55 Neuraxial Block
Introduction
In many patients, epidural and spinal blocks are routine procedures guided by loss of resistance and confirmation of free flow of cerebrospinal fluid, respectively. However, in patients with obesity or advanced age, these neuraxial blocks can be more challenging and may benefit from imaging guidance. Ultrasound imaging has been reported useful for guiding neuraxial anesthetics in patients with prior surgical instrumentation or scoliosis. Ultrasound can estimate the location and level of spinous interspaces. There also is evidence that ultrasound guidance improves the learning curve and reduces epidural failure rates of resident in training.1,2 However, there remain current limitations to the use of ultrasound technology to guide neuraxial blocks.
Selection of the correct interspace is important to the success of subarachnoid block. The interspace selected for injection of spinal anesthetic drugs affects the resultant distribution. The failure rate at lower lumbar interspaces can be as high as 7%.3,4 These failures probably relate to the site of the injection with respect to the peak of the lumbosacral curve. One of the potential benefits of ultrasound is to help establish the correct interspace for neuraxial block.
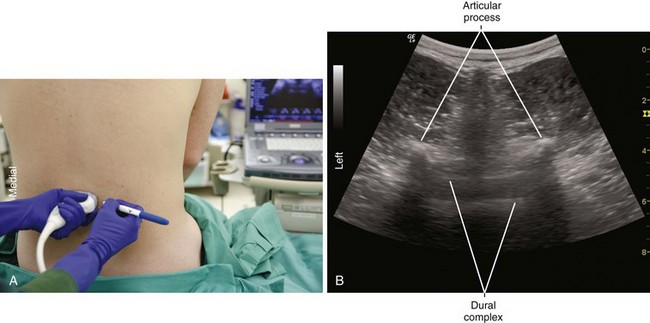
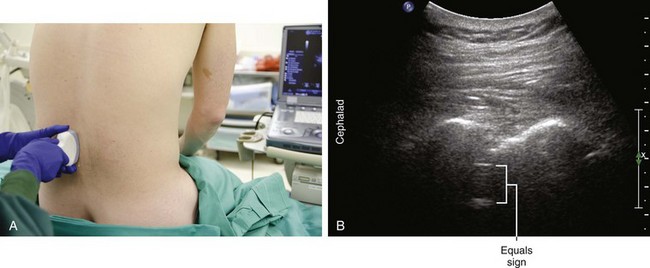
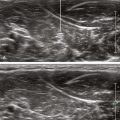
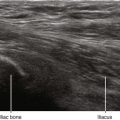
Longitudinal paramedian imaging planes provide the best visualization of neuraxial structures.5 With these views, the width of the acoustic window (the intervertebral space) is largest relative to the shadowing of the corresponding vertebral bone. Several authors have described the epidural space and adjacent bone to have a sawtooth configuration in this parasagittal view.6 The “saw sign” of longitudinal paramedian views inclines toward the skin surface in the caudal direction.
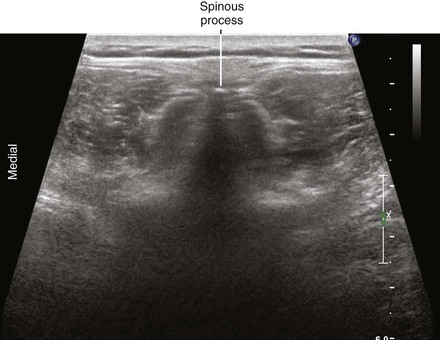
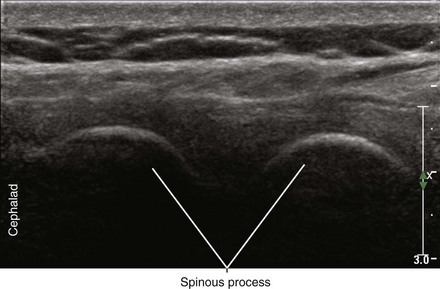
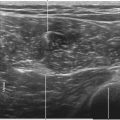
Midline transverse imaging planes are often used for offline markings for midline approaches for lumbar epidurals and spinals. This transverse view has been described as having a “flying bat” appearance.7 In this view the articular processes (the rounded mamillary processes) of the facet joints form the ears of the bat and indicate the widest part of the interlaminar space. Although transverse imaging planes most closely resemble midline approaches, this view is limited by overhanging bone of the spinous processes and shadowing by the interspinous ligaments. Transverse imaging planes have been shown to be effective at lumbar interspaces for marking the needle insertion point and estimating needle depth to loss of resistance. However, these views are not helpful at midthoracic levels because of the narrower acoustic windows across the midline produced by the steep inclination of the spinous processes. In addition, the thoracic region lacks of prominent articular processes that can serve as sonographic landmarks. It can be difficult to obtain symmetric midline transverse views of the neuraxis (in particular, the rounded articular processes) in patients with scoliosis due to rotation of the spine.
