CHAPTER 7 Neurologic disorders
General neurologic assessment
Level of consciousness
• Assess for orientation, drowsiness, inappropriate use of words, slurred speech, arousability, confusion, and amnesia.
• Close monitoring of level of consciousness (LOC) is essential to assess for determining deterioration, and even a slight change may indicate emergent intervention is needed.
• For specifics of how to assess using levels of stimulation, refer to Appendix 2, Glasgow Coma Scale (GCS).
Vital signs
Refer to specific sections for key vital sign changes specific for the type of neurologic disorder.
Key cranial nerve assessment
It is not always necessary to assess all 12 cranial nerves (see Appendix 3). Specific neurologic disorders will address cranial nerve impairments.
• Assess the nerves responsible for vision (optic), pupillary response (oculomotor), and eye movements (oculomotor, trochlear, abducens).
• Assess facial/corneal sensation and chewing (trigeminal) and facial muscle movement and taste (facial).
• All functions are evaluated bilaterally (e.g., both eyes, both sides of face, etc.).
Assess motor and cerebellar function
• If patient can walk, assess gait.
• Ask patient to walk heel to toe to check for balance and coordination.
• Perform Romberg test: Ask patient to close eyes and stand with feet close together while you stand nearby in case patient sways/falls (abnormal response indicative of cerebellar dysfunction).
• Ask patient to squeeze your hands and push feet against your hands, to assess if strength is equal on both sides.
• Note any involuntary movements (tremors, jerking, fasciculations) and general posture.
• Move the patient’s joints through passive range-of-motion (ROM) exercises, noting any tenderness of involved muscle groups.
• To further evaluate muscle strength, have patient perform active ROM exercises while you apply resistance against the movements. Use the following rating scale for muscle strength:
| MUSCLE STRENGTH RATING | |
|---|---|
| Score | Description of Strength |
| 5/5 | Patient moves joint with full ROM against normal resistance and gravity |
| 4/5 | Patient moves joint with full ROM against mild resistance and gravity |
| 3/5 | Patient moves joint with full ROM against gravity only |
| 2/5 | Patient moves joints with full ROM but not against gravity |
| 1/5 | Patient’s muscle contracts in an attempt to move joint; joint does not move |
| 0/5 | Patient does not visibly attempt to move; no muscle contraction; paralysis |
ROM, Range of motion.
• Perform specific testing for abnormalities as appropriate:
Sensory assessment
• Assess perception of touch, proprioception, pain, temperature, and vibration (if possible). Ask patient to close eyes while you apply stimuli. The patient should not be given the opportunity to anticipate your moves. Compare the same stimulus on the right side of the body to the identical location on the left side of the body. Note if patient perceives stimuli symmetrically and appropriately (sharp versus dull using a needle versus a cotton swab, or hot versus cold). Compare proximal and distal parts of arms and legs when testing pain and touch.
• Superficial and deep reflexes are tested on symmetrical sides of the body and compared noting the strength of contraction.
• Test vibratory sense (with vibrating tuning fork) distally (on the tip of big toe or finger) and ask when patient feels the vibration stop.
• For position sense, move distal joints about using very light touch and ask about the position the patient perceives of the joint.
• Two-point discrimination can be done using a bent paper clip. Note the smallest distance between the two points at which the patient senses two points are pressing on the skin. Document using a dermatome map.
Improvement in both motor and sensory perception may be seen as cerebral edema subsides.
Dysphagic screening
| Test | Purpose | Abnormal Findings |
|---|---|---|
| Cerebral angiography | Digital subtraction angiography visualizes blood flow. Involves use of intravascular catheter The gold standard for evaluating cerebral vasculature Invasive procedure with minimal risk used to visualize the cerebral blood vessels |
Areas of reduced cerebral blood flow, aneurysms, arteriovenous malformations (AVMs), vascular abnormalities Used with interventional neuroradiologic procedures such as coiling, AVM embolization (gluing) Provides specific information on the cause of stroke by identifying the blood vessel involved |
| Computed tomography (CT) of brain | Performed emergently, is the gold standard of differentiating ischemic from hemorrhagic stroke; may be done at intervals to monitor progress Assess details of structures of bone, tissue, and fluid-filled space. Detects exudate, abscesses, and intracranial pathology (e.g., tumors, brain injury) Assess for hydrocephalus. |
Shift of structures due to enlarged mass, edema, exudate, abscesses, fresh hemorrhage, hematomas, infarction, hydrocephalus Can visualize facial skeleton and soft tissue structures for abnormalities (e.g., tumors, brain injury) Within the first few hours after an acute ischemic stroke, the scan may appear normal. Intracranial hemorrhage is easily diagnosed on CT—blood appears as a bright white signal. |
| Continuous electrocardiographic (ECG) monitoring | Evaluate cardiovascular status, especially during medication administration. | Phenytoin and other AEDs can cause dysrhythmias and hypotension. |
| CT angiography | Less invasive than cerebral angiography; involves use of contrast media injection into peripheral vein and use of CT scanner | Visualize intra-arterial clot, small intracranial aneurysm, AVM |
| CT perfusion or CT-xenon scan (CTP) | Provides information related to cerebral blood flow (CBF)and volume Used to guide clinical decision making regarding the use of thrombolysis or interventional procedures |
Compromised blood flow; a limited test; cannot detect infarcted tissue |
| Electroencephalography (EEG) | Evaluate the brain’s electrical activity for ongoing seizures, even if there are no clinical signs of seizures. | Diagnosis of seizures and localization of structural abnormalities Also used as element of criteria for brain death |
| Electromyography (EMG) or nerve conduction velocity (NCV) | Assesses nerve conduction velocity deficit as a result of the demyelination of peripheral nerves | EMG and NCV demonstrate profound slowing of motor conduction velocities and conduction blocks several weeks into the illness. |
| Lumbar puncture (LP) with cerebrospinal fluid (CSF) specimen for analysis | Measures CSF pressures and obtains CSF specimen when infection, such as meningitis or neurosyphilis, is suspected May be performed when SAH is suspected and CT is normal |
Elevated protein, low glucose, elevated WBC |
| Magnetic resonance imaging (MRI) of brain | Minute oscillations of hydrogen atoms in brain create graphic image of bone, fluid, and soft tissue Provides a more detailed image MRI is most useful for ischemic patients in identifying the cause and area involved. Provides detailed information regarding the area of injury or its vascular supply (MRA) Diffusion-weighted imaging (DWI) is a measurement of edema, whereas perfusion weighted imaging (PWI) is a measurement of global CBF. |
Infarcts, areas at risk or ischemic areas, vascular defects, stenosis, occlusion |
| Positron emission tomography (PET) and single-photon emission computed tomography (SPECT) | To evaluate brain metabolism and blood flow using three-dimensional imaging produced using a radioactive tracer | Demonstrates abnormal function of the brain by revealing abnormal structures, metabolism, and perfusion Locates areas of brain causing seizures, head injury, and some disorders (e.g., Alzheimer’s) |
| Radioisotope brain scan | Examine areas of blood flow through concentration of isotope uptake in the brain. | Increased or decreased blood flow intraoperatively or assess for postoperative cerebral infarction Lack of uptake may indicate cerebral brain death. |
| Transcranial Doppler | Noninvasive and can be done serially at the bedside Evaluates the intracranial vessels and assesses the velocity of blood flow in the anterior and posterior cerebral circulation Also used to evaluate vasospasm, to determine brain death via detection of cerebral circulatory arrest, for intraoperative monitoring, and to locate emboli |
Arterial narrowing vasospasm, cerebral circulatory arrest, emboli due to vasospasm Can also be used to confirm absent blood flow in brain death |
Brain death
Pathophysiology
Brain death is defined as irreversible loss of function of the brain, including the brainstem and respiratory centers. Cardiac death is the cessation of mechanical action/pumping of the heart, resulting in absence of pulse, heart sounds, blood pressure, and respirations. Brain death is most frequently the result of increased intracranial pressure (ICP) caused by severe traumatic head injury or hemorrhagic stroke caused by ruptured cerebral aneurysm with subarachnoid hemorrhage (SAH) or intracranial hemorrhage (ICH). A significant number of patients with large acute ischemic strokes (AIS) experience cerebral edema and herniation. Hypoxic-ischemic encephalopathy with massive brain swelling after prolonged cardiopulmonary resuscitation or asphyxia and encephalopathy with cerebral edema resulting from fulminant hepatic failure may also result in increased ICP, herniation, and brain death.
When pressure in one of the two compartments (supratentorial or infratentorial) is markedly elevated, the brain structures and blood vessels within the cavity are compressed, resulting in ischemia, hypoxia, and, if uncontrolled, cerebral anoxia. When blood flow is minimal to absent, the hypoxic/anoxic brain tissues become more edematous. Eventually, no space remains for further expansion. The skull cannot expand and the tentorium expands minimally, so the brain is forced through the available openings. The movement or displacement through an opening causes further compression of blood vessels, with possible laceration and destruction, which leads to necrosis of brain tissues and brain death (see Traumatic Brain Injury, Neurologic Herniation Syndromes, p. 333).
