Chapter 20 Neutron Radiotherapy
Fast Neutron Radiotherapy
Overview
The resulting energy transfer is in the range of 20 to 100 keV/µm compared with 0.2 to 2 keV/µm for the megavoltage photons and electrons used in conventional radiotherapy. It is this higher energy transfer that gives rise to the different radiobiologic properties of fast neutrons, which are advantageous in certain clinical situations. The higher relative biologic effectiveness (RBE) accounts for the different clinical response observed with neutrons as opposed to conventional photon irradiation. Fast neutrons have RBEs in the range of 3 to 3.5 in terms of most normal tissue late effects, RBEs in the range of 4 to 4.5 in terms of damage to the central nervous system, and RBEs in the range of 8 for salivary gland malignant tumors.1–3 High–linear energy transfer (LET) radiation causes a dense chain of ionizing events to both strands of the DNA and therefore makes DNA repair more difficult. Furthermore, high-LET radiation from fast neutrons is less sensitive to the reduced cell killing effects of hypoxia, with there being approximately equal cell killing under normoxic and hypoxic conditions.
Historical Perspective
Patients were first treated with fast neutron radiotherapy in 1938 by Robert Stone and co-workers4 at the Crocker Radiation Laboratory (later to become Lawrence Berkeley Laboratories). Two hundred forty patients were treated between 1938 and 1942. Many of these patients had received prior photon irradiation. There were significant radiation sequelae in the few long-term survivors such that clinical interest in fast neutron radiotherapy dropped off significantly for the next 20 years. Brennan and Phillips5 subsequently reviewed the initial work by Stone and colleagues and showed that an inappropriate value for the neutron RBE had been used in the dose calculations. Hence, many of those initial patients had been inadvertently overdosed. With a better understanding of the RBE of fast neutron radiotherapy in different tissue types, in the 1970s Catterall and coworkers6,7,8 resumed neutron clinical trials at Hammersmith Hospital in London, England. Their results indicated that many advanced head and neck cancers exhibited a good response with acceptable side effects. The interest in fast neutron radiotherapy grew so that at one point there were 39 different centers in North America, Europe, Asia, and Africa treating patients with fast neutrons. Clinical utility has been further elucidated through clinical trials and single-institution experience, and neutron radiotherapy now has fairly well defined and limited indications. There are currently only seven operating fast neutron radiotherapy centers throughout the world. These are listed in Table 20-1, along with some of their more important characteristics.
TABLE 20-1 Location of Operating Fast Neutron Radiotherapy Centers, 2009
| Location | Beam Reaction | Comments |
|---|---|---|
| United States | ||
| University of Washington Medical Center, Seattle, Washington | 50 MeV p→Be | Isocentric gantry and multileaf collimator |
| Fermi Laboratories, Batavia, Illinois | 66 MeV p→Be | Horizontal beam, fixed collimators with blocking inserts |
| Europe | ||
| Tomsk Polytechnical University, Tomsk, Russia | Cyclotron | Mean neutron energy 6.3 MeV |
| Technisch Universtät Munich, Munich, Germany | Fission neutrons | FRMII reactor, horizontal beam with multileaf collimator, mean neutron energy 1.9 MeV |
| Institute of Physics and Power Engineering, Obninsk, Russia | Fission neutrons | Horizontal beam |
| Asia | ||
| National Institute of Radiological Sciences, Chiba, Japan | 30 MeV d→Be | Vertical beam, multileaf collimator |
| Africa | ||
| National Accelerator Centre, Faure, South Africa | 66 MeV p→Be | Isocentric gantry and jaw collimator |
Be, beryllium, d, deuteron, FRMII, Forschungs-Nuetronenquelle Heinz Maier-Leibnitz; MeV, million electron volt; p, proton.
Salivary Gland Tumors
The therapeutic advantage of fast neutron radiotherapy over conventional radiotherapy is best established in salivary gland tumors. The RBE for salivary gland tumors is approximately 8, whereas for surrounding normal tissues, the RBE is 3 to 3.5.1 A typical neutron dose for a salivary gland cancer is approximately 18 to 20 nGy (neutron Gray). The approximate equivalent photon dose is 60 to 70 Gy equivalents (GyE) as far as normal tissues are concerned but it is in the range of 150 to 160 GyE as far as the tumor is concerned. The therapeutic gain factor for salivary gland tumors is therefore in the range of 2.3 to 2.6.
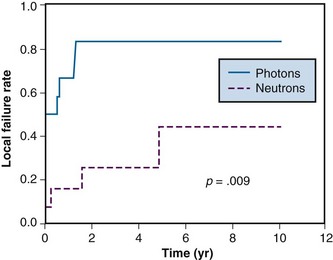
Early single-institution studies showed neutrons to be beneficial in the treatment of salivary gland tumors. These results led to a prospective randomized trial sponsored by the Radiation Therapy Oncology Group (RTOG) and the Medical Research Council (MRC) of Great Britain.9 Local control at 10 years was improved in the neutron arm (56% vs. 17%; p = .009). The final report showed a trend toward increased median survival of about 8 months in the neutron arm, but this was not statistically significant. However, the cause of death differed between the two subgroups. Metastatic disease was the main cause of death for the neutron patients, whereas local/regional tumor failure was the main cause of death for the photon patients. Of note, the study was stopped early for ethical reasons when 2-year survival data showed a strong trend in favor of the neutron patients. Local tumor control curves for this study are shown in Figure 20-1.
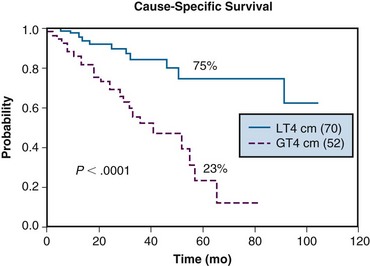
The University of Washington experience was reviewed by Douglas and colleagues.10,11 Two hundred seventy-nine patients treated with curative intent were included in the analysis. A majority (263 patients) had evidence of gross disease, with 141 having major salivary gland neoplasms and 138 having minor salivary gland neoplasms. The 6-year actuarial cause-specific survival rate was 67%. On multivariate analysis, stage I/II disease, minor salivary gland primary tumors, lack of base-of-skull involvement, and primary (rather than recurrent) disease were associated with improved survival rates. Improved locoregional control was associated with tumors having the following characteristics: greatest diameter less than 4 cm, no base-of-skull invasion, prior surgical resection, and/or no previous radiation therapy. The 6-year actuarial rate for freedom from the development of metastases was 64% for patients without lymph node involvement or base-of-skull involvement. There was a 6-year actuarial rate of 10% for developing RTOG grade 3 or 4 toxicity. Cause-specific survival curves for the major salivary gland patients as a function of tumor size are shown in Figure 20-2.
