CHAPTER 104 Neural Scarring
INTRODUCTION
Of the myriad causes of failed back surgery syndrome (FBSS), neural scarring has been reported to be the cause of failed back surgery syndrome in 6–24% of patients.1–7 In 1996, Ross et al., using a prospective, controlled, randomized, blinded, multicenter methodology, demonstrated a significant association between the gross amount of peridural scar and the occurrence of recurrent radicular pain; those with extensive epidural scarring were 3.2 times more likely to experience recurrent radicular pain than those with less scarring.7 The degree of postoperative scarring relates to the extent and magnitude of the surgery. It is undeniable that the presence of postoperative scar tissue is not solely due to the operative trauma, but is also generated as a reparative response to the herniated disc material as not all patients with scarring are symptomatic.8 Although epidural adhesions are most commonly present following spine surgery, leakage of disc material from a herniated nucleus pulposus or annular disc tear can cause an inflammatory response with fibrocyte deposition and resultant epidural adhesions in the absence of surgery.9,10 There are those who believe that neural scarring such as epidural and/or intraneural fibrosis do not ever cause symptoms. This school of thought is based upon the fact that neural scarring is a normal bodily response to injury and that all postoperative patients will have some degree of scarring, the majority of which are asymptomatic.8 However, just as there are symptomatic and asymptomatic disc protrusions, the same tenet holds true for epidural fibrosis and neural scarring. The fact that not all focal disc protrusions or epidural fibrosis, as demonstrated by magnetic resonance imaging (MRI), are clinically symptomatic does not prove that focal disc protrusions or epidural fibrosis are not pain generators and have no relationship to low back or lower limb pain preoperatively or postoperatively. Such a view is simplistic and is more a function of the lack of an effective clinical pathway in the diagnosis of symptomatic neural scarring. Historically, epidural fibrosis or arachnoiditis was an uncommon clinical entity prior to the introduction of lumbar spine surgery for the treatment of degenerative spine conditions.11 A large number of reports of epidural fibrosis found on repeat surgery led to the association of recurrent symptomatology with perineural scarring.11–13 This chapter will offer a diagnostic algorithmic approach to determine an accurate diagnosis of symptomatic neural scarring. A review of the clinical anatomy of the nerve root and its anatomic relationships is critical to understanding the basis and pathophysiology of symptomatic neural scarring.
ANATOMY
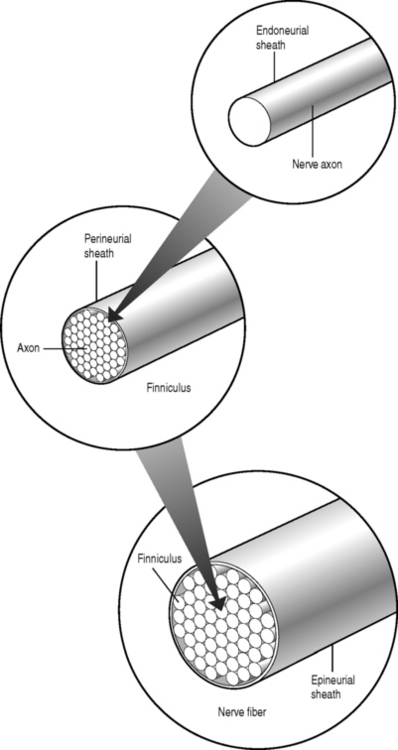
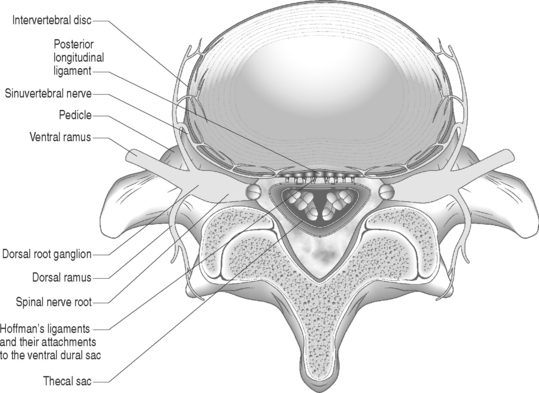
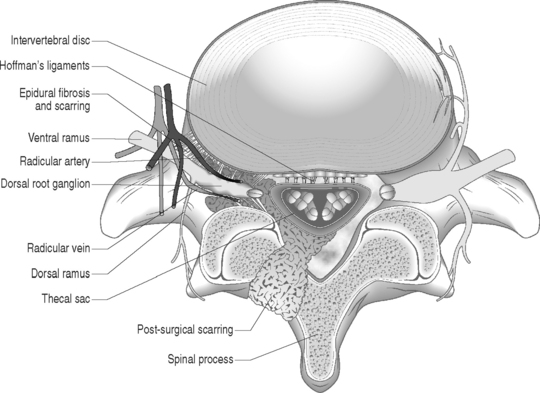
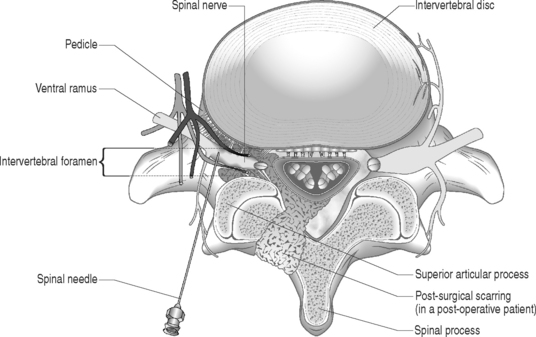
Sunderland14 described the connective tissue coverings of the nerve fiber. Each axon is surrounded by an endoneurial sheath in which multiple axons or nerve fibers form a funiculi. The endoneurial sheath resists elongation under tension. Each funiculi is invested by perineurial connective tissue. Multiple funiculi are surrounded by the epineurium which consists of alveolar connective tissue. The outer perinerium and epineurium provide some degree of protection from tensile and compressive forces (Fig. 104.1). The portions of the nerve roots which lack this outer sheath are more vulnerable to traction or compression. When there is nerve injury, fibrotic tissue may form perineurally/epidurally or intraneurally. It is important to understand the relationship between the nerve roots and the intervertebral foramen (IVF). Each intervertebral foramen is in the shape of an inverted pear that is bounded laterally by the pedicle, posteriorly by the articular facets and ligamentum flavum, and anteriorly by the intervertebral disc and vertebral bodies.15 The radicular complex of ganglia, nerve roots, spinal nerve, and surrounding sheath accounts for 20–35% of the cross-sectional area of the IVF (Fig. 104.2).15 The remainder of the IVF is occupied by loose areolar or adipose tissue, a radicular artery, and numerous venous channels that often encircle the nerve roots. The depth of the foramen varies from 4 to 9 mm.15 The lumbar nerve root complex is situated toward the upper pole of the foramen. At and beyond the ganglion, the dura loses its identity and forms the outer connective tissue sheath which represents the epineurium and perineurium of the ganglion, anterior nerve root, and the newly formed spinal nerve. The Hofmann ligaments, initially described in 1898, are fine filamentous bands of connective tissue that connect the ventral surface of the dural sac and the exiting nerve roots to the posterior longitudinal ligament and posterior vertebral periosteum (Fig. 104.3).16 The ventral ligamentous attachments within the spinal canal reduce the ability of a nerve root to be dorsally displaced by a disc herniation. The nerve root complex is mobile, but does not have unlimited motion. The lumbar roots undergo an excursion of 0.5–5 mm, depending on the anatomic level.17 There is an average excursion of 3 mm for the intrathecal portion of the L5 nerve root, while for the S1 nerve root this value ranges between 4 and 5 mm.17 Movement is induced by straight leg raising in the lumbosacral roots, nerves, and plexus, and in the intrapelvic section of the sciatic nerve.17 Since the nerve root and its dural sheath are relatively fixed within the spinal canal and the radicular foramen, they cannot easily slip away from a disc protrusion. Fibrosis and adhesions may impair the gliding capacity of the lumbar nerve roots within the radicular canals and increase the vulnerability of the extrathecal intraspinal nerve root to compression. Consequently, the nerve root is stretched and compressed, resulting in edema, ischemia, and radicular symptoms.
