24 Neurosurgery
Surgical anatomy and physiology
Blood supply
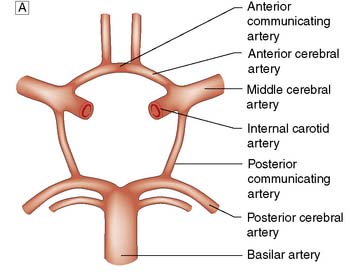
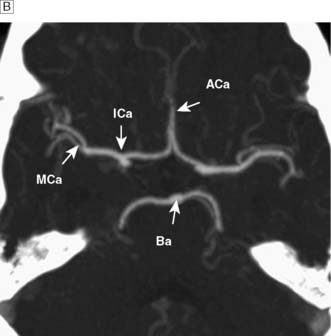
The brain requires a large blood flow (800 ml/min, 16% of cardiac output) to satisfy its oxygen and glucose requirements. The cortex receives about 50 ml/100 g/min, and white matter about 20 ml/100 g/min. Cerebral blood flow (CBF) is largely pressure-autoregulated (i.e. for mean arterial pressures between 60–140 mmHg, CBF remains constant), but is directly related to PaCO2. Other compounds, such as nitric oxide and endothelin, also regulate local CBF. The anterior and posterior circulations of the brain communicate with each other and across the midline through the circle of Willis (Fig. 24.1). In some circumstances, occlusion of a major artery can be compensated for by collateral flow.
Intracranial pressure
The brain is enclosed within a rigid bony container. Intracranial pressure (ICP) therefore depends on the relative volumes of intracranial blood, CSF and brain parenchyma. ICP also fluctuates in response to changes in intrathoracic pressure (e.g. increased by coughing, defaecation) and cardiac pulsation. These transient increases do no harm. In a normal supine adult, ICP is the same as the CSF pressure obtained at lumbar puncture (5–15 cm H2O, 4–10 mmHg). In patients with intracranial mass lesions (tumour, haemorrhage), oedema or CSF obstruction, the extra volume is at first compensated for by a reduction in cerebral blood volume and CSF volume. However, a critical point is soon reached where no further compensation is possible, and any additional volume insult will lead to exponential rises in ICP (Fig. 24.2).
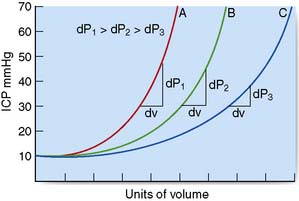
Fig. 24.2 Diagrammatic representation of the effects of a mass lesion on intracranial pressure (ICP).
Generalized or localized increases in ICP may lead to marked displacement of intracranial structures (brain herniation syndromes) and can compromise brain perfusion. The cerebral perfusion pressure (CPP) equals mean arterial pressure (MAP) less the ICP (CPP = MAP – ICP). Progressive rises in ICP lead to increases in MAP and reflex bradycardia. However, if there is a severe and sustained elevation of ICP, autoregulation will be ineffective and cerebral perfusion may be focally or generally compromised, leading to cerebral ischaemia and infarction. A CPP of > 60 mmHg is generally required to sustain adequate cerebral perfusion. Although children and young adults can tolerate lower levels, the consequences of a profound, prolonged lowering of CPP are often devastating (e.g. following severe head injury with raised ICP, or after cardiac arrest). The rate of increase in the volume of intracranial mass is crucial to the shape of the ICP pressure–volume curve (Fig. 24.2). With more chronic, slow-growing lesions such as brain tumours, abscesses or congenital abnormalities, extraordinary degrees of compensation can occur. In some situations, even massive lesions can lead to minimal symptoms and signs, despite brain herniation.
Brain herniation syndromes
Subfalcine (cingulate gyral) herniation
With a parasagittal mass, the ipsilateral cingulate gyrus may herniate beneath the free edge of the falx (Fig. 24.3A). The anterior cerebral artery may be compressed sufficiently to cause medial hemispheric infarction, but otherwise there are no obvious clinical signs except deteriorating conscious level.
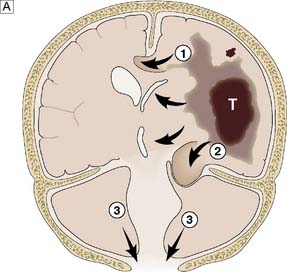
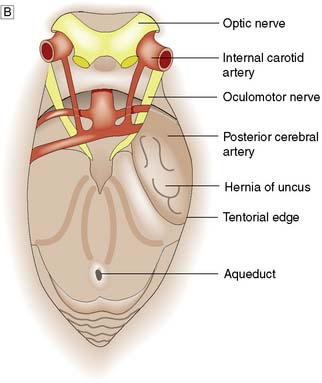
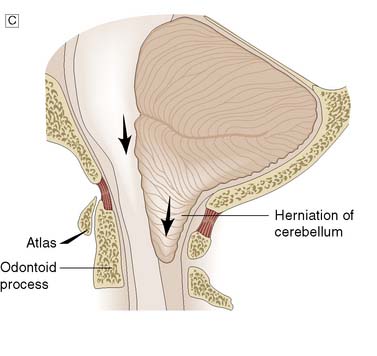
Fig. 24.3 Herniation syndromes.
