36 Neonatal Emergencies
Neonatal Physiology Related to Anesthesia
Cardiopulmonary
Oxygen Consumption
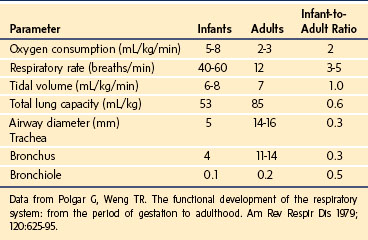
The cardiopulmonary system of the neonate is driven by the need to deliver sufficient oxygen (O2) to maintain a high metabolic rate. The O2 consumption of an average neonate is 5 to 8 mL/kg/min, whereas that of an adult is 2 to 3 mL/kg/min (Table 36-1). It is this high rate of O2 consumption that primarily leads to the rapid decrease in blood O2 partial pressures in the neonate during periods of hypoventilation. Although ventilatory gas exchange volume is nearly 10-fold greater in adults than in neonates, the tidal volume relative to body weight for both is approximately equal (6 mL/kg). In neonates, increasing the respiratory rate facilitates the elimination of carbon dioxide (CO2) generated by their relatively high metabolic processes; alveolar ventilation is approximately 130 mL/kg/min in the perinatal period, compared with 60 mL/kg/min in adulthood. In neonates, the thoracic gas volume on a weight basis is similar to that in adults. These metabolic and volume changes are consistent with those predicted by allometric scaling (see Chapter 6).1
Pulmonary Gas Exchange
Preterm neonates may have abnormalities in lung surfactant activity. Surfactant production by type II alveolar pneumocytes occurs predominantly after 32 weeks of gestation. Infants born prematurely may develop respiratory distress syndrome (RDS) because of surfactant deficiency. However, infants of mothers with gestational diabetes may develop RDS even when born near term. RDS is characterized by grunting respirations, nasal flaring, and chest retractions, developing soon after birth. Radiographic examination demonstrates decreased lung volume resulting from widespread atelectasis. The resultant intrapulmonary shunting of blood through atelectatic lung units causes intrapulmonary shunt and systemic hypoxemia and reduces O2 delivery to the tissues. Judicious application of positive end-expiratory pressure (PEEP) and treatment with exogenous surfactant may reduce intrapulmonary shunting in RDS and decrease hypoxemia.2–6 In addition, the incidence and severity of RDS has been decreased by the now routine treatment of mothers who are in preterm labor with glucocorticoids.
In the neonate, apnea decreases pulmonary gas exchange and can lead to hypoxemia and bradycardia. Conceptually, apnea is differentiated in terms of its cause: (1) central apnea, resulting from immaturity or depression of the respiratory drive; (2) obstructive apnea, caused by an infant’s inability to maintain a patent airway; and (3) mixed apnea, a combination of both central and obstructive apnea.7
Apnea of central origin may be secondary to the poor organization and integration of input from proprioceptive receptors, which are located in the diaphragm and intercostal muscles, and from medullary and peripheral chemoreceptors. Preterm infants are at greater risk for central apnea because chemoreceptor signaling is incompletely developed. Exaggerated responses to chemoreceptor signaling during periods of mild hypercarbia and hypoxia can cause apnea in preterm infants, in whom it might stimulate the respiratory rate in those born at term. Susceptibility to central apnea is also exacerbated by metabolic disturbances such as hypothermia, hypoglycemia, and hypocalcemia, even in full-term neonates. For these reasons, central apnea may be associated with anemia and sepsis in babies. Blood transfusions may decrease the incidence of apnea in preterm infants with a hematocrit less than 27%8,9; however, data suggest a poor correlation between anemia and the incidence of apnea or bradycardia episodes.10 Central apnea resulting from immaturity of the respiratory drive center is often treated with xanthine derivatives, such as caffeine and theophylline (Table 36-2).11–14 Of particular importance to the anesthesiologist is that central apnea in neonates can be exacerbated by opioids. In some cases, apnea in neonates exposed to opioids may be alleviated by treatment with naloxone. Because of these reasons, all neonates require careful continuous monitoring of blood O2 saturations and heart rate in the postoperative period.
| Cause | Treatment |
|---|---|
| Central |
Apnea of an obstructive or mixed origin is responsible for the majority of apneic episodes in preterm infants.15–17 Obstructive apnea may be due to incomplete maturation and poor coordination of upper airway musculature. These forms of apnea often respond to changes in head position, insertion of an oral or nasal airway, or placing the infant in a prone position. Application of continuous positive airway pressure (CPAP) also may reduce obstructive apnea.18
Postoperative apnea is a common morbidity associated with anesthesia in neonates with a history of prematurity, apnea, or chronic lung disease.19 Nearly 20% of such infants have apnea exacerbated by anesthesia or surgery in the postoperative period. Apnea may result from prolonged action of anesthetic agents, a shift of CO2 response curve, immaturity of respiratory control, or fatigue of respiratory muscles.20 Early studies suggested that preterm infants who were younger than 46 weeks postconceptional age (PCA*) at the time of general anesthesia require continuous cardiopulmonary monitoring in the hospital afterward until they are apnea-free.15 Kurth and colleagues21 extended these recommendations to include monitoring for premature infants younger than 60 weeks PCA for at least 12 apnea-free hours after surgery. Although several studies suggest that regional anesthesia techniques reduce the incidence of postoperative apnea, others reported that apnea may still occur if the regional technique is supplemented with a sedative (e.g., ketamine).22–25 An analysis of several hundred former preterm infants studied prospectively from four centers over 6 years revealed the following26:
 The incidence of postoperative apnea is inversely and independently related to PCA and gestational age; for example, the younger a preterm infant was born and the earlier after birth that the preterm infant undergoes surgery, the greater the incidence of apnea.
The incidence of postoperative apnea is inversely and independently related to PCA and gestational age; for example, the younger a preterm infant was born and the earlier after birth that the preterm infant undergoes surgery, the greater the incidence of apnea.
 Preterm infants younger than 56 weeks PCA are at greatest risk for apnea; the risk of postoperative apnea does not fall to less than 1% until approximately 55 weeks PCA (see Chapter 4).
Preterm infants younger than 56 weeks PCA are at greatest risk for apnea; the risk of postoperative apnea does not fall to less than 1% until approximately 55 weeks PCA (see Chapter 4).
 Even infants who were born at full term can experience postoperative apneas, although this is extremely rare.27–30
Even infants who were born at full term can experience postoperative apneas, although this is extremely rare.27–30
 Preterm infants without a history of apnea are still susceptible to the development of postoperative apnea.
Preterm infants without a history of apnea are still susceptible to the development of postoperative apnea.
 Preterm infants with anemia (hematocrit less than 30%) are particularly vulnerable even up to and possibly beyond 60 weeks PCA.31
Preterm infants with anemia (hematocrit less than 30%) are particularly vulnerable even up to and possibly beyond 60 weeks PCA.31
Apnea also might be associated with nasal obstruction in infants. Although most neonates prefer to breathe through their nose, a few are obligate nasal breathers and do not overcome nasal airway obstruction by changing to mouth breathing.32,33 In such neonates, nasal obstruction caused by choanal stenosis or atresia or a nasogastric tube may lead to apnea and cyanosis. Obstruction resulting from nasal edema may be relieved by instillation of saline or phenylephrine nose drops.
The work of breathing comprises compliance and resistive components. Although the work of breathing from compliance is 20- to 40-fold greater in the adult than in the neonate, the compliance work relative to tidal volume is nearly the same. However, the resistive work of breathing in the neonate is nearly 6 times greater than in the adult because of the relatively small airways in the neonate. Breathing through a tracheal tube especially increases resistive work in neonates because the airway resistance is inversely proportional to the fifth power of the radius of the tube and directly proportional to the length of the tracheal tube. The relatively narrow and long tracheal tubes through which neonates breathe can greatly increase their work of breathing (see Fig. 12-7). The work is also increased when a neonate breathes spontaneously through a circle system because of the inspiratory force required to open the one-way valves in the system. Although valveless ventilatory circuits, such as the Mapleson D systems, are recommended for spontaneously breathing neonates, studies suggest that a circle system may be used in these patients provided compensation is arranged for the problems associated with this circuit; that is, a neonate requires assisted ventilation and adequate inflation pressures to reduce the work of breathing and ensure adequate ventilation (see Chapter 51).34–36
Bronchopulmonary dysplasia (BPD) is an important chronic lung disease of neonates born prematurely.37 Although the use of antenatal steroids, exogenous surfactant treatment, and advanced ventilator therapies has decreased the incidence of BPD, it still afflicts nearly 10,000 premature infants in the United States every year and is a significant contributor to infant morbidity and mortality. BPD is identified in preterm infants with abnormal chest radiographs that require supplemental O2 at 36 weeks postmenstrual age (PMA). Other factors that increase the risk for developing BPD include chorioamnionitis and the persistence of a patent ductus arteriosus (PDA).38 Lung injury in the preterm infant decreases pulmonary maturation; infants who have died from BPD have impaired pulmonary alveolar and disrupted microvascular development.39 BPD is most often seen in preterm infants who are subjected to high levels of O2 and ventilation therapy and may be caused by lung injury–induced increase in cytokine activation.40 Decreased lung development in infants with BPD diminishes the surface area for pulmonary gas exchange that increases O2 requirements. Moreover, some infants with BPD have reduced lung compliance and increased airway resistance and hence have prolonged pulmonary time constants. Some infants with severe BPD have abnormal muscularization of the vessels in the periphery of their lungs, pulmonary hypertension, and right ventricular hypertrophy.
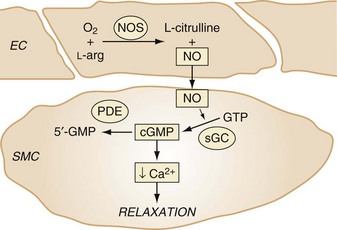
Pulmonary disease in infants with BPD is associated with abnormal pulmonary function tests and chest radiograph findings (small radiolucent cysts and hyperexpanded lungs), hypercarbia, chronic hypoxemia, and reactive airway disease.41–46 Nitric oxide (NO) is a free radical gas formed in endothelial cells and diffuses into subjacent smooth muscle cells, where it stimulates cyclic guanosine monophosphate (cGMP) production and mediates vasorelaxation (Fig. 36-1). Inhaled NO decreases abnormal cell proliferation in the injured developing lung47,48 and improves alveolar development in preterm animal models of BPD.49,50 Given the biologic plausibility and the results from animal studies, clinical trials have examined whether preterm neonates would benefit from NO. However, 14 randomized controlled trials of inhaled NO in preterm infants 34 weeks gestational age or younger failed to demonstrate benefit in survival or pulmonary or neurodevelopmental outcomes.51 It is likely that decreased NO signaling enzyme activity in the injured newborn lung reduces the effectiveness of inhaled NO in protecting pulmonary development. Emerging laboratory studies suggest that modulating cytokine signaling might improve the development of the injured lung and decrease BPD. For example, investigations in newborn animal models suggest that lung injury increases cytokine signaling. Moreover, in a mouse pup model of BPD, antibody-mediated inhibition of excess transforming growth factor-beta (TGF-β) activity was observed to improve NO signaling and pulmonary alveolar and microvascular development.52 Studies are under way to examine the mechanisms through which TGF-β modulates injured lung development.
The treatment of BPD in neonates often requires ventilatory and medical therapies.53 Early and aggressive CPAP may eliminate the need for positive-pressure ventilation. Air trapping during assisted ventilation is associated with the long lung time constants in babies with BPD and may be reduced by using a prolonged expiratory time. Respiratory infection (e.g., Ureaplasma urealyticum) may contribute to the inflammatory response. Bronchodilators such as aminophylline, albuterol, or ipratropium may be beneficial in reducing airway resistance in some infants with BPD.
Infants with BPD are often treated with diuretics. As a result of chronic furosemide treatment, metabolic abnormalities may exist. Hypercalciuria may occur from the action of furosemide on the ascending loop of Henle, leading to secondary hyperparathyroidism and nephrocalcinosis in some infants. Hydrochlorothiazide and spironolactone produce less severe metabolic abnormalities. Finally, large doses of steroids, especially dexamethasone, have been shown to provide relief for some infants with severe BPD that is refractory to other medical and ventilator therapies.54,55 However, dexamethasone treatment may result in systemic hypertension, hyperglycemia, hypertrophic cardiomyopathy, and alteration of neurologic and pulmonary development in some children.56,57 Preoperative evaluation of infants with BPD requires a very careful history and physical examination, particularly focused on the pulmonary and cardiovascular systems.
Oxygen Uptake and Circulation
Uptake of O2 in the pulmonary microvasculature of neonates is facilitated by the greater hemoglobin concentration in the neonate and the increased amount of fetal hemoglobin. Fetal hemoglobin has a reduced affinity for 2,3-diphosphoglycerate and hence a greater affinity for O2. Although this affinity facilitates fetal hemoglobin O2 uptake in the placenta, it can reduce O2 release from hemoglobin to the tissues. Red blood cells containing fetal hemoglobin have an average life span of 100 days, compared with 120 days for those containing adult hemoglobin. The higher hematocrit in neonates causes an increased bilirubin load for an immature hepatic clearance pathway. However, preterm infants frequently experience anemia of prematurity, a normocytic, normochromic hypoproliferative anemia with the hallmark of inadequate production of erythropoietin.58
The O2 delivery to systemic tissues in neonates is facilitated by a cardiac output greater than in adults on a per–kilogram body-weight basis. The relationships among myocardial preload, contractility, afterload, and heart rate determine cardiac output. Passive myocardial fiber tension is reflective of myocardial compliance, which is significantly reduced in the perinatal period.59 Active myocardial tension, reflecting contractility, is also significantly reduced in the neonate.59 At smaller end-diastolic volumes, mild increases in preload in the neonate are associated with increased cardiac output. At greater end-diastolic volumes, as a result of poor ventricular compliance in the neonatal heart, this positive effect is soon overcome and cardiac output becomes more dependent on heart rate (see Chapters 14 and 16).60–62 The heart rate of a term neonate is approximately 120 beats/min; it increases to 160 beats/min by 1 month of age.63 Parasympathetic control of heart rate in lambs, and probably in developing humans, matures earlier in gestation and to a greater extent than β-adrenergic control.64,65 For this reason, neonates tend not to respond to adrenergic signaling associated with hypovolemia or an inadequate depth of anesthesia with tachycardia. Additionally, the vagotonic response caused by succinylcholine or its metabolites (succinylmonocholine) and synthetic opioids may lead to bradycardia. These cardiac reflexes can be offset by the vagolytic effects of pancuronium or atropine.66,67
Preterm infants may have pulmonary hypertension. In utero, the lungs are not required for gas exchange; the placenta performs this function. Thus the fetal circulatory pattern consists of atria and ventricles working as units in parallel (see Fig. 16-1). As little as 10% of the fetal right ventricular output may circulate through the lungs.68 Most of the blood returned from the lower extremities and a portion of the umbilical venous blood supply passes into the pulmonary arteries and subsequently through the PDA into the systemic circulation (see Chapters 14 and 16). The superior vena caval blood supply circulates through the patent foramen ovale (PFO) into the left atria and subsequently into the systemic circulation. With expansion of the lungs during the first breath, pulmonary vascular resistance decreases and blood flow to the lungs increases, matching perfusion with new ventilation.68,69 Any factor that increases pulmonary vascular resistance, (e.g., hypoxia, hypercarbia, or acidosis) may cause the circulation to revert to a fetal circulatory pattern with shunting of deoxygenated blood from the right to the left side of the heart via the PFO or PDA. This right-to-left shunting of blood explains in part why some infants remain hypoxemic despite ventilation with 100% O2 after severe desaturation.
The ductus arteriosus may remain patent in neonates, especially in those born prematurely.70 Bounding peripheral pulses, a harsh systolic ejection murmur at the left sternal border, and widened pulse–pressure difference, suggest the presence of a PDA. The presence of a PDA and of right-to-left shunting of blood may contribute to ventilation-perfusion (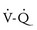 ) mismatch as deoxygenated blood is shunted from the pulmonary arteries to the systemic circulation through the PDA and differential upper and lower extremity O2 saturations result. Later, as the pulmonary vascular resistance decreases, left-to-right shunting of blood from the systemic to pulmonary circulation across the PDA may cause flow-mediated endothelial cell injury and abnormal pulmonary artery remodeling and hypertension. Infants with a PDA often require increased ventilatory support.
) mismatch as deoxygenated blood is shunted from the pulmonary arteries to the systemic circulation through the PDA and differential upper and lower extremity O2 saturations result. Later, as the pulmonary vascular resistance decreases, left-to-right shunting of blood from the systemic to pulmonary circulation across the PDA may cause flow-mediated endothelial cell injury and abnormal pulmonary artery remodeling and hypertension. Infants with a PDA often require increased ventilatory support.
