Necrotizing Enterocolitis
Necrotizing enterocolitis (NEC) is not a new disease. Reports of a disease fitting the clinical characteristics of NEC date to the 1820s in France.1 The earliest reports in the USA occurred in the early 1960s, when Santulli and colleagues published the first significant surgical experience with NEC.2,3 They described a disease of low birth weight infants with a high mortality rate, which requires early, aggressive surgical management. Many investigators have devoted careers to better define this challenging disease and improve strategies for treatment and prevention. Despite these efforts, NEC remains a difficult and elusive disease. It remains unclear which premature infants are most at risk and what are the optimal prevention and treatment strategies. Long-term sequelae are easily overlooked in the acute setting, but contribute substantially to the subsequent morbidity and mortality.
Epidemiology
Several large population-based studies have found the incidence of NEC to be approximately 1 per 1000 live births. In select populations, such as infants under 1500 g, the incidence rises to between 2.3–11% (Table 33-1). Both the incidence and case fatality rate of NEC are inversely associated with birth weight.4–9 Several studies have identified an increased incidence of NEC in black infants, particularly males, a difference that holds true even when adjusting for birth weight. Hispanic infants also show an increased incidence, though to a lesser degree.4,10
Despite improvements in other areas of neonatal care, rates of NEC have remained stable for very low birth weight infants (VLBW) infants.11 Mortality remains high, with rates ranging from 15–30%.4,5,9,10,12 Higher fatality rates are associated with lower birth weight and younger gestational age.12,13 In a study summarizing trends for mortality and NEC in the USA between 1979 and 1992, the death rate was 12.4 deaths per 100,000 live births.13 The highest mortality rate was seen in VLBW infants who were black and male.4,10,13
Although most cases of NEC are managed medically, 20–40% will require operative intervention.5,7,12,14 Mortality increases up to 50% when surgery is necessary, and has not changed significantly over the past 30 years. The highest risk for mortality in this subgroup is also in the lowest birth weight and youngest gestational age infants.15
Long-term outcomes in patients requiring operation are worse, with increased complications such as neurodevelopmental delay, growth delay and chronic gastrointestinal problems.16
Though over 90% of cases are seen in preterm infants, there are occasional reports of NEC developing in full-term infants. Although the clinical and pathologic findings are similar, the initiating factors are likely different. Term infants who develop NEC are more likely to have predisposing risk factors such as congenital heart disease, sepsis, respiratory disease, or reported hypoxic events.17–20 The common feature of these predisposing conditions is reduced mesenteric perfusion.18 The incidence of NEC in term or near term infants is approximately 0.5 per 1000 live births.20 The mortality rate for term infants with NEC appears to be similar to that of preterm infants with NEC.18
The morbidity associated with NEC translates into considerable economic burden. In one study, infants with NEC were hospitalized 60 days longer than unaffected preterm infants if operation was needed, and 20 days longer if medical treatment was successful.21 In this study, the mean hospital costs were $186,200 greater for surgically treated patients than controls and $73,700 greater for medically treated patients. In addition to the high costs associated with the initial hospitalization, these children often have significant long-term health care needs. The mean cost of care for a child with short bowel syndrome over a five-year period exceeds $1.6M.22
Pathophysiology
Despite decades of research into the pathogenesis of NEC, a complete understanding of its pathophysiology remains elusive. Classic histologic findings include inflammation, bacterial overgrowth, and coagulation necrosis, and are present in over 90% of surgical specimens.23 Radiographic findings provide insight into the pathologic process that is unfolding. Pneumatosis intestinalis, or air within the intestinal wall, is thought to be due to gas produced by overgrowth of enteric bacteria.24 Progression to portal venous or lymphatic gas suggests extension of this process along vessels draining the affected intestine. Pneumoperitoneum indicates necrosis with complete disruption of the intestinal wall.
As our understanding of the pathophysiology of NEC evolves, a working model of the multifactorial nature of this disease has emerged. The unifying concept is that NEC represents an exaggerated inflammatory response to an insult. The nature of this insult is not well defined, and may vary among affected infants. It may be a global ischemic insult from congenital heart disease, an infectious insult from abnormal bacterial colonization, an insult related to formula feeding or lack of enteral feeding, or simply the response of translocation of normal bacterial flora in a genetically predisposed host.
The Intestinal Barrier
Intestinal Motility and Digestion
Intestinal motility develops during the third trimester of pregnancy but may not be fully mature until the eighth month of gestation.25–28 In premature infants, immature motility leads to increased epithelial exposure to potentially noxious substances, and poor clearance of bacteria with subsequent overgrowth. Additionally, the immature intestine has decreased digestion and absorption, which may lead to direct epithelial injury through a lowered pH.29–31 Newborns have reduced gastric acidity and pancreatic enzyme activity, which may further contribute to impaired digestion of macromolecules and bacterial proliferation.32
An increased ileal bile acid level may play a role in the pathogenesis of NEC. Bile acids are known to be cytotoxic, resulting in the development of mucosal injury.33 In premature infants, levels of ileal bile acid-binding protein are lower, leading to increased levels of bile acids in the intestinal lumen and in enterocytes.34 Another risk factor which may contribute is formula feeding, which elicits more toxic bile acids than breast feeding.35
The Mucous Coat
The mucous coat overlying the intestinal epithelium plays a key role in the barrier function. This layer is composed of water, mucin, lipids, and peptides.36 Mucin, a glycoprotein, is secreted by goblet cells in the epithelial layer and concentrates enzymes near the intestinal surface.37,38 Mucin aids in lubrication, mechanical protection, protection against the acidity of gastric and duodenal secretions,39 and fixation of pathogens.40 The effectiveness of mucin is related to maturity.39 Mature mucins have higher viscosity, better pH buffering, and resistance to bacterial breakdown.39–41 Mucin production and composition changes with gestational age, bacterial challenges, and colonization by commensal organisms.42–44 Deficits in the production or composition of mucin may contribute to the ability of bacteria to invade the intestinal epithelium and thus contribute to the pathogenesis of NEC.32,36,37,45–47
Tight Junctions
Tight junctions create fusion points between epithelial cells, forming an intact yet semipermeable barrier. Mature tight junctions are composed of the transmembrane proteins occludin, claudin, and junctional adhesion protein; these normally present a barrier to diffusion of large molecules.32,48 Tight junctions are not static, but may be altered by disease processes.49 Immaturity in the composition of tight junctions likely plays a role in the increased permeability of the epithelium of the newborn intestine.50
Cytokines are produced in response to bacteria, and may interfere with tight junctions, promoting the translocation of bacteria.51 Inflammatory mediators such as tumor necrosis factor, interferon (IFN)-γ, and interleukin (IL)-1β further cause epithelial dysfunction by upregulating inducible nitric oxide synthase (iNOS) leading to the overproduction of nitric oxide (NO), and the generation the reactive nitrogen intermediate peroxynitrite (ONOO−). This process has been associated with increased epithelial cell apoptosis and death.52 NO has been shown to play a role in mediating the decrease in the localization and expression of tight junction proteins.49,53 Disruption of tight junctions may lead to increased intestinal permeability, allowing bacterial translocation and activation of the immune system.
Immunologic Defenses of the Gastrointestinal Tract
The gastrointestinal tract contains the largest amount of lymphoid tissue in the body and coordinates the immunologic mechanisms of the adaptive and innate immune systems.54 Gut-associated lymphoid tissue consists of several cell types which work in concert to perform antigen presentation and processing37,55
In neonates, antigen processing and presentation is less efficient, reducing the ability of the immune system to respond to pathogenic organisms. Peyer patches are fewer, smaller, and lack germinal centers.36 Paneth cell activation by bacteria or components of bacterial cell walls leads to secretion of a variety of antibacterial substances, including α-defensins and lysozyme.56,57 Production of these peptides is decreased in premature infants, and may predispose to bacterial overgrowth, allowing NEC to develop. Following recovery from NEC, Paneth cell hyperplasia occurs, suggesting these cells play an important role in NEC.56
IgA is normally synthesized by plasma cells of the lamina propria and secreted into the mucin layer where it binds bacteria and viruses, inhibiting attachment to the epithelium. The newborn lamina propria is largely devoid of the IgA-secreting plasma cells, resulting in deficient secretion until several weeks of age.58,59 Neonates can obtain IgA through passive transfer from breast milk,49 but infants who do not receive breast milk lack this important immunoglobulin and its protective effects.
Regenerating the Intestinal Barrier
The pathologic findings of NEC arise not only from alterations in the integrity of the intestinal barrier but also from an impaired ability to regenerate.60 Premature infants have a reduced capacity for intestinal repair, likely contributing to the pathogenesis of NEC.
