Chapter 13 Nerve Root, Sacral, and Pelvic Stimulation
 Stimulation of sacral nerve roots from within the spinal canal is an important technique to consider for the treatment of many forms of chronic pelvic pain, including epididymoorchialgia, vulvodynia, and interstitial cystitis; urinary control problems; and other forms of pelvic floor dysfunction and bilateral foot pain.
Stimulation of sacral nerve roots from within the spinal canal is an important technique to consider for the treatment of many forms of chronic pelvic pain, including epididymoorchialgia, vulvodynia, and interstitial cystitis; urinary control problems; and other forms of pelvic floor dysfunction and bilateral foot pain. A transforaminal placement from a cranial to caudal orientation is often the best way to obtain the stimulation pattern desired with a low migration rate.
A transforaminal placement from a cranial to caudal orientation is often the best way to obtain the stimulation pattern desired with a low migration rate. A “laterograde” approach to gain cephalad access is easier to perform and improves the learning curve.
A “laterograde” approach to gain cephalad access is easier to perform and improves the learning curve. The more lateral the entry site, the more shallow is the angle of the needle to the dura, and the easier the access.
The more lateral the entry site, the more shallow is the angle of the needle to the dura, and the easier the access. The needle is perpendicular to the skin in craniocaudal orientation—the entire angle is mediolateral.
The needle is perpendicular to the skin in craniocaudal orientation—the entire angle is mediolateral. When placing bilateral electrodes, allow the needle tips to nearly “kiss” in the midline, then turn the bevels caudally to pass the electrodes.
When placing bilateral electrodes, allow the needle tips to nearly “kiss” in the midline, then turn the bevels caudally to pass the electrodes. For transforaminal placement keep the electrode in the midline until the last minute; then roll laterally into the foramen only about a vertebral level from target.
For transforaminal placement keep the electrode in the midline until the last minute; then roll laterally into the foramen only about a vertebral level from target. In patients with prior lumbar surgery, the epidural space may be obliterated. Get appropriate imaging.
In patients with prior lumbar surgery, the epidural space may be obliterated. Get appropriate imaging. Many practitioners are less familiar with sacral radiographs. Make use of lateral films to verify level.
Many practitioners are less familiar with sacral radiographs. Make use of lateral films to verify level.Introduction
Brindley1 performed the first implantation of a sacral anterior root stimulator in a patient with multiple sclerosis who suffered from impaired bladder emptying and incontinence in 1976. Since then, sacral neuromodulation has evolved rapidly, with the development of several different anatomic approaches and Food and Drug Administration (FDA) approval of a specific device in 1997 for the treatment of urge incontinence and frequency-urgency syndrome and nonobstructive urinary retention in 1999.2–6 Sacral neuromodulation has also been shown to be efficacious in the treatment of chronic pelvic pain syndromes such as interstitial cystitis (IC), vulvodynia, prostadynia/epididymoorchialgia, sacroiliac pain, and coccygodynia.
Establishing the Diagnosis
Interstitial Cystitis
Interstitial cystitis (IC) is a chronic, often debilitating condition with symptoms of urinary urgency and frequency associated with suprapubic or pelvic pain with bladder filling in the absence of urinary tract infection (UTI) or other obvious pathology.7–12 The diagnosis of IC can be made in patients with characteristic cystoscopic findings of glomerulation or Hunner ulcers (10%) along with clinical findings.12,13 The histological findings are consistent with neurogenic inflammation.13 The prevalence varies across studies least in part because of differing diagnostic criteria, but it is in the range of 45 to 197 per 100,000 women and 8 to 41 per 100,000 men14,15 The pathogenesis and natural history are not completely understood but appear to be multifactorial,12,15 including infection, allergic, immunological, and genetic processes.12,15 The most popular theory is that a sequence of toxic reactions follows damage to the bladder epithelium, resulting in a severe inflammatory reaction that induces neurogenic pain and bladder irritation.9,12,16,17 Other chronic pelvic conditions may mimic this process with minimal differences. The European Society for the Study of Interstitial Cystitis (ESSIC) has proposed a new nomenclature and classification system with the name of painful bladder syndrome.18 Patients without classic cystoscopic or histological findings were included in these criteria.
