6 Non-ablative fractional laser rejuvenation
Summary and Key Features
• Non-ablative fractional resurfacing is a safe and effective treatment that has become the cornerstone for facial rejuvenation and acne scarring
• It is effective in treating a variety of conditions including acne scarring, mild to moderate photoaging, and some forms of dyspigmentation
• Non-ablative fractional photothermolysis (NAFR) has minimal downtime with almost no restrictions on activity immediately following treatment
• Common areas treated include the face, neck, chest, and hands
• All Fitzpatrick skin phototypes can be treated provided settings are adjusted accordingly
• The preoperative consultation is a vital component of the treatment regimen to ensure optimal outcomes
• Erythema and edema are common sequelae after treatment and resolve within a few days
• Long-term complications are exceedingly rare
• Technology in the field is changing rapidly and the selection of equipment is based on individual preference
• Home-based devices are a new frontier for lasers but will not replace office-based systems
Pathophysiology
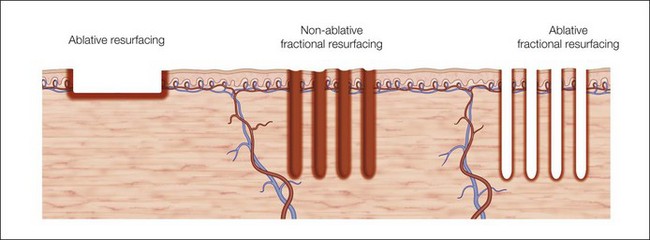
In fractional photothermolysis, a regular array of pixelated light energy creates focal areas of epidermal and dermal tissue damage or microthermal treatment zones (MTZ) (Fig. 6.1). Since its inception, several different lasers have been developed to take advantage of this technological advance. Each laser has parameters that can modify the density, depth, and size of the vertical columns of MTZs. The individual wounds created by FP are surrounded by healthy tissue resulting in a much quicker healing process when compared with traditional ablative skin resurfacing. This targeted damage with MTZ is hypothesized to stimulate neocollagenesis and collagen remodeling leading to the clinical improvements seen in scarring and photoaging. In the original study by Manstein et al., the histologic changes seen after NAFR were elegantly described. Immediately following treatment, lactate dehydrogenase (LDH) viability staining showed both epidermal and dermal cell necrosis within a sharply defined column correlating with the MTZ. There was continued loss of dermal cell viability 24 hours after treatment, but via a mechanism of keratinocyte migration, the epidermal defect had been repaired. One week after treatment, individual MTZs were still evident by LDH staining, but after 3 months there was no histologic evidence of loss of cell viability. Water serves as the target chromophore allowing for thermal damage to epidermal keratinocytes and collagen.
Equipment
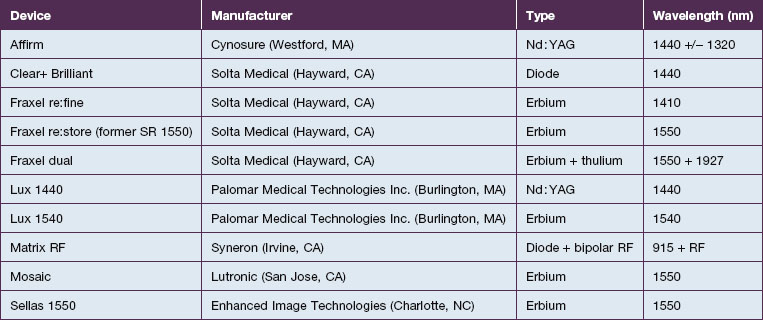
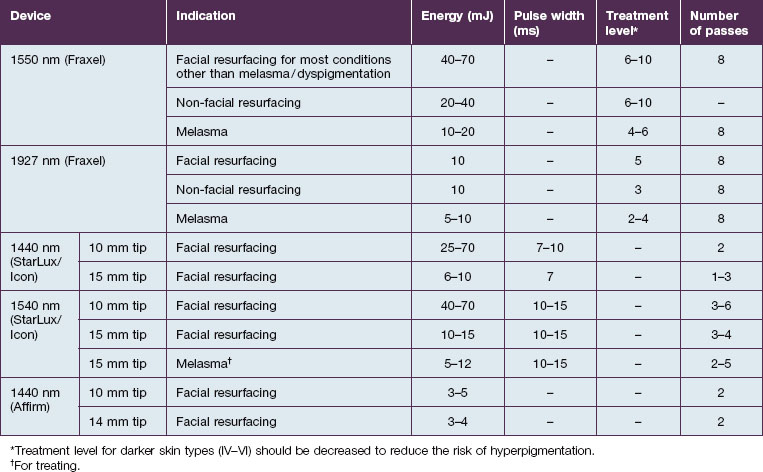
As the technology of fractional photothermolysis continues to evolve, new devices continually come to market. A list of currently available NAFR systems is given in Table 6.1. The table is not comprehensive and, as one can imagine, the devices will change constantly. This section will provide a brief description of a few of the more commonly used devices.
