Chapter 10 Nerves of the lumbar spine
Lumbar spinal nerves
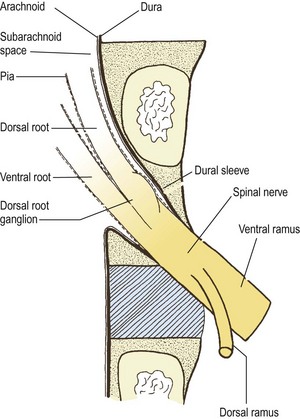
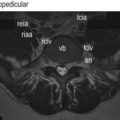
The lumbar spinal nerves lie in the intervertebral foramina and are numbered according the vertebra beneath which they lie. Thus, the L1 spinal nerve lies below the L1 vertebra in the L1–2 intervertebral foramen, the L2 spinal nerve lies below the L2 vertebra, and so on. Centrally, each spinal nerve is connected to the spinal cord by a dorsal and ventral root. Peripherally, each spinal nerve divides into a larger ventral ramus and a smaller dorsal ramus. The spinal nerve roots join the spinal nerve in the intervertebral foramen, and the ventral and dorsal rami are formed just outside the foramen. Consequently, the spinal nerves are quite short. Each is no longer than the width of the intervertebral foramen in which it lies (Fig. 10.1).
Lumbar nerve roots
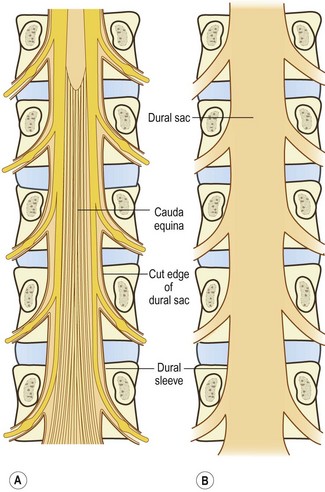
The spinal cord terminates in the vertebral canal opposite the level of the L1–2 intervertebral disc, although it may end as high as T12–L1 or as low as L2–3.1 Consequently, to reach the spinal cord, the lower lumbar (and sacral) nerve roots must run within the vertebral canal where they are largely enclosed in the dural sac (Fig. 10.2). Within the dural sac, the lumbar nerve roots run freely, mixed with the sacral and coccygeal nerve roots to form the cauda equina, and each root is covered with its own sleeve of pia mater, which is continuous with the pia mater of the spinal cord. All the roots of the cauda equina are bathed in cerebrospinal fluid (CSF), which percolates through the subarachnoid space of the dural sac.
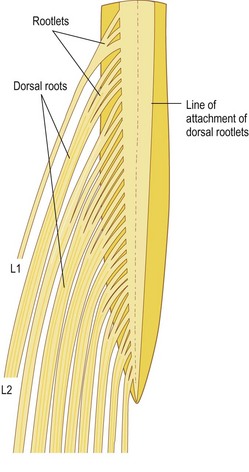
For the greater part of their course, the nerve fibres within each nerve root are gathered into a single trunk, but near the spinal cord they are separated into smaller bundles called rootlets, which eventually attach to the spinal cord. The size and number of rootlets for each nerve root are variable but in general they are 0.5–1 mm in diameter and number between two and 12 for each root.2 The rootlets of each ventral root attach to the ventrolateral aspect of the cord, while those of the dorsal roots attach to the dorsolateral sulcus of the cord, and along the ventral and dorsal surface of the cord the rootlets form an uninterrupted series of attachments (Fig. 10.3).
A pair of spinal nerve roots leaves the dural sac just above the level of each intervertebral foramen. They do so by penetrating the dural sac in an inferolateral direction, taking with them an extension of dura mater and arachnoid mater referred to as the dural sleeve (see Fig. 10.2). This sleeve encloses the nerve roots as far as the intervertebral foramen and spinal nerve, where the dura mater merges with, or becomes, the epineurium of the spinal nerve (see Fig. 10.1). The pia mater of each of the nerve roots also extends as far as the spinal nerve, as does an extension of the subarachnoid space (see Fig. 10.1). Thus, the nerve roots are sheathed with pia mater and bathed in CSF as far as the spinal nerve.
The angle at which each pair of nerve roots leaves the dural sac varies from above downwards. The L1 and L2 roots leave the dural sac at an obtuse angle but the dural sleeves of the lower nerve roots form increasingly acute angles with the lateral margins of the dural sac (see Fig. 10.2). The angles formed by the L1 and L2 roots are about 80° and 70°, respectively, while the angles of the L3 and L4 roots are each about 60°, and that of the L5 roots is 45°.3
The level of origin of the nerve root sleeves also varies from above downwards. In general, the sleeves arise opposite the back of their respective vertebral bodies. Thus, the L1 sleeve arises behind the L1 body, the L2 sleeve behind the L2 body, and so on. However, successively lower sleeves arise increasingly higher behind their vertebral bodies until the sleeve of the L5 nerve roots arises behind the L4–5 intervertebral disc.3
Relations of the nerve roots
The relations of the nerve roots are of critical importance in the pathology of nerve root compression, for space-occupying lesions of any of the tissues intimately, or even distantly, related to the nerve roots may encroach upon them. In this regard, the majority of structures related to the nerve roots have already been described (see Ch. 5), although the anatomy of the spinal blood vessels is described in detail in Chapter 11.
The most intimate relation of the nerve roots are the meninges. The roots of the cauda equina are enclosed in the dural sac and bathed in CSF. Beyond the dural sac, individual pairs of roots are sheathed by pia, arachnoid and dura in the nerve root sleeves (Figs. 10.1, 10.4). The relevance of this relationship is that tumours or cysts of the dura or arachnoid can at times form space-occupying lesions that compress the roots. Running within the root sleeves are the radicular arteries and veins (see Ch. 11), and the relevance of this relationship is described in Chapter 15.
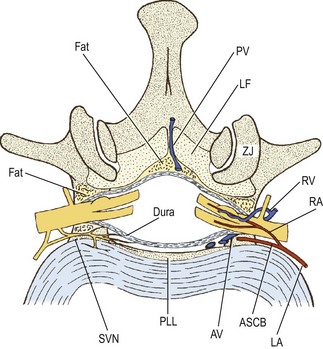
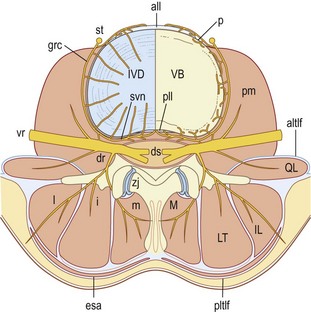
As a whole, the dural sac rests on the floor of the vertebral canal (see Ch. 5). The anterior relations of the dural sac, therefore, are the backs of the vertebral bodies and the intervertebral discs, and covering these structures is the posterior longitudinal ligament (see Fig. 10.4). Running across the floor of the vertebral canal, and therefore anterior to the dural sac, are the anterior spinal canal arteries (see Ch. 11) and the sinuvertebral nerves (see below). Posteriorly, the dural sac is related to the roof of the vertebral canal, the laminae and ligamenta flava (see Ch. 5).
