CHAPTER 106 NEUROREHABILITATION
This chapter provides an overview of the rehabilitation principles used to improve function and facilitate recovery in patients with neurological injury. Numerous and varied neurological conditions manifest unique impairments. Neurorehabilitation is a diverse topic. This chapter presents a systematic approach to evaluation and implementation of general rehabilitation interventions for neurological injury. The paradigms for treatment presented focus on spinal cord injury (SCI) and brain injury models, although the treatment approaches can be applied to the rehabilitation of patients with other disorders with similar neurological sequelae. Rehabilitation programs typically consist of two parts: (1) skilled therapeutic exercise, to maximize function, and (2) prescription and incorporation of specific adaptive equipment, to facilitate optimal function, mobility, and independence.
ASSESSMENT
Initial Evaluation
Numerous disease states can cause injury to the spinal cord, including trauma, spondylosis, demyelinating diseases, tumor, neurodegenerative diseases, infection, vascular injury, and toxic metabolic disorders. There are approximately 11,000 new cases of traumatic SCI per year in the United States.1 About 700,000 people suffer a stroke each year, and stroke is currently the leading cause of serious long-term disability in the United States.2 However, numerous other disorders of brain function cause significant disability, and these patients may also benefit from directed rehabilitation therapies. Examples include encephalopathies, neurodegenerative diseases, hydrocephalus, demyelinating disease, and primary and metastatic brain malignancies. Many different types of rehabilitation programs are available for patients and are stratified in Table 106-1 according to level of medical acuity (the need for close medical supervision or nursing services) and intensity.
| Program | Medical Acuity* | Hours of Rehabilitation |
|---|---|---|
| Acute inpatient rehabilitation | High | 3 (minimum/day) |
| Day therapy (outpatient) | Low-moderate | 3-5 (usually 5 days/week) |
| Subacute rehabilitation | Moderate | 1-3 (usually 3-5 days/week) |
| Home therapy | Low | 1 (usually 2-3 times/week) |
| Outpatient therapy | Low | 1-2 (usually 2-3 times/week) |
| Extended care facility | Moderate | 1 (usually 2-3 times/week) |