Applications
While NAFR is currently approved by the US Food and Drug Administration for the treatment of benign epidermal pigmented lesions, periorbital rhytides, skin resurfacing, melasma, acne and surgical scars, actinic keratoses, and striae, it has been reported to be used in many other clinical settings (Box 6.1).
Box 6.1
Clinical indications for non-ablative fractional resurfacing
Photoaging
With their seminal study in 2004 using a prototype non-ablative fractional resurfacing device, Manstein and colleagues first demonstrated the clinical effectiveness of fractional photothermolysis by showing improvement in periorbital rhytides. Three months after four treatments with the fractionated device, 34% of patients had moderate to significant improvements and 47% had improvement in texture as rated by blinded investigators. Overall, 96% were noted to be ‘better’ post-treatment. The skin tightening seen after non-ablative fractional resurfacing is similar to ablative resurfacing with tightening within the first week after treatment, apparent relaxation at 1 month, and retightening at 3 months (Case study 1).
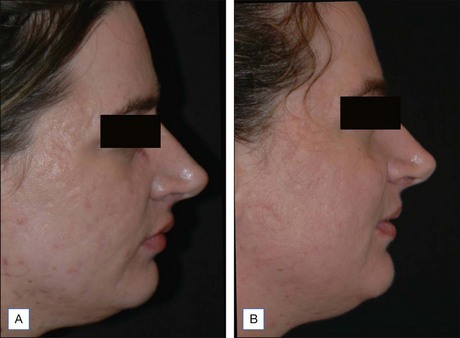
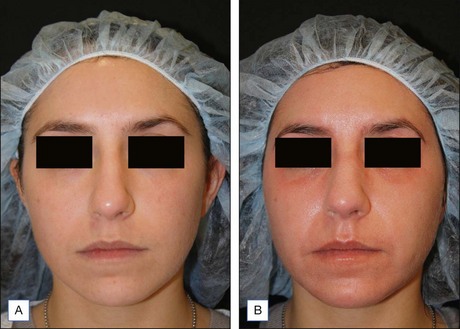
Subsequent reports have confirmed the efficacy of NAFR beyond just periorbital lines. Wanner and colleagues showed statistically significant improvement in photodamage of both facial and non-facial sites with 73% of patients improving at least 50%. In 2006, Geronemus also reported his experience with fractional photothermolysis, finding it to be effective in treating mild to moderate rhytides. Figures 6.2 and 6.3 show typical improvement in rhytides and pigmentation after treatment with non-ablative fractional resurfacing. For deeper rhytides, such as the vertical lines of the upper lip, improvement is also seen but not nearly to the same degree as in ablative approaches.
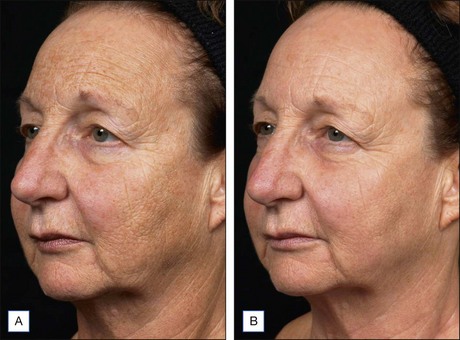
Figure 6.2 Improvement in moderate rhytides 1 month after two treatments with Fraxel 1927 nm.