Neurologic assessment: brain death
History and risk factors
• Severe traumatic head injury (motor vehicle accident, gunshot/other assault, recreational/industrial accidents)
• Ruptured cerebral aneurysm with SAH
• ICH resulting in intracerebral hematoma
• Large AIS resulting in massive cerebral edema and/or brain herniation
• Prolonged cardiopulmonary resuscitation
• Asphyxia (asthmatic cardiac arrest, drug overdose, hanging, carbon monoxide poisoning, drowning, meningitis)
Vital signs
• Mild hypothermia: Core temperature must be greater than 32°C (90°F), but less than 36.5°C (97°F).
• Hypotension: With mechanical ventilation in place, blood pressure is greater than 90 mm Hg. Without mechanical ventilation, the heart rate will decrease, resulting in hypotension and eventually asystole.
• Apnea: No spontaneous respirations when mechanical ventilation is suspended. A formal apnea test is required to confirm the absence of respirations.
Observation/inspection/palpation
• Coma or unresponsiveness: Patient does not respond to verbal stimuli, touch, or deep pain induced by pressure exerted on nail beds, the supraorbital area of the skull, or the temporomandibular joint or rubbing the sternum.
• Brainstem reflexes/cranial nerve function: Absent
• Apnea: No spontaneous respirations. Structured testing is required for diagnosis.
• Euvolemia: Patient does not exhibit signs of dehydration. If dehydration is present, patient must be hydrated prior to structured apnea testing.
Screening labwork
• Toxicology screen: Evaluates for presence of toxic doses of recreational drugs, medications, or poisons (see Drug Overdose, p. 868)
• Basic metabolic panel/blood chemistry: Identifies electrolyte imbalance, including hypoglycemia, hyperglycemia, and acidosis (using bicarbonate/CO2). May also reflect patient’s volume status, including dehydration and hypovolemia (see Fluid and Electrolyte Disturbances, in p. 37).
• Arterial blood gas (ABG) analysis: Evaluates for hypoxia, acidosis, and hypercapnia (see Acid-Base Balance, p. 1).
Making the diagnosis of brain death
The three cardinal signs/symptoms in brain deat h are coma, absent brainstem reflexes, and apnea according to the AAN guidelines. Patients must have all three findings to be considered dead (brain dead patients are dead). There should be at least two separate clinical examinations, preferably by a neurologist, with at least 2 hours between examinations. If clinical uncertainty is present, two different physicians, preferably neurologists, should examine the patient following completion of appropriate diagnostic testing to ensure patient has not overdosed on a drug; has ingested a poison; has undiagnosed endocrine system dysfunction, electrolyte imbalance, or dehydration; and is free of untreated significant hypoxia, hypercapnia, or acidosis. Adherence to the AAN guidelines varies among the large medical centers in the United States. Diabetes insipidus, myxedema coma, and adrenal crisis may result from loss of the hypothalamic/pituitary regulatory axis as part of brain death. Large amounts of dextrose-containing IV fluids and insulin resistance may prompt hyperglycemia.
| Test | Purpose | Abnormal Findings |
|---|---|---|
| Arterial blood gas (ABG) analysis | Assesses for acidosis resulting from abnormal gas exchange or compensation for metabolic derangements | Low pH: Acidosis may reflect respiratory failure or metabolic crisis. Carbon dioxide: Elevated CO2 or hypercapnia reflects respiratory failure; decreased CO2 may reflect compensation for metabolic acidosis. Hypoxemia: PaO2 less than 80 mm Hg Oxygen saturation: SaO2 less than 92% Bicarbonate: HCO3−less than 22 mEq/L Base deficit: less than –2 |
| Apnea test
• The ventilator is disconnected, the patient placed on 100% oxygen via T-tube and observed for apnea. Vital signs must be stable (mild hypothermia, normotensive BP, euvolemia, with normal Pao2 and Pco2) to begin the test. PaO2, PCO2, and pH are measured after approximately 8 minutes; the ventilator is reconnected after the ABG sample is drawn. |
The apnea test is positive if:
• No spontaneous chest or abdominal excursions that produce reasonably normal, effective tidal volumes occur.
• The arterial Pco2 is either more than 60 mm Hg or increased 20 mm Hg from the baseline Pco2.
• The ventilator must be reconnected before 8 minutes due to instability/intolerance of test.
The apnea test is negative if:
Electroencephalographic Society criteria for those with suspected brain death.
Positive findings indicate very high vascular resistance resulting from markedly increased ICP.
Collaborative management
| Once brain death has been diagnosed, the organ removal team may begin preparations for organ removal within 5 minutes under ideal conditions. If the death was unanticipated, or organ donation was not discussed or controversial, additional time is needed for approaching the donor’s family/significant other(s) regarding donation. The parameters below must be managed in order to provide the best opportunity to recover viable organs from the donor. | |
| Physiologic Parameter | Intervention |
| Maintain blood pressure. | MAP 60 to 70 mm Hg: maintain euvolemia; administer vasopressor agents (e.g., norepinephrine) if needed. |
| Monitor organ perfusion. | Monitor urine output and lactate level; consider hemodynamic monitoring with a pulmonary artery catheter. |
| Balance electrolytes. | Monitor electrolytes (Na+, K+) every 2 to 4 hours; correct to normal range. |
| Control diabetes insipidus. | Suspected diabetes insipidus (urine output more than 200 ml/hr, rising serum sodium): administer DDAVP (e.g., 2 to 4 mcg IV in adults) and replace volume loss with 5% dextrose. |
| Manage hyperglycemia. | Treat hyperglycemia: keep blood glucose 100 to 180 mg/dl. |
| Control hypothermia. | Keep temp greater than 35°C. Early use of warming blankets to prevent declining temperature is helpful; hypothermia is difficult to reverse once developed. |
| Ventilate and oxygenate. | Provide ongoing respiratory care: frequent suctioning, positioning/turning, PEEP, alveolar recruitment strategies. |
| Manage anemia. | Maintain hemoglobin at greater than 8 g/dl. |
| Control hormonal imbalances causing hemodynamic instability. | Consider hormonal replacement therapy if volume resuscitation and low-dose inotropes are ineffective for maintaining BP and/or if cardiac ejection fraction is less than 45%. Typical regimens include: Triiodothyronine (T3): 4 mcg IV bolus, then 4 mcg/hr by IV infusion Arginine vasopressin (AVP): 0.5 to 2.4 units/hr to maintain MAP 60 to 70 mm Hg Methylprednisolone: 15 mg/kg IV single bolus |
From the Australasian Transplant Coordinators Association Inc: National guidelines for organ and tissue donation, ed 3. 2006. http://www.atca.org.au/files/ATCAguidelinesonlineoct06.pdf
Care priorities
2. Allay doubts about the diagnosis:
• Spontaneous movement of limbs: spinal reflex movements may occur.
• Respiratory-like movements of the chest and abdomen: shoulder elevation and adduction, back arching, intercostal expansion, which do not produce effective tidal volume
• Sweating, blushing, and tachycardia: residual autonomic responses
• Normal blood pressure without vasopressors or sudden increases in blood pressure: residual autonomic responses
• Absence of diabetes insipidus: does not occur in some patients
• Reflexes are present: deep tendon, superficial abdominal, triple flexion, Babinski
• Words to avoid: harvest, cadaver, remains, breathing, respirator, corpse, or any complex medical terminology
• Phrases to avoid: artificial life support, will live on in others, deeply comatose
• Words to include: the deceased patient’s name, ventilator, procurement, retrieval, donation, dead
• Phrases to include: time of death, wishes regarding organ donation, reasons for declining the opportunity, religious beliefs on organ donation
CARE PLANS FOR BRAIN DEATH
Decreased intracranial adaptive capacity
Tissue Perfusion: Cerebral; Neurological Status: Consciousness.
1. Consult with physician or midlevel practitioner to determine hemodynamic parameters.
2. Maintain hemodynamics within set parameters.
3. Administer osmotic diuretics/rheologic agents (e.g., mannitol, dextran) as ordered.
4. Administer vasopressin as ordered if diabetes insipidus ensues.
5. Keep blood glucose level within ordered range, avoiding hyperglycemia unless using medications which induce osmotic diuresis.
6. Avoid neck flexion or extreme hip/knee flexion.
7. Consult with physician regarding optimal elevation of the head of bed.
1. Monitor neurologic status closely and compare with baseline.
2. Monitor respiratory status: rate, rhythm, depth of respirations, PaO2, PCO2, pH, and bicarbonate.
3. Monitor ICP and cerebral perfusion pressure (CPP) at rest and in response to patient care activities. Minimize activities that result in further increases in ICP.
1. Monitor pupillary size, shape, symmetry, and reactivity.
2. Assess LOC, orientation, and trend of Glasgow Coma Scale score.
3. Monitor vital signs: temperature, blood pressure (BP), pulse, and respirations (RR).
4. Monitor for corneal reflex, cough and gag reflexes.
5. Monitor EOMs and gaze characteristics.
7. Monitor for Cushing response; a late indicator of increased ICP.
Decision Making; Information Processing; Dignified Life Closure; Acceptance: Health Status
1. If cerebral perfusion promotion measures fail and brain death ensues, provide care appropriate for the dying.
2. Encourage family to share feelings about death.
3. Monitor deterioration of patient’s physical (and mental) capabilities.
4. Facilitate obtaining spiritual support for the family/significant others.
1. Assess the impact of the patient’s life situation on roles and relationships within family/support system.
2. Use a calm, reassuring approach.
3. Provide factual information concerning diagnosis, treatment, and prognosis.
4. Seek to understand the family’s perception of the stressful situation.
5. Acknowledge the patient and significant others’ religious, spiritual, and cultural beliefs surround death, dying, and organ donation.
