12
Neurovascular Healing and Thromboembolic Disease
2. Discuss the vascular supply to nerve tissue.
3. Understand the mechanical behavior of nerve tissue.
4. Identify the causes and classification of nerve injury.
5. Discuss intrinsic nerve healing.
6. Describe methods of surgical repair of nerve injury.
7. Identify structure and composition of vascular tissue.
8. Discuss the vascular response to injury.
9. Explain the various signs and symptoms of vascular injury.
10. Discuss the pathophysiology of thromboembolic disease.
11. Recognize risk factors of deep vein thrombosis and pulmonary emboli.
PERIPHERAL NERVE INJURY
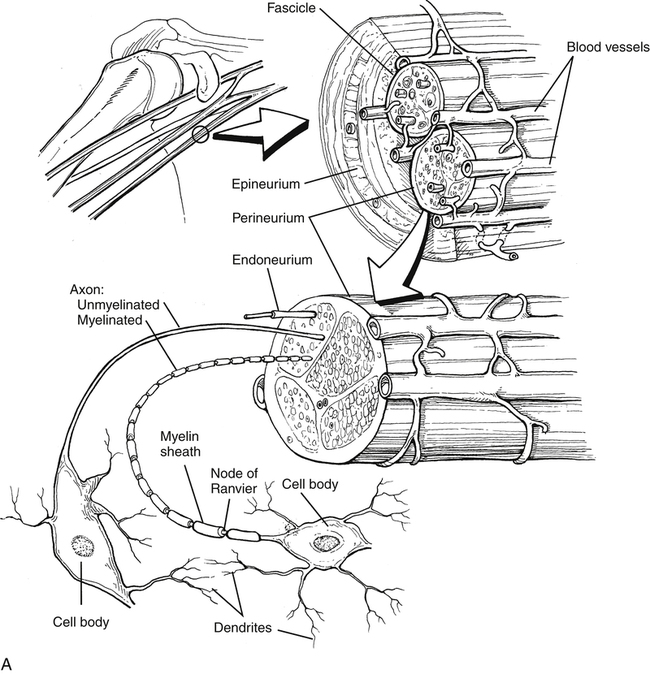
Vascular Supply
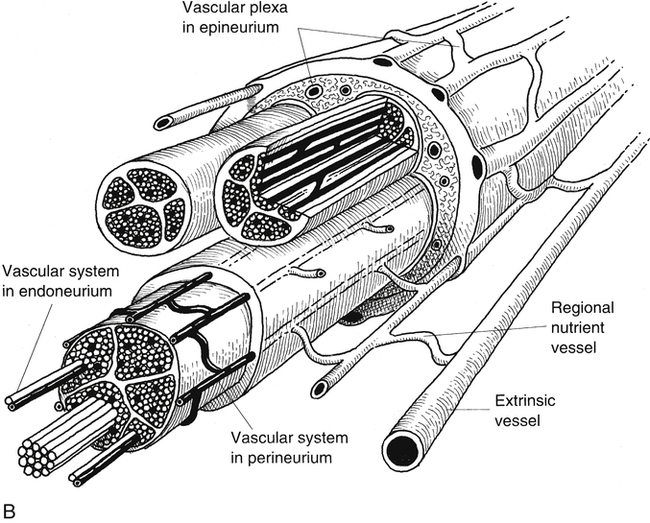
Peripheral nerve has a complex and extensive blood supply. The PNS requires an ongoing nutritive energy supply for maintenance of nerve conduction. Longitudinal extrinsic vessels connect with regional feeding vessels that form a vascular plexus within the epineurium, perineurium, and endoneurium connective tissue network within the nerve fiber (Fig. 12-1).2,9
The initial response to nerve trauma is a predictable response like that seen after vascular tissue trauma. After a brief period of vasoconstriction, vascular permeability increases because of the release of potent chemical mediators such as serotonin and histamine.11 The result is edema within the nerve fiber’s connective tissue barriers (epineurium, perineurium, endoneurium). The dramatic change in fluid pressure and tissue edema adversely affects oxygen transport, nutrition, ion content of nerve cells, and conductivity of the traumatized nerve fiber.9
Mechanical Behavior of Nerve Tissue
Nerve tissue is highly deformable, expressing relatively similar viscoelastic mechanical behavior as other soft tissue. Essentially, two load deformation terms are used to quantify a tissue’s ability to adapt structurally and mechanically to time-dependent forces. Creep is a term used to describe the tissue’s ability to change or “creep” to a new length in response to a constant, applied load. The greater the load (stress), the faster the tissue will deform or creep. Stress relaxation is similar to creep, in that it is a time-dependent phenomenon. It occurs when a material is elongated (strained) to a given dimension and then maintained at that length. In this situation, there is a reduction in the amount of stress required to maintain the fixed length. Peripheral nerve tissue responds with this viscoelastic behavior by showing ultimate load-to-failure values of 20% to 60%. Although peripheral nerve tissue may tear when the nerve is elongated to approximately 20% more than its resting length, ischemic changes, which profoundly affect nerve function, may occur when a nerve is stretched less than or equal to 15% of its resting length.1,12
Causes and Classification of Nerve Injuries
Trauma to peripheral nerve comes from mechanical, thermal, chemical, and vascular injury. Mechanical sources cause contusion, concussion, stretch, compression, laceration, and transection.8,9 Classification of nerve injury provides concise and anatomic descriptions. However, the clinical reliability of this system is debatable.1,6,9 Many injuries cannot be classified into a single grade. The three most common categories of nerve injury are neurapraxia, axonotmesis, and neurotmesis.6,8,9
