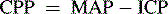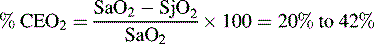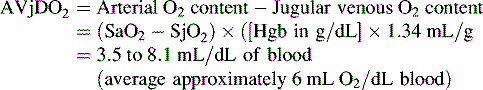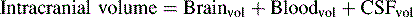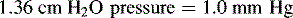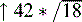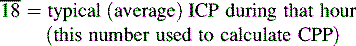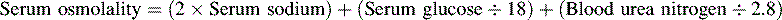11 Neurologic Disorders
Pearls
• The major goals of therapy in the treatment of traumatic brain injury (TBI) are preservation of cardiopulmonary and cerebral function and the prevention of secondary brain injury.
• The 5 H’s responsible for secondary brain injury in the pediatric patient are: hypotension, hypoxia, hyperthermia, hypo-/hyperglycemia, and hyponatremia.
• Seizures result from abnormal discharges or firing of cerebral neurons that produce alterations in motor function, behavior and consciousness.
• It is important to recognize the difference between posturing and seizure activity. Decorticate and decerebrate posturing typically occur in response to a stimulus, such as a painful stimulus, while seizures can occur at any time and may produce a variety of movements or other evidence of neurologic discharges (e.g., changes in heart rate).
• Decorticate posturing indicates damage along the corticospinal tract, the pathway between the cortex and spinal cord. Decerebrate posturing indicates deterioration of the structures of the nervous system, particularly the upper brain stem; decerebrate posturing has a less favorable prognosis than decorticate posturing.
• Stroke and cerebral vascular disease are among the top 10 causes of childhood death.
• Therapeutic hypothermia has been shown to improve morbidity and mortality following adult cardiac arrest and neonatal hypoxic-ischemic insult. In the pediatric population with TBI, hypothermia remains a controversial therapy. Clinical trials are underway to evaluate the effects of hypothermia in pediatric TBI and hypoxic-ischemic injury.
• When using an extraventricular drainage (EVD) device for CSF diversion, the nurse must maintain the drain at the precise level ordered. If the drain is placed too high, increased ICP may develop before drainage occurs. If the drain is placed too low, excessive drainage of CSF can lead to upward herniation and/or ventricular collapse and intraventricular hemorrhage.
Essential anatomy and physiology
The Axial Skeleton
Blood vessels and cranial nerves enter and leave the skull through small openings, or foramina. It is useful to know the course of the cranial nerves so that clinical signs and symptoms can be correlated with areas of cranial injury (Fig. 11-1). The posterior fossa contains a large foramen, the foramen magnum, through which the brainstem and spinal cord join. Lesions in this area, such as those produced by cervical neck trauma, can interrupt vital brain functions and nerve pathways to and from the brain. Cerebrospinal fluid (CSF) flows through the foramen magnum as it passes from the brain to the spinal cord and back again, and the vertebral arteries enter the skull through the foramen magnum.
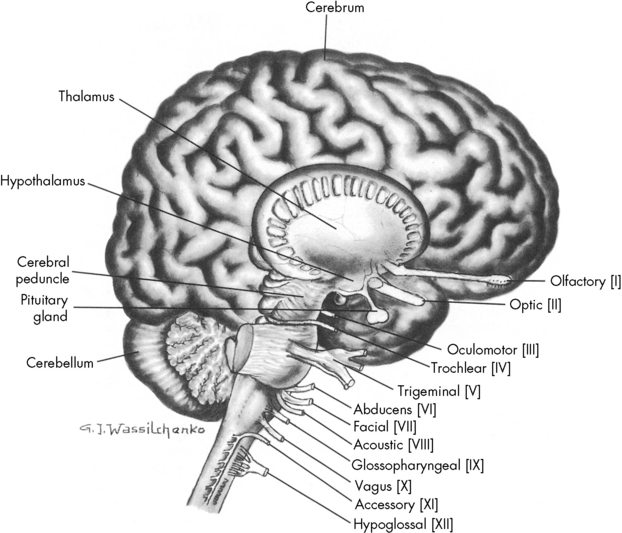
Fig. 11-1 Lateral view of the brain depicting origin of cranial nerves.
(From Rudy EB: Advanced neurological and neurosurgical nursing, St Louis, 1984, Mosby.)
At birth, the skull plates are not fused; they are separated by nonossified spaces called fontanelles. The anterior fontanelle is the junction of the coronal, sagittal, and frontal bones. The posterior fontanelle represents the junction of the parietal and occipital bones (see Evolve Fig. 11-1 in the Chapter 11 Supplement on the Evolve Website). Normally, the posterior fontanelle closes at approximately 2 months of age, and the anterior fontanelle closes at approximately 16 to 18 months of age. If the brain does not grow, such as in patients with microcephaly, the cranial bones can fuse early. Conversely, premature fusion of cranial bones, known as craniosynostosis, can result in microcephaly unless surgery is performed, because brain growth is inhibited by the restricted intracranial space.
At birth, the brain is approximately 25% of the adult volume.76 By 2 years of age, approximately 75% of adult brain volume has been achieved. The cranium itself continues to expand until approximately 7 years of age, when most brain differentiation is complete. This growth of the brain can be assessed indirectly through measurements of the head circumference. These measurements should always be plotted on a growth chart, because they can aid in the detection of excessive or inadequate head and brain growth that may reflect neurologic disease.
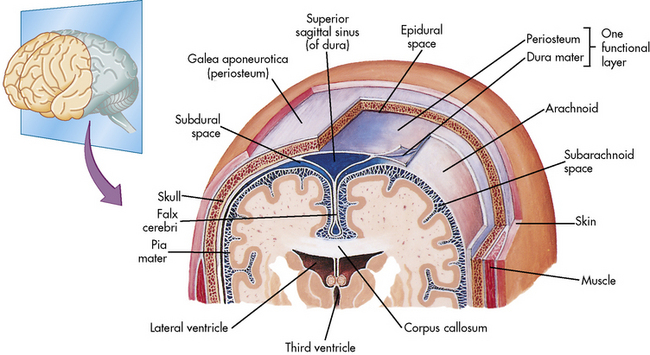
The Meninges
Three highly vascular membranes surround the brain and spinal column; the three membranes collectively are called the meninges. The outermost membrane, the dura mater, consists of tough connective tissue that lines the endocranial vault (Fig. 11-2). The dura mater is folded into tents of tissue immediately underneath the skull cap (the periosteum). The most familiar of the many dural folds is the fold that roofs the posterior fossa; this is called the tentorium cerebelli. This fold serves as an anatomic landmark; intracranial lesions are divided into those that occur above the tentorium cerebelli (supratentorial lesions) and those that occur below the tentorium cerebelli (infratentorial lesions). The dura not only lines the endocranium, but it also lines the vertebral column. It descends through the foramen magnum to the level of the second sacral vertebra and ends as a blind sac.
The Brain
The brain is contained within the cranial vault and extends through the foramen magnum. It is composed of distinct structures, each having a specific function. The brain can be considered in three major functional areas: the cerebrum, the brainstem, and the cerebellum (see Fig. 11-1). The cerebrum (from the embryologic forebrain) consists of the cerebral hemispheres, the thalamus, hypothalamus, basal ganglia, and the olfactory and optic nerves. The brainstem consists of the midbrain, pons, and medulla. The cerebellum is the final major division of the brain. The brainstem and cerebellum develop from the embryologic hindbrain. Table 11-1 lists the divisions of the brain and their major functions. Each of these brain divisions is presented separately in the following pages.
| Structure | Division | Function |
| Cerebrum | Cerebral hemispheres | Integration of sophisticated sensory and motor activities and thoughts |
| Cerebral cortex | ||
| Frontal lobes | Reception of smell, memory banks, and higher intellectual processes | |
| Parietal lobes | Sensory discrimination, localization of body awareness (spatial relationships), and speech | |
| Temporal lobes | Auditory functions and emotional equilibrium | |
| Occipital lobes | Vision and memory of events | |
| Limbic lobes | Primitive behavior, moods, and instincts | |
| Basal ganglia | Transmission of motor tracts, linking pyramidal pathways | |
| Corpus callosum | Provision of intricate connection between cerebral hemispheres | |
| Brain stem | Midbrain | Hypothalamic response to neuroendocrine stimuli |
| Pons | Origin of cranial nerves V, VI, VII, VIII | |
| Medulla | Vital center activity (cardiac, vasomotor, respiratory centers); origin for cranial nerves IX, X, XI | |
| Cerebellum | White and gray matter | Muscle and proprioceptive activity, balance, and dexterity |
The Cerebrum
The Thalamus and Hypothalamus
The thalamus surrounds the third ventricle and is composed of tracts of gray matter. The thalamus is a major integrating center for afferent impulses from the body to the cerebral cortex.85 The thalamus integrates and modifies messages that come from the basal ganglia and cerebellum and then transmits information up to the cerebral cortex. All sensory impulses, with the exception of those from the olfactory nerve, are received by the thalamus. These impulses are then associated, synthesized, and relayed through thalamocortical tracts to specific cortical areas. The thalamus is the center for the primitive appreciation of pain, temperature, and tactile sensations.
Injury to or disease of the hypothalamus or the pituitary can produce a wide variety of neuroendocrine problems and can result in fluid and electrolyte imbalance and growth disturbances (see Chapter 12).
The Brainstem
The midbrain is a short segment between the hypothalamus and the pons. It contains the cerebral peduncles and the corpus quadrigemina. The midbrain consists of fibers that join the upper and lower brainstem; it is the origin of the oculomotor and trochlear cranial nerves. The midbrain is the center for reticular activity and assimilates all sensory input from the lower neurons before it is relayed to the cortex (see Evolve Fig. 11-2 in the Chapter 11 Supplement on the Evolve Website). It is because of this relay that the cortex can maintain consciousness, arousal, and sleep.
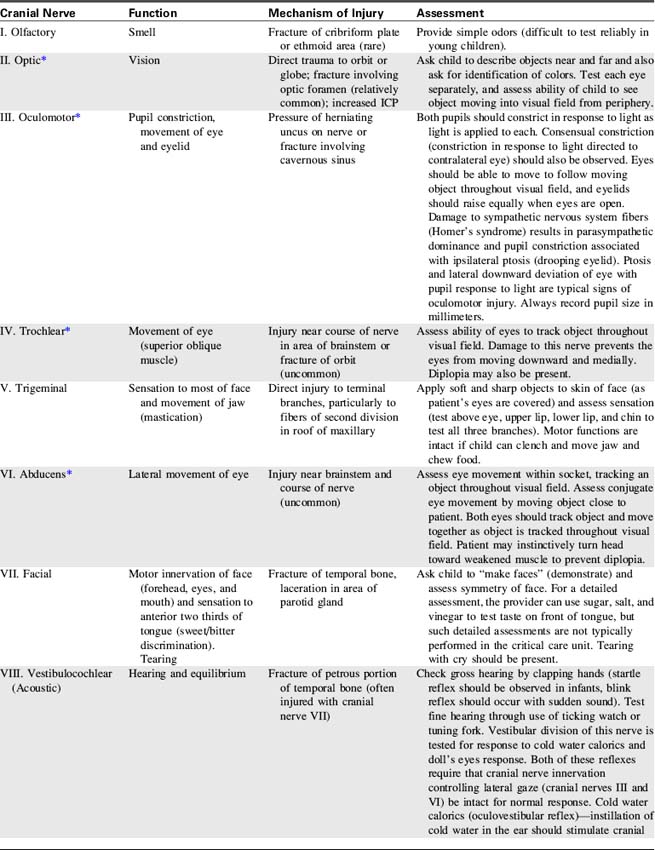
The Cranial Nerves
The cranial nerves are 12 pairs of peripheral nerves that arise from the brain; each has a specific motor or sensory function, and four cranial nerves have parasympathetic functions.111 Cranial nerve function can be lost as a result of lesions near the origin of the cranial nerve or following direct injury to the cranial nerve itself.
Identification of lost cranial nerve functions can help determine the location and severity of CNS disease or injury. For example, pupil inequality and a unilateral sluggish pupil response to light can develop with uncal herniation of the brain (lateral herniation of the temporal lobe through the tentorial notch) and with compression and stretching of the oculomotor nerve. Assessment of cranial nerve function becomes extremely important when the patient is comatose or unresponsive. (Table 11-2 lists cranial nerve origins and functions; see Fig. 11-1).
