CHAPTER 12 Neuro-ophthalmology of Meningiomas
INTRODUCTION
Neuro-ophthalmology is a complex, broad-ranging area of ophthalmology and one of the most important fields that can help in diagnosis and follow-up in neuro-oncology. Recognizing subtle physical signs, such as pupil abnormalities, cranial nerve palsies, or visual field defects may be the key to finding significant intracranial meningioma pathology. Correct diagnosis and referral to neuroimaging all depend on a thorough understanding of neuroanatomy and neuro-ophthalmologic examination. Symptoms may be expressed simply as loss of vision or double vision, or as complex syndromes depending on the location of lesion. A firm understanding of the neuroanatomy and neurophysiology of the eye is essential for correct diagnosis. Neuro-ophthalmologic findings of meningiomas depend on the tumor size and its location along the neuro-ophthalmologic tract. Meningiomas arise from arachnoidal cells, and they may be attached to any of the three layers of the meninges. Problems may occur anywhere along the visual pathway, including the brain stem, cavernous sinus, cerebral cortex, subarachnoid space, and orbital apex, and may affect adjacent structures also. Meningiomas with primary ophthalmic importance are located mainly in the cavernous sinus, tuberculum sella, optic nerve sheath, orbita, sphenoid wing and olfactory groove.1 Other meningiomas located in convexity, falx and parasagittal area, middle cranial fossa, cerebellopontine angle, clivus, foramen magnum, tentorium cerebelli, and ventricles usually present with seizures or focal neurologic signs before they produce neuro-ophthalmologic signs.2
CAVERNOUS SINUS MENINGIOMAS
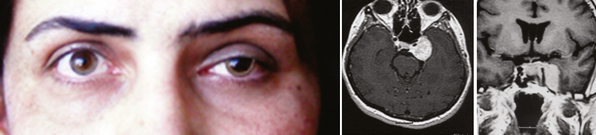
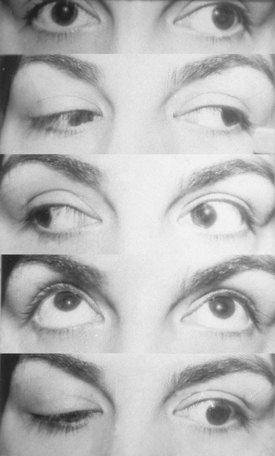
The clinical picture of cavernous sinus meningiomas (CSMs) can arise from compression or damage to one or more of the ocular motor nerves and vessels.3 Patients with cavernous sinus tumors most frequently present with ocular motor deficits: ptosis, diplopia, anisocoria, or complete ophthalmoplegia. The most common symptom is diplopia, resulting from paresis of the ocular motor nerves. Damage to the oculomotor nerve within the cavernous sinus may produce a partial or complete oculomotor nerve palsy. The pupil is usually involved in complete third nerve palsy, and is mydriatic and not reactive to light or accommodation (Fig. 12-1). In partial third nerve palsy, the pupil may be normal and initially may be mistaken for the ophthalmoparesis produced by myasthenia gravis.4 If the pupil is smaller than normal, damage to the oculosympathetic pathway should be considered. Slowly progressive compressive lesions such as meningiomas and aneurysms may cause primary aberrant regeneration between the fibers of the oculomotor nerve.5 The features of aberrant regeneration may be one or a combination of the following: adduction on attempted upgaze, constriction of pupil on lateral gaze and elevation of the upper lid on down gaze (Fig. 12-2).
Sixth nerve palsy is rarely isolated. Undiagnosed chronic isolated sixth nerve paresis or remitting sixth nerve palsy associated with CSM were described mostly before modern imaging techniques were available.6–8 The oculosympathetic pathway runs for a short distance with the abducens nerve in the cavernous sinus; therefore ipsilateral postganglionic Horner syndrome may be associated with unilateral abducens nerve paresis.1 Some patients may complain of dysesthesia and pain in and around the eye, the orbit, or the upper face if the trigeminal nerve is involved. Intermittent exotropia and neuroparalytic keratopathy has been reported as a rare presenting signs of CSM.9,10 Ocular neuromyotonia is an intermittent ocular deviation due to spasm of one or more eye muscles innervated by the same nerve and usually involves the oculomotor nerve. Typically, spasm is triggered by sustained extremes of gaze. Although the radiotherapy remains the most frequent cause, it has been described in patients with CSM who has not received radiotherapy.11,12
The patient may have visual loss and visual field deficits if the optic nerve is damaged by growth of the tumor into the orbit through the superior orbital fissure or by extension of the tumor to involve the intracranial or intracanalicular portions of the nerve. Gaze-evoked amaurosis is a transient monocular loss of vision occurring in a particular direction of eccentric gaze associated with orbital tumors, optic nerve sheath meningioma, and CSM.13 Possible mechanisms for gaze evoked amaurosis include inhibition of axonal impulses or transient optic nerve ischemia.
The decision regarding treatment is often difficult because of poor accessibility and the frequent involvement of the cavernous carotid artery. Cranial nerve morbidity is a critical issue on the resection of cavernous sinus meningiomas. Extraocular muscle functions may be impaired because of cranial nerve III, IV, and VI injuries. The reported incidences of permanently impaired extraocular muscle function developing after resection of cavernous sinus meningioma is 14% to 58%.14–18 Initially, with the advent of skull base approaches, the authors thought that these tumors could be removed with an acceptable cranial nerve morbidity. However, surgical treatment of these tumors with radical excision using all the modern skull-base surgery techniques is usually an unnecessary effort that leads to complications and decreases the quality of life, which could have been avoided with more conservative strategies. Extraocular muscle impairment is so disturbing that it becomes the main determining factor for the quality of life in the postoperative period. With time, authors favored more conservative strategies with cytoreduction of extracavernous tumor to protect brain function and to contain the tumor within the cavernous sinus to preserve cranial nerve function.19–21 Repair of the third through sixth cranial nerves injured during surgery can be pursued in suitable patients.22
Gamma-knife radiosurgery was found to be an effective low morbidity–related tool for the treatment of cavernous sinus meningioma.23 Although it cannot eradicate the neoplasm, it makes a favorable change in its natural history and helps clinical healing or stabilization of the symptoms. From a clinical perspective, the authors indicated stable or improved neurologic status in more than 90% of cases.24–27 Postoperative cranial nerve palsies are extremely rare (0% to 1% post-radiosurgery cranial nerve palsy).25,26 However, radiation sensitivity of the optic apparatus is one of the radiosurgery-limiting issues in the management of cavernous sinus meningiomas. Duma and colleagues28 reported 2 of 34 patients with radiation-induced neuropathy after radiosurgery. Removing the extracavernous part near the optic nerves also may eliminate this problem; a distance may be maintained between the radiated tumor margin and the closest optical anatomic structure to preserve optic nerve function.