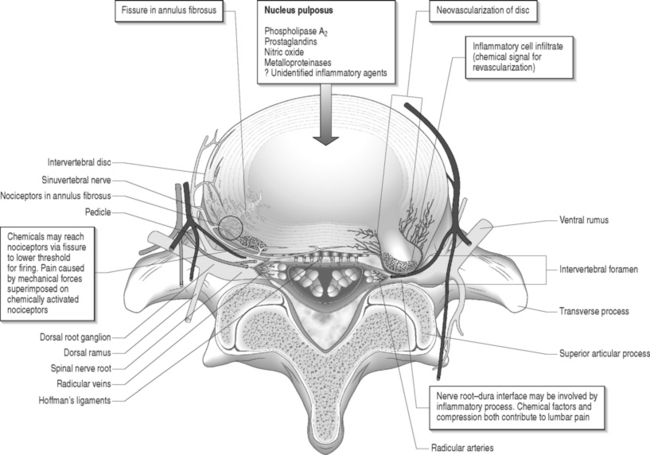
Fig. 104.2 Nerve root complex with dorsal root ganglion, nerve root, and its arterial and venous blood supply.
ADHESIVE ARACHNOIDITIS
Arachnoiditis is inflammation of the pia–arachnoid membrane that covers the spinal cord, cauda equina, nerve roots, or a combination. The extent of scarring varies from mild membrane thickening to severe scarring that can obliterate the subarachnoid space and obstruct cerebrospinal fluid (CSF) flow. The etiology of arachnoiditis is unclear, but typically the intrathecal contents are exposed to an inciting agent such as lumbar spine surgery, intraoperative dural tears, postoperative infections, intrathecal pharmacologic medications, and injection of oil-based contrast agents. Arachnoiditis is more common in patients who undergo extensive or bilateral surgical procedures, and repeat surgeries.
Pathophysiology
Adhesive arachnoiditis causes proliferation of soft tissue leading to filmy adhesions and later to a dense fibrotic matrix. Inflammatory changes are seen microscopically. It is thought that the severity and duration of arachnoiditis is directly correlated to the degree of inflammation and tissue remodeling associated with wound healing. A vascular etiology has also been proposed whereby venous obstruction and dilatation leads to endothelial damage, fibrin deposition, intravascular thromboses, and ultimately fibrosis of the neural elements.18 In the subarachnoid space, the nutritional support of nerve roots is dependent upon its limited vascular supply and the circulation of CSF. The tenuous blood supply and nutrition to the nerve roots within the subarachnoid space can easily be interrupted by neural scarring with subsequent ischemia possible. The exact mechanism of adhesive arachnoiditis is still unknown.
EPIDURAL AND INTRANEURAL FIBROSIS
Epidural fibrosis refers to scar tissue formation outside the dura, on the cauda equina or directly on the nerve roots. Epidural fibrosis develops when epidural fat is replaced by the hematoma that typically forms in the path of surgical dissection. The hematoma is gradually absorbed and simultaneously replaced by granulation tissue that matures into fibrotic connective tissue. Scar tissue development in postoperative patients is not solely due to the operative trauma, but is also a physiologic and reparative response to a herniated disc.8 Epidural scar tissue may constrict the neural elements and cause postoperative pain. Intraneural fibrosis is the formation of scar tissue within the nerve root as opposed to epidural fibrosis, which refers to scar tissue formation outside the nerve root or directly on the nerve root. Intraneural fibrosis cannot be detected by any imaging study and can only be confirmed by pathologic examination under microscopy.
Pathophysiology
Mechanical compression
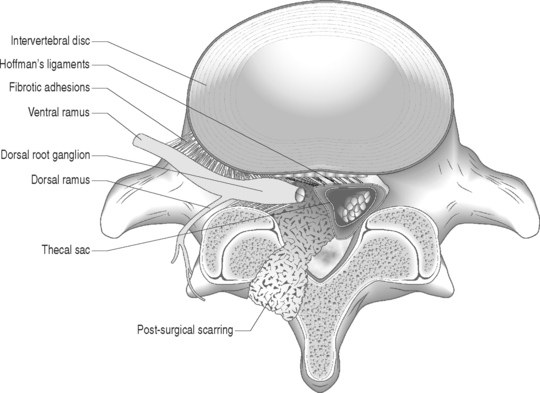
Nerve fibers encased in collagenous scar tissue suffer an increase in neural tension, impairment of axoplasmic transport, and restriction of arterial supply and venous return. Spinal nerve roots and dorsal root ganglia are particularly sensitive to mechanical deformation due to intraspinal disorders.19 The importance of axonal stretching and deformation in response to a mechanical force and the consequent morphological changes within the nervi nervorum were first described by Horsley et al. in 1883.20 A chronic compressive force decreases blood perfusion causing ischemia and limits CSF circulation which leads to endoneural hyperemia21 and further intraneural damage.22 Since a nerve root lacks a perineurium and has a poorly developed epineurium, it may be more susceptible to compression from intraspinal disorders such as epidural fibrosis or a disc herniation. It has been shown that compression of a peripheral nerve will induce direct mechanical effects on the nerve tissue such as deformation of nerve fibers, displacement of nodes of Ranvier, decreased intraneural microcirculation, and neurophysiologic changes of conduction block. Olmarker et al.19 reported increased permeability of the endoneurial capillaries of the nerve roots resulting in edema from mechanical compression. Intraneural edema may impair capillary blood flow and compromise nutritional transport, causing ischemia. Compression and edema may lead to intraneural fibrosis. These processes have important implications as intraneural damage causes inflammation and eventual fibrotic tissue formation after healing leading to intraneural fibrosis. Similarly, perineural or epidural fibrotic tissue in response to surgical trauma or part of the normal reparative process, in response to inflammation or vascular compromise, may mechanically compress the nerve roots and initiate the above cascade, leading to intraneural fibrosis. Tension or stretching of nerve segments may lead to similar anatomic alterations. This may occur if a segment of the spinal nerve root is fixed (e.g. intervertebral foramen) or tethered by fibrotic epidural scar tissue (Fig. 104.4.) Ebeling et al.2,23 demonstrated nerve root immobilization as a result of epidural fibrosis causing nerve root impingement from small recurrent disc fragments embedded in fibrotic tissue. This finding occurred in 23% of cases during second operation following lumbar disc surgery in 92 patients.
