Non-colorectal hepatic metastases
Introduction
The development of liver metastases was previously considered a preterminal event with treatment limited to palliation; however, the success of hepatectomy in improving outcomes in metastatic CRC has generated renewed enthusiasm in considering resection of liver metastases from non-colorectal primary cancers. Liver resection has become the standard of care for CRC liver metastases and many centres have adopted an increasingly aggressive approach, with reported 5-year survival rates exceeding 50%.1,2 The complementary use of portal vein embolisation, radiofrequency ablation and staged resection strategies has increased the proportion of patients eligible for resection. At the same time, advances in surgical technique and knowledge of liver anatomy have reduced significantly the morbidity and mortality associated with liver resection to less than 20% and 5%, respectively.2,3
Liver metastases of non-colorectal origin constitute a diverse group of tumours, most commonly arising from gastrointestinal sites. These tumours can be broadly divided into neuroendocrine and non-neuroendocrine malignancies, encompassing unique and markedly varied natural histories. Neuroendocrine tumours (NETs) have historically been described as indolent malignancies with hepatectomy for NET liver metastases associated with 5- and 10-year survival rates of 77.4% and 50.4%, respectively.4 While hepatectomy is an increasingly accepted management strategy for NETs, it is performed less frequently for non-neuroendocrine tumours.
The evidence regarding hepatectomy for non-colorectal metastases originates largely from retrospective reviews spanning several decades of experience.5–8 Many studies fail to distinguish between NET and non-NET metastases, and when that distinction is made, the non-NET metastases are usually considered a single entity despite comprising a heterogeneous set of pathologies. Reports focusing on a single tumour type are usually based on small case series. With advances in surgical techniques and promising results observed in CRC and NET hepatic metastases, the role of surgical treatments in non-NET liver tumours has once again become an area of active research.
Pathophysiology and molecular basis of liver metastases
The rationale behind a surgical approach to metastatic disease is based on the concept of site-specific metastases. First proposed by Paget in 1889, this ‘seed and soil’ hypothesis argues that solid tumours have a distinct pattern of distant organ involvement created by the target organ microenvironment. Ewing proposed a ‘mechanical’ theory in which the metastatic pattern is determined by the venous drainage of the primary tumour.10 Neither theory takes into account the complexity of the metastatic process, which requires that a cancer cell gains specific invasion and metastatic potential before it can disseminate. The clonal selection model of the metastatic process suggests that heterogeneity develops within a population of cancer cells through mutational events, allowing a subpopulation to randomly acquire the necessary traits to disseminate successfully.11 Alternatively, it has been argued that within cancers of the same pathological type, i.e. breast cancer, some tumours are a priori more likely to develop metastases than others. This is supported by gene expression data where specific molecular signatures have been found to predict accurately prognosis in breast cancer,12 ovarian cancer13 and melanoma.14 Similarly, in CRC the genotype of microsatellite instability correlates with a decreased likelihood of metastatic spread.15
A recent refinement to Paget’s hypothesis, based on molecular genetic research, suggests that the primary tumour is itself capable of preparing the soil by creating a ‘premetastatic niche’.16 Every cancer has a type-specific pattern of cytokine expression that appears to direct both malignant and non-malignant cells to specific distant organs. The influx and clustering of bone-marrow-derived haematopoietic cells is one of the earliest events in the development of a metastatic deposit. This is closely followed by local inflammation and the release of matrix metalloproteinases. These local events appear to mediate remodelling of the extracellular matrix, creating a more permissive microenvironment for the eventual deposition and growth of malignant cells.17 Thus, the primary tumour both chooses and alters the sites to which it metastasises. For reasons not yet understood, many solid tumours metastasise preferentially to the liver.
If the site-specific hypothesis of metastatic spread is correct, complete surgical excision of liver metastases can remove the only site of disease and offers a chance for cure. Nonetheless, residual micrometastatic disease may exist within the liver, and hepatic recurrences are a common cause of treatment failure following hepatectomy. Even in the presence of micrometastases, the removal of all macroscopic disease may have immunological benefits. The immune-suppressing effects of cancers are well accepted: malignant cells can induce both adaptive and innate immune suppression, facilitating tumour growth.18 The degree of immune suppression correlates with the tumour burden19 and if all gross metastatic disease can be removed, host defences may attack micrometastatic deposits more effectively. The use of neoadjuvant or adjuvant chemotherapy may improve cure rates by controlling micrometastases.20,21
The advent of next generation sequencing technologies and high-density oligonucleotide arrays has further deepened our understanding of the metastatic process. Whereas the ability of a cancerous cell to metastasise was once believed to occur following the accumulation of multiple somatic mutations in many cancer-causing genes, new findings, specifically in pancreatic cancer, have challenged this belief. Studies by Yachida et al.22 and Campbell et al.23 describe the existence of multiple subclones within a primary pancreas cancer tumour, each containing a unique genetic signature corresponding to an eventual site of metastastic spread. These subclones are present many years before an eventual metastasis is clinically detected, when disease is at an early stage. Furthermore, metastases seen in different organs share many common genetic mutations as well as site-specific changes that confer a selective growth advantage in the respective tissue. Future studies on the biology of metastases are likely to improve our understanding of this complex process, translating into more efficacious therapy.
Clinical approach to non-colorectal liver metastases
Some patients can be assessed for recurrence using more targeted techniques and biochemical markers (i.e. CA-125 for ovarian cancer, chromogranin A for NETs). Nuclear imaging can detect NETs expressing somatostatin receptors with 80–90% sensitivity. Whole-body PET scanning using a new somatostatin analogue, [68Ga]DOTA-TOC, has been found to be accurate for the detection of new metastases in NETs following radionuclide therapy.24 Occasionally, the original presentation of an NET will be a liver metastasis from an unidentified primary, and the investigative focus is aimed at localisation of the primary tumour.
Certain tumours, such as gastric, breast and ovarian cancer, have a predilection for intraperitoneal spread. Although CT is the preferred modality for diagnosing peritoneal carcinomatosis, its accuracy is still limited by histological type, the anatomical site of spread and the size of tumour deposits.25 For many of these equivocal cases, diagnostic laparoscopy has been recommended. Routine laparoscopy with laparoscopic ultrasound for patients with potentially resectable non-colorectal liver metastases has been found to result in a change in management in 20% of cases and may be used in preoperative staging.26
Treatment strategies
Several treatment modalities exist for metastatic disease, and the therapeutic approach must be tailored to the tumour type, the performance status of the patient and the extent of disease, determined in the setting of a multidisciplinary conference. Ablative strategies and systemic or locally delivered chemotherapy can be used as adjuncts to resection. Radiofrequency ablation (RFA) has been reported to be safe and successful at achieving local control in patients with liver metastases from breast cancer,27 ovarian cancer28 and NETs,29 but its major limitation is the difficulty of achieving complete necrosis for tumours larger than 3 cm.