Neurapraxia is the reduction in nerve conduction at the site of injury, usually due to compression. The lesion is local, the axon’s continuity is maintained, and all pathologic changes associated with neurapraxia generally are reversible if the cause is removed.6,8,9 Functional recovery occurs within weeks or months.9
In axonotmesis, the epineurium remains intact, while damage to the perineurium and endoneurium occurs to varying degrees. Because the epineurium is undamaged, functional recovery without surgery may occur. However, as greater amounts of perineurium and endoneurium become involved, surgery is required to achieve the most functional recovery.9
Neurotmesis is diagnosed when the entire nerve trunk is transected or ruptured. The total loss of nerve continuity requires surgical adaptation and coaptation. Prognosis depends on the nature of the injury, as well as local and general factors, such as patient age and timing of the repair.1,6
In a very broad sense, a pure motor nerve is a greater risk for injury than a pure sensory nerve. A gross prediction is that peripheral nerves usually fail to conduct impulses related to motion (first), proprioception, touch, temperature and pain (last). Recovery of these abilities occurs in the reverse order.1,8
Compression and Traction Neuropathy
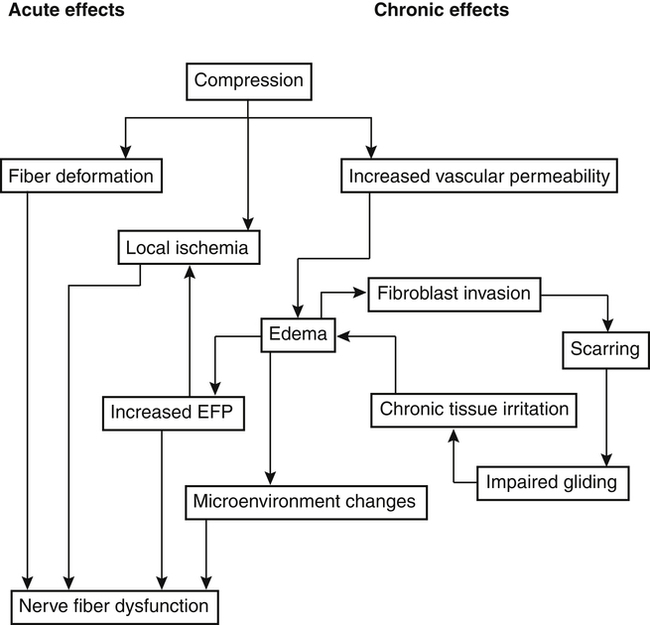
Nerve compression injuries are acute or chronic in origin. In both cases, the physiologic consequence of compression or traction on peripheral nerve tissue is mechanical disruption of the nerve fiber and ischemia (Fig. 12-2).9 Anatomically, certain peripheral nerves are at risk for compression neuropathy because of the surrounding arrangement of soft tissue and bone, which limits the nerve’s three-dimensional motion. Specifically at risk in the lower extremity are the common peroneal nerve behind the fibular head and the lateral femoral cutaneous nerve within the inguinal ligament (meralgia paresthetica).1 In the upper extremity, nerves most susceptible to mechanical disruption are the radial nerve within the spiral groove of the humerus, and the median nerve within the soft tissue confines of the carpal tunnel arch. Spinal nerve roots are more susceptible to compression injury than peripheral nerves because spinal nerve roots have no epineurium.9
The biological responses of the PNS to acute and chronic compression include obstruction of intraneural blood vessels, tissue anoxia, local ischemia, nerve fiber deformation, increased vascular permeability, intraneural edema, and fibroblastic proliferation with resultant decreased nerve gliding.9
Traction or stretch neuropathy is classified as acute or chronic with the magnitude of injury classified as neurapraxia, axonotmesis, and neurotmesis as described. A practical and common description of acute traction neurapraxia is the “burner” or “stinger” experienced by athletes, in which the shoulder is depressed with concomitant contralateral head and neck flexion that creates a brachial plexus stretch.2
Occasionally with lengthy surgical procedures, prolonged wide surgical field exposure leads to inadvertent stretch of surrounding peripheral nerve tissue. Surgical procedures may also require prolonged tourniquet application, with cuff pressures occluding neural blood supply.8,9