A space intervenes between the dural sac and the osseoligamentous boundaries of the vertebral canal; this space is referred to as the epidural space. This space, however, is quite narrow, for the dural sac is applied very closely to the osseoligamentous boundaries of the vertebral canal. It is almost a ‘potential space’, and the term ‘epidural region’ has been advocated as an alternative description to avoid the connotation of a wide, empty space (see Fig. 10.4).4
The epidural space is principally filled by a thin layer of areolar connective tissue, which varies from diaphanous to pseudomembranous in structure.4 Some investigators, however, consider this to be a substantive structure which they call the epidural membrane.5 The membrane surrounds the dural sac and lines the deep surface of the laminae and pedicles. Ventrally, opposite the vertebral bodies, the membrane lines the back of the vertebral body and then passes medially deep to the posterior longitudinal ligament, where it attaches to the anterior surface of the deep portion of the ligament.5 The membrane does not cover the back of the anulus fibrosus; it is prevented from doing so by the posterior longitudinal ligament as it expands laterally over the back of the disc. Consequently, the membrane blends with the upper and lower borders of the anulus fibrosus but in a plane just anterior to that of the posterior longitudinal ligament. Opposite the intervertebral foramen, the membrane is drawn laterally to form a circumneural sheath around the dural sleeve of the nerve roots and spinal nerve.5
Running within the areolar tissue of the epidural membrane are the anterior and posterior internal vertebral venous plexuses (see Ch. 11), and located within it are collections of fat. The epidural fat is not distributed uniformly throughout the epidural space but is concentrated around the nerve roots in the intervertebral foramina and in collections wrapped in areolar tissue and lodged in the midline recesses between the ligamenta flava at each segmental level.4
Within the vertebral canal, the dural sac and the nerve root sleeves are tethered to the vertebral column by condensations of the epidural fascia that have been referred to as dural ligaments or meningovertebral ligaments or the ligaments of Hofmann.4,6–8 Although the first term is the more traditional, the second is a better description, in that the tissue is not an extension of dura but a connection between the meninges and the vertebral column.
The ventral meningovertebral ligaments pass from the ventral surface of the dura to the posterior longitudinal ligament. They are most evident when the dura is drawn backwards and the ligaments are tensed. At rest, they are barely distinguishable from the epidural membrane. When tensed, they form a discontinuous septum in the median or paramedian plane. Individual ligaments may form single bands, bands that bifurcate in a Y shape towards the posterior longitudinal ligament, or two or more paramedian bands that skirt the posterior longitudinal ligament and attach to the periosteum of the lateral recesses.6,7 These ligaments are variably developed at the L1–L4 levels but are well developed at L5.8
Lateral meningovertebral ligaments pass from the lateral surface of the dural sac to blend with the periosteum of the pedicles and with the capsule of the zygapophysial joint.6 Posteriorly, the dural sac is attached to the roof of the vertebral canal by occasional, weak pseudoligamentous connections,4 which represent dorsal meningovertebral ligaments.6
The nerve root sleeves are tethered both within the vertebral canal and in the intervertebral foramen. At the proximal end of the root sleeve, the meningovertebral ligaments tether the dura to the posterior longitudinal ligament and the periosteum of the adjacent pedicle.8,9 In the intervertebral foramen, the root sleeve is surrounded by the circumneural sheath, which indirectly binds the nerve roots and spinal nerve to the margins of the foramen, but mainly to the capsule of the zygapophysial joint dorsally.8,9 At the outer end of the intervertebral foramen, the spinal nerve may be related to a transforaminal ligament when one is present (see Ch. 4). As a rule, the spinal nerve lies below most forms of transforaminal ligaments but emerges above the inferior transforaminal variety (see Ch. 4).10
The relative size of the spinal nerve and nerve roots within the intervertebral foramen varies from level to level and is important with respect to the risk of spinal nerve and nerve root compression. As an approximate rule, the cross-sectional area of an intervertebral foramen increases from L1–2 to L4–5, but the L5–S1 foramen is conspicuously smaller than the rest,11 yet, paradoxically, the L5 spinal nerve is the largest of the lumbar nerves.11 Consequently, the L5 spinal nerve occupies about 25–30% of the available area in an intervertebral foramen, while the other lumbar nerves occupy between 7% and 22%, making the L5 nerve the most susceptible to foraminal stenosis.
Anomalies of the nerve roots
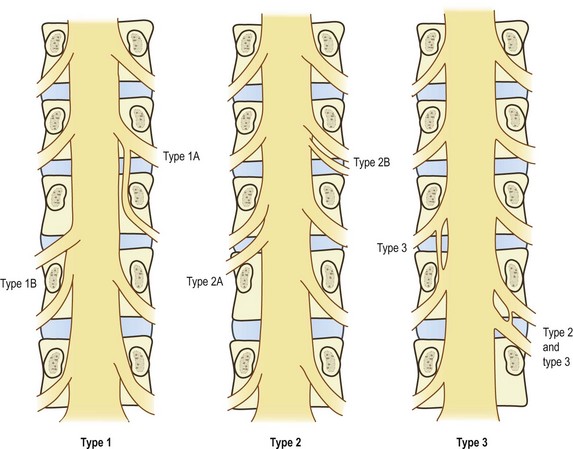
The clinically most significant anomalies of the lumbar nerve roots are aberrant courses and anastomoses between nerve roots;12–18 the morphology of these anomalies is summarised in Figure 10.5.
Fortunately, symptomatic nerve root anomalies are not common, and such confusing considerations do not regularly complicate clinical practice. The incidence of anomalies has been estimated at about 8.5%,19 but when symptomatic the major types are readily recognised in myelograms.2 Nonetheless, nerve root anomalies should be borne in mind and considered as a possibility in patients with unusual distributions of neurological signs.
