoma of the lung: a clinicopathologic correlation of 78 cases. Cancer. 1994;73:2936-2945.
33 Franks T.J., Galvin J.R. Sarcomatoid carcinoma of the lung: histologic criteria and common lesions in the differential diagnosis. Arch Pathol Lab Med. 2010;134:49-54.
34 Mochizuki T., Ishii G., Nagai K., et al. Pleomorphic carcinoma of the lung: clinicopathologic characteristics of 70 cases. Am J Surg Pathol. 2008;32(11):1727-1735.
35 Martin L.W., Correa A.M., Ordonez N.G., et al. Sarcomatoid carcinoma of the lung: a predictor of poor prognosis. Ann Thorac Surg. 2007;84:973-980.
36 Nappi O., Swanson P.E., Wick M.R. Pseudovascular adenoid squamous cell carcinoma of the lung: clinicopathologic features of three cases and comparison with true pleuropulmonary angiosarcoma. Hum Pathol. 1994;25:373-378.
37 Ritter J.H., Mills S.E., Nappi O., Wick M.R. Angiosarcoma-like neoplasms of epithelial organs: true endothelial tumors or variants of carcinoma? Semin Diagn Pathol. 1995;12:270-282.
38 Chang Y.L., Wu C.T., Shih J.Y., Lee Y.C. New aspects in clinicopathologic and oncogene studies of 23 pulmonary lymphoepithelioma-like carcinomas. Am J Surg Pathol. 2002;26:715-723.
39 Brambilla E., Moro D., Veale D., et al. Basal cell (basaloid) carcinoma of the lung: a new morphologic and phenotypic entity with separate prognostic significance. Hum Pathol. 1992;23:993-1003.
40 Koss M.N., Travis W.D., Moran C.A., Hochholzer L. Pseudomesotheliomatous adenocarcinoma: a reappraisal. Semin Diagn Pathol. 1992;9:117-123.
41 Dessy E., Pietra G.G. Pseudomesotheliomatous adenocarcinoma of the lung: an immunohistochemical and ultrastructural study of three cases. Cancer. 1991;68:1747-1753.
42 Lin J.I., Tseng C.H., Tsung S.H. Pseudomesotheliomatous carcinoma of the lung. South Med J. 1980;73:655-657.
43 Moran C.A. Pulmonary adenocarcinoma: the expanding spectrum of histologic variants. Arch Pathol Lab Med. 2006;130:958-962.
44 DaCosta N., Sivararnan A., Kinare S.G. Carcinoma of lung with special reference to adenocarcinoma: an autopsy study of 122 cases. Ind J Cancer. 1993;30:42-47.
45 Iezumi K., Masunaga A., Kadofuku T., et al. Combined small cell carcinoma with pulmonary blastoma and adenocarcinoma: case report and clonality analysis. Pathol Res Pract. 2006;202:895-899.
46 Solis L.M., Raso M.G., Kalhor N., Behrens C., Wistuba I.I., Moran C.A. Primary oncocytic adenocarcinomas of the lung: a clinicopathologic, immunohistochemical, and molecular biologic analysis of 16 cases. Am J Clin Pathol. 2010;133:133-140.
47 Weidner N. Pulmonary adenocarcinoma with intestinal-type differentiation. Ultrastruct Pathol. 1992;16:7-10.
48 Hayashi H., Kitamura H., Nakatani Y., et al. Primary signet-ring-cell carcinoma of the lung: histochemical and immunohistochemical characterization. Hum Pathol. 1999;30:378-383.
49 Moran C.A., Hochholzer L., Fishback N., Travis W.D., Koss M.N. Mucinous (so-called colloid) carcinomas of lung. Mod Pathol. 1992;5:634-638.
50 Gaffey M.J., Mills S.E., Ritter J.H. Clear cell tumors of the lower respiratory tract. Semin Diagn Pathol. 1997;14:222-232.
51 Nakatani Y., Kitamura H., Inayama Y., et al. Pulmonary adenocarcinomas of the fetal lung type: a clinicopathologic study indicating differences in histology, epidemiology, and natural history of low-grade and high-grade forms. Am J Surg Pathol. 1998;22:399-411.
52 Li H.C., Schmidt L., Greenson J.K., Chang A.C., Myers J.L. Primary pulmonary adenocarcinoma with intestinal differentiation mimicking metastatic colorectal carcinoma: case report and review of literature. Am J Clin Pathol. 2009;131:129-133.
53 Colby T.V., Koss M.N., Travis W.D. Tumors of the lower respiratory tract. In: Atlas of Tumor Pathology, Series 3, Fascicle 13. Washington, DC: Armed Forces Institute of Pathology; 1995:203-234.
54 Higashiyama M., Doi O., Kodama K., et al. Extramammary Paget’s disease of the bronchial epithelium. Arch Pathol Lab Med. 1991;115:185-188.
55 Ordonez N.G. Immunohistochemical diagnosis of epithelioid mesothelioma: a critical review of old markers and new markers. Hum Pathol. 2002;33:953-967.
56 Liebow A.A. Bronchioloalveolar carcinoma. Adv Intern Med. 1960;10:329-358.
57 Clayton F. Bronchioloalveolar carcinomas: cell types, patterns of growth, and prognostic correlates. Cancer. 1986;57:1555-1564.
58 Clayton F. The spectrum of significance of bronchioloalveolar carcinomas. Pathol Annu. 1988;23(2):361-394.
59 Feldman E.R., Eagan R.T., Schaid J. Metastatic bronchioloalveolar carcinoma and metastatic adenocarcinoma of the lung: comparison of clinical manifestations, chemotherapeutic responses, and prognosis. Mayo Clin Proc. 1992;67:27-32.
60 Grover F.L., Piantadosi S. Recurrence and survival following resection of bronchioloalveolar carcinoma of the lung – the Lung Cancer Study Group experience. Ann Surg. 1989;209:779-790.
61 Lozowski W., Hajdu S.I. Cytology and immunocytochemistry of bronchioloalveolar carcinoma. Acta Cytol. 1987;31:717-725.
62 Manning J.T., Spjut H.J., Tschen J.A. Bronchioloalveolar carcinoma: the significance of two histopathologic types. Cancer. 1984;54:525-534.
63 Marcq M., Galy P. Bronchioloalveolar carcinoma: clinicopathological relationships, natural history, and prognosis in 29 cases. Am Rev Resp Dis. 1973;107:621-629.