* The need for close medical supervision or nursing services.
Assessment Scales
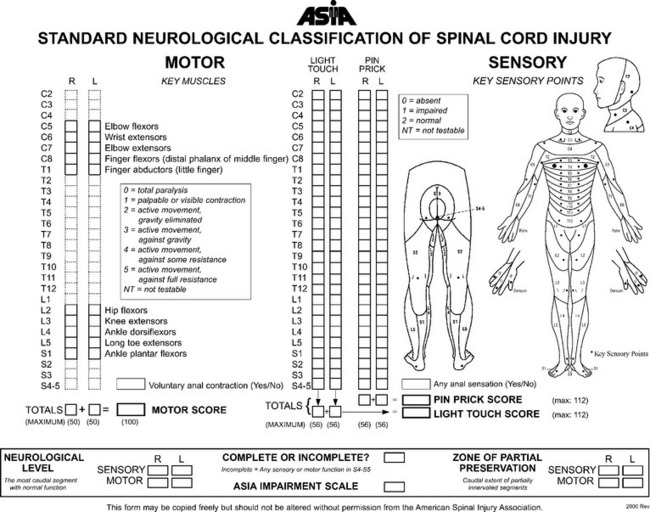
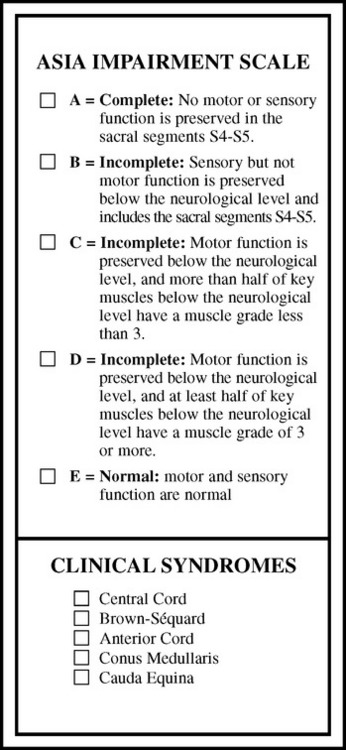
SCI assessment starts with a determination of the level and the completeness of the spinal injury. The American Spinal Injury Association has published standards for classification of SCI level (Fig. 106-1) and a grading system for completeness of neurological injury (Fig. 106-2).3 Incomplete injuries have a much better prognosis for motor recovery than do complete injuries. Since 2000, the most frequent SCI neurological category at rehabilitation hospital discharge of persons reported to the National Spinal Cord Injury database is incomplete tetraplegia (34.3%), followed by complete paraplegia (25.1%), complete tetraplegia (22.1%), and incomplete paraplegia (17.5%).1
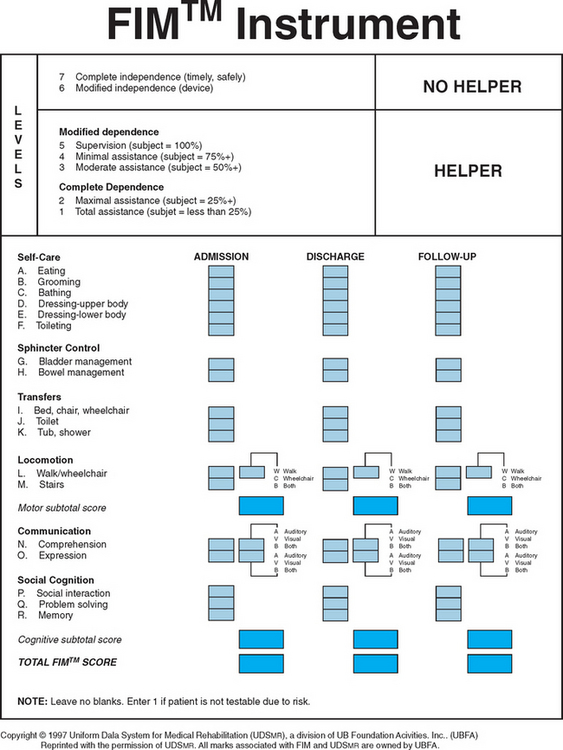
A variety of scales and assessment tools exist for evaluation of function after neurological injury. The primary initial assessment scale administered in traumatic brain injury is the Glasgow Coma Scale. The Glasgow Coma Scale numerical score reflects the depth of unconsciousness and is one of the most significant initial predictors of outcome and recovery.4 Table 106-2 summarizes some of the more commonly used scales in rehabilitation medicine. The Functional Independence Measure instrument is probably the most widely used functional assessment tool in the inpatient rehabilitation setting and can be applied irrespective of diagnosis (Fig. 106-3).
| Scale Type | Scale Name |
|---|---|
| Spinal cord injury impairment | ASIA Impairment Scale |
| Stroke deficit | NIH Stroke Scale |
| Canadian Neurologic Scale | |
| Level of consciousness/cognitive function | Glasgow Coma Scale |
| (Ranchos) Level of Cognitive Function Scale | |
| Galveston Orientation and Amnesia Test | |
| Motor function | Fugl-Meyer Scale |
| Spasticity | Modified Ashworth Scale |
| Basic ADLs and mobility | Barthel Index |
| Functional Independence Measure | |
| Instrumental ADLs | Lawton & Brody Instrumental ADL Scale |
| Katz ADL Scale | |
| Depression | Beck Depression Scale |
| Hamilton Depression Scale | |
| Quality of life | Sickness Impact Profile |
| Health status | Short-Form 36 |
ADL, activity of daily living; ASIA, American Spinal Injury Association; NIH, National Institutes of Health.