IC is typically associated with other chronic debilitating conditions such as irritable bowel syndrome (IBS), systemic lupus erythematosus (SLE), migraine, fibromyalgia, asthma, incontinence, or vulvodynia, and is commonly associated with a history of abuse.12,14,19,20 The differential diagnosis includes a variety of similar conditions such as overactive bladder (OAB), chronic pelvic pain (CPP), vulvodynia, UTI, or even endometriosis.15
Vulvodynia
Vulvodynia is a chronic stinging, burning, itching, or irritating pain in the vaginal region without evidence of infectious, inflammatory, or neoplastic causes or any underlying neurologic disorder.21–23 The estimated prevalence is more than 2 million women in the United States.24 This chronic condition has debilitating effects on both physical and psychological aspects of the patient’s life.22 The pathology is not well understood. Hormonal and immunologic factors that stimulate nociceptive nerve endings directly or lead to local inflammatory signals can cause neuropathic pain of this kind.22 Clinical symptoms occur in episodes with irregular intervals. The diagnosis is one of exclusion. Both a gynecologist and urologist should evaluate the patient to make this diagnosis. The mainstays of therapy are dietary changes, physical therapy, psychological and sexual counseling, and oral or local medications under the guidance of pain specialists.22,23,25
Chronic Testicular Pain
Chronic testicular pain is pain in the scrotal or testicular area lasting for more than 3 months and interfering with daily activities and quality of life. It is also called chronic orchialgia, orchiodynia or chronic scrotal pain syndrome.26,27 It typically occurs in patients with infectious, inflammatory, vascular, postsurgical, or neoplastic conditions. Detailed examination by a urologist with proper investigations to rule out treatable causes is the primary initial focus in patient evaluation.
Prostadynia
Prostadynia is defined as chronic genital or perineal pain associated with urinary urgency and dysuria. It is seen in 5 of every 10,000 outpatient visits. National Institutes of Health (NIH) criteria differentiate this from CPP syndrome based on the presence or absence of leukocytes in expressed prostatic secretions, postprostatic massage urine, or seminal fluid analysis.13 Some authors suggest that this pathological process in males is similar to IC in females.28
Coccygodynia
Coccygodynia is a painful syndrome limited to the coccyx; it may be aggravated by sitting or standing up. The process is commonly attributed to fractures or soft tissue injuries, but many cases are idiopathic.29 Coccygodynia is extremely challenging to treat. Options include rubber ring cushions, sacrococcygeal rhizotomy, physiotherapy, local injections, coccygectomy, and neuromodulation.13
Basic Science
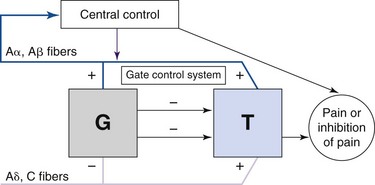
Spinal cord or nerve root neuromodulation for pain was initially explored based on the gate control theory introduced by Melzack and Wall,30 which suggested that activation of large-diameter afferent fibers (Aβ) suppressed pain signals travelling to the brain (Fig. 13-1). Since its adoption for widespread clinical use, it has become clear that for electrical neuromodulation to be successful, the following criteria must be met:
 The anatomical distribution of the paresthesia is primarily determined by the location of the cathode, or negative contact, often referred to as the active contact.
The anatomical distribution of the paresthesia is primarily determined by the location of the cathode, or negative contact, often referred to as the active contact. Alternative positions of the anode, or positive contact, can “pull” the perceived stimulation in a desired direction or “shield” other areas by hyperpolarizing them.
Alternative positions of the anode, or positive contact, can “pull” the perceived stimulation in a desired direction or “shield” other areas by hyperpolarizing them.Shaker and colleagues31 suggested that neuromodulation acts by inhibiting signal transmission to the central nervous system (CNS) via C fibers, but chronic neuropathic pain conditions can also induce new neural activity in second-order neurons in the CNS, shifting the focus of activity.13 Matharu and associates32 showed that successful stimulation may change thalamic metabolism and remodel the intrinsic pain circuits. Thus neuromodulation can be applied to many neuropathic pain conditions.
Pelvic pain syndromes appear to be neuropathic in nature, with characteristics of hyperpathia and allodynia.13 Histological findings in both IC and vulvodynia suggest neurogenic inflammation.33–35 In addition, 40% of women with IC have a history of hysterectomy, suggesting that injury may lead to transient inflammation, which resembles reflex sympathetic dystrophy.13
Imaging
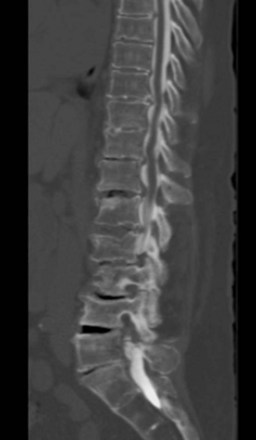
No specific imaging techniques are available that demonstrate the pathological changes that cause neuropathic pain disorders such as CPP and coccygodynia. Imaging is directed at ruling out other causes of the symptoms such as infection or spinal pathology and for delineating anatomical difficulties in applying neuromodulation techniques such as spina bifida, previous surgical sites where the epidural space is obliterated, severe spondylolisthesis, or severe stenosis, which would make placing epidural electrodes more difficult or impossible (Fig. 13-2).
