Neonatal Resuscitation
Perspective
Approximately 10% of newborns require some resuscitative assistance at birth, and approximately 1% require extensive resuscitative measures.1 Appropriate equipment and preparation, knowledge of neonatal physiology and response to stress, and skill in performing the necessary procedures are essential to successful resuscitation. Preparation for neonatal resuscitation requires an understanding of how it differs from pediatric and adult resuscitation, as follows:
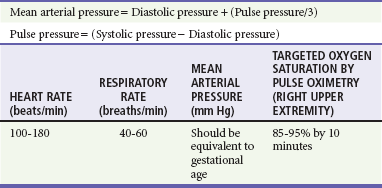
1. Newborns have rapidly changing cardiopulmonary physiology, their own range of normal vital signs (Table 11-1), and unique responses to stress.2,3
2. The approach to newborn resuscitation focuses almost entirely on respiratory, not cardiac, management.2
3. Because of their small size, infants require special equipment.
Pathophysiology
Transition from Fetal to Extrauterine Life
The successful transition from the fetal to the extrauterine environment requires two major cardiorespiratory changes: (1) removal of fluid from unexpanded alveoli to allow ventilation and (2) redistribution of cardiac output to provide lung perfusion. Failure of the development of either adequate ventilation or adequate perfusion leads to shunting, hypoxia, and ultimately reversion to fetal physiology.2
The fetal lung is poorly perfused. Because the pulmonary arterial bed is intensely vasoconstricted, the fetal lung receives only 40% of the right ventricular cardiac output; most of the right ventricular output is shunted from the pulmonary artery through the ductus arteriosus to the descending aorta.4 After the first few breaths, with exposure to alveolar oxygen, pulmonary vascular resistance decreases. The fetal shunt through the ductus arteriosus reverses as systemic vascular resistance increases; then the shunt usually ceases by 15 hours of age as the ductus also constricts. This reversal of flow allows all right ventricular output to perfuse the lungs. If hypoxia or severe acidosis occurs, however, the muscular pulmonary vascular bed constricts again, and the ductus may reopen. The reinstitution of fetal circulation, with its attendant shunting, leads to ongoing hypoxia and is termed persistent fetal circulation.2,4 Resuscitation facilitates the first few breaths, prevents and reverses ongoing hypoxia and acidosis, and assists the newborn in the transition to extrauterine life.
Neonatal Responses
The newborn’s clinical response to severe hypoxia is unique. In utero or intrapartum asphyxia (pathologic lack of oxygen to the fetus before or during delivery) precipitates a sequence of events termed primary apnea and secondary apnea. After initial hypoxia, the infant gasps rapidly, followed by cessation of respirations (primary apnea) and a decreasing heart rate (HR). At this point, only simple stimulation is needed to reverse bradycardia and assist the development of ventilation. With ongoing asphyxia, however, the infant takes several final deep, gasping respirations, followed by cessation of respirations (secondary apnea), worsening bradycardia, and decreasing blood pressure. In secondary apnea, more vigorous and prolonged resuscitation is needed to restore ventilation and an adequate circulation.2
The presence of respirations may not ensure adequate ventilation. In addition, signs of hypoxia (e.g., cyanosis, lethargy, unresponsiveness) may have other causes. Bradycardia in the newborn (HR <100 beats/min) almost always reflects inadequate ventilation and oxygenation. Bradycardia is a major indicator of hypoxia.1,2 Pulse oximetry may assist in determining hypoxemia, but it may take several minutes for a reliable waveform to be achieved.5,6
Hypothermia
The newborn’s inability to maintain body temperature (>36.5° C) has severe physiologic consequences. The newborn cannot generate heat by shivering, cannot retain heat due to low fat stores, and has a relatively large surface-to-volume area. In addition, the newborn is at risk for heat loss because of a high metabolic rate, wet amniotic fluid covering, and exposure to a relatively cool environment, especially in contrast to intrauterine temperature. The body temperature easily and rapidly decreases, and low body temperatures can lead to metabolic acidosis, increased oxygen consumption, hypoglycemia, and apnea.1–3 Drying and warming the newborn are vital to initial resuscitation.
