Chapter 29 Neurogenic Bowel
Dysfunction and Rehabilitation
Gastrointestinal dysfunction is most often characterized by a conglomeration of symptoms that most often indicate lower gastrointestinal impairment, including constipation, diarrhea, and fecal incontinence (FI). It can also present as upper gastrointestinal impairment heralded by bloating, nausea, early satiety, burning, and gaseousness. Neurogenic bowel dysfunction can be a clinically elusive impairment. It is often eclipsed by other, more noticeable associated motor deficits. Neurogenic bowel dysfunction itself can be particularly life-limiting if it is not thoroughly assessed and treated using rehabilitation principles. Interdisciplinary rehabilitative interventions focus on establishing a total management plan for bowel function, termed a bowel program, and for assisted defecation, known as bowel care.107 Sensation and mobility might be limited, affecting a person’s ability to anticipate the need for and to physically perform independent bowel care and hygiene.
In spite of many abilities regained during the rehabilitation process, bowel care capabilities at the time of discharge are not always comparable to other skills that would be expected for a given level of function. Bowel incontinence is one of the greatest predictors of return to home for stroke survivors.29 In fact, bowel management has been found to be one of the areas of least competence among rehabilitated persons with spinal cord injury (SCI).14,50 More than one third of surveyed persons with SCI rated bowel and bladder dysfunction as having the most significant effect on their lives after SCI.51 In a recent Swedish review of medical problems after SCI, 41% of subjects rated bowel dysfunction as a moderately to severely life-limiting problem.74
Epidemiology
Gastrointestinal dysfunction is frequently seen in persons with neurologic diseases who require rehabilitation. Besides the direct effects of neurologic disease on gut function, other factors can also play a huge role in the development of enteric problems, including debility, insufficient fluid intake, and the use of anticholinergics and other medications. Digestive tract problems in stroke, brain injury, multiple sclerosis, Parkinson’s disease, neuromuscular diseases, dysautonomias, peripheral nerve injuries, SCIs, and other neurologic disorders have been shown to be difficult and challenging to manage. Neurogenic bowel difficulties can be a primary disabling and handicapping feature for patients with SCI, stroke, amyotrophic lateral sclerosis, multiple sclerosis, diabetes mellitus, myelomeningocele, and muscular dystrophy.∗
Neurogenic bowel dysfunction results from autonomic and somatic denervation, and produces FI, constipation, and difficulty with evacuation (DWE). These symptoms are common. The prevalence of FI and fecal impaction ranges from 0.3% to 5.0% in the general population. The prevalence of DWE ranges from 10% to 50% among the hospitalized or institutionalized elderly.100,115 Although many gastrointestinal disorders can contribute to FI or DWE, disorders that impair the extrinsic (sympathetic, parasympathetic, or somatic) nervous control of the bowel and anorectal mechanisms are more common among the patient populations seen by physiatrists.
Impact
FI decreases the return-to-home rates for stroke patients.49 Almost one third of persons with SCI report or exhibit worsening of bowel function 5 years beyond their injury, with 33% developing megacolon, suggesting inadequate long-term management.52,110 Recent evidence has shown some improvement in outcomes for SCI bowel management.67 When restoring normal defecation is not possible, social continence becomes the goal. Social continence is defined as predictable, scheduled, adequate defecations without incontinence at other times. It is often achievable by persons with neurogenic bowel dysfunction. Embarrassment and humiliation from FI frequently result in extreme vocational and social disability. Vocational disability and excessive institutionalization add substantial costs to the care related to neurogenic bowel dysfunction. Nursing home costs are higher for patients with FI.115 A 1983 report estimated that $8 billion per year is spent in the United States for the care of fecally incontinent institutionalized patients.100
Neuroanatomy and Physiology of the Gastrointestinal Tract
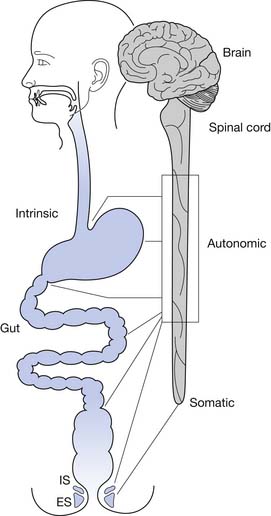
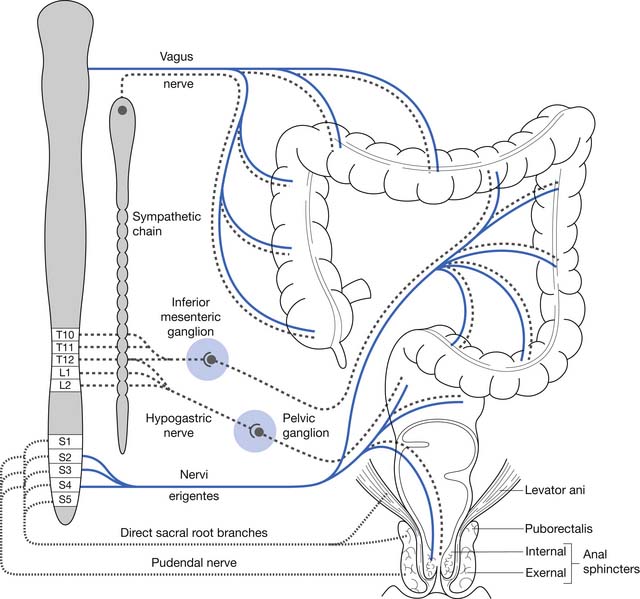
Normal functioning of the stomach and intestines entails coordination of muscle contraction, digestion and absorption of nutrients, and regulation of blood flow. The neural schema of the gastrointestinal tract is much more complex than previously thought. Neural control of the gastrointestinal tract is an extremely organized and integrated hierarchy of mechanisms that involve the central nervous system (CNS; brain and spinal cord), autonomic nervous system (sympathetic and parasympathetic), and enteric nervous system (ENS) (Figures 29-1 and 29-2).123,126
Enteric Nervous System
The ENS is a distinct system that has its own set of neurons that coordinate sensory and motor functions. In the ENS the ganglia are interconnected, which allows for integration and processing of data (as opposed to autonomic ganglia, which only serve as relay centers for stimuli transmitted from the CNS). There are three different types of neurons in the ENS based on function: sensory neurons, interneurons, and motor neurons.123,126 Sensory neurons perceive thermal, chemical, or mechanical stimuli and transform these sensations into action potentials that are conducted to the nervous system. Interneurons serve as conduits between the sensory and motor neurons. The numerous synapses between interneurons create a highly organized circuitry that processes sensory input from the gut and other parts of the nervous system, and integrates and generates reflex responses to these stimuli. Motor neurons are the final common pathway. They receive and translate signals to the gut (mucosa, muscle, vasculature) that affect digestive, interdigestive, and emetic functions based on the transmitters released.123,126
Automatic feedback control is present in the ENS, with the neurons being in close proximity to the stomach and intestines. This can be manifested as reflex circuits that systematize reflex responses to sensory signals, as integrative circuits that coordinate motor patterns (migrating motor complex, digestive activity, giant migratory contractions),123,126 or as a pattern-generating activity occurring when a “command neuron” is actuated with a resulting rhythmic, repetitive behavior.11,126
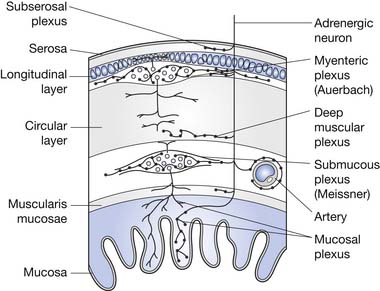
The ENS is the key to proper functioning of the entire gastrointestinal tract. This collection of highly organized neurons is situated in two primary layers: the submucosal (Meissner’s) plexus and the intramuscular myenteric (Auerbach’s) plexus. These plexi have an estimated 10 to 100 million neurons, plus two to three glial cells per neuron. The ENS glial cells resemble CNS astrocytes and are much less abundant than the 20 to 50 glial cells per neuron in the CNS.46 The coordination of segment-to-segment function is largely regulated by the ENS.121 The ENS also has its own blood-nerve barrier, similar to the blood-brain barrier of the CNS.24
Gastrointestinal Neurosensory System
Enteric Nervous System Sensory Neurons
The ENS relays chemical, mechanical, and thermal sensory information to the CNS through vagal afferents and spinal afferents. Vagal and spinal sensory neuron endings supply the muscle, mucosa, and ganglia of the ENS. Spinal sensory neurons also supply the serosa and mesentery and their blood vessels.11,47
Vagal afferent nerve endings act as chemical, thermal, and mechanical receptors. These directly monitor various changes in the gastrointestinal tract such as chemical milieu, temperature, muscle tension, and mucosal brushing.11,47
Chemical receptors are located in the mucosal epithelium of the stomach and intestinal lumen in close proximity with the lamina propria, constantly determining acidity, osmolarity, and the concentration of glucose, fatty acids, and amino acids.11,47 The existence of nociceptors (C or Aδ fibers) in the gastrointestinal tract has not been established.21,22,126 Temperature change in the lumen is detected by thermoreceptors that play a role in the brain’s perception and regulation of core temperature.126
Mechanical receptors are classified as intramuscular arrays or intraganglionic laminar endings. Intramuscular arrays are adjacent to the intramuscular interstitial cells of Cajal and run contiguously with the long muscle layers.43,47 Intraganglionic laminar endings that follow circular and longitudinal muscle fibers provide data on distention or contraction, and connect with myenteric ganglia.47,129 They are also found in rectal muscles and contribute to pelvic innervation.47,78
Spinal afferent nerve endings are distributed extensively throughout the bowel, relaying chemical, thermal, and mechanical information. In the mucosa they detect chemical changes related to injury, ischemia, infection, or inflammation that contribute to pain and discomfort.47,66 Spinal nerve endings express receptors for bradykinin, adenosine triphosphate (ATP), adenosine, prostaglandins, leukotrienes, histamine, mast cell proteases, and serotonin 5-hydroxytryptamine3 (5-HT3). In the serosa and mesentery, they recognize visceral distention and contraction.70,82,83 Those that innervate the blood vessels and ENS ganglia release neurotransmitters that affect gastrointestinal blood flow, motility, and secretory reflexes.47,83
Mechanoreceptors (whether derived from vagal or spinal afferents) are either low or high in threshold.101 Low-threshold receptors detect normal nonconscious and conscious sensations such as satiety, hunger, gaseousness, and nausea. High-threshold receptors detect distention or contraction beyond a set threshold and perceive painful stimuli, eliciting acute, sharp visceral pain. Actuation of both low- and high-threshold receptors contributes to the range of symptoms that are felt along the gastrointestinal tract.21,22,44,47 In the setting of inflammation or ischemia, chronic pain can develop from sensitization of both types of receptors and activation of silent receptors (notable only in pathologic states). Vagal afferents are primarily implicated in the emetic response, and spinal afferents are primarily implicated in the sensation of nausea.44,47
Enteric Nervous System Relationship to the Spinal Cord and Brain
Sensory information from vagal afferents in the ENS is relayed to the nodose ganglia (caudal ganglion of the vagus) and consequently to the nucleus tractus solitarius (NTS) and area postrema in the medullary area of the brainstem. The NTS and the area postrema send signals to the rostral centers in the brain.123,126 The brain processes the data and projects descending connections to the dorsal vagal complex. The brain also participates in vago-vagal reflex circuits that continuously monitor and promptly modify responses to changes in the chemical, thermal, and mechanical environment in the whole gut (mostly in the esophagus, stomach, duodenum, gallbladder, and pancreas). Sensory stimulation through this vagal circuit does not appear to reach the level of consciousness.123,126
Besides making connections with the NTS and the area postrema in the brainstem, vagal afferents synapse with the dorsal motor nucleus of the vagus and the nucleus ambiguus, creating the dorsal vagal complex. The incoming sensory information is integrated and shared with the forebrain and brainstem by the dorsal vagal complex. Brain coordination and influences (conscious and nonconscious) are translated to the dorsal vagal complex, where the dorsal motor nucleus of the vagus and nucleus ambiguus represent the efferent arm of the reflex pathway. It is the final common pathway from the brain responsible for precise control of muscular, glandular and circulatory responses of the gastrointestinal tract.123,124,126 Multiple neurotransmitters are involved in the intricate conduction of impulses between the neuronal circuits in the dorsal vagal complex. Approximately 30 are identified and include acetylcholine, biogenic amines, amino acids, nitric oxide, and peptides.123,126
