CHAPTER 24 Neuromuscular Scoliosis
General Principles
Natural History and Associated Complications
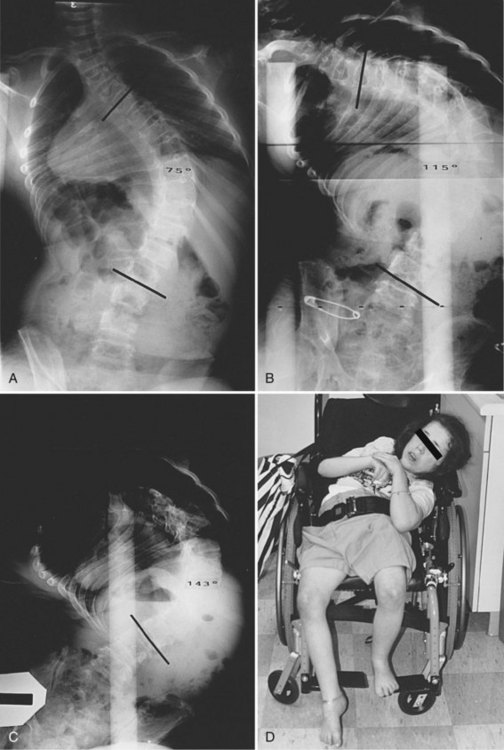
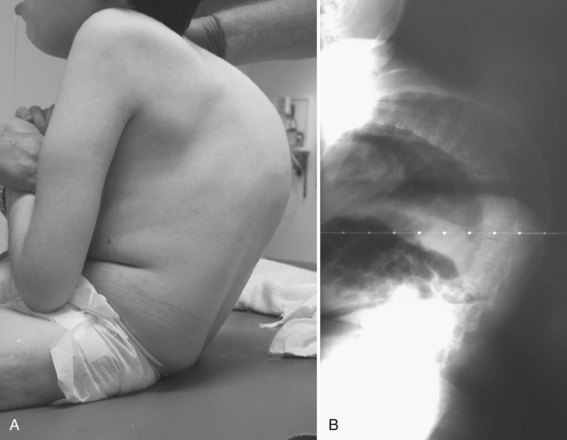
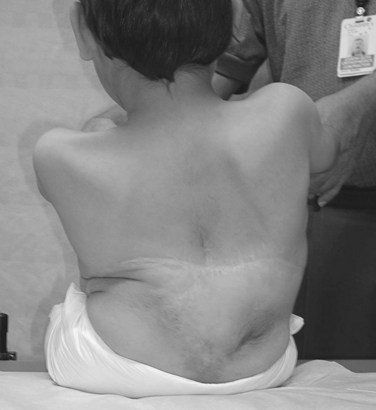
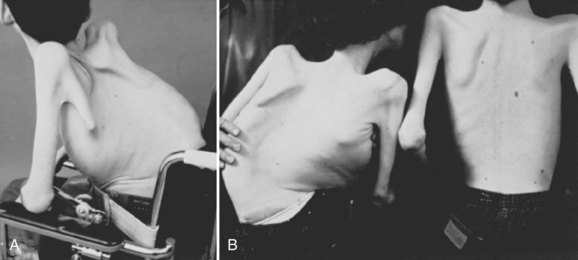
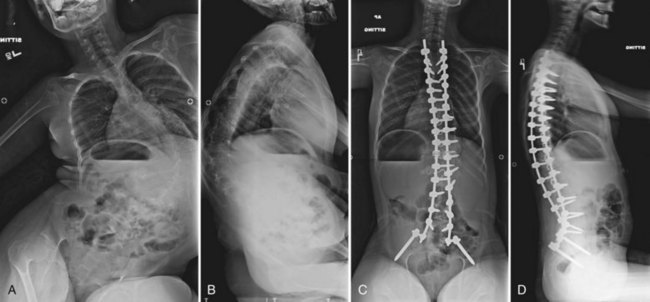
Neuromuscular scoliosis generally begins early in life, is rapidly progressive, and causes significant morbidity. Some patients are capable of ambulation, although many lose their ability to walk early in life or never achieve ambulatory status at all. The use of a wheelchair affords these patients educational and social opportunities that enrich their lives. Spinal deformity can impair comfortable sitting and dramatically reduce the individual’s quality of life. Unbalanced curves and significant pelvic obliquity make wheelchair positioning difficult and may cause uneven distribution of weight that may lead to pressure sores (Fig. 24–1). Prominences created by the convexity of a curve may result in skin breakdown; creases within the concavity of the trunk deformity are susceptible to skin maceration and infection (Fig. 24–2). Majd and colleagues1 showed a correlation between deformity size, functional decline, and decubitus. Large rigid curves restrict lung volume and impair respiration in patients who often already have limited pulmonary capacity. Treatment of neuromuscular scoliosis can also help the caretakers of these patients, improving the ease of transfers, positioning, feeding, and hygiene. The ultimate goal of treatment of patients with neuromuscular scoliosis is the maintenance of as much independence and function as possible. When patients with neuromuscular scoliosis lose the ability to sit comfortably, their quality of life is dramatically decreased. The natural history for a given patient is largely determined by the specific underlying neuromuscular condition and the degree of involvement.
Nonoperative Treatment
Medical Treatment
Spinal Muscle Atrophy
Various medications have been tested to improve the musculoskeletal function of patients with neuromuscular disorders. Randomized placebo-controlled trials have been conducted investigating the efficacy of several medical treatments for SMA, including creatine, phenylbutyrate, gabapentin, and thyrotropin-releasing hormone.2–5 None of these compounds has proven to be an efficacious drug treatment for SMA.2
Cerebral Palsy
Several medical therapies have been investigated for the treatment of spasticity in patients with cerebral palsy. Botulinum toxin has gained a growing acceptance as a treatment of upper and lower limb spasticity. Initial reviews of the literature by the Cochrane Collaboration and others yielded inconclusive evidence that could neither confirm nor deny the efficacy of botulinum toxin in the treatment of spasticity.6 Inclusion of more recent randomized controlled trials into the analysis has provided evidence that supports the use of botulinum toxin to provide a time-limited benefit to decrease muscle tone in children with upper and lower limb spasticity associated with cerebral palsy.7 Although evidence for the use of botulinum toxin is not yet conclusive, the evidence trend is in favor of using this therapy to reduce spasticity associated with cerebral palsy.
Intrathecal baclofen is a well-established treatment that has been shown to provide significant benefits in controlling spasticity in patients with cerebral palsy. Intrathecal baclofen has been shown to reduce the need for orthopaedic lower extremity procedures and the rate of postoperative complications associated with these procedures.8 Concerns have been raised, however, regarding its impact on the progression of scoliosis in patients with spastic quadriplegia. In a retrospective review, Ginsburg and Lauder9 found a sixfold increase in the rate of scoliosis curve progression at 2-year follow-up in a group of 19 spastic quadriplegic cerebral palsy patients. Caird and colleagues10 showed a significantly higher rate of complications associated with posterior spinal fusion and instrumentation in a group of 20 spastic cerebral palsy patients with intrathecal baclofen pumps compared with a matched control group. This study was limited by its relatively small sample size and lack of a control group. Shilt and colleagues11 found no difference in curve progression at 3-year follow-up between 50 cerebral palsy patients treated with intrathecal baclofen and 50 matched control cerebral palsy patients. Based on the current evidence, no significant conclusions can be drawn about the impact of intrathecal baclofen pumps on the progression or treatment of spinal deformity in patients with cerebral palsy. Baclofen can provide significant relief of spasticity, and this evidence must be considered in the context of any potential side effects.
Duchenne Muscular Dystrophy
Advances in general care, glucocorticoid treatment, noninvasive ventilatory support, cardiomyopathy management, and scoliosis management have significantly changed the course of Duchenne muscular dystrophy (DMD). Survival into adulthood is now a realistic possibility for many patients who received optimal treatment.12 Although gene-based and cellular-based therapies are currently under development for the treatment of DMD, the efficacy of glucocorticoid steroids has been evaluated by several randomized controlled trials. In their Cochrane review and meta-analysis, Manzur and colleagues13 concluded there is evidence that muscle function and strength are improved in the short-term (6 months to 2 years) with corticosteroid therapy. The authors based their conclusion on six randomized controlled trials and observed that the most effective prednisolone dose seemed to be 0.75 mg/kg/day, given daily.13 Markham and colleagues14 showed that glucocorticoid therapy provides the added benefit of retarding the anticipated development of ventricular dysfunction if begun before ventricular dysfunction in their series of 14 DMD patients treated with steroids compared with 23 DMD patients treated without steroids.
Genetic and Family Counseling
Because of the complexity of the medical and psychosocial issues associated with neuromuscular disorders and spinal deformity, care needs to be coordinated with a multidisciplinary team. The primary care physician should be well informed of all orthopaedic issues and play a central role in managing care. Psychosocial support for patients and parents is also vital. Patient advocacy groups have proved to be very useful in helping families cope with the illness and associated surgical care. Physicians may wish to provide information regarding clinical trials or refer families to clinical trial websites (www.clinicaltrials.gov provides a current listing of open clinical trials). Patients and parents may need to be referred for genetic counseling to confirm the patient’s diagnosis and aid in family planning.
Bracing
Bracing is a controversial treatment method in idiopathic and neuromuscular scoliosis. Bracing in neuromuscular scoliosis may be used for postural support, although there is incomplete evidence of its efficacy in limiting curve progression (Fig. 24–3). The etiology of the patient’s scoliosis and the patient’s muscle tone have an impact on the practicality of brace treatment. Patients with spastic disorders generally do not tolerate rigid brace treatment, whereas patients with flaccid paresis are more apt to be compliant with brace treatment. The type of orthoses may play a role in the outcome of the treatment.
Kotwicki and colleagues15 followed 45 nonambulatory patients with neuromuscular scoliosis treated with a suspension trunk orthosis (STO) and found that the STO slowed curve progression in 23 patients. The STO construction functions contrary to the classic thoracolumbosacral orthosis (TLSO), with the STO not resting against the patient’s pelvis but rather directly against the seat. The evidence supporting STO use to prevent curve progression is limited, however, and skin intolerance found in 36 patients complicates its clinical practicality. Although there is limited research on the results of the STO brace, there are numerous studies investigating the TLSO brace. In a study of 15 patients, Shoham and colleagues16 found that a TLSO reduced scoliotic deformity and pelvic obliquity leading to reduced sitting pressure. These results are contrary to other studies reported in the literature. In a study of 23 patients, Miller and colleagues17 followed 23 patients with cerebral palsy who wore a rigid Wilmington TLSO for an average of 67 months and concluded that the bracing did not slow progression of their deformity. Olafsson and colleagues18 followed 90 patients with various neuromuscular conditions treated with a soft Boston orthosis for an average of 3 years after brace treatment. They concluded that brace wear was indicated only in a limited subset of patients—ambulatory patients with hypotonia and short thoracolumbar curves (<40 degrees). In all other patients, brace wear was ineffectual in altering progression but did provide assistance in sitting.
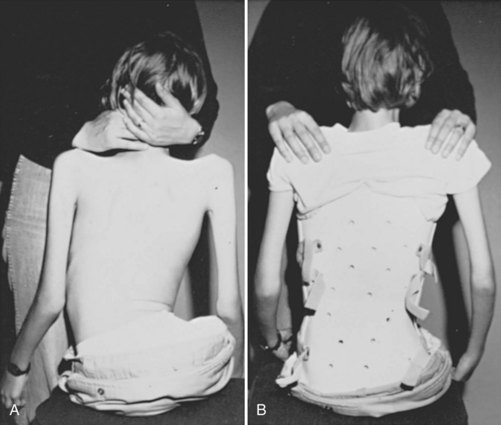
Patients with neuromuscular scoliosis may lack sensate skin to feel pressure from the brace or the muscular control to pull away from the sides of the brace. These patients rarely tolerate the rigid braces often used in idiopathic scoliosis. Patients tend to tolerate soft TLSOs designed to provide improved sitting stability and head and trunk control, while limiting discomfort and skin breakdown (Fig. 24–4).19
The impact of the orthosis on pulmonary function is another important factor to consider when contemplating a TLSO for patients with neuromuscular scoliosis. The effect of bracing on pulmonary dysfunction seems to depend on the level of muscle spasticity. Flaccid patients are more amenable to rigid bracing, although this bracing may significantly decrease chest expansion leading to compromised pulmonary function.20 Spastic patients seem to be more amenable to soft bracing, which does not seem to compromise pulmonary function,21 although this bracing has been shown only to enhance seating comfort.17 Olafsson and colleagues18 and Bunnell and MacEwen22 suggested that a subset of patients with minimum deformity and muscle hypotonia or mild spasticity may experience slowing of curve progression by bracing without a negative impact on pulmonary function.
Other factors to evaluate when choosing orthotic treatment for neuromuscular scoliosis include ease of application and obstructions. A bivalved brace may be easier for a caregiver to place, although it cannot provide as much corrective strength as a single opening brace. Winter and Carlson23 found the two-piece bivalved brace to be a useful support in children with myelomeningocele and SMA. Patients with a stoma or gastrostomy tube require modification of the brace to accommodate these features.
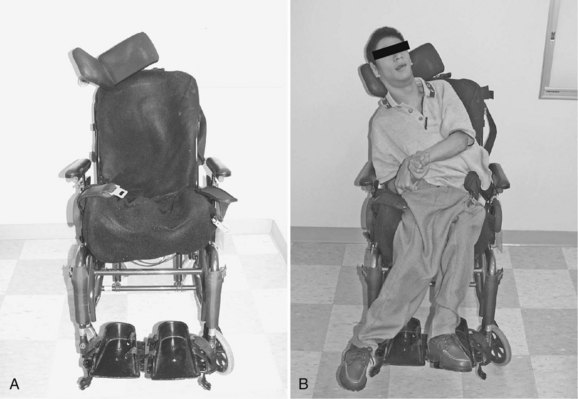
If a patient is not a candidate for bracing or surgery, wheelchair modifications can aid in providing a more comfortable seating position. Modular seating systems can be configured for optimal support of an individual patient (Fig. 24–5). A biomechanical evaluation of seating insert configurations by Holmes and colleagues24 concluded that three-point force application provides significant sitting support and static correction of scoliosis. Patients with more severe deformity may benefit from custom-molded seatbacks, although these items are expensive, and younger patients may outgrow them quickly.
Operative Treatment
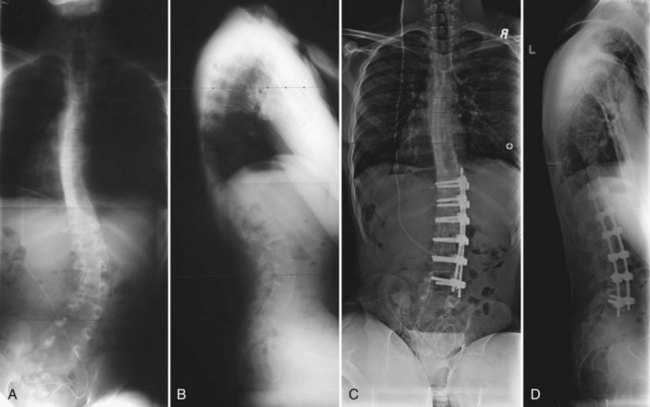
The timing for operative treatment is influenced by curve severity, underlying neuromuscular pathology, and other factors. The curve severity guidelines are loosely based on, but less aggressive than, the guidelines used in idiopathic scoliosis. Fusion should be considered as coronal deformity approaches 40 to 60 degrees. DMD may be an important exception to this concept: Surgery has been advocated when the deformity reaches 20 degrees because of pulmonary considerations.25 Patients with severely limited respiratory function have been shown to have good outcomes in spinal surgery, however. The sagittal profile is another important consideration because lordotic and kyphotic deformities can also impair sitting balance and pulmonary capacity (Fig. 24–6). Other factors that play a role in the decision to operate include patient age, nutritional status, cardiac function, curve progression, patient comorbidities, and family and caretaker support.
