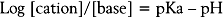CHAPTER 17 Neurophysiology of Diagnostic Injections
INTRODUCTION
In the patient presenting with symptoms in a classic dermatomal pattern and a corroborative imaging study, the diagnosis may not be in question. However, cervical radicular pain may not radiate in a classic dermatomal distribution, creating doubt in diagnosis.1 Furthermore, patients often do not present with classic symptoms. A patient presenting with posterior arm pain radiating into the radial aspect of the forearm to the wrist in the presence of multilevel cervical foraminal stenosis may have involvement of either the C6 or C7 nerve root. The ability to selectively anesthetize a specific nerve root would be helpful to determine the involved nerve root and confirm the diagnosis.
A variety of structures are potential pain generators for those patients presenting with axial pain. Potential pain generators include bone, muscle, tendon, ligament, intervertebral disc, zygapophyseal joint, and sacroiliac joint. Bone pathology can typically be diagnosed by imaging studies. Magnetic resonance imaging (MRI) has been disappointing for diagnosing discogenic pain as disc pathology can be seen in asymptomatic individuals.2–5 Furthermore, annular fissures seen on discography has been reported after normal MRI.6 Discography has been utilized to diagnose a painful disc and is discussed in greater detail in Chapter 25.
History and physical examination has not been reliable to diagnose pain of Z-joint or sacroiliac joint etiology.7–9 Imaging studies have not been helpful in diagnosing pain from the Z-joint or sacroiliac joint.10–14 Diagnosis has been based upon anesthetizing the joint.15–19 The diagnosis of mechanical low back pain has been reported to be elusive.20 However, with the advent of diagnostic injections, the etiology of mechanical low back pain can frequently be determined. With a more specific diagnosis, more specific treatment may be rendered.
PREMISE OF DIAGNOSTIC INJECTIONS
Diagnostic injections are performed to confirm or exclude a pain generator. Diagnostic injections may be utilized prior to surgery or therapeutic interventional spine management. A diagnostic injection is indicated when the diagnosis is in question despite less invasive testing and further invasive treatment is indicated.21 More specifically, when history, physical examination, imaging studies, and electrodiagnostic testing have failed to elucidate the etiology of the patient’s symptoms, a diagnostic injection may be indicated. Additionally, the patient should be a candidate for more invasive treatment such as interventional or surgical procedures. If the diagnostic injection is not going to affect treatment, the injection should not be performed.
NEUROANATOMY
The epineurium at the dorsal root ganglion consists of collagen fibrils and fibroblasts heavier than peripheral nerve as it nears transition with the thicker dura mater.22 The perineurium has multiple layers with basement membrane separating epi- and endoneurium. The endoneurium has finer collagen fibrils in the dorsal root ganglion compared to peripheral nerve.22,23
The subarachnoid angle marks the lateral border of the subarachnoid space. The dorsal root proximal to the spinal ganglion continues with epi-, peri- and endoneurium until 170 microns from the subarachnoid angle in the rat model.22 In the subarachnoid region, cells bordering the subarachnoid space may either reflect back onto itself or attach to the root sheath with punctate junctions at the subarachnoid angle. The epineurium in the subarachnoid space becomes the dura mater. The outer layers of the perineurium continue between the dura mater and arachnoid membrane. The inner layers of the perineurium becomes highly irregular. For the ventral root, highly hydrated cells, lacking basement membrane, replace the inner layers of the perineurium. In the dorsal root, the perineurium at the subarachnoid angle loses continuity with irregular tissues. In the subarachnoid space, loosely arranged cells overly the root sheath, which has endoneurial tissue similar to peripheral nerve. The basement membrane of perineurium serves as a diffusion barrier to substances such as anesthetic agents. The discontinuity of perineurium at the subarachnoid angle for the dorsal root, and lack of basement membrane for ventral root, allow easier penetration of substances to the nerve sheath.22
In the subarachnoid region, the nerve root arachnoid tissue is not as effective a barrier as perineurium to anesthetic substances. Hence, lower dosages will result in block compared to the epidural space.23 However, the subarachnoid angle may also allow quicker diffusion of anesthetics because of the discontinuity of the perineurium.
The axolemma is formed by a mosaic bilayer of primarily phospholipids with lesser amounts of glycolipids and cholesterol. The outer layers of the membrane contain the hydrophobic portion of the lipid molecule while the inner layers consist of the hydrophilic portion. Interspersed within the membrane are proteins, many of which are glycosylated. The protein moieties are fixed within the membrane and compose the ion pores or channels. Various ions such as Na+, K+, Ca+, and Cl− pass through these pores that traverse the width of the membrane. In myelinated nerve, the Na+ channels are located at the nodes of Ranvier with the K+ channels interspersed between the nodes.24 In unmyelinated nerves, the Na+ and K+ channels are not selectively located.25 The flow of ions through these channels is dependent upon various factors such as ion concentration gradient, voltage gradient, and configuration of the channel. The channels may exist in an open state, closed resting state, or closed inactivated state. The channels are ion specific, primarily only allowing the passage of a specific ion. For example, voltage-gated Na+ channels allow predominately only Na+ to pass through the channel. The voltage-gated Na+ channel is typically closed at the resting membrane potential of −60 mV, but with chemical or electrical depolarization, the transmembrane potential may reach threshold of −45 mV, resulting in opening of these voltage-gated Na+ channels. With opening, extracellular Na+ flows rapidly into the axon, resulting in an action potential with subsequent depolarization of adjacent membrane. The wave of depolarization is then propagated down the axon. In a closed state, the Na+ cannot traverse this channel.
NEUROPHYSIOLOGY
Positively and negatively charged ions are present in the intracellular and extracellular neuronal environment. Intracellularly, there is an overall negative charge and extracellularly an overall positive charge. This separation of charges results in a resting potential across the membrane. The major ions responsible for the charge are Na+, Cl−, K+, and organic anions. The organic anions, such as amino acids, remain intracellular and are not permeable to the axolemma. The concentrations of Na+ and Cl− are higher extracellularly and K+ concentrations higher intracellularly. Typically, ions will diffuse from a higher concentration to a lower concentration. Electrically, positively charged ions will tend to diffuse to the more negative side. These forces interact until equilibrium develops between the electropotential and concentration gradient. This has been termed the equilibrium potential and for each ion is dependent upon concentration gradients, electropotential gradients, ion charge, and permeability. The neural membrane affects these factors.26
A Na+–K+ pump regulates the flow of these ions with three Na+ ions pumped extracellularly to every two K+ ions pumped intracellularly. The energy-dependent pump is driven by the hydrolysis of ATP. The pump accounts for maintenance of the ion gradient across the membrane and maintains the resting potential. Without the pump, Na+ accumulates intracellularly and K+ extracellularly until the electropotential gradients for both become zero. The pump maintains the potential difference at a metabolic cost – hydrolysis of ATP. The Na+–K+ pump, high permeability of K+, and low permeability of Na+ results in a zero net influx:efflux of ions, maintaining the resting potential.26
Membrane phospholipids have an insulating property separating the negatively charged axoplasma from the positively charged extracellular fluid. The charge is separated and maintained with a negative charge on the inner membrane and positive charge on the outer membrane with a potential difference. The membrane phospholipids bilayer serves as a capacitor.27
Another factor that will affect electrical conduction longitudinally down the axon is axon diameter. With a larger axoplasmic core, more ions are available for transmission of the current, resulting in a lower resistance. Smaller axons have higher resistance. For a given voltage potential across the membrane, a higher resistance will result in lower conductance (I=V/R). Another factor is the length constant. The length constant is the length of axon that a voltage potential can spread passively. The decay of voltage down the length of axon is exponential and related to the loss of current through the membrane and resistance to current in the axoplasmic core. The higher the membrane resistance the longer the length constant will be as there is less decay in the potential. Conversely, the lower the axoplasmic resistance the longer the length constant will be. A longer length constant allows spatial summation of impulses.27 The length constant is important in allowing the propagation of a depolarizing current to adjacent sections of axon without decay.