Spinal afferents (splanchnic and pelvic) send sensory impulses to the dorsal root ganglia (or prevertebral sympathetic ganglia) that are then conducted to the dorsal horn (laminae I, II, V, X) of the spinal cord and the dorsal column nuclei.47,88 The dorsal column is thought to play a greater role in nociceptive transmission of messages from the gut than the spinothalamic or spinoreticular tracts. The conscious perception of pain in the digestive tract is largely mediated through spinal afferents. The spinal cord modulates the conduction of neural messages (both nociceptive and nonnociceptive) to the higher brain.47 Other somatic and visceral sensory stimuli from the vagina, uterus, bladder, colon, and rectum are conveyed through the dorsal horn and dorsal column in the spinal cord as well. Likewise, somatic afferents supply sensory input from the muscles of the pelvic floor through the pudendal nerve to the sacral region of the spinal cord.10,47,88,89
Brain centers modulate the nociceptive impulses from the bowel that are relayed to the dorsal horn of the spinal cord. These descending pathways are facilitatory, inhibitory, or both, based on the visceral stimulus, and can alter the perception of pain in the digestive tract.47,97 The neurotransmitters serotonin, noradrenalin, and dopamine are released by these descending pathways as they synapse with the spinal cord.126
The secondary somatosensory cortex and, to a lesser extent, the paralimbic and limbic areas (anterior insular, anterior and posterior cingulate, prefrontal and orbitofrontal cortices) mediate emotional, volitional, and psychologic responses to sensory input from the gut. These are manifested by abdominal pain, anorexia, nausea, vomiting, hyperphagia, constipation, or diarrhea.47,73,104 The somatosensory cortex regulates the awareness and recognition of pain, and the paralimbic and limbic areas contribute to the cognitive and affective aspects of pain.47,104,126
Data from the brain and descending vagal pathways are conducted to the spinal cord, and then with the preganglionic neurons in the thoracolumbar area (modulates the sympathetic response) and the sacral area (modulates the parasympathetic response). Sympathetic or parasympathetic output is translated to the neurons in the ENS circuitry, which can be excitatory or inhibitory to motor neurons, gastric or digestive glands, and secretory mechanisms.47,126
Gastrointestinal Neuromotor System
The muscles of the gastrointestinal tract carry out essential functions throughout the gut, including propulsion, grinding, mixing, absorption, storage, and disposal. These muscles are composed of “self-excitable” smooth muscles that contract in an all-in-one manner. These smooth muscles spontaneously responds to stretch and can be independent of neural or endocrine control. The interstitial cells of Cajal act like a pacemaker and allow propagation of electrical slow waves into the circular muscle layer, which generates spreading muscle contraction. These smooth muscles act like an electrical syncytium where action potentials are conducted in three dimensions from one smooth muscle fiber to another through gap junctions.47,126
The gastrointestinal tract muscles respond to influences of the vagal efferents and the ENS microcircuitry based on excitatory or inhibitory innervation of motor neurons. Contraction is mediated by the release of excitatory neurotransmitters by vagal afferents at the neuromuscular junctions, acetylcholine (at the muscarinic receptor), and substance P (at the neurokinin-1 receptor).26,47,82,126 Conversely, the release of nitric oxide, ATP, and vasoactive intestinal peptide from the inhibitory motor neurons (which express the serotonergic 5-HT1 receptor) impedes contractile activity and facilitates relaxation. The aboral direction of propulsive activity throughout the digestive tract is achieved by segmental inactivation of inhibitory motor neurons distally.15,16,47,58,126 Contraction can only occur in the segments where the inhibitory motor neurons are inactivated. With passing of the food bolus or stool, the esophageal and internal anal sphincters (smooth muscle sphincters), and inhibitory motor neurons are usually shut off and are inactivated. During vomiting, however, the inhibitory motor neurons are deactivated in the opposite direction.47,126
The sympathetic and parasympathetic nervous systems seem to modulate the ENS, rather than directly controlling the smooth muscles of the bowel.121 The smooth muscles of the bowel also have their own electromechanical automaticity, which is directly modulated by the inhibitory control of the ENS.24,46 Sympathetic nervous system stimulation tends to promote the storage function by enhancing anal tone and inhibiting colonic contractions, although little clinical deficit occurs from bilateral sympathectomy.32 Parasympathetic activity enhances colonic motility, and its loss is often associated with DWE, including impactions and functional obstructions, such as Ogilvie’s pseudoobstructive syndrome.32
The ENS and sympathetic postganglionic neurons transmit excitatory or inhibitory messages to secretomotor neurons. These secretomotor neurons release acetylcholine and vasoactive intestinal polypeptide when there is excitatory activation from paracrine stimulation by mucosal and submucosal cells such as enterochromaffin cells, mast cells, and other immune and inflammatory cells. Acetylcholine and vasoactive intestinal polypeptide are released at the neuroepithelial and neurovascular junctions. This promotes secretion into the gut of water, sodium chloride, bicarbonate, and mucus drawn from the intestinal glands. In addition, dilatation and an increase in blood flow occur with the release of nitric oxide from the vascular endothelium.6,13,47 The inhibitory influence reduces neuronal firing from secretomotor neurons by hyperpolarization of membranes. Sympathetic release of norepinephrine from nerve endings of the α2-noradrenergic receptors inhibits secretomotor actuation, preventing the release of excitatory neurotransmitters. As a result, there is a decrease in secretion of water and electrolytes into the lumen and a congruent shunting of blood from the splanchnic to systemic circulation.47,76,91
Gastric Motility
Based on its motility pattern the stomach is divided into an upper and lower portion. The upper portion (fundus) has sustained, low-frequency contractions and has a tonic pattern. The lower portion (antrum) has intermittent, powerful contractions and has a phasic pattern. The fundus acts as reservoir and accommodates incoming food, which inhibits contraction and allows the stomach to stretch without a significant increase in pressure. The antrum is a mixer that generates propulsive waves that accelerate as food is propagated towards the pylorus. The amount and consistency of food in the fundus regulates excitatory and inhibitory influences and adjustments to volume and pressure.47,69
Intestinal Motility
The ENS is designed to control the various patterns of motility in the intestinal tract. The interdigestive migrating motor complex pattern occurs during fasting in the stomach and the small intestine.47,120 It seems to be influenced by the hormone motilin, and is responsible for removal of waste from the intestinal lumen throughout the fasting period.47,77 When a meal is ingested, the postprandial segmentation (“mixing”) pattern of motility commences as digestion transpires. The brainstem sends signals that are transmitted to vagal efferents, which convert migrating motor complex motility to segmentation motility with the increase in bulk and nutrients, especially lipids (medium-chain triglycerides). This subsequently becomes peristaltic motility, which is propagated through brief segments of intestine at a time.30,47 Peristaltic activity gradually evolves into powerful contractions sustained through long portions of circular muscle along the small and large bowel. These “giant migratory contractions” (GMCs) propel waste through the lumen, particularly in the large intestine.47,59,99
Motility of the Anus, Rectum, and Pelvic Floor
Normal defecation and maintenance of fecal continence entail a highly coordinated mechanism that involves the levator ani, puborectalis, and the external (EAS) and internal anal sphincter (IAS) muscles. The pelvic floor is composed of the levator ani, the underlying sheets of which form a sling. The levator ani, puborectalis, and EAS are skeletal muscles that constantly maintain tone and sustain pelvic organs in place against the forces of gravity.35,47 Simultaneous contraction of these muscles prevents the involuntary loss of stool and helps maintain the regular pattern of defecation.41,47
Physiology of Normal Defecation
The colon is a reservoir for food waste until it is convenient for elimination. It also acts as a storage device as long as the colonic pressure is less than that of the anal sphincter mechanism. Fecal elimination occurs when colonic pressure exceeds that of the anal sphincter mechanism. Other functions of the colon are to reabsorb fluids (up to 30 L/day can be reabsorbed from the large and small bowel walls, with typically only 100 mL of water loss in feces) and gases (90% of the 7 to 10 L of gases produced by intracolonic fermentation is absorbed rather than expelled). The colon also provides an environment for the growth of bacteria needed to assist in digestion, and serves to absorb certain bacterial breakdown products as well.48 The layers of the colon wall are depicted in Figure 29-3.
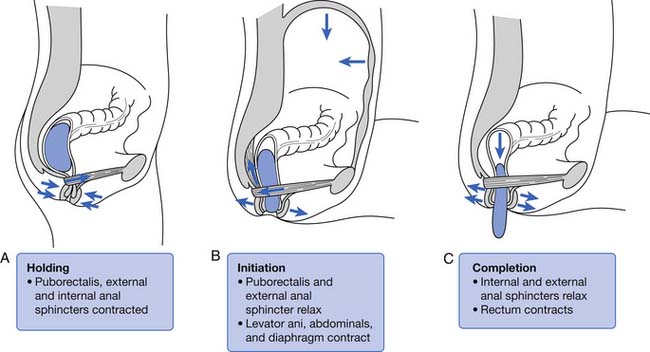
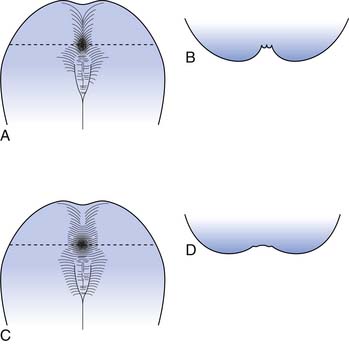
The rectum is usually empty until just before defecation. Perception of rectal contents and pressures105 is essential for signaling voluntary contraction of the anal sphincter. Normal defecation begins with reflexes triggered by rectosigmoid distention produced by approximately 200 mL of feces (Figure 29-4).94 A rectorectal reflex occurs in which the bowel proximal to the distending bolus contracts and the bowel wall distally relaxes, serving to propel the bolus further caudad. Reflex relaxation of the IAS also occurs, which is enhanced by, but does not require, an extrinsic nerve supply. This relaxation, called the rectoanal inhibitory reflex, correlates with the urge labeled “the call to stool.”121 One can then volitionally contract the levator ani to open the proximal anal canal and relax the EAS and puborectalis muscles. This allows a straighter, shorter, and more open anorectal passage (see Figure 29-4), which permits the bolus to pass. Increasing the intraabdominal pressure by squatting and by Valsalva’s maneuver assists bolus elimination. For 90% of normal individuals, only the contents of the rectum are expulsed, whereas 10% will clear the entire contents of the left side of the colon from the splenic flexure distally.33
One can elect to defer defecation, however, by volitionally contracting the puborectalis muscle and EAS. The reflexive IAS relaxation subsequently fades, usually within 15 seconds, and the urge resolves until the IAS relaxation is again triggered. The rectal wall accommodates to the bolus by decreasing its wall tension with time, resulting in less sensory input and less reflex triggering from that particular accommodated bolus. This continence and reflex process is somewhat analogous to the function of the striated external urethral sphincter in volitional control of urinary voiding (see Chapter 28).