The surgical significance of nerve root anomalies relates to the mobility of anomalous nerve roots, the care necessary when operating in their vicinity, and the types of procedures that can be carried out to decompress them. These issues are explored in the surgical literature.2,16
Another feature of nerve roots, which is not an anomaly but rather a variation, is intrathecal anastomoses. Within the dural sac, bundles of nerve fibres may pass from one nerve root to the next, and such communications have an incidence of 11–30%.20 They usually occur close to the spinal cord and may vary in size from small filaments to substantial bundles.20 Since they occur proximal to the regions where nerve roots are liable to compression, these anastomoses are not of diagnostic clinical significance, but they are of relevance to neurosurgeons operating on the proximal ends of nerve roots.20,21
Dorsal rami
The L1– L4 dorsal rami are short nerves that arise almost at right angles from the lumbar spinal nerves.22 Each nerve measures about 5 mm in length23 and is directed backwards towards the upper border of the subjacent transverse process. The L5 dorsal ramus differs, in that it is longer and travels over the top of the ala of the sacrum (Fig. 10.6).23
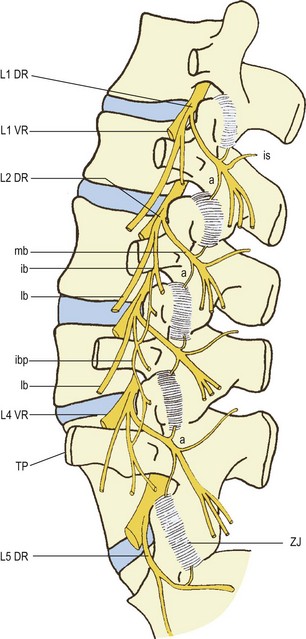
Figure 10.6 A left posterior view of the lumbar spine showing the branches of the lumbar dorsal rami. (Based on Bogduk et al. 1982.23) DR, dorsal ramus: ib, intermediate branch; ibp, intermediate branch plexus; lb, lateral branch; mb, medial branch. TP, transverse process; a, articular branch; is, interspinous branch. VR, ventral ramus. ZJ, zygapophysial joint.
As they approach their transverse processes, the L1–4 dorsal rami divide into two or three branches (see Fig. 10.6). A medial branch and a lateral branch are always represented at every level. The variable, third branch is the intermediate branch. Although this branch is always represented, it frequently arises from the lateral branch instead of the dorsal ramus itself.23 The L5 dorsal ramus forms only a medial branch and a branch that is equivalent to the intermediate branches of the other lumbar dorsal rami.
The lateral branches of the lumbar dorsal rami are principally distributed to the iliocostalis lumborum muscle, but those from the L1, L2 and L3 levels can emerge from the dorsolateral border of this muscle to become cutaneous. Cutaneous branches of these pierce the posterior layer of thoracolumbar fascia and descend inferolaterally across the iliac crest to innervate the skin of the buttock, over an area extending from the iliac crest to the greater trochanter.24 When crossing the iliac crest, these nerves run parallel to one another with those from lower levels lying most medial.
Variations occur in the regularity with which branches of the L1, L2 and L3 dorsal rami become cutaneous.25,26 In embryos and fetuses, the L1 lateral branch always becomes cutaneous, the L2 in 90% of cases, the L3 in 70%; the L4 lateral branch reaches the skin in 40%.26 In dissections of adults a similar pattern emerges, except that cutaneous branches from L4 appear to be uncommon.25 Most commonly, only the L1 lateral branch becomes cutaneous. This occurs in some 60% of individuals. Both L1 and L2 become cutaneous in about 27% of cases, and all three levels furnish cutaneous branches in only 13% of cases. Regardless of its segmental origin, the lowest and most medial nerve that crosses the iliac crest does so approximately 7–8 cm from the midline.25
The intermediate branches of the lumbar dorsal rami have only a muscular distribution to the lumbar fibres of the longissimus muscle and within this muscle they form an intersegmental plexus (see Fig. 10.6).22,23 The intermediate branch of the L5 dorsal ramus supplies the lowest fibres of longissimus which arise from the L5 transverse process and attach to the medial aspect of the iliac crest (see Ch. 9).
It is the medial branches that are of paramount clinical relevance because of their distribution to the zygapophysial joints. The medial branches of the L1–L4 dorsal rami run across the top of their respective transverse processes and pierce the dorsal leaf of the intertransverse ligament at the base of the transverse process (see Fig. 4.7). Each nerve then runs along bone at the junction of the root of the transverse process with the root of the superior articular process (see Fig. 10.6). Hooking medially around the base of the superior articular process, each nerve is covered by the mamillo-accessory ligament (see Ch. 4). Finally, it crosses the vertebral lamina, where it divides into multiple branches that supply the multifidus muscle, the interspinous muscle and ligament, and two zygapophysial joints.
Each medial branch supplies the zygapophysial joints above and below its course (see Fig. 10.6).22,23,27–30 An ascending articular branch arises from the nerve just beyond the mamillo-accessory ligament where the nerve starts to cross the lamina. A descending articular branch arises slightly more distally and courses downwards to the joint below.
The muscular distribution of the medial branches of the lumbar dorsal rami is very specific. Each medial branch supplies only those muscles that arise from the lamina and spinous process of the vertebra with the same segmental number as the nerve.23,31 Thus, for example, the L1 medial branch supplies only those fibres from the L1 vertebra; the L2 nerve supplies only those muscles from the L2 vertebra, and so on. This relationship can be stated more formally as follows:
The same applies for the interspinous ligaments. This relationship indicates that the principal muscles that move a particular segment are innervated by the nerve of that segment (see Ch. 9).
Histology
Histological studies have shown that capsules of the lumbar zygapophysial joints are richly innervated with encapsulated, unencapsulated and free nerve endings.27,32,33 These joints are therefore endowed with the appropriate sensory apparatus to transmit proprioceptive and nociceptive information. Modern studies have ventured to characterise the nerve fibres in the zygapophysial joints according to their transmitter substance but this has yielded curious results. Nerves containing substance P and calcitonin gene-related peptide (CGRP) were encountered in very few specimens but nerves containing neuropeptide Y were often encountered.34 This suggests either that the majority of nerves in the zygapophysial joints are sympathetic efferent fibres and not sensory fibres, or that technical problems still impede obtaining accurate profiles of neuropeptides in human material obtained at operation.
Nerve fibres and nerve endings also occur in the subchondral bone of the zygapophysial joints. They occur in erosion channels extending from the subchondral bone to the articular cartilage.35 Such fibres might provide a pathway for nociception from these joints other than from their capsules.