Physical therapy (e.g., range of motion [ROM] exercise) after prolonged joint immobilization may cause traction neurapraxia due to premature and intensive stretch. During the protection phase of postoperative care or during prolonged immobilization, slow, controlled stretch, devoid of high velocity or force protects nerve from unwanted injury.8,12
Some patients may experiences signs and symptoms of peripheral nerve compression or entrapment at more than one level of the same nerve. The term double crush syndrome is used to describe these signs and symptoms. The most common example is carpal tunnel median nerve compression neuropathy and cervical nerve root injury. Another example is nerve entrapment neuropathy at the elbow along with cervicothoracic root lesion. To explain this syndrome, it is hypothesized that a compression lesion at one level of the nerve makes the same nerve more susceptible to injury at another site.1,9 It is suggested that compression reduces nerve conduction, blood supply, and the amount of plasma membrane proteins. These reductions adversely influence other segments, making them more sensitive to mechanical or compressive forces.9
Methods of Peripheral Nerve Repair
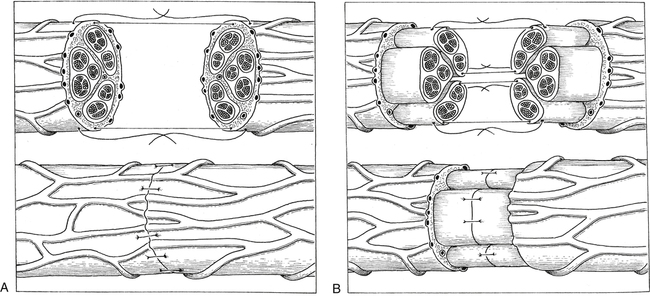
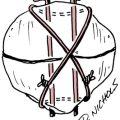
The term neurorrhaphy is synonymous with direct coaptation or surgical apposition of corresponding nerve stumps or fascicles.8,9 This specific intervention is reserved for neurotmesis with complete disruption of nerve continuity. The basic objectives for surgical repair (neurorrhaphy) are to maximize the number of axons that regenerate across the lesion and to reinnervate distal sites accurately, for example, that proximal motor axons reach distal motor axons (Fig. 12-3).1,8,9
Skeletal stability and a well-vascularized tissue bed are essential for effective, direct coaptation. The procedure requires that appropriate tension be applied at the injury site.8,9 Animal studies show that repairs subject to minimal tension produce better results than tension-free repairs.8,9 Overall, four steps are identified as prerequisites for direct coaptation: clean preparation of traumatized nerve stumps, manual approximation of tissue stumps with correct tension, direct connection between nerve fascicles at both nerve endings or interposing nerve graft, and maintenance of coaptation with sutures, fibrin glue, or interstitial clot.8,9
When primary repair cannot be achieved without undue tension on nerve endings, autografts may be used for indirect coaptation. The most common autograft source for peripheral nerve repair is the sural nerve.6,8,9 Other sources are the anterior branch of the medial antebrachial cutaneous nerve, the lateral femoral cutaneous nerve, and the superficial radial nerve. The use of autografts must be carefully weighed because their use sacrifices healthy nerves and puts the donor site at risk for morbidity and local disturbances of sensory function.9
Without repair, denervated tissues undergo many changes. Denervated bone becomes osteoporotic, joint capsule and periarticular soft tissues become fibrotic, and muscle atrophies leading to decreased muscle volume. During the first month after unrepaired nerve injury, muscle loses up to 30% of its weight, and approximately 60% is lost by the end of the second month of denervation.8
