Neoplasia
Overview
Hepatic neoplasms constitute approximately 2% of all childhood tumors and approximately 6% of pediatric abdominal neoplasms. Two thirds of liver tumors in children are malignant, and they represent the third most common intraabdominal malignancy in the pediatric age group after Wilms tumor and neuroblastoma. The most common hepatic malignant tumors in order of frequency are hepatoblastomas, hepatocellular carcinomas (HCC), undifferentiated embryonal sarcomas, angiosarcomas, and embryonal rhabdomyosarcomas.1 Benign hepatic tumors in pediatrics include tumors specific to children such as vascular tumors and mesenchymal hamartomas, as well as entities that also are seen in adults, such as focal nodular hyperplasia (FNH), hepatocellular adenoma, and nodular regenerative hyperplasia (NRH).
A differential diagnosis of liver tumors can be obtained based on the age of the patient, laboratory findings such as serum alpha fetoprotein (AFP) levels, and imaging characteristics (Table 92-1). However, the serum AFP level is normally elevated at birth (25,000-50,000 ng/mL) and does not reach adult levels (<25 ng/mL) until 6 months of age.2,3
Malignant Hepatobiliary Tumors
Overview: Hepatoblastoma is the most common primary malignant liver tumor in infants and children. Sixty-eight percent of cases are seen in the first year of life, and 90% occur in patients younger than 5 years, with a male predominance of 2 : 1.3 Four percent of cases are congenital.1,3–6
Etiology: Predisposing conditions include Beckwith-Wiedemann syndrome, familial adenomatous polyposis, type 1A glycogen storage disease, Gardner syndrome, fetal alcohol syndrome, Wilms tumor, and trisomy 18. It also is reported in premature infants, low-birth-weight infants, and in infants born of mothers taking oral contraceptives. A strong association exists between low birth weight and hepatoblastoma, raising the issue of potential contribution by a variety of iatrogenic exposures in the neonatal intensive care unit.7
Clinical Presentation: Hepatoblastoma usually presents as a palpable mass in the right upper quadrant and may be confused with hepatomegaly. Nonspecific clinical symptoms include pain, weight loss, irritability, vomiting, and infrequently, jaundice and precocious puberty (related to the secretion of chorionic gonadotropins). Distant metastases are present in fewer than 10% of cases at diagnosis, with the lungs being the most common site, followed by the lymph nodes, bone, brain, eye, and ovary. Compression or invasion of the hepatic vasculature and inferior vena cava may occur. Serum AFP, which is markedly elevated in approximately 90% of patients with hepatoblastoma, can be used to monitor therapy and detect recurrence.
Hepatoblastoma is classified into two histologic types: the epithelial type, which represents the majority of these tumors, and the mixed epithelial mesenchymal type.1–3 Hepatoblastoma is usually solitary, but these tumors may be multifocal or, less commonly, diffusely infiltrating. Multifocal disease may consist of a dominant mass with satellite nodules or multiple small masses. When it is solitary, hepatoblastoma is most commonly located in the right hepatic lobe (60% of cases).8
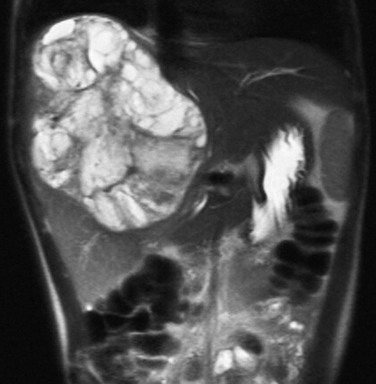
Imaging: Plain radiographs may show hepatomegaly or a mass with or without calcifications. On sonography, hepatoblastomas are most often well-defined and hyperechoic relative to adjacent liver (Fig. 92-1 and e-Fig. 92-2). Epithelial-type hepatoblastomas are more homogeneous, whereas mixed tumors are more heterogeneous and often contain hyperechoic foci with acoustic shadowing indicating calcifications and hypoechoic or anechoic foci representing necrosis or hemorrhage.9–11 Intravascular tumor thrombus may be seen within the hepatic or portal veins. Flow within the thrombus on color Doppler imaging is useful to differentiate neoplastic from nonneoplastic thrombus. Infiltrative hepatoblastomas show diffuse heterogeneous echogenicity, with loss of normal parenchymal architecture.9–11
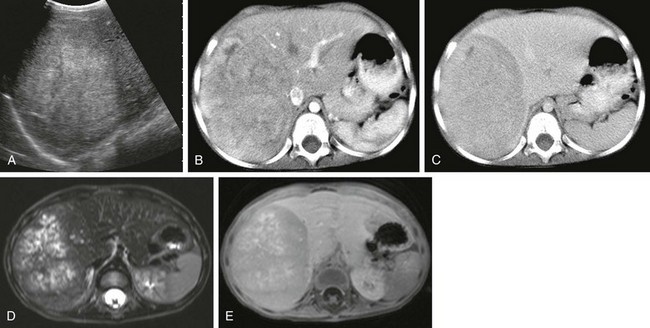
Figure 92-1 Hepatoblastoma.
A, A longitudinal abdominal sonogram in a 3-year-old boy shows a well-defined lesion that is hyperechoic relative to the liver. B, A computed tomography (CT) scan in the early arterial postcontrast phase demonstrates a heterogeneous mass that is hyperdense compared with the liver. C, A CT scan in the delayed contrast phase shows a hypodense lesion. D, An axial T2-weighted magnetic resonance (MR) image shows hyperintense nodules with intervening hypointense septa. E, An axial T1-weighted MR image after intravenous administration of gadolinium demonstrates enhancement of the septa and capsule.
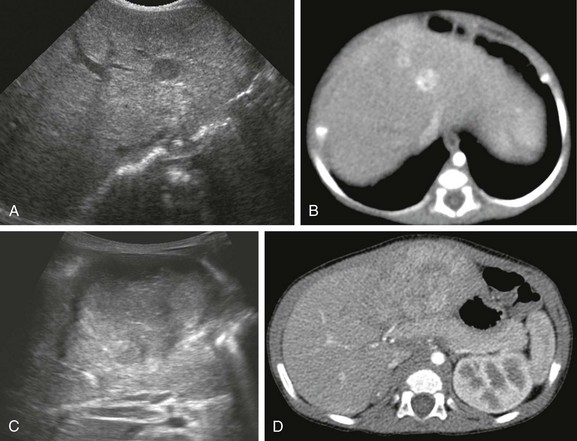
e-Figure 92-2 Hepatoblastoma.
A, A sonogram of a 71-day-old girl with Beckwith-Wiedemann syndrome shows a hypoechoic lesion in segment IV-A of the liver. B, An enhanced computed tomography (CT) scan reveals marked enhancement of the lesion. C, Eight months after the first ultrasound, a transverse ultrasound image shows a large hypoechoic mass with a lobular margin. D, An enhanced CT scan shows a circumscribed heterogeneous lesion with lobular enhancement.
Unenhanced CT typically shows a well-circumscribed mass with decreased attenuation relative to the surrounding liver. Speckled or amorphous calcifications are seen in more than 50% of cases.9 On contrast-enhanced computed CT, the tumor enhances in a heterogeneous fashion and may be hyperdense compared with liver parenchyma in the early arterial postcontrast phase; it usually is isodense or hypodense on delayed images. Peripheral enhancement can be seen. CT angiography can help define vascular invasion when present and assess its potential to be resected.
On MRI, epithelial-type hepatoblastomas are homogeneously hypointense on T1-weighted images and hyperintense on T2-weighted images relative to adjacent liver, and they enhance after intravenous (IV) administration of gadolinium contrast material.9 Mixed-type tumors show more heterogeneous signal intensity. However, areas of calcification, necrosis, hemorrhage, and septation may influence the signal intensity.9–11 Hemorrhage is most often hyperintense on T1-weighted images and bands of fibrosis or septation are hypointense on T1- and T2-weighted sequences.9 Vascular invasion is demonstrated with gradient-echo sequences; tumor thrombus appears as a high signal on T1-weighted images and as a signal void on gradient-echo images. On postgadolinium arterial and venous phase images, the tumor thrombus enhances and shows a filling defect, respectively. MR angiography is useful for preoperative evaluation of the relationship of the tumor to the hepatic vasculature.
Hepatic scintigraphy currently is not performed in persons with a hepatoblastoma but may demonstrate increased activity on the initial angiographic phase as a result of tumor vascularity and photopenia on the delayed images. Rarely, increased uptake of the radiopharmaceutical agent may be seen on delayed images, which is a finding more typical of focal nodular hyperplasia. Currently, catheter angiography is rarely performed and usually shows tumor hypervascularity except in avascular areas of necrosis.9–11
Treatment: The treatment for hepatoblastoma is surgical resection. However, in about 40% to 60% of cases, the tumor cannot be resected at diagnosis.12 Initial treatment with chemotherapy permits up to 85% of these tumors to become resectable.3 The overall survival rate is reported to be 65% to 70%. 1,9–11 Imaging is crucial for assessment of the surgical resectability of hepatoblastomas at presentation and after the patient undergoes chemotherapy. Disseminated tumors have been treated successfully with chemotherapy and multiple resections of metastases. Radiofrequency ablation may be a promising treatment for recurrence.13 Liver transplantation can be useful in lesions that are considered unresectable; the presence of pulmonary metastases is not considered an absolute contraindication to liver transplantation because of their sensitivity to chemotherapy.9 Poor prognostic factors include AFP levels less than 100 ng/mL or more than 1,000,000 ng/mL, vascular invasion, and aneuploid nuclear content. Factors associated with favorable prognosis include single lobe involvement, pure fetal histologic composition, and AFP levels between 100 and 1,000,000 ng/mL.9
Hepatocellular Carcinoma
Overview: HCC, the second most common liver tumor in children after hepatoblastoma, accounts for 35% of primary pediatric hepatic malignancies.14 It affects two age peaks in childhood: 4 to 5 years and (more commonly) 12 to 14 years. HCCs, like hepatoblastoma, occur more often in the right than in the left lobe of the liver and demonstrate a high propensity for vascular invasion, which is seen in approximately 75% of cases. Neoplastic cells vary from very well-differentiated to poorly differentiated. The most helpful histologic feature in distinguishing HCC from metastases is the presence of bile canaliculi or bile pigment.1 Kupffer cells also may be present.9
Etiology: In nonendemic areas of the world, approximately half of HCCs arise in patients with underlying liver disease. Predisposing conditions include entities leading to cirrhosis, such as biliary atresia, infantile cholestasis, Alagille syndrome, hemochromatosis, hereditary tyrosinemia, glycogen storage disorder, α1-antitrypsin deficiency, Wilson disease, galactosemia, and viral hepatitis (hepatitis B and C).
