CHAPTER 177 Neoplasms of the Posterior Fossa
Approach to Diagnosis of Neoplasms of the Posterior Fossa
Posterior fossa skull base neoplasms may be grouped into common CPA lesions, including lesions of the internal auditory canal; petrous apex lesions; rare CPA lesions; and intra-axial lesions. The signs, symptoms, and diagnostic procedures for tumors within the designated categories (Box 177-1) are described next.
Common Cerebellopontine Angle Neoplasms
In published series of CPA neoplasms, acoustic neuromas are the most common tumors, accounting for more than 90%. The remaining primary tumors were meningiomas (3%), primary cholesteatomas (2.5%), and facial nerve schwannomas (1%), with less common tumors composing the remaining lesions.1 When secondary tumors also are considered, paragangliomas constitute up to 10% of CPA neoplasms.2
Acoustic Neuroma
Type 2 neurofibromatosis, NF2, is the central form of the disease, characterized by bilateral acoustic neuromas in up to 96% of patients. Schwannomas of the other cranial nerves, meningiomas, and ependymomas also are observed much more commonly in patients with NF2.3 The precise frequency of NF2 is unknown, but it is far less frequent than NF1. The gene responsible for the condition encodes a membrane protein (merlin or schwannomin) and is located on chromosome 22. Acoustic neuromas in NF2 are characterized by an onset early in life, often before the age of 21 years, as opposed to unilateral lesions, most of which are diagnosed between the ages of 40 and 60 years. Thus, acoustic neuromas appearing before the age of 30 years mandate particularly close evaluation of the contralateral ear. Although the acoustic neuromas in NF1 and NF2 resemble the lesions in nonhereditary acoustic neuromas, they are technically more challenging to remove because of a tendency to adhere to nearby structures. The clinical presentation of acoustic neuroma in neurofibromatosis is identical to that of unilateral acoustic neuroma.
Malignant schwannomas may rarely occur. They often are associated with neurofibromatosis, but they also may occur with solitary schwannomas. Another very uncommon variant is a pigmented schwannoma.4
Natural History
The growth rate of acoustic neuromas is extremely variable. These tumors generally are slow-growing, with average reported growth rates of 0.2 cm per year; however, growth rates in excess of 2 cm per year have been documented. Acoustic neuromas that are not treated are potentially lethal. Gradual enlargement can lead to indentation of the brainstem, increased intracranial pressure, and death during a course of 5 to 15 years.5
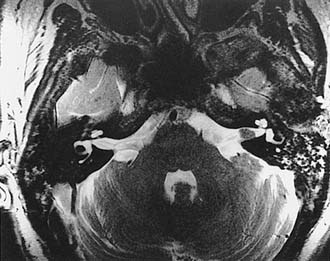
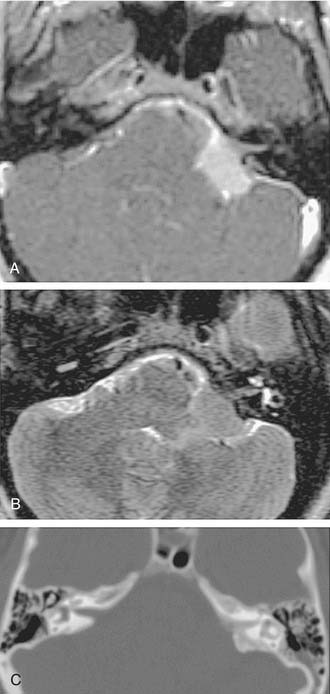
Growth of acoustic neuromas generally occurs in three phases: internal auditory canal, cisternal, and brainstem compression (Figs. 177-1, 177-2, and 177-3). Growth within the internal auditory canal results in acoustic and facial nerve compression and attenuation. Displacement of the seventh cranial nerve, the acoustic (eighth) nerve, and the anterior inferior cerebellar artery occurs just medial to the porus acusticus (cisternal portion). Medial tumor growth augments the blood supply of the tumor with bridging vessels from the brainstem surface. Fourth ventricle shift often occurs when the CPA component reaches 2 to 3 cm and total ventricle obstruction with resulting hydrocephalus occurs with continued tumor growth. Trigeminal compression occurs at approximately the 3-cm stage, which permits the superior portion of the tumor to abut the fifth cranial nerve. Massive tumor growth with hydrocephalus, brainstem compression, and cerebellar tonsil herniation may occur even with modern imaging. The otolaryngologist should remain vigilant for signs and symptoms suggestive of retrocochlear pathology.
Signs and Symptoms
Sensorineural hearing loss (SNHL), tinnitus, dysequilibrium, and facial hypesthesia are, in descending order, the most common symptoms of acoustic neuroma. Although progressive unilateral SNHL is the most common symptom, loss of speech discrimination is characteristic of retrocochlear dysfunction of the cochlear nerve presumably from pressure on the auditory nerve. The symptoms usually are slowly progressive, with a median duration of 2 years in one series.6,7 However, up to 20% of affected patients experience sudden SNHL, although complete recovery of hearing has been reported.8 Overall, only 1% of patients with sudden SNHL are found to have acoustic neuroma, whereas up to 5% of patients with acoustic neuroma have normal hearing.9 Thus, any patient with asymmetric or sudden hearing loss (even after total recovery) should be considered for further evaluation for a possible retrocochlear lesion.
Diagnostic Studies
Audiometry
Routine air, bone, and speech discrimination studies may suggest the possibility of an acoustic neuroma. Asymmetric SNHL or impairment of speech discrimination disproportionate to the pure tone loss requires specific investigation for a retrocochlear lesion. In the past, an extensive audiometric test battery was necessary for timely tumor diagnosis.6,10 In the magnetic resonance imaging (MRI) era, specialized audiometric tests are not sensitive or specific enough to detect small acoustic neuromas consistently.11
Auditory Brainstem Response Testing
Until recently, auditory brainstem (evoked) response (ABR) testing was considered the most sensitive modality for the detection of even small tumors, with detection rates of 95% to 100%.12 The brainstem response to an 83-dB broadband click is recorded while the contralateral ear is masked by 78-dB white noise.13 The latency for detection of wave V for the two ears is compared, and an adjusted interaural latency for wave V greater than 0.2 msec is considered abnormal. An approximately 10% false-positive rate has been reported for patients with SNHL who do not have a CPA tumor. Since the advent of gadolinium-enhanced MRI, however, the ABR false-negative rate (missing tumor diagnosis) has been 18% to 30% for intracanalicular tumors.14 The possibility of false-negative results with intracanalicular acoustic neuromas has lessened the role of ABR testing for routine screening.15 Stacked ABR testing is a modified ABR technique that offers improved sensitivity for detection of intracanalicular acoustic neuromas. It consists of testing the audiometric spectrum in a frequency-specific fashion followed by temporal alignment of the results; amplitude changes thus detected are much more sensitive for diagnosing small acoustic tumors than with routine ABR.16,17 Nonetheless, the technique has not yet been verified in widespread clinical practice.
Vestibular Tests
Vestibular testing is not a useful screening test for acoustic neuroma. Electronystagmography (ENG) and infrared video caloric testing are helpful in defining whether the tumor arises from the superior or the inferior vestibular nerve.18 Such information is valuable when hearing preservation surgery for acoustic neuromas is under consideration, because theoretically better results may be obtained with resection of tumors of the superior vestibular nerve.
Imaging
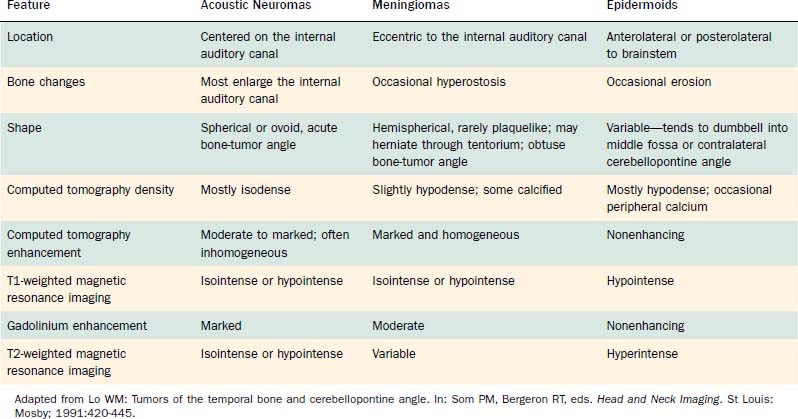
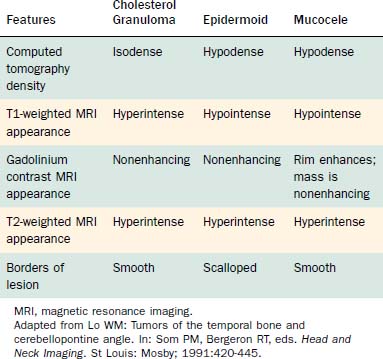
Computed tomography (CT) and MRI with the paramagnetic intravenous contrast agent gadopentetate (gadolinium-DTPA) are the principal imaging modalities for CPA lesions. Lo19 proposed a scheme for the CT or MRI differential diagnosis of CPA lesions by anatomic location (extra-axial, extradural, or intra-axial) and by incidence (rare or common) (Table 177-1). In adults and teenagers acoustic neuromas, meningiomas, and epidermoids are the three most common lesions. Acoustic neuromas are very rare in children. Instead, brainstem gliomas (which can enlarge the internal auditory canal) are the most common CPA lesion in younger children.20 Distinguishing among the three most common CPA lesions is based on specific imaging characteristics on CT and MRI (Table 177-2).
Table 177-1 Differential Diagnosis of Cerebellopontine Lesions by Imaging Location* and Incidence
| Location | Incidence | Type |
|---|---|---|
| Extra-axial | Most common | Acoustic neuroma |
| Common | Meningioma | |
| Common | Epidermoid (and other cysts: arachnoid, cysticercal, dermoid) | |
| Rare | Nonacoustic neuromas (cranial nerves V, VII, IX, X, XI, XII) | |
| Rare | Vascular lesions (loops, aneurysms, malformations) | |
| Extradural | Common | Paraganglioma (glomus jugulare, glomus vagale) |
| Rare | Bone lesions (benign or malignant; primary or metastatic) | |
| Intra-axial | Rare | Astrocytoma, ependymoma, papilloma, hemangioblastoma, metastasis |
* Computed tomography or magnetic resonance imaging.
Adapted from Lo WM. Tumors of the temporal bone and cerebellopontine angle. In: Som PM, Bergeron RT, eds. Head and Neck Imaging. St Louis: Mosby; 1991:420-445.
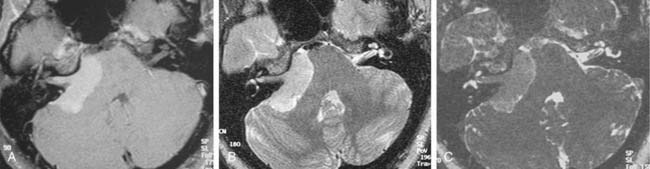
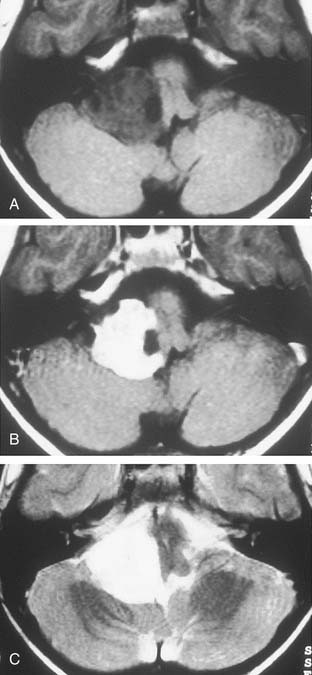
Although CT revolutionized the diagnosis of CPA tumors, gadolinium contrast MRI is sensitive and specific for detection of acoustic neuroma and is the diagnostic imaging technique of choice for the evaluation of acoustic neuroma and other CPA lesions (see Fig. 177-2).21,22 Acoustic neuroma images on MRI are isointense or mildly hypointense to brain on T1-weighted images and mildly hyperintense to brain on T2-weighted images.19 MRI for investigation of possible acoustic neuroma should be performed with intravenous contrast. Gadolinium increases the diagnostic sensitivity of MRI on T1-weighted images, and detection of lesions as small as 3 mm has been reported.21 In addition to improving sensitivity in diagnosing acoustic neuromas, MRI is noninvasive, and the patient incurs no radiation exposure.
