CHAPTER 151 Noise-Induced Hearing Loss
One of the most common causes of permanent hearing impairment is exposure to excessive sounds. Millions of individuals worldwide have noise-induced hearing loss (NIHL), resulting in a reduced quality of life because of social isolation, and possible inexorable tinnitus and impaired communication with family members, coworkers, and friends.1 The costs in terms of compensation and early retirement payments for work-related NIHL are immense. The U.S. Department of Veterans Affairs spends approximately $700 million a year on disability compensation and treatments for NIHL. NIHL is the single largest disability expenditure of the Veterans Benefits Administration.2
Measurement of Noise
The term noise is commonly used to designate an undesirable sound. In the scientific and clinical fields that deal with hearing, this term has come to mean any excessively loud sound that has the potential to harm hearing. The temporal patterns of environmental noise are typically described as continuous, fluctuating, intermittent, or impulsive.3 Continuous or steady-state noise remains relatively constant, whereas fluctuating noise increases and decreases in level over time, and intermittent sounds are interrupted for varying time periods. Impulsive or impact noises caused by explosive or metal-on-metal mechanical events have rapidly changing pressure characteristics consisting of intense, short-lasting (i.e., milliseconds) wave fronts, followed by much smaller reverberations and echoes that occur over many seconds. The amount of noise, usually referred to as the sound pressure level (SPL), is conventionally measured by a sound-level meter in decibel (dB) units using a frequency-weighting formula called the A-scale. The dBA-scale metric of sound level essentially mimics the threshold-sensitivity curve for the human ear, so the low-frequency and high-frequency components are given less emphasis as auditory hazards. Standard sound-level meters have electronic networks designed to measure noise magnitude automatically in dBA, whereas to measure impulse or impact noise, a more intricate peak-reading sound-level meter is needed that is capable of accurately measuring sounds with essentially instantaneous onset times.
Nature of the Hearing Loss
In PTS, the elevation in hearing thresholds is irreversible because lasting structural damage occurs to the critical elements of the cochlea. The precise relationship between the TTS and PTS stages of hearing loss caused by noise exposure is unknown. Although it seems logical to assume that repeated episodes of TTS would eventually lead to PTS, experimental findings imply that the fundamental processes underlying the development of reversible versus permanent NIHL are unrelated. Nordmann and colleagues4 using a survival fixation approach showed that the histopathologic manifestations of TTS and PTS noise damage to the chinchilla cochlea are distinct. Specifically, TTS was correlated with a buckling of the supporting pillar cell bodies in the frequency region of the maximal exposure effect. The morphologic abnormality that was consistently correlated with PTS was a focal loss of hair cells, and a complete degeneration of the corresponding population of nerve fiber endings. Because PTS eventually develops from repeated exposures to stimuli that initially produce only TTS, it is likely that the latter condition is also associated with subtle changes to the sensitive outer hair cell (OHC) system that go undetected by conventional light microscopy.
Acoustic trauma was previously a relatively rare event that was typically associated with accidental explosions in industrial settings. Military servicemen and servicewomen caught in roadside bomb explosions in the current armed conflicts in Iraq and Afghanistan are returning home in epidemic numbers, however, with profound permanent hearing losses and tinnitus.5 Consequently, acoustic trauma is a hearing problem that is increasing, at least in combat troops. Because many of these postdeployment cases are being treated in the private sector, all otolaryngologists may see acoustic trauma in increasing numbers.
Irreversible NIHL is a specific pathologic state exhibiting a recognized set of symptoms and objective findings.6 NIHL includes (1) a permanent sensorineural hearing loss with damage principally to cochlear hair cells, and primarily to OHCs; (2) a history of a long-term exposure to dangerous noise levels (i.e., >90 dBA for 8 hours/day) sufficient to cause the degree and pattern of hearing loss described by audiologic findings; (3) a gradual loss of hearing over the first 5 to 10 years of exposure; (4) a hearing loss involving initially the higher frequencies from 3 to 8 kHz before including frequencies less than or equal to 2 kHz; (5) speech-recognition scores that are consistent with the audiometric loss; and (6) a hearing loss that stabilizes after the noise exposure is terminated.
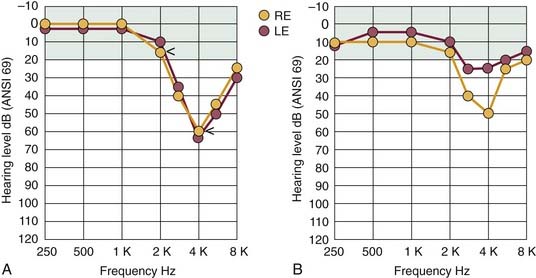
A patient with NIHL commonly consults a physician because of difficulties in hearing and understanding ordinary speech, especially in the presence of background noise. Many variations can be found in the detailed configuration of the audiogram of a noise-damaged ear, depending on the temporal and spectral distribution of the noise stimulus, and on the stage of hearing loss. The pattern of hearing loss most commonly associated with the earlier stages of NIHL is illustrated in Figure 151-1A. The beginning region of impairment involves the sensitive midfrequency range, primarily 3 to 6 kHz, and the corresponding hearing loss is classically described as the “4-kHz notch.” This pattern of maximal hearing loss, with little or no loss at less than 2 kHz, typically occurs regardless of the noise-exposure environment. The audiogram results in Figure 151-1A also show the sensorineural aspect of NIHL in that thresholds for bone-conducted stimuli are essentially identical to the thresholds for air conduction. The profile of noise-induced threshold hearing is usually symmetric for both ears, particularly for individuals who have been working in noisy industrial settings in which there are “surround” sounds.
Commonly, other forms of noxious sound, such as the gunfire associated with sport shooting, cause an asymmetric pattern of hearing loss similar to the one illustrated in Figure 151-1B. In this case, the ear pointed toward the source of noise (gun barrel), which is the right ear of the left-handed shooter depicted in Figure 151-1B, would have worse hearing than the ear directed away from the source (in this example, the left or protected ear) by 15 to 30 dB or more, and particularly at higher frequencies because of the absence of the protective head-shadow effect.
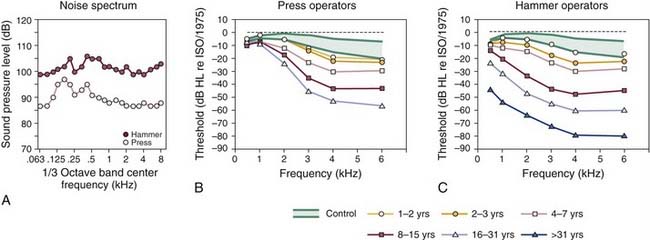
The development of a hearing loss caused by habitual exposure to moderately intense levels of noise typically consists of two stages. Initially, the middle to high frequencies exhibit the resulting hearing loss. As the length of time of exposure to loud noise increases, hearing loss becomes greater and begins to affect adjacent higher and lower frequencies. In a classic cross-sectional study of occupational NIHL, Taylor and colleagues7 showed the gradual loss of hearing sensitivity in workers caused by habitual exposure to the intense sounds of dropforging tools that are commonly used in foundries. Figure 151-2 illustrates the progressive effects of exposure to the wideband noise (Fig. 151-2A) of two types of forging tools, presses and hammers, on the magnitude of hearing loss as the length of exposure increased. For the operators of the press and hammer equipment (Fig. 151-2B and C), the approximate 10- to 20-dB threshold shifts, typically observed at the higher frequencies during the first 1 to 2 years of exposure, grew to be a 20-dB or greater loss, from 3 to 6 kHz, after a 3-year exposure. There was a 40-dB or greater threshold shift after an 8-year exposure.
Detailed comparisons of the NIHL growth curves of Figure 151-2B and C reveal that with continuing exposure, hearing loss worsened at the higher frequencies and spread to the lower frequencies. In addition, for average exposure times of less than 10 years, hearing levels for the press and hammer operators who were exposed to mean levels of 108 and 99 dB SPL deteriorated similarly. For long-term exposures of 10 years or more, the results of Taylor and colleagues7 indicated that hearing losses resulting from the hammer-induced impact noise were greater than the losses resulting from the more continuous noise of the press equipment. Finally, a characteristic feature of NIHL, which is clearly documented in Figure 151-2B and C, is that hearing levels are increased rarely beyond about 70 to 90 dB of hearing loss, on average, even after more than 31 years of continuous noise exposure.
Cochlear Damage
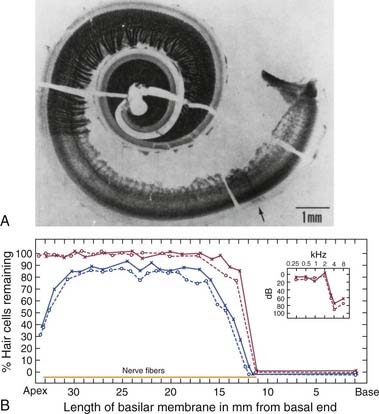
Johnsson and Hawkins8 were among the first investigators to describe the typical patterns of cochlear injury for humans with chronic exposure to different types of loud sound. The photomicrograph of the cochlear tissue in Figure 151-3A depicts some common histopathologic consequences of NIHL for a patient with a lengthy history of noise exposure. The authors reported that the 50-year-old patient had worked intermittently over a 5- to 6-year period in an automotive body–stamping plant, and that he had a long history of using recreational firearms. The sharp transition in the basal end (following the uncoiling to the right) from the normal-looking organ of Corti (a darkish stripe corresponding to the region of inner hair cells and OHCs), with its dense network of nerve fibers, to the complete absence of hair cells and their corresponding nerve fibers (the much lighter adjacent area) can be noted. Figure 151-3B graphically reconstructs the histopathologic features of this cochlea as a cytocochleogram by depicting the number of remaining hair cells, in the form of percentages, averaged over 1-mm sections. A typical finding in individuals exposed to the occupational noise exemplified in this case is the almost symmetric pattern of degeneration observed for the two ears. The inset at the top right of the cytocochleogram shows the patient’s audiogram obtained about 1 year before his death, which shows the severity of the anatomic damage in functional terms by revealing an abrupt hearing loss for test frequencies greater than 2 kHz.