Lipopolysaccharide
Lipopolysaccharide (LPS) is the endotoxin portion of the Gram-negative bacterial cell wall, and is one of the most abundant proinflammatory stimuli. LPS is seen in high levels in NEC.24 LPS impairs intestinal barrier function by inhibiting repair and promoting the release of signaling molecules and proinflammatory mediators such as NO, IFN-γ, cyclooxygenase-2 (COX-2) and RhoA from enterocytes which promote intestinal injury.49,60–62 LPS causes increased expression and function of integrins on the cell surface, resulting in increased cell adhesion to the basement membrane,63 and compounds the effects of platelet-activating factor (PAF).64,65
Nitric Oxide
NO is a key mediator of numerous physiologic and pathologic systems, but has been shown to have a paradoxical role in NEC. Low levels of NO are important for maintaining vasodilation; conversely, sustained overproduction of NO can have profound cytopathic effects. The cytopathic effects of NO are believed to be due to toxic nitrogen intermediates, such as ONOO−.32
NO is a highly reactive free radical formed by the conversion of arginine to citrulline by NO synthase (NOS) which exists in three forms: the constitutive form (nNOS), the inducible isoform (iNOS), and the constitutive endothelial isoform (eNOS).66 The presence of the constitutive forms of NOS in the gastrointestinal tract suggests that NO has a normal physiologic role in gut function. The eNOS isoform maintains intestinal homeostasis by enhancing mucosal blood flow and maintaining microvascular tone.67
When produced by iNOS under inflammatory conditions, the NO level increases up to a million fold,67 which can lead to cellular damage and failure of the intestinal barrier. Excess NO overwhelms local scavenging mechanisms and reacts with superoxide anion (O2−) to produce the highly toxic ONOO−.67–69 These effects may be compounded in the presence of high levels of LPS, which leads to increased iNOS expression and function within the intestine.69–70 Studies have linked NO with the pathogenesis of NEC. The expression of iNOS has been shown to be upregulated in critically ill patients and in patients with NEC.52 Conversely, expression is down regulated by the anti-inflammatory cytokine interleukin-10.71 Excess NO may also inhibit intestinal restitution by blocking enterocyte migration and proliferation.32,49,72
Platelet-Activating Factor
PAF is potent phospholipid inflammatory mediator that is produced by most cells and tissues.73,74 The cytotoxic effects of PAF are due to initiation of the inflammatory cascade. PAF-induced bowel injury is associated with the production of oxygen-derived free radicals as well as leukocyte migration, activation, and capillary leakage resulting in apoptosis in affected enterocytes.75
Various studies have shown the importance of PAF in the pathogenesis of NEC. Higher concentrations of PAF have been found in NEC patients compared with controls.75–77 PAF-acetylhydrolase (AH) activity has been shown to be deficient in sick infants with NEC, and the administration of PAF-AH or a PAF receptor antagonist in animal models of NEC reduces the degree of intestinal injury.74,76,78 PAF-AH is present in maternal breast milk, which may contribute to the protective effect against NEC it provides.74
Maintaining Intestinal Barrier Homeostasis
Epidermal Growth Factor
Epidermal growth factor (EGF) is a peptide secreted into the intestinal lumen.79 It plays an important role in the development, maturation, and maintenance of gut homeostasis, being active in processes from intestinal repair and adaptation to cell movement and prevention of bacterial translocation.80–86 EGF has been shown to support maintenance of the intestinal barrier, as well as being active in the down regulation of inflammatory cytokines.79,86
EGF is believed to play an important role in the pathogenesis of NEC. Decreased levels of EGF have been demonstrated in the saliva and serum of premature infants with NEC.87 Furthermore, studies have shown that salivary levels of EGF in the first two weeks of preterm life may have a predictive value for the occurrence of NEC.88 A potentially therapeutic role for EGF was reported in an infant suffering from intestinal necrosis resembling NEC who received a continuous infusion of EGF resulting in complete recovery of the damaged intestine.89 These investigators subsequently treated a small group of neonates with stage II and III NEC in a randomized, double-blind, prospective trial with recombinant EGF and found that repair of the intestinal epithelium was seen at four, seven, and 14 days.90
Heparin-binding EGF (HB-EGF) is a member of this family of growth factors, and is found in amniotic fluid and breast milk.91 In animal models of NEC, administration of HB-EGF has been shown to reduce the incidence of bowel injury by 50%, more than double survival,92–95 and preserve the integrity of the intestinal barrier.96 Animals with overexpression of HB-EGF have decreased susceptibility to NEC,97 while animals with deletion of the HB-EGF gene have increased susceptibility.86,97 These effects seem to be at least in part due to the cytoprotective effects of HB-EGF, which serves to protect intestinal stem cells from injury.86
Neonatal Vasculature and the Pathogenesis of NEC
Newborn intestinal circulation is characterized by a low resting vascular resistance.98,99 This results in increased blood flow and oxygen delivery. Control of vascular resistance involves intrinsic and extrinsic control mechanisms.100 Extrinsic mechanisms are mediated by the autonomic nervous system. The intrinsic regulation is mediated by two vascular effector mechanisms produced and released within the intestine—one vasoconstrictive and one vasodilatory.101,102 Endothelin (ET)-1 is the primary vasoconstrictor stimulus in the newborn intestine and is produced by the endothelium.103,104 Although constitutively produced, it can also be stimulated by decreased flow, hypoxia, and various inflammatory cytokines.105–107 The production of ET-1 is age specific, being greater in younger subjects.103
NO is the primary vasodilator stimulus.98,99 eNOS is also continuously produced, but like ET-1 the rate of production can be increased in response to a variety of stimuli.101 In the neonate, the balance of these two products favors vasodilation, generating the characteristic low vascular resistance. In disease states, endothelial dysfunction leads to ET-1 mediated vasoconstriction, causing compromised blood flow, intestinal ischemia, and injury. The vasoconstrictor ET-1 has been linked to intestinal tissue injury in several studies.103,108 Increased expression of ET-1 has been found in intestine removed from infants with NEC, and the amount of ET-1 increased proportionally to the degree of intestinal injury.102
Bacterial Colonization
Abnormal colonization may alter the balance of pathogenic and beneficial bacteria, favoring an increase in pathogenic bacteria and resulting in a loss of the beneficial role of commensal bacteria. Furthermore, the immature immune system of premature infants may not be able to respond appropriately to normal colonization of bacteria, much less abnormal flora.109
Clinical Diagnosis
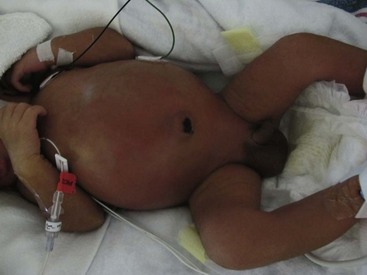
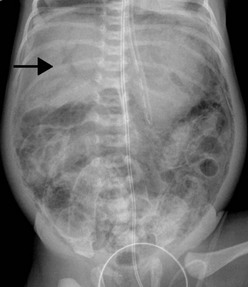
Gastrointestinal signs progress from abdominal distention to tenderness suggestive of peritoneal irritation (Fig. 33-1). Palpable loops of intestine may become evident. Localized disease may progress to generalized peritonitis or may worsen in a focal area, including discoloration of the skin and the development of an abdominal mass. When present, the findings of a fixed abdominal mass and erythema of the abdominal wall are strongly predictive of dead bowel; however, these findings occur in only 10% of patients with NEC.110 A sudden need for increased ventilatory support may also serve as a harbinger of NEC.111 This is due to increased metabolic requirements combined with increased intra-abdominal pressure.
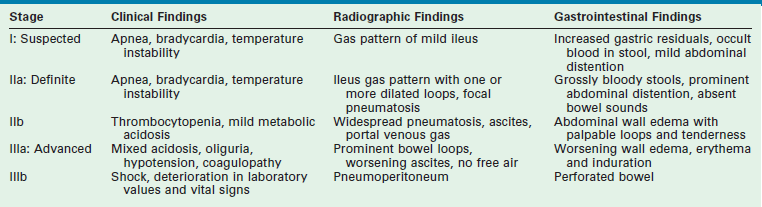
Confirmation of the diagnosis of NEC combines signs and symptoms with radiologic findings. These findings have been combined into the clinical staging system proposed by Bell that aids in describing the severity of disease (Table 33-2).112,113
Laboratory Studies
Laboratory studies reveal nonspecific indicators of an inflammatory or infectious process such as leukocytosis with bandemia. Thrombocytopenia and metabolic acidosis are also common. A rapid fall in platelet count is a poor prognostic factor.114
Several studies have tried to identify an accurate biochemical marker to identify neonates at risk for NEC, avoiding prolonged periods without enteral nutrition as well as the use of unnecessary tests and antibiotics.115 Serum acute phase proteins and cytokines have been investigated for an association between high levels and the severity of NEC. Increased levels of IL-6, IL-10, and C-reactive protein (CRP) have been documented in premature infants with NEC, with the highest levels of IL-10 in those patients who did not survive.116 CRP has also been associated with NEC when the levels rose quickly after the diagnosis was suspected. A failure of the levels to return to normal has been found to be associated with complications, including abscesses, strictures, and sepsis.117 In a prospective study, CRP levels were elevated in infants with stage II and III NEC and may be useful in discriminating between stage II NEC and other gastrointestinal disorders.118
Multiple other potential markers have been studied—gastrointestinal tonometry, urinary D-lactate levels, exhaled breath hydrogen, endotoxin elevations in stool, plasma intestinal fatty acid-binding proteins—but none of these has yielded the sensitivity or specificity required for a diagnostic tool.61,119–121 Currently, no biochemical markers have been adequately predictive of the patient’s clinical course or outcome to be clinically useful.
Radiographic Findings
Plain Films
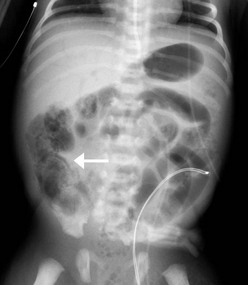
The cornerstone of the radiographic diagnosis of NEC relies on plain radiographs. The most specific radiographic finding is pneumatosis intestinalis, as seen in Figure 33-2. Other radiographic findings include air–fluid levels, gas-filled loops of bowel, persistently dilated loops of bowel, thickened bowel walls, portal venous gas, and pneumoperitoneum. Although most commonly seen in NEC, pneumatosis intestinalis has also been reported in cases of Hirschsprung enterocolitis, severe diarrhea, and carbohydrate intolerance. Portal venous gas (Fig. 33-3) is a less common radiographic finding but is generally considered a poor prognostic sign. This finding is associated with twice the incidence of diffuse or ‘pan’ necrosis and a significantly lower survival rate.122 Nevertheless, many patients with portal venous gas recover fully with medical management.
Other Imaging Modalities
Studies have examined ultrasonography (US) as an adjunctive measure for the diagnosis and management of infants with NEC. Abdominal ultrasound evaluation emerged as a potential modality in the treatment of NEC after a report in 2005 that assessed bowel viability using color Doppler imaging in neonates with NEC.123 This publication established critical data for bowel wall thickness, echogenicity, peristalsis, and perfusion in both normal neonates and those with NEC. Additional studies corroborated the usefulness of ultrasound as a means of diagnosing NEC.124,125 ultrasound offers some potential advantages over plain films in that it can depict bowel wall thickness and echogenicity, free and focal fluid collections, peristalsis, and the presence or absence of bowel wall perfusion by using Doppler imaging.126,127
The presence of pneumatosis on plain abdominal radiographs helps clinch the diagnosis, but mild findings, such as the lack of intramural gas, makes the diagnosis more difficult. ultrasound may be a useful adjunct in this population because it may allow detection of small amounts of intramural gas not visible on plain films or changes in bowel wall thickness, peristalsis, or perfusion that could confirm or exclude the diagnosis of NEC.128 The time frame for when to perform ultrasound initially or when to use it during follow-up has not been established.