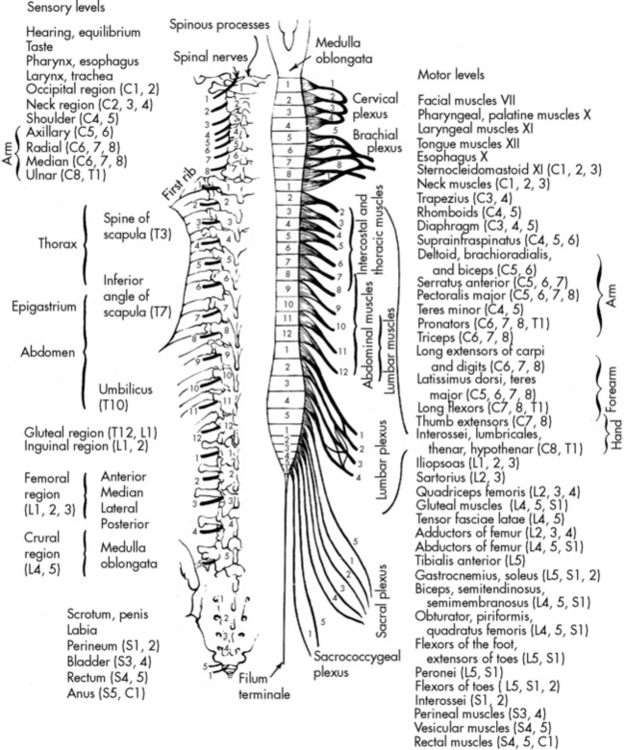
The Spinal Cord
The spinal cord is a cylindrical structure composed of neurons and nerve fibers. It joins the medulla at the foramen magnum and extends to the level of the second lumbar vertebra. There are 31 pairs of spinal nerves, which are distributed along the entire spinal cord (Fig. 11-3). These spinal nerves are all multifibered and transmit impulses between the CNS and the rest of the body. When a portion of the spinal cord is viewed in cross section, the cord fills only part of the vertebral column; it is surrounded by the pia mater, the CSF, the arachnoid, and the dura mater.
Central Nervous System Circulation and Perfusion
The Cerebral Circulation
The brain requires a constant supply of oxygen and substrates (perfusion) to metabolize carbohydrates as an energy source. Adequate perfusion is also necessary to remove carbon dioxide and other metabolites from the brain. The brain requires approximately 20% of the child’s cardiac output. A healthy child’s brain consumes 5.5 mL of oxygen per 100 g of brain tissue per minute.37 As a result, if the brain is deprived of oxygen for even a few minutes, brain ischemia can develop and result in permanent neurologic dysfunction or brain death.32
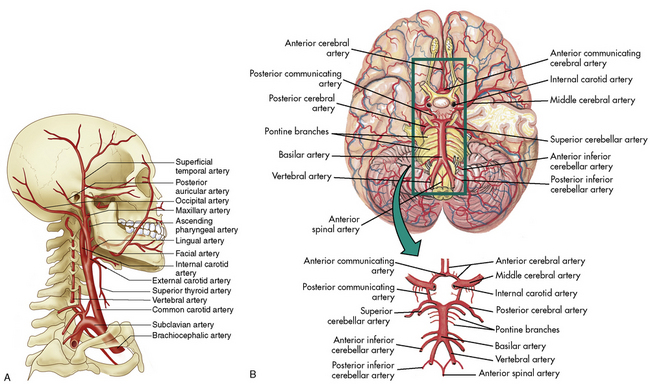
The cerebral arterial blood flow is provided by the two vertebral arteries and the right and left internal carotid arteries. The internal carotid arteries enter the skull anteriorly and end in the anterior cerebral and the middle cerebral arteries; they supply approximately 85% of cerebral blood flow. The vertebral arteries enter the skull posteriorly and join to form the basilar artery. The basilar artery bifurcates to form two posterior communicating arteries (Fig. 11-4).
The circle of Willis at the base of the brain is formed by a junction of the two internal carotid arteries, the two anterior and two posterior cerebral arteries, and the posterior and anterior communicating arteries (see Fig. 11-4, B). This arterial configuration, present in approximately half of all adults,85 maintains effective cerebral perfusion despite a reduction in flow from any single contributory artery. Patients with an alternative form of arterial circulation are considered to have anomalous cerebral circulation, although their arterial circulation typically is not significantly different from that which is considered normal. Congenital anomalies of one or both carotid arteries or of the internal carotid system have been documented. In many of these patients, the development of collateral circulation early in life prevents any compromise in cerebral perfusion.29
The cerebral venous circulation is unique in that the cerebral veins have no valves, and they do not follow the course of the cerebral arteries.85 Venous drainage from the brain flows primarily into large vascular channels within the dura, known as dural sinuses, that ultimately drain into the internal jugular veins. Occlusion of the jugular vein can obstruct cerebral venous return.
Cerebral Blood Flow and Regulation
Cerebral Blood Flow
Normal CBF in adults is approximately 60 mL per 100 g brain tissue per minute. The normal quantity of CBF in children is unknown, but is thought to be approximately 50 to 100 mL per 100 g brain tissue per minute or more. The absolute quantity of CBF is not as important as the relationship between cerebral oxygen, substrate delivery, and cerebral metabolic requirements. It is essential that CBF is adequate to maintain effective cerebral oxygenation to sustain cerebral functions. Under normal circumstances, CBF, metabolism, and oxygen extraction are closely interrelated.133
Autoregulation
CBF normally is maintained at a constant level by cerebral autoregulation, which is the constant adjustment of the tone and resistance in the cerebral arteries in response to local tissue biochemical changes.72 Autoregulation is essential to the maintenance of cerebral perfusion and function over a wide variety of clinical conditions. If systemic arterial pressure increases, cerebral arterial constriction will prevent a rise in the cerebral arterial pressure to maintain CBF at a constant level. Conversely, if systemic arterial pressures falls, cerebral vasodilation will minimize the effects on CBF. Severe alterations in systemic arterial blood pressure will exceed the limits of autoregulatory compensation, however, and will be associated with changes in CBF.
Autoregulation may be compromised or destroyed with severe traumatic or anoxic brain injury. If cerebral autoregulation is lost, CBF becomes related passively to the mean arterial pressure (MAP) so that a fall in MAP will result in a decrease in cerebral flow and perfusion. Recent studies suggest that impaired autoregulation in traumatic brain injury may correlate with severity of injury and young age.126
Effects of Arterial Blood Gases on Cerebral Blood Flow
CBF is affected by significant changes in arterial oxygen and carbon dioxide tensions.
Carbon Dioxide Response
Normoventilation is the accepted method of management of patients with severe head injury and other causes of increased ICP. Mild hyperventilation to a PaCO2 of 30 to 35 mm Hg should be reserved for acute rises in ICP that are refractory to other medical management (e.g., sedation, hyperosmolar therapy) and that are thought to be associated with acute (impending) herniation syndrome. Although the reduction in CBF caused by hyperventilation can transiently reduce intracranial hypertension, it may worsen ischemia by decreasing oxygen delivery to an already compromised brain.9
When increased ICP is present, routine care such as suctioning must be performed skillfully to prevent the development of hypercarbia and cerebral vasodilation and an increase in ICP.9 The vasoconstrictive response to hypocarbia is unpredictable in patients after traumatic brain injury and the requires careful monitoring of clinical effects of any changes in PaCO2.9
Cerebral Perfusion Pressure
The normal range of CPP is thought to be approximately 50 to 150 mm Hg in healthy adults, with a goal of 70 mm Hg following traumatic brain injury. There is a paucity of information to identify the normal range of CPP in children; it is thought to be approximately 40 to 60 mm Hg, but normal ranges vary with age.9,48 A CPP of at least 40 mm Hg is thought to be necessary for effective cerebral perfusion; however, this number is not absolute because perfusion is determined by blood flow, not blood pressure. It is likely that a CPP of 40 mm Hg is acceptable in an infant, but a CPP of 50 to 65 mm Hg is likely to be necessary in older children and adolescents.
The calculated CPP will fall if the mean systemic arterial pressure falls, if the mean ICP rises, or if both occur simultaneously. The calculated CPP can be maintained despite a rise in ICP if the MAP rises commensurately with a rise in ICP. It is important to note that such compensation may or may not be associated with effective CBF and actual cerebral perfusion (for a Case Study of calculation of CPP, see the Chapter 11 Supplement on the Evolve Website); a normal CPP (40-50 mm Hg or more) has been recorded after brain death was pronounced.16
Evaluation of Cerebral Blood Flow
Jugular Venous Oxygen Saturation
The SjO2 will rise, typically above 75%,127 if oxygen delivery to the brain rises in excess of cerebral oxygen consumption. This rise is unlikely to be caused by decreased cerebral oxygen consumption unless a drug such as a barbiturate is administered. The SjO2 will rise with the development of hypercarbia and associated cerebral vasodilation. An unexpected rise in SjO2 can indicate hyperemia (excessive CBF) that may signal a loss of cerebral autoregulation.
Several calculations can be made using the SjO2 (Box 11-1). Providers can calculate the cerebral extraction of oxygen (normally 20%-42%) and the cerebral arteriovenous oxygen content difference (normally 3.5-8.1 mL/dL of blood).127 A rise in these variables indicates a decrease in oxygen delivery versus demand, with increased oxygen extraction or uptake that may signal decreased CBF. A rise in the arterial-jugular lactate difference can indicate a compromise in CBF.
Box 11-1 Calculations Using Jugular Venous Bulb Oxyhemoglobin Saturation
Cerebral extraction of oxygen (normally 20%-42%):
Cerebral arteriovenous oxygen content difference (cerebral AVjDO2)*:
Note that calculations derived from SjO2 monitoring reflect only global brain oxygenation and cannot identify areas of regional ischemia. In addition, these calculations do not provide absolute values for cerebral metabolic rate and CBF. Sources of inaccurate values include a shift in the oxyhemoglobin dissociation curve—which will change the relationship between oxyhemoglobin saturation and partial pressure of oxygen, thus altering oxygen content at a given saturation—and technical errors related to positioning and calibration, especially in the pediatric population.96
Doppler Flow Velocity
Doppler flow velocity can be measured at the carotid artery or through an open fontanelle. In addition, transcranial Doppler flow velocity studies can be performed in older children and adolescents to evaluate cerebral flow velocity within the middle cerebral artery, anterior cerebral artery, and basal artery. Technical limitations of transcranial Doppler include inadequate visualization through available bone windows, the ability to view only medium to large vessels, variations in measurements between studies, and poor correlation with other indices of CBF.53 There are also limited data regarding pediatric norms. For further information regarding the interpretation of changes in mixed venous oxygen saturations and Doppler flow studies, see the final section of this chapter and Chapters 6 and 8.96
Partial Pressure of Oxygen in Brain Tissue
The cerebral tissue oxygen tension (PbtO2) can be monitored using a probe placed through the skull into brain tissue. The probe can be placed in healthy tissue or in an area of the brain in or near a lesion, to monitor trends in oxygenation. Normal PbtO2 is approximately 20 to 35 mm Hg, and critical values are those less than 15 mm Hg. The PbtO2 can be altered by factors that alter CBF and by those that shift the oxyhemoglobin dissociation curve and alter release of oxygen to the tissues.40
Noninvasive Near-Infrared Spectroscopy
Noninvasive techniques for detection of trends in CBF, including the near-infrared spectroscopy, have been used more frequently in recent years. These techniques require placement of a light source on the scalp to transmit light through the skin and skull into the brain, and then quantify light reflection from the brain. Light reflection will be affected by tissue or hemoglobin oxygenation. These devices can help to identify trends in cerebral perfusion (see Chapter 21).
The Blood-Brain Barrier
Oxygen, carbon dioxide, and lipid-soluble drugs readily cross the blood-brain barrier. The blood-brain barrier is also freely permeable to water, so rapid changes in intravascular osmolality can affect cerebral function (see Chapter 12). The major factor affecting transport across the blood-brain barrier is lipid solubility; the more lipid-soluble the drug is, the more easily it will cross the blood-brain barrier. Many drugs, including some water-soluble contrast agents and some antibiotics, do not cross the blood-brain barrier.
The immature brain does not have adequate development of glial cells; therefore, the blood-brain barrier is incomplete in the preterm infant.64 This incomplete barrier is thought to contribute to increased risk of intracranial hemorrhage in preterm infants. It also makes the neonatal brain more vulnerable to some circulating drugs and toxins.