The EAS generally tenses in response to small rectal distentions via a spinal reflex, although reflexive relaxation of the EAS occurs in the presence of greater distentions.105 These spinal cord reflexes are centered in the conus medullaris and are augmented and modulated by higher cortical influences. When cortical control is disrupted, as by SCI, the EAS reflexes usually persist and allow spontaneous defection. During sleep the colonic activity, anal tone, and protective responses to abdominal pressure elevations are all decreased, while rectal tone increases.24,121
The gastrocolonic response or gastrocolic reflex refers to the increased colonic activity (GMCs and mass movements) in the first 30 to 60 minutes after a meal. This increased colonic activity appears to be modulated both by hormonal effects, from release of peptides from the upper gastrointestinal tract (gastrin, motilin, cholecystokinin) that increase the contractility of the colonic smooth musculature, and by a reduction in the threshold for spinal cord–mediated vesicovesical reflexes.24 Upper gastrointestinal receptor stimulation also results in increased activity in the colon, possibly because of reflexively increased parasympathetic efferent activity to the colon. The possibility of a purely ENS-mediated activation exists, although the small bowel and colon motor activities do not seem to be synchronized. In persons with SCI the measured increase in colonic activity after a meal is blunted as compared with that in normal subjects.24 The gastrocolonic response is often used therapeutically, even in patients with SCI, to enhance bowel evacuation during this 30- to 60-minute postprandial time frame.1,34 Occasionally certain foods can serve as trigger foods that are especially likely to induce bowel evacuation shortly after consumption.
The resting anal canal pressure is largely determined by the angulation and pressure at the anorectal junction by the puborectalis sling and smooth muscle IAS tone. Continence is maintained by the anal sphincter mechanism,81,107 which consists of the IAS, EAS, and puborectalis muscle.81 Only about 20% of the anal canal pressure is due to the static contraction of the somatically innervated striated EAS.9 The EAS and puborectalis muscle are the only striated skeletal muscles whose normal resting state is tonic contraction, and these muscles consist mainly of slow-twitch, fatigue-resistant type I fibers (unlike the situation in nonupright animals such as the cat or dog, in which these muscles consist of predominately type II fibers).9 Anal pressure can be increased volitionally by contracting the EAS and puborectalis muscles. Maximum volitional squeeze pressures, however, are not as high as can be generated reflexively against Valsalva pressure. The EAS is physically larger than the IAS, and its contraction is under both reflex and volitional control. The volitional control is learned during the course of normal maturation. Normal baseline reflex action of the anorectal mechanism allows spontaneous stool elimination.102 The EAS is innervated by the S2 through S4 nerve roots via the pudendal nerve, and the puborectalis muscle is innervated by direct branches from the S1 to S5 roots (see Figures 29-1 and 29-2).92 The remarkable degree of learned EAS coordination allows the selective discrete passage of gas while juggling a variable mixture of solids, liquids, and gases.
Pathophysiology of Gastrointestinal Dysfunction
A whole range of neurologic diseases affecting central, peripheral, and intrinsic enteric nervous innervation can cause disorders that affect various segments of the bowel. These are predominantly characterized by disturbances in gastroesophageal, small or large intestinal motility and by disturbances in sensation. Symptoms of dysphagia, vomiting, bloating, abdominal discomfort and pain, constipation, and incontinence have been described in individuals with neurologic ailments.5,18,19,93
Nausea, Vomiting, and Bloating
The syndrome of nausea, vomiting, bloating, and early satiety in the setting of neurologic conditions without mechanical obstruction can herald motility problems in the gastrointestinal tract. Neurologic dysfunction that affects the inhibitory motor neurons in the ENS at any level of the neural axis from the brain, spinal cord, afferent nerves, or efferent nerves can lead to spasticity of the gastric or intestinal and colonic musculature. The digestive muscles perform as an electrical syncytium. Inhibitory motor neurons allow propagation of contractile activity in an organized, segmental, and aboral pattern. When inhibitory motor neurons are inactivated or destroyed by disease, the circular muscles contract continuously and nonsystematically. These contractions are incapable of forward propulsion, causing functional obstruction.123,125 This can be manifested as dysphagia, gastroparesis, or chronic intestinal or colonic pseudoobstruction, which might be associated with anorexia, abdominal pain, and diarrhea and constipation (these symptoms can occur together). Inhibitory motor neurons can be affected by autonomic neuropathy, dysfunction of neurons in the myenteric plexus, or degeneration of smooth muscle.∗
Abdominal Pain and Discomfort
Abdominal pain and discomfort arise from gastrointestinal tract distention and powerful contractions. High-threshold and silent mechanoreceptors sense severe distention and intense contractions when there is ischemia, injury, or inflammation. Mechanical and chemical irritants stimulate mechanoreceptors in the ENS and translate signals to the brain and spinal cord from muscle stretching and contractions.11,89,125
Diarrhea
Neurologic dysfunction can present with frequent passage of watery stools. Diarrhea occurs when there is overstimulation of secretomotor neurons by histamine from inflammatory and immune-mediated cells in the mucosa and submucosa, or overstimulation by vasoactive intestinal peptide and serotonin from mucosal enterochromaffin cells, or overstimulation by all three substances. Moreover, these substances influence presynaptic inhibitory receptors to block the release of norepinephrine from the postganglionic sympathetic fibers that inhibit secretomotor neurons.124,125 Bacterial overgrowth in the gut can be a factor in chronic inflammatory states presenting with diarrhea.7,27
Defecation Dysfunction
Constipation
Constipation can be a huge enigma in neurologic states. Infrequent, incomplete emptying of hard stools is due to decreased water and electrolyte secretion into the lumen, resulting from reduced excitation of the secretomotor neurons in the ENS. Norepinephrine released by sympathetic stimulation inhibits the firing of secretomotor neurons by hyperpolarization. Release of excitatory neurotransmitters is reduced in the secretory epithelium, decreasing the secretion of water and electrolytes.125,126 A lack of rectal sensation and a decreased urge to defecate can be strongly associated with constipation in various conditions that present with lesions in the brain, spinal cord, sacral nerves, and hypogastric and pudendal nerves. Outlet obstruction can ensue because of delayed colonic transit times and lack of perineal and rectoanal sensation.125
Fecal Incontinence
True FI, described as an unconscious loss of stool, often occurs in neurologic conditions with lesions affecting the lumbar spinal cord, cauda equina, S2–S4 nerves, pudendal nerve, and pelvic floor nerves. Denervation leads to impaired perineal and rectoanal sensation, aberrant contraction, loss of tone, and weakening of the pelvic floor muscles and the EAS. These contribute to unexpected loss of stool and abnormal defecation, and diminished support for pelvic structures.25,47 Parasympathetic augmentation can occur and might further complicate matters, since it contributes to weakness in the IAS and increases the risk for incontinence. It is always important to rule out overflow incontinence resulting from constipation.
Upper Motor Neurogenic Bowel
Any destructive CNS process above the conus, from SCI to dementia, can lead to the upper motor neurogenic bowel (UMNB) pattern of dysfunction. Spinal cortical sensory pathway deficits lead to a decreased ability to sense the urge to defecate. Most persons with SCI, however, sense a vague discomfort when excessive rectal or colonic distention occurs. It has been reported that 43% of persons with SCI have chronic complaints of a vague discomfort caused by abdominal distention that eases with bowel evacuation.80,110 These sensations might be mediated by autonomic nervous system afferent fibers bypassing the zone of SCI via the paraspinal sympathetic chain, or by means of vagal parasympathetic afferents.
Colonic compliance and sphincter tone102 have been experimentally evaluated in subjects with SCI. Studies of colonic compliance in response to a continuous infusion of saline initially suggested rapid pressure rises and a hyperreflexic response.87,117 More recent studies have demonstrated normal colonic compliances in subjects with SCI who have UMNB.79,90 Passive filling of the rectum leads to increases in the resting sphincter tone.114 These increases are associated with increased EAS pressure development resulting from sacral reflexes that can be abolished by pudendal block.9 This form of rectal sphincter dyssynergia has unfortunately been labeled decreased colonic compliance, even though intermittent or slow filling in the rectum appears to be associated with normal bolus accommodation and pressure relaxation.79,100 This contrasts with “true” decreased rectal wall compliance caused by fibrosis resulting from ischemia or inflammation, where there is no accomodation and relaxation of the rectal wall regardless of flow rates.
Colonic motility and stool propulsion are known to be affected by SCI. De Looze et al.29 used a questionnaire method to study subjects with SCI levels above L2, and found 58% of subjects with chronic SCI had constipation (defined as two or less bowel movements per week or the requirement for digital evacuation). Only 30% (p = 0.002) of patients with paraplegia below T10 and above L2, however, were prone to constipation. Actual stool propulsion was studied later by Krogh et al.71 using swallowed markers and serial radiographs. In subjects with chronic SCI with supraconal lesions, transit times were significantly prolonged in the ascending, transverse, and descending colon and rectosigmoid. Total gastrointestinal transit time averaged 3.93 days (control subjects, 1.76 days) for chronic complete SCI above the conus. In an attempt to demonstrate a difference that might have been conferred by sympathetic innervation, mean total gastrointestinal transit times were compared in patients with lesions above T9 (2.92 ± 2.41 days) and in those with lesions from T10 to L2 (2.84 ± 1.93 days). No significant differences could be found even with comparison of transit times for individual colonic segments.
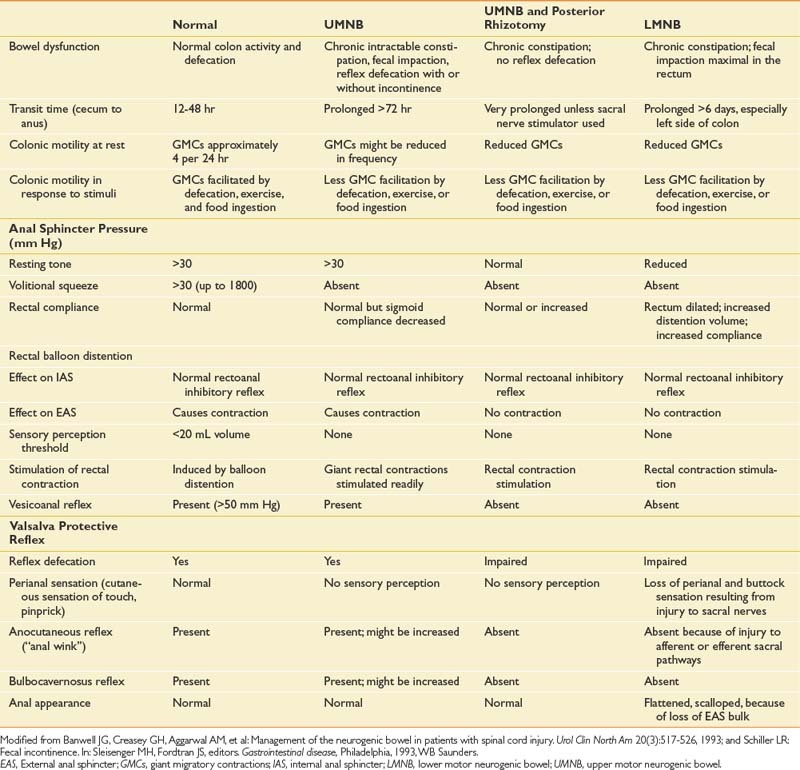
Subjects with SCI who had complete upper motor neuron bowel lesions were studied during the acute phase (5 to 21 days) after SCI. These same patients were reevaluated 6 to 14 months later. Total gastrointestinal transit time was longer during the acute rather than the chronic phase. The upper motor neuron neurogenic colon tended to have slower transit throughout the colon, with less severe rectosigmoid dysfunction.71 Patients with UMNB have spared reflex arc control of the rectosigmoid and pelvic floor. IAS relaxation on rectal distention occurs in persons with SCI as well as in neurologically intact persons. Sufficient rectal distention might cause the EAS to completely relax, resulting in expulsion of the fecal bolus. Rectal sphincter dyssynergia does not necessarily correlate with bladder sphincter dyssynergia, but it often results in DWE.92 The protective vesicorectal reflex, in which the EAS pressure increases in response to increased intraabdominal pressure, is usually intact (Table 29-1).9 Patients with UMNB also have normal or increased anal sphincter tone, intact anocutaneous (or “anal wink”) and bulbocavernosus reflexes,107 a palpable puborectalis muscle sling, and normal anal verge appearance(Figure 29-5).