The benefit of scoliosis surgery in this population is a topic of much debate. Many of these patients are poor operative candidates and risk much undergoing involved corrective surgery. Preoperatively, patients may have compromised pulmonary function, limited cardiac capacity, poor bone stock, and high risk for aspiration, which put them in danger of intraoperative or postoperative complications. Correction of large deformities requires extensive exposures and long procedures that can lead to blood loss greater than one to two patient blood volumes. Although many patients already have neurologic compromise, they are still at risk for further compromise because of intraoperative spinal column manipulation. More powerful instrumentation systems have led to less postoperative decompensation and pseudarthrosis; however, there remains a considerable risk of curve progression, sometimes necessitating revision surgery.26–28
Despite the risk of surgery, the benefits of corrective scoliosis surgery for many of these patients are substantial. Halting or slowing curve progression has a positive impact on the functional ability, comfort, and overall quality of life of these patients. Lonstein and Akbarnia29 reported that more than 50% of patients treated had functional improvement after surgery. In a study of 79 patients with total body spastic cerebral palsy, Comstock and colleagues27 found that 85% of caretakers surveyed were satisfied with the surgery, reporting improved comfort, sitting ability, and cosmesis for the patients. Bridwell and colleagues26 found similar trends in a study of 54 patients with neuromuscular disorders with all caretakers reporting benefit from the surgery, specifically in the areas of ease of patient care, skin breakdown, patient comfort, pulmonary complications, and quality of life. Askin and colleagues30 evaluated 20 patients with neuromuscular scoliosis preoperatively and 6, 12, and 24 months after corrective spinal surgery. The authors noted decreased physical ability at the 6-month time point followed by a return to preoperative function by 12 months and concluded that scoliosis surgery in these patients can stabilize, but not improve, function; however, 75% of patients or caregivers were extremely pleased with the cosmetic results of the surgery. Although most of these patients have deteriorating courses, the correction of spinal deformity seems to improve their function and quality of life.
Although these positive results make a strong case for spine surgery in patients with neuromuscular scoliosis, several review studies have been unable to show a clear benefit of surgical intervention for the patient. Mercado and colleagues31 evaluated 198 publications and graded their results on the concept of Grades of Recommendation introduced in the Journal of Bone and Joint Surgery.32 These authors concluded that the current literature shows there is poor-quality evidence that spinal fusion improves the quality of life in patients with cerebral palsy or DMD.31 In a Cochrane Collaboration review, Cheuk and colleagues33 found that there were no randomized controlled clinical trials available to evaluate the effectiveness of scoliosis surgery in patients with DMD, and so no evidence-based recommendations could be made. Although the practicality of conducting a randomized controlled trial of this nature is questionable, the literature does not provide sufficient evidence to support the role of spinal surgical treatment in patients with neuromuscular scoliosis. It is recommended that the decision for surgical intervention be made based on the needs of the individual patient in consultation with the multidisciplinary neuromuscular care team.
Preoperative Considerations
Neurologic
Many patients with neuromuscular scoliosis are on long-term seizure therapy, which has some important operative ramifications. Antiepileptic medications such as phenytoin and valproate have been linked to decreased bone turnover and decreased intestinal absorption of calcium resulting in osteopenia, which may affect implant fixation and should be considered in the selection of construct components.34,35 In addition, valproate is associated with decreased von Willebrand factor and an increased bleeding time. If possible, consideration should be given to weaning the patient off of this medication, or at least the surgeon should prepare for increased blood loss by having supplementary blood products available during surgery. Preoperative screening of complete blood count, prothrombin time, and partial thromboplastin time may not be predictive of intraoperative coagulopathy.36
Pulmonary
Full pulmonary assessment should be conducted by a pulmonologist and include a chest radiograph, arterial blood gases, and pulmonary function tests if the patient’s developmental age is at least 4 years old. Vital capacity that exceeds 500 mL and peak expiratory flow greater than 180 mL/min are associated with decreased perioperative pulmonary complications. Although surgery may be considered in appropriately selected patients with preexisting respiratory failure, Gill and colleagues37 showed that patients with a forced vital capacity (FVC) of 20% of predicted value can safely be operated on for deformity correction. This prospective observational study followed eight patients on noninvasive night ventilation for respiratory failure with 48 months after surgery and found that all patients recovered well with no major complications. If a patient cannot be assessed with formal pulmonary function tests, other signs of ventilatory capacity must be used, including crying, laughing, and other vocalizations.38–40
Nutritional
Proper nutritional balance is crucial for successful surgical outcomes in patients with neuromuscular scoliosis. Many patients are malnourished secondary to a combination of reflux, low calorie intake, and high metabolic demand from frequent illness. Malnourished patients are more prone to perioperative complications such as wound dehiscence, wound infection, and pulmonary complications. Conversely, older patients may be obese, presenting further operative complications associated with their body habitus. Nutritional status should be assessed preoperatively with albumin and total blood lymphocyte levels. Albumin should be greater than 3.5 g/L, and total lymphocyte count should be greater than 1.5 g/L40; in a study of 44 patients, Jevsevar and Karlin41 found that patients had a lower incidence of postoperative infections if they met these criteria.
Cardiovascular
Patients may have cardiac problems secondary to their deformity and other cardiac issues that are comorbidities of the primary disorder. Thoracic cage deformity resulting from scoliosis can cause hypoventilation and subsequent increased pulmonary vascular resistance; this increased vascular resistance can cause right ventricular hypertrophy and eventually cor pulmonale. Patients with DMD may have cardiomyopathy and arrhythmias. The complications associated with arrhythmias may be alleviated with glucocorticoid steroid treatment.14 Patients with myotonic dystrophy may also have cardiac arrhythmias. Left ventricular hypertrophy can be associated with Friedreich ataxia.
Hematologic
Studies have shown that patients with neuromuscular scoliosis have greater blood loss than patients with idiopathic scoliosis undergoing similar procedures. In this neuromuscular group, the underlying disorder plays a major role in determining the extent of blood loss. In a review article, Shapiro and Sethna42 found that patients with DMD had the greatest mean levels of blood loss. Much of this difference is due to the requirement for larger fusions in patients with neuromuscular scoliosis, although osteopenia in these patients may also play a role.43,44 Preparation for major blood loss—sometimes exceeding 200% of a patient’s blood volume—is essential.38 Often, these patients have already had major surgery, and previous blood loss experience can be used as a guideline for preoperative preparation. Patients should have partial thromboplastin time, prothrombin time, and platelet function evaluated as a part of their preoperative blood work; a more aggressive coagulopathy workup should be conducted if the patient has previously shown a tendency toward excessive blood loss.
In a prospective, double-blinded, placebo control study of 40 pediatric patients, Neilipovitz and colleagues45 found that tranexamic acid administration significantly reduced perioperative blood transfusions. These results have been supported by a meta-analysis by Gill and colleagues,46 which found that tranexamic acid and aminocaproic acid are effective in minimizing blood loss and transfusion in patients undergoing spine surgery. The side effects for tranexamic acid and aminocaproic acid are minor but should be discussed with the patient before using these agents. The surgeon and the anesthesiologist should familiarize themselves with these agents and make a collaborative decision on their use based on the needs and concerns of the individual patient.
Radiographic Assessment
Patients should have a preoperative anteroposterior and lateral film taken of the entire spine preferably in an upright (sitting or standing) position. For assessment of skeletal maturity, a separate anteroposterior radiograph of the pelvis should be considered because scoliosis films often truncate the anatomy necessary to determine skeletal maturity. To assess spinal flexibility, supine bending films or traction films are used. Accurate measurements of the coronal Cobb angle, sagittal Cobb angle, and pelvic obliquity are crucial for complete preoperative planning and postoperative evaluations. In a more recent analysis of the interobserver and intraobserver variability of radiographic measurements of patients with neuromuscular scoliosis, Gupta and colleagues53 found that neuromuscular radiographs can be reliably analyzed with the use of coronal Cobb angle. Patients who may have congenital spinal anomalies or spinal tethering, such as patients with myelomeningocele, should undergo magnetic resonance imaging (MRI) to evaluate the neural elements fully before surgery. Computed tomography (CT) may also be useful in some patients with severe deformity or in patients with a congenital malformation of the vertebrae.
History of Instrumentation in Neuromuscular Scoliosis
In 1942, Haas47 published one of the first references to surgical intervention in neuromuscular scoliosis: a case report describing muscle and fascial transfers to obtain complete and permanent correction in one patient. With the introduction of the Harrington rod in 1962, use of this instrumentation with fusion of the spine in patients with neuromuscular scoliosis became the standard. Series using only Harrington rods and posterior spinal fusion have been associated with high incidences of pseudarthrosis (19% to 40%), moderate initial correction (20% to 57%), and loss of correction ranging from 14% to 28%.29,48 After Harrington rod instrumentation, most patients required bed rest and bracing or casting for up to 1 year.
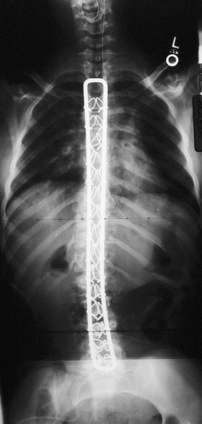
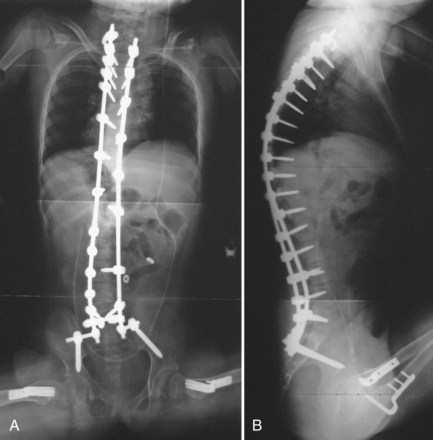
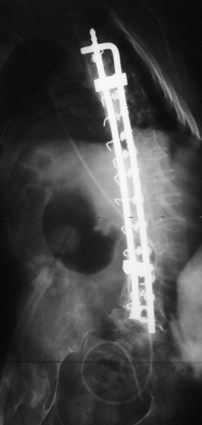
The introduction of segmental spinal instrumentation by Luque49 in 1976 led to major advances in the biomechanical stability and correction of these very deformed spines (Fig. 24–7). Several studies revealed that Luque segmental sublaminar wire fixation had fewer complications than Harrington instrumentation and was stable enough so that most patients required no brace or cast postoperatively.48,50,51 Using the Luque method, the only patients with cerebral palsy requiring postoperative bracing may be patients with athetosis or poor fixation because of severe osteopenia. This is a tremendous advantage because postoperative casting carries the potential for skin and pulmonary complications. Because of these attributes, the Luque technique became the standard method for posterior spinal instrumentation in patients with neuromuscular spinal deformities.
Contouring Luque spinal rods after the technique introduced by Allen and Ferguson52 (Galveston technique) allowed the rods to be fixed to the pelvis, providing surgeons with a more effective method of controlling pelvic obliquity. Bell, Moseley, and Koreska developed the unit rod, a precontoured U-shaped rod that includes the Galveston portion for pelvic fixation (Fig. 24–8). Studies of patient outcomes with unit rod fixation have revealed excellent correction and maintenance of correction.54–56 Bulman and colleagues57 compared the unit rod with double Luque rods and reported superior correction of sagittal and coronal alignment and pelvic obliquity with the unit rod constructs. Tsirikos and colleagues56 evaluated 287 children treated with unit rod instrumentation to the pelvis with 2-year follow-up and concluded that it offers the advantages of good correction of deformity and pelvic obliquity, a low complication rate, and a 96% caretakers’ survey satisfaction rate. Unit rod instrumentation has also been shown to have good results in ambulatory patients, with excellent deformity correction and preservation of ambulatory function at 2.9-year follow-up in 24 patients.58 Additionally, biomechanical studies have shown that the addition of an L5 pedicle screw increases the construct stiffness and the strength-reducing complications associated with the loss of fixation.59
Through a desire to achieve similar correction as the unit rod construct, without the need for pelvic fixation, the U-rod was investigated by McCall and Hayes.60 This rod is an outgrowth of the unit rod concept except the rod terminates in pedicle screws at the L5 level relying on the iliolumbar ligaments to achieve correction of the pelvic obliquity. In their comparison study of 30 patients with unit rod instrumentation and sacral fusion and 25 patients with U-rod instrumentation and L5 fusion, McCall and Hayes60 found that the U-rod provided comparable correction of scoliosis and pelvic obliquity in curves with less than 15 degrees L5 tilt at 4 years of follow-up. Regardless of whether the precontoured unit rod or double Luque rods are used, segmental sublaminar wire instrumentation provides simple, inexpensive, and fairly powerful correction of coronal plane deformity. Segmental sublaminar wire instrumentation has limitations in the maintenance of sagittal plane alignment, however, because the sublaminar wiring fails to fix spinal length, and the vertebrae can slide along the smooth rod construct, particularly during trunk flexion (Fig. 24–9A).
Multihook segmental systems such as Cotrel-Dubousset (CD) and Isola have also been shown to be efficacious in patients with neuromuscular scoliosis.61–63 The comparative efficacy of these two different constructs is inconclusive. In a study of 47 patients with neuromuscular scoliosis, Yazici and colleagues64 concluded that the Isola instrumentation combined with Galveston pelvic fixation provided correction and maintenance of pelvic obliquity superior to Luque Galveston, unit rod, or CD instrumentation. The results of this study are in contrast to the work of Wimmer and colleagues,63 who found that there was no difference between Luque Galveston and Isola instrumentation in radiographic outcomes, patient satisfaction, or complication rate. The selection of either of these two instrumentation systems is a choice that relies on surgeon experience and the needs of the individual patient.
Pelvic and Sacral Fixation
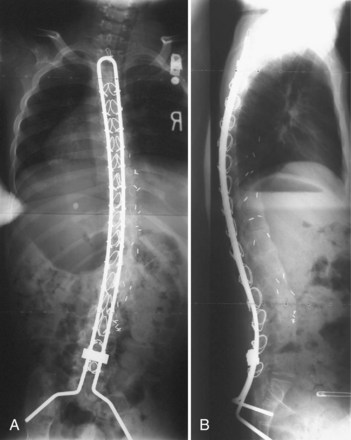
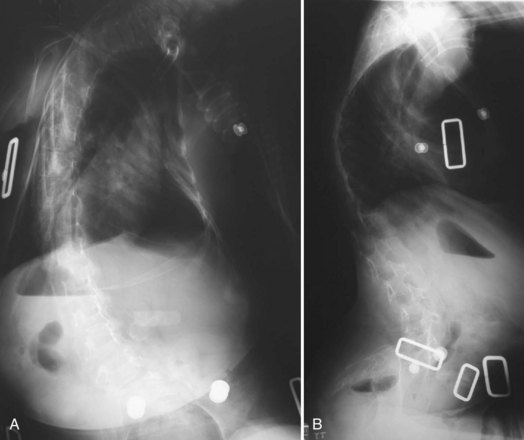
Severe pelvic obliquity secondary to unbalanced scoliotic curves and lower extremity contractures is common and progressive in patients with neuromuscular scoliosis. A solid spinal fusion to the pelvis aids in sitting comfort and balance1,65; however, achieving this goal can be troublesome (Fig. 24–9B). Controlling the motion across the lumbosacral joint requires secure fixation to the pelvis to prevent a pseudarthrosis.
