CHAPTER 103 Neoplasms of the Hypopharynx and Cervical Esophagus
Anatomy of the Hypopharynx and Cervical Esophagus
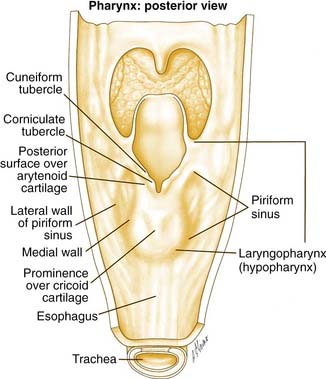
The hypopharynx is the region of the pharynx that extends from the oropharynx superiorly to the cervical esophagus inferiorly. The cervical esophagus is that portion of the esophagus that extends to the thoracic inlet. The superior extent of the hypopharynx is approximately at the level of the hyoid bone or at the level of the pharyngoepiglottic folds. Inferiorly, the hypopharynx tapers to the esophageal introitus at the cricopharyngeus muscle. It is bordered anteriorly by the larynx and posteriorly by the retropharyngeal space. It is subdivided into three regions: the pyriform sinuses, the postcricoid region, and the posterior pharyngeal wall (Fig. 103-1).
The pyriform sinuses (one on each side) are composed of anterior, medial, and lateral walls that form an inverted pyramid with the base of the pyramid at the level of the pharyngoepiglottic fold and the apex extending to just below the cricoid cartilage. This lower region is lateral to the aryepiglottic folds and medial to the thyroid lamina. Because of this close relationship, tumors of the hypopharynx often extend to invade the larynx.1 The medial pyriform mucosa forms the posterior wall of the paraglottic space and is separated from the endolarynx by the aryepiglottic folds and the lateral cricoarytenoid muscles. Hypopharyngeal tumors extending medially from the medial wall of the pyriform sinus can, therefore, invade into the larynx.
The aryepiglottic folds separate the endolarynx from the medial wall of the pyriform sinus bilaterally and form what has been termed the “marginal area.” Although the aryepiglottic folds are actually part of the supraglottic larynx, tumors arising at this site behave aggressively like hypopharyngeal cancers and not supraglottic carcinomas.2
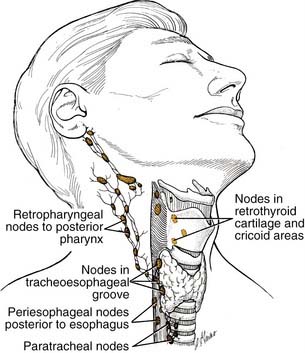
Lymphatic drainage from the pyriform sinuses passes through the thyrohyoid membrane primarily to the jugulodigastric lymph node and to the midjugular and spinal accessory chains (Fig. 103-2). Lymphatic vessels from the inferior portion of the hypopharynx and from the postcricoid region also drain to the paratracheal and paraesophageal nodes and to nodes in the supraclavicular fossa. Lymphatic drainage from the posterior hypopharyngeal wall is to the retropharyngeal nodes and to the midjugular chain. The retropharyngeal nodes are divided into a medial and lateral group. Lateral retropharyngeal nodes, also known as the nodes of Rouvière, are present at the level of the skull base.
Incidence
The incidence of cancer of the hypopharynx varies depending on worldwide geographic location. In addition, the incidence of cancer arising from specific subsites within the hypopharynx may also vary by geographic location. In the United States, three large database reviews shed light on the epidemiology of hypopharyngeal carcinoma. Neoplasms specific to the cervical esophagus are not mentioned in the first two of these three studies. Canto and Devesa3 analyzed data from 1975 to 1988 in nine registries of the Surveillance, Epidemiology, and End Results (SEER) database. Although these data are not a random sample because each registry is from a specific geographic location, they represent approximately 10% of the U.S. population. The rates of cancer per 100,000 person-years vary with race and sex. It is also not clear from this study why the sites are listed separately as hypopharynx and pyriform. Whether there is overlap in these numbers is not stated in this study. For white males the rate is 0.4 and 0.9 for the hypopharynx and pyriform sinus, respectively. For African American males the rate is 0.8 and 2.3 for the hypopharynx and pyriform sinus, respectively. For white females the rate is 0.2 for both the hypopharynx and pyriform sinus. For African American females the rate is 0.2 and 0.5 for the hypopharynx and pyriform sinus, respectively.
Hoffman and colleagues4 conducted a second major database review of the National Cancer Data Base (NCDB) report, which is a comprehensive hospital-based registry of all cancer cases encountered. The data cover the years 1985 to 1994 and reveal that hypopharyngeal malignancy comprised 4.3% of all head and neck cancers, which included salivary gland and thyroid tumors in this study. Ninety-five percent of the hypopharyngeal tumors were squamous cell carcinomas with the remainder being adenocarcinomas, lymphoma, and other pathologies. Stage III and IV tumors comprised 77.3% of cancers, demonstrating the advanced stage of hypopharyngeal tumors at presentation. The survival data in this study were noteworthy in that hypopharyngeal cancers had the worst 5-year survival compared with any other primary site in the head and neck. The 5-year disease-specific survival was 31.4%.
Finally, Davies and Welch5 analyzed the updated SEER database for the years 1999 to 2001 and found an average of 75,000 new cases of head and neck cancer. Interestingly, compared with the average 1975 statistics, this study found that incidence and mortality rates for hypopharyngeal and cervical esophageal cancer were decreasing. The one caveat to consider in saying that this trend was a definitive finding is that the rates of these tumors were below 1 per 100,000. Notwithstanding this statistic, this study shows a continued significant incidence of these deadly cancers within all head and neck squamous cell carcinomas.
The statistics outside of the United States reveal similar epidemiologic data. Wahlberg and colleagues6 analyzed the comprehensive Swedish databases, covering 1960 to 1989, and found rates of 1.22/100,000 for men and 0.45/100,000 for women, similar to rates described by Canto and Devesa.3 During this time the rates for men increased, but the rates for women decreased. The authors suggest that this may reflect a decrease in Plummer-Vinson syndrome, the sideropenic anemia common in Northern Sweden that predisposes to development of postcricoid carcinoma.
The data on cervical esophageal carcinoma are much more limited. Esophageal carcinoma causes approximately 12,000 deaths per year in the United States.7,8 In one series, cervical esophageal carcinoma represented only 5.3% (9/168 patients) of all esophageal cancers.9 Given this low frequency, the data are limited on the incidence and optimal treatment of this cancer. Additionally, these tumors are often included in statistics regarding all esophageal cancers.
Clinical Evaluation
PATIENT SYMPTOMS
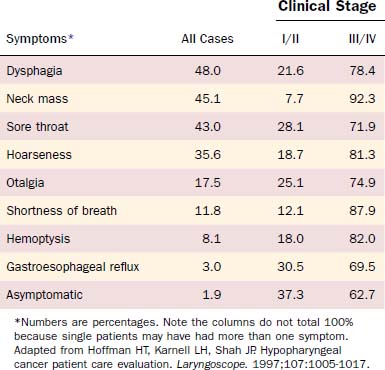
Patients with lesions in the hypopharynx and cervical esophagus usually present at an advanced stage of disease. Lesions in this area can grow unabated to a size larger than most other head and neck sites because the anatomic boundaries of neighboring structures are not as limiting as for other sites (e.g., the larynx). Thus a disturbance of function is not seen until the disease is advanced. Because this area also has a rich lymphatic drainage, a neck mass is not an uncommon finding, especially in the advanced-stage disease. Hoffman and colleagues10 analyzed presenting symptoms in 2939 cases from the Patient Care Evaluation study of the American College of Surgeons Commission on Cancer (Table 103-1). For stage I/II disease, gastroesophageal reflux, a common and nonspecific symptom, was most common (30.5%) followed closely by sore throat (28.1%). Importantly, 37.3% of patients with early-stage disease were asymptomatic at presentation. For stage III/IV disease, the most common symptom was a neck mass (92.3%) followed by shortness of breath (87.9%). Dysphagia occurred in 21.6% of early-stage and 78.4% of advanced-stage lesions. Additionally, referred otalgia was present in 25.1% of early-stage and 74.9% of advanced-stage lesions.
PHYSICAL FINDINGS
The overall appearance of these patients will likely reveal a malnourished individual. A complete examination of the head and neck should be performed with a focus on the mucosa of the upper aerodigestive tract to evaluate the extent of the primary tumor and to assess for second primaries. Synchronous tumors were present in a significant proportion of asymptomatic stage I/II patients in the Hoffman and colleagues’ study.10 In the same study a significant portion of patients presented with a neck mass; thus clinical staging of the neck to define the nodal stage is also critical. In the clinic, fiberoptic laryngoscopy should be used to evaluate the airway because impending airway distress is a possibility with advanced tumors. Immobility of the vocal cord(s) may reveal whether the larynx has been invaded.
In defining the options for managing a patient with hypopharyngeal and cervical esophageal cancer, the following must be addressed. The extent of tumor and lymph node involvement must be defined by office examination, operative endoscopy, and imaging. For tumors involving the posterior pharyngeal wall, invasion of the prevertebral fascia needs to be assessed clinically and radiographically to ensure an adequate surgical margin. Because distant metastases in certain series are the highest for hypopharyngeal cancers,11 a metastatic workup by imaging and laboratory testing should also be completed. Comorbidities of the patient are also a significant contributing factor in managing these patients. For example, if a conservation surgical procedure is planned, the pulmonary function status of the patient needs to be evaluated because these patients are at significant risk for aspiration and must have adequate pulmonary reserve.
Imaging
In the treatment of hypopharyngeal malignancies, the role of imaging involves the pretreatment evaluation of the extent of the primary tumor and possible metastases, as well as the evaluation of the patient following treatment. Hypopharyngeal tumors have a propensity for submucosal spread that may be undetectable on clinical or radiographic examination.12 Causes for surgical failure include submucosal extension, involvement of the thyroid gland, and metastasis to paratracheal and upper mediastinal lymph nodes.13 Thus imaging is essential to assess tumor extent or recurrence.
Although the barium swallow has been historically used to identify malignancies in this region,14 its use is now more limited. Some centers continue to use the swallow to evaluate for second primaries in the esophagus. In the case of a cervical esophageal cancer where an endoscope cannot be passed distally, the barium swallow can be used for imaging the distal extent of disease. Another use of the barium swallow study is in the postoperative setting to examine the deglutition process or to identify anastomotic problems such as stricture or fistula.
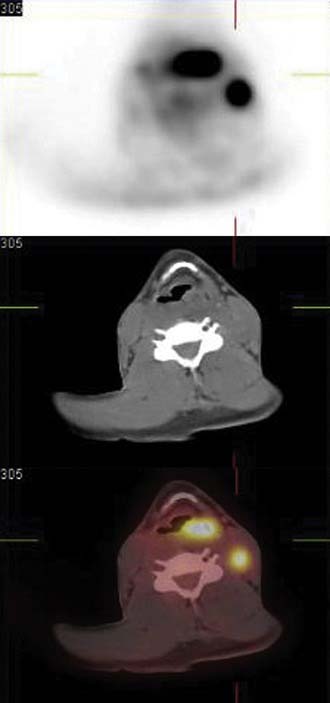
The primary imaging modality for pretreatment evaluation of the hypopharynx is cross-sectional imaging with computed tomography (CT) or magnetic resonance (MRI). In multiple studies examining the impact of cross-sectional imaging on the staging of hypopharyngeal and/or esophageal cancer, the clinical tumor stage was upstaged in up to 90% of patients.15 Accuracy of tumor staging, determined by comparison to pathologic findings, is 58% for clinical examination, 80% for CT, and 85% for MRI.16 CT is often preferred to MRI to assess cartilage invasion; however, an experienced radiologist looking for an increased T2 and decreased T1 signal intensity on MRI, which indicates cartilage involvement, can achieve a high degree of sensitivity (89% to 100%).15 The specificity of MRI for cartilage invasion, however, is inferior to CT (62% vs. 84%).17 A wide variability in specificity reflects the variability in ossification and the degree of inflammation, edema, and/or fibrosis at the tumor site. The specificity for cartilage invasion is lowest for the thyroid cartilage (57%), highest for the arytenoid cartilages (95%), and intermediate for the cricoid cartilage (87%).16 Figure 103-3 demonstrates a T4 left pyriform sinus squamous cell cancer that has invaded the arytenoid cartilage and the thyroid lamina. The incidence of a second neoplasm of the aerodigestive tract, either synchronous or metachronous to an index tumor of the hypopharynx, has been reported to be 16% to 18%11; therefore a thorough radiographic evaluation of the entire aerodigestive tract including the esophagus is necessary.18,19
There are no available data supporting the routine use of imaging with CT or MRI in the post-treatment period to follow disease-free status. Typically patient symptoms or clinical endoscopic findings will dictate the utilization of specific imaging. The role of positron emission tomography (PET) has been explored as a modality to assist in the detection of locoregional recurrence and/or persistent disease. Several studies have reported on the improved sensitivity of PET over MRI in the evaluation of response to therapy.20,21 Although this modality appears to have an increased sensitivity (86%) for recurrence over CT/MRI (57%), there is a lower measured specificity with PET (75% vs. 92% for CT/MRI).21 An improvement in specificity with PET may be achieved as newer tracers are studied. For example, Chesnay and colleagues22 described the successful use of 11C-methionine to follow post-chemotherapy tumor response. The combination of PET and CT scans (PET/CT) provides significant advantages due to the ability to correlate fluorodeoxyglucose (FDG) avid lesions with anatomic findings and is increasingly used in all phases of hypopharyngeal tumor management.23 Figure 103-4 illustrates the use of PET/CT in a patient with an advanced left pyriform sinus squamous cell carcinoma with cervical lymphadenopathy. The fused scan not only shows the primary FDG avid lesion but also highlights the left cervical lymph node with obvious tumor involvement. Thus the combination of CT, MRI, and PET scanning can be used in all phases of the management of hypopharyngeal and cervical esophageal carcinoma.
Pathology
Although the vast majority (95% in the Hoffman and colleagues’ study10) of neoplasms of the hypopharynx and cervical esophagus are squamous cell carcinomas, other rare pathologic entities have been described (Fig. 103-5). In addition, variants of squamous cell carcinoma have also been described.
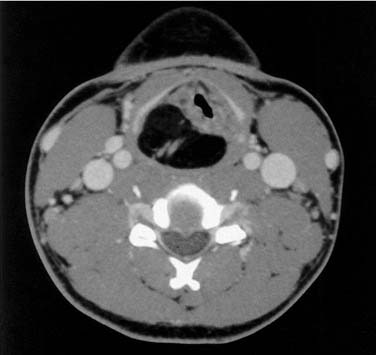
Figure 103-5. Axial computed tomography scan of a patient with a hypopharyngeal lipoma displacing the larynx.