Currently, good to excellent results can be expected in approximately 50% of surgically repaired peripheral nerves. A few generalizations can be made concerning reconstruction. Better results occur in younger patients, patients with prompt repairs, more distal repairs, and those with shorter grafts.8,9
Recovery after Peripheral Nerve Injury and Repair
Repair site protection is the most important factor after peripheral nerve coaptation or autograft. Overzealous ROM exercise during early recovery can easily disrupt fragile healing of fascicle sutures and axonal regeneration. Typically, postoperative splinting is used to maintain appropriate tension and mechanical properties of the repaired nerve.8,12 Compressive dressings help to decrease venous congestion and edema. ROM exercises, used cautiously, encourage venous return, muscle activation, and lymphatic flow, and reduce the potential for muscle and connective tissue adhesions.8,12 Vascular supply to the healing and repaired nerve tissue must be continuously enhanced to influence axonal regeneration. Superficial heat application may encourage peripheral vascular blood flow to the healing tissues. Electrical stimulation may be of some benefit to initiate muscle contraction, increase blood flow, and stimulate removal of cellular debris.9 Neuromuscular and sensory reeducation exercise are initiated early and progressed continuously during all phases of peripheral nerve recovery after injury, with or without surgical repair. Continuous stimulation of distal target sites of reinnervation is essential to redirect axonal regeneration and to facilitate reeducation. Various sensory stimulation tactics are used to decrease hypersensitivity. Reintroduction of light touch, pressure, vibration, thermal stimuli, texture, and stimulation of the mechanoreceptor system (proprioception and kinesthesia) are critical components of functional recovery of nerve injury or repair.8
Not only do changes occur at the site of injury and distal to it, but central changes also occur. The somatosensory cortex, even in adults, is rapidly altered after injury. Therefore the correct amount of sensory input from the affected area is necessary to reexpand cortical representation from the injured extremity. Animal studies show that the injured part needs repeated and substantial practice of functional movements to, perhaps, induce expansion of the contralateral cortical area that controls movement of the affected extremity.9
Assessment of Functional Recovery
To document the quality and quantity of motor and sensory nerve recovery after injury or surgical repair, an objective grading system is used to determine the level of functional recovery. The Mechanical Research Council Grading System is used for continued assessment of both motor and sensory recovery.8,9 Motor recovery is graded M0 through M5, and sensory recovery S0 through S4. A parallel analogy is manual muscle strength testing:
With an excellent result after nerve repair, a grade of M5, S4 describes full motor reinnervation and complete sensory recovery. A good result is described as M3, S3; a fair result is M2, S2; and a poor result is M0 to M1, S0 to S1.