Transarterial embolisation (TAE) takes advantage of the differential blood supply of liver metastases, which depend mainly on the hepatic arteries, and the normal parenchyma, which relies more heavily on the portal vein. Transarterial chemoembolisation (TACE) involves the local delivery of a drug prior to occluding the artery and allows prolonged exposure of the tumour to the agent without increasing systemic toxicity. Both TAE and TACE have been well described for the treatment of unresectable hepatocellular carcinoma30 and the symptomatic relief of NETs.31
Neuroendocrine tumours
Most NETs of gastrointestinal origin demonstrate ‘indolent’ growth. Despite such a benign description, 46–93% of patients with NETs will have liver involvement at the time of diagnosis, with 5-year untreated survival of 0–20%.32 Systemic chemotherapy with platinum-based regimens has shown a response rate of up to 67% in poorly differentiated NETs. Nevertheless, the survival benefit of chemotherapy is limited and associated with significant toxicity.33 Somatostatin analogues such as octreotide can achieve symptomatic relief in 70–80% of patients, but an antiproliferative effect is seen in less than 10% of cases.34 Furthermore, newer agents such as the receptor tyrosine kinase inhibitor sunitinib, the mammmalian target of rapamycin (mTOR) inhibitor everolimus, and the anti-vascular endothelial growth factor (anti-VEGF) bevacizumab have shown promise in PNETs.33
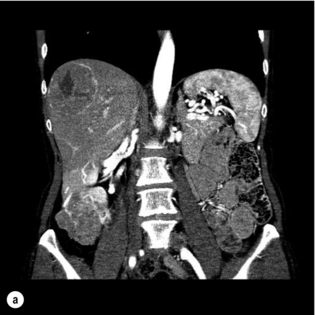
NETs metastasise preferentially to the liver, and in many patients the liver remains the only site of metastatic disease for a prolonged period of time. The majority of patients have multifocal, bilobar disease, of which less than 20% are candidates for surgery32 (Fig. 7.1a,b). Liver resection may be performed with curative intent, symptom control or prolongation of survival in the palliative setting.
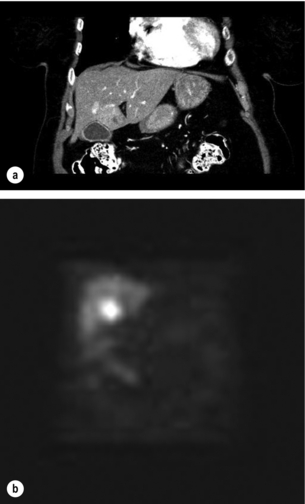
Figure 7.1 (a) A 67-year-old female with a node-positive distal jejunal carcinoid tumour and synchronous solitary liver metastasis in segment 4B. (b) Octreotide scan of the same patient. Transaxial single-photon emission computed tomography (SPECT) demonstrates abnormal activity in segment 4B corresponding to known metastasis on CT.
The choice of treatment for NET hepatic metastases is largely dependent on underlying tumour biology and pattern of metastatic spread.35 According to the 2010 World Health Organization guidelines for the management of NETs, pathological grade (1-3) has been highlighted as an important marker for underlying tumour biology affecting survival.36 Pathologic grade is determined microscopically by the number of cellular mitoses per high powered field (hpf) and through Ki-67 labelling of tumours. NETs with <2mitoses/10hpf and <3% Ki-67 index are classified as low grade (G1) well-differentiated tumours whereas NETs with >20/10hpf and >20% Ki-67 labelling are denoted as high grade (G3) and poorly differentiated. Recent studies have shown that G3 NETs exhibit a poor prognosis following surgical management for hepatic metastases and are better treated non-operatively with chemotherapy.36
The metastatic pattern of spread in the liver for NETs also has prognostic implications and is categorized into three morphological subtypes:35,36 (I) “restricted metastases” involving one lobe or two adjacent segments; (II) “dominant lesion with bilobar metastases” whereby a single major focus is accompanied by multiple contralateral satellite lesions; (III) diffuse, multifocal liver metastases affecting multiple segments within and between lobes. Patients with Type I or II (25% and 15% of cases respectively) disease, in the absence of metastases at distant extrahepatic sites are considered for curative surgical resection.35,36
The aim of liver resection with curative intent in NETs is to leave no residual disease (R0 resection) in both primary and secondary sites, and this may be associated with 5-year survival rates of up to 85%.31,32 Surgical indications include the presence of a resectable well-differentiated NET without extra-abdominal metastases or peritoneal carcinomatosis, in a patient without right-sided cardiac dysfunction.35 Optimal cytoreduction aims to reduce tumour volume by at least 90%.32 Although there are no data from randomised trials, large series using historical controls or contemporary cases matched for stage have demonstrated that liver resection with optimal cytoreduction results in improved survival.37–39
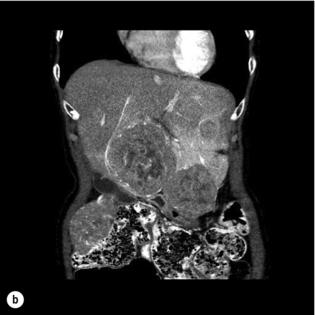
Most series of hepatic resection for metastatic NETs include an occasional case with an unknown primary, despite thorough imaging and endoscopy. Although survival data are sparse, an aggressive resectional approach for these patients is reasonable (Fig. 7.2b).
Non-surgical treatment modalities include RFA, TAE and TACE. RFA in isolation can achieve symptomatic relief and local control of variable duration in up to 80% of NET patients with hepatic metastases. Although studies comparing RFA to other modalities are limited, RFA has been advocated in patients with bilobar disease with up to 14 hepatic lesions of less than 7 cm in diameter, encompassing up to 20% of liver volume.33,39 TAE and TACE appear to deliver comparable results and thus one modality is not favoured over the other. Embolisation is usually indicated for more extensive hepatic disease or for tumours in close proximity to biliary structures precluding RFA.39 Duration of response is routinely short as the tumour rapidly develops collaterals and thus repeat treatments are often required.41 Embolisation is contraindicated in patients with 50–75% liver involvement due to the risk of precipitating acute hepatic failure.
