Neurotrophic Keratopathy
Introduction
The cornea is one of the most richly innervated organs. Corneal innervation not only provides sensation but is integral in the maintenance of the structure and function of the cornea. Normal innervation helps the cornea to regulate epithelial integrity, proliferation and wound healing. Neurotrophic keratopathy (NK) is a degenerative condition of the cornea, characterized by lack of or decreased corneal sensation. This results in increased susceptibility of the corneal surface to injury and compromised healing. In severe cases this can lead to corneal ulceration, stromal melt and perforation. The cornea is supplied by the long ciliary nerve, derived via the nasociliary nerve from the ophthalmic branch of the trigeminal nerve. Any ocular, localized or systemic condition affecting the nerve function along this course can create corneal anesthesia, resulting in NK (Box 27.1).1 Management of NK can be formidable, with the aim of therapy being prevention of disease progression, preservation of globe integrity and promotion of ocular surface repair.
Pathogenesis
Most of the corneal nerves are derived from the ophthalmic division of the trigeminal nerve, via the anterior ciliary nerves and to a lesser degree from the maxillary nerve. The limbus and the peripheral cornea also receive autonomic sympathetic innervation from the superior cervical ganglion.2
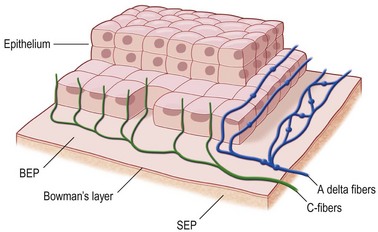
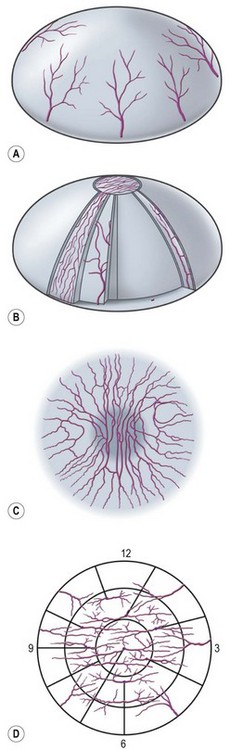
Nerves enter the cornea in the middle third of the stroma and run forward anteriorly in a radial fashion towards the center where they lose their myelin sheath approximately 1 mm from the corneal limbus, giving rise to branches that innervate the anterior and mid-stromal layers. In the interface between Bowman’s layer and the anterior stroma, the stromal nerves form the subepithelial nerve plexus. They perforate Bowman’s layer and form the sub-basal epithelial nerve plexus, providing innervation to the basal epithelial cell layer, to terminate finally within the superficial epithelial layers (Fig. 27.1).3 Thin branches of the subepithelial plexus ascend and penetrate Bowman’s layer, bending almost at a right angle to form the sub-basal nerve plexus at the basal epithelial cell layer (Fig. 27.2).4
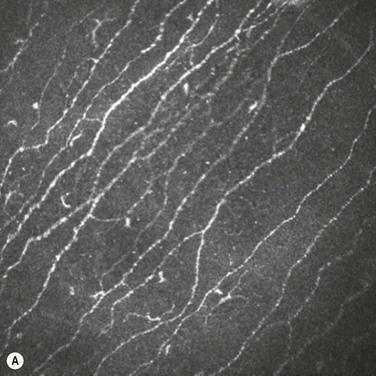
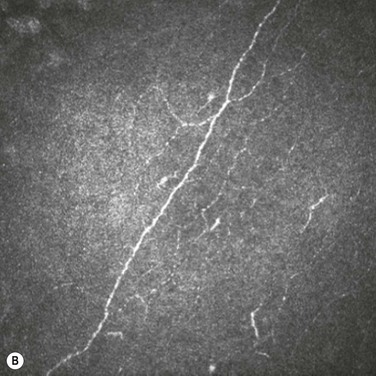
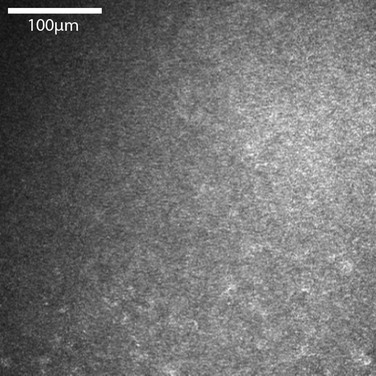
In vivo confocal microscopy has emerged as a powerful tool to image the corneal cellular structure, including the corneal nerves (Fig. 27.3). The density of the sub-basal plexus varies depending on the method of examination and method of analysis. However, when compared to normal controls, the density of the plexus is reduced in NK and several predisposing disease states, including diabetes mellitus and viral keratitis (Fig. 27.4 and Fig. 27.5).5