Inflammatory etiology
Marshall24 suggested that leakage of breakdown products from degenerating nucleus pulposus may induce a chemical radiculitis. In animal studies, Bobechko and Hirsch25 demonstrated an autoimmune response to the nucleus pulposus of rabbits. This was confirmed by Gertzbein et al.26 in 1975. Using human nucleus pulposus tested with rabbit sera, they demonstrated the presence of a cellular immune response in 73% of patients whose discs were found to be sequestered at the time of surgery and 26% in those patients whose discs were herniated.
McCarron et al.9 showed that there was an inflammatory response to nucleus pulposus injectate in the epidural fat and dura. Saal et al.27 demonstrated that high concentrations of phospholipase A2, the rate-limiting enzyme for the inflammatory cascade of prostaglandins and leukotrienes, were present in human lumbar herniated nucleus pulposus and degenerative discs. Franson et al.28 reported that extracted PLA2 form human lumbar disc has powerful inflammatory activity in vivo. Byrod et al.29 reported that epidural application of substances can have a direct transport route to the axons of the spinal nerve roots. PLA2 may act to excite nociceptors within the anulus or within the epidural space. Direct contact with a nerve root may cause neural injury either by enzymatic activity on the membrane phospholipids or by production of inflammatory mediators. Human disc PLA2 has been shown to cause perineural inflammation, conduction block, and axonal injury by extrathecal application to animal nerve.30 Leakage of PLA2 or another neurotoxic chemical within the disc may irritate small unmyelinated nerve fibers in the anulus or nearby structures such as spinal nerve roots. It has been suggested that radicular pain results from the ectopic firing within sensory fibers injured by the inflammatory chemical mediators released from degenerated disc tissue.31,32 Perineural inflammation or demyelination induced by phospholipase A2 may be responsible for the hypersensitivity of a nerve root to mechanical stimulation.33
Herniated intervertebral disc material can cause inflammatory changes which may lead to epidural or intraneural fibrosis. It has also been suggested that ventrally located epidural scar tissue that adheres to the dorsal aspect of the disc can create injury via mechanical tension followed leakage of inflammatory enzymes.34
Vascular etiology
Disc degeneration has been known to be closely associated with abnormalities in the anatomy and physiology of the adjacent nerve roots. Holt and Yates35 correlated the histology of cervical disc degeneration with adjacent spinal nerve root fibrosis in their cadaver study. Lindahl and Rexed36 demonstrated intraneural fibrosis in 78% of dorsal nerve root biopsies taken at the time of surgery in patients operated on for herniated intervertebral discs. Jayson37 found that there is a statistically significant relationship between the extent of disc degeneration and prolapse with evidence of epidural venous obstruction, perineural/intraneural fibrosis, focal demyelination and neuronal atrophy. A fibrinolytic defect was found in patients with back pain, suggesting that a decreased ability to clear fibrin may be responsible for chronic tissue damage.38 Epidural fibrotic tissue may induce vascular compromise and ischemia with resultant intraneural fibrosis (Fig. 104.5).
DIAGNOSIS
Imaging
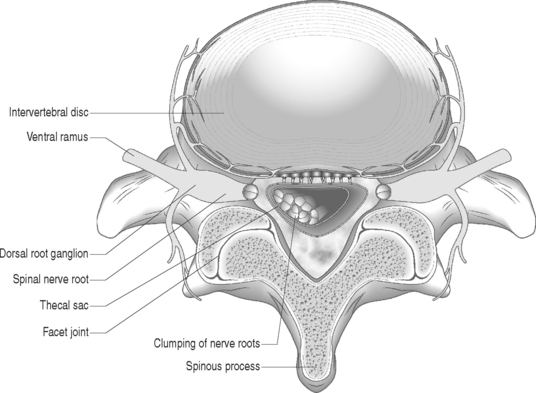
Gadolinium-enhanced MRI is the imaging study of choice when evaluating for epidural fibrosis.7,34 Epidural fibrosis can only be seen on MRI performed with gadolinium dye. Gadolinium enhances vascularized fibrotic scar tissue and distinguishes epidural fibrosis from a recurrent disc protrusion in a postoperative spine patient. MRI is the imaging study of choice in diagnosing arachnoiditis and differentiating other causes of FBSS such as epidural fibrosis, retained or recurrent disc protrusion, lateral recess stenosis, and infection.7,34 MRI has demonstrated excellent correlation with CT-myelography in the diagnosis of adhesive arachnoiditis without the additional risks of an intrathecal injection.39 The characteristic central clumping or clustering of nerve roots within the thecal sac is best seen on axial T2-weighted images (Fig. 104.6).40,41 Peripheral adhesions or severe thecal sac distortion can also be seen. These findings are due to the adhesive nature of the inflamed pia–arachnoid membranes. There is still an occasional role for CT-myelography in a patient with clinically suspected adhesive arachnoiditis and a normal MRI. The ability of CT-myelography to delineate the intrathecal nerve root anatomy may be useful in those patients with limited, localized areas of arachnoiditis involvement.42 It is important to note that findings consistent with arachnoiditis on an imaging study may be clinically asymptomatic.
Clinical assessment
The diagnosis of postoperative low back and limb pain due to neural scarring is a diagnosis of exclusion. Any possible cause for the radicular symptoms other than scar formation has to be excluded.43 The common etiologies of FBSS must be algorithmically eliminated. This concept has been discussed by Jerome Schofferman in the preceding chapter.