In addition to assisting with the diagnosis in difficult cases, ultrasound has been suggested as an adjunct modality in two other groups of patients: those in whom the evolution of changes in the radiographs does not match the clinical course and those whose condition is deteriorating without evidence of pneumatosis on plain films.128 Finally, ultrasound may be useful in helping to decide the appropriate time to re-initiate and advance feeding.123 However, at this time, ultrasound does not yet have a well-defined or established role in the management of NEC.
In the acute setting, contrast examinations of the gastrointestinal tract, computed tomography, and magnetic resonance imaging have not been found to be useful modalities in clinical practice.129–133
Differential Diagnosis
A subset of premature infants presents with bowel perforation while not exhibiting other symptoms of NEC nor pneumatosis on radiographs. Some investigators have defined this as spontaneous, isolated, or focal intestinal perforation (FIP). FIP tends to occur in low birth weight infants, usually the first seven to ten days of life, and is sometimes associated with indomethacin treatment.134–140 Whether these infants have a limited form of NEC or a distinct entity is controversial. Some reports contend that FIP is a different disease than NEC, but definitive evidence is lacking.139–142 As expected, neonates with an isolated bowel perforation have better outcomes in the absence of extensive disease.138,143–145
Medical Management
Medical management of NEC begins with bowel rest, gastric decompression, intravenous fluid resuscitation, and broad-spectrum antibiotic therapy, including anaerobic coverage. Blood, urine, and sputum cultures should be obtained before the initiation of antibiotic therapy. A critical component of medical management is ongoing close observation with serial abdominal examinations and radiographs. As long as the clinical situation is stable or improving, expectant management can continue. Clinical deterioration or worsening radiographic features may indicate the need to consider surgical intervention.
Experimental Medical Treatments: HB-EGF
Endogenous HB-EGF is increased in response to hypoxia, stress, and during wound healing.146–151 HB-EGF mRNA is induced after intestinal ischemia/reperfusion injury in vivo152 and is involved in epithelial cell repair, proliferation, and regeneration in the early stages after injury.153 Based on these findings, it has been theorized that exogenous HB-EGF may also play a role protecting the intestinal mucosa from injury.
Multiple studies have demonstrated that exogenous administration of HB-EGF can protect cells and organs from injury both in vitro and in vivo. HB-EGF can protect enterocytes from proinflammatory cytokine-induced apoptosis.154 Intestinal epithelial cells pretreated with HB-EGF before hypoxia showed less necrosis with maintenance of the cytoskeletal structure and improved recovery ability.155 HB-EGF also downregulates the production of NO156,157 and blocks NF-κB activation in intestinal epithelial cells after cytokine stimulation.157 In a neonatal rat model of NEC, the administration of HB-EGF reduced the severity and incidence of NEC with preservation of gut barrier integrity.92 Studies have also shown that treatment with HB-EGF decreases the overproduction of IL-18 and increases the production of anti-inflammatory IL-10.158 HB-EGF is the only compound with imminent plans for investigation in humans. A host of other therapeutic agents have shown promise but not yet reached the stage of clinical testing.
Surgical Management
Although many infants can be managed medically, 20–40% will require operative intervention. In some cases, indication for operation develops during the medical management, while in others it is found at presentation. The only absolute indication for drainage or exploration is evidence of intestinal perforation either on an abdominal radiograph (Fig. 33-4) or via paracentesis that is positive for stool or bile.159 Relative indications for operation include deterioration in the infant’s clinical condition despite maximal medical management. Such signs can include oliguria, hypotension, worsening metabolic acidosis, worsening thrombocytopenia, leukopenia or leukocytosis, and ventilatory failure. Relative radiographic indicators for operation include portal venous gas or persistently abnormal ‘fixed’ loops of bowel on serial radiographs.
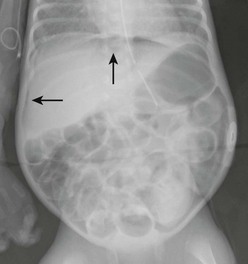
FIGURE 33-4 Free air (arrows) is seen on this radiograph. This finding is an indication of perforation, and is considered an absolute indication for intervention, whether drainage or exploration.
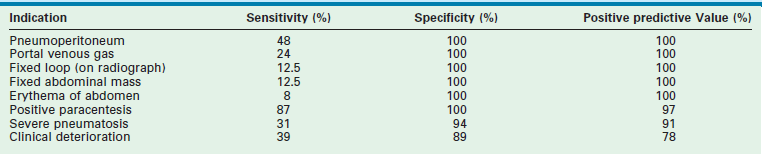
Ideally, surgical intervention would occur when intestinal gangrene is imminent but before actual perforation or necrosis actually occurs. However, this ideal time for intervention is often difficult to identify. One study has tried to evaluate the sensitivity and specificity of 12 different findings to identify early indicators for operation.110 Three findings had a specificity and positive predictive value (PPV) close to 100% with prevalence greater than 10%. These findings were deemed the ‘best’ indications and included portal venous gas and a positive paracentesis (Table 33-3). Three indicators had specificity and PPV close to 100% but prevalence less than 10% and were considered ‘good’ indicators, including a fixed loop on an abdominal radiograph, erythema of the abdominal wall, and a palpable abdominal mass. One indicator, severe pneumatosis, was deemed fair because it had a specificity and PPV above 90% and 20% prevalence. The five remaining indicators were considered poor because the specificities were less than 90% and the PPVs less than 80%. This probability analysis may be useful in the complex decision-making process when an operation is being considered.
NEC can affect any segment of the gastrointestinal tract. Most commonly, both large and small bowel are involved.23 Isolated small intestinal lesions occur with the next greatest frequency. It is as common to have a single affected area as to have multiple-segment disease.23,160,161 A small subgroup of NEC patients develop massive necrosis of the entire intestine, known as ‘NEC totalis.’160
Peritoneal drainage was first reported in 1977 as salvage treatment for perforation in VLBW infants who were believed to be too unstable for laparotomy (Fig. 33-5).162 Initially intended as a temporizing procedure in the sickest and smallest patients, this treatment has evolved into a widely utilized option as primary treatment of perforated NEC. After many years of conflicting results comparing outcomes of the two approaches, a meta-analysis was attempted to synthesize these disparate data. This study found such significant bias in the assignment of patients to one treatment or another that the two options could not be adequately compared.163 A need existed for a prospective randomized controlled trial.
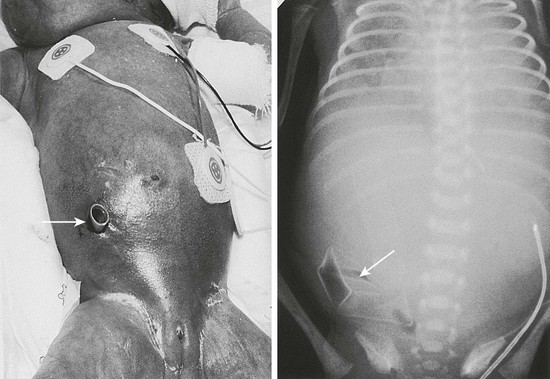
FIGURE 33-5 A micropremature infant with NEC and perforation is shown with her corresponding abdominal radiograph. A percutaneous drain (arrows) has been placed in the right lower quadrant for drainage of the intestinal perforation. Note that the drain is placed at a position below the level of the umbilicus to avoid injury to the lower edge of the right lobe of the liver.
Three prospective studies have compared laparotomy to peritoneal drainage. The NICHD Neonatal Research Network conducted a prospective observational cohort study at 16 centers.164 This study included 156 infants with either NEC or FIP. Overall 50% (n = 78) of the patients died and 72% (n = 112) either died or had some element of neurologic impairment at 18 to 22 months. The babies in this study were not randomized to their treatment groups. The treating surgeons and neonatologists chose which therapy to use for each infant. However, unlike other nonrandomized studies, extensive prospective data were collected, allowing for risk-adjusted multivariable regression analyses. This strategy enabled the investigators to account for the differences between the treatment groups. The odds ratio for death after adjusting for differences in the two treatment groups was 0.97 for laparotomy compared with peritoneal drainage (95% confidence interval [CI]: 0.43–2.20). The odds ratio for the combined outcome of death or neurodevelopmental impairment at 18 to 22 months was 0.44 for laparotomy compared with drainage (95% CI: 0.16–1.2). Although not statistically significant, there is some suggestion in this study that overall outcomes at 18 to 22 months of age may be improved by laparotomy rather than drainage.
The first randomized trial evaluating laparotomy versus peritoneal drainage was the NECSTEPS trial.165 In this trial, 117 VLBW infants at 15 North American tertiary care centers were randomized to either treatment group. The primary outcome variable was mortality at 90 days. There was no difference in mortality at 90 days between the two treatment groups (34.5% vs 35.5%). Need for parenteral nutrition at 90 days and the length of hospitalization were also similar between the two groups. This study focused on short-term outcomes; within those limits, results suggest that the method of surgical intervention does not impact the outcome.
The second randomized trial comparing laparotomy and peritoneal drainage in infants with perforated NEC was the NET trial.166 This trial was a multinational trial conducted at 31 centers in 13 countries. The primary outcome variable was mortality at 1 and 6 months. Sixty-nine patients weighing less than 1000 g were enrolled and randomized. There was a trend toward better survival in the laparotomy group (65% survival) compared with the drainage group (51%), with a relative risk of mortality of 0.5 (95% CI: 0.2–1.5). These findings were not statistically significant. The authors concluded that there was no evidence from the trial to support the benefit of primary peritoneal drainage in extremely low birth weight (LBW) infants with intestinal perforation.