A PDA in the neonate may be treated with indomethacin or ibuprofen. If the PDA persists despite medical treatment, surgical ligation may be required.71–73 Treatment with indomethacin is associated with renal and platelet dysfunction. Although experience suggests that infants who have received large volumes of intravascular fluid have a greater incidence of PDA,74 the mechanism behind this is unknown, and the relationship may not be causal. In fact, if the PDA is associated with ventricular dysfunction and poor organ perfusion, it is imperative to provide adequate intravascular volume and to improve cardiac output rather than expose the infant to hypotension mediated by left-to-right shunting across the PDA. Occasionally, systemic perfusion in infants with PDA may be improved with a dopamine infusion and judicious intravenous fluid administration.
In neonates, increased pulmonary vascular resistance causes shunting of desaturated blood through the PFO and PDA and thereby causes severe systemic hypoxemia. Although the cause of persistent pulmonary hypertension of the newborn (PPHN) is incompletely understood, it is associated with increased muscularization of pulmonary arterial vessels75 and sepsis and aspiration syndromes.76 PPHN is suspected in severely hypoxic neonates who do not have a significant increase in postductal O2 saturation when they breathe 100% O2. A difference in preductal and postductal O2 saturations supports the diagnosis because it reflects the extrapulmonary right-to-left shunting of deoxygenated blood via the PDA. PPHN is diagnosed when pulmonary hypertension and no other structural heart lesions are observed by cardiac ultrasound.
Treatment of PPHN is directed at decreasing pulmonary vascular resistance and the extrapulmonary shunting of deoxygenated blood. In cases of pneumonia and aspiration syndromes, in which airway disease can lead to atelectasis and intrapulmonary shunt, PEEP and exogenous surfactant are sometimes used to recruit alveoli. To treat the pulmonary vasoconstriction that is pathognomonic of PPHN, hyperoxia and alkalosis therapies have been used. Although inspired O2 is a potent vasodilator, maximum dilation of the pulmonary vasculature is achieved by relatively low levels of O2. For this reason, increasing the Fio2 often does not improve gas exchange in PPHN. Through a mechanism that is incompletely understood, alkalosis causes pulmonary vasodilation. In many infants with PPHN, alkalosis induced by hyperventilation or the infusion of base decreases pulmonary vascular resistance and increases systemic O2 partial pressures. In general, the vasodilation induced by alkalosis occurs when the arterial pH is 7.55 or greater. With the advent of inhaled NO, alkalosis has been largely replaced with avoiding acidemia, but not inducing marked alkalosis. Intravenous vasodilator drugs cause inconsistent vasodilation in infants with pulmonary hypertension.77 Unfortunately, because these agents dilate the systemic as well as the pulmonary vasculature, they often cause severe systemic hypotension.
Inhaled NO selectively decreases pulmonary vascular resistance and increases systemic O2 partial pressures in infants with PPHN.78–80 NO is a gas that is synthesized by NO synthase from l-arginine and O2 in endothelial cells and diffuses into subjacent smooth muscle cells, where it stimulates soluble guanylate cyclase to increase cGMP levels. It is probably through cGMP’s stimulation of cGMP-dependent protein kinase I and the phosphorylation of several cytoplasmic targets that regulate intracellular calcium levels and vasomotor protein function that NO causes vascular relaxation (see Fig. 36-1). Inhaling small concentrations of NO caused selective pulmonary vasodilation, decreased right-to-left shunting of blood, and increased systemic O2 partial pressures in neonate animals and infants with pulmonary hypertension.81–83 Acutely breathing up to 80 parts per million (ppm) by volume of NO rapidly and selectively decreases pulmonary vascular resistance and increases systemic O2 partial pressures by decreasing extrapulmonary shunting of deoxygenated blood. Although methemoglobin is produced during the metabolism of NO, no important increases in methemoglobin were observed in infants breathing low concentrations of NO. Large multicenter studies suggest that breathing 20 ppm NO decreases the requirement for extracorporeal membrane oxygenation (ECMO). Although ECMO can be lifesaving for severely hypoxemic neonates,84–86 it is expensive and invasive, sometimes causes important complications, and is not available at most hospitals. For these reasons, inhaled NO is used in most intensive care nurseries to treat the severe hypoxemia associated with PPHN.
Studies also suggest that chronic NO inhalation protects the neonatal lung from injury.47 In cell cultures, NO has been observed to decrease pulmonary smooth muscle cell proliferation and increase programmed cell death (apoptosis). Inhaled NO has been observed to decrease pulmonary artery neomuscularization in animals with lungs injured by breathing gases with low concentrations of inspired O2. Although inhaled NO may decrease lung artery remodeling by preventing hypoxic pulmonary vasoconstriction, studies suggest that it prevents abnormal pulmonary artery remodeling in injured lungs without hypertension.78 Although the protective mechanism of inhaled NO is unknown, it does decrease smooth muscle cell proliferation in vitro,87 possibly by altering the expression of transcriptional regulators and the progression of the cell cycle.88–90 It is likely that studies that identify the protective mechanisms of NO/cGMP signaling will guide the development of novel therapies to prevent pulmonary disease in the injured lung.
Appreciation has been increasing of the potential harmful effects of even brief periods of hyperoxia.91 Six randomized controlled clinical studies have shown no apparent advantage of initiating resuscitation of neonates with 100% supplemental O2 instead of 21% O2. In fact, some studies suggest slightly greater mortality rates with 100% O2. In light of these findings, the 2010 Neonatal Resuscitation Program has been updated to recommend initiating resuscitation of full-term neonates with 21% O2 and that pulse oximetry be used if supplemental O2 is needed.92
Temperature Regulation
The neonatal body habitus favors heat loss. The large surface area of the head relative to that of the body of neonates increases heat dissipation. A head cover may significantly reduce temperature loss.93,94 Neonates do not shiver or sweat effectively to maintain body temperature and rely primarily on brown fat metabolism to maintain body heat. Brown fat cells begin to differentiate at 26 to 30 weeks of gestation and hence are not available in extremely preterm infants to provide fat for metabolism and heat generation.95 Warming the operating room to 85° F (30° C), using radiant warming units, forced-air heating pads, and adding humidity to the inspired gases in the ventilator circuits help maintain the neonate’s temperature in the neutral thermal range.96–98 The sources of heat loss in infants is 39% radiation, 37% convection, 21% evaporation, and 3% conduction. Warming intravenous and irrigation fluids before they are used may also be beneficial.
Renal and Metabolic Function
Renal Function
The placenta acts as an excretory organ for the fetus. A neonate’s kidneys are not fully developed at birth, and development is closely related to PCA.98,99 At full-term birth, the glomerular filtration rate (GFR) is only 30% of normal adult rates (see Chapter 6).100 The GFR reaches adult values at approximately 1 year of age (see Figs. 6-10 and 6-11). The kidneys’ tubular function, and hence sodium-retaining ability, does not develop until about 32 weeks of gestation.101,102 The immaturity of the kidney at birth also affects the metabolism of many drugs in the neonate The renal excretion of medications such as penicillin, gentamicin, and some neuromuscular blocking drugs (NMBDs), such as pancuronium, may be prolonged, resulting in increased duration of action or the development of excessive blood concentrations. This effect is particularly important when administering medications to an extremely preterm infant. Thus the use of NMBDs that do not require renal function are most advantageous (e.g., cisatracurium).
The total body water content in neonates is greater than it is in infants, children, or adults. In the preterm infant 75% to 85% of body weight is water; in general, the less the PCA of the neonate, the greater the percentage of water. In a term infant, 70% of body weight is water.103 By 6 to 12 months of age, 50% to 60% of body weight is water (see also Figs. 6-7 and 6-8). Differences between the total body water, renal maturity, and serum protein concentrations (see also Fig. 6-6) in a neonate affect the volume of distribution of many medications. Because of the increase in the volume of distribution of drugs confined to the extracellular fluid, the initial doses of some medications (e.g., NMBDs, aminoglycosides) may be greater on a weight basis in neonates than for adults to achieve the desired blood concentration. In contrast, because of immaturity of renal function, the interval between doses of these drugs may be increased (see also E-Fig. 6-4).
Fluid Management
The basic principles of fluid maintenance in neonates are similar to those in older children and adults. The highly variable body fluid composition, degree of renal maturity,104 neuroendocrine control of intravascular fluid status, and insensible fluid loss with age105 make precise estimates of fluid requirements in neonates very difficult (see Chapter 8). Urine volume and concentration may be difficult to determine intraoperatively and may not always correlate with volume status. Moreover, blood pressure and heart rate may not correlate with intravascular volume status in preterm infants, and anesthetics may mask subtle cardiovascular changes that occur with changes in intravascular volume. Increased insensible fluid loss, which often occurs in the operating room environment, requires judicious titration of intravenous fluids. Congenital abnormalities (e.g., gastroschisis, omphalocele) may markedly increase insensible fluid loss through exposure of large mucosal surfaces. The use of humidified gas mixtures reduces insensible fluid loss through the respiratory tract. However, overzealous intraoperative administration of fluids can result in pulmonary complications and worsened third-spacing of fluids.
Methods of Intravenous Access and Monitoring
Infants who are dehydrated after a prolonged period of fasting or vomiting or because of increased insensible fluid losses may require special procedures for intravenous access. With severe hypovolemia, scalp and peripheral veins may be difficult to cannulate. Many of the superficial veins may be thrombosed from prior use. Fiberoptic light sources or ultrasound may help visualize deeper veins and peripheral arteries. Femoral and axillary veins may be accessed percutaneously or via a surgical approach for the delivery of fluids and medications.106–108 Knowledge of femoral artery and vein anatomy decreases the incidence of accidental injury of the femoral head joint and possible septic arthritis (see Fig. 48-5).109 The external or internal jugular veins can also provide alternative sites for venous access.
In neonates, fluids and many medications may be infused through the umbilical vessels. The tip of umbilical arterial lines should be placed either in a “low” position, at the level of the bifurcation of the femoral arteries (L3-4), or in a “high” position, in the descending aorta above the diaphragm (T6-9). The catheter tip of the umbilical artery catheter should not be left in the descending aorta in the area of the renal or mesenteric arteries (L1-2) because renal or mesenteric artery thrombosis might result (see Figs. 48-8 and 48-9). Aortic thrombosis occurs in approximately 1% of umbilical artery catheterizations, although thrombi can be detected with radiologic techniques in 20% to 95% of infants.110–112 Infants with renal artery thrombosis may present with hypertension, oliguria, hematuria, and elevated blood creatinine concentrations. The tip of an umbilical venous catheter should rest in the inferior vena cava above the level of the ductus venosus and hepatic veins so that solutions are not directly infused into the liver parenchyma. The delivery of hypertonic solutions into the liver might result in liver damage and portal cirrhosis. Aspiration of arterial oxygenated blood suggests that the venous catheter has entered the left atrium through the foramen ovale. Catheters in the left atrium should be pulled back to the level described earlier.
Some drugs may be delivered through the tracheal tube. Rapid uptake and minimal effects on gas exchange or the pulmonary parenchyma occur with epinephrine, atropine, and lidocaine (see Chapter 39). The doses of some drugs administered through the tracheal tube are increased in comparison with that delivered intravenously (e.g., epinephrine 0.05 to 0.1 mg/kg via the tracheal tube versus 0.01 to 0.03 mg/kg intravenously).92 It is important to understand that administration of solutions via the endotracheal tube can cause adverse effects. For example, complications associated with the tracheal administration of exogenous surfactant include occasional transient hypoxia and bradycardia and are likely due to the relatively large volume of fluid (2.5 to 6 mL/kg) associated with the proper dose of surfactant.
Intraosseous cannulation of the tibia with a special intraosseous infusion needle (e.g., Easy-IO, Vidacare, San Antonio, Tex.) or a styleted spinal needle provides a rapid route for emergency fluid and drug administration and can be lifesaving (see Fig. 48-6 and E-Fig. 48-1, A through E).
Glucose Homeostasis
Although the placenta allows the delivery of glucose from the maternal circulation to the fetus, the development of significant glycogen stores does not occur until late in gestation. Various conditions may lead to hypoglycemia in a neonate. Preterm and small-for-gestational-age (SGA) infants have very high glucose requirements; they require glucose infusion rates of 8 to 10 mg/kg/min. In full-term infants, a glucose infusion rate of 5 to 8 mg/kg/min prevents hypoglycemia. Full-term infants who have been excessively fasted, SGA infants, and infants of diabetic mothers are all prone to develop hypoglycemia. Although hypoglycemia may result in respiratory distress, apnea, cyanosis, seizures, tremors, high-pitched cry, irritability, limpness, lethargy, eye-rolling, poor feeding, temperature instability, and sweating, the signs and symptoms in infants are often blunted and nonspecific.113,114 In infants younger than 24 hours old, a plasma glucose concentration of less than 40 mg/dL is a cause for concern and should be treated.115 After 24 hours of life, plasma glucose values less than 45 mg/dL should be considered abnormally low. Although hypertonic glucose administration has been used to treat hypoglycemia in neonates, studies reveal that administration of hypertonic sodium bicarbonate solutions increases the incidence of intraventricular hemorrhage in preterm infants.116 For this reason, it is prudent to avoid bolus administration of hypertonic glucose to treat hypoglycemia to prevent sudden changes in blood tonicity and hyperglycemia. A bolus of 2 to 4 mL/kg of D10W (0.2 to 0.4 g/kg of glucose) and an increase in the basal glucose infusion are prudent measures to treat hypoglycemia. It is extremely important to reassess the blood glucose concentration after these treatments to determine the effectiveness of the therapy.
Infants undergoing surgical procedures often require less glucose supplementation.117 This reduced need may be attributed to hormonal responses that decrease glucose uptake as a result of catecholamine release in excess of insulin activity, as well as a decrease in metabolic demand from the effects of the anesthetic agents.118–120 Nevertheless, it is important to administer glucose-containing solutions using a constant-infusion device to avoid large fluctuations in blood glucose values and monitor blood glucose values in critically ill neonates. All other fluids (e.g., to replace third-space losses, blood loss, and fluid deficits) should be glucose-free to avoid hyperglycemia.117 Infants treated with high levels of glucose via total parental nutrition (TPN) may develop severe hypoglycemia if the infusion rate is abruptly lowered; thus it is important to continue these infusions (possibly at a slightly reduced rate) during surgery and to check the serum glucose concentrations.
Calcium Homeostasis
Calcium exists in the serum in three fractions: (1) protein bound; (2) chelated to bicarbonate, phosphate, and citrate; and (3) free or ionized calcium (iCa2+). The ionized fraction is the physiologically active component. The serum calcium concentration is mainly regulated by the action of parathyroid hormone (PTH) and vitamin D metabolites. PTH acts directly in the bone and kidneys and indirectly through calciferol in the gut.121
Hypocalcemia has been observed in nearly 40% of critically ill neonates.122 Causes of hypocalcemia include (1) PTH insufficiency and peripheral resistance to PTH, (2) inadequate calcium supplementation, and (3) altered calcium metabolism caused by transfusion with citrated blood products (see also Fig. 10-9), bicarbonate administration, or diuretics (furosemide). Hypocalcemia may be asymptomatic or accompanied by nonspecific symptoms (e.g., seizures and tremors). Thus the diagnosis rests on the determination of total and iCa2+ levels. In critically ill children, total calcium concentrations do not accurately reflect the iCa2+ concentrations; therefore the diagnosis of hypocalcemia in these infants should be determined by direct measurement of iCa2+ with an ion-specific electrode.123 Neonatal hypocalcemia may be defined as a serum iCa2+ less than 1 mmol/L in full-term infants and less than 0.75 mmol/L in preterm infants. Persistent hypocalcemia necessitates determination of magnesium, phosphorus, PTH, and vitamin D concentrations.
Treatment of hypocalcemia is not effective in the presence of hypomagnesemia. In this situation, administration of supplemental magnesium and calcium and treatment of the underlying cause of hypocalcemia are necessary.122 Symptomatic hypocalcemia is treated with 100 mg/kg calcium gluconate (10%) by slow intravenous infusion (5 minutes) in a patent intravenous line. Thereafter, maintenance calcium is administered at 100 to 200 mg/kg/day elemental calcium and the clinical response and serum iCa2+ levels are carefully monitored.
