Chapter 9 Neurostimulation in Complex Regional Pain Syndrome
 CRPS is a regional pain disorder of uncertain etiology with likely inflammatory and neuropathic components.
CRPS is a regional pain disorder of uncertain etiology with likely inflammatory and neuropathic components. Management centers on functional rehabilitation. Psychiatric and pain medicine interventions are often critical to management and facilitation of functional restoration.
Management centers on functional rehabilitation. Psychiatric and pain medicine interventions are often critical to management and facilitation of functional restoration. One randomized controlled trial showed that SCS is effective in long-term pain relief (2 years) in refractory CRPS patients.
One randomized controlled trial showed that SCS is effective in long-term pain relief (2 years) in refractory CRPS patients. Retrospective data and case series suggest potential effectiveness of peripheral nerve stimulation and motor cortex stimulation.
Retrospective data and case series suggest potential effectiveness of peripheral nerve stimulation and motor cortex stimulation. Before offering neurostimulation to patients with CRPS, less invasive options are usually tried, and patients need to have careful psychological screening and preferably an interdisciplinary committee recommendation for neuromodulation.
Before offering neurostimulation to patients with CRPS, less invasive options are usually tried, and patients need to have careful psychological screening and preferably an interdisciplinary committee recommendation for neuromodulation. It is critical to stress to patients that neurostimulation is only one component of the management of CRPS and that it may only offer the patient a window of improved pain control to facilitate rehabilitation.
It is critical to stress to patients that neurostimulation is only one component of the management of CRPS and that it may only offer the patient a window of improved pain control to facilitate rehabilitation. Although expensive upfront, SCS is cost-effective in the management of refractory pain in CRPS patients.
Although expensive upfront, SCS is cost-effective in the management of refractory pain in CRPS patients. Recent technological improvements in SCS devices may result in lower complication rates than those that have been reported in the literature to date.
Recent technological improvements in SCS devices may result in lower complication rates than those that have been reported in the literature to date. Diagnosis of the syndrome using the available IASP criteria is the first step in clinical management.
Diagnosis of the syndrome using the available IASP criteria is the first step in clinical management. SCS may be part of a treatment plan that focuses on rehabilitation through desensitization and active functional improvement strategies.
SCS may be part of a treatment plan that focuses on rehabilitation through desensitization and active functional improvement strategies. Meticulous surgical technique and careful placement of the SCS leads may improve outcomes and limit revisions.
Meticulous surgical technique and careful placement of the SCS leads may improve outcomes and limit revisions. Placement of a spinal cord stimulator device involves surgery along with the risks and benefits associated with it.
Placement of a spinal cord stimulator device involves surgery along with the risks and benefits associated with it. Lead migration, unwanted stimulation, and discomfort at the generator site may lead to loss of analgesia and multiple revisions may be necessary. Surgical site infection is another complication that curbs effectiveness of the therapy early on.
Lead migration, unwanted stimulation, and discomfort at the generator site may lead to loss of analgesia and multiple revisions may be necessary. Surgical site infection is another complication that curbs effectiveness of the therapy early on.Introduction
Complex Regional Pain Syndrome History and Nomenclature
Complex regional pain syndrome (CRPS) is the newer nomenclature encompassing the clinical entities of reflex sympathetic dystrophy (RSD) and causalgia.1 It is characterized by intractable pain usually affecting one or more extremities. Even though it was originally described over a hundred years ago, much debate lingers over the clinical and basic pathophysiological characteristics of this condition. Named as causalgia (from Greek, kausos [heat], algos [pain]), it was initially described in 1864 during the American Civil War by Silas Weir Mitchell from the observation of soldiers developing chronic pain following traumatic nerve injuries.2 Since its original description, it has been given a number of different names such as algodystrophy, posttraumatic dystrophy, sympathetic-maintained pain syndrome, hand-shoulder syndrome, Sudeck atrophy, and other names. Early in the twentieth century Paul Sudeck3 described a syndrome with predominantly trophic symptoms that developed following distal bone fractures not affecting directly peripheral nerves. Patients experiencing Sudeck syndrome obtained significant pain relief by sympathetic block, thus suggesting at the time a central role for the autonomic nervous system in the pathophysiology of the condition. An articulation of the belief in a central role of sympathetic system was the term reflex sympathetic dystrophy (RSD), coined by Evans in 1946 to label all syndromes characterized by excessive chronic pain following injury, responsive to sympathetic blocks and as such driven by the sympathetic system.4 As understanding of the condition evolved, it was clear that sympatholytic interventions and sympathetically maintained pain (SMP) were not specific to RSD but common in other neuropathic pain disorders. In addition, dystrophic changes were not always observed, and there was no evidence that the condition was a reflex. As such, a working group of the International Association for the Study of Pain (IASP) developed a consensus definition in 1994 and proposed a new terminology reflecting a more accurate description of the condition. The term CRPS type I replaces RSD; the term CRPS type II, which requires demonstrable peripheral nerve injury, replaces the term causalgia.1 Various diagnostic tests have been proposed (without much success) to confirm the diagnosis of CRPS, including among others radiological studies, triple-phase bone scans, quantitative sensory testing, quantitative sudomotor axon reflex test (QSART), and limb thermography with or without sympathetic block. However, diagnosis of CRPS remains a clinical process relying mostly on history and potentially on physical examination. The current IASP diagnostic criteria define CRPS type I as a syndrome that usually develops following a trauma, fracture, surgery, or immobilization, with pain that is disproportionate to the inciting event in a regional/nondermatome pattern (not limited to the distribution of a single peripheral nerve or nerve root). CRPS II requires the same set of descriptive criteria; however, an identifiable nerve injury is required for diagnosis. Although these diagnostic criteria had a high sensitivity (98%), their specificity was poor (36%), resulting in a correct diagnosis in as few as 40% of patients.5 The lack of an objective test that serves as a gold standard for diagnosis has led to extensive efforts to validate a set of bedside diagnostic criteria to improve the accuracy of CRPS diagnosis. The new proposed diagnostic criteria (Box 9-1) do not imply at all the pathogenesis of the disease; however, they supply a set of descriptive signs and symptoms that are adequately sensitive and specific in diagnosing CRPS6 (Table 9-1). The same set of criteria would be applied with varied stringency, depending on the intent, and thus defined as research criteria or clinical diagnostic criteria. These newer criteria have not yet been ratified by the taxonomy committee of the IASP and will undergo further validation studies before full adoption.5–8
Box 9-1
Revised International Association for the Study of Pain Diagnostic Criteria
| Diagnosis of CRPS (Clinical) Requires: | Diagnosis of CRPS (Research) Requires: |
From Harden et al: Proposed new diagnostic criteria for complex regional pain syndrome, Pain Med 8:326-331, 2007.
Table 9-1 Criteria and Decision Rules Considered for Complex Regional Pain Syndrome Diagnosis
| Criteria/Decision Rules for Proposed Criteria | Sensitivity | Specificity |
|---|---|---|
| 2+ sign categories and 2+ symptom categories | 0.94 | 0.36 |
| 2+ sign categories and 3+ symptom categories | 0.85 | 0.69 |
| 2+ sign categories and 4 symptom categories | 0.70 | 0.94 |
| 3+ sign categories and 2+ symptom categories | 0.76 | 0.81 |
| 3+ sign categories and 3+ symptom categories | 0.70 | 0.83 |
| 3+ sign categories and 4 symptom categories | 0.86 | 0.75 |
From Harden et al: Proposed new diagnostic criteria for complex regional pain syndrome, Pain Med 8:326-331, 2007.