An additional randomized trial is currently underway. The NEST trial is designed to compare long-term outcomes in extremely LBW infants (≤1000 g) with necrotizing enterocolitis or isolated intestinal perforation treated by either laparotomy or peritoneal drainage. The primary outcome is death or neurodevelopmental impairment at 18–22 months corrected gestational age. Results are expected in the fall of 2015.167
When laparotomy is performed, stomas are usually created. Because of concerns about the high morbidity associated with enterostomies (Box 33-1), a few centers have advocated primary anastomosis at the time of initial laparotomy. The data to support such management are nonrandomized and retrospective. In actuality, the majority of stomal complications are easily managed and early closure is well tolerated.168 One study found that survival was 72% with intestinal diversion but only 48% in those undergoing primary anastomosis.169
Diffuse intestinal involvement poses the most difficult situation for the surgeon. Those infants who survive may develop short bowel syndrome and have some level of dependency on total parenteral nutrition given the extensive amount of affected bowel. Surgical strategies focus on trying to preserve as much intestine as possible while still resecting enough bowel to stabilize the patient. Second-look laparotomies have been proposed as a way to minimize the amount of bowel resected.170 The ‘clip and drop-back’ technique is another option with a similar strategy.171 All nonviable intestine is resected initially but no ostomies or anastomoses are created. Blind-ending segments are left in the abdomen, and continuity is restored or ostomies are created on re-exploration in 48 to 72 hours. Proximal diversion alone has also been used to treat ‘pan’ necrosis without reported worsened survival and with recovery of much of the bowel by the time of ostomy closure.172 None of these approaches has been prospectively evaluated; therefore, no single technique can be strongly advocated.
Outcomes
Recurrence
NEC recurs in approximately 5% of cases.173,174 There is no apparent correlation between the site of disease and the site of recurrence. Usually recurrent disease can be managed nonoperatively.159,174–179
Length of Hospitalization
Hospital stays are longer for infants who suffer from NEC when compared with other infants of the same gestational age. Furthermore, those who require operation tend to have even longer hospitalization. Several studies have shown hospitalizations averaging two to three months for medically treated NEC and four to five months for surgically treated NEC.180–182 There was no difference in length of hospitalization between the two treatment groups in the NECSTEPS trial.165
Mortality
Estimates of mortality from NEC have remained steady over the past two decades at 15–30% despite the fact that the postsurfactant era has led to a rise in the incidence of disease.4,5,9,10,12,13,180,181,183,184 Operative mortality has improved with a decline from 70% mortality in the 1960s185 to more recent rates of 20–50%.5,10,13,180,183,186,187 The main predictor of mortality in NEC is gestational age. The highest mortality occurs in the youngest, smallest infants, and black male VLBW infants are at greatest risk. Additionally, infants with a greater extent of bowel affected by the disease tend to also have a higher mortality rate.188,189
Gastrointestinal Outcomes
Short Bowel Syndrome
NEC is the leading disease responsible for short bowel syndrome (SBS) in children, accounting for half of all pediatric cases. Furthermore, SBS develops in a fourth of all patients who suffer from NEC.190 SBS develops when an infant is left with inadequate functional intestine to absorb the nutrients required for growth. This can result from resection at the time of operation or from poor function of the remaining intestine. Traditional teaching based on early reports suggests that a minimum of 40 cm of small intestine is required for a patient to have a chance of weaning from total parenteral nutrition.191 Despite these observations, experience has shown that the function of the intestine is much more important to this disease than the specific length of intestine.
The portion of intestine resected is also important for subsequent gastrointestinal functioning. Patients with ileal or jejunal disease have a higher mortality rate than those with colonic disease.187,188,192,193 Patients with extensive jejunal resection fare better than those with extensive ileal resection. This is due to the differing abilities of the intestinal regions to undergo adaptation. The ileum has the greatest capacity to adapt and increase its absorptive capacity.
Preservation of the ileocecal valve has been considered important for minimizing the risk of SBS.194 Some studies have suggested that dependence on parenteral nutrition is lessened when the ileocecal valve was preserved,195–198 but others have found no difference.180,199–202 It appears that the actual length and functional capacity of the remaining ileum is far more important than the presence of the valve itself.
Stoma Complications
Creation of a properly constructed stoma can be life-saving in the management of NEC. Stomas are used for both decompression and diversion. Enterostomies can be fraught with early and late complications. A number of strategies have been proposed for optimal stoma creation, including what type of stoma to create as well as how to exteriorize the stoma. End stomas, double-barrel (Mikulicz) stomas, and loop enterostomy have all been advocated. Small studies comparing complication rates between these various strategies have not found differences in complications, including retraction, prolapse, hernias, or wound infections.203–205 Many surgeons exteriorize the stoma and mucous fistula through the incision, some at one end, others at opposite ends of the incision. Others advocate a separate incision, citing concerns about increased wound infection and difficulties attaching stoma appliances. Another consideration for a separate incision is whether the stoma needs to remain for a prolonged period of time. Most surgeons do not recommend maturing a stoma owing to potential interference with an already tenuous blood supply.203
Stomal complications can lead to significant morbidity. Studies have shown complication rates exceeding 50%.187,206–209 The most serious complications include prolapse, stricture, and retraction, all of which may require surgical intervention. Proximal jejunostomies can cause significant electrolyte and fluid losses that can lead to problems with fluid balance and weight gain.194,210 Fluid losses from jejunostomies can also cause peristomal skin complications. Despite these problems, with an aggressive approach to fluid and electrolyte replacement and meticulous skin care, proximal jejunostomies can be a viable option for the management of NEC.194,211
The timing of enterostomy closure remains controversial. Recommendations vary from as early as one month to as late as four months after operation.212–215 Most suggest waiting one to two months after the initial operation, and until a weight of 2000 g is reached, as long as adequate feeding and growth is being maintained.194,210,213 Earlier closure may be necessary with very proximal stomas due to fluid and electrolyte losses and the inability to gain weight. Coexisting medical problems must also be considered in determining the optimal time for closure.
Intestinal Strictures
Intestinal strictures are a common occurrence after NEC. The incidence of stricture has been reported from 12–35%, and occurs in patients who have been managed medically or surgically.131,207,216–221 This incidence does not differ between patients treated by primary anastomosis or enterostomies.159,186,187,199,206,207,212,215–227 In one series of patients treated for severe NEC by proximal diverting enterostomy, the incidence was 55%.217 Most post-NEC strictures occur in the colon, specifically the left colon (Fig. 33-6).160,212,219,226,228
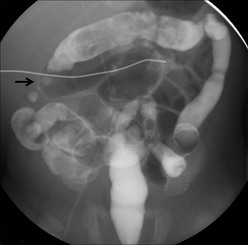
FIGURE 33-6 This contrast enema demonstrates a stricture (arrow) in the ascending colon after nonoperative management for NEC. NEC strictures may occur anywhere in the intestinal tract, but are most common in the left colon. These can develop whether the baby was treated medically or surgically.
Resection of strictures is usually needed, although not all lesions are symptomatic and spontaneous resolution has been reported.131,224,229,230 Other approaches have been proposed, including close radiographic follow-up of asymptomatic patients.131 Balloon dilatation is an option for focal, nonobstructing lesions, but has not been widely utilized.230
Patients treated by laparotomy and stoma for NEC should undergo routine imaging of the distal intestine before enterostomy closure to evaluate for a possible stricture. Patients managed medically, by peritoneal drainage, or with primary anastomosis may also develop strictures. Some patients remain asymptomatic and others present acutely in distress due to perforation.219 Thus, some surgeons advocate contrast studies in all NEC patients.131,218,219,221 The potential for false-negative results and the invasiveness of the procedure prevent this practice from being commonly used.
Little is known about the long-term impact of stricture formation after NEC. Infants who require ostomy closure may have strictures addressed at that time. For those managed without ostomies, stricture resection requires a new operation with subsequent prolongation of recovery and time to full enteral feeding. Resection of additional bowel may also ultimately impact gastrointestinal outcomes.
Growth
Several small observational studies have shown that children treated for NEC fall below the 50th percentile for height and weight even into their grade-school years.231–233 This growth retardation seems particularly to affect those who suffered from stage III NEC.234,235 The problem is much more severe in children who develop SBS as a result of NEC. Long-term evaluations of growth are required to evaluate the impact that birth weight, NEC severity, operative strategy, and subsequent outcomes may play in ultimate growth outcomes for these children.
Neurodevelopmental Outcomes
In infants surviving NEC, adverse neurodevelopmental outcomes remain an important challenge. In 1980, a groundbreaking study reported that less than half of children surviving NEC were neurodevelopmentally normal at three-year follow-up.236 Subsequently, multiple observational studies have cited intellectual delays,237 moderate-to-severe developmental delay with speech and motor impairment,183 developmental delay requiring special educational classes,231 and delays in locomotor, hearing and speech, intellectual performance, and social skills.234
A large multicenter cohort study from the NICHD Neonatal Research Network evaluated neurodevelopmental outcomes in 1100 extremely LBW survivors.238 This study confirmed that NEC is associated with increased odds of having a delayed score on psychomotor developmental assessment as well as increased odds of cerebral palsy. This study found that almost all of these abnormalities occurred in patients who required an operation for NEC.239
Two systematic reviews have confirmed the increased risk of neurodevelopmental impairment in VLBW infants who develop NEC (Fig. 33-7).240,241 The risk for those treated surgically is nearly twice the risk for those treated medically. Most infants with NEC who are treated medically develop like age-matched premature infants without NEC, whereas those requiring an operation have an increased risk of poor neurodevelopmental outcomes.
Prevention
Human Milk
Four small (n = 36–81 patients) randomized or quasi-randomized studies in the 1980s evaluated the incidence of NEC in infants fed either human milk or formula.242–245 None of these studies showed a statistically significant difference in NEC between the two groups, though all had a lower incidence in infants who received human milk.
Another randomized study was done as part of a larger prospective observational trial in the UK.246 The study included 159 infants who were fed exclusively either donor breast milk or preterm formula. The incidence of NEC was slightly lower in the breast milk group, but the odds ratio did not reach significance. These investigators looked at all 926 patients who were part of the prospective observational study. They divided the patients into three groups based on what they had been fed: formula only, formula plus mother’s milk, and human milk only. The formula group had a 10% incidence of NEC versus 4% in the human milk only group. When only considering confirmed cases of NEC, the formula group had 7% versus 1% in the human milk group.
Two systematic reviews and meta-analyses have subsequently been published which found that human milk reduces the risk of NEC with findings of a relative risk of 0.21 (95% CI: 0.06–0.76) in one and 0.25 (95% CI: 0.06–0.98) in the other.247,248 However, these findings must be evaluated with some caution. The studies included in the meta-analyses had significant variation in the incidence of NEC (0–20%), the type of human milk (donor, maternal, term, or preterm), and the timing of feeding. Furthermore, one of the included studies was not truly randomized but used an alternate assignment allocation and none of the studies was blinded to allocation or outcome. Also, the criteria for NEC was not uniform among the studies.