Hypoglycemia
The newborn is at risk for developing hypoglycemia when stressed because of poor glycogen stores and immature liver enzymes. Hypoglycemia is common in premature or small-for-gestational-age infants and in infants born to diabetic mothers. It also develops in response to respiratory illness, hypothermia, polycythemia, asphyxia, and sepsis. Hypoglycemia may be asymptomatic or may cause an array of symptoms, including apnea, color changes, respiratory distress, lethargy, jitteriness, seizures, acidosis, and poor myocardial contractility.7,8 Low blood glucose has been associated with adverse neurologic outcomes in both animal and clinical studies.7 Neonatal hypoglycemia is generally defined as less than 40 mg/dL, although this number is not scientifically proven. Newborns showing symptoms of hypoglycemia together with glucose level less than 40 mg/dL should be treated with intravenous glucose. Of note, bedside glucometers generally result in lower glucose readings than plasma glucose levels by approximately 10 mg/dL.8
Indications for Resuscitation
Any infant born outside of the controlled environment of the delivery room should be considered in need of resuscitation.1–3 Minimal intervention may be required, but a standardized approach as described in this chapter should be followed. Adequate ventilation and warming are essential to a successful resuscitation. Some specific conditions such as the ones presented here increase the likelihood that resuscitation will be required.
Medications given to the mother or illicit drugs taken before delivery can lead to respiratory depression in the newborn. Maternal opioid administration or opioid use should be considered in any newborn with isolated respiratory depression that persists after successful initial resuscitation. As in adults, 0.1 mg/kg of naloxone administered to newborns may reverse respiratory depression caused by opioids. Naloxone may have a shorter half-life than the original maternal opioid; therefore the neonate should be monitored closely for recurrent apnea or hypoventilation, and subsequent doses of naloxone may be required.9,10 However, use of naloxone may precipitate acute withdrawal, seizures, or both in infants who have had prolonged intrauterine opioid exposure, and the overall effect and side effects of naloxone in the newborn are not fully known.9,10 Therefore naloxone is not recommended as a first-line treatment in the initial resuscitation efforts. Support of ventilation may be preferable to reversal with naloxone.
No reliable set of parameters has been identified for newborns who should not receive resuscitative efforts.11 Currently, resuscitation is not recommended for neonates with confirmed gestational age less than 23 weeks; those with birth weight less than 400 g; and those with confirmed anencephaly, trisomy 13, or trisomy 18.11,12 If unsure of gestational age or birth weight limitations, the recommendation is to initiate resuscitation. In the out-of-hospital setting or in the emergency department, every attempt should be made to stabilize the neonate until it is clear that attempted or continued resuscitation would not improve the patient’s chance of survival. Infants with no signs of life (no heart beat and no respiratory effort) after 10 minutes of resuscitation show either a high mortality rate or severe later developmental delay. After 10 minutes of continuous and adequate resuscitative efforts, discontinuation may be justified if there are no signs of life.12,13 It is important to communicate openly with the parent(s) and to acknowledge their opinions regarding risk of morbidity, including participation in the decision of whether or not to continue resuscitation.
Specific Disorders
Meconium in the amniotic fluid is a sign of in utero distress, and the presence of thick or particulate meconium before or at delivery should raise concern about the potential for aspiration. Aspiration of meconium and its consequences can be avoided by rapid intervention. Previous recommendations included suctioning meconium from the infant’s airway after delivery of the head but before delivery of the shoulders (intrapartum suctioning). However, evidence from a large multicenter trial did not show benefit from intrapartum suctioning.14–16 Therefore current recommendations no longer advise routine intrapartum suctioning for infants born to mothers with meconium-stained fluid. The decision to perform endotracheal intubation with tracheal suctioning after delivery of the infant should be made based on the vigor of the infant, rather than on the presence or consistency of the meconium (e.g., thick or particulate vs. thin). Infants with meconium-stained fluid and with any of the following are candidates for tracheal suctioning: (1) absent or depressed respirations, (2) poor muscle tone, or (3) HR less than 100 beats/min.2 In such newborns, a meconium aspirator should be attached to the ETT and connected to wall suction at 100 mm Hg or less. The ETT is withdrawn as suction is being applied. After intubation, the ETT with meconium aspirator serves as the ideal suction catheter. Because of its narrower width, a suction catheter placed in the ETT does not suction meconium effectively. Reintubation and suction should be repeated until the meconium clears. Two passes of intubation and suctioning are usually sufficient. When these steps have been completed, the resuscitation should continue, beginning with the steps at the top of the neonatal flow algorithm (Fig. 11-1). If bradycardia or apnea continues beyond two passes, resuscitation should include bag-mask ventilation and consideration of intubation with ETT to secure the airway.