In general, aggressive multimodal therapy with embolic, ablative and systemic strategies is recommended to debulk or downstage metastatic NETs.41 Despite complete resection, hepatic recurrence occurs in up to 84% of patients at 5 years post-surgery.39 Recurrence is suspected by the elevation of tumour markers such as 5-hydroxyindoleacetic acid (5-HIAA) and chromogranin A. Chromogranin A is more sensitive than 5-HIAA in identifying disease progression and high levels have been shown to predict poorer outcomes. A reduction in chromogranin A levels of > 80% predicts a good outcome following cytoreductive hepatectomy, even when complete resection has not been achieved.42
Liver transplantation has been advocated for patients with extensive, unresectable liver metastases with no extrahepatic disease. A recent retrospective study of 150 patients who underwent transplantation for metastatic NETs reported 5-year survival comparable to patients with hepatocellular carcinoma (HCC).43 Of those transplanted, patients under the age of 55 without the need for concurrent major resection of the primary tumour had the best overall survival.9 Therefore, liver transplantation does appear to confer long-term survival in carefully selected patients and should be considered in the management of NETs.44
Gastrointestinal stromal tumours
Gastrointestinal stromal tumours (GISTs) are the most common gastrointestinal mesenchymal malignancies originating from the interstitial cells of Cajal. Approximately 70–80% of GISTs harbour a mutated c-Kit proto-oncogene, which results in the constitutive activation of the receptor tyrosine kinase and unregulated cell growth. Two thirds of c-Kit mutations are located on exon 11.45 C-Kit exon 9 and PDGFRA mutations, encompassing a wild-type kinase domain that modulates receptor inhibitor sensitivity, account for another 5–10% of GISTs.46
Primary GISTs represent 1% of all gastrointestinal malignancies, and arise in the stomach (55%), small intestine (35%), colon/ rectum (10%) and oesophagus (5%), with the remainder found in various other sites (gallbladder, appendix or mesentery).47 The primary tumour is usually classified into four prognostic categories, ranging from very low risk to high risk, according to site of the lesion, size of the lesion and the number of mitotic figures identified.48 Surgery remains the gold standard for the treatment of primary GISTs.
Imatinib mesylate is a selective tyrosine kinase inhibitor that has revolutionised the treatment of unresectable GISTs.44 Response to imatinib is greatest in tumours that harbour the c-Kit exon 11 mutation, with resistance rates higher in patients harbouring exon 9 or platelet-derived growth factor receptor α (PDGFRA) mutations.44 Despite complete surgical resection with microscopic negative margins, recurrence (local or distant) occurs in 50% of patients.48 The use of imatinib in the adjuvant setting was investigated in the phase III ACOSOG placebo controlled trial (Z9001) for patients with resected GISTs 3 cm or greater in size. A statistically significant 1-year recurrence-free survival (RFS) of 98% in the treatment group versus 83% in the placebo group was observed, prompting the inclusion of imatinib as an adjuvant treatment modality.48 Currently, many nomograms have emerged to guide patient selection for those believed to be at highest risk of recurrence.
The treatment of metastatic GISTs has similarly been transformed by imatinib. Recurrence of GISTs most commonly occur with one of two metastatic patterns: local recurrence with peritoneal disease or intraparenchymal liver metastases.49 Most patients with recurrent metastatic GISTs will receive imatinib as first-line treatment, with a clinical response demonstrated in 80%. This response is durable with a median survival of 48 months.50 However, many patients develop imatinib resistance and disease progression caused by the development of secondary mutations.51 Second- (e.g. sunitinib) and third-line agents (e.g. nilotinib and masitinib) have shown promise in patients resistant to imatinib.52
A subset of patients with GISTs develop a pattern of disease progression where isolated nodular foci progress within a pre-existing tumour mass in a patient already on imatinib. Such cases of partial progression have the same median survival as patients who meet standard criteria for disease progression.54 There is currently no rationale for resection in this group. The benefit of surgical resection in the group of patients with disease that is stable or responding to imatinib is not clear.55
Breast cancer
Although breast cancer is common, isolated liver lesions in metastatic breast cancer are seen in only 7% of patients.56 Sakamoto et al.57 reported only 34 patients with resectable liver metastases among 11 000 breast cancer patients treated over an 18-year period. Selection criteria for such metastases are inconsistent in surgical series, with some centres considering resection only to disease confined to the liver while others advocate a more liberal approach. In short, there are no clear selection criteria for resection.
Despite heterogeneous selection criteria, 5-year survival rates fall into two groups. Several reports describe 5-year overall survival of approximately 25%;57,59 however, others report 5-year survival between 45% and 60%.60,61 These disparate results cannot be explained by differences in study design or treatment factors. Outcomes following hepatic resection may therefore merely reflect differences in tumour biology, or publication bias. Furthermore, 5-year disease-free survival rates are much lower than overall survival rates, suggesting that liver resection may function as a cytoreductive rather than curative procedure in these highly selected patients.
Ovarian cancer
Epithelial ovarian cancer represents the most common malignancy of the ovary, of which surgery and platinum-based chemotherapy remain the mainstay of treatment. Unfortunately, most develop chemoresistance after 24–36 months and median survival for advanced (stage III–IV) disease is 3.5 years.62 Aggressive surgical debulking is advocated in advanced cases, with optimal cytoreduction targeted at <1 cm of residual disease.63 Intraperitoneal (i.p.) chemotherapy has been demonstrated to further improve survival compared to intravenous therapy, and this is the current aim of treatment in many large centres. To be eligible for i.p. chemotherapy, patients must undergo maximal debulking.64 Successful cytoreduction is thus a crucial step in the management of advanced ovarian cancer.
