Neoplasms, Neoplasm-like Lesions, and Infections of the Skull
Primary Neoplasms
Primary neoplasms of the skull are rare. The most commonly encountered lesions in children with a solitary nontraumatic lump on the head are dermoid tumors of the scalp (61%), cephalhematoma deformans (9%), Langerhans cell histiocytosis (LCH) (7%), and occult meningoceles and encephaloceles (4%).1,2
Osteochondromas may arise from the cartilaginous bones of the skull base. Osteoblastomas have been reported in the calvaria of infants, and aneurysmal bone cysts have been noted in the skull base and the calvaria (Fig. 21-1). Osteomas are small and usually limited to the outer table, although osteoid osteomas may occur in the diploë. Osteoid osteomas may present with the appearance of a button sequestrum. Malignant bone tumors are unusual, but osteogenic sarcoma and Ewing sarcoma have been reported (e-Fig. 21-2).3–5
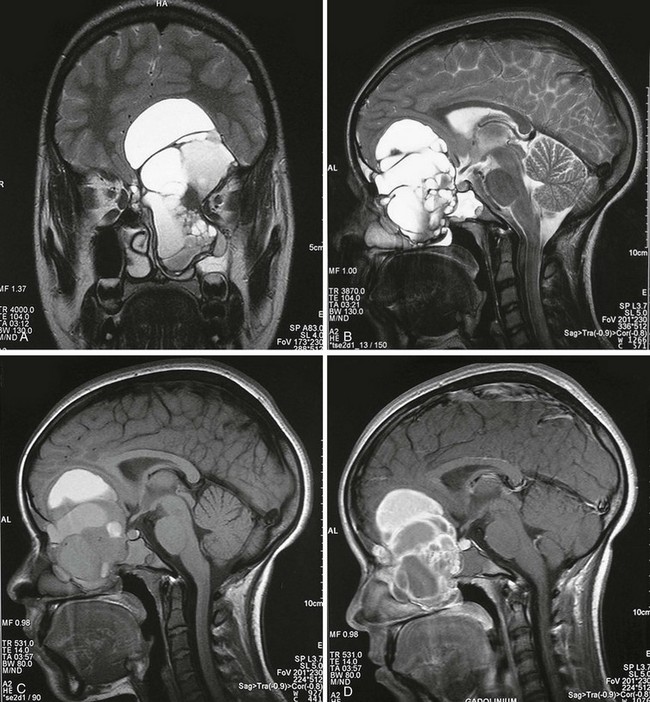
Figure 21-1 Aneurysmal bone cyst of the anterior skull base.
A, A coronal T2-weighted magnetic resonance image shows a multicystic expansile lesion of the anterior skull base. The lesion extended into the anterior cranial fossa, left ethmoid bone, and clivus. Sagittal T2-weighted (B), T1-weighted (C), and gadolinium-enhanced (D) images reveal the multiple fluid levels and inhomogeneous enhancement of the central septations and periphery. Only 1% of aneurysmal bone cysts are in the skull and usually involve the cranial vault. (A and B From Theron S, Steyn F. An unusual cause of proptosis: aneurysmal bone cyst of the anterior skull base. Pediatr Radiol. 2006;36:997.)
e-Figure 21-2 An osteogenic sarcoma of the skull.
An axial computed tomography scan with a bone window reveals a right occipital soft tissue mass with bony permeation and spiculation from tumor new bone formation.
Angiomas and neurofibromas of the scalp may affect the underlying skull and cause deformities, bony defects, and regional hyperostoses. Plain film findings of neurofibromatosis (NF) include lytic defect in the lambdoid suture, absence of the orbital roof and floor, elevated lesser sphenoid wing, enlarged middle cranial fossa, enlarged cranial nerve foramina, unilateral orbital enlargement, and J-shaped sella turcica (Fig. 21-3).6,7
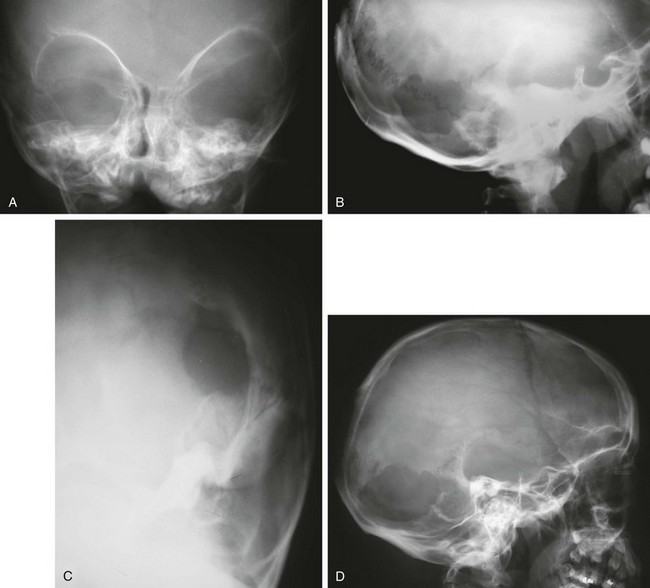
Figure 21-3 Skull changes of neurofibromatosis (NF) type 1.
A, A frontal view of the orbits reveals an elevated sphenoid wing (bilateral) and hypoplasia of the left sphenoid bone. B and C, A 5-year-old boy with NF has a defect in the left lambdoid suture seen on lateral (B) and oblique (C) views. D, A lateral view of another child with NF who has a large skull defect seen in the left lambdoid suture. (Courtesy Peter Strouse, MD, Ann Arbor, MI.)
