chapter 36 Neurology
INTRODUCTION AND OVERVIEW
This chapter discusses the more common neurological symptoms and disorders encountered in general practice, and some of the less-common classic neurological problems that are rarely seen in general practice. It is far from comprehensive—more detailed information can be found in textbooks of neurology.1,2
HEADACHE
Headache is probably one of the most common reasons for a patient to consult a general practitioner (GP). Many patients are fearful that they may have a serious neurological disorder, such as a brain tumour, whereas in reality this is rare. In primary care, the risk of brain tumour with a headache presentation is less than 0.1%.3 The most common causes of headache seen by a GP are primary headaches such as those associated with fever, tension headache and, less often, migraine. The challenge for the GP is to identify the very rare but more sinister causes of headache, such as subarachnoid haemorrhage (SAH), brain tumour, cranial arteritis, meningitis and so on. There are some clinical features that should increase the suspicion of a secondary cause for headache, such as:
MANAGEMENT
Headache of sudden onset
Tension headache and chronic daily headache
This is the most common type of headache encountered by the GP. The headache can be generalised, or focal, unilateral or bilateral. Focal neurological symptoms, nausea and systemic symptoms are absent. The headache typically commences some time after awakening and lasts most of the day, and fluctuation in severity during the day is characteristic. The patient may retire with the headache but usually awakens in the morning free of headache. Chronic daily headache is by definition the presence of headaches on a daily basis for six or more months. Although worsening of daily headache is regarded as a ‘red flag’ for a possible more sinister secondary cause of headache, in fact worsening in terms of increased duration and intensity of headache is not uncommon in these patients, particularly in the setting of increased use of analgesics, a syndrome referred to as the ‘analgesic overuse syndrome’. This is a vicious cycle, where increasing use of analgesics (even aspirin or paracetamol) leads to increasing severe headaches, leading to the use of even more of analgesia. A clue is that the patient often states: ‘I am taking all these pain killers and they do not seem to work any more.’ Many patients also have neck discomfort and when examined may have tenderness in the trapezius and sternocleidomastoid muscles; this often leads to a diagnosis of cervicogenic headache. Here the headache is assumed to be arising from disease in the cervical spine, and yet most patients do not have any demonstrable pathology on imaging or have osteoarthritis that may be an incidental finding, particularly in older patients where it is often present and asymptomatic. Relief of headache can be seen after massage to the neck muscles, physiotherapy or chiropractic, but the benefit may be short-term only.
A multifaceted approach to the treatment of tension-type headache, employing psychological, physiological and pharmacological therapies is recommended.4 The treatment for the analgesic overuse syndrome is for the patient to withdraw analgesia, which they often find difficult. The use of a tricyclic antidepressant for a short period has been shown to be beneficial.5
Migraine
Migraine is a complex headache disorder that has many manifestations. The aetiology is unknown. Patients experience recurrent headaches occasionally associated with recognisable precipitating factors. The diagnosis is entirely dependent on the history of the headache and associated features if present, as currently there are no tests to confirm the diagnosis. The patient with ‘classic migraine’ rarely presents a diagnostic problem. The headache is preceded by visual phenomena or neurological symptoms, referred to as the aura, that commence in one part of the body or visual field and spread, over a period of minutes (on average lasting approximately 30 minutes). The headache is usually throbbing in nature, and commences after the onset of the aura and increases in severity over minutes to hours. It is almost invariably associated with nausea, vomiting, photophobia and phonophobia. The headache persists for hours to days. The associated visual symptoms can vary from photopsia (positive phenomena such as flashing lights or bright dots) to patches of loss of vision, referred to as scotomata, that may or may not be surrounded by positive visual phenomena or fortification spectra. The diagnosis is more difficult in patients with recurrent headaches in which gastrointestinal, visual and neurological symptoms are absent. The diagnosis of probable migraine can be made if the patient experiences recurrent headaches that persist for hours on end and are either present on awakening or awaken the patient from their sleep. Rare forms of migraine include hemiplegic migraine and basilar artery migraine. Some patients can experience the aura of a migraine without the subsequent development of a headache, referred to ‘migraine without headache’ or ‘migraine equivalents’. Migraine headaches that occur only at the time of menstruation are referred to as ‘menstrual migraine’ and are due to the drop in the oestradiol level just prior to menstruation (see ch 52, Gynaecology).
Initial standard medical treatment often consists of simple analgesia, including non-steroidal anti-inflammatory drugs (NSAIDs) with an antiemetic drug such as prochlorperazine or metoclopramide. Ergot preparations, oral, rectal and subcutaneous, or the triptans, have all been shown to be effective.6,7
INTEGRATIVE MANAGEMENT OF HEADACHE/MIGRAINE
Prevention
Herbs and supplements
Magnesium
Intracellular magnesium levels are often lower in people with migraine headaches and magnesium deficiency is common. Two randomised controlled trials (RCTs)8,9 found that high-dose magnesium reduced the frequency and duration of migraine headaches. Magnesium can also help prevent migraine in women with cyclic headaches related to periods, and is also used in treatment of conditions involving muscle spasm or tension, fibromyalgia, anxiety states and tension headaches.
Riboflavin (vitamin B2)
Regular riboflavin may help reduce the frequency and shorten the duration of migraine headaches through anti-nociceptive and anti-inflammatory effects.10 Dose: 400 mg/day for at least 3 months, to assess effect in combination with feverfew and magnesium.
Feverfew (Tanacetum parthenium)
A systematic review of six RCTs11 concluded that evidence favours feverfew as an effective preventative treatment against migraine, and that it is well tolerated. Note that there is significant variation in the amount of active ingredient in commercially available feverfew preparations.
Physical therapies
Chiropractic
In one study12 including 127 people with migraine headaches, 22% of those who received chiropractic manipulation reported more than a 90% reduction of migraines and 49% reported a significant reduction of the intensity of each episode. There is some evidence to suggest that patients with tension headache and headache related to neck pain will benefit from chiropractic, although overall the evidence is less strong that chiropractic is beneficial for migraine.13
Physiotherapy
A review article14 evaluating nine studies that tested spinal manipulative therapy for tension or migraine headaches concluded that this technique is comparable to medications used to try to prevent either of these two types of headaches.
Acupuncture
Acupuncture is well studied as an effective treatment for various types of headache. Results from a study published in 200315 suggest that having an acupuncture treatment when migraine symptoms first begin is as effective as sumatriptan. Later in the course of the migraine attack, however, the medication works better than acupuncture. A 2001 Cochrane review16 found 26 trials on acupuncture for headaches. Of these, 16 trials investigated migraine headaches, six tension-type headaches and four various headaches. The majority, although not all, showed acupuncture to be better than placebo/sham acupuncture for tension/migraine headaches, although the quality of many trials was suboptimal. A more recent literature review by Endres and colleagues17 discussed 10 trials of acupuncture for migraine and concluded that a 6-week course of acupuncture was at least not inferior to a 6-month course of prophylactic treatments, and had a role in the integrated management of migraine headaches.
Drugs for prevention
Cranial arteritis
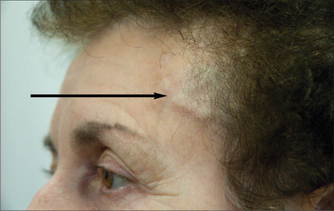
Cranial arteritis should be suspected in older patients, typically 75 years of age and older—it is rarely seen in patients under the age of 50 years. The headache is of insidious onset of days to weeks, often with systemic ill health, fever, polymyalgia rheumatica, scalp tenderness and jaw claudication, with the latter two virtually pathognomonic. The ESR is raised, often as high as 100, but may be normal early in the course of the illness, and therefore the ESR should be repeated once or twice per week when the diagnosis is suspected and the initial ESR is not raised. Occasionally the diagnosis can be made at the bedside by detecting thrombosed temporal arteries (see Fig 36.1). If the clinical suspicion is high and despite a normal ESR, the patient should be treated with high-dose corticosteroids and a temporal artery biopsy obtained as a matter of urgency. Delays in treatment can result in blindness and, less frequently, cerebral infarction.
FUNNY TURNS
INVESTIGATIONS
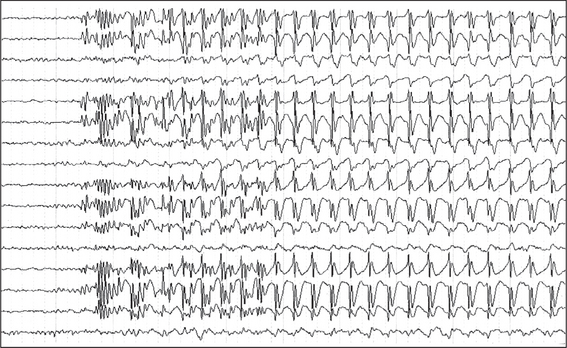
The investigations already alluded to rarely, if ever, establish a diagnosis in patients with intermittent disturbances of function. If seizures are frequent or can be precipitated by sleep deprivation or hyperventilation, then video-electroencephalographic monitoring may occasionally detect a seizure (see Fig 36.2). Holter monitoring can on rare occasions detect complete heart block in patients with suspected Stokes-Adams attacks. Symptoms related to hyponatraemia, hypokalaemia or hypocalcaemia are so rare that routine electrolyte estimation is rarely helpful. A full blood examination could detect anaemia or evidence of an infection, but intermittent symptoms related to either of these entities are very rare. Imaging such as CT scan or MRI scan is usually normal, except for patients with focal seizures.
MANAGEMENT
Epilepsy
Tonic-clonic seizure
Tonic-clonic seizure may or may not be preceded by an aura. An aura suggests a focal onset. The patient stiffens and may ‘cry out’—the so-called ‘cri de chat’—the eyes remain open, and this tonic phase, lasting up to 30 seconds, is followed by the clonic phase, with repeated flexing movements of all four limbs. Tongue or cheek biting and incontinence of urine and faeces may also occur. There is a period of post-ictal drowsiness and confusion. Most episodes last less than 2–3 minutes, rarely as long as 20 minutes.19
Diagnosis
The diagnosis is essentially a clinical one and based almost entirely on the history of the episode, as only rarely do patients have a seizure while undergoing electro-encephalography (EEG). Current recommendations advocate an EEG, CT or MRI brain scan in all patients presenting with a first unprovoked seizure. The presence of epileptic discharges on an EEG in patients with their first seizure increases the risk of recurrence. Many patients presenting with their first apparent seizure have, on detailed questioning, had unrecognised seizures in the past.20
Treatment of seizures and epilepsy
Most seizures last only a matter of 1–2, rarely 5, minutes, and the initial management is simply to prevent the patient from harming themselves and to lie them on their side when the seizure is finished. Prolonged seizures and status epilepsy require prompt intervention. Intravenous (IV) lorazepam is the drug of choice in both adults and children.21
Carbamazepine is the drug of choice for partial or focal seizures, and valproic acid for generalised seizures.22 However, carbamazepine interferes with the oral contraceptive pill and causes drowsiness; valproic acid can result in hair loss and obesity, side effects that young women are often not willing to contemplate. There are many guidelines23 and these are frequently evolving and should be consulted on a regular basis.
Integrative management of epilepsy
The adverse effects of antiepileptic pharmaceuticals make it difficult to attain optimal dosage levels in order to prevent seizures in many cases.24 Current evidence suggests that 25–50% of individuals with epilepsy have tried some form of CAM therapy.25–27 It is important to note that these therapies are not a substitute for medication; however, depending on the effectiveness of the intervention(s), dosage reductions under medical supervision may be possible.28
Mind–body medicine
A systematic review assessing several different meditative practices (meditation, meditative prayer, yoga, relaxation) has revealed some evidence for the efficacy of meditation in the treatment of epilepsy.29 In particular, studies have demonstrated that transcendental meditation (TM) may be a potential antiepileptic treatment. However, further trials are required, as many of the EEG recordings during TM (increased alpha, theta, gamma frequencies with increased coherence and synchrony) are similar to the neuronal activity that occurs during seizures.30
Sleep
It has long been known that there is a close relationship between the physiological state of sleep and the pathological process underlying epileptic seizures, but the connection is still not well understood.31 Sufferers of epilepsy often demonstrate multiple sleep abnormalities, such as increased sleep latency, fragmented sleep, increased awakenings and stage shifts and an increase in stages 1 and 2 of non-REM sleep.32 It is important that any sleep disorders are identified and managed as soon as possible, as sleep deprivation has been shown to increase the frequency of epileptiform discharges and seizures.33–35 Sleep quality, as well as daytime alertness and neurocognitive function, can be improved by anticonvulsant medications, ketogenic dietary principles and vagus nerve stimulation.33 However, it should be noted that poor sleep can also be an adverse effect of some anticonvulsant drugs.24,36
Sunshine
Individuals with epilepsy who have been on long-term treatment with various anticonvulsants may develop hypocalcaemia, osteomalacia and osteopenia that is independent of vitamin D metabolism. It has been demonstrated that there are regional variances in the incidence of drug-induced bone disease and this has been attributed to variances in sunlight exposure, with the majority of reports coming from areas of low sunshine or from institutionalised patients.37,38
Exercise
In times past, exercise was restricted for those with epilepsy, rather than encouraged, as it is today. As many people with epilepsy still fear suffering an exercise-induced seizure, they often lead a sedentary life and have poor physical fitness.39 Although there have been rare cases of exercise-induced seizures, both clinical and experimental data have shown that exercise can reduce the frequency of seizures as well as improving cardiovascular and psychological health.40,41 Indeed, current evidence demonstrates that regular exercise may have a moderate seizure-preventative effect in 30–40% of this population.39 Most sporting activities, including contact sports, are safe to participate in, according to current medical recommendations. Supervised water sports and swimming are also considered safe, provided seizures are well controlled. However, sports such as hang-gliding and scuba diving are not recommended, as there is a high risk of severe injury or death if a seizure were to occur.40 It is imperative that medical advice regarding exercise is individualised for each patient, taking into account their seizure type and frequency.40
Yoga
Recent studies have shown that yoga can reduce seizure index and increase quality of life in people with epilepsy.42 Yoga has also been shown to significantly improve parasympathetic parameters, suggesting that it may have a role in the treatment of autonomic dysfunction in patients with refractory epilepsy.43
Diet
Maintaining good blood sugar balance, identifying and eliminating allergenic foods and avoiding suspected triggers such as alcohol, artificial sweeteners, diet soft-drinks, energy drinks and MSG are important in the management of epilepsy.28,44,45 Growth retardation is common among children with epilepsy and this appears to be secondary to poor dietary intake. Dietary intake of vitamins D, E and K, folate, calcium, linoleic acid and alpha-linolenic acid has been found to be below the recommended daily allowance (RDA) in approximately 30% of children with intractable epilepsy.46
Studies of dietary treatments for epilepsy suggest that approximately 50% of children have a 50% reduction in seizures after 6 months. Approximately one-third will achieve more than 90% reduction in their seizures. Such diets maintain their efficacy when provided continuously for several years. Furthermore, long-term benefits may be seen even when the diet is ceased after only a few months, indicating neuroprotective effects.47 Other dietary guidelines for the management of epilepsy are given below. Recommending such diets in the management of epilepsy needs to be balanced with the overall nutritional needs and health of the patient.
