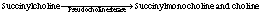23 Neuromuscular blocking agents
Action Potential: The passage of an electric impulse at any point on the nerve fiber where the inside becomes positive and the outside becomes negative; also referred to as action current.
Anticholinesterase: A drug that inhibits or inactivates the action of acetylcholinesterase.
Antimuscarinic (Anticholinergic): A drug that blocks the effects of acetylcholine receptors and results in the inhibition of the transmission of parasympathetic nerve impulses.
Clinical Duration: In reference to the use of neuromuscular blocking agents, the time from the administration of the drug to 25% recovery of the train-of-four twitch response.
Defasciculation: A result of the administration of a subclinical dose of a nondepolarizing skeletal muscle relaxant for prevention of the skeletal muscle twitches that occur after the administration of the depolarizing skeletal muscle relaxant succinylcholine.
Depolarizing Skeletal Muscle Relaxant: A skeletal muscle relaxant that after administration produces skeletal muscle twitches by stimulating the nicotinic receptors on the neuromuscular end plate and remaining on the end plate for 3 to 5 minutes, which leads to muscle paralysis. Skeletal muscle function returns as the pseudocholinesterase metabolizes the succinylcholine, usually between 3 and 5 minutes.
End Plate Potential (EPP): One action potential at the myoneural junction.
Excitation-Contraction (E-C) Coupling: The entire process of muscle contraction, starting with the electric and then the chemical stimulus to the process of the release of calcium in the sarcoplasmic reticulum, which causes the muscle fibers (actin and myosin) to slide and thus contract.
Extraocular Muscles: The six sets of muscles that control the movement of the eyeball.
Fasciculations: Skeletal muscle twitches.
Muscarinic: Subset receptors of the parasympathetic nervous system.
Myopathy: An abnormal condition of skeletal muscle characterized by muscle weakness and wasting.
Neurohumoral Transmission: Combined electric and chemical transmission of an impulse.
Nicotinic: Subset of the parasympathetic nervous system.
Nondepolarizing Agents: Drugs that cause paralysis of skeletal muscle by blocking neural muscular transmission at the myoneural junction.
Onset Time: In reference to the use of neuromuscular blocking agents, the time from the administration of the drug to maximum effect.
Pseudocholinesterase: An enzyme that acts like cholinesterase and metabolizes acetylcholine.
Recovery Index: In reference to the use of neuromuscular blocking agents, the time from the train-of-four twitch index of 25% to 75% recovery of the twitch response.
Total Duration of Action: In reference to the use of neuromuscular blocking agents, the time from drug administration to 90% recovery of the train-of-four twitch response.
Train-of-Four Ratio: A term used in reference to neuromuscular blocking agents in which a comparison is made between the fourth twitch of the train-of-four with the first twitch; when the fourth twitch is 90% of the first twitch, recovery from the neuromuscular blocking agent is indicated.
Neuromuscular blocking drugs, or muscle relaxants, have been used in clinical anesthesia since the early 1940s. Significant advances have been made in understanding the physiology of neuromuscular transmission and the pharmacology of muscle relaxants and have contributed greatly to clinical anesthesia as it is currently practiced. Interestingly, muscle relaxants have taken the same path as the inhalational anesthetic agents—rapid onset and a short duration of action. Muscle relaxants are not used exclusively in the field of anesthesia; in postanesthesia care units (PACUs), intensive care units, and emergency department settings, these drugs may be needed to enhance patient care. Muscle relaxants are used: (1) for facilitation of endotracheal intubation; (2) for procedures that necessitate muscle relaxation, such as intraperitoneal and thoracic surgery; (3) in ophthalmic surgery for relaxation of the extraocular muscles; (4) for termination of laryngospasm and elimination of chest wall rigidity, which can occur after rapid intravenous injection of a potent opioid; and (5) for facilitation of mechanical ventilation with production of total paralysis of the respiratory muscles.
Physiology of neuromuscular transmission
Because of the frequent and routine intraoperative and postoperative use of drugs that alter neuromuscular function, a review of the anatomy and physiology of the neuromuscular system is important, with an emphasis on the chemical changes that occur at the receptor sites. Activation of skeletal muscle is both an electric and a biochemical event. The term conduction refers to the passage of an impulse along an axon to a muscle fiber. Transmission applies to passage of a neurotransmitter substance across a synaptic cleft (neuromuscular junction). The combined electric and chemical event is called neurohumoral transmission.1
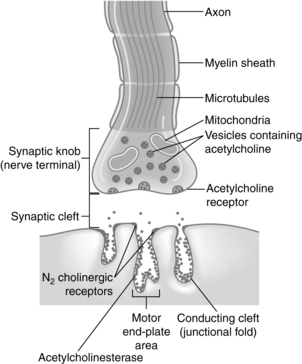
As the fine terminal branch of a motor neuron approaches the muscle fiber, it loses its myelin sheath and forms an expanded terminal that lies close to a specialized area of muscle membrane called the end plate (Fig. 23-1). Between the end of the muscle fiber and the end plate is the synaptic cleft, or neuromuscular junction. This space between the nerve and muscle fibers is approximately 20 nm wide. Acetylcholine is the biochemical neurotransmitter involved in the initiation of muscle contraction. Acetylcholine or cholinergic receptors are classified as nicotinic and muscarinic, respectively. The acetylcholine receptors are stimulated by acetylcholine. Anticholinesterase drugs such as neostigmine (Prostigmin), edrophonium chloride (Tensilon, Enlon), and pyridostigmine (Regonol) produce an increase in acetylcholine at the acetylcholine receptor. Therefore the pharmacologic effects of the anticholinesterase drugs are on both the nicotinic and muscarinic receptors. The nicotinic receptors are further classified as either N1 or N2 receptors. The N1 receptors are located at the presynaptic cleft and influence the release of acetylcholine. The N2 receptors are situated on the postsynaptic cleft in the neuromuscular junction and, when occupied by acetylcholine, open their channels to allow the flow of ions down the cell membrane, thus resulting in the skeletal muscle contraction. The nondepolarizing neuromuscular blocking agents such as pancuronium (Pavulon) produce a block of the N2 receptor and thus cause an inability of the channel to conduct ions, which results in skeletal muscle paralysis. Extrajunctional nicotinic receptors are located throughout the skeletal muscles. Their activity is normally suppressed by normal neural activity. However, when a patient has prolonged sepsis, inactivity, denervation, or burn trauma in the skeletal muscles, a proliferation of these extrajunctional nicotinic receptors results. As a result, these patients usually have an exaggerated hyperkalemic response when succinylcholine is administered.1
The muscarinic receptors are also subdivided into M1 and M2 receptors. M1 receptors are located in the autonomic ganglia and the central nervous system, and M2 receptors are located in the heart and salivary glands. Atropine and glycopyrrolate (Robinul) block both the M1 and the M2 receptors.2
Acetylcholine is formed in the body of the nerve cell and the cytoplasm of the nerve terminal and is stored in the small membrane-enclosed vesicles for subsequent release. A quantum is the amount of acetylcholine stored in each vesicle and represents approximately 10,000 molecules of acetylcholine. The presynaptic membrane contains discrete areas of specialization that are thought to be sites of release of the transmitter. These presynaptic active zones lie directly opposite the N2 cholinergic receptors, which are located on the postsynaptic membrane. This alignment ensures that the acetylcholine diffuses directly to the N2 receptors on the postsynaptic membrane quickly and in a high concentration. The N2 receptor, which responds to the neurotransmitter acetylcholine, is a glycoprotein that is an integral part of the postsynaptic membrane of the neuromuscular junction (see Fig. 23-1). New evidence indicates that a positive feedback mechanism also exists at the neuromuscular junction. Acetylcholine has a presynaptic action; therefore acetylcholine receptors are located on the presynaptic membrane. This positive feedback mechanism enhances the mobilization and release of acetylcholine. Finally, the enzyme that hydrolyzes acetylcholine is acetylcholinesterase, which is located in the neuromuscular junction.3
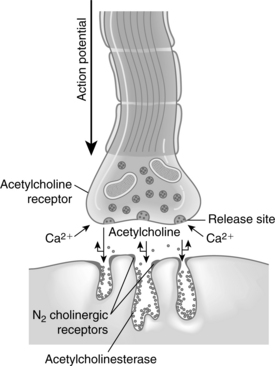
The initiation of skeletal muscle contraction occurs as a result of applying a threshold stimulus. An action potential that travels down the axon causes depolarization of the presynaptic membrane. As a result of this depolarization, the membrane permeability for calcium ions is increased and the calcium enters, or influxes, into the presynaptic membrane. Calcium acts to unite the vesicle to the presynaptic membrane and causes the rupture of that coalesced membrane, thus releasing acetylcholine into the fluid of the synaptic cleft (Fig. 23-2).