Before MRI, CT was the principal study for CPA lesions. Now the role of CT is to provide adjunctive information regarding the osseous structures surrounding a CPA lesion. It also can be the primary imaging modality in patients who cannot undergo MRI because of medical reasons (e.g., cardiac pacemaker or cochlear implant) or unmanageable phobic reactions. The CT finding characteristic of an acoustic tumor is an ovoid lesion centered on the internal auditory canal with moderate enhancing qualities. The tumor often is not homogeneous, exhibiting areas of lesser and greater enhancement. Approximately 85% of acoustic neuromas show acute angles at the bone-tumor interface, in contrast with meningiomas, in which the interface is obtuse in 75% of tumors.19
Intravenous contrast CT can fail to detect acoustic neuromas smaller than 1.5 cm in greatest dimension. Oxygen cisternography has been used to diagnose small, mainly intracanalicular lesions. In this technique, 4 mL of oxygen was introduced into the subarachnoid space through the opening afforded by a lumbar puncture, to highlight the structures of the internal auditory canal. This technique was very sensitive for detection of small lesions; however, it was limited by a false-positive rate of 5% and the need for a lumbar puncture.23
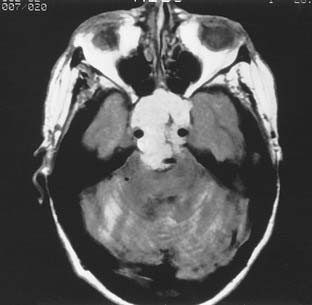
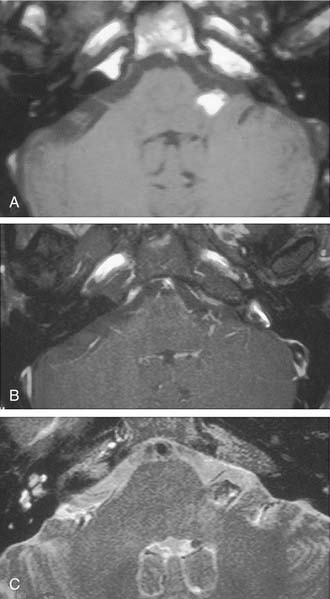
The improved availability of MRI has led to the development of MRI-based internal auditory canal screening protocols, which in many centers are comparable in cost to ABR-based internal auditory canal screening.11 Special T2-weighted fast-spin echo sequences may achieve the accuracy of T1-weighted sequences with gadolinium without the need for contrast (see Fig. 177-1).24 Although the software for these special sequences is not generally available, initial findings with this technique are very encouraging.
Meningiomas
Meningiomas represent up to 18% of all intracranial tumors and approximately 3% of CPA tumors.25 The cells lining the arachnoid villi are the cells of origin. These cells are distributed throughout the intracranial space predominantly in relation to veins and dural sinuses. Meningiomas are benign but locally aggressive tumors, which occur at different anatomic sites in the following order of frequency: parasagittal region, falx, convexity, olfactory groove, tuberculum sellae, sphenoid ridge, petrous face (CPA), tentorium, lateral ventricle, clivus, and others.26
The gross appearance typically is that of a globular mass firmly adherent to the dura mater, with characteristic speckles scattered throughout the tumor that correspond to the microscopic psammoma bodies. The tumor displaces but does not invade adjacent neural tissue and has a thin investing capsule. Meningiomas can invade bone without destruction by extension along haversion canals. Adjacent bone is hyperostotic in 25% of cases.26
Many histopathologic classifications have been proposed, but a single, widely used system distinguishes among syncytial, transitional, fibrous, angioblastic, and sarcomatous types.27 The specific histopathologic subtypes and growth characteristics are reviewed elsewhere.28 In the posterior fossa, meningiomas usually arise on the posterior surface of the petrous bone, away from or at the edge of the internal auditory canal, or along the sigmoid sinus. Because they usually arise outside of the canal, these tumors may become large before producing signs and symptoms of cranial nerve VIII compression. Most meningiomas eventually involve cranial nerve VIII.
Signs and Symptoms
Audiovestibular symptoms usually are the first indication of a posterior fossa meningioma. Among patients presenting to neurosurgeons, a higher proportion first experienced symptoms referable to the trigeminal nerve.29 The signs and symptoms of meningiomas are similar to those of acoustic neuromas. Small tumors produce hearing loss, tinnitus, and imbalance. Larger tumors also produce signs and symptoms of involvement of other cranial nerves and hydrocephalus.
Diagnostic Studies
Imaging
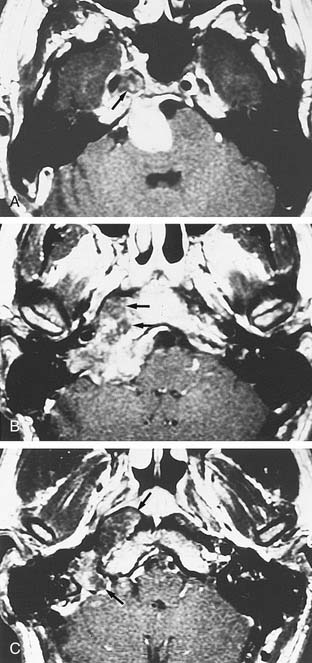
Table 177-2 summarizes features of meningiomas that assist in differentiating them from acoustic neuromas. Unlike acoustic neuromas, meningiomas usually are eccentric to the porus. Whereas acoustic neuromas seldom herniate into the middle fossa, approximately 60% of meningiomas extend to the middle fossa.19 Meningiomas usually are hemispheric because of their broad-based attachment to the posterior petrous wall, accounting for the obtuse bone-tumor angles in 75% of meningiomas. Unlike the site of origin of acoustic neuromas, that of posterior fossa meningiomas is varied (Figs. 177-4 and 177-5).
On MRI, meningiomas are of extremely variable intensity on T2-weighted images and isointense or slightly hypointense to brain on T1-weighted images. The different signal intensities among meningiomas correspond to different histopathologic subtypes.27 Surface flow voids on MRI correspond to marginal pial blood vessels, and arborizing flow voids represent active feeders to the tumor. Calcification and cystic foci cause heterogeneity on MRI images of meningiomas.
Primary Cholesteatomas
Diagnostic Studies
Auditory Testing
Auditory testing does not show any distinguishing features. As with other retrocochlear lesions, speech discrimination is poorer than would be expected from the degree of pure-tone loss. ABR also is frequently abnormal in primary cholesteatomas.30
Imaging
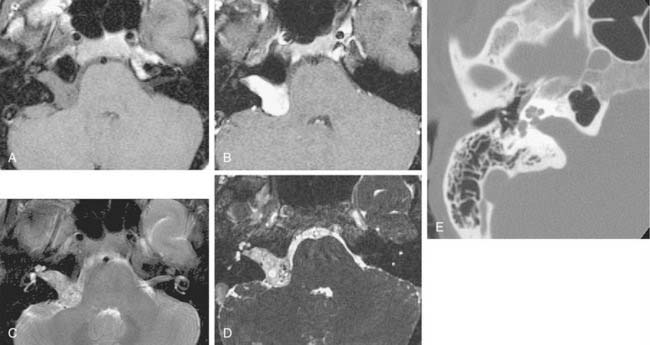
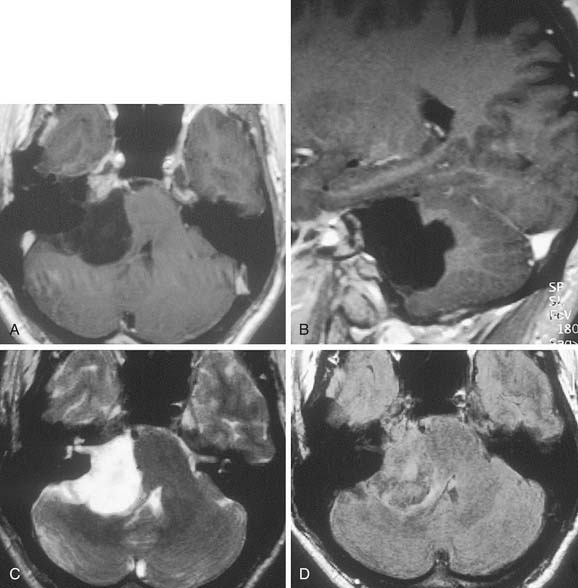
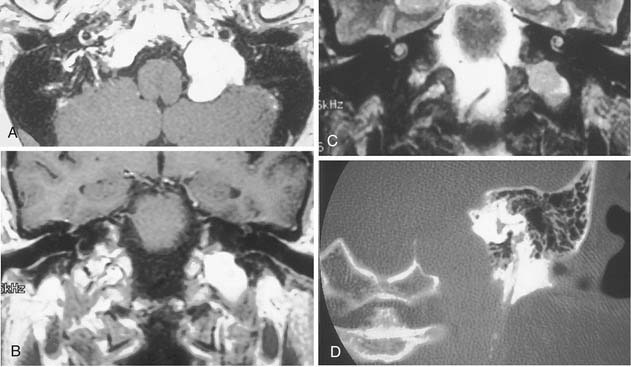
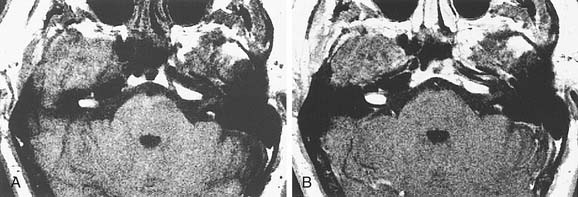
On CT, primary cholesteatomas are less dense than brain (having a density approximating that of cerebrospinal fluid [CSF]) and exhibit no enhancement with intravenous contrast. These lesions have irregular margins and are eccentric to the porus acusticus. Enhancing components suggest an associated malignancy.31 With MRI, primary cholesteatomas are inhomogeneous and hypointense to brain on T1-weighted images, and homogeneous and isointense or hyperintense to brain on T2-weighted images (Fig. 177-6). Schwannomas, meningiomas, and chondromas are similar to primary cholesteatomas by intensity criteria; however, epidermoids are differentiated because they are nonenhancing.
Arachnoid cysts of the CPA are difficult to distinguish from epidermoids on CT and MRI. Both lesions are of CSF density and nonenhancing; compared with primary cholesteatomas, however, arachnoid cysts have smoother surfaces, and special MRI sequences such as diffusion-weighted images (DWIs) may differentiate the intensity of the epidermoid from the CSF intensity of the arachnoid cyst (Fig. 177-7).