Unusual malignant lesions include lymphomas, which can present in the hypopharynx as the primary site or can secondarily involve the hypopharynx and cervical esophagus after systemic presentation. Some of the subtypes include angiocentric T cell lymphomas, non-Hodgkin’s extranodal lymphomas, and mucosa-associated lymphoid tissue (MALT) lymphomas.24 A high index of suspicion for lymphoma should be maintained in patients with AIDS and a hypopharyngeal mass.25 Lymphomas need to be extensively staged and, depending on subtype, are treated with a combination of radiation and/or chemotherapy. The neuroendocrine tumors of the larynx and hypopharynx are a diverse subset of small cell tumors.26 Depending on the histologic subtype, treatment varies from chemotherapy and radiation to surgical therapy.
Adenocarcinomas are another rare malignant entity seen in the hypopharynx and cervical esophagus. These lesions may initiate in the minor salivary glands within the hypopharynx or ectopic gastric mucosa in the cervical esophagus. The outgrowth of adenocarcinomas from ectopic gastric mucosa is a rare event.27 Thyroid malignancies may secondarily involve the hypopharynx or cervical esophagus via direct invasion.28 Other extremely rare malignancies include sarcomas29 such as liposarcomas,30 angiosarcomas,31 and synovial sarcomas.32
Of the squamous cell cancers, there are three histologic subtypes that deserve special attention. First is basaloid squamous cell carcinoma,33 which is a bimorphic variant of squamous cell carcinoma that has distinct pathologic features. Clinically, these lesions appear primarily in the supraglottis, pyriform sinus, and tongue base and have a distinctly more aggressive clinical course.34 Second, lymphoepitheliomas are the hypopharyngeal counterpart of nasopharyngeal cancers.35,36 These tumors are treated with radiation and chemotherapy, similar to nasopharyngeal carcinoma. Finally, the adenosquamous carcinoma is another rare variant, which also behaves clinically in an aggressive manner.37
Location of Tumors and Patterns of Spread
An understanding of the site of initiation and patterns of spread of hypopharyngeal carcinoma is critical in the management of these tumors. Substantial data exist regarding the origin of the carcinoma within specific subsites of the hypopharynx. Kirchner1 described experience at the Yale University hospitals in managing hypopharyngeal carcinoma. In their patient population, 152 (86%) carcinomas arose in the pyriform sinus, 17 (10%) were in the posterior pharyngeal wall, and 8 (4%) were located in the postcricoid region. In the series of Carpenter and colleagues,38 117 (72%) carcinomas arose in the pyriform sinus, 37 (23%) in the posterior pharyngeal wall, and 8 (5%) were located in the postcricoid region. Saleh and colleagues39 described their series of patients from Egypt and found that postcricoid cancers were the majority of presenting lesions (50.1%) followed by pyriform sinus (26.5%) and finally posterior pharyngeal wall tumors (23.4%). A European cancer registry review40 demonstrated highest rates of pyriform sinus cancer in France (78%) and lowest rates in Sweden (5%). Although difficult to identify the precise reason for these subsite differences, one explanation is the possibility that an assignment of the exact origin of these tumors may be difficult except in the earliest stages.
Regarding the histologic spread of tumor, Kirchner1 performed whole organ serial sections on 51 surgically resected pyriform sinus carcinoma specimens and described several interesting findings. Tumors were able to infiltrate the larynx and behave as transglottic cancers. They may invade the lateral and posterior pharyngeal walls and they may spread into the supraglottis and base of tongue. Invasion inferiorly into the cervical esophagus was not a common finding in these specimens. Twenty-two specimens invaded the thyroid cartilage at its posterior border, and all were found to have lateral pharyngeal wall involvement. Kirchner demonstrated in this study that the exclusion of tumors with pyriform apex involvement from conservation surgery was indeed appropriate because all of these tumors had laryngeal framework invasion.
A distinct feature of hypopharyngeal carcinoma that was noted early in the surgical treatment of this disease was its tendency to spread in a submucosal fashion.13,41 This is an often-cited feature of hypopharyngeal cancer, especially in discussing its poor prognosis. Ho and colleagues12 performed a detailed analysis of submucosal extension by serial sectioning of 57 specimens of hypopharyngeal cancer. Three classes of submucosal extension were identified: type 1 was a direct submucosal extension visible on gross inspection as elevated mucosa, type 2 was direct submucosal extension that was only visible on histologic examination, and type 3 was a true “skip” lesion with no connection to the primary. Only 1 of 57 specimens had type 3 extension; however, 33 (58%) patients did have submucosal extension of some type. In contradiction to the findings of Harrison,13 significant inferior extension toward the cervical esophagus was observed. Patients treated with preoperative radiotherapy had significantly increased amounts of type 2 extension. Interestingly, in comparing overall 5-year survival, no differences were found between the group that had submucosal extension and the one that did not. Additionally, submucosal extension was not associated with increased locoregional recurrences. Thus although it is true that submucosal extension occurs in a significant number of patients with hypopharyngeal cancer, most of it is detectable clinically and a poorer prognosis is not seen in patients with submucosal extension of tumor.
Included in the data from serial sectioning by Ho and colleagues and Wei12,42 was the finding that submucosal extension was greatest in the inferior direction, followed by lateral extension, and finally superior extension. On the basis of these data, they recommended that resection margins should be 3 cm inferiorly, 2 cm laterally, and 1.5 cm superiorly in patients who have not received previous radiation. For those with previous radiation, these margins were recommended to be 4 cm, 3 cm, and 2 cm, respectively. The deep margin was recommended to be greater than 1 mm in all patients.43
Etiology and Biology
The causal relationships among alcohol and tobacco intake, genetic predisposition, diet, and socioeconomic conditions in the development of squamous cell cancers of the head and neck apply as well to hypopharyngeal cancer.44–46 In examining these factors, two specific components appear to be more closely associated with hypopharyngeal cancer. The first is alcohol intake, which appears to be more common in patients with hypopharyngeal cancer, at least when compared with laryngeal cancer.10,47–49 What the specific effect of alcohol may be in hypopharyngeal cancer development is unclear. Alcohol may have direct carcinogenic effects, may be more of a promoter of tobacco’s carcinogenic effects, or may have both functions. Two recent studies found that specific chewing tobacco products or exposure to wood smoke were associated with hypopharyngeal cancers in India.50,51
A condition specifically associated with postcricoid carcinoma is the Plummer-Vinson or Paterson-Brown-Kelly syndrome, which primarily affects women (85% of the cases).52 This syndrome represents the combination of dysphagia, iron deficiency anemia, and hypopharyngeal and esophageal webs. It is hypothesized that chronic irritation results in the hypopharyngeal webs that then progress to carcinoma. The syndrome is also geographically biased in that patients are predominantly located in the United States, Wales, and Sweden. The etiology is believed to be due to nutritional deficiency and since these issues have been addressed, the incidence of postcricoid carcinomas has declined in Sweden.6
Staging
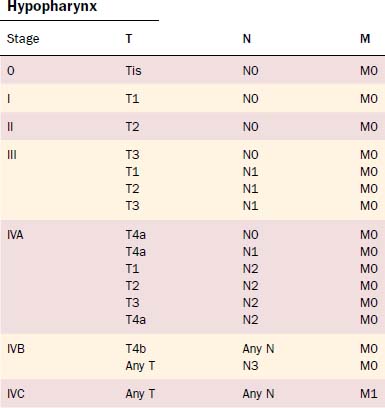
The TNM system continues to be the standard by which the morphologic extent of the tumor is described. The American Joint Commission on Cancer updated the TNM staging in 2002, and the relevant hypopharyngeal and cervical esophageal system is listed in Tables 103-2 and 103-3.53 The TNM system’s major advantages are that it allows for comparing end results, communicating about patients, determining prognosis, and selecting treatments.54 However, it is also acknowledged to have deficiencies due to inconsistencies; inaccuracies; observer variability; problems with various T, N, and M classification criteria; and exclusion of host factors. Piccirillo and colleagues from our institution have examined the role of host factors including patient symptom severity and comorbidities on outcomes of treatment. Piccirillo and colleagues55,56 demonstrated that comorbidities are an independent prognostic factor in outcomes of patients with head and neck cancer. The most recent AJCC Cancer Staging Manual53 recommends comorbidity assessment but does not yet incorporate these factors into staging.
| Primary Tumor (T) | |
| Hypopharynx | |
| TX | Primary tumor cannot be assessed |
| T0 | No evidence of primary tumor |
| T1 | Tumor limited to one subsite of hypopharynx and ≤ 2 cm in greatest dimension |
| T2 | Tumor invades more than one subsite of hypopharynx or an adjacent site, or measures > 2 cm but not > 4 cm in greatest dimension without fixation of hemilarynx |
| T3 | Tumor > 4 cm in greatest dimension or with fixation of hemilarynx |
| T4a | Tumor invades thyroid/cricoid cartilage, hyoid bone, thyroid gland, esophagus, or central compartment soft tissue (including prelaryngeal strap muscles and subcutaneous fat) |
| T4b | Tumor invades prevertebral fascia, encases carotid artery, or involves mediastinal structures |
| Cervical Esophagus | |
| TX | Primary tumor cannot be assessed |
| T0 | No evidence of primary tumor |
| Tis | Carcinoma in situ |
| T1 | Tumor invades lamina propria or submucosa |
| T2 | Tumor invades muscularis propria |
| T3 | Tumor invades adventitia |
| T4 | Tumor invades adjacent structures |
| Regional Lymph Nodes (N) | |
| Hypopharynx | |
| NX | Regional lymph nodes cannot be assessed |
| N0 | No regional lymph node metastasis |
| N1 | Metastasis in a single ipsilateral lymph node, ≤ 3 cm in greatest dimension |
| N2a | Metastasis in a single ipsilateral lymph node > 3 cm but not > 6 cm in greatest dimension |
| N2b | Metastasis in multiple ipsilateral lymph nodes, none > 6 cm in greatest dimension |
| N2c | Metastasis in bilateral or contralateral lymph nodes, none > 6 cm in greatest dimension |
| N3 | Metastasis in a lymph node > 6 cm in greatest dimension |
| Cervical Esophagus | |
| NX | Regional lymph nodes cannot be assessed |
| N1 | No regional lymph node metastasis |
| N2 | Regional lymph node metastasis |
| Distant Metastasis (M) | |
| MX | Distant metastasis cannot be assessed |
| M0 | No distant metastasis |
| M1 | Distant metastasis present |
Therapeutic Modalities—Surgical Options Including Techniques
Cheever57 first described the lateral pharyngotomy in combination with a mandibulotomy approach in 1878. Resection of a large tonsil tumor and a node plucking were performed; however, the patient recurred with local and regional metastases. With no anesthesia, attempts at control of the local recurrence were made with 18 applications of white-hot irons. Not surprisingly, the patient’s tumor continued to grow. Other highlights in the development of hypopharyngeal cancer treatment include Sebileau’s (1904) description of the lateral retrothyroid pharyngectomy; Trotter’s (1913) development of the lateral pharyngectomy; the supracricoid hemilaryngopharyngectomy (SCHLP) described by Andre, Pinel, and Laccourreye (1962); and Ogura’s (1965) development of the extended supraglottic laryngectomy. Many other surgeons contributed to the development of other techniques including Orton, Alonso, and Harrison.
The available surgical options can be divided among the organ-preservation procedures and more radical operations (Table 103-4). The conservation surgical procedures are limited to T1, T2, or highly selected T3 lesions, except for the endoscopic approach used by Steiner and colleagues.58 The more radical operations typically require extensive reconstruction of the alimentary tract. The options and the algorithm for deciding on a specific reconstruction are discussed in a separate section.
| Procedure | T Stage | Reconstruction |
|---|---|---|
| Partial pharyngectomy | T1, T2 | Primary closure |
| Partial laryngopharyngectomy | T1, T2, T3 | Regional or free flap |
| Supracricoid hemilaryngectomy | T1, T2, T3 | Primary closure |
| Endoscopic CO2 laser resection | T1, T2 (possible T3, T4) | Secondary intention |
| Total laryngectomy with partial/total pharyngectomy | T3, T4 | Primary closure vs. regional or free flap |
| Total pharyngo-laryngo-esophagectomy | T4 | Gastric pull-up |
PARTIAL PHARYNGECTOMY
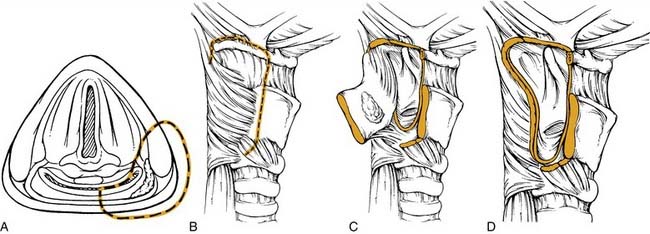
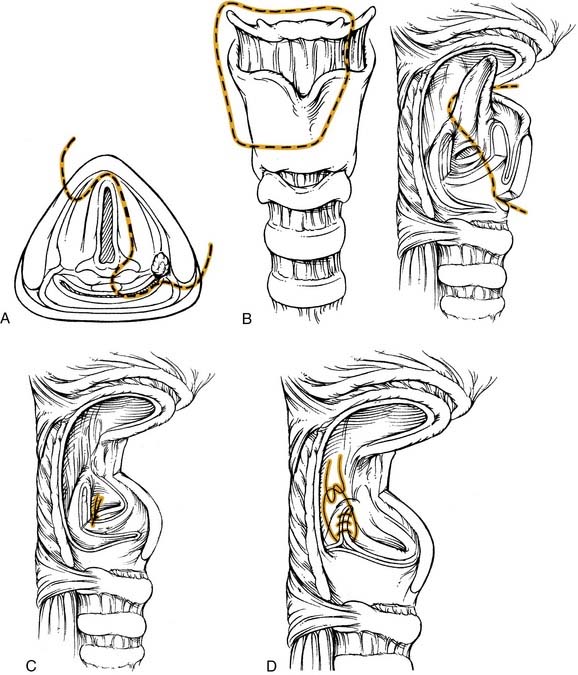
A tumor that more extensively involves the lateral hypopharyngeal wall needs to be addressed by wider margin. This can be accomplished with a lateral transthyroid pharyngotomy (Fig. 103-6) by a wider resection that involves the posterior portions of the thyroid cartilage and the hyoid bone.59 In this variation, instead of approaching the tumor through the pyriform, an entry through the vallecula is chosen. Once the neck dissection is completed, a medial and inferiorly based perichondrial flap is elevated off the thyroid cartilage. The hyoid bone is exposed from lesser cornu extending laterally. The hyoid bone is then cut through the lesser cornu, and cartilage cuts are made in a vertical fashion at the junction of the anterior two thirds and posterior one third of the thyroid cartilage. The hypopharynx is then visualized by entering through the vallecula. A blunt retractor placed into the vallecula assists entry. The incision is then carried laterally and inferiorly through the cartilage cuts. As these cuts are made, visualization through the initial incision ensures a safe margin around the tumor. The lesion is reflected laterally and the other required circumferential cuts are completed. Frozen sections for control of the margins are obtained. Reapproximating the mucosa and suturing the perichondrial flap as a second layer closes the defect.