By establishing objective documentation relating to functional motor and sensory nerve recovery after injury or surgical repair, appropriate physical therapy interventions can be explored to stimulate, regulate, or enhance specific motor or sensory functions. Patients should be encouraged to identify daily stresses that fall under a continuum of low to extreme stress on the injured or repaired extremity. Through activity modification, education, and physical therapy intervention, the patient may be able to achieve a better balance of stresses and improve functional recovery or prevent further injury or reinjury.12
VASCULAR INJURY
Structure and Composition
Grossly, vessels consist of three basic components: endothelial cells, smooth muscles, and connective tissue. Arteries and veins consist of three identifiable concentric layers (lamina) that form the lumen or “walls.” The innermost layer is the tunica intima (interna), consisting of connective tissue, endothelial cells, and a basement membrane. The middle layer is the tunica media, composed of smooth muscle cells and connective tissue. The outermost layer is the tunica adventitia (externa), made up of fibrous connective tissue that blends with loose surrounding connective tissue. Between the tunica media and externa is an external elastic membrane (elastic externa). The internal elastic lamina (elastica interna) lies between the internal and intermediate tunicae. The outermost walls of large vessels contain their own microvascular system. Nerves of the sympathetic nervous system innervate the arterial tunicae.11
Although veins contain the same three layers of tunicae, the relative percentage of smooth muscle and elastic components is less than that of arteries. In addition, the amount of connective tissue in veins is much greater than in arteries. These anatomic distinctions contribute to the vasomechanical differences between the highly elastic muscular arteries and the relatively less elastic veins. The biochemical composition within the vascular system varies greatly depending on regional requirements. Generally, the peripheral vascular system is composed of extracellular matrix, collagens, elastin, proteoglycans, squamous epithelial (endothelial), and smooth muscle cells.11
Vascular Response to Injury
As with other types of soft tissue, injuries to vessels incite an organized, predictable inflammatory response with distinct overlapping sequential events: coagulation, inflammation, fibroplasia, and remodeling and maturation. The most significant histochemical event in the vascular response is intimal hyperplasia, where smooth muscle cells proliferate after arterial injury. Depending on the nature and severity of injury, the cell proliferation of the damaged lumen may actual thicken vessel walls, reducing blood flow.11
Mechanisms of Injury
Vascular injury from traction, avulsion, compression or penetration can occur during orthopedic surgery (Table 12-1). Total knee arthroplasty (TKA) poses a risk to the popliteal artery as well. Total hip arthroplasty may put the common or external iliac artery at risk for injury. Identifiable arterial injury can occur because of shoulder dislocation and humeral neck fractures, resulting in axillary artery injury. Supracondylar humeral fractures and elbow dislocation can lead to brachial artery injury. Posterior knee dislocation and supracondylar femoral fracture can result in popliteal artery injury (Table 12-2).4
Table 12-1
Arterial Injuries Associated with Orthopedic Operative Procedures
| Body Area | Orthopedic Procedure | Artery Injured |
| Upper extremity | Clavicular compression plate/screw | Subclavian artery |
| Anterior approach to the shoulder | Axillary artery | |
| Closed reduction humeral fracture | Brachial artery | |
| Lower extremity | Total hip arthroplasty | Common or external iliac artery |
| Nail or nail-plate fixation of intertrochanteric or subtrochanteric hip fracture | Profunda femoris artery | |
| Subtrochanteric osteotomy | Profunda femoris artery | |
| Total knee arthroplasty | Popliteal artery | |
| Anterior or posterior cruciate reconstruction | Popliteal artery | |
| External fixator pin | Superficial femoral, profunda femoris, popliteal, or tibial arteries | |
| Spine | Anterior spinal fusion | Abdominal aorta |
| Lumbar spine fixation device | Abdominal aorta | |
| Resection of nucleus pulposus | Right common iliac artery and vein, inferior vena cava |
From Browner BD, Jupiter JB, Levine AM, et al: Skeletal trauma, ed 3, Philadelphia, 2003, Saunders.