ACUTE ILLNESS REHABILITION
Rehabilitation protocols should begin immediately after neurological injury, even if the patient is critically ill. One of the main goals is to minimize the effects of prolonged immobility that can be associated with severe neurological injury. In addition, quick identification of other body systems that may have been adversely affected facilitates immediate implementation of treatment schemes and thus minimizes the potential for morbidity. Table 106-3 outlines the various areas that require assessment.
| Rehabilitation Measure | Principle |
|---|---|
| Musculoskeletal | Prevention of contractures with range-of-motion exercise, stretching, positioning, splints, and footboards |
| Pulmonary | Incentive spirometry, chest percussive therapy, pulmonary toilet |
| Swallowing | Bedside or video swallowing evaluation to assess aspiration risk |
| Skin | Frequent repositioning (every 2 hours), padding of bony prominences, pressure-reducing mattresses, and specialized beds |
| Bowl | Assessment of continence, constipation, neurogenic bowl |
| Bladder | Assessment of continence, check for infection, implementation of indwelling or intermittent catheterization |
| DVT prophylaxis | Subcutaneous heparin, sequential leg compression devices, compression garments, encouraging mobility and active calf exercises |
| Confusion | Reducing sedating medications, installing restraints or net bed for patient safety |
DVT, deep vein thrombosis.
FUNDAMENTAL REHABILITATION INTERVENTIONS
Flexibility
Flexibility is an integral component of most rehabilitation schemes. Flexibility exercises can reduce contractures, reduce muscle pain, and decrease spasticity. Prolonged gentle passive stretching is preferred over aggressive forced or ballistic stretching,5 which can tear myofibrils and supportive connective tissue, leading to increased pain. The pain and microscopic tearing associated with aggressive stretching techniques reduces the potential for progressive gains in flexibility. Heat application before stretching or a gentle aerobic warmup (if the patient is able to perform this) improves blood flow to the muscle and improves tissue elasticity. Ideally, stretches should be held for at least 30 seconds6 and, for optimal results, repeated twice a day.
Strengthening
For patients with profound motor impairment, neuromuscular electrical stimulation can be used to facilitate strengthening, and in patients with SCI, such stimulation has been demonstrated to reduce disuse-related atrophy.7 However, caution should be exercised with use of neuromuscular electrical stimulation in patients with myopathy because it may result in exhaustion of the myopathic muscle.
The primary physiological processes by which normal muscles achieve greater strength are muscle hypertrophy and enhanced neuromuscular control. In the initial 2 weeks of any strengthening program, the improvements in strength are related less to muscle hypertrophy than to enhanced neuromuscular control8; thereafter, muscle hypertrophy is the predominant factor. In the patient with neurological injury as the cause of weakness, improvements in strength also depend greatly on the processes of neurological recovery. The mechanisms of recovery vary, depending on the precise nature and extent of neurological injury.9 For example, central reorganization of motor control (neural plasticity) occurs after stroke, and collateral sprouting of motor unit nerve endings occurs in peripheral neurological injury.
Balance, Coordination, and Neuromuscular Reeducation
Rehabilitation of an extremity where injury has significantly impaired voluntary motor control is likewise challenging. Selection of a rehabilitation strategy depends, in part, on whether the limb is flaccid or spastic. Treatment in the flaccid extremity focuses on exploiting synergy patterns of muscle activation to facilitate voluntary movement. In the spastic extremity, techniques such as the Bobath neurodevelopmental training method or proprioceptive neuromuscular facilitation are used along with gentle prolonged stretching to decrease spasticity and improve motor control by using specific tone-reducing postures and movement patterns.10,11 Biofeedback with surface electromyography can be used to facilitate neuromuscular reeducation.11 Orthoses and adaptive equipment are commonly used thoroughout the rehabilitation process to facilitate improvements in extremity function. These are discussed in more detail in subsequent sections.