With both these approaches, complications include pharyngocutaneous fistula with wound breakdown and dysphagia. Fistula problems are addressed by attempting to decrease or divert salivary contamination, meticulous wound care, and the possibility of secondary flap closure. A cricopharyngeal myotomy performed intraoperatively is one prophylactic measure to address possible dysphagia, although one study has shown no benefit of this maneuver in head and neck cancer patients.60
The anterior transhyoid pharyngotomy (Fig. 103-7) is another approach for lesions that primarily involve the posterior pharyngeal wall. Thus T1 or T2 lesions are good candidates for this approach. A tracheotomy is first performed. Again, a collar incision is used to identify the hyoid bone. The hyoid may be resected or the approach may be above or below the hyoid. The vallecula is identified, and sharp dissection through this mucosa with subsequent tongue base retraction provides access to the posterior hypopharyngeal wall. The tumor is removed with the prevertebral fascia as the deep margin. After all margins are assessed by frozen section, a split-thickness skin graft can be used to cover the defect. The wound is closed by reapproximating the vallecular mucosa. The primary limitation to this procedure is that there is limited visualization of the full hypopharynx. Thus choosing the appropriate tumor for this approach is critical. Complications of this approach include pharyngocutaneous fistula and dysphagia.
PARTIAL LARYNGOPHARYNGECTOMY
This operation combines the classic hemilaryngectomy operation with a partial pharyngectomy.61 In certain cases the laryngeal remnant is still able to maintain speech, swallowing, and airway protection. The tumor must fit strict criteria including involvement of the medial pyriform sinus. The lesion may be a marginal aryepiglottic fold tumor. Extension into the base of tongue, lateral pyriform sinus, and vallecula can potentially be included in the resected specimen. Contraindications include (1) involvement of the pyriform apex, (2) extension to the postcricoid region, (3) ipsilateral true vocal cord paralysis, or (4) encroachment into the cricopharyngeus.
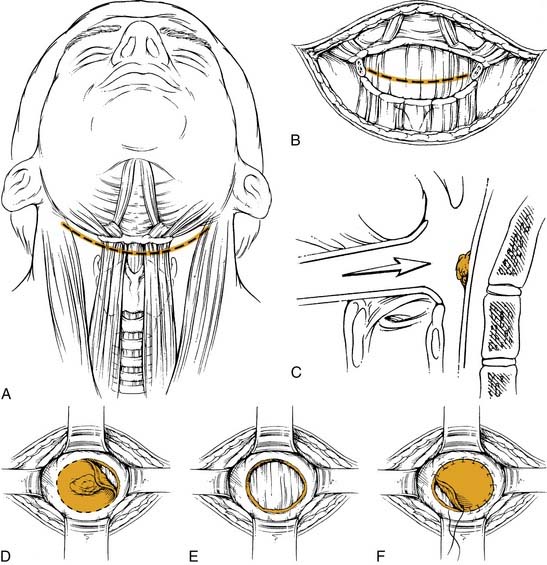
The procedure (Fig. 103-8) is begun after the completion of the tracheotomy and the neck dissection(s). For a lesion involving the medial wall of the pyriform sinus, minimal extension onto the epiglottis, and encroachment onto the arytenoid, the final resected specimen will contain the tumor on the medial wall, a portion of the epiglottis, the ipsilateral arytenoid, the ipsilateral half of the hyoid bone and the superior two thirds of the ipsilateral thyroid cartilage. After transection of the suprahyoid musculature, a superiorly based perichondrial flap is elevated off the thyroid cartilage. The thyroid cartilage is cut in the midline in an incision extending approximately two thirds of its height. This cut is then extended horizontally to the posterior border of the cartilage. The ipsilateral hyoid is freed and the vallecula is entered. Extension of this incision along with visualization of the epiglottis allows better exposure. At this point the epiglottis is grasped and retracted, providing visualization of the tumor. The epiglottis is then divided with a safe margin around the tumor and this cut is brought to, but does not include, the anterior commissure. One blade of the scissors is placed into the ventricle, and the other blade approximates the horizontal thyroid cartilage cut. This incision is made to the arytenoid, which is spared only if there is no involvement; however, it has to be sacrificed with tumor involvement. The vallecular incision is extended inferiorly with a good margin around the tumor. This is where a portion of the lateral pyriform/pharyngeal wall can be included in the resection if involved with tumor. The medial and lateral cuts meet at the arytenoid. If tumor involves more of the supraglottic area, a standard supraglottic laryngectomy can be performed to resect the entire epiglottis, pre-epiglottic space, and/or a portion of the base of tongue.
SUPRACRICOID HEMILARYNGOPHARYNGECTOMY
The major proponent of this technique is the French group of Laccourreye.62–65 This procedure extends the partial laryngopharyngectomy procedure to include the entire ipsilateral supracricoid hemilarynx along with the pyriform sinus. Thus contraindications to this approach include involvement of the pyriform apex, ipsilateral cord fixation, postcricoid involvement, or posterior pharyngeal wall invasion.
ENDOSCOPIC CO2 LASER RESECTION
The technique for all tumors involves use of a bivalved laryngopharyngoscope, an operating microscope, and a CO2 laser as the dissecting instrument. As opposed to conventional open procedures, the tumor is often cut through during endoscopic approaches to provide a direct view of tumor depth and/or to assess cartilage invasion. Under microscopic vision, tumor margins are taken up to 10 mm and in this fashion the entire tumor is removed. The specimen is usually resected in multiple pieces, which are all oriented appropriately by the surgeon. A typical sequence of resection for a pyriform sinus primary is an initial cut through medial tumor, transverse to the plane of the aryepiglottic fold to assess extent of laryngeal extension, followed by posterior ± arytenoid, anterior, inferior posterolateral, and then superior posterolateral excisions. Margins are checked by frozen sections and a step serial technique.66 Cartilage may be exposed or resected during the surgery and to avoid perichondritis antibiotics are given prophylactically. The wound bed is allowed to heal by secondary intention. In Steiner’s management, delayed neck dissections are performed as indicated by the tumor size, location, and lymph node status. Postoperatively the patients are started on an oral diet, as early as day 1, unless extensive resections are undertaken.
TOTAL LARYNGECTOMY WITH PARTIAL/TOTAL PHARYNGECTOMY
For T3 (those that are judged inappropriate for conservation therapy) and T4 lesions of the hypopharynx, combining a standard laryngectomy with excision of the involved pyriform walls completes this operation. In addition, total laryngopharyngectomy (TLP) is increasingly used as a salvage procedure for failures of chemoradiation protocols. Figure 103-9 shows a TLP of the patient whose PET/CT is shown in Figure 103-4 after failed chemoradiation. Reconstruction of these defects with primary closure can be accomplished if an adequate remnant of pharyngeal mucosa remains. Myocutaneous or free flap coverage may be required. Postoperative complications can include pharyngocutaneous fistulas with rates as high as 40% in patients previously treated with chemotherapy and/or radiation.67
TOTAL PHARYNGO-LARYNGO-ESOPHAGECTOMY
This procedure is indicated for patients with involvement of the cervical esophagus due to either primary involvement or regional extension from the hypopharyngeal subsites. As for the TLP, this procedure is also used in cases of chemoradiation failure. Harrison recommended this operation for patients with postcricoid carcinoma because of the possibility of skip lesions.68
The procedure is begun after completion of bilateral neck dissections including paratracheal lymph node dissections and a total thyroidectomy with parathyroid autotransplantation. A total laryngo-pharyngectomy is performed as described earlier. A transhiatal pull-through esophagectomy is then performed to complete the inferior resection. Reconstruction is performed with the gastric pull-up technique as described later. The morbidity (20% to 60%) and mortality (5% to 20%) rates are significant with this combined procedure.69 Larynx-sparing esophagectomy has been described in certain case reports for lesions limited to the cervical esophagus.70,71
Therapeutic Modalities—Options for Reconstruction
The principles of reconstruction that are used for hypopharyngeal reconstruction are the same as elsewhere in the head and neck region. These include the restoration of form and function with minimal donor site morbidity. In addition, to be able to achieve these goals in a single setting without need for secondary procedures is ideal. For the conservation procedures, a primary closure can be performed so long as there is no compromise of the pharyngeal lumen that could result in significant dysphagia. Primary closure can be performed safely when the pharyngeal lumen measures between 28 and 32 French. Skin grafts are the next available alternatives and are primarily used in the posterior pharyngeal wall resections. In addition, mucosal and dermal grafts, local rotation flaps, and tongue flaps have been described for closure.72 Regional flaps that have been described include the platysma, deltopectoral, and latissimus dorsi flaps.73 These flaps are typically used in the event of failure of the more popular options discussed later.
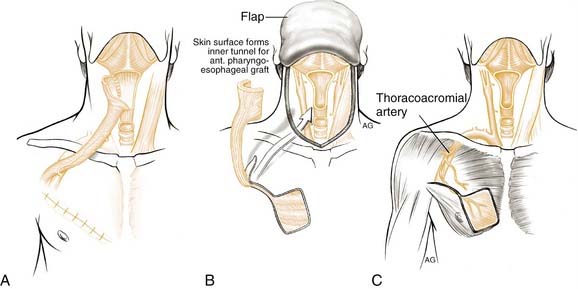
PECTORALIS MYOCUTANEOUS FLAP
The pectoralis major myocutaneous flap has been the “work-horse” pedicled flap for head and neck reconstruction since its introduction by Ariyan in 1979.74 The advantages of its use to close partial defects of the hypopharynx include its reliability and ease of harvest (Fig. 103-10). Disadvantages include the bulky nature of the flap, which can lead to postoperative stenosis, fistula problems, dysphagia, and difficulties of harvest in women with pendulous breasts. Partial defects are best closed with this flap when there is a strip of pharyngeal mucosa available for direct suturing. Although possible, tubing of this flap is not facile and should be avoided. Fabian described use of this flap for repair of partial defects73,75 and others have extended his earlier reports.76,77
CIRCUMFERENTIAL DEFECTS
The reconstruction of circumferential defects of the hypopharynx and cervical esophagus is a controversial topic because a single flap has not been identified that necessarily gives the best functional outcome with the least morbidity. The choice of reconstruction is also limited by the surgeon’s experience and availability of options at a particular institution. The literature on reconstruction in the 1980s and 1990s primarily focused on the jejunal free flap or the gastric pull-up as viable options. More recently, fasciocutaneous flaps that are tubed around a salivary stent have gained favor.78–80
GASTRIC PULL-UP
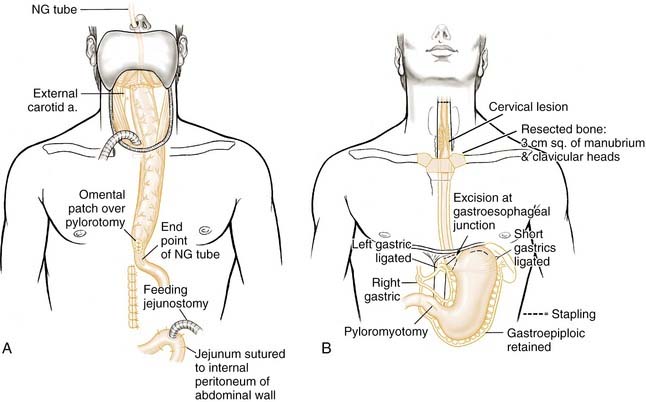
This option can be used when the inferior margin of tumor ablation extends past the cervical-thoracic junction of the esophagus or when a safe margin requires total esophagectomy. One school of thought advocates a complete esophagectomy along with the hypopharyngeal resection to address possible second primary malignancies for all hypopharyngeal cancer. Harrison, who originally advocated this concept, stated: “Total removal of the esophagus not only ensures complete resection of the lower limits of the tumor but also areas of potential malignancy and unexpected, undiagnosed second tumors.”68 He based these comments on whole-organ serial sections, which showed second primaries in the esophagus consistent with the field cancerization idea of Slaughter and coworkers.81 Most recently, Martins made a similar case for total laryngo-pharyngo-esophagectomy in patients with advanced-stage hypopharyngeal cancer.82
Turner originally described this operation for esophagectomy using stomach as the reconstruction (Fig. 103-11).83 However, it was Ong and Lee84 who popularized this reconstructive method for laryngo-pharyngo-esophagectomy defects. The operation is carried across three compartments: neck, thorax, and abdomen. After completion of the laryngo-pharyngo-esophagectomy, the stomach is mobilized on the basis of the right gastric and right gastroepiploic artery. A pyloromyotomy is performed, and the stomach is then passed to the neck through the posterior mediastinum. A pharyngogastric anastomosis is then completed. As described earlier, the combination of total laryngo-pharyngo-esophagectomy with gastric pull-up is associated with high morbidity and mortality.69
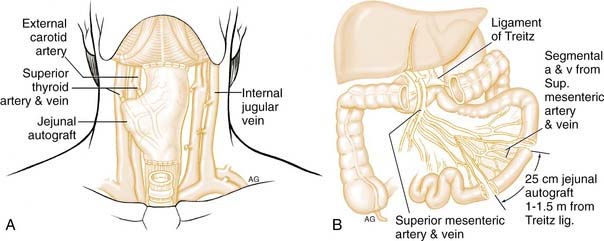
JEJUNAL FREE FLAP
Seidenberg and colleagues first described the jejunal free flap in 1959 (Fig. 103-12).85 The advantages to this procedure are that a large segment can be harvested for anastomosis as high as the nasopharynx. Concomitantly with the harvest, the primary tumor resection can be performed in the neck. Additionally, the intrinsic mucus production of the jejunum is hypothesized to assist in swallowing. The disadvantages are poor donor vessels, a short ischemia time, dysphagia from the intrinsic peristalsis of the jejunum,86 poor vocal rehabilitation using a tracheoesophageal puncture, and morbidity from the abdominal surgery. Despite these potential problems, the jejunal free flap is a popular method of reconstruction for these defects.
While tumor resection is being performed in the neck, a second team performs the harvest. A segment of jejunum is harvested with associated vessels. Typically the second vascular arcade of vessels after the ligament of Treitz has a caliber that matches those of donor vessels in the neck. An exteriorized segment can be used to monitor flap viability. The jejunum usually matches the lumen on the side of the cervical esophagus. The flap is oriented such that isoperistaltic contraction will aid normal swallowing. At the proximal anatomosis, cutting the antimesenteric border to provide a better match with the oropharynx or nasopharynx expands the jejunum. A jejunostomy distal to the bowel anastomosis is then placed. The major disadvantage to this procedure is the requirement for a laparotomy, which can lead to complications in the debilitated and malnutritioned cancer patient. Recent efforts with minimally invasive laparoscopic harvest may revitalize the interest of surgeons who may have turned away from this option.87,88 Two studies described successful harvest of the required jejunal segment. Gherardini and colleagues87 performed the bowel anastomosis via a mini-laparotomy, whereas all patients in the study by Wadsworth and colleagues88 underwent laparoscopic bowel anastomosis. The laparoscopic approach avoids the complications of postoperative ileus and other abdominal complications.