6. Encourage gradual mastery of the situation if resistance or denial is impacting the family’s ability to accept the diagnosis of brain death.
7. Ensure the family understands brain dead patients are dead. Patients are no longer able to breathe without mechanical ventilation, will experience cardiac death when removed from mechanical ventilation, will never regain consciousness, will never interact with others, and have no ability to experience joy related to human life.
8. Explain the difference between brain death, persistent vegetative state, and cardiac death. The family and significant others may have difficulty understanding why brain dead patients are different than those in coma who can recover from their insult/injury and those in a vegetative state who can recover brainstem function to begin breathing spontaneously. Families may not be able to comprehend why when the brain is dead, the heart still functions unless mechanical ventilation is removed. Guilt may be associated with removal of mechanical ventilation since the patient appears “alive” with mechanical ventilation in place.
Additional nursing diagnoses
As appropriate, see nursing diagnoses and interventions in Nutritional Support (p. 117), Acute Respiratory Failure (p. 383), Mechanical Ventilation (p. 99), Prolonged Immobility (p. 149), and Emotional and Spiritual Support of the Patient and Significant Others (p. 200).
Cerebral aneurysm and subarachnoid hemorrhage
Pathophysiology
The critical care nurse may care for a patient with an unruptured aneurysm or a patient who is post rupture and has a diagnosis of subarachnoid hemorrhage (SAH). Unruptured aneurysms may be asymptomatic, but nearly half of the affected population experiences some warning sign or symptom prior to rupture as a result of expansion of the lesion and compression of cerebral tissue. When rupture occurs, an SAH into the subarachnoid space (SAS) and basal cisterns results. If the patient survives the initial compromise of cerebral circulation from the force of hemorrhaging arterial blood, with sharply increased ICP, the next challenge is the possibility of rebleeding and cerebral arterial vasospasm. The greatest incidence of rebleeding is between 3 and 11 days after SAH, with the peak at day 7. Mortality is about 70% overall from aneurysmal SAH. Theories regarding the cause(s) of rebleeding involve the normal process of clot dissolution coupled with fluctuations in arterial pressure.
The surge of ADH from the posterior pituitary results in SIADH, which may include hyponatremia caused by cerebral salt-wasting syndrome, or a combination of factors influencing sodium and water metabolism (Table 7-1). Fluid management strategies in this patient population may be difficult (see Syndrome of Inappropriate Antidiuretic Hormone, p. 734). Hyponatremia may occur in 10% to 50% of patients with SAH. Untreated hyponatremia may lead to intracranial hypertension, cerebral ischemia, seizures, coma, and death.
Table 7-1 CLINICAL PRESENTATION WITH CEREBRAL SALT-WASTING SYNDROME VS SYNDROME OF INAPPROPRIATE ANTIDIURETIC HORMONE (SIADH)
| Cerebral Salt-Wasting Syndrome | SIADH |
|---|---|
| Hypotension | Normotension |
| Postural hypotension | Normotension |
| Tachycardia | Normal pulse rate or bradycardia |
| Elevated hematocrit | Normal or low hematocrit |
| Decreased glomerular filtration rate | Increased glomerular filtration rate |
| Normal or elevated BUN and creatinine | Normal or decreased BUN and creatinine |
| Normal or low urine output | Normal or low urine output |
| Hypovolemia | Normovolemia or hypervolemia |
| Dehydration | Normal hydration |
| True hyponatremia | Dilutional hyponatremia |
| Hypo-osmolality | Hypo-osmolality |
| Decreased body weight | Increased body weight |
BUN, Blood urea nitrogen.
Neurologic assessment: cerebral aneurysm(s) and subarachnoid hemorrhage
Goal of system assessment
Evaluate for key nursing diagnoses requiring emergent intervention: alteration in cerebral tissue perfusion due to vasospasm of cerebral vessels and increased ICP due to decreased intracranial adaptive capacity, risk for seizure activity with potential impairment of cerebral tissue perfusion, impaired gas exchange or ineffective airway clearance due to altered level of consciousness, potential need for management of hyperglycemia, and fluid volume imbalance and potential for aspiration.
History and risk factors
Hunt and hess classification system:
Grade I: Asymptomatic, alert, and oriented
Grade II: Alert, oriented, headache, and stiff neck
Grade III: Lethargic or confused; minor focal deficit such as hemiparesis
Grade IV: Stuporous, moderate to severe focal deficits, hemiplegia, possible early decerebrate rigidity, and vegetative disturbances
Grade V: Deep coma, decerebrate rigidity, moribund appearance
Intracranial pressure
Blood in the SAS can produce acute, subacute, or chronic hydrocephalus by blocking pathways for the resorption of CSF and leading to ventricular enlargement and nonfocal neurologic deterioration. Intraventricular extension at the time of aneurysm rupture can result in symptoms of acute hydrocephalus and will require temporary external ventricular drainage for management. Nuchal rigidity may be present even in the absence of hydrocephalus. Indicators of increased ICP are listed in Box 7-1.
Box 7-1 INDICATORS OF INCREASED INTRACRANIAL PRESSURE
• Alterations in consciousness: increasing restlessness, confusion, irritability, disorientation, increasing drowsiness, and lethargy
• Increasing systolic blood pressure with a widening pulse pressure
• Irregular respiratory patterns (e.g., Cheyne-Stokes, ataxic, apneustic, central neurogenic, hyperventilation)
• Hemisensory changes and hemiparesis or hemiplegia: caused by involvement of hemispheric sensory and motor pathways
• Dysconjugate gaze and inability to move one eye beyond midposition: caused by involvement of cranial nerves III, IV, and VI
• Involvement of other cranial nerves: depends on the severity of neurologic insult
Note: If these indicators of increased ICP are left untreated, the patient will undergo irreversible brain damage or death. If these indicators occur suddenly, there will be displacement of brain substance (herniation), which will progress rapidly to permanent brain damage or death. For additional information about herniation syndromes, see Traumatic Brain Injury (p. 331). BP, Blood pressure.
Indicators of hydrocephalus
• Acute: Persistent or sudden onset of coma with loss of pupillary reflexes within 24 hours of SAH
• Subacute: Gradual onset of confusion, drowsiness, lethargy, or stupor within 1 to 7 days of SAH
• Delayed: Gradual onset of confusion, incontinence, or impaired balance, mobility, and gait; intellectual impairment (slowness, mutism); lack of affect; and presence of the grasp and sucking frontal lobe reflexes (abnormal in adults), at about 10 days following SAH
Observation and functional assessment
Fluctuating hemiparesis or aphasia with increasing confusion can be clinical symptoms of vasospasm. Hydrocephalus is generally not associated with focal neurologic deficits. Anxiety, confusion, agitation, disorientation, lethargy, stupor, and coma may indicate hydrocephalus, vasospasm, or early hyponatremia. Anorexia, nausea, vomiting, abdominal pain, cold and clammy skin, generalized weakness, and lower extremity muscle cramps are late signs of untreated hyponatremia. Flushing, diaphoresis, pupillary dilation, decreased gastric motility, increased serum glucose, fever, hypertension, tachycardia, cardiac dysrhythmias, ischemia, and infarction can be due to increased circulating catecholamines.
Screening labwork
• CSF analysis may be performed to confirm the presence of blood in the CSF in patients with symptoms suggestive of SAH but with no clear abnormalities detected on the CT scan. CSF pressure, normally 0 to 15 mm Hg (75 to 180 mm H2O), may be elevated. The pressure is proportionate to the amount of bleeding. Protein may increase to 80 to 130 mg/dl (normal is 15 to 50 mg/dl). Note: Performance of lumbar puncture in the patient with SAH and increased ICP carries substantial risk of herniation and rebleeding; thus, it is not a routine study in this patient population. In patients with SAH and an external ventricular drain for the management of hydrocephalus, CSF may be sampled as part of a workup of infectious causes of sustained fever.
• Electrolytes and glucose levels should be monitored at least daily to detect hyponatremia and hyperglycemia. Fluid management in SAH after an aneurysm is secured can be associated with hypokalemia, hypomagnesemia, and hypophosphatemia so these electrolytes should be also monitored on at least a daily basis.
• ABG analysis: To detect hypoxemia and hypercapnia and to determine appropriate respiratory therapy.
Collaborative management
Care priorities
• Calcium channel blocker: Nimodipine (Nimotop) inhibits calcium influx across the cell membrane of vascular smooth muscles. The resulting decrease in peripheral vascular resistance and vasodilation is believed to increase perfusion in cerebral vessels. While nimodipine does not prevent vasospasm, its use has been shown to be associated with improved long-term outcomes in patients who experience vasospasm. Nimodipine is given as 60 mg enterally every 4 hours for 21 days (the recommended course of therapy). Some patients experience significant decreases in BP with nimodipine and may require a dosing schedule of 30 mg every 2 hours. Intravenous (IV) administration of calcium antagonists is not supported in evidence at this time.