NONSURGICAL TREATMENT
Medical rehabilitation and interventional physiatric treatment of FBSS for those patients with recurrent radicular leg pain greater than back pain and with radiologic abnormalities limited to the presence of epidural fibrosis have not shown long-term success.6,44 The majority of the current literature examining FBSS secondary to epidural/intraneural fibrosis has investigated surgical outcomes.6,45–48 Treatment is multidisciplinary. This may include a combination of physical therapy and modalities, pharmacologic agents of various classes, fluoroscopically guided selective nerve root blocks, activity modifications, education, and pain counseling.
Physical therapy
Bed rest is not indicated and the patient is encouraged to be as active as possible. Postoperative rehabilitation and physical therapy is necessary to treat the deconditioned lumbar spine stabilizers, pelvic girdle musculature, and lower limb muscles. Although there are no randomized, controlled studies investigating the effectiveness of physical therapy and modalities such as transcutaneous electrical nerve stimulation (TENS) in the treatment of symptomatic neural scarring, current studies to date do show that these patients may show improvement in their overall functional ability and even in their subjective complaints of pain.49 Nerve root glide maneuvers may also be helpful to slacken the tethered nerve roots from fibrotic tissue.
Pharmacologic agents
Medications including nonsteroidal antiinflammatory drugs (NSAIDs), neuropathic agents such as tricyclic antidepressants (TCAs) or anticonvulsants, muscle relaxants, and opioid pain medications can be tried. These medications should be given in a step-wise fashion with NSAIDs as first-line treatment. Neuropathic agents are membrane stabilizers and should be initiated if there is a significant component of limb pain with neurogenic symptoms such as dysesthesias, paresthesias, and burning or lancinating pain. Gabapentin, one of the most commonly used agents for neuropathic pain, has been reported to be effective in the management of symptomatic limb pain due to epidural fibrosis.50 Muscle relaxers, as a pharmacologic class, are not consistently effective for the treatment of muscle spasms but may be initiated if the spasms are a primary complaint. Most muscle relaxers are also sedatives and should be best taken in the evenings. Medications to restore normal sleep cycle are important for those patients with impaired sleep. Opioid pain medications are primarily used for analgesia but do have secondary effects of membrane stabilization.
Selective nerve root block
There are few studies that have examined the nonsurgical outcomes of FBSS secondary to epidural/intraneural fibrosis treated with selective nerve root blocks (SNRB). Devulder51 demonstrated good pain relief in 12 out of 20 patients with FBSS treated with SNRB consisting of local anesthetic diluted in 1500 U hyaluronidase and 40 mg of methylprednisolone. However, this retrospective pilot study lacked stringent inclusion criteria, used only verbal pain scores, and had a follow-up period of only 3 months. Devulder and colleagues52 found no statistical difference between three patient groups treated with SNRB using a combination of either bupivacaine, hyaluronidase, methylprednisolone, or saline in 60 patients with documented fibrosis and a follow-up period of 6 months. Unfortunately, this study also lacked stringent inclusion criteria and used verbal pain scales as the sole outcome measure. A preliminary study by Slipman et al.53 reported epidemiologic data and outcomes of patients with FBSS secondary to epidural or intraneural fibrosis treated nonsurgically with fluoroscopic guided therapeutic selective nerve root blocks using stringent inclusion criteria and multiple outcome measures. The preliminary study reported successful outcomes from the selective nerve root blocks; however, the frequency of a successful outcome was less than 25% in the small sample size of subjects.
In contrast, several studies have demonstrated the efficacy of fluoroscopically guided transforaminal selective nerve root blocks in the treatment of painful radiculopathy due to a focal disc protrusion. Although selective nerve root blocks may provide reduction of painful lower limb symptoms in the presence of epidural or intraneural fibrosis, the outcomes are much poorer. The injection approach and technique are critical for the successful performance of the selective nerve root block. A fluoroscopically guided SNRB or transforaminal injection is preferably employed instead of an interlaminar or translaminar injection because this facilitates ventral epidural flow to the involved nerve root complex or the posterior surface of the intervertebral discs. The therapeutic agents injected with the posterior translaminar injection approach may remain in the dorsal epidural space without spreading to the affected nerve root or intervertebral disc in the ventral epidural space.54 Furthermore, it is inappropriate to use an interlaminar or translaminar injection technique in patients who have had prior lumbar spine surgery. Surgery alters the normal spinal anatomy with postoperative scar tissue obliterating the normal posterior epidural space and obstructing the flow of contrast dye or medication to the target structures. A transforaminal injection circumvents this problem by placing the needle just inside the neural foramen at the disc–nerve root interface in the ventral epidural space (Fig. 104.7). In addition, the needle track of a transforaminal injection, as opposed to an interlaminar injection, will not be impeded by postoperative scar tissue. Confirmation of proper needle position during a fluoroscopically guided diagnostic SNRB occurs when contrast outlines the targeted nerve root and extends extraforaminally without evidence of epidural flow. The rationale for employing an SNRB is that both corticosteroids and local anesthetic agents have been shown to stabilize neural membranes.55–58 Local anesthetic agents also improve intraradicular blood flow.55 These effects may reduce intraneural or epidural fibrosis and/or the endoneurial edema, compression, or ischemia of the nerve roots. Glucocorticoids suppress inflammatory cytokines and inhibit inflammatory cells.59,60 By modulating cytokines controlling proteolytic enzymes such as collagenase, corticosteroids may affect collagen formation and the organization of the epidural or intraneural fibrotic tissue. The combination of an oral membrane stabilizer, a selective nerve root block with corticosteroids, and physical therapy with nerve root gliding techniques may help to reorganize dense fibrotic scar toward looser connective tissue. If such a process occurs, nerve root excursion within the neural foramen may be restored to a more normal state. Due to the sparse literature, no definitive judgment can currently be made regarding the efficacy of therapeutic SNRB for the treatment of radicular pain caused by epidural or intraneural fibrosis. Based upon personal observation and communications with experienced colleagues, these injections typically do not cure the painful symptoms but may relieve these symptoms for varying periods when they are used in isolation.