Alteration in the density of sub-basal nerves has been shown to alter the concentration of several neuromediators and growth factors on the ocular surface.6,7 These neuromediators contribute to the homeostatic cycle that maintains a healthy ocular epithelial surface, in addition to altering tear production rate. These include substance P, calcitronin gene-related peptide, neuropeptide Y, vasoactive intestinal peptide, galanin, methionine-enkephalin and acetylcholamine.6–8
Deficiencies in such mediators have been shown to reduce mitosis rates in epithelial cells, leading to epithelial thinning and surface breakdown. Limbal stem cells fail to replace central epithelium, leading to a persistent epithelial defect. There is also a reduction in microvilli on the epithelial surface, resulting in poor tear adherence to the cornea.1 Tear composition is also altered by a reduction in goblet cell density within the conjunctival epithelium.9
Nerve growth factors (NGF) are a family of circulating mediators that are known to be essential for development and maintenance of both sympathetic and sensory nerves. NGF has also been shown to play a role in the production of acetylcholine and substance P.10 The cornea and conjunctiva are known to possess specific NGF receptors and it is likely that it plays a multifactorial role in maintaining normal corneal nerve density and preservation of the epithelium.11 NGF and neuromediators have been the target for several therapeutic agents for neurotrophic keratopathy.
Clinical Presentation
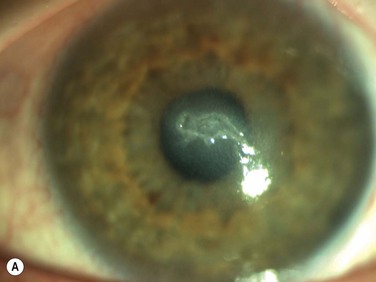
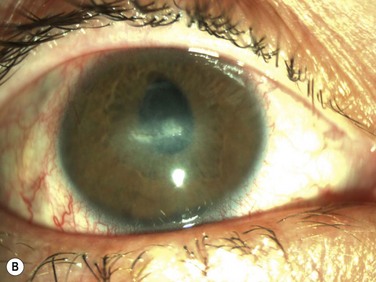
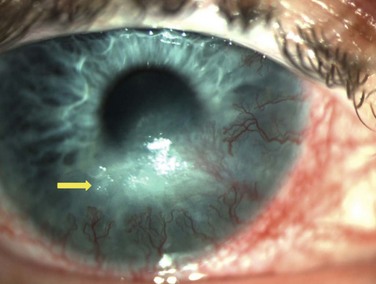
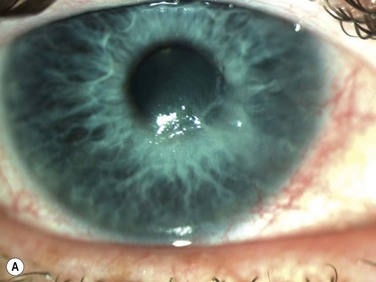
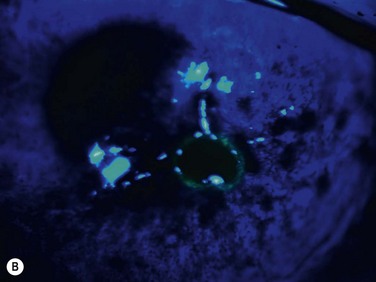
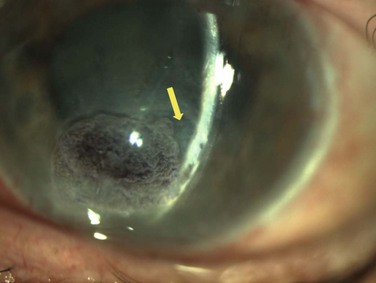
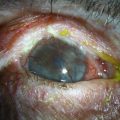
Neurotrophic keratopathy (NK) can present with varied clinical signs. Groos divided the clinical sings into three stages (Box 27.2).12 Stage 1, the least severe, is characterized by punctate keratopathy, altered tear viscosity with shorter tear break time, peripheral corneal vascularization and punctate staining of the palpebral conjunctiva. Stage 2 is characterized by a compromised corneal epithelial surface, with the defect often bordered by edematous and loose epithelium that forms a smooth edge. Compromise of the epithelium can also lead to stromal edema and visible folds in Descemet’s membrane (Fig. 27.6 – Fig. 27.8). Stage 3, the most severe form, is characterized by stromal melting and eventually corneal perforation (Fig. 27.9).