• Antihypertensives: Antihypertensive therapy is used cautiously in this patient population because allowing hypertension is a significant element of standard therapeutic management in aneurysmal SAH. Hydralazine hydrochloride (Apresoline), labetalol (Normodyne), or nicardipine may be administered to control BP both prior to definitive securing of the ruptured aneurysm and after clipping or coiling to maintain BP in desired parameters.
• Osmotic diuretics: Mannitol (Osmitrol), urea (Ureaphil), and glycerin (Glycerol) may be used to reduce ICP and treat cerebral edema via diuresis to remove fluid from the brain. Patients should be monitored for electrolyte imbalances, other systemic side effects, and adverse reactions related to fluid shifting.
2. Surgical/endovascular intervention:
Recent studies confirm improved patient outcomes when the ruptured aneurysm is secured within the first 24 to 72 hours for patients with grade I or II symptoms (Hunt and Hess Scale). Early intervention may prevent rebleeding, an often fatal complication, and allows for the management of vasospasm without risk of rebleeding. Securing of the aneurysm during the time period associated with the highest risk of development of cerebral arterial vasospasm has been shown to be associated with increased morbidity and mortality, so if the aneurysm is not secured within 24 to 72 hours of rupture, repair should be delayed until the peak time for vasospasm (7 to 10 days after SAH) has passed. Patients with grades III to V symptoms are generally considered poor interventional risks, especially in the period immediately after SAH. If these patients are clinically unstable, they may be treated medically until they improve or stabilize enough for endovascular or surgical intervention. Surgery is considered for a patient with a large intracranial clot causing life-threatening, intracranial brain shifting. Intervention is delayed for a patient with cerebral vasospasm until the vasospasm subsides.
3. Management of hydrocephalus:
• External ventricular drainage (EVD): Hydrocephalus develops in 20% to 25% of patients with SAH from a ruptured cerebral aneurysm. Patients with symptomatic hydrocephalus generally require placement of an external ventricular drainage system for management of their hydrocephalus. For those with massive hydrocephalus, coma and Hunt-Hess classification of III or IV, placement of an external ventriculostomy drain can decompress the ventricles enough to produce significant improvements in the patient’s neurologic function and make the patient a candidate for intervention to secure the ruptured aneurysm.
• Ventricular shunt: Most patients do not develop chronic hydrocephalus following SAH. For those who do develop a chronic problem, the percentage of those who initially require extraventricular drainage who progress to needing a shunt is not clearly reflected in the literature. When a shunt is necessary, one end of a small catheter is positioned into a ventricle, with the other end draining into a body cavity or space (e.g., SAS, cistern, peritoneum, vena cava, pleura). Major complications include infection and malfunction. If the shunt has a valve for the purpose of controlling drainage or preventing reflux of CSF, the surgeon may request that the valve be pumped periodically to ensure proper functioning. For nursing interventions after shunt placement, see Box 7-2.
Box 7-2 NURSING INTERVENTIONS AFTER SHUNT PLACEMENT
• After the shunting, assess patient for indicators of increased ICP (see Box 7-1) caused by either the disease itself or shunt malfunction.
• Position patient on side opposite the insertion site, either flat or with head elevated slightly (as prescribed) to prevent pressure on shunt mechanism.
• Assess vital signs; LOC (orientation to time, place, and person); papillary light reflex; and motor function.
• Monitor I&O, and limit fluids as prescribed.
• Avoid severe head and neck rotation, flexion, or hyperextension to prevent kinking, compression, or twisting of the shunt catheter, which would impede CSF flow.
• If the shunt has a valve for controlling drainage or preventing reflux of CSF, pump the valve to ensure proper functioning, according to surgeon’s directive. Usually the valve is located behind or above the ear and is the approximate diameter of a fingertip. Pumping involves gentle, serial compressions of the tissue over the shunt. If the valve is working properly, the emptying and refilling of the valve will be felt with palpation.
• Assess for indicators of meningitis including peritonitis and sepsis, caused by presence of shunt mechanism. (See Peritonitis, p. 805, and SIRS, Sepsis, and MODS, p. 924.)
CARE PLANS FOR CEREBRAL ANEURYSM AND SUBARACHNOID HEMORRHAGE
Risk for ineffective cerebral tissue perfusion
1. Bed rest, with aneurysm precautions and prevention of ICP
2. Subarachnoid precautions are instituted while the patient is awaiting definitive management of a ruptured cerebral aneurysm. Try to keep patient quiet and calm in a soothing environment, with lowered lights and noise level.
3. Active ROM and isometric exercises are restricted during acute and preoperative stages to prevent ICP.
4. Passive ROM is prescribed to prevent formation of thrombi, with subsequent pulmonary emboli.
5. Bowel management program is essential to prevent straining at stool. Instruct patient to avoid activities using isometric muscle contractions (e.g., pulling or pushing side rails, pushing against the foot board), which raise SBP, with resultant increased ICP.
Instruct patient to avoid coughing because increased intrathoracic pressure increases ICP.
Intracranial pressure monitoring
1. Increased ICP is common after SAH, but its manifestations range from minimal (e.g., persistent headache or drowsiness) to severe (e.g., coma or death). Elevated ICP that does not respond to treatment has been associated with poor patient outcomes.
2. In patients who require invasive devices to manage their ICP, the critical care nurse strives to maintain normal ICP (0 to 10 mm Hg with an upper limit of 15 mm Hg) and CPP 60 to 80 mm Hg. Calculate CPP by means of the formula: CPP = MAP (mean arterial BP) − ICP. CPP less than 30 mm Hg causes cerebral anoxia.
3. HOB elevation: A 30- to 45-degree angle facilitates venous outflow from the intracranial cavity and lowers ICP. Head should be kept in straight alignment to prevent increased ICP secondary to obstruction of jugular venous outflow. Values of 180 to 220 mm Hg as prescribed end points.
Impaired gas exchange or ineffective airway clearance due to altered level of consciousness
Respiratory Status: Gas Exchange
1. Supplemental oxygen, maintenance of patent airway, possible intubation and ventilation if needed. Serial ABG tests are performed to identify hypoxemia (PaO2 less than 80 mm Hg) and hypercapnia (PaCO2 greater than 45 mm Hg). Hypercapnia is a potent cerebral vasodilator that can increase ICP in patients who are already at risk.
related to the potential impact of hyperglycemia
Glucose levels are controlled within normalized parameters throughout all phases of treatment.
1. Studies have shown that admission hyperglycemia or perioperative hyperglycemia is associated with poor outcome after aneurysmal SAH. Daily glucose monitoring is encouraged. More frequent monitoring and intervention are required in patients with persistent hyperglycemia.
2. Further research is underway to determine the critical timing for strict glucose control, effect on neurological outcome, and how serum glucose levels impact brain glucose concentrations.
Risk for imbalanced fluid volume
related to initiation of measures to maintain hypervolemia
1. Fluid balance is maintained based on CVP, weight, and monitoring of intake/output (I&O) balance.
2. Electrolytes should be replaced on the basis of the patient’s laboratory values.
3. Hyponatremia is often seen in this patient population. Standard fluid management can make it difficult to determine whether the underlying cause is cerebral salt wasting or SIADH. Regardless of the underlying cause, hyponatremia is treated with salt repletion since the fluid restriction commonly used in other patient populations to manage SIADH is contraindicated in SAH patients who are still at high risk for vasospasm and cerebral ischemia. In mild hyponatremia, initial repletion is oral (e.g., salt tablets with meals). If hyponatremia does not respond to oral replacement, IV use of hypertonic saline (1.8% or 3%) is initiated. Hyponatremia requires frequent monitoring of laboratory values to assess effectiveness of therapy. Once the patient’s sodium normalizes, therapy is slowly tapered to assess the patient’s ability to maintain a normal serum sodium level.
4. Triple H therapy may lead to fluid volume overload and must be closely monitored. Multiple electrolyte abnormalities are often seen with triple H therapy, and serum magnesium and phosphorus levels should be monitored regularly along with standard blood chemistries.
5. Maintain adequate nutritional intake using enteral feedings, oral intake, parenteral nutrition, or lipid emulsions as indicated by patient’s neurologic status. Initially patients may present with severe nausea and vomiting following aneurysmal rupture, but this generally resolves in first 24 hours.
Additional nursing diagnoses
As appropriate, see nursing diagnoses and interventions in Nutritional Support (p. 117), Mechanical Ventilation (p. 99), Alterations in Consciousness (p. 24), Prolonged Immobility (p. 119), Emotional and Spiritual Support of the Patient and Significant Others (p. 200), Diabetes Insipidus (p. 703), and Syndrome of Inappropriate Antidiuretic Hormone (p. 734).