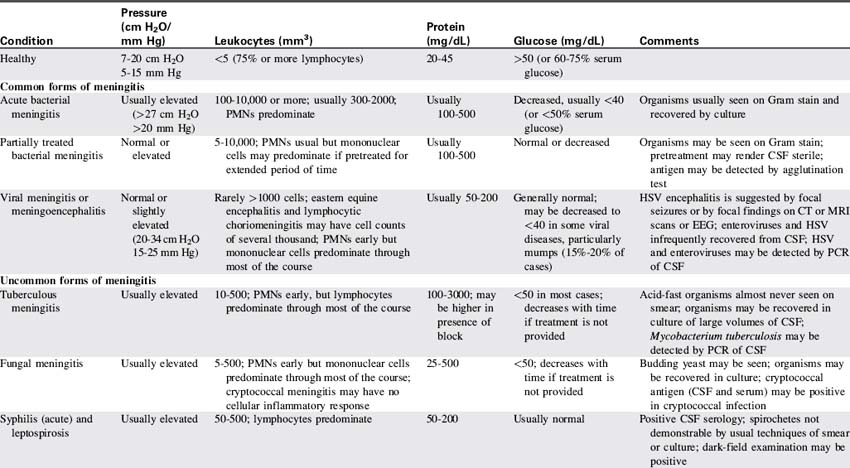
Cerebrospinal Fluid and Its Circulation
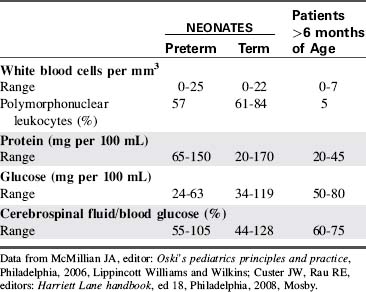
CSF is not merely a filtrate of plasma. It contains water, oxygen, carbon dioxide, sodium, potassium, chloride, glucose, a small amount of protein, and an occasional lymphocyte (Table 11-3). The CSF glucose is normally approximately 75% of the serum glucose concentration, which is approximately 50 to 80 mg/dL. The normal protein concentration is in the range of 20 to 45 mg/dL (higher normal values, up to 125 mg/dL, are present in neonates), and there are usually less than five white blood cells per cubic millimeter present in children. Again, slightly higher numbers may be normal in the neonate.58
Red blood cells are present in a CSF sample only if a traumatic spinal tap was performed or if the patient has suffered a cerebral hemorrhage. Generally, CSF is hypertonic to blood, but changes in CSF osmolality will parallel those of blood (i.e., an increase in serum osmolality will soon be followed by an increase in CSF osmolality). Abnormalities of CSF composition can aid in the diagnosis of some CNS diseases (Table 11-4).
In healthy children, the rate of CSF production is approximately 20 mL per hour.58 The amount of CSF formed is affected by cerebral metabolism, CPP, blood pressure, and changes in the serum osmolality. An increase in the CPP or systemic arterial pressure usually results in an increase in CSF formation.
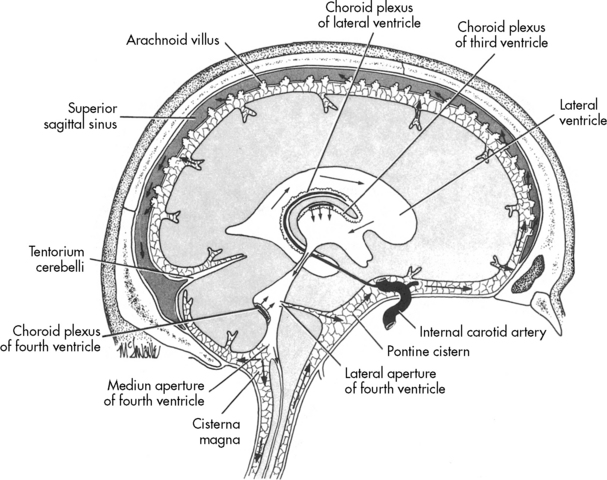
Once formed, the CSF flows from both lateral ventricles through the foramen of Monro into the third ventricle. From there the fluid passes through the cerebral aqueduct, known as the Sylvian aqueduct (or aqueduct of Sylvius), into the fourth ventricle. Some CSF then passes through the two lateral Luschka’s foramina into the subarachnoid space to bathe the brain. The remaining CSF passes through Magendie’s foramen and enters the subarachnoid space to circulate around the spinal cord. Most CSF ultimately is reabsorbed by venous sinuses that project into the subarachnoid space; these are known as the arachnoid villi (Fig. 11-5). Inflammation (e.g., meningitis) or blood in the ventricular system may obstruct CSF flow, often in the narrow aqueduct of Sylvius between the third and fourth ventricle. Subarachnoid hemorrhage can prevent normal reabsorption of CSF.
Intracranial Pressure and Volume Relationships
Normal Intracranial Pressure
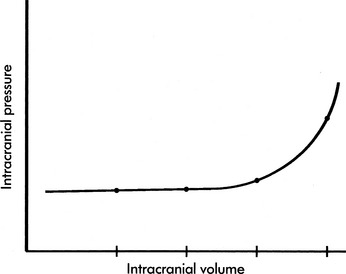
If the brain, cerebral blood, or CSF volume increases without a compensatory decrease in other intracranial components, the intracranial volume increases. Initially, however, the ICP does not rise (Fig. 11-6). This ability to tolerate an increase in the volume of one intracranial component results from the compensatory displacement of venous capacitance blood or CSF from the intracranial vault. In addition, intracranial compliance (including a small amount of brain compression) allows for some increase in intracranial volume without an increase in ICP. However, there is a limit to this compliance. If the brain, blood, or CSF volume continues to increase, ICP ultimately will rise. Once the limits of compliance have been reached, progressively smaller incremental increases in intracranial volume will be associated with progressively more significant increases in ICP (see Common Clinical Conditions, Increased Intracranial Pressure).
Common clinical conditions
Nursing care of any child with an actual or potential neurologic problem requires careful and repeated assessments over time. For this reason, before presentation of common clinical conditions themselves, this section begins with a summary of critical bedside neurologic assessment (Box 11-2).
Box 11-2 Summary of Bedside Nursing Assessment of Neurologic Function
• Airway, Ventilation and Respiratory Pattern, Oxygenation
 Monitor for abnormal respiratory patterns (see Fig. 11-7). An irregular respiratory rate or apnea may develop with increased intracranial pressure.
Monitor for abnormal respiratory patterns (see Fig. 11-7). An irregular respiratory rate or apnea may develop with increased intracranial pressure. Evaluate vital signs in light of age and clinical condition. Tachycardia is nonspecific sign of distress, so further assessment is required.
Evaluate vital signs in light of age and clinical condition. Tachycardia is nonspecific sign of distress, so further assessment is required. Cushing triad (resulting from increased intracranial pressure and impending brainstem herniation) includes: bradycardia, increased systolic blood pressure (with widened pulse pressure), and irregular respiratory pattern (including possible apnea).
Cushing triad (resulting from increased intracranial pressure and impending brainstem herniation) includes: bradycardia, increased systolic blood pressure (with widened pulse pressure), and irregular respiratory pattern (including possible apnea).• Pupil Size and Response to Light: notify on-call provider immediately if pupils dilate or have decreased constriction to light
 Oculomotor (III): pupil constriction in response to light (note size); injury can cause ptosis and lateral downward deviation of eye
Oculomotor (III): pupil constriction in response to light (note size); injury can cause ptosis and lateral downward deviation of eye Abducens (VI): lateral eye movement within socket and conjugate eye movement to track object across visual field
Abducens (VI): lateral eye movement within socket and conjugate eye movement to track object across visual field Facial (VII): movement of facial muscles (especially forehead and around eyes and mouth), sensation on anterior tongue, tearing with cry
Facial (VII): movement of facial muscles (especially forehead and around eyes and mouth), sensation on anterior tongue, tearing with cry• Glasgow Coma Scale Score (see Table 11-6)
 To evaluate ability to obey commands: Ask the child to hold up two fingers, stick out his or her tongue or (unless spinal cord injury present) wiggle toes.
To evaluate ability to obey commands: Ask the child to hold up two fingers, stick out his or her tongue or (unless spinal cord injury present) wiggle toes. To evaluate ability to localize painful stimulus: Rub the child’s sternum or pinch trapezius (child’s hand should reach toward the site of stimulus).
To evaluate ability to localize painful stimulus: Rub the child’s sternum or pinch trapezius (child’s hand should reach toward the site of stimulus). To evaluate withdrawal in response to pain in each extremity: Pinch the medial aspect of each extremity (child should abduct each extremity, pulling outward from medial stimulus).
To evaluate withdrawal in response to pain in each extremity: Pinch the medial aspect of each extremity (child should abduct each extremity, pulling outward from medial stimulus). To assess for decorticate or decerebrate posturing (see Fig. 11-9) or no response: Rub the sternum or pinch the trapezius (observe response).
To assess for decorticate or decerebrate posturing (see Fig. 11-9) or no response: Rub the sternum or pinch the trapezius (observe response).Neurologic assessment and support includes assessment and support of airway, oxygenation, ventilation, and circulation, as well as evaluation of level of consciousness, pupil size and response to light, cranial nerve function, Glasgow Coma Scale score and additional evaluation of motor activity, reflexes, and movement. General neurologic assessment is summarized in Box 11-2. To ensure clear and consistent communication and to facilitate rapid identification of clinical changes, all members of the healthcare team must use consistent terminology and assessment tools and must apply them in a consistent fashion.
Evaluation of Respiratory Pattern
When intracranial injury or insult occurs, some characteristic breathing patterns may be noted that help identify the level of intracranial problem. Such breathing patterns include Cheyne-Stokes breathing, central neurogenic hyperventilation, apneusis, cluster breathing and ataxic breathing (Fig. 11-7). If the ICP rises to a point that brainstem compression occurs and cerebral herniation is imminent, the Cushing reflex is initiated, producing an abnormal breathing pattern that often includes apnea.
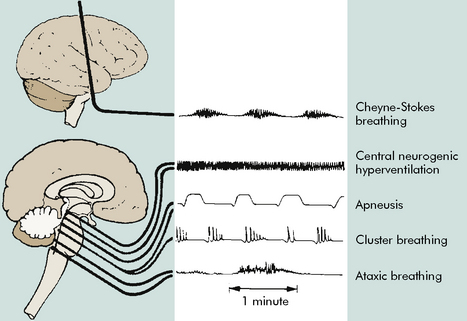
Fig. 11-7 Abnormal respiratory patterns with corresponding level of central nervous system activity.
(From Boss BJ: Alterations in cognitive systems, cerebral hemodynamics, and motor function. In McCance KE, Huether SE, editors: Pathophysiology: the biologic basis for disease in adults and children, ed 6, Philadelphia, 2010, Mosby, p. 531, fig. 16-1.)
Evaluation of Systemic Perfusion
Vital signs are evaluated in light of the patient’s clinical condition (see pages inside front cover for tables of normal heart rates, respiratory rates, and blood pressures in children). Tachycardia and tachypnea are usually more appropriate in critically ill children than are normal heart and respiratory rates. In children who are 1 to 10 years old and of average height, hypotension is present if the child’s systolic blood pressure is less than 70 mm Hg plus twice the patient’s age in years.31,56 Hypotension is also present if the MAP is less than 40 mm Hg plus one and one-half times the child’s age in years.56
The Cushing Reflex
The Cushing reflex is a late and ominous result of increased ICP and ischemia of the vasomotor center.83 The Cushing reflex indicates profound compromise in brainstem perfusion and may develop only when cerebral brainstem herniation is imminent. This reflex produces the clinical triad of bradycardia, an increase in systolic arterial blood pressure with widened pulse pressure, and abnormal breathing pattern. This clinical triad is referred to as the Cushing triad, a term often used interchangeably with the Cushing reflex. The abnormal breathing pattern that is part of the Cushing triad may consist of an abnormal or irregular respiratory effort or apnea.83
Evaluation of Level of Consciousness
If acute coma is present, members of the healthcare team should use consistent terminology (Table 11-5) to describe the child’s level of response. Coma is present when the patient demonstrates no eye opening or verbal response to any stimuli, demonstrating only motor response to painful or noxious stimuli. Stupor is present when only vigorous and repeated stimulation produces arousal.
| State | Definition |
| Confusion | Loss of ability to think rapidly and clearly; impaired judgment and decision making |
| Disorientation | Beginning loss of consciousness; disorientation to time followed by disorientation to place and impaired memory; self-recognition is last to be lost |
| Lethargy | Limited spontaneous movement or speech; easy arousal with normal speech or touch; may not be oriented to time, place, or person |
| Obtundation | Mild to moderate reduction in arousal (consciousness) with limited response to the environment; falls asleep unless stimulated verbally or tactilely; answers questions with minimal response |
| Stupor | A condition of deep sleep or unresponsiveness from which the person may be aroused or caused to open eyes only by vigorous and repeated stimulation; response is often withdrawal or grabbing at stimulus |
| Coma | No verbal response to the external environment or to any stimuli; noxious stimuli such as deep pain or suctioning yields motor movement |
| Light coma | Associated with purposeful movement on stimulation |
| Deep coma | Associated with unresponsiveness or no response to any stimulus |
From Boss BJ: Alterations in cognitive systems, cerebral hemodynamics, and motor function. In McCance KE, Huether SE, editors: Pathophysiology: the biologic basis for disease in adults and children, ed 6, Philadelphia, 2010, Mosby-Elsevier, p. 530, table 16-4.