The acetylcholine molecules released from the nerve terminal into the synaptic cleft are subject to two main processes: (1) attachment to N2 cholinergic receptors located on the postsynaptic membrane, which leads to an opening of calcium channels that results in the movement of sodium into the region and generates an end plate potential (EPP); and (2) attachment of acetylcholine to the presynaptic nicotinic receptor, which enhances the release of more acetylcholine. When enough EPPs are generated, an action potential is propagated and spreads throughout the muscle and causes a change in the ionic permeability of the muscle sarcolemma. This process results in the release of calcium from the sarcoplasmic reticulum with a resultant increase in free calcium concentration in the muscle fiber. The process of excitation-contraction (E-C) coupling then takes place within that skeletal muscle cell. The physiologic outcome of E-C coupling is the contraction of the skeletal muscle. The increased concentration of calcium in the muscle fiber leads to an interaction between troponin-tropomyosin and actin. This interaction causes the active sites on actin to be exposed and interact with myosin and slide together, thus resulting in muscle contraction. This sliding of actin and myosin is sometimes called the ratchet effect.3 The contraction of the muscle fibers is terminated when calcium is pumped back into the sarcoplasmic reticulum of the muscle fibers. The calcium is stored in the sarcoplasmic reticulum for use when another action potential is generated.
Regulation and control of skeletal muscle contraction are also based on the enzymatic breakdown of acetylcholine. As previously discussed, the stimulus must be strong enough to release enough acetylcholine to bind to the postsynaptic N2 cholinergic receptor. This process of competition between the postsynaptic N2 receptor and acetylcholinesterase allows for some degree of regulation of the excitation process and for the recovery of the muscle cell membrane. The molecules of acetylcholine either diffuse in a random fashion to the N2 receptor or are destroyed by acetylcholinesterase. As the concentration gradient begins to decrease because of the destruction of acetylcholine by acetylcholinesterase, the N2 receptor gives up its acetylcholine, which is then destroyed, and the skeletal muscle relaxes. A small portion of the acetylcholine can escape the acetylcholinesterase in the synaptic cleft and migrate into the extracellular fluid and from there into the plasma. Acetylcholine within the plasma is then destroyed by plasma acetylcholinesterase, or pseudocholinesterase, which is produced in the liver.1
Pharmacologic overview of the skeletal muscle relaxants
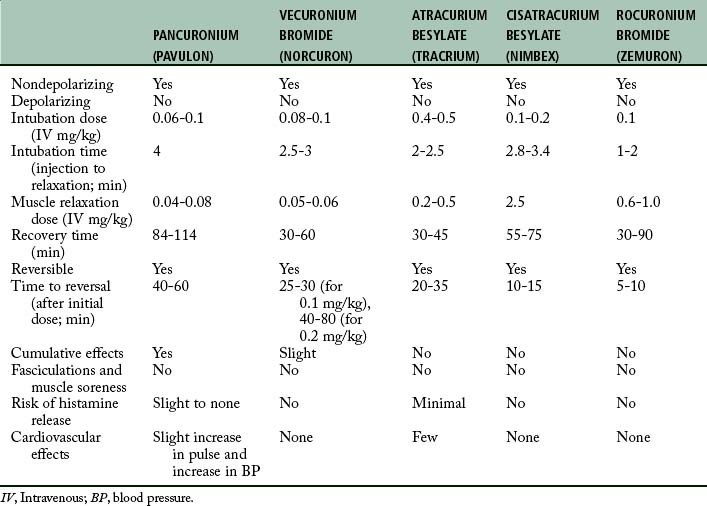
With the anatomy and physiology of neuromuscular transmission as background, the principal pharmacologic actions of the nondepolarizing and depolarizing skeletal muscle relaxants are discussed. Table 23-1 presents a pharmacologic overview of the commonly used skeletal muscle relaxants.
The prototypical nondepolarizing skeletal muscle relaxants are pancuronium and vecuronium (Norcuron). Pancuronium is an inhibitor of acetylcholine, is chemically viewed as two acetylcholine-like fragments, and has a bulky inflexible nucleus. This drug attaches to the N2 cholinergic receptors on the postsynaptic membrane and prevents depolarization. The skeletal muscle relaxant vecuronium has a chemical structure that is similar to a monoquaternary compound. The principal pharmacologic action of this drug is to block the postsynaptic N2 cholinergic receptor; in this way, it stops acetylcholine from binding to the receptor, which results in a competitive neuromuscular blockade. The nondepolarizing skeletal muscle relaxants also block the presynaptic cholinergic receptor and thus result in binding of the acetylcholine and thereby prevent activation of the positive feedback mechanism.4
The pharmacologic actions of the nondepolarizing skeletal muscle relaxants can be reversed with anticholinesterase drugs such as neostigmine. In effect, these drugs increase the quantum of acetylcholine at the postsynaptic membrane by preventing destruction of the acetylcholine by acetylcholinesterase. This process promotes a more effective competition by the released acetylcholine with the nondepolarizing skeletal muscle relaxant that is occupying the N2 receptor. Because of the increased availability and mobilization of the acetylcholine, the concentration gradients favor acetylcholine and remove the nondepolarizing agents from the N2 receptor, with the resultant return to normal contraction of the skeletal muscle.
The principal depolarizing skeletal muscle relaxant is succinylcholine (Anectine, Sucostrin). The molecular structure of this drug resembles two acetylcholine molecules back to back. Because of this structure, succinylcholine has the same effects as acetylcholine. Like acetylcholine, the succinylcholine molecule has a quaternary ammonium portion that is positively charged. This positively charged molecule is attracted by electrostatic action to the negatively charged N2 receptor. When the succinylcholine attaches to the receptor, a brief period of depolarization occurs that is manifested by transient muscular fasciculations. Succinylcholine also attaches to and activates the presynaptic acetylcholine receptor. This activation has an immediate effect of increased mobilization of acetylcholine in the motor nerve terminals, which explains why fasciculations are commonly observed after the administration of an intravenous bolus of succinylcholine. After the depolarization of the N2 receptor takes place, succinylcholine promotes and maintains the receptor in a depolarized state and prevents repolarization. Succinylcholine has a brief duration of action because of its rapid hydrolysis of the succinylcholine by the enzyme pseudocholinesterase, which is contained in the liver and plasma. The actions of succinylcholine cannot be pharmacologically reversed.5
Nondepolarizing neuromuscular blocking agents
Long-acting nondepolarizing skeletal muscle relaxants
Pancuronium bromide
Pancuronium bromide (Pavulon) was introduced into clinical anesthesia in 1972. This drug has shown value (particularly in terms of its safety, cardiovascular stability, and skeletal muscle relaxant properties) and is receiving widespread clinical use.
Chemically, pancuronium bromide is a biquaternary aminosteroid and is related to the androgens; however, it has no hormonal activities. Pancuronium is reversible with an anticholinesterase agent, such as neostigmine, that is administered in combination with an anticholinergic such as glycopyrrolate or atropine. This particular skeletal muscle relaxant has been shown clinically to be extremely difficult to reverse pharmacologically within the first 20 to 30 minutes after injection. In the PACU, if a skeletal muscle relaxant is needed for a short duration, another reversible skeletal muscle relaxant, such as vecuronium or atracurium, should be chosen. Approximately 30 to 40 minutes after injection, pancuronium is easily reversed with the combination of an anticholinesterase and anticholinergic drug preparation. Pancuronium is best suited for surgical procedures that last more than 1 hour; it is well suited for patients who need complete muscle relaxation with continuous mechanical ventilation. The dose for adults is approximately 0.08 to 0.1 mg/kg body weight. Relaxation lasts 60 to 85 minutes. If relaxation is necessary past this initial period, subsequent doses should be decreased to 0.02 to 0.04 mg/kg body weight.
Pancuronium bromide does not produce ganglionic blockade, but it does block the M2 cholinergic receptors in the heart. Consequently, when pancuronium bromide is administered, a slight 10% to 15% increase in heart rate is observed.6 Pancuronium activates the sympathetic nervous system by promoting the release of norepinephrine and blocking its uptake at the adrenergic nerve endings. After administration of this drug, a modest increase in mean arterial pressure and cardiac output is produced. Although isolated cases of histamine release have been reported, pancuronium can probably be used in patients who have a marginal allergy history. Pancuronium bromide is compatible with anesthetic agents used clinically and is safe for use in most patients when a nondepolarizing skeletal muscle relaxant is indicated. However, pancuronium bromide is not indicated when a nondepolarizing muscle relaxant is to be used with caution. In addition, pancuronium should not be used in patients who are undergoing chronic digitalis therapy because cardiac dysrhythmias have been reported. Finally, myocardial ischemia has been reported in patients with coronary artery disease when pancuronium is used. This ischemia is probably associated with the cardiac acceleration properties of the drug.