Cavernous hemangiomas of the skull are characterized by rounded areas of diminished density in which there may be a honeycomb or radial pattern of greater density caused by spiculation. Calvarial hemangiomas (e-Fig. 21-4) usually thicken the outer table externally and are radially striated. They do not displace the inner table.
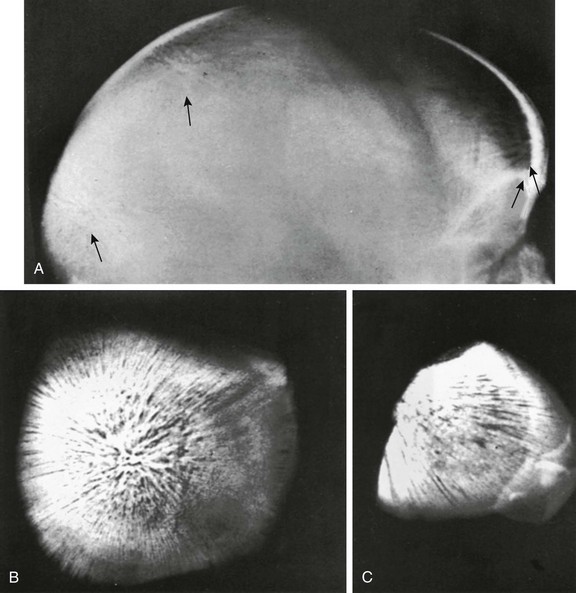
e-Figure 21-4 Radially striated hemangiomas (arrows) in the frontal and occipital squamosae and in both parietal bones of a child who also had hemangiomas of the neck, shoulder, and upper portion of the thorax.
A, A lateral radiograph of the head. B and C, Necropsy specimens from the occipital squamosa. (Courtesy Marie Capitanio, MD.)
Epidermoids are ectodermal rests or inclusions that may be located in the scalp, in the diploic spaces, or between the internal surface of the inner table and the dura. Epidermoids are usually benign and grow slowly. If they protrude into the cranial cavity, they may be the source of cerebral symptoms. When epidermoids grow within the bone or impinge on it, they produce local destruction of bone that appears radiographically as a sharply demarcated lucency surrounded by a smooth sclerotic margin (Fig. 21-5), which sometimes may be scalloped. The margin is due to flaring of the edge of the bone into a marginal ridge. Most cases are found in children younger than 3 years.8 The lesions usually disappear within a few years of discovery.
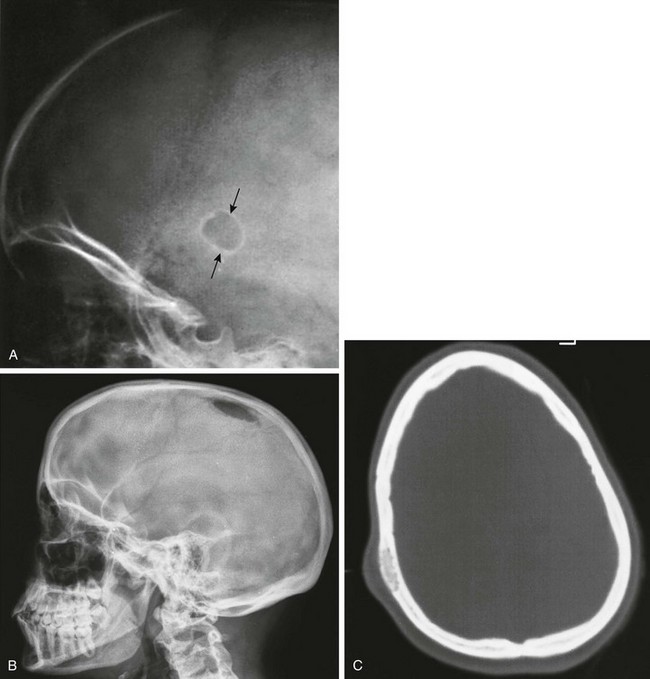
Figure 21-5 An epidermoid in a 1-year-old infant.
A, Arrows point to a small oval defect in the parietal bone with a sharply defined sclerotic border. B and C, An epidermoid in another child shows that these lesions may not be as well demarcated by sclerotic edges (B). A computed tomography scan reveals the lesion and soft tissue swelling (C).
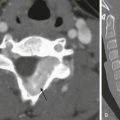
Meningiomas are rare in children. Radiographic changes include hyperostosis, an increase in the caliber and number of grooves for regional blood vessels, and calcifications in the meningioma itself. The hyperostosis is composed of normal reactive bone and is a complication rather than a part of the neoplasm. Occasionally, the overlying bone is destroyed, giving rise to radiolucent patches. Rarely, interosseous meningiomas occur (e-Fig. 21-6). Multiple meningiomas sometimes occur, most commonly in association with NF. Calvarial lesions, similar to the defects in NF, can occur in persons with congenital generalized fibromatosis.9
e-Figure 21-6 An interosseous meningioma.
A and B, Frontal and lateral scout computed tomography scans performed for a right hard mass on the skull reveal enlarged spiculated bony growth (arrow). C, A sagittal enhanced magnetic resonance image of the bone and extraaxial mass eloquently shows the enhanced spiculated bone. (Courtesy Tina Young Poussaint, MD, Boston, MA.)
Chordomas are infrequent in children; they occur predominantly in the clivus with clinical signs of diplopia, palatal or tongue weakness, and headaches.10,11 Torticollis occasionally is present. Magnetic resonance imaging (MRI) shows the location and extent of the chordoma. The tumor is inhomogeneous on T1-weighted and T2-weighted images and shows septations (Fig. 21-7).