Gastrointestinal and Hepatic Function
The fetal gut is not functionally developed until late in gestation. In full-term neonates, the maturation of esophageal function occurs soon after birth. However, in comparison with adults, gastric emptying in neonates is prolonged and lower esophageal sphincters are incompetent, making reflux of stomach contents common. Early feeding of hypertonic formulas in preterm infants increases intestinal energy demands and is associated with bowel ischemia and necrotizing enterocolitis.124 On the other hand, the use of hypocaloric or trophic feeds in preterm infants is associated with subsequent feeding intolerance, indirect hyperbilirubinemia, cholestatic jaundice, and metabolic bone disease.125,126
After birth, increased concentrations of unbound serum bilirubin introduce the risk of kernicterus, particularly in infants who are preterm, hypoxemic, and acidotic and have low serum protein concentrations.127 Highly protein-bound agents such as furosemide, sulfonamides, ceftriaxone, and benzyl alcohol (found as a preservative in many drugs such as diazepam) may displace bilirubin and increase the possibility of kernicterus.128 Hepatic metabolism is immature in neonates and particularly in preterm infants. Drug metabolism may be prolonged as a result of both immaturity of enzymatic processes and a relatively low hepatic perfusion (less drug delivered to the liver). Any factor that further compromises hepatic blood flow (e.g., increased intraabdominal pressure) may have profound adverse effects on drugs with perfusion-limited hepatic clearance.129 Therefore careful titration of these drugs (e.g., opioids, propofol) is required to optimize therapeutic effects and prevent toxicity. Just as consideration is given to immature renal function, the use of NMBDs that do not require hepatic metabolism can be advantageous, (e.g., cisatracurium). The use of remifentanil during the procedure followed by a low dose of longer-acting opioid or regional block at the end of the procedure might facilitate early extubation.
Neurologic Development
The central nervous system is incompletely developed at birth. However, early in gestation, the pain pathways are integrated with the somatic, neuroendocrine, and autonomic systems. The hormonal responses to pain and stress may be exaggerated in neonates,118,119 although the clinical significance of this has not been defined. The potential lack of autoregulation of cerebral blood flow and an infant’s fragile cerebral blood vessels may be important factors in the development of intraventricular hemorrhage.130,131 Although an association has been noted between the incidence of intraventricular hemorrhage and fluctuations in blood pressure,130,132 it is difficult to confirm any causal relationship. This association has been a concern during “awake” or nonanesthetized laryngoscopy and intubation of neonates; however, one study reported no significant change in blood pressure or heart rate in neonates even after awake intubation.133 In addition, another study questioned the lack of autoregulation of cerebral blood flow in preterm infants. Using near infrared spectroscopy, investigators found that preterm infants could maintain adequate cerebral perfusion at a mean arterial blood pressure in the range of 23 to 39 mm Hg.134
In neonates, the spinal cord extends to a lower segment of the spine than in older children and adults (see E-Fig. 41-3). The volume of cerebrospinal fluid and the spinal surface area are proportionally larger in neonates (see also E-Fig. 41-4), whereas the amount of myelination is less than in older children and adults.135,136 These factors may account in part for the increased amount of local anesthetics (milligram per kilogram) required for successful spinal anesthesia in infants.
Hyperoxia has been associated with retinopathy of prematurity (ROP).137–139 Although two case reports of ROP associated with anesthesia in preterm infants implicated hyperoxia as a primary etiologic factor, the cause of ROP appears to be multifactorial140–149; the association between arterial O2 tension and ROP is unclear (see also Chapter 32). ROP begins as retinal vascular narrowing and obliteration followed by increased vascularity (neovascularization), hemorrhage, and, in the most severe cases, retinal detachment and blindness (see also Fig. 32-1). In two clinical trials (the SUPPORT150 and BOOST II151 trials) infants born at less than 28 weeks gestation were randomized to saturation ranges of either 85% to 89% or 91% to 95%. In the SUPPORT trial, for every 11 infants treated in the lower saturation range, one case of severe ROP was prevented (results for the BOOST II trial are not yet available). However, in both the SUPPORT and BOOST II trials, the latter of which was terminated early, the mortality was greater in infants who were randomized to the 85% to 89% range than those in the 91% to 95% range. Although the optimal saturation range has not been defined in preterm infants, it seems prudent to target the O2 saturation in the range of 91% to 95% and avoid a saturation less than 89%.
Preparation for Surgery
The Operating Room
Conditions that require emergency surgery in neonates are often accompanied by medical problems, and, as a consequence, management and monitoring considerations can be complex. Routine standard monitoring equipment includes an electrocardiograph, chest or esophageal stethoscope, blood pressure monitor, temperature probe, pulse oximeter and a CO2 analyzer. Rapid decreases in arterial O2 content in neonates after brief periods of ventilatory compromise, coupled with the possible risk of ROP as a result of hyperoxia, dictate the need for continuous O2 saturation monitoring. A pulse oximeter probe placed in a preductal position (right hand) can be compared with one placed in a postductal position (foot or left hand ) to determine the severity of extrapulmonary shunting of deoxygenated blood via the PDA. Pulse oximetry may prove to be particularly useful for infants in whom the risk of intraarterial monitoring cannot be justified.152 This device can diagnose hypoxemia but not hyperoxia; however, maintaining the O2 saturation at 91% to 95% (preductal) places most infants on the steep portion of the O2 hemoglobin dissociation curve and avoids severe hyperoxia.153
Expired CO2 can be measured using capnography. However, dilution of the exhaled gases with those of the dead space of the tracheal tube or by the fresh gas may underestimate the true alveolar CO2 partial pressure. If desired, a more accurate estimate of expired CO2 concentration may be obtained by using a special tracheal tube with a sample port located at its tip, by inserting a narrow catheter through the CO2 sampling port in the elbow and into the lumen of the tracheal tube or by using a needle introduced through the side wall of the tracheal tube 2 to 3 cm distal to the 15-mm tube connector (see Chapter 51).154,155 Using a circle system provides a reasonably accurate measurement of expired CO2 concentrations because the fresh gas flows are small and less mixing of exhaled and inhaled gases occurs. However, regardless of the circuit configuration, the value obtained by the capnograph may not accurately reflect Paco2 in the presence of congenital heart disease (e.g., cyanotic heart disease or a mixing shunt) or significant intrapulmonary shunting.156
In neonates, changes in blood pressure, heart rate, and the intensity of heart sounds are excellent indicators of cardiac function, intravascular volume status, and depth of anesthesia. Under most circumstances, a urinary catheter permits adequate determination of urine output and aids in the monitoring of fluid balance during prolonged operations. In cases in which major blood or fluid losses are expected, or the physiology is complicated by the presence of cardiac disease, a central venous catheter is warranted. Any neonate with significant underlying cardiovascular instability should have an arterial catheter placed for continuous monitoring of blood pressure and to provide the means to obtain arterial blood samples for determination of blood pH and gas levels and serum glucose and electrolyte concentrations. Many neonates arrive in the operating room from the intensive care unit (ICU) with an umbilical artery line in place. Because of the risk of renal artery thrombosis with these lines, it is important to verify the location of the tip of the umbilical artery catheter. Infrahepatic umbilical venous lines may not be reliable under operative conditions because they may become wedged in the liver. In this position, infusion of hypertonic solutions may lead to parenchymal necrosis and ultimately fibrosis.157,158
Nonrebreathing (open) circuits are simple and effective for delivering anesthetic agents to infants weighing less than 10 kg. The system must have provisions for a humidifier to warm and hydrate the cold, dry anesthetic gases. However, a trend exists away from the use of nonrebreathing circuits to save money and reduce air pollution.34–3659 With the increasing need for cost savings there appears to be no significant disadvantage to using circle systems, which allow the use of small fresh gas flows. This substitution should only be made if the provider has a clear understanding of the marked increase in compression volume/compliance volume losses (see also Fig. 51-7) compared with nonrebreathing circuits.159 The anesthesia machine should also provide compressed air in addition to O2 and NO. The use of air allows regulation of inspired O2 concentrations during cases in which NO treatment is contraindicated and a high Pao2 is not desired. Table 36-3 lists basic equipment for conducting emergency anesthesia on a neonate.
Emergency Surgery
Respiratory Problems
Abnormalities of the Airway
Choanal Atresia and Stenosis
Not all neonates are able to change to oral breathing when nasal obstruction occurs.32,33 Choanal atresia can present as cyanosis at rest that resolves with crying or placement of an oral airway. Choanal atresia and stenosis result from the failure of the bone or membranous portion of the nasopharynx to undergo regression during development. The incidence is approximately 1 in 8000 births. Unilateral lesions are seldom symptomatic and may escape early detection. Although bilateral lesions may be asymptomatic, occasionally they may lead to respiratory distress.160 Choanal atresia and stenosis are generally not associated with other craniofacial anomalies. Choanal atresia may be found as part of a constellation of congenital anomalies, the CHARGE association: coloboma, heart disease (tetralogy of Fallot, PDA, double-outlet right ventricle with atrioventricular canal, ventricular septal defect, atrial septal defect, right-sided aortic arch), atresia choanae, retarded growth (including other central nervous system anomalies), genital anomalies (hypogonadism), and ear anomalies.161,162 These neonates may develop airway obstruction during anesthetic induction. Early placement of an oral airway, facilitated by preinduction topical application of viscous lidocaine to the tongue, aids in airway management.
Laryngeal and Upper Tracheal Obstruction
Obstruction at the level of the larynx or upper trachea may be due to laryngeal and tracheal webs or subglottic lesions. Subglottic lesions may be due to congenital stenosis, hemangioma, web, or vascular ring (Figs. 36-2 and 36-3; see also Videos 12-1, 12-2, and 12-5)![]() . Anesthetic management of obstructive lesions in older children and adults usually includes an inhalation anesthetic induction while maintaining spontaneous respirations. This method may be difficult in neonates for the following reasons: (1) the combined effects of inhalation anesthetic agents and immature ventilatory drive regulation may predispose to hypoventilation; (2) hypoventilation leads to an increased alveolar CO2, which displaces alveolar O2; and (3) anesthetic agents decrease intercostal muscle function, resulting in decreased functional residual capacity (FRC).163,164 The hypoventilation and reduced FRC combined with high O2 consumption predispose infants to desaturation during anesthetic induction while the infant maintains spontaneous ventilation.
. Anesthetic management of obstructive lesions in older children and adults usually includes an inhalation anesthetic induction while maintaining spontaneous respirations. This method may be difficult in neonates for the following reasons: (1) the combined effects of inhalation anesthetic agents and immature ventilatory drive regulation may predispose to hypoventilation; (2) hypoventilation leads to an increased alveolar CO2, which displaces alveolar O2; and (3) anesthetic agents decrease intercostal muscle function, resulting in decreased functional residual capacity (FRC).163,164 The hypoventilation and reduced FRC combined with high O2 consumption predispose infants to desaturation during anesthetic induction while the infant maintains spontaneous ventilation.
Webs.
Laryngeal or tracheal webs generally produce incomplete fibrous membranes that lead to obstruction of the airway with acute respiratory distress or stridor shortly after birth (Fig. 36-2).165 Complete airway obstruction occasionally results in an emergency in the delivery room. A tracheal tube may be used as a stent to open an incomplete lesion. If tracheal intubation is not possible, an intravenous catheter passed through the cricothyroid membrane may allow oxygenation and be lifesaving (see Figs. 12-25 through 12-27).166 A tracheostomy is then established.
Congenital Subglottic Stenosis.
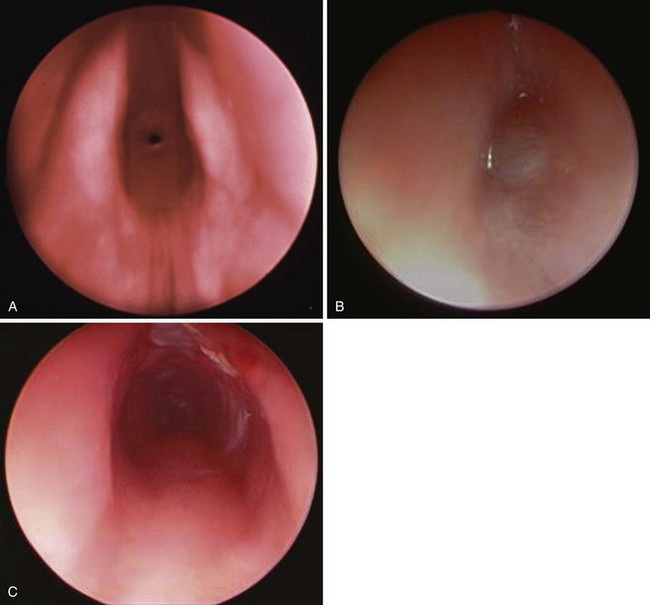
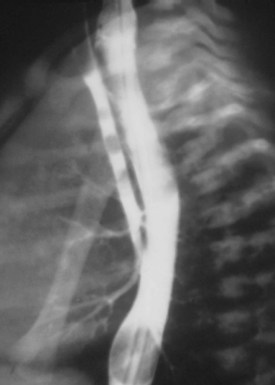
The severity of the symptoms resulting from congenital subglottic stenosis depends on the degree of airway occlusion (see Fig. 36-3, A). Treatment depends on the location and length of stenosis. For severe narrowing, a tracheostomy is placed and a series of dilations attempted.167 Tracheal dilations may cause airway disruption, pneumomediastinum, and pneumothorax. Anesthesia may be induced using an inhalation technique with a facemask and appropriate ventilatory assistance. If a tracheostomy is considered, a small-diameter tracheal tube may be placed to facilitate ventilation until a tracheostomy is performed. This tracheal tube should be of smaller diameter so that it passes beyond the level of obstruction. As resistance increases inversely with the airway radius to the fifth power, ventilatory assistance is required to overcome this substantial increase in the work of breathing. The greater time constants that result from the increased airway resistance require a greater time for expiration to avoid gas trapping.
Subglottic Hemangioma.
Subglottic hemangioma may produce respiratory distress during the first few weeks of life as it rapidly increases in size (see Fig. 36-3, B and C ). The presence of other hemangiomas on an infant’s body, especially on the face, is a clue that a subglottic hemangioma may be the source of the respiratory distress.168 These infants often present with symptoms of upper airway obstruction, which may be life-threatening when additional exacerbating factors such as upper respiratory tract infections or other causes of inspissated mucus result in further airway compromise.169 Any infant who is younger than 3 months of age and presents with symptoms of “croup” must be considered to have causes other than infection as a source of the airway obstruction. If tracheal intubation is required, it should be carried out as gently as possible because bleeding may occur secondary to trauma from the intubation procedure.
Esophageal Atresia and Tracheoesophageal Fistula.
Esophageal atresia and tracheoesophageal fistula (TEF) are often associated with other congenital abnormalities, in particular the VACTERL association (vertebral abnormalities, imperforate anus, congenital heart disease, tracheoesophageal fistula, and renal abnormalities and limb abnormalities (typically radial atresia).170,171 The specific cause of these associations is unknown. Although esophageal atresia may be an isolated occurrence, in 90% of cases it is associated with TEF.
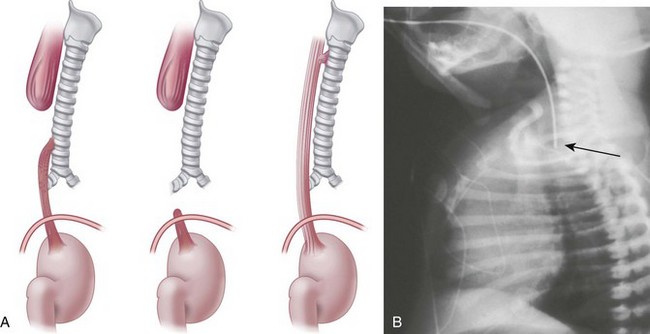
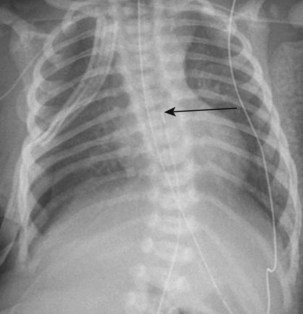
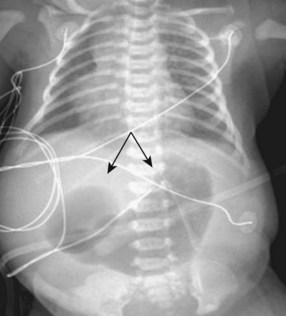
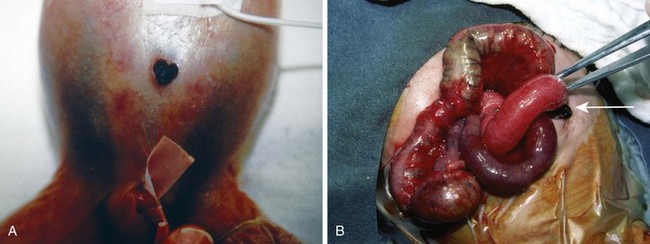
Affected neonates usually present with excessive oral secretions, regurgitation of feedings, and occasionally respiratory distress exacerbated by feedings; recurrent pneumonia is associated with an H-type TEF (∼2% to 6% of cases) and is usually diagnosed later in life. The diagnosis of esophageal atresia is confirmed by the inability to pass a moderately rigid orogastric tube into the stomach or the demonstration of a blind esophageal pouch by air contrast or radiopaque dye and radiographic studies. The presence of bowel gas suggests a TEF; on occasion, the abdominal distention may be severe enough to cause atelectasis and impede ventilation. In the most common form of TEF, the esophagus ends in a blind proximal pouch with the distal end of the esophagus connected to the trachea (usually posteriorly) just above the carina. In the less common form of isolated TEF without esophageal atresia, radiologic studies may be inconclusive (Fig. 36-4 and E-Fig. 36-1).