The poorer outcome of salivary tumors with base-of-skull involvement appears to be the result of the need to reduce the dose to portions of tumors that are adjacent to critical central nervous system structures (recall that the RBE for fast neutrons for central nervous system tissue is 4 to 4.5). With a standard 20-nGy dose to the involved site, the dose to the temporal tips could be approximately 80 photon GyE. Reducing the temporal lobe dose to a safe level sometimes necessitated blocking portions of tumor. In an attempt to compensate for the reduced neutron dose, a stereotactic radiosurgical boost with photon radiation has been instituted at the University of Washington. At 40 months there was improved local control compared with historically treated patients with skull base invasion who did not receive a stereotactic radiosurgical boost (82% vs. 39%; p = .04).12 Complications appear to be acceptable.
The use of both neutrons and photons in a composite “mixed beam” treatment has been explored by Huber and associates13 in a series of patients with advanced adenoid cystic carcinomas treated at the Heidelberg neutron facility. They found a 5-year local control rate of 75% for patients treated with neutrons alone compared with 32% for patients treated with “mixed beam” irradiation or with photons alone. It appears that patients who have a greater portion of their treatment with neutrons fare better. Distant metastatic disease once again prevented improved local control from translating into an increase in overall survival rates.
The neutron facility in Orleans has reported similar results for 59 patients treated with fast neutron radiotherapy.14 The 5-year local control rate was 69.5%, with a crude 5-year survival rate of 66% and a 5-year tumor-free survival rate of 64.5%.
Prostate Cancer
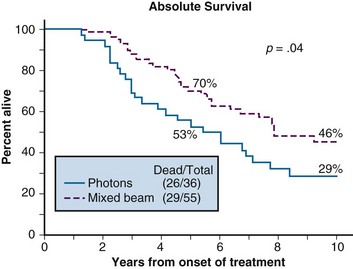
Prostate cancer is another tumor for which there has been significant clinical work using fast neutron radiotherapy. The National Cancer Institute (through RTOG 77-04) conducted a randomized trial for patients with T3, N0/N1 disease.15 One arm received a mixed beam (neutron/photon) dose to 70 GyE versus a second arm that received standard photons to 70 Gy. At the 10-year endpoint, survival rates (46% vs. 29%, p = .04) were improved in the mixed beam arm. There was also improved locoregional control (70% vs. 58%, p = .03) for the mixed beam subgroup. There was no difference in the rate of significant complications between the two arms. Survival curves for the patients in this study are shown in Figure 20-3.
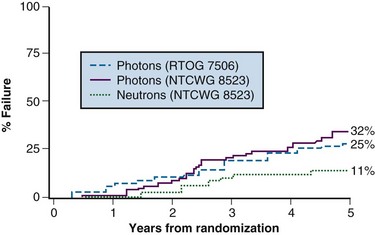
A follow-up randomized trial was conducted that compared 70-Gy standard photon irradiation with 20.4-nGy fast neutron therapy. This was carried out by the National Cancer Institute (NCI)–sponsored Neutron Therapy Collaborative Working Group (NTCWG) and included patients with high-grade T2 tumors or T3 to T4, N0 to N1, M0 tumors of any grade.16 With a median follow-up of 68 months, the 5-year actuarial locoregional failure rate was 11% for neutrons versus 32% for photons (p <.01). Actuarial overall survival and cause-specific survival rates were statistically equivalent. The incidence of severe complications was higher in the neutron group than in the photon group (11% vs. 3%). Complications were less severe and fewer in the centers that had more sophisticated beam-shaping abilities. Local failure curves for the patients in this study are shown in Figure 20-4.
Following the randomized trials, additional studies were conducted at several centers in the United States,17,18,19,20,21 Europe,22,23 and Asia.24,25 These single-institution results showed good local and regional control and appeared to corroborate the results of the randomized trials described earlier.
Sarcomas
Sarcomas are generally considered to be among the more “radioresistant” histologic types of tumors and so would fall into the category of tumors thought to be more responsive to high-LET radiation.1 A retrospective review by Schwarz and colleagues26 reports on 1171 soft tissue sarcoma patients from 11 European centers treated with fast neutron radiotherapy. In those patients with either inoperable tumors or gross disease after surgery, the local control rate was approximately 50%. Toxicity was higher than what was generally seen with photon irradiation. The local control rates were thought by the authors to be better than what would have been expected with standard radiotherapy. This conclusion is supported by other single-institution studies.27
Other Tumor Types
Fast neutron radiation has been tested on a variety of other tumor types. Generally, the rationale for the use of fast neutrons came from trying to overcome a perceived limitation of standard photon irradiation. A good example of this is malignant glioma of the brain. Dose-escalation studies with photons have failed to increase overall survival rates.28 By definition, glioblastoma multiforme tumors have associated areas of necrosis that are often surrounded by regions of hypoxia, which might make the tumors more radioresistant. Studies of malignant gliomas in the 1970s and 1980s used neutrons either alone or in combination with photons.29 None of these initial trials showed an improvement in overall survival rates with the addition of neutrons. However, with relatively high doses of fast neutrons alone, autopsy and second-look surgical data indicated that tumor cells were sterilized in the treated region.30 Unfortunately, there was also significant coagulative necrosis caused by the neutron irradiation, which indicated too much damage to normal brain tissue. A more recent study used fluorodeoxyglucose–positron emission tomography (FDG-PET) scanning to determine the final boost volume that received 18 nGy.31 Only 10 patients were treated and all exhibited tumor progression by 39 weeks, perhaps because of the restricted fields that were employed. Gliosis and microvascular sclerosis consistent with radiation injury were noted in regions of the contralateral brain receiving 6 to 10 nGy. Much of the current interest in neutron radiotherapy for malignant gliomas is focused on BNCT, which is discussed later in this chapter.