(Photo courtesy of Solta Medical.)
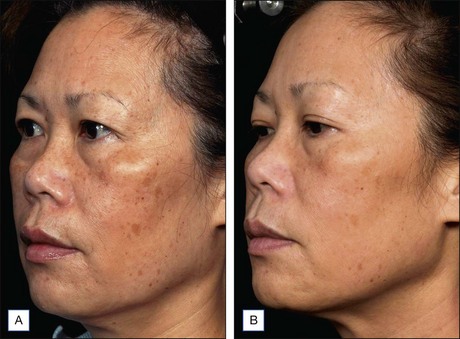
Figure 6.3 Improvement in rhytides and dyspigmentation 1 month after three treatments with Fraxel re:store.
(Photo courtesy of Solta Medical.)
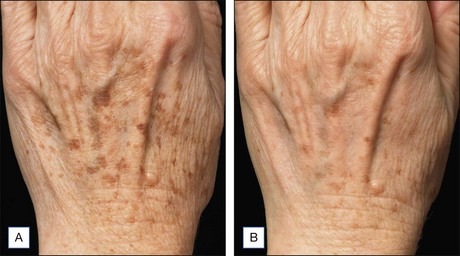
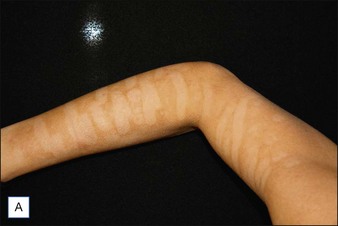
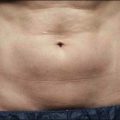
NAFR is also considered to be an effective and safe treatment modality for photoaging off the face including the neck, chest, arms, hands (Fig. 6.4), legs, and feet. These body sites are typically very challenging to treat with other treatment modalities given either increased risks of complications (e.g. scarring) associated with ablative technologies or lack of efficacy that has been previously observed with other non-ablative devices. Jih et al reported statistically significant improvement in pigmentation, roughness, and wrinkling of the hands in ten patients treated with non-ablative fractional resurfacing. In our experience, we have found NAFR to be very safe when settings are adjusted accordingly.
Scarring
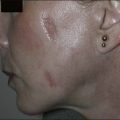
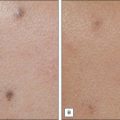
Scarring can induce a tremendous psychological, physical, and cosmetic impact on individuals. Previous therapeutic modalities in scar treatment include surgical punch grafting, subcision, dermabrasion, chemical peeling, dermal fillers, as well as laser resurfacing with ablative and non-ablative devices. Published studies have demonstrated that NAFR can be successfully utilized in the treatment of various forms of scarring, including acne scarring, with a very favorable safety profile (Fig. 6.5). Mechanistically, fractional photothermolysis allows controlled amounts of high energy to be delivered deep within the dermis resulting in collagenolysis and neocollagenesis, which smoothes the textural abnormalities of acne scarring. In a large clinical study, Weiss showed a median 50–75% improvement of acne scars using a 1540 nm fractionated laser system after three treatments at 4-week intervals with 85% of patients rating their skin as improved. Alster showed similarly impressive results in a study of 53 patients with mild to moderate acne scarring; 87% of patients who received three treatments at 4-week intervals showed at least 51–75% improvement in the appearance of their acne scars. Non-ablative fractional resurfacing, in our estimation, is the treatment of choice for facial acne scarring.
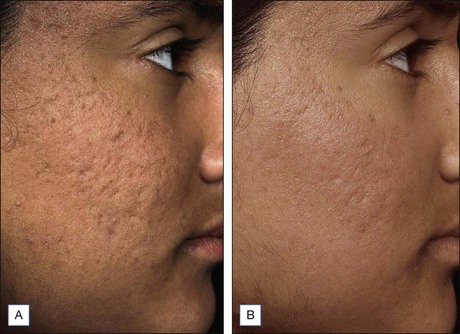
NAFR can also be safely used to treat acne scarring in darker-pigmented patients (Fig. 6.6). A study of 27 Korean patients with skin types IV or V that were treated with three to five non-ablative fractional resurfacing treatments revealed no significant adverse effects, specifically pigmentary alterations. Furthermore, all forms of acne scarring including ice-pick, boxcar, and rolling scars improved with eight patients (30%) reporting excellent improvement, 16 patients (59%) significant improvement, and three patients (11%) moderate improvement. With such a good efficacy and safety profile, many clinicians prefer NAFR to ablative fractional photothermolysis when it comes to treating acne scarring.