Examination of human temporal bone specimens by numerous laboratories8–10 has yielded documentation of the progressive stages of noise damage as depicted by epidemiologic data such as those of Figure 151-2. First, in the predictable sequence of events, a small region of hair cell and nerve fiber degeneration appears bilaterally at a cochlear region corresponding to the 4-kHz notch. Typically, these discrete lesions gradually grow in the basal-ward direction (i.e., toward the high-frequency extent of the cochlea) to involve a greater portion of the organ of Corti. Finally, as exposure to noise continues over years, the remaining sensory and neural elements in the basal end of the cochlea are destroyed, resulting in an abrupt loss of midfrequency to high-frequency hearing, such as that depicted by the clinical audiogram of Figure 151-3.
Research on Noise-Induced Hearing Loss
Scientific interest in the damaging effects of excessive sound on hearing has a long history for many reasons. First, the experimental strategy of exposing animals to noise and examining their ears for the sites of the resulting acoustic injury was used in the past as the independent variable in establishing some of our basic knowledge about hearing. Particularly, with the use of intense tones as the damaging agents, frequency information relating physical distance along the basilar membrane to the “best” frequency of the injured region provided an initial basis for understanding the tonotopicity of the cochlea and the central projection of such frequency-related information.11 Additionally, noise-damage strategies have been used to contribute to our understanding of the function of inner hair cells and OHCs by permitting differences in their central terminations in the ventral and dorsal cochlear nuclei to be distinguished.12
Anatomic Mechanisms Underlying Noise Damage
Experimental studies have led to an increased understanding of some major features of NIHL. It is well-accepted that the origin of the 4-kHz notch in the NIHL audiogram is related to the resonator function of the external auditory ear canal,13 rather than to indeterminable innate properties of the cochlea, such as a reduced vascular supply to this region of the organ of Corti.14 The primary research interest has always been, however, in the fundamental mechanism by which the sensory cell degenerates or is damaged after exposure. Numerous mechanisms15 have been proposed, including mechanical injury caused by severe motion of the basilar membrane, metabolic exhaustion of activated cells, activity-induced vascular narrowing that causes ischemia, and ionic poisoning from interruption of the normal chemical gradients of the cochlea owing to minuscule disruptions in the organization of sensory and supporting cells.
Although the many years of experimental research have not yet produced a major breakthrough in understanding of damage mechanisms, the current most convincing morphologic evidence supports a combination of the mechanochemical theories. First, at the ultrastructural level, it is likely that alterations in the stereocilia in the form of shortened or broken rootlets are involved in the initial pathologic processes that lead to TTS and, if such injuries are not repaired, then PTS.16–18 More recent findings showed that hair bundles are capable of rebuilding their ultrastructure from top to bottom over a 48-hour period.19,20 If damage is so severe that it overwhelms this self-repair mechanism as exposure continues, a discrete but direct mechanical disruption likely results in a toxic mixing of endolymph and perilymph through microbreaks in the structural framework of the cochlear duct,21 which leads to secondary effects, including loss of hair cells and their corresponding nerve fibers.
New Knowledge about Cellular and Molecular Mechanisms of Noise-Induced Hearing Loss
Hair Cell Regeneration and Repair
In the late 1980s, several seminal reports on hair cell regeneration in avian species established that hair cells in neonatal and adult birds regenerate after exposure to either damaging levels of sound22–24 or ototoxic antibiotics.25 Additionally, follow-up studies showed that recovery of cochlear function accompanied the cellular-recovery process.26–28 In the best-studied model for hair cell regeneration, the neonatal chick, it was shown that new hair cells arose as progeny from an otherwise nondividing supporting-cell population that was induced to proliferate by the damaging insult.29
Generally, noise-induced hair cell loss in the mammalian cochlea is irreversible. Experimental findings using in vitro cultures of neonatal mouse cochleas showed, however, that overexpression of mammalian atonal homolog 1 (Atoh1), also known as Math 1, a basic helix-loop-helix transcription factor known to be necessary for hair cell differentiation during development, leads to an increase in the production of extranumerary hair cells.30 Consequently, it seems that certain cells in the mammalian organ of Corti, at least in young animals, can be redirected toward a hair cell fate by the overexpression of Math 1.31 In addition, a most exciting finding was the discovery that new hair cells can be grown in a mature mammalian ear using the Math 1 gene. Kawamoto and colleagues32 injected an adenovirus carrying the Math 1 gene into the endolymph of mature guinea pigs. Using scanning electron microscopy, 1 to 2 months after the inoculation of the Math 1 gene insert, immature hair cells within the nonsensory supporting cells of the organ of Corti were detected. Most importantly, follow-up studies in the deafened guinea pig showed that not only did Math 1 induce regeneration of hair cells in the adult, deafened guinea pig, but also substantially improved hearing thresholds were measured.33 These findings that Math 1 (Atoh1) can direct hair cell differentiation and induce hearing function in mature nonsensory cells support the notion that adenoviral gene therapy based on expressing crucial developmental genes for cellular and functional restoration in damaged auditory epithelium may lead some day to a new treatment for NIHL.
Protection from Conditioning the Cochlear Efferent System
The outcomes of other experiments indicate that the mammalian cochlea may be capable of actively adapting to certain high-level sounds by receiving “exposure experience.” The notion that the cochlea can become resistant over time to the consequences of excessive sound was reported initially by noting “conditioning” effects in several animal models.34–37 The typical conditioning paradigm consists of providing a pre-exposure training experience using a moderate-level stimulus that, at a more intense level, becomes the subsequent overexposure stimulus. Together, these findings in animal models suggested that the mammalian cochlea might be capable under certain conditions of dynamically adapting to excessive sound.
For humans, the practical implications of the capacity to develop a “resistance” to loud sounds are obvious. A follow-up study in teenagers used a TTS-type paradigm to show the relevance of resistance training to humans. In this experiment,38 the investigators provided a pre-exposure training period in which the young subjects were exposed to 6 hours of pop/rock music at around 70 dBA. Threshold shifts in response to a 10-minute exposure to a 105-dB SPL, one-third octave-band noise centered at 1 kHz were compared for pretraining versus post-training intervals. The major result was that the “trained” ears exhibited significant decreases in TTS compared with their baseline values, showing that the “conditioning” effect, or the development of “resistance,” can be shown in human subjects, at least under brief TTS-exposure conditions.
Such human studies of the relationship between noise exposure and permanent hearing loss are more difficult to perform for many reasons including the ethical issues involved in the deliberate exposure of study subjects to noises, even though they are thought to be reversibly damaging. More recent research into the protective role of cochlear efferents in NIHL has depended more on the benefits of diagnostic testing with evoked otoacoustic emissions (OAEs) to predict potential susceptibility to the aftereffects of noise exposure.39 This simple, noninvasive, and objective procedure is based on the systematic measurement of a class of cochlear responses (i.e., the OAEs) that are primarily generated by the OHCs.40 Not only are OHCs exquisitely sensitive to the initial effects of acoustic overstimulation, which makes them excellent indicators of sound-induced ultrastructural damage, but also the final common pathway of the descending auditory-efferent system preferentially innervates the OHCs.
To take advantage of the ability of OAEs to measure efferent activity, several experimental paradigms were developed including a common one41 that uses contralateral acoustic stimulation to elicit medial olivocochlear-induced reductions of OAEs from the ipsilateral test ear. The other procedure42 is based on measurements of a subtype of evoked emissions represented by distortion product OAEs (DPOAEs) at 2f1-f2. In this strategy, long-lasting f1 and f2 primary tones of approximately 1 second are applied binaurally to elicit an efferent-based fast adaptation response that tests the ability of the combined medial and lateral cochlear-efferent systems to suppress DPOAEs in the test ear.
Experiments in guinea pigs documented the ability of the fast-adaptive DPOAE response to predict vulnerability to acoustic injury based on the vigor of efferent activity.43 The robustness of the olivocochlear efferents was inversely correlated with the degree of cochlear dysfunction after subsequent noise exposure, in that animals exhibiting large adaptive effects showed smaller postexposure losses than animals displaying small amounts of efferent-induced adaptation. It is clear from these findings that a modification of this assay could easily be applied to human populations to screen for individuals most at risk in noisy environments.