Nerve fibres are distributed to the intra-articular inclusions of the zygapophysial joints.36–38 These fibres contain substance P,37,39 but it remains contentious whether these nerves are nociceptive37 or predominantly vasoregulatory.39
Nerve fibres are plentiful in the interspinous ligaments,40–43 where they give rise to Ruffini endings, paciniform endings and free nerve endings.43 The Ruffini endings are sparse towards the centre of the ligament but more numerous towards its lateral surfaces.40 These endings are mechanoreceptors and probably convey proprioceptive information from the ligament. Paciniform endings are uniformly distributed across the ligament but appear to be associated with blood vessels.40 This intriguing juxtaposition requires an explanation for the function of the paciniform endings. Free nerve endings are located near the attachment of the ligament to the spinous processes.43
The supraspinous ligaments and adjacent thoracolumbar fascia are well innervated and contain nerve fibres, Ruffini endings and paciniform endings.41,43,44 The ligamentum flavum appears to be sparsely innervated. Some studies have found no nerves,34 or only a few nerves,41 in this ligament. Others have found nerve endings only in the outermost layers of the dorsal surface of the ligament.42
Variations
Variations have been reported in the number and nature of branches of the lumbar dorsal rami that innervate the lumbar zygapophysial joints. Lazorthes and Juskiewenski28 reported that, occasionally, an articular branch may arise from the dorsal ramus proper and innervate the ventral aspect of the adjacent joint. A similar branch was described by Auteroche,45 who also described multiple articular branches arising from the spinal nerve, the lateral branch of the dorsal ramus, and from the entire length of the medial branch. Such a plethora of articular nerves has not been observed in any other study.22,23,28–30 The study by Auteroche was based solely on dissection using magnifying glasses; the nature of the putative articular branches was not confirmed histologically. Under such conditions it is possible to mistake collagen fibres for articular nerves. Studies using a dissecting microscope and histological corroboration do not support his generous description of articular branches. Similarly, ascending articular branches from the root of the medial branch, as described by Paris,46 have not been confirmed histologically nor have they been seen in previous studies,21,22,27–29 and indeed they have been explicitly denied in subsequent studies.47
Ventral rami
The ventral rami of the lumbar spinal nerves emerge from the intervertebral foramen by piercing the ventral leaf of the intertransverse ligament (see Ch. 4). Therefore, they enter the space in front of the ligaments and lie within the substance of the psoas major muscle. Within the muscle, they enter into the formation of plexuses. The L1 to L4 ventral rami form the lumbar plexus, and the L4 and L5 ventral rami join to form the lumbosacral trunk, which enters the lumbosacral plexus. Because these plexuses are not particularly relevant to the pathology or physiology of lumbar spinal disorders, their anatomy will not be further explored. They are adequately described in other textbooks of anatomy.48
The one exception to this exclusion relates to the course of the L5 ventral ramus. This nerve crosses the ala of the sacrum, below the L5 transverse process, and in this location can be trapped between these two bones. This phenomenon has been called the ‘far out syndrome’ and is described fully elsewhere.49
Dermatomes
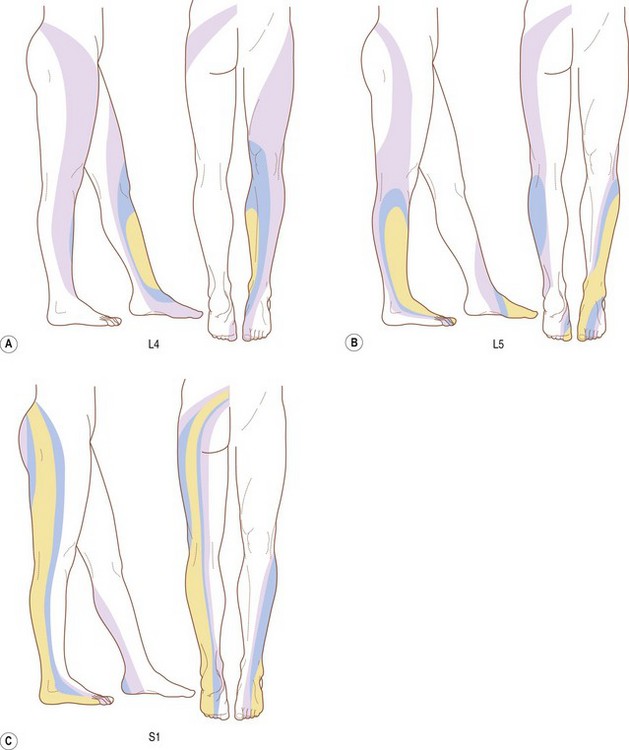
The dermatomes of the L4, L5 and S1 spinal nerves vary from individual to individual with respect to their total extent but nonetheless exhibit a consistent concentric pattern between individuals.50 Each dermatome can extend from the posterior midline of the back, across the buttock and into the lower limb (Fig. 10.7). However, only a minority of individuals exhibits such an extensive distribution for L4 and L5. For L4, the majority of individuals exhibit an area centred on the medial aspect of the lower leg; for L5 the central area extends from the medial aspect of the foot, across the dorsum of the foot, and onto the lateral aspect of the lower leg (Fig. 10.7A,B). A more extensive distribution is characteristic for S1. Its area extends as a band from the posterior sacrum, along the entire length of the lower limb posteriorly to the lateral aspect of the foot (Fig. 10.7C).
These latter figures are inconsistent with traditional and contemporary anatomical data, which acknowledge a cutaneous distribution of the S1 dorsal ramus but deny such a distribution for L5. A 40% incidence of a cutaneous branch from L4 is consistent with embryological data26 but not with dissection data.25 The presence of a cutaneous distribution of L5 is inconsistent with both embryological and dissection data.
The results of nerve blocks indicate that traditional anatomical wisdom may need to be reappraised. Overtly, some 40% of individuals have either an L4 or L5 dorsal cutaneous branch, or both. A distribution from L5 might be expected from its communication with the dorsal sacral plexus,26 but how branches of the L4 dorsal ramus get to the skin remains a mystery. Nerve block data, however, stipulate that they do, in 40% of individuals.
