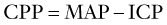10 Neurologic Emergencies
Status Epilepticus
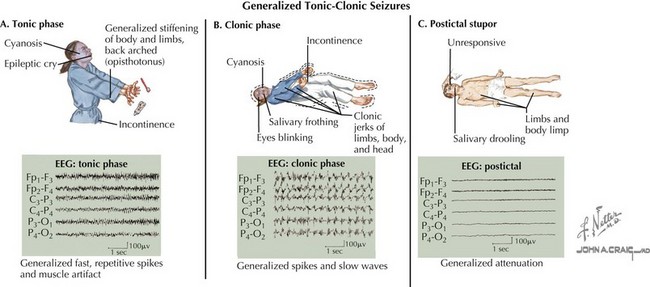
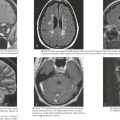
A seizure is defined as a transient, involuntary alteration of consciousness, behavior, motor activity, sensation, or autonomic function as a result of hypersynchrony and increased rate of cerebral neural discharges (Figure 10-1). Between 3% and 6% of children have at least one seizure in the first 16 years of life. Many seizures are associated with fever. Seizures can occur in individuals with underlying tendencies to seize (i.e., epilepsy) or secondary to other processes that primarily or secondarily affect the central nervous system. Seizures are discussed in detail in Chapter 74.
Etiology and Pathogenesis
Common to the pathophysiology of all seizures is the hypersynchrony of neuronal discharges. The inciting cause varies and may include metabolic, anatomic, infectious, and primary neurologic processes (see Chapter 74). Uncovering the cause of SE is essential because it guides subsequent evaluation and management of a patient in SE.
Clinical Presentation
History
A brief, focused history should be obtained in the initial evaluation of a seizing patient with the goal of uncovering the precipitating event(s). Important historical features to obtain include recent head trauma, illnesses, fever, exposure to toxins, and current medications. It is important to know if the patient has epilepsy or has seized before, and if so, what antiseizure medications are prescribed, adherence to this regimen, and recent changes to the treatment regimen. Finally, it is important to know what medications, if any, have been given to stop the current seizure. It is important to know whether the seizure is associated with fever because febrile seizures are unique to children 6 months to 6 years of age and may be evaluated and treated differently than seizures not associated with fever (see Chapter 74).
Physical Examination
Other than seizure type, the physical examination in a child in SE should focus on eliciting the cause of the seizure. Fever may be a sign of infection. Meningismus and a toxic appearance can be suggestive of meningitis. A toxidrome may lead the clinician to look for potential toxic ingestions (see Chapter 9). Significant hypertension implies hypertensive encephalopathy. Although a complete neurologic examination is difficult in a seizing patient, focal neurologic signs can suggest intracranial or spinal lesions. The entire body should be examined for signs of trauma. Dysmorphic features may be associated with nervous system abnormalities.
Differential Diagnosis
There are other paroxysmal events that can mimic seizures in children that must be considered in the differential diagnosis. Breath-holding spells occur in children 6 months to 4 years of age and consist of a period of crying resulting from an inciting event, such as trauma, followed by breath holding and ensuing pallor or cyanosis. The child becomes rigid and may have twitching movements but quickly returns to a full level of alertness. Syncope, a brief loss of consciousness and muscle tone, can be differentiated from seizure by history and physical examination (see Chapter 48). Sleep disorders, such as night terrors, benign myoclonus, and sleep paralysis, can mimic seizures.
Evaluation and Management
Benzodiazepines are the first-line anticonvulsant medications for treating children with SE (Table 10-1). IV lorazepam is usually preferred, but if IV access is not available, midazolam can be given via the buccal or intramuscular route or diazepam can be administered rectally. Although these agents have similarly rapid onsets of action, lorazepam lasts much longer than other benzodiazepines (≤12-24 hours). As a result, one must be mindful to administer another agent for long-term seizure control when using other benzodiazepines as a first-line agent. If the patient does not have seizure remittance after benzodiazepine administration, phenytoin (or fosphenytoin) is widely considered the next anticonvulsant agents to use. Although phenobarbital has been used as a second-line agent in SE, phenytoin is preferred because by acting on voltage-gated sodium channels, its mechanism of action is different than lorazepam. Lorazepam and phenobarbital, on the other hand, are both GABA (γ-aminobutyric acid) receptor agonists. Phenobarbital and some newer anticonvulsant medications, such as levetiracetam, are considered third-line agents for SE. Consultation with a pediatric neurologist and/or pediatric intensivist are warranted when treatment beyond benzodiazepines is used.