More recently, a randomized multi-institutional study compared a human milk-based diet with a bovine milk-based diet in preterm infants. Mother’s milk was used when available. Otherwise donor breast milk was used and fortified with human milk fortifier when enteral intake reached either 40 mL/kg/day or 100 mL/kg/day. These infants were compared to infants receiving bovine milk-based formula, with fortification started once intake was 100 mL/kg/day. A total of 207 infants were enrolled. Duration of parenteral nutrition, length of hospital stay, sepsis and growth were not significantly different between groups; however, the incidence of NEC was significantly lower with a human milk-based diet (5.8% vs 15.9%, p = 0.02). The odds ratio for developing NEC on an exclusively human milk-based diet was 0.23 (95% CI 0.8–0.66; p = 0.007) or a relative risk reduction of 77%.249 Other significant findings included a lower combined risk of death or NEC, and a lower risk of NEC requiring operation with human milk.
Fortification of human milk is commonly practiced to improve caloric and nutrient content of the human milk received. Fortification has been shown to result in increases in weight, length, and head circumference. A Cochrane review did not show an increase in NEC in the group fed fortified milk.250 Different proprietary preparations of fortifier exist, and little research has been done to compare outcomes or to determine the optimal composition of fortifiers.
Feeding Strategies
Early initiation of enteral feedings in preterm infants helps to promote growth and decreases the need for parenteral nutrition. There have been concerns that early feeding may be associated with an increased risk of NEC. A systematic review found only two small, randomized studies with a total of 82 infants.251 In these studies, early feeding had no effect on the incidence of NEC. Given the small sample size, important effects of either strategy may have been missed. In one large prospective study, early feeding of human milk appeared protective against NEC while early feeding of formula was linked to an increased incidence.246 These investigators found that for each day earlier formula feeds were started, the risk of developing NEC increased 20%. Among those infants who received breast milk, there was no association between the day of life that feeding was initiated and the risk of NEC.
After starting enteral feeding, the rate at which to advance feeds is another controversy. Rapid advancement is advocated by some for infants to quickly regain their birth weight and achieve full enteral nutrition.252 In one randomized trial, the study was terminated early due to a higher incidence of NEC in the group that had their feeds rapidly advanced.253 Results from this study were confounded by a questionable randomization model, an unusually high incidence of NEC, early termination of the study, and exclusion of four patients who died or developed intestinal perforation. A systematic review of rapid versus slow advancement of feeds examined 372 patients in three separate trials and did not find any difference in the relative risk of developing NEC.252 The ideal rate of feeding advancement remains unclear.
Amino Acid Supplementation
Arginine is the sole substrate for nitric oxide synthase. NO plays an important role in proper functioning of the gastrointestinal tract. Supplementation with arginine has been considered as a potential preventive measure for avoiding NEC. One randomized controlled trial of 152 preterm infants found a significant reduction in NEC in those infants receiving this supplementation.254 This small study was unable to evaluate stage II and III NEC independently; further research on this possible preventive measure needs to be performed to confirm these results.255
A deficiency of serum glutamine has also been correlated with NEC.256 Glutamine is fuel for enterocytes and promotes the growth and integrity of the intestinal epithelium. Glutamine has also been postulated as having protective effects against NEC. Two large multicenter randomized trials have failed to show a benefit for those who received glutamine.257,258 A Cochrane systematic review of five trials also did not show any benefit to the infants receiving glutamine.259
Oral Antibiotics
Given the role of bacteria in the pathogenesis of NEC, enteral antibiotics have been considered as possible NEC prophylaxis. The use of antibiotics also increases the potential for the development of resistant bacteria. Five randomized trials have examined the effects of prophylactic therapy with enteral antibiotics. No individual study found a significant reduction in NEC; however, when the five were combined together in a meta-analysis, there was a significant reduction in the incidence of NEC in those who received the antibiotics.260 Unfortunately, these studies did not report on the potential harmful effects of this use of enteral antibiotics. Without sufficient evidence regarding the safety of using enteral antibiotics as prophylaxis, an endorsement for this prophylactic measure cannot be made.
Probiotics
Probiotics are live microbial supplements that colonize the gastrointestinal tract. They have been proposed as a means of protecting against NEC. These supplements contain potentially beneficial bacteria or yeasts, most commonly Lactobacillus, Bifidobacterium, and Streptococcus strains.261 They can enhance the mucosal barrier by reducing permeability, increasing mucus production, inhibiting bacterial translocation, and strengthening tight junctions.42,262–267 Colonization with these organisms can reduce the ability of pathogenic bacteria to adhere to the intestinal mucosa.268,269 Probiotics have also been shown to increase the production of mucosal IgA and short-chain fatty acids that help the immature immune system.270–272 Additionally, they decrease intestinal inflammation through the reduction of proinflammatory cytokines, the increase of anti-inflammatory cytokines, and the increase of cytokine production by T-cells.273–276
Studies have examined the ability of probiotics to normalize intestinal flora and to prevent NEC. One randomized controlled trial showed that administration of Bifidobacterium breve within the first 24 hours and continued for 28 days can change the intestinal colonization rates, with increased levels of Lactobacillus and decreased counts of Enterobacter.277 Another study showed that administering Bifidobacterium probiotics to preterm infants lowered the levels of pathogenic species such as Enterobacter and Clostridium in their intestines compared with controls who did not receive the probiotics.278 These studies all suggest that the use of probiotic supplementation can influence intestinal colonization.
Once it was shown that such changes in intestinal flora could be manipulated, the next step lay in determining what clinical effect this might have on these preterm infants. One large prospective cohort study using historical controls evaluated whether newborns given Lactobacillus acidophilus and Bifidobacterium infantis would have reduced rates of NEC.279 They studied 1237 infants over one year with a mean gestational age of 35 weeks and mean birth weight of 2040 g. These infants were treated with each probiotic daily until discharge. The results were compared with historical controls from the preceding year. During the treatment year, the incidence of NEC was 3% compared with 6.6% the year before (p < 0.0002). Furthermore, no side effects were noted.
Subsequently, there have been many studies analyzing the effects of probiotic supplementation in preterm infants. The first meta-analysis in 2007 reviewed seven randomized controlled trials involving a total of 690 infants who received no treatment and 703 who received probiotics (Table 33-4).280 The relative risk for NEC in the group that received probiotics was 0.36 (95% CI: 0.20–0.65). These results must be considered with caution because this meta-analysis combined studies that had many significant differences. This heterogeneity normally would preclude the method of meta-analysis.291 Considerable variability among the studies in the demographics of the patients, the age at commencement of treatment, and the type, dose, and duration of probiotic treatment existed. Though this meta-analysis found no increased risk of sepsis in the treatment group, side effects of probiotics were not adequately addressed due to lack of power to detect serious infections. Given the high-risk population in which these probiotics would be used, the issue of safety is crucial.
TABLE 33-4
Clinical Trials Evaluating Probiotics and The Incidence of Necrotizing Enterocolitisa
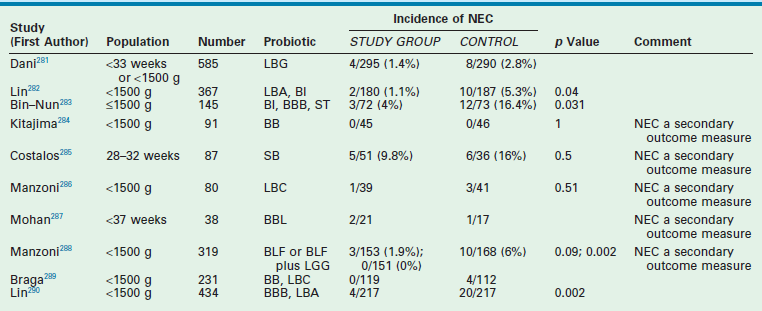
aSee references for further information.
A 2012 meta-analysis included 20 studies with a total of 3816 patients. For preterm VLBW patients receiving probiotic supplements, the incidence of stage II or higher NEC was 3% compared to 7.4% in the placebo group, with a RR of 0.33 (95% CI, .024–0.46; p < 0.00001).292 Due to differences in the type of probiotics used in the studies, subgroup analysis was performed; the three main probiotic agents (Bifidobacteria, Lactobacillus, and Bifidobacteria and Lactobacillus) all showed a significant risk reduction. Overall mortality was reduced in the group receiving probiotics as well: RR of 0.56 (95% CI 0.43–0.73; p < 0.0001). In regards to safety of probiotics, fourteen of the trials reported data for culture positive sepsis. In VLBW infants receiving probiotics, there was no difference in the risk of culture positive sepsis.
These studies suggest that probiotics are safe and effective in reducing the incidence of NEC and mortality without increasing the risk of sepsis. Despite the many studies available in the literature, there is heterogeneity in type of probiotic, timing of therapy, and dosing. Long-term effects are also not well studied. While probiotics are a very promising preventative strategy, questions still need to be addressed.
Epidermal Growth Factor
As discussed earlier, EGF plays an important role in the pathogenesis of NEC. Premature infants in general, and infants with NEC specifically, have been shown to have decreased salivary concentrations of EGF.87 Because EGF is known to support the maintenance of the intestinal barrier and downregulate proinflammatory cytokines,122 its use has been postulated to help prevent NEC. A preliminary study of neonates diagnosed with NEC has shown that administration of EGF will promote the repair of the intestinal epithelium. In animal models, supplementation with EGF has decreased the incidence of NEC.293 HB-EGF has been shown to have similar effects. Trials to test both EGF and HB-EGF as preventive strategies are planned.294
Conclusion
Despite many advances in the care of premature infants, NEC remains a challenging disease with a relatively constant incidence rate over the past four decades. The only clearly established risk factor is prematurity. Insight has been gained into the pathophysiology of this disease, with a unifying hypothesis emerging: an excessive and uncontrolled inflammatory response by the neonatal intestine after exposure to an inciting event. Future research efforts will focus on further elucidating the underlying causes and the molecular mechanisms that occur early in this pathogenic process. Because clinical parameters alone have not helped identify which children are at risk for developing the disease and progressing to serious disease,295 advanced approaches using proteomic and genomic techniques should be considered to compare those who develop NEC with those who do not, as well as those who progress to severe disease with those who have a mild course. Novel treatment strategies such as growth factors should be evaluated. Any trial of treatment should include evaluation of both short- and long-term outcomes. Finally, because no treatment for NEC will be uniformly effective once the disease is established, research efforts should focus on approaches to prevent this disease.