Table 29-1 Features of Colorectal Function in Normal Subjects and in Those With UMNB, UMNB With Posterior Rhizotomy, and LMNB
Lower Motor Neurogenic Bowel
Polyneuropathy, conus medullaris or cauda equina lesions, pelvic surgery, vaginal delivery, or even chronic straining during defecation can impair the somatic innervation of the anal sphincter mechanism. Persons with benign joint hypermobility syndrome might be more predisposed to these lesions.84 These conditions can also produce sympathetic and parasympathetic innervation deficits. If an isolated pudendal insult has occurred, colonic transit times are normal and FI predominates. Distal colonic sluggishness can occur as a result of loss of parasympathetic supply. Segmental stool transit studies demonstrate prolonged transit through the rectosigmoid segments resulting from the lack of direct innervation from the conus.71 The addition of constipation and DWE to FI compounds difficulties. This is an especially problematic combination because the accumulation of a large amount of hard stool that can result from such colonic inertia can overstretch the weakened anal mechanism. This can result in a gaping, patulous, incompetent anal orifice, often with associated rectal prolapse. The denervation, atrophy, and overstretching of the EAS and IAS lead to loss of the protective IAS tone, which can result in stool soilage from the increased abdominal pressures associated with everyday activities. Rectal distention leads to the expected IAS relaxation, but attenuated or absent EAS protective contractions can result in FI or fecal smearing whenever boluses are present at the rectum. The presence of a large bolus in the rectal vault can further compromise the rectoanal angulation at the pelvic floor and contribute to paradoxical liquid incontinence around a low impaction, called the ball-valve effect.9,112,127
Patients with lower motor neurogenic bowel (LMNB) dysfunction have decreased anal tone because of the smooth muscle that makes up the IAS. If no tone is found initially on inserting the examining finger, the examiner should wait up to 15 seconds to allow IAS reflex relaxation to recover and restore tone. Chronic overstretching has probably occurred if tone does not return. The anal-to-buttock contour typically appears flattened and “scalloped” (see Figure 29-5) because of atrophy of the pudendal-innervated pelvic floor muscles and EAS.9 The anocutaneous reflex is absent or decreased (depending on the completeness of the lesion). The bulbocavernosus reflex is also weak if present (see Table 29-1). The anal canal is shortened (as compared with the normal 2.5- to 4.5-cm length), and the puborectalis muscle ridge might not be palpable. Excessive perineal descent and even rectal prolapse can occur with Valsalva’s maneuver.
Gastrointestinal Dysfunction in Common Neurologic Disorders
Brain Disorders
Strokes (ischemic or hemorrhagic), cerebral trauma, masses, infections, and other lesions cause a whole range of neurologic damage and complications to many areas of the brain. These disorders commonly present with dysphagia, delays in gastric emptying and ileus (intestinal pseudoobstruction), constipation, and FI. Brainstem or cranial nerve involvement often causes dysphagia. Studies report that dysphagia occurs in approximately 45% of patients with stroke during the acute phase. Cerebral edema and increased intracranial pressure play a role in gastrointestinal ulceration, gastroparesis, and ileus through unknown mechanisms. Depending on the severity of the cerebral or brainstem involvement, dysphagia and ileus progressively improve. About 23% of patients with stroke were shown to have FI in stroke research, and constipation has been observed to be very common in clinical practice.5,19,53,93
Parkinson’s Disease and Parkinson’s-Plus Diseases
Parkinson’s disease and Parkinson’s-plus diseases (multiple system atrophy, progressive supranuclear palsy, corticobasal degeneration) are neurodegenerative diseases that affect various parts of the nervous system including the cerebral cortex, basal ganglia, brainstem, cerebral cortex, cerebellum, and spinal cord. They are characterized by dementia, muscle rigidity, bradykinesia, resting tremor, dystonia, shuffling gait, and dysphagia. They also have dysautonomia, which is manifested as orthostatic hypotension, sexual incompetence, gastric dysmotility, and constipation (see Chapter 51). Up to 50% of persons with Parkinson’s disease have been found to have dysphagia and problems with defecation in a study of 98 persons. In this group, 52.1% had dysphagia, 28.7% had constipation, and 65% had problems with defecation. These gastrointestinal problems can be severe enough to compromise nutrition and cause severe morbidity.25,36–38,72,95
Multiple Sclerosis
Multiple sclerosis is a demyelinating disease caused by an autoimmune process that involves different regions of the brain and spinal cord (see Chapter 52). Presentation can be any neurologic sign or symptom, which can consist of paresis, spasticity, ataxia, paresthesias, visual problems, cognitive deficits, mood disorders, dysphagia, gut dysmotility, urinary incontinence or retention, FI, diarrhea, or constipation. In a study of patients with multiple sclerosis, 68% were reported to have gastrointestinal problems, with 43% having constipation and 53% having FI. The bowel and bladder problems in multiple sclerosis are believed to be caused by supraspinal or spinal lesions associated with autonomic sympathetic and parasympathetic system impairment.19,25,42,118
Spinal Cord Disorders
Trauma, masses, infection, hemorrhage, or ischemia involving the spinal cord and causing tetraplegia or paraplegia, primarily affects colonic motility and perineal and anorectal sensation and function during the acute and chronic phases (see Chapter 55). Upper gastrointestinal tract problems resulting from disorders of the spinal cord, however, are increasingly being recognized. Depending on the level of the injury, brain and autonomic modulation of the ENS is impaired. The bowel dysfunction is principally one of motility rather than secretion or absorption. The upper digestive tract is more involved in persons with tetraplegia rather than with paraplegia, and is characterized by gastroparesis, impaired gastric emptying, and delayed gastrointestinal times. Dysphagia is common in persons with cervical cord lesions during the acute phase, and often eventually resolves. Gastric and duodenal ulcerations have been prevalent as well in persons with myelopathy. Across all levels of injury, there is a loss of voluntary control over bowel movements, with a scenario of constipation or FI. For lesions occurring above the conus medullaris (above T12), rectoanal reflexes are preserved with the sphincters being spastic. This is usually called the UMNB. Lesions below the conus medullaris (below T12) display flaccidity of the sphincters with loss of reflexes. This is referred to as the lower motor neuron bowel (LMNB).∗
Peripheral Neuropathy
Peripheral neuropathy has multiple causes including metabolic, viral, traumatic, toxic, metastatic, and genetic (see chapter 47). Sensory and motor deficits are associated with autonomic involvement. Disturbances in gastrointestinal transit are most often caused by peripheral neuropathies. Motility problems might result in diarrhea (from bacterial overgrowth) or in constipation. Well-known complications of diabetes include gastroparesis and decreased intestinal motility primarily from autonomic dysfunction. In paraneoplastic syndromes caused by small cell carcinoma of the lung or pulmonary carcinoid, immunoglobulin G antibody against enteric neurons has been found to be responsible for esophageal dysmotility, gastroparesis, and constipation. Injury to the pudendal nerve (from childbirth or persistent and protracted straining), S2–S4 nerve root lesions, or cauda equina compromise under FI and abnormal defecation.5,18,24,86
Comprehensive Evaluation
History
A comprehensive investigation for nausea, vomiting, bloating, early satiety, abdominal pain, diarrhea, constipation, and FI must be completed.27,50,109 The gastrointestinal history should not only review for cardinal symptoms, but should also address the patient’s general neuromuscular and gastrointestinal function. A detailed review of the patient’s bowel program includes an assessment of fluids, diet, activity, medications, and aspects of bowel care.65 Careful consideration must be given to drugs that seriously decrease gastrointestinal motility such as opiates, anticholinergics, tricyclics, antihistamines, calcium channel blockers, and phenothiazines.27,50,109 A review of the technique and outcome of bowel care should include a description of schedule, initiation method (chemical or mechanical stimulation), facilitative techniques, time requirements, and characteristics of stool results.107 The history should include premorbid bowel pattern information such as defecation frequency, typical time(s) of the day, associated predefecatory activities, bowel medications and techniques or trigger foods, and stool consistency. It is important to elicit any history of premorbid gastrointestinal disease or dysfunction. The presence of gastrointestinal sensations or pain, warning sensations for defecation, a sense of urgency, and an ability to prevent stool loss during Valsalva activities such as laughing, sneezing, coughing, or transfers should be noted. Excessively large-caliber, hard stool can be ascertained by a history of toilet plugging.86 The patient’s goals and willingness to alter prior bowel patterns or management need to be established.63
The approach to the problems associated with neurogenic bowel is the same as for all issues that confront the patient in the rehabilitation process. All aspects of impairment and disability that limit a person’s ability to maintain continence and volitionally defecate must be assessed within the perspective of the entire person. This includes an appraisal of the patient’s activity level, bowel pattern, and dietary habits. All aspects of personal performance should be addressed in person-centered rehabilitation, with the overall goal of maximizing independence in bowel management or direction of a bowel program.98,100
Physical Examination
The physical examination should include the gastrointestinal system and the associated parts of the musculoskeletal and nervous systems required for independent management of the bowel program. The examination should be completed at the onset and then annually for SCI.65 The purpose of the examination is to detect functional changes, screen for complications, and identify any new masses or lesions.
The abdomen should be inspected for distention, hernias, and other abnormalities. Careful assessment for the presence or absence of bowel sounds should be done. Percussion and auscultation should precede palpation for masses and tenderness. With the abdomen relaxed, the examiner transabdominally palpates the colon for hard stool. Palpable hard stool should not be present on the right side of the abdomen (ascending colon). Gastroparesis and intestinal pseudoobstruction present with a distended abdomen that is tympanitic with hypoactive bowel sounds, and with generalized abdominal tenderness (which can be accompanied by autonomic dysreflexia in persons with SCI). The patient can show signs of malnutrition and dehydration, including loss of weight, pale skin, dry mucous membranes, poor skin turgor, orthostatic hypotension, and tachycardia.33,65,117
Physical examination continues with inspection of the anus. A patulous, gaping orifice suggests a history of overdistention and trauma by a previous regimen. A normal anal-buttock contour (see Figure 29-5) suggests an intact EAS muscle mass, whereas its loss results in a flattened, fanned-out, scalloped-appearing anal region. The patient should perform a Valsalva maneuver while the examiner observes the anus and perineum for excessive descent.113 Perianal cutaneous sharp stimulation normally results in an externally visible anal sphincter reflexive contraction. This is the anocutaneous reflex, mediated by the inferior hemorrhoidal branch of the pudendal nerve (S2–S5). Sensation to pinprick is tested at the same time. The tone and voluntary squeeze strength of the EAS and tone of the IAS should be assessed. Integrity of the pelvic floor muscles can be examined by the ability to contract and relax. The length of the anus, where pressure is sensed, is normally 2.5 to 4.5 cm. The point where the pressure decreases marks the anorectal junction. Along the posterior wall, 1.5 to 2.5 cm from the anal verge, the puborectalis muscular sling can be palpated as a ridge that will push the finger forward as the subject resists defecation. No palpable ridge or push suggests puborectalis atrophy or dysfunction. A shortened length of anal pressure zone suggests EAS muscle atrophy. With the examiner’s finger in place, the bulbocavernosus reflex can be elicited by rapidly tapping or squeezing the clitoris or glans penis. The response can be delayed up to a few seconds in pathologic conditions. A consistent response to the stimulus indicates an intact bulbocavernosus reflex, although the reflex extinguishes when repeatedly elicited. Insertion of the finger in the anal canal occasionally triggers IAS relaxation, but more often triggers a tightening squeeze that is efferently equivalent to the bulbocavernosus reflex. Ask the patient to volitionally squeeze the anus before removing the finger (“resist defecation”) to check for volitional EAS and puborectalis tone and control. In addition, ask the patient to bear down and relax alternately to evaluate contraction, relaxation, and coordination of the pelvic muscles.
Diagnostic Testing
The history and physical examination provide most of the necessary information. The clinical cause of neurogenic bowel dysfunction in most patients who are referred to physiatrists is readily apparent. Additional objective laboratory testing can be helpful when the cause of FI or DWE is obscure, the history appears doubtful, conservative interventions fail, or surgical interventions are contemplated. Table 29-2 lists some of the many tests available.