Evaluation of Pupil Response and Cranial Nerve Function
The pupils are normally of equal size, and they both should constrict briskly in response to light. This constriction reflects function of the third cranial (oculomotor) nerve. Hippus is a spasmodic, rhythmic papillary movement, that is often a normal variant.50 When increased ICP develops, the oculomotor nerve is compressed by general expansion of the brain, by the intracranial lesion, or by uncal herniation; such compression can produce pupil dilation and decreased or absent pupil constriction in response to light. When the intracranial herniation or lesion is unilateral, the pupil dilation will typically occur on the same side as the lesion. The child also may complain of blurred vision or diplopia.
Some clinical conditions or medications can modify pupil size or response to light. When the patient has unilateral blindness, the involved pupil will not constrict in response to light. Pupil constriction (miosis) can result from hemorrhage in the pons, poisoning, or administration of large doses of opioids. Pupil dilation can be present with significant pain or hypothermia and can result from administration of atropine or of extremely large doses of sympathomimetic drugs such as dopamine or epinephrine. Pupil dilation will also be present following administration of mydriatic drops to dilate the pupils for examination, so administration of such drugs should be well-documented. Changes in the pupil size and response to light with altered levels of consciousness are summarized in Fig. 11-8.
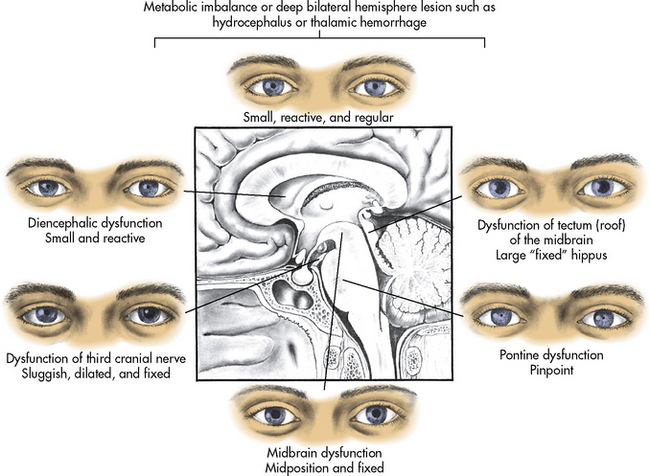
Fig. 11-8 Appearance of pupils associated with common causes of neurologic dysfunction.
(From Boss BJ: Alterations in cognitive systems, cerebral hemodynamics, and motor function. In McCance KE, Huether SE, editors: Pathophysiology: the biologic basis for disease in adults and children, ed 6, Philadelphia, 2010, Mosby-Elsevier, p. 532, fig. 16-2.)
Evaluation of the function of most cranial nerves can be performed during routine nursing care (see Box 11-2). If the child is verbal and able to count, you can ask the child to tell you the number of fingers you hold in front of the child’s face; if the child can correctly identify the number of fingers, the optic nerve (cranial nerve II) is probably intact. Consensual pupil constriction in response to light requires the oculomotor nerve (cranial nerve III). If the child can track objects across a visual field, the oculomotor, trochlear, and abducens nerves (cranial nerves III, IV, and VI, respectively) are functioning. If wrinkling of the forehead is noted during cough, the facial nerve (VII) is probably intact. If noise startles the child, the acoustic nerve (cranial nerve VIII) is functioning. A gag reflex (cranial nerve X) should be observed during suctioning or insertion of a nasogastric tube, even if the child is comatose. Movement of the shoulders and upper extremities indicates function of the spinal accessory nerve (cranial nerve XI). The hypoglossal nerve (cranial nerve XII) provides tongue movement (e.g., the child can stick out his or her tongue).
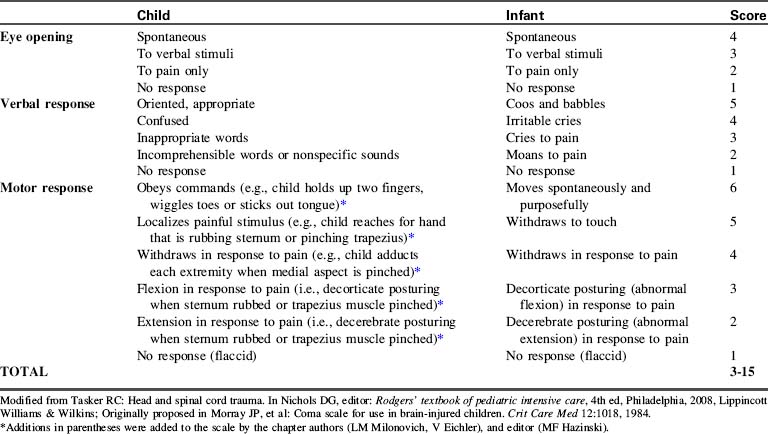
Glasgow Coma Scale Scoring of Neurologic Function
The GCS was validated in adults, and the original GCS verbal section cannot be used in the care of the preverbal or intubated patient. For these reasons, modifications of the GCS have been developed and found to be useful for pediatric patients with neurologic injury or disease (Table 11-6).34a,83,90,106
Decorticate posturing is characterized by flexion of the elbows, wrists, and fingers and by extension of the legs and ankles with plantar flexion of the feet. During decorticate posturing, the legs are tightly adducted. The development of decorticate posturing or rigidity (Fig. 11-9, A) indicates ischemia of or damage to the cerebral hemispheres.
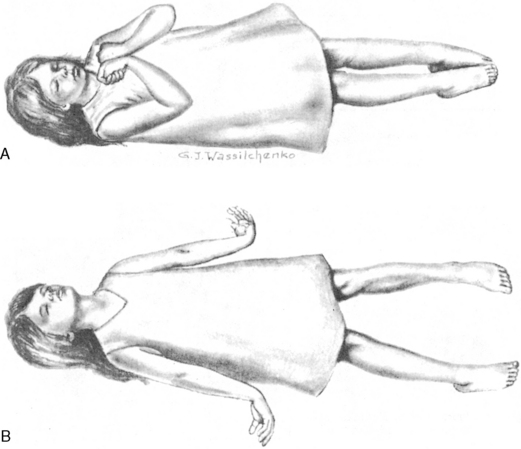
Fig. 11-9 Abnormal posturing. A, Decorticate posturing and rigidity. B, Decerebrate posturing and rigidity.
(From Whaley LF, Wong DL: Nursing care of infants and children, ed 2, St. Louis, 1983, Mosby.)
Decerebrate posturing is a lower level of reflexive response to painful stimulation than decorticate posturing. It is characterized by extension and slight abduction (movement outward from midline) of the arms and legs. The development of decerebrate posturing or rigidity (see Fig. 11-9, B) indicates the presence of a diffuse metabolic cerebral injury or the development of ischemia of or damage to more primitive areas of the brain, including the diencephalon, midbrain, or pons. In general, a progression from decorticate rigidity to decerebrate posturing usually indicates progression of the neurologic dysfunction and should be reported to an on-call provider immediately (while obtaining additional information including vital signs, pupil response to light, and ICP). Patients occasionally can alternate between decorticate and decerebrate posturing if there is variability in CBF to the brainstem and the cerebral hemispheres.
Additional Assessment of Motor Function and Reflexes
Neurologic disease or insult can result in abnormal appearance of some other reflexes.
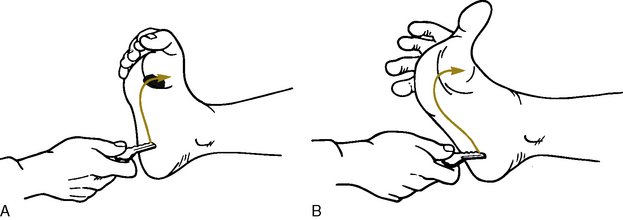
Babinski’s reflex is present if the toes fan out and if the great toe extends when the sole of the foot is stroked from the heel to the toes and around to the ball of the foot (Fig. 11-10). Although Babinski’s reflex is normally present before the infant or toddler learns to walk, the reflex is abnormal after a child has begun walking, and it may indicate the presence of increased ICP or neurologic dysfunction.
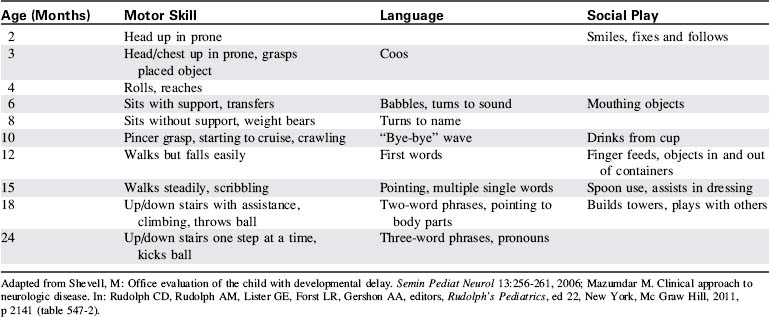
Development of incontinence in a child who previously demonstrated bowel and bladder control is a worrisome sign and can indicate significant neurologic deterioration. Deterioration can affect the child’s ability to coordinate movement or to follow simple commands. Changes in the child’s motor skills will be more readily identified if the nurse is familiar with the normal sequence of achievements of developmental milestones (Table 11-7) and motor skills.
Increased Intracranial Pressure
Etiology
Increased Brain Volume
Vasogenic cerebral edema is characterized by increased cerebral capillary permeability and disruption of the blood brain barrier in the absence of neuronal injury.71 The increased permeability often is caused by inflammatory conditions such as encephalitis and meningitis. When capillary permeability is increased, proteins leak from the vascular space. Because proteins exert osmotic force, when they move from the intravascular to the extravascular space they pull water from the intravascular to the extravascular space, worsening edema and creating further ischemia and further edema.
Cytotoxic cerebral edema or cellular swelling usually is associated with severe neural cell damage. It occurs across the spectrum of brain injuries, but particularly in traumatic brain injury and ischemic injury. The edema occurs secondary to dysfunction of intracellular mechanisms, which promote sodium and water accumulation in the cells, with resultant cell swelling.71,108
Osmotic cerebral edema results from an acute fall in serum (and therefore extracellular) osmolality that results in an acute free water shift from the intravascular/extracellular space to the intracellular space. Interstitial cerebral edema results from impaired absorption of CSF.108 Most commonly, this form of cerebral edema is observed in patients with obstructive hydrocephalus.
Increased Cerebral Blood Volume
Arteriovenous malformations are the most common nontraumatic cause of intracranial hemorrhage in children.55 Head trauma also can result in accumulation of blood in the subdural or epidural space, or in an intracerebral hematoma. Intraventricular hemorrhage can occur after a traumatic injury or in premature infants.
Mass Lesions
Brain tumors are the most common solid tumor of childhood.55 The tumors are typically infratentorial, located near or in the brainstem, and most contain glial cells (see Intracranial Tumors in the Specific Diseases section of this chapter). Intracranial hypertension can result from the tumor mass itself, from edema generated by the tumor presence, or from obstruction of CSF flow.