Sympathetic nerves
The lumbar sympathetic trunks descend through the lumbar region along the anterolateral borders of the lumbar vertebral column. Each trunk is applied to the vertebral column next to the medial edge of the attachment of the psoas major muscle. The number of ganglia on the trunks varies from one to six,51 but most commonly four are present.52
Branches of the lumbar sympathetic trunks are distributed to abdominal and pelvic blood vessels and viscera, and some direct branches pass into the psoas major muscle,52 but the principal branches are the rami communicantes to the lumbar ventral rami. White rami communicantes are distributed to the L1 and L2 ventral rami, and grey rami communicantes are distributed to every lumbar ventral ramus. The number of rami communicantes to each lumbar nerve varies from one to three, and exceptionally may be as high as five.52
In general, the rami communicantes reach the ventral rami by passing through the tunnels deep to the psoas muscle that lie along the concave lateral surfaces of the lumbar vertebral bodies (see Ch. 9). These tunnels direct them to the lower borders of the transverse processes where the rami communicantes join the ventral rami just outside the intervertebral foramina. Rami communicantes may also reach the ventral rami by penetrating the substance of psoas.52,53
Sinuvertebral nerves
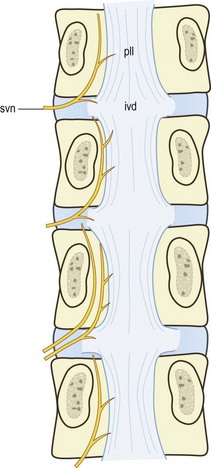
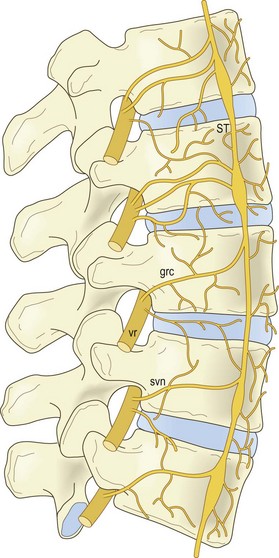
The sinuvertebral nerves are recurrent branches of the ventral rami that re-enter the intervertebral foramina to be distributed within the vertebral canal.27,28,53–56 They are mixed nerves, each being formed by a somatic root from a ventral ramus and an autonomic root from a grey ramus communicans. Although traditionally portrayed as a single nerve, the sinuvertebral nerve may be represented by a series of filaments that pass through the intervertebral foramen, or by an identifiable single trunk accompanied by additional fine filaments.57 The filamentous sinuvertebral nerves may not be evident to the naked eye or even under a dissecting microscope.
In the intervertebral foramina the lumbar sinuvertebral nerves run across the back of the vertebral body, just below the upper pedicle (Fig. 10.8). Within the vertebral canal, each nerve forms an ascending branch which passes rostrally, parallel to the posterior longitudinal ligament, to which it sends branches, and ends in the next higher intervertebral disc, which it also supplies. A shorter descending branch ramifies in the disc and ligament at the level of entry of the parent nerve (see Fig. 10.8).
In addition to this skeletal distribution, each lumbar sinuvertebral nerve is distributed to the blood vessels of the vertebral canal and to the ventral aspect of the dura mater. In the dura mater each sinuvertebral nerve forms ascending and descending meningeal branches.54,58 The descending branches are the longer, extending up to two segments caudally, while the ascending branch ascends up to one segment.54 The dura mater is in fact covered with a dense plexus of nerves on its ventral surface.59 This plexus extends around the lateral aspect of the dural sac but attenuates dorsally. The paramedian portion of the dorsal aspect of the dural sac is distinctly devoid of nerve fibres.58,59
Innervation of the lumbar intervertebral discs
Whether or not the lumbar intervertebral discs receive an innervation has long been a controversial issue. Early studies failed to demonstrate nerve fibres or nerve endings within the discs,56,60,61 and the results of these studies have been used to promulgate the conclusion that the lumbar discs lack an innervation.62–64 However, other studies identified nerve fibres in the superficial layers of the anulus fibrosus,32,33,65,66 and in a painstaking study, Malinsky67 demonstrated a variety of free and complex endings in the outer third of the anulus. Malinsky’s findings have been confirmed in studies by Rabischong et al.68 and by Yoshizawa et al.69 The latter workers studied specimens of intervertebral discs removed at operation for anterior and posterior lumbar interbody fusion. They found abundant nerve endings with various morphologies throughout the outer half of the anulus fibrosus.
Histology
In the prenatal period, nerves are abundant in the anulus fibrosus, where they form simple free endings, and they increase in number in older fetuses.67 During the postnatal period, various types of unencapsulated receptors emerge, and in adult material five types of nerve terminations can be found: simple and complex free nerve endings; ‘shrubby’ receptors; others that form loops and mesh-like formations; and clusters of parallel free nerve endings.67 On the surface of the anulus fibrosus, various types of encapsulated and complex unencapsulated receptors occur. They are all relatively simple in structure in neonates, but more elaborate forms occur in older and mature specimens.
Within a given disc, receptors are not uniformly distributed. The greatest number of endings occurs in the lateral region of the disc, and nearly all the encapsulated receptors are located in this region.67 Following postnatal development, there is a relative decrease in the number of receptors in the anterior region, such that in adults the greatest number of endings occurs in the lateral regions of the disc, a smaller number in the posterior region, and the least number anteriorly.
The varieties of nerve endings found in adult discs include free terminals, often ending in club-like or bulbous expansions or complex sprays, and, less commonly, terminals forming convoluted tangles or glomerular formations that were occasionally demarcated by a ‘capsule-like’ condensation of adjacent tissue.67–69 Modern immunohistochemical techniques have revealed endings resembling Golgi tendon organs, Ruffini endings and paciniform endings in the outer lamellae of the anulus fibrosus that contain CGRP, substance P and vasoactive intestinal polypeptide.70 Nerve endings are also frequent in the anterior and posterior longitudinal ligaments,32,61,66 many of which contain substance P.71 In the anulus fibrosus, the substance P neurones are distributed with blood vessels that express NK1 receptors.72 This suggests a vasoactive role for substance P in the disc.