Table 29-2 Diagnostic Tests for Gastrointestinal Dysfunction
| Techniques for Delivery of Gastrointestinal Tract Sensory Stimulation | |
|---|---|
| Category of Stimulus | Experimental Technique |
| Mechanical∗ (pressure or volume) | |
macronutrients, specific dietary components, and so forth
transcutaneous delivery of drugs, peptides, and so forth
| Invasive Techniques for Measurement of Gastrointestinal Tract Motility | ||
|---|---|---|
| Category of Technique | Physiologic Measure | Main Region (Application) |
| Manometry∗ | Contractile activity | |
| (intraluminal pressure) | ||
| Water perfused | All regions | |
| Solid state | All regions | |
| Berostat∗ | Tone or compliance | All regions |
| Tensostat | Wall tension | All regions |
| Electromyography | Myoelectric activity | |
| Intraluminal or needle | Stomach | |
| Anal sphincter (pelvic floor) | ||
| Intraluminal gas perfusion | Gas transit | Small bowel and colon |
| Intraluminal impedance | Bolus transit | Esophagus, antropyloroduodenum |
| (multichannel) | Esophageal reflux (liquid, gas) | |
| Noninvasive Techniques for Measurement of Gastrointestinal Tract Motility | ||
| Category of Technique | Physiologic Measure | Main Region (Application) |
| Imaging | Transit, wall motion, volume | |
| Scintigraphy∗ | All regions | |
| Barium radiopaque markers and radiography∗ | Colon (transit) | |
| Videofluoroscopy∗ | Esophagus (transit or bolus movement) | |
| Anorectum (defecography) | ||
| Ultrasonography | Gastroduodenum (wall motion) | |
| Antrum or proximal stomach (area or volume) | ||
| MRI | Stomach (wall motion) | |
| SPECT | Stomach (volume or accommodation) | |
| Other marker techniques | Transit | |
| C-substrate breath tests | Stomach (gastric emptying) | |
| Acetaminophen (plasma) | Stomach (gastric emptying) | |
| Lactulose breath test | Small bowel (orocecal transit) | |
| Sulfasalazine (plasma) | Small bowel (orocecal transit) | |
| Elecytromyography | Myoelectric activity | |
| Surface | Stomach | |
MRI, Magnetic resonance imaging; SPECT, three-dimensional single photon emission computed tomography.
∗ Most commonly used techniques.
Modified from Kellow JE: Principles of motility and sensation testing, Gastroenterol Clin North Am 32:733-750, 2003
Basic laboratory tests complement the physical examination. A stool guaiac test is helpful to rule out the presence of blood in the stool. False-positive results are common after SCI because of hemorrhoids, as well as from anal trauma secondary to bowel care.107 A flat plate radiograph of the abdomen can be helpful to rule out impaction,127 megacolon, obstruction, and a perforated viscus.
Management
Management of Nausea, Vomiting, Bloating, and Early Satiety
Nausea, vomiting, bloating, and early satiety typically indicate gastroparesis or a small intestinal or colonic pseudoobstruction in persons with neurologic disease. Acute or chronic episodes of subocclusion can occur. During episodes of acuity, there are findings of distended bowel loops and air-fluid levels requiring gastrointestinal decompression with nasogastric and rectal tubes. The patient must be placed on bowel rest and take nothing by mouth. Nutritional, fluid, and electrolyte support must be provided with an intravenous infusion. Drugs such as opiates, anticholinergics, tricyclics, antihistamines, calcium channel blockers, and phenothiazines (all of which profoundly diminish motility) must be discontinued or minimized. Once decompression is achieved, feeding is slowly advanced to a general diet. Prokinetic agents such as metoclopramide and domperidone (dopamine antagonists) and erythromycin (macrolide antibiotic, motilin receptor agonist) have been found to be beneficial in facilitating gastric emptying and intestinal motility and improving symptoms. Bethanechol and neostigmine have been shown to have limited success. Effects of tegaserod (Zelnorm) and cisapride (Propulsid), 5-HT4 receptor agonists, were more favorable, but these drugs were pulled out of the U.S. market because of serious cardiovascular adverse events, including myocardial ischemia, acute angina, arrhythmias, and stroke.∗ For chronic subocclusive states, enteral nutrition is preferred in patients with satisfactory digestive function. Meals should be small; frequent; liquid; contain polypeptide and hydrolyzed protein; be low in fat, fiber, and lactose; and contain multivitamins (iron, folate, calcium, vitamins D, K, and B12). If oral intake is not tolerated, enterostomies can be helpful to optimize nutrition. Total parenteral nutrition to satisfy nutritional requirements should be reserved for very severe cases. It generates various complications such as pancreatitis, glomerulonephritis, liver abnormalities, and septicemia or thrombosis from long-term catheter use.†
Management of Diarrhea
Occurrence of diarrhea in neurologic disease is commonly caused by bacterial overgrowth. Treatment with 4 weeks of antibiotics is helpful in relieving diarrhea, thus preventing malnutrition. Alternating 1-week courses (for a period of 2 to 4 weeks) of various antibiotics, including metronidazole, co-trimoxazole, ciprofloxacin, doxycycline, and amoxicillin-clavulanate, is recommended. Broad-spectrum antibiotics have been shown to be effective in a limited number of patients.7,33,117
Management of Defecation Dysfunction: Constipation and Fecal Incontinence
A bowel program is a comprehensive, individualized patient-centered treatment plan focused on preventing incontinence, achieving effective and efficient colonic evacuation, and preventing the complications of neurogenic bowel dysfunction.107 The subcomponents of a bowel program address diet, fluids, exercise, medications, and scheduled bowel care. Bowel care is the individually developed and prescribed procedure for defecation that is carried out by the patient or attendant.107
Goals of the Bowel Program
Fully appreciating an individual’s premorbid “normal” bowel function is important in the planning and goal setting for a new neurogenic bowel program. A wide range of “normal” bowel patterns exist. Ninety-five percent of individuals have a frequency of between three times per day and three times per week.33,96 Stool consistencies vary from liquid to pudding, pasty, semisolid, soft-formed, medium-formed, and hard-formed.
Bowel programs and techniques for bowel care training can be more effectively pursued during inpatient rehabilitation. Some patients need attendants to help with bowel care, and the attendants must also be well trained.108,109 The burden of care for persons with neurogenic bowel is much higher if continence is not achieved or if bowel care evacuation times are excessive.118,127 It is crucial that the patient takes a decisive leadership role in designing a bowel program that includes a convenient bowel care schedule. Educating patients about their altered neurogenic bowel physiology empowers them with options and techniques to construct a bowel care regimen compatible with their daily routine. Making them independent with their bowel care is an important aspect of the overall rehabilitation process.
Dietary Considerations
Food and fluid choices are important when colonic transit time is prolonged, as in SCI, where the transit time is often 96 hours versus the 30 hours typically found in normal subjects.9,12,95 The main goal of dietary choices is to achieve soft but well-formed, bulky stools. Excessive fluid resorption can result in stool hardening and subsequent constipation. Gases and liquids are propelled 30 to 100 times faster than solids by the colon. Stools that have lost their plasticity might not be kneaded and folded properly by the haustra, impeding the transit time. Stool softeners, both docusate and food fiber, have been used to maintain a more fluid content. Fiber increases stool bulk and plasticity, especially the more physically coarse forms of fiber, which also tend to decrease colonic pressures.9 Control of excessive stool hardness requires higher fiber foods in preference to lower residual foods. It is important to remember, however, that sufficient intake of fluids (preferably water) is imperative when consuming a high-fiber diet. Drinking at least 2 to 3 L of fluids per day is recommended. Otherwise, a high-fiber diet with insufficient intake of fluids can promote constipation. It should also be remembered that highly caffeinated fluids such as coffee, tea, and energy drinks can lead to dehydration from diuresis.
A diet that contains at least 15 g of fiber daily is recommended.65 Aside from fiber from vegetables, fruits, and grains, artificial fiber products such as psyllium (Metamucil, Fiberall), calcium polycarbophil (Fibercon), and methylcellulose (Citrucel) can be used. Increases in the fiber content of the diets of persons with SCI do not decrease colonic transit time,17 but enhance the rectoanal inhibitory reflex.33 The effects of fiber intake on stool consistency, frequency, and efficacy of evacuation should be evaluated in each individual patient, and fiber intake should be titrated accordingly. High pressures involved in moving solid feces probably contribute to the 90% incidence of hemorrhoids in Americans, and to premature diverticula formation and hemorrhoidal complications in more than 70% of patients with SCI.110 Constant straining at stool can contribute to peripheral neuropathic deficits in the anal sphincteric musculature.33 Acceptance of softer stools from a higher fiber diet might help reduce these complications and is often recommended for their treatment.
Treatment Approaches and Rationale
To select the best treatment approach, the UMNB must be delineated from the LMNB. Brain disease and spinal cord disorders above T12 present with UMNB, and peripheral neuropathy and spinal cord disorders below T12 present with LMNB. Colonic transit time and fecal elimination are enhanced by softer stool. If the stool becomes too liquid, however, the protective angle provided by the puborectalis becomes less effective, and greater EAS pressures are required to maintain continence. Neurogenic bowel resting anal pressures are usually normal to slightly decreased, but are unable to develop the protective increases in EAS tone needed to control more liquid stool.9 Some degree of stool firmness must be tolerated to prevent incontinence. To avoid incontinence on straining, more firmness (medium-formed) is required for the weaker anal sphincter mechanism of the LMNB than for the UMNB (semiformed to soft-formed).
Docusate is often used to try to increase fluid content and plasticity of the stool, although its clinical efficacy should be individually monitored. Fiber more consistently softens stool but also adds bulk. Bulkier stools can help stimulate the defecatory response more easily in LMNB, although less stimulus is needed in UMNB.41,83 The presentation of stool to the rectum, triggering defecation, can be associated with GMCs and mass movements more than with the slow accumulation of sufficient rectal stool to trigger reflex defecation. The GMC might be what is actually habituated.100
Choosing long intervals between elimination allows more fluid reabsorption, resulting in harder stools, which can worsen DWE. Because 95% of unimpaired persons defecate three or more times per week, choosing a frequency of at least as often as every other day would seem more physiologic and less likely to contribute to constipation.40,71,105 One study of patients with well-managed SCI found frequencies of bowel care to be chosen as daily by 24%, every other day by 46%, and more often than three times a week by 85%.67 The desire to avoid the unpleasant task of stool elimination leads some to elect longer intervals between bowel care sessions, but this carries the attendant risk for impaction or sphincter damage caused by rectal distention by larger-caliber, harder stools.
Management of Upper Motor Neuron Defecatory Dysfunction
Various rectally administered medications are used to trigger and sustain reflex defecation. These are usually inserted into the rectum approximately 30 minutes before the intended bowel program, followed by digital rectal stimulation. The rationale for this prescription is to use the least irritating and most easily inserted and retained medication. Suppositories that are typically used include glycerine, vegetable oil–based bisacodyl (Dulcolax), and polyethylene glycol–based (PGB) bisacodyl (Magic Bullet). Options for minienema-triggered bowel care include small-volume phospho-soda enema, bisacodyl enema, and Enemeez. Clinical efficacy studies have been conducted in attempts to measure efficiency and effectiveness of bowel care using various rectally administered triggering medications. In a randomized blinded study, hydrogenated vegetable oil–based (HVB) bisacodyl suppositories were compared with PGB suppositories and the Therevac minienema.57 Subjects with UMNB dysfunction were studied with the events and intervals of bowel care.57 Bowel care trials with PGB suppositories showed an average time to defection of approximately 22 minutes, and a total bowel care time of 50 minutes. This was much shorter than the average time to defecation of 40 minutes and total bowel care time of 85 minutes observed with HVB bisacodyl suppository trials. The use of minienemas had an efficiency similar to that of PGB suppositories and have been since replaced by Enemeez. Results vary in individual patients, and the use of digital stimulation alone is efficient for many SCI persons with UMNB.