A recent phase II trial investigating combined i.p. carboplatin with i.v. paclitaxel in stage II–IV disease is under way, with preliminary results showing minimal toxicity and appropriate response in patients with suboptimal (>2 cm) surgical debulking.67 Furthermore, various non-randomised observational studies have reported a benefit with varying degrees of cytoreductive surgery combined with hyperthermic intraperitoneal chemotherapy (HIPEC) in peritoneal carcinomatosis. The preliminary nature of these results precludes any definitive management recommendations.25
Survival following primary surgical debulking is inversely correlated with volume of residual disease, disease stage and tumour differentiation. Similarly, survival following hepatectomy for metastatic disease is dependent on optimal cytoreduction, negative margin status, greater pelvic than abdominal disease and a longer recurrence-free interval.68 TACE offers a potential future therapeutic option in achieving local control in patients with unresectable hepatic disease.69
Renal cell carcinoma
Renal cell carcinoma (RCC), often termed the ‘internist’s tumour’, represents the deadliest urological malignancy. Approximately 20–30% of patients with RCC present with synchronous metastatic disease and another 20–40% of patients with previous nephrectomy will develop more advanced disease.70 Fewer than 5% of patients have metastases restricted to the liver.71 Whereas interleukin-2 and interferon-α were previously used as first-line therapy for metastatic RCC, current regimens employ sunitinib, which has displayed a higher progression-free survival in phase III trials.72
The available data on hepatic resection for RCC metastases are limited to retrospective reports. A recent study from the Netherlands examined 33 patients who underwent resection or ablative therapy for RCC hepatic metastases. The study documented no operative mortality with 5-year disease-free and overall survival of 11% and 43%, respectively. The median overall survival was 33 months. Metachronous metastases and complete resection were highlighted as prognostic factors.70
Staehler et al. reported a 12-year retrospective comparative analysis of patients with metastatic RCC to the liver. In the study, 68 patients underwent surgery and were compared to a cohort of 20 patients who were eligible but refused an operation. Disease in these patients was mostly confined to the liver. Overall 5-year survival in the treatment arm was 62% in comparison to 29% in the control group. Prognostic features included complete resection of liver lesions, negative margins, length of disease-free interval from resection of the primary and a left-sided primary lesion.73 With ongoing improvements in surgical techniques coupled with an increasingly aggressive approach to metastatic disease in the liver, future prospective studies examining the role of hepatectomy in RCC should provide clearer treatment algorithms. Furthermore, an evidence-based approach to surgery combined with sunitinib or surafenib will hopefully be forthcoming.
Melanoma
The prognosis for patients with metastatic melanoma is poor and the median survival for patients with American Joint Committee on Cancer (AJCC) stage IV melanoma is 6–9 months.74 Gastrointestinal and liver metastases occur in 2–4% of individuals with stage IV disease,75 and palliative radiotherapy and systemic chemotherapy have largely been ineffective in conferring a survival advantage. Although biological agents such as interferon-α and interleukin-2 have yielded promising response rates, these are rarely durable and are associated with significant toxicity.74 Favourable results in patients undergoing metastasectomy in the lung, soft tissues or abdomen have provided some enthusiasm for surgery in a selected patient population.
The available evidence for hepatectomy for metastatic melanoma is limited and consists largely of subset analyses from larger series of patients with non-colorectal liver metastases. A recent retrospective study evaluated all patients who presented with metastatic melanoma over the last decade at a single Australian institution. In this series, 13 of 23 patients underwent resection for liver metastases. Disease-free interval from resection of the primary was a median of 49 months. Overall 3-year survival was 40% with a median survival of 21 months, influenced largely by the number of metastases and the presence of multiple sites involved. The median disease-free interval observed prior to recurrence was 14 months.75 Nevertheless, the authors have outlined the potential bias in their study, including only those patients who were most likely to achieve complete surgical resection in the operative cohort.
Recently, liver resection with postoperative tumour infiltrating lymphocyte (TIL) therapy has been explored. TIL involves the resection of metastatic lesions followed by extraction and culture of infiltrating lymphocytes ex vivo with interleukin-2. A direct comparison was performed between patients with complete surgical resection versus those with residual hepatic disease receiving postoperative TIL. The observed 3-year overall survival was 53% in the TIL cohort, with prognosis largely favoured by lack of extrahepatic disease and a single hepatic metastasis.76
The biological behaviour of metastatic melanoma depends in part on the site of origin of the primary tumour.77 Cutaneous melanoma is more common than ocular melanoma.78 While both metastasise to the liver, they appear to do so with distinct patterns and natural history. Ocular melanoma metastasises to the liver more frequently, but is more likely to be associated with isolated liver metastases than cutaneous melanoma.77,78 Survival following hepatectomy appears to be more favourable in the highly selected but rare group of patients with melanoma of ocular origin. Pawlik et al. reported 5-year survival of 21% for liver resection for ocular primaries, with no 5-year survivors when the initial site of disease was cutaneous. However, 75% of resected patients in this study developed recurrent disease, and the rate of recurrence was similar between the ocular and cutaneous groups.78
Non-colorectal gastrointestinal adenocarcinoma
Metastatic oesophageal cancer is usually widely disseminated and is associated with a 5-year survival of 3–5% when multiple sites of disease are present and 7–8% when disease is limited to the liver.79 Two case reports in the English-language literature describe hepatectomy for isolated, synchronous liver metastases.80,81 In both cases hepatectomy was performed simultaneously with oesophagectomy and was followed by hepatic arterial chemotherapy. Both patients developed multiple liver metastases at 680 and 781 months postoperatively. These recurrences responded partially to systemic chemotherapy, and patients were alive with disease at 1480 and 1881 months following hepatectomy. Thus, although rarely feasible, hepatectomy followed by hepatic arterial chemotherapy may provide a limited survival benefit in chemosensitive oesophageal cancer with isolated liver metastases.
Gastric adenocarcinoma is the second most common cause of cancer-related death worldwide, and the liver is a major site of spread in 9% of cases, generally in a bilobar distribution.82 Overall 5-year survival in patients with liver metastases ranges from 0% to 10% and the role of surgery is unclear in this setting. A recent study of patients with isolated synchronous or metachronous liver metastases reported that surgery was performed if the tumour burden was deemed completely resectable, while lesions <5 cm were considered for RFA. Overall 5-year, survival in this cohort was 27% with a median survival of 48 months. In a comparison of patients who were not offered the above treatment modalities, no patients survived to 5 years, with a median survival of 9 months.82 These results appear comparable to previously published studies.83 Data regarding independent predictors of survival are limited and appear to correlate with absence of serosal invasion of the primary tumour and the presence of a solitary liver metastasis.