Preparation
To maximize the effectiveness of resuscitation, the emergency department should have a prestocked drug pack, standardized equipment (Box 11-1), and staff familiar with newborn resuscitation.1,3 The pediatric length-based resuscitation tape (Broselow-Luten tape) has a section that can be used to determine equipment size and drug dosages for newborn resuscitation for infants weighing 3 kg or more.18,19
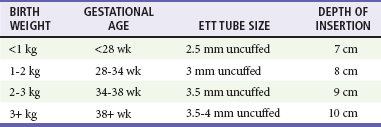
It is crucial to use universal precautions and to wear gown, gloves, and eye protection during neonatal resuscitation. For adequate preparation, the heat source is turned on early, and the resuscitation table should be warm when the newborn is placed on it. Equipment of proper size is essential (especially respiratory equipment because it is most likely to be used), and ventilation is the key to most resuscitative efforts. Appropriately sized self-inflating devices and ventilation masks decrease complications from overventilation and prevent injury or inability to ventilate due to improper mask fit. See Table 11-2 for recommended ETT sizes by birth weight and gestational age of the newborn.
If available, additional information may be helpful in preparing for resuscitation (Box 11-2). The estimated gestational age provides information about possible prematurity and associated complications. Multiple births require more equipment and personnel, and the newborns are at greater risk for prematurity and its complications. A history of maternal vaginal bleeding increases the likelihood of hypovolemic shock and respiratory distress in the newborn. A history of medication administration or drug use may provide clues to the cause of respiratory depression in the neonate.
Management
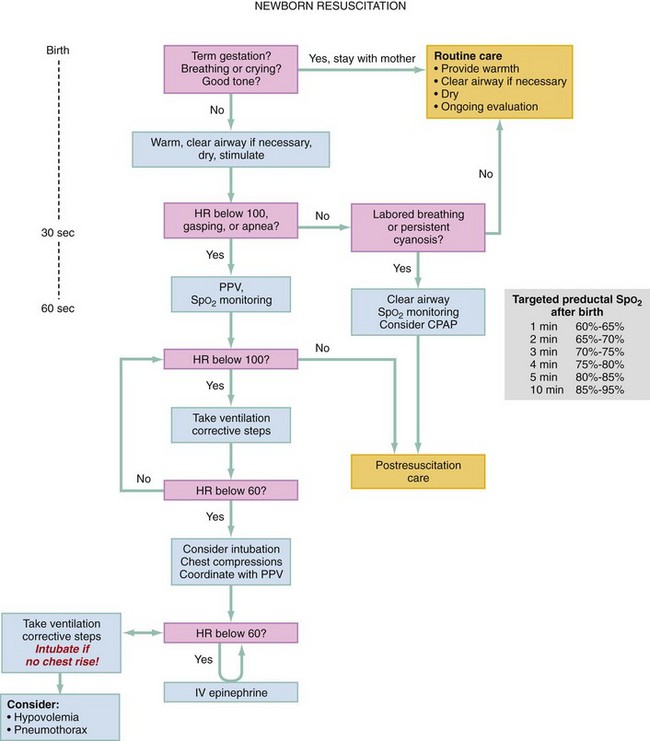
An organized approach is key to successful resuscitation both outside and inside the delivery room. The American Heart Association and the American Academy of Pediatrics have published a neonatal flow algorithm (see Fig. 11-1)—a stepwise approach to neonatal resuscitation. These steps are discussed in this section.1–3
Dry, Warm, Position, Suction, Stimulate, Assess Need for Further Intervention
If meconium is present and the newborn has poor tone, poor respirations, or bradycardia (HR <100 beats/min), the trachea should be suctioned with an ETT with meconium aspirator attachment before any other intervention is performed. If no meconium is present but the newborn has poor respirations and obvious obstruction from secretions, the newborn may be suctioned with bulb or mechanical suction (approximately 100 mm Hg wall suction). The mouth should be suctioned first, followed by the nose. The mouth is suctioned first to avoid aspiration of material if the infant takes in a breath after suctioning of the nose. Vigorous or deep suctioning can lead to a vagal response with subsequent bradycardia or apnea and should be avoided.2,20 There is no benefit to suctioning of the oropharynx in newborns without respiratory distress, bradycardia, or poor tone.