Indications/Contraindications
General Indications
Sacral intraspinal NRS is commonly used for36:
The transforaminal approach is commonly used for36:
Absolute Contraindications
 Any process that obliterates the epidural space is a contraindication to epidural placement over that location (e.g., previous laminectomy at that level). Computed tomography (CT) scan best shows bony removal from previous operation; magnetic resonance imaging (MRI) with gadolinium is the best test to show scar formation.
Any process that obliterates the epidural space is a contraindication to epidural placement over that location (e.g., previous laminectomy at that level). Computed tomography (CT) scan best shows bony removal from previous operation; magnetic resonance imaging (MRI) with gadolinium is the best test to show scar formation.Equipment
In principle nearly any stimulator could be used to deliver sacral NRS. In practice many centers prefer a cephalocaudal intraspinal approach using standard spinal cord stimulators with percutaneous leads.37–39 Two devices have received FDA approval for sacral NRS.
 In 1997 the Medtronic InterStim (Medtronic, Inc., Minneapolis, Minn) (Fig. 13-3) received the Food and Drug Administration (FDA) approval for the treatment of urge incontinence.
In 1997 the Medtronic InterStim (Medtronic, Inc., Minneapolis, Minn) (Fig. 13-3) received the Food and Drug Administration (FDA) approval for the treatment of urge incontinence. In 1999 the FDA approved Medtronic InterStim (Medtronic, Inc., Minneapolis, Minn) for the treatment of urinary retention and urgency-frequency.
In 1999 the FDA approved Medtronic InterStim (Medtronic, Inc., Minneapolis, Minn) for the treatment of urinary retention and urgency-frequency. In 2002 the FDA approved the revised Medtronic InterStim (Medtronic, Inc., Minneapolis, Minn) to include the term overactive bladder.
In 2002 the FDA approved the revised Medtronic InterStim (Medtronic, Inc., Minneapolis, Minn) to include the term overactive bladder. The FDA approved Renew radiofrequency (RF)-powered generator (St. Jude Medical, Inc., Plano, Tex) (Fig. 13-4) in 1999 for the treatment of chronic pain of the trunk and limbs.
The FDA approved Renew radiofrequency (RF)-powered generator (St. Jude Medical, Inc., Plano, Tex) (Fig. 13-4) in 1999 for the treatment of chronic pain of the trunk and limbs.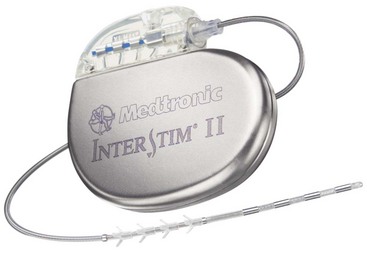
Fig. 13-3 Neurostimulation system for sacral nerve root stimulation.
With permission of Medtronic, Inc. 2010.
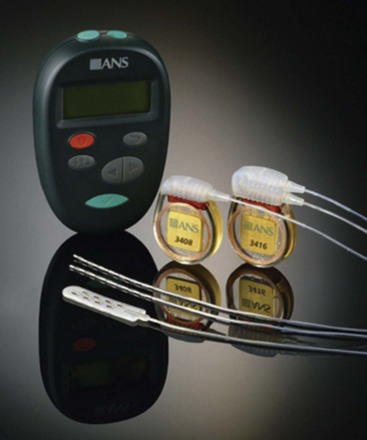
Fig. 13-4 Radio frequency–powered generator with an external power source.
With permission of St. Jude Medical, Inc., Plano, Tex.