Some studies44 simply relating the presence or absence of the contralateral acoustic stimulation–induced reduction of emissions in human ears have reported such suppression is absent in most industrial workers who otherwise have normal OAEs. One implication of such observations is that a lack of efferent-related activity may be an early indication of cochlear damage from exposure to noise. Experimental studies such as these will eventually determine whether inherent efferent processes can account for and predict the remarkable individual variation in susceptibility to NIHL. The use of efferent-induced suppression of evoked OAEs in predicting susceptibility to NIHL, or in monitoring of noise effects in hearing-conservation programs, represents a promising line of future investigation.
Pharmacologic Protection from Noise-Induced Hearing Loss
It has long been recognized that hypoxia is a major pathogenic factor in NIHL. Based on the assumption that oxidative stress plays a substantial role in the genesis of noise-induced cochlear injuries that lead to permanent hearing loss, numerous pharmacologic strategies have been developed primarily in animal models to enhance the intrinsic defense mechanisms of the cochlea against this condition.45 Kopke and colleagues46 postulated several causes of noise-induced oxidative stress, all of which are amenable to pharmacologic treatments. Specifically, these investigators proposed that noise-induced oxidative stress leading to cochlear injury was related to (1) impaired mitochondrial function with respect to bioenergetics and biogenesis; (2) glutamate-induced excitotoxicity, which is the main excitatory neurotransmitter in the peripheral and central auditory systems; and (3) depletion of glutathione (GSH), an antioxidant that protects cells from toxins such as free radicals.
Related experimental work showed a reduction in NIHL and hair cell loss in the chinchilla model after application of pharmacologic agents specific to these oxidative stress–related states. Acetyl-L-carnitine, an endogenous mitochondrial membrane compound that helps maintain mitochondrial bioenergetics and biogenesis in the face of oxidative stress; carbamathione, which acts as a glutamate antagonist for cochlear N-methyl-D-aspartate (NMDA) receptors; and the GSH-repletion drug, D-methionine (MET), all improved hearing in noise-exposed animals as indexed by auditory brainstem responses, along with reductions in inner hair cell and OHC loss compared with counterpart measures in saline-treated control subjects.46
Based on the assumption that a potential mechanism of NIHL is the generation of damaging free radicals through the activation of reactive oxygen species such as hydrogen peroxide, hydroxyl radicals, and superoxide, many other studies have tested the influence of antioxidants and related compounds on noise-induced cochlear dysfunction and organ of Corti morphology. Such candidate substances include GSH,47 the xanthine oxidase blocker allopurinol,48 N–L-acetylcysteine (L-NAC),49 and GSH peroxidase and superoxide dismutase.50 Together, all these findings support the notion that combating cellular oxidative stress, principally with prophylactic administration of antioxidant compounds, eliminates noise-induced cochlear injury in animal models. More recently, Kopke and colleagues51 reported on some promising results using L-NAC in military recruits to reduce the NIHL sometimes associated with the basic training related to military operations. The success of such a pharmacologic approach essentially ensures the development in the near future of a therapeutic intervention that reduces NIHL clinically.
Susceptibility
A long-standing observation in NIHL has been that some ears are more easily damaged by noise than others. Individually varying susceptibilities to noise-induced hearing impairment have been found in humans and research animals. Because of the widespread interest in NIHL and its prevention, it is important to develop valid and reliable indices to predict human susceptibility to various noise levels. It is commonly assumed that such variability in susceptibility is a manifestation of biologic factors unique to each subject. A genetically based imperfection in the physical characteristics of the cochlea (e.g., stiffness of the cochlear partition) and variability in cochlear ultrastructure (e.g., density of hair cells) have been proposed as contributing to susceptibility.52
Identifying individual differences among various factors has long been a focus of interest. Numerous potentially important variables that have been investigated in the past and continue to be examined include age53; gender54; race55; previous damage to the cochlea56; efficiency of the acoustic reflex57; smoking habits58; and the influence of certain disease states such as hypercholesterolemia59 or hypertriglyceridemia,60 diabetes,61 and cardiovascular disease represented by hypertension.62 Although many pertinent factors have been uncovered, most data are inconclusive. In addition, although a few factors, such as pigmentation,63 seem to have some relationship to potentiating noise damage, others, such as age, simply produce additive effects,64 although this latter relationship remains controversial.65 Possibly, as McFadden and Wightman66 suggested in their review of the contribution of the psychoacoustic method toward understanding the symptoms of clinical hearing disorders, the orthogonally based research approach, which assumes a causal relationship between factors, will not reveal meaningful relationships. Perhaps the uncovering of interrelationships among myriad individual differences by application of a multivariate test battery would be more successful in identifying the basic factors that predict susceptibility to NIHL.
One of the most exciting areas in current experimental research is aimed at examining the genetic origin of differences in susceptibility to noise damage using the mouse model. A clear benefit of the mouse as an experimental animal is the great amount of knowledge that exists about the mouse genome. Additionally, mice from the same inbred strain are considered to be genetically identical. Consequently, assessing individual variability to noise damage in subjects that are homozygous at all chromosomal loci offers a unique opportunity to isolate genetic factors responsible for susceptibility to NIHL. To date, several studies67,68 have shown that the inbred, mutant C57BL/6J (C57) strain, often used as a model of early age-related hearing loss, is more susceptible to noise damage than the CBA/CaJ (CBA) strain, which is frequently used as a model of normal hearing. Other observations supporting the potentiation of noise damage by the age-related hearing loss gene, Ahl, relate to the finding that when C57 (B6) mice are backcrossed with a mouse strain (e.g., CAST/Ei) exhibiting normal aging, the B6/CAST progeny display neither age-related hearing loss nor susceptibility to noise exposure aftereffects.69
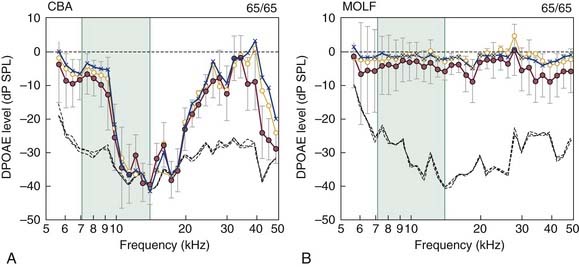
Just as susceptible mutant mouse strains are readily available, there are also strains with normal cochlear function that are exceptionally resistant to acoustic overstimulation, such as wild-type inbred MOLF/Ei (MOLF) mice. Figure 151-4 compares the effects of an intense noise exposure (105-dB SPL, octave-band noise centered at 10 kHz, for 8 hours) on control CBA and MOLF mice at 2 months of age.70 Although CBA mice showed only an expected minor recovery of DPOAEs to baseline levels at 2 days and 1 and 2 weeks postexposure, MOLF DPOAEs had essentially returned to their pre-exposure levels by 1 to 2 weeks postexposure. In combination, findings in inbred mouse models of NIHL provide the basis for applying suitable molecular techniques that permit the mapping of an NIHL gene to specific chromosomal loci (e.g., identifying differentially expressed genes using DNA microarrays and suppressive subtractive hybridization). Successful identification of this gene and perhaps its related modifier genes would have great implications for developing a diagnostic indicator of the susceptibility of a particular human ear to the adverse effects of sound overexposure.
Genetic association studies on oxidative stress genes have identified the first heritable factors that likely influence an individual’s susceptibility to NIHL. Konings and coworkers71 investigated whether variations in the form of single nucleotide polymorphisms of the catalase gene, which is one of the genes involved in oxidative stress, influenced noise susceptibility. By comparing audiometric data and DNA samples from the 10% most susceptible and 10% most resistant Swedish and Polish noise-exposed workers, significant interactions were observed between noise-exposure levels and several single nucleotide polymorphisms in both populations. These findings indicate that catalase is potentially a noise susceptibility gene.
The importance of performing longitudinal field studies of communities or particular population segments, such as elderly individuals, children, and chronically ill individuals, who are habitually exposed to loud environmental noises produced by road traffic or aircraft, is obvious to determine effective health criteria for controlling such noises. Most notably, a great disadvantage in gaining a more complete understanding of NIHL in humans is that there have been no more recent studies of the hearing status of contemporary workers, at least in the countries of North America and Europe. Reliance on data collected 30 or more years ago, such as those illustrated in Figure 151-2,7 has likely resulted in underestimates of the amount of hearing loss that is due to occupational noise, especially for individuals with intermittent or impulsive noise exposures.
Early Detection of Noise-Induced Hearing Loss
The diagnostic technique incorporating OAEs is ideal for assessing the normality of cochlear processing in ears suspected of being overstimulated by loud sound because of its well-recognized sensitivity to the OHC class of receptor cell, which is primarily involved in the initial stages of NIHL. The functional status of OHCs in established instances of NIHL has been well described in many more recent studies on the practical applicability of evoked OAE testing in the audiology clinic.72 Numerous reports in the literature have shown that in groups of noise-exposed humans who have been followed serially, reductions in OAE levels are more sensitive than pure-tone audiometric thresholds in detecting the early states of permanent noise-induced cochlear damage.73–77 In these studies, decreases in emission magnitudes were measured in the absence of any change in the corresponding audiometric threshold frequencies.