Clinical Presentation: The clinical symptoms and presentation are similar to those of hepatoblastoma, in that patients often present with an abdominal mass, abdominal pain, fever, and cachexia. The serum AFP level is markedly elevated in 70% of patients.14,15
Imaging: HCCs have three main growth patterns: solitary, multifocal, and diffuse/infiltrative.9 The ultrasound findings of HCC are variable. With respect to liver parenchyma, smaller lesions may be isoechoic or hyperechoic, although most tend to be hypoechoic9,10; larger lesions are heterogeneous. Internal areas of increased echogenicity may represent acute hemorrhage, fat, or calcifications (which is less common than in hepatoblastoma), whereas areas of decreased echogenicity may represent necrosis. If a capsule is present, it may be detected as a thin halo of decreased echogenicity. Doppler demonstrates high-velocity arterial flow and is useful for identifying vascular invasion by showing blood flow within the substance of tumoral thrombus.9
On unenhanced CT, an HCC appears as a solitary mass or as multiple well-defined or poorly defined, hypointense to isodense masses.10 The tumor shows variable enhancement after IV administration of contrast material and may contain low-attenuation regions of necrosis. The tumor capsule may also show a rim of low attenuation on unenhanced images that enhances on the delayed phase after injection of contrast material (Fig. 92-3).9 Vascular tumor thrombi may be seen as intraluminal filling defects with a surrounding meniscus of contrast and can be better evaluated with angiographic sequences. When the tumor arises in a cirrhotic liver, differentiation from regenerating nodules may be difficult.
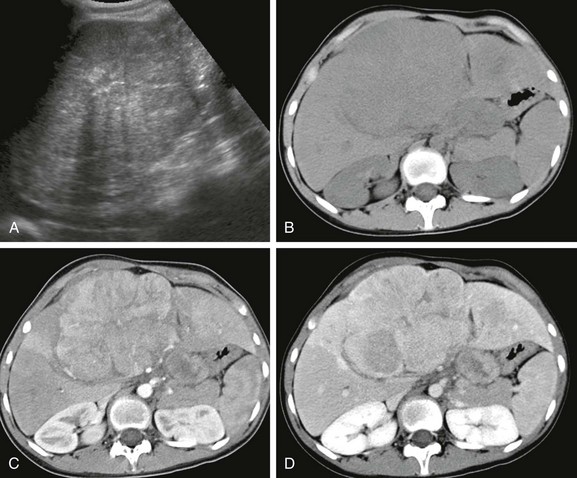
Figure 92-3 Hepatocellular carcinoma in a 14-year-old girl without underlying liver disease.
A, An abdominal sonogram shows a heterogeneous hepatic lesion. B, An unenhanced computed tomography scan demonstrates the liver mass, with slightly decreased attenuation compared with adjacent normal liver parenchyma. Postcontrast arterial (C) and venous phase (D) images show enhancement during the arterial phase, which decreases during the venous phase. After administration of contrast material, the tumor margins are more conspicuous.
With MRI, the tumor typically is slightly hyperintense on T2-weighted images and hypointense on T1-weighted images, although the latter presentation tends to be more variable; hyperintense areas of fat or hemorrhage may be seen on T1-weighted images. If a fibrous pseudocapsule is present, MRI shows low signal intensity on T1- and T2-weighted pulse sequences.16 After administration of gadolinium, in the early arterial phase, the lesion demonstrates enhancement and washes out with relatively low signal intensity during the portal venous phase. Vascular invasion appears as lack of a signal on spin-echo images and as an intravascular arterial enhancing mass with a delayed filling defect on dynamic gadolinium-enhanced images.17
Nuclear scintigraphy is rarely performed in patients with HCC and usually shows decreased uptake. Gallium scans, however, are characteristic and may help distinguish HCC, which is gallium-avid, from regenerating nodules, which are not gallium-avid. Fluorodeoxyglucose (FDG) positron emission tomography (PET) is useful in evaluating the degree of tumor differentiation. FDG uptake is variable in HCCs; uptake may be normal in well-differentiated tumors, with markedly elevated uptake usually seen in poorly differentiated tumors. FDG-PET may be useful in the staging of HCC or in distinguishing regenerating nodules in cirrhotic livers from HCC. Combining FDG-PET and gallium scintigraphy can be worthwhile when grading tumors.18 For example, low-grade tumors typically show normal uptake on PET and increased uptake on gallium scans. However, gallium uptake also is seen in other processes, such as metastatic disease (lymphoma) and hepatic adenoma.
Treatment: The treatment for HCC is complete surgical resection when possible, but approximately two thirds of children present with unresectable tumors as a result of multifocal or massive involvement of the liver, major vascular involvement, or metastases. HCC is relatively insensitive to systemic chemotherapy.19 The impact of chemotherapy is unclear, with no evidence that it offers additional benefit in children with resectable localized HCC. Liver transplantation has been reported for an unresectable tumor but remains controversial.14 Radiofrequency ablation and intraarterial chemotherapy have been reported, but the benefits require further investigation.20,21 The prognosis is variable and is directly related to the resectability and histology of the lesion. In tumors with favorable histology and complete resection, the 2-year survival rate may exceed 97%. However, without complete resection and with unfavorable histology, the 2-year survival rate may be less than 20%.14 In places where the prevalence of tyrosinemia is especially high, routine neonatal screening and immediate treatment of positive cases by 2-(2-nitro-4-3 trifluoromethylbenzoyl)-1,3-cyclohexanedione (NTBC) have resulted in marked decrease in the prevalence of HCC in this population.22 In Quebec, where the prevalence of tyrosinemia is especially high, routine neonatal screening and immediate treatment of positive cases with NTBC have resulted in 100% prevention of subsequent HCC to date in patients treated at birth. However, patients with delayed diagnosis who are treated with NTBC are at risk of having HCC develop.
Fibrolamellar Carcinoma
Overview: Fibrolamellar carcinoma (FLC) is a variant of HCC that occurs in patients without underlying hepatic disease. It has distinctive clinical and pathologic features and represents approximately 5% to 8% of all cases of HCC. FLC tends to occur in younger patients, with a peak in the late teens; approximately 85% of patients with FLC are younger than 35 years, and it may be diagnosed in children as young as 10 years. The incidence is similar in males and females.23
Etiology: The etiology of FLC is unknown. It has been reported in association with syndromes, including Wilms, Carney, Fanconi anemia, and familial adenomatous polyposis; these associations have been implicated in shared molecular pathways. FLC also has been associated with FNH, but currently evidence does not support such an association.23
Clinical Presentation: Clinically, patients present with abdominal symptoms or pain and sometimes a palpable mass. Uncommon presentations are gynecomastia, jaundice, and venous compression or thrombosis.9 Metastatic lymphadenopathy is seen in 70% of cases.24 AFP levels generally are normal.16 In 80% to 90% of patients, the gross pathological appearance is one of a large, circumscribed, and nonencapsulated mass.16 Other patterns can be seen, including satellite lesions, multiple diffuse masses, or a bilobed mass. Fibrous tissue within a central scar is common (appearing in 30% of cases) and typically does not enhance after administration of contrast material. Calcifications are seen in 35% to 55% of tumors and are localized in the central scar.16
Imaging: Plain radiographs may demonstrate hepatomegaly or calcifications. Ultrasound shows a well-defined mass with heterogeneous echo texture and isoechoic or hyperechoic areas.25 If it is present, the central scar appears hyperechoic and may contain shadowing hyperechoic calcifications.
CT shows a hypoattenuating, well-defined, lobulated mass with calcifications in 30% to 55% of cases and a central scar in 45% to 60% of cases (Fig. 92-4). Adjacent lymphadenopathy often is present in the hepatic hilum at diagnosis. After IV injection of contrast material, in the early arterial phase, the tumor is hyperattenuating relative to the adjacent liver with variable attenuation during the portal venous phase. The central scar is hypoattenuating with little or no enhancement on CT.
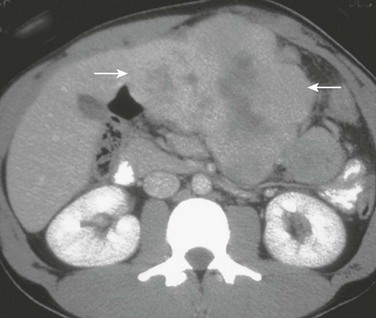
Figure 92-4 Fibrolamellar carcinoma in an 18-year-old girl.
Axial contrast-enhanced computed tomography shows a lobulated mass (arrows) in the left lobe of the liver with a hypoattenuating central scar.
On T1-weighted MRI, the tumor is hypointense (86%) to isointense (14%), and it is slightly hyperintense (85%) to isointense (15%) on T2-weighted images. The fibrous scar is hypointense on T1-weighted images and hypointense on T2-weighted images, and it does usually not enhance after IV administration of contrast material.9,11,15,25,26 These features are useful in distinguishing FLC from FNH, in which the central scar has increased signal on T2-weighted sequences and enhances with IV administration of contrast material.