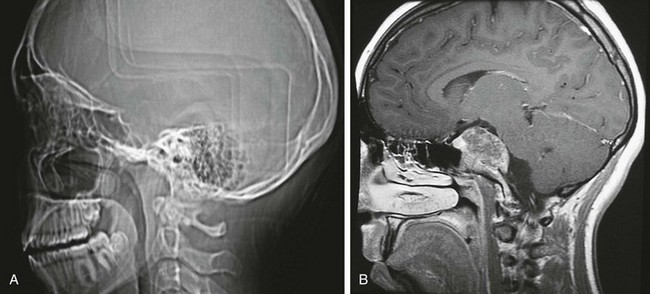
Figure 21-7 A chordoma.
A, A computed tomography scout image reveals the absence of the clivus. B, A contrast-enhanced magnetic resonance image shows the huge enhancing chordoma, which is involving the clivus and sphenoid, pushing on the brainstem.
Melanotic neuroectodermal tumor of infancy (melanotic progonoma, retinal anlage) is a rare tumor of the skull. The tumor is believed to be of neuroectodermal origin.12–15 More than 90% of cases involve the head and neck region; 70% occur in the maxilla, and about 13% occur in the calvarium, where the tumor has a predilection for the region of the anterior fontanelle. About 6% of cases occur in the mandible. In the calvarium, the tumor usually begins during the first year of life as a movable scalp nodule that subsequently invades the bone, becomes fixed to it (often adhering to the dura), and grows very rapidly. The bone is destroyed, but reactive spicules develop internally and externally, producing a sunburst appearance tangential to the mass on films (e-Fig. 21-8). Occasionally the mass appears as a soft tissue density. Local recurrences may occur after surgical removal. Although malignancy has been noted in extracalvarial tumors, it has not been reported for tumors in the calvarium.
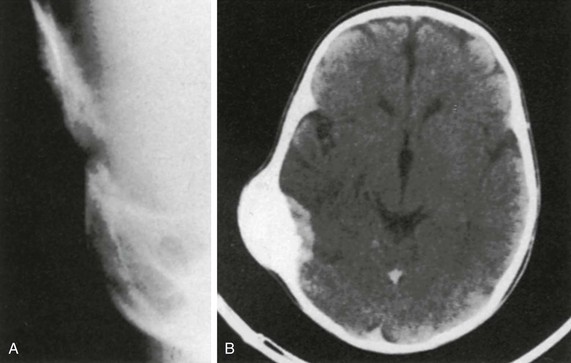
e-Figure 21-8 A melanotic neuroectodermal tumor of infancy.
A, Tangential projection of the posterolateral fontanelle shows the typical bone reaction and speculation. B, A nonenhanced computed tomography scan shows the internal expansion of the tumor and the massive bone reaction. (From Jones HH, Parker BR, Ballerio CG, et al. Case report 617: melanotic neuroectodermal tumor of infancy in the calvaria. Skeletal Radiol. 1990;19:317-318.)
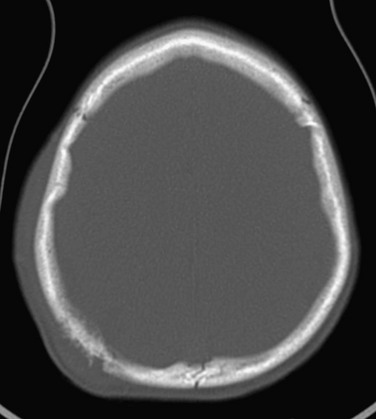
“Doughnut lesions” are rounded or oval radiolucent calvarial defects with a surrounding sclerotic halo, central bone density, or both.16 Multiple radiographic “doughnut lesions” have been described.17 Microscopic features in the calvarial lesions included fibrous tissue with clusters of foam cells or histiocytes and surrounding sclerotic bone. Familial doughnut lesions (Fig. 21-9) of the skull have been reported, and similar radiographic changes have been found in persons with sickle cell anemia. A malignant calvarial doughnut lesion caused by a metastatic carcinoma has been reported with features of a button sequestrum.18
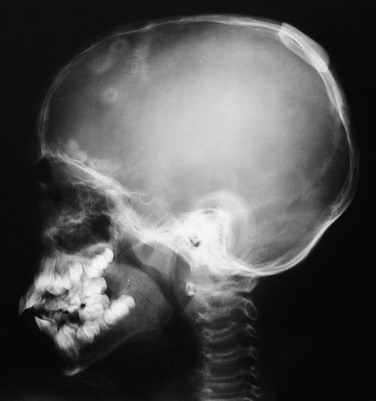
Figure 21-9 A calvarial doughnut lesion in a well child.
Secondary Neoplasms
Secondary tumors of the calvarium are more common than primary tumors and include leukemia, neuroblastoma, small round cell tumors, and histiocytosis (Figs. 21-10 through 21-12 and e-Fig. 21-13). The differential diagnosis of a permeative pattern includes osteomyelitis.