Patient Demographics and Risk Factors
There are only two population-based epidemiological studies of CRPS in the general population. One reported the population-based incidence rate in North America,9 and the other in Europe (Netherlands).10 The reported incidence rates are different; the U.S. study reporting an incidence of 5.6 per 100,000 person-years; the more recent European study reported a rate of 26.2. The inclusion criteria for both studies were different, which could be one of the factors accounting for varying results. CRPS affects females more than males at a ratio almost 4 : 1,11 and the majority of CRPS cases in females occur in the postmenopausal stage of life, suggesting a potential hormonal etiological role in CRPS. The observed mean age of diagnosis in these studies of CRPS is 50 to 70 years old with a mean of 52.7 years old.9,10,12 This age peak is higher than is generally expected and observed in some nonpopulation-based investigations.13 Before the mid-1980s there were only scattered case reports of RSD in children. However, over the last 10 to 15 years it has become apparent that CRPS does occur in children, with a mean age of onset of about 12.5 years (range 3 to 18 years),14 particularly following sports injuries.
No single causative factor has been found that explains the development of this complex disorder, but an inciting event often precedes the onset of CRPS. Initial observations correlated CRPS with wounds and crushing limb injuries. Fractures are the most common trigger, wrist fractures in particular.15 Cast immobilization appears to be another condition associated with development of CRPS.16 During cast immobilization increased pressure and early complaints of tightness are predictive risk factors for the onset of CRPS. On the other hand, CRPS cases developing as a consequence of remote processes such as stroke,17 spinal cord injury, and myocardial infarction18 have been reported. The risk for developing CRPS may depend on susceptibility to exaggerated responses, probably through genetic predisposition to basic pain-related mechanisms such as inflammation and sensitization. This has led to a search for gene polymorphisms that could predict development of CRPS. In a study from Herlyn and colleagues19 a single nucleotide polymorphism within the α-adrenoceptor appears to be a risk factor for the development of CRPS I after distal radius fracture. Polymorphisms in the human leukocyte antigen (HLA) system have been studied, and loci from all three HLA classes reportedly have been associated with CRPS onset.20 Studies on co-occurrence of disorders such as migraine, osteoporosis, menstrual cycle–related problems, and neuropathies with CRPS21,22 can potentially give clues to shared etiologic factors and reveal risk factors.
Complex Regional Pain Syndrome Pathophysiology
The pathophysiology of CRPS is not fully understood; however, based on animal and human studies several hypothesized mechanisms appear to play an important role. In the acute (early) stage as described by Veldman and associates13 CRPS presents with skin discoloration, edema, increased nail or hair growth, temperature difference, limited movement, or reported sweating. Traditional sequential staging of CRPS into acute inflammatory, subacute dystrophic, and chronic atrophic stages has been largely supplanted by classifying the condition based on limb appearance and warmth. Thus CRPS has been more recently subdivided into a “warm and a cold form.”13,23 The difference in temperature between affected and unaffected extremities has led to the use of thermography, albeit with low specificity for either diagnosis or prognosis.24,25 Symptoms such as edema, trophic changes, sweating, and vasomotor-related changes have been considered signs of autonomic system dysregulation (sympathetic); pain responding favorably to sympathetic blocks is considered sympathetically maintained pain (SMP). However, the role of the sympathetic system in CRPS has been debated since the vasomotor instability can be explained by other mechanisms26–28 such as abnormal sensitivity of adrenergic receptors to normal sympathetic outflow.29 Moreover, α-adrenoceptors appear to be overexpressed in hyperalgesic skin from CRPS-affected limbs.30 The reverse hypothesis of diminished sympathetic stimulation has been postulated as an underlying cause of adrenergic receptor up-regulation and sensitization in CRPS patients.31 A generally acknowledged view today is that SMP and sympathetic dysregulation can be important but not obligatory components of CRPS.
Aseptic neuroinflammation may be a mechanism that is active early in the establishment of CRPS.32 Trauma-related events could lead to activation and sensitization of primary neuronal afferents to cytokines and neuropeptides released in the affected body region, mainly substance P (SP) and calcitonin gene-related peptide (CGRP).32 Evidence of a neuroinflammatory process is also obvious from analysis of fluid derived from artificially produced blisters on CRPS-affected extremities. Analysis of blister fluid with a multiplex array testing for 25 different cytokines revealed a strong proinflammatory expression profile, with increased markers for activated monocytes and macrophages.33 Recently neuropeptide Y and angiotensin-converting enzyme (ACE) have been also suggested as potential modulators of neuroinflammatory responses.34 Despite the commonly found increase in proinflammatory cytokines in human studies, there is a lack of correlation between cytokine expression and severity and duration of CRPS, suggesting that neuroinflammation is only partly involved in the pathophysiology of CRPS.35
Pain and hyperalgesia are the predominant symptoms in CRPS. Persistent peripheral nociceptive input in CRPS results in spinal cord central sensitization with features of mechanical hyperalgesia and allodynia.36,37 A hallmark of central sensitization is spreading of hyperalgesia, which goes far beyond the initial site of injury. This expansion of nociceptive receptive fields occurs as a result of neuroplasticity changes in the central nervous system (CNS) between the dorsal horn (DH) of the spinal cord and the somatosensory cortex. At the spinal level DH central pain-projecting neurons are pathologically activated by N-methyl-d-aspartate (NMDA) receptor–mediated processes, which leads to hyperexcitability and central sensitization.38 Furthermore, changes in central representation of somatosensory input in the thalamus and cortex have been found by various studies.39,40 This cortical reorganization correlates linearly with the amount of CRPS pain and is reversed following pain relief as confirmed by magnetoencephalography (MEG) studies.40
Recently the hypothesis of progressive small-fiber degeneration as the basis for CRPS has gained some ground. This has primarily resulted from the work of Oaklander and Fields.41 Oaklander and colleagues42 demonstrated for the first time through a morphometric analysis performed on skin biopsies that CRPS I is associated with small-fiber axonal degeneration.
Electrical Neurostimulation for Complex Regional Pain Syndrome
Management of Complex Regional Pain Syndrome
A number of treatment approaches are available for CRPS. These approaches can be categorized as pharmacological, interventional, physical/occupational therapy, and psychological techniques. Physical therapy is the first-line and the mainstay treatment for CRPS. However, it is often limited by the pain itself, and pain-control interventions are often essential to enable full patient participation.43,44 Interventional approaches are very useful and are applied usually in combination with pharmacological measures to enhance patient compliance with physical therapy (PT). Various sympathetic blocks, intravenous regional blocks, and epidural blocks can be provided on an outpatient basis. However, the response to sympathetic blocks varies and appears to be more effective than placebo in duration but not magnitude of pain relief.45 In general, pharmacological pain treatment is similar to that of managing neuropathic pain and would include antidepressants (particularly tricyclics and serotonin-noradrenalin reuptake inhibitors), antiepileptics (e.g., gabapentin), and occasionally muscle relaxants and topical analgesics.37 Opioids may have a limited role in refractory CRPS patients. Steroids, given their anti-inflammatory function, may be effective in improving inflammatory signs, especially early on.46 Antioxidants and free radical scavengers may be effective given that hypoxic phenomena in the affected limb can enhance the production of free radicals. In the Netherlands free radical scavengers such as dimethylsulfoxide (DMSO)47 and N-acetylcysteine (NAC) are widely applied in the treatment of CRPS. Bisphosphonates have shown promise to significantly improve symptoms of CRPS in randomized clinical trials.48,49 By reducing local acceleration of bone remodeling, bisphosphonates may alleviate pain by effects on nociceptive primary afferents in bone. Psychological treatment in CRPS involves cognitive behavioral techniques and biofeedback and relaxation training. Even though there are no studies to support its use in CRPS, in the general chronic pain population psychological treatment is an effective treatment modality,37 and it may be used in CRPS to improve coping skills and facilitate rehabilitation.