Time is of the essence in resuscitating a newborn. Within the first 30 seconds of life, the newborn should be assessed and warmed, dried, and stimulated; the airway is cleared if necessary; and HR is counted (see Fig. 11-1). If the HR is below 100 beats/min or if the newborn has apnea or respiratory distress, positive pressure ventilation (PPV) and pulse oximetry should be initiated within the next 30 seconds. If bradycardia (HR <60 beats/min) persists despite adequate ventilation after 60 seconds total, then chest compressions are initiated. The reason for the persistent bradycardia is likely inadequate ventilation. If chest compressions are initiated, intubation is recommended at that point.
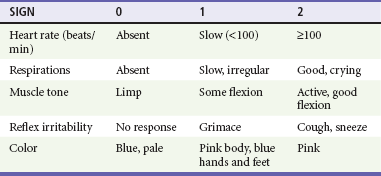
Apgar scores can also be counted at 1 and 5 minutes of life. The Apgar score is composed of scores for HR, respiratory effort, muscle tone, reflex irritability, and color. The score has been used as a prognostic indicator in newborns (Table 11-3) but is not useful in initial resuscitation management.21 Muscle tone and reflex irritability do not aid in the assessment of the newborn during resuscitation.21,22 HR and respiratory effort are the important indicators of hypoxia and should be monitored continuously. Further resuscitative efforts are required if respiratory effort is insufficient or if the HR is less than 100 beats/min. Evidence suggests that skin color may be a poor indicator for oxyhemoglobin status during the first several minutes of life as the transition from fetal to infant circulation is established. Pulse oximetry may be a useful tool to assess the oxygenation status of the newborn in the first few minutes of life.5,6,23–25
Ventilation, Oxygen, Intubation
Any infant who is cyanotic or appears to be in respiratory distress (grunting, nasal flaring, tachypnea) should be given respiratory assistance with either continuous positive airway pressure (CPAP) or PPV. If the infant is apneic, appears to be in severe respiratory distress, or has an HR of less than 100 beats/min, bag-mask ventilation (with a manometer, if available) should be initiated. The initial breaths require higher pressure (30-40 mm Hg) to remove lung fluid, and adequacy of ventilation is assessed by chest rise. Subsequent breaths generally require 20 mm Hg.1,2 Excessive pressures (defined as more pressure than is needed to achieve chest rise) should be avoided because barotrauma may result. The primary measure of adequate initial ventilation is a prompt improvement in HR. An appropriately sized mask with a good seal (covering the mouth and nose but not the eyes), proper positioning of the infant, and use of appropriate pressure to attain chest wall movement are essential for effective ventilation. The newborn should be ventilated at 40 to 60 breaths/min unless blood gases dictate otherwise. If bag-mask ventilation is required for more than 2 minutes, an orogastric tube should be placed to prevent respiratory compromise from stomach distention.2
Resuscitation with 100% oxygen was previously recommended, but more recent studies support the effectiveness of room air for resuscitation and bag-mask ventilation.26–29 Guidelines now recommend initiating resuscitation with room air, then blending in increasing oxygen concentration as needed. Use of 100% oxygen for resuscitation occurs only if the newborn has a persistent HR below 60 after 90 seconds. Attempts to restore adequate ventilation are more useful than increasing oxygen concentration. Increasing evidence points to lower initial preductal oxygen saturations in healthy uncomplicated newborns, which may contribute to the appearance of cyanosis. Figure 11-1 shows that targeted oxygen saturation after birth may not reach 90% or more until 10 minutes of life. Studies suggest that neurologic survival rates are improved when resuscitation of neonates is initiated with room air versus 100% oxygen because of the reduction in creation of free radicals in the brain.1,3,26–29
Endotracheal intubation may be indicated at several points during neonatal resuscitation: when tracheal suctioning for meconium is needed, if bag-mask ventilation is ineffective or prolonged, when chest compressions are performed, and for extremely low-birth-weight infants or infants with anatomic anomalies (e.g., diaphragmatic hernia). Box 11-1 lists the equipment needed for endotracheal intubation. Confirmation of proper ETT placement should include detection of expired carbon dioxide.30–32
Acute deterioration (bradycardia, decreased oxygen saturation) after intubation should suggest one of the following problems (DOPE)33:
1. Dislodgment: The ETT is no longer in the trachea (right mainstem bronchus or esophagus).
2. Obstruction: Secretions are obstructing airflow through the ETT.
4. Equipment: Oxygen is not being delivered to the patient, or an air leak is present (check equipment).
The LMA has been shown to be effective for ventilating full-term newborns34–36; however, there are limited data on LMA use in preterm infants (<2000 g or <34 weeks’ gestation). The usefulness of the LMA has not been evaluated in meconium aspiration syndrome or during cardiopulmonary resuscitation (CPR).