Ketogenic diet
The ketogenic diet is a high-fat, low-carbohydrate, high-protein diet used to treat medically refractory epilepsy.48 At least 15–20% of patients on the ketogenic diet experience more than 50% reduction in seizure frequency. For this reason, the ketogenic diet should be considered an early treatment for drug-resistant epilepsy, not a ‘last resort’.49–51 The ketogenic diet may also be a valuable therapeutic option for children with drug-resistant focal epilepsy, particularly those with recent deterioration of seizure control.52,53
Modified Atkins diet
The modified Atkins diet is a less-restrictive ketogenic diet. It has no restrictions on calories, fluids or protein, and does not involve fasting. As for the ketogenic diet, high-fat foods are encouraged, with 10 g/day of carbohydrate allowed in children and 15 g/day in adults. Approximately 45% of patients have 50–90% seizure reduction, with 28% having more than 90% seizure reduction.54 The ketogenic diet appears to exert its effects more quickly, although by 6 months the difference is no longer considered significant.50
Low glycaemic index diet
A study in 2009 reported on the efficacy, safety and tolerability of the low glycaemic index diet in paediatric intractable epilepsy. More than 50% reduction from baseline seizure frequency was seen in 42%, 50%, 54%, 64% and 66% children at 1, 3, 6, 9 and 12 months respectively.55
Polyunsaturated fatty acids
Increased concentrations of both ketone bodies and polyunsaturated fatty acids (PUFAs) have been found in the cerebrospinal fluid and plasma of patients on the ketogenic diet, and it is thought that a high dietary intake of PUFAs may be one mechanism responsible for the potent anticonvulsant effects of the ketogenic diet.56,57 Supplementation with omega-3 fatty acids has also been shown to prevent status epilepticus-associated neuropathological changes in animal studies.58 Although PUFAs have been shown to reduce seizures in several animal models, available data regarding the effects of supplementation in epileptic patients (1–3 g EPA/DHA daily) reveal mixed results with respect to seizure frequency.59–61 More research is therefore required before PUFAs can be definitively recommended as a treatment option for epilepsy.56
Amino acids
Current research suggests that serotonin has an antiepileptic effect, and serotonin receptors are expressed in almost all networks involved in epilepsies. Some SSRIs, such as fluoxetine, have been found to improve seizure control, and some antiepileptic drugs have recently been found to increase endogenous serotonin levels.62–64
Tryptophan
Tryptophan is an essential amino acid and the only brain precursor of serotonin. It has been estimated that patients with epilepsy have approximately 30% lower brain intake of tryptophan compared with controls. Whey proteins that are high in tryptophan but lower in other large neutral amino acids are currently being trialled in combination with antiepileptic medications.62
Taurine
Taurine is one of the most abundant free amino acids found mainly in excitable tissues. Taurine is necessary for the production of GABA, one of the major inhibitory neurotransmitters in the limbic system.65 For this reason, drugs that target GABA receptors are the mainstay of treatment of seizures, with the recent transplantation of GABA-producing cells effectively reducing seizures in several well-established models.66,67
There have been multiple animal studies demonstrating that taurine-fed animals have increased levels of GABA and a higher threshold for seizure onset compared to controls.65 Furthermore, increased taurine levels in the hippocampus improve membrane stabilisation, significantly reducing neuronal cell death and favouring recovery after neuronal hyperactivity.68,69
Carnosine
Carnosine is an amino acid with many of the features of a neurotransmitter. It has the ability to act as both a neuromodulator and a neuroprotective agent, and it may indirectly influence neuronal excitability by modulating the effects of zinc and copper.70–72 Carnosine may have a significant anticonvulsant effect and, as such, it may prove to be a potential anticonvulsant treatment for epilepsy in the future.73
Antioxidants
Excessive production of free radicals with neuronal hyperexcitability have been strongly implicated in the pathogenesis of idiopathic epilepsy. There is therefore a possible role of antioxidants in combination with antiepileptic drugs for better seizure control,74–76 although, as with other chronic illnesses, it is likely that a nutritious diet rich in antioxidants such as vitamins A, C and E is likely to be far more effective than a poor diet supplemented by antioxidants. Results have thus far been mixed for vitamin E supplementation, and more studies are required before definitive recommendations can be made.77
Selenium deficiency has been implicated in the pathogenesis of epilepsy.78 Anticonvulsants may further deplete total body selenium stores, and failure to advise appropriate selenium supplementation, especially to pregnant women taking sodium valproate, may increase the risk of neural tube defects or other free radical mediated damage.79
Zinc
Altered zinc homeostasis appears to be associated with epilepsy; however, the definitive role of zinc as a neuromodulator in synapses is still uncertain.80,81
Other vitamins and minerals
Many anticonvulsant medications are known to reduce folic acid levels, subsequently raising homocysteine levels. Homocysteine, however, is known to be a convulsing agent that can result in increased seizure recurrence and intractability to medications. In addition, anticonvulsant medications can disturb lipid metabolism, creating hypercholesterolaemia, dyslipidaemia and altered uric acid metabolism. As such, routine supplementation with folic acid, vitamin B12, B6, C, E and beta-carotene for all those on anticonvulsant medications is important.82
A Cochrane review has found that vitamin B1 improves both neuropsychological and cognitive functions in patients with epilepsy. In the same review, vitamin D was also found to improve bone mineral density in those taking anticonvulsant medications.28,77
Manganese deficiency has also been associated with seizures in both animals and humans, according to a systematic review. More research is needed, as it is currently unclear as to whether this is a cause or an effect of the convulsions.83
Herbs
Herbal medicine has been used for centuries in many cultures for the treatment of epilepsy and some patients may be self-medicating with herbal medicines. It is therefore important for the GP to ask about the patient’s use of herbal preparations, as many herbs can increase the risk of seizures by affecting cytochrome-P450 enzymes and P-glycoproteins, or through possible contamination by heavy metals.84,85
Well-designed clinical trials of herbal therapies for epilepsy are scarce. However, based on animal studies and numerous anecdotal observations of clinical benefits in humans, further research is certainly warranted.84,85
The flavonoid derivatives from Scutellaria baicalensis Georgi, Artemesia herba-alba, Melissa officinalis and Salvia triloba have all been found to exert anticonvulsant effects by exhibiting significant affinity for the GABA(A) receptor benzodiazepine binding site.86,87 Ginkgo biloba appears to have the capacity to both induce and inhibit seizures.88
Interestingly, Curcumin has also recently been shown to significantly prevent generalisation of electroclinical seizure activity as well as the pathogenesis of iron-induced epileptogenesis.89,90 Further studies are required.
Various Chinese herbs, such as Chaihu-longu-muli-tang, appear to have antiepileptic properties, and it also appears that the reduction in seizure frequency may be related to the antioxidant effects of the herbs.91 Shitei-To is another TCM formulation that may have therapeutic effects in the prevention of secondarily generalised seizures. It is made from three medicinal herbs: Shitei (calyx of Diospyros kaki L.f), Shokyu (rhizome of Zingiber officinale Roscoe) and Choji (flower bud of Syzygium aromaticum).92
Prevention
Lifestyle measures that may be important in the management of epilepsy have been discussed previously. Furthermore, living as ‘cleanly’ as possible is important for individuals with epilepsy, as there are numerous documented toxic causes of seizures. These include substance abuse (alcohol and recreational drugs), exposure to various industrial and household products, occupational nickel and other heavy metals.94 Animal studies have demonstrated that blood lead levels similar to those found in humans living in urban areas with high pollution levels can lower seizure threshold.95
Research on hospital admissions of patients in status epilepticus reveals several environmental factors that are either protective or precipitative. It appears that there is a significant diurnal pattern, with the majority of admissions occurring between 4 pm and 5 pm, and least admissions in the early morning hours. Admissions also vary significantly throughout the lunar cycle, with the peak at day 3 after the new moon and trough at 3 days before the new moon. Admissions have been noted to be highest on bright, sunny days, whereas dark days, high humidity and high temperature appear to be significantly protective factors.96
MOTOR WEAKNESS
EXAMINATION
Central nervous system (CNS) causes of weakness produce a classic ‘upper motor neuron’ pattern of weakness with finger extension, shoulder abduction and elbow extension in the upper limbs and hip flexion, weakness of dorsiflexion of the feet and knee flexion in the lower limbs. This pattern of weakness would most often be associated with increased tone (with or without clonus), increased reflexes and up-going plantar responses. If the facial muscles are affected with CNS, then the forehead is not affected. In disorders of the peripheral nervous system (PNS), the pattern of weakness will indicate whether the problem is in the anterior horn cell (usually associated with significant wasting and fasciculations), motor nerve root (usually associated with significant radicular pain), plexus, peripheral nerve, neuromuscular junction (fluctuating weakness with exercise) or muscle. The presence of sensory signs excludes disorders of the anterior horn cell, motor nerve root, neuromuscular junction or muscle. In disorders of the PNS, the tone may be normal or decreased, the reflexes are often absent, plantar responses are down-going and, if present, sensory signs will reflect the site of the problem in the PNS. Disorders of muscle are usually associated with preserved reflexes (rare exceptions to this include myotonic dystrophy and inclusion body myositis). If the weakness affects the face, it is important to see whether the muscles that wrinkle the forehead (frontalis muscle) are affected; if so, this points to a lower motor neuron facial palsy. If the forehead is not affected but there is weakness around the mouth, this is an upper motor neuron facial weakness.
MANAGEMENT
Management is disease specific and is discussed below.
Facial weakness
Bell’s palsy
Corticosteroids and aciclovir have been recommended in the belief that functional outcome is improved.97 However, a 2009 Cochrane review concluded that corticosteroids and aciclovir have not been proved to be beneficial.98 Subsequent to this review, a large randomised controlled trial99 demonstrated complete recovery at 3 months in 83% of patients treated with prednisolone (50 mg per day for 10 days), as opposed to 63.6% for patients treated with placebo. At 9 months, 94.4% of the prednisolone-treated group had completely resolved, compared to 81.6% for the placebo. No benefit was found from aciclovir.
Acute and chronic inflammatory demyelinating neuropathy
The term acute inflammatory demyelinating neuropathy (AIDP) has replaced the term (Landry) Guillain-Barré (Strohl) syndrome. This presents with the rapid onset of weakness usually affecting the limbs, with proximal weakness in the lower limbs and distal weakness in the upper limbs initially, subsequently becoming generalised weakness of the limbs. There is almost invariably neck flexion and occasionally neck extension weakness. The cranial nerves may also be affected, resulting in bilateral facial weakness, palatal weakness or weakness of the extra ocular muscles, resulting in variable patterns including a pseudo-internuclear ophthalmoplegia (INO). The reflexes are absent and there may or may not be peripheral sensory loss. Some patients develop involvement of the autonomic nervous system, resulting in arrythmias, periods of hypertension and hypotension and disturbances of bladder and bowel function. The respiratory muscles are involved in severe cases, and patients will often require long-term ventilation. In addition to supportive measures, either intravenous immunoglobulin (IVIG) or plasma exchange is used in the acute phase.100–102 IVIG is probably the treatment modality of choice, for ease of use. The prognosis is variable, with approximately 75% of patients making an excellent recovery; the remainder have mild or moderate impairment, with severe disability occurring in less than 5% of patients.
Chronic inflammatory demyelinating neuropathy (CIDP) can cause the same patterns of weakness as AIDP but evolves more slowly (by definition over more than 6 weeks). IVIG or corticosteroids should be considered in sensory and motor CIDP. IVIG should be considered as the initial treatment in pure motor CIDP.103
Motor neuron disease
Motor neuron disease (MND), also referred to as amyotrophic lateral sclerosis (ALS), presents with weakness and fatigue associated with significant muscle wasting and fasciculations in the absence of any sensory symptoms. Muscle cramps can occur. Four distinct clinical phenotypes have been identified, with different rates of progression and survival. Patients presenting with global involvement have the worst prognosis, while patients presenting with a flail arm survive longer.104 When MND affects the bulbar musculature, patients present with a characteristic dysarthria, with wasting and fasciculations of the tongue. At present there is no specific treatment, but these patients should be cared for in specialised centres with expertise in MND.
Myasthenia gravis
Myasthenia gravis is a very rare autoimmune disease and most GPs are not likely to see a case. The characteristic feature is a weakness that is exacerbated by use of the muscle and reduced in intensity by rest. Myasthenia gravis can remain confined to the extra ocular muscles, where it presents with intermittent ptosis and variable horizontal and vertical diplopia—for example, when reading or watching television. Myasthenia affecting muscles in the rest of the body is referred to as generalised myasthenia. Myasthenia affecting the bulbar musculature (the lower four cranial nerves) will present with dysarthria exacerbated by talking, dysphagia and difficulty chewing, exacerbated by eating. When it affects the muscles of the arms and legs, patients will complain of increasing weakness when they are trying to do activities such as hanging the washing on the line or washing their hair, or they may complain of increased difficulty walking, and of having to rest. The diagnosis can be made at the bedside by asking the patient to repeatedly exercise and by demonstrating increased weakness; or, if it affects the extra ocular muscles, by asking the patient to look up, and observing increased ptosis and the development of increasing diplopia, the longer the patient is looking up. Applying ice to the ptosed eyelid can lead to a dramatic resolution of the ptosis.105 Another bedside test is the Tensilon test, where edrophonium hydrochloride is injected and a transient improvement or resolution of symptoms can be observed. The ice test and the Tensilon test both have a high sensitivity (92–95%) and specificity (97%). Antibodies directed against the acetylcholine receptor (ACHR) are highly specific (98%) but the sensitivity varies from one report to another, from as low as 54% to as high as 88%.106 Electrodiagnostic studies include repetitive nerve stimulation, where a decremental response is observed and the variability of stimulation of the acetylcholine receptor by acetylcholine can be observed with single fibre electromyography (SFEMG)—this is referred to as increased jitter. All patients with myasthenia gravis should have a CT scan of the chest to look for thymic hyperplasia (usually seen in younger patients) or a thymoma (in older patients); these can be either benign or locally malignant. Ocular myasthenia gravis sometimes responds to the cholinesterase inhibitor pyridostigmine.107 Thymectomy is recommended for patients with thymic hyperplasia108 or thymoma.109 IVIG is very effective in the treatment of myasthenic crisis and can be used preoperatively prior to thymectomy or prior to any surgery in patients with generalised myasthenia gravis.101 Corticosteroids, other immunosuppressive agents and plasma exchange are used in patients who fail to respond to thymectomy.110,111
Polymyositis and dermatomyositis
Polymyositis and dermatomyositis are very rare inflammatory disorders of muscle. Dermatomyositis is more common in younger patients but may also occur in patients with malignancy. Patients present with proximal weakness in their arms and legs, with normal reflexes. Neck flexion weakness is common; rarely the bulbar or respiratory muscles may be affected. The characteristic skin changes seen to imitate myositis include a violaceous discolouration of the eyelids, scaly erythema over the joints on the dorsal aspect of the hands, macular erythema on the posterior neck and shoulders or on the anterior neck and chest, or a violaceous erythema associated with increased pigment and telangiectasia on the anterior neck, chest, posterior shoulders, back and buttocks. Electromyography demonstrates myopathic changes with brief small amplitude polyphasic potentials (BSAPPs) and full recruitment with minimal effort, but also demonstrates fibrillation potentials and positive sharp waves. The definitive diagnosis is established by muscle biopsy. There are few randomised controlled trials regarding treatment; it is largely empirical. Corticosteroids remain the agent of first choice, with other immunosuppressive agents such as azathioprine, cyclophosphamide and cyclosporin used in patients who fail to respond to 3 months of corticosteroids. Prednisolone is commenced at a dose of 1–2 mg per kilogram of body weight and is continued for 2–4 weeks, and then the dose is slowly reduced to a maintenance level over the next 6 months.112
PARAESTHESIA AND NUMBNESS
EXAMINATION
Proprioception is tested in the index finger and big toe. Younger patients can appreciate minute changes; older patients can also detect even small movements of a finger or target. Marked impairment of proprioception in the lower limbs can also be tested by asking the patient to put their feet together and close their eyes—this is referred to as Rhomberg’s test. An abnormal test is where the patient becomes unstable with their eyes closed. Vibration is also initially tested on the index finger or big toe using a 128 Hz tuning fork. It is important to remember that vibration sense diminishes in the feet with advancing age, and may even be absent in older patients. Temperature sensation can be tested with either the cold tuning fork or, in patients whose limbs are cold, a warm object such as the examiner’s hands. Pain sensation is tested using a sharp object, but a hypodermic needle should not be used. A simple drawing pin or the specific things referred to as ‘sharps’ can be used. In patients who have objective sensory loss, it is important to test sensation in 360 degrees until the exact distribution of sensory loss is established, and then one can consult a sensory chart to see which part of the nervous system is likely to be affected.