Boron Neutron Capture Therapy
Historical Perspective
The basic idea for BNCT dates to 1936, when Locher32 proposed treating malignant tumors with low-energy thermal neutrons via a capture process with 10B. A few years later, Kruger33 and Zahl and colleagues34 experimentally verified some of the basic tenets of this concept and demonstrated the high RBE of the resulting helium-4 (4He) and lithium-7 (7Li) fission fragments. The theoretical advantage of “tagging” the tumor-specific carrier with an innocuous substance such as 10B and then activating it, rather than “tagging” it with a toxic compound such as ricin, is that the “binary approach” is more forgiving of other body tissues that might also display some avidity for the carrier agent. In the real world, the thermal and epithermal beams produced by nuclear reactors are contaminated with gamma rays and fast neutrons, which can cause considerable normal tissue damage even in the absence of a high 10B concentration.35 Moreover, even in the absence of such beam contaminants, nuclear species such as 1H, nitrogen-14 (14N), and chlorine-35 (35Cl), which are commonly found in tissue, interact with thermal neutrons via capture events of their own, and their reaction products cause biologic damage to non–10B-containing normal tissues. Although the respective capture cross sections for these commonly found nuclei are much lower than for 10B and other nuclear species of interest for neutron capture therapy, the concentrations of these commonly found nuclei are many orders of magnitude greater than the concentrations of 10B. Hence, reactions involving these nuclides can result in an appreciable tissue dose, even in the absence of 10B. The key to BNCT as a practical therapy is the development of compounds that localize specifically in tumors and deliver sufficient 10B concentrations to yield a significant therapeutic advantage compared with the radiation doses delivered to normal tissues.
Early Clinical Studies
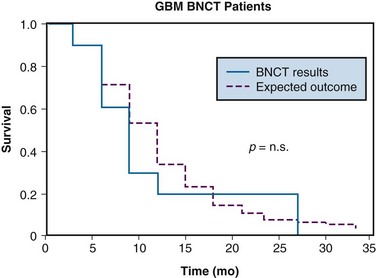
The first set of human clinical trials was carried out in the 1950s at Brookhaven National Laboratory using 10B-enriched, boric acid derivatives.36,37 These compounds yielded high concentrations of 10B in the blood at the time of irradiation and unfortunately produced relatively low 10B levels in the tumors. This resulted in considerable blood vessel endothelial damage and no therapeutic benefit from treatment either in these trials or in subsequent trials that were carried out in the 1960s.38,39,40 Interest in the technique waned until Hatanaka and others in Japan41 reinstituted BNCT for malignant gliomas using a different 10B-carrier, 10B-sodium-mercaptoundecahydrododecaborate (BSH). This compound produced better tumor-to-blood boron ratios than the compounds used in the prior Brookhaven National Laboratory studies and the treatments caused less damage to normal brain tissue. Favorable reports regarding the efficacy of BSH41,42,43 led to renewed worldwide interest in the technique. Many patients have received BNCT treatments using thermal neutron beams from various reactors in Japan, but to date, no randomized clinical trials have been carried out. An analysis of the RTOG database by Curran and colleagues44 has demonstrated that there are some subsets of patients with high-grade gliomas who will do well with conventional photon irradiation. A few years ago, an analysis of a subset of patients from the United States who were treated with BNCT in Japan showed no improvement over that which would have been expected with conventional therapy after correcting for the appropriate prognostic factors.45 A comparison of the survival rates of these patients with a pseudo-matched group from the RTOG data base is shown in Figure 20-5. The prognostic factors identified by Curran and colleagues44 were used to generate these curves. There is no statistical difference between the two curves. Moreover, review of the data for the entire group of evaluable patients showed that in all except one patient there was a documented tumor recurrence within the primary radiation field.45 The only long-term survivor in this group of American patients had an anaplastic astrocytoma and fell into the most favorable category I of Curran and coworkers.44
Modern Clinical Trials
In Japan, there were three reactor facilities involved in patient treatments: (1) KUR in Osaka, (2) MITR in Musashi, and (3) JRR-4 at the Japan Atomic Energy Research Institute in Ikargi.46 Although most patients have been treated using thermal neutron beams, the KUR facility has been modified to produce a higher-energy epithermal beam allowing treatment through the intact skull. The majority of the patients have been treated for various types of brain tumors using BSH as the 10B-carrier agent and with a single fraction of neutron radiation. A report by Nakagawa and colleagues47 shows that in the subset of glioblastoma multiforme patients, 12% of treated patients lived longer than 2 years. Depending on other prognostic factors, this may or may not be better than expected with more conventional forms of treatment. Currently only the KUR and JRR-4 reactors are treating patients.
There were several reactor facilities in Europe with BNCT clinical programs for malignant brain tumors, all using epithermal neutron beams. The program that treated the most patients was based at the HFR reactor in Petten (the Netherlands) and was directed by the European Collaboration on Boron Neutron Capture Therapy. BSH at 100 mg/kg was used as the 10B-carrier. Researchers conducted a phase I dose-searching study restricted to the subsets of glioblastoma multiforme patients whose expected median survival time with conventional radiotherapy would be less than 10 months. Unlike the other ongoing treatment regimens, which employed a single BNCT treatment, the Petten program used four fractions requiring multiple administrations of the boron-carrier compound. BNCT dose reporting is complex, and the Petten group has chosen to specify each component of the radiation field separately rather than state a Gray-equivalent dose.48 The starting dose from the 10B component was set at 80% of the dose that produced neurologic changes in a dog brain study, DB = 8.6 Gy. The other components of the radiation dose were limited to Dn < 0.9 Gy, DN < 1.1 Gy, and Dg < 5.8 Gy, where Dn is the neutron-absorbed dose delivered by the thermal, epithermal, and fast neutrons and the charged particles produced by their reactions, Dg is the gamma ray absorbed dose, and DN is the absorbed dose from the protons produced by the 14N capture reaction. Twenty-five patients were entered into the initial dose arm of the study, with 21 actually receiving BNCT treatment.49 The median survival time of the BNCT-treated patients was 31 weeks, with 6 patients alive at the time of a preliminary report. All 21 patients exhibited tumor recurrence. A second BNCT program is based at the FiR No. 1 reactor in Helsinki, Finland. The initial protocol was a phase I dose-searching study that used L–para-boronophenylalanine (BPA) in a fructose solution at 290 mg BPA/kg as the B-carrier. Only a single treatment was given using two radiation fields. Thus far, 10 patients have been treated with tumor doses between 35.1 and 66.7 GyE. This effective dose has been determined using weighting factors for each of the radiation components (see Kankaanranta and colleagues50 for specific details). With a median follow-up of 9 months, 7 of 10 patients had recurrent or persistent tumor, with the median time to tumor progression being 8 months.50 The toxicity in both of the European trials was thought to be acceptable, making future escalation of the radiation dose possible. Small numbers of patients were also treated at Studsvik Medical AB in Uppsala University51 and at the Nuclear Research Institute in the Czech Republic.52 Currently, only the FiR No. 1 reactor is treating patients.
