105 Neonatal Infections
Torch Infections
Etiology and Pathogenesis
Clinical Presentation
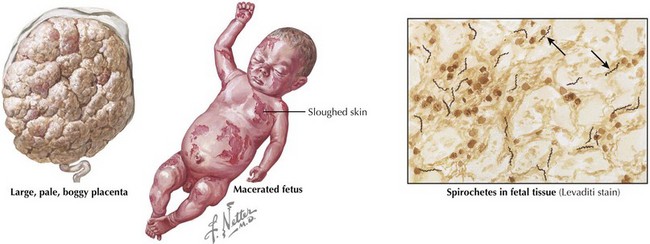
Congenital syphilis is similar to the acquired form of syphilis in that it presents with early and late manifestations. Most cases are detected by routine maternal screening. Early syphilis presents within the first 2 years of life, with one-third of patients symptomatic at birth. Symptoms can affect almost any organ system and include the following: hepatosplenomegaly, jaundice, Coombs’-negative hemolytic anemia, thrombocytopenia, rhinitis (snuffles), condylomatous skin or mucous membrane lesions, diffuse mucocutaneous rash involving the palms and soles, osteochondritis presenting as pain and refusal to move the affected limb (pseudoparalysis of Parrot), periosteitis, chorioretinitis, failure to thrive, or renal disease (Figure 105-1). Late manifestations are not detailed here but result from chronic tissue inflammation, most commonly involving the central nervous system (CNS), bones, and teeth, and present within the first 2 decades of life.
Evaluation and Management
Toxoplasmosis
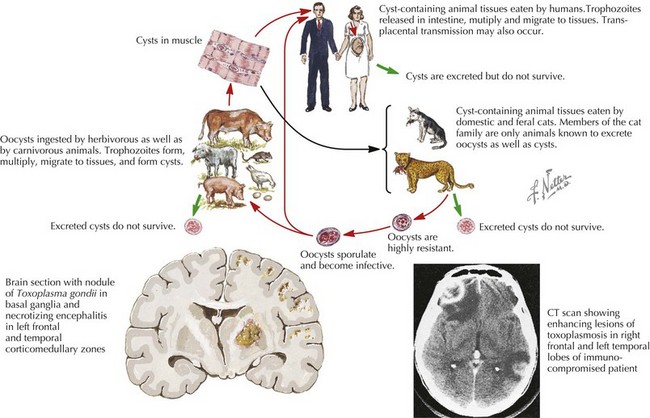
If in utero infection is suspected based on prenatal ultrasonographic findings, amniotic fluid can be sent for Toxoplasmosis polymerase chain reaction (PCR). Postnatally, the recommended workup includes ophthalmologic, neurologic, and audiologic evaluations, as well as CSF PCR testing for Toxoplasma and head computed tomography (CT). For infected newborns, treatment with pyrimethamine, sulfadiazine, and leucovorin is the most widely accepted regimen and results in improved ocular outcomes. All pregnant women should be counseled to avoid changing cats’ litter boxes and to wear gloves when in contact with soil (Figure 105-2).
Other Infections
Clinical Presentation
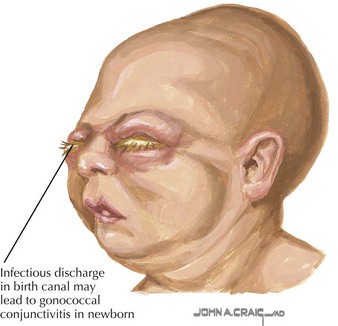
Gonococcal infection generally presents as purulent unilateral or bilateral eye discharge and conjunctival erythema, termed ophthalmia neonatorum (Figure 105-3). Scalp abscess after fetal monitoring has also been noted. Less commonly, meningitis or disseminated disease can present and should be included in the differential diagnosis of a septic-appearing infant. Similarly, chlamydial disease presents as purulent eye discharge and may form a membrane on the conjunctivae. Neonatal pneumonia is another classic presentation of neonatal chlamydial infection, presenting with staccato cough, tachypnea, and occasionally nasal congestion within the first 2 to 3 months of life.
Baker MD, Bell LM, Avner JR. Outpatient management without antibiotics of fever in selected infants. N Engl J Med. 1993;329(20):1437-1441.
Gordon A, Jeffery HE. Antibiotic regimens for suspected late onset sepsis in newborn infants. Cochrane Database Syst Rev (3):CD004501, 2005.
Hammerschlag MR, Cummings C, Roblin PM, et al. Efficacy of neonatal ocular prophylaxis for the prevention of chlamydial and gonococcal conjunctivitis. N Engl J Med. 1989;320(12):769-772.
Kimberlin DW, Lin CY, Jacobs RF, et al. Natural history of neonatal herpes simplex virus infections in the acyclovir era. Pediatrics. 2001;108(2):223-229.
Lee C, Gong Y, Brok J, Boxall EH, Gluud C. Effect of hepatitis B immunisation in newborn infants of mothers positive for hepatitis B surface antigen: systematic review and meta-analysis. BMJ. 2006;332(7537):328-336.
Lopez A, Dietz V, Wilson M, Narvin T, Jones JL. Preventing congenital toxoplasmosis. MMWR. Morbid Mortal Wkly Rep. 2000;49(RR02):57-75.
Mofenson LM. U.S. Public Health Service Task Force recommendations for use of antiretroviral drugs in pregnant HIV-1 infected women for maternal health and for interventions to reduce perinatal HIV-1 transmission in the United States. MMWR Morbid Mortal Wkly Rep. 2002;51(RR18):1-38.
Pickering LK, editor. Red Book: 2009 Report of the Committee on Infectious Diseases, ed 27, Elk Grove Village, IL: American Academy of Pediatrics, 2009.
Schrag SJ, Gorwitz R, Fultz-Butts K, Schuchat A. Prevention of perinatal group B streptococcal disease. Revised guidelines from CDC. MMWR. Morbid Mortal Wkly Rep. 2002;51(RR-11):1-22.
Schrag SJ, Zell ER, Lynfield R, et al. A population-based comparison of strategies to prevent early-onset group B streptococcal disease in neonates. N Engl J Med. 2002;347(4):233-239.