MANAGEMENT
Carpal tunnel syndrome
Carpal tunnel syndrome is almost certainly the most common cause of intermittent sensory symptoms. Although in theory these sensory symptoms should be confined to the distribution of the median nerve distal to the wrist, it is not uncommon for patients to complain of altered sensation affecting more proximal parts of the limb. Carpal tunnel syndrome should be suspected in patients who have numbness or tingling precipitated by holding objects, driving, mowing the lawn but in particular in patients who awaken in the middle of the night with numbness in one or both hands that is relieved by either moving the hands and arms or hanging the arms out of the side of the bed. Two simple bedside tests are Phalen’s sign, where sensory symptoms are precipitated with forced reflection of the wrist, and Tinel’s sign, where a transient electric shock-like sensation or paraesthesia is precipitated by tapping the median nerve in the carpal tunnel using a tendon hammer. Nerve conduction studies may be normal if symptoms have been present for only a short period of time. On the other hand, in older patients, nerve conduction studies are often very abnormal, even in patients who have only recently noticed symptoms. Nerve conduction studies can also assess the severity of the carpal tunnel syndrome. Symptoms occurring during pregnancy or in the setting of obesity may result following the pregnancy or after weight loss. Patients with mild symptoms may elect to simply tolerate the symptoms; some will use splints of their wrists at night to reduce the intensity of the symptoms. Some patients with mild carpal tunnel syndrome may respond to a corticosteroid injection into the carpal tunnel.113 Surgical decompression is the definitive treatment in patients with more severe carpal tunnel.114
Meralgia paraesthetica
Numbness, paraesthesia or dysaesthesia (unpleasant sensory symptoms) affecting the lateral aspect of the thigh (see Fig 36.3) is typical of the condition referred to as meralgia paraesthetica, and relates to compression of the lateral cutaneous nerve of the thigh beneath the inguinal ligament. Most patients do not seek treatment once the benign nature of the problem is explained to them. Patients with dysaesthesia, on the other hand, can be offered a corticosteroid injection into the site of compression, formal decompression of the nerve, or avulsion of the nerve that results in a resolution of the dysaesthesia, but this is replaced by permanent numbness.
DISTURBANCES OF VISION
MANAGEMENT
Management is disease specific.
Monocular impairment of vision
Optic and retrobulbar neuritis
Optic neuritis and retrobulbar neuritis both result in the gradual onset, over hours to days, of severe visual loss, often associated with pain on movement of the eye. There is an afferent pupillary defect (Marcus-Gunn) in the affected eye in both, but the optic disc is swollen with optic but not retrobulbar neuritis. While the most common cause is multiple sclerosis, it can also be a manifestation of connective tissue disease.115
CEREBRAL VASCULAR DISEASE
The challenge for the clinician is to:
and then choose the appropriate treatment in a particular patient, taking into account their past history, social history and listed medications, all of which can influence the choice of therapy. The diagnosis of stroke presents little difficulty when an elderly patient presents with the sudden onset of a focal neurological deficit in the setting of multiple recognised risk factors for stroke. On the other hand, many patients awaken with the neurological deficit and therefore one cannot be certain that the onset was sudden. And stroke can occur in young patients and in patients without recognised risk factors. Some symptoms, such as diplopia or vertigo, will always develop suddenly and may not represent cerebral vascular disease. Obtaining a history from patients is not always possible, because of the presence of speech disturbance or cognitive problems related to the stroke.
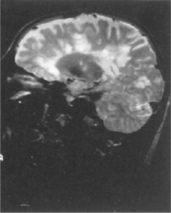
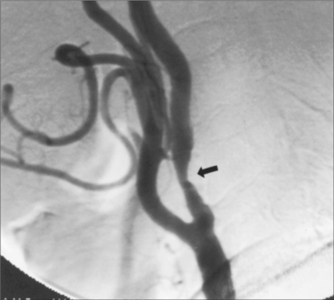
Cerebral infarction (CI) rather than intracerebral haemorrhage (ICH) is more common in the era of better management of hypertension. The main causes of CI are atherosclerotic vascular disease with or without stenosis of the major extracranial and, to a lesser extent, intracranial vessels, small vessels (lacunar) infarction related to hypertension and diabetes, and AF. In younger patients (< 45 years of age), alternative causes of stroke need to be considered, such as extracranial artery dissection (see Fig 36.4), a patent foramen ovale, or lupus anticoagulant anticardiolipin antibody syndrome.
FIGURE 36.4 Internal carotid artery dissection, a rare but important cause of stroke in young patients
PRIMARY PREVENTION
Optimal management of treatable risk factors such as hypertension, smoking, hypercholesterolaemia, obesity and diabetes is the cornerstone of both primary116 and secondary prevention.
The old adage that a normal blood pressure is 100 plus the patient’s age has clearly been shown to be incorrect. The Progress Study117 demonstrated that the blood pressure should be as close to 120/80 as possible although, in reality, most elderly patients cannot achieve this level of blood pressure due to side effects of medication.
Anticoagulation with warfarin (PT INR 2–3) for patients in AF with a CHADS score118 of two or more significantly reduces the risk of sudden stroke. There is a 70% relative risk reduction with less than 1% mortality and a 1–2% major bleeding risk.119 Absolute contraindications include bleeding peptic ulcer or other pathology that would predispose to bleeding. A risk of falls and cognitive impairment are relative contraindications, and the slightly increased risk of warfarin in these patients needs to be balanced with the risk of stroke using the CHADS score (Table 36.1).
| Score | Annual risk of stroke | 95% CI |
|---|---|---|
| 1 | 2.8 | 2.0–3.8 |
| 2 | 4.0 | 3.1–5.0 |
| 3 | 5.9 | 4.6–7.3 |
| 4 | 8.5 | 6.3–11.0 |
| 5 | 12.5 | 8.2–17.5 |
| 6 | 18.2 | 10.5–29.4 |
The CHADS score is calculated as 1 point each for recent (< 3 months) congestive cardiac failure (C), history of hypertension (H), age > 75 (A) and diabetes (D), and 2 points for prior TIA or stroke (S).
The absolute risk reduction with carotid endarterectomy for patients with asymptomatic carotid stenosis of greater than 50% is 1% per annum.120
HISTORY
The neurological history should also elicit the presence of risk factors for stroke, which will influence the choice of secondary prevention measures. It will also need to examine the social history, which can have a significant impact on the subsequent management of patients with cerebral vascular disease. Many patients with cerebral vascular disease have multiple other medical problems and are taking a large number of drugs, which will also influence subsequent choice of therapy. Details of past medical problems should be obtained, as many of these will also influence the subsequent choice of therapy—for example, a history of peptic ulcer disease or epistaxis.
INVESTIGATIONS
Initial investigations that should be performed as a matter of urgency include:
In patients with either a TIA or a minor stroke in the anterior circulation, ischaemia should have an urgent Doppler carotid ultrasound, as 50% of subsequent strokes after a TIA will occur within the first 24 hours.121 It is important to remember that a normal CT of the brain does not exclude cerebral ischaemia, particularly in the first 6 hours after onset, but also in patients with ischaemia related to small vessel disease or in the brainstem.
Subsequent investigations
MRI, particularly diffusion weighted MRI (dwMRI), can detect cerebral infarction in some patients with transient ischaemic attacks, and can detect cerebral ischaemia as early as 1.5 hours after the onset of symptoms.122 dwMRI has a sensitivity of more than 90–95% for detecting early (within the first 6 hours after onset) ischaemic changes.122 It must be performed within the first week, as these changes are transient. It can also detect very small (lacunar) infarcts in both cerebral hemispheres and the brainstem. Magnetic resonance angiography (MRA) and magnetic resonance venography (MRV) enable imaging of the cerebral circulation. Very rarely, a stroke can relate to cerebral venous thrombosis.
Fasting lipids should be performed, as many patients have hypercholesterolaemia.
Transthoracic and/or trans-oesophageal echocardiography is indicated in patients with a cardiac source for cerebral embolism (in essence, patients with ischaemia in the distribution of the major vessels in the absence of demonstrable vascular disease in those vessels). Patients admitted to stroke units should have cardiac monitoring, in order to detect intermittent AF.123
MANAGEMENT
Management of ischaemic TIA and stroke
Acute treatment of ischaemic stroke
Ideally, patients should be referred to centres of expertise, as the development of stroke units has led to a reduction in mortality and morbidity. Aspirin in the first 24 hours reduces mortality in the first few weeks after stroke.124 All patients should have a dysphagia screen and placed on nil orally if they fail, until a detailed speech therapist assessment. Cardiac monitoring is essential, not only to detect AF but also to monitor for other arrhythmias that may occur in the setting of acute myocardial infarction, which occurs in a small percentage of patients with stroke in the early days after stroke. Intravenous fluids are essential to maintain hydration, but 5% glucose should be avoided. Deep vein thrombosis (DVT) prophylaxis with antithrombotic stockings and either unfractionated heparin or low molecular weight heparin should be instituted from day one. Patients should be checked daily for possible aspiration pneumonia.
Thrombolysis
Thrombolysis with tissue plasminogen activator (t-PA) is now a well-established treatment for patients with ischaemic stroke of less than 4.5 hours’ duration and a NIHSS score of > 4, provided there are no contraindications.125 Exclusion criteria include the following:125
Secondary prevention
Antiplatelet therapy
In addition to the management of risk factors as discussed above, antiplatelet therapy in the form of aspirin, aspirin and persantin,126 or clopidogrel127 is recommended for patients with ischaemic stroke not related to AF or another cardiac source for embolism.
Anticoagulation
Anticoagulation with warfarin (optimal PT INR 2.0–2.5128) is recommended for patients with AF or other potential cardiac sources for embolism, such as a cardiomyopathy or artificial heart valve.129 Recently, dabigatran, a direct thrombin inhibitor, was found at a dose of 110 mg twice a day to be as effective as warfarin130 although some concerns have been expressed about the methodology of the trial. Despite numerous trials confirming the effectiveness of warfarin, many practitioners are reluctant to prescribe, because of perceived risks. Each area of medicine associated with AF and warfarin has its own perspective. Neurologists see the strokes that could have been prevented by warfarin, neurosurgeons see the complications of warfarin therapy and cardiologists see hundreds of patients with AF who have a very low risk of stroke. As discussed above, the CHADS score allows stratification of risk.
Carotid endarterectomy
Carotid endarterectomy is probably the most potent therapy available for secondary prevention in patients with a symptomatic stenosis of 70% or greater (Fig 36.6). It is associated with a 17% absolute risk reduction over 2 years, with numbers needed to treat (NNT) of only 15.131 As 50% of strokes after TIA occur in the first 24 hours121 urgent assessment for carotid stenosis with a Doppler carotid ultrasound is essential, although most of these recurrent events may well relate to small vessel disease—the so-called capsular warning syndrome.132
ABNORMAL MOVEMENTS AND DIFFICULTY WITH MOVEMENT
EXAMINATION
The various examination findings that differentiate one condition from another are discussed below.
MANAGEMENT
Management is disease specific and is discussed below.
Abnormal movements
Tremor (benign essential, benign familial, resting tremor of Parkinson’s)
Benign essential and benign familial tremors are identical except that, in the latter, there is a positive family history. This is the patient who complains that the cup rattles in the saucer. This is not a tremor at rest; it occurs with action. The severity of the tremor may be reduced by alcohol, and this can be a diagnostic clue. There is no alteration in tone, nor is there any bradykinesia. Walking is not affected. Hyperthyroidism should be excluded. If the trend is mild, no treatment is required. Primidone and propanolol are the drugs of choice. Alprazolam, atenolol, gabapentin (monotherapy), sotalol and topiramate are probably effective.134
The tremor of Parkinson’s, on the other hand, is present at rest and not when a patient holds an object. In resistant cases, chronic deep brain stimulation or thalamotomy is effective.134 The tremor may also be noticed when the patient is walking and the arm is hanging down by their side. The tremor is not influenced by alcohol, and in the early stages may be the only manifestation of Parkinson’s disease. There are some patients who may only experience tremor, and this is referred to as the benign tremulous form of Parkinson’s disease. Treatment of the tremor in Parkinson’s disease can be very difficult, and it does not always respond as well as the other manifestations to the various levodopa preparations.