Table 12-2
Arterial Injuries Associated with Fractures and Dislocations
| Body Area | Fracture or Dislocation | Artery Injured |
| Upper extremity | Fracture of clavicle or first rib | Subclavian artery |
| Anterior dislocation of shoulder | Axillary artery | |
| Fracture of neck of humerus | Axillary artery | |
| Fracture of shaft or supracondylar area of humerus | Brachial artery | |
| Dislocation of elbow | Brachial artery | |
| Lower extremity | Fracture of shaft of femur | Superficial femoral artery |
| Fracture of supracondylar area of femur | Popliteal artery | |
| Posterior dislocation of the knee | Popliteal artery | |
| Fracture of proximal tibia or fibula | Popliteal artery, tibioperoneal trunk, tibial artery, or peroneal artery | |
| Fracture of distal tibia or fibula | Tibial or peroneal artery |
From Browner BD, Jupiter JB, Levine AM, et al: Skeletal trauma, ed 3, Philadelphia, 2003, Saunders.
Signs and Symptoms of Vascular Injury
General signs and symptoms of peripheral artery injury are classified either as “hard” or “soft.” Hard signs and symptoms include the traditional indications of pulselessness, pallor, paresthesias, pain, and paralysis. Overt signs of bleeding and a rapidly spreading hematoma are additional signs that warrant immediate surgical repair.4
Soft signs include a possible history of arterial bleeding, hematoma over a peripheral artery, and a neurological deficit originating in a nerve adjacent to the injured artery. After specific fractures, dislocation, and selected surgical procedures (e.g., glenohumeral or posterior knee dislocation, TKA), continuous reassessment of peripheral pulses (radial or dorsalis pedis) is essential to identify potential vascular injury. Also routinely assessed are skin color, temperature, presence of edema, and digital capillary refill.4
Diagnostic Studies
Noninvasive diagnosis of arterial occlusion uses Doppler flow detection or duplex ultrasonography. The latter is a combination of ultrasound imaging and pulsed Doppler flow detection. With Doppler flow, a comparison is made with the noninvolved extremity. In this way, an arterial pressure index (API) is calculated by dividing the Doppler systolic pressure of the involved extremity by that of the noninvolved extremity. Arterial injury can be predicted with 97% accuracy when the API is lower than 0.90.3 Percutaneous arteriography is the gold standard and most commonly used invasive technique for diagnosis of suspected arterial injury. This technique requires injection of dye proximal (antegrade) or distal (retrograde) to the suspected injury, then multiple sequential plain film radiographs are taken of the area.10
Methods of Vascular Repair
An analogy of repair techniques can be drawn from peripheral nerve repair. Generally, direct vascular repair, including arteriorrhaphy or venorrhaphy or anastomosis, involves direct suturing of the traumatized vessel. Interpositional grafting uses an autograph or synthetic graft material (polytetrafluoroethylene [PTFE]) or Dacron).10
THROMBOEMBOLIC DISEASE
Thromboembolic disease, caused by a deep vein thrombosis (DVT) that may propagate to pulmonary embolism (PE), is one of the most common causes of mortality and morbidity in hospitalized patients. Hip and knee arthroplasties place patients at increased risk of developing thromboembolic disease. The probability of a TKA patient developing DVT, without additional risk factors or prophylactic anticoagulation, is between 40% and 70%.7
Risk Factors
Several intrinsic and extrinsic conditions are identified as additive risk factors in the potential development of thromboembolic disease. Surgery, trauma, obesity, pregnancy, age older than 40 years, use of oral contraceptives, and immobility are well-known causative factors. In addition, history of DVT and varicose veins, smoking, family history, and congestive heart failure contribute as risk factors for DVT development. The type of surgery has little effect on the incidence of DVT. Rather, it is other factors, such as heart disease and length of immobilization, that determine the incidence.10
Pathophysiology of Thromboembolism
Most thrombi start in the valve cusps of the deep lower leg veins. Critical components of the coagulation cascade are prothrombin, thrombin, fibronectin, and fibrin. The result of this cascade is stimulation of platelet adherence to vessel walls. Eventually, this buildup of blood cells creates a thrombus. A dislodged clot from the thrombus is referred to as an embolus. PE is a life-threatening consequence in which the lower lobes of the lungs are involved four times more often than the upper lobes.11
Virchow Triad