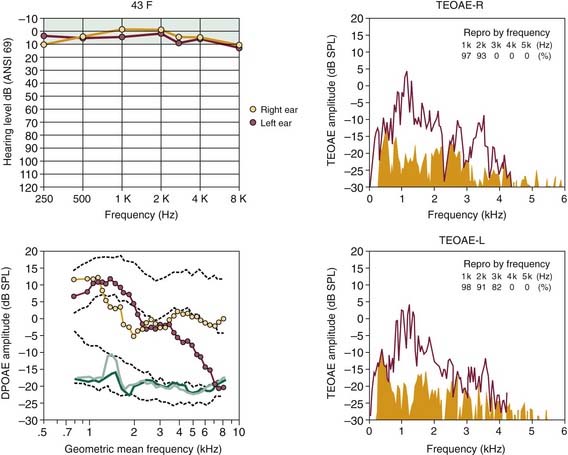
In Figure 151-5, the ability of the two major types of evoked OAEs, the DPOAE, in its DP-gram form depicting emission level as a function of the f2 test frequency (lower left in Fig. 151-5), and the transient-evoked OAE (TEOAE), elicited by clicks, in its spectral form (right plots in Fig. 151-5), to describe the configuration of a developing NIHL is illustrated. The 43-year-old woman in this example had participated in recreational rifle shooting for 3 years before OAE testing, and she claimed to have worn protective headphone devices continuously during this period. This right-shouldered shooter presented in the otology clinic with complaints of hearing loss, tinnitus, muffled hearing, and difficulty in understanding speech in background noise. It is clear from the test results that the magnitude and frequency extents of the TEOAEs reflected the pattern of normal hearing illustrated by the clinical audiogram at top left of Figure 151-5. The DP-gram clearly shows abnormal activity, however, with the right ear showing below-average response levels and the left ear exhibiting a significant reduction in emitted responses for frequencies greater than 3 kHz. True to classic principles, the left ear, which was exposed more to the muzzle end of the gun, went unprotected by the head-shadow effect. This example of the ability of evoked OAEs to detect cochlear pathology caused by noise exposure attests to the potential usefulness of emission procedures in identifying the primary site of pathology associated with hearing complaints secondary to a sensorineural loss, and in monitoring the development of potential hearing impairments in hearing-conservation programs.
Other evidence supporting the usefulness of evoked OAEs in examining patients with noise damage is illustrated in Figures 151-6 and 151-7. In Figure 151-6, the results of OAE testing in a 21-year-old man, who had just completed 3 years of military service in the infantry, show the precision of DPOAEs in particular in tracking the asymmetric pattern of hearing loss for this left-handed shooter. Additionally, the OAE findings presented in Figure 151-7 show the ability of evoked emissions to reflect accurately the magnitude and frequency extent of the more severe, but essentially symmetric hearing loss experienced by a 49-year-old man who had been working for more than 20 years in a fish cannery.
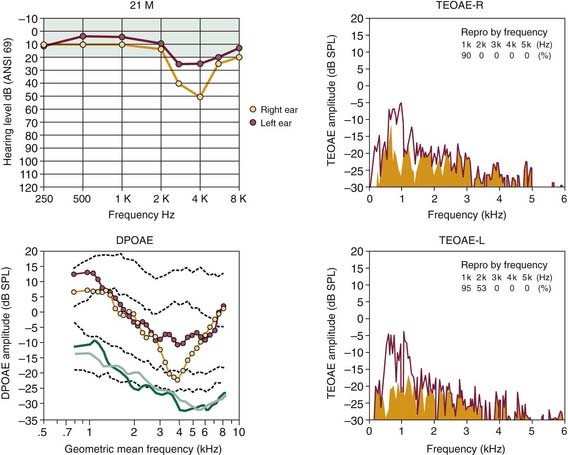
Figure 151-6. Early stages of noise-induced hearing loss in a 21-year-old military veteran, who had just completed a 3-year tour of duty. For this left-handed infantryman, note greater hearing loss from approximately 3 to 4 kHz for the right ear (yellow circles) depicted in the audiogram (top left). In corresponding DP-gram, note abnormally low levels of distortion product otoacoustic emission (DPOAE) activity for frequencies greater than 1.5 kHz, which were poorer for the right ear. Click-evoked otoacoustic emission spectra on the right (transient-evoked otoacoustic emission [TEOAE]) also show poorer emitted responses from 1 to 2 kHz and lower “Repro” values for the right (R) ear. There is no emitted response for either ear greater than 2 kHz. See legend for Figure 151-5 for an explanation of click-evoked spectra and two sets of dashed lines in DP-gram.
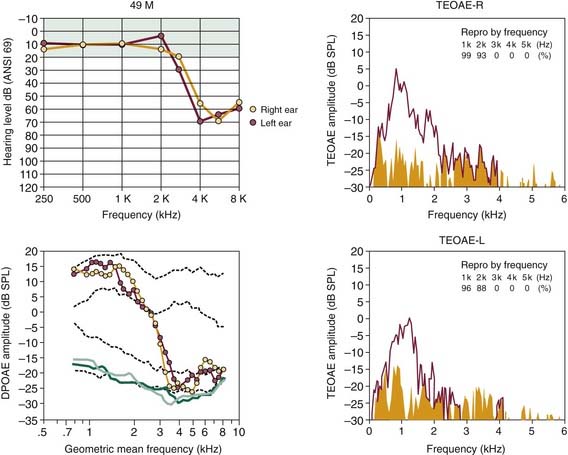
Figure 151-7. More advanced stage of noise-induced hearing loss for a 49-year-old man, who had worked for more than 20 years in a fish cannery. Audiograms at top left exhibit symmetric hearing loss for frequencies greater than 2 kHz. DP-grams indicate symmetric pattern of dysfunction in a similar manner. Click-evoked otoacoustic emission spectra on the right (transient-evoked otoacoustic emission [TEOAE]) also show a lack of emitted responses for frequencies greater than 2 kHz. See legend for Figure 151-5 for an explanation of click-evoked spectra and two sets of dashed lines in DP-grams. DPOAE, distortion product otoacoustic emission.
Also, especially important to hearing conservation is the correct identification of individuals exhibiting pseudohypacusis, who seek monetary compensation for a purported work-related hearing impairment. An example of this application is illustrated in Figure 151-8 for a 42-year-old man who was referred to the audiology clinic for assessment by a local worker’s compensation committee. In this case, the patient claimed to have poor hearing caused by on-the-job exposure to intense noise to the extent that he could not hear frequencies greater than about 4 kHz. According to the OAE findings, it is likely that the patient, a wood-lathe operator, had an asymmetric noise-related hearing loss that was worse in the ear (right) that was more exposed to the tool. Based on the type of hearing-loss data presented in Figure 151-1A for an industrial worker, however, it is unlikely that lathe-related noises could cause such a profound deafness for frequencies greater than 4 kHz. Additionally, the normal levels of DPOAEs and TEOAEs for frequencies less than 2 kHz do not support the patient’s claim that the elevated audiometric hearing levels over the low-frequency to midfrequency regions were due to noise overexposure. Findings such as these support the use of objective tests of evoked OAEs in determining the authenticity of compensation claims for work-related hearing problems.
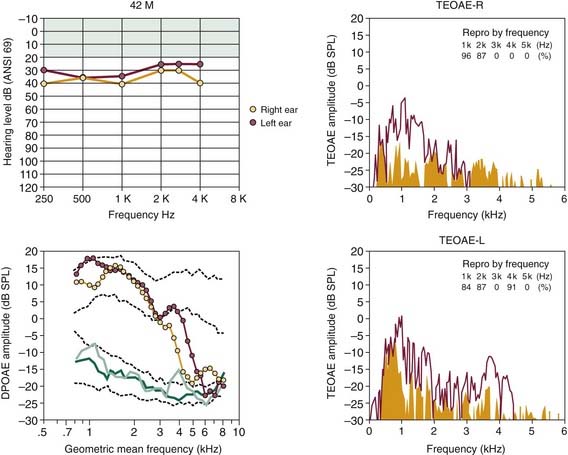
Figure 151-8. A possible case of pseudohypacusis in a 42-year-old male factory worker, who operated a wood lathe. Audiograms at top left show a relatively flat hearing loss up to approximately 4 kHz. For frequencies greater than 4 kHz, the patient claimed that test tones were inaudible. DP-grams show normal-like function up to approximately 3 to 4 kHz, when reductions in distortion product otoacoustic emission (DPOAE) levels are evident, occurring initially for the right ear (yellow circles), which was more exposed to the lathe. Spectral plots on the right (transient-evoked otoacoustic emission [TEOAE]) also show poorer click-evoked otoacoustic emissions for the right ear. Together, normal levels of evoked emissions for frequencies less than 3 kHz and asymmetric pattern of reduction in otoacoustic emissions suggest that exposure to noise and aging contributed to decreased levels of activity for frequencies greater than 3 kHz. See legend for Figure 151-5 for an explanation of click-evoked spectra and two sets of dashed lines in DP-grams.