64 Rosenblatt M.B., Lisa J.R., Collier F. Primary and metastatic bronchioloalveolar carcinoma. Chest. 1967;52:147-152.
65 Schulze E.S., Mattia A.R., Chew F.S. Bronchioloalveolar carcinoma. Am J Roentgenol. 1994;162:1294.
66 Sutton L.N., Morrison J.F., Rees M.R. Radiographic features and prognosis in bronchioloalveolar carcinoma: a local experience. Resp Med. 1989;83:471-477.
67 Fukui T., Mitsudomi T. Small peripheral lung adenocarcinoma: clinicopathological features and surgical treatment. Surg Today. 2010;40:191-198.
68 Mizutani Y., Nakajima T., Morinaga S., et al. Immunohistochemical localization of pulmonary surfactant apoproteins in various lung tumors, with special reference to lung adenocarcinoma subtypes. Cancer. 1988;61:532-537.
69 Sorensen J.B., Hirsch F.R., Gazdar A., Olsen J.E. Interobserver variability in histopathologic subtyping and grading of pulmonary adenocarcinoma. Cancer. 1993;71:2971-2976.
70 Yokose T., Suzuki K., Nagai K., Nishiwaki Y., Sasaki S., Ochiai A. Favorable and unfavorable morphological prognostic factors in peripheral adenocarcinoma of the lung 3 cm or less in diameter. Lung Cancer. 2000;29:179-188.
71 Sakurai H., Maeshima A., Watanabe S., et al. Grade of stromal invasion in small adenocarcinoma of the lung: histopathological minimal invasion and prognosis. Am J Surg Pathol. 2004;28:198-206.
72 Barsky S.H., Grossman D.A., Ho J., Holmes E.C. The multifocality of bronchioloalveolar lung carcinoma: evidence and implications of a multiclonal origin. Mod Pathol. 1994;7:633-640.
73 Grotte D., Stanley M.W., Swanson P.E., et al. Reactive type II pneumocytes in bronchoalveolar lavage fluid from acute respiratory syndrome can be mistaken for cells of adenocarcinoma. Diagn Cytopathol. 1990;6:317-322.
74 Ritter J.H., Wick M.R., Reyes A.R., Coffin C.M., Dehner L.P. False-positive interpretations of carcinoma in exfoliative respiratory cytology: report of two cases and a review of underlying disorders. Am J Clin Pathol. 1995;104:133-140.
75 Mori M., Chiba R., Takahashi T. Atypical adenomatous hyperplasia of the lung and its differentiation from adenocarcinoma: characterization of atypical cells by morphometry and multivariate cluster analysis. Cancer. 1993;72:2331-2340.
76 Hegg C.A., Flint A., Singh G. Papillary adenoma of the lung. Am J Clin Pathol. 1992;97:393-397.
77 Stenhouse G., Fyfe N., King G., Chapman A., Kerr K.M. Thyroid transcription factor 1 in pulmonary adenocarcinoma. J Clin Pathol. 2004;57:383-387.
78 Beasley M.B. Immunohistochemistry of pulmonary and pleural neoplasia. Arch Pathol Lab Med. 2008;132:1062-1072.
79 Ueno T., Linder S., Elmberger G. Aspartic proteinase napsin is a useful marker for diagnosis of primary lung adenocarcinoma. Br J Cancer. 2003;88:1229-1233.
80 Bishop J.A., Sharma R., Illei P.B. Napsin A and thyroid transcription factor-1 expression in carcinomas of the lung, breast, pancreas, colon, kidney, thyroid, and malignant mesotheliomas. Hum Pathol. 2010;41:20-25.
81 Yamato Y., Tsuchida M., Watanabe T., et al. Early results of a prospective study of limited resection for bronchioloalveolar adenocarcinoma of the lung. Ann Thorac Surg. 2001;71:971-974.
82 Watanabe S., Oda M., Tsunezuka Y., Go T., Ohta Y., Watanabe G. Peripheral small-sized (2 cm or less) non-small cell lung cancer with mediastinal lymph node metastasis; clinicopathologic features and patterns of nodal spread. Eur J Cardiothorac Surg. 2002;22:995-999.
83 Koike T., Yamato Y., Yoshiya K., et al. Criteria for intentional limited pulmonary resection in cT1N0M0 peripheral lung cancer. Jpn J Thorac Cardiovasc Surg. 2003;51:515-519.
84 Ishiwa N., Ogawa N., Shoji A., et al. Correlation between lymph node micrometastasis and histologic classification of small lung adenocarcinomas, in considering the indication of limited surgery. Lung Cancer. 2003;39:159-164.
85 Rusch V.W., Tsuchiya R., Tsuboi M., Pass H.I., Grunenwald D., Goldstraw P. Surgery for bronchioloalveolar carcinoma and “very early” adenocarcinoma: an evolving standard of care? J Thorac Oncol. 2006;1(suppl 9):S27-S31.
86 Kitamura H., Kameda Y., Ito T., et al. Atypical adenomatous hyperplasia of the lung: implications for the pathogenesis of peripheral lung adenocarcinoma. Am J Clin Pathol. 1999;111:610-622.
87 Miller R.R., Nelerris B., Evans K.G., et al. Glandular neoplasia of the lung: a proposed analogy to colonic tumors. Cancer. 1988;61:1009-1014.
88 Gupta R., McKenna R.Jr, Marchevsky A.M. Lessons learned from mistakes and deferrals in the frozen section diagnosis of bronchioloalveolar carcinoma and well-differentiated pulmonary adenocarcinoma: an evidence-based pathology approach. Am J Clin Pathol. 2008;130:11-20.
89 Sheikh H.A., Fuhrer K., Cieply K., Yousem S. p63 expression in assessment of bronchioloalveolar proliferations of the lung. Mod Pathol. 2004;17:1134-1140.
90 Saad R.S., Liu Y.L., Silverman J.F. Distribution of basal-myoepithelial markers in benign and malignant bronchioloalveolar proliferations of the lung. Appl Immunohistochem Mol Morphol. 2010;18:219-225.
91 Huang M.S., Colby T.V., Goellner J.R., et al. Utility of bronchoalveolar lavage in the diagnosis of drug-induced pulmonary toxicity. Acta Cytol. 1989;33:533-538.
92 Rao S.K., Fraire A.E. Alveolar cell hyperplasia in association with adenocarcinoma of the lung. Mod Pathol. 1995;8:165-169.