Primary small-bowel malignancies represent an exceedingly rare but histologically diverse subgroup accounting for 2% of all GI malignancies.84 Small-bowel adenocarcinoma (SBA) represents the majority of these tumours and is seen in up to 5% of patients with familial adenomatous polyposis (FAP). By virtue of its non-specific clinical presentation and the limitations of radiological and endoscopic diagnostic modalities to examine the small bowel, approximately 80% of patients present with advanced disease. In addition, the low prevalence of SBAs limits our understanding of the natural history of tumour spread, restricting the development of clear treatment guidelines. A French multicentre retrospective study examining the efficacy of chemotherapy in 93 patients with advanced SBA compared various chemotherapeutic regimens for progression-free survival (PFS) and overall survival (OS). Median PFS and OS were 6.6 and 15.1 months, respectively, with best outcomes seen with FOLFOX therapy.85 Negative prognostic factors include a poor baseline WHO performance status, elevated carbohydrate antigen (CA) 19-9/carcinoembryonic antigen (CEA) levels and the presence of a duodenal primary. The ability of surgery to prolong PFS in hepatic SBA metastases has only been described in a single case report of an FAP patient with a PFS of 3 years following neoadjuvant chemotherapy and surgery.84 As such, future studies examining liver resections in metastastic SBA will provide further guidance as to its role in this disease.
Pancreatic ductal adenocarcinoma (PDAC) accounts for 90% of all histological subtypes of pancreatic cancer and confers the worst overall prognosis.86 Over the last 50 years, PDAC has continued to rank as the tenth most common cancer in the western world and the fourth leading cause of cancer death. PDAC presents in a non-specific manner, often when disease is already at an advanced stage. Improvements in chemotherapy, surgical technique and knowledge of tumour biology have translated into marginal improvements in survival. Currently, only 15–20% of patients present with disease amenable to curative resection, of which 20% are alive at 5 years.86 The overall average 5-year survival for unresectable PDAC is 5%, with a median survival of 6–9 months. Due to the dismal prognosis in patients with localised resectable disease, surgery in metastatic PDAC has been contraindicated. Yamada et al. examined the role of partial hepatectomy in non-neuroendocrine pancreatic cancer, including five patients with PDAC, one with adenosquamous carcinoma and one with cystadenocarcinoma.87 Patients were chosen for surgery if complete excision of intrahepatic disease was deemed feasible, reliable control of the primary disease was possible and the liver was the only site of spread. Overall 5-year survival in this cohort was 16.7%; however, five patients experienced a recurrence and subsequently died of their disease within 4–52 months. Prognostic factors appear to correlate with disease-free interval from primary to metastasis and the presence of negative surgical margins at metastasectomy. Although the authors highlight the potential role of liver resection in metastatic PDAC, they acknowledge the need for future studies to clarify the true benefit of this approach.
Testicular cancer
Metastasectomy is well established in the management of disseminated non-seminomatous germ cell testicular carcinoma that does not completely respond to chemotherapy. Although it can be difficult to differentiate active residual tumour from post-treatment fibrosis or necrosis, the probability of achieving cure by surgical resection is high. Residual teratoma has the potential for sarcomatous transformation and thus lymphadenectomy and visceral resection are performed whenever there is radiographic evidence of residual disease. The overall 10-year survival is 62% from diagnosis of hepatic metastasis.88
A single institution experience of 57 liver resections performed over the last two decades has demonstrated that surgery for hepatic metastases is safe and efficacious, depending on the histopathological characteristics of the resected specimen. Based on the presence and type of tumour in the liver, 40–70% of patients remain disease free at 20 months.89 Negative prognostic indicators included viable tumour in the resected specimen, metastases greater than 3 cm in diameter and pure embryonal carcinoma in the primary lesion.
Urothelial cancer
Data for metastasectomy in the management of disseminated urothelial cancer are sparse, and no studies specifically address the role of hepatectomy. Of those patients treated for primary urothelial cancer 30% will recur, of which 75% will be with distant spread. Five-year survival of 28% has been reported following resection of lung, brain, adrenal, small-bowel or lymph node metastases with variation in the use of adjuvant chemotherapy.90 Metastasectomy has also been employed for palliation.
Lung cancer
The management of metastatic lung cancer is largely restricted to radiation and chemotherapy. Although the surgical management of hepatic metastasis remains controversial, most cases have been reviewed within the broader context of NCRNNET. Hepatic metastases appear most commonly in right-sided non-small-cell lung tumours with concomitant bone metastases. A small case series of highly selected patients with one to two liver lesions has shown that surgery may confer a marginal survival benefit.91 Nevertheless, the role of surgery as well as other treatment modalities (RFA, TAE/TACE) cannot be definitively made with current evidence.
Adrenocortical tumours
Adrenocortical tumours with liver metastases are rare, and literature on the management of this disease scenario is mostly anecdotal. Case reports have provided no clear guidance regarding the role of surgical or ablative strategies. It is possible that patients who develop metachronous liver metastases with a disease-free interval >1 year from primary to metastasis may derive benefit from surgery.92
Endometrial cancer
Metastatic endometrial cancer is usually multifocal and rarely managed operatively. A recent single-centre report described the results in five patients who developed metastatic disease to the liver ranging from 11 months to 10 years after primary resection. All patients underwent hepatic surgery, with disease-free survival between 8 and 66 months. Based on these results, the authors advocate referral to a hepatobiliary specialist with the intent of pursuing surgery.93 Other isolated reports of long-term survivors exist within the context of larger studies focused on NCRNNET hepatic metastasis.
References
1. Nguyen, K.T., Laurent, A., Dagher, et al, Minimally invasive liver resection for metastatic colorectal cancer: a multi-institutional, international report of safety, feasibility and early outcomes. Ann Surg 2009; 250:842–848. 19806058
2. Nanji, S., Cleary, S.P., Ryan, P., et al, Up front hepatic resection for metastatic colorectal cancer results in favorable long-term survival. Ann Surg Oncol. 2013;20(1):295–304. 23054102
3. Abad, A., Massuti, B., Anton, A., et al. Colorectal cancer metastasis resectability after treatment with the combination of oxaliplatin, irinotecan and 5-fluorouracil. Final results of a phase II study. Acta Oncol. 2007; 22:1–7.