Gait and Mobility
Specific techniques taught to patients to facilitate transfers include sliding board transfers and standing pivot transfers. Good trunk stability and triceps strength are requirements for sliding board transfers when patients have significant lower extremity weakness. Standing pivot transfers require good trunk stability, hip girdle strength, and quadriceps strength. Parallel bars and hemibars (a single bar for stroke rehabilitation) are used for gait training. The focus is on trying to achieve a fluid reciprocal gait. Specialized harness and treadmill apparatuses have been developed for gait training, including that for patients with significant impairments such as hemiplegia, paraplegia, and gait abnormalities associated with Parkinson’s disease.12,13 Adaptive equipment and lower extremity orthoses are widely used throughout the rehabilitation process to facilitate improvements in mobility and ambulation.
Activities of Daily Living
Activities of daily living (ADLs) can be classified as basic ADLs and instrumental ADLs. Basic ADLs include such activities as feeding, grooming, bathing, dressing, and toilet use. Instrumental ADLs include higher level functions such as housekeeping, bookkeeping, shopping, telephoning, and managing medications.
Adaptive Equipment
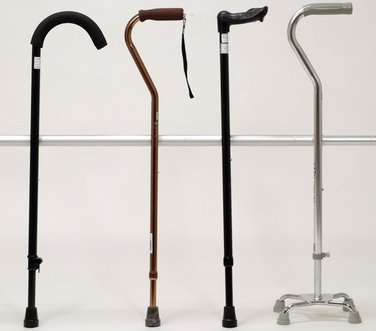
As previously mentioned, rehabilitation of the neurologically impaired patient consists of two parts: (1) skilled therapeutic exercise to maximize function (as detailed in the preceding sections) and (2) prescription and incorporation of specific adaptive equipment to facilitate optimal function, mobility, and independence. Table 106-4 outlines some of the more commonly prescribed orthoses and their primary rehabilitation applications. Figures 106-4 to 106-9 depict common ambulatory aids. It is important for the physician and physical therapist to ensure that the prescribed orthosis or ambulatory aid is properly fitted and has the desired effect on function. Proper training with the assistive device to promote maximal function is particularly important with the prescription of ambulatory aids.
TABLE 106-4 Commonly Prescribed Orthoses and Their Primary Applications
| Orthoses Type | Primary Application |
|---|---|
| Wrist-hand orthosis | Prevents contracture and reduces tone in a spastic upper extremity |
| Lumbrical bar | Prevents hyperextension of metacarpophalangeal joints and improves grip in a hand with loss of intrinsic muscle function |
| Short thumb shell | For median nerve injury: improves pincer grip |
| Wrist tenodesis splint | Facilitates passive finger flexion and grasping when the wrist is placed in extension by the brace |
| Universal cuff | Assists with feeding and grooming by holding utensils |
| Balanced forearm orthosis | Improves active elbow motion by supporting the upper arm in an abducted position and thereby eliminating the effects of gravity |
| Arm sling or tray | Supports the elbow, thus reducing shoulder pain in patients with stroke |
| Ankle-foot orthosis | Prevents footdrop, improves gait mechanics |
| Long leg brace | Supports weak quadriceps and ankle to facilitate ambulation |
| Reciprocating gait orthosis | Supports weak hip girdle and leg muscles to facilitate ambulation |
| Cane, crutch, or walker (many varieties) | Improves balance, prevents falls, reduces arthritic hip and knee pain, reduces energy demands of walking |
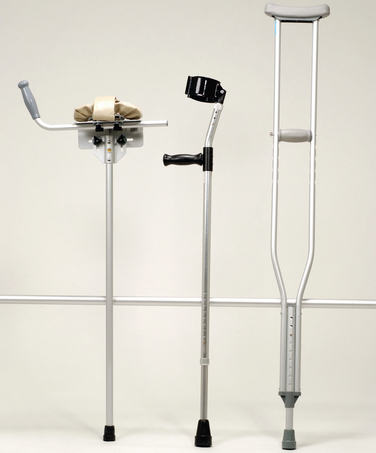
Figure 106-5 Crutches. Left to right, Platform crutch with arm tray and hand grip, Lofstrand crutch, and axillary crutch.