Pathophysiology
Intracranial Compliance
Intracranial compliance is affected by time. If the intracranial volume increases gradually, as occurs with a slow-growing brain tumor, a significant volume may be accommodated without a rise in ICP because physiologic compensatory mechanisms have time to develop. In contrast, if the intracranial volume increases rapidly, as occurs following an intracranial hemorrhage, there is little time for compensation, and the ICP is likely to rise rapidly.103
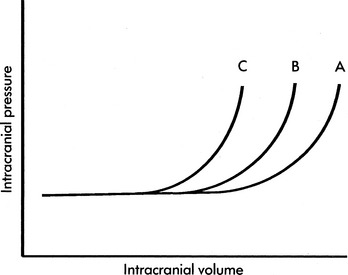
Intracranial compliance also is affected by previous rises in intracranial volume and pressure. Frequent spikes in the ICP will decrease intracranial compliance, resulting in a rise in ICP with subsequently smaller and smaller increases in intracranial volume. For example, consider the child with a head injury who develops an increase in CBF and CBV associated with hypercarbia, hypoxemia or seizures. During the initial episode of hypercarbia or hypoxemia or an initial seizure, the increase in CBF and CBV may be tolerated with only a mild rise in ICP. Subsequent similar episodes of hypercarbia or hypoxemia or additional seizures are likely to cause more substantial increase in ICP. A change in compliance alters the relationship between intracranial volume and pressure (Fig. 11-11).
The volume pressure resistance index or response was described by Guertin et al. in 1982.54 Under a research protocol involving children with ICP monitoring in place, physicians gently instilled a known quantity of normal saline (typically 1 mL) into the child’s lateral ventricle. The greater the rise in ICP produced by the 1-mL instillation, the lower the intracranial compliance. For example, if instillation of 1 mL of saline produced an approximately 1 mm Hg rise in ICP, the patient’s intracranial compliance was characterized as high, as depicted on the relatively horizontal part of an intracranial pressure-volume curve. If, however, instillation of 1 mL of normal saline produced a rise in ICP of 7 to 10 mm Hg, the patient’s intracranial compliance was characterized as very low, as depicted by the relatively vertical part of an intracranial pressure-volume curve. In patients with low intracranial compliance, instillation of progressively smaller quantities of normal saline resulted in progressively greater rises in ICP (such as shown in Fig. 11-11, curves B and C).
Complications of Increased Intracranial Pressure
Transtentorial herniation occurs when part of the brain herniates downward around the tentorium cerebelli. This herniation can occur in the anterior or posterior portions of the brain, and it may be unilateral or bilateral. Transtentorial herniation initially will produce pupil dilation (unilateral or bilateral) with a decreased response to light and impeded upward gaze. It also may produce obstruction of the Sylvian aqueduct with resulting CSF accumulation and a progressive rise in ICP. If large portions of the brain herniate across the tentorial notch, compression of vital brain structures causes death (see Evolve Fig. 11-3 in the Chapter 11 Supplement on the Evolve Website for illustrations of herniation).
Clinical Signs and Symptoms
The child with increased ICP characteristically demonstrates altered level of consciousness; pupil dilation with decreased reactivity to light; alterations in heart rate, blood pressure, and respiratory rate or pattern; and abnormal motor activity and reflexes. The child may complain of headache or nausea and may vomit, particularly in the morning or after moving from a reclining to an upright position. Because the infant or very young child and the child with decreased level of consciousness will be unable to articulate symptoms, the nurse must be able to recognize signs and symptoms of increased ICP (Box 11-3).
Box 11-3 Signs of Increased Intracranial Pressure in Children
Level of Consciousness
Evaluation of the child’s level of consciousness is probably the single most important aspect of neurologic assessment of the child with increased ICP. The level of consciousness must be evaluated on a regular basis and whenever changes are observed in the child’s clinical appearance, or cardiopulmonary function or in the monitored ICP or CPP (see Box 11-2).
Pupil Response and Cranial Nerve Function
Evaluation of the function of most cranial nerves can be performed during routine nursing care (see Table 11-2 and Box 11-2). If the child demonstrates a loss of previously demonstrated cranial nerve function, notify the on-call provider immediately. When cerebral perfusion is compromised and cessation of brain function occurs, cranial nerve function will disappear (e.g., the child who had a gag reflex will no longer cough or have a gag during suctioning).
Changes in Motor Function and Reflexes
Increased ICP will produce decreased motor function and possible abnormal posturing or reflexes, such as decorticate or decerebrate posturing (see Fig. 11-9). With progressive neurologic deterioration, flaccid paralysis will result.
Alterations in Respiratory Pattern
The patient with increased ICP may demonstrate a wide variety of respiratory patterns (see Fig. 11-7). When ICP rises and the Cushing reflex is initiated, respirations become irregular, and apnea then respiratory arrest can develop. However, other breathing patterns also may be noted before the Cushing reflex develops.
The Cushing Reflex
The Cushing reflex produces the Cushing triad, three late and ominous signs of increased ICP that result from ischemia of the vasomotor center.83 The Cushing triad may appear only when cerebral brainstem herniation is imminent. This triad consists of bradycardia, an increase in systolic arterial blood pressure (as an attempt to maintain CPP) with widened pulse pressure, and abnormal breathing pattern, typically apnea.83
Scoring Neurologic Function
The most widely used neurologic scoring system is the GCS. This scale evaluates motor activity, verbal responses and motor responses, with a total scale range of 3 to 15 (see Table 11-6)
The GCS was validated in adults, so the verbal section cannot be used as written in the care of the preverbal patients. As a result, modifications of the GCS have been developed and found to be useful for pediatric patients with neurologic injury or disease (see Table 11-6).34a,83,93,106 The verbal section can’t be used as written for intubated patients. When the patient is intubated, it is very important for healthcare providers to identify a consistent approach to either modified application or omission of the verbal portion of the GCS.
When the child is comatose, careful evaluation of the motor response is extremely important. However, this section of the GCS is often applied inconsistently or incompletely. For this reason, details about assessment of motor function are listed under the GCS Score in Table 11-6 and Box 11-2.
Other Signs of Increased Intracranial Pressure
The verbal child with intracranial hypertension may complain of headache, nausea, vomiting, blurred vision, or diplopia. The child may demonstrate mood swings and also may be more lethargic, with periods of confusion. Slurred speech is common. Clinical signs of increased ICP are summarized in Box 11-3.
Neurogenic pulmonary edema occasionally complicates increased ICP. This pulmonary edema can develop suddenly and without warning. The mechanism of the pulmonary edema is unclear, but it appears to be related to development of increased systemic and pulmonary artery pressure in response to the intracranial hypertension. The pulmonary edema usually produces respiratory failure (i.e., hypoxemia with decreased lung compliance and increased respiratory effort). For further information about pulmonary edema, refer to Chapter 9.
Helpful Diagnostic Tests
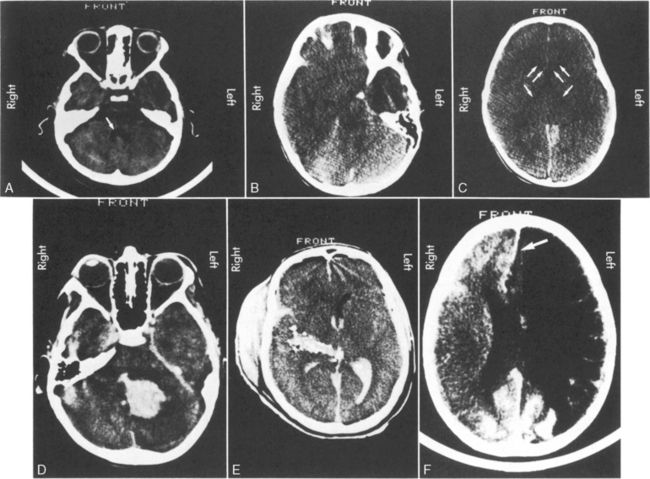
Computed tomography (CT) is extremely helpful in localizing mass lesions or intracranial bleeding or in determining the presence of diffuse cerebral edema or infarction. (See the Common Diagnostic Tests section, later in this chapter, for further discussion of EEG and CT). The CT scan may be used in the acute management of the patient with increased ICP to evaluate potential causes of deterioration and to assess for the presence and severity of cerebral edema. If the third and fourth ventricles are widely patent and visible on a CT scan, the patient’s intracranial volume has not yet reached the limits of intracranial compensation (Fig. 11-12, A). If the third and fourth ventricles are collapsed and the basilar cisterns are obliterated, CSF has been displaced from the intracranial vault and the patient probably is reaching the limits of intracranial compensation, so intracranial compliance is low. Further increase in intracranial volume is likely to produce a significant increase in ICP, and cerebral herniation is possible. Collapse of the lateral ventricles (in the absence of an external CSF drain) also indicates that intracranial compliance is low (see Fig. 11-12, B and C).
Management
Anoxic cerebral injury produces cytotoxic cerebral edema. Signs of increased ICP often do not develop for approximately 48 to 72 hours or longer after the anoxic insult—too late to reverse the damage. In fact, longitudinal studies of drowning victims have demonstrated that a rise in ICP following an anoxic insult is generally an indication of overwhelming neurologic insult, and patients who develop such an increase in ICP may deteriorate despite efforts to control the ICP.26,27,43
Assessment and Support of Airway and Ventilation
Whenever the child is at risk for increased ICP, the healthcare team must continually monitor the child’s airway and ventilation. If the child is obtunded or demonstrates a decreased response to painful stimulation, intubation with mechanical ventilation are indicated to prevent possible airway obstruction or respiratory arrest (Box 11-4). Intubation is also indicated if the child demonstrates hypoventilation or if signs of increased ICP are present.
Box 11-4 Indications for Intubation in the Child with Increased Intracranial Pressure
Intubation of the child with increased ICP requires coordination of team activities to provide adequate preoxygenation, prevent development of hypoxia or hypercarbia during the attempt, and verify correct tube placement. Medications chosen for intubation should suppress awareness of and reflexes during direct laryngoscopy, to prevent a cough or gag that will further increase ICP. Succinylcholine is generally not the neuromuscular blocking agent of choice, because it can increase CBF and ICP. Other rapid onset neuromuscular blocking agents such as rocuronium are preferred (see Chapter 9 for additional information about Rapid Sequence Intubation).
If the child coughs frequently or struggles against the ventilator, peak and mean airway pressures will rise and will impede cerebral venous return. If the child is struggling “against” the ventilator, verify oxygenation and tube patency and the position and appropriateness of ventilator settings. If oxygenation, airway patency and ventilator settings are adequate, sedation, analgesia, and possibly neuromuscular blocking agents with sedation may be needed to ensure effective control of ventilation (see Chapter 5).
Intracranial Pressure Monitoring
Methods
The ICP can be monitored in a variety of ways (for additional information, see Chapter 21). The most accurate method of ICP monitoring is through an intraventricular catheter (Fig. 11-13) using a fiberoptic or standard intraventricular catheter inserted into a lateral ventricle. Intraventricular monitoring is typically performed in combination with drainage of CSF.
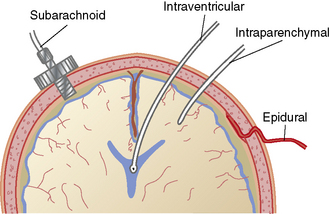
Fig. 11-13 Locations for intracranial pressure monitoring.
(From Kee KR, Hoff JT: Youman’s neurological surgery, ed 4, Philadelphia, 1996, Saunders.)
Complications of intraventricular monitoring include infection, excessive drainage, difficulty in placement, and technical complications of a fluid filled system. Drains that are in place longer than 5 days increase the risk of infectious complications (this risk is about 5%).123 Ventricular bleeding may develop during catheterization. Aspiration of the ventricular catheter is not recommended, because application of suction can tear or injure the choroid plexus, producing intracranial hemorrhage.
The intraparenchymal fiberoptic catheter is easier to place than the intraventricular catheter and offers accuracy that rivals intraventricular pressure monitoring, with a similar risk of infection. One disadvantage of this catheter is that it cannot be readjusted to a zero reference point after insertion, and its zero reference has a tendency to drift over time.123
Nursing Responsibilities
When an ICP monitor is in place, the nurse is responsible for monitoring trends in the ICP, identifying worrisome changes in ICP, evaluating accuracy of the ICP measurements, and ensuring safety of the system (see Chapter 21 for information regarding the insertion and maintenance of ICP monitors). Throughout ICP monitoring, the nurse should immediately notify the appropriate physician or on-call provider of any deterioration in the child’s clinical status, an increase in the child’s ICP above the threshold reporting request, dampening of the ICP waveform, or malfunction of the ICP system.