Minimally invasive procedures
Percutaneous lysis of epidural adhesions has been described to be an effective treatment for chronic low back and/or leg pain secondary to epidural fibrosis.10,61–66 Racz initially described the technique involving epidurography, adhesiolysis, and injection of local anesthetic agents, corticosteroids, and hyaluronidase followed by injection of hypertonic saline.10,64,65,67,68 One-year outcome studies reported 25–47% good outcomes.62,64,65 However, there are devastating consequences such as cauda equina syndrome, spinal cord compression, paraplegia, myelopathy, and arachnoiditis if not performed properly.10,69–71 Retinal hemorrhages due to increased intracranial pressure from rapid high-volume injections72–76 and catheter shearing with retention in the epidural space have also been reported.10,77 Gabor Racz addresses these issues in much greater detail in Chapter 106. Implantable intrathecal morphine pumps are an option if a patient responds to oral opioid treatment but requires high dosages due to tolerance. The major advantage of a morphine pump is that a small dosage intrathecally can provide the same or better clinical analgesia when compared to oral opioid treatment.78 A drawback to the morphine pump is that the intrathecal agent may act as another insult to the neural elements and worsen arachnoiditis or neural scarring. A detailed discussion of implantable pain pumps including indication, contraindications, and outcomes is covered by Joshua Prager in Chapter 108. The use of TENS units can sometimes reduce painful neurogenic symptoms. Implantable dorsal column stimulators are a more invasive form of TENS, but may provide significant reduction of painful neurogenic lower limb symptoms in carefully selected patients. A more detailed review of this treatment modality is provided by Robert Windsor in Chapter 107.
ALGORITHMIC APPROACH
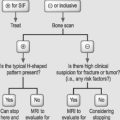
When approaching a postoperative patient with persistent lower limb pain who has failed a reasonable trial of conservative treatment consisting of physical therapy and medications, one must first determine the location of the lower limb pain and its time course in relation to the surgery. The time course characteristically involves onset of radicular symptoms within 1 year of postoperative pain relief. Nerve root pain due to epidural or intraneural fibrosis classically follows a radicular pattern of the involved nerve root and is within all or part of the radicular symptom distribution prior to surgery. The radicular lower limb pain is generally more intensely painful than axial low back pain. Gadolinium-enhanced MRI of the lumbar spine must demonstrate epidural fibrosis around the suspected nerve root without evidence of other pathology such as neuroforaminal stenosis or a recurrent disc protrusion. If the physical examination reveals a new myotomal strength deficit or reflex change correlating with the distribution of radicular pain and MRI findings, a fluoroscopically guided therapeutic selective nerve root block is recommended to treat the involved nerve root. In the absence of these physical examination findings, a positive electrodiagnostic evaluation identifying new acute changes consistent with the involved nerve root level will diagnose the radiculopathy. A therapeutic selective nerve root block is then offered to treat the radiculopathy. An EMG is considered positive when the involved nerve root level has abnormal spontaneous activity at rest in the form of positive sharp waves and fibrillation potentials in the associated paraspinal musculature and in at least two muscles from the same myotomal, but different peripheral nerve innervation with neuropathic recruitment abnormalities.79 Although a positive EMG provides the diagnosis of radiculopathy, a negative EMG does not rule out nerve injury. It is not uncommon for patients with radicular pain to have normal electrodiagnostic findings or instances where only chronic changes are identified.80 If the electrodiagnostic study and physical examination are both negative in the setting of an abnormal imaging study with radicular complaints, a fluoroscopically guided diagnostic selective nerve root block is required.80–82 The diagnostic selective nerve root block is performed only with a small aliquot (1 cc or less) of local anesthetic to directly anesthetize only the suspected nerve root. Care must be taken to ensure that contrast only outlines the nerve root without epidural spread. Otherwise nearby structures in the epidural space will be inadvertently anesthetized, thus comprising the selective nature of this block and removing its diagnostic reliability. If there is at least an 80% or greater reduction of the post-block visual analog scale (VAS) when compared to the pre-block VAS several minutes post-injection, the diagnostic selective nerve root block is positive.81,83,84 This confirms that the radicular lower limb pain is emanating from the suspected nerve root, and a fluoroscopically guided therapeutic steroid selective nerve root block is then offered. The high sensitivity, ranging 99–100%,85,86 and high specificity, ranging 87–100%,85–90 of diagnostic selective nerve root blocks have been well documented by several studies. If the patient fails to improve with a fluoroscopically guided therapeutic steroid SNRB and seeks further treatment for persistent radicular lower limb pain due to epidural fibrosis, percutaneous epidural adhesiolysis under fluoroscopy is recommended by some. A dorsal column stimulator trial is reserved as the last resort if the patient is refractory to the aforementioned treatments.
SURGERY
Most authors do not recommend reoperation when fibrosis is suspected to be the only cause of FBSS because the probability of long-term success after reoperation is low.1–7,43–48,91,92 Surgical outcomes have been reported to be between 10% and 30%.6,48 In a prospective case series, Jonssson and Stromqvist47 demonstrated that 62% of patients with FBSS secondary to neural fibrosis after discectomy undergoing reoperation were unchanged or worse. Neurolysis for epidural fibrosis not only yields dismal results and more expansive fibrosis, but it also increases the risk of nerve root injury.45,48,93 The overall failure rate for neurolysis has been reported to be 62–83%.91,92,94–96
Outcomes are poor for eliminating scar tissue and significantly reducing pain from adhesive arachnoiditis, and may exacerbate symptoms by further damaging the neural elements.97,98 Exploratory surgery is not indicated in the absence of progressive neurologic deficit nor in patients whose pain can be controlled with nonsurgical treatment. Patients with high-grade arachnoiditis and those with preoperative dysesthesias have a worse prognosis for surgical success.99–102 In recent years, much attention has been focused on various biologic, pharmacologic, and synthetic materials to inhibit neural scarring and its intraoperative applications. Several materials have been used intraoperatively to inhibit scar; however, research related to their efficacy has been via animal models, and clinical results are not convincing. The ideal agent for scar inhibition remains to be identified.
Fat free grafts have had the longest history of use and are generally accepted as a way to avoid possible postsurgical fibrosis. Again, its usefulness in epidural and perineural fibroses inhibition is uncertain. Fat graft is readily available with no additional cost, and it can become vascularized, nourishing the dura. The possible disadvantages of using this graft include seroma formation, indentations, and the fact that it is a space-occupying mass that may cause neural compression. The size of the graft is also important. A large graft may possibly result in neural compression, whereas if too thin it may be ineffective as the fat graft shrinks in size over time.103,104 Migration of the graft is also a concern and a few cases of cauda equina syndrome believed to result from migration have been reported.105,106 Generally speaking, surgeons prefer fat grafts, as they are easily available without additional costs and do protect the dura without excessive formation of fibrous tissue. Alternatives to fat grafts include a number of materials. Gelfoam is widely used as a hemostatic agent, but has also been shown to prevent scar adhesion when used in the epidural space after a laminectomy.107 Another gelatin-derived product is ADCON-L. It was FDA approved in 1998 for inhibition of postsurgical fibrosis. Several cases of dural tears after use of ADCON-L in posterior lumbar surgeries were reported which has limited the widespread use of this material.108 There are several scar-inhibiting agents that are currently being evaluated in clinical trials.