Before discharge from inpatient rehabilitation centers, patients should have their home environments thoroughly assessed. Some patients may require significant modification of their homes and need additional durable medical equipment to ensure a safe environment and provide the patient with maximal independence. Table 106-5 lists some of the more common equipment needs.
TABLE 106-5 Common Adaptive Equipment and Durable Medical Equipment Needs
| Environment | Equipment |
|---|---|
| Home entrance | Ramp, railing |
| Living room | Lift chair, reaching device |
| Bedroom | Electric hospital-type bed, bedside commode, urinal |
| Bathroom | Toilet seat riser, bath bench or shower chair, grab bars, handheld shower |
| Kitchen | Built-up utensils |
| General | Scooter, power wheelchair |
COMMON MEDICAL AND REHABILITATION ISSUES
Spasticity
Numerous therapeutic strategies exist for the management of spasticity in patients with upper motor neuron syndrome, including rehabilitation strategies, oral medications, intrathecal medications, focal chemodenervation, and surgery. Rehabilitation treatment strategies are variably effective14 and are summarized in Table 106-6. Spasticity scales, such as the Modified Ashworth Scale (Table 106-7), are helpful in monitoring response to treatment.
TABLE 106-6 Rehabilitation Strategies for Spasticity Management
TABLE 106-7 The Modified Ashworth Scale
| 0 | No increase in muscle tone |
| 1 | Slight increase in tone with a catch and release or minimal resistance at end of range of motion |
| 1+ | Slight increase in tone with a catch, followed by minimal resistance throughout the remaining range of motion |
| 2 | More marked increase in tone throughout range of motion |
| 3 | Considerable increase in tone, difficulty with passive movement |
| 4 | Rigidity of affected part |
Before aggressive medical or interventional therapies for spasticity are initiated, the patient should undergo a detailed assessment to determine which functional limitations or possible functional benefits their hypertonicity creates. For example, Ashworth grade 3 tone in the lower extremities of a paraparetic patient with multiple sclerosis may impair lower extremity dressing and personal hygiene. However, the spasticity may make standing pivot transfers easier, particularly if the patient has very poor lower extremity strength. The patient and physician may desire to reduce the spasticity through treatment; however, if therapy is successful and tone is significantly reduced, the patient may lose the ability to perform standing pivot transfers. Thus, both positive and negative effects on function must be considered before treatment, particularly if irreversible treatments such as neurolysis and surgery are being considered.15
The evaluation of a patient with worsening spasticity starts with a thorough assessment of potential noxious stimuli that may create amplification of muscle tone. Common causes of worsening spasticity in patients with SCI include bowel impaction, urinary tract infection, decubitus ulceration, acute abdomen, and pathological fracture. Rapid identification and treatment of these problems can result in a quick return to baseline tone.15
Neurogenic Bowel
Neurogenic bowel associated with SCI and other upper motor neuron disorders typically results in constipation and difficulty with stool evacuation. Rehabilitation interventions are aimed at bowel retraining and establishment of a routine bowel regimen.16,17 The goals of a bowel program are to use either digital rectal stimulation or chemical stimulation (mini-enema or suppository) to trigger reflex evacuation of stool in the distal colon. For this to work appropriately, stools must be soft but well formed so that peristalsis can propagate the stool toward the rectum. Bulking agents such as fiber supplements and stool softeners aid in establishing optimal stool consistency. In a bowel program, patients typically evacuate daily or every other day.
Patients who have sustained lower motor neuron injury to the sacral segments face a more challenging task in maintaining stool continence.18 With partial nerve injuries, bowel retraining is focused predominantly on strengthening the pelvic floor, especially the puborectalis and the external anal sphincter. Persistent and significant fecal incontinence reduces patient dignity and may lead to avoidance of socialization. For severe persistent problems that jeopardize skin care, surgical sling procedures or colostomy are options.