4. Glazer, E.S., Tseng, J.F., Al-Refaie, W., et al, Long-term survival after surgical management of neuroendocrine hepatic metastases. HPB (Oxford) 2010; 12:427–433. 20662794
5. Adam, R., Chiche, L., Aloia, T., et al, Hepatic resection for noncolorectal nonendocrine liver metastases: analysis of 1,452 patients and development of a prognostic model. Ann Surg 2006; 244:524–535. 16998361
6. O’Rourke, T.R., Tekkis, P., Yeung, S., et al, Long-term results of liver resection for non-colorectal, non-neuroendocrine metastases. Ann Surg Oncol 2008; 15:207–218. 17963007
7. Lendoire, J., Moro, M., Andriani, O., et al, Liver resection for non-colorectal, non-neuroendocrine metastases: analysis of a multicenter study from Argentina. HPB (Oxford) 2007; 9:435–439. 18345290
8. Weitz, J., Blumgart, L.H., Fong, Y., et al, Partial hepatectomy for metastases from noncolorectal, nonneuroendocrine carcinoma. Ann Surg 2005; 241:269–276. 15650637
9. Lewis, M.A., Hubbard, J., Multimodal liver-directed management of neuroendocrine hepatic metastases: review article. Int J Hepatol 2011; 2011 452343. 22121491
10. Ribatti, D., Mangialardi, G., Vacca, A., Stephen Paget and the ‘seed and soil’ theory of metastatic dissemination. Clin Exp Med 2006; 6:145–149. 17191105
11. Fidler, I.J., The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nat Rev Cancer 2003; 3:453–458. 12778135
12. van’t Veer, L.J., Dai, H., van de Vijver, M.J., et al, Gene expression profiling predicts clinical outcome of breast cancer. Nature. 2002;415(31):530–536. 11823860
13. Spentzos, D., Levine, D.A., Ramoni, M.F., et al, Gene expression signature with independent prognostic significance in epithelial ovarian cancer. J Clin Oncol. 2004;22(23):4700–4710. 15505275
14. Winnepenninckx, V., Lazar, V., Michiels, S., et al, Gene expression profiling of primary cutaneous melanoma and clinical outcome. J Natl Cancer Inst. 2006;98(5):472–482. 16595783
15. Gryfe, R., Kim, H., Hsieh, E.T., et al, Tumor microsatellite instability and clinical outcome in young patients with colorectal cancer. N Engl J Med. 2000;342(2):69–77. 10631274
16. Kaplan, R.N., Rafii, S., Lyden, D., Preparing the “soil”: the premetastatic niche. Cancer Res. 2006;66(1):11089–11093. 17145848
17. Kaplan, R.N., Riba, R.D., Zacharoulis, S., et al, VEGFR 1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438(7069):820–827. 16341007
18. Wojtowicz-Praga, S., Reversal of tumor-induced immunosuppression by TGF-beta inhibitors. Invest New Drugs. 2003;21(1):21–32. 12795527
19. Morton, D.L., Holmes, E.C., Golub, S.H., Immunologic aspects of lung cancer. Chest 1977; 71:640–643. 852344
20. Tabernero, J., Van Cutsem, E., Diaz-Rubio, E., et al, Phase II trial of cetuximab in combination with fluorouracil, leucovorin, and oxaliplatin in the first-line treatment of metastatic colorectal cancer. J Clin Oncol. 2007;25(33):5225–5232. 18024868
21. Znajda, T.L., Hayashi, S., Horton, P.J., et al, Postchemotherapy characteristics of hepatic colorectal metastases: remnants of uncertain malignant potential. J Gastrointest Surg 2006; 10:483–489. 16627212
22. Yachida, S., Jones, S., Bozic, I., et al, Distant metastasis occurs late during the genetic evolution of pancreatic cancer. Nature. 2010;467(7319):1114–1117. 20981102
23. Campbell, P.J., Yachida, S., Mudie, L.J., et al, The patterns and dynamics of genomic instability in metastatic pancreatic cancer. Nature. 2010;467(7319):1109–1113. 20981101
24. Gabriel, M., Oberauer, A., Dobrozemsky, G., et al, 68Ga DOTA-Tyr3-octreotide PET for assessing response to somatostatin-receptor-mediated radionuclide therapy. J Nucl Med. 2009;50(9):1427–1434. 19690033
25. Sommariva, A., Pilati, P., Rossi, C.R., Cytoreductive surgery combined with hyperthermic intra-peritoneal chemotherapy for peritoneal surface malignancies: current treatment and results. Cancer Treat Rev. 2012;38(4):258–268. 21807464
26. D’Angelica, M., Jarnagin, W., Dematteo, R., et al, Staging laparoscopy for potentially resectable non-colorectal, nonneuroendocrine liver metastases. Ann Surg Oncol 2002; 9:204–209. 11888880
27. Sofocleous, C.T., Nascimento, R.G., Gonen, M., et al, Radiofrequency ablation in the management of liver metastases from breast cancer. Am J Roentgenol 2007; 189:883–889. 17885061
28. Gervais, D.A., Arellano, R.S., Mueller, P.R., Percutaneous radiofrequency ablation of ovarian cancer metastasis to the liver: indications, outcomes, and role in patient management. Am J Roentgenol 2006; 187:746–750. 16928940
29. Gillams, A., Cassoni, A., Conway, G., et al, Radiofrequency ablation of neuroendocrine liver metastases: the Middlesex experience. Abdom Imaging 2005; 30:435–441. 15759207
30. Ribero, D., Curley, S.A., Imamura, H., et al, Selection for resection of hepatocellular carcinoma and surgical strategy: indications for resection, evaluation of liver function, portal vein embolization, and resection. Ann Surg Oncol 2008; 15:986–992. 18236112
31. Yao, K.A., Talamonti, M.S., Nemcek, A., et al, Indications and results of liver resection and hepatic chemoembolization for metastatic gastrointestinal neuroendocrine tumors. Surgery 2001; 130:677–685. 11602899
32. Harring, T.R., Nguyen, N.T.N., Goss, J.A., et al, Treatment of liver metastases in patients with neuroendocrine tumors: a comprehensive review. Int J Hepatol 2011; 2011 154541. 22013537
33. Strosberg, J.R., Cheema, A., Kvols, L.K., A review of systemic and liver directed therapies for metastatic neuroendocrine tumors of the gastroenteropancreatic tract. Cancer Control. 2011;18(2):127–137. 21451455
34. Faiss, S., Pape, U.F., Bohmig, M., et al, Prospective, randomized, multicenter trial on the antiproliferative effect of lanreotide, interferon alfa, and their combination for therapy of metastatic neuroendocrine gastroenteropancreatic tumors – the International Lanreotide and Interferon Alfa Study Group. J Clin Oncol. 2003;21(15):2689–2696. 12860945 Symptomatic relief but limited response to treatment.