Treatment: The primary treatment of FLC is surgical resection, with surgical resectability considered to be the most important prognostic factor.25 However, when the tumor is not resectable, orthotopic liver transplantation, systemic chemotherapy, or hepatic intraarterial chemoembolization is considered. Recent literature reports no significant difference in the prognosis of FLC compared with HCC in patients without underlying hepatic disease.23,24 Normal hepatic function, younger age, absence of vascular invasion or thrombosis, lack of lymphadenopathy, and negative surgical margins are favorable prognostic indicators. The 5-year survival rate ranges from 30% to 67%.20,21,23,24
Undifferentiated Embryonal Sarcoma
Overview: Undifferentiated embryonal sarcoma, previously called malignant mesenchymoma, embryonal sarcoma, or fibromyxosarcoma, is a rare, aggressive tumor of mesenchymal origin. Most commonly, the tumor affects children around 6 to 10 years of age with a slight male predominance.27 In one review, this was the third most common malignant pediatric liver tumor, following hepatoblastoma and HCC.28
Etiology: The etiology of undifferentiated embryonal sarcoma is uncertain. It has been linked to mesenchymal hamartoma as its malignant counterpart,3,15 and some tumors have been reported to arise in a background of mesenchymal hamartoma.29 On histology, an undifferentiated embryonal sarcoma shows primitive spindle-shaped, sarcomatous satellite cells closely packed in sheets or whorls and scattered throughout a background of loose myxoid tissue, which contains foci of hematopoiesis in 50% of cases.27
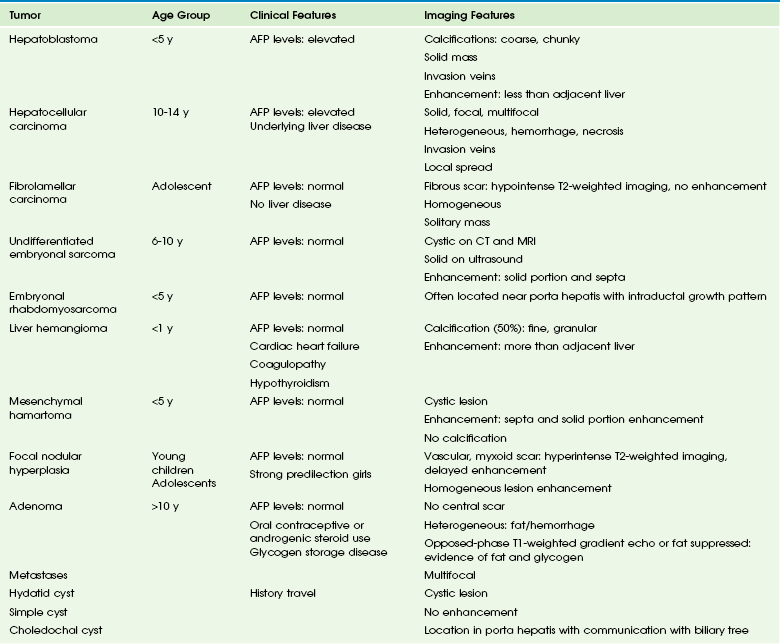
Clinical Presentation: The most common presenting symptoms include an abdominal mass, pain, and discomfort. An undifferentiated embryonal sarcoma usually is large at presentation, is solitary, involves the right lobe of the liver (in 75% of cases), and is predominantly solid with occasional cystic, necrotic, or hemorrhagic areas.27 AFP levels are normal.15
Imaging: Plain radiographs show a large, typically noncalcified mass. On sonography, the tumor is solid and isoechoic to hyperechoic relative to normal liver with small anechoic spaces that correspond to necrosis, hemorrhage, or cystic degeneration.27,30
On CT, the tumor reveals predominantly water attenuation correlating with myxoid stroma (88% of tumor volume) (e-Fig. 92-5).9,27 After administration of IV contrast material, a dense enhancing peripheral rim can be seen in relation to the pseudocapsule. Uncommon hyperattenuation regions may indicate hemorrhage and calcifications.
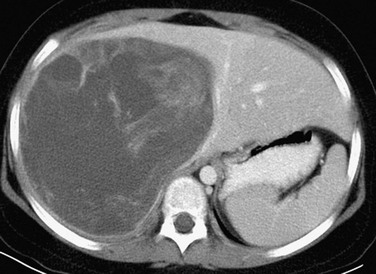
e-Figure 92-5 Undifferentiated embryonal sarcoma in a 9-year-old girl.
A postcontrast computed tomography image shows a large, well-defined, heterogeneously enhancing but low-attenuation mass in the right hepatic lobe. (Courtesy Robert P Guillerman, MD.)
With MRI, the tumor shows low signal intensity on T1-weighted sequences and increased signal intensity on T2-weighted sequences (Fig. 92-6). A hypointense rim on T1- and T2-weighted images indicates a pseudocapsule. Bright areas on T1-weighted images correspond to regions of hemorrhage. Fluid levels, internal debris, and septa may be seen on T2-weighted images.27,30 Heterogeneous enhancement of the tumor is seen after IV administration of gadolinium contrast material.31 MRI permits excellent evaluation of tumor resectability, vascular invasion, and involvement of adjacent lymph nodes. Metastases usually are to the lungs and bone.
Treatment: Treatment consists of complete tumor resection. As late as three decades ago, the prognosis was poor, with death occurring within 12 months in many cases.28 However, more recent reports of multimodal treatment show markedly improved survival rates. Patients with unresectable tumors that are not responsive to chemotherapy can be treated with liver transplantation.9
Rhabdomyosarcoma of the Biliary Tree
Overview: Although rhabdomyosarcoma can be found throughout the body, involvement of the biliary ducts is one of the rarest forms of this mesenchymal tumor. It occurs almost exclusively in the pediatric age group and often in children younger than 5 years (in 75% of cases).9
Etiology: The tumor arises from the biliary tree beneath the biliary epithelium, with which the tumor is invested,32 and grows as a polypoid mass within the biliary tree. Only the embryonal subtype of rhabdomyosarcoma arises in the biliary tree.9 The tumor most commonly involves major extrahepatic bile ducts but can arise within the intrahepatic bile ducts, gallbladder, and cystic duct. Histologically, rhabdomyosarcoma demonstrates undifferentiated blue cells with scant cytoplasm and primitive nuclei that form a firm, lobulated mass with infiltrative margins and a well-defined pseudocapsule.6,9,12,33
Clinical Presentation: Jaundice, the most frequent manifestation, can be associated with abdominal distention, pain, nausea, vomiting, or fever. Elevated levels of conjugated bilirubin and alkaline phosphatase with normal levels of AFP are typical of the laboratory workup. At diagnosis, metastases are present in 30% of cases9 and typically appear in the lung, appendicular skeleton, skull, and pericardium, although the tumor has a greater propensity to invade contiguous structures.32 The clinical presentation may resemble that of hepatitis, which may lead to delay in obtaining diagnostic tests.34
Imaging: Multiplanar imaging demonstrates a mass within the biliary ducts. The tumor most frequently involves the common bile duct, in or near the porta hepatis. In large lesions, necrosis can be seen. Ultrasound may reveal a solitary, heterogeneous, relatively hypoechoic mass or multiple hypoechoic nodules associated with biliary duct dilation and intraductal extension.9,35 Portal vein displacement without thrombosis is common.35 CT shows a homogeneous or heterogeneous hypoattenuating or hyperattenuating intraductal mass with variable contrast enhancement associated with biliary dilatation (Fig. 92-7).35,36 MRI usually shows low signal intensity on T1-weighted sequences and high signal intensity on T2-weighted sequences, with intense, heterogeneous enhancement after IV administration of contrast material.9,35 Magnetic resonance cholangiopancreatography often demonstrates a partially cystic lesion in the common bile duct and a mass adjacent to the duct causing mural irregularity. Percutaneous cholangiography displays an intraluminal polypoid mass.35–37 Gallium uptake can help localize metastatic disease.35
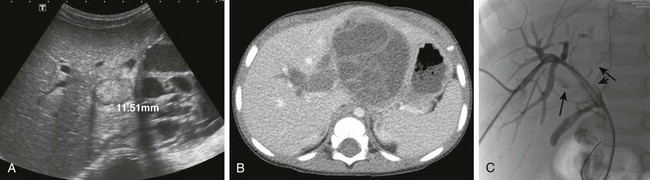
Figure 92-7 Embryonal rhabdomyosarcoma in a 20-month-old boy.
A, A transverse sonogram shows a multicystic lesion with a mass in the biliary tree (measured). B, A computed tomography scan shows the hypoattenuating tumor in the common bile duct extending into the intrahepatic and extrahepatic bile ducts. C, Percutaneous cholangiography reveals multiple filling defect of the common bile duct (short arrow) with extension in the intrahepatic bile ducts (long arrows).
Treatment: Multimodal therapy, including surgical resection, radiation, and chemotherapy, has improved outcome, with 78% survival in cases of local disease.38 Concurrent internal or external biliary drainage is essential because some chemotherapeutic agents depend on hepatobiliary excretion, and the inability to excrete the agent may result in significant systemic toxicity.38
Angiosarcoma
Overview: Angiosarcoma is a rare, aggressive, malignant vascular tumor that generally develops after the first year of life. Histologically, the spindle cell form is especially common in children.33,39 Immunohistochemical studies demonstrate reactivity of tumor cells to factor VIII–related antigen, CD31, and CD34, confirming the vascular nature of the tumor.40
Etiology: Angiosarcoma typically presents in adults who have a history of exposure to thorium dioxide (Thorotrast), arsenic, and vinyl chloride9,39; however, as exposure to these toxins becomes rarer, some of these tumors arise de novo in adults with cirrhosis or are associated with hemochromatosis or the use of anabolic steroids. As a result, this tumor is considered the “quintessential example of malignant transformation secondary to environmental exposures.”41,42 In children, the tumors are very rare and typically arise without a history of exposure. Cases of the tumor arising in children with previously diagnosed infantile hemangioendotheliomas have been reported (e-Fig. 92-8).39,43
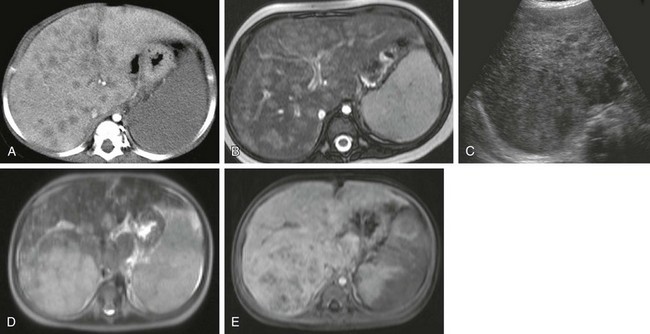
e-Figure 92-8 Angiosarcoma.