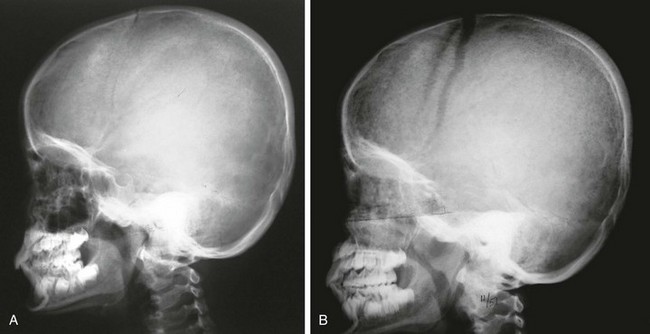
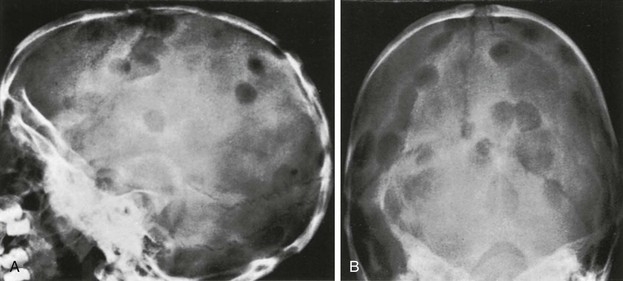
Figure 21-10 Leukemia.
A, An initial radiograph shows a mottled “salt and pepper” appearance to the frontal region of the calvaria and some spiculation of bone over the parietal region. B, Radiographic evaluation in another child with leukemia shows much more florid inhomogeneity of the skull density and widened coronal sutures indicating increased intracranial pressure.
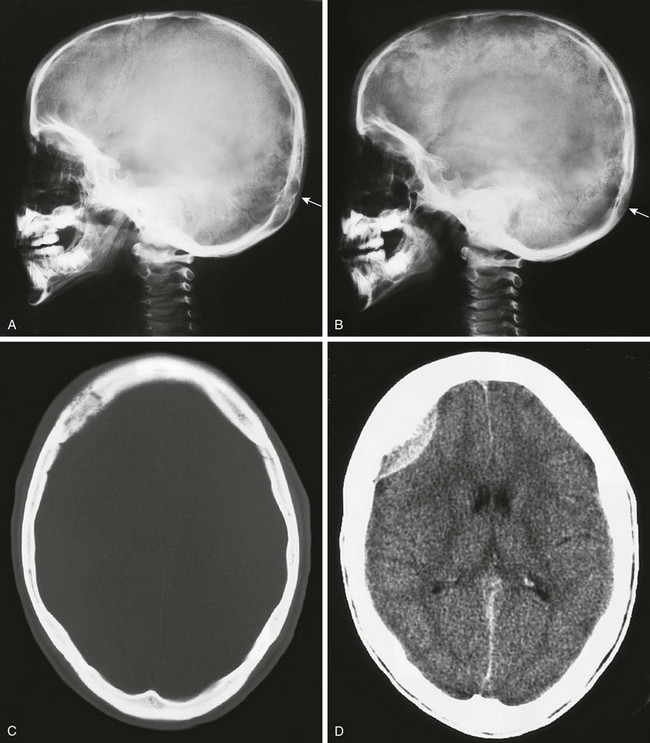
Figure 21-11 A metastatic neuroblastoma.
A and B, Plain films in a child who presented with scalp pain and a destructive lesion in the occipital lobe. A mild “salt and pepper” change of the calvaria is present in (A), seen best in the frontal and occipital regions (arrow). This change is much more pronounced in B. C and D, A computed tomography scan in another patient. A permeative right frontal lesion is present, and brain imaging shows the enhancing epidural mass.
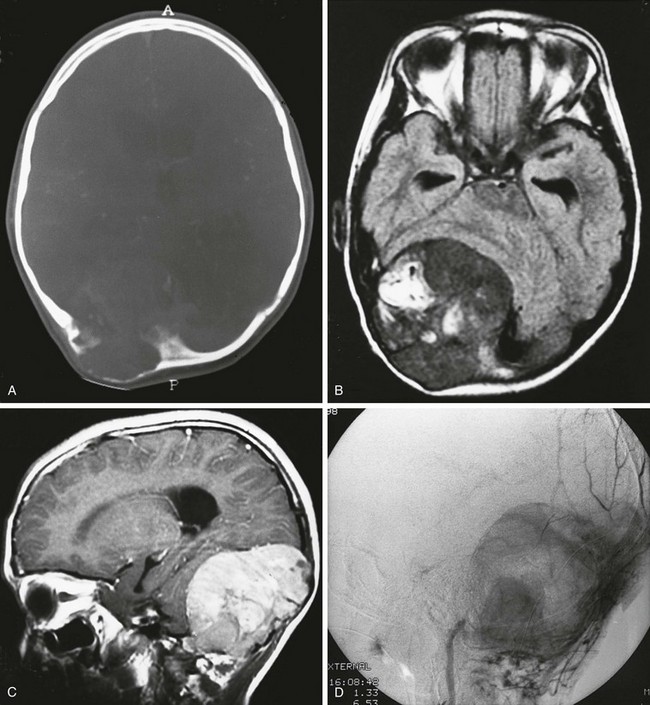
Figure 21-12 A metastatic neuroblastoma.
A, An axial computed tomography scan shows a mass in the occipital region with bone destruction. Axial nonenhanced (B) and sagittal enhanced (C) magnetic resonance imaging scans show the extradural mass. D, Angiography shows that the mass is vascular.