Assessment
Examination
The blink rate should also be assessed, as a reduction can result in a failure to evenly distribute tears over the ocular surface. This can lead to secondary dryness and exposure. There is a reduction in conjunctival goblet cell density and mucin production in neurotrophic keratopathy, resulting in tear instability. There might also be a reduction in tear production as a result of a lack of reflex tearing from corneal irritation.9 A Schirmer test should be performed as part of the work-up. Corneal sensitivity can be measured with a Cochet – Bonnet esthesiometer. A difference in corneal sensitivity in different quadrants of the cornea might indicate a local cause for anesthesia, such as viral keratitis. An alternative to an esthesiometer is the use of a wisp of a sterile cotton-tipped applicator.
Differential Diagnosis
Neurotrophic keratopathy may have a similar clinical appearance to other ocular surface disorders at each clinical stage. History is often helpful in directing the diagnosis towards neurotrophic keratopathy and the presence of an anesthetic cornea on clinical assessment is a critical sign. The presence of a surface punctate keratopathy in early neurotrophic keratopathy may lead to the incorrect diagnosis of dry eye, exposure keratopathy, surface irritation from topical therapy, or limbal stem cell deficiency. Symptoms of ocular pain or discomfort would not normally be reported in neurotrophic keratopathy and their presence might support these other diagnoses. Limbal stem cell deficiency could also be differentiated from corneal neovascularization by impression cytology, which would distinguish corneal from conjunctival epithelium.13
Treatment
The primary aim of treatment is to protect the corneal surface and to promote epithelial regrowth. The specific measures taken are dependent on the stage of the disease at the time of presentation and severity of anesthesia. In stage 1 disease, topical lubricants can be used to protect the corneal epithelium. To minimize ocular surface irritation from long-term use, preservative-free topical preparations should be considered for both lubricants and other topical preparations, if used for pre-existing ocular co-morbidity.8 Ideally, other topical medications should be discontinued if possible. A review of concurrent topical and systemic medications should be undertaken to minimize the use of therapies that might predispose to neurotrophic keratopathy. In order to increase tears on the ocular surface, punctal plugs may be considered.1 Eyelid hygiene and treatment of blepharitis optimizes meibomian gland function and reduces the risk of secondary infection. Oral doxycycline can be prescribed to treat blepharitis and reduce tear concentrations of matrix metalloproteinases (MMPs). MMPs have been implicated as a contributing factor of the surface epithelium failing to heal in RCE syndrome.14 The use of a bandage contact lens as a temporary measure to protect the corneal surface has previously been proposed. However, this may introduce infection and be a risk factor for secondary infective keratitis.15 Any exposure of the corneal surface, secondary to either an eyelid defect or lid malposition, should be addressed by surgical correction.