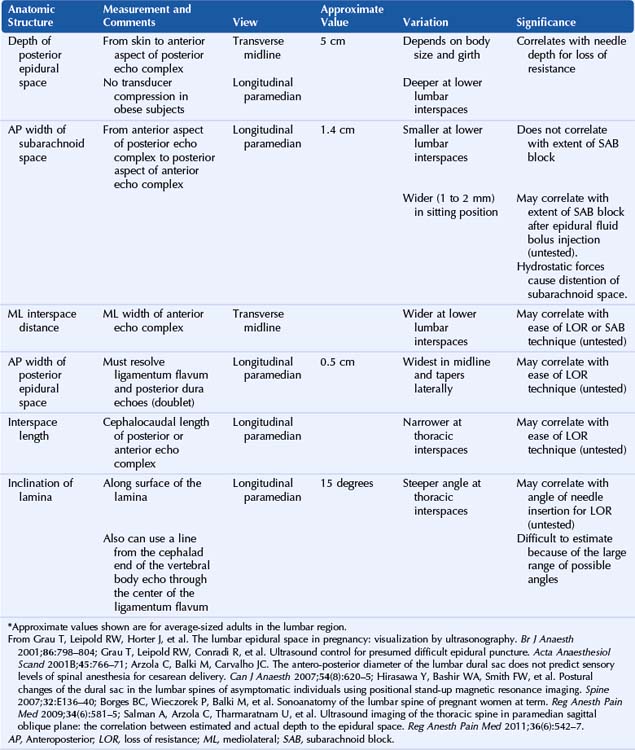
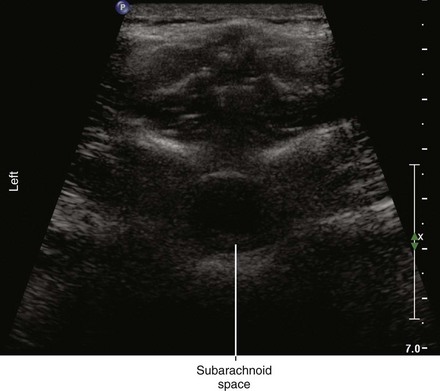
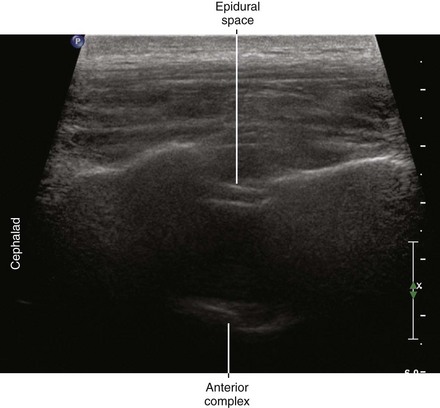
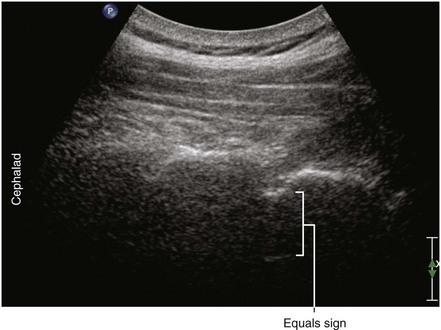
Ultrasound is an accurate imaging modality for depiction of the dura mater. The dura appears highly echogenic on ultrasound scans, defined by a single- or double-layer hyperechoic signal.8 It can be difficult to resolve the echo signals of the ligamentum flavum and posterior dura, and therefore this signal is sometimes referred to as the posterior complex (Table 55-1). The anterior complex consists of the anterior dura, posterior longitudinal ligament, and vertebral body. This produces a wider hyperechoic band that is deeper and parallel to the first band (the equals sign). Because the subarachnoid space contains few endogenous scatterers of ultrasound, it appears echo free on ultrasound scans (Table 55-2).
Table 55-1 Anatomic Structures that Comprise the Posterior and Anterior Echo Complexes as Seen in Longitudinal Paramedian View of the Neuraxis (the “Equals Sign”)*
| Ultrasound Image | Structure |
|---|---|
| Posterior complex |
* These two echo complexes appear as parallel hyperechoic bands on ultrasound scans. The equals sign is not truly symmetric because the anterior echo complex is wider than the posterior echo complex (which appears more similar to a straight line). In the thoracic region the interspaces are smaller and therefore the equals sign is not as long in its cephalocaudad dimension in comparison with the lumbar region. The equals sign indicates the spinal canal and hypoechoic subarachnoid space are correctly imaged because this is not seen with off-axis views. Structures are listed from posterior to anterior.