Neurogenic Bladder
Neurological injury can cause a variety of problems with bladder function. In the first few weeks after an acute SCI, the bladder is usually flaccid (as a result of spinal shock), and urinary retention occurs. Indwelling catheters are usually placed on initial hospitalization, but as soon as appropriate, patients should be transitioned to an intermittent catheterization schedule. Patients are typically taught to self-catheterize at a frequency of four to five times a day. Oral hydration schedules are created to balance the patient’s needs for hydration without making it necessary to self-catheterize every few hours. The aim is to prevent accumulation of more than 500 mL in the bladder at one time and prevent overdistention, as well as overflow incontinence.19
If the patient regains sacral segment sensorimotor function, bladder training ensues. Patients follow timed voiding schedules and learn techniques to facilitate bladder emptying such as suprapubic compression or reflex-stimulated voiding. Patients can also undergo more intensive electromyographically assisted bladder retraining programs to improve coordinated activation and relaxation of the muscles necessary to evacuate the bladder.19 Postvoid residuals are monitored to ensure complete emptying. When patients can consistently achieve postvoid residuals of less than 100 mL, intermittent catheterization can be discontinued.
Many patients with SCI are at risk for developing detrusor sphincter dyssynergia, which results in a high-pressure bladder. In dyssynergia, the detrusor muscle spontaneously contracts, and the urethral sphincter does not concomitantly relax to allow bladder emptying. This creates high pressures in the bladder that force urine up the ureters into the kidneys; serious renal injury can develop. Dyssynergic bladders can be diagnosed with voiding cystomyography.20 Patients with neurogenic bladder (especially high-pressure bladders) need to undergo periodic reevaluation with cystomyography and lifelong renal surveillance.21
Pressure Ulcers
Pressure ulceration of the skin can be a devastating complication of immobility. Patients with neurological injury are particularly at risk for pressure ulcers because of immobility and insensate skin. The body areas at highest risk are, first, the sacrum and then bony prominences such as the greater trochanters and heels. Furthermore, bowel and bladder incontinence can increase the risk of skin maceration and secondary infection.22
Patients with the ability to reposition themselves should do so frequently. Paraplegic patients use triceps press-ups to provide transient pressure relief to their buttocks and legs. Dependent patients must be turned by a caregiver every 2 hours and have bony prominences padded. Wheelchairs should be fitted with pressure-reducing cushions, and patients who spend considerable time in bed need a pressure-reducing mattress.22
Dysphagia
A clinical swallowing evaluation can be administered at the bedside to establish a basic understanding of a patient’s aspiration risk. A more extensive evaluation with video fluoroscopy can help determine how different food consistencies are handled at the oral, pharyngeal, and esophageal phases of swallowing.23 Patients are taught protective and compensatory techniques for swallowing, such as the supraglottic swallow and the Mendelsohn maneuver.24 Liquids may be particularly difficult to control during the oral phase and are easily aspirated. This risk can be minimized by using a flavorless gelatin to increase the consistency of the liquid without altering its taste. Avoiding foods with multiple consistencies (e.g., stews or soups) also decreases the risk of aspiration.
Communication
Speech and language disorders can be generally classified into four categories: aphasias, dysarthrias, dysphonias, and apraxia of speech. Speech-language pathologists can further characterize these disorders and formulate appropriate speech therapy interventions. Rehabilitation approaches vary according to diagnosis and target the patient’s primary impairments.25 Other difficulties in communication may also result from confusion, ventilator dependency, and cognitive impairment, among other sequelae of significant neurological injury. Further discussion on evaluation and treatment of speech and language disorders can be found in Chapter 3.