93 Miller R.R. Bronchioloalveolar cell adenomas. Am J Surg Pathol. 1990;14:904-912.
94 Nakanishi K. Alveolar epithelial hyperplasia and adenocarcinoma of the lung. Arch Pathol Lab Med. 1990;114:363-368.
95 Travis W.D., Linnoila R.I., Horowitz M., et al. Pulmonary nodules resembling bronchioloalveolar carcinoma in adolescent cancer patients. Mod Pathol. 1988;1:372-377.
96 Niho S., Yokose T., Suzuki K., et al. Monoclonality of atypical adenomatous hyperplasia of the lung. Am J Pathol. 1999;154:249-254.
97 Greenberg A.K., Yee H., Rom W.N. Preneoplastic lesions of the lung. Respir Res. 2002;3:20.
98 Garfield D.H., Cadranel J.L., Wislez M., Franklin W.A., Hirsch F.R. The bronchioloalveolar carcinoma and peripheral adenocarcinoma spectrum of diseases. J Thorac Oncol. 2006;1:344-359.
99 Morandi L., Asioli S., Cavazza A., Pession A., Damiani S. Genetic relationship among atypical adenomatous hyperplasia, bronchioloalveolar carcinoma and adenocarcinoma of the lung. Lung Cancer. 2007;56:35-42.
100 Wu M., Orta L., Gil J., Li G., Hu A., Burstein D.E. Immunohistochemical detection of XIAP and p63 in adenomatous hyperplasia, atypical adenomatous hyperplasia, bronchioloalveolar carcinoma and well-differentiated adenocarcinoma. Mod Pathol. 2008;21:553-558.
101 Raz D.J., He B., Rosell R., Jablons D.M. Current concepts in bronchioloalveolar carcinoma biology. Clin Cancer Res. 2006;12:3698-3704.
102 Inamura K., Ninomiya H., Ishikawa Y., Matsubara O. Is the epidermal growth factor receptor status in lung cancers reflected in clinicopathologic features? Arch Pathol Lab Med. 2010;134:66-72.
103 Li A.R., Chitale D., Riely G.J., et al. EGFR mutations in lung adenocarcinomas: clinical testing experience and relationship to EGFR gene copy number and immunohistochemical expression. J Mol Diagn. 2008;10:242-248.
104 Kawakami T., Nabeshima K., Makimoto Y., et al. Micropapillary pattern and grade of stromal invasion in pT1 adenocarcinoma of the lung: usefulness as prognostic factors. Mod Pathol. 2007;20:514-521.
105 Sánchez-Mora N., Presmanes M.C., Monroy V., et al. Micropapillary lung adenocarcinoma: a distinctive histologic subtype with prognostic significance– Case series. Hum Pathol. 2008;39:324-330.
106 Rudomina D.E., Lin O., Moreira A.L. Cytologic diagnosis of pulmonary adenocarcinoma with micropapillary pattern: does it correlate with the histologic findings? Diagn Cytopathol. 2009;37:333-339.
107 Meyer E.C., Leibow A.A. Relationship of interstitial pneumonia honeycombing and atypical epithelial proliferation to cancer of the lung. Cancer. 1965;18:322-351.
108 Shimosato Y., Noguchi M., Matsuno Y. Adenocarcinoma of the lung: its development and malignant progression. Lung Cancer. 1993;9:99-108.
109 Cagle P.T., Cohle S.D., Greenberg S.D. Natural history of pulmonary scar cancers: clinical and prognostic implications. Cancer. 1985;56:2031-2035.
110 Barsky S.H., Huang S.J., Bhuta S. The extracellular matrix of pulmonary scar carcinomas is suggestive of a desmoplastic origin. Am J Pathol. 1986;124:412-419.
111 Harris J.M., Johnston I.D., Rudd R., Taylor A.J., Cullinan P. Cryptogenic fibrosing alveolitis and lung cancer: the BTS study. Thorax. 2010;65:70-76.
112 Attanoos R.L., Thomas D.H., Gibbs A.R. Synchronous diffuse malignant mesothelioma and carcinomas in asbestos-exposed individuals. Histopathology. 2003;43:387-392.
113 Takamori S., Noguchi M., Morinaga S., et al. Clinical pathologic characteristics of adenosquamous carcinoma of the lung. Cancer. 1991;67:649-654.
114 Sridhar K.S., Bounassi M.J., Raub W., Richman S.P. Clinical features of adenosquamous lung carcinoma in 127 patients. Am Rev Respir Dis. 1990;142:19-23.
115 Ishida T., Kaneko S., Yokohama H., et al. Adenosquamous carcinoma of the lung: clinicopathologic and immunohistochemical features. Am J Clin Pathol. 1992;97:678-695.
116 Cakir E., Demirag E., Aydin M., Unsal E. Clinicopathologic features and prognostic significance of lung tumours with mixed histologic patterns. Acta Chir Belg. 2009;109:489-493.
117 Carter D., Patchefsky A.S. Tumors & tumor-like conditions of the lung. Philadelphia: WB Saunders, 1998;266-285.
118 Pardo J., Martinez-Penuela M., Sola J.J., et al. Large-cell carcinoma of the lung: an endangered species? Appl Immunohistochem Mol Morphol. 2009;17:383-392.
119 Carvalho L. Reclassifying bronchial-pulmonary carcinoma: differentiating histological type in biopsies by immunohistochemistry. Rev Port Pneumol. 2009;15:1101-1119.
120 Nash G., Stout A.P. Giant cell carcinoma of the lung: report of 5 cases. Cancer. 1958;11:369-376.
121 Ginsberg S.S., Buzaid A.C., Stern H., Carter D. Giant cell carcinoma of the lung. Cancer. 1992;70:606-610.
122 Attanoos R.L., Papagiannis A., Suttinont P., et al. Pulmonary giant cell carcinoma: pathological entity or morphological phenotype? Histopathology. 1998;32:225-231.
123 Sawyers C.L., Golde D.W., Quan S., Nimer S.D. Production of granulocyte-macrophage colony stimulating factor in two patients with lung cancer, leukocytosis, and eosinophilia. Cancer. 1992;69:1342-1346.
124 Vegh G.L., Szigetvári I., Soltesz I., et al. Primary pulmonary choriocarcinoma: a case report. J Reprod Med. 2008;53:369-372.
125 Shimosato Y. Lung tumors of uncertain histogenesis. Semin Diagn Pathol. 1995;12:185-192.
126 Yoshida J., Nagai K., Hasebe T., et al. Pulmonary metastasis of renal cell carcinoma resected sixteen years after nephrectomy. Jpn J Clin Oncol. 1995;25:20-24.