Interactive Effects
It is well established that noise in combination with certain chemical agents produces stronger reactions than each stimulus applied singly. The four major categories of ototoxic drugs are aminoglycoside antibiotics, platinum derivative antitumor agents, “loop” diuretics, and salicylates. The latter two classes of drug cause reversible effects, whereas aminoglycosides and platinum derivatives cause permanent damage to the inner ear and to hearing. Many laboratories have established in animal models that kanamycin, neomycin, or amikacin applied in combination with different types of noise produces a marked potentiating interaction.78,79 Other studies of the temporal aspects of interactive effects indicate that the degree of potentiating interaction is the same whether the drug is given concurrently with the noise exposure or several months later.80 Other evidence from humans81 and controlled laboratory research in a rat model82 indicates that additional loss may occur when subjects are treated with aspirin and exposed to noise concomitantly, although other findings infer that the combination of salicylate plus noise does not produce greater effects than noise alone.83 Finally, experimental evidence in several animal models showed that the heavy-metal, antineoplastic agent cis-diamminedichloroplatinum, commonly known as cisplatin, significantly increased the amount of hearing and sensory cell losses from exposure to noise.84,85
In recent years, the interactive effects of noise with chemical agents common to industry and the environment have been reported. In a series of experimental studies in the rat model, Fechter and colleagues86 discovered that the simultaneous exposure to noise and the environmental pollutants carbon monoxide or hydrogen cyanide produced more permanent hearing loss at the high frequencies than the sum of the losses produced by each agent administered alone. Various other chemicals present in the environment as commercial products or chemical intermediaries and contaminants, such as the organic solvents toluene and hexane, the pollutants methyl mercury and lead acetate, and the organic compounds trimethyltin chloride and styrene used in the manufacture of plastics and polyurethane foam and rubber, have been identified as potent ototoxic agents that potentially interact synergistically with excessive noises. The ototoxicity of environmental agents such as metals, solvents, and asphyxiants and their interaction with noise are issues that have received a great deal of attention in the literature.87 Although many of these environmental toxicants have been associated with direct injury to inner ear structures, possible additional anatomic damage to more central auditory pathways is also very likely.
Other Adverse Effects Caused by Noise
Nevertheless, some studies of industrial workers and military personnel have reported a high occurrence of vestibular symptoms and signs, and complaints of dysequilibrium.88 Such simple correlations between noise exposure and vestibular disturbances are highly controversial because of the typical low incidence of clinical vestibular symptoms associated with gradually developing NIHL.89 A study of military personnel with mild to severe NIHL using sophisticated vestibular testing in the form of a computerized rotatory-chair system along with routine electronystagmographic measures of caloric responses showed more direct correlations between hearing loss and vestibular upset.90 In combination, the results showed a symmetrically centrally compensated decrease in the response of the vestibular end organ that was associated with a symmetric hearing loss. A more recent study91 combining caloric testing with vestibular-evoked myogenic potentials in patients with chronic NIHL showed absent or delayed electrophysiologic potentials, indicating that the vestibular system, especially the sacculocollic reflex pathway, was damaged in addition to the cochlear portion of the inner ear.
Together, these findings imply that a subclinical, well-compensated malfunction of the vestibular system is associated with NIHL. Beyond the medicolegal implications is the daunting possibility that a currently asymptomatic vestibulopathy produced by noise exposure might progress to a disturbing vertigo under certain environmental conditions.92
There has been considerable interest more recently in the interaction of vibration and noise, which are common cofactors in the workplace. Although most research shows that vibration alone does not affect hearing, the results of epidemiologic studies in humans and controlled laboratory studies in animal models indicate a synergistic interaction of concomitant vibration (either whole-body or hand-arm) and noise that results in an increase in the degree of NIHL.93
Exposure to infra-frequency and ultra-frequency sounds outside of the human hearing range has also been studied. Infrasonic or vibratory stimuli are defined as sounds in the range of 0.1 to 20 Hz. There are no known instances in which infrasound alone clearly caused permanent damage to the human cochlea. Studies in the chinchilla model showed, however, that the presence of intense sounds in the audible frequency range along with simultaneous infrasound increases cochlear damage.94
In contrast, some reports have shown detrimental effects of microwaves on hearing.95 The thermal properties of ultra-high-frequency sound waves in the gigahertz range, rather than mechanical energy, seem to have produced these adverse experimental effects. Overall, it is probably safe to conclude that the auditory system’s built-in, middle ear filter limits the extreme frequencies of sound that are hazardous to the inner ear.96
Magnetic resonance imaging (MRI) systems have acoustic noise associated with their clinical scanning actions. For specific protocols that are incorporating faster and noisier sequences, peaks of 120 to 130 dBA have been measured, particularly during high-speed echoplanar imaging.97 Early on, Brummett and associates98 showed that sounds generated by MRI are sufficiently intense to cause some TTS in a significant number of patients. Their results also showed, however, that earplugs help attenuate the noise sufficiently to prevent TTS in most patients undergoing MRI evaluation. The use of the higher gradient fields that are applied at faster slew rates, which are used in current functional MRI, demands increased hearing protection systems to minimize the risk of noise trauma.99
Other nonauditory problems concern the general bothersome or fatiguing effects of noise, which may lead to nonspecific health disorders because of interference with the restorative processes associated with rest and sleep. In conditions of chronic exposure, noise is considered to act as a biologic stressor that can lead to prolonged activation of the autonomic nervous system and the pituitary-adrenal complex, resulting in general health impairment.100 Noise has also been related to disorders involving gastrointestinal motility, such as peptic ulcers,101 and, as noted earlier, to circulatory problems such as hypertension.102 Certain types of noise can be annoying and lead to emotional unrest.103 Finally, noise can have a deleterious effect on task performance, especially if speech understanding is involved.104 Generally, data relevant to the nonauditory effects of noise tend to be inconclusive because the variables are difficult to identify and isolate for objective study.
Legal Issues
One practical issue that the lawmakers of industrialized societies have had to consider concerns the balancing of two conflicting goals: How can laborers be protected against the hazards of the workplace without placing an enormous financial burden on society, either by satisfying compensatory obligations or by preventing such work-related effects through costly engineering modifications of the industrial process? Over the years, concerns about protecting the public and work force from environmental insults such as noise have been written into a combination of law and governmental regulations. Federal, state, and community legislative and regulatory actions are continually being reviewed and altered. Although the purpose of this review is not to detail such legal controls, regarding either their historical development or their current status, a brief discussion is appropriate. For a lucid analysis of governmental statutes and regulations pertaining to noise control, see the review by Dobie.6
The major difficulty encountered in composing regulatory control has been in defining in practical terms what constitutes a hazardous noise. The equal-energy principle105 embodies one popular approach toward expressing the danger of a particular noise by a simple number. The equal-energy principle assumes that permanent damage to hearing is related to total sound energy, which is a product of the noise level in dBA and the duration of exposure. One tenet of this postulate is that an equal amount of noise energy causes an equal amount of hearing loss.
Burns and Robinson,106 who investigated the hearing loss of thousands of industrial workers, concluded that the equal-energy principle could be applied to determine daily exposure doses because hearing loss caused by work-related noise exposure seemed to be a simple function of noise energy. Atherley and Martin107 extended the concept to impulse noise by application of the equivalent continuous sound level (Leq) principle. The Leq is defined as the A-weighted level of a continuous, steady sound that produces, in a specified interval, an exposure that has the same total acoustic energy as that of an actual time-varying sound over the identical interval.108 In other words, if one sound contains twice as much energy as a second sound but lasts half as long, both sounds would be characterized by the same equivalent sound level. The Leq concept holds that these two exposures produce the same damage to the ear.
Although notions such as the equal-energy principle have proved useful in the practical definition of noise-control variables, their validity has been more difficult to establish. Over the years, experimental evidence has been conflicting in that it has tended to be either for or against the equal-energy rule. Generally, it seems that the principle cannot be generalized indiscriminantly to stimuli throughout the range of noise parameters because exposure to impulse or intermittent noises may lead to more or less degeneration in the organ of Corti than would be expected according to the equal-energy principle. Some aspect of the equal-energy concept has been adopted by most industrialized nations, however, including the United States, as a means of measuring the potential hazard of a particular noise exposure. Present regulations use a 5-dB exchange rate, along with a 90-dBA time-weighted average to define the permissible exposure limit over an 8-hour work day.109 Consequently, exposure to 90 dBA for 8 hours is equivalent in sound-energy terms to an exposure of 85 dBA for half that time (i.e., 4 hours).
Currently, industries that employ laborers who work where noise is greater than 85 dB SPL implement a hearing-conservation program consisting of several components as dictated by the U.S. Occupational Safety and Health Administration (OSHA).109 First, pre-employment audiometric assessment and annual audiometric monitoring are required so that noise-induced cochlear impairment can be detected before becoming too severe. When a hearing loss is identified, the worker must be notified of the disorder and counseled about the use of personal hearing protectors. The second portion of the conservation program requires workers in areas of high-noise levels (≥85 dBA) to wear ear protectors and to participate in a noise education program that informs the employee of the hazardous effects and the correct fitting of personal protectors. An important part of hearing conservation is the otologic referral (see next), which is essential when in-plant audiometry has detected a substantial hearing loss.
Role of the Otolaryngologist
Patients with NIHL constitute a notable portion of an otolaryngologist’s patient population, particularly for otolaryngologists who are in private practice. With the increase in the population older than 65 years, the psychological, economic, and social impact of NIHL is still growing. Modern stereo systems in the form of personal music players such as the MP3 and iPod systems have sufficient noise levels and listening durations to put consumers at risk for developing NIHL.110,111 The widespread use of these devices, particularly among children and adolescents, is a growing worry,112 particularly as reported by the mainstream media. Such concern is supported by the finding uncovered in the audiometric database of a large-scale national cross-sectional survey of an accelerating prevalence of sensorineural hearing loss in young adults 20 to 29 years old.113 This growing prevalence of a high-frequency hearing loss in young adults may relate to an increase in personal stereo player use. The outcome of at least one relevant experimental study using similarly aged subjects, who were systematically exposed to music from an MP3 player at their preferred listening levels, does not agree with this explanation, however.114 In any case, otolaryngologists are expected to continue to be conspicuously involved with the problem of NIHL.