Autonomic Hyperreflexia
Autonomic hyperreflexia is a potentially life-threatening condition that can occur in patients with SCI above the T6 level. Autonomic hyperreflexia develops when a noxious stimuli creates a sudden surge in sympathetic outflow that is not appropriately modulated by descending supraspinal inhibitory signals as a result of the SCI. This results in severe hypertension, headache, facial flushing, and reflex bradycardia (caused by stimulation of carotid sinus receptors).26 Treatment consists of sitting the patient upright, quickly identifying and eliminating the noxious source, and, when necessary, administering antihypertensives such as nitrates or nifedipine.26
Heterotopic Ossification
Heterotopic ossification is aberrant bone formation that can occur with a variety of neurological injuries, such as SCI, stroke, and traumatic brain injury. It most commonly occurs about the hips, knees, shoulders, and elbows. Early clinical manifestations are warmth and swelling of the affected area. If new bone formation is exuberant, loss of range of motion and even joint ankylosis can occur. Radiographs demonstrate new bone formation; however, the most specific test for early identification is the triple-phase bone scan. The blood flow and blood pooling phases show areas of angiogenesis and matrix formation even before ossification.27 Initial treatment is institution of an aggressive range-of-motion program to prevent ankylosis of the joint. Bisphosphonates can reduce the severity of heterotopic ossification when they are initiated soon after the diagnosis is made.28
SUMMARY
Foti D, Pedretti LW, Lillie S. Activities of daily living. In: Pedretti LW, editor. Occupational Therapy Practice Skills for Physical Dysfunction. 4th ed. St. Louis: CV Mosby; 1996:463-506.
Good DC. Stroke: Promising Neurorehabilitation Interventions and Steps Toward Testing Them. Am J Phys Med Rehabil. 2003;82S:50-57.
Kirshblum S. Rehabilitation of spinal cord injury. In: DeLisa JA, Gans BM, Walsh NE, et al, editors. Physical Medicine and Rehabilitation: Principles and Practice. 4th ed. New York: Lippincott Williams & Wilkins; 2004:1715-1752.
Roth EJ, Harvey RL. Rehabilitation of stroke syndromes. In: Braddom RL, editor. Physical Medicine and Rehabilitation. 2nd ed. Philadelphia: WB Saunders; 2000:1117-1160.
Whyte J, Hart T, Laborde A, et al. Rehabilitation issues in traumatic brain injury. In: DeLisa JA, Gans BM, Walsh NE, et al, editors. Physical Medicine and Rehabilitation: Principles and Practice. 4th ed. New York: Lippincott Williams & Wilkins; 2004:1677-1714.
1 Spinal Cord Injury, Facts and Figures at a Glance, Birmingham AL. University of Alabama at Birmingham, National Spinal Cord Injury Statistical Center, August 2004. J Spinal Cord Med. 2004;27(Suppl 1):S139-S140.
2 American Heart Association. Heart Disease and Stroke Statistics—2005 Update. Dallas: American Heart Association, 2004.
3 International Standards for Neurological Classification of SCI. Atlanta: American Spinal Injury Association, 2002.
4 Vollmer DG. Prognosis and outcome of severe head injury. In: Cooper PR, editor. Head Injury. 3rd ed. Baltimore: Williams & Wilkins; 1993:553-581.
5 Bandy WD, Irion JM, Briggler M. The effect of static stretch and dynamic range of motion training on the flexibility of the hamstring muscles. Phys Ther. 1998;27:295-300.
6 Bandy WD, Irion JM. The effect of time on static stretch on the flexibility of the hamstring muscles. Phys Ther. 1994;74:845-852.
7 Yarkony GM, Roth EJ, Cybulski GR, et al. Neuromuscular stimulation in spinal cord injury II: prevention of secondary complications. Arch Phys Med Rehabil. 1992;73:195-200.
8 Moritani T, deVries HA. Neural factors versus hypertrophy in the time course of muscle strength gain. Am J Phys Med. 1979;58:115-130.