127 Bonetti F., Pea M., Martignoni G., et al. Clear cell (“sugar”) tumor of the lung is a lesion strictly related to angiomyolipoma – the concept of a family of lesions characterized by the presence of the perivascular epithelioid cell (PEC). Pathology. 1994;26:230-236.
128 Gaffey M.J., Mills S.E., Frierson H.F.Jr, Askin F.B., Maygarden S.J. Pulmonary clear cell carcinoid tumor: another entity in the differential diagnosis of pulmonary clear cell neoplasia. Am J Surg Pathol. 1998;22:1020-1025.
129 Nowak M.A., Fatteh S.M., Campbell T.E. Glycogen-rich malignant melanomas and glycogen-rich balloon cell malignant melanomas: frequency and pattern of PAS positivity in primary and metastatic melanomas. Arch Pathol Lab Med. 1998;122:353-360.
130 MacDowell E.M., Wilson T.S., Trump B.F. Atypical endocrine tumors of the lung. Arch Pathol Lab Med. 1981;105:20-28.
131 Hammond M.E., Sause W.T. Large cell neuroendocrine tumors of the lung. Cancer. 1985;56:1624-1629.
132 Piehl M.R., Gould V.E., Warren W.H., et al. Immunohistochemical identification of exocrine and neuroendocrine subsets of large cell lung carcinomas. Pathol Res Pract. 1988;183:675-682.
133 Wick M.R., Berg L.C., Hertz M. Large cell carcinoma of the lung with neuroendocrine differentiation: a comparison with large cell “undifferentiated” pulmonary tumors. Am J Clin Pathol. 1992;97:796-805.
134 Cavazza A., Colby T.V., Tsokos M., Rush W., Travis W.D. Lung tumors with a rhabdoid phenotype. Am J Clin Pathol. 1996;105:182-188.
135 Wick M.R., Ritter J.H., Dehner L.P. Malignant rhabdoid tumors: a clinicopathologic review and conceptual discussion. Semin Diagn Pathol. 1995;12:233-248.
136 Perry A., Fuller C.E., Judkins A.R., Dehner L.P., Biegel J.A. INI1 expression is retained in composite rhabdoid tumors, including rhabdoid meningiomas. Mod Pathol. 2005;18:951-958.
137 Hoot A.C., Russo P., Judkins A.R., Perlman E.J., Biegel J.A. Immunohistochemical analysis of hSNF5-INI1 distinguishes renal and extra-renal malignant rhabdoid tumors from other pediatric soft tissue tumors. Am J Surg Pathol. 2004;28:1485-1491.
138 Fuller C.E., Pfeifer J., Humphrey P., Bruch L.A., Dehner L.P., Perry A. Chromosome 22q dosage in composite extrarenal rhabdoid tumors: clonal evolution or a phenotypic mimic? Hum Pathol. 2001;32:1102-1108.
139 Hirsch F.R., Spreafico A., Novello S., Wood M.D., Simms L., Papotti M. The prognostic and predictive role of histology in advanced non-small cell lung cancer: a literature review. J Thorac Oncol. 2008;3:1468-1481.
140 West H., Lilenbaum R., Harpole D., Wozniak A., Sequist L. Molecular analysis-based treatment strategies for the management of non-small cell lung cancer. J Thorac Oncol. 2009;4(9 suppl 2):S1029-S1039.
141 Stinchcombe T.E., Socinski M.A. Current treatments for advanced stage non-small cell lung cancer. Proc Am Thorac Soc. 2009;6:233-241.
142 Tiseo M., Rossi G., Capelletti M., et al. Predictors of gefitinib outcomes in advanced non-small cell lung cancer (NSCLC): study of a comprehensive panel of molecular markers. Lung Cancer. 2010;67:355-360.
143 Miller V.A., Riely G.J., Zakowski M.F., et al. Molecular characteristics of bronchioloalveolar carcinoma and adenocarcinoma, bronchioloalveolar carcinoma subtype, predict response to erlotinib. J Clin Oncol. 2008;26:1472-1478.
144 Ansari J., Palmer D.H., Rea D.W., Hussain S.A. Role of tyrosine kinase inhibitors in lung cancer. Anticancer Agents Med Chem. 2009;9:569-575.
145 Sheth S. Current and emerging therapies for patients with advanced non-small-cell lung cancer. Am J Health Syst Pharm. 2010;67(1 suppl 1):S9-S14.
146 Takahashi T., Yamamoto N., Nukiwa T., et al. Phase II study of erlotinib in Japanese patients with advanced non-small cell lung cancer. Anticancer Res. 2010;30:557-563.
147 Chang J.W., Liu H.P., Hsieh M.H., et al. Increased epidermal growth factor receptor (EGFR) gene copy number is strongly associated with EGFR mutations and adenocarcinoma in non-small cell lung cancers: a chromogenic in situ hybridization study of 182 patients. Lung Cancer. 2008;61:328-339.
148 Kargi A., Gurel D., Tuna B. The diagnostic value of TTF-1, CK 5/6, and p63 immunostaining in the classification of lung carcinomas. Appl Immunohistochem Mol Morphol. 2007;15:415-420.
149 Baksh F.K., Dacic S., Finkelstein S.D., et al. Widespread molecular alterations present in stage I non-small cell lung carcinoma fail to predict tumor recurrence. Mod Pathol. 2003;16:28-34.
150 Kanazawa H., Ebina M., Ino-Oka N., et al. Transition from squamous cell carcinoma to adenocarcinoma in adenosquamous carcinoma of the lung. Am J Pathol. 2000;156:1289-1298.
151 Park S.H., Ha S.Y., Lee J.I., et al. Epidermal growth factor receptor mutations and the clinical outcome in male smokers with squamous cell carcinoma of lung. J Korean Med Sci. 2009;24:448-452.
152 Monaco S.E., Nikiforova M.N., Cieply K., Teot L.A., Khalbuss W.E., Dacic S. A comparison of EGFR and KRAS status in primary lung carcinoma and matched metastases. Hum Pathol. 2010;41:94-102.
153 Kalikaki A., Koutsopoulos A., Trypaki M., et al. Comparison of EGFR and K-RAS gene status between primary tumours and corresponding metastases in NSCLC. Br J Cancer. 2008;99:923-929.
154 Gow C.H., Chang Y.L., Hsu Y.C., et al. Comparison of epidermal growth factor receptor mutations between primary and corresponding metastatic tumors in tyrosine kinase inhibitor-naïve non-small-cell lung cancer. Ann Oncol. 2009;20:696-702.