A Coronal diagram of the dynamics of an intracranial mass lesion. Tumour (T) causes mass effect that compresses the midline and the ventricles (unnumbered arrows), provoking subfalcine or cingulated herniation (arrow 1), and transtentorial herniation (arrow 2). If the mass effect is uncontrolled, tonsillar herniation may also occur (arrows 3). B Transtentorial herniation in the axial plane (plane of dotted line in Fig. 24.3A). C Foraminal herniation.
Transtentorial (uncal) herniation
With large ipsilateral brain lesions, the medial part of the temporal lobe is pushed down through the tentorial notch to become wedged between the tentorial edge and the midbrain (Fig. 24.3B). The opposite cerebral peduncle is pushed against the sharp tentorial edge, and the midbrain and uncus become wedged at the tentorium. The aqueduct is compressed, obstructing CSF flow, and venous obstruction leads to midbrain haemorrhage. The clinical features of an uncal herniation, most often due to a traumatic intracranial haematoma, are:
| Eyes open |
To verbal command
Foraminal (tonsillar) herniation
With mass lesions of the posterior cranial fossa, the cerebellar tonsils and medulla are displaced downwards through the foramen magnum (Fig. 24.3A and C). Cerebellar impaction leads to medullary compression. Following traumatic or spontaneous haematomas, this can lead to a dramatic decrease in the GCS, acute hypertension, bilateral extensor responses and bilateral fixed dilated pupils, followed by sudden respiratory arrest. A similar syndrome may occur following the removal of CSF at lumbar puncture in patients with raised ICP due to a posterior fossa tumour, and is also known as ‘coning’. There is a rapid deterioration in conscious level, with decerebration. Lumbar puncture must not be performed in patients suspected of having raised ICP due to a mass lesion.
Summary Box 24.1 Intracranial pressure
• The rigid bony framework enclosing the central nervous system means that any increase in mass content increases intracranial pressure (ICP)
• Acute increases in ICP lower perfusion pressure and, if unrelieved, lead progressively to decreased Coma Score, herniation syndromes, bradycardia, hypertension, respiratory abnormalities (e.g. apnoea), vasoparalysis and death
• The principal symptoms of chronic raised ICP are headache, vomiting and visual disturbance (blurring of vision). Papilloedema may be apparent
• If ICP is due to a unilateral mass lesion, intracranial structures may be displaced. There are three major forms of herniation: subfalcine, transtentorial and foraminal.
Investigations
Plain X-ray
Summary Box 24.2 Vascular disorders and the central nervous system
• Symptoms due to occlusive vascular disease most often originate from blockage of extracranial vessels by atherosclerosis. Intracranial occlusion can be thrombotic or embolic
• Transient ischaemic attacks are associated with a high risk of major stroke within 5 years unless treatment is instituted (e.g. by carotid endarterectomy or aspirin therapy)
• Intracranial haemorrhage may be extradural, subdural, subarachnoid or intracerebral. Extradural and subdural haemorrhage are usually the result of trauma; subarachnoid bleeding is due to rupture of an aneurysm in around 70% of cases; and intracerebral bleeding is frequently associated with hypertension and amyloid angiopathy
• Patients with subarachnoid haemorrhage from an aneurysm should be considered for either clipping or coiling of the aneurysm to avoid recurrent bleeding.
vascular markings. Calcification of the pineal gland or choroid plexus may allow displacement to be detected, and abnormal calcification can develop in certain cysts and tumours. This investigation has, however, largely been superseded by computed tomography and magnetic resonance imaging.
Cerebrovascular disease
Stroke is a common major clinical disorder in clinical neuroscience practice. Occlusive disease often affects the extracranial vessels and is a common cause of stroke and transient ischaemic attack, usually managed by vascular surgeons (Ch. 25). Most forms of embolic and ischaemic stroke are dealt with by medical neurologists, whereas many large primary intracerebral haemorrhages and, more importantly, subarachnoid haemorrhage (SAH) are dealt with by the neurosurgeon. Brain tissue metabolism is vitally dependent on a consistent delivery of oxygen and glucose substrates for energy. If there is cessation of substrate delivery, the brain tissue will either die (if CBF is below a threshold of 12–15 ml/ 100 g/min) or stop functioning (if CBF is between 15 and 25 ml/100 g/min). These ischaemic thresholds are very important in terms of the extent of stroke (i.e. the amount of tissue that will die) and the penumbra (i.e. tissue that is damaged but still able to recover from these acute events). Urgent thrombolysis in ischaemic stroke is possible if the patient reaches hospital within 4 hours of onset of symptoms and can improve prognosis. Decompressive craniectomy can also save life in selected cases if raised intracranial pressure due to ischaemic brain swelling is a problem.