References
1. Obladen, M. Necrotizing enterocolitis–150 years of fruitless search for the cause. Neonatology. 2009; 96:203–210.
2. Santulli, TV, Schullinger, JN, Heird, WC, et al. Acute necrotizing enterocolitis in infancy: A review of 64 cases. Pediatrics. 1975; 55:376–387.
3. Touloukian, RJ, Berdon, WE, Amoury, RA, et al. Surgical experience with necrotizing enterocolitis in the infant. J Pediatr Surg. 1967; 2:389–401.
4. Llanos, AR, Moss, ME, Pinzon, MC, et al. Epidemiology of neonatal necrotising enterocolitis: A population-based study. Paediatr Perinat Epidemiol. 2002; 16:342–349.
5. Guthrie, SO, Gordon, PV, Thomas, V, et al. Necrotizing enterocolitis among neonates in the United States. J Perinatol. 2003; 23:278–285.
6. Horbar, JD, Badger, GJ, Carpenter, JH. Trends in mortality and morbidity for very low birth weight infants, 1991-1999. Pediatrics. 2002; 110:143–151.
7. Sankaran, K, Puckett, B, Lee, DS, et al. Variations in incidence of necrotizing enterocolitis in Canadian neonatal intensive care units. J Pediatr Gastroenterol Nutr. 2004; 39:366–372.
8. Stoll, BJ, Hansen, NI, Bell, EF, et al. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics. 2010; 126:443–456.
9. Guillet, R, Stoll, BJ, Cotten, CM, et al. Association of H2-blocker therapy and higher incidence of necrotizing enterocolitis in very low birth weight infants. Pediatrics. 2006; 117:e137–e142.
10. Holman, RC, Stoll, BJ, Curns, AT, et al. Necrotising enterocolitis hospitalisations among neonates in the United States. Paediatr Perinat Epidemiol. 2006; 20:498–506.
11. Lemons, JA, Bauer, CR, Oh, W, et al. Very low birth weight outcomes of the National Institute of Child health and human development neonatal research network, January 1995 through December 1996. NICHD Neonatal Research Network. Pediatrics. 2001; 107:E1.
12. Luig, M, Lui, K, et al. Epidemiology of necrotizing: enterocolitis: II. Risks and susceptibility of premature infants during the surfactant era: A regional study. NICUS group. J Pediatr Child Health. 2005; 41:174–179.
13. Holman, RC, Stoll, BJ, Clarke, MJ, et al. The epidemiology of necrotizing enterocolitis infant mortality in the United States. Am J Public Health. 1997; 87:2026–2031.
14. Sharma, R, Hudak, ML, Tepas, JJ, 3rd., et al. Impact of gestational age on the clinical presentation and surgical outcome of necrotizing enterocolitis. J Perinatol. 2006; 26:342–347.
15. Blakely, ML, Lally, KP, McDonald, S, et al. Postoperative outcomes of extremely low birth-weight infants with necrotizing enterocolitis or isolated intestinal perforation: A prospective cohort study by the NICHHD Neonatal Research Network. Ann Surg. 2005; 241:984–994.
16. Blakely, ML, Gupta, H, Lally, KP. Surgical management of necrotizing enterocolitis and isolated intestinal perforation in premature neonates. Semin Perinatol. 2008; 32:122–126.
17. Ng, S. Necrotizing enterocolitis in the full-term neonate [see comment]. J Paediatr Child Health. 2001; 37:1–4.
18. Lambert, DK, Christensen, RD, Henry, E, et al. Necrotizing enterocolitis in term neonates: Data from a multihospital health-care system [see comment]. J Perinatol. 2007; 27:437–443.
19. Ostlie, DJ, Spilde, TL, St Peter, SD, et al. Necrotizing enterocolitis in full-term infants. J Pediatr Surg. 2003; 38:1039–1042.
20. Bolisetty, S, Lui, K. Necrotizing enterocolitis in full-term neonates [comment]. J Pediatr Child Health. 2001; 37:413–414.
21. Bisquera, JA, Cooper, TR, Berseth, CL. Impact of necrotizing enterocolitis on length of stay and hospital charges in very low birth weight infants. Pediatrics. 2002; 109:423–428.
22. Spencer, AU, Kovacevich, D, McKinney-Barnett, M, et al. Pediatric short-bowel syndrome: the cost of comprehensive care. Am J Clin Nutr. 2008; 88:1552–1559.
23. Ballance, WA, Dahms, BB, Shenker, N, et al. Pathology of neonatal necrotizing enterocolitis: A ten-year experience. J Pediatr. 1990; 117:S6–13.
24. Hsueh, W, Caplan, MS, Qu, XW, et al. Neonatal necrotizing enterocolitis: Clinical considerations and pathogenetic concepts. Pediatr Dev Pathol. 2003; 6:6–23.
25. Sanderson, I. The physicochemical environment of the neonatal intestine. Am J Clin Nutr. 1999; 69:10285–10345.
26. Berseth, CL. Gestational evolution of small intestine motility in preterm and term infants. J Pediatr. 1989; 115:646–651.
27. Berseth, CL. Gastrointestinal motility in the neonate. Clin Perinatol. 1996; 23:179–190.
28. Milla, PJ. Intestinal motility during ontogeny and intestinal pseudo-obstruction in children. Pediatr Clin North Am. 1996; 43:511–532.
29. Lebenthal, A, Lebenthal, E. The ontogeny of the small intestinal epithelium. J Parenter Enteral Nutr. 1999; 23(Suppl. 5):S3–S6.
30. Di Lorenzo, M, Bass, J, Krantis, A. An intraluminal model of necrotizing enterocolitis in the developing neonatal piglet. J Pediatr Surg. 1995; 30:1138–1142.
31. Lin, J. Too much short chain fatty acids cause neonatal necrotizing enterocolitis. Med Hypotheses. 2004; 62:291–293.
32. Ford, HR. Mechanism of nitric oxide-mediated intestinal barrier failure: Insight into the pathogenesis of necrotizing enterocolitis. J Pediatr Surg. 2006; 41:294–299.
33. Halpern, MD, Holubec, H, Saunders, TA. Bile acids induce ileal damage during experimental necrotizing enterocolitis. Gastroenterology. 2006; 130:359–372.
34. Halpern, MD, Dvorak, B. Does abnormal bile acid metabolism contribute to NEC? Semin Perinatol. 2008; 32:114–121.
35. Hammons, JL, Jordan, WE, Stewart, RL. Age and diet effects on fecal bile acids in infants. J Pediatr Gastroenterol Nutr. 1988; 7:30–38.
36. Hunter, CJ, Upperman, JS, Ford, HR, et al. Understanding the susceptibility of the premature infant to necrotizing enterocolitis. Pediatr Res. 2008; 63:117–123.
37. McElroy, SJ, Prince, LS, Weitkamp, JH, et al. Tumor necrosis factor receptor 1-dependent depletion of mucus in immature small intestine: A potential role in neonatal necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol. 2011; 301:G656–G666.
38. Strous, GJ, Dekker, J. Mucin-type glycoproteins. Crit Rev Biochem Mol Biol. 1992; 27:57–92.
39. Allen, A, Bell, A, Mantle, M. The structure and physiology of gastrointestinal mucus. Adv Exp Med Biol. 1982; 144:115–133.
40. Montagne, L, Piel, C, Lalles, JP. Effect of diet on mucin kinetics and composition: Nutrition and health implications. Nutr Rev. 2004; 62:105–114.
41. Rhodes, JM. Colonic mucus and mucosal glycoproteins: The key to colitis and cancer? Gut. 1989; 30:1660–1666.
42. Deplancke, B, Gaskins, HR. Microbial modulation of innate defense: Goblet cells and the intestinal mucus layer. Am J Clin Nutr. 2001; 73:1131S–1141S.
43. Hackam, DJ, Upperman, JS, Grishin, A, et al. Disordered enterocyte signaling and intestinal barrier dysfunction in the pathogenesis of necrotizing enterocolitis. Semin Pediatr Surg. 2005; 14:49–57.
44. Ryley, HC, Rennie, D, Bradley, DM. The composition of a mucus glycoprotein from meconium of cystic fibrosis, healthy pre-term and full-term neonates. Clin Chim Acta. 1983; 135:49–56.
45. Corfield, AP, Myerscough, N, Longman, R, et al. Mucins and mucosal protection in the gastrointestinal tract: New prospects for mucins in the pathology of gastrointestinal disease. Gut. 2000; 47:589–594.
46. Kyo, K, Muto, T, Nagawa, H, et al. Associations of distinct variants of the intestinal mucin gene MUC3A with ulcerative colitis and Crohn’s disease. J Hum Genet. 2001; 46:5–20.
47. Vieten, D, Corfield, A, Carroll, D, et al. Impaired mucosal regeneration in neonatal necrotizing enterocolitis. Pediatr Surg Int. 2005; 21:153–160.
48. Liu, Z, Li, N, Neu, J. Tight junctions, leaky intestines, and pediatric diseases. Acta Paediatr. 2005; 94:386–393.
49. Anand, RJ, Leaphart, CL, Mollen, KP, et al. The role of the intestinal barrier in the pathogenesis of necrotizing enterocolitis. Shock. 2007; 27:124–133.
50. Muresan, Z, Paul, DL, Goodenough, DA. Occludin 1B, a variant of the tight junction protein occludin. Mol Biol Cell. 2000; 11:627–634.
51. Shen, L, Turner, JR. Role of epithelial cells in initiation and propagation of intestinal inflammation. Eliminating the static: Tight junction dynamics exposed. Am J Physiol Gastrointest Liver Physiol. 2006; 290:G577–G582.
52. Ford, H, Watkins, S, Reblock, K, et al. The role of inflammatory cytokines and nitric oxide in the pathogenesis of necrotizing enterocolitis. J Pediatr Surg. 1997; 32:275–282.