Electrical Neurostimulation for Complex Regional Pain Syndrome
The idea of electrical stimulation for pain control was based on the initial description of the gate control theory by Melzak and Wall50 whereby electrical stimulation of Aβ (A beta) fibers in dorsal columns would result in closing the “gate” and obliterating onward central transmission from peripheral nociceptors (C fibers). Varying methods of neurostimulation have been developed, depending on target tissue. In CRPS there is solid evidence for effectiveness of spinal cord stimulation (SCS) and to a lesser extent for peripheral nerve stimulation (PNS) and motor cortex stimulation (MCS).
Spinal Cord Stimulation for Complex Regional Pain Syndrome
Dorsal column electrical stimulation or SCS has been applied to a variety of pain disorders. The mechanism of action of SCS is described elsewhere in this volume. However, focusing on CRPS, SCS theoretically could act on various pathogenetic mechanisms such as (a) direct inhibitory actions on central sensitization mechanisms, (b) restoration and sustainability of blood flow (microcirculation) to the affected extremity by increasing the release of vasoactive mediators such as CGRP and SP,51 and (c) decreasing sympathetic output by antidromic effects.52 Even though there are no firm data to support these hypotheses, some recent animal data and clinical observation are emerging that could indicate how SCS could influence various biological functions in CRPS.51,53–55
Evidence of Spinal Cord Stimulation Effectiveness in Complex Regional Pain Syndrome
A plethora of reports supports the use of SCS in treating neuropathic pain conditions in general and CRPS in particular. Of note, the Neuromodulation Therapy Access Coalition found substantial evidence to recommend the use of SCS for treatment of CRPS.56 However, as of early 2010, literature search reveals only one SCS randomized controlled trial (RCT), three prospective long-term trials (Table 9-2), and 12 retrospective studies and multiple case reports and case series.
Table 9-2 Summary of Prospective Studies on Spinal Cord Stimulation for Complex Regional Pain Syndromes
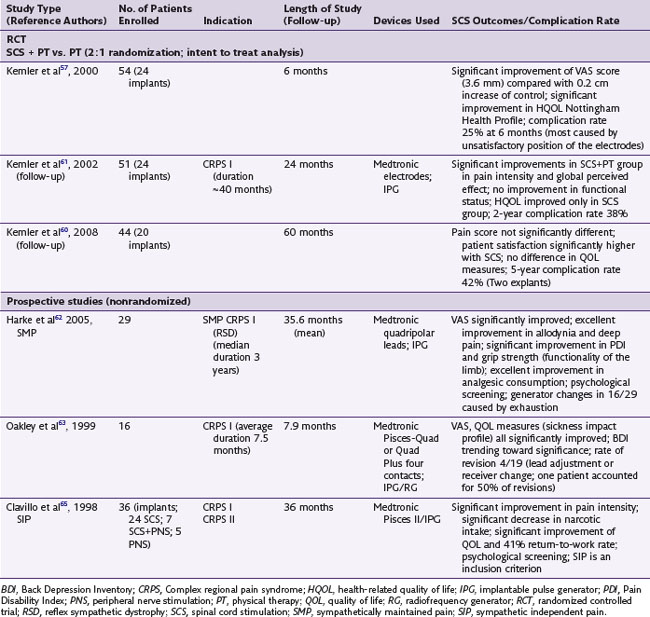
The only RCT of SCS for CRPS was performed by Kemler and associates57 and was initially presented in 2000. Investigators designed a prospective, randomized trial to examine the effects of SCS on a group of 54 patients with refractory CRPS who had persistent pain even after surgical sympathectomy.57–60 Patients were randomized into two groups in a 2 : 1 ratio to compare the outcomes of SCS and PT vs. PT alone (36 patients were randomized to receive SCS + PT as active treatment arm, and 18 patients randomized to only receive PT as control). As part of the inclusion criteria most patients recruited for this study had severe disease, with 10 patients requiring wheelchairs, 8 crutches, and 13 upper-extremity splints. CRPS had to be present for at least 6 months and affected unilaterally the entire hand or foot. Inclusion criteria also included pain intensity of at least 5 cm on the visual analog scale (VAS) scale despite pharmacological or psychological therapies. All patients had failed surgical sympathectomy. Outcome measures included quality of life measurements (the Nottingham Health Profile and Sickness Impact Profile), pain measurements (VAS and McGill pain questionnaire), and global perceived effect on a 7-point scale (1 worst ever to 7 best ever). Patients were assessed at 1, 3, and 6 months. Data were analyzed on an intent-to-treat (ITT) basis. Of the 36 patients assigned to SCS and trialed for 7 days, 24 underwent implantation, and 22 of those were available for follow-up at 6 months. At 6 months the ITT analysis results indicated a significant improvement in the 36-patient group assigned to receive SCS and PT (p < 0.001; mean reduction of pain intensity on VAS was −2.4 cm) compared with the group that received PT alone (mean change +0.2 cm). The average VAS reduction for the 24 subjects treated with SCS (as-treated analysis) was even greater (−3.6 cm). A significant improvement was also reported in the “much improved” score of the global perceived effect in the subjects assigned to SCS. The authors concluded that there is a beneficial effect of SCS in CRPS at 6 months after implant. These effects were maintained at 2-year follow up (VAS: −2.1 cm vs. 0.0 cm; p <0.001 global perceived effect “much improved” score 43% for the SCS group vs. 6% for the PT group; p = 0.001).61 However, despite a significant improvement in health-related quality of life, no clinically significant improvement in functional status was noted.61 These findings support the conclusion that SCS results in long-term pain reduction and health-related quality of life improvement in CRPS. The same authors assessed the effectiveness of SCS at 5-year follow up.60 A statistically significant difference was no longer evident between the SCS + PT group compared to the PT group using an intent-to-treat analysis (p = 0.25 at 5 years)60 with regard to pain relief and other variables. However, an as-treated subgroup analysis looking at patients that were implanted (20 patients) and compared to patients undergoing PT only (13 patients) had a p value near statistical significance (p = 0.06) for pain relief, and statistically significant improvement in global perceived effect in the “much improved” subscale (p = 0.02). The authors commented that despite the apparently diminishing effectiveness of SCS over time, 95% of implanted patients (19/20) stated they would repeat the treatment for the same result.60
In a prospective trial Harke and associates62 assessed the long-term effects of SCS on pain and improvement of functional status in CRPS patients who had SMP (i.e., their pain responded to blockade of sympathetic efferents). They studied 29 patients with a mean CRPS duration of 5.4 years. All patients received a constant-voltage, fixed-channel system, 16 cervical and 13 thoracic lead placements. The outcomes examined were VAS scores for deep pain and allodynia, changes in Pain Disability Index (PDI) to quantify impairments in activities of daily living, and changes in analgesic medication consumption during SCS therapy. Patients were followed for an average of 35.6 ± 21 months after implant. They reported that all patients had a complete resolution of allodynia at 12 months and a significant improvement in deep pain. Furthermore, nearly three quarters of the patients showed a significant decrease in the PDI with over 50% improvement in scores. However, nearly half of the patients needed battery replacement secondary to exhaustion (at the time of the study, rechargeable generator systems were not available), and 44.1% underwent surgical revision of the leads. The authors do not describe in detail the reasons for revisions. It is not clear whether the cervical or the thoracic placements were more prone to revision. Of significance, the authors placed the electrodes in the lateral aspects of the canal for cervical lead placement for coverage of shoulders, arms, hands, and midline for thoracic lead placement. Although their rationale was to obtain better coverage and stimulate with low output to conserve energy, this approach could cause stimulation of nerve roots at the entry zones that might lead to uncomfortable stimulation (discomfort threshold or recruitment of motor fibers). Even with the complications encountered, the effectiveness of SCS in CRPS with SMP type is impressive. The complete obliteration of allodynia and the significant increase in grip strength and improvement in functional status and quality of life affirm the effectiveness of SCS in CRPS I patients with SMP. In a prospective study, Oakley and coworkers63,64 reported on 19 CRPS patients treated with SCS. Sixteen were available at follow-up; 11 were still using their devices; two patients stopped using the system, reporting “no pain”; one died unrelated to the device; and two were unresponsive to therapy. Eight of the 11 patients obtained at least 50% pain relief. Complications identified were minor and were corrected without adverse effect on stimulation parameters or efficacy. In another study by Calvillo and colleagues65 36 patients with CRPS affecting the upper extremity were examined following treatment with SCS, SCS and PNS, or PNS alone. The authors report a significant reduction in VAS scores (53%) and 50% reduction in analgesic consumption at 36 months following implant when compared to baseline.65 They concluded that neurostimulation is an effective and reasonable option when alternative therapies have failed.