 The C-arm portable fluoroscopy machine—used to guide needle placement and electrode steering to the desired final location
The C-arm portable fluoroscopy machine—used to guide needle placement and electrode steering to the desired final location Electrodes, most commonly dual percutaneous quadripolar electrodes (St. Jude Medical, Inc., Plano, Tex)
Electrodes, most commonly dual percutaneous quadripolar electrodes (St. Jude Medical, Inc., Plano, Tex) Subcutaneous tunneller—used to externalize percutaneous trial lead extensions or to connect the lead to the permanent pulse generator
Subcutaneous tunneller—used to externalize percutaneous trial lead extensions or to connect the lead to the permanent pulse generatorTechnique
Intraspinal Nerve Root Stimulation
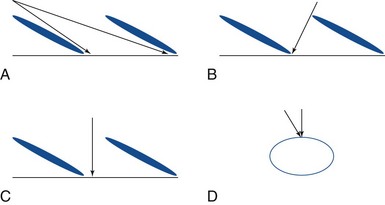
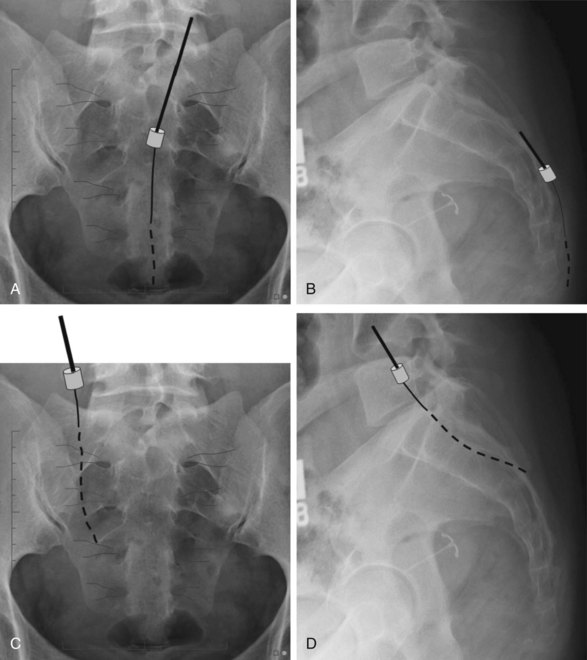
In these techniques the desired roots are stimulated from the intraspinal epidural space. This can be accomplished via an anterograde or retrograde placement. Anterograde placement is usually used for thoracic and cervical roots, with a traditional entry point in the upper lumbar spine, keeping the electrode in the midline as for a traditional dorsal column stimulator; and then rolling laterally to cover the nerve root in the lateral gutter as it approaches the foramen. This approach is not feasible for the lower lumbar or sacral roots, but entry at the sacral hiatus can be used for anterograde access to these lower roots.39 Widespread adoption of this technique has been limited by the extremely caudal area of the incision and the personal hygiene issues that are associated with it. The concern for infection with any contamination of the wound in permanent implant situations is considerable, and it is often difficult to place the connections and anchors in this region in such a way that they are not uncomfortable in a seated position when the permanent system is fully implanted. Selective NRS via the retrograde (aka cephalocaudad) percutaneous placement of electrode is also a well-established technique. It has been described by two techniques. Under fluoroscopic guidance the epidural Tuohy needle can be placed in a paramedian fashion and advanced caudally until it reaches the desired interspace. The shingling of the laminae (Fig. 13-5) can make access to the epidural space quite difficult from this approach; but, if the electrode can be introduced to the epidural space in this fashion, it can be directed retrograde from this point without difficulty. Because of the technical difficulty of this approach and the steep learning curve, several centers have moved to what is often termed the laterograde approach.40,41 In this technique the Tuohy needle begins initial approach to eventual S2-3 placement more laterally at the L2-L3 interspace, with an entry angle nearly perpendicular to the skin (Fig. 13-6). The bevel is then rotated caudally, and the electrode introduced and passed caudally in the midline (Fig. 13-7).42
Retrograde Transforaminal Nerve Root Stimulation
To address limited spinal segment pathology, the transforaminal approach for spinal NRS may be used. This can be done through an open laminectomy or through a minimally invasive technique. The electrode is introduced by a retrograde technique; and, when the foramen of interest is approached, the electrode is rotated laterally and passed to just inside the foramen.42,43
Extraforaminal Nerve Root Stimulation
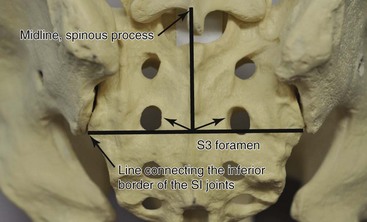
This technique is used primarily for isolated S3 stimulation, but it can be applied with modifications at higher levels when dictated by the presence of significant spinal stenosis. Two landmarks are visualized: the spinous processes and the inferior point of the sacroiliac joints. The S3 foramen is located lateral to the intersection of these two lines (Fig. 13-8). The needle is inserted under the fluoroscopic guidance 1 to 4 cm above the foramen. A small paramedian incision is made over the S3 foramen, and a finder needle is inserted. Next, an Angiocath or introducer sheath is advanced over the finder needle. The beveled end of it should not go beyond the S3 foramen. The finder needle is removed, and the permanent electrode (typically the tined Medtronic InterStim (Medtronic, Inc., Minneapolis, Minn) is inserted through the sheath, which is removed.44 This technique becomes more invasive when surgical exposure is necessary to identify the foramen.36 Retrograde lead placements have a lower rate of lead migration.13
Sacral Nerve Root Stimulation for the Treatment of Coccygodynia or Sacroiliac Joint Pain
To implant the leads over the S4 and S5 roots, a retrograde technique or the approach through the sacral hiatus is required. With a retrograde technique the needle is advanced caudally in the midline until it crosses S3. However, a transforaminal technique is not used, leaving the electrode in the midline caudally. Care must be taken to avoid placing the tip close to the sacral hiatus since undesired stimulation may result in pain (Fig. 13-9).