After approximately a 30-year hiatus, the first “modern era” BNCT clinical program in the United States was begun at the Massachusetts Institute of Technology (MIT) research reactor in collaboration with Tufts Medical Center. The first patient was treated in September 1994. This project was under the clinical direction of the New England Deaconess Hospital. Treatments were delivered using an epithermal beam with BPA as the 10B-carrier agent. Between 1996 and 1999, a total of 22 patients with intracranial lesions, either glioblastoma multiforme or metastatic melanoma, were treated.53 The initial clinical trial was a dose-searching toxicity study with the target dose ranging between 8.8 and 14.2 GyE. At the higher radiation doses, the time required to deliver the dose ranged up to 3 hours. At the higher dose levels, significant skin and mucosal reactions were observed. No adverse effects were noted due to the BPA administration, and no long-term complications relating to the BNCT treatment were noted. Some tumor responses were noted in the patients with metastatic melanoma, but there was no obvious prolongation of survival rates in either group of treated patients. This facility is no longer treating patients.
A second clinical BNCT program focusing on high-grade gliomas of the brain was initiated at Brookhaven National Laboratory. This program differed from the Japanese glioma program in two main respects: (1) a higher-energy epithermal neutron beam was used rather than a thermal neutron beam, and (2) a different 10B-carrier agent (BPA) was used rather than BSH. The epithermal beam meant that patients could be treated without recourse to a craniotomy and intraoperative irradiation and that more deeply located tumors could be given adequate doses. It was expected that BPA would produce higher tumor 10B concentrations than could be achieved with BSH. Based on 10B blood levels, it appeared that tumor 10B concentrations of approximately 35 to 50 µg/g were achieved. However, unlike BSH, BPA is not excluded from the normal brain by the blood-brain barrier, and tumor enrichment depends on other mechanisms of selective uptake (perhaps relating to an enhanced amino acid transport system). A dose-escalation approach was used. Prior to June 1996, all patients were treated using a simple appositional field with a single treatment fraction being given. A critique of this early Brookhaven National Laboratory treatment technique indicated significant deficiencies in the basic approach relating to the inability to achieve a tumor dose adequate to sterilize glioblastoma multiforme tumors using a single appositional field.54
Fifty of the 53 subjects have exhibited tumor persistence/recurrence within the treatment volume.55 The median time to progression was independent of dose. In fact, there was a significant trend toward worse local control in subjects treated using three fields. Survival as an endpoint was found to be more dependent on the aggressiveness of the postrecurrence treatment than the BNCT dose given and, as such, was not a useful indicator of treatment-dependent tumor control. The functional brain tolerance was reached in the three-field group. All except one patient treated with three fields exhibited significant acute or subacute functional neurologic toxicity at average brain doses as low as 6.7 GyE. This may indicate a “volume effect” in that multiple fields generally treat a larger volume of normal brain to a lower dose compared with when a single field is used. The early stopping criterion of a more than 20% incidence of grade 3 or 4 toxicity (RTOG and European Organization for Research and Treatment of Cancer [EORTC] criteria) was reached during this study. However, no significant tumor response was observed in the patients treated to minimum tumor doses above those predicted by Laramore and coworkers54,55 to be therapeutic. We also note that preclinical studies using a 9L rat gliosarcoma model showed significant tumor response of the tumor to BPA-fructose–mediated BNCT at comparable doses.56 Hence, neither the preclinical radiobiologic studies nor the mathematical modeling based on fast neutron data provided an accurate guide to the necessary therapeutic dose. The Brookhaven National Laboratory clinical program is no longer in operation.
In parallel with the BNCT program for malignant gliomas, a second area of major interest is the treatment of malignant melanoma metastatic to the brain. This work was instigated by Mishima and associates57,58 in Japan. They used BPA as the 10B-carrier agent with a single fraction of thermal neutrons. BPA acts as a dopamine analog in the melanin synthetic pathway and concentrates well in pigmented tumors. Initial reports describe good clinical results with this treatment,59,60 but no randomized trials have been performed. Reports from the MIT/Massachusetts General Hospital program indicate that responses are better in this setting than in the treatment of glioblastoma multiforme, which is consistent with BPA being better suited to the treatment of pigmented tumors. Under the auspices of the Atomic Energy Commission of Argentina, a program for the treatment of metastatic melanoma has been instituted at the RA-6 research reactor in San Carlos de Bariloche, and this program is still clinically active.61
A very interesting approach to the treatment of liver metastases was begun at the University of Pavia in Italy. The patient is infused with BPA, and the diseased liver is extirpated and irradiated in the reactor and then reimplanted into the patient. Two patients have been treated in this manner, with one long-term survivor at 3 years from the time of the procedure. Model calculations indicate that it may be possible to perform this treatment without first removing the liver.62
Summary
To date there is no convincing evidence that BNCT offers a therapeutic advantage over conventional treatments for glioblastoma multiforme. Reactor-based treatment centers are sufficient in number to conduct well-designed clinical trials to provide a “proof of concept” but certainly will not be adequate to treat large numbers of patients in the event that a therapeutic advantage is demonstrated. In this event, accelerator-based sources must be designed and placed in hospital settings. The main hurdle at the present time is the lack of tumor-specific 10B-carrier agents. The only compounds currently approved for human trials are BSH and BPA—both of which are suboptimal in many ways. Support of research in compound development will be key to further development of the field.63
Californium 252 Brachytherapy
Gynecologic applications of 252Cf brachytherapy were explored by Maruyama and associates,64 who developed appropriate dosing schedules. More recently, Tacev and colleagues65 reported on long-term results of a randomized trial for patients with advanced cervical cancer (stages IIB and IIIB) that compared 252Cf brachytherapy with a supplementary gamma ray boost of 16 Gy with conventional brachytherapy alone with gamma ray–emitting radium-226 (226Ra) or cesium-137 (137Cs) sources following 40-Gy external beam radiotherapy. Using an RBE of 6 for the 252Cf neutrons, point A doses of 56 GyE were given with the implants. Two hundred seventy-seven patients were entered into the study, and there was an improved 5-year survival rate in the group receiving the 252Cf implant (75.2% vs. 56.3%; p < .001).
Also of note is a pilot study using 252Cf interstitial brachytherapy in 56 patients with high-grade gliomas of the brain.66 After surgical debulking, catheters were placed under CT guidance followed by 252Cf placement. A dose of 3 Gy was given over 4 to 6 hours to the residual tumor, and then patients received 60-Gy to 70-Gy doses of conventional external beam radiotherapy. The median survival time was only 10 months, and recurrent tumor was found in all patients who died and were autopsied. Given the dose inhomogeneties and the relatively small amount of neutron radiation given, this outcome is not surprising.