Pancuronium bromide should be avoided in patients with a history of myasthenia gravis. It is contraindicated in patients with true renal disease because a major portion of the drug is excreted unchanged in the urine. This agent is contraindicated in patients known to be hypersensitive to it or to the bromide ion.
Intermediate-acting nondepolarizing skeletal muscle relaxants
Vecuronium bromide
Vecuronium (Norcuron) is a nondepolarizing skeletal muscle relaxant with a more rapid onset of action and a shorter duration of action than pancuronium. Actually, vecuronium is pancuronium without the quaternary methyl group in the steroid nucleus. Because of this structural difference, vecuronium has no effect on heart rate, arterial pressure, autonomic ganglia, or the alpha and beta adrenal receptors. The potency of vecuronium is equal to or slightly greater than that of pancuronium. Vecuronium has little or no cumulative effect. Although a portion of vecuronium is metabolized, most of the drug is excreted unchanged in the urine and bile. However, the neuromuscular blockade produced by vecuronium is not prolonged by renal failure. The duration of neuromuscular blockade produced by vecuronium is increased in patients with impaired hepatic function. Of clinical interest is that vecuronium, like atracurium, is less influenced by general inhalation anesthetics than is pancuronium.7 The pharmacologic action of this drug is easily reversed with the combination of an anticholinesterase and an anticholinergic drug.
The onset of action of vecuronium is between 2.5 and 3 minutes, with the normal dose of 0.08 mg/kg intravenously. Because of the rapid onset of action, vecuronium can be used for rapid-sequence intubation. In this instance, a doubling of the dose of vecuronium to 0.2 mg/kg can be used to achieve intubation conditions within 45 seconds to 2 minutes. Another method for use of vecuronium for intubation is the priming technique. The object of this technique is to administer a small priming dose of vecuronium several minutes before the intubation dose is given to shorten the onset of neuromuscular blockade. The usual priming dose is 0.015 mg/kg; after 3 minutes, an intubation dose of 0.1 mg/kg is administered. The onset of neuromuscular blockade should be between 70 and 90 seconds. The main drawback of this technique is the potential development of symptoms of partial neuromuscular blockade. Sensations reported are heavy eyelids, blurred vision, and difficulty in swallowing. Therefore, if this technique is used in the PACU, the nurse should warn patients of the possible symptoms, and ventilatory support should always be available.
Because of concerns about the priming technique, the timing technique was developed. In the timing technique, which is used mainly in the operating room, the patient is given vecuronium before sodium pentothal. Consequently, the induction of anesthesia is specifically timed to the onset of clinical muscular weakness. In this technique, the patient is given 0.1 to 0.2 mg/kg of vecuronium intravenously. At the onset of weakness, as determined with the peripheral nerve simulator, a 1.0- to 2.5-mg/kg bolus dose of propofol is given. Intubating conditions occur within 1 minute.4
Vecuronium is primarily used for intraoperative skeletal muscle relaxation and facilitation of mechanical ventilation in the critical care setting. Long-term infusions of this drug in the critical care situation can result in a prolonged recovery and an inability to pharmacologically reverse vecuronium, because the metabolites are still in active form. If corticosteroid therapy is being used for patients with multiorgan failure, this prolonged effect can be exacerbated.
The dose is 0.05 to 0.2 mg/kg for skeletal muscle paralysis, and the onset is 1 to 3 minutes, with a duration between 30 and 90 minutes. Prolonged skeletal muscle relaxing effects can be prolonged with patients with hepatic disease. No significant cardiac effects for this drug have been reported.
Atracurium besylate
Atracurium (Tracrium) is a nondepolarizing skeletal muscle relaxant that offers an advantage over other skeletal muscle relaxants in that it does not depend on renal or hepatic mechanisms for its elimination. In fact, this quaternary ammonium compound breaks down in the absence of plasma enzymes through what is called Hofmann elimination and, to a lesser extent, through ester hydrolysis. Hofmann elimination is a nonbiologic method of degradation that occurs at a physiologic temperature and pH.
Atracurium is less potent than pancuronium and has a rapid onset of 1 to 3 minutes and a duration of action of 30 to 45 minutes. For endotracheal intubation in the PACU setting, 0.3 to 0.5 mg/kg of atracurium should provide adequate skeletal muscle relaxation for intubation in about 2.5 minutes. For maintenance of mechanical ventilation in the PACU setting, an infusion rate of 10 mcg/kg/min of atracurium may be used. When the infusion has been discontinued, spontaneous ventilation by the patient occurs in approximately 30 minutes.1 The effects can be reversed with a combination of anticholinesterase and antimuscarinic in 12 to 15 minutes after the discontinuation of the atracurium infusion.
Atracurium has many distinct advantages, such as its neuromuscular blockade not being prolonged by renal failure or impaired hepatic function; it has little or no cumulative effect and is not influenced significantly by the specific general inhalation anesthetic dose or concentration. Finally, this drug has little or no cardiovascular effect and is easily antagonized with the combination of an anticholinesterase and an anticholinergic.
Cisatracurium besylate
Cisatracurium (Nimbex) is a stereoisomer of atracurium that is approximately threefold more potent as atracurium, but with fewer side effects; it is degraded by the same metabolic pathway as atracurium (i.e., the Hofmann elimination mechanism).
The average adult intubation dose of cisatracurium is 0.2 mg/kg and has an onset of approximately 90 seconds, a peak in 3 to 5 minutes, and a duration of action of 40 to 50 minutes. A supplemental dose of 0.03 mg/kg provides an additional 20 minutes of skeletal muscle relaxation. For maintenance of a stable state of skeletal muscle relaxation in the PACU, cisatracurium can be administered via infusion at a rate of 1 to 2 mcg/kg/min.8
This drug has all the assets of atracurium plus a great advantage over atracurium of less histamine release. It does not have any particular effect on the cardiovascular system, and because it undergoes an organ-independent clearance, it can be used in patients with hepatic or renal failure without a noticeable change in duration of action. It is well suited for many patients who undergo intermediate to long surgical procedures.
Short-acting nondepolarizing skeletal muscle relaxants
Rocuronium bromide
Rocuronium (Zemuron) is a nondepolarizing skeletal muscle relaxant with a chemical structure related to vecuronium. It has a rapid onset (1 to 1.5 minutes) and a short duration of action of 30 to 120 minutes, depending on the total dose of the drug. The onset and duration of action are not altered in obese patients when the dose is based on the actual body weight. In patients who are older than 65 years, the duration of action is slightly prolonged. In pediatrics, the onset and duration is slightly faster.
Because rocuronium has such rapid effects and short duration of action, spontaneous recovery from neuromuscular blockade is possible. However, if a patient arrives to the PACU with spontaneous ventilation after rocuronium administration during surgery that was not reversed with anticholinesterase and an anticholinergic, the patient still should be monitored in the PACU for neuromuscular function with the use of a peripheral nerve stimulator (PNS). In addition to use of the PNS, the patient should be evaluated for adequate clinical evidence of an adequate return of neuromuscular function with evaluation of the 5-second head lift, adequate phonation, ventilation, and upper airway maintenance.1
Rocuronium can be used in patients with renal failure and has a low potential for histamine release. Although rare, its actions are prolonged in patients with cirrhosis of the liver. The muscle relaxant actions of rocuronium are potentiated by the inhalation anesthetics, which makes a prediction of a total recovery from neuromuscular blockade variable. Because rocuronium produces minimal cardiovascular effects, it does not have significant histamine-releasing effects.
Rocuronium can be used as an agent of choice for nondepolarizing rapid sequence intubation in the PACU when appropriate intubation doses are used because it has such a fast onset and short duration of action and therefore is useful in intraoperative and postoperative periods. At a dose of 0.6 to 1.0 mg/kg, rocuronium provides excellent intubating conditions in 60 to 90 seconds for both children and adults. Therefore, before the intubation is attempted, the patient should undergo ventilation with 100% oxygen until appropriate paralysis of the skeletal muscle occurs to facilitate the intubation. The maintenance dose for rocuronium for adults is between 0.1 and 0.2 mg/kg and for children is 0.08 to 0.12 mg/kg intravenously. If rocuronium is to be used for continuous infusion, the initial rate is 0.01 to 0.012 mg/kg/min; at the desired level of neuromuscular blockade, the infusion of this drug can be individualized according to the patient’s twitch response as monitored with the use of the PNS. The research indicates that infusion rates can range from 0.004 to 0.016 mg/kg/min. In assessment of the maintenance dosing of rocuronium, it should be administered at 25% of control T1, which is three twitches of the train-of-four. The infusion solutions for rocuronium can be prepared in solutions of 5% glucose and water or lactated Ringer solution. When the infusion is completed, the unused portions of the infusion solutions should be discarded.4
Reversal of nondepolarizing neuromuscular blocking agents
For restoration of neuromuscular transmission, the antagonist must displace the competitive neuromuscular blocking agent from the nicotinic receptor sites and open the way for depolarization of the postjunctional membrane. The antagonist is an antiacetylcholinesterase that blocks the enzymatic action of acetylcholinesterase located in the postsynaptic clefts so that acetylcholine is not hydrolyzed. The result is a buildup of acetylcholine at the end plate at the N2 cholinergic receptor. The accumulated acetylcholine displaces the competitive neuromuscular blocking agent, which diffuses back into the plasma and thus reestablishes neuromuscular transmission.