Although a known prevention exists, it is unlikely for fiscal and technical reasons that the general loud sound levels of our environment will be reduced. Although the inhalation of hyperoxygenated air as a prophylaxis115 has been proposed, and, as noted previously, clinical trials on the safety and efficacy of certain antioxidant compounds to prevent or reverse NIHL are currently in progress, to date, no proven treatment or cure for noise damage exists. Consequently, detecting the early stages of NIHL is important to prevent further injury to the crucial sensory-cell receptors of the organ of Corti.
Although NIHL is not medically or surgically treatable, it is almost entirely preventable. Its prevention requires education, engineering, and administrative controls, and the proper use of hearing protection. One of the otolaryngologist’s most important contributions is in teaching the patient to guard against further losses.116 When recommending the use of personal hearing protectors or reinforcing compliance with ongoing hearing-protection measures, the physician should be aware that the commonly used personal protectors in the form of inserted earplugs or earmuffs vary considerably in effectiveness, and produce an attenuation that is highly frequency dependent.117,118 When sealed correctly into the ear canal, earplugs reduce the noise reaching the middle ear by 15 to 30 dB, and work best for the midfrequency to higher frequency range (i.e., 2 to 5 kHz). Earmuffs are more effective protectors, especially for frequencies between 500 Hz and 1 kHz, where noise is attenuated by 30 to 40 dB.119 In areas with extremely high noise levels, earplugs do not afford sufficient protection, and individuals should be advised to wear both earplugs and earmuffs. Also, sound energy associated with high noise levels may reach the inner ear by passing through vibrating bone and tissues adjacent to the ear. Bone-conduction and tissue-conduction thresholds set a practical limit to the possible attenuation provided by hearing-protection devices.
A final point concerning the use of hearing protectors should be impressed on the patient. When data relating the maximal protection in dBA to the percentage of time that the devices are worn are considered, it is clear that hearing protectors should be worn all the time because if they are removed for even a few minutes, their effective cumulative attenuation capability is severely reduced. Removing hearing protection for only 15 minutes of an 8-hour work shift can cut protection efficacy in half. Similarly, a poorly fitting hearing protector does not prevent hearing loss.1
Hearing impairment is the medical term used to refer to the hearing level at which individuals begin to experience difficulties in everyday life. Hearing impairment manifests in practical terms, such as a difficulty in understanding speech. By the time an individual becomes aware of decreased speech intelligibility, considerable damage to the organ of Corti likely has occurred because speech reception is not altered greatly until a hearing loss is more than 40 dB. The amount of loss at frequencies most important to speech (i.e., at 2 kHz, 3 kHz, and 4 kHz) is used by OSHA108 as a basis for calculating amounts of compensation because NIHL occurs initially at greater than or equal to 2 kHz. The measure of hearing impairment is called the hearing handicap, which is always based on the functional state of both ears. An official guide devised by the American Academy of Otolaryngology–Head and Neck Surgery120 provides a detailed explanation of how to evaluate and compute an NIHL handicap. Generally, the disability benefits of the U.S. Social Security Administration121,122 are less generous than are the benefits for either military veterans or typical worker’s compensation programs.
The physician can be particularly effective in teaching individuals how to recognize the danger signals of potential NIHL including the need to shout to be heard and the development of the sensation of pain, muffled sound, and tinnitus.123,124 The influence of otolaryngologists as hearing experts in the areas of education and motivation can be a major force in preventing NIHL.
Bogoch II, House RA, Kudla I. Perceptions about hearing protection and noise-induced hearing loss of attendees of rock concerts. Can J Public Health. 2005;96:69-72.
Carlsson PI, Van Laer L, Borg E, et al. The influence of genetic variation in oxidative stress genes on human noise susceptibility. Hear Res. 2005;202:87-96.
Darrat I, Ahmad N, Seidman K, et al. Auditory research involving antioxidants. Curr Opin Otolaryngol Head Neck Surg. 2007;15:358-363.
Davis RR, Kozel P, Erway LC. Genetic influences in individual susceptibility to noise: a review. Noise Health. 2003;5:19-28.
Dobie RA. The burdens of age-related and occupational noise-induced hearing loss in the United States. Ear Hear. 2008;29:565-577.
Fechter LD. Promotion of noise-induced hearing loss by chemical contaminants. J Toxicol Environ Health A. 2004;67:727-740.
Folmer RL, Griest SE, Martin WH. Hearing conservation education programs for children: a review. J Sch Health. 2002;72:51-57.
Kanzaki S, Kawamoto K, Oh SH, et al. From gene identification to gene therapy. Audiol Neurootol. 2002;7:161-164.
Kujawa SG, Liberman MC. Acceleration of age-related hearing loss by early noise exposure: evidence of a misspent youth. J Neurosci. 2006;26:2115-2123.
Lefebvre PP, Malgrange B, Lallemend F, et al. Mechanisms of cell death in the injured auditory system: otoprotective strategies. Audiol Neurootol. 2002;7:165-170.
Le Prell CG, Dolan DF, Schacht J, et al. Pathways for protection from noise induced hearing loss. Noise Health. 2003;5:1-17.
Lynch ED, Kil J. Compounds for the prevention and treatment of noise-induced hearing loss. Drug Discov Today. 2005;10:1291-1298.
Niu X, Canlon B. Protective mechanisms of sound conditioning. Adv Otorhinolaryngol. 2002;59:96-105.
Ohlemiller KK. Contributions of mouse models to understanding of age- and noise-related hearing loss. Brain Res. 2006;1091:89-102.
Rosenhall U. The influence of ageing on noise-induced hearing loss. Noise Health. 2003;5:47-53.
Sisto R, Chelotti S, Moriconi L, et al. Otoacoustic emission sensitivity to low levels of noise-induced hearing loss. J Acoust Soc Am. 2007;122:387-401.
Toppila E, Pyykko I, Starck J. Age and noise-induced hearing loss. Scand Audiol. 2001;30:236-244.
Wagner W, Heppelmann G, Kuehn M, et al. Olivocochlear activity and temporary threshold shift-susceptibility in humans. Laryngoscope. 2005;115:2021-2028.
Williams W. Noise exposure levels from personal stereo use. Int J Audiol. 2005;44:231-236.
1. National Institute of Occupational Safety and Health. Available at http://www.cdc.gov/NIOSH/ Accessed January 28, 2009
2. National Institute of Deafness and Other Communication Disorders. Available at http://www.nidcd.nih.gov Accessed January 28, 2009
3. Occupational Safety and Health Administration, Department of Labor. Occupational noise exposure: hearing conservation amendment. Fed Reg. 1981;46:4078-4179.
4. Nordmann AS, Bohne BA, Harding GW. Histopathological differences between temporary and permanent threshold shift. Hear Res. 2000;139:13-30.
5. Helfer TM, Jordan NN, Lee RB. Postdeployment hearing loss in U.S. Army soldiers seen at audiology clinics from April 1, 2003, through March 31, 2004. Am J Audiol. 2005;14:165-168.
6. Dobie RA. Medical-Legal Evaluation of Hearing Loss, 2nd ed. Toronto: Cengage Delmar Learning; 2001.
7. Taylor W, Lempert B, Pelmear P, et al. Noise levels and hearing thresholds in the drop forging industry. J Acoust Soc Am. 1984;76:807-819.
8. Johnsson LG, Hawkins JE. Degeneration patterns in human ears exposed to noise. Ann Otol Rhinol Laryngol. 1976;85:725-739.
9. Bredberg G. Cellular pattern and nerve supply of the human organ of Corti. Acta Otolaryngol Suppl. 1968;236:1-135.
10. McGill TJI, Schuknecht HF. Human cochlear changes in noise-induced hearing loss. Laryngoscope. 1976;86:1293-1302.
11. Ou HC, Harding GW, Bohne BA. An anatomically based frequency-place map for the mouse cochlea. Hear Res. 2000;145:123-129.
12. Morest DK, Bohne BA. Noise-induced degeneration in the brain and representation of inner and outer hair cells. Hear Res. 1983;9:145-151.
13. Caiazzo AJ, Tonndorf J. Ear canal resonance and temporary threshold shift. Otolaryngology. 1978;86:820.
14. Schuknecht HF. Pathology of the Ear. Cambridge, MA: Harvard University Press; 1974.
15. Wang Y, Hirose K, Liberman MC. Dynamics of noise-induced cellular injury and repair in the mouse cochlea. J Assoc Res Otolaryngol. 2002;3:248-268.
16. Clark JA, Pickles JO. The effects of moderate and low levels of acoustic overstimulation on stereocilia and their tip links in the guinea pig. Hear Res. 1996;99:119-128.
17. Liberman MC. Chronic ultrastructural changes in acoustic trauma: serial-section reconstruction of stereocilia and cuticular plates. Hear Res. 1987;26:65-88.
18. Liberman MC, Dodds LW. Acute ultrastructural changes in acoustic trauma: serial-section reconstruction of stereocilia and cuticular plates. Hear Res. 1987;26:45-64.