The velocity of depolarization is dependent upon membrane capacitance and axon resistance. A lower resistance allows a larger conductance. A larger capacitance will require a larger ion flow to alter the charge to change the transmembrane potential. The velocity of depolarization down an axon is dependent upon the product of axon resistance and membrane capacitance. With increasing axon diameter, the resistance is exponentially decreased with only linear increase in capacitance. This leads to higher velocity. Another way to affect velocity favorably is to increase the thickness of the capacitor, which results in decreased capacitance. Myelination achieves this goal. Myelin will also decrease the amount of ion flow across the membrane with less decay. However, the depolarization would dissipate without the node of Ranvier to allow Na+ channel conductance. This results in saltatory conduction as the action potential jumps from node to node. Sodium channels are located at the nodes. Potassium channels are primarily located along the axon between the nodes of Ranvier with few if any potassium channels at the nodes.24 The axonal membrane between nodes functions as a passive cable unless demyelination occurs. With demyelination, the bare axon becomes excitable.25 However, demyelination can result in conduction block due to decay of the propagating action potential.
Subthreshold changes in the transmembrane potential can result in intermediate opening of only a few voltage-sensitive Na+ channels which flip between open and closed states. Additionally, these subthreshold spikes may increase K+ conductance extracellularly. This can lead to some resistance to depolarization creating a higher threshold, termed accommodation.28 Once a threshold stimulus occurs, then depolarization occurs with opening of all sodium channels with development of an action potential. The action potential is then propagated down the axon. This process allows transmission along long neural pathways in the body.
Neurophysiologic effects of local anesthetics
Local anesthetics block nerve impulses by inhibiting depolarization. Local anesthetic exists in both a neutral and cation form. The cation moiety has been determined to be the active form.29–31 The cation form binds to a receptor located on the alpha subunit of the ion-conducting pore. The receptor consists of an amino acid chain within the pore.32
The cation-receptor complex alters the configuration of the sodium channel. The influx of sodium is blocked. Depolarization does not occur and the propagating impulse is blocked. The blocked segment of nerve maintains the resting potential with resultant membrane stabilization.33 Local anesthetic then dissociates from the receptor, allowing sodium conductance to resume. The cation binds and dissociates from the receptor through open channels.34 Complexed channels open and close normally but do not conduct sodium.34 Depolarizing impulses open the channels. In the presence of local anesthetic, further binding occurs leading to greater inhibition.34 Increased inhibition with depolarization has been termed phasic inhibition. With increasing discharges, more channels are opened and subject to greater blockade.35,36 The amplitude of the conditioning impulse – larger impulse with more channels open – will affect the number of channels opened and subsequent inhibition.35 Recovery from this frequency-dependent block is dependent on the concentration of anesthetic.36
Another factor that affects whether the propagating wave of the depolarizing is aborted is the length of nerve inhibited. Previously, the inhibition of depolarization at three consecutive nodes was considered the critical length for conduction block.37,38 Local anesthetic was found to result in graded reduction in nodal action potential current. Graded reduction of the sequential nodes occurred until propagation ceased.39 Complete conduction block at three consecutive nodes was found not to be necessary. However, the concept of graded reduction across sequential nodes and complete block at three consecutive nodes are not mutually exclusive.38 The graded response is dose dependent. With higher dosages complete block of sequential nodes can occur.
Various factors affect the rapidity, density, and duration of neural blockade. The onset of blockade is affected by anesthetic permeability. The anesthetic agent needs to penetrate epineurium, perineurium, endoneurium, and axolemma. In myelinated nerves, penetration would occur through the myelin sheath or at the nodes of Ranvier. Diffusion across these structures is dependent upon the ion state of the local anesthetic. The neutral form of local anesthetic is more lipophilic and can diffuse across the phospholipid bilayer of the axonal membrane.31 Alkaline pH favors the neutral form and more readily penetrates mammalian A, B, and C fiber than the cation form.30 However, the neutral form is not the active form. The neutral form needs to be converted to the cation form for blockade to occur. Conversely, the active charged cation has greater difficulty penetrating and diffusing across the axonal membrane.29,30,40
The pKa is the pH at which an anesthetic is present equally in neutral and cation form. Intracellularly, the pH is 7.4 and will favor anesthetics with a pKa lower than this – a greater concentration of the active cation form. The onset of anesthesia is faster for agents with a lower pKa.41,42 Other factors affect the ability of local anesthetics to diffuse across nerve membrane. Besides lipid solubility, permeability is additionally affected by molecular volumes, specific chemical groups, and position of chemical groups on the molecule.42
Once the neutral form of anesthetic diffuses into the phospholipid bilayer, the anesthetic agent has to desorb from the membrane into the axolemma. The benzene ring of the local anesthetic may be strongly associated to the membrane.42 The NH-C4H9 group of tetracaine is more hydrophobic than the NH2 group of procaine, with resultant slower desorption.42 The desorption of the neutral forms is inverse to the partition coefficients. The desorption rate may be the rate-limiting factor to the onset of blockade.42
Other factors may affect the degree and duration of blockade. Anesthetics with a longer alkoxyl chain have greater hydrophobia which enhances voltage-dependent block.35 The potency between anesthetic compounds is probably secondary to different kinetics within the sodium channels during the step-depolarizing pulses.35
The differences in lipid solubility among anesthetic agents relative to impulse frequency conduction block has been evaluated in frogs.36 Low and high lipid-soluble anesthetics required large number of impulses to reach maximum block in vitro.36 Intermediate lipid-soluble agents required 4–8 impulses at 40 Hz to reach maximum effect. The recovery from blockade was quicker with the intermediate lipid-soluble agents.36 Low lipid-soluble agents were the quartenary compounds. Intermediate lipid-soluble agents were procaine, lidocaine, prilocaine, and mepivacaine. High lipid-soluble agents were bupivacaine, tetracaine, etiodocaine.36
Not all nerves have the same degree of susceptibility to anesthetic agents. Differential block refers to preferential blocking of small fibers over large fibers. The smaller fibers are blocked more easily as there is less tissue for local anesthetic to diffuse across. The unmyelinated C-fibers and smaller-diameter myelinated A-delta fiber are more easily blocked than A-alpha and beta fibers. Hence, pain is preferential blocked over light touch, pressure, and motor. A higher concentration of anesthetic is needed to block these larger fibers. However, fiber size is not the only factor that leads to differential block. Another factor that may be related to differential block is frequency-dependent block. An extremely phasic sensory nerve with short, widely spaced bursts may be resistant to block.36 Nerve firing at 10–50 Hz with burst durations greater than 0.5 seconds are more apt to be blocked.36 In a painful spine condition, the C-fibers and A-delta fibers are actively firing and may be preferentially blocked.
The minimum concentration of anesthetic agent to block a nerve in vitro is the Cm. The Cm between spinal nerve and peripheral nerve is the same.41 However, the Cm is lower for subarachnoid versus epidural blockade. Various factors account for this difference. The nerve roots within the thecal sac have less tissue and barrier to anesthetic agent. Lymphatics and the venous plexus in the epidural space can carry local anesthetic away from the site. Local tissue within the epidural space can bind local anesthetic, rendering it unavailable to the spinal nerve and nerve root. Fluid within the space can dilute anesthetic agent. Any fibrous tissue between the anesthetic agent and targeted nerve serves as a barrier that anesthetic agent has to cross. Another factor that can affect the onset of blockade is the presence of a rapid transport route between the epidural space and endoneurial space.43 This rapid transport is postulated to occur through epidural venous system with retrograde flow into intraneural capillaries of the nerve roots – bypassing diffusion across the dura. Local anesthetic in the epidural space may have direct transport to the axons of the nerve roots.43 In the performance of spinal injections, the goal is to place the anesthetic agent as close as possible to the dorsal root ganglion to help minimize these other factors. Posterior epidural injections compared to selective nerve root injections would be more prone to venous absorption, lymphatic uptake, and tissue binding. Other factors that effect Cm are the pKa, metabolism, elimination, and distribution of the anesthetic.