Hemiballismus
Hemiballismus is a relatively rare movement disorder characterised by uncontrolled, random, large-amplitude movements of the limbs, likened to a large-amplitude chorea. It is usually caused by a vascular lesion that involves the contralateral subthalamic nucleus (STN) and its afferent and efferent pathways. Occasionally it can occur with infarction at other sites, and it can be a manifestation of multiple sclerosis. It is usually self-limiting and responsive to conservative treatment to prevent injury and dehydration, and the use of drugs to reduce the intensity of the abnormal movements. Tetrabenazine, haloperidol, propanolol, clonazepam, phenytoin and baclofen have all been used, with variable success. Sterotactic pallidotomy has been recommended in refractory cases.135
Tardive dyskinesia
Tardive dyskinesia (TD) results from chronic exposure to levodopa, dopamine agonists and dopamine receptor blockers. It is characterised by involuntary, repetitive, purposeless movements of the tongue, jaw, lips, face, trunk, upper extremities, lower extremities and respiratory system. Initially the mouth, lips and tongue are involved, and later the trunk and limbs may be involved. The movements are choreiform in nature. The diagnosis is a clinical one and Wilson’s disease needs to be excluded. Approximately one-third of patients will remit upon cessation of the causative agent. Persistent symptoms can occur, particularly in the elderly, those without dentures or those with underlying organic cerebral dysfunction. The incidence may be less with the newer atypical antipsychotic drugs, and these drugs may be useful in treatment, and therefore replacing typical antipsychotic drugs with atypical antipsychotic drugs may be useful. Central anticholinergic drugs that can exacerbate TD should be discontinued. Reserpine, tetrabenazine, clonazepam, baclofen and botulinum toxin injections are recommended in refractory cases.136
Chorea: Huntington’s and Sydenham’s
Huntington’s chorea is an autosomal-dominant disorder in middle age with the characteristic chorea, with the subsequent development of abnormal behaviour and dementia. It relates to an expanded and unstable trinucleotide repeat on chromosome 4. The length of the trinucleotide repeats correlates inversely with the age of onset. Genetic testing can confirm the diagnosis. Treatment is symptomatic, with the use of dopamine-depleting agents such as tetrabenazine, reserpine, dopamine agonists such as haloperidol and benzodiazepines such as clonazepam or diazepam.137
Sydenham’s chorea is a major complication of rheumatic fever due to group A beta-haemolytic streptococcal infection. It is an immune-mediated disorder, with the antibodies triggered by beta-haemolytic streptococcus cross-reacting with antigens in the brain by a molecular mimicry mechanism. Systemic lupus erythematosus (SLE), drug reactions and Wilson’s disease should be excluded. Serum antistreptococcal antibodies are also raised in Sydenham’s chorea. MRI may show an increase in basal ganglia volume and also evidence of neuronal damage in the ganglia, especially the caudate nuclei and putamina. Single proton emission computed tomography (SPECT) studies can detect both hypo- and hyper-perfusion pattern abnormalities. Valproic acid, haloperidol, carbamazepine or pimozide can be used to treat the chorea.138
Difficulty walking: Parkinson’s and apraxia of gait
Parkinson’s disease
The most common presentation of Parkinson’s disease is a resting tremor, the so-called typical ‘pill-rolling’ tremor. When Parkinson’s disease affects both lower limbs, patients present with difficulty walking, and they walk with small, shuffling steps and do not swing their arms. When there is an associated resting tremor, the diagnosis presents little difficulty. There is a characteristic ‘cogwheel’ rigidity best tested at rest by slowly flexing and dorsi-flexing the wrist. Occasionally, patients present with unilateral involvement mimicking a hemiparesis. The characteristic pathology of Parkinson’s disease is a loss of pigmentation in the substantia nigra and the presence of Lewy bodies, which leads to a deficiency in dopamine. The mainstay of treatment is levodopa replacement therapy; and to reduce side effects from the levodopa, this is combined with a decarboxylase inhibitor such as carbidopa or benserazide. Increasing duration of treatment is associated with great efficacy initially, a shorter duration of action leading to end-of-dose failure, and subsequently the ‘on–off’ phenomenon whereby periods of bradykinesia alternate with periods of dyskinesia regardless of the timing of the levodopa. Dyskinesia refers to the involuntary movements that can occur in patients with longstanding Parkinson’s on levodopa, initially as a peak dose phenomenon and subsequently the on–off phenomenon.139 Dopamine agonists such as cabergoline or pergolide have been used to manage the on–off phenomenon, but in recent years there have been concerns regarding significant side effects of cardiac valve abnormalities and pathological gambling. COMT inhibitors such as entacapone can reduce the dose when one tablet is taken with each dose of levodopa. More recently, pramipexole, a dopamine receptor agonist, has been shown to be very effective in patients with Parkinson’s—although less effective than levodopa, it has fewer motor complications. Stereotactic surgery140 and deep brain stimulation141 are other treatment modalities in drug-resistant cases.
Integrative management of Parkinson’s disease
Recent studies have placed an increasing focus on the role of environmental factors in Parkinson’s disease aetiology.142 For example, there have been many studies concerning the relationship of the neurotoxic effects of certain heavy metals to the risk of developing Parkinson’s.142 In particular, these include chronic exposure to manganese, copper and lead.143–147 Parkinson’s has also recently been characterised by high tissue iron (not currently connected to haemachromatosis) and miscompartmentalisation of zinc and copper.148,149
Epidemiological evidence indicates that exposure to pesticides (particularly organophosphates, carbamates, pyrethroids and organochlorides) may also play a significant role in the aetiology of idiopathic Parkinson’s disease.150–155 There is evidence that exposure to the common pesticides, such as maneb, paraquat and rotenone, especially at an early age, increases the risk of Parkinson’s.156,157,152 Despite this evidence, however, ethical challenges in exposure assessment make it impossible to conduct formal studies for further evaluation. Even exposure to pesticides, heavy metals and/or an iron-enriched diet while in the womb may directly reduce the number of dopaminergic neurons or cause increased susceptibility to loss of these neurons, with subsequent environmental insults or ageing.158,159
More than 1 year of antidepressant, anxiolytic or hypnotic drugs combined with a family history of Parkinson’s have been shown to be associated with significantly increased odds ratios for developing Parkinson’s.156
Previous work in electronic plants and exposure to fluorides and chlorpyrifos products have also been associated with a significantly increased risk of Parkinson’s, as has repeated traumatic episodes of consciousness loss.150,156 Conversely, cigarette smoking and alcohol intake may be associated with a reduced risk of Parkinson’s, although this could not be taken as an argument for taking up smoking.142,150
Allied healthcare referrals
Although there is growing evidence that specific allied healthcare interventions are beneficial when integrated into rehabilitation programs, recent Cochrane reviews indicate that there is not enough evidence to support or refute the use of occupational therapy, physiotherapy and speech therapy in Parkinson’s disease.160–162
Exercise
Several meta-analyses have shown that exercise is beneficial for physical functioning, quality of life, strength, balance and speed of gait for those suffering from Parkinson’s.163–165 A further systematic review has shown that these positive effects decrease when exercise is ceased.166 In particular, dancing and aerobic exercise have both demonstrated positive effects.167,168
Acupuncture
A number of studies, including meta-analyses, have demonstrated possible benefits of acupuncture in the treatment of Parkinson’s.169–171 The symptoms of tremor, rigidity and bradykinesia have especially responded with similar effects from electro-scalp acupuncture.172,173 The use of acupuncture combined with anti-Parkinson’s disease medications has also been found to be synergistic, with reduction of medication dose and reduced adverse drug effects.174,175
Herbal therapies
Ayurvedic medicine
Mucuna pruriens (MP) naturally contains levodopa and is the traditional herb used in Ayurveda to treat Parkinson’s.176,177 The pharmaceutical preparation HP-200, which contains MP combined with coenzyme Q10 and nicotine adenine dinucleotide, has been shown to be more effective than conventional levodopa in treating Parkinson’s in animal models. Unlike synthetic levodopa, HP-200 was found to significantly restore endogenous levodopa, dopamine, noradrenaline and serotonin in the substantia nigra, suggesting that it has both neurorestorative and neuroprotective effects.178,179 MP appears to be safe in the treatment of patients with Parkinson’s, although more studies are required to confirm its efficacy.180
Nutritional therapies
It has been postulated that imbalances in body metal levels may be a significant risk factor for the development of Parkinson’s as well as other neurological diseases.182 Studies have demonstrated low zinc and high iron and selenium levels in the cerebrospinal fluid of Parkinson’s patients who are taking levodopa, which may be correlated with lowered dopamine levels and increased oxidative stress.183
Low plasma, platelet and cerebral cortex levels of the antioxidant coenzyme Q10 in patients with Parkinson’s has been shown in several studies.184 A large phase III clinical trial was undertaken in 2008 to determine whether coenzyme Q10 supplementation slowed the progression of Parkinson’s.185
A meta-analysis of antioxidants has revealed that dietary intake of vitamin E may have a neuroprotective effect, attenuating the risk of Parkinson’s.186 Adequate dietary intake of vitamin B6 has also been shown to reduce the risk of developing Parkinson’s.187
Individuals with Parkinson’s treated with levodopa have been found to have significantly lower serum levels of folate and vitamin B12.188 Interestingly, those patients with Parkinson’s and depression have significantly lower folate levels, whereas those with Parkinson’s and cognitive impairment have lower levels of vitamin B12.189 Supplementation with these nutrients can lower homocysteine, which can be of particular importance to those Parkinson’s individuals at risk of vascular diseases, cognitive impairment or dementia.188
Patients with Parkinson’s have been found to have significantly lower levels of vitamin D than controls, and therefore vitamin D deficiency may be a significant risk factor in the pathogenesis of Parkinson’s.190 Animal studies have shown that supplementation with vitamin D may help to prevent dopaminergic neuron damage, but further studies on humans are needed.191
Diets high in creatine may be beneficial for those individuals with Parkinson’s who are on levodopa, to reduce the levodopa-induced dyskinesia that may occur after long-term use of the medication.192
Patients with Parkinson’s show a significantly reduced zinc status compared with controls.193 Zinc is required to synthesise superoxide dismutase (SOD), a powerful antioxidant that is normally found in high concentrations in the substantia nigra, where it protects neurons from oxidative stress. Supplementation with zinc significantly increases SOD in in vitro studies.193 Copper has also been implicated in Parkinson’s, as low copper may result in incomplete CNS development and high copper may be involved in the production of free radicals, which can result in mitochondrial damage, DNA breakage and neuronal injury.194 Magnesium deficiency has also recently been associated with neuronal degeneration in the substantia nigra and, hence, may contribute to the pathogenesis of Parkinson’s.142,195 Animal studies have shown that magnesium might protect against this, but further clinical trials are needed.196
A large-scale study of the dietary patterns of 130,000 individuals was conducted over 16 years. Results show that a diet high in fruits, vegetables, legumes, whole grains, nuts, fish and poultry combined with low saturated fat and moderate alcohol consumption was protective against Parkinson’s. In contrast, a typical Western diet high in animal and saturated fats was directly associated with an increased risk of Parkinson’s.197,198 In particular, the polyphenols in fruits such as blueberries have been shown to improve neuronal signal conduction and communication.199
The correct levels of protein intake in order to prevent Parkinson’s are currently debatable, with studies showing mixed results for both ketogenic and low-protein diets.200–202 Similarly, the consumption of dairy products has been positively associated with increased risk of Parkinson’s (especially in men); however, more studies are required to confirm this.203 The intake of caffeine is controversial; however, recent studies have shown that it may be ingredients in black tea other than caffeine that are responsible for a possible inverse associative risk with Parkinson’s.201–204
It should be noted that dietary exposure to food contaminants such as polychlorinated biphenyls (PCBs) and methylmercury (MeHg) have been positively associated with Parkinson’s, and therefore avoidance of these contaminants where possible is recommended.205
Mind–body medicine
Depression is commonly associated with Parkinson’s and it has been shown that Parkinson’s patients who are suffering from depression may experience faster deterioration in their neurological symptoms, greater cognitive decline and poorer quality of life. There have been several studies showing that therapies such as cognitive behaviour therapy may benefit these patients (and their carers); however, further larger-scale trials are warranted.206,207 A systematic review of RCTs has shown that the Alexander technique (a process of psycho-physical re-education) is effective in reducing disability in those with Parkinson’s, and earlier trials have shown that it may be beneficial in reducing depression when combined with drug therapy.208 Music therapy has also been shown to be effective on both motor and behavioural functioning in those with Parkinson’s.209,210
MULTIPLE SCLEROSIS
Multiple sclerosis (MS) is the most common neurodegenerative condition affecting young people. It is currently thought to be an immune-mediated disorder of unknown aetiology, due primarily to CD4+ T-cell-mediated immune responses to the major myelin proteins, myelin basic protein (MBP) and proteolipid protein (PLP). As with most other autoimmune conditions, MS is more common among women. In genetically susceptible individuals, factors such as Epstein-Barr virus211,212 and chlamydia infection213 have been implicated, as have vitamin D, diet, smoking and stress.214–218 The complex interplay of vulnerability, causative factors and triggers is far from fully elucidated, but they do point to potential therapies in the integrated management of MS, which will be discussed later. Humoral immune responses are also believed to contribute to the immunopathology. It results in inflammation, demyelination and subsequent gliosis in the central nervous system. MS is rare, with an incidence of approximately 0.1%. The average age of onset is 18–35 years but it may develop in patients at any age and is more common in women. There is a 30–50% increased incidence in children of parents with MS. In younger patients it may present with a clinically isolated syndrome such as optic neuritis or transverse myelitis. A relapsing–remitting course is also characteristic of MS in younger patients, which sometimes transforms to secondary progressive MS. A primary progressive form is characteristic of later-onset (40–55 years of age) MS.
INVESTIGATIONS
Investigations need to exclude other disorders that can produce multifocal involvement of the CNS, such as acute disseminated encephalomyelitis, anti-phospholipid antibody syndrome, Wilson’s disease, sarcoidosis, paraneoplastic syndromes, SLE and CNS vasculitis or lymphoma. The diagnosis is currently based on the revised MRI criteria of McDonald.219 If a patient presents with two or more attacks with objective evidence of two or more lesions, no additional information is required for the diagnosis. On the other hand, if a patient presents with two or more attacks and objective clinical evidence of only one lesion, or one attack with objective evidence of one or more lesions, the MRI scan and CSF analysis can be used to support the clinical diagnosis of MS. The MRI scan demonstrates characteristic abnormalities such as lesions arising from the corpus callosum—the so-called ‘Dawson’s fingers’ (Fig 36.6). Visual evoked responses (VER) can identify subclinical optic nerve lesions, with a prolonged P100 latency and a normal waveform typical of a demyelinating optic neuropathy.220
The finding of oligoclonal bands (OCBs) in the CSF and not the serum can improve overall diagnostic accuracy by increasing specificity and negative predictive value.221 OCBs are also found in, for example, paraneoplastic disorders and CNS infections. Most of these alternative diagnoses can be excluded on clinical grounds and analysis of the CSF. Oligoclonal bands can have a predictive value, as patients presenting with a clinically isolated syndrome (CIS) and a negative MRI are at very low risk of developing MS if their CSF examination is also normal—less than 5% after a median follow-up of 50 months.222
MEDICAL MANAGEMENT
Corticosteroids, oral or intravenous, are used in the acute relapse and have been shown to shorten the duration of relapses, but they do not alter the degree of recovery or the long-term prognosis. In recent years, disease-modifying drugs223 have been developed so rapidly that the management of MS has become very complex and should, ideally, be done in centres with expertise in this area. Drugs such as interferon-alpha, interferon-beta and glatiramer acetate have all been demonstrated to reduce the relapse rate, but their effect on long-term prognosis is uncertain. Immune suppression with azathioprine, cyclophosphamide, cladribine, methotrexate, IVIG or mitoxantrone is often employed, although the evidence of efficacy is limited.223 Natalizumab is a selective adhesion-molecule inhibitor and has been shown to reduce the risk of sustained progression and disability, and also the rate of clinical relapse in patients with relapsing–remitting MS.224 Progressive multifocal leucoencephalopathy occurs in less than 1% of patients and appears to be related to the number of infusions.