Facial Nerve Neuroma
Signs and Symptoms
Symptoms associated with these tumors depend on the portion of the nerve affected by the neoplasm. Peripheral involvement can manifest as a parotid mass, middle ear involvement can produce conductive hearing losses, and internal auditory canal or CPA involvement may result in SNHL. Unlike hemangiomas of the facial nerve, schwannomas do not produce facial weakness until they become very large. Sometimes a facial nerve tic is evident, which helps distinguish facial neuromas from acoustic neuromas but not from primary cholesteatomas. Of note, intratemporal lesions, with the possibility of neural entrapment, are more likely than CPA lesions to result in facial paralysis.32
Diagnostic Studies
Auditory Testing
The mechanisms for conductive hearing impairment and SNHL have already been described earlier in this chapter. Impedance testing may reflect motor fiber impairment on ipsilateral reflex testing or cranial nerve VIII involvement on contralateral reflex testing. ABR testing of tumors arising in the internal auditory canal shows abnormalities similar to those seen with acoustic neuroma.13
Electroneurography
Electroneurography, also known as electroneuronography (ENoG), measures the muscle response to a maximal bipolar stimulation of the facial nerve near the stylomastoid foramen. ENoG potentials may be reduced in facial nerve neuroma even when no facial weakness or tic is present, whereas ENoG findings remain normal in acoustic neuroma until the tumor becomes very large. Some reports suggest that the value of preoperative ENoG in patients with acoustic neuroma lies in predicting whether the lesion actually represents a facial nerve tumor.33,34
Imaging
Because facial nerve neuromas are histologically identical to acoustic neuromas, they have the same enhancement characteristics. It usually is impossible to identify these lesions using CT. Anterosuperior erosion of the internal auditory canal or erosion of the labyrinthine facial nerve canal if present may be the only diagnostic clue.19 More distal tumors enlarge the geniculate ganglion and fallopian canal.
As with CT, MRI of facial neuroma produces imaging characteristics identical to those of acoustic neuromas (Fig. 177-8). The preoperative diagnosis of intracranial facial neuroma is difficult. Early facial nerve symptoms are an obvious warning that a CPA lesion may rarely be a facial nerve neuroma. Patients diagnosed with CPA neuromas should be warned preoperatively of a 1% risk of facial nerve neuroma. This possibility is emphasized if findings on preoperative ENoG are abnormal on the tumor side. In some tumors, the lesion may extend from the CPA through the temporal bone course of the nerve to the extratemporal nerve in the parotid gland.
Other Cranial Nerve Neuromas
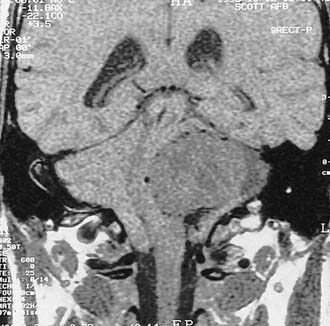
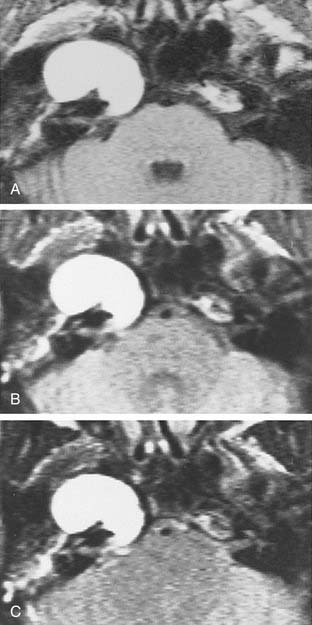
Trigeminal neuromas arise intradurally, from the nerve root in the CPA and Meckel’s cave, and extradurally, from the gasserian ganglion in the middle cranial fossa.35 Typically these lesions enlarge Meckel’s cave and produce hypesthesia of the face (Fig. 177-9).

Figure 177-9. Cystic trigeminal schwannoma, demonstrated by magnetic resonance imaging. A, T1-weighted image. B, Gadolinium T1-weighted image. C, T2-weighted image. The bulk of the tumor lies in the posterior fossa, with a small middle cranial fossa component enlarging the left Meckel’s cave (arrow). Note the similarity of the tumor to an arachnoid cyst (see Figure 177-7) on the noncontrast images (A and C). The tumor is nearly of CSF intensity because of the predominance of the intratumoral cystic components.
(From Jackler RK, Brackmann DE, eds. Neurotology. St Louis: Mosby; 1994.)
Neuromas of cranial nerves IX, X, and XI produce smooth enlargement of the jugular foramen and classically cause hypesthesia and weakness of the palate, vocal cord, and shoulder, respectively (Fig. 177-10). If they arise in the posterior fossa, these tumors may grow to a large size before producing predominantly acoustic or cerebellar signs. Accurate preoperative differentiation of these tumors from acoustic neuromas is important because residual hearing is more likely to be preserved with lower cranial nerve neuromas. Hypoglossal neuromas produce motor hemiatrophy of the tongue and enlargement of the hypoglossal canal on radiography.
Glomus Tumors
Glomus tumors (paragangliomas) are discussed in detail in Chapter 176. Because glomus jugulare tumors and glomus vagale tumors may extend into the posterior fossa, they constitute an important consideration in the differential diagnosis of skull base neoplasms.
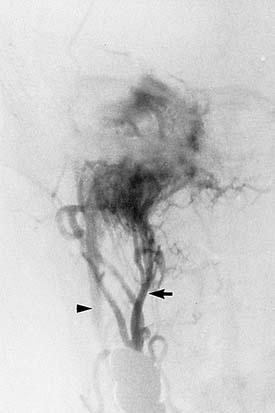
The characteristic appearance on CT with bone windows is irregular destruction of the jugular foramen, as compared with jugular foramen schwannomas, which produce smooth enlargement (Fig. 177-11). The vascular pattern of paragangliomas on angiography is characteristic (Fig. 177-12), and biopsy of these lesions is not indicated.36 Diagnostic angiography should be performed concomitantly with preoperative embolization when surgical resection is planned.
MRI produces a unique salt-and-pepper mixture of intensities on T1- and T2-weighted images. Arborizing flow voids reveal the prominent tumor vessels of this lesion. Lo19 described two limitations of MRI in evaluating paragangliomas: (1) bone changes and the relation of tumor to bone landmarks are not visualized; and (2) distinguishing tumor from bone marrow is difficult, especially on gadolinium-enhanced T1-weighted images. Thus, MRI may provide complementary information about infralabyrinthine and intracranial tumor extensions, but bone algorithm CT is the cornerstone of imaging evaluation in paragangliomas.
The combination of CT, MRI, and angiography can provide extensive information about involvement of the internal carotid artery. Magnetic resonance angiography (MRA) is an adjunct to intra-arterial catheterization and formal angiography (see Fig. 177-12). Angiography is a necessary step in preoperative embolization of glomus tumors; however, it is unlikely that MRA can replace direct intravascular angiography in glomus tumor management. For resection of skull base neoplasms involving the posterior fossa, if surgical resection requires manipulation of the artery, preoperative assessment of the adequacy of collateral flow by way of the circle of Willis is necessary. Temporary balloon occlusion of the carotid artery in combination with radioisotope imaging or xenon-enhanced CT can accurately quantify collateral blood flow.37
Arachnoid Cysts
Arachnoid cysts are thin-walled sacs that contain yellow, entrapped CSF. The current theory is that these lesions represent congenital developmental anomalies.38 Symptoms are produced by the mass effect of the cyst on surrounding structures and may be similar to the symptoms of acoustic neuromas. These patients may experience mild to profound hearing loss with a retrocochlear pattern.39
These lesions have CT and MRI characteristics similar to those of epidermoids. Enlargement of the internal auditory canal often is noted but is not characteristic of these lesions. The typical appearance is that of a smooth-surfaced lesion, which on CT approximates CSF and is nonenhancing and on MRI exhibits isointensity or hypointensity to brain on T1-weighted images and hyperintensity to brain on T2-weighted images (see Fig. 177-7). In most patients, no direct intervention is necessary. When treatment is necessary to control symptoms, management of these lesions is not total resection. Instead, surgical drainage by way of retrolabyrinthine exposure is the usual recommended therapy; however, diuretic therapy provides symptomatic relief in a few patients.
Hemangiomas
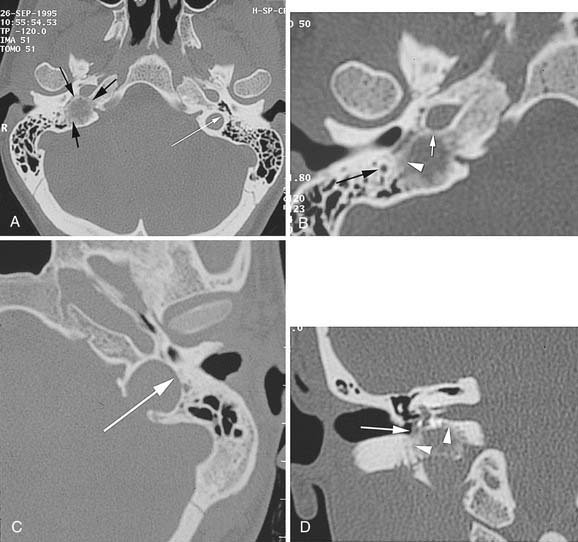
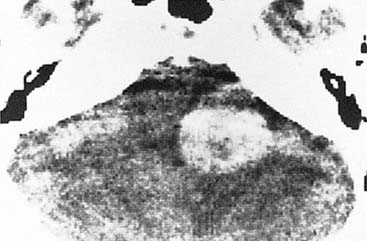
Capillary hemangiomas typically arise in the area of the geniculate ganglion in association with a perigeniculate capillary plexus.40 The lesion is characterized by a progressive facial weakness despite being much smaller than facial nerve neuromas. The expanding lesion with the production of pulsatile tinnitus may expose the upper basal turn of the cochlea. CT shows a smooth enlargement of the geniculate ganglion and enlargement of the labyrinthine portion of the fallopian canal by a soft tissue mass. Although capillary hemangiomas are enhancing, they produce facial weakness at such an early stage that only subtle findings may be apparent on CT, and a very small enhancement in the area of the labyrinthine segment may be the only finding. Other CT findings include honeycomb bone, irregular and indistinct bone margins, and intratumoral bone spicules (Fig. 177-13). These findings contrast with those in facial nerve neuromas, which are larger, more obvious lesions with sharp bone margins.
Petrous Apex Lesions
Cholesterol Granulomas
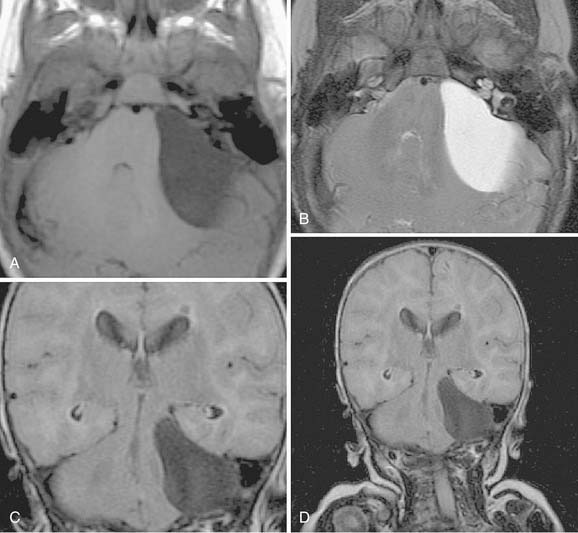
A cholesterol granuloma arises in the pneumatized spaces of the temporal bone presumably as a result of occlusion of the air cell system. Hemorrhage into the air cells results in a foreign body reaction and progressive granuloma formation. An expansile lesion of the temporal bone results, with extension into the CPA and resultant signs and symptoms of cranial nerve VIII dysfunction. A recent theory suggests that exposed bone marrow may hemorrhage into the pneumatized petrous apex air cells, producing the foreign body reaction and granuloma formation.41 This newer concept more adequately explains the presence of cholesterol granuloma in well-pneumatized temporal bones.