The molecular structure of the local anesthetic agents affects their properties. For example, exchanging the butyl group of mepivacaine for methane on the amine branch increases the protein binding affinity. This substitution creates bupivacaine which has a longer duration of neural blockade than mepivacaine secondary to improved binding to the protein receptor in the sodium channel. Changes on the aromatic head affect lipid solubility. As lipid solubility is a primary determinant of onset latency, any alteration of the aromatic head will affect the onset of neural blockade.44
Other physiologic actions of local anesthetics
Local anesthetics are primarily utilized in painful spine disorders as a diagnostic tool to determine pain generators. Local anesthetic may potentially have therapeutic benefits besides just neural blockade effects. In 1930, Evans proposed that infusing large volumes of fluid could disrupt perineural adhesions.45 However, this is unlikely, as fluids tend to flow in paths of least resistance as demonstrated by radiographic contrast flow patterns.46
Local anesthetic may have an antiinflammatory effect. Local anesthetics have been shown to inhibit peritonitis in a rat model.47 Greater inhibition occurred with lidocaine than bupivacaine. The authors of the study postulate local anesthetic antagonizes prostaglandin release, leukocyte migration, and neutrophil lysosomal release. In burn injuries, topical lidocaine resulted in local vasoconstriction, reducing albumin extravasation. However, at high dosage, lidocaine results in vasodilation.48
Lidocaine has been found to affect neutrophil function. Exposure to local anesthetic (lidocaine, tetracaine) resulted in reduced superoxide release, lysosomal release, phagocytosis, exocytosis, reduced adherence, granulocyte colony stimulating factor, and bactericidal activity.49–53 Peck et al.49 postulated the mechanism of inhibition was probably stabilization of the neutrophil membrane through sodium channel blockade. Goldstein et al.50 stated tetracaine acts on the neutrophil membrane modifying stimulus–membrane interactions and retarding membrane fusion similar to corticosteroid. Local anesthetic inhibits neutrophil NADPH oxidase activity in a dose-dependent manner, resulting in decreased superoxide release.53
Local anesthetic has been found to decrease macrophage superoxide anion release.54 Lymphocyte adherence and mobility was reduced by local anesthetic.55 Local anesthetic has been postulated to effect the cell membrane, calmodulin-dependent pathways affect calcium dependent function and inhibiting protein synthesis.55
In vitro, local anesthetic has be shown to inhibit human fibroblast, endothelial cell, and keratinocyte proliferation,56 which may potentially reduce scar formation. Another potential benefit of local anesthetics is sympathetic blockade.57,58
Central processing theories may provide an explanation for the observed therapeutic effect. One theory is the benefit occurs from a placebo response, which has been reported in one-third of all interventions.59,60 Another theory is that a neural engram of pain in the brain forms from repetitively firing of wide dynamic range neurons in the substantia gelatinosa. Each of these areas incites afferent information, thereby triggering activity. Peripheral blockade can disrupt or shut off these central processing zones, resulting in pain relief or mitigation.
DIAGNOSTIC SELECTIVE NERVE ROOT INJECTIONS
The purpose of a diagnostic selective nerve root injection is to identify the nerve root causing extremity pain. A positive selective nerve root injection identifies that specific nerve root as the cause of pain. A negative selective nerve root injection rules out that nerve root as the cause of pain. To achieve this, the nerve fibers transmitting pain must be blocked without blocking fibers from an adjacent level. For example, with L5 radicular pains the L5 and not the L4 or S1 nerve needs to be blocked. Blockade of unmyelinated C and small, myelinated A-delta fibers is the objective as these fibers transmit acute, sharp pain and delayed-onset dull aching or burning pain in radicular pain.61 The goal of a diagnostic injection is to anesthetize these fibers at the spinal nerve, dorsal root ganglion, and/or dorsal nerve root. Motor blockade does not need to be achieved and these larger myelinated fibers are more resistant to blockade than the smaller fibers conducting pain. Additionally, with a radicular pain the pain fibers may be easier to block secondary to phasic inhibition. The main diffusion barrier to the anesthetic agents reaching myelinated and unmyelinated fibers within the spinal nerve, dorsal root ganglion, and nerve root is the basement membrane of the perineurium. Once crossed, the anesthetic agent may need to diffuse across the myelin sheath or enter through open sodium channels to reach the anesthetic receptor within the ion pore. Additionally, some anesthetic agent will be absorbed by non-neural tissues in the epidural space. The venous plexus and lymphatics can absorb anesthetic molecules, carrying them away from the target site.
The interventionist spine specialist needs to deliver the anesthetic as close to the target as possible without causing neural injury. The chapter on spinal injection techniques addresses this issue (Ch. 23). Additionally, consideration must be given to the volume and concentration of anesthetic required to optimally block the targeted nerve root without spread to adjacent spinal levels. The block also needs to be rapid enough to allow assessment of pain relief within a reasonable time after injection, typically within the first 20 minutes.
North et al. utilizing 3 ml of 0.5% bupivacaine found selective nerve root injections to be non-specific.62 However, Van Akkerveeken utilizing only 1.0 cc of lidocaine found a sensitivity of 90% and positive predictive value of 95%.63 In this study, subjects with known radiculopathy secondary to either tumor or herniated nucleus pulposus underwent selective nerve root injection at multiple levels with the subject blinded to the levels injected. Van Akkerveeken was also able to determine the sensitivity of selective nerve root injections, which was 100%. The study of North et al.62 suggests that utilizing a volume of 3.0 cc is unacceptable as specificity is lost as medication travels to adjacent levels. Blockage of multiple levels is undesirable as it may not only incorrectly identify the nerve root level in an individual suffering from radiculitis but could also incorrectly miss a peripheral nerve lesion. Peripheral nerve lesions are typically composed of nerve fibers from multiple adjacent nerve root levels. Blockage of these multiple levels could potentially relieve peripheral nerve pain, as in North’s study. Blockage of a single nerve root level is theoretically less apt to result in 80% decrement in pain with pain emanating from multiple levels. For a diagnostic selective nerve root injection to be positive, an 80% decrement in pain from preinjection to postinjection must be achieved.21 The study of Van Akkerveeken suggests 1 cc of lidocaine is adequate for both sensitivity and specificity.
Besides temporary spinal nerve root blockade of pain transmission, local anesthetic may have other affects. Local anesthetics may improve blood flow. Yabuki and Kikuchi64 demonstrated increased radicular blood flow both proximal and distal to nerve root compression in dogs with nerve root infiltration of lidocaine. This effect was not seen with injection of saline. Localized ischemia has been postulated to cause symptoms in spinal stenosis.65 Additionally, in an animal model, exposure to autologous nucleus pulposus was found to impair blood flow along with intraneural edema, demyelination, and axon loss.66 Improvement in radicular blood flow by anesthetic agent may impart a therapeutic benefit. Whether increased radicular blood flow in an animal model translates to a therapeutic effect in humans has been questioned.44 One concern is whether the increased radicular blood flow is transient. Another concern is whether the increased blood flow produces a clinical effect. Hayashi et al.67 had no lasting effect from bupivacaine with chemical radiculitis in an animal model. However, Yabuki et al.,68 utilizing a different animal simulating inflammatory radicular pain from nucleus pulposus, found less inflammation and nerve injury in the animals that received lidocaine. In earlier work, Yabuki and Kikuchi64 postulated the improved radicular blood flow from lidocaine may improve intraneural tissue metabolism or simply wash away chemical inflammogens. In 1998, Yabuki et al.68 postulated the therapeutic benefit of lidocaine may be the antiinflammatory effect as opposed to improved blood flow.
The intervertebral disc may result in radicular pain via involvement of the dorsal root ganglion (DRG) or nerve root. Compression of the DRG does result in repetitive electrical discharge, which could result in continued radicular pain with foraminal stenosis or a foraminal disc protrusion. Frequently, however, disc herniations are posterolateral in location and compress the nerve root, not the dorsal root ganglion. With compression of the nerve root, only a single burst of electrical discharge occurs.69 This would not explain continuous radicular pain. Howe et al.69 noted repetitive nerve root firing when a combination of inflammation with compression occurred. Kawakami et al.70 also noted severe hyperalgesia in rats with nerve root compression from chromic suture as opposed to silk suture or clip. The chromic suture resulted in inflammation whereas silk suture or clip did not. Kawakami et al.70 postulated inflammation, not compression, was the important factor in producing radicular pain.