Reports of SCS in children and adolescents are limited. CRPS I is not uncommon in children and adolescents, affecting in particular girls with an average age of 11 to 14 years old. It could be associated with minor trauma or sports injury; occasionally a trigger cannot be identified. Although treatment centers around active PT, SCS has been used to facilitate the process by reducing pain intensity. In a case series of seven girls 11 to 14 years of age with significantly incapacitating and therapy-resistant CRPS I, SCS was implemented.66 Trial stimulation was performed, and optimal coverage was achieved in five of seven cases. Despite a 1- to 2-week delay in onset of pain relief following SCS, pain alleviation was complete in five of seven patients at 2 to 6 weeks after the intervention. SCS therapy was deemed successful in all seven patients (even in two patients in whom the SCS system was removed) since no or minor remaining symptoms were noted at 1- to 8-year follow-up and there were no severe recurrences.
Several retrospective studies have examined the effect of SCS in CRPS. Of note, Bennett and colleagues67 examined pain reduction in relation to current trends in the use of SCS to treat CRPS. The study specifically examined common factors among patients with successful SCS treatment retrospectively compared to patients implanted with single-lead, four-electrode (quadripolar) systems (with an internal battery) and with those using dual-lead, eight-electrode (octipolar) systems (which at the time required a radiofrequency unit). Data were compiled on 101 CRPS patients who had similar psychological profiles. Patients were divided into two groups: those who had single-lead quadripolar systems and those who had dual-lead octipolar systems. Both groups displayed a significant reduction in VAS pain intensity scores when compared to baseline. The overall satisfaction rate for the group with single quadripolar leads was 70%, whereas for those with dual octipolar leads the rate was 91%. Analysis of variance for improvement in pain scores showed a significantly greater improvement (F-value 56.081, P <0.0001) with dual octipolar leads. There was a mean pain improvement (ΔVAS) in the quadripolar lead group of 3.70 ± 0.79 vs. a mean pain improvement in the dual octipolar lead group of 6.00 ± 1.59. The ability to regain pain control after spontaneous lead movement was significantly different between the two groups. In the quadripolar lead group four patients (3.3%) required surgical revision as a result of spontaneous lead migration, whereas in the dual octipolar group no patients required surgical revision as a result of spontaneous lead migration. The larger number of electrodes available for programming in the dual-octipolar group allowed an increased flexibility when rostral-caudal changes in positioning occurred; patients who experienced movement of their leads were able to recapture their pain coverage with reprogramming alone. The ability to use higher frequencies (above 250 Hz) in the group with dual octipolar leads (radiofrequency generator unit) allowed some patients to “recapture” pain control. A subset of patients (15.5%) who had lost analgesia in the presence of adequate paresthesia coverage were able to regain pain control when stimulation frequency was increased above 250 Hz (mean 455 ± 104.5 Hz). Although both groups had statistically significant improvements in pain scores and overall satisfaction when compared to baseline, the dual-octipolar group showed greater improvements.67
In a case series on 12 patients with upper- and lower-extremity pain, including five CRPS I patients, and using a system delivering multiple independent constant-current output with electrical field-steering capabilities through low cervical epidural SCS leads, Hayek, Veizi, and Stanton-Hicks68 found a consistent ability to capture and maintain paresthesia coverage in all four extremities that correlated with pain relief.68 They also reported that the frequency used was relatively low (mean 45 ± 5 Hz). In a recent case report by Williams, Korto, and Cohen69 complete resolution of CRPS symptoms occurred after 1 month of SCS treatment. Based also on a previous case series reporting resolution without recurrence of CRPS in adolescent girls (11 to 14 years old) following treatment with SCS,66 the authors argued in favor of central-peripheral neuronal interface (“neuronal switch”) that could be modulated by SCS and could lead to complete “cure” of CRPS. Thus a more aggressive treatment strategy, which places neuromodulation therapies early in the treatment algorithm along with the use of more advanced SCS configurations, may prove to be more effective. This hypothesis is promising but requires further research.
Peripheral Nerve Stimulation for Complex Regional Pain Syndrome
Almost in parallel to SCS, PNS was developed in the late 1960s. Whereas SCS targets dorsal columns of the spinal cord, PNS targets fibers of the peripheral nerve along its path. Correct placement of leads close to the nerve trunks requires an incision, nerve exposure, and alignment along or wrapping of the nerve trunk, depending on the leads used. Recent placement of percutaneous leads with ultrasound guidance close to peripheral nerves was touted as potentially safer, possibly reducing risks associated with open dissection.70,71 The introduction of four electrode contacts coupled with the availability of programmable generators in the early 1980s provided a significant advancement.72 However, there have been no specific leads developed for PNS, and the procedure is not without complications and not always effective. Although the exact mechanism of action of PNS is not known, it is believed that the principle behind PNS is the same as with SCS but the target is different (peripheral nerves innervating the painful area). Constant peripheral activation of large fibers could provide a consistent block of peripheral nociceptive input allowing the neuronal plasticity to implement changes that could control CRPS-related pain. A recent study demonstrated an increase in brain activity by functional magnetic resonance imaging (fMRI) in the somatosensory cortex on median nerve stimulation, which suggests a central effect as a result of PNS.72
Several studies exist on open-dissection PNS placement for chronic pain following peripheral nerve injury. A recent study by Mobbs, Nair, and Blum73 reviewed the results of a retrospective analysis of 38 patients implanted with PNS systems and followed up for 35 months. The overall results indicated that 61% of the patients reported over 50% pain relief. This recent study compares favorably with previous reports in the literature also reviewed in the same article.73 In a meta-analysis from previous studies reported, of the 175 implanted patients (studies reviewed from 1975 to 2000), 65.4% reported a successful outcome with good-to-excellent ratings. Efficacy of PNS in CRPS cannot really be assessed with currently available studies. In fact, there is only one study published that specifically examined PNS efficacy over the long term in CRPS. In a prospective study by Hassenbusch and associates,74 32 CRPS patients were trialed with PNS systems, and 30 of those had a successful trial with significant pain relief. The study reported a 63% success rate at 3 years after implant in patients with advanced disease that had failed all other modalities except a trial of SCS. Of the 30 patients implanted with permanent PNS systems, 8 (27%) required a revision of the electrode; placement of additional electrodes was carried out in six patients. There were 11 pain relief failures, mostly early on at 1.3 ± 0.1 years. According to this study the best application of PNS appears to be for a small group of patients with localized pain along the distribution of a single major nerve. However, when CRPS spreads and the receptive pain area increases, further surgical revisions might be necessary. Although there might be a place for PNS in the treatment algorithm of CRPS, particularly type II, well-designed randomized trials are necessary to assess its long-term effectiveness.75 Furthermore, increased effectiveness of this modality may occur with improved design of electrodes.