Sources
The sources of the nerve endings in the lumbar discs are two extensive microscopic plexuses of nerves that accompany the anterior and posterior longitudinal ligaments. These plexuses cannot be discerned by dissection but are evident in whole mounts of human fetuses stained for acetylcholinesterase.57
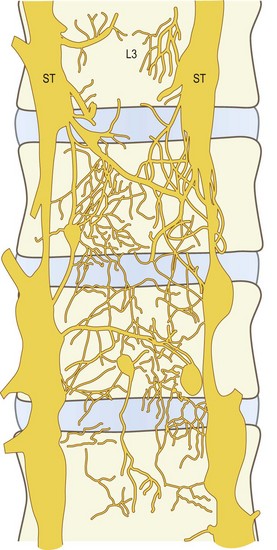
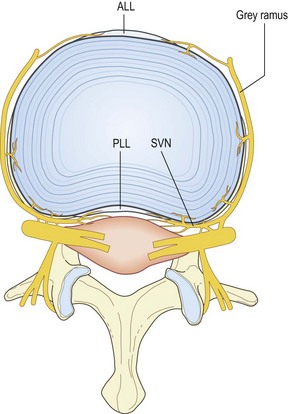
The anterior plexus bridges the two lumbar sympathetic trunks and covers the anterior longitudinal ligament (Fig. 10.9). It is formed by branches of the sympathetic trunks and branches from the proximal ends of the grey rami communicantes. The posterior plexus is derived from the sinuvertebral nerves and accompanies the posterior longitudinal ligament (Fig. 10.10). Within the posterior plexus, the sinuvertebral nerves constitute the largest and most visible elements but they are not the only components; the majority of fibres are microscopic. The anterior and posterior plexuses are connected around the lateral aspects of the vertebral bodies and discs by way of a less pronounced lateral plexus that is formed by branches of the grey rami communicantes (Fig. 10.11).
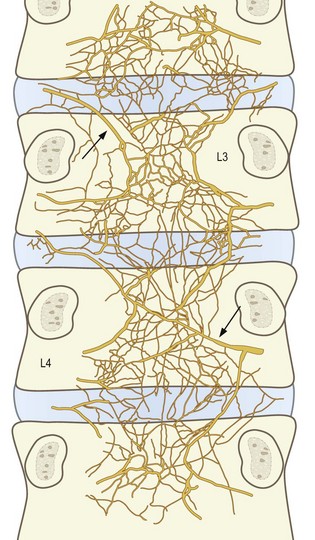
Figure 10.10 The nerve plexus accompanying the posterior longitudinal ligament at the levels of L3 and lower vertebrae, as seen in whole mounts of human fetuses. (Based on Groen et al. 1990.57) The large fibres arrowed represent what would be found, on dissection, to be the sinuvertebral nerves.
The anterior and posterior plexuses supply superficial branches that innervate the periosteum of the vertebral bodies, and long penetrating branches that enter the intervertebral discs and vertebral bodies, the latter following blood vessels as far as the centre of the bone. Through these branches the vertebral bodies and intervertebral discs are innervated around their entire circumference (Figs. 10.12, 10.13).
The discovery of the anterior and posterior plexuses explains and corrects certain previous descriptions of the source of nerves to the lumbar discs. It had previously been established, by dissection, that direct branches of the ventral rami enter the posterolateral corner of the discs.53,73 It now appears that these are not isolated, special ‘disc branches’. Rather they represent one of the several sources that contribute to the plexus that overlies the discs and vertebral bodies laterally. The other sources are the grey rami communicantes, which send branches to the discs across their lateral surface and at their posterolateral corner (see Fig. 10.11).
The fact that the lumbar intervertebral discs and their adjacent ligaments are innervated by branches of the sympathetic nervous system does not necessarily mean that afferent fibres from these structures return to the central nervous system via the sympathetic trunk. Rather, it has been suggested that somatic afferent fibres from the discs and ligaments simply use the course of the rami communicantes to return to the ventral rami.53
Within the vertebral body, nerve fibres consistently accompany the basivertebral veins and arteries, and ramify throughout the spongiosa along with the vessels.74 These nerves extend to the vertebral endplates. Nerve endings are located on the endosteal surface of the endplate and underneath the cartilaginous endplate.75 These latter endings endow the disc with an innervation additional to that in the anulus fibrosus.
Endplate innervation appears to be greater in discs that are painful, ostensibly because of a greater vascular supply.75 However, the nerve endings contain substance P and CGRP, and they are often not related to blood vessels.75 Both of these features indicate that they are sensory nerves not vasomotor nerves.
The presence of nerve endings in the lumbar intervertebral discs raises the question as to their function. Any free endings associated with blood vessels in the disc may reasonably be ascribed a vasomotor or vasosensory function53,67 but because the anulus fibrosus contains so few blood vessels (see Ch. 11) this is unlikely to be the function for the majority of the nerve fibres in the anulus fibrosus. For the encapsulated receptors on the surface of the disc, Malinsky67 postulated a proprioceptive function. Theoretically, this would be a valid, useful role for these receptors but the only study that has addressed this contention failed to find any evidence in its favour.76 However, this study was performed on cats, which are not a suitable model, for the cat is a quadrupedal animal whose vertebral column is not used for weight-bearing and may not be endowed with receptors and reflexes that would be appropriate for an upright vertebral column. Therefore, a proprioceptive role for the intervertebral disc has not been excluded.
In other tissues of the body, isolated free nerve endings are ascribed a nociceptive function, and it is presumably the case that they play a similar role in the lumbar intervertebral discs. Although there is no explicit evidence that disc pain can be ascribed to a particular type of nerve ending in the disc, there is abundant evidence that the disc can be painful. The issue of disc pain is addressed in Chapter 15.
Nerve ingrowth
Several studies have now shown that this pattern of innervation differs in certain discs. In discs shown to be painful by discography (see Ch. 15), and removed at operation, nerve fibers have been found in the deeper anulus and into the nucleus pulposus.77–79
The investigators referred to these as degenerated but it is probably more accurate to refer to them as damaged. The distinguishing feature of these discs was not that they were simply degenerated, in the sense that they were old (see Ch. 14). Rather they were discs that were painful, and sufficiently so, as to warrant surgical excision. Moreover, one study78 compared discs from the same patients: the symptomatic one and an adjacent painless one. Nerve ingrowth was far more common in the painful disc even though the control disc was the same age.
Damage, rather than degeneration, also explains the origin of the nerves. It appears that they accompany blood vessels that grow in along fissures through the anulus fibrosus.77–79 Fissuring, therefore, is a trigger for neovascularisation and neo-innervation of the disc. Fissuring is also the cardinal characteristic of internal disc disruption, which is an acquired traumatic disorder (see Ch. 15).
Summary
The lumbar spine receives an extensive innervation (see Fig. 10.13). Posteriorly, the branches of the lumbar dorsal rami are distributed to the back muscles and the zygapophysial joints. Anteriorly, the ventral rami supply the psoas major and quadratus lumborum. The vertebral bodies and intervertebral discs are surrounded by extensive plexuses of nerves that accompany the longitudinal ligaments and which are derived from the lumbar sympathetic trunks. Within the posterior plexus, larger filaments constitute the sinuvertebral nerves. Short branches innervate the vertebral periosteum, and long penetrating branches enter the vertebral body from all aspects of its circumference. Nerves enter the outer third of the anulus fibrosus from the longitudinal plexuses anteriorly, laterally and posteriorly. The posterior plexus innervates the dura mater and nerve root sleeves along their anterior and lateral aspects.