The prognosis for vision depends on the extent of compression on optic nerves. Removal of compressing part of tumor may help; however, ischemia to the optic nerve is also an issue in this regard. Delayed ischemic optic neuropathy in two cases after surgery on skull-base meningiomas have been reported and successfully treated with nimodipine and rheologic therapy.29 In addition, some patients may experience spontaneous regression of symptoms and signs.30,31
OLFACTORY GROOVE MENINGIOMAS
Olfactory groove meningiomas (OGMs) arise from the midline of the anterior fossa between the crista galli and the tuberculum sella. Although these tumors arise in the midline, they may extend predominantly to one side. They are usually bilateral but may be asymmetric and reach a large size before causing symptoms.32 They can be differentiated from tuberculum sella meningiomas because OGMs arise more anterior in the skull base and displace the optic nerve and chiasm inferiorly rather than superiorly.33 An intracranial mass is often not suspected until the patient develops symptoms and signs of increased intracranial pressure (ICP), slowly progressive visual loss from pressure on the intracranial optic nerves or chiasm, or evidence of frontal lobe compression. In some cases, OGMs grow between the optic nerves, forcing them against the internal carotid and anterior cerebral arteries. Compression of the temporal portions of the optic nerves by these vessels produces bilateral nasal field defects.34 Less commonly, acute visual loss may occur when hemorrhage develops.1 Optic atrophy is a frequent finding, which may be unilateral or bilateral and is often asymmetric. Optic atrophy associated with contralateral papilledema and anosmia is a well known triad of Foster Kennedy syndrome which is caused by optic nerve compression and increased ICP due to OGM.35 Visual loss can be bilateral with unilateral optic disc swelling. Sequential anterior ischemic optic neuropathy can produce a similar picture with acute optic disc swelling in one eye and optic atrophy on the contralateral side, called pseudo-Foster Kennedy syndrome.36
With current microsurgical techniques, the results of OGM resection are excellent, with a high rate of total resection and a low incidence of complications.33 Authors indicated high rate of visual improvement (80% to 100%)33,37,38 with no visual deterioration.
OPTIC NERVE SHEATH MENINGIOMAS
Optic nerve sheath meningiomas (ONSMs) arise either primarily from meningeal cells within the orbit or in the optic canal, or are an extension of intracranial meningiomas that are invading the optic canal. They are also known as primary optic nerve meningiomas or perioptic meningiomas. Most primary ONSMs are unilateral, and 6% are reported to be bilateral; of those, 60% were canalicular.39–41 Secondary ONSMs originate intracranially, usually in the region of the planum sphenoidale or tuberculum sella, and gradually extend into the optic canal. In many cases, it may be difficult to determine whether a meningioma began in the posterior orbit or optic canal and then spread intracranially, or whether the tumor began intracranially and spread into the optic canal.
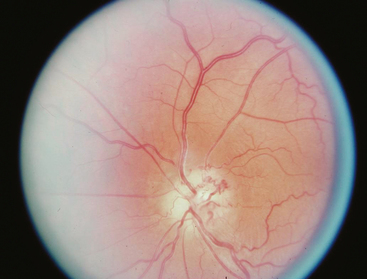
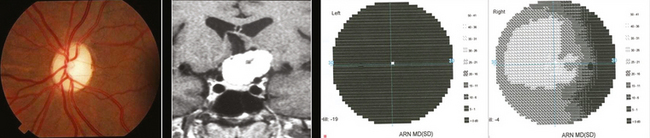
Occasionally these tumors cause no initial changes in the appearance of the optic disc. Examination of color vision, relative desaturation of red compared with the fellow eye, and relative afferent pupillary defect may give a clue to the presence of an optic neuropathy. The optic disc is usually swollen when the tumor surrounds or compresses the intraorbital portion of the nerve. When the patient gradually lose vision, the optic disc swelling begins to resolve, and optociliary shunt vessels may appear on the surface of the disc42 (Fig. 12-3). Optociliary shunt vessels are abnormal blood vessels on the disc, directing blood from retinal circulation to choroidal circulation, which indicate elevated retinal venous pressure with good compensatory circulation. The triad of visual loss, optic atrophy, and optociliary shunt veins is characteristic of an optic nerve sheath meningioma; however, it can also occur with optic nerve gliomas, sphenoid wing meningiomas, advanced glaucoma, and central retinal vein occlusion. The shunt vessels are seen in only a third of optic nerve meningiomas. They often become subtler or resolve as atrophy progresses.43,44
When the tumor originates at the apex of the orbit or within the optic canal, there is slowly progressive visual loss without orbital signs, usually with a normal-appearing optic disc. Although such patients almost always have evidence of an underlying optic neuropathy, the diagnosis may be delayed and the cause of the visual loss may be obscure. In rare cases, small tumors located within the optic canal are impossible to detect using currently available neuroimaging procedures. ONSMs should be suspected in any patient with slowly progressive, unilateral loss of vision associated with signs of optic neuropathy. Intraocular invasion can occur in ONSMs but it is rare.41,45 Metastatic tumors, lymphoma, aneurysm, and inflammatory lesions such as sarcoid or sclerosing orbital inflammation may mimic ONSMs, and these should be considered in the differential diagnosis of a patient with a presumed ONSM.40,46–48
The presence of enlarged, aerated, posterior ethmoid and sphenoid sinuses, a condition known as pneumosinus dilatans, has been reported in association with spheno-orbital meningiomas and considered to be a sign of an adjacent meningioma.49
The diagnosis of an ONSM may be made by a variety of imaging studies. High-resolution computerized tomography (CT), thin-section magnetic resonance imaging (MRI), or ultrasonography make an early diagnosis possible and obviate the need for tissue biopsy in most cases.40,50
CT scan with contrast is an excellent imaging technique for evaluation of ONSM. Thin slices (1.5–3 mm) must be taken to assess the extent of the tumor. ONSM is confined to the dura mater; therefore, it often appears as a distinct, fusiform thickening of the optic nerve. ONSMs usually enhance homogeneously. In addition, linear, diffuse, or patchy calcifications within or along an optic nerve mass are commonly seen.51 ONSMs have three main morphologic patterns on imaging: tubular, fusiform, and globular. Meningiomas surround the optic nerve. The diameter of the nerve is attenuated within the surrounding mass, giving a bull’s eye appearance on coronal images and a tram track appearance on axial images.52 It can be differentiated from an optic nerve glioma, in which the nerve itself is expanded and there is no bull’s eye appearance on coronal section. These changes are particularly well seen after intravenous injection of iodinated contrast material; however, calcifications surrounding the nerve may be masked by contrast enhancement. They are best identified on precontrast soft tissue and bone windowed images. MRI with gadolinium-enhanced fat-suppression, T1-weighted pulse sequences allow visualization of meningiomas as a localized or tubular enlargement with significant contrast enhancement. Compared to brain tissue, meningiomas may appear hypointense on T1-weighted images and hyperintense on T2-weighted images. MRI also provides sufficient tissue detail that one can assess intracranial extension. Ultrasound evaluation of an ONSM can be helpful to show an enlargement in the diameter of the nerve and shadowing from internal calcification.40,41