Neostigmine and pyridostigmine are usually the anticholinesterase drugs of choice because of their long duration of action and reliability as compared with edrophonium chloride. However, research has shown that edrophonium chloride is an effective reversal agent of neuromuscular blockades produced by vecuronium and atracurium. Atropine or glycopyrrolate, both antimuscarinic (anticholinergic) drugs, can be administered immediately before or in conjunction with the anticholinesterase for minimization of the muscarinic effects of the anticholinesterase drug. The muscarinic effects include bradycardia, salivation, miosis, and hyperperistalsis. These effects are produced at lower concentrations of the anticholinesterase-type drug when administered (acetylcholine nicotinic effects are at the autonomic ganglia and the neuromuscular junction). Consequently, when an anticholinesterase drug is administered for reversal of the nondepolarizing neuromuscular blocking agent at the N2 receptor, an antimuscarinic drug is also given for prevention of the adverse muscarinic cholinergic effects associated with the high dose of anticholinesterase. Generally, 2.5 mg of neostigmine is the maximum dose necessary for reversal; however, the suggested limit is 5 mg. The method is administration of 0.4 mg atropine or 0.2 mg glycopyrrolate intravenously over a 1-minute period, observation for an increase in pulse rate, and then administration of 0.5 mg neostigmine intravenously and monitoring for the reversal. This procedure can be repeated until reversal has been achieved or until the limit of neostigmine that can be given is reached. If edrophonium chloride is indicated for reversal, the dose is 0.5 mg/kg with 0.007 mg/kg of atropine.
Neostigmine should be administered cautiously. Cardiac monitoring is essential, especially in elderly or debilitated patients and in patients with cardiac disease. Atrioventricular dissociation and other dysrhythmias can be initiated by the anticholinesterases.7
Pyridostigmine is an analogue of neostigmine. It facilitates the transmission of impulses across the myoneural junction by inhibiting the destruction of acetylcholine by acetylcholinesterase. Clinical data indicate a lower incidence rate of muscarinic side effects with this drug than with neostigmine. Like neostigmine, pyridostigmine should be administered with caution in patients with bronchial asthma or cardiac problems. Signs of overdose are related to muscarinic and nicotinic receptor stimulation (Box 23-1). The muscarinic side effects are blocked with atropine or glycopyrrolate. Nicotinic responses can be blocked with drugs such as ganglionic or neuromuscular blocking agents. The recommended dose for reversal is 0.15 mg/kg of intravenous pyridostigmine, in combination with 0.007 mg/kg of intravenous atropine. Full recovery occurs within 15 minutes in most patients; in other patients, 30 minutes or more may be necessary.
Another parasympatholytic agent, glycopyrrolate, has been substituted for atropine in the reversal technique. Its advantages over atropine are a longer duration of action and a lower incidence rate of arrhythmias; it causes small slow changes in the heart rate, and it does not cross the blood-brain barrier. The usual reversal dose is 1 mg of neostigmine and 0.2 mg of glycopyrrolate in a 2-mL mixture. This dose can be repeated if reversal is inadequate.1
Sugammadex (Bridion) is a selective skeletal muscle relaxant binding agent that is effective in reversing the steroidal neuromuscular blocking drugs such as rocuronium, vecuronium, and pancuronium. Essentially, the mode of action of this drug is to form a tight bond with the steroidal neuromuscular blocking agents. The drug has minimal side effects and no anticholinesterase or anticholinergic drugs are required in the reversal using sugammadex. The dose for this drug is 2 to 4 mg/kg with a return to a full train-of-four approximately 3 minutes after injection. This drug appears to have significant clinical benefits compared with anticholinesterase and anticholinergic drugs for reversal.9,10 The drug is approved for use in Europe and is under consideration but not yet approved for use by the U.S. Food and Drug Administration.
Depolarizing neuromuscular blocking agents
Succinylcholine
Succinylcholine (Anectine, Quelicin, Sucostrin) represents a valuable pharmacologic advance in modern anesthesia and in critical care, areas in which resuscitation is required. This agent is usually included as one of the drugs available for emergencies, especially with endotracheal intubation. Outside the operating room, succinylcholine is used for electroshock therapy, for relief of profound laryngospasm, for control of convulsions from tetanus, for management of ventilation of the flail chest, and during reduction of fractures or dislocations.
Although succinylcholine is widely used in the United States, it has side effects and complications that can be avoided with a basic understanding of the pharmacology of the drug.
Succinylcholine acts at the N2 postsynaptic cholinergic receptor by causing a persistent depolarization of the end plate. It also acts on the presynaptic cholinergic receptor by causing an initial increase in acetylcholine at the motor end plate. This reaction is why patients who receive succinylcholine have fasciculations with initial administration. Succinylcholine is a synthetic quaternary ammonium compound with a chemical structure that closely resembles that of acetylcholine. The typical intravenous dose of succinylcholine to produce flaccid paralysis is 0.5 to 1.5 mg/kg; onset is 30 to 60 seconds with a duration of 5 to 10 minutes.1 The drug is hydrolyzed rapidly by plasma pseudocholinesterase, an enzyme produced by the liver, to succinylmonocholine and choline. Succinylmonocholine is further hydrolyzed by pseudocholinesterase and true cholinesterase, which are found in the erythrocyte, to succinic acid and choline (Box 23-2).
Advantages and uses
Succinylcholine has certain advantages that, in most instances, justify its clinical use. Its rapid onset of action, coupled with its short duration of action, has made this drug valuable when: (1) rapid intubation is necessary; (2) laryngospasm is irreversible with positive pressure; (3) the skeletal muscles are rigid and prevent good ventilatory excursion; (4) procedures require a short duration of skeletal muscle relaxation, such as reduction of dislocations and fractures; and (5) electroconvulsive therapy is used to decrease the negative effects of seizures. Continued use over a 30-year period has shown succinylcholine to produce complications that can, in most instances, be prevented if the basic pharmacodynamics of the drug are understood.
In emergencies, succinylcholine remains the major muscle relaxant for facilitation of endotracheal intubation. Succinylcholine is contraindicated in children and adolescent patients except when used for emergency tracheal intubation or in instances in which immediate securing of the airway is necessary.
When this drug is used for a rapid-sequence intubation, the succinylcholine-induced fasciculations and the associated increase in gastric pressure should be reduced or eliminated with an intravenous injection of a small amount (3 mg per 70 kg of body weight) of vecuronium. This defasciculating dose of vecuronium should be administered 1 or 2 minutes before the intravenous bolus injection of succinylcholine of 1.5 mg/kg.
Untoward reactions
Because hydrolysis of succinylcholine depends on enzymatic activity, an understanding is important of the atypical responses that may occur. Pseudocholinesterase activity in the plasma may be increased or decreased. Cases with increased activity are congenital and occur rarely. Patients with atypical pseudocholinesterase are resistant to succinylcholine and do not relax well. The reductions in pseudocholinesterase activity may be acquired or congenital. Acquired deficiencies are more important to understand because they are more common. They occur with liver disease, severe anemia, malnutrition, prolonged pyrexia, pregnancy, and recent renal dialysis. Drugs such as quinidine and propranolol (Inderal) inhibit pseudocholinesterase, as do echothiophate iodide eye drops (Phospholine). Patients with low pseudocholinesterase activity have a prolonged response to these drugs.
Atypical pseudocholinesterase occurs alone in approximately 1 in 2800 people; this atypical form is inherited.6 Patients with genetically induced deficiencies of pseudocholinesterase have been seen to remain apneic for as long as 48 hours after a usual dose of succinylcholine. These patients need mechanical ventilation and constant nursing care. Patients with documented pseudocholinesterase deficiency should be advised to wear a Medic Alert bracelet. If anesthesia is necessary, these patients should be administered nondepolarizing skeletal muscle relaxants, such as pancuronium, vecuronium, and rocuronium, because these drugs can usually be reversed.
Disadvantages and side effects
Succinylcholine can be administered via single injection or continuous infusion. The single-injection method is used when neuromuscular relaxation is needed for a short time, such as facilitation of endotracheal intubation. The usual intubation dosage of succinylcholine is 1 mg/kg intravenously. During the first intravenous injection, cardiovascular status usually remains normal. If the injection must be repeated, the patient may have profound bradycardia and various arrhythmias; therefore monitoring of the patient’s cardiovascular status with succinylcholine administration is important, especially if the dose is repeated.