Although early case series using this technique reported poor results,89,90 more recent and larger series have shown flap success rates between 95% and 97%.91–93 Complications included flap failure, fistula, stricture, and abdominal complications. Detailed description of these complications is described by Reece and colleagues,92 who found that flap failure caused by arterial thrombosis occurred in 7 of 93 patients. Postoperative fistulas, which delay oral alimentation and initiation of adjuvant therapy, have been reported to be high for jejunal flaps. Fistula rates were reported as 10% by Disa and colleagues94 and 19% by Reece and colleagues.92 Strictures as a cause of dysphagia were reported to occur in 4% to 15% of patients. Donor site morbidity consisting of dehiscence, small bowel obstruction, and infection occurred in only 1 of 90 patients in the Memorial Sloan-Kettering series and in 9 of 93 patients in the M.D. Anderson group of patients. Postoperative oral alimentation began between 11 and 14 days after surgery in two large case series. An unrestricted diet was tolerated between 53% and 92% of patients in three large case series.91–93
Although animal studies suggested that the jejunal autograft was poorly tolerant of radiation,89 patients with jejunal grafts have tolerated postoperative radiation with minimal complications.94–97 Wei and colleagues97 performed biopsies at defined intervals over a 2-year period on either cohorts of patients receiving radiation or controls who did not receive radiation after jejunal free tissue transfer. Although microscopic changes were identified, no clinically significant symptoms or signs were observed consistent with previous observations.
Vocal rehabilitation using a TEP in these patients has been described as worse compared with patients who have undergone total laryngectomy alone.98 The patients are described as having a “wet” voice. Most patients in the study by Reece and colleagues used an electro-larynx for communication with only 12 of 93 patients using a TEP.
FASCIOCUTANEOUS FREE FLAPS
Although the literature regarding hypopharyngeal reconstruction primarily discusses the jejunal free flap or gastric pull-up techniques, fasciocutaneous free flap techniques are being used more frequently. The popularity of these flaps has increased within the past decade.78 This method was first described by Harii and associates in 1985 as an alternative reconstructive option; however, the early experience with fasciocutaneous flaps showed fistula rates as high as 67%.99–101 One possible reason for strictures is that granulation tissue that forms at the fistula site ultimately may lead to stenosis at the anastomosis. However, more recent studies with modified techniques have shown more positive results.
Varvares and colleagues80 and Scharpf and Esclamado79 reported on their series of 20 and 25 patients, respectively, who underwent radial forearm free flap reconstruction of pharyngoesophageal defects. Both authors used surgical modifications to attempt improvements on past reported data on fistulae and stenosis. In seven patients of the Varvares series, the flap was completely tubed around a Montgomery salivary bypass tube and one patient developed a fistula. In Esclamado’s report a “lock and key” design at the distal anastomotic site, a slightly larger flap than the actual defect (to allow for contraction) and minimal stretching prior to inset yielded a 28% (7/25) total fistula rate and a 36% (9/25) stenosis rate. Of the fistulas, five occurred greater than 4 weeks after surgery and all healed spontaneously. The stenosis was treated satisfactorily in 5/9 patients. Recently, Rosenthal and colleagues102 reviewed their experience with 104 patients who underwent radial forearm free flap reconstruction for hypopharyngeal reconstruction. Similar to other studies, a fistula rate of 28.8% was noted in this series, which was associated with longer hospital stays and other complications. The anterolateral thigh is another effective donor site for circumferential defects and results in acceptable postoperative speech and swallowing.103,104
Therapeutic Modalities—Radiation Therapy
Although these modalities are discussed briefly here, a separate chapter in this text discusses in further detail chemotherapy, radiation therapy, and concurrent chemoradiation. The use of radiation as a single-modality therapy for hypopharyngeal carcinoma is limited to early lesions such as T1 cancers and selected T2 lesions. Favorable lesions are considered to be those that are exophytic in appearance and those lesions limited to the medial wall of the pyriform. The neck is included in the radiation field, although surgical salvage for bulky neck disease may be required.105 Additional contexts in which this modality may be used are for elderly, debilitated patients; patients with advanced lesions who refuse surgical treatment; or palliative treatment. Complications associated with radiation include mucositis, stricture, airway compromise caused by laryngeal edema, chondronecrosis, and dysphagia.
In addition to the standard delivery of the radiation dose, altered fractionation schemes have been shown to achieve improved loco-regional control in patients with head and neck cancer.106 This was demonstrated in the large Radiation Therapy Oncology Group (RTOG) trial, in which different schemes were used in a randomized trial.107 Two general types of altered fractionation schemes exist. The first is hyperfractionation, which results in a higher total dose by delivering smaller fractions more frequently (typically twice a day) without causing more late complications. The second is accelerated fractionation, which shortens the overall treatment time and attempts to achieve the same overall dose as standard fractionation. In the study by Fu and colleagues,107 both hyperfractionation and accelerated fractionation with a concomitant boost showed significantly improved locoregional control but showed no difference in overall survival compared with standard treatment. Depending on the arm, between 11% and 15% of the patients in this trial had hypopharyngeal primary tumors. Acute reactions were increased in all accelerated fractionation schemes. Thus incorporation of these techniques in the use of radiation for cure or as postoperative treatment is a further advance for treating head and neck cancer patients.
Therapeutic Modalities—Chemotherapy
Chemotherapy is not used to treat hypopharyngeal and cervical esophageal carcinoma as single-modality treatment except in palliative treatment cases. For all squamous cell carcinomas of the head and neck, the best responses are to platinum-based compounds such as cisplatin or carboplatin. In addition, 5-fluorouracil (5-FU), methotrexate, leukovorin, mitomycin C, and the taxanes have been used.108 Some of the major complications of the commonly used 5-FU and cisplatin include hearing loss, mucositis, myelosuppression, and peripheral neuropathy.
LeFebvre and colleagues109 published the first prospective, randomized study evaluating laryngeal preservation for hypopharynx carcinoma. Their protocol involved infusional chemotherapy consisting of cisplatin and 5-FU followed by endoscopy to assess tumor response. After two or three cycles, complete responders went on to receive definitive radiation and the remaining patients underwent conventional surgery.
Therapeutic Modalities—Concomitant Chemoradiotherapy
This combination modality attempts to combine the toxic effects of both agents on cancer cells to achieve therapeutic benefit. Concurrent chemotherapy also potentially radiosensitizes tumor cells in addition to direct toxic effects. These approaches use various combinations of cisplatin, 5-FU, taxanes/taxol, or hydroxyurea in conjunction with radiation. Obviously, one untoward effect of concurrent therapy is a significant increase in toxicity to patients. These toxicities are the same as those seen in patients being treated with the individual modality alone but are much more severe. Again, these include neutropenia, thrombocytopenia, mucositis, nausea, dysphagia with the need for a feeding tube, renal failure, cutaneous reactions, chondronecrosis, airway compromise, and death. Lee and colleagues110 found that hypopharyngeal or upper esophageal strictures occurred in 21% of patients (41/222) undergoing concurrent chemoradiation and that a hypopharyngeal primary was a significant risk factor for developing this complication. A separate chapter in this text provides further details on chemoradiotherapy for hypopharyngeal and cervical esophageal malignancies.
Management of the Neck
The control of regional metastasis is a critical component of the management of hypopharyngeal and cervical esophageal tumors. Although this statement can be generalized to all other head and neck tumor sites, it is particularly important in this region due to the rich lymphatic drainage and the high percentage of tumors that have regionally metastatic disease (see Fig. 103-2). As for other sites, the discussion of neck management can be divided between elective neck dissection (for N0 stage necks) and therapeutic neck dissection (for N+ necks). For necks with positive nodes, the current management is to treat both necks, either with radiation followed by salvage surgery if necessary or surgery followed by radiation. For the ipsilateral neck that is staged N0, there is compelling evidence to treat both necks for all but the very early lesions where a unilateral neck dissection alone may be adequate. The types of neck dissections are discussed in a separate chapter in this text.
Many studies demonstrate the prognostic significance of lymph node metastasis in hypopharyngeal carcinoma. We focus on two early studies that demonstrate this finding. Shah and colleagues111 observed that positive neck node(s) were a poor prognostic indicator in patients with hypopharyngeal carcinoma. In their series of 104 patients who were N0 at presentation, 61 underwent radical neck dissection and 36 did not. Nodes were positive in 25 (41%) patients who underwent neck dissection. In comparing survival with those patients whose nodes were negative after neck dissection, the 5-year survival was significantly better in the pathologically N0 group (50% vs. 32%). It is unclear whether the tumor stage was controlled for these groups (i.e., it is possible that the node-negative group involved early stages, whereas the node-positive group involved advanced stages). However, it is apparent from this overall study that positive nodes in hypopharyngeal cancer do not indicate a good prognosis. LeFebvre and colleagues112 confirmed these data by examining lymph node status in a large series of patients treated at the Centre Oscar Lambret from 1974 to 1983. Almost 70% of these patients presented with palpable cervical metastasis. Again, a significant correlation between prognosis and lymph node status (N0 vs. N+) was established.
Given this prognostic significance, what are the draining lymph node basins for hypopharyngeal carcinoma? To understand the drainage patterns, Shah113 reviewed the records of 1081 patients, 126 of whom had hypopharyngeal primaries, who underwent radical neck dissections. All the occult positive nodes in this study were in levels II and III. Interestingly, in the therapeutic neck dissection group, all five levels were found to have lymph nodes involved with tumor. On the basis of these studies, Shah recommended levels II, III, and IV neck dissection for N0 patients and comprehensive levels I to V neck dissection for node-positive patients. Shah and colleagues114 confirmed these findings on a larger group of hypopharyngeal cancer patients and again came to the conclusion that N0 necks should be treated electively removing levels II to IV and N+ necks should undergo comprehensive neck dissection.
These studies provide support for the prognostic value of neck dissections and detail the nodal basins that need to be addressed. We next address the rates of lymphatic metastasis in patients with N0 necks to describe a rationale for ipsilateral neck treatment. Multiple studies demonstrate that patients of stage N0 will have occult lymph node metastasis. In some of these studies a comparison of N0 necks in T1/T2 tumors to N0 necks in T3/T4 tumors was not performed. Clearly, one problem in attempting this comparison is the relatively few T1/T2 tumors that are encountered compared with T3/T4 tumors. Byers and colleagues115 reported on the occurrence of occult metastasis in elective neck dissections from patients with cancers at various sites in the head and neck. There were only three N0 patients with T1/T2 pyriform sinus tumors and two of these had occult metastasis. The T3/T4 group had 16 of 29 (55.2%) patients with occult metastasis. Buckley and MacLennan116 prospectively examined 16 neck dissection specimens that were N0 from primary hypopharyngeal tumors with a careful sampling technique of all nodal levels. This subset of tumors was part of a larger series of cancers examined similarly. They found 9 of 16 (56%) of the hypopharyngeal neck dissection specimens to harbor occult metastatic deposits, although the T stages were not specifically given for the positive necks. Some of these occult tumor deposits were in nodes as small as 3 mm. Both the Byers and Buckley studies illustrate the high occult metastatic rate of hypopharyngeal carcinoma. These studies in combination with the studies of Shah and colleagues support the idea of selective level II/III/IV dissections in patients with N0 neck stage.
The retropharyngeal and paratracheal level VI nodes also form part of the draining echelon of nodes for these tumors. The studies discussed earlier do not discuss these nodal basins in detail. The removal of these nodes in conjunction with the jugular chain nodes is supported by the following studies. The study of Buckley and MacLennan116 mentions that there were positive nodes in N0 necks in level VI, but no detail is given as to what primaries are or what tumor stage yielded these nodes. Harrison117 performed serial sections of laryngopharyngectomy specimens and showed that lymphatic channels pierce through the cricothyroid membrane and drain into the paratracheal lymph nodes. Thus he argued that a total thyroidectomy with paratracheal node dissection was critical in the management of these tumors. Weber and colleagues118 analyzed 141 patients from the M.D. Anderson Cancer Center who underwent paratracheal lymph node dissections for laryngeal, hypopharyngeal, or cervical esophageal cancer. Of the patients with a cervical esophageal primary, 10 of 14 (71.4%) had metastases in these nodes and 3 of 36 (8.3%) of hypopharynx primaries had positive nodes. Survival was significantly reduced in patients with positive paratracheal nodes. On the basis of these findings, the authors recommend paratracheal node dissection in all patients with primaries in these locations.
The retropharyngeal lymph nodes are not commonly addressed in hypopharyngeal and cervical esophageal squamous cell cancers and few studies have discussed management of these nodes. McLaughlin and colleagues119 analyzed pretreatment CTs or MRIs for retropharyngeal adenopathy in patients with head and neck cancers. Pharyngeal wall tumors had 19% incidence of adenopathy in this area. As a group, rates of regional relapse were highest in those patients with retropharyngeal adenopathy. Hasegawa and Matsuura120 found that 8 of 13 (62%) hypopharynx cancer patients had retropharyngeal lymphatic metastasis. Amatsu and colleagues121 performed retropharyngeal node dissections in 82 patients with hypopharyngeal and cervical esophageal carcinoma. Twenty percent (16/82) of patients had positive lymph nodes identified in the retropharynx. Thus these authors recommend bilateral retropharyngeal node dissection in all patients with hypopharyngeal and cervical esophageal cancer. Despite this data, the standard approach does not include dissection of these nodes. A recent case report highlights the involvement of retropharyngeal lymph nodes in hypopharyngeal cancer and the use of fused PET/CT in the pretreatment evaluation.122
The treatment of the N0 contralateral neck in hypopharyngeal carcinoma has been addressed by few studies. Marks and colleagues122a studied this question in a diverse group of head and neck cancer patients. Their conclusions were that (1) the risk of developing contralateral metastasis was unrelated to the size of the tumor, (2) the contralateral metastasis existed or developed in 13% of pyriform sinus tumors, and (3) the risk of contralateral metastasis was higher in patients with ipsilateral palpable disease. However, Johnson and colleagues123 compared regional nodal metastases in medial versus lateral pyriform wall tumors and found significantly higher rates of contralateral metastases in the medially based tumors. These authors recommend bilateral neck dissections for medial pyriform wall tumors and unilateral neck dissections for lateral pyriform wall tumors.123 Buckley and MacLennan116 further examined this question in their study. For N+ necks, 3 of 3 patients had positive nodes in contralateral necks. Interestingly, in the N0 cases, 7 of 15 (47%) contralateral necks were positive for occult carcinoma in the lymph nodes. The disease was always in levels II to IV or VI, and never in level I or V. Except for these studies, there are limited other data on the treatment of the contralateral neck in hypopharyngeal carcinoma. The choice to treat the contralateral neck in the majority of cases will be straightforward because so many patients present with advanced disease. Postoperative radiation is routinely administered to treat the necks if there is extracapsular spread or if more than one lymph node is involved.
Therapeutic Outcomes
There are currently no prospective, randomized reports comparing the modalities available for the management of cervical esophageal or hypopharyngeal squamous cell carcinoma. Thus no clear evidence exists for the superiority of one strategy versus the next. It appears in reviewing the literature that individual preference of the physician in charge of the patient’s care directs the therapeutic modality that is chosen. In summary, options for tumors that are staged T1 or select T2 tumors include surgery followed by radiation or radiation followed by salvage neck surgery for residual nodal disease. For any other lesions radical surgery followed by postoperative radiation is the standard practice. Laryngeal preservation, using induction chemotherapy followed by radiation, is supported by the study of Lefebvre and coworkers.109 This protocol requires an understanding by the patient that surgical salvage is part of the protocol. Also, it is incumbent on the treating physician to closely follow the patient’s tumor because prompt surgical salvage will ensure survival similar to conventional treatment.