The treatment of primary optic nerve sheath meningiomas is controversial because they rarely represent a life-threatening process. Surgical excision usually results in complete visual loss due to the damage of the perineural pial vessels of the optic nerve, causing an ischemic infarction. Patients with useful vision are generally not candidates for surgical excision. Surgical excision is considered to minimize secondary orbital problems, such as progressive proptosis, only if the tumor is confined to the orbit and the eye is blind. When intracranial extension is present the decision to excise a tumor depends on (1) whether the contralateral optic nerve or chiasm is threatened; (2) the size, location, and growth of the tumor; and (3) remaining sight in the affected eye.40,51,53,54
For tumors that have already involved the chiasm, surgery is an unacceptable risk to the patient’s remaining vision, and radiotherapy is the only treatment option. Conventional radiotherapy is not preferred because it can damage the optic nerve, retina, and pituitary gland if high doses of radiation are given and lower doses are not effective.55 Fractionated stereotactic conformal radiotherapy appears to be the treatment of choice, and has been demonstrated to improve or stabilize vision in progressive or advanced cases.56 Other options are new frameless radiosurgery devices such as the robotic CyberKnife, an image-guided radiosurgery system that can provide the conformality and accuracy of frame-based systems and at the same time can deliver radiation in several sessions.57
ORBITAL MENINGIOMAS
Orbital meningiomas that do not originate from either the optic fascicle or the intracranial meninges are exceedingly rare. Primary orbital meningiomas originate from ectopic (extradural) meningeal tissue within the orbit.58–60 Such tumors occur in an extradural location but they can compress the optic nerve, the intraorbital contents, the contents of the superior orbital fissure, the cavernous sinus, and the frontal and temporal lobes. Visual loss and proptosis are characteristic findings. Diplopia can occur due to ocular motility limitation, if the vision is intact. Chorioretinal folds are a rare presentation of a meningioma involving the right orbit and right anterior middle cranial fossa.61
Orbital meningiomas are different from those that are located within the dural sheath of the optic nerve (ONSM). Microsurgical removal with subsequent improvement in vision is more common in orbital meningiomas.1 Intraosseous orbital meningioma is a rare cause of chronic optic neuropathy and exophthalmos. Because of their unusual presentation, there are case reports initially misdiagnosed as intraosseous meningioma62 and fibrous dysplasia.63
Surgical resection is the first choice of treatment.64 Frameless computer-assisted images guide the surgeon in the safe resection of these tumors.65 Image guidance is particularly useful when resecting the osseous portion of the tumor because the tissue does not shift with respect to the calibration frame.
SPHENOID RIDGE (WING) MENINGIOMAS
Meningiomas of the sphenoid ridge are traditionally divided into three types: outer, middle, and inner (medial). Outer ridge meningiomas can grow from the pterion toward the sylvian fissure or the tumor infiltrates the dura and invades the bone of the sphenoid ridge, producing marked thickening or hyperostosis (en plaque). Differential diagnosis includes all lesions that can be associated with focal hyperostosis (e.g., osteomas, Paget disease, neurosarcoidosis, tuberculosis, lymphoma, and fibrous dysplasia).51,63
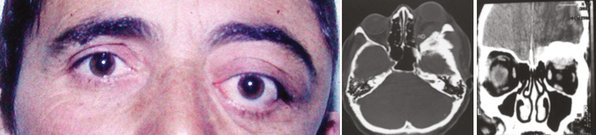
Outer ridge meningiomas often become quite large before producing any symptoms or signs. Most patients eventually present with headache, papilledema, and signs of increased ICP. Some patients develop visual hallucinations. Despite their lateral origin in the region of the pterion, they eventually spread medially across the sphenoid ridge to produce symptoms and signs identical with those produced by inner ridge tumors. The patient develops slowly progressive, unilateral proptosis with periorbital edema. A characteristic swelling in the ipsilateral temporal region develops from hyperostosis and thickening of the greater wing of the sphenoid over a period of several years (Fig. 12-4). As the tumor continues to grow and the posterior orbital bones become increasingly thickened, proptosis becomes severe, and diplopia in the lateral gaze may develop. Loss of vision from optic nerve compression is rare and occurs late. It is ultimately associated with optic disc pallor. Although optic disc swelling occasionally occurs, it is directly related to compression of the orbital portion of the optic nerve and is not papilledema caused by increased ICP.
Inner ridge meningiomas arise from the inner ridge of the sphenoid and are also known as lesser wing of the sphenoid. These tumors often produce visual symptoms and signs while still quite small because of their proximity to the optic canal, superior orbital fissure, and cavernous sinus. The characteristic syndrome of inner sphenoid ridge meningiomas is that of unilateral, progressive loss of vision associated with signs of optic neuropathy. Reduction of color vision, visual field defect, relative afferent pupillary defect, and atrophy of the optic nerve can be the early signs. Because the tumor is usually small when visual symptoms begin, patients usually have no headache or other symptoms of an intracranial mass and they may not seek medical attention. Unilateral visual loss may progress until the patient has no useful vision in the eye and there is profound optic atrophy. A rapid visual loss mimicking pituitary apoplexy has also been reported.66 Variety of visual field defects can be seen in each eye (e.g., binasal defects, central scotoma). A temporal field defect may develop in the asymptomatic contralateral eye from damage to the fibers of Willebrand’s knee in the distal portion of the ipsilateral optic nerve, from involvement of the optic chiasm. Involvement of the optic tract may produce contralateral incongruous homonymous field defect. If a tumor that causes unilateral progressive visual loss becomes large enough, it will eventually produce increased ICP. By this time, the ipsilateral optic nerve may be atrophic, and therefore papilledema may occur only in the contralateral eye (Foster Kennedy syndrome).35,67 Glaucoma, like cupping and visual field loss, can occur as the result of a compressive lesion of the anterior visual pathway.68
A second major group of visual symptoms and signs produced by inner sphenoid ridge meningiomas relates to involvement of the cavernous sinus, superior orbital fissure, and orbit. Such patients complain primarily of diplopia and proptosis. The abducens, oculomotor, and trochlear nerves may be damaged, and total ophthalmoplegia occurs in rare cases. They may interrupt the oculosympathetic pathway and produce Horner’s syndrome. If the tumor extends into the orbit through the superior orbital fissure, the optic nerve may become swollen, and visual loss may occur. The proptosis may develop due to compression to the orbital veins or invasion of the sphenoid wing by the tumor. Subretinal choroidal neovascular membranes have been reported in three cases ipsilateral to meningiomas involving the optic nerve, although the association might be coincidental.69
Two patients have been described who demonstrated intermittent involuntary monocular eyelid elevation in an eye with complete ptosis caused by partial resection of sphenoid wing meningioma.70 The lid occasionally rose spontaneously. A possible explanation was an aberrant regeneration activated by sensory stimuli.