There is a delicate balance between efficacy and safety. Serious safety issues, including death, have been identified with many of the newer agents, with potential for increased efficacy. Concerns have been identified for natalizumab, rituximab, alemtuzumab, daclizumab, cladribine and fingolimod.225
INTEGRATIVE MANAGEMENT
Smoking
There are many studies linking smoking and passive smoking to MS. Those who begin smoking at an early age have an increased risk of developing progressive MS.226,227
Environmental exposure
Although no direct links have been made, there appear to be clusters of MS, in various countries, around lead smelters, oil refineries and environments high in air pollution.228–231 Iron overload and the up-regulation of iron-binding proteins in the brain have been implicated in the pathogenesis of MS, with MRIs revealing significant and pathological iron deposition.232,233 Similarly, significantly higher urinary concentrations of aluminium have been documented in individuals with MS. Correspondingly, urinary excretions of silicon, a natural antagonist of aluminium, are lower in MS. Aluminium may therefore be another environmental factor associated with the pathogenesis of MS.233
Mind–body medicine
Excessive stress is known to exacerbate the course of neurodegenerative diseases.234 Individuals with MS have been shown to have hypothalamic–pituitary–adrenal (HPA) axis hyperactivity, and therefore any stress-relieving activity or exercise may be of benefit.235 A 2006 Cochrane review demonstrated a range of psychological interventions that may potentially help those with MS. In particular, CBT has been shown to assist those with MS and depression.236 This has been confirmed by an Australian longitudinal assessment study of depression, anxiety and fatigue in patients with MS.237 Interest in the role of mindfulness-based interventions in MS is also growing because of their stress-relieving and potentially neuroprotective neurogenic effects.
Sleep
Sleep problems are more common in those with MS (especially women) compared with the general population.238 Identifying and managing sleep disorders is an important part of an MS work-up, as fatigue is such a prevalent symptom of MS239 and sleep has such a significant effect upon mental health. Restless legs syndrome (RLS) is also significantly associated with MS, particularly in those with severe pyramidal and sensory disabilities.240 Magnesium may assist with RLS.
Sunshine
MS has always been found to be more common in areas further away from the equator. However, over the past five decades, the latitude gradient has been decreasing.241 It has been well documented that the prevalence of MS is higher in those living at higher latitudes.242 This observation has been closely associated with the insufficient vitamin D levels that are frequently found in individuals with MS.243 Lack of sun exposure has also been associated with an increased risk of developing and relapsing from MS.244,245 It has been discovered that vitamin D deficiency is endemic throughout many countries in the world, including across a wide latitude in Australia.246 In Australia, this appears to be partly related to excessive sunlight avoidance and sunscreen use.247 The emphasis should be on responsible sun exposure, not sun avoidance.
Exercise
Current evidence demonstrates that physical activity in individuals with MS counteracts depression and fatigue, and may improve mobility and quality of life.248–251 Although it is difficult to prescribe a generalised regular exercise program, activities that encourage ‘listening to your body’ and ‘perceived control over fatigue’ are recommended.252,253 Aerobic activity has been shown to improve early anomalies of posture and gait in MS patients and moderate exercise (acute cycling) has been shown to improve anxiety and other mood disturbances. Exercise therapy has been noted to be beneficial for patients with MS not experiencing a relapse, in a 2005 Cochrane review. Studies of yoga have also shown evidence of some benefit to those with MS.
Dietary modification
Despite some clinical evidence of certain dietary regimens being beneficial for individuals with MS, a 2007 Cochrane review stated that more research is necessary.254 One such regimen involves caloric restriction (under medical supervision), as this has been shown to induce anti-inflammatory, antioxidant and neuroprotective effects.255,256
Increased dietary intake of saturated fatty acids has been associated with increased risk of developing MS.257 Research on a 34-year prospective cohort study of patients with MS published in the Lancet258 gave very promising findings but received relatively little notice in the wider medical community. Swank and Dugan found that over a 34-year follow-up, only 31% of MS patients adhering to a low saturated fat diet (less than 20 g/day) died, compared with approximately 80% of patients not sticking to the diet. Further, in the group who started with a lower level of disability, only 5% died. The rates of disease progression and disability were also vastly different in the two groups, and when those who died from non-MS-related illnesses were excluded from the analysis, 95% survived and remained physically active.
Diets rich in salmon (3–4 times weekly) may provide some protection against demyelination. This is thought to be due to the high content of both omega-3 essential fatty acids and vitamin D.259
There is current controversy regarding the possible adverse effects of artificial sweeteners such as aspartame, saccharin and acesulfame-K (ASK) as contributory factors to the development of MS.260,261
There is some evidence that individuals with MS have highly significant IgA and IgG antibodies against gliadin and gluten (wheat), as well as significant antibody increases against casein (cow’s milk dairy) compared with controls.262–264 However, recent studies have shown conflicting data, and therefore no specific dietary recommendations on these dietary factors can be made at this time.265,266
Nutritional supplementation
Vitamin D
Evidence associates low levels of vitamin D with increased risk of MS and other immune-mediated disorders.267–271 As such, it is an important modifiable environmental and nutritional factor that has a potential role in both prevention and treatment of MS.243,267,272 Studies demonstrate lower levels of serum vitamin D3 in relapsing–remitting MS compared with progressive MS and controls. Furthermore, lower levels have been significantly associated with MS-related disability in women.270,273 A meta-analysis has shown that vitamin D supplementation significantly reduces all-cause mortality, and therefore it is very important to identify and promptly treat any evidence of deficiency. Those with MS may need more aggressive treatment so that levels are at least 55–70 ng/mL.271,274 It is important to note, however, that with increasing knowledge of the significance of vitamin D to health maintenance, these reference ranges are likely to be increased in the future. Long-term supplementation with both vitamin D and cod-liver oil in humans has been associated with a reduced risk of developing MS.
Antioxidants
Antioxidants may potentially benefit those with MS, as reactive oxygen species (ROS) have been demonstrated to play major roles in several events in the pathogenesis of MS.257–277 As yet, there is limited research on the beneficial effects of antioxidants on humans with MS, although studies on animal models demonstrate beneficial effect of vitamins C and E.276
PUFAs
Omega-3 essential fatty acids play a major role in the activity of the nervous system, cognitive development, memory-related learning, neuroplasticity of neural membranes, synaptogenesis and synaptic transformation.278 The human brain has the highest lipid concentration in the body after adipose tissue, and therefore dysregulated lipid metabolism is of prime importance.279 Both omega-3 and omega-6 deficiencies as well as high levels of monosaturated and saturated fatty acids have been found in individuals with MS. Conversely, both omega-3 and omega-6 supplementation have been shown to reduce clinical signs of disease.257,280 There is accumulating evidence that both fatty acids can reduce immune-cell activation and result in marked positive clinical effects by various pathways. They may therefore be indicated for the wellbeing of all individuals with MS.259,281,282 It should be noted, however, that larger clinical trials, preferably with MRI investigations, are required.
B vitamins and iron
For myelin to be continually regenerated in the human body, adequate iron, folate and vitamin B12 to enact methylation pathways are required.283 Elevated homocysteine levels are common and are associated with cognitive impairment and depression in those with MS.284,285 In Caucasian females, both serum iron and ferritin have been found to be low compared with controls. A small study of patients with relapsing–remitting MS supplemented with myelination-activating nutrients has shown a significant neurological improvement compared with those simply taking multivitamins.283
American Academy of Neurology. http://www.aan.com.
Jelinek G. Overcoming multiple sclerosis. An evidence-based guide to recovery. Sydney: Allen & Unwin, 2010.
Neurology. http://www.neurology.org/.
Overcoming multiple sclerosis. http://www.overcomingmultiplesclerosis.org/.
Therapeutic Guidelines, Neurology. http://www.tg.org.au/index.php?sectionid=46.
1 Rowland L, editor. Merrill’s neurology, 11th edn., New York: Lippincott, Williams & Wilkins, 2005.
2 Gates P. Clinical neurology: a primer. Sydney: Elsevier; 2010. Online slideshare: http://www.slideshare.net/AnnekeElsevier/clinical-neurology-a-primer-by-peter-gates-4433610.
3 Rasmussen BK. Migraine and tension-type headache in a general population: psychosocial factors. Int J Epidemiol. 1992;21(6):1138-1143.
4 Lance JW. Headache and face pain. Med J Aust. 2000;172(9):450-455.
5 Kudrow L. Cluster headache. Clinical, mechanistic, and treatment aspects. Panminerva Med. 1982;24(1):45-54.
6 Pryse-Phillips WE, Dodick DW, Edmeads JG, et al. Guidelines for the diagnosis and management of migraine in clinical practice. Canadian Headache Society. CMAJ. 1997;156(9):1273-1287.
7 Evers S, Afra J, Frese A, et al. EFNS guideline on the drug treatment of migraine—revised report of an EFNS taskforce. European Federation of Neurological Societies. Eur J Neurol. 2009;16(9):968-981.
8 Taubert K. Magnesium in migraine. Results of a multicentre pilot study. Fortschr Med. 1994;112(24):328-330.
9 Peikert A, Wilimzig C, Kohne-Völland R. Prophylaxis of migraine with oral magnesium: results from a prospective multi-center, placebo-controlled and double-blind randomized study. Cephalalgia. 1996;16(4):257-263.
10 Schoenen J. The pathophysiology of migraine: a review based on the literature and on personal contributions. Funct Neurol. 1998;13(1):7-15.
11 Ernst E, Pittler MH. The efficacy and safety of feverfew (Tanacetum parthenium): an update of a systematic review. Public Health Nutr. 2000;3(4A):509-514.
12 Tuchin PJ, Pollard H, Bonello R. A randomized controlled trial of chiropractic spinal manipulative therapy for Migraine. J Manipulative Physiol Ther. 2000;23(2):91-95.
13 Biondi DM. Physical treatments for headache: a structured review. Headache. 2005;45(6):738-746.
14 Bronfort G, Assendelft WJ, Evans R, et al. Efficacy of spinal manipulation for chronic headache: a systematic review. J Manipulative Physiol Ther. 2001;24(7):457-466.
15 Melchart D, Thormaehlen J, Hager S, et al. Acupuncture versus placebo versus sumatriptan for early treatment of migraine attacks: a randomized controlled trial. J Intern Med. 2003;253(2):181-188.
16 Melchart D, Linde K, Fischer P, et al. Acupuncture for idiopathic headache. Cochrane Database Syst Rev. 2001;1:CD001218.
17 Endres HG, Diener H-C, Molsberger A. Role of acupuncture in the treatment of migraine. Expert Rev Neurother. 2007;7:1121-1134.
18 Gates P. Clinical neurology: a primer. Sydney: Elsevier, 2010.
19 Jenssen S, Gracely EJ, Sperling MR. How long do most seizures last? A systematic comparison of seizures recorded in the epilepsy monitoring unit. Epilepsia. 2006;47(9):1499-1503.
20 King MA, Newton MR, Jackson GD, et al. Epileptology of the first-seizure presentation: a clinical, electroencephalographic and magnetic resonance imaging study of 300 consecutive patients. Lancet. 1998;352(9133):1007-1011.
21 Sofou K, Kristjánsdóttir R, Papachatzakis NE, et al. Management of prolonged seizures and status epilepticus in childhood: a systematic review. J Child Neurol. 2009;24(8):918-926.
22 Marson AG, Al-Kharusi AM, Alwaidh M, et al. The SANAD study of effectiveness of valproate, lamotrigine, or topiramate for generalised and unclassifiable epilepsy: an unblinded randomised controlled trial. SANAD Study group. Lancet. 2007;369(9566):1016-1026.
23 Aylward RL. Epilepsy: a review of reports, guidelines, recommendations and models for the provision of care for patients with epilepsy. Clin Med. 2008;8(4):433-438.
24 Perucca P, Carter J, Vahle V, et al. Adverse antiepileptic drug effects: towards a clinically and neurobiologically relevant taxonomy. Neurology. 2009;72(14):1223-1229.
25 Schachter SC. CAM therapies. Curr Opin Neurol. 2008;21(2):184-189.
26 Ricotti V, Delanty N. Use of CAM in epilepsy. Curr Neurol Neurosci Rep. 2006;6(4):347-353.
27 Gross-Tsur V, Lahad A, Shalev RS. Use of complementary medicine in children with ADHD and epilepsy. Paediatr Neurol. 2003;29(1):53-55.
28 Gaby AR. Natural approaches to epilepsy. Altern Med Rev. 2007;12(1):9-24.
29 Arias AJ, Steinberg K, Banga A, et al. Systematic review of the efficacy of meditation techniques as treatments for mental illness. J Altern Complement Med. 2006;12(8):817-832.
30 Lansky EP, St Louis EK. Transcendental meditation: a double-edged sword in epilepsy? Epilepsy Behav. 2006;9(3):394-400.
31 Rocamora R, Sanchez-Alvarez JC, Salas-Puig J. The relationship between sleep and epilepsy. Neurologist. 2008;14(6 Suppl 1):S35-S43.
32 Mendez M, Radtke RA. Interactions between sleep and epilepsy. J Clin Neurophysiol. 2001;18(2):106-127.
33 Kotagal P, Yardi N. The relationship between sleep and epilepsy. Semin Pediatr Neurol. 2008;15(2):42-49.
34 deRoos ST, Chillag KL, Keeler M, et al. Effects of sleep deprivation on the paediatric EEG. Pediatrics. 2009;123(2):703-708.
35 Sebit MB, Mielke J. Epilepsy in sub-Saharan Africa: its socio-demography, aetilogy, diagnosis and EEG characteristics in Harare, Zimbabwe. East Afr Med J. 2005;82(3):128-137.
36 Kwan P, Yu E, Leung H, et al. Association of subjective anxiety, depression and sleep disturbance with quality-of-life ratings in adults with epilepsy. Epilepsia. 2009;15(2):196-201.
37 Weinstein RS, Bryce GF, Sappington LJ, et al. Decreased serum ionised calcium and normal vitamin D metabolite levels with anticonvulsant drug treatment. J Clin Endocrin Metab. 1984;58(6):1003-1009.
38 Williams C, Netzloff M, Folkerts L, et al. Vitamin D metabolism and anticonvulsant therapy: effect of sunshine on incidence of osteomalacia. South Med J. 1984;77(7):834-836. 842.
39 Nakken KO. Should people with epilepsy exercise? Tidsskr Nor Laegeforen. 2000;120(25):3051-3053.
40 Howard GM, Radloff M, Sevier TL. Epilepsy and sports participation. Curr Sports Med Rep. 2004;3(1):15-19.
41 Arida RM, Cavalheiro EA, da Silva AC, et al. Physical activity and epilepsy: proven and predicted benefits. Sports Med. 2008;38(7):607-615.
42 Lundgren T, Dahl J, Yardi N, et al. Acceptance and commitment therapy and yoga for drug-refractory epilepsy: a randomised controlled trial. Epilepsy Behav. 2008;13(1):102-108.
43 Sathyaprabha TN, Satishchandra P, Pradhan C, et al. Modulation of cardiac autonomic balance with adjuvant yoga therapy in patients with refractory epilepsy. Epilepsy Behav. 2008;12(2):245-252.
44 Mortelmans LJ, van Loo M, de Cauwer HG, et al. Seizures and hyponatremia after excessive intake of diet coke. Eur J Emerg Med. 2008;15(1):51.
45 Iyadurai SJ, Chung SS. New-onset seizures in adults: possible association with consumption of popular energy drinks. Epilepsy Behav. 2007;10(3):504-508.