53. Han, X, Fink, MP, Delude, RL. Proinflammatory cytokines cause NO-dependent and independent changes in expression and localization of tight junction proteins in intestinal epithelial cells. Shock. 2003; 3:220–237.
54. Mowat, AM, Viney, JL. The anatomical basis of intestinal immunity. Immunol Rev. 1997; 156:145–166.
55. Neutra, MR, Mantis, NJ, Kraehenbuhl, JP. Collaboration of epithelial cells with organized mucosal lymphoid tissues. Nat Immunol. 2001; 1004–1009.
56. Puiman, PJ, Burger-Van Paassen, N, Schaart, MW, et al. Paneth cell hyperplasia and metaplasia in necrotizing enterocolitis. Pediatr Res. 2011; 69:217–223.
57. Ayabe, T, Satchell, DP, Wilson, CL, et al. Secretion of microbicidal alpha-defensins by intestinal Paneth cells in response to bacteria. Nat Immunol. 2000; 1:113–118.
58. Mayer, L. Mucosal immunity. Pediatrics. 2003; 111:1595–1600.
59. Ogra, SS, Weintraub, D, Ogra, PL. Immunologic aspects of human colostrum and milk: III. Fate and absorption of cellular and soluble components in the gastrointestinal tract of the newborn. J Immunol. 1977; 119:245–248.
60. Cetin, S, Ford, HR, Sysko, LR, et al. Endotoxin inhibits intestinal epithelial restitution through activation of Rho-GTPase and increased focal adhesions. J Biol Chem. 2004; 279:24592–24600.
61. Duffy, LC, Zielezny, MA, Carrion, V, et al. Concordance of bacterial cultures with endotoxin and interleukin-6 in necrotizing enterocolitis. Dig Dis Sci. 1997; 42:359–365.
62. Forsythe, RM, Xu, DZ, Lu, Q, et al. Lipopolysaccharide-induced enterocyte-derived nitric oxide induces intestinal monolayer permeability in an autocrine fashion. Shock. 2002; 17:180–184.
63. Grishin, A, Wang, J, Hackam, DJ, et al. p38 MAP kinase mediates endotoxin-induced expression of cyclooxygenase-2 in enterocytes. Surgery. 2004; 136:329–335.
64. Gonzalez-Crussi, F, Hsueh, W. Experimental model of ischemic bowel necrosis: The role of platelet-activating factor and endotoxin. Am J Pathol. 1983; 112:127–135.
65. Hsueh, W, Gonzalez-Crussi, F, Arroyave, JL. Platelet-activating factor-induced ischemic bowel necrosis: The effect of PAF antagonists. Eur J Pharmacol. 1986; 123:79–83.
66. Levy, RM, Prince, JM, Billiar, TR. Nitric oxide: A clinical primer. Crit Care Med. 2005; 33:S492–S495.
67. Chokshi, N, Guner, Y, Hunter, CJ, et al. The role of nitric oxide in intestinal epithelial injury and restitution in neonatal necrotizing enterocolitis. Semin Perinatol. 2008; 32:92–99.
68. Beckman, JS. Ischaemic injury mediator. Nature. 1990; 345:27–28.
69. Upperman, JS, Potoka, DA, Grishin, A. Mechanisms of nitric oxide mediated intestinal barrier failure in necrotizing enterocolitis. Semin Pediatr Surg. 2005; 14:159–166.
70. Hoffman, RA, Zhang, G, Nussler, NC. Constitutive expression of inducible nitric oxide synthase in the mouse ileal mucosa. Am J Physiol. 1997; 272(2Pt1):G383–G392.
71. Emami, CN, Chokshi, N, Wang, J, et al. Role of interleukin-10 in the pathogenesis of necrotizing enterocolitis. Am J Surg. 2012; 203:428–435.
72. Chokshi, N, Guner, Y, Hunter, CJ, et al. The role of nitric oxide in intestinal epithelial injury and restitution in neonatal necrotizing enterocolitis. Semin Perinatol. 2008; 32:92–99.
73. Snyder, F. Platelet-activating factor and related acetylated lipids as potent biologically active cellular mediators. Am J Physiol. 1990; 259:C697–C708.
74. Frost, BL, Jilling, T, Caplan, MS. The importance of proinflammatory signaling in neonatal necrotizing enterocolitis. Semin Perinatol. 2008; 32:100–106.
75. Caplan, MS, Simon, D, Jilling, T. The role of PAF, TLR, and the inflammatory response in neonatal necrotizing enterocolitis. Semin Pediatr Surg. 2005; 14:145–151.
76. Caplan, M, Hsueh, W, Kelly, A, et al. Serum PAF acetylhydrolase increases during neonatal maturation. Prostaglandins. 1990; 39:705–714.
77. Rabinowitz, SS, Dzakpasu, P, Piecuch, S, et al. Platelet-activating factor in infants at risk for necrotizing enterocolitis. J Pediatr. 2001; 138:81–86.
78. Caplan, MS, Lickerman, M, Adler, L, et al. The role of recombinant platelet-activating factor acetylhydrolase in a neonatal rat model of necrotizing enterocolitis. Pediatr Res. 1997; 42:779–783.
79. Coursodon, CF, Dvorak, B. Epidermal growth factor and necrotizing enterocolitis. Curr Opin Pediatr. 2012; 24:160–164.
80. Dvorak, B, Philips, AF, Koldovsky, O. Milk-borne growth factors and gut development. In: Zeigler E, Lucas A, Moro G, eds. Nutrition of the Very Low Birthweight Infant. Philadelphia: Lippincott Williams and Wilkins; 1999:245–255.
81. Pollack, PF, Goda, T, Colony, PC. Effects of enterally fed epidermal growth factor on the small and large intestine of the suckling rat. Regul Pept. 1987; 17:121–132.
82. Playford, R, Wright, N. Why is epidermal growth factor present in the gut lumen. Gut. 1996; 38:303–305.
83. Barnard, JA, Beauchamp, D, Russel, W. Epidermal growth factor-related peptides and their relevance to gastrointestinal pathophysiology. Gastroenterology. 1995; 108:564–580.
84. Warner, B, Warner, B. Role of epidermal growth factor in the pathogenesis of neonatal necrotizing enterocolitis. Semin Pediatr Surg. 2005; 14:175–180.
85. Hirai, C, Ichiba, H, Saito, M. Trophic effect of multiple growth factors in amniotic fluid or human milk on cultured human fetal small intestinal cells. J Pediatr Gastroenterol Nutr. 2002; 34:524–528.
86. Chen, CL, Yu, X, James, IO, et al. Heparin-binding EGF-like growth factor protects intestinal stem cells from injury in a rat model of necrotizing enterocolitis. Lab Invest. 2012; 92:331–344.
87. Shin, CE, Falcone, RA, Jr., Stuart, L, et al. Diminished epidermal growth factor levels in infants with necrotizing enterocolitis. J Pediatr Surg. 2000; 35:173–177.
88. Warner, B, Ryan, A, Seeger, K. Ontogeny of salivary epidermal growth factor and necrotizing enterocolitis. J Pediatr. 2007; 150:358–363.
89. Sullivan, PB, Brueton, MJ, Tabara, ZB. Epidermal growth factor in necrotising enteritis. Lancet. 1991; 338:53–54.
90. Sullivan, PB, Lewindon, PJ, Cheng, C. Intestinal mucosal remodeling by recombinant human EGF in neonates with severe necrotizing enterocolitis. J Pediatr Surg. 2007; 42:462–469.
91. Christensen, RD, Gordon, PV, Besner, GE. Can we cut the incidence of necrotizing enterocolitis in half–today? Fetal Pediatr Pathol. 2010; 29:185–198.
92. Feng, J, El-Assal, O, Besner, GE. Heparin-binding EGF-like growth factor (HB-EGF) and necrotizing enterocolitis. Semin Pediatr Surg. 2005; 14:167–174.
93. Feng, J, Besner, GE. Heparin-binding epidermal growth factor-like growth factor promotes enterocyte migration and proliferation in neonatal rats with necrotizing enterocolitis. J Pediatr Surg. 2007; 42:214–220.
94. Feng, J, El-Assal, ON, Besner, GE. Heparin-binding epidermal growth factor-like growth factor reduces intestinal apoptosis in neonatal rats with necrotizing enterocolitis. J Pediatr Surg. 2006; 41:742–747.
95. Feng, J, El-Assal, ON, Besner, GE. Heparin-binding epidermal growth factor-like growth factor decreases the incidence of necrotizing enterocolitis in neonatal rats. J Pediatr Surg. 2006; 41:144–149.
96. Radulescu, A, Yu, X, Orvets, ND, et al. Deletion of the heparin-binding epidermal growth factor-like growth factor gene increases susceptibility to necrotizing enterocolitis. J Pediatr Surg. 2010; 45:729–734.
97. Radulescu, A, Zhang, HY, Yu, X, et al. Heparin-binding epidermal growth factor-like growth factor overexpression in transgenic mice increases resistance to necrotizing enterocolitis. J Pediatr Surg. 2010; 45:1933–1939.
98. Nankervis, CA, Nowicki, P. Role of nitric oxide in regulation of vascular resistance in postnatal intestine. Am J Physiol. 1995; 268:G949–G958.
99. Reber, KM, Mager, GM, Miller, CE. Relationship between flow rate and NO production in postnatal mesenteric arteries. Am J Physiol. 2000; 280:G43–G50.
100. Nowicki, P. Intestinal ischemia and necrotizing enterocolitis. J Pediatr. 1990; 117:S14–S19.
101. Reber, KM, Nankervis, CA, Nowicki, PT. Newborn intestinal circulation. Physiology and pathophysiology. Clin Perinatol. 2002; 29:23–39.
102. Nowicki, P. Ischemia and necrotizing enterocolitis: Where, when and how. Semin Pediatr Surg. 2005; 14:152–158.
103. Nankervis, CA, Dunaway, DJ, Nowicki, P. Role of endothelin-1 in regulation of the postnatal intestinal circulation. Am J Physiol. 2000; 278:G367–G375.
104. Yanagisawa, M, Kurihara, H, Kimura, S. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1998; 332:411–415.
105. Kuchan, MJ, Frangos, JA. Shear stress regulates endothelin-1 release via protein kinase C and cGMP in cultured endothelial cells. Am J Physiol. 1993; 264:H150–H156.