19. Schneider ME, Belyantseva IA, Azevedo RB, et al. Rapid renewal of auditory hair bundles. Nature. 2002;418:837-838.
20. Lin HW, Schneider ME, Kachar B. When size matters: the dynamic regulation of stereocilia lengths. Curr Opin Cell Biol. 2005;17:55-61.
21. Ahmad M, Bohne BA, Harding GW. An in vivo tracer study of noise-induced damage to the reticular lamina. Hear Res. 2003;175:82-100.
22. Corwin JT, Cotanche DA. Regeneration of sensory hair cells after acoustic trauma. Science. 1988;240:1772-1774.
23. Cotanche DA. Regeneration of hair cell stereociliary bundles in the chick cochlea following severe acoustic trauma. Hear Res. 1987;30:181-196.
24. Ryals BM, Rubel EW. Hair cell regeneration after acoustic trauma in adult Coturnix quail. Science. 1988;240:1774-1776.
25. Cruz RM, Lambert PR, Rubel EW. Light microscopic evidence of hair cell regeneration after gentamicin toxicity in chick cochlea. Arch Otolaryngol Head Neck Surg. 1987;13:1058-1062.
26. McFadden EA, Saunders JC. Recovery of auditory function following intense sound exposure in the neonatal chick. Hear Res. 1989;41:205-215.
27. Smolders JW. Functional recovery in the avian ear after hair cell regeneration. Audiol Neurootol. 1999;4:286-302.
28. Tucci DL, Rubel EW. Physiologic status of regenerated hair cells in the avian inner ear following aminoglycoside ototoxicity. Otolaryngol Head Neck Surg. 1990;103:443-450.
29. Cotanche DA, Lee KH, Stone JS, et al. Hair cell regeneration in the bird cochlea following noise damage or ototoxic drug damage: a review. Anat Embryol (Berl). 1994;198:1-18.
30. Zheng JL, Gao WQ. Overexpression of Math1 induces robust production of extra hair cells in postnatal rat inner ears. Nat Neurosci. 2000;3:480-586.
31. Bermingham-McDonogh O, Rubel EW. Hair cell regeneration: winging our way towards a sound future. Curr Opin Neurobiol. 2003;13:119-126.
32. Kawamoto K, Ishimoto S, Minoda R, et al. Math1 gene transfer generates new cochlear hair cells in mature guinea pigs in vivo. J Neurosci. 2003;23:4395-4440.
33. Izumikawa M, Minoda R, Kawamoto K, et al. Auditory hair cell replacement and hearing improvement by Atoh1 gene therapy in deaf mammals. Nat Med. 2005;11:271-276.
34. Canlon B, Borg E, Flock A. Protection against noise trauma by preexposure to a low level acoustic stimulus. Hear Res. 1988;34:197-200.
35. Franklin DJ, Lonsbury-Martin BL, Stagner BB, et al. Altered susceptibility of 2f1-f2 acoustic-distortion products to the effects of repeated noise exposure in rabbits. Hear Res. 1991;53:185-208.
36. Sánchez Fernandez JM, Martínez Ibargüen A, Orbegozo Etxebarría E, et al. Distortion product otoacoustic emissions study of the noise-induced toughening effect in rats. Acta Otolaryngol. 2003;123:154-159.
37. Subramaniam M, Campo P, Henderson D. The effect of exposure level on the development of progressive resistance to noise. Hear Res. 1991;52:181-188.
38. Miyakita T, Hellström PA, Frimanson E, et al. Effect of low level acoustic stimulation on temporary threshold shift in young humans. Hear Res. 1992;60:149-155.
39. Kemp DT. Stimulated acoustic emissions from within the human auditory system. J Acoust Soc Am. 1978;64:1386-1391.
40. Brownell WE. Outer hair cell electromotility and otoacoustic emissions. Ear Hear. 1990;11:82-92.
41. Collet L, Kemp DT, Veuillet E, et al. Effect of contralateral auditory stimuli on active cochlear micro-mechanical properties in human subjects. Hear Res. 1990;43:251-261.
42. Kim DO, Dorn PA, Neely ST, et al. Adaptation of distortion product otoacoustic emission in human. J Assoc Res Otolaryngol. 2001;2:31-40.
43. Maison SF, Liberman MC. Predicting vulnerability to acoustic injury with a noninvasive assay of olivocochlear reflex strength. J Neurosci. 2000;20:4701-4707.
44. Desai A, Reed D, Cheyne A, et al. Absence of otoacoustic emissions in subjects with normal audiometric thresholds implies exposure to noise. Noise Health. 1999;1:58-65.
45. Canlon B, Agerman K, Dauman R, et al. Pharmacological strategies for preventing cochlear damage induced by noise trauma. Noise Health. 1998;1:13-23.
46. Kopke RD, Coleman JK, Liu J, et al. Candidate’s thesis: enhancing intrinsic cochlear stress defenses to reduce noise-induced hearing loss. Laryngoscope. 2002;112:1515-1532.
47. Yamasoba T, Nuttall AL, Harris C, et al. Role of glutathione in protection against noise-induced hearing loss. Brain Res. 1998;784:82-90.
48. Franzé A, Sequino L, Saulino C, et al. Effect over time of allopurinol on noise-induced hearing loss in guinea pigs. Int J Audiol. 2003;42:227-234.
49. Kopke RD, Weisskopf PA, Boone JL, et al. Reduction of noise-induced hearing loss using L-NAC and salicylate in the chinchilla. Hear Res. 2000;149:138-146.
50. McFadden SL, Ohlemiller KK, Ding D, et al. The influence of superoxide dismutase and glutathione peroxidase deficiencies on noise-induced hearing loss in mice. Noise Health. 2001;3:49-64.
51. Kopke RD, Jackson RL, Coleman JK, et al. NAC for noise: from the bench top to the clinic. Hear Res. 2007;226:114-125.
52. Ward WD. General auditory effects of noise. Otolaryngol Clin North Am. 1979;12:473-491.
53. Toppila E, Pyykkö II, Starck J, et al. Individual risk factors in the development of noise-induced hearing loss. Noise Health. 2000;2:59-70.
54. Brink LL, Talbott EO, Burks JA, et al. Changes over time in audiometric threshold in a group of automobile stamping and assembly workers with a hearing conservation program. AIHA J (Fairfax, Va). 2002;63:482-487.
55. Ishii EK, Talbott EO. Race/ethnicity differences in the prevalence of noise-induced hearing loss in a group of metal fabricating workers. J Occup Environ Med. 1998;40:661-666.
56. Cantrell RW. Physiologic effects of noise. Otolaryngol Clin North Am. 1979;12:537-549.
57. Celik O, Yalcin S, Ozturk A. Hearing parameters in noise exposed industrial workers. Auris Nasus Larynx. 1998;25:369-375.
58. Mizoue T, Miyamoto T, Shimizu T. Combined effect of smoking and occupational exposure to noise on hearing loss in steel factory workers. Occup Environ Med. 2003;60:56-69.
59. Axelsson A, Lindgren F. Is there a relationship between hypercholesterolaemia and noise induced hearing loss? Acta Otolaryngol. 1985;100:379-386.
60. Chang NC, Yu ML, Ho KY, et al. Hyperlipidemia in noise-induced hearing loss. Otolaryngol Head Neck Surg. 2007;137:603-606.
61. Ishii EK, Talbott EO, Findlay RC, et al. Is NIDDM a risk factor for noise-induced hearing loss in an occupationally noise exposed cohort? Sci Total Environ. 1992;127:155-165.
62. Ising H, Babisch W, Kruppa B. Noise-induced endocrine effects and cardiovascular risk. Noise Health. 1999;1:37-48.
63. Barrenas ML, Lindgren F. The influence of eye colour on susceptibility to TTS in humans. Br J Audiol. 1991;25:303-307.
64. Bies DA, Hansen CH. An alternative mathematical description of the relationship between noise exposure and hearing loss. J Acoust Soc Am. 1990;88:2743-2754.
65. Macrae JH. Presbycusis and noise-induced permanent threshold shift. J Acoust Soc Am. 1991;90:2513-2516.
66. McFadden D, Wightman FL. Audition: some relations between normal and pathological hearing. Ann Rev Psychol. 1983;34:95-128.
67. Erway LC, Shiau YW, Davis RR, et al. Genetics of age-related hearing loss in mice, III: susceptibility of inbred and F1 hybrid strains to noise-induced hearing loss. Hear Res. 1996;93:181-187.
68. Jimenez AM, Stagner BB, Martin GK, et al. Susceptibility of DPOAEs to sound overexposure in inbred mice with AHL. J Assoc Res Otolaryngol. 2001;2:233-245.
69. Vázquez AE, Jimenez AM, Martin GK, et al. Evaluating cochlear function and the effects of noise exposure in the B6.CAST+ahl mouse with distortion product otoacoustic emissions. Hear Res. 2004;194:87-96.
70. Candreia C, Martin GK, Stagner BB, et al. Distortion product otoacoustic emissions show exceptional resistance to noise exposure in MOLF/Ei mice. Hear Res. 2004;194:109-117.
71. Konings A, Van Laer L, Pawelczyk M, et al. Association between variations in CAT and noise-induced hearing loss in two independent noise-exposed populations. Hum Mol Genet. 2007;16:1872-1883.