Care of the patient after intracranial surgery
Cranial surgery can be performed to remove a space-occupying lesion such as a tumor, evacuate a hematoma or abscess, or remove a foreign object. A patient may have a surgical repair of a vascular abnormality, such as an aneurysm or arteriovenous malformation (AVM) or to correct skull fractures. Neurosurgeon may elect to perform a procedure as a treatment modality, such as to drain CSF from the ventricular system or to divert CSF to promote dural repair, control seizures or tremors, and reduce pain. Minimally invasive intracranial procedures using stereotactic techniques are used for some biopsies and for implantation of deep brain stimulators for control of essential tremors. Endoscopic and stereotactic aspiration is being performed for noncomatose basal ganglia hemorrhages. The type of surgical approach the neurosurgeon takes depends primarily on the location of the pathologic condition. The supratentorial approach is used to remove or correct problems in the frontal, temporal, or occipital lobes, as well as in the diencephalic area (i.e., pituitary, hypothalamus). Lesions of the cerebellum and brainstem usually require an infratentorial (i.e., suboccipital) approach. The transsphenoidal approach gains access to the pituitary gland to remove a tumor, control bone pain associated with metastatic cancer, or attempt to arrest the progression of diabetic retinopathy in a patient with diabetes mellitus.
Neurologic assessment: postoperative care
Goal of system assessment
Evaluate for several key nursing diagnoses requiring emergent intervention:
• Alteration in cerebral tissue perfusion due to increased ICP or cerebral vasospasm
• Impaired gas exchange and/or ineffective airway clearance due to altered level of consciousness
• Risk of infection at site or due to cerebral spinal fluid leak
• Fluid volume deficit or excess impaired mobility
• Altered sensory perception involving trunk, extremities, or cranial nerves
Vital signs
• BP, HR, and RR changes: May further alter cerebral tissue perfusion. Uncontrolled high BP can lead to ICH; therefore, close monitoring is important to keep the SBP less than 160 mm Hg to prevent bleeding. Hypotension reduces cerebral perfusion and can cause cerebral infarcts. Monitor rate, rhythm, and depth of respirations for changes or abnormal breathing patterns.
• Hyperthermia: May be associated with injury or irritation of the hypothalamic temperature-regulating centers, presence of blood in the CSF, or infection. Elevated temperature increases the metabolic needs of the brain, potentially leading to increased blood flow to the area, with concomitant cerebral hyperemia.
• Intracranial pressure: If ICP monitoring is utilized, the critical care nurse needs to understand the dynamics of CPP. A postoperative increase in either the volume of brain tissue (e.g., edema), cerebral spinal fluid, or blood or the addition of a hematoma can cause intracranial hypertension. The normal ICP is generally 0–10 mm Hg (up to 15 mm Hg). CPP is inversely related to ICP and in pressures less than 50 mm Hg can lead to cerebral ischemia or infarction: CPP = MAP – ICP.
Observation
• LOC: The improvement in the degree of LOC depends on preoperative damage to cerebral tissue. LOC often improves as anesthesia wears off, or as cerebral edema subsides, and then the ICP approaches normal.
• Pupillary changes: Pupillary abnormalities can indicate unilateral or bilateral brain dysfunction, interruption of sympathetic or parasympathetic pathways, damage in the brainstem, cranial nerve damage, and herniation.
• Communicative and cognitive deficits: The ability to communicate and understand spoken or written words after surgery depends on the level of preoperative dysfunction, the site of the lesion, extent of the procedure, and the degree of postoperative cerebral edema.
• CSF leakage: Assess for CSF leakage from the ear (otorrhea), from the nose (rhinorrhea) which is seen particularly with transphenoidal surgery, and also from the surgical site. The leakage of CSF indicates an open pathway to the SAS, which carries a serious risk of infection. Causes specific to craniotomy include the use of an external ventricular drainage device (which is being used as a treatment modality) and can be a source of entrance of organisms, or remote site infection, and any repeat operation. CSF leak treatment depends upon severity, site, and weighing the risk for infection and may include the use of external CSF drainage (e.g., lumbar subarachnoid drain) to divert CSF flow and thus reduce pressure, keeping patient flat (if not contraindicated), and allowing time for the dural tear to heal. Surgical intervention may be done to seal the dural leak at the origin site.
Observation and functional assessment
1. Assess motor function and sensory responses:
• Motor: Motor deficits (weakness or paralysis) are caused by injury or edema to the primary motor cortex and corticospinal (pyramidal) tracts.
• Sensory: Sensory deficits occur when the primary sensory cortex, the sensory association areas of the parietal lobe, or the spinothalamic tracts are injured or edematous. Sensory deficits include inability to distinguish objects according to characteristics (e.g., size, shape, weight) and inability to distinguish overall changes in temperature, touch, pressure, and position.
Improvement in both motor and sensory perception may be seen as cerebral edema subsides.
Screening labwork
• Sodium levels and osmolality: Important for management of SIADH and diabetes insipidus. Close monitoring during hyperosmolar therapy is important. Hyponatremia can indicate dehydration and if not managed can produce brain swelling.
• CSF analysis: Evaluates the color, white blood cell (WBC) count, differential, glucose content, and protein level, which are important whenever CSF leak develops
• Complete blood count (CBC), electrolytes, and coagulation studies: Evaluates for anemia, hypo-or hyperglycemia; potential for hemorrhage or infection
• Anticonvulsant medication levels: If patient is receiving anticonvulsant therapy, monitor for subtherapeutic and supratherapeutic levels.
Collaborative management after intracranial surgery
Care priorities
Supplemental oxygen, intubation, and mechanical ventilation as needed. In patients requiring mechanical ventilation who have potential or actual increased ICP, use of hyperventilation needs careful monitoring to avoid cerebral vasoconstriction. The Brain Tumor Foundation standard emphasizes that in absence of increased ICP, chronic hyperventilation (PaCO2 less than 25 mm Hg) should not be done the first 24 hours after traumatic brain injury. Use of prophylactic hyperventilation (PaCO2 less than 35 mm Hg) should be avoided during the first 24 hours or used only if increased ICP does not respond to other measures such as CSF drainage, sedation, or use of neuromuscular blocking agents.
• In posterior fossa surgery (infratentorial approach), the supporting muscles of the neck are altered. Patients should be turned with the neck in alignment with the head, with the head, neck, and shoulders supported.
• After hemicraniectomy, to avoid injury, the patient should not be turned to the side from which hemicraniectomy has been removed. Label head dressing, chart, and bed with location of missing bone. A head protective device such as a specially sized helmet should be worn.
• After procedures in which a large intracranial space is left after extensive surgery, to avoid a sudden shift in intracranial contents, with subsequent hemorrhage or herniation, the patient should not be positioned on operative side immediately after surgery.
• Osmotic diuretics (e.g., mannitol): To control cerebral edema causing increased ICP. Dose is usually 0.25 to 1 g/kg of 20% solution administered over 20 to 30 minutes, and ICP levels should be measured before, during, and after administration of mannitol. When the ICP reaches a desired fixed reduced level (usually within 15 minutes), the dosage of mannitol needs to be gradually reduced.
• Fluid and electrolyte management: To prevent or treat increasing cerebral edema
The method and type of nutritional support are determined by the patient’s condition and may include any of the following: oral feedings, enteral feedings, supplements, or parenteral nutrition (i.e., total parenteral nutrition [TPN], fat emulsion therapy). See Nutritional Support (p. 117).
11. Facilitate mobility and return of functions needed for activities of daily living:
• Physical medicine consultation: To evaluate patient and plan for rehabilitation: physical and occupational therapies for planning return of function
Speech therapy may be important for dysphagic screening, monitoring for meeting communication needs.
12. Implement therapeutic hypothermia:
CARE PLANS: COMPLICATIONS AFTER INTRACRANIAL SURGERY
Decreased intracranial adaptive capacity
related to possible changes in intracranial fluid or brain tissue volume following surgery
Neurological status: consciousness
1. Monitor for increased ICP with potential for herniation: Cerebral edema, hemorrhage, infection, and surgical trauma can all lead to increased ICP with herniation (see Box 7-1). Some cerebral edema is expected after intracranial surgery, and usually peaks about 72 hours after surgery (see Traumatic Brain Injury, p. 331). Postoperative uncontrolled nausea and vomiting can cause high intra-abdominal and also increased intrathoracic pressure (e.g., high PEEP ventilator settings) leading to high ICP.
2. Monitor for intracranial bleeding: Postoperative bleeding can be related to the surgical site and may be intracerebral, intracerebellar, subarachnoid, subdural, epidural, or intraventricular. Coagulation profiles and platelet counts should be monitored closely. Bleeding may be caused by the lengthy and extensive surgical procedure, high BP, prolonged anesthesia, preexisting medical problems, or medications. Contusions can develop after evacuation of epidural or subdural hematomas and may create a mass effect.
3. Control seizures: Generalized or partial seizures can occur as a result of surgical trauma, irritation of cerebral tissue by the presence of blood, cerebral edema, cerebral hypoxia, hypoglycemia, preexisting seizure disorder, or inadequate anticonvulsant levels. The use of anticonvulsants prophylactically remains controversial.
4. Monitor for hydrocephalus: May appear before surgery or occur after surgery as an acute or chronic complication. Usually it is caused by a slowing or complete stoppage of the flow of CSF through the ventricular system secondary to edema, bleeding, scarring, or obstruction. For further discussion, see Cerebral Aneurysm and Subarachnoid Hemorrhage (p. 629).
5. Assess for tension pneumocephalus: Uncommon but can occur as a result of air entering the subdural, extradural, subarachnoid, intracerebral, or intraventricular spaces and is an emergent situation May be a complication of infratentorial/posterior fossa craniotomy, burr holes for removal of chronic subdural hematoma, and transsphenoidal hypophysectomy. Rapid decompression is usually required.