An alternative technique is to place an electrode in a retrograde fashion over the posterior surface of the sacrum to provide peripheral nerve stimulation as depicted in Fig. 13-10. Generally this technique is used after mapping the pain generators with RF stimulation via a needle probe, confirmed with a stimulation-guided local anesthetic block. For coccygodynia this is treated with placement of quadripolar electrodes where stimulation leads to coccygeal paresthesia. This activates the S4-S5 roots and lateral transverse branches but avoids the potential discomfort of stimulation at the sacral hiatus. An identical technique with treatment of the lateral transverse branches of L5 to S3 is often effective in the treatment of sacroiliac joint pain. This represents a fairly simple and stable approach to sacroiliac joint pain, which is extraordinarily difficult to cover from the epidural space.
Outcome Evidence
Treatment of Interstitial Cystitis
Treatment is tailored to each individual patient. First-line treatment includes diet modifications, oral pentosan polysulfate sodium (PPS), hydroxyzine, amitriptyline, gabapentin, prednisone, and cyclosporine A. Intravesical dimethylsulfoxide (DMSO), cystoscopic bladder hydrodistention, and resection of Hunner ulcers are more aggressive options. Intramural botulinum toxin injections and hyperbaric oxygen have been shown beneficial in some patients.10,15,45 Major reconstructive surgery has been performed as well.12,45
In 1997 the FDA approved the Medtronic InterStim (Medtronic, Inc., Minneapolis, Minn) device for urinary urge incontinence, urinary urgency-frequency, and unobstructive urinary retention.46 Many reports in the literature document the efficacy of neuromodulation in IC.13,38,42,47–52 These studies have shown significant decreases in urinary urgency-frequency and pelvic pain and decreased antiproliferative factor activity. These findings were established in a multicenter trial.53 Improvements in muscular activity are clearly seen in these patients.47 These patients can experience an initial worsening of their typical symptoms with subsequent improvement,47,49 which can be treated effectively with caudal epidural block or prevented by giving the block before stimulator placement.47 It is postulated that, if administered early before the muscularis fibrosis phase, caudal block might prevent or reverse the neurogenic inflammation.47
Other centers have found that S2-S4 stimulation from an intraspinal approach, either unilateral or bilateral,47,54,55 may be more effective than isolated S3 stimulation. Alternatively Peters, Feber, and Bennett46 in a randomized, cross-over trial demonstrated that pudendal nerve stimulation leads to better improvement than S3 root stimulation.
Treatment of Vulvodynia
Because of the excellent results in patients with IC, the treatment of vulvodynia has also been explored. Nair and associates22 reported a patient with vulvodynia treated with bilateral S4 NRS with 90% improvement in symptomatology and overall quality of life.22 Ramsey, Wright, and Fischer23 demonstrated an excellent clinical outcome after S3 NRS in a patient with vulvar vestibulitis. The literature for this indication suffers from variable selection of patients, target nerve roots, stimulation systems, and surgical approaches.13,22,23 Because the disorder is uncommon and difficult to distinguish clearly from other pelvic pain conditions, prospective trials with good outcome scales are very difficult to conduct.
Treatment of Chronic Testicular Pain
Treatment with antibiotics, nonsteroidal antiinflammatory drugs, tricyclic antidepressants, and pudendal nerve blocks is considered first line for pain management.26 More invasive methods such as microsurgical denervation of the spermatic cord, epididymectomy, and vasovasostomy have been advocated as the next level of care.26 When these treatments have failed, the inguinal orchiectomy can be considered. However, sacral nerve stimulation is considered by many to be a less morbid alternative. In a single case McJunkin, Wuollet, and Lynch27 reported 80% improvement after unilateral S2, S3, and S4 NRS. Feler, Whitworth, and Fernandez13 demonstrated 75% improvement with sacral NRS at S2-S4 performed via an anterograde approach.