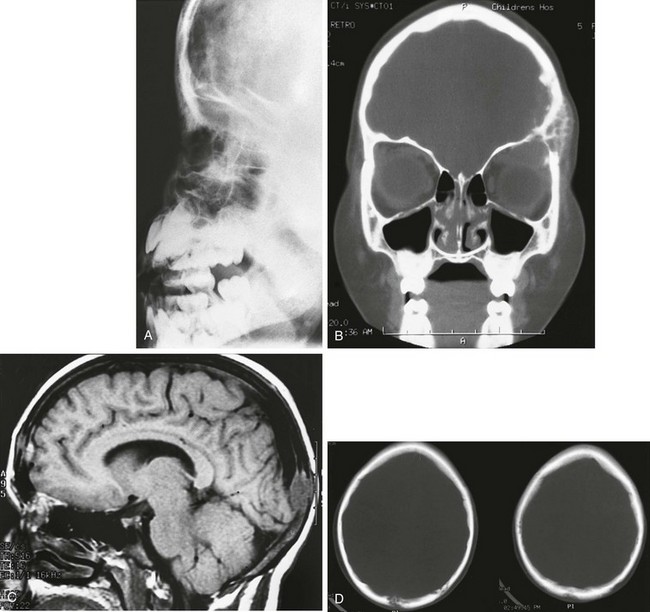
e-Figure 21-13 Ewing sarcoma metastatic to the calvarium.
A, A lateral skull film shows permeative changes of the frontal region. B, A coronal computed tomography (CT) scan shows the destruction of the frontal bone and the lateral wall and root of the left orbit. A large mass is in place superiorly within the orbit. C, A lateral magnetic resonance imaging scan of another child with a metastatic Ewing sarcoma reveals an extraaxial lesion in the occipital portion of the calvaria. D, A noncontrast CT scan demonstrates osseous destruction.
Neoplasm-like Lesions
LCH manifestations are addressed in more detail in Chapter 139; only their calvarial manifestations are presented here. Lesions in the skull are common, with a reported incidence of 28% in one large series.19 The calvarium is affected more frequently than the skull base. Multiple lesions occur more frequently in children younger than 5 years. A solitary calvarial lesion in a child older than 5 years is likely to be associated with LCH of bone (Figs. 21-14 and 21-15). Lesions also occur in the mastoid portion of the temporal bone and adjacent petrous pyramids, in the sphenoid bone, and in the bones of the orbit.20 Sphenoid bone involvement may be associated with diabetes insipidus. Exophthalmos may occur when the bones of the orbit are affected.21
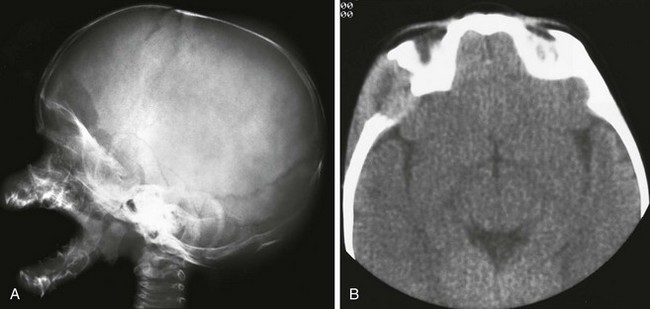
Figure 21-14 Langerhans cell histiocytosis.
A, A lateral skull film shows a lytic, well-defined lesion in the frontal region just above the orbit. B, A computed tomography scan shows the bony destruction and soft tissue mass in this region. Note the beveled appearance with the greater destruction of the outer table.
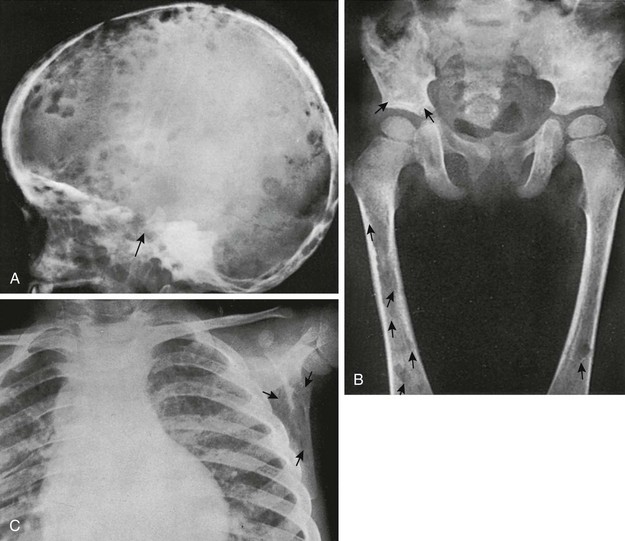
Figure 21-15 Langerhans cell histiocytosis in a  -year-old boy.
-year-old boy.
A, A lateral skull radiograph shows numerous small and large rounded defects in the calvaria and base (arrow). An autopsy disclosed replacement of a large part of the body of the sphenoid. B, Large and small defects (arrows) in the ilia and femora; the latter show erosions of the cortex, which indicates their origin in the medullary cavity and not under the periosteum. C, Defects (arrows) in the scapula and infiltration of the lungs and pleura.