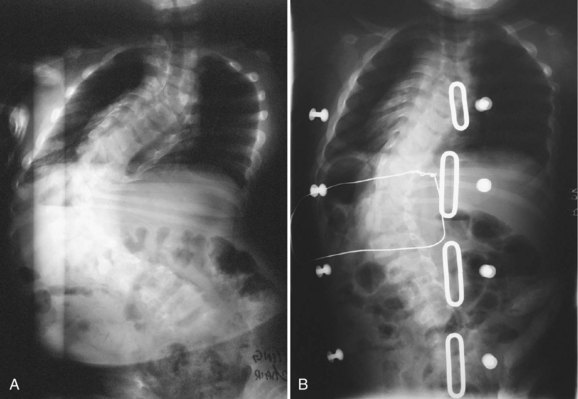
Various systems have been proposed to provide fixation to the pelvis. The Galveston technique was the first advancement to improve fusion rates and clinical success in long fusions to the sacrum.52,53 When paired with either contoured Luque rods or unit rods, it provides powerful coronal correction of pelvic obliquity. This technique places greater forces, however, on the lumbrosacral junction, and proper contouring of the rods may be difficult. The initial concern regarding the association between radiolucency around the screw tips (“windshield wiper” sign) and an increased incidence of complications is of little clinical significance (Fig. 24–10).66,67 Although a biomechanical evaluation of the Galveston technique by Sink and colleagues28 showed that this construct creates a long lever arm that places considerable cantilever forces at the lumbrosacral junction, these forces lead to a high incidence of proximal fixation pullout and distal migration of Galveston rods. The rods also require three-dimensional bending that makes it difficult to contour the rod properly.68
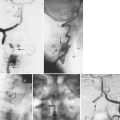
Other systems of sacropelvic fixation use an “S” bend (Dunn-McCarthy), which hooks distally over the sacral alae, while the more proximal portion is secured to the lumbar spine at L4 or above with a pedicle screw or infralaminar hook. Reviewing the results of 67 patients, McCarthy and colleagues69 found that this technique had decreased operative time compared with Galveston fixation and excellent clinical results, although in 2 of the 67 constructs there was migration of the rods into the pelvis. Other techniques of rod contouring to fix to the pelvis include the Warner-Fackler and McCall techniques, both commonly used in the treatment of myelomeningocele-associated kyphosis in which posterior elements of the lumbar or sacral spine may be absent. In the Warner-Fackler technique,70 Luque rods are bent to 90 degrees in two places at the distal end, allowing the rods to pass through the S1 foramina and lever against the front of the sacrum to provide sagittal correction (Fig. 24–11). In a slight variation of this technique, McCall71 described bending Luque rods to 20 to 40 degrees, passing them through the S1 foramina and bending the protruding portion according to the contour of the anterior sacrum. In 16 myelomeningocele patients with hyperkyphosis, McCall71 found satisfactory correction and maintenance of correction after 5 years of follow-up.
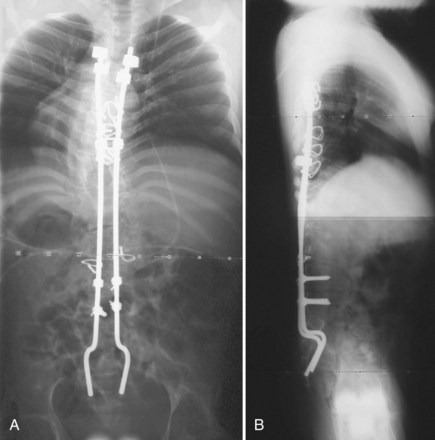
FIGURE 24–11 A and B, Warner-Fackler method of pelvic fixation was used after kyphectomy in this patient with myelodysplasia.
Improvement on the Galveston concept has been the focus of many clinical studies.69,72–74 The use of S1 screws alone was investigated, but bone quality is generally not substantial enough for successful use in patients with neuromuscular scoliosis. Early and colleagues72 compared the biomechanical properties of Galveston sacropelvic fixation versus Colorado II sacropelvic plates using S1 screws, S2 alar screws, and iliac screws and found that both methods provided similar construct stiffness with the Colorado II plate limiting L5-S1 motion in flexion-extension. These authors found that addition of a pair of L1 screws increased the construct stiffness by approximately 50% in both fixation techniques.
The use of iliac screw fixation has become a subject of several more recent articles because of its ease of implantation, avoiding the complex lumbosacral three-dimensional Galveston rod contouring. Clinical and biomechanical studies have shown an improved fusion rate and high pullout strengths after the use of iliac screws for caudad lumbosacral fixation.73,74 In a review of 50 patients treated with one or two bilateral iliac screws, Phillips and colleagues75 concluded that iliac screws provide a safe and effective means to treat neuromuscular scoliosis at 21 months of follow-up. These authors also noted that two screws in each iliac wing provided a more stable fixation with fewer implant-related complications than using a single screw. In a direct comparison of 20 patients with Galveston rod fixation versus 20 patients with iliac screw fixation, Peelle and colleagues76 found that both techniques offer similar pelvic fixation with the iliac screw construct allowing additional screw fixation points to the sacrum and lower lumbar vertebrae. The long-term impact of these screws on the sacroiliac joint was investigated in 67 adult patients by Tsuchiya and colleagues,74 and no evidence of degeneration was observed at 5- to 10-year follow-up.
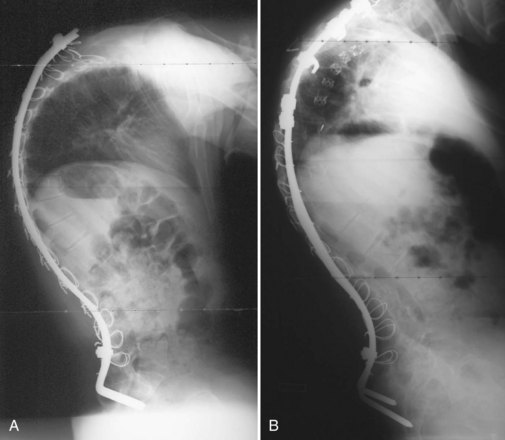
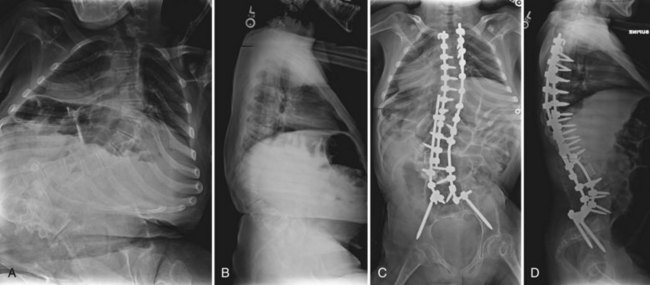
Although iliac screws provide a promising alternative to Galveston fixation, several studies have shown difficulty with implant prominence causing skin irriation.77 Peelle and colleagues76 did not observe this complication in their patient series, however, because they countersunk the screws below the superficial portion of the posterior iliac crest. The patient’s body habitus must be considered when selecting the means of sacropelvic fixation. In very small or thin patients, the authors continue to prefer a Galveston rod construct for pelvic fixation. Screw fixation is used when possible, especially in cases with a kyphotic deformity, where the Galveston rod may not optimally resist pelvic flexion (Fig. 24–12).
When patients with neuromuscular scoliosis are instrumented because of the severe obliquity of the pelvis, intraoperative halo-femoral traction may also be beneficial. Previous studies on this traction technique have been described for patients with idiopathic and congenital scoliosis. In nonambulatory patients with neuromuscular scoliosis, surgeons have relied on rods or screws inserted into the iliac wings by a cantilever method to level the pelvis. This method has the potential to weaken the bone-construct connection in patients with poor bone stock. In a study of 20 nonambulatory patients with neuromuscular scoliosis with halo-femoral traction and 20 matched patients without halo-femoral traction, Takeshita and colleagues78 found that halo-femoral traction provided significantly improved lumbar curve and pelvic obliquity correction at 2-year follow-up. These authors had no associated perioperative complications with this technique and found that unilateral femoral traction with corresponding halo traction was able to level the pelvis to an acceptable position before the surgery was begun. When a significant hip flexion contracture exists, traction results in an increase in lumbar lordosis, however, that may be undesirable.
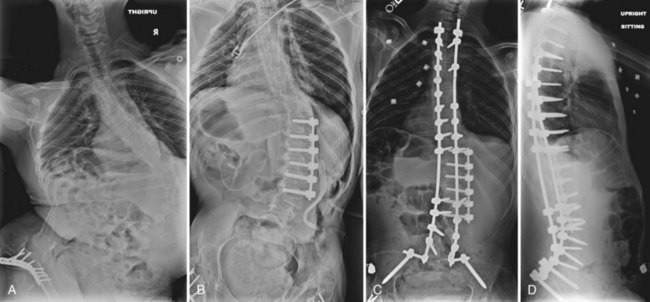
Some authors have argued that the pelvis can be left unfused in patients with slight pelvic obliquity, mild contractures, and little pelvic deformity in the sagittal plane, whereas others have argued that an ambulatory patient should never be fused to the pelvis.69,79 McCall and colleagues60 advocated that patients with less than 15 degrees of L5 tilt should be considered for a fusion to L5. These authors believed that this fusion allows greater mobility and improves the patients’ ability to carry out activities of daily living. Other studies promote fusion to the pelvis in all patients regardless of ambulatory status. A study by Tsirikos and colleagues58 of ambulatory cerebral palsy patients with severe pelvic obliquity who were treated with fusion to the pelvis by Luque-Galveston instrumentation found that 23 of 24 patients maintained their ambulatory status. Given the progressive nature of this deformity and the fragility of these patients as operative candidates, the authors generally recommend including the pelvis in the fusion mass for most neuromuscular deformities.
Anterior Spinal Release and Fusion
Indications
The addition of an anterior procedure can assist in the correction of neuromuscular spinal deformity and may be justified in several situations (Fig. 24–13). Anterior release and fusion has generally been indicated in patients with rigid scoliosis, patients with rigid kyphosis, immature patients at risk for the development of crankshaft growth, and patients at risk for pseudarthrosis owing to incompetent posterior elements (myelomeningocele or severe osteopenia).80 In assessing the rigidity of the deformity, traction and supine bending films are useful but may underrepresent the available flexibility. An anterior release of a large rigid curve increases the overall spine mobility and makes the posterior correction easier with a relatively high fusion rate. In a study by Newton and colleagues,80 the fusion rate achieved with anterior release combined with posterior corrective instrumentation and fusion was found to be comparable between adolescent patients with neuromuscular scoliosis and idiopathic scoliosis at 3-year follow-up.
Anterior instrumentation and spinal fusion alone and in combination with posterior instrumentation have also been shown to be successful techniques in a subset of patients with neuromuscular scoliosis. The authors’ current algorithm indicates an anterior procedure for “severe” curves (most often thoracolumbar). If a near-complete correction of the major curve can be predicted with anterior instrumentation after an aggressive multilevel discectomy, a single rod anterior system is included. If the curve remains rigid after an anterior release, the anterior instrumentation is skipped, and either an apical vertebrectomy is performed, or the correction is achieved after posterior osteotomies (Fig. 24–14). It is important to avoid “locking in” a poor correction with anterior instrumentation in rigid curves. The goals of achieving a level pelvis and balanced spine must be weighed against the added morbidity of an anterior release or instrumentation procedures or both.
In skeletally immature patients with idiopathic scoliosis, anterior release and fusion reduces anterior overgrowth that results in crankshaft deformity; however, whether this principle can be applied in neuromuscular scoliosis is controversial. In a study of 50 skeletally immature patients with neuromuscular scoliosis treated with posterior instrumentation only, Smucker and Miller81 noted no significant curve progression at an average of 4 years of follow-up. In contrast, Comstock and colleagues,27 after review of 60 skeletally immature patients with cerebral palsy who underwent surgical scoliosis correction, concluded that skeletally immature patients have the best correction and long-term outcomes when treated with anterior and posterior procedures.
Instrumentation
The indications for anterior instrumentation have been a subject of investigation more recently. Several studies have shown that anterior instrumentation alone provides acceptable correction without the need for posterior instrumentation in selected short flexible curves that do not include the pelvis or have less than 15 degrees of pelvic obliquity.82,83 Some studies advocate anterior release followed by posterior instrumentation, whereas others find indications for anterior disc excision and anterior instrumentation before proceeding posteriorly. Anterior instrumentation provides a very powerful means of addressing coronal plane deformities and in many cases simplifies the posterior instrumentation across the levels instrumented anteriorly.
Intraoperative Considerations
Spinal Cord Monitoring
Multimodality monitoring is a useful tool in children with neuromuscular scoliosis. Although patients with true paralysis and myelomeningocele do not benefit from this observation, the use of spinal cord monitoring is helpful for other patients to protect existing extremity function. Current evidence supports the use of transcranial motor evoked potentials (TcMEPs), somatosensory spinal evoked potentials, and 1+ reflex potentials in spinal cord monitoring.84,85 TcMEPs provide useful data on the motor function and vascular status of the spinal cord, whereas somatosensory spinal evoked potentials provide information on the integrity of the sensory pathways of the dorsal columns. TcMEP monitoring has become a vital component of spinal cord monitoring. In a study of 1121 consecutive patients with adolescent idiopathic scoliosis undergoing spine surgery, Schwartz and colleagues85 concluded that TcMEP monitoring is the most effective means to detect evolving spinal cord injury. TcMEPs monitor the anterior horn motor neurons, whose high metabolic rate is especially vulnerable to ischemic injury.86 Because of the complexity and associated hematologic issues of patients with neuromuscular scoliosis, multimodal spinal cord monitoring is highly recommended.
Blood Conservation
Antifibrinolytic agents, hypotensive anesthesia, Cell Saver, subperiosteal dissection, and electrocautery all can reduce blood loss. If an anterior approach is selected, unilateral ligation of the segmental vessels can be performed without significant risk of ischemia of the neural elements. Several studies have concluded that unilateral ligation carries no risk of causing neurologic compromise. Some authors recommend spinal cord monitoring during temporary (10 to 15 minutes) clamping of the segmental vessels before division.87–89 Periodic intraoperative coagulation panels should also be used to detect a dilutional coagulopathy, which ideally can be treated with early use of fresh frozen plasma, cryoprecipitate, and platelets before disseminated intravascular coagulopathy (DIC) develops. The hematologic status of cerebral palsy patients should be monitored very closely because they typically have increased bleeding that starts earlier in a procedure despite a normal coagulation profile.90
Bone Graft
In patients with neuromuscular scoliosis, the autologous iliac crest bone graft is often of poor quality and limited quantity. Also, harvesting may interfere with the placement of pelvic instrumentation. Because these patients need a significant volume of graft given the extent of fusion, supplemental graft in the form of freeze-dried or frozen cancellous allogeneic bone is almost always necessary to supplement local bone graft (facets, spinous process). Although allograft is generally regarded as safe and reliable for fusion augmentation in these patients,64 evaluation by Sponseller and colleagues91 of 210 patients with neuromuscular scoliosis revealed an increased risk of infection with the use of allograft. In a study by Yanci and Asher,92 the rate of pseudarthrosis in patients with neuromuscular scoliosis undergoing surgery with allograft was 2.5%.