Imaging techniques, such as CT scan and MRI, allow definitive diagnosis. The postcontrast study of either imaging technique is highly sensitive in detecting these tumors. Most of these tumors can be surgically debulked to the extent that compression of the optic nerves or chiasm is relieved.71 The closer the tumor is to the midline, the more likely that its removal will be incomplete and the more likely that complications will be encountered. Progressive proptosis without neurologic deficit was the most common symptom in a series of 15 patients who underwent neurosurgical procedures for recurrent spheno-orbital meningioma.72 Tumors close to the midline often extend to the contralateral side, which can require successive operative procedures on one side, and then the other. Stereotactic radiotherapy for these tumors is considered an attractive alternative, given the high rate of complications and postoperative neural deficits that can be expected with surgical approaches to meningiomas on the skull base.73,74
TUBERCULUM SELLA (SUPRASELLAR) MENINGIOMAS
Tuberculum sella meningiomas characteristically lie in a suprasellar subchiasmal midline position, displacing the optic chiasm posteriorly and slightly superiorly, and the optic nerves laterally. In cases with large tumors extending toward the planum sphenoidale, sella, and cavernous sinus, it can be difficult to differentiate tuberculum sella meningiomas from others originating from the planum sphenoidale, olfactory groove, clinoid process, sella, and medial sphenoid wing. Occasionally meningiomas have no attachment to the tuberculum and appear to arise from the diaphragma sella; these are called diaphragmatic meningiomas.75,76
Slowly progressing visual deterioration is the most common initial complaint. Many patients with visual loss have associated optic atrophy, but in some cases, the optic nerves appear normal or there may be subtle atrophy of the peripapillary nerve fiber layer, which causes a delay in diagnosis. The most common misdiagnosis of tuberculum sella meningiomas is retrobulbar optic neuritis.77 Growth of these masses is usually asymmetric, causing an initially monocular, fluctuating loss of vision with a central scotoma, and frequently an anterior junction syndrome of the chiasm. Such patients initially may be thought to have a retrobulbar optic neuritis, but progression of the visual loss will eventually challenge this impression. Because growth is slow and insidious in most cases, evaluation and diagnosis are delayed, and significant vision loss of one or both eyes occurs due to chiasmal compression. Pre- and postoperative ophthalmologic evaluation for both visual acuity and visual fields is mandatory. Visual evoked potentials (VEPs) can be useful for early recognition and of optic nerve compression and postoperative monitoring.78 Most of the symptoms and signs that occur in patients with tuberculum sella meningiomas develop over a long period of time, usually months to years, but rare patients may experience sudden visual difficulties. Headache is the second most common associated symptom, occurring in approximately half of the patients, and it may occasionally be the only presenting symptom.
The characteristic sign in patients with tuberculum sella meningiomas is bilateral asymmetric optic nerve dysfunction manifested by loss of vision, color deficits, visual field defects, and optic nerve pallor (Fig. 12-5). Rare patients have a unilateral optic neuropathy with a visual field defect such as a central or paracentral scotoma, temporal or nasal hemianopia, altitudinal defect, or generalized constriction.79 Other patients show evidence of a distal optic nerve syndrome, with loss of vision in one eye and a superior temporal field defect in the contralateral, asymptomatic eye (junctional scotoma). In some cases, there is binasal visual field loss from damage to the temporal fibers of the intracranial portions of both optic nerves. This occurs most frequently in patients with postfixed optic chiasms. In such cases, the tumor grows between the two nerves and pushes them laterally against the internal carotid arteries. More commonly, the field defects are bitemporal from damage to the optic chiasm. When the tumor grows posteriorly or the optic chiasm is markedly prefixed, the primary field defect may be a homonymous hemianopia from damage to the optic tract.1
The visual prognosis in patients with tuberculum sella meningiomas is variable. Most series report an improvement in visual function in 40% to 70% of cases.80–82 In addition, 50% of eyes had improvement in visual fields or maintenance of normal visual fields. Nevertheless, most investigators described worsening of visual function in 12% to 15% of patients.83
Prompt treatment is directed at preserving and improving vision. Treatment involves tumor removal and decompression of the optic chiasm.77,84 Complete monocular blindness due to tumour compressing or distorting the anterior visual pathways does not preclude recovery after timely decompressive surgery, especially when the appearance of the optic disc is normal.85 Current practice is surgical excision, although complete extirpation may not be achieved. This is particularly true of those tumors having the “en plaque” pattern of growth. The long-term visual prognosis for patients who undergo surgery for suprasellar meningioma is good, although recurrences are common (up to 50%) and often occurs more than 10 years after surgery. The frequency of recurrence is proportional to the area of dura that has been resected. Patients who develop tumor recurrence are likely to lose vision in at least one eye and subsequent surgery or radiation therapy may not improve the vision. Thus, patients with suprasellar meningiomas believed to have been completely resected should undergo long-term, serial postoperative clinical examinations and neuroimaging to allow detection and further treatment of recurrences as early as possible. Postoperative radiation therapy should be considered for patients whose suprasellar meningiomas have been incompletely resected. The most important factors affecting postoperative vision appear to be duration of visual symptoms, tumor size, and preoperative visual function. The earlier the diagnosis is made, the more likely it is that preoperative visual function will be relatively good, and tumor size will be small.
FALX AND PARASAGITTAL MENINGIOMAS
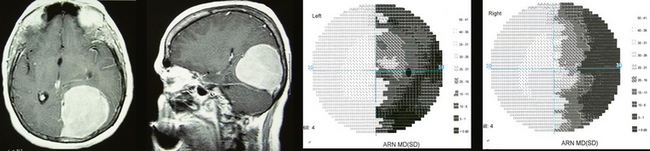
Anterior meningiomas usually compress one or both frontal lobes and may become quite large before they are discovered. At presentation, many patients have unilateral pyramidal signs and papilledema. Over the long term, they may also have optic atrophy secondary to the papilledema (Fig. 12-6). Headache is the most prominent symptom. Seizures, motor and sensory symptoms, parkinsonism, changes of mental function and behavior may also occur.
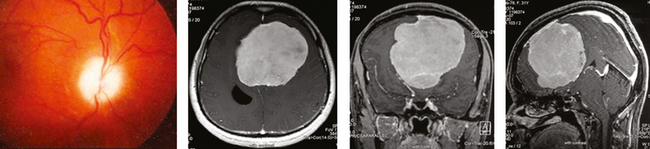
FIGURE 12-6 Funduscopic examination shows optic atrophy secondary to papilledema in an anterior parasagittal meningioma.