155 Sartori G., Cavazza A., Sgambato A., et al. EGFR and K-ras mutations along the spectrum of pulmonary epithelial tumors of the lung and elaboration of a combined clinicopathologic and molecular scoring system to predict clinical responsiveness to EGFR inhibitors. Am J Clin Pathol. 2009;131:478-489.
156 Baba M., Castillo A., Koriyama C., et al. Human papillomavirus is frequently detected in gefitinib-responsive lung adenocarcinomas. Oncol Rep. 2010;23:1085-1092.
157 Moran C.A. Primary salivary gland-type tumors of the lung. Semin Diagn Pathol. 1995;12:106-122.
158 Dowling E.A., Miller R.E., Johnson I.M., et al. Mucoepidermoid tumors of the bronchi. Surgery. 1962;52:600-609.
159 Ozlu C., Christopherson W.M., Allen J.D. Mucoepidermoid tumors of the bronchi. J Thorac Cardiovasc Surg. 1961;42:24-31.
160 Axelsson C., Burcharth F., Johansen A. Mucoepidermoid lung tumors. J Thorac Cardiovasc Surg. 1973;65:902-908.
161 Turnbull A.D., Huvos A.G., Goodner J.T., et al. Mucoepidermoid tumors of bronchial glands. Cancer. 1971;28:539-544.
162 Reichle F.A., Rosemond G.P. Mucoepidermoid tumors of the bronchus. J Thorac Cardiovasc Surg. 1966;51:443-448.
163 Klacsmann P.G., Olson J.L., Eggleston J.C. Mucoepidermoid carcinoma of the bronchus. Cancer. 1979;43:1720-1733.
164 Barsky S.H., Martin S.E., Matthews M., et al. “Low grade” mucoepidermoid carcinoma of the bronchus with “high grade” biologic behavior. Cancer. 1983;51:1505-1509.
165 Seo I.S., Warren J., Mirkin D., et al. Mucoepidermoid carcinoma of the bronchus in a 4-year-old child. Cancer. 1984;53:1600-1604.
166 Yousem S.A., Hochholzer L. Mucoepidermoid tumors of the lung. Cancer. 1987;60:1346-1352.
167 Liu X., Adams A.L. Mucoepidermoid carcinoma of the bronchus: a review. Arch Pathol Lab Med. 2007;131:1400-1404.
168 Molina J.R., Aubry M.C., Lewis J.E., et al. Primary salivary gland-type lung cancer: spectrum of clinical presentation, histopathologic and prognostic factors. Cancer. 2007;110:2253-2259.
169 Shilo K., Foss R.D., Franks T.J., DePeralta-Venturina M., Travis W.D. Pulmonary mucoepidermoid carcinoma with prominent tumor-associated lymphoid proliferation. Am J Surg Pathol. 2005;29:407-411.
170 Macarenco R.S., Uphoff T.S., Gilmer H.F., et al. Salivary gland-type lung carcinomas: an EGFR immunohistochemical, molecular genetic, and mutational analysis study. Mod Pathol. 2008;21:1168-1175.
171 Rossi G., Sartori G., Cavazza A., Tamberi S. Mucoepidermoid carcinoma of the lung, response to EGFR inhibitors, EGFR and K-RAS mutations, and differential diagnosis. Lung Cancer. 2009;63:159-160.
172 Moran C.A., Suster S., Koss M.N. Primary adenoid cystic carcinoma of the lung: a clinicopathological and immunohistochemical study of 16 cases. Cancer. 1994;73:1390-1397.
173 Ishida T., Nishino T., Oka T., et al. Adenoid cystic carcinoma of the tracheobronchial tree: clinicopathology and immunohistochemistry. J Surg Oncol. 1989;41:52-59.
174 Heilbrunn A.A., Crosby I.K. Adenoid cystic carcinoma and mucoepidermoid carcinoma of the tracheobronchial tree. Chest. 1972;61:145-149.
175 Nomori H., Kaseda S., Kobayashi K., et al. Adenoid cystic carcinoma of the trachea and main stem bronchus: a clinical, histopathologic, and immunohistochemical study. J Thorac Cardiovasc Surg. 1988;96:271-277.
176 Inoue H., Iwashita A., Kanegae H., et al. Peripheral pulmonary adenoid cystic carcinoma with substantial submucosal extension of the proximal bronchus. Thorax. 1991;46:147-148.
177 Conlan A.A., Payne W.S., Woolner L.B., et al. Adenoid cystic carcinoma (Cylindroma) and mucoepidermoid carcinoma of the bronchus. J Cardiothorac Surg. 1978;76:369-377.
178 Markel S.F., Abell M.R., Haight L., et al. Neoplasms of the bronchus commonly designated as adenomas. Cancer. 1964;17:590-604.
179 Payne W.S., Ellis F.H., Woolner L.B., et al. The surgical treatment of cylindroma (adenoid cystic carcinoma) and mucoepidermoid tumors of the bronchus. J Thorac Cardiovasc Surg. 1959;38:709-726.
180 Youkouchi H., Otsuka Y., Otoguro Y., et al. Primary peripheral adenoid cystic carcinoma of the lung and literature comparison of features. Intern Med. 2007;46:1799-1803.
181 Maygarden S.J., Detterbeck F.C., Funkhouser W.K. Bronchial margins in lung cancer resection specimens: utility of frozen section and gross examination. Mod Pathol. 2004;17:1080-1086.
182 Sterman D.H., Sztejman E., Rodriguez E., Friedberg J. Diagnosis and staging of “other bronchial tumors. Chest Surg Clin N Am. 2003;13:79-94.
183 Daneshbod Y., Modjtahedi E., Atefi S., Bedayat G.R., Daneshbod K. Exfoliative cytologic findings of primary pulmonary adenoid cystic carcinoma: a report of 2 cases with a review of the cytologic features. Acta Cytol. 2007;51:558-562.
184 Ozkara S.K., Turan G. Fine needle aspiration cytopathology of primary solid adenoid cystic carcinoma of the lung: a case report. Acta Cytol. 2009;53:707-710.
185 Chuah K.L., Lim K.H., Koh M.S., Tan H.W., Yap W.M. Diagnosis of adenoid cysticcarcinoma of the lung by bronchial brushing: a case report. Acta Cytol. 2007;51:563-566.
186 Farrell T., Chang Y.L. Basal cell adenocarcinoma of minor salivary glands. Arch Pathol Lab Med. 2007;131:1602-1604.
187 Jayakrishnan A., Elmalah I., Hussain K., Odell E.W. Basal cell adenocarcinoma in minor salivary glands. Histopathology. 2003;42:610-614.