Motor Cortex Stimulation for Complex Regional Pain Syndrome
Motor cortex stimulation (MCS) may be a promising tool for the treatment of patients with refractory neuropathic pain. The technique consists of implanting epidural electrodes over the motor strip of the parietal lobe. Epidural MCS is safer, less invasive, and easier to perform than deep brain stimulation. The analgesic effects of MCS could be caused either by neuronal activation in the cortical and thalamic areas as demonstrated by positron emission tomography (PET) studies or by activation of descending fibers originating from the motor cortex that control sensory input.76–78 Although MCS has been shown to be effective in relieving trigeminal neuropathic pain, it has only recently been reported in a few cases with recalcitrant CRPS. MCS resulted in significant relief of pain and allodynia that lasted over 12 months in a patient with CRPS type II of the upper extremity with hemi body allodynia.79 Velasco and associates78 reported a case series of five CRPS patients, four of whom benefited from MCS implant. VAS and McGill pain scales diminished significantly throughout the follow-up and worsened when MCS was turned off for 30 days (in a double-blinded fashion). In addition, there was resolution of allodynia and sympathetic signs lasting over 1 year. Nevertheless, randomized, prospective studies are required; technical improvements in areas such as electrode design and stimulation parameters are needed.
Benefits vs. Risks and Complications of Spinal Cord Stimulation in Complex Regional Pain Syndrome
In addition to analgesia, other favorable outcomes of SCS therapy in CRPS range from absence of hyperpathia to normalization of capillary filling, normalization and sustainability of temperature over time, improved quality of life, functional improvement of the diseased limb when SCS is combined with physiotherapy, and a notable decrease in analgesic medication consumption.59,60,62,80 Currently SCS is the only modality capable of providing sustained pain relief at any treatment stage for CRPS, but it is not effective in all patients. Successful analgesic outcome with SCS has been classically defined as either over 50% pain relief or significant reduction in VAS pain scores. On the basis of these parameters, the success rate of SCS in CRPS I and II derived from of all the studies reported in the literature with a follow-up of at least 6 months is 82% (from 224 cases).81 Patient satisfaction with SCS is illustrated in the long-term follow-up study of Kemler and associates.60 Despite no apparent improvement of SCS + PT vs. PT patients at 5 years after implant, 95% of the patients with CRPS I who had undergone SCS implantation stated they would repeat the procedure for similar results.
One of the main criticisms of SCS applications has arisen from the possible role of placebo. Because a patient cannot be blinded to the paresthesias induced by SCS, double-blinded studies evaluating SCS have not been conducted. However, one well-controlled study has shown effectiveness of SCS therapy when compared to placebo in angina.82
As an invasive procedure SCS placement carries surgical risks related to the procedure and technical complications related to the implanted device. Risks and complications of SCS are discussed in detail in another chapter of this series. Briefly, risks associated with the procedure that should be discussed with the patients before the trial include mechanical issues such as lead migration or generator problems, infection, spinal fluid leaks, hemorrhage, and neurological injury. Some consequences can be temporary such as cerebrospinal fluid (CSF) leaks; however, others result in explants or even worse in neurological deficits. The typical reported complication rate after SCS varies from 20% to 75% (mean, 42%). On the basis of findings from larger studies, it is known that complications necessitate removal of the system in 5% to 15% of cases.81 Technical problems (e.g., lead shifting and breaking) seem to be inherent in the treatment with reported incidence of breakage in 9.1% and shifting (migration) in 13.2% (2753 cases).81 Although complications affect treatment results, cause patient discomfort, and generate costs, these factors rarely cause permanent neurologic deficits (0.03%).81 CSF leakage occurs after accidental dural puncture with the epidural needle, guidewire, or leads during surgical procedure. A CSF leak can lead to headache, which usually occurs early in the postoperative period. In a large review of the literature (from 1981 to 2004) Cameron81 found that the incidence of infection was 3.4% (2972 cases) and CSF leak 0.3% (2972 cases).
Specific to CRPS, a varied SCS complication rate is reported. In the RCT study by Kemler and colleagues60 the complication rate at 2 years that required intervention was 38%, and overall 42% of the patients underwent reoperation 5 years after implant. Most of the complications occurred in the first 2 years after implant (79%), and the annual complication rate after 2 years was fairly low at 5%. Most reoperation in this study was caused by battery replacement, which could be avoided because the technology has advanced and rechargeable batteries are now available. On the other hand, lead migration was clearly high in the first 2 years but was noted in only two cases from the second to the fifth year. Regardless of lead location, cervical or lumbar, the complication rate was comparable.83 Oakley et al63 did not report any complication at implant. However, the follow-up complication rate was 21%, with lead repositioning/replacement being the main complication. Overall revision rate was 35% (6 out of 19 patients). In the study of 29 patients by Harke and associates62 there were 16 battery replacements (55%), 12 lead revision/replacements (41%), and two explants. The relatively high complication rate of SCS should not be an obstacle in treatment consideration but must clearly be communicated to future candidates.
Patient Selection, Trial, and Long-Term Patient Management
Patient Selection
 Applying appropriate diagnostic criteria. The valuable work from IASP special interest groups on CRPS diagnostic criteria is of paramount importance in defining the patient population for treatment clinically and for clinical research studies.
Applying appropriate diagnostic criteria. The valuable work from IASP special interest groups on CRPS diagnostic criteria is of paramount importance in defining the patient population for treatment clinically and for clinical research studies. Psychological factors and behavioral aspects are thought to contribute significantly to CRPS presentation and maintenance.84–86 But the actual association between psychological factors and CRPS remains controversial because of the lack of methodological high-quality studies. Nonetheless, it is very important that psychological evaluation be performed before patients are considered for SCS therapy.87
Psychological factors and behavioral aspects are thought to contribute significantly to CRPS presentation and maintenance.84–86 But the actual association between psychological factors and CRPS remains controversial because of the lack of methodological high-quality studies. Nonetheless, it is very important that psychological evaluation be performed before patients are considered for SCS therapy.87 Appropriate patient expectations and goal-oriented therapy. An initial clinical interview can also elicit the patient’s subjective experience of pain. Discussion with patients should involve clear explanation of the benefits, side effects, and ineffectiveness of the method. It should also be clear that SCS is a part of the complex therapeutic approach together with PT, psychological therapy, and pharmacotherapy working toward the goal of functional rehabilitation of the extremity.
Appropriate patient expectations and goal-oriented therapy. An initial clinical interview can also elicit the patient’s subjective experience of pain. Discussion with patients should involve clear explanation of the benefits, side effects, and ineffectiveness of the method. It should also be clear that SCS is a part of the complex therapeutic approach together with PT, psychological therapy, and pharmacotherapy working toward the goal of functional rehabilitation of the extremity. Since SCS has been used generally at the end spectrum of treatment, it is encouraging that many studies have shown significant pain improvement. Obviously integrating SCS with PT at earlier stages in combination with new implantable systems with enhanced capabilities (multiple electrodes and flexibility in output parameters) might eventually lead to even better outcomes.