Suggested Technique for Offline Lumbar Epidural Catheter Placement
Remove all the gel with dry gauze and then proceed with lumbar epidural catheter placement by testing for loss of resistance in the usual sterile fashion. The noted depth can be off by as much as 0.5 to 1 cm (Table 55-3). If unsuccessful on the first attempt, try redirecting the needle in a cephalocaudal fashion. This is suggested because the greatest uncertainty with the offline technique is the tilt (inclination) of the transducer.
Table 55-3 Reasons for Discrepancies Between Offline Estimates of Epidural Space Depth and Needle Depth for Loss of Resistance*
| Offline Markings | Reasons for Discrepancies in Depth Estimates |
|---|---|
| Probe compression | |
| Local anesthetic skin wheal | |
| Needle trajectory | |
| Needle advancement (soft tissue elastic properties) |
* Offline estimates of the depth of the epidural space are reasonably accurate because the skin mobility is limited and the underlying tissue (primarily bone and ligaments) is relatively firm compared with other body regions. However, there are several reasons why these depth estimates differ from those obtained from needle depths at loss of resistance.
Clinical Pearls
• The spinous processes give a long triangular shadow down the midline on transverse views using a curved array transducer. If this shadow is seen, the probe position must be adjusted.
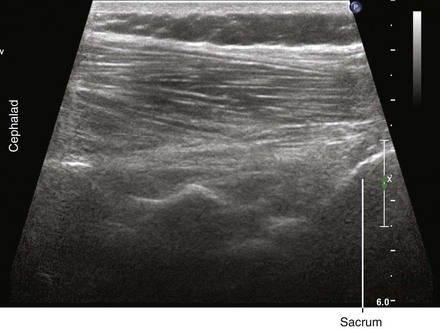
• There are many sacral morphologies, but usually the hyperechoic sacral line can be used as a reference for establishing the lumbar level. The sacrum shadow is continuous so there is no underlying equals sign.
• Probe compression in obese subjects can lead to underestimation of the distance to the epidural space.
• Because there is a strong concordance in their measurements, longitudinal paramedian views can be used to check the distances to the epidural space if transverse views are not adequate.
• Avoid epidural attempts at interspaces where the posterior echo complex is incomplete or missing (with good visualization of the articular processes). Although this may relate to acoustic shadowing by the spinous processes or interspinous ligaments, these interspaces may have a midline gap in the ligamentum flavum and may be at higher risk of dural puncture.
• Ultrasound imaging can be used to help decide when an extra-long needle is necessary for epidurals or spinals. If the posterior epidural space echo is more than 8 cm from the skin (or the spinous process deeper than 4 cm) a longer needle is chosen (more than 9 cm in length).
• Ultrasound imaging accelerates and improves the learning curve for epidural catheter placement.
1 Grau T, Bartusseck E, Conradi R, et al. Ultrasound imaging improves learning curves in obstetric epidural anesthesia: a preliminary study. Can J Anaesth. 2003;50(10):1047–1050.
2 Vallejo MC, Phelps AL, Singh S, et al. Ultrasound decreases the failed labor epidural rate in resident trainees. Int J Obstet Anesth. 2010;19(4):373–378. Epub 2010, Aug 8
3 Munhall RJ, Sukhani R, Winnie AP. Incidence and etiology of failed spinal anesthetics in a university hospital: a prospective study. Anesth Analg. 1988;67:843–848.
4 Tarkkila PJ. Incidence and causes of failed spinal anesthetics in a university hospital: a prospective study. Reg Anesth. 1991;16:48–51.
5 Grau T, Leipold RW, Horter J, et al. Paramedian access to the epidural space: the optimum window for ultrasound imaging. J Clin Anesth. 2001;13:213–217.
6 Grau T, Leipold RW, Conradi R, et al. Ultrasound control for presumed difficult epidural puncture. Acta Anaesthesiol Scand. 2001;45:766–771.
7 Carvalho JC. Ultrasound-facilitated epidurals and spinals in obstetrics. Anesthesiol Clin. 2008;26(1):145–158. vii–viii. Review
8 Rapp HJ, Folger A, Grau T. Ultrasound-guided epidural catheter insertion in children. Anesth Analg. 2005;101:333–339.