A, A 9-month-old girl with a normal liver ultrasound at 3 days of life now has numerous hypodense hepatic lesions on contrast-enhanced computed tomography. B, An axial T2-weighted magnetic resonance (MR) image demonstrates multiple hyperintense nodules. A diagnosis of infantile hemangiomas was made. After treatment with steroids and interferon, complete regression of lesions was seen. Eight months later, the patient presented with coagulopathy and an abdominal mass. C, An ultrasound scan shows multiple infiltrating nodules associated with a significantly larger lesion in the right lobe. D, An axial T2-weighted MR image shows heterogeneous signal intensity. E, Dynamic MR imaging at 5 minutes after administration of gadolinium contrast material demonstrates a heterogeneous pattern of tumor enhancement.
Clinical Presentation: Clinical symptoms include hepatomegaly associated with pain, anorexia, and weight loss, as well as thrombocytopenia and consumptive coagulopathy.42 At the time of diagnosis, 60% of cases have metastatic disease, most commonly to the lungs and spleen.41,42
Imaging: Imaging findings are variable and depend on the patterns at presentation such as a localized mass versus a multifocal or diffuse lesion (see e-Fig. 92-8).
On ultrasound, the lesion is heterogeneous and the echogenicity varies depending on the amount of hemorrhage or necrosis. With CT, nodules hypoattenuate compared with normal liver. In the presence of hemorrhage, hyperattenuating foci can occur. Contrast enhancement is variable, often showing a centripetal enhancement pattern similar to hepatic venous malformations (formerly and inappropriately called “cavernous hemangiomas”).41,44 At MRI, the masses show low signal intensity relative to the liver on T1-weighted images with hyperintense foci (related to hemorrhage) and heterogeneous signal intensity on T2-weighted images. Dynamic MRI with contrast reveals a heterogenous pattern of enhancement.42 FDG-PET/CT displays marked uptake in the tumors and is helpful for detection of metastases.
Hepatic Metastases
Wilms tumor metastases most commonly are found in the lungs and adjacent lymph nodes. Hematogenous spread of Wilms tumor to the liver occurs in 15% of cases; however, the primary renal lesion often is very large at presentation, and if it arises from the right kidney, it may abut the liver, rendering direct hepatic invasion by the primary mass difficult to exclude with certainty. Most hepatic metastatic lesions attributed to Wilms tumor are hematogenous and tend to be multifocal and heterogeneously enhancing; central necrosis, calcifications, or vascular invasion may be identified (Fig. 92-9).44,45
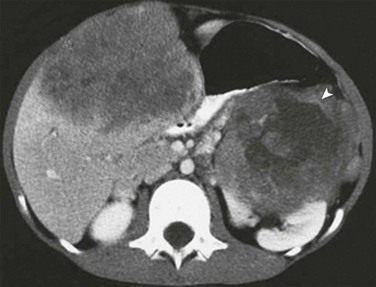
Figure 92-9 Metastatic Wilms tumor in a 4-year-old boy with clinical evidence of hepatomegaly.
A postcontrast computed tomography image demonstrates heterogeneous enhancement within a hepatic metastasis from a Wilms tumor arising in the left kidney (arrowhead).
Neuroblastoma may present with metastatic spread to bone, regional lymph nodes, liver, brain, and lungs. Metastatic neuroblastoma to the liver has two typical patterns: (1) numerous discrete lesions of variable echogenicity, attenuation, and enhancement; and (2) diffuse infiltration with distortion of the normal hepatic architecture, leading to hepatomegaly (Fig. 92-10). The latter pattern is especially common with stage IV-S disease (a primary lesion limited to the organ of origin or a primary lesion with regional spread but without extension across the midline, along with involvement of the liver, skin, or bone marrow).
Benign Hepatic Neoplasms
Overview: The International Society for the Study of Vascular Anomalies (ISSVA) has adopted terminology to address the classification of vascular anomalies, based on the classification by Mulliken and Glowacki proposed in 1982.46 In this classification, vascular anomalies are divided into vascular tumors (exhibiting cellular proliferation and hyperplasia) and vascular malformations (lesions that arise by dysmorphogenesis and exhibit normal endothelial turnover).47
Despite these changes and recent literature,47–49 improper terminology still in use leads to inappropriate description of findings in imaging studies and at times to misdiagnosis of vascular anomalies. For instance, in adults the term “liver hemangioma” continues to be erroneously applied instead of “venous malformation.” According to the ISSVA classification, the proper name for liver vascular tumors in children is “liver hemangioma.”
Liver hemangiomas are classified as infantile and congenital. Infantile hemangiomas usually begin growing after birth, although some are present at birth; they typically grow during the first year of life, enter an involuting phase between 1 and 7 years, and have an involuted phase between 8 and 12 years. Infantile hemangiomas are positive for glucose transporter-1 protein (Glut-1), a protein that facilitates the transport of glucose across erythrocyte cell membranes. Congenital hemangiomas are fully developed at birth and are characterized by Glut-1 negativity. Congenital hemangiomas in turn are subdivided into a rapidly involuting group, which involute more rapidly than infantile hemangiomas (usually within 12-14 months of age),50 and a noninvoluting group, with some overlap between these groups.47,50 The vascular tumors that appear most frequently in infancy are infantile hemangiomas. The differentiation among these tumors may be impossible at initial diagnosis because (1) there is no biopsy in most instances to identify the Glut-1 marker, and (2) contrary to skin lesions, it usually is not known whether the hepatic lesion is present and fully developed at birth. Infantile hemangioma is the most frequently occurring pediatric tumor, affecting 4% to 5% of infants.47 Other vascular tumors seen in the pediatric age group include hemangioendotheliomas, tufted angiomas, and sarcomas.
Etiology: Hemangioma is a model of the angiogenesis concept proposed by Folkman et al.,51 and its development is related to a combination of upregulation of factors that promote angiogenesis and downregulation of its inhibitors. The trigger for this abnormal angiogenesis is unknown, although a viral cause and a somatic mutation have been postulated.47
Clinical Presentation: Infantile hemangiomas occur more commonly in girls, with a female to male ratio of 3-5 : 1, and they are more common in fair-skinned individuals. Most liver hemangiomas are clinically silent and remain undetected. Others are discovered on prenatal ultrasound or postnatal imaging that is performed for various reasons. Even though most liver hemangiomas are asymptomatic, life-threatening complications may occur, especially in the setting of multifocal lesions or congestive heart failure related to an intralesional shunt, hypothyroidism resulting from overproduction of type III iodothyronine deiodinase, fulminant hepatic failure, and/or abdominal compartment syndrome. In some patients with liver hemangioma, consumptive coagulopathy may develop as a result of intralesional thrombosis, hemorrhage, and hemolysis.52,53
Liver hemangiomas may present as focal (Fig. 92-11), multifocal (e-Fig. 92-12), or diffuse lesions.52 Focal lesions, which often are found at birth in asymptomatic children, can be associated with mild thrombocytopenia and anemia. They often occur in the absence of skin hemangioma. The Glut-1 marker is negative. This focal liver hemangioma likely corresponds to the cutaneous rapidly involuting congenital hemangioma, which characteristically regresses in an accelerated fashion by 12 to 14 months.54–56
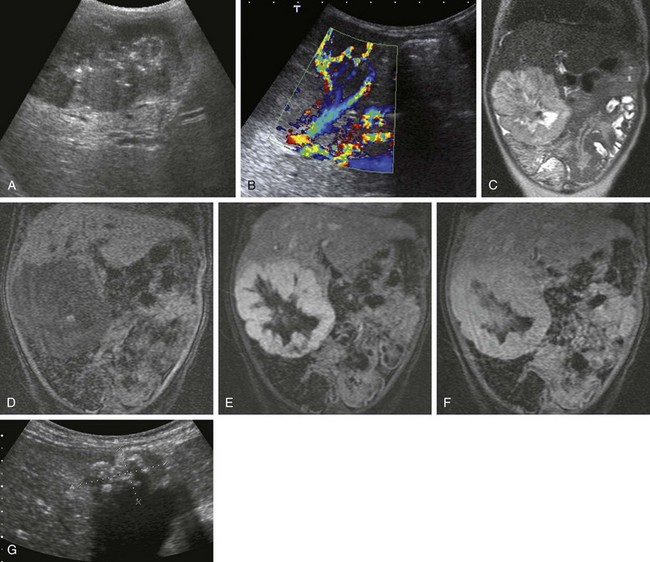
Figure 92-11 Liver hemangioma.
A, A 2-month-old girl with an abdominal mass. Ultrasound imaging shows a well-defined hypoechoic lesion with hyperechoic calcifications. B, Color Doppler ultrasound demonstrates a hypervascular lesion. C, A coronal T2-weighted magnetic resonance (MR) image reveals a well-defined hyperintense and heterogeneous lesion without peripheral edema. Dynamic gadolinium MR imaging before administration of contrast material (D) and at 1 minute (E) and 10 minutes (F) shows centripetal enhancement with central necrosis and calcifications. G, Eighteen months later, ultrasound imaging reveals calcifications with significant regression of the lesion.
e-Figure 92-12 A 14-day-old girl with multiple skin infantile hemangiomas and liver hemangioma.
A, Axial hepatic ultrasound imaging shows multiple hyperechoic lesions. B, Color Doppler ultrasound reveals hypervascular nodules. C, Coronal T2-weighted magnetic resonance imaging shows multiple hyperintense lesions.