Several approaches may be used to secure the airway in these infants. Awake intubation may be conducted with topical anesthesia of the airway or with intravenous suction if the neonate is medically stable. Alternatively, inhalational induction and spontaneous ventilation may be used until the trachea is secured, particularly if rigid bronchoscopy is used to define the anatomy of the airway before ligation of the fistula. To properly position the tracheal tube, an intentional right main-stem bronchial intubation is sometimes performed; subsequently, the tracheal tube is slowly withdrawn while auscultating the left thorax until breath sounds are heard. During this maneuver, it is key to ensure the bevel of the tube is on the left side (particularly if a Murphy eye is not present on the tube) to facilitate detection of air exchange with the left lung as soon as the tube is withdrawn above the level of the carina. At this position, the tip of the tracheal tube is just above the carina and usually below the level of the fistula. When the tracheal tube is secured in this location, less gastric insufflation will occur through the fistula. The use of fiberoptic bronchoscopy after tracheal intubation has simplified positioning and verifying the position of the tracheal tube and has reduced the need for rigid bronchoscopy. The tracheal tube should be carefully secured; if the tracheal tube is withdrawn to the position of the fistula opening, adequate pulmonary ventilation cannot be guaranteed. A stethoscope placed over the left chest (usually best in the axilla) may be helpful in detecting accidental advancement of the tracheal tube into the right main-stem bronchus. An arterial line allows monitoring of blood gas values during the procedure. Pulse oximetry is particularly helpful in detecting partial displacement of the tracheal tube. Preterm infants with poorly compliant lungs occasionally require positive-pressure ventilation.172,173
Preferential ventilation through the fistula (the path of least resistance) may result in inadequate pulmonary gas exchange because the air leak through the fistula and into the stomach might increase the intraabdominal pressure, causing the diaphragm to press on the lungs and thereby lead to atelectasis. A staged preoperative gastrostomy was commonly used in the past to avoid this complication. However, although gastrostomy may avert life-threatening gastric rupture, it also may contribute to ineffective ventilation as a result of a bronchocutaneous fistula. A spontaneous breathing technique often results in little gastric insufflation. Although positive-pressure ventilation may cause gastric dilation, ventilation is usually successful because the compliance of the lungs is greater than that of the distended stomach. Pneumoperitoneum can be managed by emergent needle decompression of the left upper abdominal quadrant. Strategies to ensure adequate ventilation include one-lung ventilation, tracheal intubation distal to the fistula, or Fogarty catheter occlusion of the fistula. If a staged gastrostomy has been performed, a Fogarty catheter passed retrograde through the gastrostomy can be used to occlude the esophagus from below. This approach offers the advantage of avoiding bronchoscopy to pass a Fogarty catheter into the fistula through the trachea in an infant with pulmonary compromise.174 An epidural catheter threaded from the caudal to the thoracic space may provide a means to give postoperative analgesia in patients who have had TEF repairs (E-Fig. 36-2).
Diseases of the Lung Parenchyma
The small diameter of a neonate’s airway makes double-lumen tracheal tubes impractical. Nevertheless, selective bronchial intubations may be successful, especially on the right. Fiberoptic bronchoscopes and guidewires aid in the placement of left-sided tracheal tubes. Bronchial blocking with 5F embolectomy catheters or the intrathoracic use of a clamp by the surgeons may allow selective ventilation of the lung.175–177
Specific Lesions
Congenital Diaphragmatic Hernia.
Failure of the diaphragm tissues to fuse appropriately most often appears as the posterolateral Bochdalek type of hernia (90%). Approximately 9% are the anterior, Morgagni type of defects. Less than 1% are bilateral hernias, which are often fatal. The incidence of CDH ranges from 1 in 2000 to 1 in 5000 live births, the variation being attributable to underdiagnosed postdelivery deaths among those neonates severely affected. Equal representation occurs among the genders, although some larger, population-based studies have demonstrated a male preponderance.178
Environmental and genetic variables (some poorly understood) contribute to the frequency and presentation of CDH. In neonates in whom the hernia is the only health issue, the risk of recurrence for future pregnancies is small (2%). Neonates with the Bochdalek type of hernia are more likely to have concurrent birth defects, including a 20% to 40% chance of congenital heart defects and a 5% to 15% chance of chromosomal abnormalities.179 CDH is associated with genitourinary and gastrointestinal malformations, as well as chromosomal anomalies, including trisomy 13, trisomy 18, tetrasomy, and 12p mosaicism.
Prenatal diagnosis of CDH has become an effective tool in furthering the treatment modalities of affected neonates and counseling expectant parents. Level 2 ultrasonography is required for antenatal diagnosis of CDH.180 Ultrasound examination findings suggestive of the presence of CDH include polyhydramnios, an intrathoracic gastric bubble, and mediastinal shift away from the herniation site. Additional diagnostic testing may include amniocentesis and karyotyping for the delineation of any coexisting chromosomal abnormalities. Abnormally low levels of maternal serum α-fetoprotein are also associated with CDH. The importance of prenatal diagnosis cannot be overemphasized because prior knowledge of the defect will permit the arrangement for delivery at a tertiary care center equipped to provide support. In addition, a handful of research centers offer advanced treatment options such as fetal-based corrective surgical procedures.181,182 Antenatal diagnosis has allowed the development of risk stratification for fetuses with CDH. The constellation of CDH with liver herniation and a small lung-to-head ratio is often indicative of high postnatal mortality. However, newer surgical techniques performed in utero have attempted to address this issue. Temporary fetoscopic tracheal plugging, performed between 25 and 28 weeks gestation, prevents the normal outflow of surfactant-rich fetal lung fluid.183 The retained volume subsequently enlarges the fetal lungs, accelerates growth, and reduces the mass effect of herniated viscera. This is coupled with another advanced technique (the ex utero intrapartum tracheoplasty [EXIT] procedure) used to unplug the trachea at the time of birth. During the EXIT procedure, placental support of the fetus continues until the airway has been secured (see Chapter 37). Only a small number of centers offer this treatment option, so only a few randomized, controlled studies have been performed to determine long-term outcomes for fetuses with CDH.183–185 Further clinical experience is needed before the comparative long-term efficacy of such fetal therapies can be determined.
Emergent surgical closure of the defect was the standard of care in the 1980s, because of the prevalent belief that reduction of the herniated viscera would facilitate lung growth and a return toward normal lung size and function. A thorough understanding of the specific pathophysiology of the defect prompted the application of new medical therapies and changed the timing of open surgical repair.186 Although the mere presence of abdominal viscera (which may include intestine, liver, spleen and stomach) in the thoracic cavity is not in itself life-threatening, the compressive effects of these viscera on the developing pulmonary structures presents a significant obstacle to the smooth transition from a fetal to neonatal circulatory pattern. The abnormal compression of pulmonary structures is the hallmark of CDH and its cardiopulmonary sequelae.187–190 Lung growth is severely restricted, especially during the pseudoglandular phase, when multiplication of proximal airway divisions and the formation of supporting pulmonary arterial vasculature usually occur. Subsequently, fewer functional alveolar units and a grossly diminished surface area for effective gas exchange in the hypoplastic lungs develop. Lung hypoplasia is also associated with a decreased number of alveolar type II cells that results in a deficiency of surfactant apoprotein A (SP-A), which can be associated with alveolar instability, atelectasis, intrapulmonary shunting of deoxygenated blood, and inadequate gas exchange. These biochemical deficiencies are only partially ameliorated with exogenous surfactant treatment. A unilateral CDH may still restrict the normal growth of both lungs depending on the degree of mass effect. The severity of the lung hypoplasia, and its associated morbidity and mortality, were negatively correlated with the gestational age at the time the hernia occurred. The abdominal mass effect, which hinders normal lung growth, also reduces the total cross-sectional area and may alter the reactivity of the arterioles, resulting in pulmonary hypertension. Decreased pulmonary blood flow prevents the normal transition from an intrauterine to extrauterine circulatory pattern. Right-to-left shunting via the PFO and PDA, with associated severe hypoxemia, poses an immediate threat to the neonate. The increased volume of intrathoracic contents may also cause caval compression, with subsequent reductions of preload and cardiac output.
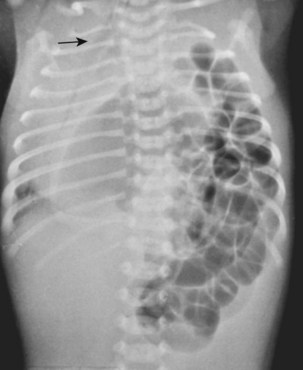
CDH most often presents as severe respiratory distress in the neonate, a direct consequence of lung hypoplasia and inadequate pulmonary gas exchange. Tachycardia, tachypnea, and cyanosis can be observed shortly after delivery. The abdomen may appear concave because of the displacement of viscera into the thorax (the classically described scaphoid abdomen). The mediastinum may be shifted as a result of the herniated viscera (E-Fig. 36-3). Neonates with the Morgagni type of hernia may present with less severe respiratory compromise but with symptoms of bowel obstruction.
The initial management of neonatal respiratory failure associated with CDH is directed at refining oxygenation and ventilation. Definitive airway control is a priority and should be achieved soon after delivery. Mask ventilation is generally avoided in an attempt to limit gastric insufflation that can cause visceral distention and increase the mass effect within the thorax. Once the airway has been secured, mechanical ventilation should be instituted at the lowest peak airway pressures to achieve clearance of CO2 and adequate oxygenation at an acceptable inspired O2 concentration. A nasogastric tube should be passed to decompress the intestinal contents. Serial arterial blood gas analysis and chest radiographs are essential in establishing the prognosis. Studies suggest that alveolar-arterial O2 differentials (A-ao2) and serial Paco2 measurements are useful predictors for survival.191,192 If the blood gas values are unacceptable, further strategies, including high-frequency ventilation, oscillation, and ECMO may be considered (see later). An echocardiogram will identify the cardiac anatomy and specific flow characteristics, permitting assessment of pulmonary hypertension and right heart dysfunction, as well as major vessels that may be used for ECMO. Concurrent congenital heart defects also may be diagnosed at this time. Cranial ultrasonography is useful to diagnose intraventricular bleeding, an important consideration for potential institution of ECMO. Appropriate intravenous access and invasive blood pressure monitoring should be established as soon as feasible.
Several ventilator strategies have been successfully used and include conventional mechanical ventilation, high-frequency oscillatory ventilation, gentle ventilation with permissive hypercapnia, and intratracheal pulmonary ventilation. All of these methods seek to avoid the harmful effects of volutrauma and conditions known to increase pulmonary vascular resistance (hypoxemia, acidosis, hypotension, and significant hypercarbia).190,193–195 CDH may have variable presentations and degrees of severity; thus no single technique may address all ventilation requirements. Settings that allow for rapid low tidal volumes and limit peak inspiratory pressures will reduce the degree of volutrauma and the potential for pneumothorax. Deliberate alkalosis may increase pulmonary blood flow and decrease 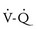 mismatch, but possibly worsening the risk of volutrauma. In neonates in whom oxygenation remains compromised, global tissue hypoxemia will result in significant metabolic acidosis, which may be treated with intravenous bicarbonate infusions, if adequate ventilation is ensured.
mismatch, but possibly worsening the risk of volutrauma. In neonates in whom oxygenation remains compromised, global tissue hypoxemia will result in significant metabolic acidosis, which may be treated with intravenous bicarbonate infusions, if adequate ventilation is ensured.
ECMO has been used in several centers to treat infants with CDH in whom other medical and ventilator therapies have not been effective. Although the mechanism whereby ECMO improves the outcome of infants with CDH is not fully understood, ECMO can act as a temporizing measure, permitting the lungs to rest and mature while providing appropriate gas exchange through membrane oxygenators.196 ECMO has also been used as a rescue technique in postoperative infants who decompensate following surgical closure (most often from the incompletely understood phenomenon of rebound pulmonary hypertension). There are multiple inclusion and exclusion criteria (e.g., the presence of high-grade intraventricular hemorrhage is a contraindication because of systemic heparinization).197 Venovenous (VV) and venoarterial (VA) are two variations of the extracorporeal circuit, and refer to the location of the inflow and outflow catheters. In VV circuits, a double-lumen catheter resides in the internal jugular vein. VA circuits require the placement of two catheters (internal jugular vein and carotid artery) and the ligation of the carotid ipsilateral artery to prevent backflow. In general, VA ECMO is used for neonates who require additional hemodynamic support and greater delivered O2 concentrations. The widespread institution of ECMO has promoted the early stabilization of infants with CDH. The procedure is invasive, however, and complications may arise as a result of systemic anticoagulation and intracranial bleeding. Emboli, infection, vascular damage, and circuit failure are additional concerns.
Currently, surgical repair is delayed until the neonate has been optimized using applicable treatment modalities. Timing of the procedure is variable, based on individual infant condition and institutional experience. A transabdominal approach will replace the herniated viscera and conclude with primary closure of the defect, or if the defect is large, a synthetic patch will be needed to augment the repair. Anesthetic management focuses on supportive care during transport and surgery. Adequate central access should be established, and ventilator settings should mimic those used in the ICU. Serial blood gas sampling will guide changes in respiratory management during closure of the diaphragm. It should be noted that the compliance of the chest actually decreases after surgical correction of the hernia and return of the displaced abdominal organs to the abdomen.198 This paradoxical effect is the result of distending the abdomen and increasing tension on the diaphragm without relief above the diaphragm (i.e., the hypoplastic lung remains unchanged and nondistensible). If the compliance decreases substantively, the outcome may be fatal.
The use of current multimodal therapies presents a special challenge to the anesthesiologist. Transporting a neonate on ECMO and other agents such as inhaled NO remains a dangerous part of the anesthetic care. Machine malfunctions and disconnects can be sudden and catastrophic. In addition, standard anesthesia machines may not be able to deliver special ventilation modes such as high-frequency oscillatory ventilation. Some tertiary care centers possess facilities for the surgical repair to be conducted within the ICU.199 Neonates receiving ECMO may require higher doses of opioids delivered via the extracorporeal circuits because of the increased volume of distribution within the circuit, altered plasma clearance rates, and variable degrees of drug adhesion to the synthetic tubing.200–203
Despite many years of clinical investigation and the introduction of multiple new approaches, the overall survival for neonates with CDH has remained essentially unchanged, with widely varying mortality rates among institutions.204 The optimal therapy for the neonate with CDH is unclear. Survivors often have medical issues that require further treatment after hospital discharge, including gastroesophageal reflux, growth impairment, developmental delay, and sensorineural hearing loss. Additional research and multicenter trials are needed to improve clinical outcomes and advance our understanding of long-term issues associated with survival.205
Congenital Bronchogenic and Pulmonary Cysts
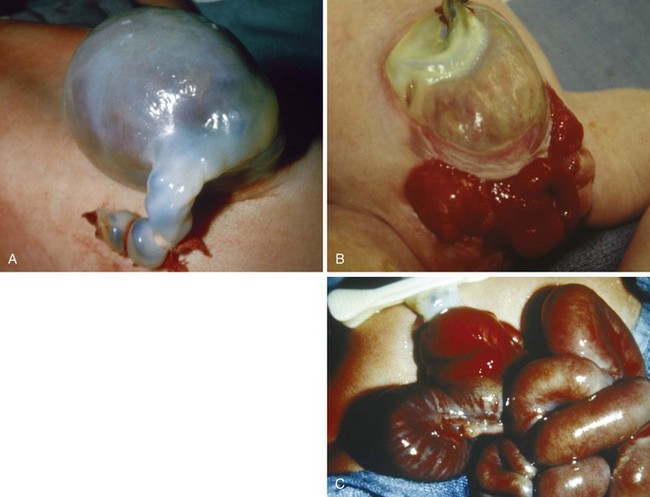
Congenital bronchogenic and pulmonary cysts represent arrests of embryologic pulmonary tissue during early fetal lung development.206 These cysts may be centrally located within the mediastinum and produce obstruction by a mass effect.207 They may also be located at the carina and cause obstruction or distal gas trapping by a ball-valve effect. Those located in the hilum, in the paratracheal region, or in the lung parenchyma may lead to chronic respiratory illness from infection and abscess formation.208,209 Congenital cysts are occasionally diagnosed only after rupture of the cyst produces hemorrhage or bronchopulmonary fistula formation.206,209
Anesthetic management is directed at minimizing further enlargement of the cyst because a communication may exist with the airway. Awake (sedated) intubation or intubation with an inhalation induction, followed by maintenance of spontaneous ventilation, if possible, until the thorax is opened, may reduce the potential for sudden enlargement of the cyst. If assisted ventilation is required, low peak inspiratory pressures should be used. Should the cyst be fluid filled or infected, selective bronchial blocking may be helpful in protecting the unaffected lung.175,176 Nitrous oxide and positive-pressure ventilation without adequate expiratory time should be avoided to decrease the possibility of enlarging the cyst. If these attempts are not successful and the cyst enlarges to the point of occluding the airway or compromising the circulation, needle aspiration to reduce the size of the cyst and facilitate oxygenation and ventilation should be considered. If this method is unsuccessful, emergency thoracostomy may be lifesaving.