Since SCS has been used generally at the end spectrum of treatment, it is encouraging that many studies have shown significant pain improvement. Obviously integrating SCS with PT at earlier stages in combination with new implantable systems with enhanced capabilities (multiple electrodes and flexibility in output parameters) might eventually lead to even better outcomes. Magnetic resonance imaging (MRI) should be performed in any suspected case of stenosis, disc herniation, or other anatomical abnormality that might explain the patient symptoms or otherwise increase the procedural risk of SCS.80 Some clinicians rely on MRI to gain information about the depth of epidural space/dorsal CSF and the position of the spinal cord, dimensions that vary among individuals and may affect electrode selection, placement, and adjustment; however, there are no data that show improved technical outcomes following MRI.
Magnetic resonance imaging (MRI) should be performed in any suspected case of stenosis, disc herniation, or other anatomical abnormality that might explain the patient symptoms or otherwise increase the procedural risk of SCS.80 Some clinicians rely on MRI to gain information about the depth of epidural space/dorsal CSF and the position of the spinal cord, dimensions that vary among individuals and may affect electrode selection, placement, and adjustment; however, there are no data that show improved technical outcomes following MRI.Spinal Cord Stimulation Trial
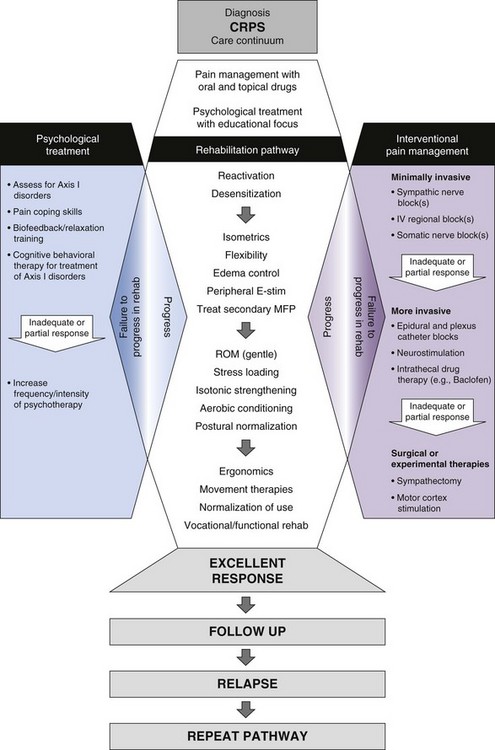
According to the Neuromodulation Therapy Access Coalition, there is excellent evidence that screening trials of SCS provide valid patient selection information.56 Indeed, one of the advantages of SCS is that trials offer both the physician and patient an opportunity to evaluate SCS before committing to it. The trial should answer two fundamental questions: Is the patient’s pain responsive to SCS therapy, and can the patient tolerate the treatment? In CRPS the trial is an opportunity to determine whether the patient can tolerate the interdisciplinary care required for functional rehabilitation (Fig. 9-1). The physician and patient should agree in advance on the goals of the trial and the measures used to assess those goals. In general, candidates should proceed to implantation if their pain can be reduced by at least 50% and they can participate in functional rehabilitation,44 the area of paresthesia is tolerable and concordant with the area of pain,88 analgesic medication intake remains stable or can be decreased, and functional improvement has been assessed (different clinics use different tools for physical evaluation). SCS is effective in nearly two thirds of the CRPS I patients.88 Generally trials last for 1 week or longer and use externalized lead wires and a temporary external transmitter. Trial period and lead selection varies and is a function of multiple variables detailed elsewhere in this volume. However, a 5- to 7-day home-testing period resulting in at least 50% pain relief is commonly accepted as appropriate. The selection criteria that are currently used do not predict which candidates will continue to respond after implantation of a SCS system. One of the predictors of successful outcomes of SCS in CRPS is the presence of brush allodynia.89
Current Treatment Paradigm for Complex Regional Pain Syndrome and the Role of Spinal Cord Stimulation
Functional recovery and rehabilitation of the limb are the essential goals of therapy in CRPS. Physiotherapy is recommended widely in the literature as the mainstay of CRPS treatment. The concurrent implementation of physiotherapy with pain management and psychological therapies is meant to facilitate a sequential progression through the steps of the rehabilitation algorithm (see Fig. 9-1). The point of entry would depend entirely on pragmatic factors such as response to therapy and clinical presentation. It is rational to treat patients who present with CRPS using a multimodal approach. What has not been given competing credence in consensus statements is the place of electrical neuromodulation therapies in CRPS. Although other therapies have been proposed as “more conservative” and therefore “initial” therapies, this rationale is not supported with favorable long-term outcome data (i.e., a change in the course of the condition or significant long-term diminution of pain and sequelae of the syndrome). Thus a time-oriented construct that relies on the concept that timely reduction of pain with normalization of blood flow may provide the best environment for functional recovery, may thus favor earlier use of SCS. Mere implantation of a device without rehabilitation is unlikely to produce good functional outcomes. After significant progress has been made, follow-up visits would be scheduled as necessary to ensure a safe and effective use of SCS.
Cost-Effectiveness of Spinal Cord Stimulation in Complex Regional Pain Syndrome
SCS is considered an expensive invasive therapy for chronic pain because of the upfront costs of the screening trial and devices. However, there is a large body of evidence that implanting an SCS system is less costly after 3 years of successful therapy. Kemler and co-workers57 looked at the economics of conventional treatment vs. SCS for the treatment of CRPS. Even though during the first year the cost of SCS therapy was $4000 higher than non-SCS therapy (PT), in the lifetime analysis SCS was more effective than conventional therapy and less expensive (by $60,000 per patient).90 These findings are similar to those in a study by Kumar, Malik, and Demeria that compared conventional chronic pain therapies vs. SCS in 104 patients with failed back surgery syndrome.91 Treatment costs with SCS were higher for the first 2.5 years; thereafter they were approximately one third lower. Furthermore, 15% of the SCS-treated group returned to work. A British trial of patients treated for CRPS I found a lifetime cost savings of approximately U.S. $60,800 for the SCS group when compared to the PT group.92,93 In a U.S. retrospective study of 222 patients (54% were CRPS I and II patients) Mekhail and colleagues93 found reduced demand for health care resources by patients receiving neurostimulation, which suggests that, despite the initial costs, PNS and SCS have substantial long-term economic benefits that translate to $17,900 savings per year.
1 Stanton-Hicks M, et al. Reflex sympathetic dystrophy: changing concepts and taxonomy. Pain. 1995;63:127-133.
2 Michell SW, Morehouse GR, Keen WW. Gunshot wounds and other injuries of nerves. Philadelphia: Lippincott; 1864.
3 Staunton H. Sudeck atrophy. Ir Med J. 2006;99:313-315.
4 Evans JA. Reflex sympathetic dystrophy; report on 57 cases. Ann Intern Med. 1947;126:4174-4226.
5 Bruehl S, et al. External validation of IASP diagnostic criteria for complex regional pain syndrome and proposed research diagnostic criteria: International Association for the Study of Pain. Pain. 1999;81:147-154.
6 Harden RN, et al. Proposed new diagnostic criteria for complex regional pain syndrome. Pain Med. 2007;8:326-331.
7 Harden RN, et al. Complex regional pain syndrome: are the IASP diagnostic criteria valid and sufficiently comprehensive? Pain. 1999;83:211-219.