46 Volpe SL, Schall JI, Gallagher PR, et al. Nutrient intake of children with intractable epilepsy compared with healthy children. J Am Diet Assoc. 2007;107(6):1014-1018.
47 Kossoff EH, Rho JM. Ketogenic diets: evidence for short- and long-term efficacy. Neurotherapeutics. 2009;6(2):406-414.
48 Weinshenker D. The contribution of noradrenaline and orexigenic neuropeptides to the anticonvulsant effect of the ketogenic diet. Epilepsia. 2008;49(Suppl 8):104-107.
49 Nordli DRJr. The ketogenic diet, four score and seven years later. Nat Clin Pract Neurol. 2009;5(1):12-13.
50 Porta N, Vallee L, Boutry E, et al. Comparison of seizure reduction and serum folic acid levels after receiving the ketogenic and modified Atkins diet. Seizure. 2009;18(5):359-364.
51 Kozak N, Csiba L. Dietary aspects of epilepsy. Ideggyogy Sz. 2007;60(5/6):234-238.
52 Villeneuve N, Pinton F, Bahi-Buisson N, et al. The ketogenic diet improves recently worsened focal epilepsy. Dev Med Child Neurol. 2009;51(4):276-281.
53 Hartman AL. Does the effectiveness of the ketogenic diet in different epilepsies yield insights into its mechanisms? Epilepsia. 2008;49(Suppl 8):53-56.
54 Kossoff EH, Dorward JL. The Modified Atkins Diet. Epilepsia. 2008;49(Suppl 8):37-41.
55 Muzykewicz DA, Lyczkowski DA, Memon N, et al. Efficacy, safety and tolerability of the low glycaemic index diet in paediatric epilepsy. Epilepsia. 2009;50(5):1118-1126.
56 Farman AH, Lossius MI, Nakken KO. Polyunsaturated fatty acids and epilepsy. Tidsskr Nor Laegeforen. 2009;129(1):26-28.
57 Xu XP, Erichsen D, Börjesson SI, et al. Polyunsaturated fatty acids and cerebrospinal fluid from children on the ketogenic diet open a voltage-gated potassium channel: a putative mechanism of antiseizure action. Epilepsy Res. 2008;80(1):57-66.
58 Ferrari D, Cysneiros RM, Scorza CA, et al. Neuroprotective activity of n-3 fatty acids against epilepsy-induced hippocampal damage: quantification with immunohistochemical for calcium-binding proteins. Epilepsy Behav. 2008;13(1):36-42.
59 Taha AY, Huot PS, Reza-Lopez S, et al. Seizure resistance in fat-1 transgenic mice endogenously synthesising high levels of n-3 polyunsaturated fatty acids. J Neurochem. 2008;105(2):380-388.
60 Pifferi F, Tremblay S, Plourde M, et al. Ketones and brain function: possible link to polyunsaturated fatty acids and availability of a new brain PET tracer, 11C-acetoacetate. Epilepsia. 2008;49(Suppl 8):76-79.
61 Bromfield E, Dworetsky B, Hurwitz S, et al. A randomised trial of polyunsaturated fatty acids for refractory epilepsy. Epilepsy Behav. 2008;12(1):187-190.
62 Mainardi P, Leonardi A, Albano C. Potentiation of brain serotonin activity may exhibit seizures, especially in drug-resistant epilepsy. Med Hypotheses. 2008;70(4):876-879.
63 Bagdy G, Kecskemeti V, Riba P, et al. Serotonin and epilepsy. J Neurochem. 2007;100(4):857-873.
64 Isaac M. Serotonergic 5-HT2C receptors as a potential therapeutic target for the design of antiepileptic drugs. Curr Top Med Chem. 2005;5(1):59-67.
65 El Idrissi A, L’Amoreaux WJ. Selective resistance of taurine-fed mice to isoniazide-potentiated seizures: in vivo functional test for the activity of glutamic acid decarboxylase. Neuroscience. 2008;156(3):693-699.
66 Galanopoulou AS. GABAA receptors in normal development and seizures: friends or foes? Curr Neuropharmacol. 2008;6(1):1-20.
67 Thompson K. Transplantation of GABA-producing cells for seizure control in models of temporal lobe epilepsy. Neurotherapeutics. 2009;6(2):284-294.
68 Baran H. Alterations of taurine in the brain of chronic kainic acid epilepsy model. Amino Acids. 2006;31(3):303-307.
69 Junyent F, Utrera J, Romera R, et al. Prevention of epilepsy by taurine treatments in mice experimental model. J Neurosci Res. 2009;87(6):1500-1508.
70 Trombley PQ, Horning MS, Blakemore LJ. Carnosine modulates zinc and copper effects on amino acid receptors and synaptic transmission. Neuroreport. 1998;9(15):3503-3507.
71 Trombley PQ, Horning MS, Blakemore LJ. Interactions between carnosine and zinc and copper: implications for neuromodulation and neuroprotection. Biochemistry (Mosc). 2000;65(7):807-816.
72 Horning MS, Blakemore LJ, Trombley PQ. Endogenous mechanisms of neuroprotection: role of zinc, copper and carnosine. Brain Res. 2000;852(1):56-61.
73 Kozan R, Sefil F, Bagirici F. Anticonvulsant effect of carnosine on penicillin-induced epileptiform activity in rats. Brain Res. 2008;1239:249-255.
74 Devi PU, Manocha A, Vohora D. Seizures, antiepileptics, antioxidants and ox stress: an insight for researchers. Expert Opin Pharmacother. 2008;9(18):3169-3177.
75 Hayashi M. Oxidative stress in developmental brain disorders. Neuropathology. 2009;29(1):1-8.
76 Gupta RC, Milatovic D, Dettbarn WD. Depletion of energy metabolites following acetylcholinesterase inhibitor-induced status epilepticus: protection by antioxidants. Neurotoxicogy. 2001;22(2):271-282.
77 Ranganathan LN, Ramaratnam S. Vitamins for epilepsy. Cochrane Database Syst Rev. 2005;2:CD004304.
78 Kutluhan S, Naziroglu M, Celik O, et al. Effects of selenium and topiramate on lipid peroxidation and antioxidant vitamin levels in blood of PTZ-induced epileptic rats. Biol Trace Elem Res. 2009;129:181-189.
79 Gutierrez-Alvarez AM, Moreno CB, Gonzalez-Reyes RE. Changes in selenium levels in epilepsy. Rev Neurol. 2005;40(2):111-116.
80 Moreno CB, Gutierrez-Alvarez AM, Gonzalez-Reyes RE. Zinc and epilepsy: is there a causal relation between them? Rev Neurol. 2008;42(12):754-759.
81 Dominguez MI, Blasco-Ibanez JM, Crespo C, et al. Neural overexcitation and implications of NMDA and AMPA receptors in a mouse model of temporal lobe epilepsy implying zinc chelation. Epilepsia. 2006;47(5):887-899.
82 Hamed SA, Nabeshima T. The high atherosclerotic risk among epileptics: the atheroprotective role of multivitamins. J Pharmacol Sci. 2005;98(4):340-353.
83 Gonzalez-Reyes RE, Gutierrez-Alvarez AM, Moreno CB. Manganese and epilepsy: a systematic review of the literature. Brain Res Rev. 2007;53(2):332-336.
84 Schacter SC. Botanics and herbs: a traditional approach to treating epilepsy. Neurotherapeutics. 2009;6(2):415-420.
85 Samuels N, Finkelstein Y, Singer SR, et al. Herbal medicine and epilepsy: proconvulsive effects and interactions with antiepileptic drugs. Epilepsia. 2008;49(3):373-380.
86 Huen MS, Leung JW, Ng W, et al. 5,7-dihydroxy-6-methoxyflavone, a benzodiazepine site ligand isolated from Scutellaria baicalensis Georgi, with selective antagonistic properties. Biochem Pharmacol. 2003;66(1):125-132.
87 Salah SM, Jager AK. Screening of traditionally used Lebanese herbs for neurological activities. J Ethnopharmacol. 2005;97(1):145-149.
88 Harms SL, Garrard J, Schwinghammer P, et al. Gingko biloba use in nursing home elderly with epilepsy or seizure disorder. Epilepsia. 2006;47(2):323-329.
89 Jyoti A, Sethi P, Sharma D. Curcumin protects against electrobehavioural progression of seizures in the iron-induced experimental model of epileptogenesis. Epilepsy Behav. 2009;14(2):300-308.
90 Sumanont Y, Murakami Y, Tohda M. Effects of manganese complexes of curcumin and diacetylcurcumin on kainic acid-induced neurotoxic responses in the rat hippocampus. Biol Pharm Bull. 2007;30(9):1732-1739.
91 Hung-Ming W, Liu CS, Tsai JJ, et al. Antioxidant and anti-convulsant effect of a modified formula of chaihu-longu-muli-tang. Am J Chin Med. 2002;30(2/3):339-346.
92 Minami E, Shibata H, Nomoto M, et al. Effect of shitei-to, a traditional Chinese medicine formulation, on pentylenetetrazol-induced kindling in mice. Phytomedicine. 2000;7(1):69-72.
93 Cheuk DK, Wong V. Acupuncture for epilepsy. Cochrane Database Syst Rev. 2008;4:CD005062.
94 Denays R, Kumba C, Lison D, et al. First epileptic seizure induced by occupational nickel poisoning. Epilepsia. 2005;46(6):961-962.
95 Arrieta O, Palencia G, Garcia-Arenas G, et al. Prolonged exposure to lead lowers the threshold of pentylenetetrazole-induced seizures in rats. Epilepsia. 2005;46(10):1599-1602.
96 Ruegg S, Hunziker P, Marsch S, et al. Association of environmental factors with the onset of status epilepticus. Epilepsy Behav. 2008;12(1):66-73.
97 Grogan PM, Gronseth GS. Practice parameter: steroids, acyclovir, and surgery for Bell’s palsy (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2001;56(7):830-836.
98 Salinas RA, Alvarez G, Ferreira J. Corticosteroids for Bell’s palsy (idiopathic facial paralysis). Cochrane Database Syst Rev. 2009;2:CD001942.
99 Sullivan FM, Swan IR, Donnan PT, et al. A randomised controlled trial of the use of aciclovir and/or prednisolone for the early treatment of Bell’s palsy: the BELLS study. Health Technol Assess. 2009;13(47):iii-iv. ix–xi, 1–130.
100 Lehmann HC, Hartung HP, Hetzel GR, et al. Plasma exchange in neuroimmunological disorders: part 2. Treatment of neuromuscular disorders. Arch Neurol. 2006;63(8):1066-1071.
101 Lehmann HC, Hartung HP, Hetzel GR, et al. Plasma exchange in neuroimmunological disorders: part 1. Rationale and treatment of inflammatory central nervous system disorders. Arch Neurol. 2006;63(7):930-935.
102 Elovaara I, Apostolski S, van Doorn P, et al. EFNS guidelines for the use of intravenous immunoglobulin in treatment of neurological diseases: EFNS task force on the use of intravenous immunoglobulin in treatment of neurological diseases. Eur J Neurol. 2008;15(9):893-908.
103 Hughes RA, Raphaël JC, Swan AV, et al. Intravenous immunoglobulin for Guillain-Barré syndrome. Cochrane Database Syst Rev. 2006;1:CD002063.
104 Talman P, Forbes A, Mathers S. Clinical phenotypes and natural progression for motor neuron disease: analysis from an Australian database. Amyotroph Lateral Scler. 2009;10(2):79-84.
105 Benatar M, Kaminski H. Medical and surgical treatment for ocular myasthenia. Cochrane Database Syst Rev. 2006;2:CD005081.
106 Sethi KD, Rivner MH, Swift TR. Ice pack test for myasthenia gravis. Neurology. 1987;37(8):1383-1385.
107 Ferreira VF, da Rocha DR, Lima Araújo KG. Advances in drug discovery to assess cholinergic neurotransmission: a systematic review. Curr Drug Discov Technol. 2008;5(3):236-249.
108 Sonett JR, Jaretzki AIII. Thymectomy for nonthymomatous myasthenia gravis: a critical analysis. Ann NY Acad Sci. 2008;1132:315-328.
109 Kondo KJ. Optimal therapy for thymoma. Med Invest. 2008;55(1/2):17-28.
110 Chavis PS, Stickler DE, Walker A. Immunosuppressive or surgical treatment for ocular myasthenia gravis. Arch Neurol. 2007;64(12):1792-1794.
111 Hart IK, Sathasivam S, Sharshar T. Immunosuppressive agents for myasthenia gravis. Cochrane Database Syst Rev. 2007;4:CD005224.
112 Wiendl H. Idiopathic inflammatory myopathies: current and future therapeutic options. Neurotherapeutics. 2008;5(4):548-557.
113 Marshall S, Tardif G, Ashworth N. Local corticosteroid injection for carpal tunnel syndrome. Cochrane Database Syst Rev. 2007;2:CD001554.
114 Gautschi OP, Land M, Hoederath P, et al. Carpal tunnel syndrome—modern diagnostic and management. Praxis (Bern 1994). 2010;99(3):163-173.
115 Cikes N, Bosnic D, Sentic M. Non-MS autoimmune demyelination. Clin Neurol Neurosurg. 2008;110(9):905-912.
116 Goldstein LB, Adams R, Alberts MJ, et alAmerican Heart Association, American Stroke Association Stroke Council. Primary prevention of ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council: cosponsored by the Atherosclerotic Peripheral Vascular Disease Interdisciplinary Working Group; Cardiovascular Nursing Council; Clinical Cardiology Council; Nutrition, Physical Activity, and Metabolism Council; and the Quality of Care and Outcomes Research Interdisciplinary Working Group. Circulation. 2006;113(24):e873-e923.
117 Jackson G. Secondary prevention of stroke: PROGRESS and the evidence for blood pressure reduction. Int J Clin Pract. 2001;55(10):655.
118 Gage BF, Fihn SD, White RH. Warfarin therapy for an octogenarian who has atrial fibrillation. Ann Intern Med. 2001;134(6):465-474.
119 Go AS, Hylek EM, Chang Y, et al. Anticoagulation therapy for stroke prevention in atrial fibrillation: how well do randomized trials translate into clinical practice? JAMA. 2003;290(20):2685-2692.
120 MacDougall NJ, Amarasinghe S, Muir KW. Secondary prevention of stroke. Expert Rev Cardiovasc Ther. 2009;7(9):1103-1115.
121 Chandratheva A, Mehta Z, Geraghty OC, et alOxford Vascular Study. Population-based study of risk and predictors of stroke in the first few hours after a TIA. Neurology. 2009;72(22):1941-1947.
122 Kucinski T, Väterlein O, Glauche V, et al. Correlation of apparent diffusion coefficient and computed tomography density in acute ischemic stroke. Stroke. 2002;33(7):1786-1791.
123 Vivanco Hidalgo RM, Rodríguez Campello A, Ois Santiago A, et al. Cardiac monitoring in stroke units: importance of diagnosing atrial fibrillation in acute ischemic stroke. Rev Esp Cardiol. 2009;62(5):564-567. [Article in English, Spanish]
124 Ansara AJ, Nisly SA, Arif SA, et al. Aspirin dosing for the prevention and treatment of ischemic stroke: an indication-specific review of the literature. Ann Pharmacother. 2010;44(5):851-862.