1 Batterman JJ, Breur K, Hare GAM, et al. Observations on pulmonary metastases in patients after single doses and multiple fractions of fast neutrons and cobalt-60 gamma rays. Eur J Cancer. 1981;17:539.
2 Hall EJ. Radiobiology for the radiologist, ed 4. Philadelphia: Lippincott; 1992. chapters 9 and 14
3 Laramore GE, Austin-Seymour MM. Fast neutron radiotherapy in relation to the radiation sensitivity of human organ systems. Adv Radiat Biol. 1992;15:153.
4 Stone RS. Neutron therapy and specific ionization. AJR Am J Roentgenol. 1948;59:771.
5 Brennan JT, Phillips TL. Evaluation of past experience with fast neutron teletherapy and its implications for future applications. Eur J Cancer Clin Oncol. 1971;7:219.
6 Catterall M. The treatment of advanced cancer by fast neutrons from the Medical Research Council’s cyclotron at Hammersmith Hospital, London. Eur J Cancer Clin Oncol. 1974;10:343.
7 Catterall M, Bewley DK, Sutherland I. Second report on results of a randomized clinical trial of fast neutrons compared with X or gamma rays in treatment of advanced tumor of head and neck. Br Med J. 1977;1:1642.
9 Laramore GE, Krall JM, Griffin TW, et al. Neutron versus photon irradiation for unresectable salivary gland tumors. Final report of an RTOG-MRC randomized clinical trial. Int J Radiat Oncol Biol Phys. 1993;27:235.
11 Douglas JG, Lee S, Laramore GE, et al. Neutron radiotherapy for the treatment of locally-advanced major salivary gland tumors. Head Neck. 1999;21:255.
12 Douglas JG, Goodkin R, Laramore GE. Gamma knife stereotactic radiosurgical boost for salivary gland neoplasms with base of skull invasion following neutron radiotherapy. Head Neck. 2008;30:492.
13 Huber PE, Debus J, Latz D, et al. Radiotherapy for advanced adenoid cystic carcinoma. Neutrons, photons, or mixed beam? Radiother Oncol. 2001;59:161.
15 Laramore GE, Krall JM, Griffin TW, et al. Fast neutron radiotherapy for locally-advanced prostate cancer. Am J Clin Oncol (CCT). 1993;16:164.
16 Russell KJ, Caplan RJ, Laramore GE, et al. Photon versus fast neutron external beam radiotherapy in the treatment of locally advanced prostate cancer. Results of a randomized prospective trial. Int J Radiat Oncol Biol Phys. 1993;28:47.
17 Chuba PJ, Maughan R, Forman JD. Three dimensional conformal neutron radiotherapy for prostate cancer. Strahlenther Onkol. 1999;175:79.
19 Forman JD, Porter AT. The experience with neutron irradiation in locally advanced adenocarcinoma of the prostate. Semin Urol Oncol. 1997;15:239.
22 Scalliet PG, Remouchamps V, Lhoas F, et al. A retrospective analysis of the results of p(65) + Be neutron therapy for the treatment of prostate adenocarcinoma at the cyclotron of Louvain-la-Neuve. Part I. Survival and progression-free survival. Cancer Radiother. 2001;5:262.
23 Schwarz R, Krull A, Heyer D, et al. Present results of neutron therapy. The German experience. Acta Oncol. 1994;33:281.
25 Tsunemoto H, Morita S, Shimazaki J. Fast neutron therapy for carcinoma of the prostate. In: Karr JP, Yamanaka H, editors. Prostate cancer: the second tokyo symposium. New York: Elsevier; 1989:383-391.
26 Schwarz R, Krull A, Heyer D, et al. Neutron therapy in soft tissue sarcomas. A review of European results. Bull Cancer/Radiother. 1996;83(Suppl):110.
27 Schwartz DL, Einck J, Bellon J, Laramore GE. Fast neutron radiotherapy for soft tissue and cartilaginous sarcomas at high risk for local recurrence. Int J Radiat Oncol Biol Phys. 2001;50:449.
28 Fitzek MM, Thornton AF, Rabinov JD, et al. Accelerated fractionated proton/photon irradiation to 90 cobalt gray equivalent for glioblastoma multiforme. Results of a phase II prospective trial. Accelerated fractionated proton/photon irradiation to 90 cobalt gray equivalent for glioblastoma multiforme. Results of a phase II prospective trial. J Neurosurg. 1999;91:251.
30 Silbergeld DL, Rostomily RC, Alvord ECJr. The cause of death in patients with glioblastoma is multifactorial. Clinical factors and autopsy findings in 117 cases of supratentorial glioblastoma in adults. J Neurooncol. 1991;10:179.
31 Stelzer KJ, Douglas JG, Mankoff DA, et al. Positron emission tomography-guided conformal fast neutron therapy for glioblastoma multiforme. Neutro-Oncology. 2008;10:88.
32 Locher GL. Biological effects and therapeutic possibilities of neutrons. Am J Roentgenol Radium Ther. 1936;36:1.
33 Kruger PG. Some biological effects of biological disintegration products on neoplastic tissue. Proc Natl Acad Sci U S A. 1940;26:181.
34 Zahl PA, Cooper FS, Dunning JR. Some in vivo effects of localized nuclear disintegration products on a transplantable mouse sarcoma. Proc Natl Acad Sci U S A. 1940;26:589.
35 Moss RL. Review of reactor-based neutron beam development of BNCT applications. In: Soloway AH, Barth RE, Carpenter DE, editors. Advances in neutron capture therapy. New York: Plenum Press; 1993:1-7.
36 Farr LE, Sweet WH, Robertson JS, et al. Neutron capture therapy with boron in the treatment of glioblastoma multiforme. AJR Am J Roentgenol. 1954;71:279.
39 Brownell GL, Murray BW, Sweet WH, et al. A reassessment of neutron capture therapy in the treatment of cerebral gliomas. In: Seventh national cancer conference proceedings. Philadelphia: Lippincott; 1973:827-837.
40 Slatkin DN. A history of boron neutron capture therapy of brain tumors. Postulation of a brain radiation dose tolerance limit. Brain. 1991;114:1609.
41 Hatanaka H. Boron neutron capture therapy for tumors. Niigata, Japan: Nishimura Press; 1986.
42 Hatanaka H, Nakagawa N. Clinical results of long-surviving brain tumor patients who underwent boron neutron capture therapy. Int J Radiat Oncol Biol Phys. 1994;28:1061.