Because children and adolescent patients are more likely than adults to have undiagnosed myopathies, a nondepolarizing skeletal muscle relaxant such as rocuronium (Zimuron) should be used for routine procedures in the PACU. More specifically, except when used for emergency tracheal intubation or in the instance in which immediate securing of the airway is necessary, succinylcholine is contraindicated in children and adolescent patients because a patient with a myopathy in this age group who is administered succinylcholine can have acute fulminating destruction of skeletal muscle (rhabdomyolysis) that results in hyperkalemia and cardiac arrest.
If succinylcholine must be administered to an adolescent or child, the patient must be monitored completely because the patient is especially prone to bradycardia, even on the initial injection of succinylcholine. This complication can be easily overcome with prior administration of glycopyrrolate or atropine sulfate, either alone or mixed with succinylcholine. This method appears to be the safest way of administering intravenous succinylcholine in this age group.
A disadvantage of the single-injection method with succinylcholine is that it causes fasciculations of the muscles. These “mini” contractions are a result of the initial depolarization of the skeletal muscle from the positive feedback mechanism of initial stimulation of the presynaptic acetylcholine receptor. These contractions frequently lead to muscle pain, which is usually noted by the patient the day after surgery. This effect is particularly true in patients who are ambulatory soon after surgery. In ambulatory patients, muscle pains (myalgia) occur in 60% to 70% of cases. The incidence rate decreases to 10% in those patients confined to bed.11 Symptoms include pain in the neck, back, and abdomen; pain when blinking the eyes; pain when smiling; and generalized pain when ambulatory. These objective symptoms are usually noticed first by the nurse in the PACU. Skeletal muscle pain around the neck area is sometimes described to the perianesthesia nurse as a sore throat caused by the endotracheal tube; in fact, the pain is caused by the myalgia from the succinylcholine. The pain usually does not require analgesics and subsides in 1 or 2 days. The fasciculations can be prevented by administering a nondepolarizing neuromuscular blocking drug at a pretreatment dose of between 5% and 10% of its normal intubation 2 to 4 minutes before the injection of succinylcholine. If a pretreatment nondepolarizing drug was administered, the dose of succinylcholine should be increased by about 70%.
When succinylcholine is administered to patients in the presence of extensive burns, severe trauma, severe abdominal infections, tetanus, neuromuscular disease, or neurologic lesions such as paraplegia and quadriplegia, a release of potassium from the damaged muscle and nerve cells can result. The common denominator appears to be either massive tissue destruction or central nervous system injury with muscle wasting. This pathophysiologic process results when denervated muscle is stimulated by succinylcholine because of the extrajunctional nicotinic receptors proliferated during the skeletal muscle destruction. After activation of the nicotinic receptor by succinylcholine, response is enhanced in the ionic channels, with a resultant increase in the release of potassium into the circulation. Elevation of the serum potassium level has been reported as high as 10 to 15 mEq/L. The result of this potassium elevation is cardiac dysrhythmias and cardiac arrest. The peak time for this reaction is 7 to 10 days after the injury. However, the critical period for these reactions is 1 to 180 days after trauma. Consequently, succinylcholine is contraindicated in patients who have injuries of major multiple traumas, extensive denervation of skeletal muscle, or upper motor neuron injury.12
Succinylcholine has been implicated as one of the trigger agents of malignant hyperthermia. Chapter 53 contains a complete description of the pathophysiology and treatment of malignant hyperthermia.
In pediatric and adult patients anesthetized with halothane combined with succinylcholine as the muscle relaxant, an unusual incidence of plasma myoglobin has occurred. Myoglobin is an intracellular muscle protein and therefore should not be released into the plasma. If myoglobin is found in the plasma, it can only mean that the muscle membrane has been injured.
Succinylcholine can be administered in a drip infusion during a procedure that requires skeletal muscle relaxation for a longer period than a single injection can provide. It is usually administered in a 0.1% to 0.2% solution. If the infusion is administered for a prolonged period, the type of block can gradually change from a depolarizing block to a characteristic nondepolarizing block. The change is always from depolarization to nondepolarization, never in the reverse direction. This type of block is called a dual or phase II block.4 The exact time relationship and the mechanism of action are still uncertain. Treatment is via mechanical ventilation and careful monitoring of the patient until the dual block disappears.
Succinylcholine increases intraocular pressure by approximately 7.5 mm Hg in both children and adults, in part because of the contraction of the extraocular muscles. When administered before succinylcholine to prevent contraction of the extraocular muscles, a nondepolarizing neuromuscular blocking drug does not completely extinguish the increase in intraocular pressure. Therefore, even if succinylcholine is used with a neuromuscular blocking drug, it is contraindicated in patients in whom an increase in intraocular pressure would be detrimental.
Factors that influence the neuromuscular blocking agents
Fluid balance
Patients who are dehydrated are reported to be extremely sensitive to skeletal muscle relaxants. This finding is probably true because: (1) dehydration decreases neuromuscular excitability; (2) the contracted extracellular fluid compartment permits an increase in the plasma concentration of the relaxant and thus intensifies the relaxant action; and (3) renal function is slowed and the elimination time of the relaxant and its metabolites is prolonged.
Sodium
A deficit of sodium may prolong the neuromuscular block. Experimental evidence indicates that a sodium deficiency itself can result in a partial neuromuscular block.
Potassium
Potassium deficiency appears to increase the blocking action of pancuronium and other nondepolarizing neuromuscular blocking agents. Alternatively, depolarizing neuromuscular blocking agents are required in larger amounts when potassium deficiency exists. Depolarization is prevented to some extent because a potassium deficiency appears to stabilize the muscle end plate. Potassium depletion can occur from decreased intake or excessive loss, such as in chronic pyelonephritis, primary aldosteronism, chlorothiazide therapy, and chronic diarrhea.
Magnesium
An increase in magnesium concentration causes a flaccid paralysis clinically similar to that caused by a nondepolarizing neuromuscular blocking agent. The principal action of magnesium is that it can enter the nerve terminal and replace or decrease the amount of calcium that enters, which stabilizes the postsynaptic membrane. Ultimately, depression of the release of acetylcholine occurs and reduces the EPP, which causes a partial neuromuscular block. Consequently, magnesium enhances a neuromuscular block produced by a nondepolarizing agent and, to a lesser extent, potentiates the block produced by succinylcholine.
Calcium
A deficiency in calcium prolongs the effects of nondepolarizing neuromuscular blocking agents by reducing the amount of acetylcholine released and by inhibiting neuromuscular transmission. The depolarizing neuromuscular blocking agents are also potentiated because a low calcium level aids depolarization. Conversely, the administration of calcium chloride solution in calcium deficiency states antagonizes the nondepolarizing effects of agents such as pancuronium. Calcium chloride has a pronounced antagonism to the respiratory depressant effects of succinylcholine.
pH and carbon dioxide
The neuromuscular blocking effect of pancuronium is intensified in acidosis and in states of elevated carbon dioxide tension. With drugs such as rocuronium and succinylcholine, the neuromuscular blocking action is diminished. Alkalosis by itself decreases the effects of pancuronium. Hyperventilation has been thought to augment the abdominal muscle relaxation produced by pancuronium. One explanation of this phenomenon is that changes in pH or plasma concentrations of pancuronium reflect a change in binding to the receptor substance.
Catecholamines
Epinephrine and ephedrine have a type of reversal effect on skeletal muscle. Clinically, an antagonism to pancuronium has been shown. This effect is caused by an increase in acetylcholine release, the inhibition of acetylcholinesterase, a decreased excitability of muscle fibers, and the release of potassium with administration of epinephrine and ephedrine.
Mycins
Several antibiotics have a nondepolarizing neuromuscular blocking property because the aminoglycoside antibiotics potentiate the neuromuscular blockade by inhibiting the presynaptic release of acetylcholine. The resulting clinical difficulties are related to a combination of factors, including large doses of antibiotics, parenteral administration into body cavities that represent a large surface area for absorption, and concomitant use of a neuromuscular blocking agent. Neomycin and streptomycin have been implicated the most frequently (Box 23-3).
Cardiac antidysrhythmic drugs
When administered intravenously, lidocaine potentiates a preexisting neuromuscular blockade that occurs because lidocaine stabilizes the postsynaptic membrane and depresses the skeletal muscle fibers. Quinidine interferes with the presynaptic release of acetylcholine at the neuromuscular junction. Consequently, it intensifies the neuromuscular blockade of both depolarizing and nondepolarizing skeletal muscle blocking agents. Finally, calcium channel blocking agents inhibit the calcium entry, with a resultant reduction in acetylcholine release followed by a reduction in neuromuscular function.