Subarachnoid haemorrhage
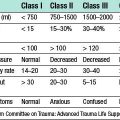
Grading of SAH depends on the coma score of the patient at time of presentation. The most widely used system, and also the easiest to use, is the World Federation of Neurosurgical Societies’ (WFNS) grading, which goes from grade 1 to grade 5 (Table 24.2). When the SAH has produced a syndrome of grade 2–5, it is quite apparent that some neurological catastrophe has occurred. However, when the syndrome is of a grade 1 haemorrhage, differential diagnosis is quite extensive and not infrequently the primary event is overlooked. SAH can mimic atypical migraines, thunderclap headache, coital cephalgia, pituitary apoplexy and meningitic-like syndromes. Sudden death is not uncommon when an aneurysm ruptures into the brain substance rather than the subarachnoid space. The focal signs depend upon the vessel affected. When symptoms and signs are mild, the differential diagnosis is quite extensive and diagnosis depends on having a high index of suspicion.
Table 24.2 World Federation of Neurosurgical Societies (WFNS) grading system for subarachnoid haemorrhage
| WFNS grade | Glasgow Coma Score | Focal neurological deficits |
|---|---|---|
| 1 | 15 | No |
| 2 | 13–14 | No |
| 3 | 13–14 | Yes |
| 4 | 9–12 | – |
| 5 | 3–8 | – |
Investigations
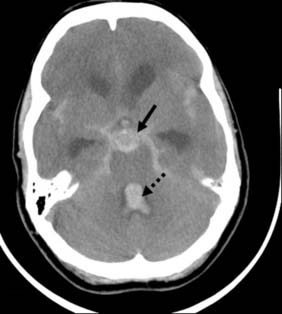
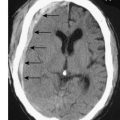
The standard investigation is CT, which characteristically shows blood in the CSF basal cisterns in the acute phase (Fig. 24.4). As CSF blood is broken down, the CT detection rate falls after the first 72 hours. If the diagnosis is in doubt, then a lumbar puncture should be performed, but only in patients whose clinical condition is good and in whom CT has excluded an intracranial mass lesion or midline shift. If this is performed early, the CSF will be uniformly blood-stained; later it will contain haem pigments that will be apparent on naked-eye inspection (xanthochromia) or can be detected by spectrophotometry.
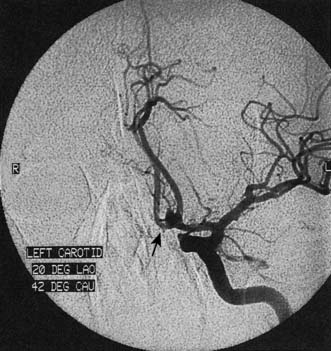
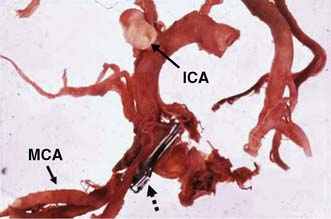
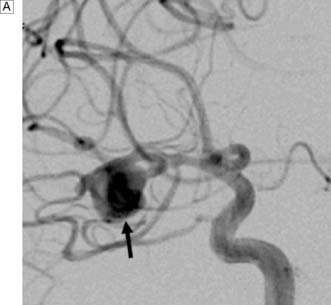
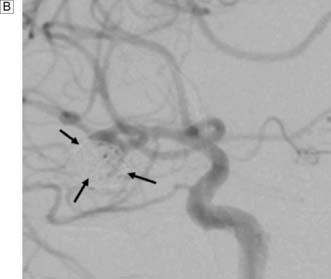
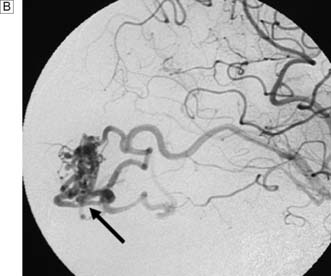
Next, a search must be made for the site of the bleeding. About 80% of aneurysms involve the anterior circulation. Conventionally, carotid or vertebral angiography has been performed (Fig. 24.5). However, CT and MR angiography are equally good at revealing aneurysms greater than 5 mm, and are non-invasive and so associated with less morbidity. As the consequences of missing a diagnosis of SAH are serious, there is a tendency to perform angiography in patients in whom the diagnosis is equivocal. For that reason, a source of bleeding will be identified in only 70% of angiograms. This is usually an aneurysm, less commonly an AVM or a cavernoma. In 30% of cases, no source is found. In the majority, this is a ‘true’ negative, and many of these patients will have a condition called perimesencephalic SAH, which is of unknown aetiology.
Management of aneurysmal SAH
The medical management of SAH includes intravenous fluids; the calcium antagonist, nimodipine (EBM 24.1); analgesia; and antiemetics. Patients in coma will usually be intubated and managed in a neurointensive care unit. Having initially been quite well, many patients begin to exhibit signs of focal or global cerebral ischaemia 4–10 days following an SAH. This has been attributed primarily to vasospasm and can be ameliorated by the prophylactic use of nimodipine; however, clinical deterioration may occur due to hydrocephalus, seizures, metabolic abnormalities and systemic infections.
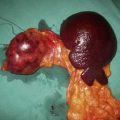
Rebleeding is a major cause of morbidity and mortality following aneurysm rupture. Until recently, standard management was occlusion of the aneurysm from the cerebral circulation by surgically clipping its neck (Fig. 24.6 and EBM 24.2). However, it is now possible to place detachable coils within the aneurysm via a catheter passed from the femoral artery into the cerebral circulation (Fig. 24.7). The coils unwind in the aneurysm and induce thrombosis. Coiling is a much less invasive procedure than open surgery and a recent prospective randomized controlled trial showed that, when an aneurysm can be treated by surgery or coiling, the latter is safer (EBM 24.3). Previously, only a proportion of aneurysms were suitable for coiling. However, improvements in coil, stent and basket technology mean most aneurysms can now be coiled.