The CT and MRI findings with this lesion are distinguishable from those with the other common lesions of the petrous apex (Table 177-3). On CT, the lesions result in a punched-out appearance of the temporal bone with an isodense mass of the petrous apex that does not enhance; however, rim enhancement is evident with intravenous contrast. On MRI, T1- and T2-weighted images are hyperintense with respect to brain (Fig. 177-14). Primary cholesteatomas are the main lesions from which cholesterol granulomas should be distinguished. Cholesterol granulomas are much more common and occur 20 times more frequently than epidermoids. Overall, cholesterol granulomas are relatively uncommon compared with acoustic neuromas. Only 1 cholesterol granuloma is identified for every 35 acoustic neuromas diagnosed. Imaging distinguishes petrous apex epidermoids from petrous apex cholesterol granulomas. On CT, epidermoids show rim enhancement. With MRI, cholesterol granulomas are hyperintense on T1- and T2-weighted images, whereas epidermoids are hyperintense only on T2-weighted images.
Total excision of cholesterol granulomas usually is unnecessary. Drainage may be achieved by a transmastoid or transcanal infralabyrinthine approach with preservation of cranial nerve function. The transcanal, infracochlear, hypotympanotomy approach is preferred because it affords dependent drainage and the possibility of revision, if necessary, through a myringotomy.42 In cases with unfavorable anatomy for infracochlear or infralabyrinthine drainage, middle fossa drainage is an option. Suprisingly, long-term follow-up imaging has demonstrated that middle fossa catheter drainage to the mastoid remains patent.43
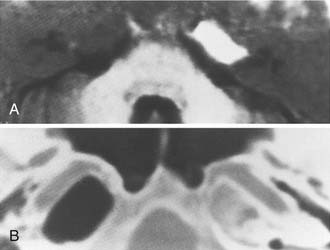
Asymmetric Petrous Apex Pneumatization
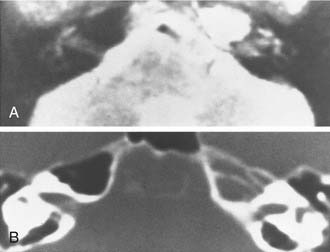
Although asymmetric pneumatization of the petrous apex does not represent a true neoplasm, this condition can be confused with and should be distinguished from true neoplasms. The fat content of bone marrow in a nonpneumatized petrous apex can produce a hyperintense appearance on T1-weighted noncontrast MRI (Fig. 177-15). The lack of bone destruction or expansion on CT, absence of contrast enhancement with gadolinium, and hypointensity on T2-weighted images distinguish this condition from a neoplasm.
Mucocele, Mucus Retention Cysts, and Petrous Apex Effusion
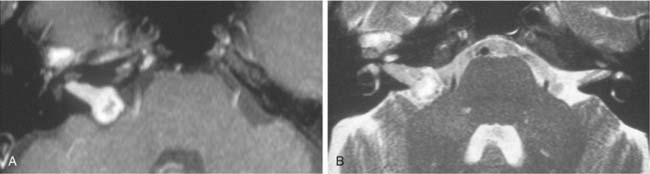
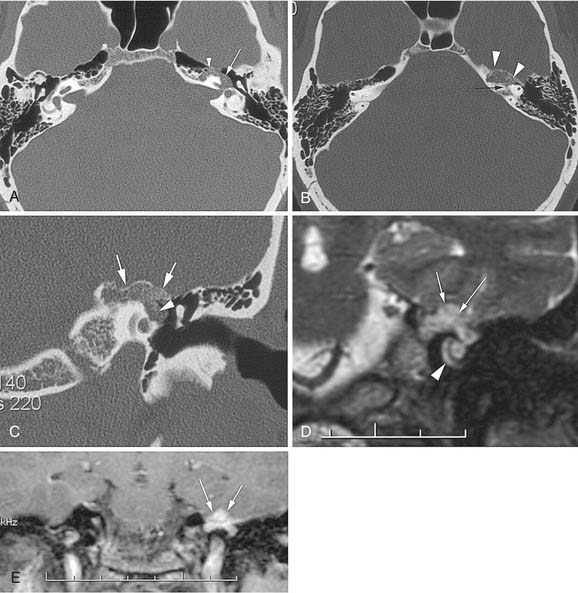
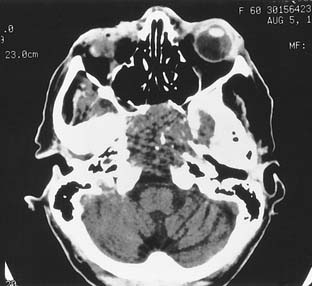
Petrous apex air cells may become obstructed, resulting in retained secretions as a mucus retention cyst in the petrous apex (Fig. 177-16) or an expansile mucocele (Fig. 177-17). CT reveals a nonenhancing lesion limited to the petrous apex air cell system. The MRI appearance is consistent with a mucus-filled lesion (hypointense on T1-weighted and hyperintense on T2-weighted images). A symptomatic mucocele behaves like chronic mastoiditis, with pressure symptoms that require drainage for relief. Occasionally, retained petrous apex fluid also can cause symptoms even without mucocele. Medical treatment as for a middle ear effusion or surgical drainage usually is therapeutic.43
Petrous Carotid Artery Aneurysms
Although aneurysms of the horizontal carotid artery are rare, they may appear as expansile, well-defined masses (Fig. 177-18). Their preoperative identification is critical for obvious reasons. Carotid aneurysms may be confused with chondrosarcomas on the basis of radiologic appearance.
Miscellaneous Cerebellopontine Angle Lesions
Metastatic Tumors
Tumors may metastasize to the CPA from other sites, including lung, breast, prostate, oropharynx, and skin (i.e., cutaneous melanomas).44 Tumors from these sites are distinguished by their rapid progression of symptoms and associated neurologic signs, in addition to hearing loss and dizziness. Associated lytic lesions in the petrous apex constitute another distinguishing feature of metastatic tumors. Rapid impairment of hearing, other cranial neuropathies, and brainstem dysfunction suggest malignant neoplasms of the posterior fossa, especially in a patient with a history of another malignancy.
Chordomas
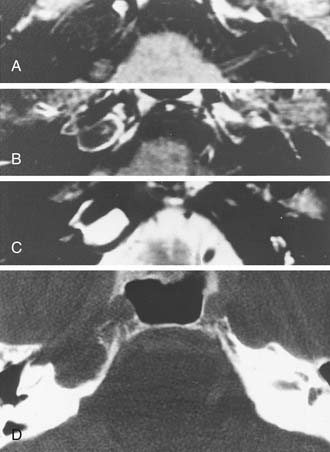
Chordomas are dysontogenetic neoplasms that arise in remnants of the embryonic notochord. Although more than one half of these tumors arise in the sacrococcygeal region, more than one third occur at the skull base in the region of the clivus or, less commonly, the upper cervical vertebrae.45 The prominent clinical characteristics are extensive bone destruction and progressive cranial nerve palsies. Extension of clivus chordomas to the petrous apex, sphenoid, or CPA is not unusual. Although fronto-orbital headache and vision complaints (e.g., limited visual fields, diplopia, loss of acuity) are more common, occasionally the initial symptoms reflect extension into the CPA. On CT, the bone destruction is readily apparent, and the masses are homogeneous with moderate enhancement and a greater density than that of bone (Fig. 177-19). MRI reveals isointensity on T1-weighted images and hyperintensity on T2-weighted images (Fig. 177-20).
Chondrosarcomas
Chondrosarcomas of the skull base also may arise in the CPA. They are clinically indistinguishable from chordomas except that they are centered more laterally. CT shows characteristic bone destruction and invasiveness. MRI shows this lesion well, which is hyperintense on T2-weighted images in the area of bone destruction in the skull base (Figs. 177-21 and 177-22).
Lipomas
Lipomas are hamartomas that are thinly encapsulated and poorly delineated. They appear as soft, multilobular masses of typical adult adipose tissue. Lipomas within the internal auditory canal can produce symptoms typical of an acoustic neuroma. Although on CT these lesions are less dense than neuromas, MRI is diagnostic. The lesions are hyperintense on T1-weighted images, nonenhancing with gadolinium, and hypointense on T2-weighted images (Fig. 177-23). Fat-suppression techniques with T1-weighted sequences are confirmatory because the previously identified lesion that was hyperintense on nonenhanced T1-weighted images is rendered hypointense with fat suppression (Fig. 177-24).
Intra-axial Tumors
Intra-axial tumors may occasionally be confused with CPA tumors as a result of extension to the CPA or compression of CPA structures. Intra-axial tumors may arise from the brainstem (gliomas), the cerebellum (medulloblastomas from the vermis or astrocytomas from the peduncles), or the fourth ventricle (choroid plexus papillomas and ependymomas). Although such lesions are highly unusual, the possibility cannot be ignored. In children, brainstem gliomas are reportedly the most common CPA neoplasms. Intra-axial tumors usually are isointense on T1-weighted MRI and hyperintense on T2-weighted images.20,21
Tumors of the Fourth Ventricle
Malignant Choroid Plexus Papillomas and Ependymomas
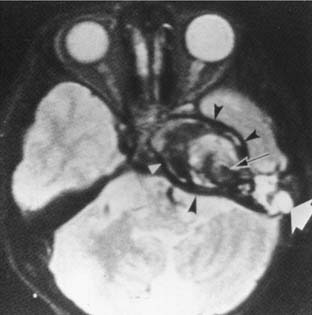
Choroid plexus papillomas and ependymomas arise from the fourth ventricle and cause CPA symptoms by growing through the foramen of Luschka. In this location, they produce early signs of cranial nerve VIII dysfunction. In malignant choroid plexus papillomas, CT shows a mass with enhancing characteristics of a schwannoma (Fig. 177-25). These tumors are distinguishable from an acoustic schwannoma by being separate from the internal auditory canal. Ependymomas may calcify, and those portions have variable imaging characteristics. At MRI, both lesions are isointense to brain on T1-weighted images and mildly hyperintense to brain on T2-weighted images (Fig. 177-26). Malignant ependymomas require multimodality therapy. The role of surgery is for biopsy, brainstem decompression, and management of hydrocephalus.
Surgery of the Posterior Cranial Fossa
This section addresses surgical management of extra-axial lesions of the posterior cranial fossa and internal auditory canal. Adequate exposure for skull base neoplasms involving the posterior fossa requires precise management of the temporal bone. The modern era for skull base surgery and transtemporal techniques began in 1961, when House introduced the operating microscope and multidisciplinary team surgery for removal of acoustic neuromas. With the vastly reduced mortality and excellent facial nerve preservation rates of the translabyrinthine approach for acoustic neuromas, House established the translabyrinthine approach as the procedure with which all other microsurgical approaches to the CPA are compared.46 Subsequently, multiple approaches to the CPA have been developed, each with its own advantages and indications.