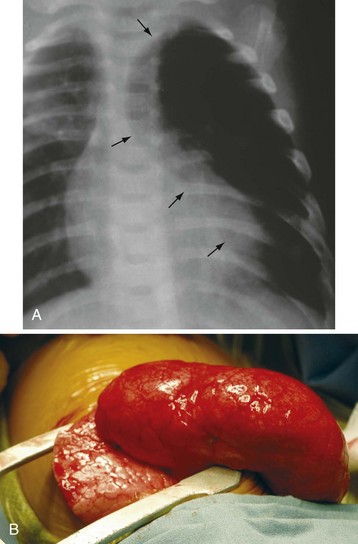
Congenital Lobar Emphysema
Congenital lobar emphysema most commonly affects the left upper lobe but may involve the entire lung (Fig. 36-5).210,211 Congenital heart disease coexists in approximately 15% of infants.212 Patients usually have with progressive respiratory failure, unilateral thoracic hyperexpansion, atelectasis of the contralateral lung, and possibly mediastinal shift with cardiovascular compromise.213 The anesthetic care is focused on minimizing expansion of the emphysema and is similar to that for patients with pulmonary cysts.
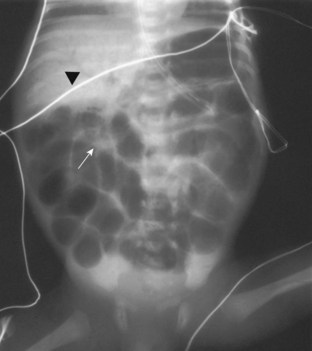
Gastrointestinal Problems
Obstructive lesions may be congenital or acquired. Congenital obstruction of the gastrointestinal tract may be suggested by an abnormal increase in maternal weight, polyhydramnios, fetal size greater than normal for gestational age, and fetal abdominal distention detected by ultrasonography. Neonates with acquired lesions may soon after birth have vomiting, abdominal distention, and late passage of meconium (Fig. 36-6). Associated findings may include aspiration pneumonia, dehydration, hypovolemia, and metabolic abnormalities. Unless life-threatening compromise of organ blood flow occurs, these lesions do not require emergent care. Instead, a priority is to reestablish euvolemia and a metabolically stable state before surgery. Neonates with obstructive lesions usually undergo general anesthesia with tracheal intubation. Induction of general anesthesia may follow an awake intubation if a difficult intubation is anticipated or if active vomiting occurs; a rapid-sequence induction may be used if no airway anomaly is apparent. A rapid-sequence intubation may proceed in a manner similar to that for children and adults. Desaturation after apnea is more rapid, however, because the O2 consumption of a neonate is twice that of an adult and the alveolar stores are reduced. If surgery is an emergency and the intravascular volume status of the neonate is tenuous, ketamine may be used for induction in place of propofol. A stylet within the tracheal tube should be used to facilitate placement during awake intubations but is infrequently needed in neonates with normal airways. If a stylet is used, it may cause injury to the airway if it extends beyond the bevel of the tube. After tracheal intubation, general anesthesia with either an inhalation (without N2O) or opioid technique and neuromuscular blockade is generally used. Air should be blended with O2 to decrease the inspired O2 concentration to a safe concentration. As described earlier, these neonates may have poor renal perfusion; therefore pancuronium and many antibiotics may have prolonged action. In addition, if hepatic blood flow is compromised, metabolism of opioids and muscle relaxants may be delayed.214,215
Specific Lesions
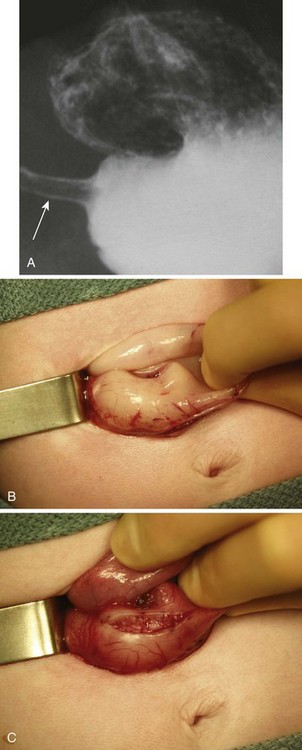
Hypertrophic Pyloric Stenosis
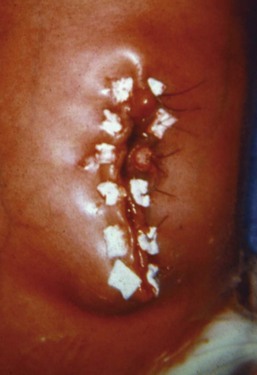
Pyloric stenosis usually manifests within weeks 2 to 6 of life with nonbilious vomiting. This lesion occurs more frequently in first-born males; the incidence is approximately 1 in 500 live births (Fig. 36-7).216 Pyloric stenosis represents hypertrophy of the muscularis layer of the pylorus and can often be palpated as an olive-shaped mass between the midline and right upper quadrant. The lesion is most commonly delineated by ultrasonography217 or rarely with a barium swallow and radiographic examination.
With protracted vomiting, these infants may become hypokalemic, hypochloremic, and alkalotic.218 The renal response to vomiting is twofold. Initially, serum pH is defended by excretion of alkaline urine with sodium and potassium loss; later, with depletion of these electrolytes, the kidneys secrete acidic urine (paradoxical aciduria), further increasing the metabolic alkalosis. Hypocalcemia may be associated with the hyponatremia. With further fluid loss prerenal azotemia may portend hypovolemic shock and metabolic acidosis. Hemoconcentration may result in polycythemia. This lesion does not mandate an emergency surgical procedure; therefore intravascular volume and metabolic stabilization and correction are a priority.
Suctioning an in situ orogastric or nasogastric tube seldom empties the stomach. A freshly inserted wide-bore orogastric tube almost always removes additional residual gastric contents; this step is especially important for infants who have had a barium contrast radiographic study. One study found nearly complete emptying of the gastric contents if the infant’s stomach is suctioned in the right and left lateral as well as the supine positions with a vented catheter (e.g., Salem sump).219 Therefore the stomach is aspirated immediately before induction using a large-bore orogastric tube as described. These infants are most commonly managed with an intravenous induction (although some centers have performed inhalational inductions for more than 30 years for this abdominal defect) because vomiting during induction might result in serious pulmonary aspiration. A rapid-sequence induction is preferred. Intermediate acting NMBDs are infrequently required. However, if the intubation is anticipated to be difficult, a sedated “awake” intubation is carried out before the induction of anesthesia. Inhalation or balanced anesthesia may then be used for maintenance. These surgeries are brief and thus the concentration of inhalational anesthetic must be carefully titrated because these infants are notoriously slow to recover from anesthesia. Infants should be extubated once fully awake and vigorous. Feedings are usually begun soon after surgery, and the postoperative course is generally uncomplicated. Local infiltration of the incision site with a long-acting local anesthetic generally provides complete analgesia.
Some surgeons will perform this procedure laparoscopically or through a periumbilical incision. Laparoscopic techniques may have profound hemodynamic and respiratory effects (see Chapter 27).220,221 One further consideration is the number of case reports describing apnea in this population222; some centers advocate postoperative apnea monitoring.
Duodenal and Ileal Obstruction
Congenital duodenal and ileal obstructions (atresia, stenosis, annular pancreas, meconium ileus) are often associated with other anomalies. For example, 20% to 30% of neonates with duodenal atresia have trisomy 21, with a high likelihood of associated cardiac lesions such as atrial septal defect, ventricular septal defect, or atrioventricular canal. Neonates with intestinal atresia are frequently preterm or may have other associated anomalies, such as malrotation of the gut, volvulus, and abdominal wall defects.223 Meconium ileus occurs in 10% to 15% of neonates with cystic fibrosis. It may present either as terminal ileal obstruction by viscid meconium or as bowel atresia. Hyperosmolar enemas are frequently administered to these infants in an effort to clear the viscid meconium plugs. These enemas may result in serious shifts in intravascular volume, leading to hypovolemia requiring aggressive treatment before anesthetic induction.
Duodenal atresia is associated with bilious vomiting beginning within the first 24 to 48 hours after birth. Radiographs of the abdomen demonstrate the pathognomonic double-bubble sign formed by air contrast of the dilated stomach and proximal duodenum; the remaining bowel is devoid of air (Fig. 36-8). With jejunoileal atresia, air–fluid levels are observed throughout the abdomen. With distal ileal obstruction, a barium enema may demonstrate a microcolon. The typical radiographic presentation of meconium ileus is a soap-bubble mass in the right lower abdomen and absence of air–fluid levels. Initial stabilization of neonates with these lesions is directed at fluid and metabolic resuscitation. Anesthetic management proceeds after suctioning of contrast agent and other stomach contents. Awake intubation may be indicated in volume-depleted or actively vomiting infants; rapid-sequence induction is used in hemodynamically stable infants with normal airway anatomy. N2O should be avoided to minimize intestinal distention. Neuromuscular blockade is generally necessary to facilitate abdominal exploration; use of a neuromuscular blocking agent also decreases or avoids the need for high concentrations of potent inhalation agents, which are poorly tolerated by hypovolemic neonates.
Infantile Hernia
Infantile hernia usually presents within the first 6 months after birth. Although the potential for incarceration through the inguinal canal is present in greater than 90% of neonates, it occurs in only 3% to 5% of full-term infants and 30% of preterm infants.224 With incarceration or strangulation of bowel or gonads, emergency correction is indicated. Incarcerated hernias with or without strangulation should be treated similarly to bowel obstruction. A rapid-sequence or awake intubation should be used at the induction of general anesthesia. An inhalation technique without N2O or an opioid technique may be used after intubation.
Elective repair of a nonincarcerated hernia can be carried out with either general or regional anesthesia when there is no acute bowel obstruction (e.g., a reduced inguinal hernia).225–228 An intravenous line should be placed before anesthetic induction if there is any concern about the volume status. Because blood pressure instability is unusual in infants undergoing spinal anesthesia,229 placement of an intravenous line in the lower extremity may follow the onset of sensory block. Similarly, blood pressure monitoring may be accomplished by using a blood pressure cuff placed on the lower (anesthetized) extremity. Swaddling and a pacifier often are all that is required for sedation.
Spinal anesthesia may be administered through a  -inch, 22-gauge spinal needle (see also Fig. 41-8). Once the block is placed, the infant should be maintained in the supine position. Leg lifting, especially during placement of the electrocautery grounding pad, should be avoided because it has been associated with a high spinal blockade (see also Fig. 41-9).230 Rather, log-roll or lift the entire infant to apply the cautery pad. Apnea or sudden cessation of crying may be the presenting sign of a high spinal blockade in a neonate.
-inch, 22-gauge spinal needle (see also Fig. 41-8). Once the block is placed, the infant should be maintained in the supine position. Leg lifting, especially during placement of the electrocautery grounding pad, should be avoided because it has been associated with a high spinal blockade (see also Fig. 41-9).230 Rather, log-roll or lift the entire infant to apply the cautery pad. Apnea or sudden cessation of crying may be the presenting sign of a high spinal blockade in a neonate.
Because hernia repair is a common operation in former preterm infants, this population is at greatest risk for postoperative life-threatening apnea.19–21,231,232 Outpatient repair of inguinal hernia in former preterm infants is contraindicated in those infants 55 weeks or younger postconceptional age (see also Fig. 4-7 and E-Fig. 4-6). Because apnea may occur even after regional anesthesia, provision for postoperative monitoring must be available.233
Imperforate Anus
Imperforate anus is generally recognized at the initial physical examination or by failure to pass meconium within the first 48 hours of life. Neonates with imperforate anus are likely to have associated anomalies of the urogenital sinus, as well as those associated with the entire spectrum of the VACTERL association.171 Some infants require a decompressive colostomy before definitive surgery. It may be prudent to perform echocardiography to rule out associated congenital heart disease before anesthetic induction. Preductal and postductal O2 saturation determinations may be of value. If these infants exhibit signs of bowel obstruction, they should undergo anesthesia in a manner similar to that in others with obstructive lesions.
Necrotizing Enterocolitis
Necrotizing enterocolitis is not an anomaly but an illness found predominantly in preterm infants.234 The incidence is 5% to 15% in infants less than 1500 grams birth weight, and the mortality rate is 10% to 30%.235 Morbidity associated with necrotizing enterocolitis includes short-bowel syndrome, sepsis, and adhesions associated with bowel obstruction. Necrotizing enterocolitis is associated with birth asphyxia, hypotension, RDS, PDA, recurrent apnea, intestinal ischemia, umbilical vessel cannulation, systemic infections, and early feedings.110,236–238
Initial signs of necrotizing enterocolitis include temperature instability, poor feeding with gastric residuals or vomiting, malabsorption of feedings (positive stool-reducing substances), lethargy, hyperglycemia, and heme-positive or overtly bloody stools. Affected infants may appear very ill and have a distended and tender abdomen. Radiographic examination may initially suggest an ileus with edematous bowel and later demonstrate gas in the intestinal wall (pneumatosis intestinalis) and in the hepatobiliary tract or portal venous system (Fig. 36-9). When perforation has occurred, free air within the abdominal cavity can be appreciated by radiologic examination of the abdomen.
Infants generally have metabolic and hematologic abnormalities, including hyperglycemia, thrombocytopenia, coagulopathy, and anemia. Hypotension, metabolic acidosis, and prerenal azotemia are other significant findings in severe cases. Initial treatment includes discontinuation of enteral feedings and decompression of the abdomen by means of low-pressure continuous nasogastric or orogastric suctioning. Wide-spectrum antibiotics are administered, although no specific bacteria are associated with necrotizing enterocolitis. Dopamine infusions may be required to increase the cardiac output and improve intestinal perfusion. Umbilical arterial lines are replaced with a peripheral arterial line so that mesenteric blood flow is not compromised. Indications for surgery can vary from surgeon to surgeon but may include abdominal viscus perforation resulting in free air, persistence of a bowel loop on serial abdominal radiographs, or persistent metabolic acidosis with clinically important hyperkalemia, indicating the presence of necrotic tissue.239
Adequate preparation before surgery is important. Blood, fresh frozen plasma, and platelets likely will be required. Dopamine infusions may be necessary to improve renal and intestinal perfusion and cardiac output. Patients often are already intubated; otherwise, rapid-sequence or awake intubation is indicated. Because these infants are septic and volume depleted, potent inhalation agents are poorly tolerated and generally avoided. An opioid and muscle relaxant technique, avoiding N2O, is preferred. These infants usually have very large volume requirements; it is common for 1 or more blood volumes of fluid infusion to be required as a result of massive third-space losses. In addition, severe coagulopathy may result in sudden, catastrophic generalized hemorrhage (Fig. 36-10). Platelet transfusion is often needed before surgical incision.