Primary intracerebral haemorrhage (ICH)
Primary ICH is becoming an increasing problem as the population of many developed countries ages. It is four times more common than SAH and, in about 30% of cases, is associated with amyloid angiopathy. Most cases are caused by hypertensive rupture of small arterioles in the basal ganglia or cerebellum which have been damaged by chronic hypertension. Many haemorrhages are small and deep; others may be very large and cause death by raising ICP and provoking uncal or tonsillar herniation syndromes. Other causes of primary ICH include the rupture of a saccular aneurysm or AVM. The onset is usually abrupt, with many patients developing a flaccid hemiparesis or brain stem-cerebellar syndrome. One-third of patients die within a few weeks and many of those who survive are permanently disabled. Emergency removal of the haematoma was not uncommon neurosurgical practice in an effort to save life. However, the results of a recent prospective multicentre international randomized controlled trial of surgery for ICH (STICH) have shown that there are no significant differences in outcome with either medical or surgical management of spontaneous, non-aneurysmal, supratentorial ICH (EBM 24.4).
Arteriovenous malformations
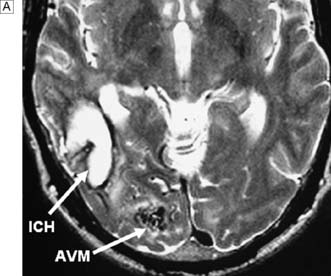
Cerebral AVMs are congenital abnormalities of the capillary system that lead to a direct arteriovenous communication (fistula). This leads to gross dilatation of the draining cerebral veins, as well as ectasia and occasionally aneurysmal dilatation of the feeding artery. AVMs can lead to cerebral ischaemia (because blood preferentially enters the venous system, bypassing the tissues), focal seizures and haemorrhage. Rupture of an AVM typically causes a spontaneous intracerebral haemorrhage rather than an SAH. As the haematoma is under less pressure, the overall prognosis is better than for aneurysmal SAH. The diagnosis is confirmed on CT or MRI and angiography (Fig. 24.8). If the AVM can be excised, then the risk of further haemorrhage is removed, and the incidence and frequency of seizures is considerably reduced. Sometimes, however, the AVM is large and in an important or inaccessible location. In these cases, endovascular ‘glueing’ or stereotactic radiosurgical treatment is appropriate. The latter is a focused form of X-ray therapy that leads to fibrosis over a 2-year period. AVMs may also affect the spine and lead to cord ischaemia. Symptoms are often progressive and include pain, weakness and, ultimately, paraplegia. Nowadays, the treatment is usually occlusion by interventional neuroradiology.
Neurotrauma
Management
Brain injury evolves over several days and the principal aim of management is to limit secondary damage due to ischaemia and brain herniation caused by raised ICP, hypoxia and hypotension. ICP is often severely elevated following neurotrauma because of oedema, haematoma, contusions, engorgement of the brain vasculature, hydrocephalus or even infection. A sustained ICP that exceeds 25 mmHg is associated with a poorer outcome. Severely brain-injured patients are therefore kept sedated and ventilated and their ICP is monitored. Hyperventilation, mannitol and barbiturates are used to reduce ICP, and the systemic blood pressure may be raised using fluids and inotropes (EBM 24.5). CBF is often directly related to MAP after head injury due to loss of autoregulation, and a CPP of > 60 mmHg is generally required to sustain adequate cerebral perfusion. Although children and young adults can tolerate lower levels, the functional consequences of profound and prolonged lowering of CPP are often devastating. Prolonged rehabilitation is required for many neurotrauma patients.
Extradural haematoma
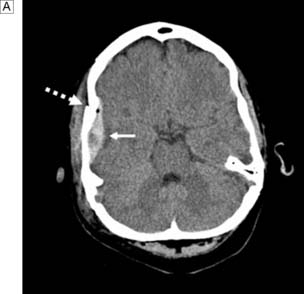
This is usually the result of a skull fracture with tearing of a meningeal artery (Fig. 24.9A). It is most common in the middle fossa after a temporal fracture and middle meningeal artery tear. The primary brain injury is often minimal, with a typical ‘lucid’ interval followed by rapid deterioration as the haematoma enlarges. The prognosis after treatment is usually good.