8 Stanton-Hicks MD, et al. An updated interdisciplinary clinical pathway for CRPS: report of an expert panel. Pain Pract. 2002;2:1-16.
9 Sandroni P, et al. Complex regional pain syndrome type I: incidence and prevalence in Olmsted county, a population-based study. Pain. 2003;103:199-207.
10 de MM, et al. The incidence of complex regional pain syndrome: a population-based study. Pain. 2007;129:12-20.
11 Schwartzman RJ, Erwin KL, Alexander GM. The natural history of complex regional pain syndrome. Clin J Pain. 2009;25:273-280.
12 de MM, et al. Referral and treatment patterns for complex regional pain syndrome in the Netherlands. Acta Anaesthesiol Scand. 2009;53:816-825.
13 Veldman PH, et al. Signs and symptoms of reflex sympathetic dystrophy: prospective study of 829 patients. Lancet. 1992;342:1012-1026.
14 Tan EC, et al. Complex regional pain syndrome type I in children. Acta Paediatr. 2008;97:875-879.
15 Atkins RM, Duckworth T, Kanis JA. Features of algodystrophy after Colles’ fracture. J Bone Joint Surg Brit. 1990;72:105-110.
16 Schwartzman RJ, McLellan TL. Reflex sympathetic dystrophy: a review. Arch Neurol. 1987;44:555-561.
17 Riedl B, et al. Autonomic failure after stroke—is it indicative for pathophysiology of complex regional pain syndrome? Acta Neurol Scand. 2001;103:27-34.
18 Wasner G, Backonja MM, Baron R. Traumatic neuralgias: complex regional pain syndromes (reflex sympathetic dystrophy and causalgia): clinical characteristics, pathophysiological mechanisms and therapy. Neurol Clin. 1998;16:851-868.
19 Herlyn P, et al. Frequencies of polymorphisms in cytokines, neurotransmitters and adrenergic receptors in patients with complex regional pain syndrome type I after distal radial fracture. Clin J Pain. 2010;26:175-181.
20 van de Beek WJ, et al. Susceptibility loci for complex regional pain syndrome. Pain. 2003;103:93-97.
21 de MM, et al. Estrogens and the risk of complex regional pain syndrome (CRPS). Pharmacoepidemiol Drug Saf. 2009;18:44-52.
22 de MM, et al. Medical history and the onset of complex regional pain syndrome (CRPS). Pain. 2008;139:458-466.
23 Janig W, Baron R. Complex regional pain syndrome: mystery explained? Lancet Neurol. 2003;2:687-697.
24 Herrick A, et al. Abnormal thermoregulatory responses in patients with reflex sympathetic dystrophy syndrome. J Rheumatol. 1994;21:1319-1324.
25 Sherman RA, et al. Stability of temperature asymmetries in reflex sympathetic dystrophy over time and changes in pain. Clin J Pain. 1994;10:71-77.
26 Eisenberg E, et al. Plasma endothelin-1 levels in patients with complex regional pain syndrome. Eur J Pain. 2004;8:533-538.
27 Groeneweg G, et al. Regulation of peripheral blood flow in complex regional pain syndrome: clinical implication for symptomatic relief and pain management. BMC Musculoskelet Disord. 2009;10:116.
28 Drummond PD. Mechanism of complex regional pain syndrome: no longer excessive sympathetic outflow? Lancet. 2001;358:168-170.
29 Chemali KR, Gorodeski R, Chelimsky TC. Alpha-adrenergic supersensitivity of the sudomotor nerve in complex regional pain syndrome. Ann Neurol. 2001;49:453-459.
30 Gibbs GF, et al. Unravelling the pathophysiology of complex regional pain syndrome: focus on sympathetically maintained pain. Clin Exp Pharmacol Physiol. 2008;35:717-724.
31 Wasner G, et al. Vascular abnormalities in acute reflex sympathetic dystrophy (CRPS I): complete inhibition of sympathetic nerve activity with recovery. Arch Neurol. 1999;56:613-620.
32 Weber M, et al. Facilitated neurogenic inflammation in complex regional pain syndrome. Pain. 2001;91:251-257.
33 Heijmans-Antonissen C, et al. Multiplex bead array assay for detection of 25 soluble cytokines in blister fluid of patients with complex regional pain syndrome type 1. Mediators Inflamm. 2006;2006:283-298.
34 Kramer HH, et al. Inhibition of neutral endopeptidase (NEP) facilitates neurogenic inflammation. Exp Neurol. 2005;195:179-184.
35 Wesseldijk F, et al. Tumor necrosis factor-alpha and interleukin-6 are not correlated with the characteristics of Complex Regional Pain Syndrome type 1 in 66 patients. Eur J Pain. 2008;12:716-721.
36 Vartiainen NV, Kirveskari E, Forss N. Central processing of tactile and nociceptive stimuli in complex regional pain syndrome. Clin Neurophysiol. 2008;119:2380-2388.
37 Yung CO, Bruehl SP. Complex regional pain syndrome. Curr Treat Options Neurol. 2003;5:499-511.
38 Woolf CJ, Thompson SW. The induction and maintenance of central sensitization is dependent on N-methyl-D-aspartic acid receptor activation; implications for the treatment of post-injury pain hypersensitivity states. Pain. 1991;44:293-299.
39 Shiraishi S, et al. Cerebral glucose metabolism change in patients with complex regional pain syndrome: a PET study. Radiat Med. 2006;24:335-344.
40 Maihofner C, et al. Cortical processing of mechanical hyperalgesia: a MEG study. Eur J Pain. 2010;14:64-70.
41 Oaklander AL, Fields HL. Is reflex sympathetic dystrophy/complex regional pain syndrome type I a small-fiber neuropathy? Ann Neurol. 2009;65:629-638.
42 Oaklander AL, et al. Evidence of focal small-fiber axonal degeneration in complex regional pain syndrome-I (reflex sympathetic dystrophy). Pain. 2006;120:235-243.
43 Oerlemans HM, et al. Adjuvant physical therapy versus occupational therapy in patients with reflex sympathetic dystrophy/complex regional pain syndrome type I. Arch Phys Med Rehabil. 2000;81:49-56.
44 Oerlemans HM, et al. Do physical therapy and occupational therapy reduce the impairment percentage in reflex sympathetic dystrophy? Am J Phys Med Rehabil. 1999;78:533-539.
45 Price DD, et al. Analysis of peak magnitude and duration of analgesia produced by local anesthetics injected into sympathetic ganglia of complex regional pain syndrome patients. Clin J Pain. 1998;14:216-226.
46 Christensen K, Jensen EM, Noer I. The reflex dystrophy syndrome response to treatment with systemic corticosteroids. Acta Chir Scand. 1982;148:653-655.
47 Perez RS, et al. The treatment of complex regional pain syndrome type I with free radical scavengers: a randomized controlled study. Pain. 2003;102:297-307.
48 Robinson JN, Sandom J, Chapman PT. Efficacy of pamidronate in complex regional pain syndrome type I. Pain Med. 2004;5:276-280.
49 Varenna M, et al. Intravenous clodronate in the treatment of reflex sympathetic dystrophy syndrome: a randomized, double blind, placebo controlled study. J Rheumatol. 2000;27:1477-1483.
50 Melzack R, Wall PD. Pain mechanisms: a new theory. Science. 1965;150:971-979.
51 Tanaka S, et al. Low intensity spinal cord stimulation may induce cutaneous vasodilation via CGRP release. Brain Res. 2001;896:183-187.
52 Wu M, Linderoth B, Foreman RD. Putative mechanisms behind effects of spinal cord stimulation on vascular diseases: a review of experimental studies. Auton Neurosci. 2008;138:9-23.