Imaging: On ultrasound, a well-defined hypoechoic or hyperechoic lesion is seen. The echotexture is sometimes heterogeneous, related to the central hemorrhage or necrosis. Color Doppler ultrasound demonstrates a variety of flow patterns depending on the presence of microshunts, portosystemic, or arteriovenous shunts, and on the stage of the lesion. These shunts are clinically significant when they are associated with cardiac failure.
On MRI, the lesion is well defined, solitary, hypointense on T1-weighted images, and hyperintense on T2-weighted images, without peripheral edema. After administration of gadolinium contrast material, the tumor shows centripetal enhancement and may contain internal vascular flow voids.46 Heterogeneous signal is seen on all sequences if hemorrhage, thrombosis, and/or necrosis are present. Calcifications may occur in about 16% of cases.
Multifocal lesions frequently are associated with multiple cutaneous infantile hemangiomas with a Glut-1 positive marker.54–56 Many of these lesions are asymptomatic; however, depending on the presence of arteriovenous or portosystemic shunts, cardiac failure may supervene and require treatment.
On MRI, multiple nodular tumors present with T1 hypointense and T2 hyperintense signal, as well as homogeneous enhancement after injection of gadolinium contrast material. Flow voids are seen within and/or adjacent to the lesions as a result of large hepatic arteries or veins. Aortic tapering distal to the celiac trunk is a good indicator of increased hepatic flow and accordingly is a predictor of cardiac overload risk.52
Diffuse lesions present with extensive hepatic involvement, with the numerous small lesions in the liver leading to severe hepatomegaly and abdominal compartment syndrome, hypothyroidism due to overproduction of type III iodothyronine deiodinase that deactivates thyroid hormone, cardiac failure, and mental retardation.53 Despite the size of the lesion, most diffuse liver hemangiomas show no cardiac failure in relation to the shunt. The enlarged infiltrated liver reveals numerous hypoechoic lesions. MRI findings are similar to those of the focal and multifocal lesions.
Treatment: Symptomatic liver hemangiomas have been treated with corticosteroids followed by interferon-α-2a or vincristine. Recently, propranolol has demonstrated excellent efficacy as a first-line treatment for life-threatening soft tissue infantile hemangioma. More time is needed to determine the efficacy of this drug in liver hemangiomas. Catheter embolization in association with medical treatment is recommended for patients with cardiac failure related to arteriovenous or portosystemic shunts.57 In patients with diffuse lesions, medical treatment is recommended, including the treatment of hypothyroidism. In selected instances, a liver transplant may be contemplated.
Mesenchymal Hamartoma
Overview: Mesenchymal hamartoma is the second most common benign liver mass in children after vascular tumors. Most of them are discovered by 5 years of age.3,58,59 Mesenchymal hamartomas affect boys slightly more often than girls and have been diagnosed on prenatal ultrasound. Histologically, they are composed of disordered, primitive, fluid-filled mesenchyma, hepatic parenchyma, and bile ducts, in addition to stromal cysts of variable size without a capsule. Sampling of the hepatocyte component can generate confusion with hepatoblastoma.60
Etiology: It is postulated that a mesenchymal hamartoma arises from primitive mesenchymal tissues through a developmental aberration of excessive and uncoordinated proliferation during embryogenesis.59 Mesenchymal hamartoma generally is considered a congenital lesion related to a developmental anomaly. However, recent reports demonstrate balanced translocations at 19q13.4 and aneuploidy in some cases, suggesting that a mesenchymal hamartoma may represent a true neoplasm.29,58,61
Clinical Presentation: The most common clinical presentation is painless abdominal distention. AFP levels are normal. The mass may be pedunculated and attached to the inferior surface of the liver.
Imaging: On ultrasound and CT, mesenchymal hamartomas typically are multicystic, heterogeneous masses with septa of variable thickness (Fig. 92-13). When the cysts are tiny, the lesion is hyperechoic and simulates a solid lesion. Low-level echoes may be seen within the fluid related to gelatinous contents or hemorrhage. On CT, mesenchymal hamartomas are complex cystic masses. Their septations and solid components enhance after IV administration of contrast material. On MRI, the cystic regions are hypointense on T1-weighted images and hyperintense on T2-weighted images. The signal intensity varies, depending on the stromal content, the amount of protein within the cyst fluid, and the presence or absence of hemorrhage within the cyst. Septa and solid portions of the lesion usually are of decreased signal on T1- and T2-weighted images, with enhancement of septa and solid components.10,11,62–64
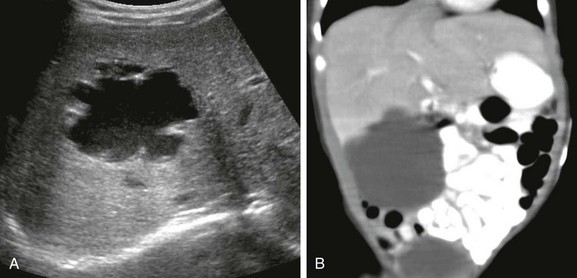
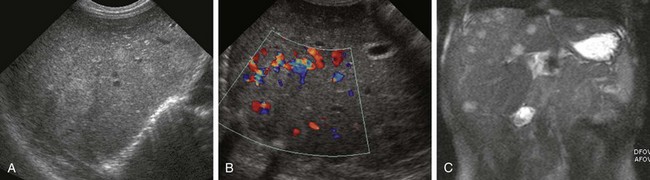
Figure 92-13 Mesenchymal hamartoma in a 1-week-old girl with a palpable abdominal mass.
A, An axial sonogram demonstrates a well-defined, lobulated, hypoechoic mass with layering internal debris. B, A coronal reformat from a contrast-enhanced computed tomography image confirms the well-defined fluid attenuation lesion.
Focal Nodular Hyperplasia
Overview: FNH is a rare benign epithelial liver tumor that occurs more often in adults; approximately 7% of reported cases of FNH are in pediatric patients,67 in whom the peak age at presentation is 2 to 5 years. FNH accounts for 2% of all primary pediatric hepatic tumors in children from birth to age 20 years with marked female predominance.60 Histologically, FNH is composed of hepatocytes, Kupffer cells, radial fibrous septa with biliary epithelium unconnected to the biliary tree, and a central vascular scar. Foci of necrosis and hemorrhage are rare compared with hepatocellular adenoma.6,60,67,68
Etiology: The etiology of FNH is not certain, but some investigators believe that FNH may represent a hyperplastic response to an underlying vascular malformation, as represented by the central scar, possibly related to vascular thrombosis, recanalization, and reperfusion.60 Recent reports have noted the occurrence of FNH in oncologic patients, which is believed to be a manifestation of injury sustained to the vascular endothelium as a result of chemotherapy and/or radiotherapy.69 Oral contraceptives and pregnancy are no longer considered risk factors for development of FNH.67 FNH is most common in the right hepatic lobe and is multiple in 20% of cases.
Clinical Presentation: FNH is usually an incidental finding. Large lesions can present with an abdominal mass, as cited in approximately 20% of patients. Patients also may present with abdominal pain, rarely with tumor rupture or hemorrhage, because the lesion has a low tendency for hemorrhagic complications. No AFP elevation occurs.6,9
Imaging: On imaging, FNH is composed of a single, well-defined, often subcapsular mass with a characteristic vascular myxomatous central scar. However, atypical imaging features are common and necessitate multiple studies to obtain the diagnosis (Fig. 92-14).
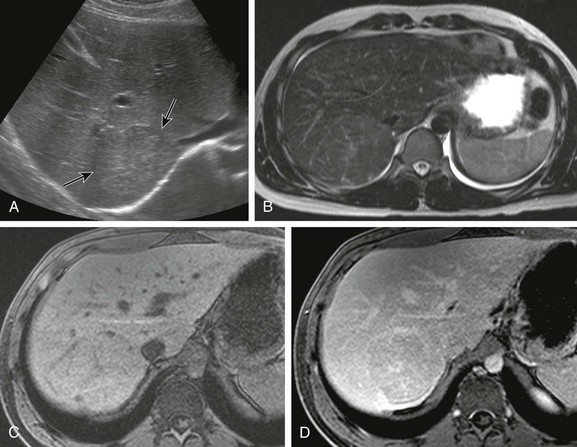
Figure 92-14 A 16-year-old boy with focal nodular hyperplasia.
A, Transverse ultrasound imaging shows a homogeneous minimally hyperechoic lesion (arrows). B, Axial T2-weighted magnetic resonance (MR) imaging demonstrates a slightly hyperintense lesion with a hyperintense central scar. Dynamic gadolinium MR imaging was performed. C, A pregadolinium image shows an isodense lesion with a very subtle central scar. D, Five minutes after injection of gadolinium, the lesion is nearly isointense with enhancement of the central scar.
On ultrasound, FNH is a well-defined homogeneous mass of variable echogenicity.70 The central scar, which is seen in approximately 33% of cases on ultrasound, appears hyperechoic relative to the remaining mass.67,70 On color Doppler ultrasound, increased blood flow is seen in the central scar extending to the periphery in a spoke-wheel pattern.71,72 The flow has been reported as arterial, in contradistinction to intratumoral venous flow, as was seen in hepatic adenomas.71
On unenhanced CT, the mass and central scar are hypodense to isodense in comparison with the liver. A central scar is found in up to 60% of cases. After the administration of contrast material, the mass enhances brightly, with delayed enhancement and washes out of the central scar.73
On MRI, FNH is isointense to slightly hypointense to the liver on T1-weighted images and isointense to slightly hyperintense on T2-weighted images. The scar is hypointense on T1-weighted images and hyperintense on T2-weighted images. Dynamic IV gadolinium contrast imaging shows uniform enhancement, which is hyperintense during the arterial phase and isointense to slightly hyperintense on the delayed images, with either parallel or divergent enhancement of the central scar over time.68,70,74 In contrast, the central scar in FLC does not enhance.15 Hepatobiliary specific contrast agents, such as Eovist, also show arterial phase enhancement which persists for an extended period due to intralesional hepatocyte uptake and malformed bile ducts.