The middle third of the falx or sagittal sinus is the most common location for parasagittal and falx meningiomas. Tumors involving this region rarely cause headache or visual symptoms due to increased ICP. Most frequently produced signs are contralateral jacksonian seizures, or sensory or motor neurologic deficit. Parasagittal meningiomas sometimes can be coincidental findings in imaging. An unusual patient was a 61-year-old man who presented with headache and found to have a large parasagittal meningioma that was operated successfully. Shortly after surgery he developed bilateral progressive visual loss and continued to have headache. He was diagnosed with carcinomatous meningitis after extensive workup. Meningioma had no contribution to his symptomatology.86
Meningiomas that involve the posterior third of the falx are the least common but the most likely to cause visual symptoms.87 They may produce unilateral or bilateral hemianopic or quadrantopic field defects from pressure on one or both occipital lobes (Fig. 12-7). They also may produce seizures characterized by unformed visual hallucinations or transient homonymous hemianopia. In addition, when such tumors obstruct the posterior third of the superior sagittal sinus, they may produce the syndrome of pseudotumor cerebri which may cause irreversible visual loss from optic atrophy due to long standing papilledema.88,89
Tumors involving the falcotentorial junction are very rare and are characterized by headache, gait disturbance, and early papilledema. Cranial nerve involvement (V, VI, VII, and VIII), homonymous hemianopias, upgaze limitation, hemiparesis, dysarthria, urinary incontinence, and mental deterioration may also occur.90
Falsely localizing signs can be seen due to distant effects of the tumor on the optic tract or radiations. Central scotomas, peripheral constriction, noncongruous hemianopia, and bitemporal hemianopia have been described.1
MIDDLE CRANIAL FOSSA MENINGIOMAS
The ocular signs of middle cranial fossa meningiomas include unilateral or bilateral optic disc swelling sometimes associated with visual loss and optic atrophy, cranial neuropathies, and homonymous hemianopia or quadrantopia. Bilateral optic disc swelling is usually related to increased ICP and there may be indirect compression of the ipsilateral optic nerve. The oculomotor and trigeminal nerves are often affected.91
POSTERIOR FOSSA MENINGIOMAS
Neurologic signs that occur in patients with cerebellopontine angle (CPA) meningiomas include neurosensory hearing loss, vestibular nystagmus, trigeminal sensory neuropathy, and cerebellar ataxia. Other patients may have abducens paresis, facial weakness, lower cranial neuropathies, altered mental status, or a combination of these deficits. Papilledema may be present in patients with increased ICP and may evolve into optic atrophy if the lesion is not removed. In some patients, optic atrophy due to papilledema is already present when the tumor is first discovered.92,93
Two patients developed gaze-evoked tinnitus in the year after removal of a cerebellopontine angle mass. The eighth nerve was transected in both cases. The noise was present with saccades, pursuit, and vestibuloocular eye movements. The tinnitus was attributed to an abnormal interaction between the vestibular and the cochlear nucleus, possibly secondary to neural sprouting.94
Relative pupil-sparing oculomotor paresis initially attributed to ischemia was reported in a case of posterior fossa meningioma. The neurologic symptoms and signs disappeared immediately after resection of the tumor. The third nerve palsy was attributed to deformation of the brain stem.95
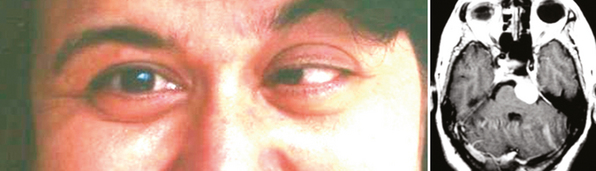
Patients with clivus meningiomas usually present with headache, followed by disturbance in gait, difficulty with hearing, vertigo, or visual difficulties, particularly double vision. Papilledema, loss of hearing, and ataxia are common findings. Cranial neuropathies are also quite common, with the trigeminal, abducens, facial, and vestibulocochlear nerves being affected most frequently1,96 (Fig. 12-8).
Meningiomas of the foramen magnum usually present with progressive neurologic signs.97 Nystagmus is often present; it may be horizontal, torsional, downbeat, upbeat, or rarely periodic alternating nystagmus. Horner’s syndrome may occur in some patients.
INTRAVENTRICULAR MENINGIOMAS
Intraventricular meningiomas often grow slowly to a substantial size before they become symptomatic. They originate from tela choroidea in the roof of the ventricles.98
Meningiomas of the third ventricle are even less common than meningiomas in the lateral ventricle.99 The clinical characteristics are nonspecific and are related to the effects of increased ICP. Intermittent headache is the major early symptom, but loss of vision or diplopia may also occur. Confusion, lethargy, disturbance of gait, and urinary incontinence may develop. Papilledema is the rule. When the tumor is primarily posterior, there may be pressure on the midbrain that produces a partial or complete dorsal mesencephalic (Parinaud’s) syndrome.
The treatment is surgical and operative route should be selected according to the tumor’s location.
VISUAL COMPLICATIONS OF VARIOUS TREATMENT MODALITIES OF MENINGIOMAS
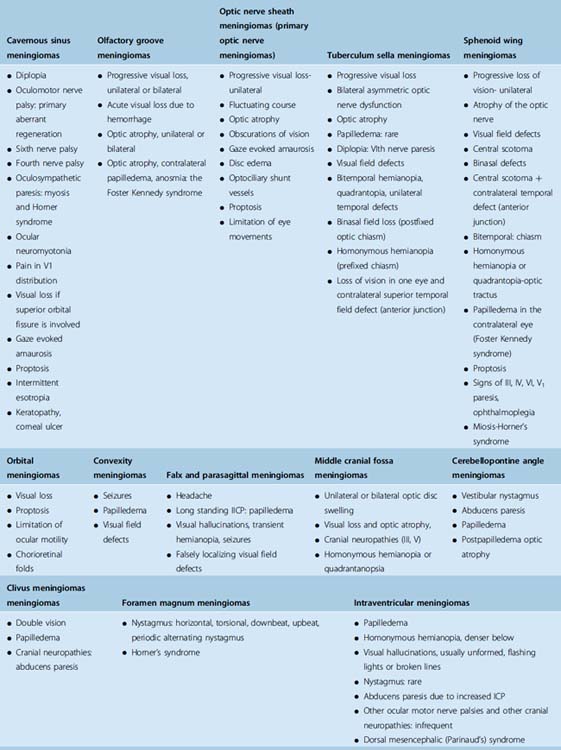
A summary of neuro-ophthalmologic symptoms and signs of all intracranial meningiomas is provided in Table 12-1.