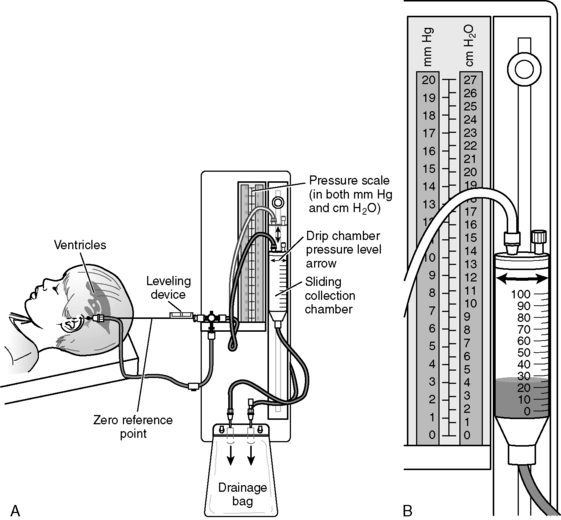
If an extraventricular drainage (EVD) system is in place, the nurse should obtain orders regarding the height for placement of the drainage chamber (above the level of the child’s lateral ventricles), whether CSF drainage is to be continuous or intermittent, and actions to take if the ICP rises. Typically, the drainage port is placed at a specified height (typically ordered in centimeters) above the patient’s lateral ventricles, and the drainage stopcock is open to allow drainage (Fig. 11-14). If the system is functioning and the stopcock is turned “open” to drainage, CSF will drain from the child’s ventricle into the collection chamber and ultimately into the drainage bag once the patient’s ICP is sufficiently high to equal or exceed the equivalent of centimeters of water (cm H2O) pressure.
Dangerous Trends in the ICP
A rise in ICP alone is not problematic. Intracranial hypertension can be harmful because it can result in a compromise in cerebral perfusion or shifting (herniation) of the brain. There is no single ICP that is universally considered to be deleterious; the level at which the ICP becomes harmful is determined by intracranial compliance, the patency of the CSF spaces, the function of cerebral autoregulation and carbon dioxide responsiveness, the cerebral metabolic rate, and the rapidity and duration of the rise in ICP. For further information, see the Intracranial Pressure section earlier this chapter (see Figs. 11-6 and 11-11).
Documenting the ICP
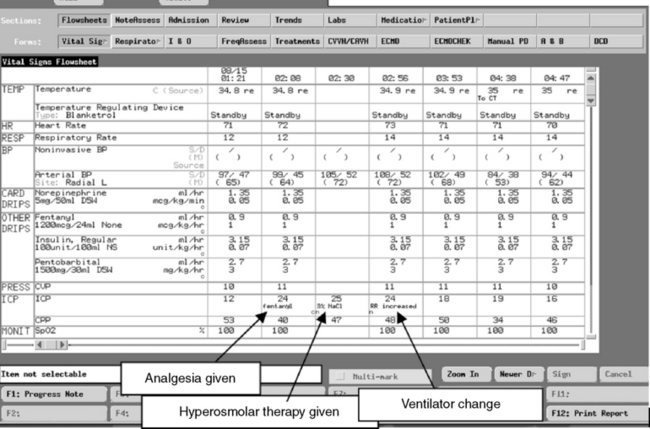
The ICP documented on the nursing flow sheet or in the electronic record should reflect the peak ICP as well as the typical ICP that hour and the CPP during a monitoring interval (see Box 11-5 for one nurse entry method and Fig. 11-15 for sample screen from an electronic record). Such documentation provides more information than simple documentation of the ICP at one point in time, particularly if the ICP varies throughout the monitoring interval. If the nurse either documents or downloads the ICP measurement at a convenient time without ensuring that the pressure is representative, it will be more challenging to identify trends in the patient’s condition. The healthcare team can also examine graphic images or trends in the ICP over hourly, daily, or longer intervals; such evaluation is better than review of isolated pressure measurements.
Box 11-5 Hourly Documentation of Intracranial Pressure
↑42 = highest ICP observed that hour
CPP, Cerebral perfusion pressure; ICP, intracranial pressure.
C waves are also rhythmic, low amplitude waves. They are related to normal changes of the systemic arterial blood pressure and ventilation, and their clinical significance is not clear.62
Reduction in Cerebral Blood Volume
An acute reduction in CBV can be achieved through hyperventilation and the creation of hypocarbia. Hyperventilation is not recommended for long-term management of increased ICP. Mild hyperventilation (to a PaCO2 of 30-35 mm Hg) can be used in the presence of an acute neurologic deterioration and fear of impending herniation.9 If the child is not intubated, the CBV may be acutely reduced using bag-mask ventilation, but intubation and mechanical ventilation are ultimately required.
Neuromuscular blockade with sedation and analgesia may be necessary to control ventilation.7 Support of ventilation is adjusted to avoid high peak inspiratory pressure and high positive end-expiratory pressure, because high intrathoracic pressure will impede cerebral venous return. Any pneumothorax must be immediately detected and decompressed to avoid impeding cerebral venous return.
Maintenance and Manipulation of Serum Osmolality
Serum osmolality exceeding 320 to 340 mOsm/L has been associated with renal dysfunction and increased mortality in patients with head injury when using mannitol as the osmotic agent.8,70 However, recent studies suggest that when using hypertonic saline solution, serum osmolality as high as 360 mOsm/L may be acceptable without adverse effects.
The healthcare team must closely monitor the child’s serum electrolytes during therapy. The osmotic diuretic effect of mannitol can produce the loss of free water and electrolytes, leading to fluid and electrolyte imbalance and possible hypotension. This hypotension can compromise cerebral perfusion, producing secondary injury in an already compromised brain. Hypertonic saline does not cause the same diuresis and may improve hemodynamics.8,70
Fluid administration
The key goals of fluid administration are to support adequate intravascular volume and systemic perfusion. If intravascular volume and systemic perfusion are adequate (i.e., euvolemia) in the child with increased ICP, fluid administration is typically calculated to provide approximately 75% of estimated maintenance fluids requirements (Table 11-8), typically administered as normal saline. This approach maintains the serum sodium and osmolality even if the child develops the syndrome of inappropriate antidiuretic hormone (see Syndrome of Inappropriate Antidiuretic Hormone in Chapter 12), a common complication of head injury and other neurologic disorders. Administration of hypotonic solutions is avoided because it can contribute to a fall in serum sodium and osmolality and development or worsening of cerebral edema.
Table 11-8 Formulas for Estimating Daily Maintenance Fluid Requirements for Children
| Daily Requirements | Hourly Requirements | |
| Fluid Requirements Estimated from Weight* | ||
| Newborn (up to 72 h after birth) | 60-100 mL/kg (newborns are born with excess body water) | – |
| Up to 10 kg | 100 mL/kg (may increase up to 150 mL/kg to provide caloric requirements if renal and cardiac function adequate) | 4 mL/kg |
| 11-20 kg | 1000 mL for the first 10 kg + 50 mL/kg for each kg over 10 kg | 40 mL for first 10 kg + 2 mL/kg for each kg over 10 kg |
| 21-30 kg | 1500 mL for the first 20 kg + 25 mL/kg for each kg over 20 kg | 60 mL for first 20 kg + 1 mL/kg for each kg over 20 kg |
| Fluid Requirements Estimated from Body Surface Area (BSA) | ||
| Maintenance | 1500 mL/m2 body surface area | – |
| Insensible losses | 300-400 mL/m2 body surface area | – |
* The “maintenance” fluids calculated by these formulas must only be used as a starting point to determine the fluid requirements of an individual patient. If intravascular volume is adequate, children with cardiac, pulmonary, or renal failure or increased intracranial pressure should generally receive less than these calculated “maintenance” fluids. The formula utilizing body weight generally results in a generous “maintenance” fluid total.
Administration of Hypertonic Saline
Although resuscitation with hypertonic saline has not been shown to improve survival when compared with use of conventional fluids in studies of adults with head trauma, in these studies the patients who received hypertonic saline required fewer interventions to treat ICP. For this reason, hypertonic saline may be used instead of mannitol for treatment of increased ICP; it can be administered intermittently or as a continuous infusion to produce an extracellular fluid shift, reduce cerebral edema, and control ICP. Use of hypertonic saline may improve CPP and brain tissue oxygenation (PbtO2). Hypertonic saline can also have additional benefits, including restoration of normal cell volume and resting membrane potential, stimulation of atrial natruretic peptide release, inhibition of inflammation, and enhancement of circulating blood volume and cardiac output.70
Potential complications of hypertonic saline administration include a rebound intracranial hypertension and higher incidence of subarachnoid hemorrhage and pontine myelinolysis.8 Hypertonic saline is a vesicant and for that reason should be administered via a large vein or central venous catheter.
Mannitol
Mannitol is widely accepted and has long been used as an osmotic agent for treatment of intracranial hypertension. Although precise mechanisms of beneficial effects have not been completely established, mannitol produces both osmotic and vasoactive effects that aide in decreasing ICP. Its initial effect on intracranial hypertension is likely related to its rheologic properties, including characteristics of flow and deformity. The osmotic effects of mannitol create a shift of water from the cellular to the extracellular (including intravascular) space that rapidly decreases blood viscosity, leading to an increase in CBF and tissue oxygen delivery. This acute fluid shift likely explains the immediate effect in reduction of ICP.8
The diuretic effect of Mannitol occurs approximately 15 to 30 minutes after administration when it is filtered out of the blood by the kidneys, causing an osmotic diuresis (i.e., it draws water with it and is excreted in urine).8 Mannitol is excreted essentially unchanged by the kidneys. Care must be taken to prevent hypovolemia and associated hypotension caused by the diuretic effects of mannitol, because these complications can lead to further cerebral ischemia.
Other Osmotic Agents
Glycerol, urea, and hypertonic glucose are no longer part of standard hyperosmolar therapy.
Drainage of Cerebrospinal Fluid
Even if CSF volume is not increased or excessive, reduction in CSF volume can be used to reduce intracranial volume and pressure. This can be accomplished using an intraventricular catheter to drain CSF. This extraventricular drain (EVD) can be intermittent or continuous (see Fig. 11-14). The collection chamber is positioned at a constant prescribed level above the patient’s lateral ventricles (leveled at the anatomic landmark of the outer canthus of the eye, the external auditory canal, or a space between these points); the higher the collection chamber above the lateral ventricles, the higher the ICP required to produce CSF drainage. Rapid drainage of a large volume of CSF is not recommended, because upward herniation of the brain can occur. If an EVD is in place, strict sterile technique is used whenever the drainage system is entered (per provider order or unit policy), for example, to change drainage bags.
CSF drainage should be performed with some caution. The most common complication of external CSF drainage is infection, although the incidence is low. The other common complication is loss of waveform transmission, typically secondary to obstruction of the catheter or drainage system. This complication can result from the presence of tissue or a clot within the system or from complete collapse or compression of the ventricular system in the setting of extremely elevated ICP. There are no pediatric data documenting the incidence of complications such as brain injury, hemorrhage, or seizure related to the placement of these drains, although the incidence of these noninfectious complications is thought to be low.6
As with the EVD, the lumbar drain collection device is leveled at a predetermined position. The level of the transducer is dependent on the goal of therapy: draining at a precise level, draining at a specified volume, or draining at an established pressure. If the lumbar drain is being utilized in conjunction with an EVD for treatment of increased ICP then the collection chamber should be raised to the same level as the drain of the EVD. The tragus is typically used as the zero reference point.125a
General Supportive Care and Control of Oxygen Requirements and Nutrients
The use of prophylactic anticonvulsants in pediatric patients with head injury is controversial. Current guidelines recommend the use of anticonvulsant therapy to prevent seizures in high-risk pediatric patients during the first week following a traumatic brain injury, noting that infants and small children are at greater risk for early posttraumatic seizure activity than are older children and adults.14 Late prophylaxis (after the first week) should not be used.
Anesthetic, sedative, analgesic, and neuromuscular blocking agents (lidocaine, fentanyl, midazolam, vecuronium) are often administered to reduce the cerebral metabolic rate and improve the cerebral oxygen delivery and supply relationship (see Chapter 5 for information about the drugs). The use of barbiturates is reviewed in “Barbiturate therapy,” below.
Gastrointestinal function should be monitored closely, and stress ulcer prophylaxis provided until feedings are initiated. Stool softeners should be administered as needed. Unless contraindicated, the head of the bed should be elevated to 30 degrees, to decrease risk of aspiration and ventilator-associated pneumonia. Regular oral care and frequent assessment of endotracheal secretions should be instituted (per protocol to prevent ventilator-associated pneumonia) to detect, prevent, or minimize the risk of pneumonia associated with aspiration (see Chapter 9).