Only FNH contains sufficient Kupffer cells to cause normal to increased uptake on technetium-99m sulfur colloid scintigraphy, a finding that is nearly pathognomonic. Sulfur colloid scans may be used to distinguish between FNH and hepatic adenoma.6,70–72
Treatment: FNH has no malignant potential, and hemorrhage or rupture are rare. Conservative management with serial hepatic ultrasound examination is recommended for asymptomatic patients. Recommendations for symptomatic patients include discontinuation of oral contraceptives, surgical resection, ablative therapy, or embolization.6
Hepatic Adenoma
Overview: Hepatic adenoma is a relatively rare, benign tumor that is termed “adenomatosis” when more than four tumors are present, which occurs more often in adults than in pediatric patients. On histology, a hepatic adenoma consists of a solitary, spherical growth of hepatocytes within a pseudocapsule. The hepatocytes contain an increased amount of fat and glycogen and are organized in sheets along with thin-walled vessels, and dysfunctional Kupffer cells. Unlike FNH, this lesion does not exhibit a central scar or radiating septa.15,60
Etiology: The lesion is associated with use of steroids, and adolescents who use oral contraceptives are the most frequent pediatric patients with liver adenomas.15,59 Hepatic adenomas also are associated with use of anabolic steroids, such as in the treatment of patients with Fanconi anemia, glycogen storage disease types I and III, galactosemia, and familial diabetes mellitus.75,76
Clinical Presentation: Patients often are asymptomatic. An association between hypervascular liver neoplasms, such as hepatic adenoma and FNH, and patients with congenital or acquired abnormal hepatic vasculature (e.g., portal vein absence or occlusion and congenital portosystemic shunts) has been reported. Intratumoral hemorrhage occurs in approximately 10% of patients and may result in abdominal pain; it rarely may result in intraperitoneal hemorrhage and hypovolemic shock.76 Liver function is normal with no elevation of AFP levels.77,78
Imaging: Imaging reveals that hepatic adenomas are solitary in approximately 80% of cases and multiple in 20% of cases. The ultrasound appearance of hepatic adenoma depends on the tumor content, such as lipid or hemorrhage. Hyperechoic masses related to the lipid content or hemorrhage may have a surrounding hypoechoic rim, and some may lack a well-defined wall (Fig. 92-15). Hypoechoic lesions to liver are more often seen in the setting of diffuse fatty infiltration and/or glycogen storage.79 Color Doppler ultrasound imaging shows central venous flow, which differs from the arterial flow seen in persons with FNH.71,80
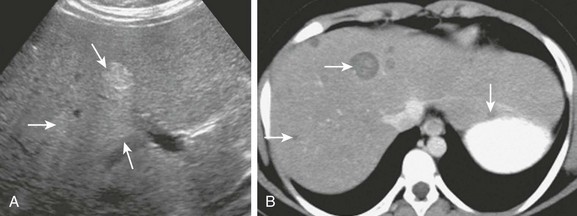
Figure 92-15 A hepatic adenomas in a 9-year-old girl with glycogen storage disease.
A, An axial sonogram reveals hyperechoic foci within the liver (arrows). B, Axial contrast-enhanced computed tomography confirms multiple well-defined, hypoattenuating masses of varied size throughout the liver (arrows).
On unenhanced CT, hepatic adenomas usually are hypodense with a low-attenuation capsule (25%) and a well-defined border; the presence of hemorrhage or fat can result in heterogeneous attenuation (7%). Homogeneous enhancement is seen during the arterial phase of contrast injection, and the mass typically becomes isodense on delayed CT images.78,79,81
On MRI, most hepatic adenomas are mildly hyperintense to liver on T1- and on T2-weighted images.78,82–84 On fat-suppressed or opposed-phase images, the signal drops out relative to the fat content.78 This finding is not specific to adenomas and also can be seen in persons with HCC. The pseudocapsule is hypointense on T1-weighted images, has a variable signal on T2-weighted images, and may enhance.
Gallium uptake in hepatic adenomas is decreased, and they usually do not take up sulfur colloid, appearing as photopenic defects on both studies. Rarely a hepatic adenoma contains enough Kupffer cells to show uptake of sulfur colloid on scintigraphy, a finding typically associated with FNH.80 With hepatobiliary agents, hepatic adenomas usually have early uptake, which persists on delayed images.85
Treatment: Treatment options include discontinuation of oral contraceptives, dietary therapy in patients with glycogen storage disorder, or surgery. Some authors recommend surgery because of the risks of hemorrhage and the fact that HCC has been reported in solitary or multiple adenomas larger than 4 cm.86 Radiofrequency ablation is an alternative to surgical resection.87
Inflammatory Pseudotumor of the Liver
Overview: Inflammatory pseudotumor refers to an inflammatory mass that typically consists of fibrous tissue with plasma cells and mononuclear leukocytes; it lacks signs of anaplasia, and, prior to histologic analysis, it is difficult to distinguish from malignancy. It occurs most commonly in the lungs. Inflammatory pseudotumor of the liver is uncommon in children. Single or multiple well-defined hepatic masses occur. Single lesions, which have been termed type I, tend to occur centrally in the liver and may lead to biliary obstruction, portal pyelophlebitis, and portal hypertension. Multiple nodules have been termed type II lesions, involve both lobes, have an appearance indistinguishable from metastatic disease, and do not tend to be associated with biliary obstruction or the development of portal hypertension.88
Etiology: The etiology of pseudotumor is unknown; inflammatory autoimmune and infectious causes have been postulated, including previous infection by the Epstein-Barr virus.89,90
Clinical Presentation: The lesion tends to be more common in males.90 Patients may present with fever, anorexia, and abdominal pain. Laboratory values may show liver enzyme elevations denoting biliary obstruction, as well as leukocytosis and elevation of the erythrocyte sedimentation rate and C-reactive protein. AFP values are normal.88,91,92
Imaging: On ultrasound the lesions are solid and tend to be hypoechoic or heterogeneous (e-Fig. 92-16). On CT they are of low density; large single lesions tend to have a lower density core related to coagulation necrosis. Findings of biliary obstruction or pyelophlebitis may be seen. On delayed phase CT, they may show greater postenhancement attenuation than surrounding liver parenchyma.93 On MRI, the lesion may be isointense to surrounding liver at both T1 and T2 weighting, necessitating IV contrast for evaluation.94 The lesion is hypoechoic on sonography and of low attenuation on CT. It is not possible to reliably distinguish inflammatory pseudotumor from hepatic malignancy with imaging.
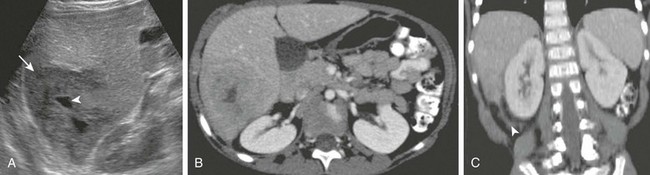
e-Figure 92-16 An inflammatory pseudotumor in a 5-year-old girl with a 1-week history of abdominal pain and low-grade fever.
A, A longitudinal sonogram of the right lobe of the liver reveals a hypoechoic mass (arrow) with small focus of central necrosis (arrowhead). Axial postcontrast (B) and reformatted coronal (C) computed tomography images confirm the mass and show inflammatory extension into the adjacent perirenal space (arrowhead in C).
Treatment: In some cases, the lesion may be resected based on a high preoperative probability of neoplasm, although a percutaneous biopsy may be diagnostic. Multiple lesions tend to be quiescent and involute.88 Despite its relative rarity, it is important for clinicians, surgeons, and radiologists to be familiar with this diagnosis and consider it in the appropriate clinical setting.
Nodular Regenerative Hyperplasia
Overview: NRH of the liver is a lesion characterized by regenerative nodules surrounded by atrophic liver in the absence of fibrosis, which is rarely seen in children.67 Histologically, regenerative nodules are composed of focal proliferation of cells resembling hepatocytes within a supporting stroma.67
Etiology: The etiology of this lesion is uncertain. This entity has been associated with small vessel vasculitis, with subsequent atrophy leading to compensatory hyperplasia of adjacent units. The entity has been linked to several other disorders such as collagen vascular diseases, hematologic disorders, cardiovascular diseases, neoplasms, metabolic disorders, and immunosuppressive or chemotherapeutic drugs.60
Clinical Presentation: The lesion may be detected incidentally in asymptomatic patients, or it may be found in patients with portal hypertension but without cirrhosis. Malignant transformation to HCC has been reported.60
Imaging: On imaging, NRH may be confused with FNH, adenomas, or metastases. Imaging findings are variable and depend on the size of the nodules.
The nodules are difficult to detect with ultrasound imaging because they are made of hepatocyte-like cells. Regenerative nodules may show heterogeneous echotexture with distortion of normal architecture. When detected, most nodules are hypoechoic, but they can be hyperechoic.95,96 The nodules may bleed or lead to portal hypertension from pressure on portal radicles, and the liver may or may not be enlarged. On CT, lesions are of decreased attenuation compared with normal liver and do not enhance significantly after administration of IV contrast material.95,96 On MRI, regenerative nodules are slightly hyperintense to adjacent liver on T1-weighted images and variable on T2-weighted images. A rim may be seen, which is hypointense or hyperintense on T1-weighted images and hyperintense on T2-weighting.97 Because of the presence of fat in the lesion, decreased signal intensity on fat-suppressed T1-weighted images may be seen. After injection of gadolinium, enhancement occurs during the portal phase such that the lesion is similar to the signal intensity of normal liver.95
Christison-Lagay, ER, Burrows, PE, Alomari, A, et al. Hepatic hemangiomas: subtype classification and development of a clinical practice algorithm and registry. J Pediatr Surg. 2007;42:62–68.
Chung, EM, Cube, R, Lewis, RB, et al. From the Archives of the AFIP. Pediatric liver masses: radiologic-pathologic correlation. Part 1. Benign tumors. Radiographics. 2010;30:801–826.