Subdural haematoma
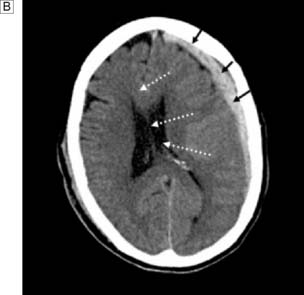
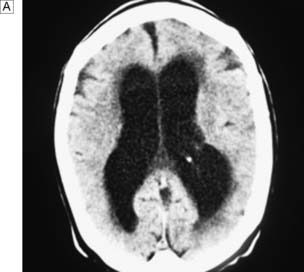
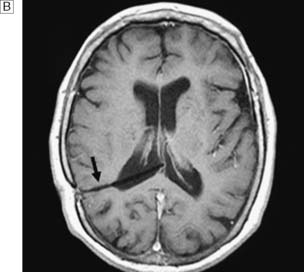
This is more common than extradural haematoma and is due to laceration of vessels (especially small cerebral veins) on the brain surface, or ‘bursting’ of the brain. CT shows a haematoma that is concave on its inner surface (Fig. 24.9B). Craniotomy is performed to remove the haematoma and arrest the bleeding. Morbidity and mortality are often high because of the severity of the primary brain injury. An increasingly common problem with the ageing population is chronic subdural haematoma (CSDH). This is a collection that varies in viscosity from breaking-down clot to bloodstained CSF-like fluid, and which can collect after relatively minor head trauma. Patients with cerebral atrophy who are on aspirin or anticoagulants are predisposed to CSDH. Because the collection can occur slowly, there may be significant midline shift and sometimes very few signs and symptoms. CSDH can mimic most neurological syndromes in their presentation. Treatment involves drainage of the collection through burr holes or mini-craniotomy, with or without drainage of the subdural space.
Diffuse axonal injury
Summary Box 24.3 Head injury
• Grading (Glasgow Coma Score, GCS): minor, GCS 13–15; moderate, GCS 8–12; severe, GCS < 8
• May cause extradural haematoma, subdural haematoma, intracerebral haematoma, cerebral contusions, diffuse axonal injury
• Avoidance of hypotension, hypoxia, hypercapnia, pyrexia and ICP > 25 mmHg minimizes secondary brain damage.
Intracranial infections
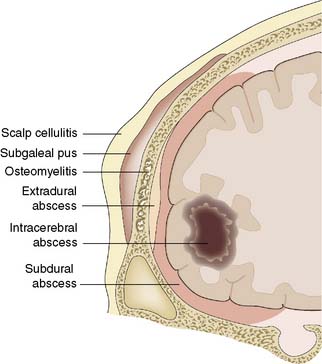
Infection of the central nervous system and its meninges acquires surgical importance if it produces a mass (abscess or oedema), hydrocephalus or osteomyelitis, or if it occurs as a result of a breach in, or absence of, the coverings of the brain. In developed countries, intracranial infections are relatively uncommon in immunocompetent patients. However, immunocompromised patients, particularly those affected with HIV, frequently suffer from a range of opportunistic organisms: for example, toxoplasmosis and tuberculosis. Infection may affect the scalp, cranium and meninges, as well as the brain itself (Fig. 24.10).
Intracranial tumours
Tumours of the skull
Gliomas
• Grade I tumours (e.g. pilocytic astrocytoma, dysembryoplastic neuroepithelial, ganglioglioma) are on the borderline between hamartomas and extremely low-grade tumours. They are often cured by surgery.
• Grade II tumours (astrocytoma, oligodendroglioma and ependymoma) have variable intrinsic malignancy and survival periods are usually long (median 8.5 years after surgery, radiotherapy and chemotherapy).
• Grade III tumours are prefixed anaplastic (e.g. anaplastic astrocytoma, anaplastic oligodendroglioma) and frequently have median survival periods of around 3 years after multimodality treatment (Fig. 24.11). As with grade II tumours, oligodendrogliomas tend to be more responsive to therapies than astrocytomas.
• Grade IV tumours are highly malignant and comprise glioblastoma and gliosarcomas.
• Grade III and grade IV tumours have features suggestive of malignant transformation (e.g. vascular endothelial proliferation, nuclear pleomorphism, high mitotic rate). Despite multimodality treatment, median survival with glioblastoma is around 9–12 months, depending on age (younger patients do better), performance status (patients in good condition do better) and history of seizures.
Meningiomas
These arise from the dura (Fig. 24.12) and account for 20% of all primary intracranial tumours. They commonly arise from the skull convexity, skull base or sagittal sinus region. They may compress the adjacent brain and cause seizures. They are generally slow-growing (90% are WHO grade I tumours) but may spread widely over the dura (‘en plaque’ tumours), and may invade the skull to form a palpable mass. The treatment is excision and prognosis is usually good. However, recurrences are not infrequent with WHO grade II (atypical) or WHO grade III (anaplastic) meningiomas, or following subtotal excision of a grade I tumour.