Temperature
Hypothermia antagonizes the action of pancuronium and potentiates the action of succinylcholine. During the recovery phase of an anesthetic, when a neuromuscular blocking agent has been administered, young infants should be specifically monitored for return of skeletal muscle tone. This rule is especially in effect when a nondepolarizing relaxant is administered to infants, who are prone to have some hypothermia because of their immature heat-regulating systems.
Inhalation anesthetics
The inhalation anesthetics produce a dose-dependent enhancement of the neuromuscular blockade of the nondepolarizing neuromuscular blocking agents. More specifically, this blockade is most pronounced when a patient has received isoflurane. Nitrous oxide only produces minimal potentiation of nondepolarizing neuromuscular blocking agents. This potentiation of the neuromuscular blockade by the inhalation anesthetics results from depression of the central nervous system and ultimately reduces skeletal muscle tone. Consequently, patients in the PACU who have received nondepolarizing neuromuscular blocking agents during surgery and have not completely emerged from the inhalation anesthetic should be closely monitored with a peripheral nerve stimulator for a reduction in skeletal muscle function. In addition, an aggressive stir-up regimen should be instituted for these patients.
Assessment of neuromuscular blockade
In a study conducted in the 1940s, injection with D-tubocurarine (a nondepolarizing skeletal muscle relaxant) first caused motor weakness and then total muscle flaccidity. The small rapidly moving muscles, such as those of the fingers, toes, eyes, and ears, are involved before the long muscles of the limbs, neck, and trunk. The intercostal muscles and finally the diaphragm become paralyzed, and respiration ceases.
The perianesthesia nurse should know the order of the return of muscle function after a patient has received a nondepolarizing muscle relaxant. The recovery of skeletal muscle function is usually in reverse order to that of paralysis; therefore the diaphragm is ordinarily first to regain function. The order of appearance of paralysis after injection with a nondepolarizing neuromuscular blocking agent can be assessed electromyographically as follows:
1. Small-sized muscle groups: oculomotor muscles, muscles of the eyelids; muscles of the mouth and face; small extensor muscles of the fingers, followed by the flexor muscles of the fingers
2. Medium-sized muscle groups: muscles of the tongue and pharynx; muscles of mastication; extensor muscles of the limb, followed by flexor muscles of the limbs
3. Large-sized muscle groups: neck muscles, shoulder muscles, abdominal muscles, dorsal muscle mass
4. Special muscle groups: intercostal muscles, larynx, diaphragm
The order of paralysis is essentially the same after injection with a depolarizing neuromuscular agent, except that the flexor muscles are paralyzed before the extensor muscles. Patients who arrive in the PACU must be evaluated for residual effects from a neuromuscular blocking agent that was administered during surgery. In most instances, the action of the nondepolarizing neuromuscular blocking agent is pharmacologically reversed at the end of the operation before the patient is admitted to the PACU; however, any patient who has received a neuromuscular blocking agent should be closely watched for signs of residual drug action. The goal of the assessment of the recovery from nondepolarizing neuromuscular blocking agents with the train-of-four is a ratio of more than 75%. The residual actions of the depolarizing muscle relaxants are similar to those of the nondepolarizing muscle relaxants, and the same nonrespiratory parameters and respiratory variables can be used in evaluation of the neuromuscular blockade. The evoked (electric stimulation) responses differ between the depolarizing and nondepolarizing neuromuscular blocking agents.
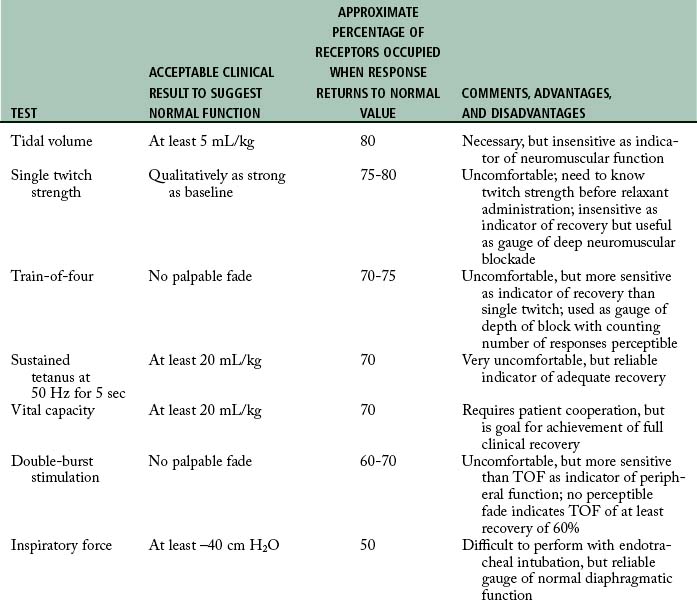
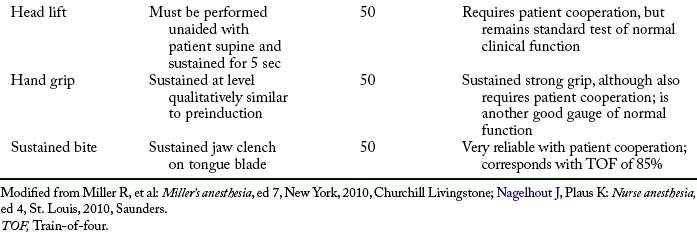
The PNS (Fig. 23-3) can be used in the PACU for assessment of the type and degree of a neuromuscular blockade. This electric device can be used to stimulate the ulnar nerve at the wrist or elbow, and, on stimulation of the ulnar nerve, the nurse can observe the contraction of the fingers. Fig. 23-4 graphically shows the train-of-four test. The assessment of the depth of neuromuscular blockade with electric stimulation is useful when more than 70% of the N2 receptors are blocked by a skeletal muscle relaxant. However, in most instances, if the patient has a normal tidal volume, vital capacity, and maximal inspiratory force and can lift the head for 5 seconds, the use of the PNS is not warranted. If identification of the type of neuromuscular blockade used (depolarizing or nondepolarizing) is needed, or if some of the aforementioned parameters are marginal, the train-of-four or sustained tetanus with the PNS can be used to provide the objective data for assessment.
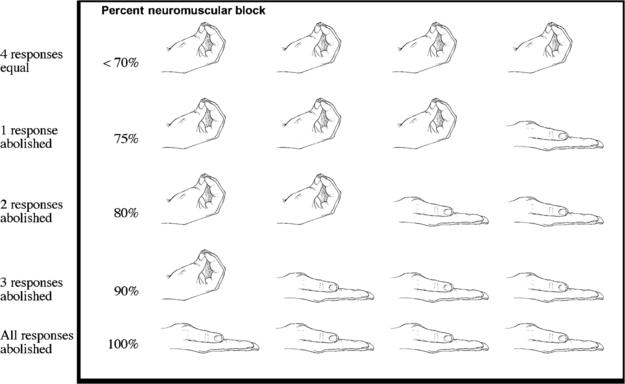
FIG. 23-4 Train-of-four suppression.
(From Nagelhout J, Plaus K: Nurse anesthesia, ed 4, St. Louis, 2010, Saunders.)
Although the mechanisms that produce the nondepolarizing block differ from the depolarizing block, the diagnostic criteria with a PNS for assessment of a nondepolarizing and phase II dual block are basically the same. The hallmark of a nondepolarizing neuromuscular blockade is an inability to sustain contraction in response to a tetanic stimulus and posttetanic potentiation. A tetanic stimulus is the usual 50 Hz of current for 5 seconds (sustained tetanus) produced by a PNS. Posttetanic facilitation is a twitch after the response to tetanic stimuli higher than the twitch immediately before tetanus. If a patient has a partial nondepolarizing neuromuscular block, an unsustained contraction called fade is seen after the initial tetanic stimulus (Fig. 23-5). Fade is caused by the decreased mobilization of acetylcholine in the nerve terminal, because the presynaptic acetylcholine receptor is blocked by the nondepolarizing muscle relaxant. The responses to electric stimulation result from the interaction of acetylcholine released and the number of N2 cholinergic receptors occupied by the relaxant. In patients with a partial nondepolarizing neuromuscular block, the first three single electric stimuli are of enough intensity to produce a twitch, but the twitch produced is not of the same magnitude as a twitch produced in a subject who has not received a nondepolarizing skeletal muscle relaxant (see Fig. 23-5). The three electric stimuli cause the normal quantum of acetylcholine to be released at the synaptic cleft; however, in this instance, the reduction in twitch magnitude is the result of the number of acetylcholine receptors being occupied by the nondepolarizing relaxant. Consequently, if the patient has had a complete nondepolarizing neuromuscular block in which all the N2 cholinergic receptors were occupied, no twitch is elicited from the three electric stimuli.