Various animal studies have been performed to understand the effect of nucleus pulposus or disc protrusions in the pathophysiology of radicular pain. Leukocytes, macrophages, and lymphocytes were found at surgically created porcine disc protrusions.71 Introduction of nucleus pulposus into the epidural space of canines resulted in edema, fibrin deposition, and marked neutrophil infiltrate at 5 and 7 days postexposure. At 14 and 21 days postexposure regional fibrosis, vascular infiltration, and marked histiocytic–lympocyte infiltration was noted. Granulation tissue was present.72 Nucleus pulposus has been demonstrated to induce leukotaxis.73
Olmarker et al.74 evaluated the effect of methylprednisolone upon porcine cauda equina exposed to nucleus pulposus. The cauda equina was found to be red and swollen in the untreated group and pale and not swollen in the methylprednisolone group. Histologic analysis demonstrated inflammatory cells in both groups. Nerve conduction velocity was slowed in the untreated group. Olmarker et al. hypothesized subcellular processes resulted in the slowed conduction since there was no difference histologically between groups.74 In another study, nerve conduction slowing was noted following exposure of the cauda equina to autologous porcine nucleus pulposus.75 Kayama et al.75 postulated the effect to structures in the membrane of the nucleus pulposus cells.
Human studies have also been performed. Inflammatory cells with a predominance of macrophages were noted in disc material obtained at surgery in subjects with disc herniation.76 Disc material in subjects suffering from radiculopathy due to disc herniation was found to have elevated levels of phospholipase A2.77 Phospholipase A2 has been shown to be neurotoxic.78 Phospholipase A2 is the rate-limiting step in the liberation of arachidonic acid and the generation of leukotrienes and prostaglandins in inflammation.
Prostaglandin E2 and E1 have been found in surgical disc specimens.79 Prostaglandin E2 is involved in sensitizing nociceptors to bradykinins. Takahashi et al.80 studied human disc material from surgical specimens in subjects with either a disc protrusion, extrusion, or sequestration. While there was no difference in the cytokines produced, there was difference in the cells that produced the cytokines. The disc protrusion group had elevated levels of chondrocytes. The disc extrusion and sequestration groups had elevated levels of histiocytes, fibroblasts, and endothelial cells with few chondrocytes. Betamethasone added to the cultures inhibited cytokine production and prostaglandin E2 levels.80 Granulation tissue with mononuclear infiltrates were found in two-thirds of samples from disc extrusions or sequestration.81 The mononuclear cells expressed interleukin-1. Interleukin-1 stimulates inflammatory mediators and proteolytic enzymes such as collaganese, stromelysis, and plasminogen activators.81
Disc material obtained from herniated disc subjects compared to scoliosis subjects demonstrated elevated levels of matrix metalloproteinase activity, nitric oxide, prostaglandin E2, and interleukin-6.82 Interleukin-1, tumor necrosing factor, interleukin-1 receptor antagonist protein (IRAP), and substance P were not found at appreciable levels in either group. Matrix metalloproteinase is involved in disc degeneration and nitric oxide in inflammation and immune regulation. Increased levels of matrix metalloproteinase-3 and tissue inhibitor of metalloproteinase-1 have been found in disc extrusions and sequestrations compared to protrusions. These substances are involved in disc degeneration, and whether there is any role in discogenic low back pain is unclear.
Various neuropeptides are also involved in disc pathology. Substance P and calcitonin gene-related peptide (CGRP) have been found in the outer anulus fibrosus and posterior longitudinal ligament.83,84 Anulus was found to have nerve fibers immunoreactive to vasoactive intestinal peptide (VIP) and C-flanking peptide.83 VIP is involved in vasodilation and possibly sensory transmission. C-flanking peptide is a vasoconstrictor. CGRP and substance P are involved in nociception. Substance P additionally increases prostaglandins, interleukin-1, collagenase, and tumor necrosing factor. Measurement of neuropeptides in the dorsal root ganglion of compressed porcine nerve root found increased substance P but not VIP, while in canines undergoing discography both substance P and VIP were elevated.85,86 Disc compression has been postulated to pump fluid into vertebral body, anulus, and posterior longitudinal ligament with stimulation of nociceptive fibers.86 Mechanical stimulation of rat DRG resulted in elevated substance P in DRG, Lissauer’s tract, and substantia gelatinosa laminae I–III.87
Chronic inflammation secondary to immune response to nucleus pulposus has been postulated.88 Elevated lymphocyte transformation test, indicating a cellular immune response, was found in disc sequestrations as opposed to contained disc protrusions.88 The sequestered nucleus pulposus is exposed to the vascular space with postulated immune response and subsequent antibody formation.88 In subjects suffering from discogenic back pain or sciatica, six of nine subjects had elevated titers of IgM.
Alteration of vascular flow has been postulated as a potential etiology of chronic low back pain.89 Cadaveric intervertebral foramen specimens in chronic low back pain sufferers demonstrated perineural fibrosis, epidural vein compression, and dilation of noncompressed veins. Direct nerve root compression by osteophyte or disc was rarely found. Venous obstruction and dilatation with anoxia, fibrosis, and neuronal atrophy is hypothesized to result from mechanical damage.89 Tissue injury can result in fibrin deposition. Tissue plasminogen activator activates plasminogen in the fibrinolytic system to cleave fibrin for clearance. Tissue plasminogen activator inhibitor balances this response. Increased levels of tissue plasminogen activator antigen and inhibitor were reported and may be involved in pain production, though further research is required.89
The pathophysiology of neurogenic claudication secondary to spinal stenosis is incompletely understood. Porter90 postulates neurogenic claudication occurs from venous pooling with subsequent impaired blood flow. This results in metabolite buildup and decreased nutrients with nerve dysfunction. Walking impairs venous flow by increased spinal canal pressure with arteriole dilation, upright position increases epidural pressures, increasing venous return from the lower extremities. Increased venous flow from the lower extremities through the pelvic veins results in engorgement of Batson’s plexus with impairment of spinal venous flow. Nerve root compression, various vascular pathology, demyelination, and loss of neurons was found in symptomatic spinal stenosis subjects postmortem.91 Watanabe and Parke91 postulate neurogenic claudication arises from avascular atrophy of nerve fibers with constriction of nerve root pia-arachnoid mater. The pia-arachnoiditis results in adherence of the nerve root with susceptibility to mechanical spinal excursions. The thickened pia-arachnoid mater impairs CSF nutrients from diffusing across to the nerve root.
Nutrient delivery to the nerve root is dependent upon both vascular system and CSF. A porcine model of compression demonstrated significant impairment of CSF flow with even low compression. CSF flow is also subject to postural changes in spinal stenosis subjects. Takahashi et al.80 evaluated epidural pressure in lumbar spinal stenosis and normal control subjects. Lumbar spinal stenosis subjects in upright position had significantly increased pressure (82 mmHg) compared to flexed position (37 mmHg) and normal upright controls (34 mmHg). There was no statistical difference between the stenosis subjects in a flexed position versus normal upright controls. Increased epidural pressure in the upright posture of spinal stenosis subjects was postulated to impair nutrition to the nerve root or cauda equina. For impaired venous flow, either two adjacent levels or levels above and below a spinal segment needs to be involved.92
The above studies suggest a role of vascular or CSF flow impairment in the pathology of spinal stenosis. However, these morphologic models do not account for radiologic findings of spinal stenosis in asymptomatic subjects.2 The successful treatment of neurogenic claudication secondary to spinal stenosis by injection of corticosteroids is not explained by the anatomic model.93–95 Biochemical studies of the role of inflammatory mediators, cytokines, and neuropeptides in neurogenic claudication are needed.
The use of local anesthetics in the performance of both diagnostic injections and therapeutic injections when mixed with corticosteroid may hypothetically provide a therapeutic benefit. Local anesthetics have been shown to have antiinflammatory activity. The affect on leukocytes could alter not only the inflammatory reaction but also the release of various neuropeptides and cytokines. The affect on local blood flow by local anesthetics may also serve a role in both herniated disc and spinal stenosis subjects. However, whether these effects are clinically relevant requires further study.