Schwannomas
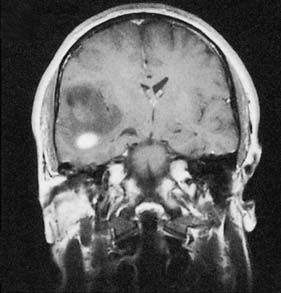
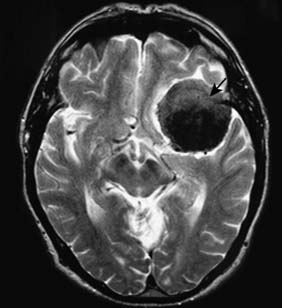
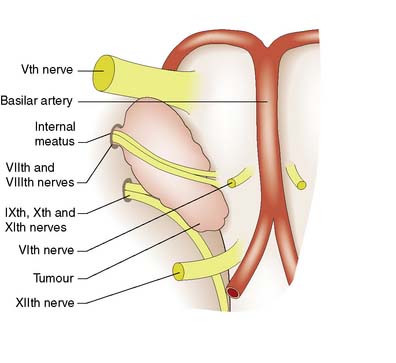
Cranial nerve tumours account for 10% of intracranial tumours and virtually all of them affect the vestibulo-cochlear nerves (acoustic neuroma or vestibular schwannoma) (Fig. 24.13). The tumour grows within, expands and erodes the internal auditory meatus. The VIIth and VIIIth nerves become stretched over its surface as it grows into the cerebellopontine angle. Early VIIIth nerve symptoms include progressive nerve deafness, tinnitus and vertigo. Larger tumours may involve the trigeminal nerve, leading to diminished facial sensation, as well as the pons and cerebellum, leading to ataxia and nystagmus. Displacement of the fourth ventricle and aqueduct may lead to hydrocephalus. Patients are often misdiagnosed as having Ménière’s disease and the tumour may reach a large size before it is discovered. Treatment options include microsurgical excision, stereotactic radiosurgery or observation.
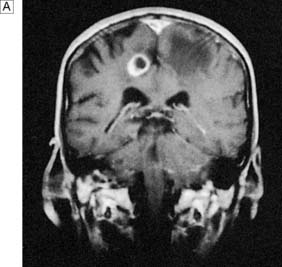
Brain metastasis
Metastatic tumours are present at postmortem in 20% of patients dying of cancer, and in 50% they are multiple (Fig. 24.14). They most commonly arise from the lung, breast, kidney, melanoma and colon. Such metastases may be the presenting feature or appear only late in the course of a previously diagnosed primary cancer. Prostate cancer classically spreads to the cranium and never involves the brain parenchyma. Conversely, gliomas very rarely spread beyond the central nervous system.
Clinical features of intracranial tumours
Diagnosis
MRI of the brain parenchyma, before and after the administration of contrast agents, usually clarifies the locality and neuropathology of neoplastic cranial lesions. In most malignant tumours, there is a neovascular capillary bed so that with anaplastic change, tumours will enhance (see Fig. 24.11). However, some low-grade tumours (e.g. pilocytic astrocytomas) also enhance, even though these are, in fact, grade I tumours. Meningiomas, which have a mesodermal origin, do not have a BBB and therefore enhance. With the better resolution of MRI, apparently solitary lesions on CT are not infrequently found to be multifocal. A feature of the anaplastic and malignant tumours is peritumoral brain oedema. This fluid occurs in the interstitial white matter due to the neoplastic endothelium not having the integrity of the normal BBB.
Management
The management principles of surgical neuro-oncology generally rely on:
• undertaking appropriate surgery to relieve patients’ signs and symptoms
Excision of the lesion is warranted to reduce mass effect, control seizures and restore lost brain function. If the preoperative neuroradiology suggests a malignant glioma, then extensive resection may provide optimal symptomatic control, a smoother course during radiotherapy and a reduction in steroid requirement. A recent phase III study has shown survival benefit for patients with malignant gliomas who have had nitrosurea-impregnated biodegradable wafers (Gliadel) placed in the resection cavity (EBM 24.6). If the lesion is a meningioma, then a total excision is generally planned, as this is a benign lesion. Similarly, if a posterior fossa lesion looks like a vestibular schwannoma, complete excision may be the treatment of choice. During excisional surgery, a whole variety of adjunctive techniques can be used, ranging from surgical ultrasonic aspirators to intraoperative localization techniques, as well as cortical stimulation of the brain of the awake patient with neurophysiological assessment. The latter technique is extremely useful in operating in areas of eloquent brain, such as the language cortex and motor region.
Summary Box 24.4 Tumours affecting the skull and its contents
• Common tumours involving the skull are osteomas and metastatic deposits
• Intracranial tumours have a bimodal age distribution (peaks at 6–7 years and the fifth decade). Almost 50% of intracranial tumours are metastatic (the most common primary sources being lung and breast)
• Primary cerebral tumours arise from the supporting cells of the brain (gliomas), from the walls of ventricles (ependymomas) and from the roof of the fourth ventricle (medulloblastomas). They constitute about 60% of intracranial tumours
• Meningiomas account for 20% of intracranial tumours (90% of meningiomas are supratentorial), grow slowly, can cause focal seizures, and are treated by excision
• Pituitary and parapituitary types account for 10% of all intracranial tumours. Pituitary tumours may be functional, both types can cause pressure effects and both types are best removed surgically
• Neurinomas of the cranial nerves account for 10% of all intracranial tumours. Acoustic neurinomas develop in the internal auditory meatus and involve the VIIth and VIIIth cranial nerves, to cause deafness, tinnitus, vertigo and facial weakness. Involvement of the Vth nerve may cause loss of facial sensation.