Table 10-1 Suggested Treatment Algorithm for Status Epilepticus
| Immediately |
IM, Intramuscular; IV, intravenous; PICU, pediatric intensive care unit; PR, per rectum.
Altered Level of Consciousness
Consciousness is the state of being awake and aware of one’s self and surroundings. Alteration of this state may signify severe, life-threatening pathology. The most extreme form of ALOC is coma, in which one has a complete lack of awareness and responsiveness. Lethargy is a depressed state of consciousness resembling deep sleep; the patient can be aroused but quickly returns to this state without stimulation. Obtundation refers to a profoundly decreased response to external stimuli. These terms are somewhat subjective, and several schemas to quantify level of consciousness are used clinically, including the AVPU (awake, verbal, pain, unresponsive) scale (see below under Management) and the Glasgow Coma Score (see Chapter 8).
Etiology and Pathogenesis
ALOC occurs when there is dysfunction of the reticular activating system in the brainstem and pons, which is responsible for wakefulness, or the cerebral hemispheres, which are responsible for awareness. For these structures to function properly, the nervous system needs to be free from abnormal irritation, body temperature needs to be in the normal range, adequate blood flow needs to exist to these areas to bring vital energy-producing substrates, and the body needs to be free of metabolic waste products or toxins. Whenever there is an alteration in one of these factors, ALOC ensues. There are myriad causes for the aforementioned alterations in consciousness. The mnemonic VITAMINS outlines the most prevalent causes (Table 10-2).
Table 10-2 Causes of Altered Level of Consciousness (“VITAMINS”)
| Vascular | Stroke, AVM, venous thrombosis |
| Infection | Meningitis, encephalitis, brain abscess, sepsis |
| Trauma | Subdural hematoma, epidural hematoma, concussion, cerebral edema, cerebral contusion |
| A lot of toxins | Opioids, anticholinergics, TCAs, salicylates, anticonvulsants, sedatives |
| Metabolic derangements | Hypoglycemia, DKA, hyperammonemia, uremia, hypo- or hypernatremia, hypo- or hypercalcemia, hypo- or hypermagnesemia, metabolic acidosis, liver failure |
| Intussusception | ALOC may predominate early in some cases |
| Neoplasm | Increased ICP, direct effect of brainstem tumors |
| Seizure | Status epilepticus, postictal phase |
ALOC, altered level of consciousness; AVM, arteriovenous malformation; DKA, diabetic ketoacidosis; ICP, intracranial pressure; TCA, tricyclic antidepressant.
Evaluation and Management
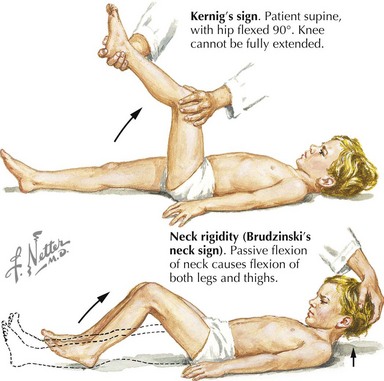
Subsequent examination of the patient begins with a global neurologic assessment using the AVPU scale or the Glasgow Coma Scale and an evaluation of vital signs, including core temperature. Pupillary response can provide important clues to the underlying cause of ALOC. A unilateral dilated pupil can indicate mass effect and increased ICP, and bilateral enlarged pupils might represent severe global intracranial dysfunction or an ingestion of sympathomimetic or anticholinergic substances. Pinpoint bilateral pupils may result from ingestion of opiates. The patient should next be examined for signs of head trauma such as scalp hematoma, retinal hemorrhage, hemotympanum, cerebrospinal fluid (CSF) otorrhea or rhinorrhea, postauricular hematoma, periorbital hematoma, and other visible signs of head injury. Evaluation for infection, especially in the setting of fever, should include testing for meningeal irritation using Kernig’s (resistance to bent knee extension with the hip in 90 degrees flexion) and Brudzinski’s (involuntary knee and hip flexion with passive neck flexion) signs (Figure 10-2). Other stigmata of infectious causes for ALOC include petechiae or purpura found in patients with meningococcal sepsis.