188 Parashar P., Baron E., Papadimitriou J.C., Ord R.A., Nikitakis N.G. Basal cell adenocarcinoma of the oral minor salivary glands: review of the literature and presentation of two cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103:77-84.
189 Aubry M.C., Heinrich M.C., Molina J., et al. Primary adenoid cystic carcinoma of the lung: absence of KIT mutations. Cancer. 2007;110:2507-2510.
190 Miettinen M., Lasota J. KIT (CD117): a review on expression in normal and neoplastic tissues, and mutations and their clinicopathologic correlation. Appl Immunohistochem Mol Morphol. 2005;13:205-220.
191 Holst V.A., Marshall C.E., Moskaluk C.A., Frierson H.F.Jr. KIT protein expression and analysis of c-kit gene mutation in adenoid cystic carcinoma. Mod Pathol. 1999;12:956-960.
192 Shimizu J., Oda M., Matsumoto I., Ararno Y., Ishikawa N., Minato H. Clinicopathologic study of surgically treated cases of tracheobronchial adenoid cystic carcinoma. Gen Thorac Cardiovasc Surg. 2010;58:82-86.
193 Fechner R.E., Bentnick B.R., Askew J.B.Jr. Acinic cell tumor of the lung: a histologic and ultrastructural study. Cancer. 1972;29:501-508.
194 Moran C.A., Suster S., Koss M.N. Acinic cell carcinoma of the lung (“Fechner Tumor”): a clinicopathologic, immunohistochemical, and ultrastructural study of five cases. Am J Surg Pathol. 1992;16:1039-1050.
195 Latz D.R., Bubis J.J. Acinic cell tumor of the bronchus. Cancer. 1976;38:830-832.
196 Gharpure K.J., Desphande R.K., Vishweshvara R.N., et al. Acinic cell tumor of the bronchus (a case report). Indian J Cancer. 1985;22:152-156.
197 Sabaratnam R.M., Anunathan R., Govender D. Acinic cell carcinoma: an unusual cause of bronchial obstruction in a child. Pediatr Dev Pathol. 2004;7:521-526.
198 Rodriguez J., Diment J., Lombardi L., Dominoni F., Tench W., Rosai J. Combined typical carcinoid and acinic cell tumor of the lung: a heretofore unreported occurrence. Hum Pathol. 2003;34:1061-1065.
199 Lee H.Y., Mancer K., Koong H.N. Primary acinic cell carcinoma of the lung with lymph node metastasis. Arch Pathol Lab Med. 2003;127:e216-e219.
200 Ukoha O.O., Quartararo P., Carter D., Kashgarian M., Ponn R.B. Acinic cell carcinoma of the lung with metastasis to lymph nodes. Chest. 1999;115:591-595.
201 Chuah K.L., Yap W.M., Tan H.W., Koong H.N. Recurrence of pulmonary acinic cell carcinoma. Arch Pathol Lab Med. 2006;130:932-933.
202 Nistal M., Garcia-Viera M., Martinez-Garcia C., et al. Epithelial-myoepithelial tumor of the bronchus. Am J Surg Pathol. 1994;18:421-425.
203 Strickler J.G., Hegstrom J., Thomas M.J., et al. Myoepithelioma of the lung. Arch Pathol Lab Med. 1987;111:1082-1086.
204 Tsuji N., Tateisha R., Ishiguro S., et al. Adenomyoepithelioma of the lung. Am J Surg Pathol. 1995;19:956-962.
205 Wilson R.W., Moran C.A. Epithelial-myoepithelial carcinoma of the lung: immunohistochemical and ultrastructural observations and review of the literature. Hum Pathol. 1997;28:631-635.
206 Pelosi G., Fraggetta F., Maffini F., et al. Pulmonary epithelial-myoepithelial tumor of unproven malignant potential: report of a case and review of the literature. Mod Pathol. 2001;14:521-526.
207 Nguyen C.V., Suster S., Moran C.A. Pulmonary epithelial-myoepithelial carcinoma: a clinicopathologic and immunohistochemical study of 5 cases. Hum Pathol. 2009;40:366-373.
208 Rosenfeld A., Schwartz D., Garzon S., Chaleff S. Epithelial-myoepithelial carcinoma of the lung: a case report and review of the literature. J Pediatr Hematol Oncol. 2009;31:206-208.
209 Moraitaki P.K., Vasilikos K., Archontovasilis F., et al. Recurrent respiratory infection and epithelial-myoepithelial carcinoma of the lung: a common presentation with a rare etiology. J BUON. 2009;14:147-148.
210 Muslimani A.A., Kundranda M., Jain S., Daw H.A. Recurrent bronchial epithelial-myoepithelial carcinoma after local therapy. Clin Lung Cancer. 2007;8:386-389.
211 Fulford L.G., Kamata Y., Okudera K., et al. Epithelial-myoepithelial carcinomas of the bronchus. Am J Surg Pathol. 2001;25:1508-1514.
212 Volm M., Hahn E.W., Mattern J., et al. Five year followup study of independent clinical and flow cytometric prognostic factors for the survival of patients with non-small cell carcinoma. Cancer Res. 1988;48:2923-2928.
213 Mountain C.F. Revisions in the International System for Staging Lung Cancer. Chest. 1997;111:1710-1717.
214 Asamura H., Naruke T. Lung carcinoma. In: Hermanek P., Gospodarowicz M.K., Henson D.E., Hutter R.V.P., Sobin L.H., editors. Prognostic Factors in Cancer. Berlin: Springer-Verlag; 1995:118-129.
215 Gallagher B., Urbanski S.J. The significance of pleural elastic invasion by lung carcinoma. Hum Pathol. 1990;21:512-517.
216 Bunker M.L., Raab S.S., Landreneau R.J., Silverman J.F. The diagnosis and significance of visceral pleural invasion in lung carcinoma: histologic predictors and the role of elastic stains. Am J Clin Pathol. 1999;112:777-783.
217 Taube J.M., Askin F.B., Brock M.V., Westra W. Impact of elastic staining on the staging of peripheral lung cancers. Am J Surg Pathol. 2007;31:953-956.
218 Butnor K.J., Vollmer R.T., Blaszyk H., Glatz K. Interobserver agreement on what constitutes visceral pleural invasion by non-small cell lung carcinoma: an internet-based assessment of international current practices. Am J Clin Pathol. 2007;128:638-647.