43 Nakagawa N. Recent study of boron neutron capture therapy for brain tumors. In: Nigg DW, Wiersema RJ, editors. Proceedings of the first international workshop on accelerator-based neutron sources for boron neutron capture therapy, Vol 1. U. S. Department of Energy Report CONF-940976; 1994:11-23.
44 Curran WJ, Scott CB, Horton J, et al. Recursive partitioning analysis of prognostic factors in three Radiation Therapy Group malignant glioma trials. J Natl Cancer Inst. 1993;85:704.
45 Laramore GE, Spence AM. Boron neutron capture therapy (BNCT) for high-grade gliomas of the brain. A cautionary note. Int J Radiat Oncol Biol Phys. 1996;36:241.
46 Kanda K. Experience of boron neutron capture therapy in Japan. In: Larsson B, Crawford JF, Higgs CE, et al, editors. Proceedings of the seventh international symposium on neutron capture therapy for cancer. Vol 1. Medicine and physics. Amsterdam: Elsevier Scientific; 1997:71-76.
47 Nakagawa Y, Kyonghon P, Kitamura K, et al. What were important factors in patients treated by BNCT in Japan? In: Larsson B, Crawford JF, Higgs CE, et al, editors. Proceedings of the seventh international symposium on neutron capture therapy for cancer. Vol 1. Medicine and physics. Amsterdam: Elsevier Scientific; 1997:65-70.
48 Sauerwein W, Rassow J, Mijnheer B. Considerations about specification and reporting of dose in BNCT. In: Larsson B, Crawford JF, Higgs CE, et al, editors. Proceedings of the seventh international symposium on neutron capture therapy for cancer. Vol 2. Chemistry and biology. Amsterdam: Elsevier Scientific; 1997:531-534.
49 Sauerwein W, Hideghety K, de Vries MJ, et al. Preliminary clinical results from the EORTC 11961 trial at the Petten irradiation facility. In: Program and abstracts. Osaka, Japan: Ninth International Symposium on Neutron Capture Therapy for Cancer; 2000:15-16.
51 Capala J, Stenstam BH, Skold K, et al. Boron neutron capture therapy for glioblastoma multiforme. Clinical studies in Sweden. J Neurooncol. 2003;62:1354.
54 Laramore GE, Wheeler FJ, Wessol DE, et al. A tumor control curve for malignant gliomas derived from fast neutron radiotherapy data. Implications for treatment delivery and compound selection. In: Larsson B, Crawford JF, Higgs CE, et al, editors. Proceedings of the seventh international symposium on neutron capture therapy for cancer. Vol 1. Medicine and physics. Amsterdam: Elsevier Scientific; 1997:580-587.
55 Diaz AZ. Assessment of the results from the phase I/II boron neutron capture therapy trials at the Brookhaven National Laboratory from a clinician’s point of view. J Neurooncol. 2003;62:101.
56 Coderre JA, Button TM, Micca PL, et al. Neutron capture therapy of the 9L rat gliosarcoma using the p-boronophenylalanine-fructose complex. Int J Radiat Oncol Biol Phys. 1994;30:643.
59 Mishima Y. Melanoma and non-melanoma neutron capture therapy using gene therapy: overview. In: Larsson B, Crawford JF, Higgs CE, et al, editors. Proceedings of the seventh international symposium on neutron capture therapy for cancer. Vol 1. Medicine and physics. Amsterdam: Elsevier Scientific; 1997:10-25.
60 Ichihasi I. Boron neutron capture therapy for malignant melanoma—retrospective and perspective of clinical trials. In: Program and abstracts. Osaka, Japan: Ninth International Symposium on Neutron Capture Therapy for Cancer; 2000:3-4.
61 Gonzalez SJ, Bonomi MR, Santa Cruz GA, et al. First BNCT treatment of a skin melanoma in Argentina. Dosimetric analysis and clinical outcome. Appl Radiat Isotop. 2004;61:1101.
62 Koivunoro H, Bleul DL, Nastasi U, et al. BNCT dose distribution in liver with epithermal D-D and D-T fusion based neutron beams. Appl Radiat Isot. 2004;61:853.
63 Chamberlin RL. Clinical research in neutron capture therapy. NCI Radiation Research Program meeting report. Int J Radiat Oncol Biol Phys. 2002;54:992.
64 Maruyama Y, van Nagell JR, Yoneda J, et al. A review of californium-252 brachytherapy for cervical cancer. Cancer. 1991;68:1189.
65 Tacev T, Ptackova B, Strnad V. Californium-252 (252Cf) versus conventional gamma radiation in the brachytherapy of advanced cervical carcinoma. Long-term treatment results of a randomized study. Strahlenther Onkol. 2003;179(6):377-384.
66 Patchell RA, Maruyama Y, Tibbs PA, et al. Neutron interstitial brachytherapy for malignant gliomas. A pilot study. J Neurosurg. 1988;68:67.
1 Batterman JJ, Breur K, Hare GAM, et al. Observations on pulmonary metastases in patients after single doses and multiple fractions of fast neutrons and cobalt-60 gamma rays. Eur J Cancer. 1981;17:539.
2 Hall EJ. Radiobiology for the radiologist, ed 4. Philadelphia: Lippincott; 1992. chapters 9 and 14
3 Laramore GE, Austin-Seymour MM. Fast neutron radiotherapy in relation to the radiation sensitivity of human organ systems. Adv Radiat Biol. 1992;15:153.
4 Stone RS. Neutron therapy and specific ionization. AJR Am J Roentgenol. 1948;59:771.
5 Brennan JT, Phillips TL. Evaluation of past experience with fast neutron teletherapy and its implications for future applications. Eur J Cancer Clin Oncol. 1971;7:219.
6 Catterall M. The treatment of advanced cancer by fast neutrons from the Medical Research Council’s cyclotron at Hammersmith Hospital, London. Eur J Cancer Clin Oncol. 1974;10:343.
7 Catterall M, Bewley DK, Sutherland I. Second report on results of a randomized clinical trial of fast neutrons compared with X or gamma rays in treatment of advanced tumor of head and neck. Br Med J. 1977;1:1642.
8 Catterall M, Sutherland I, Bewley DK. First results of a randomized clinical trial of fast neutrons compared with X or gamma rays in treatment of advanced tumors of the head and neck. Br Med J. 1975;2:653.
9 Laramore GE, Krall JM, Griffin TW, et al. Neutron versus photon irradiation for unresectable salivary gland tumors. Final report of an RTOG-MRC randomized clinical trial. Int J Radiat Oncol Biol Phys. 1993;27:235.