Chung, EM, Lattin, GE, Jr., Cube, R, et al. From the Archives of the AFIP. Pediatric liver masses: radiologic-pathologic correlation. Part 2. Malignant tumors. Radiographics. 2011;31:483–507.
References
1. Ishak, KG, Goodman, ZD, Stocker, JT. Tumors of the liver and intrahepatic bile ducts. Washington, DC: Armed Forces Institute of Pathology; 2001.
2. Koklu, E, Gunes, T, Akcakus, M, et al. Alpha-fetoprotein levels in the neonatal period. Eur J Pediatr. 2008;167(8):961–962. [author reply 963].
3. Stocker, JT. Hepatic tumors in children. Clin Liver Dis. 2001;5(1):259–281. [viii-ix].
4. Stocker, JT, Schmidt, D. Hepatoblastoma. In: Hamilton SR, Aatonen LA, eds. Pathology and genetics of tumours of the digestive system. Lyon, France: IARC Press, 2000.
5. Woodward, PJ, Sohaey, R, Kennedy, A, et al. From the archives of the AFIP: a comprehensive review of fetal tumors with pathologic correlation. Radiographics. 2005;25(1):215–242.
6. Meyers, RL. Tumors of the liver in children. Surg Oncol. 2007;16(3):195–203.
7. Slovis, TL, Roebuck, DJ. Hepatoblastoma: why so many low-birth-weight infants? Pediatr Radiol. 2006;36(3):173–174.
8. Dachman, AH, Pakter, RL, Ros, PR, et al. Hepatoblastoma: radiologic-pathologic correlation in 50 cases. Radiology. 1987;164(1):15–19.
9. Chung, EM, Lattin, GE, Jr., Cube, R, et al. From the archives of the AFIP: pediatric liver masses: radiologic-pathologic correlation. Part 2. Malignant tumors. Radiographics. 2011;31(2):483–507.
10. Helmberger, TK, Ros, PR, Mergo, PJ, et al. Pediatric liver neoplasms: a radiologic-pathologic correlation. Eur Radiol. 1999;9(7):1339–1347.
11. Powers, C, Ros, PR, Stoupis, C, et al. Primary liver neoplasms: MR imaging with pathologic correlation. Radiographics. 1994;14(3):459–482.
12. Roebuck, DJ, Perilongo, G. Hepatoblastoma: an oncological review. Pediatr Radiol. 2006;36(3):183–186.
13. Ye, J, Shu, Q, Li, M, et al. Percutaneous radiofrequency ablation for treatment of hepatoblastoma recurrence. Pediatr Radiol. 2008;38(9):1021–1023.
14. Yu, SB, Kim, HY, Eo, H, et al. Clinical characteristics and prognosis of pediatric hepatocellular carcinoma. World J Surg. 2006;30(1):43–50.
15. Siegel, MJ, Chung, EM, Conran, RM. Pediatric liver: focal masses. Magn Reson Imaging Clin North Am. 2008;16(3):437–452. [v].
16. Levy, AD. Malignant liver tumors. Clin Liver Dis. 2002;6(1):147–164.
17. Hussain, SM, Zondervan, PE, IJzermans, JN, et al. Benign versus malignant hepatic nodules: MR imaging findings with pathologic correlation. Radiographics. 2002;22(5):1023–1036. [discussion 1037-1039].
18. Mody, RJ, Pohlen, JA, Maide, S, et al. FDG PET for the study of primary hepatic malignancies in children. Pediatr Blood Cancer. 2006;47(1):51–55.
19. Czauderna, P, Mackinlay, G, Perilongo, G, et al. Hepatocellular carcinoma in children: results of the first prospective study of the International Society of Pediatric Oncology group. J Clin Oncol. 2002;20(12):2798–2804.
20. Katzenstein, HM, Krailo, MD, Malogolowkin, MH, et al. Hepatocellular carcinoma in children and adolescents: results from the Pediatric Oncology Group and the Children’s Cancer Group intergroup study. J Clin Oncol. 2002;20(12):2789–2797.
21. Gerber, DA, Arcement, C, Carr, B, et al. Use of intrahepatic chemotherapy to treat advanced pediatric hepatic malignancies. J Pediatr Gastroenterol Nutr. 2000;30(2):137–144.
22. Van Spronsen, FJ, Bijleveld, CM, van Maldegem, BT, et al. Hepatocellular carcinoma in hereditary tyrosinemia type I despite 2-(2 nitro-4-3 trifluoro- methylbenzoyl)-1, 3-cyclohexanedione treatment. J Pediatr Gastroenterol Nutr. 2005;40(1):90–93.
23. Torbenson, M. Review of the clinicopathologic features of fibrolamellar carcinoma. Adv Anat Pathol. 2007;14(3):217–223.
24. Liu, S, Chan, KW, Wang, B, et al. Fibrolamellar hepatocellular carcinoma. Am J Gastroenterol. 2009;104(10):2617–2624. [quiz 2625].
25. McLarney, JK, Rucker, PT, Bender, GN, et al. Fibrolamellar carcinoma of the liver: radiologic-pathologic correlation. Radiographics. 1999;19(2):453–471.
26. Smith, MT, Blatt, ER, Jedlicka, P, et al. Best cases from the AFIP: fibrolamellar hepatocellular carcinoma. Radiographics. 2008;28(2):609–613.
27. Buetow, PC, Buck, JL, Pantongrag-Brown, L, et al. Undifferentiated (embryonal) sarcoma of the liver: pathologic basis of imaging findings in 28 cases. Radiology. 1997;203(3):779–783.
28. Stocker, JT, Ishak, KG. Undifferentiated (embryonal) sarcoma of the liver: report of 31 cases. Cancer. 1978;42(1):336–348.
29. Rakheja, D, Margraf, LR, Tomlinson, GE, et al. Hepatic mesenchymal hamartoma with translocation involving chromosome band 19q13.4: a recurrent abnormality. Cancer Genet Cytogenet. 2004;153(1):60–63.
30. Ros, PR, Olmsted, WW, Dachman, HH, et al. Undifferentiated (embryonal) sarcoma of the liver: radiologic-pathologic correlation. Radiology. 1986;161(1):141–145.
31. Psatha, EA, Semelka, RC, Fordham, L, et al. Undifferentiated (embryonal) sarcoma of the liver (USL): MRI findings including dynamic gadolinium enhancement. Magn Reson Imaging. 2004;22(6):897–900.
32. Davis, GL, Kissane, JM, Ishak, KG. Embryonal rhabdomyosarcoma (sarcoma botryoides) of the biliary tree. Report of five cases and a review of the literature. Cancer. 1969;24(2):333–342.
33. Suriawinata, AA, Thung, SN. Malignant liver tumors. Clin Liver Dis. 2002;6(2):527–554. [ix].
34. Ruymann, FB, Raney, RB, Jr., Crist, WM, et al. Rhabdomyosarcoma of the biliary tree in childhood. A report from the Intergroup Rhabdomyosarcoma Study. Cancer. 1985;56(3):575–581.
35. Roebuck, DJ, Yang, WT, Lam, WW, et al. Hepatobiliary rhabdomyosarcoma in children: diagnostic radiology. Pediatr Radiol. 1998;28(2):101–108.
36. Donnelly, LF, Bisset, GS, 3rd., Frush, DP. Diagnosis please. Case 2: Embryonal rhabdomyosarcoma of the biliary tree. Radiology. 1998;208(3):621–623.
37. Miller, JH, Greenspan, BS. Integrated imaging of hepatic tumors in childhood. Part I: Malignant lesions (primary and metastatic). Radiology. 1985;154(1):83–90.
38. Spunt, SL, Lobe, TE, Pappo, AS, et al. Aggressive surgery is unwarranted for biliary tract rhabdomyosarcoma. J Pediatr Surg. 2000;35(2):309–316.
39. Awan, S, Davenport, M, Portmann, B, et al. Angiosarcoma of the liver in children. J Pediatr Surg. 1996;31(12):1729–1732.
40. Ishak, KG, et al. Mesenchymal tumours of the liver. In: Hamilton SR, Aatonen LA, eds. WHO classification of tumors pathology and genetics of tumors of the digestive system. Lyon, France: IARC Press, 2000.
41. Buetow, PC, Buck, JL, Ros, PR, et al. Malignant vascular tumors of the liver: radiologic-pathologic correlation. Radiographics. 1994;14(1):153–166. [quiz 167-168].
42. Koyama, T, Fletcher, JG, Johnson, CD, et al. Primary hepatic angiosarcoma: findings at CT and MR imaging. Radiology. 2002;222(3):667–673.
43. Burrows, PE, Dubois, J, Kassarjian, A. Pediatric hepatic vascular anomalies. Pediatr Radiol. 2001;31(8):533–545.
44. Peterson, MS, Baron, RL, Rankin, SC. Hepatic angiosarcoma: findings on multiphasic contrast-enhanced helical CT do not mimic hepatic hemangioma. AJR Am J Roentgenol. 2000;175(1):165–170.
45. Sripathi, V, Muralidharan, KV, Ramesh, S, et al. Wilms’ tumor with vena caval, atrial, and middle hepatic vein tumor thrombus. Pediatr Surg Int. 2000;16(5-6):447–448.
46. Mulliken, JB, Glowacki, J. Hemangiomas and vascular malformations in infants and children: a classification based on endothelial characteristics. Plast Reconstr Surg. 1982;69(3):412–422.
47. Mulliken, JB, Fishman, SJ, Burrows, PE. Vascular anomalies. Curr Probl Surg. 2000;37(8):517–584.
48. Au, WY, Liu, CL. Growth of giant hepatic hemangioma after triplet pregnancy. J Hepatol. 2005;42(5):781.
49. Hosokawa, A, Maeda, T, Tateishi, U, et al. Hepatic hemangioma presenting atypical radiologic findings: a case report. Radiat Med. 2005;23(5):371–375.
50. Berenguer, B, Mulliken, JB, Enjolras, O, et al. Rapidly involuting congenital hemangioma: clinical and histopathologic features. Pediatr Dev Pathol. 2003;6(6):495–510.