Timing of Combined Procedures
The evidence for performing staged versus same-day anterior and posterior procedures is unclear. In a study of 45 patients who underwent combined anterior and posterior surgery, Tsirikos and colleagues93 found that same-day procedures were associated with longer operative time, greater blood loss, and a higher incidence of medical and technical complications. These results were in contrast, however, to a study by Mohamad and colleagues,94 who found no difference between single-stage and staged surgical procedures in their review of 175 patients with neuromuscular scoliosis. Further studies on staging combined procedures have focused on the use of traction and defining the curve characteristics that would be most appropriate for a staged procedure. For large, rigid curves, the use of staged surgery with anterior release and halo-pelvic traction as the first stage and posterior instrumentation and fusion as the second stage was investigated by Yamin and colleagues.95 These authors concluded that patients whose Cobb angle was greater than 80 degrees and flexibility was less than 20% should be treated with this method. Yamin and colleagues95 also recommended that patients whose spine flexibility was less than 10% with a Cobb angle that remained greater than 70 degrees after the first-stage anterior release and halo-pelvic traction should undergo pedicle subtraction osteotomies in the second-stage surgery.
It is important to anticipate the need for a staged procedure because unplanned staged procedures have a higher complication rate than planned staged procedures.94 Given the evidence, the authors recommend planning a staged procedure in larger patients with severe deformity or a history of large-volume blood loss in previous surgeries or both. Despite careful planning, hemodynamic instability may force unplanned staging. Blood loss in the posterior procedure tends to be two to three times the blood loss during the anterior procedure; if the surgeon encounters anterior blood loss greater than half the patient’s blood volume, a staged procedure should be considered.
Anterior Surgical Approaches
Thoracoscopic Approach
In the thoracic spine, thoracoscopy is an option instead of open thoracotomy. Video-assisted thoracic surgery allows exposure of the entire thoracic spine through three to five intercostal portals. The thoracoscopic approach is less invasive, sparing the chest wall musculature. Investigation has shown that there is less perioperative pulmonary dysfunction associated with this approach in adolescent patients with idiopathic scoliosis. This assertion is supported by Newton and colleagues,96 who found that comparable correction rates and pulmonary function are present at 5-year follow-up in adolescent patients with idiopathic scoliosis. Although the benefits in adolescent patients with idiopathic scoliosis have been well described, the potential benefits in respiratory function remain unclear in patients with neuromuscular scoliosis.
In a study of perioperative complications after surgical correction in 175 patients with neuromuscular scoliosis, Mohamad and colleagues94 discussed no positive or negative correlations associated with video-assisted thoracic surgery and pulmonary function. Given this lack of evidence and the technical demands of this technique, the benefits of this procedure must be weighed in the context of the experience of the surgeon and possible complications. Mastery of this less invasive approach may prove to be a valuable tool in treating these fragile patients, however.
Vertical Expandable Prosthetic Titanium Rib
The vertical expandable prosthetic titanium rib (VEPTR) has gained attention more recently for its role in the correction of early-onset scoliosis and congenital spinal deformities. Hell and colleagues97 investigated the efficacy of VEPTR in 15 children, 6 of whom were diagnosed with neuromuscular scoliosis. These authors concluded that this technique was safe, effective, and improved sitting ability and cosmesis. These results were supported by a preliminary investigation by Latalski and colleagues, who found that VEPTR considerably improved respiratory capacity in two patients with congenital spinal deformity and one patient with neuromuscular scoliosis.98 Although these initial results show promise, the clinical application of VEPTR in neuromuscular scoliosis remains a subject that requires further study.
Complications
The surgical treatment of neuromuscular scoliosis has been shown to have a higher complication rate compared with surgical treatment of idiopathic scoliosis. In the largest study to date of perioperative complications associated with neuromuscular scoliosis surgery, Mohamad and colleagues94 reported their complication rate to be 33% in 175 patients. These results are consistent with previous studies that found the complication rate to range from 24% to 75%.66,67,99,100 The factors that are associated with perioperative complications include a history of seizures, unplanned staged surgical procedures, and increased blood loss.94 Pulmonary issues have been found to be the most prevalent complication of spinal surgery in these patients and should be closely monitored by the multidisciplinary care team.
Wound Infections
The rate of wound infections in this type of surgery is higher than in surgery for adolescent idiopathic scoliosis, ranging from 8% to 15% in the literature. Superficial wound infections are more common than deep wound infections and require close monitoring because they can quickly progress in patients with neuromuscular disorders. Wound infection is also more common in nonambulatory patients with severe involvement and tends to involve multiple gram-negative organisms. A higher rate of wound infection has also been found in patients with sacropelvic fixation with a trend toward a higher rate of deep wound infection.94 The incidence of deep wound infection may be reduced in patients with neuromuscular disease undergoing spine surgery with an antibiotic-loaded bone graft. In a study of 220 children with cerebral palsy treated with unit rod instrumentation, Borkhuu and colleagues101 found that the use of gentamicin-impregnated bone graft decreased the incidence of deep wound infection by 11% compared with patients who received bone graft without antibiotics.
In patients with neuromuscular disease, a high index of suspicion must still be maintained, and any suspected infection must be aspirated from deep and superficial layers. A wound infection requires débridement and closure over drains with broad-spectrum antibiotics. Vacuum-assisted closure for deep infections has shown good results with an ease of use and a marked reduction in the need for hardware removal.102,103 When a wound has been infected, the risk for pseudarthrosis increases.91,104
Respiratory Complications
Given the baseline susceptibility of patients with neuromuscular disorders to respiratory complications and the chest wall insult and immobilization associated with scoliosis correction, respiratory complications are common in the postoperative period; the reported incidence ranges from 9% to 22%. These complications include pneumonia, pleural effusion, and atelectasis that may require prolonged intubation.66,67,100 A preoperative consultation with a pulmonologist to maximize the patient’s pulmonary health and aggressive care in the intensive care unit help to reduce the incidence of problems; however, caregivers and patients should be warned that permanent ventilator dependence is a possible sequela of surgery for some patients.
Urinary Tract Infections
The incidence reported in the literature of urinary tract infections in patients with neuromuscular scoliosis is 9% to 22%. Expeditious removal of a urinary catheter inserted for the procedure and adequate hydration may reduce the risk of urinary tract infection. Patients with myelomeningocele often have chronic colonization of bacteria in their bladder. Perioperative prophylactic antibiotics should address these organisms.66,67,99,100
Cerebral Palsy
Scoliosis in Cerebral Palsy
Scoliosis is common in cerebral palsy with a 25% incidence of spinal deformity in all cerebral palsy patients. The incidence and degree of deformity correlate with the amount of neurologic deficit and ambulatory status. In a study of 272 institutionalized patients, the highest prevalence of scoliosis was found in the most severely affected patients with 75% of spastic quadriplegic and 68% of spastic diplegic patients having at least 10 degrees of spinal deformity. In the above-mentioned study, 44% of patients who could ambulate independently, 54% of patients who could ambulate with assistance, 61% of patients who could sit independently, 75% of patients who could sit with assistance, and 76% of bedridden patients had significant deformity. Although these numbers may be inflated by the fact that the study population was composed entirely of institutionalized patients, they still reveal a trend of increased deformity with increased spasticity and decreased independent mobility.105,106
The development of scoliosis in cerebral palsy patients is thought to result partly from persistent primitive reflex patterns and asymmetrical tone in the paraspinous and intercostal muscles. Pelvic obliquity from contractures around the hip plays a role in scoliosis development; however, it is often difficult to isolate this as a contributing factor because pelvic obliquity, hip contractures, and scoliosis often develop simultaneously. Placing patients with a weak trunk and total body involvement into artificial upright sitting positions without appropriate spinal support may encourage gravity-related kyphosis and scoliosis. This suspicion was raised by Madigan and Wallace106 when they found a 75% incidence of scoliosis in a predominantly institutionalized population comprising “prop sitters” but only a 25% incidence in a population of spastic quadriplegics in which prop sitting was not pursued.
Scoliosis in cerebral palsy has been classified into four categories based on curve pattern (single vs. double) and the presence or absence of pelvic obliquity. Long C curves with pelvic obliquity generally occur in more severely involved nonambulatory patients with spasticity. S curves occur more frequently in sitting or walking patients with little spasticity. S curves seem to be more idiopathic in nature, often without associated pelvic obliquity.29 Patients with severe involvement and a developmental level less than 6 months seldom attain independent sitting balance. Lack of neuromuscular control prevents proper alignment of the head, and these patients do not develop compensatory curves to bring the shoulders and head over the pelvis.
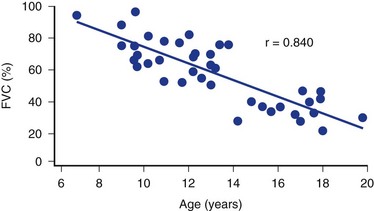
Curve progression is related to the above-mentioned risk factors and quadriplegia, poor functional status, and a single thoracolumbar curve.1,107,108 As in idiopathic scoliosis, risk of progression is also related to curve magnitude and to the amount of remaining spinal growth. Because progression of spinal deformity begins at the onset of the neuromuscular condition, patients with cerebral palsy have a much longer time to progress and have the potential for developing larger curves than patients with adolescent idiopathic scoliosis. This period of potential curve progression is prolonged further because these patients often maintain open growth plates into their late teens or early 20s. In adults, Thometz and Simon108 found that larger curves progress faster than smaller curves; they noted curve progression of 0.8 degree per year in curves less than 50 degrees and 1.4 degrees per year in curves greater than 50 degrees. Even patients who have completed growth are at risk for scoliosis progression.
Treatment Options
Bracing
The natural history of scoliosis in children with cerebral palsy is early onset with a flexible spine deformity between 3 and 10 years of age with a relatively fast progression to a rigid structural curve.107 Most of the articles in the literature do not support the use of bracing. Bracing can function as a temporizing measure, however, in patients with early-onset, flexible scoliosis or in patients with contraindications for surgery.15 Spastic cerebral palsy patients have poor tolerance for bracing because of the spasticity of their limbs inhibiting brace application, the incidence of skin pressure irritation, and sometimes increasing respiratory problems.15,17 A soft brace may be used owing to the lack of practicality of dynamic bracing, although it becomes mainly a sitting support rather than a treatment modality. For patients who cannot tolerate bracing, wheelchair seating systems can facilitate upright posture and allow patients to participate in more activities with greater social interaction.
Surgical Management
Various techniques and instrumentation exist for surgical scoliosis correction in cerebral palsy patients. In patients with hypotonia, a thoracolumbar or lumbar curve that does not extend to the pelvis, and a pelvic obliquity less than 15 degrees, fusion to L5 without sacropelvic fixation may occasionally be appropriate.60 For most cerebral palsy patients with spinal deformity, an extensive posterior fusion with instrumentation from the upper thoracic level (T1 or T2) to the pelvis is indicated. The most common instrumentation systems used have been the combination of Luque wires and a unit rod or two Galveston rods. Luque-Galveston constructs maybe modified with iliac screw fixation to aid in the ease of insertion and to provide safe, reliable correction.74,75 Several studies have shown the efficacy of these methods in cerebral palsy patients. Boachie-Adjei and colleagues100 retrospectively reviewed 45 patients treated with Luque-Galveston constructs and found 53% correction of scoliosis and 50% correction of pelvic obliquity. The correction was maintained at an average of 3 years of follow-up despite a 6.5% pseudarthrosis rate. Benson and colleagues99 reported on a cohort of 50 patients also treated with Luque-Galveston instrumentation who showed a 65% scoliosis correction rate with maintenance of correction at 40 months of follow-up. Only one patient in this series had a pseudarthrosis (2%).
Other studies have reported successful correction using the unit rod system in cerebral palsy patients. Bulman and colleagues57 compared 15 patients instrumented with Luque-Galveston constructs to 15 patients with unit rod instrumentation and concluded that the unit rod provided significantly improved correction and maintenance of correction of both scoliosis and pelvic obliquity at 2 years after surgery. Although the power of this study is limited by its small size and short follow-up, the findings are echoed in the results of other studies. Westerlund and colleagues109 reported 66% scoliosis correction and 75% pelvic obliquity correction that was maintained at an average of 5 years’ follow-up. Tsirikos and colleagues56 retrospectively reviewed 287 cerebral palsy patients treated with unit rod instrumentation and found a 68% correction of scoliosis and 71% correction in pelvic obliquity at 2-year follow-up.
More recently, there has been a trend toward using more hooks and screws in neuromuscular constructs. Segmental screw fixation common in idiopathic scoliosis may result in improved fixation with a reduced complication rate.110 Although this instrumentation shows great promise, follow-up studies are insufficient to clarify this issue.
Surgical correction of scoliosis and pelvic obliquity may also be improved with the use of intraoperative traction. In a comparison study of patients with and without asymmetrical intraoperative halo-pelvic traction, Vialle and colleagues111 concluded that intraoperative traction resulted in reduced anesthetic duration and improved correction of scoliosis and pelvic obliquity. These results are supported by Takeshita and colleagues,78 who found that halo-femoral traction improved scoliosis and pelvic obliquity surgical correction in nonambulatory patients with neuromuscular disorders.
Sagittal plane deformities are also common problems in cerebral palsy patients with scoliosis and require a different approach than that required for coronal plane deformities. Lumbar hyperlordosis and thoracic hyperkyphosis can impair sitting balance and comfort. Hyperkyphosis can be exacerbated by pelvic obliquity and gravity in a patient who sits unsupported propped up and is associated with an increased incidence of instrumentation pullout and failure.28 Sagittal plane kyphotic deformities require treatment similar to treatment of the deformity in Scheuermann disease with or without an anterior release and a hybrid system of hooks and pedicle screws to apply segmental compression across the kyphotic segments.
Authors’ Recommendations
All cerebral palsy patients with limited ambulatory capacity should be fused inferiorly to include the pelvis, even if their pelvic obliquity is correctable, because of the potential for increased obliquity owing to persistent muscle imbalances (Fig. 24–15). Most patients do not require postoperative immobilization. In patients with poor bone stock or a motion disorder undergoing fusion to the pelvis, a brace is considered for 3 to 6 months after surgery.
Myelodysplasia
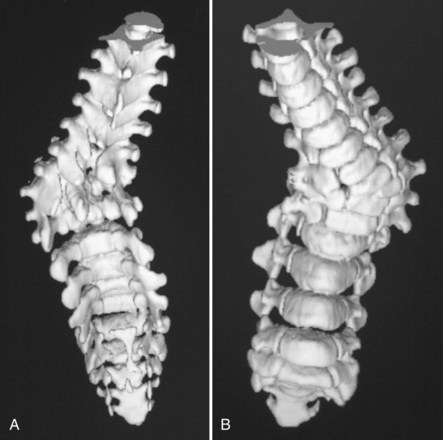
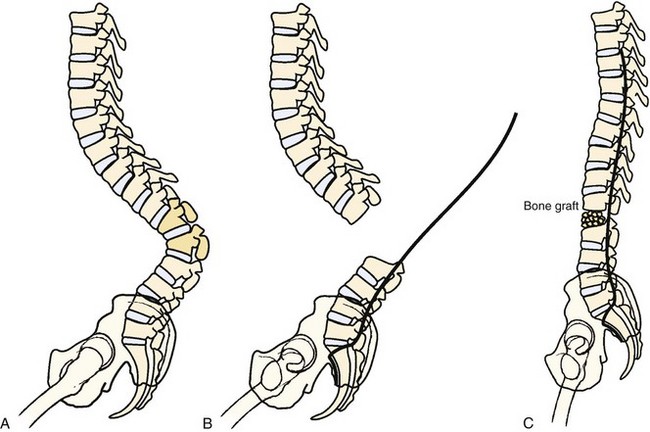
Myelodysplasia is characterized by a persistent open neural arch owing to failed proliferation of neuroectodermal cells in early embryonic development and has been associated with maternal hyperthermia and folate deficiency. The spinal deformity comes from a congenital malformation of levels at the defect, often resulting in a regional hyperkyphosis, and from the muscle imbalance around the spine distal to the lesion (Fig. 24–16).