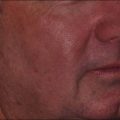
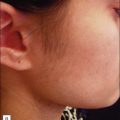
Traditionally, treatment of hypopigmented scars has shown variable efficacy, but current evidence suggests that NAFR may be particularly useful. A pilot study of NAFR laser treatment showed a 51–75% improvement in hypopigmented scars in 6 of 7 patients treated. The mechanism of action for this repigmentation has been hypothesized to be secondary to migration of melanocytes from the pigmented, normal skin into the scar, resulting in blending of the border (Fig. 6.7).
Patient selection
To achieve satisfactory results with NAFR, a series of four to six treatments is required. Typically these procedures are spaced out every 4 weeks and thus, an entire treatment regimen may take 6 months or more to complete (Case study 2). Those patients who prefer to have dramatic results after only one session are not the right candidates for non-ablative fractional resurfacing. Instead, these patients may benefit from fractional ablative resurfacing, which typically requires fewer treatment sessions, but has a much longer recovery. The procedure is painful, but topical anesthesia and forced-air cooling make the procedure tolerable in most. Redness and swelling last an average of 3 days.
Patients who should not be treated with fractional resurfacing are women who are pregnant or lactating, those with active infection, particularly herpes simplex, and patients with a history of isotretinoin use in the past 6 months (Box 6.2). Furthermore, individuals with unrealistic expectations should not be treated.
The ideal patient for treatment is a fair skin (Fitzpatrick types I–III), motivated patient who desires attainable results with a few days downtime and has realistic expectations (Case study 3).
Pretreatment
Pre-treatment with oral antiviral medications is recommended in those with a history of herpes simplex virus (HSV) infection (Box 6.3). Although some practitioners advocate the routine use of HSV prophylaxis regardless of previous infection, we do not feel that this is necessary. Firoz et al recently reported the first three cases of herpes zoster within the distribution of the trigeminal nerve after NAFR. The patients all had a history of chicken pox and none had received prophylaxis prior to treatment. Patients with a family history of herpes zoster and who have not had shingles themselves may thus benefit from the use antiviral treatment prior to treatment. Prophylactic antibiotics are not needed for non-ablative fractional resurfacing.
Box 6.3
Procedural steps in NAFR
On the day of the first procedure, serial standardized photographs should be taken to allow patients to observe their treatment progress. Topical anesthesia should be applied 1 hour prior to starting the treatment. Several different anesthetic agents are currently available including 5% lidocaine, 7% lidocaine/7% tetracaine, 23% lidocaine/7% tetracaine, and 30% lidocaine. In our experience, anesthetic agents with tetracaine induce significant erythema leading to patient dissatisfaction. We use 30% lidocaine in our practice as it provides the most comfort with the least erythema. To minimize the risk of systemic toxicity from the topical anesthetics, areas no greater than 300–400 cm2 should be treated during each session. Before the treatment, all of the anesthetic should be thoroughly washed off (Case study 4). Oral anxiolytics and analgesics may be required in a small fraction of patients who cannot tolerate the procedure with topical anesthesia alone. In those who find the procedure too uncomfortable with topical anesthesia, we first add ketorolac (Toradol®). In more anxious or intolerant individuals we have had success with diazepam and IM meperidine (Demerol®). IV sedation is not needed for this procedure. Metal eye protection is advised for the patient. All individuals in the treatment room should also wear eye protection. When it comes to selection of treatment parameters, a number of factors need to be considered including clinical indication, anatomical site, as well as skin phototype of the patients. In general, studies demonstrate that post-inflammatory pigmentation is less common when fractional resurfacing of darker skin is performed using lower density settings, fewer passes, and longer treatment intervals. Treatment of non-facial sites should be done with slightly lower density and fluence settings.