Chest Compressions
Bradycardia (HR <100 beats/min) is the major indicator of hypoxia. Most infants respond promptly to effective ventilation. If a neonate has an HR of less than 60 beats/min despite oxygen and adequate ventilation (good air movement and chest rise) for at least 30 seconds, chest compressions should also be provided.1,2,37 Chest compressions should be performed at a rate of 90 per minute with 30 breaths/min for a total of 120 events per minute. The preferred neonatal resuscitation ratio is 3 : 1 (three compressions to one ventilation). If the resuscitation provider is sure that the bradycardia/cardiac arrest has a cardiac and not a respiratory cause, a compression : ventilation ratio of 15 : 2 may be considered.1,3 The preferred method for performing chest compressions is as follows: the fingers of both hands encircle the chest and support the back. The thumbs of both hands are placed side by side or one over the other on the sternum just below the nipple line. The depth of compression is one third the anteroposterior diameter of the chest.2,38,39
Respirations and HR should be reassessed about every 30 seconds, and coordinated chest compressions and ventilation should continue until the spontaneous HR is greater than or equal to 60 beats/min.1,2
Medications
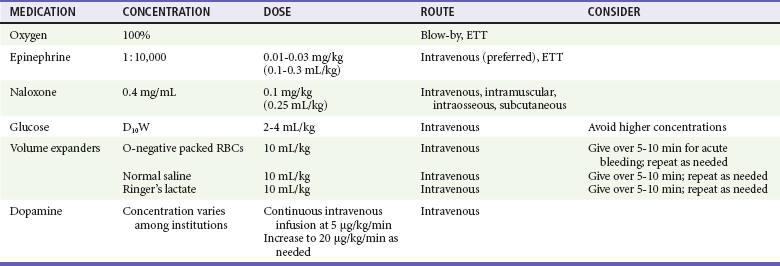
Few neonates require the use of medications during resuscitation.40 Medications are indicated for bradycardia or asystole that does not respond to effective ventilation and chest compressions, and for hemorrhage (maternal, fetal, or placental) that necessitates fluid resuscitation.1–3
The only medications that should be used during the early phase of neonatal resuscitation are oxygen, epinephrine, and volume expanders (Table 11-4). Albumin has been removed as an initial resuscitation drug. Dopamine should be reserved for prolonged hypotension and extended resuscitation. Naloxone should be used only after the neonate is adequately ventilated. Sodium bicarbonate should not be used in most resuscitations; it may be beneficial in the neonatal intensive care setting when ventilation is known to be adequate.1,41 Atropine or calcium are not routinely recommended in neonatal resuscitation, but calcium may be given if hypocalcemia is documented.
Vascular access is a challenge in neonatal resuscitation. The preferred route of immediate vascular access is the umbilical vein because it is easily identified and cannulated. Because of potentially serious complications (infection, portal vein thrombosis), the umbilical vein cannula should be removed immediately after stabilization and other access has been attained. Other vascular access routes include peripheral veins, peripherally inserted central catheter (PICC), and femoral vein. Intraosseous access can be problematic in neonates (especially premature infants) because of bone fragility and the small size of the intraosseous space. If vascular access cannot be achieved, some drugs such as epinephrine can be given through the ETT. The medication should be injected directly into the ETT, followed by a 1-mL saline flush and several positive pressure ventilations.2
Oxygen
Indications for oxygen use include persistent bradycardia (<60 beats/min) and respiratory distress (nasal flaring, grunting, tachypnea, apnea) beyond 90 seconds. Oxygen should be blended with room air if possible and titrated to achieve desirable targeted oxygen saturation (see Fig. 11-1) or to raise the HR to greater than 60 beats/min. Establishing effective ventilation is important because increased concentration of oxygen will not be delivered if ventilation is not adequate.