Paediatric neuro-oncology
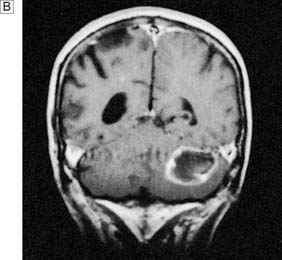
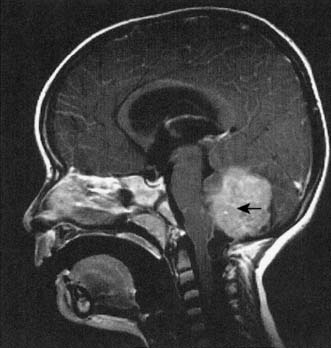
Tumours of the central nervous system are the second most common tumours of childhood after leukaemia. The incidence of paediatric central nervous system tumours in the UK is 15 per million of the paediatric population. In children under the age of 2 years, the most common tumours are teratomas, astrocytomas or primitive neuroectodermal tumours (PNET), and these can occur anywhere in the neuraxis. Between the ages of 2 and 15 years, the most common site for tumours is the posterior fossa, and most tumours are PNETs (also known as medulloblastomas (Fig. 24.15) when found in the posterior fossa), astrocytomas and ependymomas.
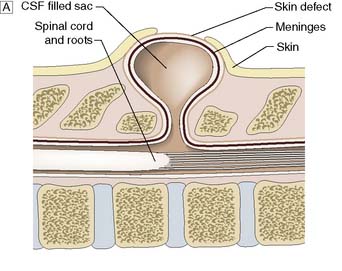
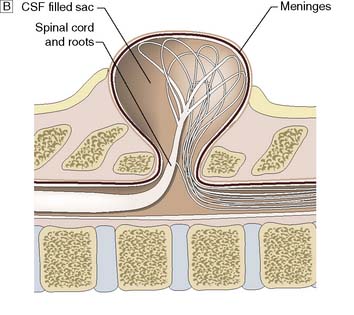
Spinal dysraphism
This is a congenital abnormality of the spinal axis, with or without abnormalities of the spinal cord, meninges and nerves, owing to failure of the neural tube to close (Fig. 24.16). Closure usually begins in the mid-dorsal region and extends cranially and caudally. Thus, thoracic defects are rare and cervical defects uncommon, and most affect the lumbar/lumbosacral region. Fortunately, maternal folate supplementation and prenatal screening for raised serum α-fetoprotein at 16 weeks’ gestation have reduced the incidence of myelomeningocoele. There are two categories of dysraphism: open and closed.
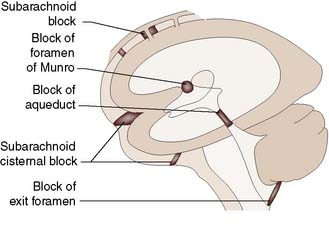
Hydrocephalus
Aetiology and clinical features
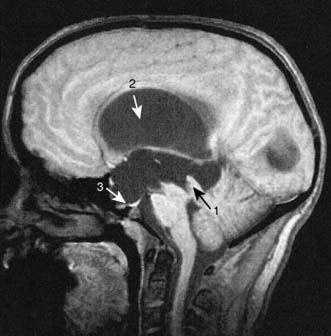
Hydrocephalus is the accumulation of CSF within the ventricles or over the surface of the brain. This may rarely be due to overproduction of CSF (because of a choroid plexus papilloma), but the vast majority are due to reduced drainage secondary to obstruction of normal CSF flow (Fig. 24.17). Obstruction may be congenital, such as in aqueduct stenosis (Fig. 24.18), or acquired, as a result of tumour or arachnoidal adhesions and fibrosis secondary to intraventricular or subarachnoid haemorrhage. Hydrocephalus due to obstruction of flow within the ventricular system, leading to dilatation of the ventricles, is termed ‘internal’, ‘non-communicating’ or ‘obstructive’. In ‘external’ or ‘communicating’ hydrocephalus, the ventricular system is patent but there is reduced flow through the basal cisterns or absorption of CSF by the arachnoid granulations. This is commonly due to fibrosis following meningitis or subarachnoid haemorrhage, or to sagittal sinus thrombosis. In this type, the ventricles and the CSF spaces around the surface of the brain will be enlarged. In adults, chronic hydrocephalus may cause the ‘normal pressure hydrocephalus’ syndrome of gait ataxia, incontinence and cognitive decline. Diagnosis is often difficult in the elderly because brain atrophy causes ex-vacuo dilatation of the ventricles due to loss of brain substance, mimicking hydrocephalus. Cognitive decline can be asso ciated with Alzheimer-like pathology or cerebrovascular disease, and urinary disturbances may be related to prostate problems.
Management and prognosis
Treatment consists of relieving the pressure by bypassing the block to CSF drainage. In some cases of aqueduct stenosis, this can be done in a minimally invasive way by endoscopic third ventriculostomy. In this procedure, using an endoscope a small hole is formed in the floor of the third ventricle, allowing CSF to flow into the basal cisterns. In most cases, however, a ventriculo-peritoneal (VP) shunt will have to be inserted (Fig. 24.19). This consists of a catheter in the lateral ventricle, which drains CSF through a valve (that sits on the skull under the scalp) into the peritoneal cavity. The risk of bleeding into the ventricular system or brain during the insertion or removal of a VP shunt is of the order of 1–2%. There is also a risk of early infection, usually with skin commensal organisms such as Staphylococcus epidermidis. Shunts can become blocked or malfunction, causing a rapid return of symptoms, and this can be a medical emergency. Shunts can also become infected many months or years after insertion, in which case the shunt has to be removed and reinserted once the infection has cleared. Occasionally, a shunt will over-drain the ventricles, leading to premature closure of the cranial sutures and microcephaly. Over-drainage may be symptomatic, with headache and vomiting. It also predisposes to blockage of the ventricular catheter.