SUMMARY
The pathophysiology of radicular pain caused by epidural or intraneural fibrosis may be the result of inflammation, vascular compromise, mechanical compression, or tension of the spinal nerve roots. An accurate diagnosis of symptomatic neural scarring can be determined with a diagnostic algorithmic pathway incorporating a detailed history, physical examination, electrodiagnostic testing, and fluoroscopically guided diagnostic selective nerve root blocks. Injection of glucocorticoids and local anesthetic agents has been proposed since these agents can indirectly stabilize neural membranes, reduce the local cellular immune response, inhibit inflammatory cytokines, decrease intraneural edema, and increase intraradicular blood flow. Nonsurgical and surgical outcomes for symptomatic neural scarring are poor. Treatment necessitates a multidisciplinary approach that also involves the patient’s social support network.
1 Burton CV, Kirkaldy-Willis WH, Yong-Hing K, et al. Causes of failure of surgery on the lumbar spine. Clin Orthopaed Rel Res. 1981;157:191-199.
2 Ebeling U, Kalbarcyk H, Reulen HJ. Microsurgical reoperation following lumbar disc surgery. Timing, surgical findings, and outcome in 92 patients. J Neurosurg. 1989;70(3):397-404.
3 Slipman CW, Shin CH, Patel RK, et al. Etiologies of failed back surgery syndrome. Pain Med. 2002;3(3):200-214.
4 Long DM. Failed back surgery syndrome. Neurosurg Clin N Am. 1991;2(4):899-919.
5 Simmons ED. North American Spine Society annual abstracts, 1998.
6 North RB, Campbell JN, James CS, et al. Failed back surgery syndrome: 5-year follow-up in 102 patients undergoing repeated operation. Neurosurgery. 1991;28(5):685-690.
7 Ross JS, Robertson JT, Frederickson RC, et al. Association between peridural scar and recurrent radicular pain after lumbar discectomy: magnetic resonance evaluation. ADCON-L European Study Group. Neurosurgery. 1996;38(4):855-861.
8 Annertz M, Jonsson B, Stromquist B, et al. No relationship between epidural fibrosis and sciatica in the lumbar postdiscectomy syndrome. A study with contrast-enhanced magnetic resonance imaging in symptomatic and asymptomatic patients. Spine. 1995;4(20):449-453.
9 McCarron R, et al. The inflammatory effect of nucleus pulposus: a possible element in the pathogenesis of low back pain. Spine. 1987;12:760-764.
10 Racz GB, Holubec JT. Lysis of adhesions in the epidural space. In: Racz GB, editor. Techniques of neurolysis. Boston: Kluwer Academic; 1989:57-72.
11 Prawl RP. Arachnoiditis and epidural fibrosis: the relationship to chronic pain. Curr Rev Pain. 1998;2:93-99.
12 Barsa JE, Charlton JE. Diagnosis of epidural scarring and its possible contribution to chronic low back pain syndrome. Pain. 1984;S4:376.
13 Cook SD, Prewett AB, Dalton JE, et al. Reduction in perineural scar formation after laminectomy with polyactive membrane sheets. Spine. 1994;19:1815-1825.
14 Sunderland S. Meningeal–neural relations in the intervertebral foramen. J Neurosurg. 1974;40:756-763.
15 Hoyland JA, Freemont AJ, Jayson MI. Intervertebral foramen venous obstruction. A cause of periradicular fibrosis? Spine. 1989;14(6):558-568.
16 Spencer DL, Irwin GS, et al. Anatomy and significance of fixation of the lumbosacral nerve roots in sciatica. Spine. 1983;8:672-679.
17 Goddard, Reid JD. Movement induced by straight leg raising in the lumbosacral roots, nerves, and plexus, and in the intrapelvic section of the sciatic nerve. J Neurol Neurosurg Psychiatry. 1965;28:12-18.
18 Parke WW, Watanabe R. The intrinsic vasculature of the lumbosacral spinal nerve roots. Spine. 1985;10:508-515.
19 Olmarker K, et al. Edema formation in spinal nerve roots induced by experimental study of the pig cauda equina. Spine. 1989;14:579. 561
20 Horsley V, Sugar O, John Marshall. Nerve stretching, and the nervi nervorum. Surg Neurol. 1990;34(3):184-187.
21 Cornefjord M, Sato K, Olmarker K, et al. A model for chronic nerve root compression studies. Presentation of a porcine model for controlled, slow-onset compression with analyses of anatomic aspects, compression onset rate, and morphologic and neurophysiologic effects. Spine. 1997;22(9):946-957.
22 Garfin SR, Rydevik B, et al. Spinal nerve root compression. Spine. 1995;20:1810-1820.
23 Ebeling U, Reichenberg W, Reulen HJ. Results of microsurgical lumbar discectomy. Review on 485 patients. Acta Neurochir (Wien). 1986;81(1–2):45-52.
24 Marshall LL. The lumbar disc. Med J Australia. 1975;2(1):10-12.
25 Bobcheko WP, Hirsch C. Autoimmune response to nucleus pulposus in the rabbit. JBJS. 1965;47B:574.
26 Gertzbein SD, et al. Autoimmunity in degenerative disc disease of the lumbar. Spine. 1975;6(1):67-73.
27 Saal JS, et al. High levels of inflammatory phospholipase A2 activity in lumbar disc herniations. Spine. 1990;15:674-678.
28 Franson RC, Saal JS, Saal JA. Human disc phospholipase A2 is inflammatory. Spine. 1992;17(6 Suppl):S129-S132.
29 Byrod G, Rydevik B, Johansson BR, et al. Transport of epidurally applied horseradish peroxidase to the endoneurial space of dorsal root ganglia: a light and electron microscopic study. J Periph Nerv Sys. 2000;5(4):218-226.
30 Saal JS. The role of inflammation in lumbar pain. Spine. 1995;20(16):1821-1827.
31 Chen C, Cavanaugh JM, et al. Effects of phospholipase A2 on lumbar nerve root structure and function. Spine. 1997;22:1057-1064.
32 Muramoto T, Atsuta Y, et al. The action of prostaglandin E2 and triamcinolone acetonide on the firing activity of lumbar nerve roots. Int Orthop. 1997;21:172-175.
33 Ozaktay AC, Kallakuri S, et al. Phospholipase A2 sensitivity of the dorsal root and dorsal root ganglion. Spine. 1998;23:1297-1306.
34 Ross JS, Blaser S, et al. Gd-DTPA ehancement of posterior epidural scar: an exprimental model. AJNR. 1989;10:1083-1088.