Three factors generally lead to DVT development. Categorically referred to as Virchow triad, the factors are hypercoagulability, venous stasis (caused by immobilization, obesity, heart disease), and venous injury, especially with endothelial damage (caused by surgery, trauma, and previous DVTs). Damage exposes the vessel walls to collagen and basement membrane, and von Willebrand factor (vWF) increases platelet activity and number. The initial trauma shifts the balance more toward coagulation than fibrinolysis and ultimately DVT formation.10,11
Vessel wall injury and stasis are directly related to orthopedic surgical procedures, such as total joint arthroplasty (TJA), and repair of pelvic and femoral fractures. Once a DVT has formed, without treatment, one of three processes occurs. The thrombus undergoes partial or complete lysis with complete or near-complete recannulization of the thrombosed blood vessel. The thrombus becomes more organized, resulting in further proximal vessel occlusion. The thrombus dislodges, completely or partially escaping to a proximal site in the vascular system as an embolus.10
Signs and Symptoms of Deep Vein Thrombosis
Very high levels of suspicion must accompany all complaints of proximal thigh, inguinal and lower leg pain after TJA, pelvic or femoral fractures, spinal surgery, and general trauma. Some signs and symptoms are nonspecific, such as diffuse complaints of leg pain and tenderness. Others are more specific, including edema, palpable warmth, skin discoloration, prominent superficial veins, and presence of Homans sign (pain in the calf upon ankle dorsiflexion). Approximately 50% of DVT cases are asymptomatic. Both extremities require assessment because studies show that thrombosis is generally present bilaterally, even when injury is limited to only one lower extremity.10
Diagnostic Studies
Duplex ultrasonography is a common noninvasive diagnostic technique for suspected DVT. This test combines venous system Doppler flow detection and ultrasound imaging with manual compression of the suspected vein. Veins that do not easily compress with normal pressure on the transducer are considered positive for DVT. Clinical probability estimates of physical examination alone in the presence of Homans sign, edema, and palpable firmness are approximately 50% for incidence of DVT. Generally, thrombi in the inguinal area, deep proximal thigh, and popliteal area are considered more dangerous than deep calf vein thrombi, because distal clots are smaller and less frequently associated with major complications.10
Pulmonary Emboli
Pulmonary emboli are a result and complication of DVT. A dislocated deep vein thrombus may travel to the pulmonary artery or obstruct pulmonary blood supply. The result is hypoxia from constriction of the bronchioles, mediated by vasoactive substances such as serotonin, histamine, and prostaglandins. The effect is pulmonary infarction, shock, right heart failure, and occasionally, death.5,10
Signs and Symptoms
Generally, tachypnea (>18 breaths/minute) is the most common sign of PE. Dyspnea is the most frequent symptom. The patient may also complain of pleuritic pain, develop a cough or hemoptysis, or report feeling apprehensive. These signs and symptoms are usually related to the size of the embolus and the patient’s cardiopulmonary status.5,10
Treatment of Thromboembolic Disease
Selective use of prophylactic anticoagulants decreases the incidence of DVT in elective surgery patients without altering the operative plan. Patients undergoing TJA are treated with heparin, or low-molecular-weight heparin (LMWH). The judicious use of LMWH has proven to be both safe and effective in DVT prevention and treatment. The patient’s response to LMWH is more predictable than the response to heparin, and the rate of bleeding complications is less than with heparin.10
If prophylactic anticoagulants are not administered preoperatively, administration of anticoagulation medication begins 12 to 24 hours postoperatively. Warfarin (Coumadin), an oral anticoagulant, is used simultaneously with heparin or during the transition from intravenous or subcutaneous anticoagulation. Coumadin is indicated for prophylaxis and treatment of DVT or PE. The duration of therapy may be 3 to 6 months postoperatively, depending on patient’s risk factors and possibility of recurrence.5,10
Nonpharmacologic treatment of DVT includes early mobilization and judicious exercise after surgery, use of antiembolism stockings, or intermittent pneumatic compression. Contraindications for use of antiembolism stockings are arterial compromise or peripheral neuropathy. Use of pneumatic compression devices is inadvisable in the presence of local ulceration, cellulitis, and arterial insufficiency. The bent knee and semisitting positions increase the incidence of postoperative thromboembolic complications. Patients should be as mobile as possible as early as possible after their surgery.5