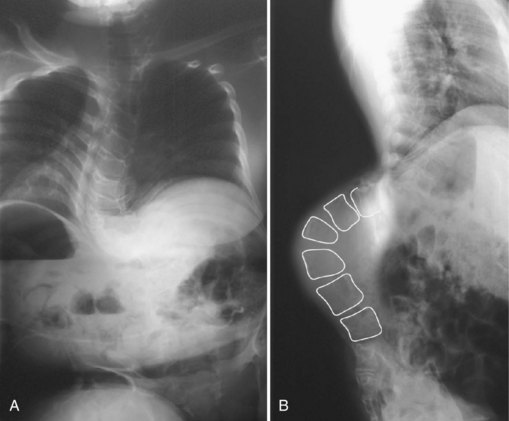
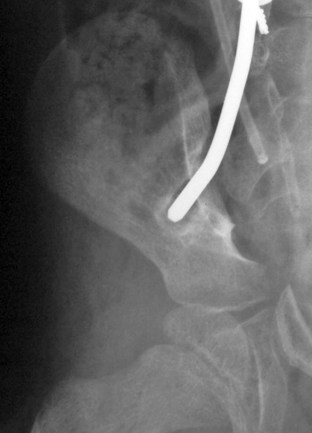
FIGURE 24–16 A and B, Severe progressive kyphosis in this patient with thoracic level myelomeningocele caused skin breakdown problems and difficulty with self-catheterization before she underwent kyphectomy and instrumentation as shown in Figure 24–11.
Scoliosis in Myelodysplasia
The incidence of scoliosis in patients with myelodysplasia (spina bifida) varies in the literature from 52% to 90%.112,113 In a more recent study of 141 patients by Trivedi and colleagues,113 the authors defined scoliosis in myelodysplasia as curvature greater than 20 degrees and concluded that new curvature could develop as late as 15 years of age.112 In this cohort of patients, 89% of patients with the last intact laminar arch located in the thoracic region had scoliosis, whereas 44% and 12% had scoliosis if their last intact laminar arch was in the upper and lower lumbar regions. From this trend, Trivedi and colleagues113 concluded that the last intact laminar arch was the most useful early predictor of scoliosis risk, although ambulatory status and clinical motor levels were also useful predictors.
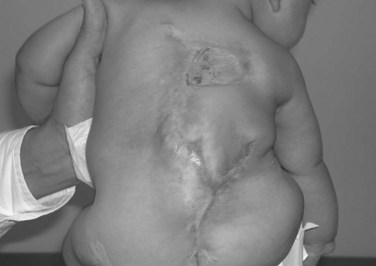
Myelodysplastic spine is one of the most difficult treatment dilemmas in scoliosis management. These patients present with congenital malformations (Fig. 24–17), often in the form of severe kyphosis preventing comfortable sitting and causing skin breakdown that may necessitate separate and early intervention. The risk of deformity from weak, spastic paraspinal musculature and asymmetrical hip contractures remains, often requiring more definitive surgical correction in the preteen or teen years. Surgical correction for myelodysplasia presents unique problems because of the absent posterior elements, increasing the dependence on anterior fusion and fixation. In addition, the posterior approach for surgical correction traverses the poor-quality skin and scar tissue that results from the defect and the early neurosurgical repair (Fig. 24–18).
There may be an association between myelodysplasia patients with a tethered spinal cord and rapidly progressive scoliosis.114 Most of these patients have some variation of cord tethering as a residuum of the defect and its neurosurgical closure. In patients with unexpected rapid curve progression or increased trunk spasticity (hypertonicity), a tethered cord with or without hydromyelia should be considered. Studies such as MRI or myelography may help define the anatomy; however, the literature does not support a causal relationship between scoliosis and Chiari malformation or syringomyelia.114 Thoughtful teamwork with a neuroradiologist, neurologist, and neurosurgeon helps clarify these issues. Surgical release of a tethered cord can result in scoliosis stabilization, maintenance of motor function, and decreased back pain.115 Pierz and colleagues116 evaluated 21 cases of de-tethering, however, and concluded that improvement or stabilization of scoliosis after de-tethering is less likely in patients with curves greater than 40 degrees or thoracic level defects or both. Given the high incidence of scoliosis and the tendency for rapid progression in these patients, annual follow-up evaluations with posteroanterior and lateral radiographs should closely track coronal and sagittal spinal deformity and hip contractures and trunk spasticity.
Treatment Options
Bracing
Bracing in myelodysplasia is even more difficult and less successful than in other neuromuscular disorders. Studies show that braces offered as a temporizing measure rarely yield effective curve control. Although Muller and Nordwall,117 after evaluating 21 myelodysplasia patients treated with bracing, concluded that deformity progression can be halted in patients with curves less than 45 degrees, a more recent study by Olafsson and colleagues18 reviewed outcomes in 20 patients and reported a successful outcome in only 20% of patients. Bracing is difficult in these patients because of pressure sores, especially prevalent over kyphotic deformities at the level of the neurologic defect. Obesity, poor-quality and insensate skin, decreased vital capacity, and obstructing stomal bags also contribute to the difficulty of brace wear in these patients. In the study by Olafsson and colleagues,18 half of the patients stopped wearing their braces because of discomfort. Custom-molded or modular wheelchair inserts offer another temporizing measure for comfortable upright posture until surgery is conducted.
Surgical Management
Anterior Fusion
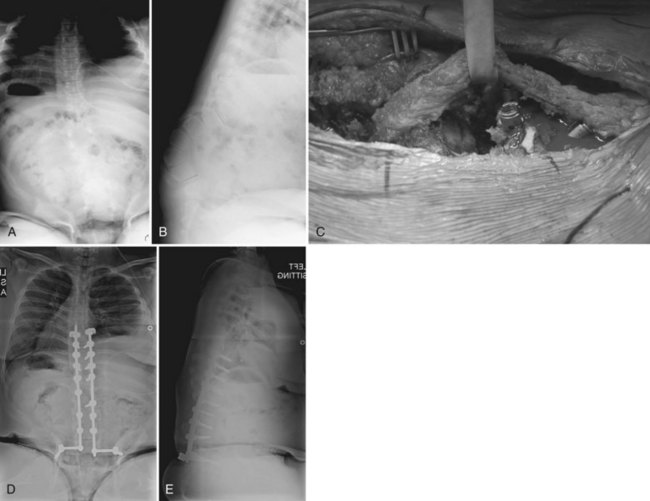
As previously discussed, anterior fusion is very important in myelodysplasia because the anterior spine provides a large surface for bony fusion in the lumbar spine, in contrast to the deficient posterior elements at these levels. A thoracoabdominal retroperitoneal approach from the convex side of the curve and division of the diaphragm allows anterior disc excision and bone grafting; this may be combined with posterior instrumentation to provide curve correction in select patients (Fig. 24–19). Use of anterior instrumentation alone has a specific role in the treatment of myelodysplasia scoliosis. In a study of 14 myelodysplasia patients treated with a Texas Scottish Rite Hospital anterior construct alone, Sponseller and colleagues118 concluded that anterior instrumentation was successful in a select group of patients with thoracolumbar curves less than 75 degrees, compensatory curves less than 40 degrees, no hyperkyphosis, and no syrinx in the spinal cord. Basobas and colleagues82 found excellent surgical correction and maintenance with an anterior instrumentation and fusion procedure in their study of 11 myelodysplasia patients at 5-year follow-up. In patients with a lumbar curve and fixed pelvic obliquity, anterior instrumentation provides a powerful means of correcting the deformity (Fig. 24–20).
Posterior Fusion
Although essential to a solid fusion in most myelodysplasia patients, the posterior procedure is difficult because of the poor quality of overlying tissue, decreased paraspinal muscle vascularity, and missing posterior elements. Posterior fusion in combination with anterior fusion is recommended by several authors. In a study of 50 patients, Banit and colleagues119 concluded that posterior fusion alone yielded a much higher pseudarthrosis rate of 16% than rates reported for anterior and posterior fusion combined. Parsch and colleagues120 reviewed results of 54 myelomeningocele patients treated surgically and also concluded that anterior and posterior instrumented fusion resulted in the best correction and lowest complication rate.
Fusion to the Pelvis
There is considerable controversy over whether the pelvis should be included in the fusion mass. Lindseth and colleagues121 contended that this is necessary only if there is a component of kyphosis in the lumbar spine or if pelvic obliquity is greater than 15 degrees. They noted an increased incidence of ischial ulcers in patients left with residual pelvic obliquity and fusion to the pelvis probably because the rigid, long, curved segment prevents easy shifting of weight between ischial tuberosities. A prospective evaluation of 11 myelomeningocele patients treated with anterior and posterior instrumented fusion to the lumbar spine revealed good correction of coronal and sagittal plane deformities with fair maintenance of correction. From this experience, the authors of the study concluded that, with the advent of more stable segmental instrumentation, the pelvis could be spared to allow more lumbosacral mobility and to avoid the morbidities associated with pelvic fusion.122 Similar results have been found in a select group of myelodysplasia patients with anterior-only instrumentation and fusion where pelvic obliquity can be corrected without pelvic fixation.82
Various options are available for instrumentation to the pelvis. The Galveston modification of Luque rods is a commonly used system, although it may not provide as much stability as the Warner-Fackler modification in correcting kyphosis. As previously described, the Warner-Fackler method involves two 90-degree bends in the distal end of a Luque rod allowing the rod to pass through the S1 foramina and lever against the anterior aspect of the sacrum (see Fig. 24–11).70 In a small series of nine patients treated with kyphectomy and this method of pelvic fixation, Thomsen and colleagues123 reported excellent correction and maintenance of kyphosis correction at an average of 28 months of follow-up. The authors described two instances of complications (loss of rod connection and migration) both at about 32 months after surgery and concluded that this technique, although effective, should be limited to patients weighing less than 30 kg. McCall described a similar technique with Luque rods passed through the S1 foramina and bent once to 20 to 40 degrees depending on sacral inclination. In his series of 16 patients, McCall71 reported good correction of kyphosis and excellent maintenance after 57 months of follow-up. Iliac screw fixation has also been shown to afford equivalent maintenance of pelvic obliquity and scoliosis correction compared with the Galveston technique.75,76
True fusion to the pelvis (to the wing of the ilium rather than just to the sacrum) can be achieved by suturing the detached iliac crest apophysis to the transverse process of the spine at L3 or L4 and filling the created triangle with bone graft (Fig. 24–21). Pseudarthrosis is common in fusions to the pelvis, and the authors still advise a conservative immobilization protocol.
FIGURE 24–21 This bone grafting technique increases the chance of stable fusion by including the wing of the ilium and the sacrum.
Major and minor complications are more common in myelomeningocele patients than in other patients with neuromuscular scoliosis. Reports of the incidence of wound infections range from 19% to 43%.124 Many patients have minor complications, such as urinary tract infections or minor wound dehiscence. Major complications, including massive blood loss and instrumentation failure, are more common in myelomeningocele scoliosis than in idiopathic scoliosis or other neuromuscular conditions.119,124 Pseudarthrosis rates range from 16% to 50% in the literature.119,125 In addition, the proximity of procedures to the dural sac and the abnormal anatomy increase the risk of shunt compromise and failure. By attending to detail and applying all that is currently known about myelomeningocele scoliosis surgery (careful anterior and posterior fusion, segmental attachment, cautious remobilization), the major complication rate can be reduced to 15% or less.
Kyphosis Treatment
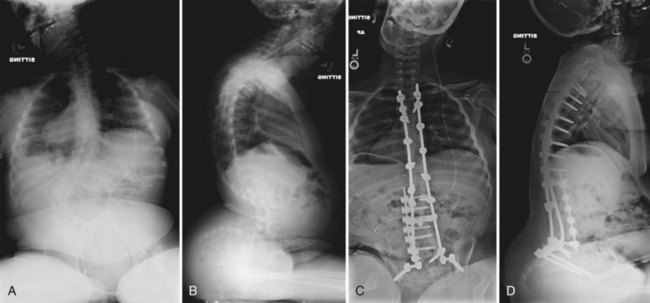
As already noted, bracing is extremely difficult in the severe kyphosis associated with myelomeningocele. Kyphosis is relentlessly progressive (3 to 8 degrees per year) and can become pathologic in 20% of patients. Because surgical correction is complex and difficult, many children are left untreated and seem to function reasonably well, although skin breakdown over the kyphosis, pressure on abdominal contents, and loss of trunk height remain problems. Indications for surgery include progressive kyphosis, skin breakdown over the kyphosis, respiratory compromise, and concern regarding the effect of severe trunk shortening (Fig. 24–22).71,126
Basic principles include a long midline posterior approach with exposure of five or six levels of normal closed laminae proximally and distal exposure down to the mid-sacral level. The sac and cord are retracted or resected, with care being taken to repair the dura slightly distal to and separate from the cord transection to avoid tying off the central canal and disturbing cerebrospinal fluid dynamics.127 In most cases, retracting the scarred thecal sac allows sufficient exposure.126 The spine is prepared for segmental attachment by passing laminar wires or hooks through all normal laminae proximally. The distal segments can be attached by pedicle screw fixation or by screws placed directly into the vertebral bodies. Because the bleeding encountered with osteotomy may force a rapid finish, complete preparation is essential for a successful instrumentation.
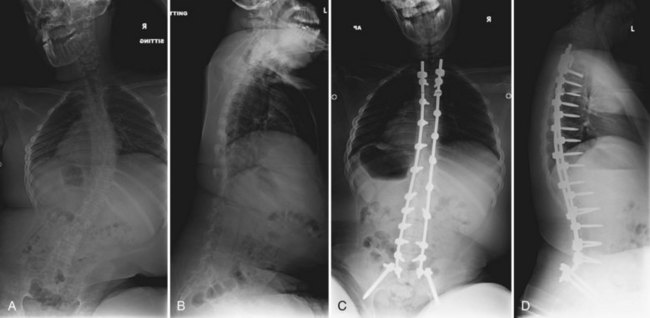
The vertebral bodies making up the cranial two thirds of the kyphosis are excised, allowing one to “fold in” the remaining proximal and distal spinal segments (Fig. 24–23). Care must be taken when the vertebrectomy is performed because the kidneys may be present in the concavity of the deformity and may sustain inadvertent injury. Often ribs 10 to 12 must be transected bilaterally to free the proximal segment enough so that the spine folds inward. The rods are positioned posterior to the infolded segments and secured in place. Posterolaterally placed polyaxial screws may be used to augment wiring and provide greater construct rigidity (Fig. 24–24). In a study of seven myelomeningocele patients, Kocaoglu and colleagues128 found that the addition of polyaxial screws provided greater correction capacity and a low instrumentation profile in patients with a kyphectomy and Luque instrumentation. The excised vertebrae provide adequate bone graft, applied anteriorly and posteriorly.