Results Summary
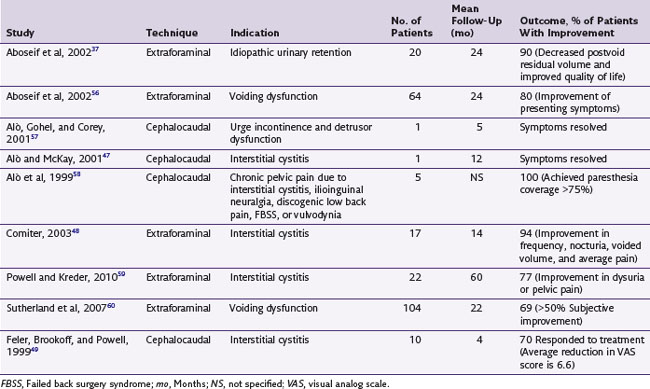
There is a paucity of level I evidence literature on pelvic and sacral NRS for the treatment of CPP and coccygodynia. A review of the literature is summarized in Table 13-1.37,47–49,56–60
Risk and Complication Avoidance
Lead migration, which is a common problem with percutaneous electrodes in general, seems to be somewhat less problematic with intraspinal cephalocaudal placement than with direct extraforaminal techniques, which rely on “tines” on the electrode to prevent backing out (see Fig. 13-3) since they pass directly through a significant amount of muscle tissue just before reaching the target. A loss of coverage that is not associated with extremely high impedance (which would indicate a broken lead) is usually caused by migration of the lead. Careful attention to anchoring and formation of a relaxing loop at the midline incision are the technique points to help prevent this complication.61,62 Particularly with bending and twisting motions of the lower lumbar area, a tension is exerted on the lead or lead extension as it traverses the distance between the entry point to the epidural space and the pulse generator. If this tension is applied repetitively, it can cause lead migration. The mechanics of this process are best avoided by careful attention to the relaxing loop and meticulous attention to anchoring technique. A tension relief loop of at least several centimeters should always be left at the spinal incision. The anchor should attach the lead to the fascia just as it exits the fascia and directly in line with this exit site. When the spine moves in flexion and extension, the distance between the target space and the exit from the fascia changes. When there is a substantial gap between the exit site and the anchor, the electrode may be slightly withdrawn during such movements, but it is quite unlikely to be pushed back through the fascia into position when the movement reverses. This ratcheting phenomenon can lead to electrode migration; but, if the anchor is placed perfectly in line with the exit site and just at the point of fascial exit, these factors are minimized. Some newer anchors have flexible extensions that can be passed through the fascial defect to further minimize this risk.
These devices can generally be used safely but cautiously in conjunction with cardiac pacemakers. When used with a cardiac device, they should generally be programmed in bipolar mode, with a frequency of at least 20 Hz. Using caution, with electrocardiogram monitoring during stimulator programming, is advised.63
1 Brindley GS. History of the sacral anterior root stimulator, 1969-1982. Neurourol Urodyn. 1993;12:481-483.
2 Brindley GS, Polkey CE, Rushton DN. Sacral anterior root stimulators for bladder control in paraplegia. Paraplegia. 1982;20:365-381.
3 Brindley GS, et al. Sacral anterior root stimulators for bladder control in paraplegia: the first 50 cases. J Neurol Neurosurg Psychiatry. 1986;49:1104-1114.
4 Cardozo L, et al. Urodynamic observations on patients with sacral anterior root stimulators. Paraplegia. 1984;22:201-209.
5 Kohli N, Rosenblatt PL. Neuromodulation techniques for the treatment of the overactive bladder. Clin Obstet Gynecol. 2002;45:218-232.
6 Sauerwein D, et al. Extradural implantation of sacral anterior root stimulators. J Neurol Neurosurg Psychiatry. 1990;53:681-684.
7 Abrams P, Hanno P, Wein A. Overactive bladder and painful bladder syndrome: there need not be confusion. Neurourol Urodyn. 2005;24:149-150.
8 Bogart LM, Berry SH, Clemens JQ. Symptoms of interstitial cystitis, painful bladder syndrome and similar diseases in women: a systematic review. J Urol. 2007;177:450-456.
9 Butrick CW. Interstitial cystitis and chronic pelvic pain: new insights in neuropathology, diagnosis, and treatment. Clin Obstet Gynecol. 2003;46:811-823.
10 Hanno P, Nordling J, van Ophoven A. What is new in bladder pain syndrome/interstitial cystitis? Curr Opin Urol. 2008;18:353-358.
11 Marinkovic SP, Gillen LM. Sacral neuromodulation for multiple sclerosis patients with urinary retention and clean intermittent catheterization. Int Urogynecol J Pelvic Floor Dysfunct. 2010;21:223-228.