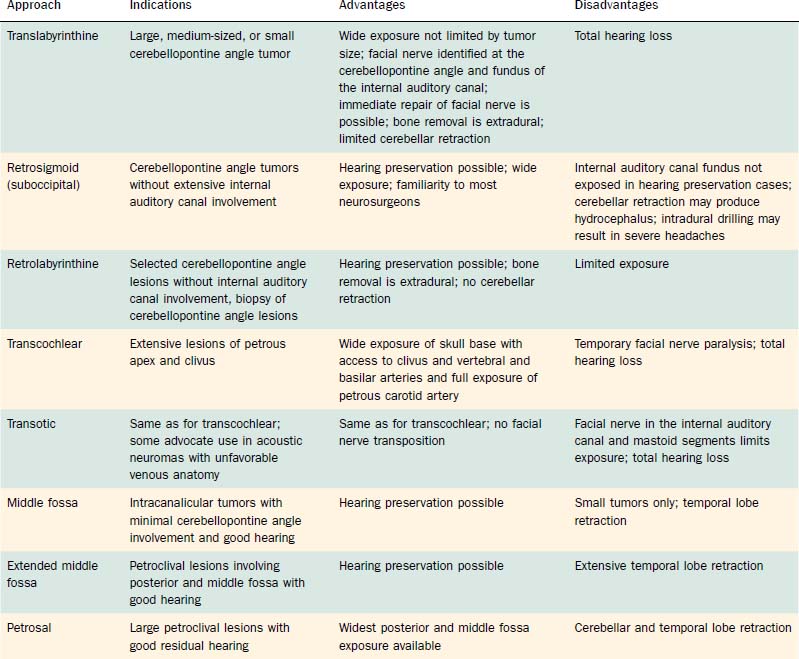
This section details the basic approach, specific indications, techniques, and special features of the translabyrinthine, retrosigmoid, suboccipital, retrolabyrinthine, transcochlear, transotic, middle fossa, extended middle fossa, and petrosal approaches for skull base neoplasms involving the posterior fossa. Table 177-4 summarizes the basic indications for and drawbacks of the various approaches for resection of these tumors.
Table 177-4 Surgical Approaches for Resection of Posterior Fossa Skull Base Neoplasms: Indications, Advantages, and Disadvantages
Translabyrinthine Approach
Basic Approach
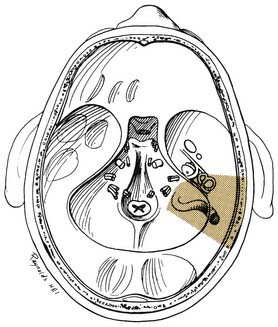
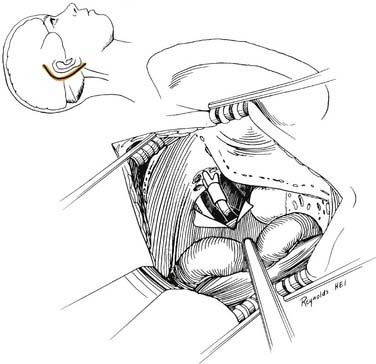
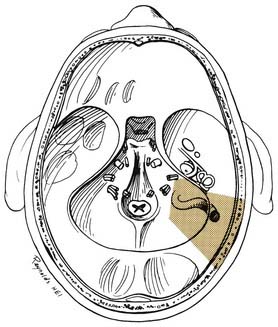
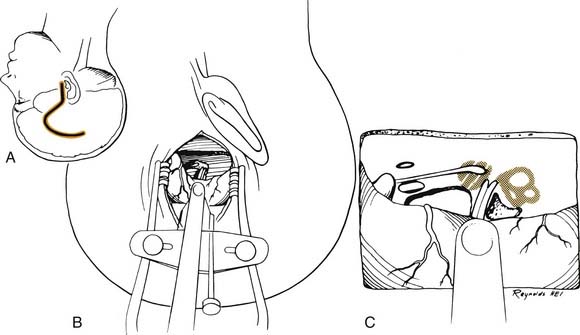
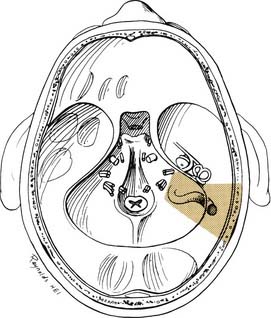
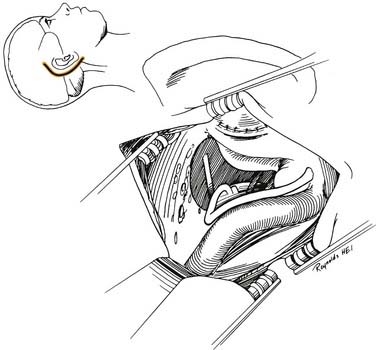
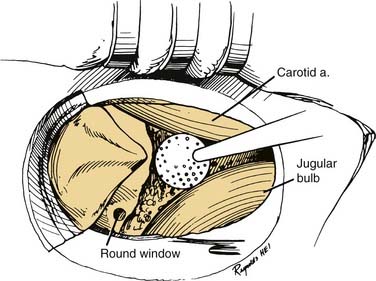
A transmastoid labyrinthectomy and skeletonization of the sigmoid sinus and posterior fossa dura permit identification of the facial nerve with wide exposure of the internal auditory canal and CPA (Fig. 177-27).
Technique
The bone removal is accomplished in four stages and is performed using the operating microscope, a high-speed drill, and continuous suction-irrigation.5 Most of the drilling is performed with cutting burrs; however, diamond burrs are used when drilling is performed on the dura or venous sinuses. The first stage is a complete mastoidectomy. Posteriorly, the bone is removed beyond the sigmoid sinus, and the sinus is skeletonized. The extent of bone removal posterior to the sinus and decompression of the posterior fossa depends on the size of the tumor. Greater posterior removal provides greater exposure of the CPA. Superiorly, the middle fossa plate is identified and thinned. Anteriorly, the facial nerve is identified in its vertical segment but left covered with bone for protection against inadvertent burr trauma.
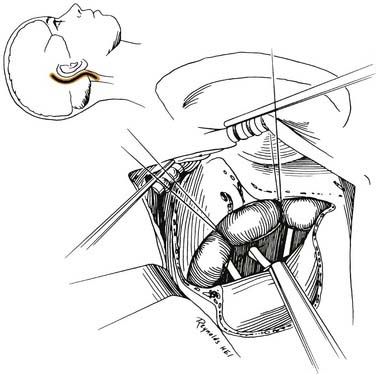
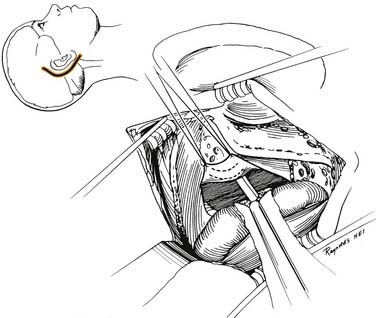
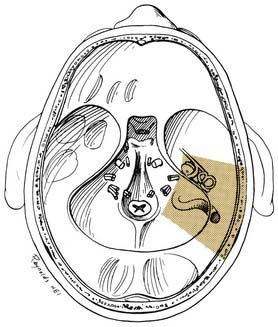
The final stage of bone removal consists of skeletonization of the internal auditory canal. The orientation of this structure is roughly parallel to the external auditory canal. Thus, the fundus of the canal is just medial to the floor of the vestibule, which was exposed during the labyrinthectomy. By contrast, identification of the porus requires extensive bone removal as the internal auditory canal is dissected medially. The basic principle of exposing the internal auditory canal is that all bone removal should be accomplished before the dura is opened, and complete bone removal requires decompression of 270 degrees around the circumference of the canal to avoid obscuring any ledges of bone. By completing bone removal before opening the dura, the risk of accidental injury to the nerves of the internal auditory canal is minimized. The inferior border of the canal is skeletonized first. This edge of the canal is identified by gradually enlarging a trough between the inferior edge of the vestibule and the jugular bulb. The cochlear aqueduct usually is identified as the dissection proceeds anteromedially. This structure becomes the inferior limit of the dissection, thereby protecting the lower cranial nerves from injury. After the inferior border of the internal auditory canal has been identified, the bone overlying the porus acusticus is thinned with cutting and diamond burrs. The superior border of the canal is identified last, because the facial nerve is more susceptible to injury in this area. A burr size that will fit between the middle fossa dura and the superior border of the internal auditory canal is used to expose the superior border. The entrance of the facial nerve into the canal can be positively identified just medial to a vertical crest of bone, “Bill’s bar,” along the superior aspect of the fundus. At this point, the bony exposure has been completed, and tumor removal can begin (Fig. 177-28).
Tumor Removal
After tumor removal, eustachian tube closure is achieved by removing the incus, transecting the tensor tympani tendon, and filling the eustachian tube with Surgicel. The temporalis muscle closes the eustachian tube. By opening the facial recess during drilling, the surgeon obtains wide exposure for eustachian tube occlusion. The edges of the dura are approximated with sutures, and the mastoidectomy defect is filled with strips of abdominal fat. The ends of the strips are placed through the dural defect to plug the dural opening. Cranioplasty with use of hydroxyapatite cement or titanium mesh is a useful technique to reduce the incidence of CSF fistula postoperatively and to enhance long-term wound healing and the cosmetic result.47,48 The wound is closed in layers, and a compressive dressing is applied.
Special Features
The fundamental advantage of the translabyrinthine approach, particularly in medium-sized and large tumors, is that it permits positive identification of the facial nerve, laterally at the fundus and medially at the brainstem. Thus, the tumor can be dissected from either direction with optimal control of the facial nerve. Such medial and lateral identification of the nerve has permitted anatomic preservation of the facial nerve in greater than 98.5% of 759 acoustic neuromas removed in one large surgical series.49 Furthermore, if the facial nerve is transected, as occurs in cases of CPA facial nerve neuroma, the tympanic and mastoid portions of the nerve are available for rerouting and reanastomosis or grafting.
The disadvantage of this approach is that hearing cannot be preserved. In a series of 300 consecutive cases of acoustic neuromas diagnosed before the advent of gadolinium-enhanced MRI, only 5% of patients, using the broadest criteria, could be considered candidates for hearing conservation surgery.50 The advent of gadolinium-enhanced MRI resulted in an increased percentage of acoustic neuromas among those detected, which are diagnosed at a small size before hearing is affected. Thus, hearing conservation procedures are becoming a more frequent consideration.
Retrosigmoid (Suboccipital) Approach
Basic Approach
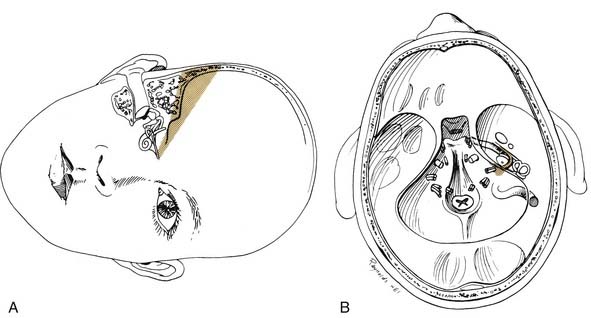
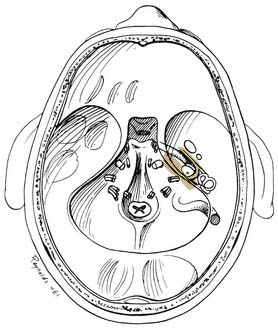
Transmastoid decompression of the sigmoid sinus and a retrosigmoid craniotomy permit access to CPA tumors without disturbing the labyrinth. Intradural removal of the posterior wall of the internal auditory canal permits direct access to the medial two thirds of the canal (Fig. 177-29).
Technique
The dura is incised posterior to the sigmoid sinus, with care taken to avoid injury to the underlying vessels that may be adherent to the dura. Adequate drainage of CSF is necessary to minimize the need for cerebellar retraction. The cerebellum is exposed and supported with retractors to allow visualization of the tumor in the CPA. The neurosurgical techniques for tumor removal at the brainstem are the same regardless of the approach chosen. If the tumor is too large to permit proximal identification of the facial nerve, debulking of the tumor is performed. Once the debulking has been accomplished, the tumor is dissected from the facial nerve toward the porus acusticus (Fig. 177-30).