10 Douglas JG, Koh W, Austin-Seymour M, Laramore GE. Treatment of salivary gland neoplasms with fast neutron radiotherapy. Arch Otolaryngol Head Neck Surg. 2003;129:944.
11 Douglas JG, Lee S, Laramore GE, et al. Neutron radiotherapy for the treatment of locally-advanced major salivary gland tumors. Head Neck. 1999;21:255.
12 Douglas JG, Goodkin R, Laramore GE. Gamma knife stereotactic radiosurgical boost for salivary gland neoplasms with base of skull invasion following neutron radiotherapy. Head Neck. 2008;30:492.
13 Huber PE, Debus J, Latz D, et al. Radiotherapy for advanced adenoid cystic carcinoma. Neutrons, photons, or mixed beam? Radiother Oncol. 2001;59:161.
14 Breteau N, Wachter T, Kerdraon R, et al. Use of fast neutrons in the treatment of tumors of the salivary glands. Rationale, review of the literature and experience in Orleans. Cancer Radiother. 2000;4:181.
15 Laramore GE, Krall JM, Griffin TW, et al. Fast neutron radiotherapy for locally-advanced prostate cancer. Am J Clin Oncol (CCT). 1993;16:164.
16 Russell KJ, Caplan RJ, Laramore GE, et al. Photon versus fast neutron external beam radiotherapy in the treatment of locally advanced prostate cancer. Results of a randomized prospective trial. Int J Radiat Oncol Biol Phys. 1993;28:47.
17 Chuba PJ, Maughan R, Forman JD. Three dimensional conformal neutron radiotherapy for prostate cancer. Strahlenther Onkol. 1999;175:79.
18 Forman JD, Duclos M, Sharma R, et al. Conformal mixed neutron and photon irradiation in localized and locally advanced prostate cancer. Preliminary estimates of the therapeutic ratio. Int J Radiat Oncol Biol Phys. 1996;35:259.
19 Forman JD, Porter AT. The experience with neutron irradiation in locally advanced adenocarcinoma of the prostate. Semin Urol Oncol. 1997;15:239.
20 Haraf DJ, Rubin SJ, Sweeney P, et al. Photon neutron mixed-beam radiotherapy of locally advanced prostate cancer. Int J Radiat Oncol Biol Phys. 1995;33:3.
21 Maughan RL, Brambs B, Porter AT, et al. The cost-effectiveness of mixed beam neutron-photon radiation therapy in the treatment of adenocarcinoma of the prostate. Strahlenther Onkol. 1999;175:104.
22 Scalliet PG, Remouchamps V, Lhoas F, et al. A retrospective analysis of the results of p(65) + Be neutron therapy for the treatment of prostate adenocarcinoma at the cyclotron of Louvain-la-Neuve. Part I. Survival and progression-free survival. Cancer Radiother. 2001;5:262.
23 Schwarz R, Krull A, Heyer D, et al. Present results of neutron therapy. The German experience. Acta Oncol. 1994;33:281.
24 Fuse H, Katayama T, Akimoto S, et al. Radiotherapy of prostatic carcinoma. Hinyokika Kiyo. 1991;37:801. [in Japanese]
25 Tsunemoto H, Morita S, Shimazaki J. Fast neutron therapy for carcinoma of the prostate. In: Karr JP, Yamanaka H, editors. Prostate cancer: the second Tokyo symposium. New York: Elsevier; 1989:383-391.
26 Schwarz R, Krull A, Heyer D, et al. Neutron therapy in soft tissue sarcomas. A review of European results. Bull Cancer/Radiother. 1996;83(Suppl):110.
27 Schwartz DL, Einck J, Bellon J, Laramore GE. Fast neutron radiotherapy for soft tissue and cartilaginous sarcomas at high risk for local recurrence. Int J Radiat Oncol Biol Phys. 2001;50:449.
28 Fitzek MM, Thornton AF, Rabinov JD, et al. Accelerated fractionated proton/photon irradiation to 90 cobalt gray equivalent for glioblastoma multiforme. Results of a phase II prospective trial. Accelerated fractionated proton/photon irradiation to 90 cobalt gray equivalent for glioblastoma multiforme. Results of a phase II prospective trial. J Neurosurg. 1999;91:251.
29 Griffin BR, Berger MS, Laramore GE, et al. Neutron radiotherapy for malignant gliomas. Am J Clin Oncol. 1989;12:311.
30 Silbergeld DL, Rostomily RC, Alvord ECJr. The cause of death in patients with glioblastoma is multifactorial. Clinical factors and autopsy findings in 117 cases of supratentorial glioblastoma in adults. J Neurooncol. 1991;10:179.
31 Stelzer KJ, Douglas JG, Mankoff DA, et al. Positron emission tomography-guided conformal fast neutron therapy for glioblastoma multiforme. Neutro-Oncology. 2008;10:88.
32 Locher GL. Biological effects and therapeutic possibilities of neutrons. Am J Roentgenol Radium Ther. 1936;36:1.
33 Kruger PG. Some biological effects of biological disintegration products on neoplastic tissue. Proc Natl Acad Sci U S A. 1940;26:181.
34 Zahl PA, Cooper FS, Dunning JR. Some in vivo effects of localized nuclear disintegration products on a transplantable mouse sarcoma. Proc Natl Acad Sci U S A. 1940;26:589.
35 Moss RL. Review of reactor-based neutron beam development of BNCT applications. In: Soloway AH, Barth RE, Carpenter DE, editors. Advances in neutron capture therapy. New York: Plenum Press; 1993:1-7.
36 Farr LE, Sweet WH, Robertson JS, et al. Neutron capture therapy with boron in the treatment of glioblastoma multiforme. AJR Am J Roentgenol. 1954;71:279.
37 Godwin JT, Farr LE, Sweet WH, et al. Pathological study of eight patients with glioblastoma multiforme treated by neutron capture therapy using boron 10. Cancer. 1956;8:601.
38 Asbury AK, Ojemann R, Nielsen SL, et al. Neuropathologic study of fourteen cases of malignant brain tumors treated by boron-10 slow neutron capture therapy. J Neuropathol Exp Neurol. 1972;31:278.
39 Brownell GL, Murray BW, Sweet WH, et al. A reassessment of neutron capture therapy in the treatment of cerebral gliomas. In: Seventh national cancer conference proceedings. Philadelphia: Lippincott; 1973:827-837.