35. Steinmuller, T., Kianmanesh, R., Falconi, M., et al, Conscensus guidelines for the management of patients with liver metastases from the digestive (neuro)endocrine tumors: foregut, midgut, hindgut and unknown primary. Neuroendocrinology 2008; 87:47–62. 18097131
36. Jagannath, P., Chhabra, D., Shrikhande, S., et al, Surgical treatment of liver metastases in Neuroendocrine neoplasms. Int J Hepatol 2012; 2012:782672. 22319653
37. Touzios, J.G., Kiely, J.M., Pitt, S.C., et al, Neuroendocrine hepatic metastases: does aggressive management improve survival? Ann Surg. 2005;241(5):776–785. 15849513
38. Chamberlain, R.S., Canes, D., Brown, K.T., et al, Hepatic neuroendocrine metastases: does intervention alter outcomes? J Am Coll Surg 2000; 190:432–445. 10757381
39. Karabulut, K., Akyildiz, Y., Lance, C., et al, Multimodality treatment of neuroendocrine liver metastases. J Surg. 2011;150(2):316–325. 21801968
40. Osborne, D.A., Zervos, E.E., Strosberg, J., et al, Improved outcome with cytoreduction versus embolization for symptomatic hepatic metastases of carcinoid and neuroendocrine tumors. Ann Surg Oncol 2006; 13:572–581. 16511671
41. Hodul, P., Malafa, M., Choi, J., et al, The role of cytoreductive hepatic surgery as an adjunct to the management of metastatic neuroendocrine carcinomas. Cancer Control 2006; 13:61–71. 16508628
42. Jensen, E.H., Kvols, L., McLoughlin, J.M., et al, Biomarkers predict outcomes following cytoreductive surgery for hepatic metastases from functional carcinoid tumors. Ann Surg Oncol 2007; 14:780–785. 17146740
43. Gedaly, R., Daily, M.F., Davenport, D., et al, Liver transplantation for the treatment of liver metastases from neuroendocrine tumors: an analysis of the UNOS database. Arch Surg. 2011;146(8):953–958. 21844436
44. van Vilsteren, F.G., Baskin-Bey, E.S., Nagorney, D.M., et al, Liver transplantation for gastroenteropancreatic neuroendocrine cancers: defining selection criteria to improve survival. Liver Transpl 2006; 12:448–456. 16498656
45. Florman, S., Toure, B., Kim, L., et al, Liver transplantation for neuroendocrine tumors. J Gastrointest Surg 2004; 8:208–212. 15036197
46. Corless, C.L., Barnett, C.M., Heinrich, M.C., Gastrointestinal stromal tumors: origin and molecular oncology. Nat Rev Cancer. 2011;11(12):865–878. 22089421
47. Stamatakos, M., Douzinas, E., Stefanaki, C., et al, Review: gastrointestinal stromal tumors. World J Surg Oncol 2009; 7:61. 19646278
48. Pisters, P.W.T., Colombo, C., Adjuvant imatinib therapy for gastrointestinal stromal tumors. J Surg Oncol 2011; 104:896–900. 22069174
49. DeMatteo, R.P., Lewis, J.J., Leung, D., et al, Two hundred gastrointestinal stromal tumors: recurrence patterns and prognostic factors for survival. Ann Surg 2000; 231:51–58. 10636102
50. Zhu, J., Yang, Y., Zhou, L., et al, A long-term follow-up of the imatinib mesylate treatment for the patients with recurrent gastrointestinal stromal tumor (GIST): the liver metastasis and the outcome. BMC Cancer 2010; 10:199. 20465813
51. Gorre, M.E., Mohammed, M., Ellwood, K., et al, Clinical resistance to STI-571 cancer therapy caused by BCR-ABL gene mutation or amplification. Science (New York) 2001; 293:876–880. 12351679
52. Kim, E.J., Zalupski, M.M., Systemic therapy for advanced gastrointestinal stromal tumors: beyond imatinib. J Surg Oncol 2011; 104:901–906. 22069175
53. Xia, L., Zhang, M.M., Ji, L., et al, Resection combined with imatinib therapy for liver metastases of gastrointestinal stromal tumors. Surg Today 2010; 40:936–942. 20872196
54. Desai, J., Shankar, S., Heinrich, M.C., et al, Clonal evolution of resistance to imatinib in patients with metastatic gastrointestinal stromal tumors. Clin Cancer Res. 2007;13(18 Pt 1):5398–5405. 17875769
55. Gronchi, A., Fiore, M., Miselli, F., et al, Surgery of residual disease following molecular-targeted therapy with imatinib mesylate in advanced/metastatic GIST. Ann Surg 2007; 245:341–346. 17435538
56. Rubino, A., Doci, R., Foteuh, J.C., et al, Hepatic metastases from breast cancer. Updates Surg 2010; 62:143–148. 21052894
57. Sakamoto, Y., Yamamoto, J., Yoshimoto, M., et al, Hepatic resection for metastatic breast cancer: prognostic analysis of 34 patients. World J Surg 2005; 29:524–527. 15770377
58. Lermit, E., Marzano, E., Chereau, E., et al, Surgical resection of liver metastases from breast cancer. J Surg Oncol 2010; 19:e79–e84. 19592234
59. Selzner, M., Morse, M.A., Vredenburgh, J.J., et al, Liver metastases from breast cancer: long-term survival after curative resection. Surgery 2000; 127:383–389. 10776428
60. Vlastos, G., Smith, D.L., Singletary, S.E., et al, Long-term survival after an aggressive surgical approach in patients with breast cancer hepatic metastases. Ann Surg Oncol 2004; 11:869–874. 15342348
61. Carlini, M., Lonardo, M.T., Carboni, F., et al, Liver metastases from breast cancer. Results of surgical resection. Hepatogastroenterology 2002; 49:1597–1601. 12397744
62. Chi, D.S., McCaughty, K., Diaz, J.P., et al, Guidelines and selection criteria for secondary cytoreductive surgery in patients with recurrent, platinum-sensitive epithelial ovarian carcinoma. Cancer. 2006;106(9):1933–1939. 16572412
63. Ellatar, A., Bryant, A., Winter-Roach, B.A., et al, Optimal primary surgical treatment for advanced epithelial ovarian cancer. Cochrane Database Syst Rev. 2011;(8) CD007565. 21833960
64. Armstrong, D.K., Bundy, B., Wenzel, L., et al, Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med. 2006;354(1):34–43. 16394300
65. Bristow, R.E., Montz, F.J., Lagasse, L.D., et al, Survival impact of surgical cytoreduction in stage IV epithelial ovarian cancer. Gynecol Oncol 1999; 72:278–287. 10053096