Special Features
The retrosigmoid approach, in selected cases, offers an opportunity for hearing preservation, with success rates ranging from 30% to 65%, depending on the selection criteria used for hearing preservation surgery.51–53 This approach is particularly useful for removal of CPA tumors smaller than 2 cm in patients with good hearing and limited involvement of the internal auditory canal. The principal limitation of this approach is that the fundus of the canal is not directly visualized. This approach is being used more frequently as small tumors of the CPA are diagnosed with enhanced MRI and minimal auditory symptoms.
Bill’s bar in the fundus of the internal auditory canal cannot be used to identify the facial nerve laterally in the retrosigmoid approach. Thus, this step is more difficult in the retrosigmoid approach and relies more heavily on the nerve monitor. Although the rates of facial nerve preservation are comparable for the translabyrinthine and the retrosigmoid techniques, in our experience, facial nerve preservation rates are better with the translabyrinthine approach because it allows positive identification of the facial nerve in medial and lateral directions.52,54,55
A 10% incidence of severe postoperative headaches has been reported with this approach.56 This symptom may be related to the intradural spread of bone dust, which occurs as a result of drilling the posterior lip of the internal auditory canal. Unlike in the translabyrinthine approach, drilling should be intradural in the retrosigmoid approach. Replacing the retrosigmoid bone flap with microplates or cranioplasty with synthetic materials has reduced the incidence of headaches with retrosigmoid surgery, suggesting that scar and muscle adherence to the dura also is an important factor.
Middle Fossa Approach
Basic Approach
A temporal craniotomy permits exposure of the internal auditory canal after identification of the superior semicircular canal and geniculate ganglion (Fig. 177-31).
Indications
The middle fossa approach is ideally suited for resection of intracanalicular acoustic neuromas. It provides limited exposure for surgery of lesions involving the CPA. CPA involvement beyond 1 cm is a relative contraindication to this approach. Decompression of the internal auditory canal in cases of acoustic nerve tumors in only hearing ears can be accomplished in this fashion.57
Technique
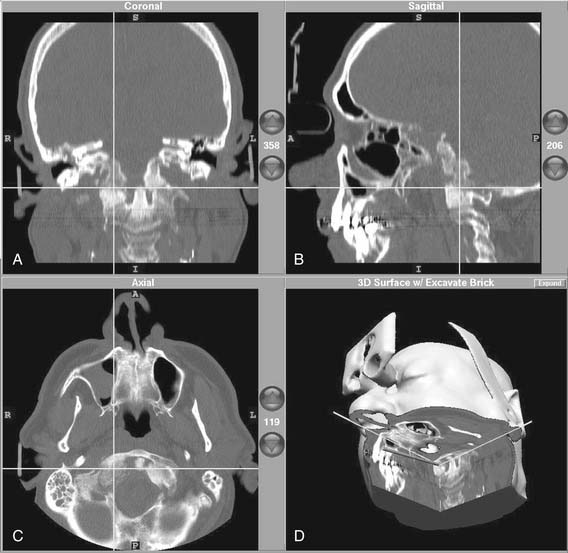
The basic surgical exposure is illustrated in Figure 177-32A and B. Mannitol and furosemide are used in addition to hyperventilation for brain relaxation. A lumbar drain placed at the beginning of the procedure can facilitate dural elevation before open drainage of the cerebrospinal fluid. A preauricular incision is extended along the temporal region. The temporalis muscle may be reflected inferiorly or split and retracted to expose the squamosa of the temporal bone. A 5 cm × 5 cm craniotomy is centered over the zygomatic root.
The floor of the middle fossa is exposed by dissecting and elevating the dura from posterior to anterior to avoid injury to the geniculate ganglion, which can be dehiscent in up to 15% of patients. The principal landmarks for orientation are the greater superficial petrosal nerve and the arcuate eminence; however, these can be deceptive. Antidromic stimulation of the greater superficial petrosal nerve with the facial nerve stimulator can be helpful for orientation.58 In addition, preoperative coronal CT can reveal the extent of pneumatization over the internal auditory canal and labyrinth.
Special Features
The success of hearing preservation in selected patients is more than 70%; however, the limiting factor is the cochlear blood supply.59 Just as in retrosigmoid hearing preservation cases, the rate of cochlear nerve preservation is substantially higher than that of actual hearing preservation. The approach is not usually recommended in patients older than 65 years because in this age group, the dura is more adherent and fragile.
Retrolabyrinthine Approach
Basic Approach
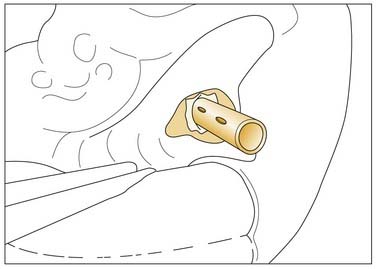
A wide mastoidectomy with skeletonization of the posterior semicircular canal permits access to the CPA with preservation of the seventh and eighth cranial nerves (Fig. 177-33).
Indications
The principal indication for the retrolabyrinthine approach is vestibular nerve section for intractable vertigo or for neurotomy in tic douloureux.60 With posterior fossa neoplasms, the retrolabyrinthine approach affords adequate exposure and hearing preservation in selected cases of arachnoid cysts, meningiomas, and metastatic lesions of the CPA. In situations in which tissue diagnosis is required before proceeding with definitive surgery, the retrolabyrinthine approach permits wide exposure with minimal morbidity for exploration of lesions of the CPA.
Technique
The dura is opened as a flap that begins just medial to the sigmoid sinus and preserves the endolymphatic sac. The flap is held anteriorly with sutures and tumor dissection can be performed in the posterior fossa (Fig. 177-34). Once the CPA is entered and CSF is released, the cerebellum falls away. Accordingly, cerebellar retractors are not necessary to expose the cranial nerves in the CPA. Instead, the sigmoid is collapsed posteriorly with fenestrated suction.
Transcochlear Approach
Basic Approach
As an extension of the translabyrinthine approach, the transcochlear approach affords additional exposure of the skull base. This is obtained by displacement of the facial nerve and removal of the cochlea to provide surgical access to lesions of the petrous tip and clivus (Fig. 177-35).
Indications
Petroclival meningiomas and epidermoids of the petrous tip are the principal lesions for which the transcochlear approach has been applied.61 However, extensions of this approach have been used with glomus jugulare tumors, temporal bone carcinomas, and extensive nonacoustic neuromas of the CPA.
Technique
The facial nerve is decompressed from the stylomastoid foramen to the geniculate ganglion. After sectioning of the chorda tympani nerve using an extended facial recess approach and transection of the greater superficial petrosal nerve, the facial nerve can be transposed posteriorly. Transposition of the nerve removes the obstacle to extending the exposure anteriorly. The transcochlear approach has been modified to include transection of the external auditory canal and two-layered closure of the meatus. This modification permits removal of the posterior wall of the external auditory canal, thus allowing wider anterior exposure in the areas of the jugular bulb and internal carotid artery. Next, the incus and stapes are removed. The cochlea is removed, thereby exposing the carotid artery anteriorly, the jugular bulb and inferior petrosal sinus inferiorly, and the superior petrosal sinus superiorly. The bony exposure leaves a dura-covered window extending from the superior petrosal sinus to the inferior petrosal sinus and reaching the clivus medially (Fig. 177-36).
Transotic Approach
Indications
The transotic approach is indicated for removal of lesions of the CPA extending inferiorly into the jugular foramen or anteriorly into the clivus. Some proponents of this approach use the additional exposure anteriorly in cases of high jugular bulbs or anteriorly placed sigmoid sinuses in small to medium-sized acoustic neuromas.62 The translabyrinthine approach with wide decompression of the sigmoid sinus and posterior fossa dura provides adequate exposure of the internal auditory canal and CPA, even with large acoustic neuromas with unfavorable venous anatomy.
Technique
Through a postauricular incision, the ear is reflected anteriorly, and the external auditory meatus is transected and closed in two layers. Essentially the same procedure as that described for the transcochlear approach is performed, with the exception that the facial nerve is not completely freed from the fallopian canal. Rather, the nerve is skeletonized and left in its canal. At the conclusion of the exposure, the facial nerve is suspended, traversing the surgical field with a bony cover. The tympanic ring is removed, with systematic exposure of the jugular bulb, jugular foramen, and carotid canal. By working around the facial nerve, the surgeon has access to lesions of the internal auditory canal, CPA, clivus, and jugular foramen (Fig. 177-37). Closure is performed with obliteration of the defect with abdominal fat and optional placement of hydroxyapatite cement or titanium mesh.
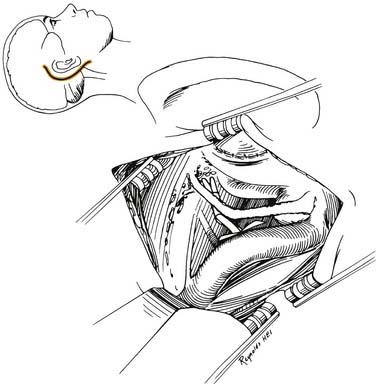
Figure 177-37. Surgeon’s view of the transotic approach to the skull base. Note that the approach is identical to the transcochlear approach except that the facial nerve is not transposed. Instead, the nerve is left in its bony canal suspended across the surgical field. The in situ facial nerve limits the exposure in its internal auditory canal and mastoid segments (compare with Fig. 177-36). Inset shows skin incision in relation to the cerebellopontine angle.
Extended Middle Fossa Approach
Basic Approach
For the extended middle fossa approach, the skull base surrounding the otic capsule is carefully removed through a wide temporal craniotomy. This approach exposes lesions extending from the posterior fossa through the tentorium and incisura and anteriorly up to the region of foramen lacerum and the posterolateral aspect of the cavernous sinus (Fig. 177-38).
Technique
The patient is positioned in the supine position with the head turned to the side. Mannitol, furosemide, and hyperventilation are used for brain relaxation. After performing a wide temporal craniotomy, the temporal lobe is progressively retracted to permit identification of the middle meningeal artery anteriorly, the petrous ridge posteriorly, and the petroclival junction medially. The internal auditory canal is uncovered using the standard middle fossa technique as described. The extension in the exposure is accomplished by removing the petrous ridge and posterior aspects of the temporal bone up to the labyrinth. Anteriorly, the temporal bone is removed up to the foramen lacerum and the internal carotid artery (see Fig. 177-32C). The area of Meckel’s cave is clearly identified, and the posterolateral portions of the cavernous sinus are accessible.
Combined Techniques (Petrosal Approach)
Technique
The usual procedures in the petrosal approach combine extended middle fossa exposure with full retrosigmoid and retrolabyrinthine exposure63 (Fig. 177-39). The middle cranial fossa, internal auditory canal, posterior fossa, and clivus all are exposed while the otic capsule is preserved. By contrast, the translabyrinthine or transcochlear approach also can be combined with the extended medial fossa approach to provide the widest possible exposure.
Experimental Approaches
Attempted hearing preservation with partial or complete labyrinthine resection has been reported. McElveen64 and Molony65 and their colleagues reported systematic sealing of the vestibule during labyrinthectomy with successful hearing preservation after translabyrinthine tumor removal. To expose the lateral internal auditory canal with the retrosigmoid approach, Arriaga and Gorum66 reported posterior semicircular canal resection with successful hearing preservation and total tumor removal in two of three enhanced retrosigmoid procedures. In extensive skull base lesions, labyrinthine procedures can be converted to partial labyrinthine resection procedures with concomitant opportunity for preserved hearing.67
Approaches for Skull Base Lesions Requiring Drainage
Translabyrinthine Approach
If hearing and vestibular function are absent, the translabyrinthine approach provides the most direct route for aeration from the mastoid through the surgical defect to the petrous apex.68 Usually, however, this approach is inappropriate because patients often have excellent hearing despite large cholesterol granulomas.