219 Travis W.D., Brambilla E., Rami-Porta R., et al. Visceral pleural invasion: pathologic criteria and use of elastic stains: proposal for the 7th edition of the TNM classification for lung cancer. J Thorac Oncol. 2008;3:1384-1390.
220 Shim H.S., Park I.K., Lee C.Y., Chung K.Y. Prognostic significance of visceral pleural invasion in the forthcoming (seventh) edition of TNM classification for lung cancer. Lung Cancer. 2009;65:161-165.
221 Elson C.E., Roggli V.L., Vollmer R.T., et al. Prognostic indicators for survival in stage I carcinoma of the lung: a histologic study of 47 surgically resected cases. Mod Pathol. 1988;1:288-291.
222 Haque A.K., Adegboyega P., Sanchez R.L. Vascular invasion in carcinoma of the lung. Mod Pathol. 1993;6:131A.
223 Miyoshi K., Moriyama S., Kunitomo T., Nawa S. Prognostic impact of intratumoral vessel invasion in completely resected pathologic stage I non-small cell lung cancer. J Thorac Cardiovasc Surg. 2009;137:429-434.
224 Jones D.R., Daniel T.M., Denlinger C.E., Rundall B.K., Smolkin M.E., Wick M.R. Stage IB nonsmall cell lung cancers: are they all the same? Ann Thorac Surg. 2006;81:1958-1962.
225 Lee Y.C., Wu C.T., Kuo S.W., Tseng Y.T., Chang Y.L. Significance of extranodal extension of regional lymph nodes in surgically resected non-small cell lung cancer. Chest. 2007;131:993-999.
226 Brown R.N.V., Fraire A.E., Roggli V., Cagle P.T. Assessment of proliferative fraction by PCNA in stage I non-small cell lung cancer. Mod Pathol. 1993;6:129A.
227 Miyarnoto H., Flarada M., lsobe A., et al. Prognostic value of nuclear DNA content and expression of the ras oncogene product in lung cancer. Cancer Res. 1991;51:6346-6350.
228 Gazdar A.F. Molecular markers for the diagnosis and prognosis of lung cancer. Cancer. 1992;69:1592-1599.
229 Noguchi M., Hirohashi S., Hara F., et al. Heterogeneous amplification of myc family oncogenes in small cell lung carcinomas. Cancer. 1990;66:2053-2058.
230 Ritter J.H., Dresler C.M., Wick M.R. Expression of bcl-2 protein in stage T1N0M0 non-small cell lung carcinoma. Hum Pathol. 1995;26:1227-1232.
231 Ponder T.B., Wick M.R., Dresler C.M., Ritter J.H. Expression of ABH antigen, p53 protein, bcl-2 protein, and tumor grade as prognostic factors in T1N0M0 non-small cell lung carcinoma. Am J Clin Pathol. 1996;105:493.
232 Ponder T.B., Wick M.R., Dresler C.M., Ritter J.H. Microvessel counts and lymphovascular invasion as prognostic indicators in T1N0M0 non-small cell lung carcinoma. Am J Clin Pathol. 1996;106:402-403.
233 Haque A.K., Abegboyega P., Al-Salalmeh A., Vrazel D.P., Zwischenberger J. p53 and p-glycoprotein expression do not correlate with survival in non-small cell lung cancer: a long-term study and literature review. Mod Pathol. 1999;12:1158-1166.
234 Hashimoto T., Tokuchi Y., Hayashi M., et al. p53 null mutations undetected by immunohistochemical staining predict a poor outcome with early-stage non-small cell lung carcinomas. Cancer Res. 1999;59:5572-5577.
235 Tomizawa Y., Kohno T., Fujita T., et al. Correlation between the status of the p53 gene and survival in patients with stage I non-small cell lung carcinomas. Oncogene. 1999;18:1007-1014.
236 Dosaka-Akita H., Hu S.X., Fujino M., et al. Altered retinoblastoma protein expression in non-small cell lung cancer: its synergistic effects with altered ras and p53 protein status on prognosis. Cancer. 1997;79:1329-1337.
237 Maitra A., Amirkhan R.H., Saboorian M.H., Frawley W.H., Ashfaq R. Survival in small cell lung carcinoma is independent of bcl-2 expression. Hum Pathol. 1999;30:712-717.
238 Fontanini G., Vignati S., Bigini D., et al. Recurrence and death in non-small cell lung carcinomas: a prognostic model using pathological parameters, microvessel count, and gene protein products. Clin Cancer Res. 1996;2:1067-1075.
239 Zhu C.Q., Shih W., Ling C.H., Tsao M.S. Immunohistochemical markers of prognosis in non-small cell lung cancer: a review and proposal for a multiphase approach to marker evaluation. J Clin Pathol. 2006;59:790-800.
240 Petty R.D., Nicolson M.C., Kerr K.M., Collie-Duguid E., Murray G.I. Gene expression profiling in non-small cell lung cancer: from molecular mechanisms to clinical application. Clin Cancer Res. 2004;10:3237-3248.
241 Girard N., Ostrovnaya I., Lau C., et al. Genomic and mutational profiling to assess clonal relationships between multiple non-small cell lung cancers. Clin Cancer Res. 2009;15:5184-5190.
242 Potti A., Mukherjee S., Petersen R., et al. A genomic strategy to refine prognosis in early-stage non-small-cell lung cancer. N Engl J Med. 2006;355:570-580.
243 Hoang C.D., Guillaume T.J., Engel S.C., Tawfic S.H., Kratzke R.A., Maddaus M.A. Analysis of paired primary lung and lymph node tumor cells: a model of metastatic potential by multiple genetic programs. Cancer Detect Prev. 2005;29:509-517.
244 Chen H.Y., Yu S.L., Chen C.H., et al. A five-gene signature and clinical outcome in non-small-cell lung cancer. N Engl J Med. 2007;356:11-20.
245 Xi L., Lyons-Weiler J., Coello M.C., et al. Prediction of lymph node metastasis by analysis of gene expression profiles in primary lung adenocarcinomas. Clin Cancer Res. 2005;11:4128-4135.
246 Kotoulas C.S., Foroulis C.N., Kostikas K., et al. Involvement of lymphatic metastatic spread in non-small cell lung cancer accordingly to the primary cancer location. Lung Cancer. 2004;44:183-191.
247 Wrage M., Ruosaari S., Eijk P.P., et al. Genomic profiles associated with early micrometastasis in lung cancer: relevance of 4q deletion. Clin Cancer Res. 2009;15:1566-1574.