ZYGAPOPHYSEAL JOINT PAIN
In vivo studies have been performed provoking Z-joint pain referral patterns.96–98 Intra-articular Z-joint instillation of local anesthetic has provided relief of low back pain.99 These studies suggest the Z-joint as a potential source of low back pain. To be a cause of pain, the structure should have nociceptive fibers. Afferent impulses are propagated from the zygapophyseal joints through medial branch of the posterior primary ramus. Complex unencapsulated nerve fibers probably involved in nociception have been found in the joint capsule.100 However, speculation existed as to whether these small fibers were just accompanying blood vessels or involved in Z-joint nociception. Plica synovial tissue obtained from surgical specimens demonstrated staining of substance P, CGRP, and PGP 9.5 but perivascularly.101 Plica tissue was felt not to be painful and the nerve fibers identified were postulated to be involved in vascular regulation.101 However, nerve fibers have been found in the Z-joint synovial folds not associated with vascular structures.102 Small unmyelinated and myelinated fibers were found corresponding to C and A-delta fibers, respectively.102 Other afferents have been found and studied in the Z-joint.
The facet joint contains group III and IV mechanosensitive units which respond to joint movement.103,104 Group II units are involved in proprioception and correspond to A-beta fibers found in adjacent musculotendinous units. The Group III units are involved in nociception. Mechanical strain may activate these group III receptors, causing pain. In vitro evaluation of neuronal discharge from mechanosensitive afferents were evaluated in rabbits subjected to mechanical forces.103 Various types of mechanoreceptors were found in the capsule of Z-joints. High-threshold type III and IV mechanoreceptors were found with neuronal discharges responding to load postulated to be noxious.103 Group III fibers were found to correspond to A-delta fibers.
Human surgical specimens have been evaluated to elucidate the pathophysiology of Z-joint pain. Z-joints specimens obtained in human subjects undergoing fusion demonstrated cartilage fibrillation, cartilage loss, exposed subchondral bone, fissuring, chondrocyte clustering, subchondral cysts, and erosion channels from subchondral bone through the tidemark region to the articular cartilage. These erosion channels contained small blood vessels and granulation tissue.105 The presence of granulation tissue suggests an inflammatory process.
Surgical specimens of Z-joints were obtained from subjects undergoing surgery for spinal stenosis and herniated disc.106 The specimens were evaluated for B and T lymphocytes involved in inflammation or immune reactions. The specimens revealed primarily monocytes and collagen-producing fibroblasts. Inflammatory cells were not verified in this study. The authors concluded mechanical irritation leads to fibroblastic collagen production and not inflammation. However, this study has a serious flaw. The Z-joints studied were not in subjects suffering from Z-joint pain but from spinal stenosis and disc pathology. Since the specimens were not from symptomatic Z-joints the pathophysiology of Z-joint from these specimens is suspect. The authors did state inflammation could not be excluded.
Various neuropeptides have been isolated from Z-joint specimens.102,105,107 C-flanking peptide of neuropeptide Y (CPON) is a potent vasoconstrictor. Substance P, VIP, and CGRP have been found and suggest a potential for pain production.102,105,107 Increased levels of prostaglandin E2 and F1-alpha have been demonstrated in Z-joints obtained from human subjects undergoing lumbar fusion.83 The affect of phospholipase A2 on Z-joint nerve fibers obtained in rabbits has been studied.78 Histologic and electrophysiologic analysis demonstrated phospholipase A2 induced an inflammatory reaction with subsequent neurotoxicity.78
Pain emanating from the Z-joint can be determined by use of local anesthetics. If the joint can be anesthetized, then pain emanating from the joint should be eliminated. Diagnostic injections utilizing local anesthetics have been performed to diagnose Z-joint pain. These injections have demonstrated specificity and validity for pain emanating from the Z-joint.14–19,108 The Z-joint can be anesthetized through neural blockade of the medial branch of the posterior primary ramus.16–18 Alternatively, the joint can be anesthetized through intra-articular instillation of local anesthetic.15,108–113 Blockade of the joint would require anesthetizing A-delta and C fibers along with A-alpha and A-gamma fibers.114 In joint injections, the amount of anesthetic should be small enough to avoid leakage to adjacent structures and sufficient enough for neural blockade. The chapter on spinal injection techniques addresses concentration and amounts of local anesthetics to achieve this, along with placement at the targeted structure.
Local anesthetics may additionally have a therapeutic effect in Z-joint pain. Local anesthetics have been shown to have an antiinflammatory effect and alter leukocyte function which may reduce neuropeptide and cytokine concentrations in the joint.47,49–55 Whether this effect is clinically relevant has not been demonstrated. However, there are individuals who present with lasting relief following a diagnostic injection. While this can potentially be a placebo effect, one can not discount the possibility of a therapeutic effect of the local anesthetic.
SACROILIAC JOINT
The sacroiliac joint (SIJ) is an auricular-shaped diarthrodial joint with joint capsule, synovial fluid, hyaline cartilage on the sacral side, and fibrocartilage on the iliac side.115 The sacroiliac joint is partially innervated by the posterior rami of the lumbosacral roots.116 The anterior joint receives innervation from L3–S2 and the superior gluteal nerve.117 The posterior joint innervation has been reported from S1–2117 and L4–S3.118 The S1 level may be the major contributor to the joint.119 Possible autonomic contribution adds to the complexity of innervation of the sacroiliac joint.120,121 The lumbosacral trunk is just anterior to the joint in the lower third.122,123 The L4 and L5 nerve roots are 1 cm medial to sacroiliac joint at the level of the pelvic brim.124 The L4 and L5 nerve roots are 23 and 26 mm medial to sacroiliac joint, respectively, and 4.0 cm above the pelvic brim.124 The innervation of the joint is not completely understood at this time. As the innervation is unknown, utilization of nerve blockade as in medial branch blockade for Z-joint pain cannot be recommended for diagnosing SIJ pain.
Injection of contrast into the SIJ was found to result in pain, suggesting the joint as a potential source of pain.125 Additionally, infection and rheumatologic condition affecting the joint have been found to be painful.126–131 The pathophysiology of sacroiliac joint pain is not known at this time. With normal aging the joint capsule thickens, plaques develop along the cartilage surface, erosions develop, fibrous interconnections develop, the joint surface becomes irregular, and eventual ankylosis can occur.115,132–134
Infectious and rheumatologic conditions have affected the joint, resulting in pain. Fluoroscopic injection of corticosteroid into the joint of seronegative spondyloarthropathy sufferers has been reported to result in greater than 70% pain relief in 79.2% of subjects.131 At a mean follow-up of 22.9 months a 50% decrement in VAS score occurred in those with positive diagnostic SIJ injection treated with SIJ corticosteroid injection and physical therapy. This may suggest an inflammatory component to SIJ syndrome. However, this is an uncontrolled study. The pathophysiology of SIJ syndrome is unknown.
Anesthetic agent has been shown to relieve SIJ pain.9,135,136 The specificity and sensitivity for diagnostic SIJ injection is unknown. The double-block paradigm has been performed, suggesting a false-positive rate of 47%.136 To maintain specificity, a volume of no more than 2.0 cc is recommended as joint capsule leakage through rents in the joint capsule may occur with larger volumes.135 Leakage of anesthetic can anesthetize the lumbosacral plexus resulting in false positives. False-negative diagnostic injections can occur even with correct placement due to loculations within the joint preventing anesthetic agents from reaching the target.137 To avoid inadvertent extravasation through rents in the joint capsule, injection under live fluoroscopy of combined nonionic contrast agent and local anesthetic has been proposed.138 If leakage outside the joint begins to occur the injection is stopped.
1 Slipman CW, Plastaras CT, Palmitier RA, et al. Symptom provocation of dermatomal map? Spine. 1998;23:2235-2242.
2 Boden SD, Davis DO, Dina TS, et al. Abnormal magnetic-resonance scans of the lumbar spine in asymptomatic subjects. J Bone Joint Surg [Am]. 1990;72:403-408.
3 Jensen MC, Brant-Zawadski MN, Obuchowski N, et al. Magnetic resonance imaging of the lumbar spine in people without back pain. N Engl J Med. 1994;331:69-73.