51. Folkman, J, Mulliken, JB, Ezekowitz, R. Angiogenesis and hemangiomas. In: Oldham K, Colombani P, Foglio R, eds. Surgery of infants and children: scientific principles and practice. Philadelphia: Lippincott-Raven, 1997.
52. Christison-Lagay, ER, Burrows, PE, Alomari, A, et al. Hepatic hemangiomas: subtype classification and development of a clinical practice algorithm and registry. J Pediatr Surg. 2007;42(1):62–67. [discussion 67-68].
53. Huang, SA, Tu, HM, Harney, JW, et al. Severe hypothyroidism caused by type 3 iodothyronine deiodinase in infantile hemangiomas. N Engl J Med. 2000;343(3):185–189.
54. Drut, RM, Drut, R. Extracutaneous infantile haemangioma is also Glut1 positive. J Clin Pathol. 2004;57(11):1197–1200.
55. Mo, JQ, Dimashkieh, HH, Bove, KE. GLUT1 endothelial reactivity distinguishes hepatic infantile hemangioma from congenital hepatic vascular malformation with associated capillary proliferation. Hum Pathol. 2004;35(2):200–209.
56. Hernández, F, Navarro, M, Encinas, JL, et al. The role of GLUT1 immunostaining in the diagnosis and classification of liver vascular tumors in children. J Pediatr Surg. 2005;40(5):801–804.
57. Kassarjian, A, Zurakowski, D, Dubois, J, et al. Infantile hepatic hemangiomas: clinical and imaging findings and their correlation with therapy. AJR Am J Roentgenol. 2004;182(3):785–795.
58. Ishak, KG, Goodman, ZD, Stocker, JT. Benign mesenchymal tumors and pseudotumors. In: Rosai J, Sobin L, eds. Atlas of tumor pathology. Washington, DC: Armed Forces Institute of Pathology, 2001.
59. Stocker, JT, Ishak, KG. Mesenchymal hamartoma of the liver: report of 30 cases and review of the literature. Pediatr Pathol. 1983;1(3):245–267.
60. Chung, EM, Cube, R, Lewis, RB, et al. From the archives of the AFIP: Pediatric liver masses: radiologic-pathologic correlation part 1. Benign tumors. Radiographics. 2010;30(3):801–826.
61. Rajaram, V, Knezevich, S, Bove, KE, et al. DNA sequence of the translocation breakpoints in undifferentiated embryonal sarcoma arising in mesenchymal hamartoma of the liver harboring the t(11;19)(q11;q13.4) translocation. Genes Chromosomes Cancer. 2007;46(5):508–513.
62. Mortele, KJ, Ros, PR. Benign liver neoplasms. Clin Liver Dis. 2002;6(1):119–145.
63. Ros, PR, Goodman, ZD, Ishak, KG, et al. Mesenchymal hamartoma of the liver: radiologic-pathologic correlation. Radiology. 1986;158(3):619–624.
64. Ye, BB, Hu, B, Wang, LJ, et al. Mesenchymal hamartoma of liver: magnetic resonance imaging and histopathologic correlation. World J Gastroenterol. 2005;11(37):5807–5810.
65. Stringer, MD, Alizai, NK. Mesenchymal hamartoma of the liver: a systematic review. J Pediatr Surg. 2005;40(11):1681–1690.
66. Lauwers, GY, Grant, LD, Donnelly, WH, et al. Hepatic undifferentiated (embryonal) sarcoma arising in a mesenchymal hamartoma. Am J Surg Pathol. 1997;21(10):1248–1254.
67. Bouyn, CI, Leclere, J, Raimondo, G, et al. Hepatic focal nodular hyperplasia in children previously treated for a solid tumor. Incidence, risk factors, and outcome. Cancer. 2003;97(12):3107–3113.
68. Vilgrain, V, Fléjou, JF, Arrivé, L, et al. Focal nodular hyperplasia of the liver: MR imaging and pathologic correlation in 37 patients. Radiology. 1992;184(3):699–703.
69. Ishak, KG, Goodman, ZD, Stocker, JT. Benign hepatocellular tumors. In: Rosai J, Sobin L, eds. Atlas of tumor pathology. Washington, DC: Armed Forces Institute of Pathology, 2001.
70. Shamsi, K, De Schepper, A, Degryse, H, et al. Focal nodular hyperplasia of the liver: radiologic findings. Abdom Imaging. 1993;18(1):32–38.
71. Bartolozzi, C, Lencioni, R, Paolicchi, A, et al. Differentiation of hepatocellular adenoma and focal nodular hyperplasia of the liver: comparison of power Doppler imaging and conventional color Doppler sonography. Eur Radiol. 1997;7(9):1410–1415.
72. Kehagias, D, Moulopoulos, L, Antoniou, A, et al. Focal nodular hyperplasia: imaging findings. Eur Radiol. 2001;11(2):202–212.
73. Choi, CS, Freeny, PC. Triphasic helical CT of hepatic focal nodular hyperplasia: incidence of atypical findings. AJR Am J Roentgenol. 1998;170(2):391–395.
74. Marti-Bonmati, L, Casillas, C, Dosda, R. Enhancement characteristics of hepatic focal nodular hyperplasia and its scar by dynamic magnetic resonance imaging. MAGMA. 2000;10(3):200–204.
75. Velazquez, I, Alter, BP. Androgens and liver tumors: Fanconi’s anemia and non-Fanconi’s conditions. Am J Hematol. 2004;77(3):257–267.
76. Resnick, MB, Kozakewich, HP, Perez-Atayde, AR. Hepatic adenoma in the pediatric age group. Clinicopathological observations and assessment of cell proliferative activity. Am J Surg Pathol. 1995;19(10):1181–1190.
77. Brancatelli, G, Federle, MP, Vullierme, MP, et al. CT and MR imaging evaluation of hepatic adenoma. J Comput Assist Tomogr. 2006;30(5):745–750.
78. Grazioli, L, Federle, MP, Brancatelli, G, et al. Hepatic adenomas: imaging and pathologic findings. Radiographics. 2001;21(4):877–892. [discussion 892-894].
79. Hussain, SM, van den Bos, IC, Dwarkasing, RS, et al. Hepatocellular adenoma: findings at state-of-the-art magnetic resonance imaging, ultrasound, computed tomography and pathologic analysis. Eur Radiol. 2006;16(9):1873–1886.
80. Golli, M, Van Nhieu, JT, Mathieu, D, et al. Hepatocellular adenoma: color Doppler US and pathologic correlations. Radiology. 1994;190(3):741–744.
81. Ichikawa, T, Federle, MP, Grazioli, L, et al. Hepatocellular adenoma: multiphasic CT and histopathologic findings in 25 patients. Radiology. 2000;214(3):861–868.
82. Arrivé, L, Fléjou, JF, Vilgrain, V, et al. Hepatic adenoma: MR findings in 51 pathologically proved lesions. Radiology. 1994;193(2):507–512.
83. Chung, KY, Mayo-Smith, WW, Saini, S, et al. Hepatocellular adenoma: MR imaging features with pathologic correlation. AJR Am J Roentgenol. 1995;165(2):303–308.
84. Paulson, EK, McClellan, JS, Washington, K, et al. Hepatic adenoma: MR characteristics and correlation with pathologic findings. AJR Am J Roentgenol. 1994;163(1):113–116.
85. Chaib, E, Gama-Rodrigues, J, Ribeiro, MA, Jr., et al. Hepatic adenoma. Timing for surgery. Hepatogastroenterology. 2007;54(77):1382–1387.
86. Micchelli, ST, Vivekanandan, P, Boitnott, JK, et al. Malignant transformation of hepatic adenomas. Mod Pathol. 2008;21(4):491–497.
87. Rocourt, DV, Shiels, WE, Hammond, S, et al. Contemporary management of benign hepatic adenoma using percutaneous radiofrequency ablation. J Pediatr Surg. 2006;41(6):1149–1152.
88. Lee, SL, DuBois, JJ. Hepatic inflammatory pseudotumor: case report, review of the literature, and a proposal for morphologic classification. Pediatr Surg Int. 2001;17(7):555–559.
89. Arber, DA, Kamel, OW, van de Rijn, M, et al. Frequent presence of the Epstein-Barr virus in inflammatory pseudotumor. Hum Pathol. 1995;26(10):1093–1098.
90. Horiuchi, R, Uchida, T, Kojima, T, et al. Inflammatory pseudotumor of the liver. Clinicopathologic study and review of the literature. Cancer. 1990;65(7):1583–1590.
91. Lyons, TJ, Benbow, EW, Taylor, PM, et al. Inflammatory pseudotumour of the liver: antecedent causes and clinical experience. J Hepatol. 1993;19(2):273–278.
92. Swinney, R, Sadri, R, Muenchow, S. Nonresectable inflammatory pseudotumor of the porta hepatis. J Pediatr Surg. 2006;41(4):e21–e23.
93. Fukuya, T, Honda, H, Matsumata, T, et al. Diagnosis of inflammatory pseudotumor of the liver: value of CT. AJR Am J Roentgenol. 1994;163(5):1087–1091.
94. Yan, FH, Zhou, KR, Jiang, YP, et al. Inflammatory pseudotumor of the liver: 13 cases of MRI findings. World J Gastroenterol. 2001;7(3):422–424.
95. Casillas, C, Marti-Bonmati, L, Galant, J. Pseudotumoral presentation of nodular regenerative hyperplasia of the liver: imaging in five patients including MR imaging. Eur Radiol. 1997;7(5):654–658.
96. Dachman, AH, Ros, PR, Goodman, ZD, et al. Nodular regenerative hyperplasia of the liver: clinical and radiologic observations. AJR Am J Roentgenol. 1987;148(4):717–722.
97. Siegelman, ES, Outwater, EK, Furth, EE, et al. MR imaging of hepatic nodular regenerative hyperplasia. J Magn Reson Imaging. 1995;5(6):730–732.