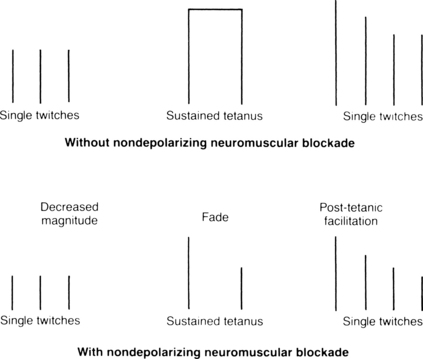
FIG. 23-5 Magnitude of posttetanic facilitation without and with nondepolarizing neuromuscular blockade.
(Adapted from Donati F: Monitoring neuromuscular blockade. In Saidman L, Smith NT, editors: Monitoring in anesthesia, ed 3, Woburn, Mass, 1993, Butterworth-Heinemann.)
In a healthy subject, when a tetanic stimulus is applied for 5 seconds, the quantum of acetylcholine that is released decreases during the stimulus period. In addition, only a fraction of nicotinic cholinergic receptors are activated at any one time to trigger an action potential. The excess in nicotinic cholinergic receptors is the safety margin of neuromuscular transmission. Consequently, in the healthy subject who receives a tetanic stimulus, the magnitude of the twitch response is maintained because of the large nicotinic cholinergic receptor pool; however, if 75% of the nicotinic receptors are occupied by a nondepolarizing neuromuscular relaxant, for example, the twitch response is not maintained and fade (unsustained contraction) occurs because the usual margin of safety of excess acetylcholine receptors has been abolished. Between the termination of a tetanic stimulus and the first single-twitch stimulus, a buildup of acetylcholine occurs in the presynaptic knob. Thus, after sustained tetanus, when the first electric stimulus is administered, the height of the first twitch is greater than the pretetanic twitches. These large posttetanic twitches (posttetanic facilitation) return to the pretetanic height as the acetylcholine mobilization also returns to the pretetanic level. Finally, more than 70% of the nicotinic cholinergic receptors must be occupied before this tetanic stimulation test is sensitive enough for detection of neuromuscular blockade.
The major drawback to the delivery of a 50-Hz tetanic stimulus to an awakened patient in the PACU is pain and general discomfort. For the patient who is awake and reactive, the train-of-four stimulation is better for assessment of the degree of neuromuscular blockade caused by nondepolarizing skeletal muscle relaxants. In this test, the ulnar nerve is used, and four supramaximal electrical stimuli—2 Hz, 0.05 seconds apart—are administered with a PNS. This test, which produces minimal discomfort to the awakened patient, is sensitive only when more than 70% of the nicotinic cholinergic receptors are occupied. The index of neuromuscular blockade in this test is the ratio of the fourth to the first twitch amplitude. More specifically, when the fourth response is abolished, a 75% block exists (Fig. 23-6). When the third and second responses to stimulation are abolished, the respective reductions in neuromuscular blockade are 80% and 90%. Finally, when all four twitch responses are absent, a 100%, or complete, block exists.
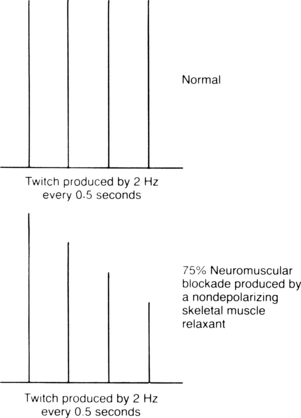
FIG. 23-6 Diagrammatic illustration of train-of-four in normal response and 75% neuromuscular blockade produced by nondepolarizing skeletal muscle relaxant.
The depolarizing neuromuscular blockade is characterized by an absence of posttetanic potentiation, a decreased response to a single impulse, a decreased amplitude (but sustained response to a tetanic stimulus), and, if present, a train-of-four ratio between the first and fourth stimulus that is greater than 70%.12 Refer to Table 23-2 for a complete overview of the commonly used monitoring tests and Table 23-3 for some key points related to the neuromuscular reversal.
Table 23-2 Neuromuscular Monitoring Methods
| MONITORING TEST | DEFINITION | COMMENTS |
|---|---|---|
| Single twitch | Single supramaximal electric stimulus ranging from 0.1-1 Hz | Requires baseline before drug administration; generally used as qualitative rather than quantitative assessment |
| Train-of-four | Series of four twitches at 2 Hz every half second for 2 sec | Reflects blockade from 70% to 100%; useful during onset, maintenance, and emergence |
| Tetanus | Generally consists of rapid delivery of 30-, 50-, or 100-Hz stimulus for 5 sec | Should be used sparingly for deep block assessment; painful |
| Posttetanic count | 50-Hz tetanus for 5 sec, 3-sec pause, then single twitches of 1 Hz | Used only when train-of-four or double-burst stimulation response is absent; count less than eight indicates deep block, and prolonged recovery is likely |
| Double-burst stimulation | Two short bursts of 50-Hz tetanus separated by 0.75 sec | Similar to train-of-four; useful during onset, maintenance, and emergence; may be easier for detection of fade than with train of four; tactile evaluation |
From Nagelhout J, Plaus K: Nurse anesthesia, ed 4, St. Louis, 2010, Saunders.
Special problems in the postanesthesia care unit
A prolonged response to succinylcholine sometimes occurs because a patient does not possess the proper blood level of pseudocholinesterase. Other causes of a prolonged response include: (1) overdose; (2) temperature changes; (3) acid-base imbalance; (4) carcinoma; (5) antitumor agents; (6) antibiotics; (7) myasthenia gravis; and (8) liver disease.
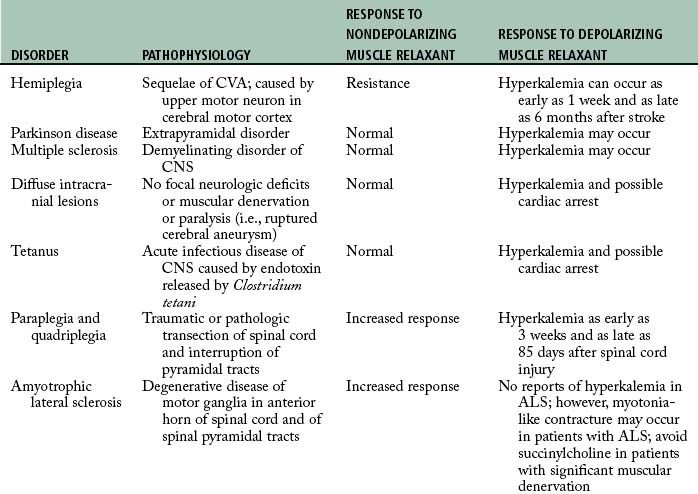
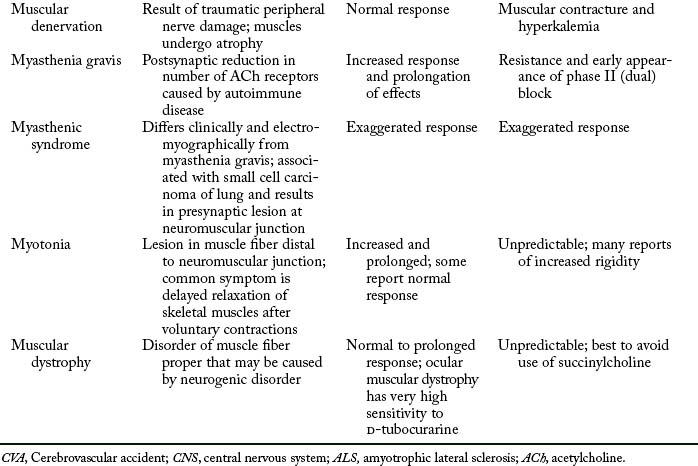
If a patient who arrives in the PACU is apneic, controlled respiration must be initiated and maintained as long as necessary. Careful monitoring of vital signs and evaluation of renal function are important. The neuromuscular block can be identified with the PNS. If electrical stimulation results in vigorous contractions, the apnea is unlikely to be the result of residual neuromuscular block. Consideration must then be given to other agents that may have caused the apneic state. The response of patients with neuromuscular disorders to muscle relaxants may include resistance, increased response, hyperkalemia, and even cardiac arrest. Table 23-4 is presented as a summary of the possible untoward responses in the clinical setting.
Dual or phase II block
Various terms are used to describe the different types of blocks that succinylcholine is able to produce. The phase I block is synonymous with the depolarizing block the drug ordinarily produces. It is characterized by a dose-dependent reduction in a single twitch without fade after a well-sustained tetanus and no posttetanic facilitation when the PNS is used.