248 Moriya Y., Iyoda A., Kasai Y., et al. Prediction of lymph node metastasis by gene expression profiling in patients with primary resected lung cancer. Lung Cancer. 2009;64:86-91.
249 Cordes C., Bartling B., Simm A., et al. Simultaneous expression of Cathepsins B and K in pulmonary adenocarcinomas and squamous cell carcinomas predicts poor recurrence-free and overall survival. Lung Cancer. 2009;64:79-85.
250 Chang J.W., Yi C.A., Son D.S., et al. Prediction of lymph node metastasis using the combined criteria of helical CT and mRNA expression profiling for non-small cell lung cancer. Lung Cancer. 2008;60:264-270.
251 Lu Y., Lemon W., Liu P.Y., et al. A gene expression signature predicts survival of patients with stage I non-small cell lung cancer. PLOS Med. 2006;3:e467.
252 Guo L., Ma Y., Ward R., Castranova V., Shi X., Qian Y. Constructing molecular classifiers for the accurate prognosis of lung adenocarcinoma. Clin Cancer Res. 2006;12:3344-3354.
253 Xi L., Coello M.C., Litle V.R., et al. A combination of molecular markers accurately detects lymph node metastasis in non-small cell lung cancer patients. Clin Cancer Res. 2006;12:2484-2491.
254 Pfeifer J.D., editor. Molecular Genetic Testing in Surgical Pathology. Lippincott-Williams & Wilkins, Philadelphia, 2005;1-50.
255 Flieder D.B. Commonly encountered difficulties in pathologic staging of lung cancer. Arch Pathol Lab Med. 2007;131:1016-1026.
256 Vansteenkiste J.F., De Belie B., Deneffe G.J., et al. Practical approach to patients presenting with multiple synchronous suspect lung lesions: a reflection on the current TNM classification based on 54 cases with complete follow-up. Lung Cancer. 2001;34:169-175.
257 Girard N., Ostrovnaya I., Lau C., et al. Genomic and mutational profiling to assess clonal relationships between multiple non-small cell lung cancers. Clin Cancer Res. 2009;15:5184-5190.
258 Xu W., Chung J.H., Jheon S., et al. The accuracy of frozen section diagnosis of pulmonary nodules: evaluation of inflation method during intraoperative pathology consultation with cryosection. J Thorac Oncol. 2010;5:39-44.
259 Sanli M., Isik A.F., Tuncozgur B., et al. The reliability of mediastinoscopic frozen sections in deciding on oncological surgery in bronchogenic carcinoma. Adv Ther. 2008;25:488-495.
260 Suda T., Mizoguchi Y., Hasegawa S., Negi K., Hattori Y. Frozen-section diagnosis of small adenocarcinoma of the lung for intentional limited surgery. Surg Today. 2006;36:676-679.
261 Orki A., Tezel C., Kosar A., Ersev A.A., Dudu C., Arman B. Feasibility of imprint cytology for evaluation of mediastinal lymph nodes in lung cancer. Jpn J Clin Oncol. 2006;36:76-79.
262 Yoshida J., Nagai K., Yokose T., et al. Limited resection trial for pulmonary ground-glass opacity nodules: fifty-case experience. J Thorac Cardiovasc Surg. 2005;129:991-996.
263 Mair S., Lash R.H., Suskin D., Mendelsohn G. Intraoperative surgical specimen evaluation: frozen section analysis, cytologic examination, or both? A comparative study of 206 cases. Am J Clin Pathol. 1991;96:8-14.
264 Wick M.R. Intraoperative consultations in pathology: a current perspective. Am J Clin Pathol. 1995;104:239-242.
265 Herbst J., Jenders R., McKenna R.J.Jr, Marchevsky A.M. Evidence-based criteria to help distinguish metastatic breast cancer from primary lung adenocarcinoma on thoracic frozen section. Am J Clin Pathol. 2009;131:122-128.
266 Gupta R., Dastane A., McKenna R.J.Jr, Marchevsky A.M. What can we learn from the errors in the frozen section diagnosis of pulmonary carcinoid tumors? An evidence-based approach. Hum Pathol. 2009;40:1-9.
267 Darvishian F., Ginsberg M.S., Klimstra D.S., Brogi E. Carcinoid tumorlets simulate pulmonary metastases in women with breast cancer. Hum Pathol. 2006;37:839-844.
268 Marchevsky A.M., Changsri C., Gupta I., Fuller C., Houck W., McKenna R.J.Jr. Frozen section diagnoses of small pulmonary nodules: accuracy and clinical implications. Ann Thorac Surg. 2004;78:1755-1759.
269 Butcher D.N., Goldstraw P., Ladas G., Dusmet M.E., Sheppard M.N., Nicholson A.G. Thyroid transcription factor-1 immunohistochemistry as an intraoperative diagnostic tool at frozen section for distinction between primary and secondary lung tumors. Arch Pathol Lab Med. 2007;131:582-587.
270 Kammerer U., Kapp M., Gassel A.M., et al. A new rapid immunohistochemical staining technique using the EnVision antibody complex. J Histochem Cytochem. 2001;49:623-630.
271 Eagan R.T., Maurer L.H., Forcier R.J., Tulloh M. Small-cell carcinoma of the lung: staging paraneoplastic syndromes, treatment, and survival. Cancer. 1974;33:527-532.
272 Lim E., Belcher E., Yap Y.K., Nicholson A.G., Goldstraw P. The role of surgery in the treatment of limited-disease small cell lung cancer: time to reevaluate. J Thorac Oncol. 2008;3:1267-1271.
273 Schreiber D., Rineer J., Weedon J., et al. Survival outcomes with the use of surgery in limited-stage small cell lung cancer: should its role be reevaluated? Cancer. 2010;116:1350-1357.
274 Colby T.V., Deschamps C. The lung and pleura. In: Banks P.M., Kraybill W.G., editors. Pathology for the Surgeon. Philadelphia: WB Saunders; 1996:155-168.
275 Butnor K.J., Beasley M.B., Cagle P.T., et al. Protocol for the examination of specimens from patients with primary non-small-cell carcinoma, small-cell carcinoma, or carcinoid tumor of the lung. Arch Pathol Lab Med. 2009;133:1552-1559.
276 Association of Directors of Anatomic & Surgical Pathology. Recommendations for the reporting of resected primary lung carcinomas. Am J Clin Pathol. 1995;104:371-374.
277 Greene F.L., Page D.L., Fleming I.D., et al, editors. AJCC Cancer Staging Manual, 6th ed. Springer, New York, 2002;165-184.