Uncontrolled endogenous or exogenous hyperglycemia can be harmful to critically ill patients and can increase complications and decrease survival. Hyperglycemia is associated with a worse outcome in children with head injury and following cardiac arrest, but it is unclear whether the hyperglycemia is the cause or a symptom of the problem. Insulin infusion to prevent hyperglycemia has been associated with reduced ICU mortality in adult studies and in one multicenter pediatric study,128 but it will increase hypoglycemic episodes. The relative risk of hyperglycemia and potential harm from hypoglycemia must be considered when contemplating insulin infusion for glucose control. If an insulin infusion is provided to control hyperglycemia, it is typically used during the first 12 to 18 hours of critical care, with careful monitoring of serum glucose concentration (using point of care testing, if possible). A glucose infusion may be added and titrated to prevent significant hypoglycemia during the insulin infusion.
Barbiturate Therapy
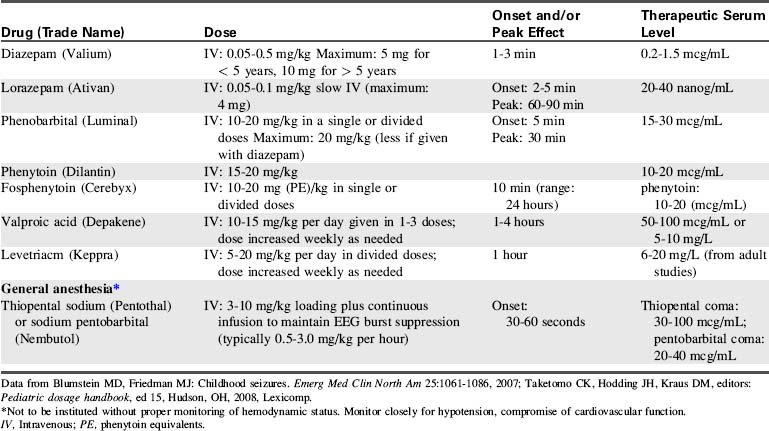
Thiopental, phenobarbital, or pentobarbital may be administered to induce coma. Thiopental is used most often for short-term anesthesia. Pentobarbital usually is preferred for continuous infusion because it has a shorter half-life than phenobarbital, so a shorter time is needed between cessation of therapy and drug elimination (i.e., drug concentration becomes subtherapeutic—and the patient should awaken—more rapidly after infusion is discontinued). An initial pentobarbital loading dose of 10 to 15 mg/kg is administered, followed by a continuous infusion of 1 mg/kg per hour.10,120 The dose is increased until coma is achieved.
A rare complication of pentobarbital infusion is propylene glycol toxicity. Propylene glycol is the vehicle in which pentobarbital intravenous (IV) formulation is prepared. If large amounts of pentobarbital are administered in a short period of time, propylene glycol toxicity may result. Initial signs of toxicity include hyperosmolarity and lactic acidosis, which can cause renal dysfunction and multisystem organ failure.24,88 During barbiturate therapy, the healthcare team should monitor for signs of toxicity.
Once medical therapy is maximized the only additional alternative to control increased ICP is decompressive craniectomy. This procedure is controversial and not employed in all centers. However, for patients with severe traumatic brain injury and refractory intracranial hypertension the procedure lowers ICP and may improve outcome. Removal of a portion of the skull, especially over the injured area allows additional room for the brain to swell without further restricting blood flow to the parenchyma. A decompressive craniectomy may be most beneficial in young patients with severe TBI and refractory intracranial hypertension from inflicted head trauma or those with some or all of the following: diffuse edema on CT scan, <48 hours post injury, no episodes of ICP >40 mm Hg prior to surgery and a GCS of >3 at some point after injury.11a
Additional Controversial Therapies
Steroid administration does not improve survival or recovery from increased ICP caused by trauma or ischemia. It can, however, reduce cerebral edema in patients with mass lesions, specifically brain tumors or discrete hematoma12; these problems remain the only indications for the use of steroids in the treatment of increased ICP. Complications of steroid administration include gastrointestinal bleeding, hyperglycemia, and increased susceptibility to infection. When discontinuing steroid therapy that has been used for more than a few days, taper the dose gradually.
The use of therapeutic hypothermia is controversial for pediatric patients with increased ICP following traumatic brain injury. There are currently no data to support the use of therapeutic hypothermia except as treatment to be considered in cases of refractory intracranial hypertension.11 There is some support for the use of therapeutic hypothermia in the adult and neonatal patients with presumed hypoxic-ischemic injury following cardiopulmonary resuscitation; its role in the pediatric population remains unclear and is currently under investigation.63
Psychosocial Support
If the child will require rehabilitative therapy, such therapy should be initiated as soon as possible so that the child’s progress or discharge from the unit is not delayed unnecessarily. Physical and occupational therapists should be consulted on admission to assist with prevention of contractures, foot drop, and other complications of long-term immobility. Prior to initiating oral intake, speech therapists should be consulted for evaluation of swallow. If the child is not expected to recover, each member of the healthcare team should be aware of the prognosis, the information provided to the family, and the family’s response to this information, so that consistent and constructive intervention can be planned (see the Brain Death and Organ Donation section in this chapter and see Chapter 3).
Coma
Etiology
Normal responsiveness to environmental stimuli requires normal functioning of the cerebral hemispheres and the reticular system. A normal state of consciousness is present when the patient is aware of the environment, can be aroused from sleep, and is oriented (as age appropriate) to time, place, and person. A decreased level of consciousness is present if the child is abnormally lethargic or confused or if the child (as age-appropriate) is not oriented to time and place. Stupor is a state of decreased consciousness from which the child can be aroused only through the application of vigorous and repeated external stimuli. Coma is a state of decreased consciousness in which the child demonstrates no verbal response despite the provision of strong external stimuli (see Table 11-5). Children in coma may, however, demonstrate motor response to noxious stimuli (e.g., deep pain or suctioning).
It is probably helpful to divide the causes of coma into structural and toxic or metabolic problems. Structural coma results from actual physical injury to the brain. Treatment is aimed at preventing or limiting cerebral swelling or edema and preventing or treating increased ICP. Structural coma may also be caused by a space occupying lesion or an intracranial hemorrhage. Toxic or metabolic coma results from electrolyte or acid-base imbalances, liver or renal failure, or the ingestion of toxic substances. Treatment of this type of coma is aimed at removing or neutralizing the toxin.102
Pathophysiology
Coma is a lack of consciousness. Consciousness is a set of neural processes that allow perception and comprehension of and action on the internal and external environment.23,100,102 Comatose patients lack two neurophysiologic processes that occur in consciousness: arousal and awareness.
Clinical Signs and Symptoms
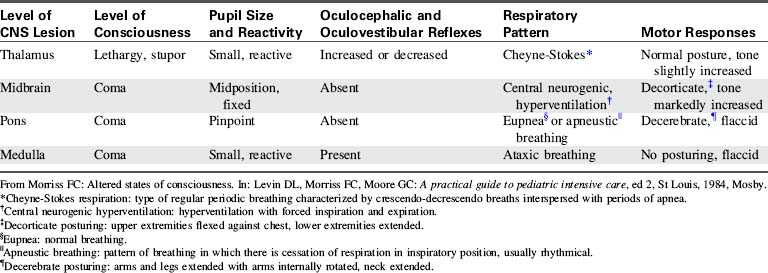
Assessment of comatose children should include all aspects presented in the section on the clinical signs and symptoms of increased ICP. This includes frequent evaluation of the child’s level of consciousness and neurologic function. The child’s pupil size and reaction to light should be assessed hourly and whenever the clinical condition changes. Although there are no characteristic changes in pupil response associated with coma, characteristic changes may occur as a result of the underlying cerebral insult or the development of increased ICP (see Fig. 11-8 and Table 11-9). The use of a standardized tool such as the GCS (see Table 11-6) will facilitate consistent, objective scoring by different providers and identification of changes over time.
The assessment of cranial nerve function is important, because the evaluation of higher brain function is impossible in unresponsive children. Thorough cranial nerve evaluation is typically performed by the physician or advanced practice nurse. The bedside nurse will also evaluate cranial nerve function. The nurse assesses function of the oculomotor nerve (third cranial nerve) when evaluating pupil constriction to light, the acoustic nerve (eighth cranial nerve) when monitoring the child’s response to voice or noise, and the glossopharyngeal and vagus nerves (ninth and tenth cranial nerves that produce a gag reflex) when suctioning the child’s airway. (See Table 11-2 for a list of cranial nerve functions.)
Deep tendon reflexes, such as the patellar reflex, can be thoroughly checked by the physician or advanced practice nurse. The bedside nurse or provider can attempt to elicit clonus by briskly flexing the wrists and ankles. Clonus is present if the extremities then rhythmically flex and contract. Exaggerated deep tendon reflexes, sustained clonus, or spasticity may be present if the child is fatigued, but they also may indicate the presence of upper motor neuron lesions (cerebral cortex injury).111
The nurse will also evaluate and document the child’s posture and limb movements. The development of decorticate rigidity or decerebrate posturing should be reported to an on-call provider. Decerebrate posturing usually indicates damage to lower (more basic) brain centers; however, some comatose patients demonstrate alternating decorticate rigidity and decerebrate posturing (see Fig. 11-9).
The nurse caring for the child in coma must carefully assess the child’s respiratory rate and pattern and the effectiveness of the child’s airway, oxygenation and ventilation. Potential abnormalities in respiratory pattern have been summarized previously (see Alterations in Respiratory Pattern in the Clinical Signs and Symptoms section of Increased Intracranial Pressure). The most common respiratory patterns observed in the comatose patient include Cheyne-Stokes respirations (alternating bradypnea and hyperpnea), central neurogenic hyper-ventilation (constant rapid respiratory rate in the absence of hypercapnia or hypoxemia), apneustic breathing (pauses after inspiration and possibly after expiration), cluster breathing (irregular breathing associated with irregular pauses), and ataxic breathing (extremely irregular rate, rhythm and depth of breaths). Table 11-9 correlates abnormal breathing patterns with levels of cerebral injury (see, also, Fig. 11-7 for an illustration of abnormal breathing patterns).
Management
Support of Vital Functions
When the child is comatose or when pharmacologic coma is induced, venous pooling of blood occurs41 and can produce a relative hypovolemia. The administration of additional IV fluids may be required to maintain an adequate central venous pressure and systemic perfusion.
If the comatose child is always kept in the recumbent position, orthostatic hypotension can result when the child initially resumes the upright position.41 In addition, the recovering child may be extremely frightened when immobilized in the supine position, with caretakers looming overhead. If possible the child should be placed in the semi-Fowler’s position several times each day. This position provides the recovering child with a different view of the unit, helps to prevent the development of orthostatic hypotension, and allows maximal diaphragm excursion and chest expansion. This positioning can be provided for the small infant through the elevation of the head of the crib mattress or the use of an upright infant’s seat.
Maintenance of Adequate Nutrition
If enteral feedings are attempted, small amounts of formula are initially provided, and the amount and concentration is then advanced slowly as tolerated. When continuous nasogastric feeding is tolerated, the child can be advanced to small bolus feedings. During this time, the nurse should measure and record the residual formula remaining in the stomach before feeding and should assess the child’s abdominal girth and firmness throughout the feeding. The comatose child might not tolerate enteral feedings, because immobilization may produce a paralytic ileus. If gastric distension, diarrhea, vomiting, or gastric reflux develops, enteral feedings should be discontinued, and parenteral alimentation should be instituted (see Parenteral Nutrition in Chapter 14).
Prevention of the Complications of Immobility
The insertion of multiple invasive monitoring lines and drains and other tubes increases the patient’s risk of hospital-acquired infection. Good hand washing technique is one of the best ways to avoid the transmission of infection. Strict hand washing technique must be used when handling the patient catheters and tubes. Aseptic technique must be used when suctioning the ET tube. For additional measures to prevent ventilator associated pneumonias, see Chapter 9.
All skin puncture sites require inspection at least once each shift, with wound drainage, wound fluctuance, erythema, or odor reported to an on-call provider. Other signs of infection include an elevation in white blood cell count and fever. In small children, a decrease in platelet count can indicate the presence of sepsis and the development of disseminated intravascular coagulation (see Chapters 6 and 16). If infection is suspected, obtain appropriate wound, serum, or catheter cultures per order or protocol, and administer antibiotics as needed.