1 Louis R. Topographic relationships of the vertebral column, spinal cord, and nerve roots. Anat Clin. 1978;1:3-12.
2 Bouchard JM, Copty M, Langelier R. Preoperative diagnosis of conjoined roots anomaly with herniated lumbar disks. Surg Neurol. 1978;10:229-231.
3 Bose K, Balasubramaniam P. Nerve root canals of the lumbar spine. Spine. 1984;9:16-18.
4 Parkin IG, Harrison GR. The topographical anatomy of the lumbar epidural space. J Anat. 1985;141:211-217.
5 Wiltse LL, Fonseca AS, Amster J, et al. Relationship of the dura, Hofmann’s ligaments, Batson’s plexus, and a fibrovascular membrane lying on the posterior surface of the vertebral bodies and attaching to the deep layer of the posterior longitudinal ligament; an anatomical, radiologic, and clinical study. Spine. 1993;18:1030-1043.
6 Scapinelli R. Anatomical and radiologic studies on the lumbosacral meningovertebral ligaments of humans. J Spinal Disord. 1990;3:6-15.
7 Wadhwani S, Loughenbury P, Soames R. The anterior dural (Hofmann) ligaments. Spine. 2004;29:623-627.
8 Spencer DL, Irwin GS, Miller JAA. Anatomy and significance of fixation of the lumbosacral nerve roots in sciatica. Spine. 1983;8:672-679.
9 Peretti F, Micalef JP, Bourgeon A, et al. Biomechanics of the lumbar spinal nerve roots and the first sacral root within the intervertebral foramina. Surg Radiol Anat. 1989;11:221-225.
10 Golub BS, Silverman B. Transforaminal ligaments of the lumbar spine. J Bone Joint Surg. 1969;51A:947-956.
11 Hasue M, Kikuchi S, Sakuyama Y, et al. Anatomic study of the interrelation between lumbosacral nerve roots and their surrounding tissues. Spine. 1983;8:50-58.
12 Cannon BW, Hunter SE, Picaza JA. Nerve root anomalies in lumbar disc surgery. J Neurosurg. 1962;19:208-214.
13 Ethelberg S, Rishede J. Malformation of lumbar spinal nerve roots and sheaths in the causation of low backache and sciatica. J Bone Joint Surg. 1952;34B:442-446.
14 Keon-Cohen B. Abnormal arrangements of the lower lumbar and first sacral nerve roots within the spinal canal. J Bone Joint Surg. 1968;50B:261-266.
15 McElverry RT. Anomalies of the lumbar spinal cord and roots. Clin Orthop. 1956;8:61-64.
16 Neidre A, MacNab I. Anomalies of the lumbosacral nerve roots. Spine. 1983;8:294-299.
17 Postacchini F, Urso S, Ferro L. Lumbosacral nerve-root anomalies. J Bone Joint Surg. 1982;64A:721-729.
18 Rask MR. Anomalous lumbosacral nerve roots associated with spondylolisthesis. Surg Neurol. 1977;8:139-140.
19 Hasner E, Schalintzek M, Snorrason E. Roentgenological examination of the function of the lumbar spine. Acta Radiol. 1952;37:141-149.
20 D’Avella D, Mingrino S. Microsurgical anatomy of lumbosacral spinal roots. J Neurosurg. 1979;51:819-823.
21 Pallie W. The intersegmental anastomoses of posterior spinal rootlets and their significance. J Neurosurg. 1959;16:187-196.
22 Bradley KC. The anatomy of backache. Aust NZ J Surg. 1974;44:227-232.
23 Bogduk N, Wilson AS, Tynan W. The human lumbar dorsal rami. J Anat. 1982;134:383-397.
24 Johnston HM. The cutaneous branches of the posterior primary divisions of the spinal nerves, and their distribution in the skin. J Anat Physiol. 1908;43:80-91.
25 Maigne JY, Lazareth JP, Surville HG, et al. The lateral cutaneous branches of the dorsal rami of the thoracolumbar junction. Surg Radiol Anat. 1989;11:289-293.
26 Pearson AA, Sauter RW, Buckley TF. Further observations on the cutaneous branches of the dorsal rami of the spinal nerves. Am J Anat. 1966;119:891-904.
27 Bogduk N. The innervation of the lumbar spine. Spine. 1983;8:286-293.
28 Lazorthes G, Juskiewenski S. Etude comparative des branches postérieures des nerfs dorsaux et lombaires et leurs rapports avec les articulations interapophysaires vertébrales. Bulletin de l’Association des Anatomistes, 49e Reunion. 1964:1025-1033.
29 Lewin T, Moffet B, Viidik A. The morphology of the lumbar synovial intervertebral joints. Acta Morphol Neerlando-Scand. 1962;4:299-319.
30 Pedersen HE, Blunck CFJ, Gardner E. The anatomy of lumbosacral posterior rami and meningeal branches of spinal nerves (sinuvertebral nerves): with an experimental study of their function. J Bone Joint Surg. 1956;38A:377-391.
31 Macintosh JE, Valencia F, Bogduk N, et al. The morphology of the lumbar multifidus muscles. Clin Biomech. 1986;1:196-204.
32 Hirsch C, Ingelmark BE, Miller M. The anatomical basis for low back pain. Acta Orthop Scand. 1963;33:1-17.
33 Jackson HC, Winkelmann RK, Bickel WH. Nerve endings in the human lumbar spinal column and related structures. J Bone Joint Surg. 1966;48A:1272-1281.
34 Ashton IK, Ashton BA, Gibson SJ, et al. Morphological basis for back pain: the demonstration of nerve fibres and neuropeptides in the lumbar facet joint capsule but not in ligamentum flavum. J Orthop Res. 1992;10:72-78.
35 Beaman DN, Graziano GP, Glover RA, et al. Substance P innervation of lumbar spine facet joints. Spine. 1993;18:1044-1049.
36 Giles LGF. Human lumbar zygapophyseal joint inferior recess synovial folds: a light microscope examination. Anat Rec. 1988;220:117-124.
37 Giles LGF, Harvey AR. Immunohistochemical demonstration of nociceptors in the capsule and synovial folds of human zygapophyseal joints. Br J Rheumatol. 1987;26:362-364.