Omphalocele and Gastroschisis
Neonates with omphalocele and gastroschisis have defects in the abdominal wall and may present with impaired blood supply to the herniated organs, intestinal obstruction, and major intravascular fluid deficits. The differences between omphalocele and gastroschisis are summarized in Table 36-4.
| Comparison Factors | Omphalocele | Gastroschisis |
|---|---|---|
| Cause | Failure of gut migration from yolk sac into abdomen | Occlusion of omphalomesenteric artery |
| Location | Within umbilical cord | Periumbilical |
| Associated lesions | Beckwith-Wiedemann syndrome (macroglossia, gigantism, hypoglycemia, hyperviscosity) Congenital heart disease Exstrophy of bladder |
Exposed gut inflammation, edema, dilation, and foreshortened |
Omphalocele represents a failure of the gut to migrate from the yolk sac into the abdomen during gestation (Fig. 36-11, A).240,241 It occurs in 1 in 6000 births.242 Infants with omphalocele may have associated genetic, cardiac, urologic (exstrophy of the bladder; see Fig. 36-11, B), and metabolic abnormalities (Beckwith-Wiedemann syndrome with visceromegaly, hypoglycemia, polycythemia).243 The herniated viscera emerge from the umbilicus and are covered with a membranous sac; the bowel is morphologically and usually functionally normal.
Gastroschisis develops as a result of occlusion of the omphalomesenteric artery during gestation.241,244 It occurs in 1 in 15,000 births and is usually not associated with other congenital anomalies. The herniated viscera and intestines are periumbilical, usually on the right, and are exposed to air after delivery, resulting in inflammation, edema, and dilated, foreshortened, and functionally abnormal bowel (see Fig. 36-11, C ).245, 246
Management of these lesions from birth until surgery is directed at maintaining perfusion to the herniated viscera and reduction of fluid loss from exposed visceral surfaces by covering the mucosal surfaces with sterile, saline-soaked dressings. A plastic wrap further decreases evaporative volume losses and the tendency to develop hypothermia. These anomalies represent a wide spectrum of pathology and require individualized assessment of intravascular volume status and fluid replacement. When these infants arrive in the ICU, fluid resuscitation is instituted; neonates with gastroschisis lose more fluid than those with omphalocoeles and thus require repeat boluses of 20 mL/kg isotonic fluids to replace evaporative and third-space losses.247
Anesthetic management is directed at continued volume resuscitation and measures to prevent hypothermia. Aspiration of stomach contents should be performed. Rapid-sequence induction or awake intubation is carried out. Neuromuscular blockade facilitates reduction of the eviscerated organs and bowel; however, abdominal closure may be associated with markedly increased intraabdominal pressure (E-Fig. 36-4). The effects of increased intraabdominal pressure are twofold: (1) decreased organ perfusion and (2) decreased ventilatory reserve. The increase in intraabdominal pressure may lead to decreased intestinal, renal, and hepatic perfusion and secondarily impaired organ function. This may lead to markedly altered drug metabolism and prolonged drug effect. The bowel may become edematous, and urine output may be reduced as a result of renal congestion. Venous return from the lower body also may be reduced, resulting in lower extremity congestion and cyanosis. Blood pressure and pulse oximetry determinations from a lower extremity may be different from those in the upper extremity. Significantly decreased diaphragmatic function and bilateral lower lobe atelectasis may occur, leading to respiratory failure.248 Transduction of intragastric or bladder pressures may be a diagnostic adjunct.249
If complete reduction is not possible, a staged reduction is carried out. The intestine is covered with a silastic pouch, and the size of the pouch is subsequently reduced in stages either in the operating room or intensive care unit, thus allowing the abdominal cavity gradually to accommodate the increased mass without severely compromising ventilation or organ perfusion.250,251
Malrotation and Midgut Volvulus
Malrotation and midgut volvulus result from abnormal migration or incomplete rotation of the intestines from the yolk sac back into the abdomen.252 Rotation of the intestine around the mesentery may produce the abnormal location of the ileocecal valve in the right upper quadrant and kinking or compression of its vascular supply. If the malrotation occurs during development, atretic segments of bowel are formed. If the kinking or compression occurs after the bowel is normally developed, bowel necrosis may result.
Hirschsprung Disease and Large-Bowel Obstruction
Hirschsprung disease is the most common cause of neonatal colon obstruction and consists of the absence of parasympathetic ganglion cells (Auerbach and Meissner plexus) in the large intestine.253,254 This deficiency creates a nonperistaltic segment of variable length, a tonically contracted anorectal sphincter, and delayed passage of meconium. Functional obstruction occurs at the level of the affected segment. The bowel may occasionally become distended to the point at which its blood supply is compromised; perforation may occur, with resultant peritonitis. If the condition is not recognized and is left untreated, enteric bacteria may invade the bowel wall and subsequently enter the bloodstream, producing the toxic megacolon syndrome. Infants thus affected present with a distended, tender abdomen and hypotension and require massive volume replacement and vasopressor support (Fig. 36-12).
1 West GB, Brown JH, Enquist BJ. A general model for the origin of allometric scaling laws in biology. Science. 1997;276:122–126.
2 Fujiwara T, Maeta H, Chida S, et al. Artificial surfactant therapy in hyaline-membrane disease. Lancet. 1980;1:55–59.
3 Hallman M, Merritt TA, Jarvenpaa AL, et al. Exogenous human surfactant for treatment of severe respiratory distress syndrome: a randomized prospective clinical trial. J Pediatr. 1985;106:963–969.
4 Gitlin JD, Soll RF, Parad RB, et al. Randomized controlled trial of exogenous surfactant for the treatment of hyaline membrane disease. Pediatrics. 1987;79:31–37.
5 Lang MJ, Hall RT, Reddy NS, Kurth CG, Merritt TA. A controlled trial of human surfactant replacement therapy for severe respiratory distress syndrome in very low birth weight infants. J Pediatr. 1990;116:295–300.
6 Soll RF, Hoekstra RE, Fangman JJ, et al. Multicenter trial of single-dose modified bovine surfactant extract (Survanta) for prevention of respiratory distress syndrome. Ross Collaborative Surfactant Prevention Study Group. Pediatrics. 1990;85:1092–1102.
7 Martin RJ, Miller MJ, Carlo WA. Pathogenesis of apnea in preterm infants. J Pediatr. 1986;109:733–741.
8 Joshi A, Gerhardt T, Shandloff P, Bancalari E. Blood transfusion effect on the respiratory pattern of preterm infants. Pediatrics. 1987;80:79–84.
9 DeMaio JG, Harris MC, Deuber C, Spitzer AR. Effect of blood transfusion on apnea frequency in growing premature infants. J Pediatr. 1989;114:1039–1041.
10 Keyes WG, Donohue PK, Spivak JL, Jones MDJ, Oski FA. Assessing the need for transfusion of premature infants and role of hematocrit, clinical signs, and erythropoietin level. Pediatrics. 1989;84:412–417.
11 Kuzemko JA, Paala J. Apnoeic attacks in the newborn treated with aminophylline. Arch Dis Child. 1973;48:404–406.
12 Shannon DC, Gotay F, Stein IM, et al. Prevention of apnea and bradycardia in low-birthweight infants. Pediatrics. 1975;55:589–594.
13 Muttitt SC, Tierney AJ, Finer NN. The dose response of theophylline in the treatment of apnea of prematurity. J Pediatr. 1988;112:115–121.
14 Brouard C, Moriette G, Murat I, et al. Comparative efficacy of theophylline and caffeine in the treatment of idiopathic apnea in premature infants. Am J Dis Child. 1985;139:698–700.
15 Dransfield DA, Fox WW. High incidence of apnea with airway obstruction in recovering premature infants. Pediatr Res. 1980;14:595.
16 Thach BT, Brouillette RT, Abu-Osba YK, Wilson SL, Mathew OP. Prevalence of mixed and obstructive apneic spells in preterm infants. Pediatr Res. 1980;14:637.
17 Milner AD, Boon AW, Saunders RA, Hopkin IE. Upper airways obstruction and apnoea in preterm babies. Arch Dis Child. 1980;55:22–25.
18 Miller MJ, Carlo WA, Martin RJ. Continuous positive airway pressure selectively reduces obstructive apnea in preterm infants. J Pediatr. 1985;106:91–94.
19 Steward DJ. Preterm infants are more prone to complications following minor surgery than are term infants. Anesthesiology. 1982;56:304–306.
20 Gregory GA, Steward DJ. Life-threatening perioperative apnea in the ex-“premie”. Anesthesiology. 1983;59:495–498.
21 Kurth CD, Spitzer AR, Broennle AM, Downes JJ. Postoperative apnea in preterm infants. Anesthesiology. 1987;66:483–488.
22 Spear RM, Deshpande JK, Maxwell LG. Caudal anesthesia in the awake, high-risk infant. Anesthesiology. 1988;69:407–409.
23 Welborn LG, Rice LJ, Hannallah RS, et al. Postoperative apnea in former preterm infants: prospective comparison of spinal and general anesthesia. Anesthesiology. 1990;72:838–842.
24 Cox RG, Goresky GV. Life-threatening apnea following spinal anesthesia in former premature infants. Anesthesiology. 1990;73:345–347.
25 Krane EJ, Haberkern CM, Jacobson LE. Postoperative apnea, bradycardia, and oxygen desaturation in formerly premature infants: prospective comparison of spinal and general anesthesia. Anesth Analg. 1995;80:7–13.
26 Coté CJ, Zaslavsky A, Downes JJ, et al. Postoperative apnea in former preterm infants after inguinal herniorrhaphy: a combined analysis. Anesthesiology. 1995;82:809–822.
27 Grylack LJ, Williams AD. Apparent life-threatening events in presumed healthy neonates during the first three days of life. Pediatrics. 1996;97:349–351.
28 Coté CJ, Kelly DH. Postoperative apnea in a full-term infant with a demonstrable respiratory pattern abnormality. Anesthesiology. 1990;72:559–561.
29 Tetzlaff JE, Annand DW, Pudimat MA, Nicodemus HF. Postoperative apnea in a full-term infant. Anesthesiology. 1988;69:426–428.
30 Noseworthy J, Duran C, Khine HH. Postoperative apnea in a full-term infant. Anesthesiology. 1989;70:879–880.
31 Welborn LG, Hannallah RS, Luban NLC, Fink R, Ruttimann UE. Anemia and postoperative apnea in former preterm infants. Anesthesiology. 1991;74:1003–1006.
32 Rodenstein DO, Perlmutter N, Stanescu DC. Infants are not obligatory nasal breathers. Am Rev Respir Dis. 1985;131:343–347.
33 Miller MJ, Carlo WA, Strohl KP, Fanaroff AA, Martin RJ. Effect of maturation on oral breathing in sleeping premature infants. J Pediatr. 1986;109:515–519.
34 Badgwell JM, Swan J, Foster AC. Volume-controlled ventilation is made possible in infants by using compliant breathing circuits with large compression volume. Anesth Analg. 1996;82:719–723.
35 Stevenson GW, Tobin M, Horn B, et al. An adult system versus a Bain system: comparative ability to deliver minute ventilation to an infant lung model with pressure-limited ventilation. Anesth Analg. 1999;88:527–530.
36 Tobin MJ, Stevenson GW, Horn BJ, et al. A comparison of three modes of ventilation with the use of an adult circle system in an infant lung model. Anesth Analg. 1998;87:766–771.
37 Kinsella JP, Greenough A, Abman SH. Bronchopulmonary dysplasia. Lancet. 2006;367:1421–1431.
38 Cordero L, Coley BD, Miller RL, Mueller CF. Bacterial and Ureaplasma colonization of the airway: radiologic findings in infants with bronchopulmonary dysplasia. J Perinatol. 1997;17:428–433.
39 Coalson JJ. Pathology of new bronchopulmonary dysplasia. Semin Neonatol. 2003;8:73–81.
40 Jobe AH, Ikegami M. Mechanisms initiating lung injury in the preterm. Early Hum Dev. 1998;53:81–94.
41 Baraldi E, Filippone M, Trevisanuto D, Zanardo V, Zacchello F. Pulmonary function until two years of life in infants with bronchopulmonary dysplasia. Am J Respir Crit Care Med. 1997;155:149–155.
42 Calder NA, Williams BA, Smyth J, et al. Absence of ventilatory responses to alternating breaths of mild hypoxia and air in infants who have had bronchopulmonary dysplasia: implications for the risk of sudden infant death. Pediatr Res. 1994;35:677–681.
43 Cano A, Payo F. Lung function and airway responsiveness in children and adolescents after hyaline membrane disease: a matched cohort study. Eur Respir J. 1997;10:880–885.
44 Carey BE, Trotter C. Bronchopulmonary dysplasia. Neonatal Netw. 1996;15:73–77.
45 Chernick V. Long-term pulmonary function studies in children with bronchopulmonary dysplasia: an ever-changing saga. J Pediatr. 1998;133:171–172.
46 Giacoia GP, Venkataraman PS, West-Wilson KI, Faulkner MJ. Follow-up of school-age children with bronchopulmonary dysplasia. J Pediatr. 1997;130:400–408.
47 Roberts JD, Jr., Roberts CT, Jones RC, Zapol WM, Bloch KD. Continuous nitric oxide inhalation reduces pulmonary arterial structural changes, right ventricular hypertrophy, and growth retardation in the hypoxic newborn rat. Circ Res. 1995;76:215–222.
48 Roberts JD, Jr., Chiche JD, Weimann J, et al. Nitric oxide inhalation decreases pulmonary artery remodeling in the injured lungs of rat pups. Circ Res. 2000;87:140–145.
49 Bland RD, Albertine KH, Carlton DP, MacRitchie AJ. Inhaled nitric oxide effects on lung structure and function in chronically ventilated preterm lambs. Am J Respir Crit Care Med. 2005;172:899–906.
50 McCurnin DC, Pierce RA, Chang LY, et al. Inhaled NO improves early pulmonary function and modifies lung growth and elastin deposition in a baboon model of neonatal chronic lung disease. Am J Physiol Lung Cell Mol Physiol. 2005;288:L450–L459.
51 Cole FS, Alleyne C, Barks JD, et al. NIH Consensus Development Conference: Inhaled Nitric Oxide Therapy for Premature Infants. NIH Consens State Sci Statements. 2010:27.
52 Nakanishi H, Sugiura T, Streisand JB, Lonning SM, Roberts JD, Jr. TGF-β neutralizing antibodies improve pulmonary alveologenesis and vasculogenesis in the injured newborn lung. Am J Physiol Lung Cell Mol Physiol. 2007.
53 Rush MG, Hazinski TA. Current therapy of bronchopulmonary dysplasia. Clin Perinatol. 1992;19:563–590.
54 Kari MA, Heinonen K, Ikonen RS, Koivisto M, Raivio KO. Dexamethasone treatment in preterm infants at risk for bronchopulmonary dysplasia. Arch Dis Child. 1993;68:566–569.
55 Rastogi A, Akintorin SM, Bez ML, Morales P, Pildes RS. A controlled trial of dexamethasone to prevent bronchopulmonary dysplasia in surfactant-treated infants. Pediatrics. 1996;98:204–210.
56 Brand PL, van Lingen RA, Brus F, Talsma MD, Elzenga NJ. Hypertrophic obstructive cardiomyopathy as a side effect of dexamethasone treatment for bronchopulmonary dysplasia. Acta Paediatr. 1993;82:614–617.
57 Haney I, Lachance C, van Doesburg NH, Fouron JC. Reversible steroid-induced hypertrophic cardiomyopathy with left ventricular outflow tract obstruction in two newborns. Am J Perinatol. 1995;12:271–274.
58 Bishara N, Ohls RK. Current controversies in the management of the anemia of prematurity. Semin Perinatol. 2009;33:29–34.
59 Friedman WF. The intrinsic physiologic properties of the developing heart. In: Friedman WF, Lesch M, Sonnenblick EH, eds. Neonatal heart disease. Philadelphia: Grune & Stratton; 1973:21–49.
60 Heymann MA, Rudolph AM. Effects on increasing preload on right ventricular output in fetal lambs in utero. Circulation. 1973;48:IV37.
61 Kirkpatrick SE, Pitlick PT, Naliboff J, Friedman WF. Frank-Starling relationship as an important determinant of fetal cardiac output. Am J Physiol. 1976;231:495–500.
62 Brinkman CR, Johnson GH, Assali NS. Hemodynamic effects of bradycardia in the fetal lamb. Am J Physiol. 1972;223:1465–1469.
63 Southall DP, Richards JM, Johnstone PGB, Shinebourne EA. Study of cardiac rhythm in healthy newborn infant. Br Heart J. 1979;41:382.
64 Walker AM, Cannata J, Dowling MH, Ritchie B, Maloney JE. Sympathetic and parasympathetic control of heart rate in unanaesthetized fetal and newborn lambs. Biol Neonate. 1978;33:135–143.
65 Vapaavouri EK, Shinebourne EA, Williams RL, Heymann MA, Rudolph AM. Development of cardiovascular responses to autonomic blockade in intact fetal and neonatal lambs. Biol Neonate. 1973;22:177–188.