[1] Cockerham K.P., Maron J.C., Bejjani G.K. Tumors of the meninges and related tissues: meningiomas and sarcomas. In: Neil R., Miller N.J.N., editors. Walsh & Hoyt’s Clinical Neuro-Ophthalmology. Lippincott Williams & Wilkins; 2005:1484-1518.
[2] www.brighamandwomens.org/neurosurgery/meningioma.
[3] Golnik K.C., Miller N.R., Long D.M. Rate of progression and severity of neuro-ophthalmologic manifestations of cavernous sinus meningiomas. Skull Base Surg. 1992;2(3):129-133.
[4] Shechtman D.L., Woods A.D., Tyler J.A. Pupil sparing incomplete third nerve palsy secondary to a cavernous sinus meningioma: challenges in management. Clin Exp Optom. 2007;90(2):132-138.
[5] Schatz N.J., Savino P.J., Corbett J.J. Primary aberrant oculomotor regeneration. A sign of intracavernous meningioma. Arch Neurol. 1977;34(1):29-32.
[6] Gurinsky J.S., Quencer R.M., Post M.J. Sixth nerve ophthalmoplegia secondary to a cavernous sinus lesion. J Clin Neuroophthalmol. 1983;3(4):277-281.
[7] Volpe N.J., Lessell S. Remitting sixth nerve palsy in skull base tumors. Arch Ophthalmol. 1993;111(10):1391-1395.
[8] Galetta S.L., Smith J.L. Chronic isolated sixth nerve palsies. Arch Neurol. 1989;46(1):79-82.
[9] White W.A., Mohney B.G., Woog J.J. Cavernous sinus meningioma presenting as intermittent exotropia in a 2–year-old girl. Can J Ophthalmol. 2007;42(2):341.
[10] Vantieghem G., Maudgal P.C. Neuroparalytic keratopathy as the first sign of a cerebral meningioma. Bull Soc Belge Ophtalmol. 2007;303:81-86..
[11] Jacob M., Vighetto A., Bernard M., Tilikete C. Ocular neuromyotonia secondary to a cavernous sinus meningioma. Neurology. 2006;66(10):1598-1599.
[12] Ela-Dalman N., Arnold A.C., Chang L.K., Velez F.G., Lasky J.L.3rd. Abducens nerve ocular neuromyotonia following non-sellar or parasellar tumors. Strabismus. 2007;15(3):149-151.
[13] Koch M.U., Houtman A.C., de Keizer R.J. Gaze-evoked amaurosis associated with cavernous sinus meningioma. Eye. 2006.
[14] Al-Mefty O., Smith R.R. Surgery of tumors invading the cavernous sinus. Surg Neurol. 1988;30(5):370-381.
[15] DeMonte F., Smith H.K., Al-Mefty O. Outcome of aggressive removal of cavernous sinus meningiomas. J Neurosurg. 1994;81(2):245-251.
[16] Knosp E., Perneczky A., Koos W.T., Fries G., Matula C. Meningiomas of the space of the cavernous sinus. Neurosurgery. 1996;38(3):434-442. discussion 442–434
[17] O’Sullivan M.G., van Loveren H.R., Tew J.M.Jr. The surgical resectability of meningiomas of the cavernous sinus. Neurosurgery. 1997;40(2):238-244. discussion 245–237
[18] Klink D.F., Sampath P., Miller N.R., Brem H., Long D.M. Long-term visual outcome after nonradical microsurgery patients with parasellar and cavernous sinus meningiomas. Neurosurgery. 2000;47(1):24-31. discussion 31–22
[19] Blake P.Y., Miller N.R., Long D.M. Treatment of cavernous sinus meningiomas. J Neurosurg. 1995;82(4):702-703.
[20] Klink D.F., Sampath P., Miller N.R., Brem H., Long D.M. Long-term visual outcome after nonradical microsurgery in patients with parasellar and cavernous sinus meningiomas. Am J Ophthalmol. 2000;130(5):689.
[21] Lee J.H. Meningiomas: Diagnosis, Treatment, and Outcome. New York: Springer, 2008.
[22] Sekhar L.N., Lanzino G., Sen C.N., Pomonis S. Reconstruction of the third through sixth cranial nerves during cavernous sinus surgery. J Neurosurg. 1992;76(6):935-943.
[23] Hasegawa T., Kida Y., Yoshimoto M., Koike J., Iizuka H., Ishii D. Long-term outcomes of Gamma Knife surgery for cavernous sinus meningioma. J Neurosurg. 2007;107(4):745-751.
[24] Roche P.H., Pellet W., Fuentes S., Thomassin J.M., Regis J. Gamma knife radiosurgical management of petroclival meningiomas results and indications. Acta Neurochir (Wien). 2003;145(10):883-888. discussion 888
[25] Nicolato A., Foroni R., Alessandrini F., Maluta S., Bricolo A., Gerosa M. The role of Gamma Knife radiosurgery in the management of cavernous sinus meningiomas. Int J Radiat Oncol Biol Phys. 2002;53(4):992-1000.
[26] Iwai Y., Yamanaka K., Ishiguro T. Gamma knife radiosurgery for the treatment of cavernous sinus meningiomas. Neurosurgery. 2003;52(3):517-524. discussion 523–514
[27] Pendl G., Schrottner O., Eustacchio S., Ganz J.C., Feichtinger K. Cavernous sinus meningiomas–what is the strategy: upfront or adjuvant gamma knife surgery? Stereotact Funct Neurosurg. 1998;70(Suppl. 1):33-40.
[28] Duma C.M., Lunsford L.D., Kondziolka D., Harsh G.R., Flickinger J.C. Stereotactic radiosurgery of cavernous sinus meningiomas as an addition or alternative to microsurgery. Neurosurgery. 1993;32(5):699-704. discussion 704–5
[29] Van Lindert E., Hassler W., Saletta A.D. Delayed ischemic optic neuropathy after surgery on skull base meningiomas successfully treated with nimodipine and rheological therapy: report of two cases. Skull Base Surg. 2000;10(4):207-210.
[30] Burde R.M., Smith J.L. Spontaneous reduction of growth rate of a large intracranial meningioma. J Clin Neuroophthalmol. 1984;4(2):137-138.
[31] Pless M., Lessell S. Spontaneous visual improvement in orbital apex tumors. Arch Ophthalmol. 1996;114(6):704-706.
[32] Slavin M.L. Acute, severe, symmetric visual loss with cecocentral scotomas due to olfactory groove meningioma. J Clin Neuroophthalmol. 1986;6(4):224-227.
[33] Hentschel S.J., DeMonte F. Olfactory groove meningiomas. Neurosurg Focus. 2003;14(6):e4.
[34] O’Connell J.E., Du Boulay E.P. Binasal hemianopia. J Neurol Neurosurg Psychiatry. 1973;36(5):697-709.