Respiratory Status: Gas Exchange
1. ![]() Monitor for partial or complete airway obstruction caused by accumulation of secretions, improper positioning, or change in level of consciousness.
Monitor for partial or complete airway obstruction caused by accumulation of secretions, improper positioning, or change in level of consciousness.
2. Assess for increased crackles caused by neurogenic pulmonary edema resulting from a sudden increase in ICP.
3. Assess for changes in level of consciousness caused by cerebral edema that causes compression of brainstem respiratory centers.
4. Discourage vigorous coughing, as it increases ICP.
5. Encourage deep breathing to help prevent atelectasis and pneumonia.
6. Following institutional protocol for venous thromboembolism (VTE)/DVT prophylaxis to help prevent pulmonary embolism.
1. Monitor for a central nervous system (CNS) infection: can be caused by a preoperative event such as organisms introduced at the time of injury (e.g., gunshot wound) or a break in sterile technique or due to nature of the surgical procedure involving opening of the dura. (See Meningitis, p. 644.)
2. Monitor for a ventriculostomy-related infection (VRI): A ventriculostomy may be performed with introduction of an intraventricular catheter to monitor and manage postoperative ICP or to provide external ventricular drainage for CSF diversion secondary to dural leaks. Extended duration of catheterization has been correlated with increasing risk of CSF infections.
Deficient fluid volume or excess fluid volume
related to hormonal or electrolyte imbalances
1. Monitor for sodium imbalance secondary to diabetes insipidus and SIADH: results from disturbance of the hypothalamus or posterior lobe of the pituitary gland. ADH is produced in the hypothalamus and stored in the posterior pituitary.
2. Assess for hypovolemic shock: may occur as a result of general fluid loss associated with treatment using osmotic diuretics; therefore, close monitoring is essential. Critical care nurses need to be observing patients for development of DI postoperatively, because severe dehydration and hypovolemic shock can occur if fluid balance is not restored.
3. Monitor for gastrointestinal (GI) bleeding: GI bleeding associated with cerebral trauma and the postoperative period after neurosurgery can cause fluid volume deficit. Although the cause is unclear, stress from the trauma or the surgery can produce continuous vagal stimulation leading to a hyperacidic state resulting in gastric erosion, ulceration, and ultimately hemorrhage. These conditions also result from medications, especially corticosteroids (see Acute Gastrointestinal Bleeding, p. 751). Other GI conditions may occur, such as constipation, after neurologic surgery. Decreased or absent peristalsis results from prolonged anesthesia, immobility, trauma, electrolyte deficiencies, and mechanical obstruction (e.g., obstipation).
related to prolonged bed rest or motor dysfunction
1. Thrombophlebitis, DVT, and pulmonary embolism: May result from prolonged bed rest and immobility after intracranial surgery. Other factors such as a prolonged surgical procedure, preexisting hypercoagulable states, and other blood dyscrasias may influence the postoperative complications. VTE is the most frequent complication following craniotomy for removal of brain tumors. Prophylactic management standards have been developed for the prevention of DVT.
related to headache or discomfort secondary to surgical intervention
1. Medicate and intervene appropriately to keep patient’s pain controlled and maintain comfort level. Use of an evidence-based pain scale measurement such as a numerical rating scale (1 to 10) or a behavioral pain scale may be appropriate for patients with impaired communication ability.
2. Assess level of sedation appropriately. Overmedication with analgesics or sedatives in postoperative patients with altered level of consciousness can produce impaired gas exchange and compromised airway.
Additional nursing diagnoses
See also nursing diagnoses and interventions in Traumatic Brain Injury (p. 331), Cerebral Aneurysm and Subarachnoid Hemorrhage (p. 629) Status Epilepticus, Meningitis (p. 644), Diabetes Insipidus (p. 703), Syndrome of Inappropriate Antidiuretic Hormone (p. 734), Nutritional Support (p. 117), Prolonged Immobility (p. 149), and Emotional and Spiritual Support of the Patient and Significant Others (p. 200).
Meningitis
Pathophysiology
Meningitis is an inflammation of the brain and spinal cord (CNS) affecting the meninges (i.e., dura, arachnoid, pia), brain surface, and cranial nerves. There are several types of meningitis, broadly classified as bacterial (pyogenic), viral, aseptic, tuberculous, fungal, and parasitic. Meningitis is most commonly community acquired or the result of direct contamination. Unfortunately, the incidence of nosocomial meningitis is on the rise. The causative agent usually travels in the bloodstream from various sources before entering the CSF. The CSF is deficient in mounting any antibacterial response as it lacks immunoglobulins and complement. Therefore, when contamination of the CSF occurs, phygocytosis and opsonization of the bacteria do not occur. Bacterial meningitis, a consequence of bacterial invasion, progresses through four interconnected phases: (1) invasion of host leading to CNS infection, (2) inflammation of the subarachnoid and ventricular space as bacteria multiply, (3) pathophysiologic changes consistent with progression of inflammation, and (4) neuronal damage.
Viral meningitis
Enteroviruses are the most common cause of viral meningitis in the spring and fall seasons. The condition generally lasts 7 to 10 days and, although serious, is rarely fatal in people with normal immune systems. Herpesviruses, including herpes simplex viruses (the cause of chickenpox, Epstein-Barr virus, and shingles), measles, and influenza may lead to viral meningitis. Mosquitoes and other insects spread arboviruses, which cause infections that precede viral meningitis.
Neurologic assessment: meningitis
Goal of system assessment
A complete neurologic examination should be performed to establish the patient’s baseline neurologic function. One or more tests for meningitis usually are positive (seeTable 7-2). Examination of associated systems (head, eye, ear, nose, and throat [HEENT] and pulmonary) provides additional data.
| Test/Description | Positive Findings |
|---|---|
| Stiff neck sign (nuchal rigidity): Raise patient’s head by flexing the neck and attempting to make the patient’s chin touch the sternum. | Pain and resistance to neck motion |
| Brudzinski sign: Assess for nuchal rigidity. | Flexion of the hips and knees when the examiner flexes the patient’s neck |
| Kernig sign: Flex the patient’s leg at the knee and hip when the patient is supine, and then attempt to straighten the leg. | Pain in the lower back and resistance to straightening the leg |
Vital signs
• BP, HR, and RR changes: May further alter cerebral tissue perfusion. Monitor rate, rhythm, and depth of respirations for changes or abnormal breathing patterns.
• Hyperthermia: May be associated with injury or irritation of the hypothalamic temperature-regulating centers, presence of blood in the CSF, or infection. Elevated temperature increases the metabolic needs of the brain, potentially leading to increased blood flow to the area, with concomitant cerebral hyperemia.
Observation
Meningeal signs (see Table 7-2):
• S. pneumoniae: The classic presentation of pneumococcal meningitis is fever, headache, meningismus, and altered mental status that progresses quickly to coma. Nuchal rigidity and Kernig or Brudzinski sign are present. Nausea, vomiting, profuse sweats, weakness, myalgia, seizures, and cranial nerve palsies also may be present.
• N. meningitidis: Patients may quickly deteriorate, beginning with fever and early macular erythematous rash that progresses rapidly to petechial and purpuric states, conjunctival petechiae, and aggressive behavior. Dysfunctions of cranial nerves VI, VII, and VIII (see Appendix 4) and aphasia, ventriculitis, subdural empyema, cerebral venous thrombosis, and disseminated intravascular coagulation (DIC) may occur.
• H. influenzae: The most distinguishing sign is early development of deafness, which can occur within 24 to 36 hours after onset. A morbilliform or petechial rash may be present.
• ![]() L. monocytogenes: Seizures and focal deficits such as ataxia, cranial nerve palsies, and nystagmus are seen early in the course of infection. Conclusive diagnosis may require serology testing.
L. monocytogenes: Seizures and focal deficits such as ataxia, cranial nerve palsies, and nystagmus are seen early in the course of infection. Conclusive diagnosis may require serology testing.
• ![]() Gram-negative species: In older adults, fever may be absent or low grade and headache may not be reported. Meningeal signs may be subtle, but confusion, severe mental status changes, and pneumonia are commonly reported. Nuchal rigidity in older adults must be differentiated from degenerative changes of the cervical spine.
Gram-negative species: In older adults, fever may be absent or low grade and headache may not be reported. Meningeal signs may be subtle, but confusion, severe mental status changes, and pneumonia are commonly reported. Nuchal rigidity in older adults must be differentiated from degenerative changes of the cervical spine.
• B. burgdorferi: The symptoms of meningitis may be preceded by symptoms of Lyme disease, which occur in three stages. The first stage is a “bull’s eye” rash within a few days of the tick bite followed by headache, stiff neck, lethargy, irritability, and changes in mental status, especially memory loss. Stage two, weeks to months after the tick bite, causes persistent headache, nausea, vomiting, malaise, irritability, cranial nerve deficits, mental status changes, peripheral neuropathies, and myalgias. In the last or third stage, arthritic types of symptoms and brain parenchymal changes are apparent.
• Acute meningitis with negative Gram stain: Fever and neck stiffness are the most frequent findings. The Gram stain for bacteria is negative, but CSF WBC count is elevated. Symptoms are similar to those for other types of meningitis.