Oral bowel stimulants can be used, however, when digital rectal stimulation and rectal medications are not sufficient to achieve the goals of the bowel program. Senna (Senokot), bisacodyl, polyethylene glycol (Miralax), and magnesium derivatives (Milk of Magnesia, magnesium citrate) are commonly used. Determining the most appropriate oral medication is usually done by trial and error based on type, dosage, quantity, and duration of efficacy. Constantly being aware of the goals of the bowel program assists with titration, revision, and timing of intake of oral medications. Judicious use of these medications is warranted because certain stimulants, especially in the anthraquinone family (senna, cascara, aloes), have been shown to damage myenteric neurons with chronic use and can cause atonic “cathartic bowel” syndrome.35,116 It has not been definitively established whether late complications from chronic use of oral bowel stimulant medications occur in those with neurogenic bowel dysfunction. Approaches that appear effective initially need longer-term studies to verify their continued benefits, especially because there is a high incidence of late gastrointestinal problems reported in an initially successfully managed SCI population.110
Management of Lower Motor Neuron Defecatory Dysfunction
One approach to initiating neurogenic bowel training is outlined in Box 29-1. Each step is added only after 2 weeks’ consistent trial of the previous step has been ineffective. In this stepwise approach, obtaining elimination at the desired time is emphasized as the first step, and usually precedes development of complete continence by several weeks. This regimen is designed to enhance responsiveness and emptying at the habituated time, with less stool presentation between bowel care sessions.
BOX 29-1 Protocol for Progressive Steps in Bowel Habituation Program
Intrinsic loss of the ENS in any segment, or surgical reanastomosis, can result in loss of the rectoanal inhibitory reflex, causing DWE. Oral laxative abuse can cause dysfunction of the ENS.35,116 If bowel training is not accomplishing defecation at the desired times, or if repeated involuntary incontinence occurs, further diagnostic evaluation might be indicated (see Table 29-2).
When neurogenic bowel deficits are incomplete and some degree of control and sensation is present, biofeedback might offer a means of enhancing the patient’s residual sensory and motor abilities. Improved sensory awareness after biofeedback training is an indicator of success. This typically requires only a few sessions, and most patients improve after just one session.100 Among more severely impaired nonselected children with myelomeningocele, biofeedback and behavioral training are equally effective in restoring continence.100 For selected individuals with some degree of volitional EAS activation and some degree of anorectal sensation, biofeedback can be a tool to help restore not just social continence, but also normal defecatory control.
Surgical Options
Gastric Electrical Stimulation
Treatment of gastroparesis with electrical pacemakers has been studied. The most promising device was surgically implanted in nine subjects with gastroparesis in whom conservative pharmacologic treatment had been unsuccessful. The causes of gastroparesis were diabetes in 5 subjects, idiopathic causes in 3, and postoperative complications in 1 subject. This device delivered high-energy electric pulses at a frequency higher than normal slow-wave impulses. Results showed improvement in gastric emptying and discontinuation of enterostomal feedings. Unfortunately, the device was too large to be implanted and was impractical for long-term use. Research is ongoing for a more feasible device.60,62,129
Gastrostomies and Enterostomies
Optimization of nutrition via gastrostomies and enterostomies has been found to be beneficial in patients with gastroparesis or intestinal or colonic pseudoobstruction. It not only helps prevent malnutrition, it also alleviates discomfort from nausea, vomiting, and abdominal distention. For persons receiving parenteral nutrition, a venting enterostomy provides symptomatic relief from gaseous distention and bloating.∗
Surgeries for Chronic Intestinal or Colonic Pseudoobstruction
During acute subocclusive episodes, colonoscopic decompression is successful in 75% to 90% of cases where nasogastric or rectal tubes were ineffective. A colonic decompression tube with suctioning can be used for those requiring repeated decompression. Decompression of the gastrointestinal tract also improves forward motility and coordinated propulsion. For cases in which conservative treatment has been unsuccessful, subtotal colectomy with ileorectostomy has been found to be the most effective treatment for chronic colonic pseudoobstruction. Intestinal transplantation is another option for patients with complete small intestinal failure who cannot receive total parenteral nutrition. Intestinal transplantation is often accompanied by liver transplantation for patients with liver failure that developed because of total parenteral nutrition. These patients have to receive lifelong immunosuppressive therapy. Survival rates have improved with the use of tacrolimus (FK506) rather than cyclosporine for immunosuppressive therapy.7,33,65,117
Pelvic Floor Sling
Sacral nerve deficits interfere with the action of the puborectalis, levator ani, and EAS (see Figure 29-2). The resulting pelvic floor descent impairs the protective puborectalis sling angle and decreases the efficacy of protective EAS contractions. Some patients have benefited from transposition of innervated gracilis, adductor longus, gluteus maximus, or free muscle graft palmaris longus to replace puborectalis function and restore the acute anorectal junction angle that this sling provides. Chronic electrical stimulation to enhance development of fatigue resistance is used with these transplants. Sensory deficits are not improved, but continence is somewhat restored with the ability to inhibit defecation if some degree of sensation remains.88,110
Internal Anal Sphincter and Partial External Anal Sphincter Myotomy
Incomplete EAS relaxation during defecation (dyssynergia) results in a functional outlet obstruction and DWE. A prolonged descending colon transit time occurs, which improves with an IAS and partial EAS myotomy.32 This procedure relieves constipation in 62% of patients, but results in FI in 16%. As a consequence of the FI complication, it has not become a popular option.85
Electroprosthesis
Stimulation of anterior sacral roots S2, S3, and S4 by transrectal electric stimulation or via a stimulator surgically placed for micturition has been performed.17,27,50,86 Stimulation of S2 tends to promote nonperistaltic, low-pressure colorectal motor activity. Stimulation of S3 causes occasional high-pressure peristaltic waves, especially with repetitive stimulation. Stimulation of S4 increases both rectal and anal tone.86,128 Electrodefecation has been obtainable by sacral root stimulation in up to 50% of patients, but remains unpredictable.8,17,27,29 A reliable electroprosthesis for defecation remains an elusive goal.8,50 An electroprosthesis for both bowel and bladder control has been found to be cost-effective in suprasacral spinal cord–injured persons, although it is unclear how much of this benefit was from improved bowel control.28 Artificial anal sphincters with a subcutaneous pump reservoir similar to urinary artificial sphincters have high complication rates and poor outcomes, and their use has only been investigative.81
Antegrade Continence Enema
The options of the antegrade continence enema should be considered in clinical scenarios of prolonged bowel care time, recurrent fecal impactions, or poor or intermittent response to rectal medications to initiate bowel care.29,76,91,125 This is an alternative method of antegrade enema delivery that requires the surgical construction of a catheterizable appendicocecostomy stoma. The appendix and the right side of the colon are mobilized through a horizontal right lower quadrant incision and brought against the abdominal wall. The tip of the appendix is then amputated, and the opening into the appendix lumen is modified into a catheterizable stoma on the abdominal wall. This procedure can now also be performed laparoscopically.60 This stoma can be catheterized and infused with 200 to 600 mL of tap water to trigger a propulsive colonic peristalsis and defecation within 10 to 20 minutes.128 Bowel care can then be additionally facilitated with digital stimulation in the usual fashion.
Colostomy
Colostomy has been shown to reduce bowel care time, especially when offered to those with chronic DWE.107,122 It can be indicated in four general scenarios:
Although diversion for pressure ulcer healing is usually anticipated to be reversed, those with neurogenic bowel often elect to maintain the colostomy even after the pressure ulcer has healed.99,111 Colostomy carries a surgical risk, is cosmetically disfiguring, and is seldom necessary to achieve adequate social continence. It remains as a procedure of last resort for the treatment of FI or DWE.8,31,65,107,110
Complications
For persons with gastroparesis or intestinal pseudoobstruction, the primary complications are chronic malnutrition, dehydration, and electrolyte imbalance. It is important to optimize nutrition and hydration, and monitor weight, vitamin, and electrolyte levels regularly. Oral and enteral feedings must provide adequate calories, protein, electrolytes, and vitamins despite being liquid in consistency; low in fat, fiber, and lactose; high in polypeptides and hydrolyzed protein; and received in small, frequent amounts.33,62,117,129
In acute subocclusive episodes of colonic pseudoobstruction, it is important to achieve decompression as soon as possible. Failure to do so can result in progressive distention and cecal ischemia, causing perforation. The risk for perforation is increased when cecal diameters are 12 cm or greater. Colonic perforation requires emergent exploratory laparotomy and intestinal resection. The mortality rate is approximately 36% to 44% and is determined by age, diameter of the cecum, delay of decompression, and comorbidities.10,109
Significant bowel complications requiring medical treatment or lifestyle alterations are reported by 27% of persons with SCI by 5 years or greater beyond their injury, even if bowel management was satisfactory during the first 5 years. More than 80% of persons with SCI reported bowel impactions, and 20% had chronic bowel impaction and DWE problems.110 Impactions have been reported to be complicated by perforation or even death.127 Impactions have a morbidity rate ranging up to 6% in the “normal” population, being higher in the cognitively impaired elderly.127 Other late gastrointestinal complications reported by patients with SCI include gastroesophageal reflux, premature diverticulosis, and autonomic dysreflexia.45,68,110 Morbidity from colonic perforation by enema use has also been reported.75
Hemorrhoids are more symptomatic when patients have intact sensation, but in one study, rectal bleeding caused by hemorrhoids was reported by 74% of patients with SCI.110 Hemorrhoids develop because of frequent high pressures in the anorectal marginal veins and are associated with constipated hard stool passage. Stool softening is the best preventive and chronic treatment measure, but it should be balanced with the requirement to modulate stool consistency to maintain continence.
An overstretched, patulous, noncompetent sphincter associated with rectal prolapse often is the end result of chronic passage of very large, hard stools through a weakened anorectal mechanism in LMNB. Overdistention of the weakened neurogenic anal mechanism should be avoided by use of stool softening and gentle care to dilate the sphincter whenever manual disimpaction is required, to minimize trauma to these denervated structures. Although the anus can be significantly dilated to accommodate two fingers for breaking up low impactions, anorectal overdistention has been hypothesized to lead to atonic segments similar to bladder overdistention. The bowel, however, cannot be as easily decompressed and rested to allow recovery as can the bladder. The IAS is smooth muscle that will shorten and remodel to eventually regain competent closure if the overstretching can be eliminated. Unfortunately, this might require months of incontinent, liquid to soft pasty stools, which is seldom tolerated.50,67 Should the patient require a temporary colostomy for some other disease process, it might be possible after many months to then train toward social continence with the decompressed and restored IAS. Such patients have usually had long courses of constant soiling, however, and often prefer to keep their colostomy and continence rather than pursue surgical reversal and training.98,111
Autonomic dysreflexia occurs in patients with SCI who have lesions at or above the midthoracic region. FI is a common and potentially dangerous cause of autonomic dysreflexia because of the substantial time that can be required for its clearance (see Chapter 55).12 If manual disimpaction is required, lubrication with lidocaine gel is recommended to decrease additional nociceptive sensory input from the richly innervated anal region.
Bloating and abdominal distention are common complaints of patients with neurogenic bowel dysfunction.40,54 These complaints can be reduced in patients with SCI by increasing the frequency of bowel care. This complaint can be especially severe in those with hyperactive EAS protective responses to rectal distention, which can preclude the passing of flatus. Digital release of flatus might be required, in addition to diet modification to eliminate foods that produce excessive gases. The workup should also include assessment for any contributing aerophagia (air swallowing).
Treatment Outcomes
Bowel habituation training in children with myelomeningocele by means of suppositories, digital stimulation, or both, resulted in 83% of compliant patients having less than one incontinent stool per month.64 The continence catheter enema, which has a distal rectal balloon to avoid immediate enema expulsion, when used daily or every other day, reduced FI to fewer than three episodes per month in children with myelomeningocele.39,103
Nursing home residents with FI and dementia evaluated to have UMNB were treated by medically constipating them (with codeine) and giving biweekly enemas. Those with diagnosed LMNB had their stools softened with lactulose and received weekly enemas. Fecal continence was restored in 80% of those who were consistently treated with these protocols.115
Although all patients with complete SCI have episodic FI,80 this is a chronic problem for only 2%.108 DWE appears to be a progressive problem that develops 5 years or more after SCI. This is rarely reported after training in the first 4 years but occurs in 20% by a mean of 17 years after injury.108 Gastrointestinal problems in SCI are not merely nuisances, because they also account for 10% of SCI late mortality.45
Patients with multiple sclerosis, Parkinson’s disease, or muscular dystrophy have also been helped by methods to enhance bowel storage or elimination in the setting of deteriorating neuromuscular and anorectal function.8,20,119 Colostomy can also provide a means of achieving social continence in these patients. As noted above, colostomy complications include embarrassing gas problems, appliance loosening and leakage, and cosmetic difficulties.