In more advanced disease when an epithelial defect occurs, the above conservative measures should also be administered. All topical treatment for other ocular conditions should be discontinued, as there is significant risk of stromal lysis and globe perforation. Topical collagenase inhibitors may be considered. A lateral tarsorrhaphy can be performed to reduce the palpebral aperture and corneal exposure.16 A measure that may be considered as an alternative to tarsorrhaphy, is inducing a temporary ptosis by injection of botulinum toxin A into the levator muscle.17
While the mainstay of topical therapy is lubrication, there are several adjunctive topical therapies available to promote both corneal epithelial and nerve regrowth. Treatment with autologous serum eye drops has been shown to promote epithelial regeneration and increase nerve density in the sub-basal plexus.18 Umbilical cord serum eye drops have also been shown to promote healing in a similar manner.19 Both preparations have been found to have significantly higher concentrations of neuromediators that play a role in corneal epithelial healing than human tears.19 Use of adjunctive therapy may be performed in combination with punctal plugging or a bandage contact lens. Preparations of individual neuromediators have also been studied as a treatment of neurotrophic keratopathy. This includes substance P in combination with insulin-like growth factor-1, epidermal growth factors, fibronectin and NGF.20–22 NGF has been found in one prospective study of 45 eyes with either stage 2 or 3 neurotrophic disease to promote persistent epithelial defect resolution, an associated improvement in visual acuity and an increase in corneal sensitivity long after cessation of treatment.20 A persisting epithelial defect only recurred in three patients who had had trigeminal nerve resection and this resolved with a further course of NGF therapy.
In cases of unilateral neurotrophic keratopathy, surgical replacement of the dysfunctional nerve in the form of corneal neurotization has been described.23 Branches of the contralateral supraorbital and supratrochlear nerves originating from the contralateral supraorbital foramen are isolated, tunneled over the bridge of the nose to the lid crease before being directed through the lid, conjunctiva, Tenon’s space, and attached at the limbus of the anesthetic cornea.
In stage 3 disease, the cornea is at significant risk of perforation. Immediate intervention is required to halt stromal lysis. In such cases, preservation of the globe integrity rather than preservation of vision takes precedent. In addition to the above measures to protect the corneal surface, either an amniotic membrane graft or conjunctival pedicle graft may be considered.24,25 In the presence of small perforations, corneal glue may be used followed by insertion of a bandage contact lens.26 In larger perforations, both lamellar keratoplasty and penetrating keratoplasty have been reported.1 Lamellar keratoplasty must only be performed if it is deemed that enough endothelium is preserved so to avoid decompensation. However, as cases of neurotrophic keratitis are characterized by altered innervation and there is invariably limbal neovascularization, performing keratoplasty is associated with a high failure rate and recurrence of an epithelial defect. The use of a Boston keratoprosthesis (Kpro) has also been suggested as an alternative to keratoplasty in the treatment of an anesthetic cornea, but the risk of device extrusion from keratolysis and inflammatory factors must be considered prior to Kpro surgery.27
References
1. Bonini, S, Rama, P, Olzi, D, et al. Neurotrophic keratitis. Eye (Lond). 2003;17:989–995.
2. Marfurt, CF, Murphy, CJ, Florczak, JL. Morphology and neurochemistry of canine corneal innervation. Invest Ophthalmol Vis Sci. 2001;42:2242–2251.
3. Guthoff, RF, Wienss, H, Hahnel, C, et al. Epithelial innervations of human cornea: a three-dimensional study using confocal laser scanning fluorescence microscopy. Cornea. 2005;24:608–613.
4. Muller, LJ, Vrensen, GF, Pels, L, et al. Architecture of human corneal nerves. Invest Ophthalmol Vis Sci. 1997;38:985–994.
5. Hamrah, P, Cruzat, A, Dastjerdi, MH, et al. Corneal sensation and subbasal nerve alterations in patients with herpes simplex keratitis: an in vivo confocal microscopy study. Ophthalmology. 2010;117:1930–1936.
6. Garcia-Hirschfeld, J, Lopez-Briones, LG, Belmonte, C. Neurotrophic influences on corneal epithelial cells. Exp Eye Res. 1994;59:597–605.