• M. tuberculosis: A slow-onset process that causes neurologic damage before treatment is sought. Symptoms include headache, lethargy, confusion, nuchal rigidity, cranial nerve abnormalities, SIADH, weight loss, and night sweats. Kernig and Brudzinski signs are present. The chest radiographic results may be clear, and purified protein derivative (PPD) may be nonreactive.
• Cryptococcus neoformans: Because the infection is subacute, fever and headache may have a subtle pattern lasting for weeks while other symptoms of meningitis occur, including positive meningeal signs (Table 7-2), alterations in mental status (e.g., hyperactivity, bizarre behavior, emotional lability, poor judgment), photophobia, focal cranial nerve deficits, nausea, vomiting, and (rarely) seizures.
• Aseptic meningitis syndrome: Fever, headache, stiff neck, fatigue, anorexia, and altered LOC are seen several hours after ingestion of causative drug. Severity varies with amount of drug taken and previous exposures. CSF glucose may be slightly elevated.
Functional assessment
1. Assess motor function and sensory responses.
• Motor: Assess motor movement in extremities related to strength, symmetry of movement, and coordination. Assess for abnormal motor movements unilaterally and bilaterally, such as decorticate posturing (abnormal flexion), decerebrate posturing (abnormal extension), or flaccidity. Motor deficits (weakness or paralysis) are caused by injury or edema to the primary motor cortex and corticospinal (pyramidal) tracts.
• Sensory: Assess perception of touch, proprioception, pain, temperature, and vibration (if possible). Superficial and deep reflexes are tested on symmetrical sides of the body and compared noting the strength of contraction. Sensory deficits occur when the primary sensory cortex, the sensory association areas of the parietal lobe, or the spinothalamic tracts are injured or edematous. Sensory deficits include inability to distinguish objects according to characteristics (e.g., size, shape, weight) and inability to distinguish overall changes in temperature, touch, pressure, and position. Improvement in both motor and sensory perception may be seen as cerebral edema subsides.
Diagnostic tests
Bacterial meningitis presents with classic symptoms of fever, altered mental status, headache, and nuchal rigidity. Immediate diagnosis and isolation of the organisms are paramount in this life-threatening disease. Delay in obtaining the necessary information needed to diagnosis and treat the underlying organism will increase morbidity and mortality.
| Test | Purpose | Abnormal Findings |
|---|---|---|
| Imaging | ||
| Computed tomography (CT) of brain Do not delay lumbar puncture or administration of antibiotics for the CT scan, especially if the history does not support traumatic injury or an expanding intracranial lesion. |
Assess details of structures of bone, tissue, and fluid-filled spaces. Detects exudate, abscesses, and intracranial pathology (e.g., tumors, brain injury). Assess for hydrocephalus. | Shift of structures due to enlarged mass, edema, exudate, abscesses, fresh hemorrhage, hematomas, infarction, hydrocephalus. Can visualize facial skeleton and soft tissue structures for abnormalities (e.g., tumors, brain injury). |
| Magnetic resonance imaging (MRI) of brain | Minute oscillations of hydrogen atoms in brain create graphic image of bone, fluid, and soft tissue. Provide a more detailed image. | MassesDetects exudate, abscesses, and intracranial pathology (e.g., tumors, brain injury). |
| Laboratory Testing | ||
| Serum CBC with WBC count and differential | Assesses for presence of infection | Elevated WBCs |
| CSF analysis An LP should not be done following head injury, if focal neurologic deficits or papilledema are present, since these signs indicate increased ICP (see Box 7-1). Antibiotic therapy should not be delayed if CSF samples cannot be obtained. Polymerase chain reaction (PCR) assays antibody titers A DNA-based CSF test to check for the presence of certain causes of meningitis includes HSV1, HSV2, VZV, HIV, Epstein-Barr virus (EBV), West Nile virus, cytomegalovirus (CMV), HHV-6. Other CSF studies Venereal Disease Research Laboratories (VDRL) Fluorescent Treponemal Antibody-Absorption (FTA-ABS) (evaluates for syphilis) |
The most important laboratory test for diagnosing meningitis. CSF may be obtained through an intraventricular catheter, ventriculostomy and reservoir via cervical approach, or lumbar puncture(LP). Note: Clinical signs of improvement rather than repeat CSF analysis is a better indicator of treatment response. However, repeat LP if: 1) there is no clinical improvement within 24–72 hrs after treatment is initiated; 2) it is performed 2-3 days after initiation of treatment if microorganisms are resistant to standard therapy; and 3) fever persists for greater than 8 days. |
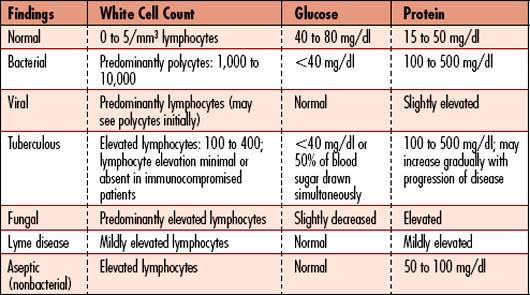
The CSF is analyzed for cell count with white cell differential, glucose, protein, Gram’s stain, acid-fast stain, culture, and sensitivity (Table 7-3). CSF studies include the following:
• Cultures: Bacterial, viral, fungal, M. tuberculosis cultures with sensitivities, and aerobic and anaerobic cultures • Latex agglutination: For bacterial antigens of N. meningitidis, S. pneumoniae, E. coli, influenza type B (Hib), group B strep • Antigen: Cryptococcal and Histoplasma polysaccharide antigen (bacterial antigen testing rarely useful) • Antibodies: Coccidioides immitis complement fixation antibodies, herpes simplex virus (HSV), and varicella zoster (VZV) antibodies |
Collaborative management
Care priorities
• Antibiotic therapy: There are two major caveats to treating bacterial meningitis. First, the bactericidal agent must be effective against the organism and second, the agent must achieve a bacteriocidal effect within the CSF. Only IV antibiotics should be used, except for rifampin which is useful as a synergistic agent.
• Rapid sterilization of the CSF via appropriate pharmacologic therapy (Table 7-4): Prophylaxis, using appropriate antimicrobials for people exposed to N. meningitidis (rifampin or spiramycin) or H. influenzae meningitis, is recommended.
Table 7-4 COMMON DRUG THERAPY FOR THE MANAGEMENT OF MENINGITIS
| Causative Agent | Characteristic | Therapy |
|---|---|---|
| Bacterial Meningitis | ||
| S. pneumoniae | Gram-positive cocci | Penicillin (PCN), ceftriaxone, cefotaxime, vancomycin with ceftriaxone if beta-lactam resistance; chloramphenicol for PCN allergies |
| H. influenzae | Gram-negative bacilli | Cefotaxime or ceftriaxone, add rifampin if pharyngeal colonization |
| N. meningitides | Gram-negative cocci | Penicillin G, add rifampin, fluoroquinolones, or cephalosporin if pharyngeal colonization; alternative is third-generation cephalosporin (cefotaxime) |
| L. monocytogenes | Gram-positive bacilli | Penicillin G or ampicillin with gentamycin for synergy; if allergy to PCN, then trimethoprim-sulfamethoxazole |
| M. tuberculosis | Acid-fast bacteria | Isoniazid, rifampin, ethambutol, pyrazinamide |
| B. burgdorferi | Spirochete | Ceftriaxone or penicillin G |
| Fungal | ||
| C. neoformans | Fungus | Amphotericin B + flucytosine, fluconazole, or itraconazole |
| Cocci | ||
| Gram-positive | Vancomycin + penicillin G + aminoglycosides | |
| Gram-negative | Penicillin G | |
| Bacilli | ||
| Gram-positive | Ampicillin, penicillin + aminoglycosides | |
| P. aeruginosa, Klebsiella, E. coli, Citrobacter, Acinetobacter, Enterobacter, Serratia marcescens | Gram-negative | High doses of third-generation cephalosporins + aminoglycosides |
Data from Fekete T, Quagliarello V: Treatment and prevention of bacterial meningitis in adults. http:www.uptodate.com ; Friedman N, Sexton D: Epidemiological and clinical features of Gram-negative bacillary meningitis. http:www.uptodate.com; and Frankel and Hartman (2004)
2. Reduce inflammation with adjunctive pharmacologic therapies:
Dexamethasone may decrease inflammation by reducing cytokines produced by bacterial products. In recent study results, improvement in outcome, decrease in neurologic sequelae, and reduction in mortality with the use of dexamethasone have been reported. Recommended dosing is 0.15 mg/kg every 6 hours for 4 days. Start dose with or just prior to first antibiotic dose.
9. Evaluate the need for support services:
Evaluate the need for home health care, support groups, and social services.
CARE PLANS: MENINGITIS
Decreased intracranial adaptive capacity
related to altered fluid dynamics secondary to brain and spinal cord inflammation
1. Assess neurologic status at least hourly. Monitor pupils, LOC, and motor activity; perform cranial nerve assessments (see Appendix 3). Early indicators of increased ICP and possible herniation include decreased LOC, changes in pupillary size and reaction, a decreased motor function (weakness, posturing), and cranial nerve palsies.
2. Monitor patient and report physical indicators of increased ICP (see Box 7-2) to the physician.
3. Monitor vital signs at least every 15 minutes if patient has signs of increased ICP. Be alert to changes in respiratory pattern, fluctuations in BP and pulse, widening pulse pressure, and slow HR.