Fibrous Dysplasia
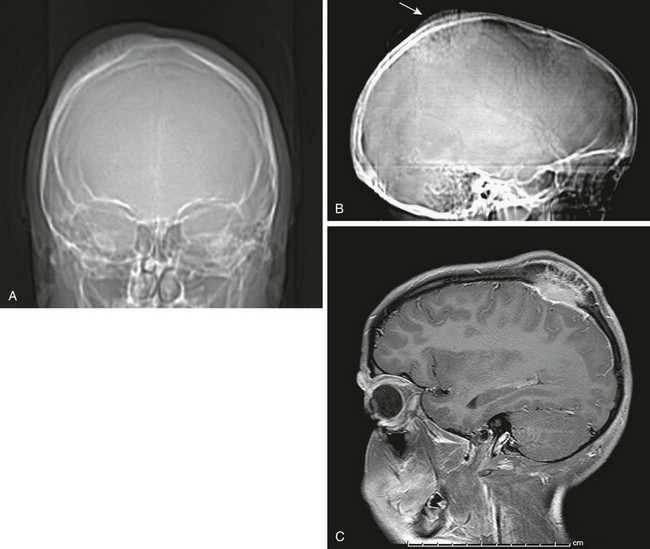
Fibrous dysplasia of the calvarium in children usually involves the frontal, sphenoid, and ethmoid bones (Fig. 21-16)22 and may manifest as painless progressive bony bulges or masses. Children rarely present with a change in vision or blindness as a result of compression of the orbital apex and ischemia of the optic nerve.23
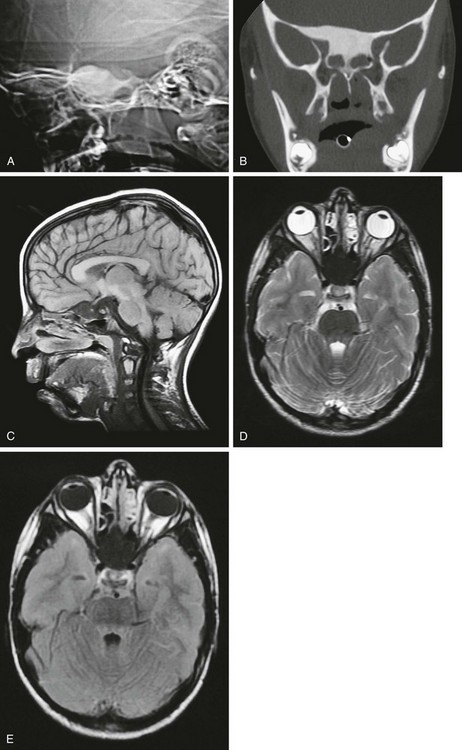
Figure 21-16 Fibrous dysplasia of the skull.
A, A scout computed tomography (CT) film shows dense sphenoid bone. B, A coronal CT scan shows the extent of the lesion. C, D, and E, Magnetic resonance imaging shows the lesion to be hypointense on T1-weighted (C), T2-weighted (D), and fluid attenuated inversion recovery (E) sequences.
CT findings of fibrous dysplasia consist of three types: the ground-glass pattern (56%), the homogeneously dense pattern (23%), and the cystic variety (21%).24 The MR signal intensity of fibrous dysplasia is usually low on T1-weighted images and intermediate on T2-weighted images.25,26 Fibrous tissue may demonstrate marked enhancement on MRI, simulating a tumor.
Infections of the Calvaria
Osteomyelitis of the skull is rare in children and is a complex disease with many different etiologies.27 Infection at the pin site in children with halo insertion may occur when pins become loose.28 Trauma is a common precursor.29 Systemic diseases can alter the body’s defense and predispose to infections. Bones adjacent to the paranasal sinuses may become infected from direct extension of sinusitis.
During the early stages of osteomyelitis, the radiographic findings are negative. When areas of inflammatory necrosis of sufficient size develop, they can be identified as areas of diminished density involving the inner and outer tables and diploic spaces. These lesions may be single or multiple and are more common in the frontal and parietal bones than elsewhere. Localized osteomyelitis may spread by contiguity or through the diploic veins. Remote lesions first appear as fine lytic foci or rarefactions that enlarge and coalesce, sometimes giving rise to a moth-eaten rarefaction that involves the entire bone or even the entire calvarium. In infants, the avascular sutures act as barriers to spreading. The differential diagnosis of this permeative destructive pattern includes osteomyelitis, leukemia, metastatic neuroblastoma, and metastatic small round cell tumors (Ewing sarcoma, medulloblastoma, and retinoblastoma).30 Clival osteomyelitis resulting from the spread of infection through the fossa navicularis magna has been reported after retropharyngeal abscess.31 In chronic osteomyelitis, sclerotic changes usually are present; in disease resulting from paranasal sinusitis, the bony walls of the affected sinus often are sclerotic.
Pott puffy tumor, described in 1760 by Sir Percival Pott, consists of a subperiosteal abscess and osteomyelitis of the frontal bone.32,33 It may result from acute frontal sinusitis and trauma. The valveless diploic veins drain the sinuses, and septic emboli can lead to abscess formation (e-Fig. 21-17). Sonography may demonstrate a subgaleal abscess. CT and MRI may show frontal sinusitis, frontal osteomyelitis, and a subgaleal abscess.
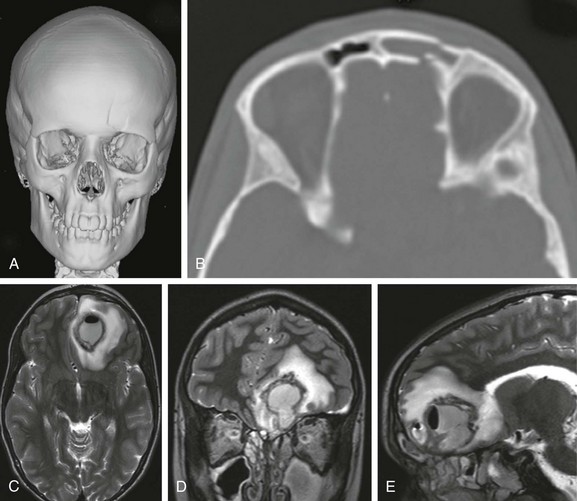
e-Figure 21-17 Frontal osteomyelitis complicated by a brain abscess in a 12-year-old who was in a sledding accident.