38 Giles LGF, Taylor JR. Innervation of lumbar zygapophyseal joint synovial folds. Acta Orthop Scand. 1987;58:43-46.
39 Gronblad M, Korkola O, Konttinen Y, et al. Silver impregnation and immunohistochemical study of nerves in lumbar facet joint plical tissue. Spine. 1991;16:34-38.
40 Jiang H, Russell G, Raso J, et al. The nature and distribution of the innervation of human supraspinal and interspinal ligaments. Spine. 1995;20:869-876.
41 Rhalmi S, Yahia L, Newman N, et al. Immunohistochemical study of nerves in lumbar spine ligaments. Spine. 1993;18:264-267.
42 Yahia LH, Newman NA. A light and electron microscopic study of spinal ligament innervation. Z mikroskop anat Forsch Leipzig. 1989;103:664-674.
43 Yahia LH, Newman N, Rivard CH. Neurohistology of lumbar spine ligaments. Acta Orthop Scand. 1988;59:508-512.
44 Yahia LH, Rhalmi S, Isler M. Sensory innervation of human thoracolumbar fascia: an immunohistochemical study. Acta Orthop Scand. 1992;63:195-197.
45 Auteroche P. Innervation of the zygapophyseal joints of the lumbar spine. Anat Clin. 1983;5:17-28.
46 Paris SV. Anatomy as related to function and pain. Orthop Clin North Am. 1983;14:475-489.
47 Lynch MC, Taylor JF. Facet joint injection for low back pain. J Bone Joint Surg. 1986;68B:138-141.
48 Williams PL, editor. Gray’s Anatomy, 38th ed, Edinburgh: Churchill Livingstone, 1995.
49 Wiltse LL, Guyer RD, Spencer CW, et al. Alar transverse process impingement of the L5 spinal nerve: the far-out syndrome. Spine. 1984;9:31-41.
50 Nitta H, Tajima T, Sugiyama H, et al. Study on dermatomes by means of selective lumbar spinal nerve block. Spine. 1993;18:1782-1786.
51 Bradley KC. Observations on the surgical anatomy of the thoracolumbar sympathetic system. Aust NZ J Surg. 1951;20:171-177.
52 Hovelacque A. Anatomie des Nerfs Craniens et Rachidiens et du Système Grande Sympathique. Paris: Doin; 1927.
53 Bogduk N, Tynan W, Wilson AS. The nerve supply to the human lumbar intervertebral discs. J Anat. 1981;132:39-56.
54 Kimmel DL. Innervation of spinal dura mater and dura mater of the posterior cranial fossa. Neurology. 1960;10:800-809.
55 Lazorthes G, Poulhes J, Espagno J. Etude sur les nerfs sinu-vertébraux lombaires. Le nerf de Roofe, existe-t-il? Comptes Rendus de l’Association des Anatomistes. 1947;34:317-320.
56 Wiberg G. Back pain in relation to the nerve supply of the intervertebral disc. Acta Orthop Scand. 1947;19:211-221.
57 Groen G, Baljet B, Drukker J. The nerves and nerve plexuses of the human vertebral column. Am J Anat. 1990;188:282-296.
58 Edgar MA, Nundy S. Innervation of the spinal dura mater. J Neurol Neurosurg Psychiatry. 1964;29:530-534.
59 Groen G, Baljet B, Drukker J. The innervation of the spinal dura mater: anatomy and clinical implications. Acta Neurochir. 1988;92:39-46.
60 Ikari C. A study of the mechanism of low-back pain. The neurohistological examination of the disease. J Bone Joint Surg. 1954;36A:195.
61 Jung A, Brunschwig A. Recherches histologiques des articulations des corps vertébraux. Presse Med. 1932;40:316-317.
62 Anderson J. Pathogenesis of back pain. In: Grahame R, Anderson JAD, editors. Low Back Pain, vol. 2. Westmount: Eden Press; 1980:23-32. Ch. 4
63 Lamb DW. The neurology of spinal pain. Phys Ther. 1979;59:971-973.
64 Wyke B. The neurology of low back pain. In: Jayson MIV, editor. The Lumbar Spine and Back Pain. 2nd ed. Tunbridge Wells: Pitman; 1980:265-339. Ch. 11
65 Ehrenhaft JC. Development of the vertebral column as related to certain congenital and pathological changes. Surg Gynecol Obst. 1943;76:282-292.
66 Roofe PG. Innervation of annulus fibrosus and posterior longitudinal ligament. Arch Neurol Psychiatry. 1940;44:100-103.
67 Malinsky J. The ontogenetic development of nerve terminations in the intervertebral discs of man. Acta Anat. 1959;38:96-113.
68 Rabischong P, Louis R, Vignaud J, et al. The intervertebral disc. Anat Clin. 1978;1:55-64.
69 Yoshizawa H, O’Brien JP, Thomas-Smith W, et al. The neuropathology of intervertebral discs removed for low-back pain. J Path. 1980;132:95-104.
70 Roberts S, Eisenstein SM, Menage J, et al. Mechanoreceptors in intervertebral discs: morphology, distribution, and neuropeptides. Spine. 1995;20:2645-2651.
71 Korkala O, Gronblad M, Liesi P, et al. Immunohistochemical demonstration of nociceptors in the ligamentous structures of the lumbar spine. Spine. 1985;10:156-157.
72 Ashton IK, Walsh DA, Polak JM, et al. Substance P in intervertebral discs: binding sites on vascular endothelium of the human annulus fibrosus. Acta Orthop Scand. 1994;65:635-639.
73 Taylor JR, Twomey LT. Innervation of lumbar intervertebral discs. Med J Aust. 1979;2:701-702.
74 Antonacci MD, Mody DR, Heggeness MH. Innervation of the human vertebral body: a histologic study. J Spinal Dis. 1998;11:526-531.
75 Brown MF, Hukkanen MVJ, McCarthy ID, et al. Sensory and sympathetic innervation of the vertebral endplate in patients with degenerative disc disease. J Bone Joint Surg. 1997;79B:147-153.
76 Kumar S, Davis PR. Lumbar vertebral innervation and intra-abdominal pressure. J Anat. 1973;114:47-53.
77 Coppes MH, Marani E, Thomeer RTWM, et al. Innervation of ‘painful’ lumbar discs. Spine. 1997;22:2342-2350.
78 Freemont AJ, Peacock TE, Goupille P, et al. Nerve ingrowth into diseased intervertebral disc in chronic back pain. Lancet. 1997;350:178-181.
79 Johnson WEB, Evans H, Menage J, et al. Immunohistochemical detection of Schwann cells in innervated and vascularised human intervertebral discs. Spine. 2001;26:2550-2557.