Middle Cranial Fossa Approach
The middle cranial fossa approach permits hearing preservation but requires temporal lobe retraction for exposure. Placing a catheter from the mastoid tegmen to the apex facilitates drainage, and despite its relatively long course, the catheter remains patent, with radiologic follow-up confirmation of aeration.43
Infralabyrinthine Approach
After a complete mastoidectomy, the sigmoid sinus is decompressed, the posterior semicircular canal and jugular bulb are identified, and the infralabyrinthine air cells are followed into the petrous apex. The cyst is incised, drained, and stented open into the mastoid68 (Fig. 177-40). This approach may be technically impossible in a patient with a high jugular bulb. Furthermore, although reexploration is possible in the face of an occluded stent, revision surgery requires formal reexploration of the transmastoid approach.
Transcanal Infracochlear Approach
The external auditory canal is transected and a superiorly based tympanomeatal flap is elevated. The bony external meatus is widened, and the air cell tract between the jugular bulb and carotid artery is followed into the petrous apex (Fig. 177-41). This approach permits dependent drainage of the petrous apex into the middle ear (see Fig. 177-14). If the drainage tube becomes obstructed, it may be revised through an office myringotomy. The principal disadvantages are the prolonged healing time required for the external auditory canal and the need for direct exposure of the petrous carotid artery.42
Selection of Surgical Approach
Patients with small tumors and good hearing have three options for surgical removal: the translabyrinthine approach, which destroys hearing; the middle fossa approach in young patients with small, mainly intracanalicular tumors; and the retrosigmoid approach in small tumors of the CPA that do not extend to the fundus of the internal auditory canal. In general, the best hearing and functional outcomes result from individualizing the surgical approach to patient and tumor characteristics.69
Realistic patient counseling is necessary regarding hearing conservation procedures. Gardner and Robertson51 rigorously reviewed the literature on hearing preservation in acoustic neuroma surgery. Of 621 reported cases of attempted hearing preservation, the success rate was 33%. Reported successful hearing preservation rates often refer only to measurable hearing. The rate is far lower if useful hearing (speech reception threshold [SRT] greater than 30 dB and speech discrimination less than 70%) or serviceable hearing (SRT greater than 50 dB and speech discrimination less than 50%) is considered. Furthermore, long-term follow-up studies reveal that 56% of patients experience significant loss of the preserved hearing over time.59
Patient Management and Surgical Complications
Comprehensive management of patients with skull base neoplasms of the posterior fossa requires a team of specialists familiar with the particular needs of this patient group. In addition to neuro-otologists and neurosurgeons, designated internists, anesthesiologists, radiologists, ophthalmologists,70 and critical care nurses are essential members of the team for optimal care of these patients with very specialized needs.
Intraoperative Monitoring
Although ABR monitoring often is used in hearing preservation cases, this modality provides only delayed feedback to the surgeon and cannot affect intraoperative decision-making or tissue manipulation in a meaningful way. The advent of direct eighth nerve recording systems and electrocochleography (ECoG) modifications may make a practical real-time feedback system available to the surgeon in hearing preservation cases.71
Complications
Anteroinferior Cerebellar Artery
Tumor manipulation at the brainstem may result in changes in vital signs. Such changes are related to ischemia, which usually is in the distribution of the anterior inferior cerebellar artery. Manipulation is stopped temporarily until vital sign changes have resolved. Vital sign alterations that do not resolve or that recur with any tumor manipulation constitute an indication to terminate the procedure. Complete interruption of this vessel may result in Atkinson’s syndrome, infarction of the lateral tegmental pons, which often is fatal.72 This complication can occur in a nonfatal form if branches of the anterior inferior cerebellar artery are transected.
Meningitis
Any procedure that exposes the subarachnoid space can be complicated by postoperative meningitis. The diagnosis is suspected in a patient with a toxic appearance including stiff neck, recent headache, and fever. Appropriate CSF studies will confirm the diagnosis. The mean time to onset of meningitis in patients with acoustic neuroma is 8 days postoperatively. Aggressive medical management of this complication successfully avoids any serious complications from meningitis, other than a delayed hospital discharge.73
Facial Nerve Injury
Facial nerve transection ideally is managed by immediate repair or with an interposition graft.74 In the translabyrinthine approach, access to the middle ear and mastoid portions of the nerve can provide a longer distal segment to ensure a tension-free anastomosis.75 In cases in which direct repair is impossible or in which an intact nerve does not resume function within 1 year, the facial hypoglossal anastomosis provides reliable facial reanimation.76 Temporalis muscle transfer is an alternative technique for reanimation of the paralyzed face that offers the benefit of immediate effects.
Ophthalmologic Considerations
The combination of facial paralysis and corneal insensitivity, which is more likely with surgery of large tumors, predisposes the cornea to significant trauma. Patients who are expected to have prolonged facial paralysis (6 months) are managed with a brow lift and placement of eyelid springs or gold weights. In cases of partial facial weakness or when the paralysis is expected to be short term, aggressive medical management including a regimen of lubricating drops or ointments, use of contact lenses, moisture chamber protection, and nightly taping of the affected eye is undertaken.26 In some centers, gold weight implantation in the upper lid is the preferred technique for managing corneal exposure.
Arriaga MA, Chen DA. Hydroxyapatite cement cranioplasty in translabyrinthine acoustic neuroma surgery. Otolaryngol Head Neck Surg. 2002;126:512.
Arriaga MA, Luxford WM, Atkins JSJr, et al. Predicting long-term facial nerve outcome following acoustic neuroma surgery. Otolaryngol Head Neck Surg. 1993;108:220.
Arriaga MA. Petrous apex effusion: a clinical disoder. Laryngoscope. 2007;116:1349.
Atkinson WJ. The anterior inferior cerebellar artery: its variations, pontine distribution and significance in the surgery of cerebellopontine angle tumors. J Neurol Neurosurg Psychiatry. 1949;12:137.
Brackmann DE, Anderson RG. Cholesteatomas of the cerebellopontine angle. In: Silverstein H, Norrell H, editors. Neurological Surgery of the Ear. Birmingham, Ala: Aesculapius Publishing; 1979:54-63.
Brackmann DE, Bartels LJ. Rare tumors of the cerebellopontine angle. Otolaryngol Head Neck Surg. 1980;88:555.
Brackmann DE, Hitselberger WE. Retrolabyrinthine approach: technique and newer indications. Laryngoscope. 1978;88:286.
Brackmann DE, Kwartler JA. A review of acoustic tumors: 1983-1988. Am J Otol. 1990;11:216.
Carrier D, Arriaga MA. Cost-effective evaluation of asymmetric sensorineural hearing loss with focused magnetic resonance imaging. Otolaryngol Head Neck Surg. 1997;116:567.
Daspit P, Spetzler R. The petrosal approach in otologic surgery. In: Brackmann DE, Shelton C, Arriaga MA, editors. Otologic Surgery. Philadelphia: WB Saunders; 1994:677-690.
Don M, Masuda A, Nelson R, et al. Successful detection of small acoustic tumors using the stacked derived-band auditory brainstem response amplitude. Am J Otol. 1997;18:608.
Gardner G, Robertson JH. Hearing preservation in unilateral acoustic neuroma surgery. Ann Otol Rhinol Laryngol. 1988;97:55.
Giddings NA, Brackmann DE, Kwartler JA. Transcanal infracochlear approach to the petrous apex. Otolaryngol Head Neck Surg. 1991;104:29.
Glasscock ME, Steenerson RL. A history of acoustic tumor surgery 1961-present. In: House WF, Leutje CM, editors. Acoustic Tumors. Baltimore: University Park Press; 1979:33-44.
House WF. Translabyrinthine approach. In: House WF, Leutje CM, editors. Acoustic Tumors. Baltimore: University Park Press; 1979:43-88.
House WF, De la Cruz A, Hitselberger WE. Surgery of the skull base: transcochlear approach to the petrous apex and clivus. Otolaryngology. 1978;86:770.
Jackler RK, Cho M. A new theory to explain the genesis of petrous apex cholesterol granuluma. Otol Neurotol. 2003;24:94.
Janecka IP, Sekhar LN, Horton JA, et al. General blood flow evaluation. Update II. Cummings CW, Frederickson JM, Harker JM, et al, editors. Otolaryngology—Head and Neck Surgery. St Louis: Mosby. 1990:54-63.
Kevanishvili Z. The detection of small acoustic tumors: the stacked derived-band ABR procedure. [Comment on Am J Otol. 1997;18:608.]. Am J Otol. 2000;21:148.
Lo WM. Tumors of the temporal bone and cerebellopontine angle. In: Som PM, Bergeron RT, editors. Head and Neck Imaging. St Louis: Mosby; 1991:420-445.
McElveen JTJr, Wilkins RH, Erwin AC, et al. Modifying the translabyrinthine approach to preserve hearing during acoustic tumor surgery. J Laryngol Otol. 1991;105:34.
Samii M, Turel KE, Penkert G. Management of seventh and eighth nerve involvement by cerebellopontine angle tumors. Clin Neurosurg. 1985;32:242.
Sekhar LN, Jannetta PJ. Cerebellopontine angle meningiomas: microsurgical excision and follow-up results. J Neurosurg. 1984;60:500.
Selters WA, Brackmann DE. Acoustic tumor detection with brainstem electric response audiometry. Arch Otolaryngol Head Neck Surg. 1977;103:181.
Shelton C, Hitselberger WE, House WF, et al. Hearing preservation after acoustic tumor removal: long-term results. Laryngoscope. 1990;100:115.
Thomsen J, Tos M, Harmsen A. Acoustic neuroma surgery: results of translabyrinthine removal in 300 patients. Discussion of choice of approach in relation to overall results and possibility of hearing preservation. Br J Neurosurg. 1989;3:349.
1. Brackmann DE, Bartels LJ. Rare tumors of the cerebellopontine angle. Otolaryngol Head Neck Surg. 1980;88:555.
2. Valvanis A, Schubiger O, Naidich TP. Clinical Imaging of the Cerebellopontine Angle. Berlin: Springer-Verlag; 1986.
3. Martuza RL, Eldridge R. Neurofibromatosis 2 (bilateral acoustic neurofibromatosis). N Engl J Med. 1988;318:684.
4. Brackmann DE, Gherini SE. Differential diagnosis of skull base neoplasms involving the posterior fossa. In: Cummings CW, Frederickson JM, Harker LA, Krause CJ, Schuller DE, editors. Otolaryngology—Head and Neck Surgery. St Louis: Mosby; 1986:3421-3436.
5. House WF. Translabyrinthine approach. In: House WF, Leutje CM, Doyle KJ, editors. Acoustic Tumors. San Diego: Singular Publishing; 1997:171-176.
6. Johnson EW. Results of auditory tests in acoustic tumor patients. In: House WF, Luetje CM, editors. Acoustic Tumors. Baltimore: University Park Press; 1979:209-224.
7. Kasantikul V, Netsky MG, Glasscock ME3rd, et al. Acoustic neurilemmoma: clinicoanatomical study of 103 patients. J Neurosurg. 1980;52:28.
8. Sataloff RT, Davies B, Myers DL. Acoustic neuromas presenting as sudden deafness. Am J Otol. 1985;6:349.
9. Beatty CW, Ebersold MJ, Harner SG. Residual and recurrent acoustic neuromas. Laryngoscope. 1987;97:1168.
10. Jerger J, Oliver TA, Jenkins H. Suprathreshold abnormalities of the stapedius reflex in acoustic tumors: a series of case reports. Ear Hear. 1987;8:131.
11. Carrier D, Arriaga MA. Cost-effective evaluation of asymmetric sensorineural hearing loss with focused magnetic resonance imaging. Otolaryngol Head Neck Surg. 1997;116:567.