Epinephrine
Epinephrine is indicated for asystole, and for an HR less than 60 beats/min despite effective ventilation with 100% oxygen and chest compressions. Although epinephrine may be given by ETT, the preferred administration route is intravenous. The intravenous dose is 0.01 to 0.03 mg/kg or 0.1 to 0.3 mL/kg of 1 : 10,000 solution. Repeat doses may be given every 3 to 5 minutes.1,2,42 If the endotracheal route is used, higher doses are needed. While access is being obtained, administration of 0.05 to 0.1 mg/kg of 1 : 10,000 solution through the ETT may be considered, but the safety and efficacy of this practice have not been evaluated.1,2,42,43
Naloxone
Respiratory depression induced by opioids given to or taken by the mother within 3 to 4 hours of delivery can be reversed with naloxone. Naloxone is infrequently needed in a newborn with respiratory depression. It may precipitate withdrawal seizures in an infant born to a drug-addicted mother.9,10 The priority of care is support of ventilation with bag-mask device and intubation, if necessary. Naloxone should be considered only after ventilatory support is achieved. If respiratory depression is persistently present, and if it is unclear whether the mother took opioids before delivery, reversal with naloxone may be attempted. The dose is 0.1 mg/kg intravenously (IV), intraosseously (IO), subcutaneously (SQ), or intramuscularly (IM). Administration of naloxone by ETT is not recommended in newborn resuscitation.1,44 The duration of action of naloxone is 1 to 4 hours, depending on route of administration. Repeat dosing may be necessary, and the patient should be monitored carefully.
Glucose
Hypoglycemia should be considered in a neonate undergoing resuscitation. Hypoglycemia is diagnosed with a rapid bedside glucose or serum glucose measurement. Neonates with glucose level less than 40 mg/dL and with symptoms of hypoglycemia (irritability, tremors, jitteriness, apnea, tachypnea, seizures, cyanosis, lethargy, poor feeding) should be treated with intravenous glucose. Hypoglycemia is treated with 2 mL of 10% dextrose in water (D10W) per kilogram as well as by starting a continuous infusion of D10W at 80 to 100 mL/kg/day.8 Higher concentrations of glucose (e.g., 25% dextrose in water, D25W) are hyperosmolar and should be avoided. If the newborn is able to tolerate feeds, then oral glucose solution, maternal breast milk, or formula may be given by mouth on demand. Repeat glucose measurement should be obtained 10 to 20 minutes after glucose administration. Asymptomatic neonates with hypoglycemia should be encouraged to feed more often and are treated with intravenous glucose only when the glucose level is severely low (<25 mg/dL at birth to 4 hours of age or <35 mg/dL at 4-24 hours of age).8
Volume Expanders
Volume expanders are indicated when acute bleeding is evident with signs of hypovolemia (pallor despite oxygenation, weak pulses with a rapid HR, poor response to resuscitation), when a history of maternal hemorrhage during delivery is present, when the newborn appears to be in shock, or when CPR is required.1,2,3,40 Volume expanders include packed red blood cells (Rh-negative type O blood), normal saline, or Ringer’s lactate solution. Normal saline and Ringer’s lactate (e.g., isotonic crystalloid solutions) should be readily available and may be considered the fluid of choice overall for volume expansion. Expanders are given in small intravenous boluses of 10 mL/kg over 5 to 10 minutes. When premature infants are being resuscitated, rapid administration of volume expanders should be avoided, as this has been associated with intraventricular hemorrhage.2 Higher-volume (e.g., 20 mL/kg) fluid boluses are recommended for older infants. Boluses may be repeated several times, guided by patient assessment. The use of albumin 5% with saline is currently not recommended for the initial resuscitation.
Therapeutic Hypothermia
When moderate to severe hypoxic-ischemic encephalopathy is suspected, studies now suggest that selective cerebral hypothermia in asphyxiated infants may protect against brain injury.1,45–53 Several randomized controlled trials have shown that therapeutic hypothermia of 33.5 to 34.5° C in this population leads to lower mortality and less neurologic disability at 18 months of age, compared with infants who did not have therapeutic hypothermia. Current guidelines recommend therapeutic hypothermia for patients with suspected early neonatal asphyxia. Symptoms may include abnormal levels of consciousness, seizures, hypotonia, or hyporeflexia.53 It is unknown whether cooling the head alone versus the entire body is more neuroprotective. The current studies suggest that the recommended cooling is to be initiated within 6 hours of birth and to be continued for 72 hours with only slow rewarming over at least 4 hours afterward. Therapeutic hypothermia is not without risk; it may also lead to complications such as thrombocytopenia or hypotension.45–52
References
1. Kattwinkel, J, et al. Part 15: Neonatal resuscitation: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122:S909–S919.
2. American Academy of Pediatrics and American Heart Association. Neonatal Resuscitation Textbook, 6th ed. Elk Grove Village, Ill: American Academy of Pediatrics; 2011.