9 Bach-y-Rita P, Brown AW, Lazarus JC, et al. Neural aspects of motor function as a basis of early and postacute rehabilitation. In: DeLisa JA, Gans BM, editors. Rehabilitation Medicine. 2nd ed. Philadelphia: JB Lippincott; 1993:381-403.
10 Luke C, Dodd KJ, Brock K. Outcomes of the Bobath concept on upper limb recovery following stroke. Clin Rehabil. 2004;18:888-898.
11 Albert T, Yelnik A. [Physiotherapy for spasticity]. Neurochirurgie. 2003;49(2–3, Pt 2):239-246.
12 Hesse S, Bertelt C, Jahnke MT, et al. Treadmill training with partial body weight support compared with physiotherapy in nonambulatory hemiparetic patients. Stroke. 1995;26:976-981.
13 Miyai I, Fujimoto Y, Yamamoto H, et al. Long-term effect of body weight–supported treadmill training in Parkinson’s disease: a randomized controlled trial. Arch Phys Med Rehabil. 2002;83:1370-1373.
14 Watanabe T. The role of therapy in spasticity management. Am J Phys Med Rehabil. 2004;83(10, Suppl):S45-S49.
15 Satkunam LE. Rehabilitation medicine: 3. Management of adult spasticity. CMAJ. 2003;169:1173-1179.
16 Chen D, Nussbaum SB. The gastrointestinal system and bowel management following spinal cord injury. Phys Med Rehabil Clin North Am. 2000;11:45-56.
17 Merenda L, Brown JP. Bladder and bowel management for the child with spinal cord dysfunction. J Spinal Cord Med. 2004;27(Suppl 1):S16-S23.
18 Yim SY, Yoon SH, Lee IY, et al. A comparison of bowel care patterns in patients with spinal cord injury: upper motor neuron bowel vs lower motor neuron bowel. Spinal Cord. 2001;39:204-207.
19 Andrews KL, Opitz JL. Bladder retraining. In: Sinaki M, editor. Basic Clinical Rehabilitation Medicine. 2nd ed. St. Louis: CV Mosby; 1993:195-206.
20 Rendeli C, Ausili E, Salvaggio E. Neurogenic bladder dysfunction: urodynamic evaluation. Rays. 2002;27:127-130.
21 Fowler CJ, O’Malley KJ. Investigation and management of neurogenic bladder dysfunction. J Neurol Neurosurg Psychiatry. 2003;74(Suppl 4):iv27-iv31.
22 Edlich RF, Winters KL, Woodard CR, et al. Pressure ulcer prevention. J Long Term Eff Med Implants. 2004;14:285-304.
23 Finestone HM, Greene-Finestone LS. Rehabilitation medicine: 2. Diagnosis of dysphagia and its nutritional management for stroke patients. CMAJ. 2003;169:1041-1044.
24 Noll SF, Bender CE, Nelson MC, et al. Rehabilitation of patients with swallowing disorders. In: Braddom RL, editor. Physical Medicine and Rehabilitation. 2nd ed. Philadelphia: WB Saunders; 2000:535-560.
25 Miller RM, Groher ME, Yorkston KM, et al. Speech, language, swallowing and auditory rehabilitation. In: DeLisa JA, Gans BM, Walsh NE, et al, editors. Physical Medicine and Rehabilitation: Principles and Practice. 4th ed. New York: Lippincott Williams & Wilkins; 2004:1025-1050.
26 Erickson RP. Autonomic hyperreflexia: pathophysiology and medical management. Arch Phys Med Rehabil. 1980;61:431-440.
27 Buschbacher R. Heterotopic ossification: a review. Crit Rev Phys Med Rehabil Med. 1992;4:199-213.
28 Finerman GA, Stover SL. Heterotopic ossification following hip replacement or spinal cord injury. Two clinical studies with EHDP. Metab Bone Dis Relat Res. 1981;3:337-342.