12 Marinkovic SP, et al. The management of interstitial cystitis or painful bladder syndrome in women. Br Med J. 2009;339:b2707.
13 Feler CA, Whitworth LA, Fernandez J. Sacral neuromodulation for chronic pain conditions. Anesthesiol Clin North Am. 2003;21:785-795.
14 Clemens JQ, et al. Prevalence and incidence of interstitial cystitis in a managed care population. J Urol. 2005;173:98-102. discussion 102
15 Dell JR, Mokrzycki ML, Jayne CJ. Differentiating interstitial cystitis from similar conditions commonly seen in gynecologic practice. Eur J Obstet Gynecol Reprod Biol. 2009;144:105-109.
16 Metts JF. Interstitial cystitis: urgency and frequency syndrome. Am Fam Physician. 2001;64:1199-1206.
17 Sant GR, Theoharides TC. Interstitial cystitis. Curr Opin Urol. 1999;9:297-302.
18 van de Merwe J, et al. Diagnostic criteria, classification, and nomenclature for painful bladder syndrome/interstitial cystitis: an ESSIC proposal. Eur Urol. 2008;53:60-67.
19 Peters KM, et al. Fact or fiction—is abuse prevalent in patients with interstitial cystitis? Results from a community survey and clinic population. J Urol. 2007;178:891-895. discussion 895
20 Rodriguez MA, Afari N, Buchwald DS. Evidence for overlap between urological and nonurological unexplained clinical conditions. J Urol. 2009;182:2123-2131.
21 Moyal-Barracco M, Lynch PJ. 2003 ISSVD terminology and classification of vulvodynia: a historical perspective. J Reprod Med. 2004;49:772-777.
22 Nair AR, et al. Spinal cord stimulator for the treatment of a woman with vulvovaginal burning and deep pelvic pain. Obstet Gynecol. 2008;111:545-547.
23 Ramsay LB, Wright JJr, Fischer JR. Sacral neuromodulation in the treatment of vulvar vestibulitis syndrome. Obstet Gynecol. 2009;114:487-489.
24 Reed BD. Vulvodynia: diagnosis and management. Am Fam Physician. 2006;73:1231-1238.
25 Farage MA, Galask RP. Vulvar vestibulitis syndrome: a review. Eur J Obstet Gynecol Reprod Biol. 2005;123:9-16.
26 Granitsiotis P, Kirk D. Chronic testicular pain: an overview. Eur Urol. 2004;45:430-436.
27 McJunkin TL, Wuollet AL, Lynch PJ. Sacral nerve stimulation as a treatment modality for intractable neuropathic testicular pain. Pain Physician. 2009;12:991-995.
28 Miller JL, et al. Prostatodynia and interstitial cystitis: one and the same? Urology. 1995;45:587-590.
29 Maigne JY, Guedj S, Straus C. Idiopathic coccygodynia. Lateral roentgenograms in the sitting position and coccygeal discography. Spine (Phila Pa 1976). 1994;19:930-934.
30 Melzack R, Wall PD. Pain mechanisms: a new theory. Science. 1965;150:971-979.
31 Shaker H, et al. Role of C-afferent fibres in the mechanism of action of sacral nerve root neuromodulation in chronic spinal cord injury. BJU Int. 2000;85:905-910.
32 Matharu MS, et al. Central neuromodulation in chronic migraine patients with suboccipital stimulators: a PET study. Brain. 2004;127:220-230.
33 Chadha S, et al. Histopathologic features of vulvar vestibulitis. Int J Gynecol Pathol. 1998;17:7-11.
34 Elbadawi AE, Light JK. Distinctive ultrastructural pathology of nonulcerative interstitial cystitis: new observations and their potential significance in pathogenesis. Urol Int. 1996;56:137-162.
35 Elgavish A, et al. Evidence for altered proliferative ability of progenitors of urothelial cells in interstitial cystitis. J Urol. 1997;158:248-252.
36 Haque R, Winfree CJ. Spinal nerve root stimulation. Neurosurg Focus. 2006;21:E4.
37 Aboseif S, et al. Sacral neuromodulation as an effective treatment for refractory pelvic floor dysfunction. Urology. 2002;60:52-56.
38 Chai TC, et al. Percutaneous sacral third nerve root neurostimulation improves symptoms and normalizes urinary HB-EGF levels and antiproliferative activity in patients with interstitial cystitis. Urology. 2000;55:643-646.
39 Jonas U, et al. Efficacy of sacral nerve stimulation for urinary retention: results 18 months after implantation. J Urol. 2001;165:15-19.