125 Hacke W, Kaste M, Bluhmki E, et alECASS Investigators. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359(13):1317-1329.
126 Diener HC. Secondary prevention of ischaemic stroke and TIA. Cardiovasc J Afr. 2008;19(4):229.
127 Simmons BB, Yeo A, Fung K, American Heart Association, American Stroke Association. Current guidelines on antiplatelet agents for secondary prevention of noncardiogenic stroke: an evidence-based review. Postgrad Med. 2010;122(2):49-53.
128 Odén A, Fahlén M, Hart RG. Optimal INR for prevention of stroke and death in atrial fibrillation: a critical appraisal. Throm Res. 2006;117(5):493-499.
129 Saour N, Sieck JO, Mamo LA, et al. Trial of different intensities of anticoagulation in patients with prosthetic heart valves. N Engl J Med. 1990;322(7):428-432.
130 Connolly SJ, Ezekowitz MD, Yusuf S, et alRE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361(12):1139-1151.
131 Bakoyiannis C, Economopoulos KP, Georgopoulos S, et al. Carotid endarterectomy versus carotid angioplasty with or without stenting for treatment of carotid artery stenosis: an updated meta-analysis of randomized controlled trials. E Int Angiol. 2010;29(3):205-215.
132 Donnan GA, O’Malley HM, Quang L, et al. The capsular warning syndrome: pathogenesis and clinical. Neurology. 1993;43(5):957-962.
133 Meier P, Knapp G, Tamhane U, et al. Short-term and intermediate-term comparison of endarterectomy versus stenting for carotid artery stenosis: systematic review and meta-analysis of randomised controlled clinical trials. BMJ. 2010;340:c467.
134 Zesiewicz TA, Elble R, Louis ED, et al. Practice parameter: therapies for essential tremor: report of the Quality Standards Subcommittee of the American. Academy of Neurology. 2005;64(12):2008-2020.
135 Slavin MJ, Phillips JG, Bradshaw JL, et al. Consistency of handwriting movements in dementia of the Alzheimer’s type: a comparison with Huntington’s and Parkinson’s diseases. J Int Neuropsychol Soc. 1999;5(1):20-25.
136 Margolese HC, Chouinard G, Kolivakis TT, et al. Tardive dyskinesia in the era of typical and atypical antipsychotics. Part 2: Incidence and management strategies in patients with schizophrenia. Can J Psychiatry. 2005;50(11):703-714.
137 Sharma N, Standaert DG. Inherited movement disorders. Neurol Clin. 2002;20(3):759-778. vii.
138 Gordon N. Sydenham’s chorea, and its complications affecting the nervous system. Brain Dev. 2009;31(1):11-14.
139 Miyasaki JM, Martin W, Suchowersky O, et al. Practice parameter: initiation of treatment for Parkinson’s disease: an evidence-based review: report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2002;58(1):11-17.
140 Alvarez L, Macias R, Pavón N, et al. Therapeutic efficacy of unilateral subthalamotomy in Parkinson’s disease: results in 89 patients followed for up to 36 months. Neurol Neurosurg Psychiatry. 2009;80(9):979-985.
141 Deuschl G, Schade-Brittinger C, Krack P, et al. A randomized trial of deep-brain stimulation for Parkinson’s disease. German Parkinson Study Group, Neurostimulation Section. N Engl J Med. 2006;355(9):896-908.
142 Guzeva VI, Chukhlovina ML, Chuklovin BA. Environmental factors and Parkinsonian syndrome. Gig Sanit. 2008;2:60-62.
143 Luccini R, Albini E, Benedetti L, et al. Neurological and neuropsychological features in Parkinson’s disease patients exposed to neurotoxic metals. G Ital Med Lav Ergon. 2007;29(3 Suppl):280-281.
144 Zoni S, Albini E, Luccini R. Neuropsychological testing for the assessment of manganese neurotoxicity: a review and a proposal. Am J Ind Med. 2007;50(11):812-830.
145 Yokel RA. Blood–brain barrier flux of aluminium, manganese, iron and other metals suspected to contribute to metal-induced neurodegeneration. J Alzheimers Dis. 2006;10(2/3):223-253.
146 Gorell JM, Johnson CC, Rybicki BA, et al. Occupational exposure to manganese, copper, lead, iron, mercury and zinc and the risk of Parkinson’s disease. Neurotoxicology. 1999;20(2/3):239-247.
147 Coon S, Stark A, Peterson E, et al. Whole-body lifetime occupational lead exposure and risk of Parkinson’s disease. Environ Health Perpect. 2006;114(12):1872-1876.
148 Barnham KJ, Bush AI. Metals in Alzheimer’s and Parkinson’s disease. Curr Opin Chem Biol. 2008;12(2):222-228.
149 Squitti R, Gorgone G, Binetti G, et al. Metals and oxidative stress in Parkinson’s disease from industrial areas with exposition to environmental toxins or metal pollution. G Ital Med Lav Ergon. 2007;29(3 Suppl):294-296.
150 Dhillon AS, Tarbutton GL, Levin JL, et al. Pesticide/environmental exposures and Parkinson’s disease in East Texas. J Agromedicine. 2008;13(1):37-48.
151 Hancock DB, Martin ER, Mayhew GM, et al. Pesticide exposure and risk of Parkinson’s disease: a family-based case-control study. BMC Neurol. 2008;8:6.
152 Brown TP, Rumsby PC, Capleton AC, et al. Pesticides and Parkinson’s disease—is there a link? Environ Health Perspect. 2006;114(2):156-164.
153 Li AA, Mink PJ, McIntosh LJ, et al. Evaluation of epidemiologic and animal data associating pesticides with Parkinson’s disease. J Occup Environ Med. 2005;47(10):1059-1087.
154 Bjorling-Poulson M, Anderson HR, Grandjean P. Potential developmental neurotoxicity of pesticides in Europe. Environ Health. 2008;7:50.
155 Keifer MC, Firestone J. Neurotoxicity of pesticides. 2007;12(1):17-25.
156 Dick FD, De Palma G, Ahmadi A, et al. Environmental risk factors for Parkinson’s disease and parkinsonism: the Geoparkinson study. Occup Environ Med. 2007;64(10):666-672.
157 Costello S, Cockburn M, Bronstein J, et al. Parkinson’s disease and residential exposure to maneb and paraquat from agricultural applications in the central valley of Caifornia. Am J Epidemiol. 2009;169(8):919-926.
158 Barlow BK, Cory-Slechta DA, Richfield EK, et al. The gestational environment and Parkinson’s disease: evidence for neurodevelopmental origins of a neurodegenerative disorder. Reprod Toxicol. 2007;23(3):457-470.
159 Costa LG, Giordano G, Guizzetti M, et al. Neurotoxicity of pesticides: a review. Front Biosci. 2008;13:1240-1249.
160 Dixon L, Duncan D, Johnson P, et al. Occupational therapy for patients with Parkinson’s disease. Cochrane Database Syst Rev. 2007;3:CD002813.
161 Deane KH, Jones D, Playford ED, et al. Physiotherapy for patients with Parkinson’s disease: a comparison of techniques. Cochrane Database Syst Rev. 2001;3:CD002817.
162 Deane KH, Whurr R, Playford ED, et al. A comparison of speech and language therapy techniques for dysarthria in Parkinson’s disease. Cochrane Database Syst Rev. 2001;2:CD002814.
163 Goodwin VA, Richards SH, Taylor RS, et al. The effectiveness of exercise interventions for people with Parkinson’s disease: systematic review and meta-analysis. Mov Disord. 2008;23(5):631-640.
164 Keus SH, Bloem BR, Hendriks EJ, et al. Evidence-based analysis of physical therapy in Parkinson’s disease with recommendations for practice and research. Mov Disord. 2007;22(4):451-460.
165 Crizzle AM, Newhouse IJ. Is physical exercise beneficial for persons with Parkinson’s disease? Clin J Sport Med. 2006;16(5):422-425.
166 Kwakkel G, de Goede CJ, van Wegen EE. Impact of physical therapy for Parkinson’s disease: a critical review of the literature. Parkinsonism Relat Disord. 2007;13(Suppl 3):S478-S487.
167 Hackney ME, Kantorovich S, Levin R, et al. Effects of tango on functional mobility in Parkinson’s disease: a preliminary study. J Neurol Phys Ther. 2007;31(4):173-179.
168 Burini D, Farabollini B, Iacucci S, et al. A randomised controlled cross-over trial of aerobic training versus Qigong in advanced Parkinson’s disease. Eura Medicophys. 2006;42(3):231-238.
169 Lee MS, Shin BC, Kong JC, et al. Effectiveness of acupuncture for Parkinson’s disease: a systematic review. Mov Disord. 2008;23(11):1505-1515.
170 Cheng XR, Cheng K. Survey of studies on the mechanism of acupuncture and moxibustion treating diseases abroad. Zhongguo Zhen Jiu. 2008;28(6):463-467.
171 Cristan A, Katz M, Cutrone E, et al. Evaluation of acupuncture in the treatment of Parkinson’s disease: a double-blind pilot study. Mov Disord. 2005;20(9):1185-1188.
172 Wang X, Liang XB, Li FQ, et al. Therapeutic strategies for Parkinson’s disease: the ancient meets the future—traditional Chinese medicine, electroacupuncture, gene therapy and stem cells. Neurochem Res. 2008;33(10):1956-1963.
173 Huang Y, Jiang XM, Li DJ. Effects of ESA on cerebral dopamine transporter in patients with Parkinson’s disease. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2006;26(4):303-307.
174 Chang XH, Zhang LZ, Li YJ. Observation on therapeutic effect of acupuncture combined with medicine on Parkinson’s disease. Zhongguo Zhen Jiu. 2008;28(9):645-647.
175 Ren XM. Fifty cases of Parkinson’s disease treated by acupuncture combined with Madopar. J Trad Chin Med. 2008;28(4):255-277.
176 Manyam BV, Sanchez-Ramos JR. Traditional and complementary therapies in Parkinson’s disease. Adv Neurol. 1999;80:565-574.
177 Nader T, Rothenberg S, Averbach R, et al. Improvements in chronic diseases with a comprehensive natural medicine approach: a review and case series. Behav Med. 2000;26(1):14-46.
178 Manyam BV, Dhanasekaran M, Hare TA. Effect of antiparkinson drug HP-200 (Mucuna pruriens) on the central monoaminergic neurotransmitters. Phytother Res. 2004;18(2):97-101.
179 Manyam BV, Dhanasekaran M, Hare TA. Neuroprotective effects of the anti-parkinson drug Mucuna puriens. Phytother Res. 2004;18(9):706-712.
180 Dhanasekaran M, Tharakan B, Manyam BV. Antiparkinson drug Mucuna puriens shows antioxidant and metal chelating activity. Phytother Res. 2008;22(1):6-11.
181 Li Q, Zhao D, Bezard E. TCM for Parkinson’s disease: a review of Chinese literature. Behav Pharmacol. 2006;17(5–6):403-410.
182 Takeda A. Essential trace metals and brain function. Yakugaku Zasshi. 2004;124(9):577-585.
183 Qureshi GA, Qureshi AA, Memon SA, et al. Impact of selenium, copper and zinc in on/off Parkinson’s disease patients on L-dopa therapy. J Neural Transm Suppl. 2006;71:229-236.
184 Hargreaves IP, Lane A, Sleiman PM. The coenzyme Q10 status of the brain regions of Parkinson’s disease patients. Neurosci Lett. 2008;447(1):17-19.
185 Henchcliffe C, Beal MF. Mitochondrial biology and ox stress in Parkinson’s disease pathogenesis. Nat Clin Pract Neurol. 2008;4(11):600-609.
186 Etminan M, Gill SS, Samii A. Intake of vitamin E, C and carotenoids and the risk of Parkinson’s disease: a meta-analysis. Lancet Neurol. 2005;4(6):362-365.
187 de Lau LM, Koudstaal PJ, Witteman JC, et al. Dietary folate, vitamin B12 and vitamin B6 and the risk of Parkinson’s disease. Neurology. 2006;67(2):315-318.
188 Triantafyllou NI, Nikolau C, Boufidou F, et al. Folate and vitamin B12 levels in L-dopa treated Parkinson’s disease patients: their relationship to clinical manifestations, mood and cognition. Parkinsonism Relat Disord. 2008;14(4):321-325.
189 Lamberti P, Zoccolella S, Armenise E, et al. Hyperhomocysteinaemia in L-dopa treated Parkinson’s disease patients: effect of cobalamin and folate administration. Eur J Neurol. 2005;12(5):365-368.
190 Evatt ML, Delong MR, Khazai N, et al. Prevalence of vitamin D deficiency in patients with Parkinson’s disease and Alzheimers. Arch Neurol. 2008;65(10):1348-1352.
191 Sanchez B, Relova JL, Gallego R, et al. 1,25-dihydroxyvitamin D3 administration to 6-hydroxydopamine-lesioned rats increases glial cell line-derived neurotrophic factor and partially restores tyrosine hydroxylase expression in substantia nigra and striatum. J Neurosci Res. 2009;87(3):723-732.
192 Valastro B, Dekundy A, Danysz W, et al. Oral creatine supplementation attenuates L-dopa induced dyskinesia in 6-hydroxydopamine lesioned rats. Behav Brain Res. 2009;197(1):90-96.
193 Forsleff L, Schauss AG, Bier ID, et al. Evidence of functional zinc deficiency in Parkinson’s disease. J Altern Comp Med. 1999;5(1):57-64.
194 Desai V, Kaler SG. Role of copper in human neurological disorders. Am J Clin Nutr. 2008;88(3):855S-858S.
195 Oyanagi K, Kawakami E, Kikuchi-Horie K, et al. Magnesium deficiency over generations in rats with special references to the pathogenesis of the P-dementia complex and amyotrophic lateral sclerosis of Guam. Neuropathology. 2006;26(2):115-128.
196 Hashimoto T, Nishi K, Nagasao J, et al. Magnesium exerts both preventative and ameliorating effects in an in vivo rat Parkinson’s disease model involving 1-methyl-4-phenylpyridium (MPP+) toxicity in dopaminergic neurons. Brain Res. 2008;1197:143-151.
197 Gao X, Chen H, Fung TT, et al. Prospective study of dietary pattern and risk of Parkinson’s disease. Am J Clin Nutr. 2007;86(5):1486-1494.
198 de Luis Roman D, Aller R, Castano O. Vegetarian diets: effects on health. Rev Clin Esp. 2007;207(3):141-143.
199 Lau FC, Shukitt-Hale B, Joseph JA. Nutritional intervention in brain aging: reducing the effects of inflammation and oxidative stress. Subcell Biochem. 2007;42:299-318.
200 Barichella M, Savardi C, Mauri A, et al. Diet with LPP for renal patients increases daily energy expenditure and improves motor function in parkinsonian patients with motor fluctuations. Nutr Neurosci. 2007;10(3/4):129-135.
201 Gasior M, Rogawski MA, Hartman AL. Neuroprotective and disease-modifing effects of the ketogenic diet. Behav Pharmacol. 2006;17(5–6):431-439.