Malformations of the skull
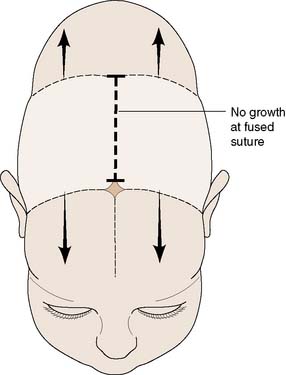
Craniosynostosis
This refers to the premature closure or absence of a cranial suture. Several intramembranous ossification centres occur in the skull vault and form plates of bone. Sutures form where these plates of bone meet each other. This is where further bone growth occurs. Overall bone growth is driven by the expanding brain. The brain has reached 85% of its adult size by the age of 2 years but continues to grow slowly after this time. The midline frontal metopic suture fuses at the age of 2 years. Premature fusion of a suture will lead to asymmetrical skull growth. Fusion of a single suture is associated with certain typical head shapes, depending on the particular suture affected (Fig. 24.20). The common ones are scaphocephaly and plagiocephaly (premature fusion of the sagittal and coronal sutures respectively). Plagiocephaly may also be caused by fusion of a lambdoid suture but this is much rarer. Many cases of plagiocephaly are due to head moulding, when the baby lies on its back to sleep. This usually resolves when the child starts to sit and walk.
Vertebral column
Spinal degenerative disease
Aetiology and clinical features
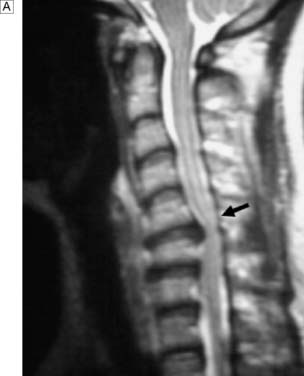
In response to acute trauma, the nucleus pulposus may protrude (herniate) through a tear in annulus (Fig. 24.21). Posterolateral protrusion usually compresses the adjacent nerve root or radicle, causing a sciatica (lumbar) or brachialgic (cervical) syndrome, with or without neurological deficit. Leg pain may be exacerbated by coughing and sneezing, and arm pain by neck movement. There is usually loss of normal lumbar lordosis, and a scoliosis often develops that is concave to the affected side. Straight leg raising is diminished. The neck may have paravertebral spasm. Tendon jerks and muscle power are diminished according to the site of the lesion Thus a L5/S1 prolapse affects the S1 nerve root and produces pain down the back of the thigh, the lateral side of the calf and the lateral border of the foot. There is sensory loss in the latter region. The ankle jerk may be diminished or absent and, as plantar flexion of the ankle is weak, the patient may have difficulty standing on tiptoe. With an L4/L5 disc prolapse, the L5 root is compressed. Pain radiates down the back of the thigh, the lateral aspect of the calf and the dorsum of the foot into the great toe. There may be accompanying sensory loss; the ankle jerk is normal but ankle dorsiflexion is weak.
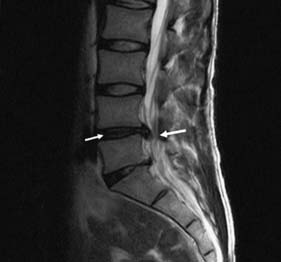
Fig. 24.21 Sagittal MRI scan of the lumbar spine showing bulging and herniation of the L4/5 disc (arrows).
A surgical emergency occurs if the disc prolapses or herniates directly into the spinal canal (Fig. 24.22). In the cervical region, this will cause a progressive myelopathy, with numb, clumsy hands and spasticity. In the lumbar region, it is usually associated with severe pain in both lower limbs, loss of foot function, urinary retention and numbness up the back of the legs and around the genitalia, anus and buttocks (saddle anaesthesia). A rectal examination should be performed to assess anal tone. These features, known as a cauda equina syndrome, may be unilateral if the disc prolapse is asymmetrical.
Summary Box 24.5 Acute lumbar disc prolapse
• The condition is most common in the fourth and fifth decades, and men are most often affected
• The annulus fibrosus ruptures, allowing protrusion of the central nucleus pulposus. The prolapse most commonly occurs posterolaterally, and compresses and angulates the spinal nerve(s) as it leaves the spinal canal. Less commonly, the disc ruptures posteriorly, with compression of the cauda equina
• The most common levels for disc prolapse are L4–5 and L5-S1
• Pain is the predominant symptom and is exacerbated by coughing and sneezing. Tendon jerks and muscle power are diminished, straight leg raising is restricted (e.g. from 80–90° to 30°) and lumbar lordosis is flattened, with scoliosis concave to the side of the lesion
• Most cases settle on conservative therapy, but those with major protrusions or persistence of symptoms beyond 6 weeks should be considered for removal of the prolapsed material
• The cauda equina syndrome (severe back pain, urinary retention and weakness bilaterally below the knees) requires urgent surgical relief.