4 Lehto IJ, Tertti MO, Komu ME, et al. Age-related MRI changes at 0.1T in cervical discs in asymptomatic subjects. Neuroradiology. 1994;36:49-53.
5 Matsumoto M, Fujimura Y, Suzuki N, et al. MRI of cervical intervertebral discs in asymptomatic subjects. J Bone Joint Surg [Br]. 1998;80:19-24.
6 Brightbill TC, Pile N, Eichelberger RP, et al. Normal magnetic resonance imaging and abnormal discography in lumbar disc disruption. Spine. 1994;19:1075-1077.
7 Jackson RP, Jacobs RR, Montesano PX. Facet joint injections in low back pain: A prospective statistical study. Spine. 1988;13:966-971.
8 Slipman CW, Sterenfeld EB, Chou LH, et al. The predictive value of provocative sacroiliac joint stress maneuvers in the diagnosis of sacroiliac joint syndrome. Arch Phys Med Rehabil. 1998;79:288-292.
9 Dreyfuss P, Michaelsen M, Pauza KJ, et al. The value of medical history and physical examination in diagnosing sacroiliac joint pain. Spine. 1996;21:2594-2602.
10 Vogel JBIII, Brown WH, Helms CA, et al. The normal sacroiliac joint: A CT study of asymptomatic patients. Radiology. 1984;151:433-437.
11 Slipman CW, Sterenfeld EB, Chou LH, et al. The value of radionuclide imaging in the diagnosis of sacroiliac joint syndrome. Spine. 1996;19:2251-2254.
12 Cohen AS, McNeill JM, Calkins E, et al. The normal sacroiliac joint. An analysis of 88 sacroiliac roentgenograms. Am J Roent Radium Ther. 1967;100:559-563.
13 Wiesel SW, Tsourmas N, Feffer HL, et al. A study of computer-assisted tomography: I. The incidence of positive CAT scans in an asymptomatic group of patients. Spine. 1984;9:549-551.
14 Schwarzer AC, Wang S, O’Driscoll D, et al. The ability of computed tomography to identify a painful zygapophyseal joint in patients with chronic low back pain. Spine. 1995;20:907-912.
15 Barnsley L, Lord S, Wallis B, et al. False-positive rates of cervical zygapophyseal joint blocks. Clin J Pain. 1993;9:124-130.
16 Barnsley L, Bogduk N. Medial branch blocks are specific for the diagnosis of cervical zygapophyseal joint pain. Reg Anesth. 1993;18:343-350.
17 Dreyfuss P, Schwarzer AC, Lau P, et al. Specificity of lumbar medial branch and L5 dorsal ramus blocks. A computed tomography study. Spine. 1997;22:895-902.
18 Kaplan M, Dreyfuss P, Halbrook B, et al. The ability of lumbar medial branch blocks to anesthetize the zygapophyseal joint. A physiologic challenge. Spine. 1998;23:1847-1852.
19 Schwarzer AC, Aprill CN, Derby R, et al. The false-positive rate of uncontrolled diagnostic blocks of the lumbar zygapophyseal joints. Pain. 1994;58:195-200.
20 Clinical Practice Guideline. No. 14. Acute low back problems in adults. Rockville, MD: US Dept. Health Human Services, Agency Health Care Policy Research, Dec 1994.
21 Huston CW, Slipman CW. Diagnostic selective nerve root blocks: indications and usefulness. Phys Med Rehabil Clin N Am. 2002;13:545-565.
22 McCabe JS, Low FN. The subarachnoid angle: an area of transition in peripheral nerve. Anat Rec. 1969;164:15-34.
23 Gamble HJ. Comparative electron-microscopic observations on the connective tissues of a peripheral nerve and a spinal nerve root in the rat. J Anat Lond. 1964;98:17-25.
24 Chiu SY, Ritchie JM. Potassium channels in nodal and internodal axonal membrane of mammalian myelinated fibres. Nature. 1980;284:170-171.
25 Bostock H, Sears TA. The internodal axon membrane: electrical excitability and continuous conduction in segmental demyelination. J Physiol. 1978;280:273-301.
26 Koester J. Resting membrane potential. In: Kandel ER, Schwartz JH, editors. Principles of neural science. New York, NY: Elsevier; 1981:27-35.
27 Koester J. Passive electrical properties of the neuron. In: Kandel ER, Schwartz JH, editors. Principles of neural science. New York, NY: Elsevier; 1981:36-43.
28 Koester J. Functional consequences of passive electrical properties of the neuron. In: Kandel ER, Schwartz JH, editors. Principles of neural science. New York, NY: Elsevier; 1981:44-52.
29 Frazier DT, Narahashi T, Yamada M. The site of action and active form of local anesthetics. II. Experiments with quaternary compounds. J Pharmacology. 1970;171:45-51.
30 Ritchie JM, Ritchie B, Greengard P. The active structure of local anesthetics. J Pharmacol Exp Ther. 1965;150:152-159.
31 Narahasi T, Frazier DT, Yamada M. The site of action and active form of local anesthetics. I. Theory and pH experiments with tertiary compounds. J Pharmacology. 1970;171:32-44.
32 Ragsdale DS, McPhee JC, Scheuer T, et al. Molecular determinants of state-dependent block of + channels by local anesthetics. Science. 1994;265:1724-1728.
33 Shanes AM. Electrochemical aspects of physiological and pharmacological action in excitable cells. Part I. The resting cell and its alteration by extrinsic factors. Pharmacol Rev. 1958;10:59-164.
34 Strichartz GR. The inhibition of sodium currents in myelinated nerve by quaternary derivatives of lidocaine. J Gen Physiology. 1973;62:37-57.
35 Wang HH, Yeh JZ, Narahashi T. Interaction of spin-labeled local anesthetics with the sodium channel of squid axon membranes. J Membrane Biol. 1982;66:227-233.
36 Courtney KR, Kendig JJ, Cohen EN. Frequency-dependent conduction block: the role of nerve impulse pattern in local anesthetic potency. Anesthesiology. 1978;48:111-117.
37 Tasaki I. Conduction of the nerve impulse. In: Field J, editor. Handbook of physiology, Vol. 1, Sec 1: The nervous system. Washington, DC: Am Physiol Soc, 1959.
38 Fink BR. The long and the short of conduction block. Anesth Analg. 1989;68:553-555.
39 Raymond SA, Steffensen SC, Gugino LD, et al. Critical exposure length for nerve block of myelinated fibers with lidocaine exceed three nodes. Reg Anesth. 1988;12:S46.
40 Ritchie JM, Ritchie B, Greengard P. The effect of the nerve sheath on the action of local anesthetics. J Pharmacol Exp Ther. 1985;150:160-164.
41 Covino BG. Pharmacology of local anaesthetic agents. Br J Anaesth. 1986;58:701.
42 Ohki S, Gravis C, Pant H. Permeability of axon membranes to local anesthetics. Biochimica Biophysica Acta. 1981;643:495-507.
43 Byrod G, Lomarker K, Konno S, et al. A rapid transport route between the epidural space and the intraneural capillaries of the nerve roots. Spine. 1995;20:138-143.
44 Slipman CW, Huston A, Shin C. Diagnostic and therapeutic injections. In: Gonzalez EG, Myers SJ, Edelstein JE, et al, editors. Downey & Darling’s physiological basis of rehabilitation medicine. 3rd edn. Boston: Butterworth Heinemann; 2001:795-813.
45 Evans W. Intrasacral epidural injection in the treatment of sciatica. Lancet. 1930;2:1225-1229.
46 Burn JM, Guyer PB, Langdon L. The spread of solutions injected into the epidural space. Br J Anaesth. 1973;45:338-345.
47 Rimback G, Cassuto J, Wallin G, et al. Inhibition of peritonitis by amide local anesthetics. Anesthesiology. 1988;69:881-886.
48 Cassuto J, Nellgard P, Stage L, et al. Amide local anesthetics reduce albumin extravasation in burn injuries. Anesthesiology. 1990;72:302-307.