[35] Massey E.W., Schoenberg B. Foster Kennedy syndrome. Arch Neurol. 1984;41(6):658-659.
[36] Lepore F.E., Yarian D.L. A mimic of the “exact diagnostic sign” of Foster Kennedy. Ann Ophthalmol. 1985;17(7):411-412.
[37] Zevgaridis D., Medele R.J., Mueller A., Hischa A.C., Steiger H.-J. Meningiomas of the sellar region presenting with visual impairment: Impact of various prognostic factors on surgical outcome in 62 patients. Acta Neurochir Suppl. 2001;143:471-476.
[38] Turazzi S., Cristofori L., Gambin R., Bricolo A. The pterional approach for the microsurgical removal of olfactory groove meningiomas. Neurosurgery. 1999;45(4):821-825. discussion 825–826
[39] Miller N.R. New concepts in the diagnosis and management of optic nerve sheath meningioma. J Neuroophthalmol. 2006;26(3):200-208.
[40] Dutton J.J. Optic nerve sheath meningiomas. Surv Ophthalmol. 1992;37(3):167-183.
[41] Hart W.M.Jr, Burde R.M., Klingele T.G., Perlmutter J.C. Bilateral optic nerve sheath meningiomas. Arch Ophthalmol. 1980;98(1):149-151.
[42] Miller N.R., Solomon S. Retinochoroidal (optociliary) shunt veins, blindness and optic atrophy: a non-specific sign of chronic optic nerve compression. Aust N Z J Ophthalmol. 1991;19(2):105-109.
[43] Imes R.K., Schatz H., Hoyt W.F., Monteiro M.L., Narahara M. Evolution of optociliary veins in optic nerve sheath meningioma. Evolution. Arch Ophthalmol. 1985;103(1):59-60.
[44] Mashayekhi A., Shields J.A., Shields C.L. Involution of retinochoroidal shunt vessel after radiotherapy for optic nerve sheath meningioma. Eur J Ophthalmol. 2004;14(1):61-64.
[45] Schittkowski M., Hingst V., Stropahl G., Guthoff R. [Optic nerve sheath meningioma with intraocular invasion–a case report]. Klin Monatsbl Augenheilkd. 1999;214(4):251-254.
[46] Fox B., Pacheco P., DeMonte F. Carcinoma of the breast metastatic to the optic nerve mimicking an optic nerve sheath meningioma: case report and review of the literature. Skull Base. 2005;15(4):281-287. discussion 287–289
[47] Selva D., Rootman J., Crompton J. Orbital lymphoma mimicking optic nerve meningioma. Orbit. 2004;23(2):115-120.
[48] Landau K., Horton J.C., Hoyt W.F., Wilson C.B. Aneurysm mimicking intracranial growth of optic nerve sheath meningioma. J Clin Neuroophthalmol. 1990;10(3):185-187.
[49] Miller N.R., Golnik K.C., Zeidman S.M., North R.B. Pneumosinus dilatans: a sign of intracranial meningioma. Surg Neurol. 1996;46(5):471-474.
[50] Eddleman C.S., Liu J.K. Optic nerve sheath meningioma: current diagnosis and treatment. Neurosurg Focus. 2007;23(5):E4.
[51] Gossman M.V. Meningioma, optic nerve sheath, 2007. Retrieved from www.emedicine.com/oph/topic671 (accessed August 15, 2009)
[52] Kanamalla U.S. The optic nerve tram-track sign. Radiology. 2003;227(3):718-719.
[53] Liu J.K., Forman S., Moorthy C.R., Benzil D.L. Update on treatment modalities for optic nerve sheath meningiomas. Neurosurg Focus. 2003;14(5):e7.
[54] Roser F., Nakamura M., Martini-Thomas R., Samii M., Tatagiba M. The role of surgery in meningiomas involving the optic nerve sheath. Clin Neurol Neurosurg. 2006;108(5):470-476.
[55] Subramanian P.S., Bressler N.M., Miller N.R. Radiation retinopathy after fractionated stereotactic radiotherapy for optic nerve sheath meningioma. Ophthalmology. 2004;111(3):565-567.
[56] Landert M., Baumert B.G., Bosch M.M., Lutolf U.M., Landau K. The visual impact of fractionated stereotactic conformal radiotherapy on seven eyes with optic nerve sheath meningiomas. J Neuroophthalmol. 2005;25(2):86-91.
[57] Romanelli P., Wowra B., Muacevic A. Multisession CyberKnife radiosurgery for optic nerve sheath meningiomas. Neurosurg Focus. 2007;23(6):E11.
[58] Miller N.R. Neuro-Ophthalmology of orbital tumors. Clin Neurosurg. 1985;32:459-473.
[59] Boulos P.T., Dumont A.S., Mandell J.W., Jane J.A.Sr. Meningiomas of the orbit: contemporary considerations. Neurosurg Focus. 2001;10(5):E5.
[60] Spraul C.W., Gareis O., Lang G.K. [Primary extradural meningioma of the orbits: a report of a patient and review of the literature]. Klin Monatsbl Augenheilkd. 1996;209(5):322-327.
[61] Yeung L., Lai C.C., Chen T.L., Wu W.C. Chorioretinal folds associated with a meningioma. Chang Gung Med J. 2005;28(8):575-580.
[62] Henchoz L., Borruat F.X. Intraosseous meningioma: a rare cause of chronic optic neuropathy and exophthalmos. Klin Monatsbl Augenheilkd. 2004;221(5):414-417.
[63] Bou-Assaly W., Illner A., Mosier K.M., Kalnin A., Pritz M.B. Intra-osseous meningioma of the orbit: an unusual presentation (2007: 5b). Eur Radiol. 2007;17(8):2192-2194.
[64] Gruber A., Bavinzski G., Killer M., Richling B. Preoperative embolization of hypervascular skull base tumors. Minim Invasive Neurosurg. 2000;43(2):62-71.
[65] Wong G.K., Poon W.S., Lam M.K. The impact of an armless frameless neuronavigation system on routine brain tumour surgery: a prospective analysis of 51 cases. Minim Invasive Neurosurg. 2001;44(2):99-103.
[66] Marano S.R., Sonntag V.K., Spetzler R.F. Planum sphenoidale meningioma mimicking pituitary apoplexy: a case report. Neurosurgery. 1984;15(6):859-862.
[67] Mariniello G., Bonavolonta G., de Divitiis E. Papilledema as a ‘false’ localizing sign. Clin Neurol Neurosurg. 2002;104(1):69-71.
[68] Kalenak J.W., Kosmorsky G.S., Hassenbusch S.J. Compression of the intracranial optic nerve mimicking unilateral normal-pressure glaucoma. J Clin Neuroophthalmol. 1992;12(4):230-235. discussion 236–7
[69] Lee M.S., Lessell S. Choroidal neovascularisation associated with meningioma. Br J Ophthalmol. 2005;89(10):1384-1386.