Pingree and colleagues124 completed a review of 1362 cases of hypopharyngeal cancer contained in the Rocky Mountain database that covered the periods 1973 to 1983. Of these, 239 (17.5%) were stage I and II and 966 (71%) were stage III and IV. Of the advanced stages 231 (23.9%) had distant metastatic disease at presentation. The hypopharyngeal tumors were predominantly located in the pyriform sinus. On the basis of treatment data, 695 of these patients who were followed for 5 years were available for analysis. The therapeutic modalities used were radiation alone, surgery alone, or combined surgery and radiation. The overall survival for all groups of patients was 25% at 5 years. The authors provide further data on stage-specific survival and therapeutic modality specific survival for all stages. Surprisingly, for all stages examined, the surgery alone or the combined modality groups had equivalent survival at 1-, 3-, and 5-year points and these were always better than radiation alone. The 5-year survival of the radiation-only group was 11.5% versus 39% for surgery only and 31.8% for combined therapy. Clearly, these data confirm that hypopharyngeal cancer has a poor prognosis. However, there are several confounding aspects of this retrospective review that must be appreciated in examining the data. First, the results against radiation in advanced-stage tumors is likely subject to selection bias because the radiation-only group likely had a significant number of advanced, inoperable patients, whereas the surgery or combined-modality therapy groups likely had patients felt to be operative candidates. Other uncontrolled or unclear variables in these analyses are what types of surgery were used, the doses of radiation, and the cause of death.
A decade later Hoffman and colleagues10 published their retrospective review of the Patient Care Evaluation (PCE) study of the American College of Surgeons. This database was subdivided between two periods, 1980 to 1985 and 1990 to 1992. There were 1317 cases in the first set of years and 1622 in the second set. Again, the majority of these were located in the pyriform sinus (64.4%) and the advanced stages (III and IV) comprised approximately 75% of the cases in both situations. A stage migration effect was clearly seen as the pathologic stage advanced a significant number of patients to stage IV disease. As 5-year survival was available only for the 1980 to 1985 cohort, an analysis of outcomes for this group was presented. Overall, disease-specific survival was 69.6% at 1 year, 39% at 3 years, and 33.4% at 5 years. Analysis of stage-specific survival revealed a gradual decrease as stage increased from 63.1% for stage I to 22% for stage IV. When analyzed for therapeutic modality, a conclusion similar to that of Pingree and colleagues124 was reached. Those patients who had surgery alone had a 50.4% five-year survival, those with combined modality had a 48% survival, and the radiation-alone group had a 14.9% survival. However, Hoffman and colleagues went on to correct for possible selection bias by matching tumors with the TNM classification and found the same pattern. The greatest difference was noted in the T3/T4N0M0 groups, in which surgery alone produced a 34.6% five-year survival and radiation alone had a 3.2% five-year survival. This would suggest that for advanced tumors radiation alone is not an adequate modality.
Wahlberg and colleagues6 addressed overall survival of patients with hypopharyngeal tumors in a retrospective review of a 30-year (1960-1989) experience. Data were analyzed from the Swedish Cancer Registry, which is a compilation with a completeness of more than 95% of all tumors in Sweden. There were 2012 total cases that not only included squamous cell carcinomas but also adenocarcinomas, malignant salivary gland tumors, and other rare tumors (these accounted for 5% to 10% of all the tumors). This study primarily examined overall survival rates, but no data describing tumor stage, actual cause of death, details of therapy, recurrences, second primaries, or status of neck disease were presented. The treatment of these patients was heterogeneous, but in general in the early years radiation was used primarily and in the 1980 to 1989 period evolved to include combined surgery followed by radiation. Incidence of cancer decreased in women at an average of 2% per year, and although not clear, may be explained by better treatment of Plummer-Vinson syndrome. The 2-year and 5-year overall survival rates were 25% and 13%, respectively. The overall survival for nonsquamous cell cancers was slightly better at 5 years.
Therapeutic Outcomes—Primary Radiation Therapy
Although outcomes of radiation are discussed in brief here, Jean Louis LeFebvre describes more extensively the use of radiation therapy in a separate chapter. The best evidence for the use of therapeutic radiation as the primary modality is for T1 and selected T2 cancers. Overall, these lesions constitute a low number of cancers from the hypopharynx. These favorable lesions are exophytic in nature, are high in the pyriform (i.e., not involving the apex), and have a low volume by CT scan measurement.125 Multiple groups have shown that actuarial survival using radiation followed by neck dissection for bulky neck disease is equivalent to conservation surgery with postoperative radiation for T1 and select T2 tumors.
The M.D. Anderson experience was first described by El-Badawi and colleagues126 and later expanded by Garden and colleagues.105 In a 1996 report, Garden and colleagues retrospectively reviewed 82 patients with T1 (19 patients) and T2 (63 patients) tumors of the hypopharynx who were treated between 1976 and 1992 with definitive radiation.105 Fifty-two percent (43/82) were node positive at the start of radiation, and of these 23 of 43 had a neck dissection performed after radiation. Radiation itself was given in a single daily dose or in divided daily doses to 40 and 42 patients, respectively. The 2- and 5-year actuarial survival rates were 72% and 52%, respectively, and overall the altered fractionation scheme gave better results. The preference of this group is to use single-modality therapy whenever possible. Thus for early tumors, the M.D. Anderson experience has been to radiate and then perform neck dissections only on those patients who still have cervical lymphadenopathy. This strategy spares a subset of patients from having surgery. Other studies that used radiation as the primary modality include the Institut Gustave-Roussy,127 which noted a 40% five-year survival of selected T1 and T2 tumors. All other tumor stages in their study of radiation as the primary modality did poorly at 5 years.
The experience of Mendenhall and colleagues at the University of Florida has been described in several reports.128–130 As opposed to the other available studies on radiation as primary treatment, Mendenhall and colleagues analyzed their data using overall stages rather than tumor stage (i.e., T1 or T2). Patients either received twice-daily fractionated therapy or daily treatments. The overall absolute and cause-specific survival rates at 5 years were 43% and 58%, respectively. A stage-specific analysis showed that the probability of cause-specific survival was 100% for stages I and II, 62% for stage III, 43% for stage IVA, and 25% for stage IVB. Acute complications occurred in two patients and included severe mucositis and hospitalization for dehydration. Complications included chondronecrosis leading to total laryngectomy in one patient. Six others had aspiration, dysphagia, or laryngeal edema leading to tracheostomy. Five of 10 patients who had to undergo total laryngectomy had significant postoperative complications including wound breakdown with fatal carotid rupture, a separate death, and fistulas.
Several groups have described radiation as primary therapy for cervical esophageal carcinoma. Mendenhall and colleagues131 described 34 patients treated in this fashion and reported a 14% five-year overall survival. Jones and colleagues132 reported on a series of 12 patients who underwent radical radiation therapy and 14 patients treated with surgery. The overall 3-year survival was 18%. Although it is difficult to draw a definitive conclusion from these small series, radiation as primary therapy appears to achieve poor control of cervical esophageal carcinoma.
Therapeutic Outcomes—Primary Surgical Therapy
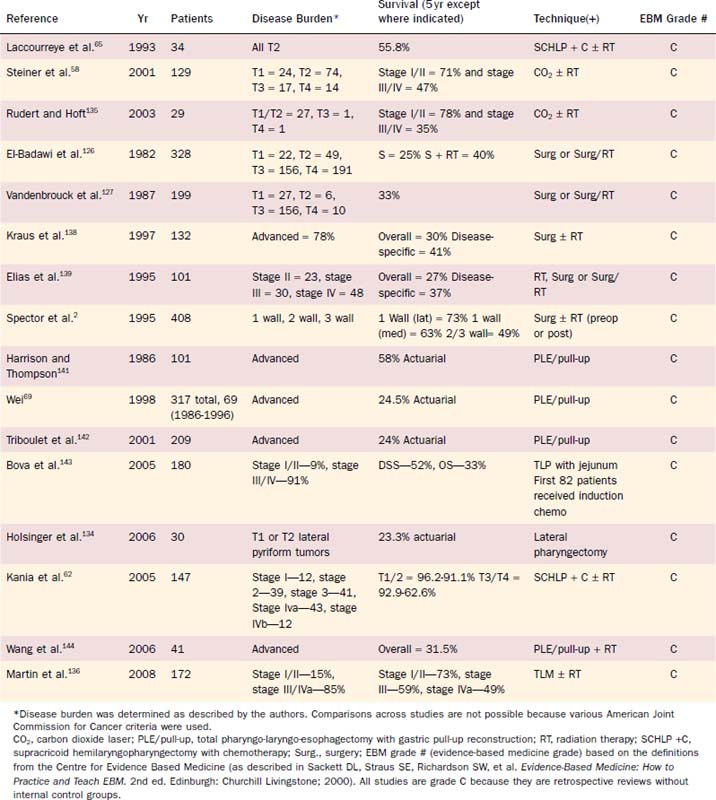
Conservation surgery for hypopharyngeal cancer became possible because of contributions from Trotter, Orton, and Alonso. In the 1960s, Ogura and associates applied lessons learned from conservation laryngeal surgery to hypopharyngeal cancers. They reported on the development of the partial laryngopharyngectomy operation and described the results of this operation.61,133 Preoperative radiation was routinely administered during this period. A summary of the major surgically oriented studies reported in the literature is shown in Table 103-5.
Holsinger and coworkers reviewed the experience of the Laccourreye group for patients with T1 or T2 lateral wall pyriform tumors that were resected using a lateral pharyngectomy with primary closure.134 A subset of these patients was treated with preoperative chemotherapy and some patients received postoperative radiation. Three postoperative deaths occurred (one related to surgery), but all patients were eventually decannulated. Surprisingly for these small tumors, this series found that the 5-year survival rate was 23.3% with four patients (13%) having local recurrences.
In addition to the lateral pharyngectomy, Laccourreye and colleagues63 used the supracricoid hemilaryngopharyngectomy (SCHLP) to treat cancers primarily of T1 to T3 staging (one patient who was a T4 was included in the series). In their retrospective review, 240 patients were treated with an ipsilateral neck dissection and the SCHLP. Deglutition was recovered in 204 of 233 patients with a 17-day average time to removal of the nasogastric tube, although the authors state that “recovery of satisfactory deglutition may take up to 1 year.” A thorough evaluation of survival data on all 192 patients was not discussed in this study. A detailed analysis of 34 selected T2 pyriform cancers was published in 1993.65 Prior to operation, most patients received chemotherapy consisting of either bleomycin (7 patients); vincristine, methotrexate, and bleomycin (14 patients); or cisplatin and 5-FU (10 patients). Postoperatively 31 of 34 patients received radiation. Of the 34 patients, 33 were decannulated and 31 of 34 patients were able to swallow after 1 month (range 13 to 26 days). Aspiration pneumonia occurred in 7 of 34 patients (20%). A completion laryngectomy was eventually performed in 3 of 34 patients due to intractable aspiration. Voice quality was initially judged as good to satisfactory in 32 of 34 patients. The 5-year actuarial survival rate was 55.8% with only one patient developing a local recurrence and two patients (one was the same, who developed local recurrence) developing neck recurrences. Cause of death was intercurrent disease in 7 of 34, second primary in 6 of 34, nodal recurrences in 1 of 34 and primary recurrence in 1 of 34. One patient died from chondroradionecrosis of the larynx, and two deaths were from unknown causes. Compared with the results with radiotherapy alone or other conservation surgical methods, these data produce similar actuarial results but appear to have a significant associated complication rate including aspiration pneumonia. However, of the several confounding factors cautioning against direct comparisons with other methods is the routine use of preoperative chemotherapy. It is unclear whether this treatment predisposed patients to the various complications described. In addition, the chemotherapy itself was given in three different regimens. An updated review of this group’s experience with SCHLP on 147 patients has been reported62,64 and demonstrates actuarial 5-year survival rates of 96.2% for T1, 91.1% for T2, 92.9% for T3, and 62.6% for T4 lesions. These rates and the functional outcomes described compare favorably with other organ-preserving approaches.
Steiner and colleagues have been strong proponents of the TLM CO2 laser endoscopic approach to excision of tumors of pharyngeal, supraglottic, glottic, and hypopharyngeal origin. The postoperative course and survival data of their group of patients are impressive and are briefly reviewed.58 Between 1981 and 1996, 129 cases of hypopharyngeal cancer underwent endoscopic excision and can be subdivided as follows: pT1:24, pT2:74, pT3:17, and pT4:14. Of these patients 68% were node positive and 75% were stage III/IV. Surgery as sole modality was used in 42% of patients, and the remainder had surgery followed by radiation. The overall survival rates were 71% and 47% for stages I/II and III/IV, respectively. These data compare favorably with outcomes using conventional treatment of these tumors. The most impressive aspect, however, of this technique is the perioperative management. Only 5 of 129 cases underwent tracheotomy during surgery and 1 required tracheotomy postoperatively due to hemorrhage. Thirty-five patients were taking an oral diet on the first postoperative day and all but two patients eventually achieved oral feeding. Only one patient developed a hypopharyngeal stenosis and was dependent on gastrostomy tube feeding. Thus although technically challenging, this procedure appears to be oncologically sound and has the significant added advantage of no tracheotomy, no reconstruction, voice preservation, and a quick return to an oral diet. For advanced-stage tumors, a definition of the inferior extent of the tumor is required because extension into the cervical esophagus would clearly limit the ability of the endoscopic surgeon.
Rudert and Hoft135 described their experience managing 29 patients with hypopharyngeal carcinoma using the endoscopic approach. Except for two patients (one T3 and one T4), the remaining 27 patients had T1 and T2 tumors. Of these patients, 9 were stage I/II and 20 were stage III/IV. No patients underwent tracheotomy at operation; however, the authors do not report on whether any required it in the postoperative period. Functionally, no surviving patients had speech or swallowing problems at 5-year follow-up. The 5-year survival rates for stage I/II were 78% and 35% for stage III/IV. Not surprisingly, node status was a significant predictor of outcome with N0 patients having 74% and node-positive patients having 34% overall survival. Locoregional recurrences occurred in 8 of 29 (27.6%) patients each. Interestingly, 7 of 8 of the local recurrences occurred below an arbitrary line drawn at the interarytenoid line. Distant metastases and second primaries occurred in 8 of 29 (27.6%) and 12 of 29 (41%) patients, respectively. These authors found the best local control with tumors of the pharyngeal walls.
Recently, Steiner and colleagues136 reported the outcomes analysis of 172 patients with hypopharyngeal tumors treated with TLM. Although the relationship between this cohort and the one reported previously is not clear from the publication, the time period of the two cohorts shows some overlap. The 5-year recurrence-free survival in this study was reported to be 73% for stages I and II, 59% for stage III, and 49% for stage IVa. These numbers compare favorably with other modalities with the major reported benefits as described earlier. Again, the outcomes of this technique need broader evaluation but deserve closer attention because of the apparent superior functional outcome and our patients’ demands of more minimally invasive techniques to handle even advanced cancers.