66 Leigh MD, McCoy DD, Belton MK, Lewis GB, Jr. Bradycardia following intravenous administration of succinylcholine chloride to infants and children. Anesthesiology. 1957;18:698–702.
67 Starr NJ, Sethna DH, Estafanous FG. Bradycardia and asystole following the rapid administration of sufentanil with vecuronium. Anesthesiology. 1986;64:521–523.
68 Dawes GS, Mott JC, Widdicombe JG. The foetal circulation in the lamb. J Physiol. 1954;126:563–587.
69 Lauer RM, Evans JA, Aoki H, Kittle CF. Factors controlling pulmonary vascular resistance in fetal lambs. J Pediatr. 1965;67:568–577.
70 Siassi B, Blanco C, Cabal LA, Coran AG. Incidence and clinical features of patent ductus arteriosus in low-birthweight infants: a prospective analysis of 150 consecutively born infants. Pediatrics. 1976;57:347–351.
71 Gersony WM, Peckham GJ, Ellison RC, Miettinen OS, Nadas AS. Effects of indomethacin in premature infants with patent ductus arteriosus: results of a national collaborative study. J Pediatr. 1983;102:895–906.
72 Hammerman C, Aramburo MJ. Prolonged indomethacin therapy for the prevention of recurrences of patent ductus arteriosus. J Pediatr. 1990;117:771–776.
73 Wessel DL, Keane JF, Parness I, Lock JE. Outpatient closure of the patent ductus arteriosus. Circulation. 1988;77:1068–1071.
74 Bell EF, Warburton D, Stonestreet BS, Oh W. Effect of fluid administration on the development of symptomatic patent ductus arteriosus and congestive heart failure in premature infants. N Engl J Med. 1980;302:598–604.
75 Haworth SG, Reid L. Persistent fetal circulation: newly recognized structural features. J Pediatr. 1976;88:614–620.
76 Roberts JD, Jr., Shaul PW. Advances in the treatment of persistent pulmonary hypertension of the newborn. Pediatr Clin North Am. 1993;40:983–1004.
77 Drummond WH, Gregory GA, Heymann MA, Phibbs RA. The independent effects of hyperventilation, tolazoline, and dopamine on infants with persistent pulmonary hypertension. J Pediatr. 1981;98:603–611.
78 Roberts JD, Jr., Chiche J-D, Weimann J, et al. Inhaled nitric oxide gas (NO) prevents pulmonary artery neovascularization in rat pups without pulmonary hypertension. Circulation. 1999;98:I-341.
79 Kinsella JP, Neish SR, Shaffer E, Abman SH. Low-dose inhalation nitric oxide in persistent pulmonary hypertension of the newborn. Lancet. 1992;340:819–820.
80 Roberts JD, Polaner DM, Lang P, Zapol WM. Inhaled nitric oxide in persistent pulmonary hypertension of the newborn. Lancet. 1992;340:818–821.
81 Roberts JD, Jr., Fineman JR, Morin FC, 3rd., et al. Inhaled nitric oxide and persistent pulmonary hypertension of the newborn. The Inhaled Nitric Oxide Study Group. N Engl J Med. 1997;336:605–610.
82 Davidson D, Barefield ES, Kattwinkel J, et al. Inhaled nitric oxide for the early treatment of persistent pulmonary hypertension of the term newborn: a randomized, double-masked, placebo-controlled, dose-response, multicenter study. The I-NO/PPHN Study Group. Pediatrics. 1998;101:325–334.
83 Kinsella JP, Truog WE, Walsh WF, et al. Randomized, multicenter trial of inhaled nitric oxide and high-frequency oscillatory ventilation in severe, persistent pulmonary hypertension of the newborn. J Pediatr. 1997;131:55–62.
84 O’Rourke PP, Crone RK, Vacanti JP, et al. Extracorporeal membrane oxygenation and conventional medical therapy in neonates with persistent pulmonary hypertension of the newborn: a prospective randomized study. Pediatrics. 1989;84:957–963.
85 Fugate JH, Ryan DP. Extracorporeal membrane oxygenation. Prob Anesth. 1989;3:271–287.
86 Dworetz AR, Moya FR, Sabo B, Gladstone I, Gross I. Survival of infants with persistent pulmonary hypertension without extracorporeal membrane oxygenation. Pediatrics. 1989;84:1–6.
87 Garg UC, Hassid A. Nitric oxide-generating vasodilators and 8-bromo-cyclic guanosine monophosphate inhibit mitogenesis and proliferation of cultured rat vascular smooth muscle cells. J Clin Invest. 1989;83:1774–1777.
88 Sarkar R, Gordon D, Stanley JC, Webb RC. Cell cycle effects of nitric oxide on vascular smooth muscle cells. Am J Physiol. 1997;272:t-8.
89 Ishida A, Sasaguri T, Kosaka C, Nojima H, Ogata J. Induction of the cyclin-dependent kinase inhibitor p21(Sdi1/Cip1/Waf1) by nitric oxide-generating vasodilator in vascular smooth muscle cells. J Biol Chem. 1997;272:10050–10057.
90 Guo K, Andres V, Walsh K. Nitric oxide-induced downregulation of Cdk2 activity and cyclin A gene transcription in vascular smooth muscle cells. Circulation. 1998;97:2066–2072.
91 Sola A. Oxygen in neonatal anesthesia: friend or foe? Curr Opin Anaesthesiol. 2008;21:332–339.
92 Kattwinkel J, Perlman JM, Aziz K, et al. Part 15: neonatal resuscitation: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122:S909–S919.
93 Marks KH, Devenyi AG, Bello ME, et al. Thermal head wrap for infants. J Pediatr. 1985;107:956–959.
94 Stothers JK. Head insulation and heat loss in the newborn. Arch Dis Child. 1981;56:530–534.
95 Schiff D, Stern L, Leduc J. Chemical thermogenesis in newborn infants: catecholamine excretion and the plasma non-esterified fatty acid response to cold exposure. Pediatrics. 1966;37:577–582.
96 Goudsouzian NG, Morris RH, Ryan JF. The effects of a warming blanket on the maintenance of body temperatures in anesthetized infants and children. Anesthesiology. 1973;39:351–353.
97 Hey E. Thermal neutrality. Br Med Bull. 1975;31:69–74.
98 Murat I, Berniere J, Constant I. Evaluation of the efficacy of a forced-air warmer (Bair Hugger) during spinal surgery in children. J Clin Anesth. 1994;6:425–429.
99 Arant BSJ. Developmental patterns of renal functional maturation compared in the human neonate. J Pediatr. 1978;92:705–712.
100 Rhodin MM, Anderson BJ, Peters AM, et al. Human renal function maturation: a quantitative description using weight and postmenstrual age. Pediatr Nephrol. 2009;24:67–76.
101 Siegel SR, Oh W. Renal function as a marker of human fetal maturation. Acta Paediatr Scand. 1976;65:481–485.
102 Ross B, Cowett RM, Oh W. Renal functions of low birth weight infants during the first two months of life. Pediatr Res. 1977;11:1162–1164.
103 Hill LL. Body composition, normal electrolyte concentrations, and the maintenance of normal volume, tonicity, and acid-base metabolism. Pediatr Clin North Am. 1990;37:241–256.
104 Siegel SR, Fisher DA, Oh W. Serum aldosterone concentrations related to sodium balance in the newborn infant. Pediatrics. 1974;53:410–413.
105 Fanaroff AA, Wald M, Gruber HS, Klaus MH. Insensible water loss in low birth weight infants. Pediatrics. 1972;50:236–245.
106 Abdulla F, Dietrich KA, Pramanik AK. Percutaneous femoral venous catheterization in preterm neonates. J Pediatr. 1990;117:788–791.
107 Kanter RK, Gorton JM, Palmieri K, Tompkins JM, Smith F. Anatomy of femoral vessels in infants and guidelines for venous catheterization. Pediatrics. 1989;83:1020–1022.
108 Dolcourt JL, Bose CL. Percutaneous insertion of Silastic central venous catheters in newborn infants. Pediatrics. 1982;70:484–486.
109 Asnes RS, Arendar GM. Septic arthritis of the hip: a complication of femoral venipuncture. Pediatrics. 1966;38:837–841.
110 O’Neill JAJ, Neblett WW, Born ML. Management of major thromboembolic complications of umbilical artery catheters. J Pediatr Surg. 1981;16:972–978.
111 Neal WA, Reynolds JW, Jarvis CW, Williams HJ. Umbilical artery catheterization: demonstration of arterial thrombosis by aortography. Pediatrics. 1972;50:6–13.
112 Goetzman BW, Stadalnik RC, Bogren HG, et al. Thrombotic complications of umbilical artery catheters: a clinical and radiographic study. Pediatrics. 1975;56:374–379.
113 Fluge G. Clinical aspects of neonatal hypoglycaemia. Acta Paediatr Scand. 1974;63:826–832.
114 Pildes R, Forbes AE, O’Connor SM, Cornblath M. The incidence of neonatal hypoglycemia: a completed survey. J Pediatr. 1967;70:76–80.
115 Srinivasan G, Pildes RS, Cattamanchi G, Voora S, Lilien LD. Plasma glucose values in normal neonates: a new look. J Pediatr. 1986;109:114–117.
116 Papile LA, Burstein J, Burstein R, Koffler H, Koops B. Relationship of intravenous sodium bicarbonate infusions and cerebral intraventricular hemorrhage. J Pediatr. 1978;93:834–836.
117 Srinivasan G, Jain R, Pildes RS, Kannan CR. Glucose homeostasis during anesthesia and surgery in infants. J Pediatr Surg. 1986;21:718–721.
118 Anand KJ, Brown MJ, Bloom SR, Aynsley-Green A. Studies on the hormonal regulation of fuel metabolism in the human newborn infant undergoing anaesthesia and surgery. Horm Res. 1985;22:115–128.
119 Anand KJ, Hickey PR. Pain and its effects in the human neonate and fetus. N Engl J Med. 1987;317:1321–1329.
120 Anand KJ, Hansen DD, Hickey PR. Hormonal-metabolic stress responses in neonates undergoing cardiac surgery. Anesthesiology. 1990;73:661–670.
121 Lynch RE. Ionized calcium: pediatric perspective. Pediatr Clin North Am. 1990;37:373–389.
122 Munoz R, Khilnani P, Ziegler J, et al. Ultrafilterable hypomagnesemia in neonates admitted to the neonatal intensive care unit. Crit Care Med. 1994;22:815–820.
123 Cardenas-Rivero N, Chernow B, Stoiko MA, Nussbaum SR, Todres ID. Hypocalcemia in critically ill children. J Pediatr. 1989;114:946–951.
124 Kosloske AM. A unifying hypothesis for pathogenesis and prevention of necrotizing enterocolitis. J Pediatr. 1990;117:S68–S74.
125 Dunn L, Hulman S, Weiner J, Kliegman R. Beneficial effects of early hypocaloric enteral feeding on neonatal gastrointestinal function: preliminary report of a randomized trial. J Pediatr. 1988;112:622–629.
126 Slagle TA, Gross SJ. Effect of early low-volume enteral substrate on subsequent feeding tolerance in very low birth weight infants. J Pediatr. 1988;113:526–531.
127 Gartner LM, Snyder RN, Chabon RS, Bernstein J. Kernicterus: high incidence in premature infants with low serum bilirubin concentrations. Pediatrics. 1970;45:906–917.
128 Lovejoy FHJ. Fatal benzyl alcohol poisoning in neonatal intensive care units: a new concern for pediatricians. Am J Dis Child. 1982;136:974–975.
129 Koehntop DE, Rodman JH, Brundage DM, Hegland MG, Buckley JJ. Pharmacokinetics of fentanyl in neonates. Anesth Analg. 1986;65:227–232.
130 Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr. 1978;92:529–534.
131 Lou HC, Lassen NA, Friis-Hansen B. Impaired autoregulation of cerebral blood flow in the distressed newborn infant. J Pediatr. 1979;94:118–121.
132 Lou HC, Lassen NA, Friis-Hansen B. Impaired autoregulation of cerebral blood flow in the distressed newborn infant. J Pediatr. 1979;94:118–121.
133 Charlton AJ, Greenhough SG. Blood pressure response of neonates to tracheal intubation. Anaesthesia. 1988;43:744–746.
134 Tyszczuk L, Meek J, Elwell C, Wyatt JS. Cerebral blood flow is independent of mean arterial blood pressure in preterm infants undergoing intensive care. Pediatrics. 1998;102:337–341.
135 Bridenbaugh PO, Greene NM. Spinal (subarachnoid) neural blockade. In: Cousins MJ, Bridenbaugh PO, eds. Neural blockade in clinical anesthesia and management of pain. 2nd ed. Philadelphia: JB Lippincott; 1988:213–252.
136 Lups S, Haan AMFH, Bailey P. The cerebrospinal fluid. Amsterdam: Elsevier; 1954.
137 Lucey JF, Dangman B. A reexamination of the role of oxygen in retrolental fibroplasia. Pediatrics. 1984;73:82–96.
138 Purohit DM, Ellison RC, Zierler S, Miettinen OS, Nadas AS. Risk factors for retrolental fibroplasia: experience with 3,025 premature infants. National Collaborative Study on Patent Ductus Arteriosus in Premature Infants. Pediatrics. 1985;76:339–344.
139 Rubaltelli DM, Hirose T. Retinopathy of prematurity update. Int Ophthalmol Clin. 2008;48:225–235.
140 Betts EK, Downes JJ, Schaffer DB, Johns R. Retrolental fibroplasia and oxygen administration during general anesthesia. Anesthesiology. 1977;47:518–520.
141 Merritt JC, Sprague DH, Merritt WE, Ellis RA. Retrolental fibroplasia: a multifactorial disease. Anesth Analg. 1981;60:109–111.
142 Bardin C, Zelkowitz P, Papageorgiou A. Outcome of small-for-gestational age and appropriate-for-gestational age infants born before 27 weeks of gestation. Pediatrics. 1997;100:E4.
143 Bossi E, Koerner F. Retinopathy of prematurity. Intensive Care Med. 1995;21:241–246.
144 Dobson V, Quinn GE. Retinopathy of prematurity. Optom Clin. 1996;5:105–124.
145 Hesse L, Eberl W, Schlaud M, Poets CF. Blood transfusion: iron load and retinopathy of prematurity. Eur J Pediatr. 1997;156:465–470.
146 Holmes JM, Duffner LA, Kappil JC. The effect of raised inspired carbon dioxide on developing rat retinal vasculature exposed to elevated oxygen. Curr Eye Res. 1994;13:779–782.
147 Holmes JM, Zhang S, Leske DA, Lanier WL. Carbon dioxide-induced retinopathy in the neonatal rat. Curr Eye Res. 1998;17:608–616.
148 Inder TE, Clemett RS, Austin NC, Graham P, Darlow BA. High iron status in very low birth weight infants is associated with an increased risk of retinopathy of prematurity. J Pediatr. 1997;131:541–544.
149 Keith CG, Doyle LW. Retinopathy of prematurity in extremely low birth weight infants. Pediatrics. 1995;95:42–45.
150 Carlo WA, Finer NN, Walsh MC, et al. Target ranges of oxygen saturation in extremely preterm infants. N Engl J Med. 2010;362:1959–1969.
151 Stenson B, Brocklehurst P, Tarnow-Mordi W. Increased 36-week survival with high oxygen saturation target in extremely preterm infants. N Engl J Med. 2011;364:1680–1682.
152 Coté CJ, Rolf N, Liu LM, et al. A single-blind study of combined pulse oximetry and capnography in children. Anesthesiology. 1991;74:980–987.
153 Bucher HU, Fanconi S, Baeckert P, Duc G. Hyperoxemia in newborn infants: detection by pulse oximetry. Pediatrics. 1989;84:226–230.
154 Badgwell JM, McLeod ME, Lerman J, Creighton RE. End-tidal PCO2 measurements sampled at the distal and proximal ends of the endotracheal tube in infants and children. Anesth Analg. 1987;66:959–964.
155 Rich GF, Sullivan MP, Adams JM. Is distal sampling of end-tidal CO2 necessary in small subjects? Anesthesiology. 1990;73:265–268.
156 Burrows FA. Physiologic dead space, venous admixture, and the arterial to end-tidal carbon dioxide difference in infants and children undergoing cardiac surgery. Anesthesiology. 1989;70:219–225.
157 Erkan V, Blankenship W, Stahlman MT. The complications of chronic umbilical vessel catheterization. Pediatr Res. 1968;2:317.