Pearl 6
Large treatment areas (>400 cm2) should be avoided to reduce the risk of lidocaine toxicity.
General technique
For facial resurfacing, our settings are individualized according to patients’ needs and tolerability. We often start with energy levels between 40–50 mJ and a treatment level of 6–8 (Table 6.2). The settings are often increased during subsequent visits if tolerated.
Post-treatment
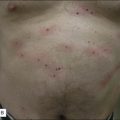
Upon completion of the treatment, patients are advised to ice their skin for several minutes and then periodically over the next few hours. Not only does this help with patient comfort, but it also reduces post-procedure swelling. Erythema develops immediately afterwards in all treated patients (Fig. 6.8) and typically resolves in 3 days. Use of non-comedogenic moisturizers is also recommended. Patients are advised to wear sun protection for several weeks after their treatment to reduce the risk of hyperpigmentation. In those with an increased risk of hyperpigmentation, hydroquinone may be started immediately after the procedure. We routinely wait to start lightening agents until we see the first signs of post-inflammatory pigmentation, which is usually around day 21 post-treatment. In a recent prospective study by Alster et al, a light emitting-diode device (Gentlewaves, Light BioSciences, Virginia Beach, VA) has been shown to decrease erythema intensity and duration following treatment, although the precise mechanism of action is unclear.
Safety and complications
While complications, especially long-term ones, are extremely rare, patients should be educated to expect typical side effects including post-treatment erythema, edema, dry skin, and desquamation (Box 6.4).
Box 6.4
Complications associated with non-ablative fractional resurfacing
| More common complications | Less common complications |
Advanced topics: treatment tips for experienced practitioners
Many patients have both the pigmentary changes and rhytides associated with photoaging. A hybrid approach combining two non-ablative fractional resurfacing wavelengths optimizes outcomes. Alternating 1927 nm with 1550 nm treatments addresses both concerns (Case study 5). An alternative approach uses intense pulsed light (IPL) to address the dyschromia and uneven pigmentation of photoaging while 1550, 1540, or 1440 nm NAFR devices address wrinkling. In a yet unpublished study that we recently performed, the addition of a MaxG IPL (Palomar Medical Technologies, Burlington, MA) treatment immediately prior to the Lux1540 non-ablative resurfacing device yielded significant improvement in photoaging scores when rated by blinded physicians.
Alexiades-Armenakas MR, Dover JS, Arndt KA. The spectrum of laser skin resurfacing: nonablative, fractional, and ablative laser resurfacing. Journal of the American Academy of Dermatology. 2008;58(5):719–737.
Bogdan Allemann I, Kaufman J. Fractional photothermolysis – an update. Lasers in Medical Science. 2010;25(1):137–144.
Geronemus RG. Fractional photothermolysis: current and future applications. Lasers in Surgery and Medicine. 2006;38(3):169–176.
Graber EM, Tanzi EL, Alster TS. Side effects and complications of fractional laser photothermolysis: experience with 961 treatments. Dermatologic Surgery. 2008;34(3):301–305.
Manstein D, Herron GS, Sink RK, et al. Fractional photothermolysis: a new concept for cutaneous remodeling using microscopic patterns of thermal injury. Lasers in Surgery and Medicine. 2004;34:426–438.
Marra DE, Yip D, Fincher EF, et al. Systemic toxicity from topically applied lidocaine in conjunction with fractional photothermolysis. Archives of Dermatology. 2006;142(8):1024–1026.
Metelitsa AI, Alster TS. Fractionated laser skin resurfacing treatment complications: a review. Dermatologic Surgery. 2010;36(3):299–306.
A good review of treatment of complications with non-ablative fractional photothermolysis.
Narurkar VA. Nonablative fractional laser resurfacing. Dermatologic Clinics. 2009;27(4):473–478.
Sherling M, Friedman PM, Adrian R, et al. Consensus recommendations on the use of an erbium-doped 1,550-nm fractionated laser and its applications in dermatologic laser surgery. Dermatologic Surgery. 2010;36(4):461–469.
Tierney EP, Kouba DJ, Hanke CW. Review of fractional photothermolysis: treatment indications and efficacy. Dermatologic Surgery. 2009;35(10):1445–1461.