7. Nishida, T. Neurotrophic mediators and corneal wound healing. Ocul Surf. 2005;3:194–202.
8. Lambiase, A, Rama, P, Aloe, L, et al. Management of neurotrophic keratopathy. Curr Opin Ophthalmol. 1999;10:270–276.
9. Heigle, TJ, Pflugfelder, SC. Aqueous tear production in patients with neurotrophic keratitis. Cornea. 1996;15:135–138.
10. Donnerer, J, Amann, R, Schuligoi, R, et al. Complete recovery by nerve growth factor of neuropeptide content and function in capsaicin-impaired sensory neurons. Brain Res. 1996;741:103–108.
11. Lambiase, A, Bonini, S, Micera, A, et al. Expression of nerve growth factor receptors on the ocular surface in healthy subjects and during manifestation of inflammatory diseases. Invest Ophthalmol Vis Sci. 1998;39:1272–1275.
12. Groos, E, Jr. Neurotrophic keratitis. In: Cornea: Fundamentals of cornea and external disease. St Loius: Mosby; 1997.
13. Elder, MJ, Hiscott, P, Dart, JK. Intermediate filament expression by normal and diseased human corneal epithelium. Hum Pathol. 1997;28:1348–1354.
14. Ramamurthi, S, Rahman, MQ, Dutton, GN, et al. Pathogenesis, clinical features and management of recurrent corneal erosions. Eye. 2006;20:635–644.
15. Kent, HD, Cohen, EJ, Laibson, PR, et al. Microbial keratitis and corneal ulceration associated with therapeutic soft contact lenses. CLAO J. 1990;16:49–52.
16. Cosar, CB, Cohen, EJ, Rapuano, CJ, et al. Tarsorrhaphy: clinical experience from a cornea practice. Cornea. 2001;20:787–791.
17. Naik, MN, Gangopadhyay, N, Fernandes, M, et al. Anterior chemodenervation of levator palpebrae superioris with botulinum toxin type-A (Botox) to induce temporary ptosis for corneal protection. Eye. 2008;22:1132–1136.
18. Rao, K, Leveque, C, Pflugfelder, SC. Corneal nerve regeneration in neurotrophic keratopathy following autologous plasma therapy. Br J Ophthalmol. 2010;94:584–591.
19. Yoon, KC, You, IC, Im, SK, et al. Application of umbilical cord serum eyedrops for the treatment of neurotrophic keratitis. Ophthalmology. 2007;114:1637–1642.
20. Bonini, S, Lambiase, A, Rama, P, et al. Topical treatment with nerve growth factor for neurotrophic keratitis. Ophthalmology. 2000;107:1347–1351.
21. Daniele, S, Gilbard, JP, Schepens, CL. Treatment of persistent epithelial defects in neurotrophic keratitis with epidermal growth factor: a preliminary open study. Graefes Arch Clin Exp Ophthalmol. 1992;230:314–317.
22. Nishida, T, Yanai, R. Advances in treatment for neurotrophic keratopathy. Curr Opin Ophthalmol. 2009;20:276–281.
23. Terzis, JK, Dryer, MM, Bodner, BI. Corneal neurotization: a novel solution to neurotrophic keratopathy. Plast Reconstr Surg. 2009;123:112–120.
24. Alino, AM, Perry, HD, Kanellopoulos, AJ, et al. Conjunctival flaps. Ophthalmology. 1998;105:1120–1123.
25. Park, JH, Jeoung, JW, Wee, WR, et al. Clinical efficacy of amniotic membrane transplantation in the treatment of various ocular surface diseases. Cont Lens Anterior Eye. 2008;31:73–80.
26. Sharma, A, Kaur, R, Kumar, S, et al. Fibrin glue versus N-butyl-2-cyanoacrylate in corneal perforations. Ophthalmology. 2003;110:291–298.
27. Pavan-Langston, D, Dohlman, CH. Boston keratoprosthesis treatment of herpes zoster neurotrophic keratopathy. Ophthalmology. 2008;115(Suppl. 2):S21–S23.