49 Peck SL, Johnston RB, Horwitz LD. Reduced neutrophil superoxide anion release after prolonged infusions of lidocaine. J Pharmacol Exp Ther. 1985;235:418-422.
50 Goldstein IM, Lind S, Hoffstein S, et al. Influence of local anesthetics upon human polymorphonuclear leukocyte function in vitro. J Exp Med. 1977;146:483-494.
51 MacGregor RR, Thorner RE, Wright DM. Lidocaine inhibits granulocyte adherence and prevents granulocyte delivery to inflammatory sites. Blood. 1980;56:203-209.
52 Cullen BF, Haschke RH. Local anesthetic inhibition of phagocytosis and metabolism of human leukocytes. Anesthesiology. 1974;40:142-146.
53 Ohsaka A, Saionji D, Sato N, et al. Local anesthetic lidocaine inhibits the effect of granulocyte colony-stimulating factor on human neutrophil functions. Exp Hematol. 1994;22:460-466.
54 Hoidal JR, White JG, Repine JE. Influence of cationic local anesthetics on the metabolism and ultrastructure of human alveolar macrophages. J Lab Clin Med. 1979;93:857-866.
55 Ramus GV, Cesano L, Barbalonga A. Different concentrations of local anesthetics have different modes of action on human lymphocytes. Agents Actions. 1983;13:333-341.
56 Martinsson T, Haegerstrand A, Dalsgaard C. Ropivacaine and lidocaine inhibit proliferation of non-transformed cultured adult human fibroblasts, endothelial cells and keratinocytes. Agents Actions. 1993;40:78-85.
57 Winnie AP, Hartman JT, Meyers JL, et al. Pain clinic II: intradural and extradural corticosteroids for sciatica. Anesth Analg Curr Res. 1972;51:990-1003.
58 El Mahdi MA, Abdel LFY, Janko M. The spinal nerve root ‘innervation’ and a new concept of the clinico-pathological interrelations in back pain and sciatica. Neurochirurgia. 1981;24:137-141.
59 Beecher HK. The powerful placebo. JAMA. 1955;159:1602.
60 Benson H, Epstein MD. The placebo effect. A neglected asset in the care of patients. JAMA. 1975;232:1225.
61 Nygaard OP, Mellgren SI. The function of sensory nerve fibers in lumbar radiculopathy. Spine. 1998;23:348-352.
62 North RB, Kidd DH, Zahurak M, et al. Specificity of diagnostic nerve blocks: a prospective, randomized study of sciatica due to lumbosacral spine disease. Pain. 1996;65:77-85.
63 Van Akkerveeken PF. The diagnostic value of nerve root sheath infiltration. Acta Orthop Scand. 1993;64:61-63.
64 Yabuki S, Kikuchi S. Nerve root infiltration and sympathetic block. An experimental study of intraradicular blood flow. Spine. 1995;20:901-906.
65 Takahashi K, Olmarker K, Holm S, et al. Double-level cauda equina compression: An experimental study with continuous monitoring of intraneural blood flow in the porcine cauda equina. J Orthoped Res. 1993;11:104-109.
66 Yabuki S, Kikuchi S, Olmarker K, et al. Acute effects of nucleus pulposus on blood flow and endoneurial fluid pressure in rat dorsal root ganglia. Spine. 1998;23:2517-2523.
67 Hayashi N, Weinstein JN, Meller St, et al. The effect of epidural injection of betamethasone or bupivacaine in rat model of lumbar radiculopathy. Spine. 1998;23:877-885.
68 Yabuki S, Kawaguchi Y, Nordborg C, et al. Effects of lidocaine on nucleus pulposus-induced nerve root injury. A neurophysiologic and histologic study of the pig cauda equina. Spine. 1998;23:2382-2390.
69 Howe JF, Loeser JD, Calvin WH. Mechanosensitivity of dorsal root ganglia and chronically injured axons: a physiological basis for the radicular pain of nerve root compression. Pain. 1977;3:25-41.
70 Kawakami M, Weinstein JN, Spratt KF, et al. Experimental lumbar radiculopathy. Immunohistochemical and quantitative demonstrations of pain induced by lumbar nerve root irritation of the rat. Spine. 1994;19:1780-1794.
71 Habtemariam A, Virri J, Gronblad M, et al. Inflammatory cells in full-thickness anulus injury in pigs. An experimental disc herniation animal model. Spine. 1998;23:524-529.
72 McCarron RF, Wimpee MW, Hudkins PG, et al. The inflammatory effect of nucleus pulposus. A possible element in the pathogenesis of low back pain. Spine. 1987;12:760-764.
73 Olmarker K, Blomquist J, Stromberg J, et al. Inflammatogenic properties of nucleus pulposus. Spine. 1994;19:2744-2751.
74 Olmarker K, Byrod G, Cornefjord M, et al. Effects of methylprednisolone on nucleus pulposus-induced nerve root injury. Spine. 1994;19:1803-1808.
75 Kayama S, Olmarker K, Larsson K, et al. Cultured autologous nucleus pulposus cells induce functional changes in spinal nerve roots. Spine. 1998;23:2155-2158.
76 Gronblad M, Virri J, Tolonen J, et al. A controlled immunohistochemical study of inflammatory cells in disc herniation tissue. Spine. 1994;19:2744-2751.
77 Saal JS, Franson RC, Dobrow R, et al. High levels of inflammatory phospholipase A2 activity in lumbar disc herniations. Spine. 1990;15:674-678.
78 Ozaktay AC, Cavanaugh JM, Blagoev DC, et al. Phospholipase A2 induced electrophysiologic and histologic changes in rabbit dorsal lumbar spine tissues. Spine. 1995;20:2659-2668.
79 Willburger RE, Wittenberg RH. Prostaglandin release from lumbar disc and facet joint tissue. Spine. 1994;19:2068-2070.
80 Takahashi H, Suguro T, Okazima Y, et al. Inflammatory cytokines in the herniated disc of lumbar spine. Spine. 1996;21:218-224.
81 Doita M, Kanatani T, Harada T, et al. Immunohistologic study of the ruptured intervertebral disc of the lumbar spine. Spine. 1996;21:235-241.
82 Kang JD, Gergescu HI, McIntyre-Larkin L, et al. Herniated lumbar intervertebral discs spontaneously produce matrix metalloproteinases, nitric oxide interleukin-6, and prostaglandin E2. Spine. 1996;21:271-277.
83 Ashton IK, Roberts S, Jaffray DC, et al. Neuropeptides in the human intervertebral disc. J Orthop Res. 1994;12:186-192.
84 Konttinen YT, Gronblad M, Antti-Poika I, et al. Neuroimmunohistochemical analysis of periodical nociceptive neural elements. Spine. 1990;15:383-386.
85 Cornefjord M, Olmarker K, Farley DB, et al. Neuropeptide changes in compressed spinal nerve roots. Spine. 1995;20:670-673.
86 Weinstein J, Claverie W, Gobson S. The pain of discography. Spine. 1988;13:1344-1348.
87 Badalamente MA, Dee R, Ghillani R, et al. Mechanical stimulation of dorsal root ganglia induces increased production of substance P: a mechanism for pain following nerve root compromise? Spine. 1987;12:552-555.
88 Getzbein SD. Degenerative disk disease of the lumbar spine. Clin Orthop Rel Res. 1977;129:61-67.
89 Jayson MIV. The role of vascular damage and fibrosis in the pathogenesis of nerve root damage. Clin Orthop Rel Res. 1992;279:40-48.
90 Porter RW. Spinal stenosis and neurogenic claudication. Spine. 1996;21:2046-2052.
91 Watanabe R, Parke WW. Vascular and neural pathology of lumbosacral spinal stenosis. J Neurosurg. 1986;64:64-70.
92 Olmarker K, Holm S, Rydevik B. Single versus double level nerve root compression: an experimental study on the porcine cauda equina with analyses of nerve impulse conduction properties. Clin Orthop. 1992;6:35-39.
93 Saal JS, Saal JA, Parthasarathy R. The natural history of lumbar spinal stenosis: the results of nonoperative treatment. Proc Tenth Annual Conf North Am Spine Soc, 1995.