12. Brackmann DE, Kwartler JA. A review of acoustic tumors: 1983-1988. Am J Otol. 1990;11:216.
13. Selters WA, Brackmann DE. Acoustic tumor detection with brainstem electric response audiometry. Arch Otolaryngol Head Neck Surg. 1977;103:181.
14. Wilson D, Hodgson RS, Gustafson MF, et al. The sensitivity of auditory brainstem response testing in small acoustic neuromas. Laryngoscope. 1992;102:961.
15. Chandrasekhar SS, Brackmann DE, Devgan KK. Utility of auditory brainstem response audiometry in diagnosis of acoustic neuromas. Am J Otol. 1995;16:63.
16. Kevanishvili Z. The detection of small acoustic tumors: the stacked derived-band ABR procedure. [Comment on Am J Otol. 1997;18:608.]. Am J Otol. 2000;21:148.
17. Don M, Masuda A, Nelson R, et al. Successful detection of small acoustic tumors using the stacked derived-band auditory brainstem response amplitude. Am J Otol. 1997;18:608.
18. Linthicum FHJr, Waldorf R, Luxford WM, et al. Infrared/video ENG recording of eye movements to evaluate the inferior vestibular nerve using the minimal caloric test. Otolaryngol Head Neck Surg. 1988;98:207.
19. Lo WM. Tumors of the temporal bone and cerebellopontine angle. In: Som PM, Bergeron RT, editors. Head and Neck Imaging. St Louis: Mosby; 1991:420-445.
20. Segall HD, Zee CS, Naidich TP, et al. Computed tomography in neoplasms of the posterior fossa in children. Radiol Clin North Am. 1982;20:237.
21. Press GA, Hesselink JR. MR imaging of cerebellopontine angle and internal auditory canal lesions at 1.5T. AJR Am J Roentgenol. 1988;150:1371.
22. Jackler RK, Shapiro MS, Dillon WP, et al. Gadolinium-DTPA enhanced magnetic resonance imaging in acoustic neuroma. Otolaryngol Head Neck Surg. 1990;102:67.
23. Barrs DM, Luxford WM, Becker TS, et al. Computed tomography with gas cisternography for detection of small acoustic tumors. Arch Otolaryngol Head Neck Surg. 1984;110:535.
24. Shelton C, Harnsberger HR, Allen R, et al. Fast spin echomagnetic resonance imaging: clinical applications in screening for acoustic neuroma. Otolaryngol Head Neck Surg. 1996;114:71.
25. Rubenstein LJ. Tumors of the central nervous system. Second Series, Fascicle 6. Firminger HI, editor. Atlas of Tumor Pathology. Washington, DC: Armed Forces Institute of Pathology. 1972:1-400.
26. Langman AW, Jackler RK, Althaus SR. Meningioma of the internal auditory canal. Am J Otol. 1990;11:201.
27. Elster AD, Challa VR, Gilbert TH, et al. Meningiomas: MR and histopathologic features. Radiology. 1989;170:857.
28. Russell DS, Rubenstein LJ. Pathology of Tumors of the Nervous System, 4th ed. Edinburgh: T & A Constable; 1977.
29. Sekhar LN, Jannetta PJ. Cerebellopontine angle meningiomas: microsurgical excision and follow-up results. J Neurosurg. 1984;60:500.
30. Brackmann DE, Anderson RG. Cholesteatomas of the cerebellopontine angle. In: Silverstein H, Norrell H, editors. Neurological Surgery of the Ear. Birmingham, Ala: Aesculapius Publishing; 1979:340-344.
31. Garcia CA, McGarry PA, Rodriguez F. Primary intracranial squamous cell carcinoma of the right cerebellopontine angle. J Neurosurg. 1981;54:824.
32. Dort JC, Fisch U. Facial nerve schwannomas. Skull Base Surg. 1991;1:51.
33. Syms CA3rd, House JR3rd, Luxford WM, et al. Preoperative electroneuronography and facial nerve outcome in acoustic neuroma surgery. Am J Otol. 1997;18:401.
34. Kartush JM, Niparko JK, Graham MD, et al. Electroneurography: preoperative facial nerve assessment for tumors of the temporal bone. Otolaryngol Head Neck Surg. 1987;97:257.
35. McCormick PC, Bello JA, Post KD. Trigeminal schwannoma. Surgical series of 14 cases with review of the literature. J Neurosurg. 1988;69:850.
36. Spector GJ, Sobol S, Thawley SE, et al. Glomus jugulare tumors of the temporal bone: patterns of invasion of the temporal bone. Laryngoscope. 1979;89:1628.
37. Janecka IP, Sekhar LN, Horton JA, et al. General blood flow evaluation. Update II. Cummings CW, Frederickson JM, Harker JM, et al, editors. Otolaryngology—Head and Neck Surgery. St Louis: Mosby. 1990:54-63.
38. Haberkamp TJ, Monsell EM, House WF, et al. Diagnosis and treatment of arachnoid cysts of the posterior fossa. Otolaryngol Head Neck Surg. 1990;103:612.
39. Pappas DG, Brackmann DE. Arachnoid cysts of the posterior fossa. Otolaryngol Head Neck Surg. 1981;89:328.
40. Balkany T, Fradis M, Jafek BW, et al. Hemangioma of the facial nerve: role of the geniculate capillary plexus. Skull Base Surg. 1991;1:59.
41. Jackler RK, Cho M. A new theory to explain the genesis of petrous apex cholesterol granuluma. Otol Neurotol. 2003;24:94-106.
42. Giddings NA, Brackmann DE, Kwartler JA. Transcanal infracochlear approach to the petrous apex. Otolaryngol Head Neck Surg. 1991;104:29.
43. Arriaga MA. Petrous apex effusion: a clinical disorder. Laryngoscope. 2007;116:1349.
44. Arriaga MA, Lo WW, Brackmann DE. Clinical and imaging characteristics of metastatic melanoma to the cerebellopontine angle. Arch Otolaryngol Head Neck Surg. 1995;121:1052.
45. Batsakis JG. Tumors of the Head and Neck, 2nd ed. Baltimore: Williams & Wilkins; 1979.
46. Glasscock ME, Steenerson RL. A history of acoustic tumor surgery 1961-present. In: House WF, Leutje CM, editors. Acoustic Tumors. Baltimore: University Park Press; 1979:33-34.
47. Arriaga MA, Chen DA. Hydroxyapatite cement cranioplasty in translabyrinthine acoustic neuroma surgery. Otolaryngol Head Neck Surg. 2002;126:512.
48. Arriaga MA, Chen DA, Burke EL. Hydroxyapatite cement cranioplasty in translabyrinthine acoustic neuroma surgery—update. Otol Neurotol. 2007;28:538.
49. Arriaga MA, Luxford WM, Atkins JSJr, et al. Predicting long-term facial nerve outcome following acoustic neuroma surgery. Otolaryngol Head Neck Surg. 1993;108:220.
50. Thomsen J, Tos M, Harmsen A. Acoustic neuroma surgery: results of translabyrinthine removal in 300 patients. Discussion of choice of approach in relation to overall results and possibility of hearing preservation. Br J Neurosurg. 1989;3:349.
51. Gardner G, Robertson JH. Hearing preservation in unilateral acoustic neuroma surgery. Ann Otol Rhinol Laryngol. 1988;97:55.
52. Harner SG, Beatty CW, Ebersold MJ. Retrosigmoid removal of acoustic neuroma: experience 1978-1988. Otolaryngol Head Neck Surg. 1990;103:40.
53. Kemink JL, LaRouere MJ, Kileny PR, et al. Hearing preservation following suboccipital removal of acoustic neuroma. Laryngoscope. 1990;100:597.
54. Devriese PP, van der Werf AJ, van der Borden J. Facial nerve function after suboccipital removal of acoustic neurinoma. Arch Otorhinolaryngol. 1984;240:193.
55. Samii M, Turel KE, Penkert G. Management of seventh and eighth nerve involvement by cerebellopontine angle tumors. Clin Neurosurg. 1985;32:242.
56. Silverstein HE, Norrell H, Wanamaker H, et al. Microsurgical posterior fossa vestibular neurectomy: an evolution in technique. Skull Base Surg. 1991;1:16.
57. Gadre AK, Kwartler JA, Brackmann DE, et al. Middle fossa decompression of the internal auditory canal in acoustic neuroma surgery: a therapeutic alternative. Laryngoscope. 1990;100:948.
58. Arriaga MA, Haid RT, Masel DA. Antidromic stimulation of the greater superficial petrosal nerve (GSPN) in middle fossa surgery. Laryngoscope. 1995;105:102.
59. Shelton C, Hitselberger WE, House WF, et al. Hearing preservation after acoustic tumor removal: long-term results. Laryngoscope. 1990;100:115.
60. Brackmann DE, Hitselberger WE. Retrolabyrinthine approach: technique and newer indications. Laryngoscope. 1978;88:286.
61. House WF, De la Cruz A, Hitselberger WE. Surgery of the skull base: transcochlear approach to the petrous apex and clivus. Otolaryngology. 1978;86:770.
62. Jenkins HA, Fisch U. The transotic approach to resection of difficult acoustic tumors of the cerebellopontine angle. Am J Otol. 1980;2:70.
63. Daspit P, Spetzler R. The petrosal approach in otologic surgery. In: Brackmann DE, Shelton C, Arriaga MA, editors. Otologic Surgery. Philadelphia: WB Saunders; 1994:677-690.
64. McElveen JTJr, Wilkins RH, Erwin AC, et al. Modifying the translabyrinthine approach to preserve hearing during acoustic tumor surgery. J Laryngol Otol. 1991;105:34.
65. Molony TB, Kwartler JA, House WF, et al. Extended middle fossa and retrolabyrinthine approaches in acoustic neuroma surgery: case reports. Am J Otol. 1992;13:360.
66. Arriaga MA, Gorum M. Enhanced retrosigmoid exposure with posterior semicircular canal resection. Otolaryngol Head Neck Surg. 1996;115:46.
67. Hirsch BE, Cass SP, Sekhar LN, et al. Translabyrinthine approach to skull base tumors with hearing preservation. Am J Otol. 1993;14:533.
68. Gherini SG, Brackmann DE, Lo WW, et al. Cholesterol granuloma of the petrous apex. Laryngoscope. 1985;95:659.
69. Arriaga MA, Chen DA, Fukushima T. Individualizing hearing preservation in acoustic neuroma surgery. Laryngoscope. 1997;107:1043.
70. Levine RE. Management of the eye after acoustic tumor surgery. In: House WF, Leutje CM, editors. Acoustic Tumors. Baltimore: University Park Press; 1979:105-152.
71. Morawski KF, Niemczyk K, Bohorquez J, et al. Intraoperative monitoring of hearing during cerebellopontine angle tumor surgery using transtympanic electrosochleography. Otol Neurotol. 2007;28:541.
72. Atkinson WJ. The anterior inferior cerebellar artery: its variations, pontine distribution and significance in the surgery of cerebellopontine angle tumors. J Neurol Neurosurg Psychiatry. 1949;12:137.
73. Brow RE. Pre- and postoperative management of the acoustic tumor patient. In: House WF, Leutje CM, editors. Acoustic Tumors. Baltimore: University Park Press; 1979:153-174.
74. Arriaga MA, Brackmann DE. The technique and outcome of facial nerve repair in cerebellopontine angle tumor surgery. Am J Otol. 1992;13:356.
75. Barrs DM, Brackmann DE, Hitselberger WE. Facial nerve anastomosis in the cerebellopontine angle: a review of 24 cases. Am J Otol. 1984;5:269.
76. Luxford WM, Brackmann DE. Facial nerve substitution: a review of sixty-six cases. Am J Otol Suppl. 1985:55.