Many solutions are yet to come for the large number of persons who cope with neurogenic bowel dysfunction. The colon will continue to be a fertile area for research.107
1. Aaronson M.J., Freed M.M., Burakoff R. Colonic myoelectric activity in persons with spinal cord injury. Dig Dis Sci. 1985;30(4):295-300.
2. Abercrombie J.F., Rogers J., Swash M. Faecal incontinence in myotonic dystrophy. J Neurol Neurosurg Psychiatry. 1998;64(1):128-130.
3. Al-Chaer E.D., Kawasaki M., Pasricha P.J. A new model of chronic visceral hypersensitivity in adult rats induced by colon irritation during postnatal development. Gastroenterology. 2000;119(5):1276-1285.
4. Al-Chaer E.D., Westlund K.N., Willis W.D. Sensitization of postsynaptic dorsal column neuronal responses by colon inflammation. Neuroreport. 1997;8(15):3267-3273.
5. Altaf M.A., Sood M.R. The nervous system and gastrointestinal function. Dev Disabil Res Rev. 2008;14(2):87-95.
6. Andriantsitohaina R., Surprenant A. Acetylcholine released from guinea-pig submucosal neurones dilates arterioles by releasing nitric oxide from endothelium. J Physiol. 1992;453:493-502.
7. Antonucci A., Fronzoni L., Cogliandro L., et al. Chronic intestinal pseudo-obstruction. World J Gastroenterol. 2008;14(19):2953-2961.
8. Banwell J.G., Creasey G.H., Aggarwal A.M., et al. Management of the neurogenic bowel in patients with spinal cord injury. Urol Clin North Am. 1993;20(3):517-526.
9. Bartolo D.C., Read N.W., Jarratt J.A., et al. Differences in anal sphincter function and clinical presentation in patients with pelvic floor descent. Gastroenterology. 1983;85(1):68-75.
10. Berkley K.J., Hubscher C.H., Wall P.D. Neuronal responses to stimulation of the cervix, uterus, colon, and skin in the rat spinal cord. J Neurophysiol. 1993;69(2):545-556.
11 Beyak M.J., Bulmer D.C.E., Jiang C.D., et al. Extrinsic sensory afferent nerves innervating the gastrointestinal tract. In Johnson L.R., Barrett K.E., Ghishan F.K., et al, editors: Physiology of the gastrointestinal tract, ed 4, San Diego: Academic Press, 2006.
12. Bittinger M., Barnert J., Wienbeck M. Autonomic dysfunction and the gastrointestinal tract. Clin Auton Res. 1999;9(2):75-81.
13. Bornstein J.C., Furness J.B. Correlated electrophysiological and histochemical studies of submucous neurons and their contribution to understanding enteric neural circuits. J Auton Nerv Syst. 1988;25(1):1-13.
14. Boss B.J., Pecanty L., McFarland S.M., et al. Self-care competence among persons with spinal cord injury. SCI Nurs. 1995;12(2):48-53.
15. Burnstock G. Physiology and pathophysiology of purinergic neurotransmission. Physiol Rev. 2007;87(2):659-797.
16. Burnstock G. The journey to establish purinergic signalling in the gut. Neurogastroenterol Motil. 2008;20(suppl 1):8-19.
17. Cameron K.J., Nyulasi I.B., Collier G.R., et al. Assessment of the effect of increased dietary fibre intake on bowel function in patients with spinal cord injury. Spinal Cord. 1996;34(5):277-283.
18. Camilleri M. Disorders of gastrointestinal motility in neurologic diseases. Mayo Clin Proc. 1990;65(6):825-846.
19. Camilleri M., Bharucha A.E. Gastrointestinal dysfunction in neurologic disease. Semin Neurol. 1996;16(3):203-216.
20. Caroscio J.T. Amyotrophic lateral sclerosis: a guide to patient care. New York: Thieme; 1986. 126
21. Cervero F., Janig W. Visceral nociceptors: a new world order? Trends Neurosci. 1992;15(10):374-378.
22. Cervero F., Laird J.M. Visceral pain. Lancet. 1999;353(9170):2145-2148.
23. Chelimsky G., Wszolek Z., Chelimsky T.C. Gastrointestinal dysfunction in autonomic neuropathy. Semin Neurol. 1996;16(3):259-268.
24. Christensen J. The motor function of the colon. In: Yamada T., editor. Textbook of gastroenterology. Philadelphia: JB Lippincott, 1991.
25. Coggrave M., Wiesel P.H., Norton C. Management of faecal incontinence and constipation in adults with central neurological diseases. Cochrane Database Syst Rev. 2006;(2):CD002115.
26. Cooke H.J. Role of the “little brain” in the gut in water and electrolyte homeostasis. FASEB J. 1989;3(2):127-138.
27. Coulie B., Camilleri M. Intestinal pseudo-obstruction. Annu Rev Med. 1999;50:37-55.
28. Creasey G.H., Dahlberg J.E. Economic consequences of an implanted neuroprosthesis for bladder and bowel management. Arch Phys Med Rehabil. 2001;82(11):1520-1525.
29. De Looze D., Van Laere M., De Muynck M., et al. Constipation and other chronic gastrointestinal problems in spinal cord injury patients. Spinal Cord. 1998;36(1):63-66.
30. De Wever I., Eeckhout C., Vantrappen G., et al. Disruptive effect of test meals on interdigestive motor complex in dogs. Am J Physiol. 1978;235(6):E661-E665.
31. Deshmukh G.R., Barkel D.C., Sevo D., et al. Use or misuse of colostomy to heal pressure ulcers. Dis Colon Rectum. 1996;39(7):737-738.
32. Devroede G., Lamarche J. Functional importance of extrinsic parasympathetic innervation to the distal colon and rectum in man. Gastroenterology. 1974;66(2):273-280.
33. Devroede G., Lamarche J. Constipation. In: Sleisenger M.H., Fordtran J.S., editors. Gastrointestinal disease. Philadelphia: WB Saunders, 1993.
34. Doughty D.B., Jackson D.B. Gastrointestinal disorders. St Louis: Mosby-Year Book; 1993.
35. Dubrovsky B., Filipini D. Neurobiological aspects of the pelvic floor muscles involved in defecation. Neurosci Biobehav Rev. 1990;14(2):157-168.
36. Edwards L.L., Pfeiffer R.F., Quigley E.M. Gastrointestinal symptoms in Parkinson’s disease. Mov Disord. 1991;6(2):151-156.
37. Edwards L.L., Quigley E.M., Harned R.K., et al. Characterization of swallowing and defecation in Parkinson’s disease. Am J Gastroenterol. 1994;89(1):15-25.
38. Edwards L.L., Quigley E.M., Pfeiffer R.F. Gastrointestinal dysfunction in Parkinson’s disease: frequency and pathophysiology. Neurology. 1992;42(4):726-732.
39. Eire P.F., Cives R.V., Gago M.C. Faecal incontinence in children with spina bifida: the best conservative treatment. Spinal Cord. 1998;36(11):774-776.
40. Fealey R.D., Szurszewski J.H., Merritt J.L., et al. Effect of traumatic spinal cord transection on human upper gastrointestinal motility and gastric emptying. Gastroenterology. 1984;87(1):69-75.
41. Filipini D.L., Dubrovsky B. Pelvic floor muscles response to graded rectal distension and cutaneous stimulation. Dig Dis Sci. 1991;36(12):1761-1767.
42. Fowler C.J., Henry M.M. Gastrointestinal dysfunction in multiple sclerosis. Semin Neurol. 1996;16(3):277-279.
43. Fox E.A., Phillips R.J., Martinson F.A., et al. Vagal afferent innervation of smooth muscle in the stomach and duodenum of the mouse: morphology and topography. J Comp Neurol. 2000;428(3):558-576.
44. Gebhart G.F. Pathobiology of visceral pain: molecular mechanisms and therapeutic implications IV. Visceral afferent contributions to the pathobiology of visceral pain. Am J Physiol Gastrointest Liver Physiol. 2000;278(6):G834-G838.
45. Gore R.M., Mintzer R.A., Calenoff L. Gastrointestinal complications of spinal cord injury, Spine (Phila Pa 1976). 1981;6(6):538-544.
46. Goyal R.K., Crist J.R. Neurology of the gut. In: Sleisenger M.H., Fordtram J.S., editors. Gastrointestinal disease. Philadelphia: WB Saunders, 1989.
47. Grundy D., Al-Chaer E.D., Aziz Q., et al. Fundamentals of neurogastroenterology: basic science. Gastroenterology. 2006;130(5):1391-1411.
48. Guyton A.C. Textbook of medical physiology, ed 8. Philadelphia: WB Saunders; 1991.
49. Hammond M.C., Umlauf R.L., Matteson B., et al. Yes you can! A guide to self-care for persons with spinal cord injury. Washington, DC: Paralyzed Veterans of America; 1989. 361
50. Han T.R., Kim J.H., Kwon B.S. Chronic gastrointestinal problems and bowel dysfunction in patients with spinal cord injury. Spinal Cord. 1998;36(7):485-490.
51. Hanson R.W., Franklin M.R. Sexual loss in relation to other functional losses for spinal cord injured males. Arch Phys Med Rehabil. 1976;57(6):291-293.
52. Harari D., Minaker K.L. Megacolon in patients with chronic spinal cord injury. Spinal Cord. 2000;38(6):331-339.
53. Harari D., Norton C., Lockwood L., et al. Treatment of constipation and fecal incontinence in stroke patients: randomized controlled trial. Stroke. 2004;35(11):2549-2555.
54. Hasler W.L. Gastroparesis: symptoms, evaluation, and treatment. Gastroenterol Clin North Am. 2007;36(3):619-647. ix
55. Hasler W.L. Gastroparesis: current concepts and considerations. Medscape J Med. 2008;10(1):16.
56. Hillsley K., Kirkup A.J., Grundy D. Direct and indirect actions of 5-hydroxytryptamine on the discharge of mesenteric afferent fibres innervating the rat jejunum. J Physiol. 1998;506(pt 2):551-561.
57. House J.G., Stiens S.A. Pharmacologically initiated defecation for persons with spinal cord injury: effectiveness of three agents. Arch Phys Med Rehabil. 1997;78(10):1062-1065.
58. Jin J.G., Murthy K.S., Grider J.R., et al. Stoichiometry of neurally induced VIP release, NO formation, and relaxation in rabbit and rat gastric muscle. Am J Physiol. 1996;271(2 pt 1):G357-G369.
59. Kamath P.S., Phillips S.F., O’Connor M.K., et al. Colonic capacitance and transit in man: modulation by luminal contents and drugs. Gut. 1990;31(4):443-449.
60. Kaname A., Kakizaki H., Machino R., et al. Laparoscopic antegrade continence enema procedure for fecal incontinence in a patient with spina bifida. Int J Urol. 2003;10:401-403.
61. Kerr T.P., Robb S.A., Clayden G.S. Lower gastrointestinal tract disturbance in congenital myotonic dystrophy. Eur J Pediatr. 2002;161(8):468-469.
62. Kiba T. Relationships between the autonomic nervous system, humoral factors and immune functions in the intestine. Digestion. 2006;74(3-4):215-227.
63. King J.C., Currie D.M., Wright E. Bowel training in spina bifida: importance of education, patient compliance, age, and anal reflexes. Arch Phys Med Rehabil. 1994;75(3):243-247.
64. King J.C., Nelson R., Tuturro T., et al. Prescriptions, referrals, and the rehabilitation team. In DeLisa J.A., editor: Rehabilitation medicine principles and practice, ed 3, Philadelphia: JB Lippincott, 1998.