3. Perlman, JM, et al. Part 11. Neonatal resuscitation. International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science with Treatment Recommendations. Circulation. 2010;122(Suppl 2):S516–S538.
4. Hamrick, SE, Hansmann, G. Patent ductus arteriosus of the preterm infant. Pediatrics. 2010;125:1020–1030.
5. Kamlin, CO, et al. Accuracy of pulse oximetry measurement of heart rate of newborn infants in the delivery room. J Pediatr. 2008;152:756–760.
6. O’Donnell, CP, Kamlin, CO, Davis, PG, Morley, CJ. Feasibility of and delay in obtaining pulse oximetry during neonatal resuscitation. J Pediatr. 2005;147:698–699.
7. Salhab, WA, Wyckoff, MH, Laptook, AR, Perlman, JM. Initial hypoglycemia and neonatal brain injury in term infants with severe fetal acidemia. Pediatrics. 2004;114:361.
8. Adamkin, D, Committee on Fetus and Newborn. Clinical report—postnatal glucose homeostasis in late-preterm and term infants. Pediatrics. 2011;127:575–579.
9. Herschel, M, Khoshnood, B, Lass, NA. Role of naloxone in newborn resuscitation. Pediatrics. 2000;106:831–834.
10. McGuire, W, Fowlie, PW. Naloxone for opiate-exposed newborn infants. Cochrane Database Syst Rev. 4, 2002.
11. Landwirth, J. Ethical issues in pediatric and neonatal resuscitation. Ann Emerg Med. 1993;22:502.
12. Paris, JJ. What standards apply to resuscitation at the borderline of gestational age? J Perinatol. 2005;25:683–684.
13. Niermeyer, S, et al. Resuscitation in newborns. Ann Emerg Med. 2001;37:S110.
14. Haddad, B, Mercer, BM, Livingston, JC, Talati, A, Sibai, BM. Outcome after successful resuscitation of babies born with Apgar scored of 0 at both 1 and 5 minutes. Am J Obstet Gynecol. 2000;182:1210.
15. Vain, NE, et al. Oropharyngeal and nasopharyngeal suctioning of meconium-stained neonates before delivery of their shoulders: Multicentre, randomised controlled trial. Lancet. 2004;364:597.
16. Wiswell, TE, et al. Delivery room management of the apparently vigorous meconium-stained neonate: Results of the multicenter, international collaborative trial. Pediatrics. 2000;105(1 Pt 1):1–7.
17. Cain, JM, Mason, LJ, Martin, RB. Airway management in two of newborns with Pierre Robin sequence: The use of disposable vs multiuse LMA for fiberoptic intubation. Pediatr Anesth. 2006;16:1274.
18. Kempley, ST, Moreiras, JW, Petrone, FL. Endotracheal tube length for neonatal intubation. Resuscitation. 2008;77:369.
19. Luten, R, Kahn, N, Wears, R, Kissoon, N. Predicting endotracheal tube size by length in newborns. J Emerg Med. 2007;32:343.
20. Cordero, L, Hon, EH. Neonatal bradycardia following nasopharyngeal stimulation. J Pediatr. 1971;78:441.
21. Papile, L. The Apgar score in the 21st century. N Engl J Med. 2001;344:519–520.
22. Laptook, AR, et al. Outcome of term infants using Apgar scores at 10 minutes following hypoxic-ischemic encephalopathy. Pediatrics. 2009;124:1619–1626.
23. Altuncu, E, Ozek, E, Bilgen, H, Topuzoglu, A, Kavuncuoglu, S. Percentiles of oxygen saturations in healthy term newborns in the first minutes of life. Eur J Pediatr. 2008;167:687–688.
24. Kamlin, CO, O’Donnell, CP, Davis, PG, Morley, CJ. Oxygen saturation in healthy infants immediately after birth. J Pediatr. 2006;148:585–589.
25. Rabi, Y, Yee, W, Chen, SY, Singhal, N. Oxygen saturation trends immediately after birth. J Pediatr. 2006;148:590–594.
26. Davis, PG, Tan, A, O’Donnell, CP, Schulze, A. Resuscitation of newborn infants with 100% oxygen or air: Systematic review and meta-analysis. Lancet. 2004;364:1329.
27. Saugstad, OD, Rootwelt, T, Aalen, O. Resuscitation of asphyxiated newborn infants with room air or oxygen—an international controlled trial: The Resair 2 study. Pediatrics. 1998;102:e1.