Spinal Muscular Atrophy
Affecting 1 in 10,000 newborns, SMA is a fairly common autosomal recessive disorder that is characterized by varying degrees of degeneration of the anterior horn cells that results in a symmetrical muscle paralysis of the trunk and proximal musculature. The etiology of SMA has been linked to mutations in the SMN1 gene, whose exact cellular function is unknown. Absence or deficiency of this inhibitory protein allows increased motor neuron death that leads to progressive muscle paralysis, eventually causing respiratory insufficiency and scoliosis (Fig. 24–25).129 Although there are more severe forms, 80% of patients live to adulthood and achieve sitting balance.130
Spinal Muscular Atrophy Types
SMA is broken down into three types. First described in the 1890s, type I is also known as Werdnig-Hoffman disease or acute infantile SMA. In this form, children are born with a normal appearance but by 6 months begin to show signs of muscle weakness with poor head control, absent reflexes, and respiratory insufficiency, which is the cause of death by 2 to 3 years of age.131 Children with type II SMA—the chronic infantile or Dubowitz form—experience the onset of muscle weakness between 6 and 19 months of age but do not have the same severity of symptoms. They attain sitting balance but rarely are able to walk independently. Although respiratory impairment is not as severe as in type I, respiratory complications are common. Type III SMA is also termed the chronic juvenile form of the disease or Kugelberg-Welander syndrome and is characterized by onset after 2 years with milder impairment; most children develop the ability to walk at some time, although many lose this ability around puberty.132
Scoliosis in Spinal Muscular Atrophy
All patients with type I SMA develop curves greater than15 degrees. Surgical intervention is not warranted in these patients because of their short life span; a body jacket provides good support for balanced and comfortable sitting. Patients with type II also develop curves greater than 15 degrees, although the onset of spinal deformity occurs later, between 2 and 4 years of age. Most of these patients do not ambulate and are likely to experience rapidly progressive scoliosis. The prevalence of spinal deformity in type III patients is varied, but it typically develops in about half of patients.133 Some of these patients are independent walkers and more likely to escape spinal deformity.131,134,135
Scoliosis in SMA tends to progress. In 52 cases of SMA, Granata and colleagues found an increase in curve magnitude of 8 degrees per year, despite brace use. They likewise showed a significant increase in curve progression when a patient with mild involvement stopped walking. In 13 nonwalking SMA patients, these investigators found a 3-degree per year curve progression. Walking patients with mild involvement showed only a 0.6-degree per year progression.136 The curve most often seen with SMA is a C-shaped thoracolumbar curve. Kyphosis can also be seen with these spinal deformities but is typically not severe.
Treatment Options
Bracing
As with other neuromuscular diseases, bracing is mainly a temporizing measure to aid in sitting while patients attain more trunk height and vertebral size before surgical intervention. The effectiveness of bracing depends on the type of SMA, the severity of the deformity, and the remaining growth of the patient. Early studies reported delay of progression in a limited subset of patients with milder forms of SMA.131,135 No studies have documented correction of deformity with dynamic bracing, however, because many patients do not have the muscular control to pull away from the sides of the brace. The evidence for brace application is still inconclusive because no current studies stratify their results based on age and SMA type. When bracing is elected to provide support for comfortable, balanced sitting before or in lieu of surgery, pulmonary function must also be considered. SMA patients are abdominal breathers because the disease affects intercostal muscle and diaphragmatic function. Because bracing significantly restricts abdominal movement and tidal volume, pretreatment pulmonary function should be a consideration in choosing this treatment modality.132
Surgical Management
Several studies have supported the use of segmental instrumentation to the pelvis for the definitive management of scoliosis in SMA. Brown and colleagues137 evaluated 40 patients with SMA who were treated with posterior fusions and concluded that there were fewer complications and better maintenance of correction with Luque instrumentation compared with Harrington instrumentation. A study by Bentley and colleagues138 reported a 51% surgical correction rate of coronal deformity in 33 SMA patients with good maintenance of correction. Fusion should be performed before the patient’s curve becomes too stiff; periodic bending films and examinations are important for the timing of fusion.
Spinal fusion almost always results in a loss of function. Evaluating 40 patients with preoperative and postoperative functional and strength testing, Furumasu and colleagues139 found that the straight but rigid spine was not always an immediate advantage to patients. The increased length of the spine creates a longer lever arm against which weakened proximal muscles have to work. These investigators reported an increased use of assistive devices, such as mobile arm supports, reachers, and lapboards. In this study, stronger patients were still able to maintain function, but weaker patients lacked the strength and flexibility to move their trunk in daily activities. Other studies have also noted a postoperative loss of function137,140; Aprin and colleagues141 noted increased preservation of preoperative function in 22 patients who underwent preoperative and postoperative physical therapy. Spinal fusion does not stabilize pulmonary function. In a study of eight patients—4 with SMA type II and 4 with SMA type III—Chng and colleagues142 reported a continued decline in pulmonary function at 44 months after spinal fusion and instrumentation. These authors noted that this decline was less marked than the natural history of SMA and that it was most likely secondary to the progressive neuromuscular weakness of the disease.
Duchenne Muscular Dystrophy
The most common hereditary neuromuscular condition, DMD is a sex-linked recessive disorder that affects 1 in 3500 boys. A deficiency of the muscle cell membrane stabilization protein dystrophin results in cell membrane leakage and gradual deterioration with fatty infiltration.143 These patients typically present with progressive muscle weakness at 3 to 5 years of age. DMD patients are young boys with delayed onset of walking, a wide-based gait, a wide-based stance with pronounced lordosis and pseudohypertrophy of the calves. Gower sign of DMD is a characteristic way in which these patients rise up from the floor using all four limbs to get into a “bear position” and then pushing off of their thighs with their upper extremities to force hip extension and right the trunk. In this manner, these children overcome their proximal muscle weakness. Elevated creatine kinase level (5000 to 15,000 U/L) is a good screening test. Definitive diagnosis is established by either a genetic analysis or a muscle biopsy specimen revealing decreased levels or absence of dystrophin.144–146
The clinical course of these patients is fairly predictable. Patients usually use a wheelchair full-time by 10 to 12 years of age. The incidence of spinal deformity and rate of scoliosis progression in these patients are controversial. Several studies have found that DMD scoliosis increases at a rate of 15 to 30 degrees yearly and may exceed 100 degrees if untreated (Fig. 24–26).147–150 In contrast, a 10-year retrospective study of 123 DMD patients by Kinali and colleagues151 found a highly variable rate of the presence and the progression of scoliosis in children with DMD. Despite this inconsistency, skeletally immature patients with a Cobb angle progression to greater than 20 degrees generally continue to progress. The association of the severity of spinal deformity and decline in pulmonary function has been well described. Respiratory failure generally leads to death in the late teens or early 20s, although advances in treatment have improved prognosis.152
Scoliosis in Muscular Dystrophy
More than any other form of neuromuscular disorder, pulmonary function and life expectancy are directly correlated with spinal deformity. FVC begins to decline at the age patients stop standing. From that time, the restrictive effects of thoracic cage deformity and loss of respiratory muscle function cause pulmonary function to decline 4% for each 10 degrees of deformity and each year of age (Fig. 24–27).153 Smith and colleagues150 reviewed the natural history of DMD in 51 boys and found that the rate of progression of scoliosis was inversely related to the age of death. Although most studies have failed to identify a protective role of spinal surgery on pulmonary function,151,152,154 Galasko and colleagues155 reported a stabilization of pulmonary function at 3-year follow-up. The study by Galasko and colleagues155 compared 32 patients who underwent spinal fusion and 23 patients who refused surgery; the FVC remained stable for 3 years postoperatively in the fused group compared with an 8% per year average decline in patients not operated on.155 Although the impact of spinal surgery on pulmonary function is unknown, the relationship between pulmonary decline and progression of scoliosis has been shown by Kennedy and colleagues154 to be 3% to 5% per year. When scoliosis progression has reached 20 to 30 degrees, many authors recommend surgery (Fig. 24–28).25,156,157 Glucocorticoid treatment seems to be affecting the natural history of this condition, but it is unclear how the surgical indications for spinal fusion should be modified.
Treatment Options
The preoperative evaluation of DMD patients must take into account other comorbidities of their disease. Pulmonary function testing should be done before surgery to assess the severity of preoperative respiratory compromise. Although more recent studies have reported successful spinal surgery at FVC of less than 35%,158 previous work associated this lowered FVC value with increased postoperative morbidity.157 Similar to the progressive decline in pulmonary function over time, the severity of cardiac dysfunction also increases with age. DMD patients are likely to develop cardiomyopathy and arrhythmias in the 2nd decade of life, and an electrocardiogram, a chest radiograph, and an echocardiogram should be done to screen for cardiac problems. Despite the fact that many of these patients are obese, they should be evaluated for malnutrition because wound healing complications and increased infections rates have been reported.159 DMD is also associated with a tendency for increased blood loss caused by poor vasoconstrictive function owing to a lack of dystrophin in vessel smooth muscle; preparation for major operative blood loss should be made.160 Additionally, patients with dystrophic myopathies have an increased risk of malignant hyperthermia.161
Surgical Management
Long fusions from T2 to either L5 or the pelvis are recommended. The distal extent of the fusion has been an area of much research and remains a controversial subject. Mubarak and colleagues157 determined that instrumentation and fusion to L5 was effective in mild deformities with pelvic obliquity less than 15 degrees at 34-month follow-up. Sengupta and colleagues162 found similar results in their review of posterior fusions in 50 patients and concluded that fusion to L5 with pedicle screw instrumentation is adequate if done soon after patients lose walking ability. These results were supported further by McCall and Hayes,60 who found that fusion to L5 was a viable alternative to fusion to the pelvis in patients with less than 15 degrees of L5 tilt.
Given the limitations of a minimal curve size and pelvic obliquity, fusion to the pelvis is the most common method of spinal fixation in DMD patients. Luque rods with the Galveston technique of pelvic fixation are a well-studied technique that offers acceptable results. Brook and colleagues163 reviewed results of Luque and Luque-Galveston instrumentation in 17 patients and found an average of 63% correction and good maintenance of correction. Bentley and colleagues138 reviewed 64 patients treated with posterior fusion and reported a 47% correction rate with good maintenance of correction. The authors recommended fusion to T4 stating that the lower level of fusion allows more freedom of movement for feeding and upper extremity use. Unit rods have also been evaluated in DMD patients and provide acceptable results; however, placement of the rods into the iliac wings makes this technique challenging.163 The Dunn-McCarthy technique with S-shaped rods looped over the sacral alae has been shown to provide good results in DMD patients. This technique has gained popularity because of its dependable fixation in patients with osteopenia and ease of placement in the kyphotic lumbar spine that is often seen in DMD patients.69,156
More recent advances in segmental pedicle screw fixation have resulted in their promotion as an alternative to more traditional hook-wire and Galveston pelvic fixation techniques. Hahn and colleagues164 reported results of 20 DMD patients treated with pedicle screw–alone fixation and concluded that pedicle screws combined with iliac screws provided a stable construct that eliminated the need for a combined anterior procedure at minimum 2-year follow-up. These authors found that this technique limited blood loss compared with sublaminar wiring, had no implant loosening, and allowed early mobilization leading to no pulmonary complications. Pedicle screw fixation with a free-hand technique has been shown to be as safe and accurate as in other conditions.165
Rett Syndrome
Rett syndrome is a developmental disorder of unknown etiology that occurs in 1 in 20,000 females. The syndrome is linked to a mutation in the MeCP2 gene located on the X chromosome, which encodes a deacetylator that regulates the transcription of certain genes. Patients appear normal at birth but begin the stepwise deterioration characteristic of Rett syndrome. The syndrome has four stages. In the first stage, generally occurring when patients are 6 to 18 months of age, signs of the syndrome include developmental stagnation, slowed head and brain growth, and generalized hypotonia. From approximately age 1 to 3 years, patients begin to regress developmentally and exhibit autisticlike symptoms. In the third stage, between age 2 and 10 years, patients have seizures, exhibit ataxia, exhibit mental retardation, and perform stereotypic gestures such as repetitive hand wringing. During this phase, scoliosis and spasticity can be seen. In the fourth stage, patients exhibit upper and lower motor neuron signs with increased rigidity and muscle wasting. Scoliosis is most likely to develop in this final stage (Fig. 24–29).166
Scoliosis in Rett Syndrome
The incidence of scoliosis increases as patients get older: 8% of girls with Rett syndrome have scoliosis at age 5, 40% have it by age 11, and 80% have it by age 20. The deformity can be rapidly progressive; Lidstrom and colleagues167 reported on 78 patients with Rett syndrome and reported progression rates of 20 to 41 degrees per year in the 10 most severe cases. Because scoliosis in Rett syndrome has this potential for progression, yearly evaluation of the spine should be conducted after the age of 5 years.168
The treatment for Rett syndrome is similar to treatment for cerebral palsy. Bracing has a limited role in treatment, although it may provide support for comfortable sitting until surgery is performed.169 As with cerebral palsy, the authors recommend that if sitting comfort and balance become compromised, treatment with segmental instrumentation is indicated. Spinal surgery is indicated when the curve exceeds 40 degrees. Long-term outcome of surgery in patients with Rett syndrome has shown good results. In a prospective study of 23 girls with Rett syndrome and neuromuscular scoliosis, Larsson and colleagues170 found improved seating position and patient satisfaction at 6-year follow-up. These authors reported that all patients who could walk preoperatively maintained ambulation postoperatively. They also noted that patients and parents reported an overall improvement in well-being with better sitting posture and improved breathing. As with all patients with neuromuscular disorders who undergo scoliosis surgery, spinal cord monitoring is advocated.171
Pearls
Pitfalls
1 Karol LA. Scoliosis in patients with Duchenne muscular dystrophy. J Bone Joint Surg Am. 2007;89:155-162.
2 Luque ER. Segmental spinal instrumentation for correction of scoliosis. Clin Orthop Relat Res. 1982;163:192-198.
3 Tokala DP, Lam KS, Freeman BJ, et al. Is there a role for selective anterior instrumentation in neuromuscular scoliosis? Eur Spine J. 2007;16:91-96.
4 Trivedi J, Thomson JD, Slakey JB, et al. Clinical and radiographic predictors of scoliosis in patients with myelomeningocele. J Bone Joint Surg Am. 2002;84:1389-1394.
1 Majd ME, Muldowny DS, Holt RT. Natural history of scoliosis in the institutionalized adult cerebral palsy population. Spine. 1997;22:1461-1466.
2 Bosboom WM, Vrancken AF, van den Berg LH, et al. Drug treatment for spinal muscular atrophy types II and III. Cochrane Database Syst Rev. 2009:CD006282.
3 Mercuri E, Bertini E, Messina S, et al. Randomized, double-blind, placebo-controlled trial of phenylbutyrate in spinal muscular atrophy. Neurology. 2007;68:51-55.
4 Miller RG, Moore DH, Dronsky V, et al. A placebo-controlled trial of gabapentin in spinal muscular atrophy. J Neurol Sci. 2001;191:127-131.
5 Tzeng AC, Cheng J, Fryczynski H, et al. A study of thyrotropin-releasing hormone for the treatment of spinal muscular atrophy: A preliminary report. Am J Phys Med Rehabil. 2000;79:435-440.