Laboratory evaluation of patients with ALOC should be determined based on the most likely causes. A bedside glucose test along with basic electrolytes, blood urea nitrogen, and creatinine can uncover correctable metabolic derangements. Blood gas analysis, complete blood count with differential, and toxicologic screening of blood and urine may also be helpful. An empiric trial of the opioid antagonist naloxone should be considered in patients with unexplained ALOC, especially with associated respiratory depression and miosis, and in toddler or adolescent patients because of their higher risk of poisoning (see Chapter 9). Brain imaging is helpful in revealing possible hemorrhage, malignancy, abscess, hematoma, cerebral edema, and hydrocephalus. A high index of suspicion for nonaccidental trauma must be maintained, especially in infants with ALOC, even in the absence of physical signs of injury. If fever or other signs of CNS infection are present, lumbar puncture is warranted, and empiric antibiotic therapy with a third-generation cephalosporin (e.g., cefotaxime) should be started, with other antibiotics, such as ampicillin or vancomycin, added if indicated by age and clinical situation. Electroencephalography (EEG) may also be necessary to evaluate for nonconvulsive seizures in patients with ALOC. Ultimately, definitive treatment depends on the results of the diagnostic evaluation.
Increased Intracranial Pressure
Clinical Manifestations
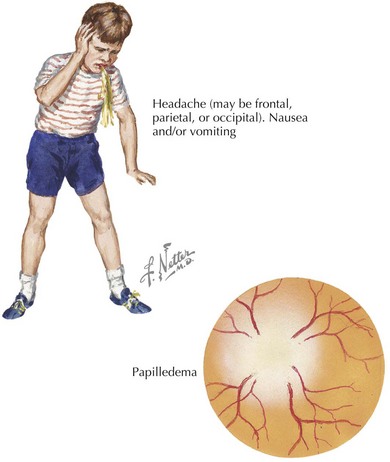
Children with increased ICP may present with headache, decreased consciousness secondary to increased pressure in the midbrain reticular formation, or vomiting. Headache is an early symptom and is typically characterized by a progressive increase in frequency and severity, nocturnal awakening, and worsening with Valsalva maneuvers (cough, defecation, micturition). Infants may present with a bulging fontanelle, poor feeding, lethargy, and flat affect. Funduscopic examination may reveal papilledema, but the absence of this finding does not rule out increased ICP, especially if it has developed acutely (Figure 10-3). The presence of retinal hemorrhages should raise suspicion for nonaccidental head trauma (e.g., shaken baby syndrome; see Chapter 12). Infants may have a “sun-setting” appearance of their eyes, split sutures, or a bulging fontanelle. Dilated pupils (unilateral or bilateral) may be present along with cranial nerve palsies (most commonly the third and sixth nerves). Such cranial nerve palsies can cause double vision and head tilt as the patient tries to correct for the visual discrepancy. Hemiparesis, hyperreflexia, and hypertonia are late signs of increased ICP. Development of Cushing’s triad (bradycardia, systemic hypertension, and irregular respirations) is a late indication of impending cerebral herniation, with bradycardia being the earliest feature.
Evaluation and Management
Abend NS, Huh JW, Helfaer MA, Dlugos DJ. Anticonvulsant medications in the pediatric emergency room and intensive care unit. Pediatr Emerg Care. 2008;24(10):705-721.
Chiang VW. Seizures. In: Fleisher GR, Ludwig S, Henretig FM, editors. Textbook of Pediatric Emergency Medicine. Philadelphia: Lippincott Williams & Wilkins; 2006:629-636.
Lewena S, Young S. When benzodiazepines fail: how effective is second line therapy for status epilepticus in children? Emerg Med Australas. 2006;18:45-50.
Lobato RD, Sarabia R, Rivas JJ, et al. Normal computerized tomography scans in severe head injury. Prognostic and clinical management implications. J Neurosurg. 1986;65(6):784-789.