28. Tan, A, Schulze, A, O’Donnell, CP, Davis, PG. Air versus oxygen for resuscitation of infants at birth. Cochrane Database Syst Rev. 18, 2005.
29. Rabi, Y, Rabi, D, Yee, W. Room air resuscitation of the depressed newborn: A systematic review and meta-analysis. Resuscitation. 2007;72:353–363.
30. Aziz, HF, Martin, JB, Moore, JJ. The pediatric disposable end-tidal carbon dioxide detector role in endotracheal intubation in newborns. J Perinatol. 1999;19:110.
31. Hosano, S, et al. A role of end-tidal CO monitoring for assessment of tracheal intubations in very low birth weight infants during neonatal resuscitation at birth. J Perinat Med. 2009;37:79–84.
32. Repetto, JE, Donohue, PA-CPK, Baker, SF, Kelly, L, Nogee, LM. Use of capnography in the delivery room for assessment of endotracheal tube placement. J Perinatol. 2001;21:284.
33. Ralston M, Hazinski MF, eds. Textbook of Pediatric Advanced Life Support. Dallas: American Heart Association, 2005.
34. Esmail, N, et al. Laryngeal mask airway versus endotracheal intubation for Apgar score improvement in neonatal resuscitation. Egypt J Anesthesiol. 2002;18:115.
35. Gandini, D, Brimacombe, JR. Neonatal resuscitation with the laryngeal mask airway in normal and low birth weight infants. Anesth Analg. 1999;9:178.
36. Grein, AJ, Weiner, GM. Laryngeal mask airway versus bag/mask ventilation or endotracheal intubation for neonatal resuscitation. Cochrane Database Syst Rev. 2004:18.
37. Perlman, JM, Risser, R. Cardiopulmonary resuscitation in the delivery room: Associated clinical events. Arch Pediatr Adolesc Med. 1995;149:20.
38. Orlowski, JP. Optimum position for external cardiac compression in infants and young children. Ann Emerg Med. 1986;15:667–673.
39. Kitamura, T, et al. Conventional and chest-compression-only cardiopulmonary resuscitation by bystanders for children who have out-of-hospital cardiac arrests: A prospective, nationwide, population-based cohort study. Lancet. 2010;375:1347–1354.
40. Wyckoff, MH, Perlman, JM, Laptook, AR. Use of volume expansion during delivery room resuscitation in near-term and term infants. Pediatrics. 2005;115:950–955.
41. Aschner, JL, Poland, RL. Sodium bicarbonate: Basically useless therapy. Pediatrics. 2008;122:831–835.
42. Perondi, MB, Reis, AG, Paiva, EF, Nadkarni, VM, Berg, RA. A comparison of high-dose and standard-dose epinephrine in children with cardiac arrest. N Engl J Med. 2004;350:1722–1730.
43. Quinton, DN, O’Byrne, G, Aitkenhead, AR. Comparison of endotracheal and peripheral intravenous adrenaline in cardiac arrest: Is the endotracheal route reliable? Lancet. 1987;1:828.
44. Guinsburg, R, Wyckoff, MH. Naloxone during neonatal resuscitation: Acknowledging the unknown. Clin Perinatol. 2006;33:121–132.
45. Gunn, AJ. Cerebral hypothermia for prevention of brain injury following perinatal asphyxia. Curr Opin Pediatr. 2000;12:111.
46. Gluckman, PD, et al. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: Multicentre randomised trial. Lancet. 2005;365:63.
47. Lin, ZL, et al. Mild hypothermia via selective head cooling as neuroprotective therapy in term neonates with perinatal asphyxia: An experience from a single neonatal intensive care unit. J Perinatol. 2006;26:180–184.
48. Shankaran, S, et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2005;353:1574–1584.
49. Eicher, DJ, et al. Moderate hypothermia in neonatal encephalopathy: Safety outcomes. Pediatr Neurol. 2005;32:18.
50. Eicher, DJ, et al. Moderate hypothermia in neonatal encephalopathy: Efficacy outcomes. Pediatr Neurol. 2005;32:11.
51. Shankaran, S, et al. Childhood outcomes after hypothermia for neonatal encephalopathy. N Engl J Med. 2012;366:2085–2092.
52. Azzopardi, DV, et al. Moderate hypothermia to treat perinatal asphyxia encephalopathy. N Engl J Med. 2009;361:1349–1358.
53. Pfister, RH, Soll, RF. Hypothermia for treatment of infants with hypoxic-ischemic encephalopathy. J Perinatology. 2010;30:S82–S87.