6 Ade-Hall RA, Moore AP: Botulinum toxin type A in the treatment of lower limb spasticity in cerebral palsy. Cochrane Database Syst Rev CD001408, 2000.
7 Lukban MB, Rosales RL, Dressler D. Effectiveness of botulinum toxin A for upper and lower limb spasticity in children with cerebral palsy: A summary of evidence. J Neural Transm. 2009;116:319-331.
8 Gerszten PC, Albright AL, Johnstone GF. Intrathecal baclofen infusion and subsequent orthopedic surgery in patients with spastic cerebral palsy. J Neurosurg. 1998;88:1009-1013.
9 Ginsburg GM, Lauder AJ. Progression of scoliosis in patients with spastic quadriplegia after the insertion of an intrathecal baclofen pump. Spine. 2007;32:2745-2750.
10 Caird MS, Palanca AA, Garton H, et al. Outcomes of posterior spinal fusion and instrumentation in patients with continuous intrathecal baclofen infusion pumps. Spine. 2008;33:E94-E99.
11 Shilt JS, Lai LP, Cabrera MN, et al. The impact of intrathecal baclofen on the natural history of scoliosis in cerebral palsy. J Pediatr Orthop. 2008;28:684-687.
12 Manzur AY, Kinali M, Muntoni F. Update on the management of Duchenne muscular dystrophy. Arch Dis Child. 2008;93:986-990.
13 Manzur AY, Kuntzer T, Pike M, et al: Glucocorticoid corticosteroids for Duchenne muscular dystrophy. Cochrane Database Syst Rev CD003725, 2008.
14 Markham LW, Kinnett K, Wong BL, et al. Corticosteroid treatment retards development of ventricular dysfunction in Duchenne muscular dystrophy. Neuromuscul Disord. 2008;18:365-370.
15 Kotwicki T, Durmala J, Czubak J. Bracing for neuromuscular scoliosis: Orthosis construction to improve the patient’s function. Disabil Rehabil Assist Technol. 2008;3:161-169.
16 Shoham Y, Meyer S, Katz-Leurer M, et al. The influence of seat adjustment and a thoraco-lumbar-sacral orthosis on the distribution of body-seat pressure in children with scoliosis and pelvic obliquity. Disabil Rehabil. 2004;26:21-26.
17 Miller A, Temple T, Miller F. Impact of orthoses on the rate of scoliosis progression in children with cerebral palsy. J Pediatr Orthop. 1996;16:332-335.
18 Olafsson Y, Saraste H, Al-Dabbagh Z. Brace treatment in neuromuscular spine deformity. J Pediatr Orthop. 1999;19:376-379.
19 Letts M, Rathbone D, Yamashita T, et al. Soft Boston orthosis in management of neuromuscular scoliosis: A preliminary report. J Pediatr Orthop. 1992;12:470-474.
20 Berven S, Bradford DS. Neuromuscular scoliosis: Causes of deformity and principles for evaluation and management. Semin Neurol. 2002;22:167-178.
21 Leopando MT, Moussavi Z, Holbrow J, et al. Effect of a Soft Boston Orthosis on pulmonary mechanics in severe cerebral palsy. Pediatr Pulmonol. 1999;28:53-58.
22 Bunnell WP, MacEwen GD. Non-operative treatment of scoliosis in cerebral palsy: Preliminary report on the use of a plastic jacket. Dev Med Child Neurol. 1977;19:45-49.
23 Winter RB, Carlson JM. Modern orthotics for spinal deformities. Clin Orthop Relat Res. 1977:74-86.
24 Holmes KJ, Michael SM, Thorpe SL, et al. Management of scoliosis with special seating for the non-ambulant spastic cerebral palsy population—a biomechanical study. Clin Biomech (Bristol, Avon). 2003;18:480-487.
25 Sussman M. Duchenne muscular dystrophy. J Am Acad Orthop Surg. 2002;10:138-151.
26 Bridwell KH, Baldus C, Iffrig TM, et al. Process measures and patient/parent evaluation of surgical management of spinal deformities in patients with progressive flaccid neuromuscular scoliosis (Duchenne’s muscular dystrophy and spinal muscular atrophy). Spine. 1999;24:1300-1309.
27 Comstock CP, Leach J, Wenger DR. Scoliosis in total-body-involvement cerebral palsy: Analysis of surgical treatment and patient and caregiver satisfaction. Spine. 1998;23:1412-1424. discussion 1424-1425, 1998
28 Sink EL, Newton PO, Mubarak SJ, et al. Maintenance of sagittal plane alignment after surgical correction of spinal deformity in patients with cerebral palsy. Spine. 2003;28:1396-1403.
29 Lonstein JE, Akbarnia A. Operative treatment of spinal deformities in patients with cerebral palsy or mental retardation: An analysis of one hundred and seven cases. J Bone Joint Surg Am. 1983;65:43-55.
30 Askin GN, Hallett R, Hare N, et al. The outcome of scoliosis surgery in the severely physically handicapped child: An objective and subjective assessment. Spine. 1997;22:44-50.
31 Mercado E, Alman B, Wright JG. Does spinal fusion influence quality of life in neuromuscular scoliosis? Spine. 2007;32:S120-S125.
32 Wright JG, Einhorn TA, Heckman JD. Grades of recommendation. J Bone Joint Surg Am. 2005;87:1909-1910.
33 Cheuk DK, Wong V, Wraige E, et al: Surgery for scoliosis in Duchenne muscular dystrophy. Cochrane Database Syst Rev CD005375, 2007.
34 Farhat G, Yamout B, Mikati MA, et al. Effect of antiepileptic drugs on bone density in ambulatory patients. Neurology. 2002;58:1348-1353.
35 Sheth RD, Wesolowski CA, Jacob JC, et al. Effect of carbamazepine and valproate on bone mineral density. J Pediatr. 1995;127:256-262.
36 Chambers HG, Weinstein CH, Mubarak SJ, et al. The effect of valproic acid on blood loss in patients with cerebral palsy. J Pediatr Orthop. 1999;19:792-795.
37 Gill I, Eagle M, Mehta JS, et al. Correction of neuromuscular scoliosis in patients with preexisting respiratory failure. Spine. 2006;31:2478-2483.
38 Pruijs JE, van Tol MJ, van Kesteren RG, et al. Neuromuscular scoliosis: Clinical evaluation pre- and postoperative. J Pediatr Orthop B. 2000;9:217-220.
39 Soudon P, Hody JL, Bellen P. Preoperative cardiopulmonary assessment in the child with neuromuscular scoliosis. J Pediatr Orthop B. 2000;9:229-233.
40 Winter S. Preoperative assessment of the child with neuromuscular scoliosis. Orthop Clin North Am. 1994;25:239-245.
41 Jevsevar DS, Karlin LI. The relationship between preoperative nutritional status and complications after an operation for scoliosis in patients who have cerebral palsy. J Bone Joint Surg Am. 1993;75:880-884.
42 Shapiro F, Sethna N. Blood loss in pediatric spine surgery. Eur Spine J. 2004;13(Suppl 1):S6-S17.
43 Kannan S, Meert KL, Mooney JF, et al. Bleeding and coagulation changes during spinal fusion surgery: A comparison of neuromuscular and idiopathic scoliosis patients. Pediatr Crit Care Med. 2002;3:364-369.
44 Meert KL, Kannan S, Mooney JF. Predictors of red cell transfusion in children and adolescents undergoing spinal fusion surgery. Spine. 2002;27:2137-2142.
45 Neilipovitz DT, Murto K, Hall L, et al. A randomized trial of tranexamic acid to reduce blood transfusion for scoliosis surgery. Anesth Analg. 2001;93:82-87.
46 Gill JB, Chin Y, Levin A, et al. The use of antifibrinolytic agents in spine surgery: A meta-analysis. J Bone Joint Surg Am. 2008;90:2399-2407.
47 Haas S. Spastic scoliosis and obliquity of the pelvis. J. Bone Joint Surg. 1942;24:774-780.
48 Sullivan JA, Conner SB. Comparison of Harrington instrumentation and segmental spinal instrumentation in the management of neuromuscular spinal deformity. Spine. 1982;7:299-304.
49 Luque ER. Segmental spinal instrumentation for correction of scoliosis. Clin Orthop Relat Res. 1982;163:192-198.
50 Herring JA, Wenger DR. Segmental spinal instrumentation: A preliminary report of 40 consecutive cases. Spine. 1982;7:285-298.
51 Taddonio RF. Segmental spinal instrumentation in the management of neuromuscular spinal deformity. Spine. 1982;7:305-311.
52 Allen BLJr, Ferguson RL. The Galveston technique for L rod instrumentation of the scoliotic spine. Spine. 1982;7:276-284.
53 Gupta MC, Wijesekera S, Sossan A, et al. Reliability of radiographic parameters in neuromuscular scoliosis. Spine. 2007;32:691-695.
54 Bell DF, Moseley CF, Koreska J. Unit rod segmental spinal instrumentation in the management of patients with progressive neuromuscular spinal deformity. Spine. 1989;14:1301-1307.
55 Dias RC, Miller F, Dabney K, et al. Surgical correction of spinal deformity using a unit rod in children with cerebral palsy. J Pediatr Orthop. 1996;16:734-740.
56 Tsirikos AI, Lipton G, Chang WN, et al. Surgical correction of scoliosis in pediatric patients with cerebral palsy using the unit rod instrumentation. Spine. 2008;33:1133-1140.
57 Bulman WA, Dormans JP, Ecker ML, et al. Posterior spinal fusion for scoliosis in patients with cerebral palsy: A comparison of Luque rod and Unit Rod instrumentation. J Pediatr Orthop. 1996;16:314-323.
58 Tsirikos AI, Chang WN, Shah SA, et al. Preserving ambulatory potential in pediatric patients with cerebral palsy who undergo spinal fusion using unit rod instrumentation. Spine. 2003;28:480-483.
59 Erickson MA, Oliver T, Baldini T, et al. Biomechanical assessment of conventional unit rod fixation versus a unit rod pedicle screw construct: a human cadaver study. Spine. 2004;29:1314-1319.
60 McCall RE, Hayes B. Long-term outcome in neuromuscular scoliosis fused only to lumbar 5. Spine. 2005;30:2056-2060.
61 Guidera KJ, Hooten J, Weatherly W, et al. Cotrel-Dubousset instrumentation: Results in 52 patients. Spine. 1993;18:427-431.
62 Neustadt JB, Shufflebarger HL, Cammisa FP. Spinal fusions to the pelvis augmented by Cotrel-Dubousset instrumentation for neuromuscular scoliosis. J Pediatr Orthop. 1992;12:465-469.
63 Wimmer C, Wallnofer P, Walochnik N, et al. Comparative evaluation of Luque and Isola instrumentation for treatment of neuromuscular scoliosis. Clin Orthop Relat Res. 2005;439:181-192.
64 Yazici M, Asher MA, Hardacker JW. The safety and efficacy of Isola-Galveston instrumentation and arthrodesis in the treatment of neuromuscular spinal deformities. J Bone Joint Surg Am. 2000;82:524-543.
65 Pritchett JW. The untreated unstable hip in severe cerebral palsy. Clin Orthop Relat Res. 1983;173:169-172.
66 Broom MJ, Banta JV, Renshaw TS. Spinal fusion augmented by Luque-rod segmental instrumentation for neuromuscular scoliosis. J Bone Joint Surg Am. 1989;71:32-44.
67 Gau YL, Lonstein JE, Winter RB, et al. Luque-Galveston procedure for correction and stabilization of neuromuscular scoliosis and pelvic obliquity: A review of 68 patients. J Spinal Disord. 1991;4:399-410.
68 Allen BLJr, Ferguson RL. The Galveston technique of pelvic fixation with L-rod instrumentation of the spine. Spine. 1984;9:388-394.
69 McCarthy RE, Bruffett WL, McCullough FL. S rod fixation to the sacrum in patients with neuromuscular spinal deformities. Clin Orthop Relat Res. 1999;364:26-31.
70 Warner WCJr, Fackler CD. Comparison of two instrumentation techniques in treatment of lumbar kyphosis in myelodysplasia. J Pediatr Orthop. 1993;13:704-708.
71 McCall RE. Modified Luque instrumentation after myelomeningocele kyphectomy. Spine. 1998;23:1406-1411.
72 Early S, Mahar A, Oka R, et al. Biomechanical comparison of lumbosacral fixation using Luque-Galveston and Colorado II sacropelvic fixation: Advantage of using locked proximal fixation. Spine. 2005;30:1396-1401.
73 Schwend RM, Sluyters R, Najdzionek J. The pylon concept of pelvic anchorage for spinal instrumentation in the human cadaver. Spine. 2003;28:542-547.
74 Tsuchiya K, Bridwell KH, Kuklo TR, et al. Minimum 5-year analysis of L5-S1 fusion using sacropelvic fixation (bilateral S1 and iliac screws) for spinal deformity. Spine. 2006;31:303-308.
75 Phillips JH, Gutheil JP, Knapp DRJr. Iliac screw fixation in neuromuscular scoliosis. Spine. 2007;32:1566-1570.
76 Peelle MW, Lenke LG, Bridwell KH, et al. Comparison of pelvic fixation techniques in neuromuscular spinal deformity correction: Galveston rod versus iliac and lumbosacral screws. Spine. 2006;31:2392-2398. discussion 2399
77 Stevens DB, Beard C. Segmental spinal instrumentation for neuromuscular spinal deformity. Clin Orthop Relat Res. 1989;242:164-168.
78 Takeshita K, Lenke LG, Bridwell KH, et al. Analysis of patients with nonambulatory neuromuscular scoliosis surgically treated to the pelvis with intraoperative halo-femoral traction. Spine. 2006;31:2381-2385.
79 Whitaker C, Burton DC, Asher M. Treatment of selected neuromuscular patients with posterior instrumentation and arthrodesis ending with lumbar pedicle screw anchorage. Spine. 2000;25:2312-2318.
80 Newton PO, White KK, Faro F, et al. The success of thoracoscopic anterior fusion in a consecutive series of 112 pediatric spinal deformity cases. Spine. 2005;30:392-398.
81 Smucker JD, Miller F. Crankshaft effect after posterior spinal fusion and unit rod instrumentation in children with cerebral palsy. J Pediatr Orthop. 2001;21:108-112.
82 Basobas L, Mardjetko S, Hammerberg K, et al. Selective anterior fusion and instrumentation for the treatment of neuromuscular scoliosis. Spine. 2003;28:S245-S248.
83 Tokala DP, Lam KS, Freeman BJ, et al. Is there a role for selective anterior instrumentation in neuromuscular scoliosis? Eur Spine J. 2007;16:91-96.
84 Costa P, Bruno A, Bonzanino M, et al. Somatosensory- and motor-evoked potential monitoring during spine and spinal cord surgery. Spinal Cord. 2007;45:86-91.
85 Schwartz DM, Auerbach JD, Dormans JP, et al. Neurophysiological detection of impending spinal cord injury during scoliosis surgery. J Bone Joint Surg Am. 2007;89:2440-2449.
86 de Haan P, Kalkman CJ, de Mol BA, et al. Efficacy of transcranial motor-evoked myogenic potentials to detect spinal cord ischemia during operations for thoracoabdominal aneurysms. J Thorac Cardiovasc Surg. 1997;113:87-100. discussion 101
87 Leung YL, Grevitt M, Henderson L, et al. Cord monitoring changes and segmental vessel ligation in the “at risk” cord during anterior spinal deformity surgery. Spine. 2005;30:1870-1874.