65. King R., Biddle A., Braunschweig C., et al. Neurogenic bowel management in adults with spinal cord injury. J Spinal Cord Med. 1998;21:248-293.
66. Kirkup A.J., Brunsden A.M., Grundy D. Receptors and transmission in the brain-gut axis: potential for novel therapies. I. Receptors on visceral afferents. Am J Physiol Gastrointest Liver Physiol. 2001;280(5):G787-G794.
67. Kirshblum S.C., Gulati M., O’Connor K.C., et al. Bowel care practices in chronic spinal cord injury patients. Arch Phys Med Rehabil. 1998;79(1):20-23.
68. Kirshblum S.C., House J.G., O’Connor K.C. Silent autonomic dysreflexia during a routine bowel program in persons with traumatic spinal cord injury: a preliminary study. Arch Phys Med Rehabil. 2002;83(12):1774-1776.
69. Koch K.L., Hong S.P., Xu L. Reproducibility of gastric myoelectrical activity and the water load test in patients with dysmotility-like dyspepsia symptoms and in control subjects. J Clin Gastroenterol. 2000;31(2):125-129.
70. Kozlowski C.M., Green A., Grundy D., et al. The 5-HT(3) receptor antagonist alosetron inhibits the colorectal distention induced depressor response and spinal c-fos expression in the anaesthetised rat. Gut. 2000;46(4):474-480.
71. Krogh K., Mosdal C., Laurberg S. Gastrointestinal and segmental colonic transit times in patients with acute and chronic spinal cord lesions. Spinal Cord. 2000;38(10):615-621.
72. Krogh K., Ostergaard K., Sabroe S., et al. Clinical aspects of bowel symptoms in Parkinson’s disease. Acta Neurol Scand. 2008;117(1):60-64.
73. Ladabaum U., Minoshima S., Hasler W.L., et al. Gastric distention correlates with activation of multiple cortical and subcortical regions. Gastroenterology. 2001;120(2):369-376.
74. Levi R., Hultling C., Nash M.S., et al. The Stockholm Spinal Cord Injury Study: 1. Medical problems in a regional SCI population. Paraplegia. 1995;33(6):308-315.
75. Liptak G.S., Revell G.M. Management of bowel dysfunction in children with spinal cord disease or injury by means of the enema continence catheter. J Pediatr. 1992;120(2 pt 1):190-194.
76. Liu S., Xia Y., Hu H., et al. Histamine H3 receptor-mediated suppression of inhibitory synaptic transmission in the submucous plexus of guinea-pig small intestine. Eur J Pharmacol. 2000;397(1):49-54.
77. Luiking Y.C., Akkermans L.M., Peeters T.L., et al. Effects of motilin on human interdigestive gastrointestinal and gallbladder motility, and involvement of 5HT3 receptors. Neurogastroenterol Motil. 2002;14(2):151-159.
78. Lynn P., Zagorodnyuk V., Hennig G., et al. Mechanical activation of rectal intraganglionic laminar endings in the guinea pig distal gut. J Physiol. 2005;564(Pt 2):589-601.
79. MacDonagh R., Sun W.M., Thomas D.G., et al. Anorectal function in patients with complete supraconal spinal cord lesions. Gut. 1992;33(11):1532-1538.
80. MacDonagh R.P., Sun W.M., Smallwood R., et al. Control of defecation in patients with spinal injuries by stimulation of sacral anterior nerve roots. BMJ. 1990;300(6738):1494-1497.
81. Madoff R.D., Parker S.C., Varma M.G., et al. Faecal incontinence in adults. Lancet. 2004;364(9434):621-632.
82. Maggi C.A., Catalioto R.M., Criscuoli M., et al. Tachykinin receptors and intestinal motility. Can J Physiol Pharmacol. 1997;75(6):696-703.
83. Maggi C.A., Meli A. The sensory-efferent function of capsaicin-sensitive sensory neurons. Gen Pharmacol. 1988;19(1):1-43.
84. Manning J., Korda A., Benness C., et al. The association of obstructive defecation, lower urinary tract dysfunction and the benign joint hypermobility syndrome: a case-control study. Int Urogynecol J Pelvic Floor Dysfunct. 2003;14(2):128-132.
85. Martelli H., Devroede G., Arhan P., et al. Mechanisms of idiopathic constipation: outlet obstruction. Gastroenterology. 1978;75(4):623-631.
86. Martelli H., Devroede G., Arhan P., et al. Some parameters of large bowel motility in normal man. Gastroenterology. 1978;75(4):612-618.
87. Meshkinpour H., Nowroozi F., Glick M.E. Colonic compliance in patients with spinal cord injury. Arch Phys Med Rehabil. 1983;64(3):111-112.
88. Ness T.J., Gebhart G.F. Visceral pain: a review of experimental studies. Pain. 1990;41(2):167-234.
89. Ness T.J., Gebhart G.F. Acute inflammation differentially alters the activity of two classes of rat spinal visceral nociceptive neurons. Neurosci Lett. 2000;281(2-3):131-134.
90. Nino-Murcia M., Stone J.M., Chang P.J., et al. Colonic transit in spinal cord-injured patients. Invest Radiol. 1990;25(2):109-112.
91. North R.A., Surprenant A. Inhibitory synaptic potentials resulting from alpha 2-adrenoceptor activation in guinea-pig submucous plexus neurones. J Physiol. 1985;358:17-33.
92. Pedersen E. Regulation of bladder and colon–rectum in patients with spinal lesions. J Auton Nerv Syst. 1983;7(3-4):329-338.
93. Perkin G.D., Murray-Lyon I. Neurology and the gastrointestinal system. J Neurol Neurosurg Psychiatry. 1998;65(3):291-300.
94. Pierce E., Cowan P., Stokes M. Managing faecal retention and incontinence in neurodisability. Br J Nurs. 2001;10(9):592-601.
95. Quigley E.M. Gastrointestinal dysfunction in Parkinson’s disease. Semin Neurol. 1996;16(3):245-250.
96. Rendtorff R.C., Kashgarian M. Stool patterns of healthy adult males. Dis Colon Rectum. 1967;10(3):222-228.
97. Saab C.Y., Park Y.C., Al-Chaer E.D. Thalamic modulation of visceral nociceptive processing in adult rats with neonatal colon irritation. Brain Res. 2004;1008(2):186-192.
98. Saltzstein R.J., Romano J. The efficacy of colostomy as a bowel management alternative in selected spinal cord injury patients. J Am Paraplegia Soc. 1990;13(2):9-13.
99. Sarna S.K. Giant migrating contractions and their myoelectric correlates in the small intestine. Am J Physiol. 1987;253(5 pt 1):G697-G705.
100. Schiller L.R. Fecal incontinence. In: Sleisenger M.H., Fordtran J.S., editors. Gastrointestinal disease. Philadelphia: WB Saunders, 1993.
101. Sengupta J.N., Gebhart G.F. Gastrointestinal afferent fibers and sensation. In Johnson L.R., Alpers D.H., Christensen J., et al, editors: Physiology of the gastrointestinal tract, ed 3, New York: Raven Press, 1994.
102. Shafik A., Shafik A.A., Ahmed I. Role of positive anorectal feedback in rectal evacuation: the concept of a second defecation reflex: the anorectal reflex. J Spinal Cord Med. 2003;26(4):380-383.
103. Shandling B., Gilmour R.F. The enema continence catheter in spina bifida: successful bowel management. J Pediatr Surg. 1987;22(3):271-273.
104. Silverman D.H., Munakata J.A., Ennes H., et al. Regional cerebral activity in normal and pathological perception of visceral pain. Gastroenterology. 1997;112(1):64-72.
105. Siproudhis L., Bellissant E., Juguet F., et al. Perception of and adaptation to rectal isobaric distension in patients with faecal incontinence. Gut. 1999;44(5):687-692.
106. Stanghellini V., Cogliandro R.F., de Giorgio R., et al. Chronic intestinal pseudo-obstruction: manifestations, natural history and management. Neurogastroenterol Motil. 2007;19(6):440-452.
107. Stiens S.A., Bergman S.B., Goetz L.L. Neurogenic bowel dysfunction after spinal cord injury: clinical evaluation and rehabilitative management. Arch Phys Med Rehabil. 1997;78(suppl 3):S86-S102.
108. Stiens S.A., Braunschweig C., Cowel F., et al. Neurogenic bowel: what you should know. A guide for people with spinal cord injury. Washington, DC: Consortium for Spinal Cord Medicine; 1997. (Vol 1999)53
109. Stiens S.A., Pidde T., Veland B., et al. Accidents stink! Bowel care 202 . Washington, DC: Paralyzed Veterans of America Education and Training Foundation; 2001.
110. Stone J.M., Nino-Murcia M., Wolfe V.A., et al. Chronic gastrointestinal problems in spinal cord injury patients: a prospective analysis. Am J Gastroenterol. 1990;85(9):1114-1119.
111. Stone J.M., Wolfe V.A., Nino-Murcia M., et al. Colostomy as treatment for complications of spinal cord injury. Arch Phys Med Rehabil. 1990;71(7):514-518.
112. Suckling P.V. The ball-valve rectum due to impacted faeces. Lancet. 1962;2(7266):1147.
113. Swash M. New concepts in the prevention of incontinence. Practitioner. 1985;229(1408):895-899.
114. Tjandra J.J., Ooi B.S., Han W.R. Anorectal physiologic testing for bowel dysfunction in patients with spinal cord lesions. Dis Colon Rectum. 2000;43(7):927-931.
115. Tobin G.W., Brocklehurst J.C. Faecal incontinence in residential homes for the elderly: prevalence, aetiology and management. Age Ageing. 1986;15(1):41-46.
116. Waseem S., Moshiree B., Draganov P.V. Gastroparesis: current diagnostic challenges and management considerations. World J Gastroenterol. 2009;15(1):25-37.
117. White J.C., Verlot M.G., Ehrentheil O. Neurogenic disturbances of the colon and their investigation by the colon metrogram: a preliminary report. Ann Surg. 1940;112(6):1042-1057.
118. Wiesel P.H., Norton C., Glickman S., et al. Pathophysiology and management of bowel dysfunction in multiple sclerosis. Eur J Gastroenterol Hepatol. 2001;13(4):441-448.
119. Wiesel P.H., Norton C., Roy A.J., et al. Gut focused behavioural treatment (biofeedback) for constipation and faecal incontinence in multiple sclerosis. J Neurol Neurosurg Psychiatry. 2000;69(2):240-243.
120. Wingate D.L. Backwards and forwards with the migrating complex. Dig Dis Sci. 1981;26(7):641-666.
121. Wingate D.L., Ewart W.R. The brain–gut axis. In: Yamada T., editor. Textbook of gastroenterology. Philadelphia: JB Lippincott, 1991.
122. Winge K., Rasmussen D., Werdelin L.M. Constipation in neurological diseases. J Neurol Neurosurg Psychiatry. 2003;74(1):13-19.
123. Wood J.D. Neuropathy in the brain-in-the-gut. Eur J Gastroenterol Hepatol. 2000;12(6):597-600.
124. Wood J.D. Enteric neuroimmunophysiology and pathophysiology. Gastroenterology. 2004;127(2):635-657.
125. Wood J.D. Neuropathophysiology of functional gastrointestinal disorders. World J Gastroenterol. 2007;13(9):1313-1332.
126. Wood J.D., Alpers D.H., Andrews P.L. Fundamentals of neurogastroenterology. Gut. 1999;45(suppl 2):II6-II16.
127. Wrenn K. Fecal impaction. N Engl J Med. 1989;321(10):658-662.
128. Yang C.C., Stiens S.A. Antegrade continence enema for the treatment of neurogenic constipation and fecal incontinence after spinal cord injury. Arch Phys Med Rehabil. 2000;81(5):683-685.
129. Zagorodnyuk V.P., Chen B.N., Brookes S.J. Intraganglionic laminar endings are mechano-transduction sites of vagal tension receptors in the guinea-pig stomach. J Physiol. 2001;534(Pt 1):255-268.