ADVANCED LARYNGOPHARYNGEAL CANCER
Goepfert and colleagues126 reported the M.D. Anderson experience in treating squamous cell carcinoma of the pyriform sinus from 1949 to 1976. An evolution in techniques affected the specific procedures used to treat patients, but the majority of lesions were treated with surgery and postoperative radiation. This factor clearly affects the analysis of the outcomes data. The major subdivisions in the treatment groups were between those patients who underwent surgery alone and those who underwent surgery and planned postoperative radiation.31 Each group had approximately equivalent numbers of patients in each T category but the nodal status was not described for each individual group. Locoregional control was superior in the combined therapy group (39% vs. 11% in the surgery-only group). The 5-year survival (not adjusted for any other factors) was 25% for the surgery-only group and 40% for the combined-therapy group. Thus as opposed to the study of Pingree and colleagues124 and Hoffman and colleagues,10 Goepfert and colleagues found that combined therapy was superior to surgery alone.
Vandenbrouck and colleagues127 described the Institut Gustave-Roussy’s experience with radical surgery followed by radiotherapy in 181 cases from 1968 to 1978. The overall 5-year survival in these patients was 39% and was not broken down by further staging or node status. Consistent with all other head and neck sites, node positivity and extracapsular spread resulted in poorer outcome. The distant metastatic rate was 27.6% and was the most common cause of failure.
Kraus and colleagues described the Memorial Sloan-Kettering experience138 in managing 132 patients with hypopharyngeal squamous cell carcinoma. Of these patients, most (78%) had advanced disease; however, none had distant metastasis at presentation. These patients were all treated by surgery and 80% then received postoperative radiation. Radiation in 76 of 106 patients was given at various outside institutions due to logistical considerations, which may be one variable in understanding the outcomes. Surgery consisted of partial laryngo-pharyngectomy, total laryngectomy with partial pharyngectomy, or total laryngopharyngectomy. The 5-year overall survival was 30% and disease-specific survival was 41%. Although T stage was not significant in outcome, the following factors were all significant: N0/N1 was better than N2/N3, stages I/II/III did better than stage IV, patients who underwent laryngectomy did worse than those who did not need one, and finally positive margins portended a poor prognosis.
Elias and colleagues139 reported their analysis of 101 patients with pyriform sinus cancer. Over a 20-year period, treatment evolved from radiation as primary therapy to surgery with postoperative radiation therapy. They reported a 5-year overall survival of 27% and a 5-year disease-free survival of 37%. Survival correlated with stage of the patient, and disease-free survival was significantly better in the combined surgery and radiation group.
Spector and colleagues140 reported a 30-year experience from our institution on 408 patients with pyriform sinus cancers. These patients were treated with a heterogeneous group of modalities: 95 patients were treated with a single modality (either radiation or surgery) and 302 were treated with preoperative radiation and then surgery or surgery followed by postoperative radiation. Survival was described by grouping these lesions into specific anatomic locations in the hypopharynx. At 5-year follow-up, survival for one-wall lesions was 73%; medial-wall lesions, 63%; and 2- or 3-wall lesions, 49%. Distant metastasis and second primary tumors occurred in 17.7% and 6.2% of patients, respectively. Interpretation of these data is complicated by the fact that there was such a diversity in therapeutic modalities. The broad experience at Washington University does, however, give an overview of survival rates in these patients.
Harrison and Thompson141reported on their series of 101 patients who underwent total pharyngolaryngectomy and esophagectomy from 1965 to 1983. They reported an 11% mortality rate and a 33% morbidity rate. The morbidities included 15 patients with pneumonia and 2 with abdominal dehiscence. Actuarial survival of 58% was achieved at a 5-year follow-up. Second primaries or distant metastasis accounted for 18 of 101 (15%) patient deaths. Harrison clearly acknowledged the contribution of comorbidities in these patients as he stated, “In comparing the relative success of the various operations … one must consider not only the intrinsic risks of the actual procedure but the age and general condition of the patients.”
Triboulet and colleagues142 performed a retrospective review of 209 patients treated from 1982 to 1999 who underwent total laryngo-pharyngectomy that in some cases included a cervical or total esophagectomy followed by reconstruction with either jejunal free flap (77 patients), gastric pull-up,92 or pharyngocolic anastamosis.121 Nearly all of these patients presented at an advanced stage or had multiple small tumors distributed between the hypopharynx and cervical esophagus, thus requiring a more radical operation. Forty-two patients were failures of preoperative treatment consisting of chemotherapy, radiation, or both. At 3- and 5-year follow-up, the overall actuarial survival rates were 32% and 24%, respectively. Survival was unrelated to the reconstruction technique used, but negative factors included positive margins, advanced primary tumor in the cervical esophagus, and not receiving postoperative radiation therapy. The mortality rate was 4.8% and the morbidity rate was 38.3%. Interestingly, patients who underwent free jejunal transfer had more complications including flap necrosis and fistulas, leading to delayed feeding. Thus these authors favor gastric pull-up over jejunum for reconstruction of the alimentary tube. Two more recent studies143,144 show similar low 5-year survival rates of approximately 30% using TLP with esophagectomy.
The group of William Wei at the University of Hong Kong has extensive experience in managing these difficult tumors.69,145 Wei and colleagues69 analyzed outcomes of 317 patients treated at the University of Hong Kong from 1966-1995. The goal of their 1998 study was to compare a group of 69 patients undergoing operations from 1986 to 1995 with earlier groups they had treated to evaluate changes in survival and morbidity and mortality of treatments. These authors exclusively used a total laryngopharyngectomy and esophagectomy for advanced tumors of the hypopharynx and cervical esophagus. In their earlier experience, this operation was also applied to advanced tumors of the larynx but was not used for these tumors from 1986 to 1995. The hospital mortality for this operation declined from 31% to 9% over 3 decades. The 5-year actuarial survival rate improved from 18% in the 1970s to 24.5% for the subset of patients from 1986 to 1995.
Laterza and colleagues146 reported their 20-year experience with the surgical management of carcinoma involving the cervical esophagus. Of 167 patients, 37 had primary cervical esophageal involvement with the others extending superiorly to involve the hypopharynx or inferiorly to involve the thoracic esophagus. The overall 5-year survival rate was only 16.6% with a mortality rate of 8.8%. Kelley and colleagues147 examined the management of 67 patients seen at the Memorial Sloan-Kettering Cancer Center from 1980 to 1993. A diverse set of protocols was used to manage these patients. Of these, 22 patients were treated with surgery with curative intent and other subsets were treated with radiation or chemotherapy in various combinations. Survival was poor in all groups (mean survival for entire group was 17 months and cumulative 5-year survival was 12%), although the surgery group had the best outcome. It is difficult in this study to compare specific treatments because of heterogeneity in therapy and the small numbers. However, Kelley and colleagues concluded that surgical therapy provided the best outcome.
THE ROLE OF CHEMOTHERAPY
Chemotherapy regimens for advanced unresectable head and neck cancers were begun in the 1970s. In the 1980s it was described that cisplatin and 5-FU were able to produce a significant number of partial and complete responders.148 These data were augmented by the interesting description of Ensley and colleagues149 that chemosensitivity was a predictor of radiosensitivity. Ultimately, this led to the landmark Veterans Affairs study on laryngeal preservation.150
In the meta-analysis reported by Pignon and colleagues151 a significant benefit of concomitant chemoradiation was observed; however, a significant confounding factor was the heterogeneity between the trials. Multiple phase III trials have attempted to use concomitant chemoradiation in the treatment of locally advanced head and neck cancers.108,152 Typically the hypopharyngeal and cervical esophageal cancers have been grouped with other subsites.153,154 In addition to concurrent protocols, some investigators have expanded on induction chemotherapy protocols for advanced head and neck cancers.155 These findings now form some of the key bases for combined chemotherapy and radiotherapy protocols for hypopharyngeal cancers. Again, the data on concomitant chemoradiotherapy application in advanced head and neck cancers are discussed in a separate chapter.
QUALITY OF LIFE STUDIES
Although survival is a critical measure of the success of therapy for all head and neck cancers, the patient’s perception of his or her quality of life (QOL) after therapy is also an important measure. As chemotherapy, radiation, and reconstructive options have advanced the management of these diseases, the question of patient perception of their lives post-treatment is one that is addressed by QOL studies. Several validated measurement tools exist for the evaluation of quality of life.156 Studies limited to QOL in patients with hypopharyngeal or cervical esophageal carcinomas are not available. However, several studies have reported QOL in patients with advanced head and neck cancers, subsets of which include hypopharyngeal carcinomas.157–159 The major limitation in these studies is the patient numbers that impede extrapolation. For example, in a large prospective evaluation of all sites for QOL, Weymuller and colleagues160 had 14 patients pretreatment and 4 post-treatment with primaries in the hypopharynx. They concluded that it is difficult to perform these types of studies from a single institution because statistical significance cannot be reached on the basis of limited numbers and because there is selection bias.160,161
Studies on QOL in laryngeal cancer patients treated with organ-sparing protocols yielded results that may be applicable to hypopharyngeal cancer patients. Terrell and colleagues162 evaluated QOL in surviving patients from the Veterans Affairs Laryngeal Cancer Study. Four different measures were used to examine patients from the surgery/radiation group or the chemotherapy/radiation group. The differences identified include better scores for the chemoradiation group in the bodily pain scores and in the mental health domain. All other scores were equivalent. Surprisingly, comparing speech domain scores in patients who underwent laryngectomy versus those with intact larynges demonstrated no significant differences and Weymuller and colleagues and Deleyiannis and colleagues161,163 confirmed these data and reported that the functional limitation imposed by a laryngectomy did not affect overall quality of life. Thus although organ-sparing protocols may appear intuitively to be superior in terms of patient QOL, these data indicate that overall QOL may not be affected by laryngectomy.
Amatsu M, Mohri M, Kinishi M. Significance of retropharyngeal node dissection at radical surgery for carcinoma of the hypopharynx and cervical esophagus. Laryngoscope. 2001;111:1099.
Burmeister BH, Dickie G, Smithers BM, et al. Thirty-four patients with carcinoma of the cervical esophagus treated with chemoradiation therapy. Arch Otolaryngol Head Neck Surg. 2000;126:205.
Clark JR, Gilbert R, Irish J, et al. Morbidity after flap reconstruction of hypopharyngeal defects. Laryngoscope. 2006;116:173.
Clayman GL, Weber RS, Guillamondegui O, et al. Laryngeal preservation for advanced laryngeal and hypopharyngeal cancers. Arch Otolaryngol Head Neck Surg. 1995;121:219.
Deleyiannis FW, Weymuller EAJr, Coltrera MD, et al. Quality of life after laryngectomy: are functional disabilities important? Head Neck. 1999;21:319.
Disa JJ, Pusic AL, Hidalgo DA, et al. Microvascular reconstruction of the hypopharynx: defect classification, treatment algorithm, and functional outcome based on 165 consecutive cases. Plast Reconstr Surg. 2003;111:652.
Ferlito A, Altavilla G, Rinaldo A, et al. Basaloid squamous cell carcinoma of the larynx and hypopharynx. Ann Otol Rhinol Laryngol. 1997;106:1024.
Forastiere AA, Goepfert H, Maor M, et al. Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med. 2003;349:2091.
Garden AS, Morrison WH, Clayman GL, et al. Early squamous cell carcinoma of the hypopharynx: outcomes of treatment with radiation alone to the primary disease. Head Neck. 1996;18:317.
Harrison DF, Thompson AE. Pharyngolaryngoesophagectomy with pharyngogastric anastomosis for cancer of the hypopharynx: review of 101 operations. Head Neck Surg. 1986;8:418.
Ho CM, Ng WF, Lam KH, et al. Submucosal tumor extension in hypopharyngeal cancer. Arch Otolaryngol Head Neck Surg. 1997;123:959.
Ho CM, Lam KH, Wei WI, et al. Squamous cell carcinoma of the hypopharynx—analysis of treatment results. Head Neck. 1993;15:405.
Kirchner JA. Pyriform sinus cancer: a clinical and laboratory study. Ann Otol Rhinol Laryngol. 1975;84:793.
Kania R, Hans S, Garcia D, et al. Supracricoid hemilaryngopharyngectomy in patients with invasive squamous cell carcinoma of the pyriform sinus. Part II: Incidence and consequences of local recurrence. Ann Otol Rhinol Laryngol. 2005;114:95.
Lee WT, Akst LM, Adelstein DJ, et al. Risk factors for hypopharyngeal/upper esophageal stricture formation after concurrent chemoradiation. Head Neck. 2006;28:808.
Lefebvre JL, Chevalier D, Luboinski B, et al. Larynx preservation in pyriform sinus cancer: preliminary results of a European Organization for Research and Treatment of Cancer phase III trial. EORTC Head and Neck Cancer Cooperative Group. J Natl Cancer Inst. 1996;88:890.
Martin A, Jackel MC, Christiansen H, et al. Organ preserving transoral laser microsurgery for cancer of the hypopharynx. Laryngoscope. 2008;118:398.
Pignon JP, Bourhis J, Domenge C, et al. Chemotherapy added to locoregional treatment for head and neck squamous-cell carcinoma: three meta-analyses of updated individual data. The Lancet. 2000;355:949.
Pingree TF, Davis RK, Reichman O, et al. Treatment of hypopharyngeal carcinoma: a 10-year review of 1,362 cases. Laryngoscope. 1987;97:901.
Triboulet JP, Mariette C, Chevalier D, et al. Surgical management of carcinoma of the hypopharynx and cervical esophagus: analysis of 209 cases. Arch Surg. 2001;136:1164.
Varvares MA, Cheney ML, Gliklich RE, et al. Use of the radial forearm fasciocutaneous free flap and Montgomery salivary bypass tube for pharyngoesophageal reconstruction. Head Neck. 2000;22:463.
Wang HW, Chu PY, Kuo KT, et al. A reappraisal of surgical management for squamous cell carcinoma in the pharyngoesophageal junction. J Surg Oncol. 2006;93:468.
Wei WI. The dilemma of treating hypopharyngeal carcinoma: more or less: Hayes Martin Lecture. Arch Otolaryngol Head Neck Surg. 2002;128:229.
Weymuller EA, Yueh B, Deleyiannis FW, et al. Quality of life in patients with head and neck cancer: lessons learned from 549 prospectively evaluated patients. Arch Otolaryngol Head Neck Surg. 2000;126:329.
Wycliffe ND, Grover RS, Kim PD, et al. Hypopharyngeal cancer. Top Magn Reson Imaging. 2007;18:243.
1. Kirchner JA. Pyriform sinus cancer: a clinical and laboratory study. Ann Otol Rhinol Laryngol. 1975;84:793.
2. Spector JG, Sessions DG, Emami B, et al. Squamous cell carcinoma of the pyriform sinus: a nonrandomized comparison of therapeutic modalities and long-term results. Laryngoscope. 1995;105:397.
3. Canto MT, Devesa SS. Oral cavity and pharynx cancer incidence rates in the United States, 1975-1998. Oral Oncol. 2002;38:610.