40 Slatkin DN. A history of boron neutron capture therapy of brain tumors. Postulation of a brain radiation dose tolerance limit. Brain. 1991;114:1609.
41 Hatanaka H. Boron neutron capture therapy for tumors. Niigata, Japan: Nishimura Press; 1986.
42 Hatanaka H, Nakagawa N. Clinical results of long-surviving brain tumor patients who underwent boron neutron capture therapy. Int J Radiat Oncol Biol Phys. 1994;28:1061.
43 Nakagawa N. Recent study of boron neutron capture therapy for brain tumors. In: Nigg DW, Wiersema RJ, editors. Proceedings of the first international workshop on accelerator-based neutron sources for boron neutron capture therapy, Vol 1. U. S. Department of Energy Report CONF-940976; 1994:11-23.
44 Curran WJ, Scott CB, Horton J, et al. Recursive partitioning analysis of prognostic factors in three Radiation Therapy Group malignant glioma trials. J Natl Cancer Inst. 1993;85:704.
45 Laramore GE, Spence AM. Boron neutron capture therapy (BNCT) for high-grade gliomas of the brain. A cautionary note. Int J Radiat Oncol Biol Phys. 1996;36:241.
46 Kanda K. Experience of boron neutron capture therapy in Japan. In: Larsson B, Crawford JF, Higgs CE, et al, editors. Proceedings of the seventh international symposium on neutron capture therapy for cancer. Vol 1. Medicine and physics. Amsterdam: Elsevier Scientific; 1997:71-76.
47 Nakagawa Y, Kyonghon P, Kitamura K, et al. What were important factors in patients treated by BNCT in Japan? In: Larsson B, Crawford JF, Higgs CE, et al, editors. Proceedings of the seventh international symposium on neutron capture therapy for cancer. Vol 1. Medicine and physics. Amsterdam: Elsevier Scientific; 1997:65-70.
48 Sauerwein W, Rassow J, Mijnheer B. Considerations about specification and reporting of dose in BNCT. In: Larsson B, Crawford JF, Higgs CE, et al, editors. Proceedings of the seventh international symposium on neutron capture therapy for cancer. Vol 2. Chemistry and biology. Amsterdam: Elsevier Scientific; 1997:531-534.
49 Sauerwein W, Hideghety K, de Vries MJ, et al. Preliminary clinical results from the EORTC 11961 trial at the Petten irradiation facility. In: Program and abstracts. Osaka, Japan: Ninth International Symposium on Neutron Capture Therapy for Cancer; 2000:15-16.
50 Kankaanranta L, Seppala T, Kallie M, et al. First clinical results on the Finnish study on a BPA-medicated BNCT in glioblastoma. In: Program and abstracts. Osaka, Japan: Ninth International Symposium on Neutron Capture Therapy for Cancer; 2000:31-32.
51 Capala J, Stenstam BH, Skold K, et al. Boron neutron capture therapy for glioblastoma multiforme. Clinical studies in Sweden. J Neurooncol. 2003;62:1354.
52 Honova H, Safanda M, Petruzelka L, et al. Neutron capture therapy in the treatment of glioblastoma multiforme. Initial experience in the Czech Republic. Cas Lek Cesk. 2004;143:44.
53 Busse PM, Harling OK, Palmer MR, et al. A phase-I clinical trial for cranial BNCT at Harvard-MIT. In: Program and abstracts. Osaka, Japan: Ninth International Symposium on Neutron Capture Therapy for Cancer; 2000:27-28.
54 Laramore GE, Wheeler FJ, Wessol DE, et al. A tumor control curve for malignant gliomas derived from fast neutron radiotherapy data. Implications for treatment delivery and compound selection. In: Larsson B, Crawford JF, Higgs CE, et al, editors. proceedings of the seventh international symposium on neutron capture therapy for cancer. Vol 1. Medicine and physics. Amsterdam: Elsevier Scientific; 1997:580-587.
55 Diaz AZ. Assessment of the results from the phase I/II boron neutron capture therapy trials at the Brookhaven National Laboratory from a clinician’s point of view. J Neurooncol. 2003;62:101.
56 Coderre JA, Button TM, Micca PL, et al. Neutron capture therapy of the 9L rat gliosarcoma using the p-boronophenylalanine-fructose complex. Int J Radiat Oncol Biol Phys. 1994;30:643.
57 Mishima Y, Honda C, Ichihashi M, et al. Treatment of malignant melanoma by single neutron capture treatment with melanoma-seeking +10B-compound. Lancet. 1989;11:388.
58 Mishima Y, Honda C, Ichihashi M, et al. Selective melanoma thermal neutron capture therapy for lymph node metastases. In: Soloway AH, Barth RF, Carpenter DE, editors. Advances in neutron capture therapy. New York: Plenum Press; 1993:705-710.
59 Mishima Y. Melanoma and non-melanoma neutron capture therapy using gene therapy: overview. In: Larsson B, Crawford JF, Higgs CE, et al, editors. Proceedings of the seventh international symposium on neutron capture therapy for cancer. Vol 1. Medicine and physics. Amsterdam: Elsevier Scientific; 1997:10-25.
60 Ichihasi I. Boron neutron capture therapy for malignant melanoma—retrospective and perspective of clinical trials. In: Program and abstracts. Osaka, Japan: Ninth International Symposium on Neutron Capture Therapy for Cancer; 2000:3-4.
61 Gonzalez SJ, Bonomi MR, Santa Cruz GA, et al. First BNCT treatment of a skin melanoma in Argentina. Dosimetric analysis and clinical outcome. Appl Radiat Isotop. 2004;61:1101.
62 Koivunoro H, Bleul DL, Nastasi U, et al. BNCT dose distribution in liver with epithermal D-D and D-T fusion based neutron beams. Appl Radiat Isot. 2004;61:853.
63 Chamberlin RL. Clinical research in neutron capture therapy. NCI Radiation Research Program meeting report. Int J Radiat Oncol Biol Phys. 2002;54:992.
64 Maruyama Y, van Nagell JR, Yoneda J, et al. A review of californium-252 brachytherapy for cervical cancer. Cancer. 1991;68:1189.
65 Tacev T, Ptácková B, Strnad V. Californium-252 (252Cf) versus conventional gamma radiation in the brachytherapy of advanced cervical carcinoma. Long-term treatment results of a randomized study. Strahlenther Onkol. 2003;179(6):377-384.
66 Patchell RA, Maruyama Y, Tibbs PA, et al. Neutron interstitial brachytherapy for malignant gliomas. A pilot study. J Neurosurg. 1988;68:67.