66. Naik, R., Nordin, A., Cross, P.A., et al, Optimal cytoreductive surgery is an independent prognostic indicator in stage IV epithelial ovarian cancer with hepatic metastases. Gynecol Oncol 2000; 78:171–175. 10926798
67. Fujiwara, K., Nagao, S., Kigawa, J., et al, Phase II study of intraperitoneal carboplatin with intravenous paclitaxel in patients with suboptimal residual epithelial ovarian or primary peritoneal cancer: a Sankai gynecology cancer study group study. Int J Gynecol Cancer 2009; 19:834–837. 19574769
68. Roh, H.J., Kim, D.Y., Joo, W.D., et al, Hepatic resection as part of secondary cytoreductive surgery for recurrent ovarian cancer involving the liver. Arch Gynecol Obstet 2011; 284:1223–1229. 21132314
69. Vogl, T.J., Naguib, N.N., Lehnert, T., et al, Initial experience with repetitive transarterial chemoembolization (TACE) as a third line treatment of ovarian cancer metastasis to the liver: Indications, outcomes and role in patient’s management. Gynecol Oncol. 2012;124(2):225–229. 22079359
70. Ruys, A.T., Tanis, P.J., Iris, N.D., et al, Surgical treatment of renal cell carcinoma liver metastases: a population based study. Ann Surg Oncol 2011; 18:1932–1938. 21347794
71. Dekernion, J.B., Ramming, K.P., Smith, R.B., The natural history of metastatic renal cell carcinoma: a computer analysis. J Urol 1978; 120:148–152. 78992
72. Motzer, R.J., Hutson, T.E., Tomczak, P., et al, Sunitinib versus interferon alfa in metastatic renal cell carcinoma. N Engl J Med 2007; 356:115–124. 17215529
73. Staehler, M.D., Kruse, J., Haseke, N., et al, Liver resection for metastatic disease prolongs survival in renal cell carcinoma: a 12-year result from a retrospective comparative analysis. World J Urol 2010; 28:543–547. 20440505
74. McGettigan, S. A review of treatments for patients with metastatic melanoma. Continuing Education Modules: The Abramson Cancer Center of the University of Pennsylvania 2009. http://www.oncolink.org/resources/article.cfm?c=16&s=59&ss=224&id=972. [[accessed 04.10.12]].
75. Chua, T.C., Saxena, A., Morris, D.L., Surgical metastasectomy in AJCC stage IV M1c melanoma patients with gastrointestinal and liver metastases. Ann Acad Med Singapore 2010; 39:634–639. 20838706
76. Ripley, R.T., Davis, J.L., Klapper, J.A., et al, Liver resection for metastatic melanoma with postoperative tumor-infiltrating lymphocyte therapy. Ann Surg Oncol 2010; 17:163–170. 19777192
77. Albert, D.M., Ryan, L.M., Borden, E.C., Metastatic ocular and cutaneous melanoma: a comparison of patient characteristics and prognosis. Arch Ophthalmol 1996; 114:107–108. 8540843
78. Pawlik, T.M., Zorzi, D., Abdalla, E.K., et al, Hepatic resection for metastatic melanoma: distinct patterns of recurrence and prognosis for ocular versus cutaneous disease. Ann Surg Oncol 2006; 13:712–720. 16538410
79. Daly, J.M., Karnell, L.H., Menck, H.R., National Cancer Data Base report on esophageal carcinoma. Cancer. 1996;78(8):1820–1828. 8859198
80. Yamamoto, T., Tachibana, M., Kinugasa, S., et al, Esophagectomy and hepatic arterial chemotherapy following hepatic resection for esophageal cancer with liver metastasis. J Gastroenterol 2001; 36:560–563. 11519836
81. Hanazaki, K., Kuroda, T., Wakabayashi, M., et al, Hepatic metastasis from esophageal cancer treated by surgical resection and hepatic arterial infusion chemotherapy. Hepatogastroenterology 1998; 45:201–205. 9496513
82. Dittmar, Y., Altendorf-Hofmann, A., Rauchfuss, F., et al, Resection of liver metastases is beneficial in patients with gastric cancer: a report on 15 cases and review of the literature. Gastric Cancer. 2012;15(2):131–136. 21892617
83. Koga, R., Yamamoto, J., Ohyama, S., et al, Liver resection for metastatic gastric cancer: experience with 42 patients including eight long term survivors. Jpn J Clin Oncol 2007; 37:836–842. 17928333
84. Eigenbrod, T., Kullman, F., Klebl, F., Resection of small bowel adenocarcinoma liver metastasis combined with neoadjuvant and adjuvant chemotherapy results in extended disease free period – a case report. Int J Gastrointest Cancer 2006; 37:94–97. 17827529
85. Zaanan, A., Costes, L., Gauthier, M., et al, Chemotherapy of advanced small-bowel adenocarcinoma: a multicenter AGEO Study. Ann Oncol. 2010;21(9):1786–1793. 20223786
86. Samuel, N., Hudson, T.J., The molecular and cellular heterogeneity of pancreatic ductal adenocarcinoma. Nat Rev Gastroenterol Hepatol. 2011;9(2):77–87. 22183185
87. Yamada, H., Hirano, S., Tanaka, E., et al, Surgical treatment of liver metastases from pancreatic cancer. HPB (Oxford) 2006; 8:85–88. 18333251
88. You, Y.N., Leibovitch, B.C., Hepatic metastasectomy for testicular germ cell tumors: is it worth it? J Gastrointest Surg 2009; 13:595–601. 19190967
89. Mallucio, M., Einhorn, L.H., Goulet, R.J., Surgical therapy for testicular cancer metastatic to the liver. HPB (Oxford) 2007; 9:199–200. 18333222
90. Lehmann, J., Suttmann, H., Albers, P., et al, Surgery for metastatic urothelial cancer with curative intent: the German experience. Eur Urol. 2009;55(6):1293–1299. 19058907
91. Ercolani, G., Ravaioli, M., Grazi, G.L., et al, The role of liver resections for metastases from lung carcinoma. HPB (Oxford) 2006; 8:114–115. 18333258
92. Di Carlo, I., Toro, A., Sparatore, F., et al, Liver resection for hepatic metastases from adrenocortical carcinoma. HPB (Oxford) 2006; 8:106–109. 18333256
93. Knowles, B., Bellamy, C.O.C., Oniscu, A., Wigmore, S.J., Hepatic resection for metastatic endometrioid carcinoma. HPB (Oxford) 2010; 12:412–417. 20662792