4. Hoffman HT, Karnell LH, Funk GF, et al. The National Cancer Data Base Report on Cancer of the Head and Neck. Arch Otolaryngol Head Neck Surg. 1998;124:951.
5. Davies L, Welch HG. Epidemiology of head and neck cancer in the United States. Otolaryngol Head Neck Surg. 2006;135:451.
6. Wahlberg PCG, Andersson KEH, Biorklund AT, et al. Carcinoma of the Hypopharynx: analysis of incidence and survival in Sweden over a 30-year period. Head Neck. 1998;20:714.
7. Devesa SS, Blot WJ, Fraumeni JFJr. Changing patterns in the incidence of esophageal and gastric carcinoma in the United States. Cancer. 1998;83:2049.
8. Ilson DH. New developments in the treatment of esophageal cancer. Curr Oncol Rep. 2002;4:213.
9. Lee DJ, Harris A, Gillette A, et al. Carcinoma of the cervical esophagus: diagnosis, management, and results. South Med J. 1984;77:1365.
10. Hoffman HT, Karnell LH, Shah JP, et al. Hypopharyngeal cancer patient care evaluation. Laryngoscope. 1997;107:1005.
11. Spector JG, Sessions DG, Haughey BH, et al. Delayed regional metastases, distant metastases, and second primary malignancies in squamous cell carcinomas of the larynx and hypopharynx. Laryngoscope. 2001;111:1079.
12. Ho CM, Ng WF, Lam KH, et al. Submucosal tumor extension in hypopharyngeal cancer. Arch Otolaryngol Head Neck Surg. 1997;123:959.
13. Harrison DF. Pathology of hypopharyngeal cancer in relation to surgical management. J Laryngol Otol. 1970;84:349.
14. Gedgaudas-McClees RK, McClees EC. The value of the barium-swallow technique in the evaluation of the hypopharynx. Mt Sinai J Med. 1984;51:455.
15. Schmalfuss IM. Imaging of the hypopharynx and cervical esophagus. Magn Reson Imaging Clin N Am. 2002;10:495.
16. Becker M. Larynx and hypopharynx. Radiol Clin North Am. 1998;36:891.
17. Zbaren P, Becker M, Lang H. Pretherapeutic staging of hypopharyngeal carcinoma. Clinical findings, computed tomography, and magnetic resonance imaging compared with histopathologic evaluation. Arch Otolaryngol Head Neck Surg. 1997;123:908.
18. Erkal HS, Mendenhall WM, Amdur RJ, et al. Synchronous and metachronous squamous cell carcinomas of the head and neck mucosal sites. J Clin Oncol. 2001;19:1358.
19. Leon X, Quer M, Diez S, et al. Second neoplasm in patients with head and neck cancer. Head Neck. 1999;21:204.
20. Chaiken L, Rege S, Hoh C, et al. Positron emission tomography with fluorodeoxyglucose to evaluate tumor response and control after radiation therapy. Int J Radiat Oncol Biol Phys. 1993;27:455.
21. Nowak B, Di Martino E, Janicke S, et al. Diagnostic evaluation of malignant head and neck cancer by F-18-FDG PET compared to CT/MRI. Nuklearmedizin. 1999;38:312.
22. Chesnay E, Babin E, Constans JM, et al. Early response to chemotherapy in hypopharyngeal cancer: assessment with (11)C-methionine PET, correlation with morphologic response, and clinical outcome. J Nucl Med. 2003;44:526.
23. Wycliffe ND, Grover RS, Kim PD, et al. Hypopharyngeal cancer. Top Magn Reson Imaging. 2007;18:243.
24. Wenzel C, Fiebiger W, Dieckmann K, et al. Extranodal marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue of the head and neck area: high rate of disease recurrence following local therapy. Cancer. 2003;97:2236.
25. Shapiro AL, Shechtman FG, Guida RA, et al. Head and neck lymphoma in patients with the acquired immune deficiency syndrome. Otolaryngol Head Neck Surg. 1992;106:258.
26. Ferlito A, Barnes L, Rinaldo A, et al. A review of neuroendocrine neoplasms of the larynx: update on diagnosis and treatment. J Laryngol Otol. 1998;112:827.
27. Ishoo E, Busaba NY. Ectopic gastric mucosa in the cervical esophagus. Am J Otolaryngol. 2002;23:181.
28. Lawson W, Som HL, Biller HF. Papillary adenocarcinoma of the thyroid invading the upper air passages. Ann Otol Rhinol Laryngol. 1977;86:751.
29. Perch SJ, Soffen EM, Whittington R, et al. Esophageal sarcomas. J Surg Oncol. 1991;48:194.
30. Mouret P. Liposarcoma of the hypopharynx. A case report and review of the literature. Rev Laryngol Otol Rhinol (Bord). 1999;120:39.
31. Pisani P, Krengli M, Ramponi A, et al. Angiosarcoma of the hypopharynx. J Laryngol Otol. 1994;108:905.
32. Dei Tos AP, Dal Cin P, Sciot R, et al. Synovial sarcoma of the larynx and hypopharynx. Ann Otol Rhinol Laryngol. 1998;107:1080.
33. Barnes L, Ferlito A, Altavilla G, et al. Basaloid squamous cell carcinoma of the head and neck: clinicopathological features and differential diagnosis. Ann Otol Rhinol Laryngol. 1996;105:75.
34. Ferlito A, Altavilla G, Rinaldo A, et al. Basaloid squamous cell carcinoma of the larynx and hypopharynx. Ann Otol Rhinol Laryngol. 1997;106:1024.
35. Ferlito A, Weiss LM, Rinaldo A, et al. Clinicopathological consultation. Lymphoepithelial carcinoma of the larynx hypopharynx, and trachea. Ann Otol Rhinol Laryngol. 1997;106:437.
36. Zbaren P, Borisch B, Lang H, et al. Undifferentiated carcinoma of nasopharyngeal type of the laryngopharyngeal region. Otolaryngol Head Neck Surg. 1997;117:688.
37. Sanderson RJ, Rivron RP, Wallace WA. Adenosquamous carcinoma of the hypopharynx. J Laryngol Otol. 1991;105:678.
38. Carpenter RJ3rd, DeSanto LW, Devine KD, et al. Cancer of the hypopharynx. Analysis of treatment and results in 162 patients. Arch Otolaryngol. 1976;102:716.
39. Saleh EM, Abdullwahab AA, Kammal MM. Age and sex incidence of hypopharyngeal tumours in upper Egypt: Assuit University experience. J Laryngol Otol. 1995;109:737.
40. Berrino F, Gatta G. Variation in survival of patients with head and neck cancer in Europe by the site of origin of the tumours. European Journal of Cancer. 1998;34:2154.
41. Hiroto I, Nomura Y, Sueyoshi K, et al. Pathological studies relating to neoplasms of the hypopharynx and the cervical esophagus. Kurume Med J. 1969;16:127.
42. Wei WI. The dilemma of treating hypopharyngeal carcinoma: more or less: Hayes Martin Lecture. Arch Otolaryngol Head Neck Surg. 2002;128:229.
43. Ho CM, Ng WF, Lam KH, et al. Radial clearance in resection of hypopharyngeal cancer: an independent prognostic factor. Head Neck. 2002;24:181.
44. Flanders WD, Rothman KJ. Interaction of alcohol and tobacco in laryngeal cancer. Am J Epidemiol. 1982;115:371.
45. Ribeiro UJr, Posner MC, Safatle-Ribeiro AV, et al. Risk factors for squamous cell carcinoma of the oesophagus. Br J Surg. 1996;83:1174.
46. Tuyns AJ, Esteve J, Raymond L, et al. Cancer of the larynx/hypopharynx, tobacco and alcohol: IARC international case-control study in Turin and Varese (Italy), Zaragoza and Navarra (Spain), Geneva (Switzerland) and Calvados (France). Int J Cancer. 1988;41:483.
47. Lefebvre JL, Buisset E, Coche-Dequeant B, et al. Epilarynx: pharynx or larynx? Head Neck. 1995;17:377.
48. Maier H, Sennewald E, Heller GF, et al. Chronic alcohol consumption—the key risk factor for pharyngeal cancer. Otolaryngol Head Neck Surg. 1994;110:168.
49. Shah JP, Karnell LH, Hoffman HT, et al. Patterns of care for cancer of the larynx in the United States. Arch Otolaryngol Head Neck Surg. 1997;123:475.
50. Sapkota A, Gajalakshmi V, Jetly DH, et al. Indoor air pollution from solid fuels and risk of hypopharyngeal/laryngeal and lung cancers: a multicentric case-control study from India. Int J Epidemiol. 2008;37:321.
51. Sapkota A, Gajalakshmi V, Jetly DH, et al. Smokeless tobacco and increased risk of hypopharyngeal and laryngeal cancers: a multicentric case-control study from India. Int J Cancer. 2007;121:1793.
52. McNab Jones RF. The Paterson-Brown-Kelly Syndrome. J Laryngol Otol. 1961;75:529.
53. Greene FL, Page DL, Fleming ID, et al. The AJCC Cancer Staging Manual, 6th ed, New York: Springer-Verlag, 2002.
54. Piccirillo JF. Purposes, problems, and proposals for progress in cancer staging. Arch Otolaryngol Head Neck Surg. 1995;121:145.
55. Piccirillo JF. Importance of comorbidity in head and neck cancer. Laryngoscope. 2000;110:593.
56. Piccirillo JF, Lacy PD, Basu A, et al. Development of a new head and neck cancer-specific comorbidity index. Arch Otolaryngol Head Neck Surg. 2002;128:1172.
57. Cheever DW. Cancer of the tonsil: removal of the tumor by external incision (A second case). Boston Med Surg Journal. 1878;99:133.
58. Steiner W, Ambrosch P, Hess CF, et al. Organ preservation by transoral laser microsurgery in piriform sinus carcinoma. Otolaryngol Head Neck Surg. 2001;124:58.
59. Cracovaner AJ, Chodosh PL. The lateral approach to the larynx and hypopharynx. Arch Otolaryngol. 1960;71:8.
60. Jacobs JR, Logemann J, Pajak TF, et al. Failure of cricopharyngeal myotomy to improve dysphagia following head and neck cancer surgery. Arch Otolaryngol Head Neck Surg. 1999;125:942.
61. Ogura JH, Jurema AA, Watson RK. Partial Laryngopharyngectomy and neck dissection for pyriform sinus cancer. Laryngoscope. 1960;70:1399.
62. Kania R, Hans S, Garcia D, et al. Supracricoid hemilaryngopharyngectomy in patients with invasive squamous cell carcinoma of the pyriform sinus. Part II: Incidence and consequences of local recurrence. Ann Otol Rhinol Laryngol. 2005;114:95.
63. Laccourreye H, Lacau St Guily J, Brasnu D, et al. Supracricoid hemilaryngopharyngectomy. Analysis of 240 cases. Ann Otol Rhinol Laryngol. 1987;96:217.
64. Laccourreye O, Ishoo E, de Mones E, et al. Supracricoid hemilaryngopharyngectomy in patients with invasive squamous cell carcinoma of the pyriform sinus. Part I: technique, complications, and long-term functional outcome. Ann Otol Rhinol Laryngol. 2005;114:25.
65. Laccourreye O, Merite-Drancy A, Brasnu D, et al. Supracricoid hemilaryngopharyngectomy in selected pyriform sinus carcinoma staged as T2. Laryngoscope. 1993;103:1373.
66. Ambrosch P, Brinck U, Fischer G, et al. [Special aspects of histopathologic diagnosis in laser microsurgery of cancers of the upper aerodigestive tract]. Laryngorhinootologie. 1994;73:78.
67. Kraus DH, Pfister DG, Harrison LB, et al. Salvage laryngectomy for unsuccessful larynx preservation therapy. Ann Otol Rhinol Laryngol. 1995;104:936.
68. Harrison DF. Surgical management of hypopharyngeal cancer. Particular reference to the gastric “pull-up” operation. Arch Otolaryngol. 1979;105:149.
69. Wei WI, Lam LK, Yuen PW, et al. Current status of pharyngolaryngo-esophagectomy and pharyngogastric anastomosis. Head Neck. 1998;20:240.
70. Horvath OP, Cseke L, Kalmar K, et al. Larynx-preserving pharyngo-esophagectomy after chemoradiation in the treatment of cancer of the pharyngo-esophageal junction. Ann Thorac Surg. 2001;72:2146.
71. Omura K, Urayama H, Kanehira E, et al. Larynx-preserving resection of the cervical esophagus for cervical esophageal carcinoma limited to the submucosal layer. J Surg Oncol. 1998;69:113.
72. Lore JMJr, Klotch DW, Lee KY. One-stage reconstruction of the hypopharynx using myomucosal tongue flap and dermal graft. Am J Surg. 1982;144:473.
73. Fabian RL. Reconstruction of the laryngopharynx and cervical esophagus. Laryngoscope. 1984;94:1334.
74. Ariyan S. The pectoralis major myocutaneous flap. A versatile flap for reconstruction in the head and neck. Plast Reconstr Surg. 1979;63:73.
75. Fabian RL. Pectoralis major myocutaneous flap reconstruction of the laryngopharynx and cervical esophagus. Laryngoscope. 1988;98:1227.
76. Spriano G, Pellini R, Roselli R. Pectoralis major myocutaneous flap for hypopharyngeal reconstruction. Plast Reconstr Surg. 2002;110:1408.
77. Spriano G, Piantanida R, Pellini R. Hypopharyngeal reconstruction using pectoralis major myocutaneous flap and pre-vertebral fascia. Laryngoscope. 2001;111:544.
78. Robb GL, Lewin JS, Deschler DG, et al. Speech and swallowing outcomes in reconstructions of the pharynx and cervical esophagus. Head Neck. 2003;25:232.
79. Scharpf J, Esclamado RM. Reconstruction with radial forearm flaps after ablative surgery for hypopharyngeal cancer. Head Neck. 2003;25:261.
80. Varvares MA, Cheney ML, Gliklich RE, et al. Use of the radial forearm fasciocutaneous free flap and montgomery salivary bypass tube for pharyngoesophageal reconstruction. Head Neck. 2000;22:463.
81. Slaughter DP, Southwick HW, Smejkal W. “Field cancerization” in oral stratified squamous epithelium. Cancer. 1953;6:963.
82. Martins AS. Multicentricity in pharyngoesophageal tumors: argument for total pharyngolaryngoesophagectomy and gastric transposition. Head Neck. 2000;22:156.
83. Turner GG. Excision of the thoracic esophagus for carcinoma with reconstruction of an extrathoracic gullet. Lancet. 1933;2:1315.
84. Ong GB, Lee TC. Pharyngo-gastric anastamosis after esophagopharyngectomy for carcinoma of the hypopharynx and cervical esophagus. Br J Surg. 1960;48:193.
85. Seidenberg B, Rosenak S, Hurwitt E, et al. Immediate reconstruction of the cervical esophagus by a revascularized isolated jejunal segment. Ann Surg. 1959;142:162.