158 Brans YW, Ceballos R, Cassady G. Umbilical catheters and hepatic abscesses. Pediatrics. 1974;53:264–266.
159 Stevenson GW, Tobin MJ, Horn BJ, et al. The effect of circuit compliance on delivered ventilation with use of an adult circle system for time cycled volume controlled ventilation using an infant lung model. Paediatr Anaesth. 1998;8:139–144.
160 Beligere N, Caldarelli D, Pruzansky S. Bilateral congenital choanal atresia and absence of respiratory distress. Cleft Palate J. 1976;13:342–349.
161 Hall BD. Choanal atresia and associated multiple anomalies. J Pediatr. 1979;95:395–398.
162 Ferguson JL, Neel HB. Choanal atresia: treatment trends in 47 patients over 33 years. Ann Otol Rhinol Laryngol. 1989;98:110–112.
163 Motoyama EK, Brinkmeyer SD, Mutich RL, Walczak SA. Reduced FRC in anesthetized infants: effect of low PEEP. Anesthesiology. 1982;57:A418.
164 Tusiewicz K, Bryan AC, Froese AB. Contributions of changing rib cage: diaphragm interactions to the ventilatory depression of halothane anesthesia. Anesthesiology. 1977;47:327–337.
165 Holinger PH, Brown WT. Congenital webs, cysts, laryngoceles and other anomalies of the larynx. Ann Otol Rhinol Laryngol. 1967;76:744–752.
166 Coté CJ, Eavey RD, Todres ID, Jones DE. Cricothyroid membrane puncture: oxygenation and ventilation in a dog model using an intravenous catheter. Crit Care Med. 1988;16:615–619.
167 Othersen HBJ. The technique of intraluminal stenting and steroid administration in the treatment of tracheal stenosis in children. J Pediatr Surg. 1974;9:683–690.
168 Lampe I, Latourette HB. Management of hemangiomas in infants. Pediatr Clin North Am. 1959;6:511–528.
169 Leikensohn JR, Benton C, Cotton R. Subglottic hemangioma. J Otolaryngol. 1976;5:487–492.
170 Barry JE, Auldist AW. The VATER association: one end of a spectrum of anomalies. Am J Dis Child. 1974;128:769–771.
171 Rittler M, Paz JE, Castilla EE. VACTERL association, epidemiologic definition and delineation. Am J Med. Gen. 1996;63:529–536.
172 Broemling N, Campbell F. Anesthetic management of congenital tracheoesophageal fistula. Pediatr Anesth. 2011;21:1092–1099.
173 Knottenbelt G, Costi D, Stephens P, Beringer R, Davidson A. An audit of anesthetic management and complications of tracheo-esophageal fistula and esophageal atresia repair. Pediatr Anesth. 2012;22:268–274.
174 Karl HW. Control of life-threatening air leak after gastrostomy in an infant with respiratory distress syndrome and tracheoesophageal fistula. Anesthesiology. 1985;62:670–672.
175 Hogg CE, Lorhan PH. Pediatric bronchial blocking. Anesthesiology. 1970;33:560–562.
176 Rao CC, Krishna G, Grosfeld JL, Weber TR. One-lung pediatric anesthesia. Anesth Analg. 1981;60:450–452.
177 Hammer GB. Single-lung ventilation in infants and children. Paediatr Anaesth. 2004;14:98–102.
178 Torfs CP, Curry CJ, Bateson TF, Honore LH. A population-based study of congenital diaphragmatic hernia. Teratology. 1992;46:555–565.
179 Langham MR, Jr., Kays DW, Ledbetter DJ, et al. Congenital diaphragmatic hernia: epidemiology and outcome. Clin Perinatol. 1996;23:671–688.
180 Adzick NS, Harrison MR, Glick PL, et al. Diaphragmatic hernia in the fetus: prenatal diagnosis and outcome in 94 cases. J Pediatr Surg. 1985;20:357–361.
181 Onal E, Lopata M, O’Connor TD. Diaphragmatic and genioglossal electromyogram responses to isocapnic hypoxia in humans. Am Rev Respir Dis. 1981;124:215–217.
182 Deprest J, Jani J, Van SD, et al. Current consequences of prenatal diagnosis of congenital diaphragmatic hernia. J Pediatr Surg. 2006;41:423–430.
183 Harrison MR, Sydorak RM, Farrell JA, et al. Fetoscopic temporary tracheal occlusion for congenital diaphragmatic hernia: prelude to a randomized, controlled trial. J Pediatr Surg. 2003;38:1012–1020.
184 Harrison MR, Keller RL, Hawgood SB, et al. A randomized trial of fetal endoscopic tracheal occlusion for severe fetal congenital diaphragmatic hernia. N Engl J Med. 2003;349:1916–1924.
185 Deprest J, Jani J, Cannie M, et al. Prenatal intervention for isolated congenital diaphragmatic hernia. Curr Opin Obstet Gynecol. 2006;18:203–215.
186 Van MK, Lou SB. Congenital diaphragmatic hernia: the neonatologist’s perspective. Pediatr Rev. 1999;20:e79–e87.
187 Wung JT, Sahni R, Moffitt ST, Lipsitz E, Stolar CJ. Congenital diaphragmatic hernia: survival treated with very delayed surgery, spontaneous respiration, and no chest tube. J Pediatr Surg. 1995;30:406–409.
188 Sigalet DL, Tierney A, Adolph V, et al. Timing of repair of congenital diaphragmatic hernia requiring extracorporeal membrane oxygenation support. J Pediatr Surg. 1995;30:1183–1187.
189 Clark RH, Hardin WD, Jr., Hirschl RB, et al. Current surgical management of congenital diaphragmatic hernia: a report from the Congenital Diaphragmatic Hernia Study Group. J Pediatr Surg. 1998;33:1004–1009.
190 Wilson JM, Thompson JR, Schnitzer JJ, et al. Intratracheal pulmonary ventilation and congenital diaphragmatic hernia: a report of two cases. J Pediatr Surg. 1993;28:484–487.
191 Harrington J, Raphaely RC, Downes JJ. Relationship of alveolar-arterial oxygen tension difference in diaphragmatic hernia of the newborn. Anesthesiology. 1982;56:473–476.
192 Bohn DJ, James I, Filler RM, et al. The relationship between PaCO2 and ventilation parameters in predicting survival in congenital diaphragmatic hernia. J Pediatr Surg. 1984;19:666–671.
193 Reyes C, Chang LK, Waffarn F, et al. Delayed repair of congenital diaphragmatic hernia with early high-frequency oscillatory ventilation during preoperative stabilization. J Pediatr Surg. 1998;33:1010–1014.
194 Clark RH, Slutsky AS, Gerstmann DR. Lung protective strategies of ventilation in the neonate: what are they? Pediatrics. 2000;105:112–114.
195 Boloker J, Bateman DA, Wung JT, Stolar CJ. Congenital diaphragmatic hernia in 120 infants treated consecutively with permissive hypercapnea/spontaneous respiration/elective repair. J Pediatr Surg. 2002;37:357–366.
196 Ford JW. Neonatal ECMO: current controversies and trends. Neonatal Netw. 2006;25:229–238.
197 Bahrami KR, Van Meurs KP. ECMO for neonatal respiratory failure. Semin Perinatol. 2005;29:15–23.
198 Sakai H, Tamura M, Hosokawa Y, et al. Effect of surgical repair on respiratory mechanics in congenital diaphragmatic hernia. J Pediatr. 1987;111:432–438.
199 Lago P, Meneghini L, Chiandetti L, et al. Congenital diaphragmatic hernia: intensive care unit or operating room? Am. J Perinatol. 2005;22:189–197.
200 Geiduschek JM, Lynn AM, Bratton SL, et al. Morphine pharmacokinetics during continuous infusion of morphine sulfate for infants receiving extracorporeal membrane oxygenation. Crit Care Med. 1997;25:360–364.
201 Buck ML. Pharmacokinetic changes during extracorporeal membrane oxygenation: implications for drug therapy of neonates. Clin.Pharmacokinet. 2003;42:403–417.
202 Peters JW, Anderson BJ, Simons SH, Uges DR, Tibboel D. Morphine pharmacokinetics during venoarterial extracorporeal membrane oxygenation in neonates. Intensive Care Med. 2005;31:257–263.
203 Peters JW, Anderson BJ, Simons SH, Uges DR, Tibboel D. Morphine metabolite pharmacokinetics during venoarterial extra corporeal membrane oxygenation in neonates. Clin Pharmacokinet. 2006;45:705–714.
204 Irish MS, Holm BA, Glick PL. Congenital diaphragmatic hernia: a historical review. Clin Perinatol. 1996;23:625–653.
205 Moya FR, Lally KP. Evidence-based management of infants with congenital diaphragmatic hernia. Semin Perinatol. 2005;29:112–117.
206 Kirwan WO, Walbaum PR, McCormack RJ. Cystic intrathoracic derivatives of the foregut and their complications. Thorax. 1973;28:424–428.
207 Eraklis AJ, Griscom NT, McGovern JB. Bronchogenic cysts of the mediastinum in infancy. N Engl J Med. 1969;281:1150–1155.
208 Buntain WL, Isaacs HJ, Payne VCJ, Lindesmith GG, Rosenkrantz JG. Lobar emphysema, cystic adenomaloid malformation, pulmonary sequestration, and bronchogenic cyst in infancy and childhood: a clinical group. J Pediatr Surg. 1974;9:85–93.
209 Crawford TJ, Cahill JL. The surgical treatment of pulmonary cystic disorders in infancy and childhood. J Pediatr Surg. 1971;6:251–255.
210 Murray GF. Congenital lobar emphysema. Surg Gynecol Obstet. 1967;124:611–625.
211 Hendren WH, McKee DM. Lobar emphysema of infancy. J Pediatr Surg. 1966;1:24–39.
212 Pierce WS, DeParedes CG, Friedman S, Waldhausen JA. Concomitant congenital heart disease and lobar emphysema in infants: incidence, diagnosis, and operative management. Ann Surg. 1970;172:951–956.
213 Coté CJ. The anesthetic management of congenital lobar emphysema. Anesthesiology. 1978;49:296–298.
214 Meretoja OA. Is vecuronium a long-acting neuromuscular blocking agent in neonates and infants? Br J Anaesth. 1989;62:184–187.
215 Koehntop DE, Rodman JH, Brundage DM, Hegland MG, Buckley JJ. Pharmacokinetics of fentanyl in neonates. Anesth Analg. 1986;65:227–232.
216 Guzzetta PC, Randolph JG, Anderson KD, Boyajian M, Eichelberger M. Surgery of the neonate. In: Avery GB, ed. Neonatology: pathophysiology and management of the newborn. Philadelphia: JB Lippincott; 1987:944–984.
217 Tunell WP, Wilson DA. Pyloric stenosis: diagnosis by real time sonography, the pyloric muscle length method. J Pediatr Surg. 1984;19:795–799.
218 Touloukian RJ, Higgins E. The spectrum of serum electrolytes in hypertrophic pyloric stenosis. J Pediatr Surg. 1983;18:394–397.
219 Cook-Sather SD, Liacouras CA, Previte JP, Markakis DA, Schreiner MS. Gastric fluid measurement by blind aspiration in paediatric patients: a gastroscopic evaluation. Can J Anaesth. 1997;44:168–172.
220 Kim SS, Lau ST, Lee SL, et al. Pyloromyotomy: a comparison of laparoscopic, circumumbilical, and right upper quadrant operative techniques. J Am Coll Surg. 2005;201:66–70.
221 Leclair MD, Plattner V, Mirallie E, et al. Laparoscopic pyloromyotomy for hypertrophic pyloric stenosis: a prospective, randomized controlled trial. J Pediatr Surg. 2007;42:692–698.
222 Andropoulos DB, Heard MB, Johnson KL, Clarke JT, Rowe RW. Postanesthetic apnea in full-term infants after pyloromyotomy. Anesthesiology. 1994;80:216–219.
223 Bishop HC. Small bowel obstructions in the newborn. Surg Clin North Am. 1976;56:329–348.
224 Boocock GR, Todd PJ. Inguinal hernias are common in preterm infants. Arch Dis Child. 1985;60:669–670.
225 Abajian JC, Mellish RWP, Browne AF, et al. Spinal anesthesia for surgery in the high risk infant. Anesth Analg. 1984;63:359–362.
226 Harnik EV, Hoy GR, Potolicchio S, Stewart DR, Siegelman RE. Spinal anesthesia in premature infants recovering from respiratory distress syndrome. Anesthesiology. 1986;64:95–99.
227 Mahe V, Ecoffey C. Spinal anesthesia with isobaric bupivacaine in infants. Anesthesiology. 1988;68:601–603.
228 Gallagher TM, Crean PM. Spinal anaesthesia for infants born prematurely. Anaesthesia. 1989;44:434–436.
229 Dohi S, Naito H, Takahashi T. Age-related changes in blood pressure and duration of motor block in spinal anesthesia. Anesthesiology. 1979;50:319–323.
230 Wright TE, Orr RJ, Haberkern CM, Walbergh EJ. Complications during spinal anesthesia in infants: high spinal blockade. Anesthesiology. 1990;73:1290–1292.
231 Liu LM, Coté CJ, Goudsouzian NG, Ryan JF, et al. Life-threatening apnea in infants recovering from anesthesia. Anesthesiology. 1983;59:506–510.
232 Coté CJ, Zaslavsky A, Downes JJ, et al. Postoperative apnea in former preterm infants after inguinal herniorrhaphy: a combined analysis. Anesthesiology. 1995;82:809–822.
233 Cox RG, Goresky GV. Life-threatening apnea following spinal anesthesia in former premature infants. Anesthesiology. 1990;73:345–347.
234 Kliegman RM, Fanaroff AA. Neonatal necrotizing enterocolitis: a nine-year experience. Am J Dis Child. 1981;135:603–607.
235 Holman RC, Stehr-Green JK, Zelasky MT. Necrotizing enterocolitis mortality in the United States, 1979-85. Am J Public Health. 1989;79:987–989.
236 Kliegman RM, Fanaroff AA. Necrotizing enterocolitis. N Engl J Med. 1984;310:1093–1103.
237 Kosloske AM. Pathogenesis and prevention of necrotizing enterocolitis: a hypothesis based on personal observation and a review of the literature. Pediatrics. 1984;74:1086–1092.
238 Frantz ID, L’Heureux P, Engel RR, Hunt CE. Necrotizing enterocolitis. J Pediatr. 1975;86:259–263.
239 Ricketts RR. Surgical treatment of necrotizing enterocolitis and the short bowel syndrome. Clin Perinatol. 1994;21:365–387.
240 deVries PA. The pathogenesis of gastroschisis and omphalocele. J Pediatr Surg. 1980;15:245–251.
241 Thomas DF, Atwell JD. The embryology and surgical management of gastroschisis. Br J Surg. 1976;63:893–897.
242 Kim SH. Omphalocele. Surg Clin North Am. 1976;56:361–371.
243 Combs JT, Grunt JA, Brandt IK. New syndrome of neonatal hypoglycemia: association with visceromegaly, macroglossia, microcephaly and abnormal umbilicus. N Engl J Med. 1966;275:236–243.
244 Hoyme HE, Higginbottom MC, Jones KL. The vascular pathogenesis of gastroschisis: intrauterine interruption of the omphalomesenteric artery. J Pediatr. 1981;98:228–231.
245 King DR, Savrin R, Boles ETJ. Gastroschisis update. J Pediatr Surg. 1980;15:553–557.
246 Moore TC. Gastroschisis and omphalocele: clinical differences. Surgery. 1977;82:561–568.
247 Philippart AI, Canty TG, Filler RM. Acute fluid volume requirements in infants with anterior abdominal wall defects. J Pediatr Surg. 1972;7:553–558.
248 Ein SH, Rubin SZ. Gastroschisis: primary closure or Silon pouch. J Pediatr Surg. 1980;15:549–552.
249 Yaster M, Buck JR, Dudgeon DL, et al. Hemodynamic effects of primary closure of omphalocele/gastroschisis in human newborns. Anesthesiology. 1988;69:84–88.
250 Schuster SR. A new method for the staged repair of large omphaloceles. Surg Gynecol Obstet. 1967;125:837–850.
251 Schwartz MZ, Tyson KR, Milliorn K, Lobe TE. Staged reduction using a Silastic sac is the treatment of choice for large congenital abdominal wall defects. J Pediatr Surg. 1983;18:713–719.
252 Andrassy RJ, Mahour GH. Malrotation of the midgut in infants and children: a 25-year review. Arch Surg. 1981;116:158–160.
253 Martin LW, Torres AM. Hirschsprung’s disease. Surg Clin North Am. 1985;65:1171–1180.
254 Kapur RP. Hirschsprung disease and other enteric dysganglionoses. Crit Rev Clin Lab Sci. 1999;36:225–273.