72. Lonsbury-Martin BL, Martin GK, Balkany T. Clinical applications of otoacoustic emissions. In: Lucente FE, editor. Highlights of the Instructional Courses—1994. St Louis: Mosby–Year Book; 1994:343-355.
73. Engdahl B, Woxen O, Arnesen AR, et al. Transient evoked otoacoustic emissions as screening for hearing losses at the school for military training. Scand Audiol. 1996;25:71-78.
74. Konopka W, Pawlaczyk-Luszczynska M, Sliwinska-Kowalska M, et al. Effects of impulse noise on transiently evoked otoacoustic emission in soldiers. Int J Audiol. 2005;44:3-7.
75. Lapsley Miller JA, Marshall L, Heller LM, et al. Low-level otoacoustic emissions may predict susceptibility to noise-induced hearing loss. J Acoust Soc Am. 2006;120:280-296.
76. Murray NM, LePage EL, Mikl N. Inner ear damage in an opera theatre orchestra as detected by otoacoustic emissions, pure tone audiometry and sound levels. Aust J Audiol. 1998;20:67-78.
77. Seixas NS, Goldman B, Sheppard L, et al. Prospective noise induced changes to hearing among construction industry apprentices. Occup Environ Med. 2005;62:309-317.
78. Brummett RE, Fox KE, Kempton JB. Quantitative relationships of the interaction between sound and kanamycin. Arch Otolaryngol Head Neck Surg. 1992;118:498-500.
79. Tan CT, Hsu CJ, Lee SY, et al. Potentiation of noise-induced hearing loss by amikacin in guinea pigs. Hear Res. 2001;161:72-80.
80. Ryan AF, Bone RC. Non-simultaneous interaction of exposure to noise and kanamycin intoxication in the chinchilla. Am J Otolaryngol. 1982;3:264-272.
81. McFadden D, Plattsmier HS. Aspirin can potentiate the temporary hearing loss induced by intense sounds. Hear Res. 1983;9:295-316.
82. Carson SS, Prazma J, Pulver SH, et al. Combined effects of aspirin and noise in causing permanent hearing loss. Arch Otolaryngol Head Neck Surg. 1989;115:1070-1075.
83. Spongr VP, Boettcher FA, Saunders SS, et al. Effects of noise and salicylate on hair cell loss in the chinchilla cochlea. Arch Otolaryngol Head Neck Surg. 1992;118:157-164.
84. Gratton MA, Salvi RJ, Kamen BA. Interaction of cisplatin and noise on the peripheral auditory system. Hear Res. 1990;50:211-223.
85. Laurell GF. Combined effects of noise and cisplatin: short- and long-term follow-up. Ann Otol Rhinol Laryngol. 1992;101:969-976.
86. Fechter LD, Chen GD, Rao D. Chemical asphyxiants and noise. Noise Health. 2002;4:49-61.
87. Morata TC, Little MB. Suggested guidelines for studying the combined effects of occupational exposure to noise and chemicals on hearing. Noise Health. 2002;4:73-87.
88. Ylikoski J, Juntunen J, Matikainen E, et al. Subclinical vestibular pathology in patients with noise-induced hearing loss from intense impulse noise. Acta Otolaryngol. 1988;105:558-563.
89. Pyykko I, Aalto H, Ylikoski J. Does impulse noise induce vestibular disturbances? Acta Otolaryngol Suppl. 1989;468:211-216.
90. Shupak A, Bar-El E, Podoshin L, et al. Vestibular findings associated with chronic noise induced hearing impairment. Acta Otolaryngol. 1994;114:579-585.
91. Wang YP, Young YH. Vestibular-evoked myogenic potentials in chronic noise-induced hearing loss. Otolaryngol Head Neck Surg. 2007;137:607-611.
92. Golz A, Westerman ST, Westerman LM, et al. The effects of noise on the vestibular system. Am J Otolaryngol. 2001;22:190-196.
93. Zhu S, Sakakibara H, Yamada S. Combined effects of hand-arm vibration and noise on temporary threshold shifts in healthy subjects. Int Arch Occup Environ Health. 1997;69:433-436.
94. Harding GW, Bohne BA, Lee SC, et al. Effect of infrasound on cochlear damage from exposure to a 4 kHz octave band of noise. Hear Res. 2007;225:128-138.
95. Chou CK, Guy AW, Galambos R. Auditory perception of radio-frequency electromagnetic fields. J Acoust Soc Am. 1982;71:1321-1334.
96. Rosowski JJ. The effects of external- and middle-ear filtering on auditory threshold and noise-induced hearing loss. J Acoust Soc Am. 1991;90:124-135.
97. Foster JR, Hall DA, Summerfield AQ, et al. Sound-level measurements and calculations of safe noise dosage during EPI at 3 T. J Magn Reson Imaging. 2000;12:157-163.
98. Brummett RE, Talbot JM, Charuhas P. Potential hearing loss resulting from MR imaging. Radiology. 1988;169:539-540.
99. Ravicz ME, Melcher JR. Isolating the auditory system from acoustic noise during functional magnetic resonance imaging: examination of noise conduction through the ear canal, head, and body. J Acoust Soc Am. 2001;109:216-231.
100. Matheson MP, Stansfeld SA, Haines MM. The effects of chronic aircraft noise exposure on children’s cognition and health: 3 field studies. Noise Health. 2003;5:31-40.
101. Doring HJ, Hauf G, Seiberling M. Effects of high-intensity sound on the contractile function of the isolated ileum of guinea pigs and rabbits. In: Tobias JV, Jansen G, Ward WD, editors. Noise as a Public Health Problem: Proceedings of the Third International Congress. Rockville, MD: American Speech-Language-Hearing Association; 1980:288-293.
102. Narlawar UW, Surjuse BG, Thakre SS. Hypertension and hearing impairment in workers of iron and steel industry. Indian J Physiol Pharmacol. 2006;50:60-66.
103. Passchier-Vermeer W, Passchier WF. Noise exposure and public health. Environ Health Perspect. 2000;108:123-131.
104. Larsby B, Hällgren M, Lyxell B, et al. Cognitive performance and perceived effort in speech processing tasks: effects of different noise backgrounds in normal-hearing and hearing-impaired subjects. Int J Audiol. 2005;44:131-143.
105. Ward WD, Nelson DA. On the equal-energy hypothesis relative to damage-risk criteria in the chinchilla. In: Robinson DW, editor. Occupational Hearing Loss. London: Academic Press; 1971:225-231.
106. Burns W, Robinson DW. Hearing and Noise in Industry. London: Her Majesty’s Stationery Office; 1970.
107. Atherley GRC, Martin AM. Equivalent-continuous noise level as a measure of injury from impact and impulse noise. Ann Occup Hyg. 1971;14:11-28.
108. Goldstein J. Fundamental concepts in sound measurement. In: Lipscomb DM, editor. Noise and Audiology. Baltimore: University Park Press; 1978:3-58.
109. Occupational Safety and Health Administration. Available at http://www.osha.gov Accessed January 28, 2009
110. Fligor BJ, Cox LC. Output levels of commercially available portable compact disc players and the potential risk to hearing. Ear Hear. 2004;25:513-527.
111. De Santis E, Ordonez R, Reuter K, et al. Effects of personal stereo use: pilot results from 20 university students. J Acoust Soc Am. 2008;123:3254.
112. Vogel I, Brug J, Hosli EJ, et al. MP3 players and hearing loss: adolescents’ perceptions of loud music and hearing conservation. J Pediatr. 2008;152:400-404.
113. Agrawal Y, Platz EA, Niparko JK. Prevalence of hearing loss and differences by demographic characteristics among US adults: data from the National Health and Nutritional Examination Survey, 1999-2004. Arch Intern Med. 2008;168:1522-1530.
114. Hodgetts WE, Rieger JM, Szarko RA. The effects of listening environment and earphone style on preferred listening levels of normal hearing adults using an MP3 player. Ear Hear. 2007;28:290-297.
115. Vavrina J, Muller W. Therapeutic effect of hyperbaric oxygenation in acute acoustic trauma. Rev Laryngol Otol Rhinol (Bord). 1995;116:377-380.
116. Lusk SL. Preventing noise-induced hearing loss. Nurs Clin North Am. 2002;37:257-262.
117. Abel SM, Rockley T, Goldfarb D. Outer ear canal shape and its relation to the effectiveness of sound attenuating earplugs. J Otolaryngol. 1990;19:91-95.
118. Neitzel R, Somers S, Seixas N. Variability of real-world hearing protector attenuation measurements. Ann Occup Hyg. 2006;50:679-691.
119. Giardino DA, Durkt GJr. Evaluation of muff-type hearing protectors as used in a working environment. Am Ind Hyg Assoc J. 1996;57:264-271.
120. American Academy of Otolaryngology–Head and Neck Surgery. Available at http://entnet.org Accessed January 28, 2009
121. Social Security Administration. Disability evaluation under Social Security. SSA Publication No. 64-039, 2003.
122. Social Security Administration. The Social Security Quick Calculator page. Available at http://www.ssa.gov/OACT/quickcalc Accessed January 28, 2009
123. Folmer RL. The importance of hearing conservation instruction. J Sch Nurs. 2003;19:140-148.
124. Peters RJ. The role of hearing protectors in leisure noise. Noise Health. 2003;5:47-55.