35 Holt S, Yates PO. Cervical spondylosis and nerve root lesions. Incidence at routine necropsy. J Bone Joint Surg. 1966;48(3):407-423.
36 Lindhal O, Rexed B. Histological changes in spinal nerve roots of operated cases of sciatica. Acta Orthop Scand. 1951;20:215-225.
37 Jayson MI. The role of vascular damage and fibrosis in the pathogenesis of nerve root damage. Clin Orthopaed Rel Res. 1992;279:40-48.
38 Jayson MI, Keegan A, Million R, et al. A fibrinolytic defect in chronic back pain syndromes. Lancet. 1984;2(8413):1186-1187.
39 Delamarter RB, Ross JS, Masaryk TJ, et al. Diagnosis of lumbar arachnoiditis by magnetic resonance imaging. Spine. 1990;15:304-310.
40 Ross JS, Masaryk TJ, Modic MT, et al. Lumbar spine: postoperative assessment with surface-coil MR imaging. Radiology. 1987;164:851-860.
41 Ross JS, Masaryk TJ, Modic MT, et al. MR imaging of lumbar arachnoiditis. Am J Roentgenol. 1987;149:1025-1032.
42 Hueftle MG, Modic MT, Ross JS, et al. Lumbar spine: postoperative MR imaging with gadolinium-DPTA. Radiology. 1988;167:817-824.
43 Fritsch EW, Heisel J, Rupp S. The failed back surgery syndrome: reasons, intraoperative findings, and long-term results: a report of 182 operative treatments. Spine. 1996;21(5):626-633.
44 Nykvist F, Hurme M, et al. Severe sciatica: A 13-year follow-up of 342 patients. Eur Spine J. 1995;4:335-338.
45 Finnegan WJ, Delin JM, et al. Results of surgical intervention in the symptomatic multiply-operated back patient. J Bone Joint Surg [Am]. 1979;61A:1077.
46 Law JD, Lehman RAW, et al. Reoperation after lumbar intervertebral disc surgery. J Neurosurg. 1978;48:259.
47 Jonsson B, Stromqvist B. Repeat decompression of lumbar nerve roots. A prospective two-year evaluation. J Bone Joint Surg. 1993;75B(6):894-897.
48 Waddell G, Kummel EG, Lotto WN, et al. Failed lumbar disc surgery and repeat surgery following industrial injury. J Bone Joint Surg [Am]. 1969;61:201-207.
49 Wynn Parry CB, Girgis F. The assessment and management of the failed back, Part II. Int Disabil Stud. 1988;10(1):25-28.
50 Braverman DL, Slipman CW, Lenrow DA. Using gabapentin to treat failed back surgery syndrome caused by epidural fibrosis: A report of 2 cases. Arch Phys Med Rehabil. 2001;82(5):691-693.
51 Devulder J. Transforaminal nerve root sleeve injection with corticosteroids, hyaluronidase, and local anesthetic in the failed back surgery syndrome. J Spinal Disord. 1998;11(2):151-154.
52 Devulder J, Deene P, De Laat M, et al. Nerve root sleeve injections in patients with failed back surgery syndrome: a comparison of three solutions. Clin J Pain. 1999;15(2):132-135.
53 Slipman CW, Chow DW, et al. Outcomes of therapeutic selective nerve root block for painful symptoms of epidural and/or intraneural fibrosis following discectomy for a herniated disc: a preliminary report. (Submitted for publication.)
54 Kraemer J, Ludwig J, Bickert U, et al. Lumbar epidural perineural injection: a new technique. Eur Spine J. 1997;6:357-361.
55 Yabuki S, Kikuchi S. Nerve root infiltration and sympathetic block. An experimental study of intraradicular blood flow. Spine. 1995;20(8):901-906.
56 Cullen BF, Haschke RH. Local anesthetic inhibition of phagocytosis and metabolism of human leukocytes. Anesthesiology. 1974;40:142-146.
57 Hoidal JR, White JG, Repine JE. Influence of cationic local anesthetics on the metabolism and ultrastructure of human alveolar macrophages. J Lab Clin Med. 1979;93:857.
58 Goldstein IM, Lind S, et al. Influence of local anesthetics upon human polymorphonuclear leukocyte function in vitro. J Exp Med. 1977;146:483-494.
59 Takahashi H, et al. Inflammatory cytokines in the herniated disc of lumbar spine. Spine. 1996;21:218-224.
60 Doita M, et al. Immunohistologic study of the ruptured intervertebral disc of the lumbar. Spine. 1996;21:235-241.
61 Manchikanti L, Pakanati R, Bakhit CE, et al. Role of adhesiolysis and hypertonic saline neurolysis in management of low back pain. Evaluation of modification of Racz protocol. Pain Digest. 1999;9:91-96.
62 Manchikanti L, Pampati V, Fellows B, et al. Role of one day epidural adhesiolysis in management of chronic low back pain: a randomized clinical trial. Pain Phys. 2001;4(2):153-166.
63 Manchikanti L, Pampati V, Bakhit CE, et al. Non-endoscopic endoscopic adhesiolysis in post lumbar laminectomy syndrome. A one-year outcome study and cost effective analysis. Pain Phys. 1999;2:52-58.
64 Racz GB, Heavner JE, Raj PP. Percutaneous epidural neuroplasty. Prospective one-year follow up. Pain Digest. 1999;9:97-102.
65 Heavner JE, Racz GB, Raj P. Percutaneous epidural neuroplasty. Prospective evaluation of 0.9% NaCl versus 10% NaCl with or without hyaluronidase. Reg Anesth Pain Med. 1999;24:202-207.
66 Arthur J, Racz G, Heinrich R, et al. Epidural space. Identification of filling defects and lysis of adhesions in the treatment of chronic painful conditions. In: Abstracts, 7th World Congress on Pain. Paris: IASP Publications; 1993:557.
67 Racz GB, Sabaonghy M, Gintautas J, et al. Intractable pain therapy using a new epidural catheter. JAMA. 1982;248:579-581.
68 Racz GB, Heavner JE, Raj PP. Epidural neuroplasty. Semin Anesthesia. 1997:302-312.
69 Aldrete JA, Zapata JC, Ghaly R. Arachnoiditis following epidural adhesiolysis with hypertonic saline report of two cases. Pain Digest. 1996;6:368-370.
70 Kim RC, Porter RW, Choi BH, et al. Myelopathy after intrathecal administration of hypertonic saline. Neurosurgery. 1988;22:942. 922
71 Lucas JS, Ducker TB, Perot PL. Adverse reactions to intrathecal saline injections for control of pain. J Neurosurg 197; 42:557–561.