A, A three-dimensional computed tomography (CT) image shows a fracture through the left frontal sinus. B, An axial CT scan demonstrates the frontal sinus fracture. C, D, and E, Three weeks later axial, coronal, and sagittal contrast-enhanced magnetic resonance imaging T2-weighted fast spin echo images show a large gas -containing left frontal lobe abscess with surrounding edema. Sinus disease is also present.
Petrous apicitis (Gradenigo syndrome) must be considered in the setting of middle ear disease with headache, cranial neuropathy, and an elevated erythrocyte sedimentation rate. Characteristic CT and MRI findings include petrosal marrow T1 hypointensity, soft tissue abnormalities, and bone destruction. Cochlear implants are an increasing cause of mastoid inflammatory disease and osteomyelitis. Lytic lesions of the skull have been seen with cat scratch fever, and the frontal and sphenoid bones have been involved in chronic recurrent multifocal osteomyelitis.34
Tuberculosis
Tuberculosis of the calvaria usually manifests as a painless subgaleal scalp swelling with a discharging sinus. The lesions are usually either small, circumscribed, punched-out lytic areas (Fig. 21-18); or spreading, circumscribed sclerotic areas; or a combination of the two.35
Sarcoidosis
Sarcoidosis of the diploic space is associated with large radiolucent patches in the frontal, parietal, and occipital bones (e-Fig. 21-19).36
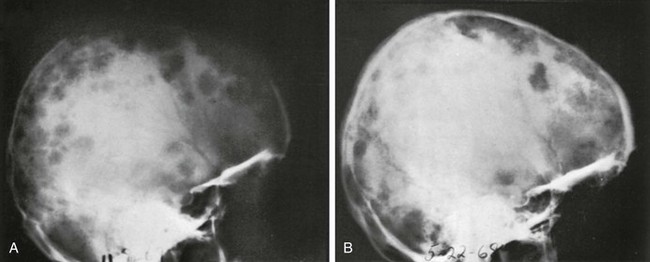
e-Figure 21-19 Sarcoidosis of the diploic space in a 14-year-old girl—lateral projections.
A, Before treatment, multiple large and small radiolucent defects are scattered widely in the frontal, parietal, and occipital bones. Microscopically, the radiolucent patches represent focal destruction of internal and external tables that overlie expanding sarcoid granulations in the diploic space. B, After 7 months of steroid therapy, some of the radiolucent patches have disappeared and others have shrunk, presumably as a result of the treatment. (From Toomey F, Bautista A. Rare manifestations of sarcoidosis in children. Radiology. 1970;94:569-573.)
Mycotic Osteomyelitis
The cranium sometimes is involved in chronic fungal infections, including actinomycosis, blastomycosis, coccidiomycosis, cryptococcosis, and candidiasis.37 The radiographic appearance of the lesions is similar to that of tuberculous, syphilitic, and chronic purulent osteomyelitis, and diagnosis requires identification of the responsible organism. Underlying immunodeficiency often is present.38
Arico, M, Egeler, PM. Clinical aspects of Langerhans cell histiocytosis. Hematol Oncol Clin North Am. 1998;12:247–258.
Atkinson, GO, Davis, PC, Patrick, LE, et al. Melanotic neuroectodermal tumour of infancy: MR findings and a review of the literature. Pediatr Radiol. 1989;20:20–22.
Calliauw, L, Roels, H, Caemaert, J. Aneurysmal bone cysts in the cranial vault and base of the skull. Surg Neurol. 1985;23:93–98.
Koch, BL. Imaging extracranial masses of the pediatric head and neck. Neuroimaging Clin N Am. 2000;10:193–214.
Lui, YW, Dasari, SB, Young, RJ. Sphenoid masses in children: radiologic differential diagnosis with pathologic correlation. AJNR Am J Neuroradiol. 2011;32:617–626.
Miyazaki, S, Tsubokawa, T, Katayama, Y, et al. Benign osteoblastoma of the temporal bone in an infant. Surg Neurol. 1987;27:277–285.
Posnick, JC, Wells, MD, Drake, JM, et al. Childhood fibrous dysplasia presenting as blindness: a skull base approach for resection and immediate reconstruction. Pediatr Neurosurg. 1993;19:260–266.
References
1. Ruge, JR, Tomila, T, Naidich, TP, et al. Scalp and calvarial masses of infants and children. Neurosurgery. 1988;22:1037–1042.
2. Moron, FE, Morriss, MC, Jones, JJ, et al. Lumps and bumps on the head of children: use of CT and MR imaging in solving the clinical diagnostic dilemma. Radiographics. 2004;24:1655–1674.
3. Salvati, M, Ciapetta, P, Capone, R, et al. Osteosarcoma of the skull in a child: case report and review of the literature. Childs Nerv Syst. 1993;9:437–439.
4. Sato, J, Himi, T, Tamakawa, M. Osteosarcomatosis involving craniofacial bones presenting with cranial nerve palsies. J Laryngol Otol. 2000;114:214–217.
5. Sharma, A, Garg, A, Mishra, NK, et al. Primary Ewing’s sarcoma of the sphenoid bone with unusual imaging features: a case report. Clin Neurol Neurosurg. 2005;107:528–531.
6. Jacquemin, C, Bosley, TM, Svedberg, H. Orbit deformities in craniofacial neurofibromatosis type 1. AJNR Am J Neuroradiol. 2003;24:1678–1682.
7. Joffe, N. Calvarial bone defects involving the lambdoid suture in neurofibromatosis. Br J Radiol. 1965;381:23–27.
8. Holthusen, W, Lassrich, MA, Steiner, C. Epidermoids and dermoids of the calvarian bones in early childhood: their behavior in the growing skull. Pediatr Radiol. 1983;13:189–194.