94 Ciocon JO, Galindo-Ciocon D, Amaranath L, et al. Caudal epidural blocks for elderly patients with lumbar canal stenosis. JAGS. 1994;42:593-596.
95 Botwin KP, Gruber RD, Bouchlas CG, et al. Fluoroscopically guided lumbar transformational epidural steroid injections in degenerative lumbar stenosis: an outcome study. Am J Phy Med Rehabil. 2002;12:898-905.
96 Mooney V, Robertson J. The facet syndrome. Clin Orthop Rel Res. 1976;115:149-156.
97 McCall JS, Park WM, O’Brien JP. Induced pai referral from posterior lumbar elements in normal subjects. Spine. 1979;4:441-446.
98 Bogduk N. Lumbar dorsal ramus syndrome. Med J Aust. 1980;2:537-541.
99 Schwarzer AC, Aprill CN, Derby R, et al. Clinical features of patients with pain stemming from the lumbar zygapophyseal joints. Is the lumbar facet syndrome a clinical entity? Spine. 1994;19:1132-1137.
100 Jackson HC, Winkelmann RK, Bickel WH. Nerve endings in the human lumbar spinal column and related structures. J Bone Joint Surg [Am]. 1966;48A:1272-1281.
101 Gronblad M, Korkala O, Konttinen YT, et al. Silver impregnation and immunohistochemical study of nerves in lumbar facet joint plical tissue. Spine. 1991;16:34-38.
102 Giles LGF, Harvey AR. Immunohistochemical demonstration of nociceptors in the capsule and synovial folds of human zygapophyseal joints. Brit J Rheum. 1987;26:362-364.
103 Avramov A, Cavanaugh JM, Ozaktay CA, et al. The effects of controlled mechanical loading on group II, III, and IV afferent units from the lumbar facet joint and surrounding tissue. J Bone Joint Surg [Am]. 1992;74A:1464-1471.
104 Yamashita T, Cavanaugh JM, El-Bhoy AA, et al. Mechanosensitive afferent units in the lumbar facet joint. J Bone Joint Surg [Am]. 1990;72A:865-870.
105 Beaman DN, Graziano GP, Glover RA, et al. Substance P innervation of lumbar spine facet joints. Spine. 1993;18:1044-1049.
106 Knottinen YT, Gronblad M, Korkala O, et al. Immunohistochemical demonstration of subclasses of inflammatory cells and active, collagen-producing fibroblasts in the synovial plicae of lumbar facet joints. Spine. 1990;15:387-390.
107 Ashton IK, Ashton BA, Gibson SJ, et al. Morphological basis for back pain: the demonstration of nerve fibers and neuropeptides in the lumbar facet joint capsule but not in ligamentum flavum. J Orthop Res. 1992;10:72-78.
108 Dreyfuss P, Tibiletti C, Dreyer S, et al. Thoracic zygapophyseal joint pain: A review and description of an intra-articular block technique. Pain Digest. 1994;4:46-54.
109 Bogduk N, Marsland A. The cervical zygapophyseal joints as a source of neck pain. Spine. 1988;13:610-617.
110 Carrera GF. Lumbar facet joint injection in low back pain and sciatica. Description of technique. Radiology. 1980;137:661-664.
111 Carrera GF. Lumbar facet joint injection in low back pain and sciatica. Preliminary results. Radiology. 1980;137:665-667.
112 Fairbanks JCT, Park WM, McCall IW, et al. Apophyseal injection of local anesthetic as a diagnostic aid in primary low-back pain syndromes. Spine. 1981;6:598-605.
113 Fortin JD, Mckee MJ. Thoracic facet blocks: bent needle technique. Pain Phys. 2003;6:513-516.
114 LaMotte RH, Campbell JN. Comparison of responses of warm and nociceptive C-fiber afferents in monkey with human judgements of thermal pain. J Neurophysiol. 1978;41:509-528.
115 Bowen V, Cassidy JD. Macroscopic and microscopic anatomy of the sacroiliac joint from embryonic life until the eighth decade. Spine. 1981;6:620-628.
116 Steindler A, Luck JV. Differential diagnosis of pain low in the back. Allocation of the source of pain by the procaine hydrochloride method. J Am Med Assoc. 1938;110:106-113.
117 Solonen KA. The sacroiliac joint in the light of anatomical, roentgenological and clinical studies. ACTA Orthop Scand (Suppl). 1957;27:1-127.
118 Bertrand TNJr, Cassidy JD. The sacroiliac joint syndrome. Pathophysiology, diagnosis, and management. In: Frymoyer JW, Ducker TB, Hadler NM, et al, editors. The adult spine. Principles and Practice. New York, NY: Raven Press; 1991:2107-2130.
119 Greenman PE. Clinical aspects of sacroiliac joint function in walking. J Man Med. 1990;5:25-130.
120 Norman GF, May A. Sacroiliac conditions simulating intervertebral disc syndrome. West J Surg. 1956;64:461-462.
121 Pitkin HC, Pheasant HC. Sacroarthrogenetic telalgia. 1: a study of referred pain. J Bone Joint Surg [Am]. 1988;70A:31-40.
122 Albee FH. A study of the anatomy and the clinical importance of the sacroiliac joint. JAMA. 1909;LIII:1273-1276.
123 Hershey CD. The sacro-iliac joint and pain of sciatic radiation. JAMA. 1943;122:983-986.
124 Ebraheim NA, Padanilam TG, Waldrop JT, et al. Anatomic consideration in the anterior approach to the sacro-iliac joint. Spine. 1994;19:721-725.
125 Fortin JD, Dwyer AP, West S, et al. Sacroiliac joint: pain referral maps upon applying a new injection/arthrography technique. Part I: Asymptomatic volunteers. Spine. 1994;19:1475-1482.
126 Engelsbel S, Swartjes JM, Schutte MF. Case report. Pyogenic sacro-iliitis, a rare cause of peripartum pelvic pain. Eur J Obstetrics Gyn Reproductive Bio. 1995;62:125-126.
127 Brand C, Warren R, Luxton M, et al. Cryptococcal sacroiliitis. Case report. Annals Rheum Dis. 1985;44:126-127.
128 Reginato AJ, Ferreiro-Seoane JL, Falasca G. Unilateral sacroiliitis in secondary syphilis. J Rheum. 1988;15:717-719.
129 Pouchot J, Vinceneux P, Barge J, et al. Tuberculosis of the sacroiliac joint: Clinical features, outcome, and evaluation of closed needle biopsy in 11 consecutive cases. Am J Med. 1988;84:622-628.
130 Dunn EJ, Bryan DM, Nugent JT, et al. Pyogenic infections of the sacro-iliac joint. Clin Ortho Rel Res. 1976;118:113-117.
131 Maugars Y, Mathis C, Vilon P, et al. Corticosteroid injection of the sacroiliac joint in patients with seronegative spondylarthropathy. Arthritis Rheum. 1992;35:564-568.
132 Stewart TD. Pathologic changes in aging sacroiliac joints. A study of dissecting-room skeletons. Clin Orthop Rel Res. 1984;183:188-196.
133 Sashin D. A critical analysis of the anatomy and the pathologic changes of the sacro-iliac joints. J Bone Joint Surg [Am]. 1930;12A:891-910.
134 MacDonald GR, Hunt TE. Sacro-iliac joints. Observations on the gross and histological changes in the various age groups. Canad M A J. 1952;66:157-163.
135 Schwarzer AC, Aprill CN, Bogduk N. The sacroiliac joint in chronic low back pain. Spine. 1995;20:31-37.
136 Maigne JY, Aivaliklis A, Pfefer F. Results of sacroiliac joint double block and value of sacroiliac pain provocation test in 54 patients with low back pain. Spine. 1996;21:1889-1892.
137 Miskew DB, Block RA, Witt PF. Aspiration of infected sacro-iliac joints. J Bone Joint Surg [Am]. 1979;61A:1071-1072.
138 Slipman CW, Huston CW. Diagnostic sacroiliac joint injections. In: Manchikanti L, Slipman CW, Fellows B, editors. Interventional pain management. Low back pain: Diagnosis and treatment. Paducah, KY: ASIPP; 2002:269-274.