Phase II block is also known as a dual block, desensitization, or open channel block. This block is caused by a conformational change in the presynaptic and postsynaptic cholinergic receptors. This anatomic change results in desensitization to the stimulation of acetylcholine because of the prolonged depolarization at the motor end plate. The characteristics of this block as shown with the PNS are fade in response to tetanic stimuli, posttetanic potentiation, and antagonism with drugs such as neostigmine. The same clinical features are seen when vecuronium is used; however, this similarity does not mean that the two blocks are the same, because good evidence suggests that the vecuronium and succinylcholine phase II blocks differ in several respects. Some clinicians believe that the phase II block produced by succinylcholine can be reversed with anticholinesterases, such as edrophonium, which has a shorter duration of action than neostigmine. Edrophonium is used in this situation because it either reverses or potentiates the block. If potentiation occurs, it is of a shorter duration than if neostigmine were used. Most experts believe that routine reversal to antagonize the dual block is unwarranted. Ventilation of the patient with a wait for return of the normal neuromuscular transmission is more advisable. See Box 23-4 for a summary of the differentiating characteristics of a depolarizing (phase I) block and a nondepolarizing (phase II) block.
BOX 23-4 Summary of the Differentiating Characteristics of Phase I and Phase II Blocks
Depolarizing (phase I) block
• Muscle fasciculation preceding the onset of neuromuscular blockade
• Sustained response to titanic stimulation
• Absence of posttetanic potentiation, stimulation, or facilitation
• Lack of fade to train-of-four or double-burst stimulation
• Block antagonized by prior administration of nondepolarizer as pretreatment (approximately 20% more succinylcholine necessary)
Nondepolarizing (phase II) block
• Absence of muscle fasciculation
• Appearance of tetanic fade and posttetanic potentiation, stimulation, or facilitation
• Train-of-four and double-burst fade
• Reversal with anticholinesterase drugs
• In rare cases, may be produced by an overdose and desensitization with succinylcholine at doses more than 6 mg/kg
From Nagelhout J, Plaus K: Nurse anesthesia, ed 4, St. Louis, 2010, Saunders.
Residual paralysis
Residual paralysis is the reappearance after surgery of the pharmacologic actions of a nondepolarizing skeletal muscle relaxant that was administered during surgery.11 Other terms that are associated with this are compromised ventilation, partial reversal, or reduced train-of-four. This complication arises when renal insufficiency exists. The interesting facet of this complication is that even when a nondepolarizing skeletal muscle relaxant is reversed sufficiently at the end of the anesthetic, residual paralysis may still occur for as long as 8 hours. This reappearance may be partly caused by the fading of the effect of the neostigmine.
Symptoms of residual paralysis include clinical evidence showing a reduction of the return of neuromuscular function with evaluation of the 5-second head lift, adequate phonation, ventilation, and upper airway maintenance along with a poor twitch response with the PNS.
If symptoms appear, the required level of the reversal agent (neostigmine) must be maintained until that portion of the nondepolarizing skeletal muscle relaxant has been eliminated so that symptoms do not reappear. If residual paralysis occurs, the postoperative use of morphine and similar opioids should be avoided because these agents enhance a residual neuromuscular block sufficiently to make it clinically significant. Finally, because the patient becomes extremely fearful, constant verbal reassurance by the PACU nurse helps to reduce stress and anxiety.
Bradycardia
Another problem that occurs in the PACU is the appearance of bradycardia when a patient has received an atropine-neostigmine combination at the end of the anesthetic. The bradycardia is usually the result of the longer duration of action of neostigmine compared with that of atropine.12 The treatment for this problem is glycopyrrolate. Glycopyrrolate should not be administered, however, until other causes of bradycardia are eliminated, such as pain, hypoventilation, and a full bladder.
Summary
The neuromuscular blocking agents used in modern perianesthesia practice produce excellent results with fewer disadvantages compared with the drugs presented in the previous edition of this book. It is remarkable how the art and science of anesthesia has narrowed down the number of agents to be used in clinical practice to the most popular and those that possess excellent advantages over the neuromuscular agents used in the past. An overview of the process of excitation-contraction coupling was presented as were the current drugs in use. An in-depth approach was used in the discussion of the pharmacologic reversal of the nondepolarizing neuromuscular agents and in a discussion of the various methods of monitoring a patient who has received a skeletal muscle relaxant during surgery. Finally, an overview of the PACU nursing care was presented (an in-depth discussion on this topic can be found in Chapter 28).
Neuromuscular blocking agents are useful agents in the operating room for facilitation of the optimum surgical field for the physician to use life-saving skills effectively. In the PACU, neuromuscular blocking agents can be used for life-saving situations and facilitation of mechanical ventilation. In the PACU, these agents should only be administered by a practitioner skilled in airway management. For patients in the PACU who require mechanical ventilation and need neuromuscular blocking agents to enhance ventilatory care, the PACU nurse should be acutely aware that, besides the skillful use of the neuromuscular blocking agent, the patient also needs appropriate sedation and verbal support during that most fearful time of care.
1. Stoelting R. Pharmacology and physiology in anesthetic practice, ed 4. Philadelphia: Lippincott Williams & Wilkins; 2005.
2. Barrett K, et al. Ganong’s review of medical physiology, ed 23. New York: McGraw-Hill Medical; 2009.
3. Hall J. Guyton and Hall textbook of medical physiology, ed 12. Philadelphia: Saunders; 2011.
4. Nagelhout J, Plaus K. Nurse anesthesia, ed 4. St. Louis: Saunders; 2010.
5. Longnecker D, et al. Anesthesiology. New York: McGraw-Hill Medical; 2007.
6. Miller R, Pardo M. Basics of anesthesia, ed 6. Philadelphia: Saunders; 2011.
7. Barash P, et al. Clinical anesthesia, ed 6. Philadelphia: Lippincott Williams & Wilkins; 2009.
8. Aitkenhead A, et al. Textbook of anesthesia, ed 5. Philadelphia: Churchill Livingstone; 2007.
9. Kopman A. Sugammadex: A revolutionary approach to neuromuscular antagonism. Anesthesiology.2006;104:631–633.
10. Naguib M. Sugammadex: another milestone in clinical neuromuscular pharmacology. Anesth Analg.2007;104(3):575–581.
11. Atlee J. Complications in anesthesia, ed 2. Philadelphia: Saunders; 2007.
12. Fisher L. Anesthesia and uncommon diseases, ed 5. Philadelphia: Saunders; 2007.
American Association of Critical-Care Nurses: Core curriculum for progressive care nursing. Philadelphia: Saunders; 2010.
Alspach J. Core curriculum for critical care nursing, ed 6. Philadelphia: Saunders; 2005.
Ball C. Unraveling the mystery of malignant hyperthermia. Anaesth Intensive Care. 2007;35(Suppl 1):26–31.
Bready L, et al. Decision making in anesthesiology, ed 4. St. Louis: Mosby; 2007.
Brunton L, et al. Goodman and Gilman’s the pharmacological basis of therapeutics, ed 12. New York: McGraw-Hill Professional; 2010.
Conlay L, et al. Case files anesthesiology. New York: McGraw-Hill Medical; 2011.
Cope T, Hunter J. Selecting neuromuscular-blocking drugs for elderly patients. Drugs Aging. 2003;20(2):125–140.
Davis P, et al. Smith’s anesthesia for infants and children, ed 8. St. Louis: Mosby; 2011.
Drake R, et al. Gray’s anatomy for students, ed 2. Philadelphia: Churchill Livingstone; 2009.
Deutschman C, Netigan P. Evidence-based practice of critical care. Philadelphia: Saunders; 2010.
Dorsch J, Dorsch S. Understanding anesthesia equipment, ed 5. Philadelphia: Lippincott Williams & Wilkins; 2007.
Gallager C, Issenberg B. Simulation in anesthesia. Philadelphia: Saunders; 2007.
Hines R, Marschall K. Handbook for Stoelting’s anesthesia and co-existing disease, ed 3. Philadelphia: Saunders; 2013.
Kaplan J, et al. Cardiac anesthesia. New York: Churchill Livingstone; 2011.
Kervin W, et al. Residual neuromuscular blockade in the immediate postoperative period. J Perianesth Nurs. 2002;17:152–158.
Kier L, Dowd C. The chemistry of drugs for nurse anesthetists. Chicago: AANA Publishing, Inc; 2004.
Pasero C, McCaffery M. Pain assessment and pharmacologic management. St. Louis: Mosby; 2011.
Schick L, Windle PE. Perianesthesia nursing core curriculum: preprocedure, phase I and phase II PACU nursing. ed 2. St. Louis: Saunders; 2010.
Schreiber J, et al. Prevention of succinylcholine-induced fasciculation and myalgia. Anesthesiology. 2005;1037:877–884.
Shorten G, et al. Postoperative pain management: an evidence-based guide to practice. Philadelphia: Saunders; 2006.
Sieber F. Geriatric anesthesia. New York: McGraw-Hill Medical; 2006.
Vincent J, et al. Textbook of critical care, ed 6. Philadelphia: Saunders; 2011.