72 Kushner FH, Olson JC. Retinal hemorrhage as a consequence of epidural steroid injection. Arch Ophthalmol. 1995;113:309-313.
73 Ling C, Atkinson PL, Munton CG. Bilateral retinal hemorrhages following epidural injection. Br J Ophthalmol. 1993;77:316-317.
74 Purdy EP, Ajimal GS. Vision loss after lumbar epidural steroid injection. Anesth Analg. 1998;86:119-122.
75 Usubiaga JE, Wikinski JA, Usubiaga LE. Epidural pressure and its relation to spread of anesthetic solution in epidural space. Anesth Analg. 1967;46:440-446.
76 Morris DA, Henkind P. Relationship of intracranial, optic-nerve sheath, and retinal hemorrhage. Am J Ophthalmol. 1967;64:853-859.
77 Manchikanti L, Bakhit CE. Removal of torn Racz catheter from lumbar epidural space. Reg Anesth. 1997;22:579-581.
78 York M, Paice JA. Treatment of low back pain with intraspinal opioids delivered via implanted pumps. Orthop Nurs. 1998;17(3):61-69.
79 Dumitru D. Electrodiagnostic medicine. Philadelphia: Hanley & Belfus, 1997;231.
80 Slipman CW, Chow DW, Whyte WS, et al. An evidenced-based algorithmic approach to cervical spinal disorders. Crit Rev Phys Rehab Med. 2001;13(4):283-299.
81 Huston CW, Slipman CW. Diagnostic selective nerve root blocks: indications and usefulness. Phys Med Rehabil Clin N Am. 2002;13(3):545-565.
82 Slipman CW, Chow DW. Therapeutic spinal corticosteroid injections for the management of radiculopathies (chapter 12). In: Dillingham TR, ed. Cervical, thoracic, and lumbosacral radiculopathies: update in diagnosis and management. PM&R Clin N Am; 2002.
83 Anderberg L, Annertz M, Brandt L, et al. Selective diagnostic cervical nerve root block – correlation with clinical symptoms and MRI pathology. Acta Neurochir (Wien). 2004;146(6):559-565.
84 Slipman CW, Plastaras CT, Palmitier RS, et al. Symptom provocation of fluoroscopically guided cervical nerve root stimulation: Are dynatomal maps identical to dermatomal maps? Spine. 1998;23(20):2235-2242.
85 Haueisen DC, Smith BS, Myers SR, et al. The diagnostic accuracy of spinal nerve injection studies. Clin Orthopaed Rel Res. 1983;198:179-183.
86 Van Akkerveeken PF. The diagnostic value of nerve root sheath infiltration. Acta Ortho Scand. 1993;64:61-63.
87 Dooley JF, McBroom RJ, Taguchi T, et al. Nerve root infiltration in the diagnosis of radicular pain. Spine. 1994;19:1125-1131.
88 Krempen JF, Smith BS. Nerve root injection: a method for evaluating the etiology of sciatica. J Bone Joint Surg [Am]. 1974;56A:1435-1444.
89 Schutz H, Lougheed WM, Wortzman G, et al. Intervertebral nerve-root in the investigation of chronic lumbar disc disease. Canadian J Surg. 1973;16:217-221.
90 Stanley D, McLaren MI, Euinton HA, et al. A prospective study of nerve root infiltration in the diagnosis of sciatica: A comparison with radiculography, computed tomography, and operative findings. Spine. 1990;6:540-543.
91 Ozgen S, Naderi S, Ozek MM, et al. Findings and outcome of revision lumbar disc surgery. J Spinal Disord. 1999;12(4):287-292.
92 Benoist M, Ficat C, Baraf P, et al. Postoperative lumbar epiduro-arachnoiditis. Diagnostic and therapeutic aspects. Spine. 1980;5(5):432-436.
93 Johnston J, Matheny J. Microscopic lysis of lumbar adhesive arachnoiditis. Spine. 1978;3:36-39.
94 Thomalske G, Galow W, Ploke G. Critical comments on a comparison of two series of lumbar disc surgery. Adv Neurosurg. 1977;4:22-27.
95 Johnston J, Matheny J. Microscopic lysis of lumbar adhesive arachnoiditis. Spine. 1978;3:36-39.
96 Grane P. The post-operative lumbar spine. A radiological investigation of the lumbar spine after discectomy using MR imaging and CT. Acta Radiol Suppl. 1998;414:1-23.
97 Quiles M, Machisello PJ, Tsairis P. Lumbar adhesive arachnoiditis: Etiologic and pathologic aspects. Spine. 1978;3:45-50.
98 Coventry MB, Stauffer RN. The multiply operated back. In: American Academy of Orthopaedic Surgeons: Symposium on the Spine. St. Louis: CV Mosby; 1999:132-142.
99 Dolan RA. Spinal adhesive arachnoiditis. Surg Neurol. 1993;39:479-484.
100 Johnston J, Matheny J. Microscopic lysis of lumbar adhesive arachnoiditis. Spine. 1978;3:36-39.
101 Roca J, Moreta D, Ubierna MT, et al. The results of surgical treatment of lumbar arachnoiditis. Int Orthop. 1993;17:77-81.
102 Wilkinson HA, Schuman N. Results of surgical lysis of lumbar adhesive arachnoiditis. Neurosurgery. 1979;4:401-409.
103 Kanamori M, Kawaguchi Y, Ohmori K, et al. The fate of autogenous free-fat grafts after posterior lumbar surgery: part 1. A postoperative serial magnetic resonance imaging study. Spine. 2001;26(20):2258-2263.
104 Kanamori M, Kawaguchi Y, Ohmori K, et al. The fate of autogenous free-fat grafts after posterior lumbar surgery: part 2. Magnetic resonance imaging and histologic studies in repeated surgery cases. Spine. 2001;26(20):2264-2270.
105 Mayer PJ, Jacobsen FS. Cauda equina syndrome after surgical treatment of lumbar spinal stenosis with application of free autogenous fat graft. J Bone Joint Surg [Am]. 1989;71A:1090-1093.
106 Prusik VR, Lint DS, Bruder WJ. Cauda equina syndrome as a complication of free epidural fat-grafting. J Bone Joint Surg [Am]. 1998;70A:1256.
107 LaRocca H, Macnab I. The laminectomy membrane: studies in its evolution, characteristics, and prophylaxis in dogs. J Bone Joint Surg [Br]. 1974;56B:545-550.
108 Le AX, Rogers DE, Dawson EG, et al. Unrecognized durotomy after lumbar discectomy: a report of four cases associated with the use of ADCON-L. Spine. 2001;26(1):115-117.