86. Kerlin P, McCafferty G, Robinson D, et al. Function of a free jejunal “conduit” graft in the cervical esophagus. Gastroenterology. 1986;90:1956.
87. Gherardini G, Gurlek A, Staley CA, et al. Laparoscopic harvesting of jejunal free flaps for esophageal reconstruction. Plast Reconstr Surg. 1998;102:473.
88. Wadsworth JT, Futran N, Eubanks TR. Laparoscopic Harvest of the Jejunal Free Flap for Reconstruction of Hypopharyngeal and Cervical Esophageal Defects. Arch Otolaryngol Head Neck Surg. 2002;128:1384.
89. Biel MA, Maisel RH. Free jejunal autograft reconstruction of the pharyngoesophagus: review of a 10-year experience. Otolaryngol Head Neck Surg. 1987;97:369.
90. Ferguson JL, DeSanto LW. Total pharyngolaryngectomy and cervical esophagectomy with jejunal autotransplant reconstruction: complications and results. Laryngoscope. 1988;98:911.
91. Disa JJ, Pusic AL, Hidalgo DA, et al. Microvascular reconstruction of the hypopharynx: defect classification, treatment algorithm, and functional outcome based on 165 consecutive cases. Plast Reconstr Surg. 2003;111:652.
92. Reece GP, Schusterman MA, Miller MJ, et al. Morbidity and functional outcome of free jejunal transfer reconstruction for circumferential defects of the pharynx and cervical esophagus. Plast Reconstr Surg. 1995;96:1307.
93. Theile DR, Robinson DW, Theile DE, et al. Free jejunal interposition reconstruction after pharyngolaryngectomy: 201 consecutive cases. Head Neck. 1995;17:83.
94. Barrett WL, Gluckman JL, Aron BS. Safety of radiating jejunal interposition grafts in head and neck cancer. Am J Clin Oncol. 1997;20:609.
95. McCaffrey TV, Fisher J. Effect of radiotherapy on the outcome of pharyngeal reconstruction using free jejunal transfer. Ann Otol Rhinol Laryngol. 1987;96:22.
96. Petruzzelli GJ, Johnson JT, Myers EN, et al. The effect of postoperative radiation therapy on pharyngoesophageal reconstruction with free jejunal interposition. Arch Otolaryngol Head Neck Surg. 1991;117:1265.
97. Wei WI, Lam LK, Yuen PW, et al. Mucosal changes of the free jejunal graft in response to radiotherapy. Am J Surg. 1998;175:44.
98. Mendelsohn M, Morris M, Gallagher R. A comparative study of speech after total laryngectomy and total laryngopharyngectomy. Arch Otolaryngol Head Neck Surg. 1993;119:508.
99. Anthony JP, Singer MI, Mathes SJ. Pharyngoesophageal reconstruction using the tubed free radial forearm flap. Clin Plast Surg. 1994;21:137.
100. Harii K, Ebihara S, Ono I, et al. Pharyngoesophageal reconstruction using a fabricated forearm free flap. Plast Reconstr Surg. 1985;75:463.
101. Nakatsuka T, Harii K, Asato H, et al. Comparative evaluation in pharyngo-oesophageal reconstruction: radial forearm flap compared with jejunal flap. A 10-year experience. Scand J Plast Reconstr Surg Hand Surg. 1998;32:307.
102. Andrades P, Pehler SF, Baranano CF, et al. Fistula analysis after radial forearm free flap reconstruction of hypopharyngeal defects. Laryngoscope. 2008;118:1157.
103. Clark JR, Gilbert R, Irish J, et al. Morbidity after flap reconstruction of hypopharyngeal defects. Laryngoscope. 2006;116:173.
104. Lewin JS, Barringer DA, May AH, et al. Functional outcomes after laryngopharyngectomy with anterolateral thigh flap reconstruction. Head Neck. 2006;28:142.
105. Garden AS, Morrison WH, Clayman GL, et al. Early squamous cell carcinoma of the hypopharynx: outcomes of treatment with radiation alone to the primary disease. Head Neck. 1996;18:317.
106. Ang KK. Altered fractionation trials in head and neck cancer. Semin Radiat Oncol. 1998;8:230.
107. Fu KK, Pajak TF, Trotti A, et al. A Radiation Therapy Oncology Group (RTOG) phase III randomized study to compare hyperfractionation and two variants of accelerated fractionation to standard fractionation radiotherapy for head and neck squamous cell carcinomas: first report of RTOG 9003. Int J Radiat Oncol Biol Phys. 2000;48:7.
108. Al-Sarraf M. Treatment of locally advanced head and neck cancer: historical and critical review. Cancer Control. 2002;9:387.
109. Lefebvre JL, Chevalier D, Luboinski B, et al. Larynx preservation in pyriform sinus cancer: preliminary results of a European Organization for Research and Treatment of Cancer phase III trial. EORTC Head and Neck Cancer Cooperative Group. J Natl Cancer Inst. 1996;88:890.
110. Lee WT, Akst LM, Adelstein DJ, et al. Risk factors for hypopharyngeal/upper esophageal stricture formation after concurrent chemoradiation. Head Neck. 2006;28:808.
111. Shah JP, Shaha AR, Spiro RH, et al. Carcinoma of the hypopharynx. Am J Surg. 1976;132:439.
112. Lefebvre JL, Castelain B, De la Torre JC, et al. Lymph node invasion in hypopharynx and lateral epilarynx carcinoma: a prognostic factor. Head Neck Surg. 1987;10:14.
113. Shah JP. Patterns of cervical lymph node metastasis from squamous carcinomas of the upper aerodigestive tract. Am J Surg. 1990;160:405.
114. Candela FC, Kothari K, Shah JP. Patterns of cervical node metastases from squamous carcinoma of the oropharynx and hypopharynx. Head Neck. 1990;12:197.
115. Byers RM, Wolf PF, Ballantyne AJ. Rationale for elective modified neck dissection. Head Neck Surg. 1988;10:160.
116. Buckley JG, MacLennan K. Cervical node metastases in laryngeal and hypopharyngeal cancer: a prospective analysis of prevalence and distribution. Head Neck. 2000;22:380.
117. Harrison DF. Thyroid gland in the management of laryngopharyngeal cancer. Arch Otolaryngol. 1973;97:301.
118. Weber RS, Marvel J, Smith P, et al. Paratracheal lymph node dissection for carcinoma of the larynx, hypopharynx, and cervical esophagus. Otolaryngol Head Neck Surg. 1993;108:11.
119. McLaughlin MP, Mendenhall WM, Mancuso AA, et al. Retropharyngeal adenopathy as a predictor of outcome in squamous cell carcinoma of the head and neck. Head Neck. 1995;17:190.
120. Hasegawa Y, Matsuura H. Retropharyngeal node dissection in cancer of the oropharynx and hypopharynx. Head Neck. 1994;16:173.
121. Amatsu M, Mohri M, Kinishi M. Significance of retropharyngeal node dissection at radical surgery for carcinoma of the hypopharynx and cervical esophagus. Laryngoscope. 2001;111:1099.
122. Allen AM, Haddad RI, Tishler RB. Retropharyngeal nodes in hypopharynx cancer on positron emission tomography. J Clin Oncol. 2007;25:599.
122a. Marks JE, Devineni VR, Harvey J, et al. The risk of contralateral lymphatic metastases for cancers of the larynx and pharynx. Am J Otolaryngol. 1992;13:34.
123. Johnson JT, Bacon GW, Myers EN, et al. Medial vs lateral wall pyriform sinus carcinoma: implications for management of regional lymphatics. Head Neck. 1994;16:401.
124. Pingree TF, Davis RK, Reichman O, et al. Treatment of hypopharyngeal carcinoma: a 10-year review of 1,362 cases. Laryngoscope. 1987;97:901.
125. Pameijer FA, Mancuso AA, Mendenhall WM, et al. Evaluation of pretreatment computed tomography as a predictor of local control in T1/T2 pyriform sinus carcinoma treated with definitive radiotherapy. Head Neck. 1998;20:159.
126. El Badawi SA, Goepfert H, Fletcher GH, et al. Squamous cell carcinoma of the pyriform sinus. Laryngoscope. 1982;92:357.
127. Vandenbrouck C, Eschwege F, De la Rochefordiere A, et al. Squamous cell carcinoma of the pyriform sinus: retrospective study of 351 cases treated at the Institut Gustave-Roussy. Head Neck Surg. 1987;10:4.
128. Mendenhall WM, Parsons JT, Cassisi NJ, et al. Squamous cell carcinoma of the pyriform sinus treated with radical radiation therapy. Radiother Oncol. 1987;9:201.
129. Mendenhall WM, Parsons JT, Devine JW, et al. Squamous cell carcinoma of the pyriform sinus treated with surgery and/or radiotherapy. Head Neck Surg. 1987;10:88.
130. Mendenhall WM, Parsons JT, Stringer SP, et al. Radiotherapy alone or combined with neck dissection for T1-T2 carcinoma of the pyriform sinus: an alternative to conservation surgery. Int J Radiat Oncol Biol Phys. 1993;27:1017.
131. Mendenhall WM, Parsons JT, Vogel SB, et al. Carcinoma of the cervical esophagus treated with radiation therapy. Laryngoscope. 1988;98:769.
132. Jones AS, Roland NJ, Hamilton J, et al. Malignant tumours of the cervical oesophagus. Clin Otolaryngol. 1996;21:49.
133. Ogura JH, Marks JE, Freeman RB. Results of conservation surgery for cancers of the supraglottis and pyriform sinus. Laryngoscope. 1980;90:591.
134. Holsinger FC, Motamed M, Garcia D, et al. Resection of selected invasive squamous cell carcinoma of the pyriform sinus by means of the lateral pharyngotomy approach: the partial lateral pharyngectomy. Head Neck. 2006;28:705.
135. Rudert HH, Hoft S. Transoral carbon-dioxide laser resection of hypopharyngeal carcinoma. Eur Arch Otorhinolaryngol. 2003;260:198.
136. Martin A, Jackel MC, Christiansen H, et al. Organ preserving transoral laser microsurgery for cancer of the hypopharynx. Laryngoscope. 2008;118:398.
137. Reference not cited in text.
138. Kraus D, Zelefsky M, Brock H, et al. Combined surgery and radiation therapy for squamous cell carcinoma of the hypopharynx. Otolaryngology—Head Neck Surg. 1997;116:637.
139. Elias MM, Hilgers FJ, Keus RB, et al. Carcinoma of the pyriform sinus: a retrospective analysis of treatment results over a 20-year period. Clin Otolaryngol. 1995;20:249.
140. Spector JG, Sessions DG, Emami B, et al. Squamous cell carcinomas of the aryepiglottic fold: therapeutic results and long-term follow-up. Laryngoscope. 1995;105:734.
141. Harrison DF, Thompson AE. Pharyngolaryngoesophagectomy with pharyngogastric anastomosis for cancer of the hypopharynx: review of 101 operations. Head Neck Surg. 1986;8:418.
142. Triboulet JP, Mariette C, Chevalier D, et al. Surgical management of carcinoma of the hypopharynx and cervical esophagus: analysis of 209 cases. Arch Surg. 2001;136:1164.
143. Bova R, Goh R, Poulson M, et al. Total pharyngolaryngectomy for squamous cell carcinoma of the hypopharynx: a review. Laryngoscope. 2005;115:864.
144. Wang HW, Chu PY, Kuo KT, et al. A reappraisal of surgical management for squamous cell carcinoma in the pharyngoesophageal junction. J Surg Oncol. 2006;93:468.
145. Ho CM, Lam KH, Wei WI, et al. Squamous cell carcinoma of the hypopharynx—analysis of treatment results. Head Neck. 1993;15:405.
146. Laterza E, Mosciaro O, Urso US, et al. Primary carcinoma of the hypopharynx and cervical esophagus: evolution of surgical therapy. Hepatogastroenterology. 1994;41:278.
147. Kelley DJ, Wolf R, Shaha AR, et al. Impact of clinicopathologic parameters on patient survival in carcinoma of the cervical esophagus. Am J Surg. 1995;170:427.
148. Weaver A, Fleming S, Vandenberg H, et al. cis-platinum and 5-fluorouracil as initial therapy in advanced epidermoid cancers of the head and neck. Head Neck Surg. 1982;4:370.
149. Ensley JF, Jacobs JR, Weaver A, et al. Correlation between response to cisplatinum-combination chemotherapy and subsequent radiotherapy in previously untreated patients with advanced squamous cell cancers of the head and neck. Cancer. 1984;54:811.
150. Induction chemotherapy plus radiation compared with surgery plus radiation in patients with advanced laryngeal cancer. The Department of Veterans Affairs Laryngeal Cancer Study Group. N Engl J Med. 1991;324:1685.
151. Pignon JP, Bourhis J, Domenge C, et al. Chemotherapy added to locoregional treatment for head and neck squamous-cell carcinoma: three meta-analyses of updated individual data. The Lancet. 2000;355:949.
152. Urba SG. Concurrent chemoradiotherapy in head and neck cancer. Curr Oncol Rep. 1999;1:105.
153. Adelstein DJ, Saxton JP, Lavertu P, et al. Maximizing local control and organ preservation in stage IV squamous cell head and neck cancer with hyperfractionated radiation and concurrent chemotherapy. J Clin Oncol. 2002;20:1405.
154. Brizel DM, Albers ME, Fisher SR, et al. Hyperfractionated irradiation with or without concurrent chemotherapy for locally advanced head and neck cancer. N Engl J Med. 1998;338:1798.
155. Posner MR, Hershock DM, Blajman CR, et al. Cisplatin and fluorouracil alone or with docetaxel in head and neck cancer. N Engl J Med. 2007;357:1705.
156. Ringash J, Bezjak A. A structured review of quality of life instruments for head and neck cancer patients. Head Neck. 2001;23:201.
157. Magne N, Marcy PY, Chamorey E, et al. Concomitant twice-a-day radiotherapy and chemotherapy in unresectable head and neck cancer patients: a long-term quality of life analysis. Head Neck. 2001;23:678.
158. Paleri V, Stafford FW, Leontsinis TG, et al. Quality of life in laryngectomees: a post-treatment comparison of laryngectomy alone versus combined therapy. J Laryngol Otol. 2001;115:450.
159. Relic A, Mazemda P, Arens C, et al. Investigating quality of life and coping resources after laryngectomy. Eur Arch Otorhinolaryngol. 2001;258:514.
160. Weymuller EA, Yueh B, Deleyiannis FW, et al. Quality of life in patients with head and neck cancer: lessons learned from 549 prospectively evaluated patients. Arch Otolaryngol Head Neck Surg. 2000;126:329.
161. Weymuller EAJr, Yueh B, Deleyiannis FW, et al. Quality of life in head and neck cancer. Laryngoscope. 2000;110:4.
162. Terrell JE, Fisher SG, Wolf GT. Long-term quality of life after treatment of laryngeal cancer. The Veterans Affairs Laryngeal Cancer Study Group. Arch Otolaryngol Head Neck Surg. 1998;124:964.
163. Deleyiannis FW, Weymuller EAJr, Coltrera MD, et al. Quality of life after laryngectomy: are functional disabilities important? Head Neck. 1999;21:319.