53 Gao J, et al. Effects of spinal cord stimulation with “standard clinical” and higher frequencies on peripheral blood flow in rats. Brain Res. 2010;1313:53-61.
54 Wu M, et al. Extracellular signal-regulated kinase (ERK) and protein kinase B (AKT) pathways involved in spinal cord stimulation (SCS)-induced vasodilation. Brain Res. 2008;1207:73-83.
55 Wu M, et al. Sensory fibers containing vanilloid receptor-1 (VR-1) mediate spinal cord stimulation-induced vasodilation. Brain Res. 2006;1107:177-184.
56 North R, et al. American Academy of Pain Medicine: practice parameters for the use of spinal cord stimulation in the treatment of chronic neuropathic pain. Pain Med. 2007;8(suppl 4):S200-S275.
57 Kemler MA, et al. Spinal cord stimulation in patients with chronic reflex sympathetic dystrophy. N Engl J Med. 2000;343:618-624.
58 Kemler MA, et al. Pain relief in complex regional pain syndrome due to spinal cord stimulation does not depend on vasodilation. Anesthesiology. 2000;92:1653-1660.
59 Kemler MA, et al. Impact of spinal cord stimulation on sensory characteristics in complex regional pain syndrome type I: a randomized trial. Anesthesiology. 2001;95:72-80.
60 Kemler MA, et al. Effect of spinal cord stimulation for chronic complex regional pain syndrome type I: five-year final follow-up of patients in a randomized controlled trial. J Neurosurg. 2008;108:292-298.
61 Kemler MA, et al. The effect of spinal cord stimulation in patients with chronic reflex sympathetic dystrophy: two years’ follow-up of the randomized controlled trial. Ann Neurol. 2004;55:13-18.
62 Harke H, et al. Spinal cord stimulation in sympathetically maintained complex regional pain syndrome type I with severe disability: a prospective clinical study. Eur J Pain. 2005;9:363-373.
63 Oakley J, Weiner RL. Spinal cord stimulation for complex regional pain syndrome: a prospective study of 19 patients at 2 centers. Neuromodulation. 1999;2:47-50.
64 Burchiel KJ, et al. Prospective, multicenter study of spinal cord stimulation for relief of chronic back and extremity pain. Spine. 1996;21:2786-2794.
65 Calvillo O, et al. Neuroaugmentation in the treatment of complex regional pain syndrome of the upper extremity. Acta Orthop Belg. 1998;64:57-63.
66 Olsson GL, Meyerson BA, Linderoth B. Spinal cord stimulation in adolescents with complex regional pain syndrome type I (CRPS-I). Eur J Pain. 2008;12:53-59.
67 Bennett DS, et al. Spinal cord stimulation for complex regional pain syndrome I (RSD): a retrospective multicenter experience from 1995 to 1998 of 101 patients. Neuromodulation. 1999;2:202-210.
68 Hayek SM, Veizi IE, Stanton-Hicks M. Four-limb neurostimulation with neuroelectrodes placed in the lower cervical epidural spac. Anesthesiology. 2009;110:681-684.
69 Williams KA, Korto K, Cohen SP. Spinal cord stimulation: “neural switch” in complex regional pain syndrome type I. Pain Med. 2009;10:762-786.
70 Huntoon MA, et al. Feasibility of ultrasound-guided percutaneous placement of peripheral nerve stimulation electrodes and anchoring during simulated movement: part two, upper extremity. Reg Anesth Pain Med. 2008;33:558-565.
71 Huntoon MA, Burgher AH. Ultrasound-guided permanent implantation of peripheral nerve stimulation (PNS) system for neuropathic pain of the extremities: original cases and outcomes. Pain Med. 2009;10:1369-1377.
72 Slavin KV. Peripheral nerve stimulation for neuropathic pain. Neurotherapeutics. 2008;5:100-106.
73 Mobbs RJ, Nair S, Blum P. Peripheral nerve stimulation for the treatment of chronic pain. J Clin Neurosci. 2007;14:216-221.
74 Hassenbusch SJ, et al. Long-term results of peripheral nerve stimulation for reflex sympathetic dystrophy. J Neurosurg. 1996;84:415-423.
75 Maleki J, et al. Patterns of spread in complex regional pain syndrome, type I (reflex sympathetic dystrophy). Pain. 2000;88:259-266.
76 Maihofner C, et al. The motor system shows adaptive changes in complex regional pain syndrome. Brain. 2007;130:2671-2687.
77 Swart CM, Stins JF, Beek PJ. Cortical changes in complex regional pain syndrome (CRPS). Eur J Pain. 2009;13:902-907.
78 Velasco F, et al. Motor cortex electrical stimulation applied to patients with complex regional pain syndrome. Pain. 2009;147:91-98.
79 Son UC, et al. Motor cortex stimulation in a patient with intractable complex regional pain syndrome type II with hemibody involvement: case report. J Neurosurg. 2003;98:175-179.
80 North R, et al. American Academy of Pain Medicine: Practice parameters for the use of spinal cord stimulation in the treatment of chronic neuropathic pain. Pain Med. 2007;8(suppl 4):S200-S275.
81 Cameron T. Safety and efficacy of spinal cord stimulation for the treatment of chronic pain: a 20-year literature review. J Neurosurg. 2004;100:254-267.
82 Eddicks S, et al. Thoracic spinal cord stimulation improves functional status and relieves symptoms in patients with refractory angina pectoris: the first placebo-controlled randomised study. Heart. 2007;93:585-590.
83 Forouzanfar T, et al. Spinal cord stimulation in complex regional pain syndrome: cervical and lumbar devices are comparably effective. Br J Anaesth. 2004;92:348-353.
84 Szeinberg-Arazi D, et al. A functional and psychosocial assessment of patients with post-Sudeck atrophy amputation. Arch Phys Med Rehabil. 1993;74:416-418.
85 Galer BS, et al. Course of symptoms and quality of life measurement in complex regional pain syndrome: a pilot survey. J Pain Symptom Manage. 2000;20:286-292.
86 Eisenberg E, et al. Evidence for cortical hyperexcitability of the affected limb representation area in CRPS: a psychophysical and transcranial magnetic stimulation study. Pain. 2005;113:99-105.
87 Beltrutti D, et al. The psychological assessment of candidates for spinal cord stimulation for chronic pain management. Pain Pract. 2004;4:204-221.
88 Oakley JC. Spinal cord stimulation for the treatment of chronic pain. In: Follet KA, editor. Neurosurgical Pain Management. Philadelphia: Saunders; 2004:131-144.
89 van EF, et al. Brush-evoked allodynia predicts outcome of spinal cord stimulation in complex regional pain syndrome type 1. Eur J Pain. 2010;14:164-169.
90 Kemler MA, Furnee CA. Economic evaluation of spinal cord stimulation for chronic reflex sympathetic dystrophy. Neurology. 2002;59:1203-1209.
91 Kumar K, Malik S, Demeria D. Treatment of chronic pain with spinal cord stimulation versus alternative therapies: cost-effectiveness analysis. Neurosurgery. 2002;51:106-115.
92 Taylor RS, Van Buyten JP, Buchser E. Spinal cord stimulation for complex regional pain syndrome: a systematic review of the clinical and cost-effectiveness literature and assessment of prognostic factors. Eur J Pain. 2006;10:91-101.
93 Mekhail NA, Aeschbach A, Stanton-Hicks M. Cost benefit analysis of neurostimulation for chronic pain. Clin J Pain. 2004;20:462-468.