Psychosocial Support
Significant work has been completed in recent years to develop helpful tools to predict functional outcome after severe brain injury. These tools may be helpful in discussing long term outcomes with families.1
As a rule, children demonstrate faster and more complete recovery from coma than do adults. However, it is important to note that the child’s brain is growing rapidly (particularly through the age of seven), making it extremely difficult to predict the degree of permanent neurologic damage that will result from a neurologic injury. Neurologic sequelae often are not manifest for months or years after the insult has occurred.1 Recovery, too, can occur over years.
Status Epilepticus
Etiology
Status epilepticus is defined as a continuous seizure lasting longer than 30 minutes or more than two seizures without return to a baseline level of consciousness between events.119 The most common causes of status epilepticus in children include high fever secondary to a non-CNS infection and sudden discontinuation of an anticonvulsant drug.46 Approximately 20% of children with status epilepticus have a chronic encephalopathy or seizure disorder. In approximately half of children who develop status epilepticus, no specific cause can be identified. The remaining causes of status epilepticus in children include acute encephalopathy (such as that resulting from meningitis or encephalitis), metabolic disorders (including hypoxia, acidosis, sepsis, dehydration, hypocalcemia, hypoglycemia, hyponatremia, and hypernatremia), ingestion of toxic substances, and head injury.119
Causes of status epilepticus vary somewhat depending on the age of the child. The most common cause of seizures in the neonatal period is intrauterine or perinatal hypoxia. Metabolic disorders, intraventricular hemorrhage, and infection can also cause neonatal seizures. High fever, toxic ingestion, and preexisting seizure disorders are more common causes of status epilepticus during later infancy and early childhood. Status epilepticus during adolescence often is caused by metabolic disorders, toxic ingestions, and head injury.78 Status epilepticus is most common in children younger than 3 years; the incidence then decreases with advancing age.25
Pathophysiology
Some clinical conditions may predispose the child to the development of seizures. These conditions include fatigue, pain, specific photic stimuli (usually rapidly and regularly flickering lights or images), or abrupt changes (particularly a fall) in serum sodium concentration (see Hyponatremia in Chapter 12).
Clinical Signs and Symptoms
Generalized myoclonic, or tonic-clonic, status epilepticus is characterized by bilateral extensor or flexor (or both) muscular contractions that occur continuously or in a series lasting for hours or days. This form of status epilepticus is typically related to degenerative brain disease, toxic encephalopathy, or anoxic brain damage. The EEG usually reveals the presence of many spikes with slow background activity.112,119
Generalized absence status epilepticus is associated with periods of confusion and with a decreased level of consciousness or stupor that is not associated with any abnormal muscle activity. This form of status epilepticus most commonly develops in children with persistent petit mal seizures. It is rarely associated with acute CNS pathology. The EEG shows bilateral, regular, generalized, symmetrical spikes.112,119
Focal motor status epilepticus is produced by a localized area of cortical injury or by metabolic disease. It produces rapid, focal, clonic movements of one part or one side of the body without loss of consciousness. The EEG reveals focal spikes.112,119
Management
Support of Vital Functions
Throughout the episode of status epilepticus, carefully assess and support systemic perfusion. Treat hypotension or bradycardia promptly because they can result in inadequate systemic and cerebral perfusion (see Treatment of Shock in Chapter 6). If vascular access is not present, insert a large-bore venous catheter as quickly as possible to allow the infusion of IV fluids and medications. Obtain blood samples for analysis of arterial blood gases, electrolytes, glucose, calcium, and BUN, and to evaluate serum concentration of anticonvulsants, as indicated. If the child has just been admitted to the critical care unit, obtain a urine specimen for toxicology screening.
Rapid correction of any existing metabolic derangement is needed and may abolish the seizure activity. Because hypoglycemia is a relatively common and rapidly treatable cause of seizures in critically ill children, evaluate the serum glucose concentration, using point-of-care testing if available. Administration of hypertonic glucose (D25:1.0-2.0 mL/kg or D50:0.5-1.0 mL/kg), may be ordered empirically after blood samples are drawn, but before the results of serum electrolyte and glucose analyses are available. Hyponatremia, hypernatremia, hypocalcemia, or hypomagnesemia should be treated if present (see Chapter 12).119
Anticonvulsant Therapy
Lorazepam (Ativan) is the first-line treatment for all types of status epilepticus beyond the neonatal period.119 It has a rapid onset of action and stops seizure activity in most patients within 2 to 5 minutes.112 Its effects last longer than those of diazepam; therefore, it may be preferable to diazepam. It is administered intravenously in a dose of 0.05 to 0.1 mg/kg (maximum single dose: 4 mg) over 2 to 5 minutes; the dose may be repeated in 10 to 15 minutes if needed. This drug may produce respiratory depression, although the incidence of this complication is lower than with diazepam. The nurse must be prepared to institute respiratory support if needed.
Diazepam (Valium) is used commonly in the initial treatment of seizures. The initial dose of 0.05 to 0.5 mg/kg (maximum: 5 mg for children under 5 years if age, and 10 mg for children over 5 years of age) is typically administered by slow IV push over several (3–5) minutes. The drug should be effective within minutes; peak diazepam concentrations are reached in the brain within 1 to 5 minutes. Because the serum half-life of the drug is relatively short, seizures often return and may require repeat dosing at 15-minute intervals. It is often necessary to add a second, longer acting anticonvulsant at this point.112
Fosphenytoin (Cerebyx), the water soluble prodrug of phenytoin, is often used in the management of status epilepticus and is preferred to phenytoin because it is less caustic to the veins and is compatible with most IV fluids.126 It is given as a loading dose of 10 to 20 mg phenytoin equivalents (PE) followed by a maintenance dose of 5 to 7 mg PE/kg per day in two to three divided doses. The therapeutic serum level is 10 to 20 mcg/mL. Side effects are similar to those of phenytoin and include hypotension, if the drug is administered too rapidly, bradycardia, tachycardia, and vasodilation.120
Phenobarbital is used widely in the treatment of seizures in children, particularly infants, because it has a relatively long serum half-life and a wide therapeutic range. If phenobarbital is administered without diazepam, a loading dose of 10 to 20 mg/kg (given as a single dose or in divided doses; maximum total loading dose, 20 mg/kg) is administered intravenously at a rate no faster than 1 mg/kg per minute (or maximum rate, 50 mg/minute).112,120
Peak concentrations of phenobarbital can develop in the brain within approximately 5 minutes, although the drug usually is not maximally effective for approximately 30 minutes. Side effects of phenobarbital include respiratory depression, bronchospasm, apnea, bradycardia, hypotension, and sedation. Occasionally, phenobarbital produces CNS irritability in children.120 The major disadvantage of this drug is the long-term sedation it produces, making further neurologic evaluation difficult.
Valproic acid (valproate, Depakene) has long been used in the treatment of seizures refractory to first line agents. However, new information suggests that IV valproate may have a role in initial treatment of status epilepticus and may be as effective as phenytoin or fosphenytoin.124,130 It is available in oral and IV formulations. A rectal formulation can be prepared by diluting valproate syrup (250 mg per 5 mL) with tap water in a 1:1 ratio.
Levetiracetam (Keppra) is used primarily for the treatment of partial onset seizures, but more recently it has been used in the treatment of status epilepticus refractory to first line agents.130 The usual dose is 5 to 20 mg/kg per day divided into two doses per day, with weekly increases of 10 mg/kg per day to a maximum dose of 60 mg/kg per day. Dose recommendations for status epilepticus vary and firm recommendations do not currently exist; therefore, providers will need to titrate doses and closely monitor patient response.124,130
Barbiturate Coma
Once adequate monitoring and support are established, phenobarbital or pentobarbital is administered in doses sufficient to induce coma. Thiopental sodium often is preferred because it has a shorter half-life, so that the effects will disappear in a shorter period of time following discontinuation of the drug. A loading dose is required, followed by a continuous infusion or hourly dose titrated to maintain burst suppression (associated with a reduced cerebral metabolic rate) on the EEG.120 The child’s blood pressure and systemic perfusion must be monitored closely as the barbiturate dose is increased, and appropriate therapy must be provided to maintain systemic perfusion.
Table 11-10 includes a list of common anticonvulsant therapies for status epilepticus. Once the child’s seizures are controlled, long-term anticonvulsant prophylaxis and patient and family education is planned.112
Non-pharmacologic Management of Seizures
Several forms of ketogenic diets have been used for centuries to reduce seizures. These diets have gained in popularity in recent years, particularly for patients who are refractory to antiepileptic agents. The precise mechanism by which the diet inhibits seizures is unclear.132 The diet induces ketosis by providing most daily calories from fat sources, so the classic diet strictly limits carbohydrate and protein intake to minimum daily quantities. Recently, more liberal forms of the diet have emerged, allowing for slightly more protein and carbohydrate intake.
Vagal Nerve Stimulators
Wound care is similar to that needed following cardiac pacemaker implantation. In addition, electromagnetic and electrostimulation devices, including magnetic resonance imaging are avoided.73,115
Surgical Interventions
Care for these patients is similar to general postoperative neurosurgical critical care (see the Postoperative Care of the Pediatric Neurosurgical Patient, later in this chapter). Anticonvulsants are generally continued in the initial postoperative period and then gradually weaned as tolerated. The nursing care plan must include a description of the patient’s baseline seizure activity, the procedure performed, and any anticipated postoperative deficits.94,95
Endocrinopathies Associated with Neurologic Insults
Diabetes insipidus (DI), the syndrome of inappropriate antidiuretic hormone (SIADH), and cerebral salt wasting (CSW) are common endocrine complications of neurologic injury or surgical procedures. CSW associated with head injury or neurosurgical procedures usually develops later (2-7 days) after injury. Please see Chapter 12 for a complete discussion of the pathophysiology, clinical presentation and management of these disorders. Table 12-7 compares and contrasts the presentation and effects of DI, SIADH, and CSW.
Brain Death and Organ Donation
Etiology
Traditionally, the pronouncement of brain death was required before transplantation of solid organs from the donor. However, donation after cardiac death protocols do not require brain death pronouncement. The Joint Commission (formerly the Joint Commission on Accreditation of Healthcare Organizations [JCAHO]) now requires that hospitals have policies regarding donation after cardiac death. This requirement is expected to increase the number of potential organ donors by as much as 20%.84 Care of the donor presents a number of unique challenges for the healthcare team; these are discussed in Chapters 3 and 24.
The declaration of brain death requires appropriate clinical examination and testing per institutional policy. Documentation of the pronouncement of brain death must be provided in the patient’s chart. Federal legislation (Box 11-6) requires that the local federally funded organ procurement agency be informed about the presence of a potential organ donor, and that the family of a potential organ donor be informed about the option of organ and tissue donation.42 This notification must also be documented in the patient chart.
Box 11-6 Public Law 99-509: “Required Request” Law
I. Hospitals receiving federal funding must establish written protocols for identification of potential donors that:
II. Local organ procurement agencies must abide by rules and requirements of Organ Procurement and Transplantation Network
Every state has a law or a precedent set by the appellate court that recognizes the cessation of brain function as a legal definition of death. In addition, every hospital receiving Medicaid reimbursement is required to have protocols in place for the identification of potential organ donors to the local organ procurement agency. Each nurse must be familiar with state law and hospital policy regarding the pronouncement of brain death and responsibilities in discussing potential organ and tissue donation with the family.59
Criteria for Pronouncement of Brain Death
The pronouncement of brain death has two requirements: (1) an irreversible condition and (2) complete cessation of clinical evidence of brain function. The cause of brain death should be known. A variety of criteria for brain death pronouncement in children have been proposed, but most are consistent in the clinical indications of brain death. These criteria differ in their requirements of adjunctive tests.2,3,16,17,121 The healthcare team must apply these criteria consistently.
Physical Examination to Document the Absence of Brainstem Function
The child must have no voluntary movement or response to stimuli in the absence of neuromuscular blockade. All brain stem function must be absent. The cranial nerve functions will be evaluated as possible through the testing of the following reflexes: oculocephalic, oculovestibular, cough and gag, corneal and pupil response to light. The pupils must be dilated or fixed in midposition, and apnea must be present. Flaccid paralysis must be present (Box 11-7).

 Be sure to check out the supplementary content available at
Be sure to check out the supplementary content available at