[70] Egan R.A., Warner J.E. Intermittent reversal of complete ptosis associated with sphenoid wing meningiomas. Can J Ophthalmol. 2006;41(4):497-499.
[71] De Jesus O., Toledo M.M. Surgical management of meningioma en plaque of the sphenoid ridge. Surg Neurol. 2001;55(5):265-269.
[72] Maroon J.C., Kennerdell J.S., Vidovich D.V., Abla A., Sternau L. Recurrent spheno-orbital meningioma. J Neurosurg. 1994;80(2):202-208.
[73] Peele K.A., Kennerdell J.S., Maroon J.C., Kalnicki S., Kazim M., Gardner T., et al. The role of postoperative irradiation in the management of sphenoid wing meningiomas. A preliminary report. Ophthalmology. 1996;103(11):1761-1766. discussion 1766–1767
[74] Elia A.E., Shih H.A., Loeffler J.S. Stereotactic radiation treatment for benign meningiomas. Neurosurg Focus. 2007;23(4):E5.
[75] Chi J.H., McDermott M.W. Tuberculum sellae meningiomas. Neurosurg Focus. 2003;14(6):e6.
[76] Kinjo T., al-Mefty O., Ciric I. Diaphragma sellae meningiomas. Neurosurgery. 1995;36(6):1082-1092.
[77] Lin M.C., Bee Y.S., Sheu S.J. A suprasellar meningioma simulating atypical retrobulbar optic neuritis. J Chin Med Assoc. 2003;66(11):689-692.
[78] Hershenfeld S.A., Sharpe J.A. Monocular temporal hemianopia. Br J Ophthalmol. 1993;77(7):424-427.
[79] Hidajat R.R., McLay J.L., Goode D.H., Hidayat J.R. The value of VEP in the diagnosis and post-operative monitoring of meningioma. Doc Ophthalmol. 2006;113(3):165-169.
[80] Yasargil M. Microneurosurgery. Stuttgart-NewYork: Thieme Verlag, 1996.
[81] Pamir M.N., Ozduman K., Belirgen M., Kilic T., Ozek M.M. Outcome determinants of pterional surgery for tuberculum sellae meningiomas. Acta Neurochir (Wien). 2005;147(11):1121-1130. discussion 1130
[82] Goel A., Muzumdar D., Desai K. Tuberculum Sellae Meningioma: A Report on Management on the Basis of a Surgical Experience with 70 Patients. Neurosurgery. 2002;51(6):1358-1364.
[83] Chicani C.F., Miller N.R. Visual outcome in surgically treated suprasellar meningiomas. J Neuroophthalmol. 2003;23(1):3-10.
[84] Fahlbusch R., Schott W. Pterional surgery of meningiomas of the tuberculum sellae and planum sphenoidale: surgical results with special consideration of ophthalmological and endocrinological outcomes. J Neurosurg. 2002;96(2):235-243.
[85] Bampoe J., Ranalli P., Bernstein M. Postoperative reversal of complete (monocular) blindness in skull base meningioma: case report. Can J Neurol Sci. 2003;30(1):72-74.
[86] Arsava E.M., Cikrikci B.I., Mocan G., Tekkok I., Kansu T. Postoperative progressive visual loss. Surv Ophthalmol. 2004;49(5):509-512.
[87] Mooney A.J., Carey P., Ryan M., Bofin P. Parasagittal parieto-occipital meningioma with visual hallucinations. Am J Ophthalmol. 1965;59:197-205.
[88] Marr W.G., Chambers J.W. Occlusion of the cerebral dural sinuses by tumor simulating pseudotumor cerebri. Am J Ophthalmol. 1966;61(1):45-49.
[89] Repka M.X., Miller N.R. Papilledema and dural sinus obstruction. J Clin Neuroophthalmol. 1984;4(4):247-250.
[90] Quinones-Hinojosa A., Chang E.F., McDermott M.W. Falcotentorial meningiomas: clinicalneuroimaging, and surgical features in six patients. Neurosurg Focus. 2003;14(6):e11.
[91] Smolin G. Middle cranial fossa meningioma. Causing unilateral loss of visual acuity. Am J Ophthalmol. 1966;61(4):798-802.
[92] Granick M.S., Martuza R.L., Parker S.W., Ojemann R.G., Montgomery W.W. Cerebellopontine angle meningiomas: clinical manifestations and diagnosis. Ann Otol Rhinol Laryngol. 1985;94(1 Pt 1):34-38.
[93] Voss N.F., Vrionis F.D., Heilman C.B., Robertson J.H. Meningiomas of the cerebellopontine angle. Surg Neurol. 2000;53(5):439-446. discussion 446–437
[94] Wall M., Rosenberg M., Richardson D. Gaze-evoked tinnitus. Neurology. 1987;37(6):1034-1036.
[95] Winterkorn J.M., Bruno M. Relative pupil-sparing oculomotor nerve palsy as the presenting sign of posterior fossa meningioma. J Neuroophthalmol. 2001;21(3):207-209.
[96] Mayberg M.R., Symon L. Meningiomas of the clivus and apical petrous bone. Report of 35 cases. J Neurosurg. 1986;65(2):160-167.
[97] Akalan N., Seckin H., Kilic C., Ozgen T. Benign extramedullary tumors in the foramen magnum region. Clin Neurol Neurosurg. 1994;96(4):284-289.
[98] Bhatoe H.S., Singh P., Dutta V. Intraventricular meningiomas: a clinicopathological study and review. Neurosurg Focus. 2006;20(3):E9.
[99] Brunette J.R., Walsh F.B. Neurophthalmological aspects of tumours of the third ventricle. Can Med Assoc J. 1968;98(25):1184-1192.
[100] Kunikata H., Tamai M. Cilioretinal artery occlusions following embolization of an artery to an intracranial meningioma. Graefes Arch Clin Exp Ophthalmol. 2006;244(3):401-403.
[101] Stelzer K.J. Acute and long-term complications of therapeutic radiation for skull base tumors. Neurosurg Clin N Am. 2000;11(4):597-604.
[102] Levi L. Radiation retinopathy after therapy for meningioma. Ophthalmology. 2005;112(8):1484.
[103] Girkin C.A., Comey C.H., Lunsford L.D., Goodman M.L., Kline L.B. Radiation optic neuropathy after stereotactic radiosurgery. Ophthalmology. 1997;104(10):1634-1643.
[104] Miller N.R., Lee A.G. Adult-onset acquired oculomotor nerve paresis with cyclic spasms: relationship to ocular neuromyotonia. Am J Ophthalmol. 2004;137(1):70-76.
[105] Carvounis P.E., Katz B. Gamma knife radiosurgery in neuro-ophthalmology. Curr Opin Ophthalmol. 2003;14(6):317-324.