4. Optimize cerebral oxygenation: Keep patient’s head in neutral alignment, maintain a patent airway, and provide supplemental oxygen as prescribed. Ensure that patient’s neck is not constricted by tracheostomy ties and oxygen tubing.
5. Avoid overhydration, which increases cerebral edema. Ensure precise delivery of IV fluids and timely delivery of medications prescribed for the prevention of sudden increases or decreases in ICP, BP, HR, or RR.
6. Teach patient to avoid activities that increase ICP: coughing, straining, and bending over.
7. If patient shows evidence of increased ICP, implement measures to decrease ICP.
![]() Cerebral Edema Management; Cerebral Perfusion Promotion; Intracranial Pressure (ICP) Monitoring: Neurologic Monitoring
Cerebral Edema Management; Cerebral Perfusion Promotion; Intracranial Pressure (ICP) Monitoring: Neurologic Monitoring
related to headache, photophobia, and fever secondary to meningeal irritation
1. Monitor patient for pain and discomfort. Devise a pain rating scale with patient. Administer analgesics as prescribed. (See Pain, p 135.)
2. Monitor temperature every 2 hours and as needed. Administer tepid baths or cooling blanket and prescribed antipyretics/antibiotics to keep temperature within prescribed limits.
3. Maintain an environment of comfort for each individual patient.
4. Provide care and visiting hours to allow for uninterrupted periods (at least 90 minutes) of rest. If ICP is elevated, clustering care is contraindicated.
5. Darken patient’s room or provide blindfold to minimize the discomfort of photophobia.
related to possible cross-contamination secondary to communicable bacterial and aseptic meningitis
Other patients, staff members, and patient’s significant others do not exhibit evidence of having acquired meningitis: diminished LOC, confusion, fever, headache, nuchal rigidity, and other signs (see previous sections for Meningitis on Neurologic Assessment, p. 623, and Diagnostic Tests, p. 649).
Additional nursing diagnoses
See Risk for Trauma (Oral and Musculoskeletal) in Status Epilepticus (p. 672). Because these patients are at risk for SIADH, see Syndrome of Inappropriate Antidiuretic Hormone, p. 734. See SIRS, Sepsis, and MODS, p. 924, since these patients are at risk for septic shock; Nutritional Support, p. 117, Prolonged Immobility, p. 149, and Emotional and Spiritual Support of the Patient and Significant Others (p. 200).
Neurodegenerative and neuromuscular disorders
Pathophysiology
Neuromuscular disorders include the neurodegenerative diseases, which affect voluntary movements. Communication between the nervous system and muscles is not possible when nerves are destroyed. Muscles weaken and atrophy due to disuse. Weakness may also be associated with muscle twitching, cramps, and pain, along with joint and movement deficits. These disorders may affect the heart and respiratory muscles. Many neuromuscular disorders are genetic, while others are immune mediated, associated with an immunologic disorder. Myasthenia gravis (MG) Guillain-Barr´e Syndrome (GBS) and muscular dystrophy are several of the more commonly recognized conditions. Most of the diseases are incurable. The goal of treatment is to improve symptoms, increase mobility, and lengthen life. Patients with MG may experience difficulties with medication management resulting in a crisis, which is rather easily corrected with the proper medication adjustment. Patients with other neuromuscular disorders may require more elaborate treatments including high-dose corticosteroids, plasmapheresis, and more prolonged hospitalization.
Myasthenia gravis
Pathophysiology
A myasthenic or cholinergic crisis may occur rapidly or incipiently. Myasthenic crisis can occur as part of the natural course of myasthenia gravis or may result from other factors, including infection, tapering of immunosuppressive medications, administration of various other medications, pregnancy, childbirth, or following a surgical procedure, ultimately resulting in respiratory failure from weakness of the respiratory muscles. Severe weakness of the oropharyngeal muscles (bulbar signs) is often associated with respiratory muscle weakness, resulting in dysphagia and aspiration. Endotracheal (ET) intubation with mechanical ventilation may be needed. A cholinergic crisis results from excessive dosing of anticholinesterase medications and rarely occurs if the dose of medications remains within the normally prescribed range. The patient is acutely aware of all sensations. Crisis is dramatic and frightening.
Assessment
Symptom progression
Myasthenic and cholinergic crises:
• Myasthenic crisis: Occurs when the patient needs increased medication as a result of drug tolerance or an exacerbation of the disease. Signs and symptoms: Increasing muscle weakness despite normal or increased drug dosage, increasing anxiety and apprehension, severe ocular and bulbar weakness, with rapid onset of respiratory muscle weakness, which can lead to respiratory arrest.
• Cholinergic crisis: Results from an overdose of anticholinesterase medication, causing a depolarizing neuromuscular blockade. Signs and symptoms: Increasing muscle weakness, increasing anxiety and apprehension, fasciculations (twitching) around the eyes and mouth, diarrhea and cramping, sweating, pupillary constriction, sialorrhea (excessive salivation), and difficulty breathing and swallowing.
Auscultation
| Test | Purpose | Abnormal Findings |
|---|---|---|
| Tensilon (edrophonium) test With MG, weakness and muscle fatigue will improve within 30 to 60 seconds of receiving IV Tensilon injection (2 to 10 mg), and improvement will last up to 5 minutes. |
Identifies the type of crisis. Tensilon is a short-acting anticholinesterase agent that delays hydrolysis of acetylcholine, permitting the acetylcholine released by the nerve to act repeatedly over a longer period. | Myasthenic crisis: Weakness improves with edrophonium chloride (Tensilon) versus Cholinergic crisis: Symptoms worsen with Tensilon. Test is done by a neurologist who assesses the patient’s immediate response. |
| Caution: Have atropine sulfate at the bedside during Tensilon test to reverse the effects of Tensilon if the patient is in cholinergic crisis. | ||
| Serum antibody titer Correlation between titer and disease severity and course has not been proved. | Assesses for presence of serum antibodies against acetylcholine receptors | Elevated serum antibodies against are present in 80% to 90% of cases of generalized MG. |
| Electromyography (EMG) Muscle action potentials are recorded from selected skeletal muscles. | Tests muscle action potentials, reflective of ability to contract | The amplitude of the evoked muscle action potentials falls rapidly in persons with MG. |
| Mediastinal magnetic resonance imaging (MRI) of the thymus gland or mediastinoscopy | To evaluate for thymic abnormalities, present in 80% of patients with MG | 65% to 90% have thymic hyperplasia, whereas 10% to 15% have gross or microscopic thymomas. |
| Thyroid studies Thyroid abnormalities are often present in young women. |
Evaluate for hyperthyroidism. | MG is associated with Hashimoto thyroiditis, an autoimmune thyroid disorder. |
| Other laboratory studies: Creatine phosphokinase (CPK), erythrocyte sedimentation rate (ESR), and antinuclear antibody levels: There is a frequent concurrence of other immunologic disorders with MG. | ||
Collaborative management
Care priorities for patients with myasthenia gravis
1. Manage respiratory failure:
ET intubation with mechanical ventilation may be necessary, depending on the degree of involvement of the respiratory muscles. (See Mechanical Ventilation, p. 99.) Bilevel positive pressure ventilation (BiPAP) may also be used effectively in a subset of patients, if able to breathe somewhat effectively.
3. Initiate nutritional support:
If patient has dysphagia, enteral or parenteral feedings may be needed. (See Nutritional Support, p. 117.)
4. Manage pharmacotherapy during noncrisis periods:
• Cholinesterase inhibitors: Pyridostigmine bromide (Mestinon), neostigmine bromide (Prostigmin), and ambenonium chloride (Mytelase) are used to inhibit the hydrolysis of ACh by acetylcholinesterase at the neuromuscular junction. Pyridostigmine is often used, since it has fewer side effects and is longer acting. The patient is given a dose every 3 hrs during the day, and the dose is adjusted based on effects. Sustained-release preparations usually are given at bedtime to maintain the patient’s strength throughout the night and early morning hours.
• Immunosuppression: Glucocorticosteroids (e.g., ACTH and prednisone) and other immunosuppressive agents: Glucocorticosteroids are used alone or in conjunction with anticholinesterase drugs. They provide clinical improvement for 70% to 100% of patients with MG who refuse thymectomy and have weakness uncontrolled by anticholinesterase drugs. Although the mechanism of action of steroids is uncertain, studies indicate they directly influence neuromuscular transmission, suppress the action of the immune system by decreasing the size of the thymus gland and lymphatic tissue, decrease circulating lymphocytes, and decrease antireceptor reactivity of peripheral lymphocytes. Treatment is continued indefinitely. Glucocorticosteroids produce favorable results in all patients with muscle involvement, from ocular to severe respiratory impairment. Azathioprine (Imuran) may be used alone or in combination with other therapies in situations in which response to steroids is poor. Side effects of azathioprine include toxic hepatitis, thrombocytopenia, leukopenia, leukemia, lymphoma, infections, vomiting, and teratogenic effects.
• Immune globulin: Routine use of human immune globulin (IG) is not recommended, but administration of IV immunoglobulin (IVIg) may be considered in patients with severe MG for whom other treatments have been unsuccessful or are contraindicated.