202 Ma L, Zhang L, Gao XH, et al. Dietary factors and smoking as risk factors for Parkinson’s disease in a rural population in China: a nested case-control study. Acta Neurol Scand. 2006;113(4):278-281.
203 Chen H, O’Reilly E, McCullough ML, et al. Consumption of dairy products and risk of Parkinson’s disease. Am J Epidemiol. 2007;165(9):998-1006.
204 Tan LC, Koh WP, Yuan JM, et al. Differential effects of black vs green tea on risk of Parkinson’s disease in the Singapore Chinese Health Study. Am J Epidemiol. 2008;167(5):553-560.
205 Petersen MS, Halling J, Bech S, et al. Impact of dietary exposure to food contaminants on the risk of Parkinson’s disease. NeuroToxicology. 2008;29(4):584-590.
206 Dobkin RD, Menza M, Bienfait KL. CBT for the treatment of depression in Parkinson’s disease: a promising nonpharmacological approach. Expert Rev Neurother. 2008;8(1):27-35.
207 Cole K, Vaughan FL. The feasibility of using CBT for depression associated with Parkinson’s disease: a literature review. Parkinsonism Relat Disord. 2005;11(5):269-276.
208 Ernst E, Canter PH. The Alexander technique: a systematic review of controlled clinical trials. Forsch Komplementarmed Klass Nuturheilkd. 2003;10(6):325-329.
209 Paccetti C, Mancini F, Agliera R, et al. Active music therapy in Parkinson’s disease: an integrative method for motor and emotional rehabilitation. Psychosom Med. 2000;62(3):386-393.
210 Paccetti C, Aglieri R, Mancini F, et al. Active music therapy and Parkinson’s disease: methods. Funct Neurol. 1998;13(1):57-67.
211 Marrie RA. When one and one make three: HLA and EBV infection in MS. Neurology. 2008;70(13 part 2):1067-1068.
212 Levin LI, Munger KL, Rubertone MV, et al. Temporal relationship between elevation of EBV antibody titres and initial onset of neurological symptoms in MS. JAMA. 2005;293(20):2496-2500.
213 Frykholm B. On the question of infectious aetiologies for MS, schizophrenia and chronic fatigue syndrome and their treatment with antibiotics. Med Hypotheses. 2009;72(6):736-739.
214 Ascherio A, Munger K. Epidemiology of MS: from risk factors to prevention. Semin Neurol. 2008;28(1):17-28.
215 Fujihara K. Update on the aetiology and pathogenesis of MS and neuromyelitis optica. Nippon Rinsho. 2008;66(6):1087-1091.
216 Holmey T, Hestvik AL. MS: immunopathogenesis and controversies in defining the cause. Curr Opin Infect Dis. 2008;21(3):271-278.
217 Pugliatti M, Harbo HF, Holmey T, et al. Environmental risk factors in MS. Acta Neurol Scand Suppl. 2008;188:34-40.
218 Giovannoni G, Ebers G. MS: the environment and causation. Curr Opin Neurol. 2007;20(3):261-268.
219 Polman CH, Reingold SC, Edan G, et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the ‘McDonald Criteria’. Ann Neurol. 2005;58(6):840-846.
220 Chiappa KH. Use of evoked potentials for diagnosis of multiple sclerosis. Neurol Clin. 1988;6(4):861-880.
221 Zipoli V, Hakiki B, Portaccio E, et al. The contribution of cerebrospinal fluid oligoclonal bands to the early diagnosis of multiple sclerosis. Mult Scler. 2009;15(4):472-478.
222 Tintoré M, Sastre-Garriga JJ. New treatment measurements for treatment effects on relapses and progression. Neurol Sci. 2008;274(1/2):80-83.
223 Goodin DS, Frohman EM, Garmany GPJr, et al. Disease-modifying therapies in multiple sclerosis: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology and the MS Council for Clinical Practice Guidelines. Neurology. 2002;58(2):169-178.
224 Gold R, Jawad A, Miller DH, et al. Expert opinion: guidelines for the use of natalizumab in multiple sclerosis patients previously treated with immunomodulating therapies. J Neuroimmunol. 2007;187(1/2):156-158.
225 Klawiter EC, Cross AH, Naismith RT. The present efficacy of multiple sclerosis therapeutics: is the new 66% just the old 33%? Neurology. 2009;73(12):984-990.
226 Di Pauli F, Reindl M, Ehling R, et al. Smoking is a risk factor for early conversion to clinically definite MS. Mult Scler. 2008;14(8):1026-1030.
227 Pittas F, Ponsonby AL, van der Mei IA, et al. Smoking is associated with progressive disease course and increased progression in clinical disability in a prospective cohort of people with MS. J Neurol. 2009;256(4):577-585.
228 Williamson DM. Studies of multiple sclerosis in communities concerned about environmental exposures. J Womens Health. 2006;15(7):810-814.
229 Henry JP, Williamson DM, Schiffer DM, et al. Investigation of a cluster of multiple sclerosis in two elementary school cohorts. J Environ Health. 2007;69(10):34-38.
230 Neuberger JS, Lynch SG, Sutton ML, et al. Prevalence of multiple sclerosis in a residential area bordering an oil refinery. Neurology. 2004;63(10):1796-1802.
231 Turabelidze G, Schootman M, Zhu BP, et al. MS: prevalence and possible lead exposure. J Neurol Sci. 2008;269(1/2):158-162.
232 Abo-Krysha N, Rashed L. The role of iron dysregulation in the pathogenesis of multiple sclerosis: an Egyptian study. Mult Scler. 2008;14(5):602-608.
233 Exley C, Mamutse G, Korchazhkina O, et al. Elevated urinary excretion of aluminium and iron in multiple sclerosis. Mult Scler. 2006;12(5):533-540.
234 Esch T, Stafano GB, Fricchione GL, et al. The role of stress in neurodegenerative diseases and mental disorders. Neuro Endocrinol Lett. 2002;23(3):199-208.
235 Ysrraelit MC, Gaitan MI, Lopez AS, et al. Impaired hypothalamic-pituitary-adrenal axis activity in patients with multiple sclerosis. Neurology. 2008;71(24):1948-1954.
236 Thomas PW, Thomas S, Hillier C, et al. Psychological interventions for multiple sclerosis. Cochrane Database Syst Rev. 2006;1:CD004431.
237 Brown RF, Valpiani EM, Tennant CC, et al. Longitudinal assessment study of anxiety, depression and fatigue with multiple sclerosis. Psycholog Psychother. 2009;82(Pt 1):41-56.
238 Bamer AM, Johnson KL, Amtmann D, et al. Prevalence of sleep problems in individuals with multiple sclerosis. Mult Scler. 2008;14(8):1127-1130.
239 Merlino G, Frattici L, Lenchig C, et al. Prevalence of poor sleep among patients with multiple sclerosis: an independent predictor of mental and physical status. Sleep Med. 2009;10(1):26-34.
240 Moreira NC, Damasceno RS, Medieras CA, et al. Restless leg syndrome, sleep quality and fatigue in multiple sclerosis. Braz J Med Biol Res. 2008;41(10):932-937.
241 Alonso A, Hernan MA. Temporal trends in the incidence of multiple sclerosis: a systematic review. Neurology. 2008;71(2):129-135.
242 Ascherio A, Munger KL. Environmental risk factors for multiple sclerosis. Part II: Non-infectious factors. Ann Neurol. 2007;6196:504-513.
243 Niino M, Fukazawa T, Kikuchi S, et al. Therapeutic potential of vitamin D for multiple sclerosis. Curr Med Chem. 2008;15(5):499-505.
244 Tremiett H, van der Mei IA, Pittas F, et al. Monthly ambient sunlight, infections and relapse rates in multiple sclerosis. Neuroepidemiology. 2008;31(4):271-279.
245 Dwyer T, van der Mei I, Ponsonby AL, et al. MC1R genotype, past environmental sun exposure and risk of multiple sclerosis. Neurology. 2008;71(8):583-589.
246 Huotari A, Herzig KH. Vitamin D and living in the northern latitudes—an endemic risk area for vitamin D deficiency. Int J Circumpolar Health. 2008;67(2/3):164-178.
247 van der Mei IA, Ponsonby AL, Engelson O, et al. The high prevalence of vitamin D insufficiency across Australian populations is only partly explained by season and latitude. Environ Health Perspect. 2007;115(8):1132-1139.
248 Motl RW, Snook EM, Wynn DR, et al. Physical activity correlates with neurological impairment and disability in multiple sclerosis. J Nerv Ment Dis. 2008;196(6):492-495.
249 Waschbisch A, Tallner A, Pfeifer K, et al. Multiple sclerosis and exercise: effects of physical activity on the immune system. Der Nervenartz. 2009;80(6):688-692.
250 Fragaso YD, Santana DL, Pinto RC. The positive effects of a physical activity program for multiple sclerosis patients with fatigue. NeuroRehabilitation. 2008;23(2):153-157.
251 Motl RW, McAuley E, Snook EM, et al. Physical activity and quality of life in multiple sclerosis: intermediary roles of disability, fatigue, mood, pain, self-efficacy and social support. Psychol Health Med. 2009;14(1):111-124.
252 Smith C, Hale L, Olson K, et al. How does exercise influence fatigue in people with multiple sclerosis? Disabil Rehabil. 2009;31(9):685-692.
253 Alsano M, Dawes DJ, Arafah A, et al. What does a structured review of the effectiveness of exercise interventions for persons with multiple sclerosis tell us about the challenges of designing trials? Mult Scler. 2009;15(4):412-421.
254 Farinotti M, Simi S, Di Pietrantonj C, et al. Dietary interventions for multiple sclerosis. Cochrane Database Syst Rev. 2007;1:CD004192.
255 Piccio L, Stark JL, Cross AH. Chronic caloric restriction attenuates experimental autoimmune encephalomyelitis. J Leukoc Biol. 2008;84(4):940-948.
256 Fernandes G. Progress in nutritional immunology. Immunol Res. 2008;40(3):244-261.
257 van Meeteren ME, Teunissen CE, Dijkstra CD, et al. Antioxidants and polyunsaturated fatty acids in multiple sclerosis. Eur J Clin Nutr. 2005;59(12):1347-1361.
258 Swank RL, Dugan BB. Effect of low saturated fat diet in early and late cases of multiple sclerosis. Lancet. 1990;336(8706):37-39.
259 Liuzzi GM, Latronico T, Rossano R, et al. Inhibitory effect of polyunsaturated fatty acids on MMP-9 from microglial cells: implications for complementary multiple sclerosis treatment. Neurochem Res. 2007;32(12):2184-2193.
260 Whitehouse CR, Boullata J, McCauley LA. The potential toxicity of artificial sweeteners. AAOHN J; 56(6):251–259.
261 Bandyopadhyay A, Ghoshal S, Mukherjee A. Genotoxicity testing of low-calorie sweeteners: aspartame, ASK and saccharin. Drug Chem Toxicol. 2008;31(4):447-457.
262 Frisullo G, Nociti V, Iorio R, et al. Increased expression of T-bet in circulating B cells from a patient with multiple sclerosis and celiac disease. Hum Immunol. 2008;69(12):837-839.
263 Reichelt KL, Jensen D. IgA antibodies against gliadin and gluten in multiple sclerosis. Acta Neurol Scand. 2004;110(4):239-241.
264 Pengiran Tengah CD, Lock RJ, Unsworth DJ, et al. Multiple sclerosis and occult gluten sensitivity. Neurology. 2004;62(12):2326-2327.
265 Borhani Haghighi A, Ansari N, Mokhtari M, et al. Multiple sclerosis and gluten sensitivity. Clin Neurol Neurosurg. 2007;109(8):651-653.
266 Nicoletti A, Patti F, Lo Fermo S, et al. Frequency of coeliac disease is not increased among multiple sclerosis patients. Mult Scler. 2008;14(5):698-700.
267 Raghuwanshi A, Joshi SS, Christakos S. Vitamin D and multiple sclerosis. J Cell Biochem. 2008;105(2):338-343.
268 Szodoray P, Nakken B, Gaal J, et al. The complex role of vitamin D in autoimmune diseases. Scand J Immunol. 2008;68(3):261-269.
269 Smolders J, Damoiseaux J, Menheere P, et al. Vitamin D as an immune modulator in multiple sclerosis: a review. Neuroimmunol. 2008;194(1/2):7-17.
270 Correale J, Ysrraelit MC, Gaitan MI. Immunomodulatory effects of vitamin D in multiple sclerosis. Brain. 2009;132(Part 5):1146-1160.
271 van der Mei IA, Ponsonby AL, Dwyer T, et al. Vitamin D levels in people with multiple sclerosis and community controls in Tasmania, Australia. J Neurol. 2007;254(5):581-590.
272 Cantorna MT. Vitamin D and multiple sclerosis: an update. Nutr Rev. 2008;66(10 Suppl 2):S135-S138.
273 Kragt J, van Amerongen B, Killestein J, et al. Higher levels of 25(OH)D are associated with a lower incidence of multiple sclerosis only in women. Mult Scler. 2009;15(1):9-15.
274 Cannell JJ, Hollis BW. Use of vitamin D in clinical practice. Altern Med Rev. 2008;13(1):6-20.
275 Carlson NG, Rose JW. Antioxidants in multiple sclerosis: do they have a role in therapy? CNS Drugs. 2006;20(6):433-441.
276 Mirshafiey A, Mohsenzadegan M. Antioxidant therapy in multiple sclerosis. Immunopharmacol Immunotoxicol. 2009;31(1):13-29.
277 Schreibeit G, van Horssen J, van Rossum S, et al. Therapeutic potential and biological role of endogenous antioxidant enzymes in multiple sclerosis pathology. Brain Res Rev. 2007;56(2):322-330.
278 Mazza M, Pomponi M, Janiri L, et al. Omega-3 fatty acids and antioxidants in neurological and psychiatric diseases: an overview. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(1):12-26.
279 Adibhatia RM, Hatcher JF. Altered lipid metabolism in brain injury and disorders. Subcell Biocem. 2008;49:241-268.
280 Aupperle RP, Denney DR, Lynch SG, et al. Omega-3 fatty acids and multiple sclerosis: relationship to depression. J Behav Med. 2008;31(2):127-135.
281 Mehta LR, Dworkin RH, Schwid SR. Polyunsaturated fatty acids and their potential therapeutic role in multiple sclerosis. Nat Clin Pract Neurol. 2009;5(2):82-92.
282 Harbige LS, Sharief MK. Polyunsaturated fatty acids in the pathogenesis and treatment of multiple sclerosis. Br J Nutr. 2007;98(Suppl 1):S46-S53.
283 van Rensburg SJ, Kotze MJ, Hon D, et al. Iron and the folate/vitamin B12 methylation pathway in multiple sclerosis. Metab Brain Dis. 2006;21(2/3):121-137.
284 Russo C, Morabito F, Luise F, et al. Hyperhomocysteinaemia is associated with cognitive impairment in multiple sclerosis. J Neurol. 2008;255(1):64-69.
285 Triantafyllou N, Evangelopoulos ME, Kimiskidis VK, et al. Increased plasma homocysteine levels in patients with multiple sclerosis and depression. Ann Gen Psychiatry. 2008;7:17.