88 Tsirikos AI, Howitt SP, McMaster MJ. Segmental vessel ligation in patients undergoing surgery for anterior spinal deformity. J Bone Joint Surg Br. 2008;90:474-479.
89 Winter RB, Lonstein JE, Denis F, et al. Paraplegia resulting from vessel ligation. Spine. 1996;21:1232-1233. discussion 1233-1234
90 Sarwark J, Sarwahi V. New strategies and decision making in the management of neuromuscular scoliosis. Orthop Clin North Am. 2007;38:485-496. v
91 Sponseller PD, LaPorte DM, Hungerford MW, et al. Deep wound infections after neuromuscular scoliosis surgery: A multicenter study of risk factors and treatment outcomes. Spine. 2000;25:2461-2466.
92 Yazici M, Asher MA. Freeze-dried allograft for posterior spinal fusion in patients with neuromuscular spinal deformities. Spine. 1997;22:1467-1471.
93 Tsirikos AI, Chang WN, Dabney KW, et al. Comparison of one-stage versus two-stage anteroposterior spinal fusion in pediatric patients with cerebral palsy and neuromuscular scoliosis. Spine. 2003;28:1300-1305.
94 Mohamad F, Parent S, Pawelek J, et al. Perioperative complications after surgical correction in neuromuscular scoliosis. J Pediatr Orthop. 2007;27:392-397.
95 Yamin S, Li L, Xing W, et al. Staged surgical treatment for severe and rigid scoliosis. J Orthop Surg. 2008;3:26.
96 Newton PO, Upasani VV, Lhamby J, et al. Surgical treatment of main thoracic scoliosis with thoracoscopic anterior instrumentation: A five-year follow-up study. J Bone Joint Surg Am. 2008;90:2077-2089.
97 Hell AK, Hefti F, Campbell RMJr. [Treatment of congenital scoliosis with the vertical expandable prosthetic titanium rib implant]. Orthopade. 2004;33:911-918.
98 Latalski M, Fatyga M, Gregosiewicz A. The vertical expandable prosthetic titanium rib (VEPTR) in the treatment of scoliosis and thoracic deformities: Preliminary report. Orthop Traumatol Rehabil. 2007;9:459-466.
99 Benson ER, Thomson JD, Smith BG, et al. Results and morbidity in a consecutive series of patients undergoing spinal fusion for neuromuscular scoliosis. Spine. 1998;23:2308-2317. discussion 2318
100 Boachie-Adjei O, Lonstein JE, Winter RB, et al. Management of neuromuscular spinal deformities with Luque segmental instrumentation. J Bone Joint Surg Am. 1989;71:548-562.
101 Borkhuu B, Borowski A, Shah SA, et al. Antibiotic-loaded allograft decreases the rate of acute deep wound infection after spinal fusion in cerebral palsy. Spine. 2008;33:2300-2304.
102 Canavese F, Gupta S, Krajbich JI, et al. Vacuum-assisted closure for deep infection after spinal instrumentation for scoliosis. J Bone Joint Surg Br. 2008;90:377-381.
103 Gabriel A, Heinrich C, Shores J, et al. Outcomes of vacuum-assisted closure for the treatment of wounds in a paediatric population: Case series of 58 patients. J Plast Reconstr Aesthet Surg. 2009;62:1428-1436.
104 Szoke G, Lipton G, Miller F, et al. Wound infection after spinal fusion in children with cerebral palsy. J Pediatr Orthop. 1998;18:727-733.
105 Kalen V, Conklin MM, Sherman FC. Untreated scoliosis in severe cerebral palsy. J Pediatr Orthop. 1992;12:337-340.
106 Madigan RR, Wallace SL. Scoliosis in the institutionalized cerebral palsy population. Spine. 1981;6:583-590.
107 Saito N, Ebara S, Ohotsuka K, et al. Natural history of scoliosis in spastic cerebral palsy. Lancet. 1998;351:1687-1692.
108 Thometz JG, Simon SR. Progression of scoliosis after skeletal maturity in institutionalized adults who have cerebral palsy. J Bone Joint Surg Am. 1988;70:1290-1296.
109 Westerlund LE, Gill SS, Jarosz TS, et al. Posterior-only unit rod instrumentation and fusion for neuromuscular scoliosis. Spine. 2001;26:1984-1989.
110 Teli M, Elsebaie H, Biant L, et al. Neuromuscular scoliosis treated by segmental third-generation instrumented spinal fusion. J Spinal Disord Tech. 2005;18:430-438.
111 Vialle R, Delecourt C, Morin C. Surgical treatment of scoliosis with pelvic obliquity in cerebral palsy: The influence of intraoperative traction. Spine. 2006;31:1461-1466.
112 Dunteman RC, Vankoski SJ, Dias LS. Internal derotation osteotomy of the tibia: Pre- and postoperative gait analysis in persons with high sacral myelomeningocele. J Pediatr Orthop. 2000;20:623-628.
113 Trivedi J, Thomson JD, Slakey JB, et al. Clinical and radiographic predictors of scoliosis in patients with myelomeningocele. J Bone Joint Surg Am. 2002;84:1389-1394.
114 Dias MS. Neurosurgical causes of scoliosis in patients with myelomeningocele: An evidence-based literature review. J Neurosurg. 2005;103:24-35.
115 Sarwark JF, Weber DT, Gabrieli AP, et al. Tethered cord syndrome in low motor level children with myelomeningocele. Pediatr Neurosurg. 1996;25:295-301.
116 Pierz K, Banta J, Thomson J, et al. The effect of tethered cord release on scoliosis in myelomeningocele. J Pediatr Orthop. 2000;20:362-365.
117 Muller EB, Nordwall A. Brace treatment of scoliosis in children with myelomeningocele. Spine. 1994;19:151-155.
118 Sponseller PD, Young AT, Sarwark JF, et al. Anterior only fusion for scoliosis in patients with myelomeningocele. Clin Orthop Relat Res. 1999;364:117-124.
119 Banit DM, Iwinski HJJr, Talwalkar V, et al. Posterior spinal fusion in paralytic scoliosis and myelomeningocele. J Pediatr Orthop. 2001;21:117-125.
120 Parsch D, Geiger F, Brocai DR, et al. Surgical management of paralytic scoliosis in myelomeningocele. J Pediatr Orthop B. 2001;10:10-17.
121 Lindseth RE, Dias LS, Drennan JC. Myelomeningocele. Instr Course Lect. 1991;40:271-291.
122 Wild A, Haak H, Kumar M, et al. Is sacral instrumentation mandatory to address pelvic obliquity in neuromuscular thoracolumbar scoliosis due to myelomeningocele? Spine. 2001;26:E325-E329.
123 Thomsen M, Lang RD, Carstens C. Results of kyphectomy with the technique of Warner and Fackler in children with myelodysplasia. J Pediatr Orthop B. 2000;9:143-147.
124 Geiger F, Parsch D, Carstens C. Complications of scoliosis surgery in children with myelomeningocele. Eur Spine J. 1999;8:22-26.
125 Ward WT, Wenger DR, Roach JW. Surgical correction of myelomeningocele scoliosis: A critical appraisal of various spinal instrumentation systems. J Pediatr Orthop. 1989;9:262-268.
126 Nolden MT, Sarwark JF, Vora A, et al. A kyphectomy technique with reduced perioperative morbidity for myelomeningocele kyphosis. Spine. 2002;27:1807-1813.
127 Ko AL, Song K, Ellenbogen RG, et al. Retrospective review of multilevel spinal fusion combined with spinal cord transection for treatment of kyphoscoliosis in pediatric myelomeningocele patients. Spine. 2007;32:2493-2501.
128 Kocaoglu B, Erol B, Akgulle H, et al. Combination of Luque instrumentation with polyaxial screws in the treatment of myelomeningocele kyphosis. J Spinal Disord Tech. 2008;21:199-204.
129 Nicole S, Diaz CC, Frugier T, et al. Spinal muscular atrophy: Recent advances and future prospects. Muscle Nerve. 2002;26:4-13.
130 Dubowitz V. Benign infantile spinal muscular atrophy. Dev Med Child Neurol. 1974;16:672-675.
131 Evans GA, Drennan JC, Russman BS. Functional classification and orthopaedic management of spinal muscular atrophy. J Bone Joint Surg Br. 1981;63:516-522.
132 Tangsrud SE, Carlsen KC, Lund-Petersen I, et al. Lung function measurements in young children with spinal muscle atrophy: A cross sectional survey on the effect of position and bracing. Arch Dis Child. 2001;84:521-524.
133 Sucato DJ. Spine deformity in spinal muscular atrophy. J Bone Joint Surg Am. 2007;89(Suppl 1):148-154.
134 Merlini L, Granata C, Bonfiglioli S, et al. Scoliosis in spinal muscular atrophy: Natural history and management. Dev Med Child Neurol. 1989;31:501-508.
135 Schwentker EP, Gibson DA. The orthopaedic aspects of spinal muscular atrophy. J Bone Joint Surg Am. 1976;58:32-38.
136 Granata C, Merlini L, Magni E, et al. Spinal muscular atrophy: Natural history and orthopaedic treatment of scoliosis. Spine. 1989;14:760-762.
137 Brown JC, Zeller JL, Swank SM, et al. Surgical and functional results of spine fusion in spinal muscular atrophy. Spine. 1989;14:763-770.
138 Bentley G, Haddad F, Bull TM, et al. The treatment of scoliosis in muscular dystrophy using modified Luque and Harrington-Luque instrumentation. J Bone Joint Surg Br. 2001;83:22-28.
139 Furumasu J, Swank SM, Brown JC, et al. Functional activities in spinal muscular atrophy patients after spinal fusion. Spine. 1989;14:771-775.
140 Phillips DP, Roye DPJr, Farcy JP, et al. Surgical treatment of scoliosis in a spinal muscular atrophy population. Spine. 1990;15:942-945.
141 Aprin H, Bowen JR, MacEwen GD, et al. Spine fusion in patients with spinal muscular atrophy. J Bone Joint Surg Am. 1982;64:1179-1187.
142 Chng SY, Wong YQ, Hui JH, et al. Pulmonary function and scoliosis in children with spinal muscular atrophy types II and III. J Paediatr Child Health. 2003;39:673-676.
143 Pennisi E. Genetics: Hopping to a better protein. Science. 2008;322:1454-1455.
144 Ashton EJ, Yau SC, Deans ZC, et al. Simultaneous mutation scanning for gross deletions, duplications and point mutations in the DMD gene. Eur J Hum Genet. 2008;16:53-61.
145 Biggar WD, Klamut HJ, Demacio PC, et al. Duchenne muscular dystrophy: Current knowledge, treatment, and future prospects. Clin Orthop Relat Res. 2002;401:88-106.
146 McDonald CM, Abresch RT, Carter GT, et al. Profiles of neuromuscular diseases: Duchenne muscular dystrophy. Am J Phys Med Rehabil. 1995;74:S70-S92.
147 Cambridge W, Drennan JC. Scoliosis associated with Duchenne muscular dystrophy. J Pediatr Orthop. 1987;7:436-440.
148 Miller F, Moseley CF, Koreska J. Spinal fusion in Duchenne muscular dystrophy. Dev Med Child Neurol. 1992;34:775-786.
149 Robin GC, Brief LP. Scoliosis in childhood muscular dystrophy. J Bone Joint Surg Am. 1971;53:466-476.
150 Smith AD, Koreska J, Moseley CF. Progression of scoliosis in Duchenne muscular dystrophy. J Bone Joint Surg Am. 1989;71:1066-1074.
151 Kinali M, Messina S, Mercuri E, et al. Management of scoliosis in Duchenne muscular dystrophy: A large 10-year retrospective study. Dev Med Child Neurol. 2006;48:513-518.
152 Miller F, Moseley CF, Koreska J, et al. Pulmonary function and scoliosis in Duchenne dystrophy. J Pediatr Orthop. 1988;8:133-137.
153 Kurz LT, Mubarak SJ, Schultz P, et al. Correlation of scoliosis and pulmonary function in Duchenne muscular dystrophy. J Pediatr Orthop. 1983;3:347-353.
154 Kennedy JD, Staples AJ, Brook PD, et al. Effect of spinal surgery on lung function in Duchenne muscular dystrophy. Thorax. 1995;50:1173-1178.
155 Galasko CS, Delaney C, Morris P. Spinal stabilisation in Duchenne muscular dystrophy. J Bone Joint Surg Br. 1992;74:210-214.
156 Karol LA. Scoliosis in patients with Duchenne muscular dystrophy. J Bone Joint Surg Am. 2007;89(Suppl 1):155-162.
157 Mubarak SJ, Morin WD, Leach J. Spinal fusion in Duchenne muscular dystrophy—fixation and fusion to the sacropelvis? J Pediatr Orthop. 1993;13:752-757.
158 Harper CM, Ambler G, Edge G. The prognostic value of pre-operative predicted forced vital capacity in corrective spinal surgery for Duchenne’s muscular dystrophy. Anaesthesia. 2004;59:1160-1162.
159 Ramirez N, Richards BS, Warren PD, et al. Complications after posterior spinal fusion in Duchenne’s muscular dystrophy. J Pediatr Orthop. 1997;17:109-114.
160 Noordeen MH, Haddad FS, Muntoni F, et al. Blood loss in Duchenne muscular dystrophy: Vascular smooth muscle dysfunction? J Pediatr Orthop B. 1999;8:212-215.
161 Flick RP, Gleich SJ, Herr MM, et al. The risk of malignant hyperthermia in children undergoing muscle biopsy for suspected neuromuscular disorder. Paediatr Anaesth. 2007;17:22-27.
162 Sengupta DK, Mehdian SH, McConnell JR, et al. Pelvic or lumbar fixation for the surgical management of scoliosis in Duchenne muscular dystrophy. Spine. 2002;27:2072-2079.
163 Brook PD, Kennedy JD, Stern LM, et al. Spinal fusion in Duchenne’s muscular dystrophy. J Pediatr Orthop. 1996;16:324-331.
164 Hahn F, Hauser D, Espinosa N, et al. Scoliosis correction with pedicle screws in Duchenne muscular dystrophy. Eur Spine J. 2008;17:255-261.
165 Modi HN, Suh SW, Fernandez H, et al. Accuracy and safety of pedicle screw placement in neuromuscular scoliosis with free-hand technique. Eur Spine J. 2008;17:1686-1696.
166 Guidera KJ, Borrelli JJr, Raney E, et al. Orthopaedic manifestations of Rett syndrome. J Pediatr Orthop. 1991;11:204-208.
167 Lidstrom J, Stokland E, Hagberg B. Scoliosis in Rett syndrome: Clinical and biological aspects. Spine. 1994;19:1632-1635.
168 Bassett GS, Tolo VT. The incidence and natural history of scoliosis in Rett syndrome. Dev Med Child Neurol. 1990;32:963-966.
169 Keret D, Bassett GS, Bunnell WP, et al. Scoliosis in Rett syndrome. J Pediatr Orthop. 1988;8:138-142.
170 Larsson EL, Aaro S, Ahlinder P, et al. Long-term follow-up of functioning after spinal surgery in patients with Rett syndrome. Eur Spine J. 2009;18:506-511.
171 Master DL, Thompson GH, Poe-Kochert C, et al. Spinal cord monitoring for scoliosis surgery in Rett syndrome: Can these patients be accurately monitored? J Pediatr Orthop. 2008;28:342-346.