9. Briselli, MF, Soule, EH, Gilchrist, DS. Congenital fibromatosis: report of 18 cases of solitary and 4 cases of multiple tumors. Mayo Clin Proc. 1980;55:554–562.
10. Matsumoto, J, Towbin, RB, Ball, WS, Jr. Cranial chordomas in infancy and childhood: a report of two cases and review of the literature. Pediatr Radiol. 1989;20:28–32.
11. Nolte, K. Malignant intracranial chordoma and sarcoma of the clivus in infancy. Pediatr Radiol. 1979;8:1–6.
12. Cutler, LS, Chaudhry, AP, Topazian, R. Melanotic neuroectodermal tumor of infancy: an ultrastructural study, literature review, and reevaluation. Cancer. 1981;48:257–270.
13. Nishio, S, Morioka, T, Murakami, N, et al. Melanotic neuroectodermal tumour of infancy at the anterior fontanelle. Neuroradiology. 1999;41:202–204.
14. Saleem, S, Altinok, D. Melanotic neuroectodermal tumor of the calvarium. Pediatr Radiol. 2010;40(suppl 1):S159.
15. Smith, AB, Rushing, EJ, Smirniotopoulos, JG. Pigmented lesions of the central nervous system: radiologic-pathologic correlation. Radiographics. 2009;29:1503–1524.
16. Keats, TE, Holt, JF. The calvarial “doughnut lesion”: a previously undescribed entity. Am J Roentgenol Radium Ther Nucl Med. 1969;105:314–318.
17. Royen, PM, Ozonoff, MB. Multiple calvarial “doughnut” lesions: a case report. Am J Roentgenol Radium Ther Nucl Med. 1974;121:112–123.
18. Som, PM, Anderson, PJ, Wolf, BS. A malignant calvarial doughnut lesion (“button sequestrum”?). Mt Sinai J Med. 1978;45:390–393.
19. Kirks, DR, Taybi, H. Histiocytosis X. In: Parker BR, Castellino RA, eds. Pediatric oncologic radiology. St Louis: Mosby-Year Book, 1977.
20. Koch, BL. Langerhans histiocytosis of temporal bone: role of magnetic resonance imaging. Top Magn Reson Imaging. 2000;11:66–74.
21. Prayer, D, Grois, N, Prosch, H, et al. MR imaging presentation of intracranial disease associated with Langerhans cell histiocytosis. AJNR Am J Neuroradiol. 2004;25:880–891.
22. DiRocco, C, Marchese, E, Velardi, F. Fibrous dysplasia of the skull in children. Pediatr Neurosurg. 1992;18:117–126.
23. Maher, CO, Friedman, JA, Meyer, FB, et al. Surgical treatment of fibrous dysplasia of the skull in children. Pediatr Neurosurg. 2002;37:87–92.
24. Brown, EW, Megerian, CA, McKenna, MJ, et al. Fibrous dysplasia of the temporal bone. AJR Am J Roentgenol. 1995;164:679–682.
25. Chong, VFH, Khoo, JBK, Fan, Y-K. Fibrous dysplasia involving the base of the skull. AJR Am J Roentgenol. 2002;178:717–720.
26. Utz, JA, Kransdorf, MJ, Jelinek, JS, et al. MR appearances of fibrous dysplasia. J Comput Assist Tomogr. 1989;13:845–851.
27. Pincus, DJ, Armstrong, MB, Thaller, SR. Osteomyelitis of the craniofacial skeleton. Semin Plast Surg. 2009;23:73–79.
28. Limpaphayom, N, Skaggs, DL, McComb, G, et al. Complications of halo use in children. Spine (Phila PA 1976). 1990;34:779–784.
29. Hanifan, WC, Olivero, WC, Duff, JJ, et al. Lawn dart injury in children: report of two cases. Pediatr Emerg Care. 1986;2:247–249.
30. Ozgen, B, Oguz, KK, Cila, A. Diffusion MR imaging features of skull base osteomyelitis compared with skull base malignancy. AJNR Am J Neuroradiol. 2001;32:179–184.
31. Prabhu, SP, Zinkus, T, Cheng, AG, et al. Clival osteomyelitis resulting from spread of infection through the fossa navicularis magna in a child. Pediatr Radiol. 2009;39:995–998.
32. Rabiu, TB. Pott’s puffy tumour in the paediatric population. Childs Nerv Syst. 2010;26:729.
33. Tsai, BY, Lin, KL, Lin, TY, et al. Pott’s puffy tumor in children. Child Neuro. 2010;26:53–60.
34. Khanna, G, Satot, S, Ferguson, P. Imaging of chronic recurrent multifocal osteomyelitis. Radiographics. 2009;29(4):1159–1177.
35. Ramdurg, SR, Gupta, DK, Suri, A, et al. Calvarial tuberculosis: uncommon manifestation of common disease—a series of 21 cases. Br J Neurosurg. 2010;24:572–577.
36. Toomey, F, Bautista, A. Rare manifestations of sarcoidosis in children. Radiology. 1970;94:569–573.
37. Budenz, CL, Tajudeen, BA, Roehm, PC. Actinomycosis of the temporal bone and brain: case report and review of the literature. Ann Otol Rhinol Laryngol. 2010;119:313–318.
38. Marsot-Dupuch, K, Quillard, J, Meyohas, MC. Head and neck lesions in the immunocompromised host. Eur Radiol. 2004;14:E155–E167.