40 Richter EO, Abramova MV, Alò KM. Percutaneous cephalocaudal implantation of epidural stimulation electrodes over sacral nerve root—a technical note on the importance of the lateral approach, Neuromodulation—Technology at the Neural Interface. Neuromodulation. 2011;14:62-67.
41 Alò KM. Selective nerve root stimulation: facilitating the cephalocaudal “retrograde” method of electrode insertion. In: Deer T, editor. Atlas of implantable therapies for pain management. New York: Springer; 2011:107-114.
42 Alò K, Feler CA: Retrograde peripheral nerve root stimulation for interstitial cystitis: update of clinical results, Worldwide pain conference, San Francisco, California, July 18, 2000.
43 Schmidt RA, et al. Sacral nerve stimulation for treatment of refractory urinary urge incontinence: Sacral Nerve Stimulation Study Group. J Urol. 1999;162:352-357.
44 Chai TC, Mamo GJ. Modified techniques of S3 foramen localization and lead implantation in S3 neuromodulation. Urology. 2001;58:786-790.
45 Fall M, Oberpenning F, Peeker R. Treatment of bladder pain syndrome/interstitial cystitis 2008: can we make evidence-based decisions? Eur Urol. 2008;54:65-75.
46 Peters KM, Feber KM, Bennett RC. A prospective, single-blind, randomized crossover trial of sacral vs pudendal nerve stimulation for interstitial cystitis. BJU Int. 2007;100:835-839.
47 Alò K, McKay E. Selective Nerve root stimulation (SNRS) for the treatment of intractable pelvic pain and motor dysfunction: case report. Neuromodulation. 2001;4:19-23.
48 Comiter CV. Sacral neuromodulation for the symptomatic treatment of refractory interstitial cystitis: a prospective study. J Urol. 2003;169:1369-1373.
49 Feler CA WL, Brookoff D, Powell R. Recent advances: sacral nerve root stimulation using a retrograde method of lead insertion for the treatment of pelvic pain due to interstitial cystitis. Neuromodulation. 1999;2:211-216.
50 Maher CF, et al. Percutaneous sacral nerve root neuromodulation for intractable interstitial cystitis. J Urol. 2001;165:884-886.
51 Peters KM. Neuromodulation for the treatment of refractory interstitial cystitis. Rev Urol. 2002;4(suppl 1):S36-43.
52 Peters KM, Konstandt D. Sacral neuromodulation decreases narcotic requirements in refractory interstitial cystitis. BJU Int. 2004;93:777-779.
53 Whitmore KE, et al. Sacral neuromodulation in patients with interstitial cystitis: a multicenter clinical trial. Int Urogynecol J Pelvic Floor Dysfunct. 2003;14:305-308. discussion 308-309
54 Steinberg AC, Oyama IA, Whitmore KE. Bilateral S3 stimulator in patients with interstitial cystitis. Urology. 2007;69:441-443.
55 Zabihi N, et al. Short-term results of bilateral S2-S4 sacral neuromodulation for the treatment of refractory interstitial cystitis, painful bladder syndrome, and chronic pelvic pain. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19:553-557.
56 Aboseif S, et al. Sacral neuromodulation in functional urinary retention: an effective way to restore voiding. BJU Int. 2002;90:662-665.
57 Alò K, Gohel R, Corey C. Sacral nerve root stimulation for the treatment of urge incontinence and detrusor dysfunction utilizing a cephalocaudal intraspinal method of lead insertion: a case report. Neuromodulation. 2001;4:53-58.
58 Alò K, et al. A study of electrode placement at the cervical and upper thoracic nerve roots using an anatomic trans-spinal approach. Neuromodulation. 1999;2:222-227.
59 Powell CR, Kreder KJ. Long-term outcomes of urgency-frequency syndrome due to painful bladder syndrome treated with sacral neuromodulation and analysis of failures. J Urol. 2010;183:173-176.
60 Sutherland SE, et al. Sacral nerve stimulation for voiding dysfunction: One institution’s 11-year experience. Neurourol Urodyn. 2007;26:19-28. discussion 36
61 Oh MY, et al. Peripheral nerve stimulation for the treatment of occipital neuralgia and transformed migraine using a c1-2-3 subcutaneous paddle style electrode: a technical report. Neuromodulation. 2004;7:103-112.
62 Alò KM. Technical tips: percutaneous lead anchoring techniques—drain suture technique, Pain Relief News. Medtronic Neurological. 2005;l1(Issue 2):7.
63 Stojanovic MP. Stimulation methods for neuropathic pain control. Curr Pain Headache Rep. 2001;5:130-137.