106. Kourembanas, S, Marsden, PA, McQuillan, LP. Hypoxia induces endothelin gene expression and secretion in cultured human endothelium. J Clin Invest. 1991; 88:1054–1057.
107. Woods, M, Mitchel, JA, Wood, EG. Endothelin-1 is induced by cytokines in human vascular smooth muscle cells: Evidence for intracellular endothelin converting enzyme. Mol Pharmacol. 1999; 55:902–909.
108. Ito, Y, Doelle, S, Clark, JA. Intestinal microcirculatory dysfunction during the development of experimental necrotizing enterocolitis. Pediatr Res. 2007; 61:180–184.
109. Lin, PW, Nasr, TR, Stoll, BJ. Necrotizing enterocolitis: Recent scientific advances in pathophysiology and prevention. Semin Perinatol. 2008; 32:70–82.
110. Kosloske, AM. Indications for operation in necrotizing enterocolitis revisited. J Pediatr Surg. 1994; 29:663–666.
111. Dolgin, SE, Shlasko, E, Levitt, MA, et al. Alterations in respiratory status: Early signs of severe necrotizing enterocolitis. J Pediatr Surg. 1998; 33:856–858.
112. Bell, MJ. Neonatal necrotizing enterocolitis. N Engl J Med. 1978; 298:281–282.
113. Kliegman, RJ, Walsh, MC. Neonatal necrotizing enterocolitis: Pathogenesis, classification and spectrum of disease. Curr Probl Pediatr. 1987; 26:327–344.
114. Kafetzis, DA, Skevaki, C, Costalos, C. Neonatal necrotizing enterocolitis: An overview. Curr Opin Infect Dis. 2003; 16:349–355.
115. Noerr, B. Current controversies in the understanding of necrotizing enterocolitis: I. Adv Neonatal Care. 2003; 3:107–120.
116. Romagnoli, C, Frezza, S, Cingolani, A, et al. Plasma levels of interleukin-6 and interleukin-10 in preterm neonates evaluated for sepsis. Eur J Pediatr. 2001; 160:345–350.
117. Isaacs, D, North, J, Lindsell, D, et al. Serum acute phase reactants in necrotizing enterocolitis. Acta Paediatr Scand. 1987; 76:923–927.
118. Pourcyrous, M, Korones, S, Yang, W, et al. C-reactive protein in the diagnosis, management, and prognosis of neonatal necrotizing enterocolitis. Pediatrics. 2005; 116:1064–1069.
119. Edelson, MB, Bagwell, CE, Rozycki, HJ. Circulating pro- and counterinflammatory cytokine levels and severity in necrotizing enterocolitis. Pediatrics. 1999; 103:766–771.
120. Kosloske, AM. Epidemiology of necrotizing enterocolitis. Acta Paediatr Suppl. 1994; 396:2–7.
121. Chandler, JC, Hebra, A. Necrotizing enterocolitis in infants with very low birth weight. Semin Pediatr Surg. 2000; 9:63–72.
122. Kennedy, J, Holt, CL, Ricketts, RR. The significance of portal vein gas in necrotizing enterocolitis. Am Surg. 1987; 53:231–234.
123. Faingold, R, Daneman, A, Tomlinson, G. Necrotizing enterocolitis: Assessment of bowel viability with color Doppler ultrasound. Radiology. 2005; 235:587–594.
124. Kim, WY, Kim, WS, Kim, IO, et al. Sonographic evaluation of neonates with early-stage necrotizing enterocolitis. Pediatr Radiol. 2005; 35:1056–1061.
125. Kim, WY, Kim, IO, Kim, WS. Bowel sonography in necrotizing enterocolitis: Histopathologic correlation in experimental studies. Pediatr Radiol. 2005; 35(Suppl):S51.
126. Azarow, K, Connolly, B, Babyn, P, et al. Multidisciplinary evaluation of the distended abdomen in critically ill infants and children: The role of bedside sonography. Pediatr Surg Int. 1998; 13:355–359.
127. Silva, CT, Daneman, A, Navarro, OM, et al. Correlation of sonographic findings and outcome in necrotizing enterocolitis. Pediatr Radiol. 2007; 37:274–282.
128. Epelman, M, Daneman, A, Navarro, OM, et al. Necrotizing enterocolitis: Review of state-of-the-art imaging findings with pathologic correlation. RadioGraphics. 2007; 27:285–305.
129. Buonomo, C. The radiology of necrotizing enterocolitis. Radiol Clin North Am. 1999; 37:1187–1198.
130. Kao, SC, Smith, WL, Franken, EA, Jr., et al. Contrast enema diagnosis of necrotizing enterocolitis. Pediatr Radiol. 1992; 22:115–117.
131. Schwartz, MZ, Hayden, CK, Richardson, CJ, et al. A prospective evaluation of intestinal stenosis following necrotizing enterocolitis. J Pediatr Surg. 1982; 17:764–770.
132. Rencken, IO, Sola, A, al-Ali, F, et al. Necrotizing enterocolitis: Diagnosis with CT examination of urine after enteral administration of iodinated water-soluble contrast material. Radiology. 1997; 205:87–90.
133. Maalouf, EF, Fagbemi, A, Duggan, PJ, et al. Magnetic resonance imaging of intestinal necrosis in preterm infants. Pediatrics. 2000; 105:510–514.
134. Meyer, CL, Payne, NR, Roback, SA. Spontaneous, isolated intestinal perforations in neonates with birth weight less than 1,000 g not associated with necrotizing enterocolitis. J Pediatr Surg. 1991; 26:714–717.
135. Mintz, AC, Applebaum, H. Focal gastrointestinal perforations not associated with necrotizing enterocolitis in very low birth weight neonates. J Pediatr Surg. 1993; 28:857–860.
136. Aschner, JL, Deluga, KS, Metlay, LA, et al. Spontaneous focal gastrointestinal perforation in very low birth weight infants. J Pediatr. 1988; 113:364–367.
137. Azarow, KS, Ein, SH, Shandling, B, et al. Laparotomy or drain for perforated necrotizing enterocolitis: Who gets what and why? Pediatr Surg Int. 1997; 12:137–139.
138. Rovin, JD, Rodgers, BM, Burns, RC, et al. The role of peritoneal drainage for intestinal perforation in infants with and without necrotizing enterocolitis. J Pediatr Surg. 1999; 34:143–147.
139. Okuyama, H, Kubota, A, Oue, T, et al. A comparison of the clinical presentation and outcome of focal intestinal perforation and necrotizing enterocolitis in very-low-birth-weight neonates. Pediatr Surg Int. 2002; 18:704–706.
140. Tarrado, X, Castanon, M, Thio, M, et al. Comparative study between isolated intestinal perforation and necrotizing enterocolitis. Eur J Pediatr Surg. 2005; 15:88–94.
141. Pumberger, W, Mayr, M, Kohlhauser, C, et al. Spontaneous localized intestinal perforation in very-low-birth-weight infants: A distinct clinical entity different from necrotizing enterocolitis. J Am Coll Surg. 2002; 195:796–803.
142. Buchheit, JQ, Stewart, DL. Clinical comparison of localized intestinal perforation and necrotizing enterocolitis in neonates [see comment]. Pediatrics. 1994; 93:32–36.
143. Cass, DL, Brandt, ML, Patel, DL, et al. Peritoneal drainage as definitive treatment for neonates with isolated intestinal perforation. J Pediatr Surg. 2000; 35:1531–1536.
144. Lessin, MS, Luks, FI, Wesselhoeft, CW. Peritoneal drainage as definitive treatment for intestinal perforation in infants with extremely low birth weight (<750 gms). J Pediatr Surg. 1998; 33:370–372.
145. Gordon, PV, Swanson, JR, Attridge, JT, et al. Emerging trends in acquired neonatal intestinal disease: Is it time to abandon Bell’s criteria. J Perinatol. 2007; 27:661–671.
146. Cribbs, RK, Harding, PA, Luquette, MH. Endogenous production of heparin-binding EGF-like growth factor during murine partial thickness burn wound healing. J Burn Care Rehabil. 2002; 23:116–125.
147. Jin, K, Mao, XO, Sun, Y. Heparin-binding epidermal growth factor-like growth factor: Hypoxia-inducible expression in vitro and stimulation of neurogenesis in vitro and in vivo. J Neurosci. 2002; 22:5365–5373.
148. Frank, GD, Mifune, M, Inagami, T. Distinct mechanisms of receptor and nonreceptor tyrosine kinase activation by reactive oxygen species in vascular smooth muscle cells: Role of metalloprotease and protein kinase C-delta. Mol Cell Biol. 2003; 23:1581–1589.
149. Kayanoki, Y, Higashiyama, S, Suzuki, K. The requirement of both intracellular reactive oxygen species and intracellular calcium elevation for the induction of heparin-binding EGF-like growth factor in vascular endothelial cells and smooth muscle cells. Biochem Biophys Res Commun. 1999; 259:50–55.
150. Marikovsky, M, Breuing, K, Liu, PY. Appearance of heparin-binding EGF-like growth factor in wound fluid as a response to injury. Proc Natl Acad Sci U S A. 1993; 90:3889–3893.
151. McCarthy, DW, Downing, MT, Brigstock, DR. Production of heparin-binding epidermal growth factor-like growth factor (HBEGF) at sites of thermal injury in pediatric patients. J Invest Dermatol. 1996; 106:49–56.
152. Xia, G, Rachfal, AW, Martin, AE. Upregulation of endogenous heparin-binding EGF-like growth factor (HB-EGF) expression after intestinal ischemia reperfusion injury. J Invest Surg. 2003; 16:57–63.
153. El-Assal, O, Besner, GE. Heparin-binding epidermal growth factor-like growth factor and intestinal ischemia-reperfusion injury. Semin Pediatr Surg. 2004; 13:2–10.
154. Michalsky, MP, Kuhn, A, Mehta, V. Heparin-binding EGF-like growth factor decreases apoptosis in intestinal epithelial cells in vitro. J Pediatr Surg. 2001; 36:1130–1135.
155. Pillai, SB, Turman, MA, Besner, GE. Heparin-binding EGF-like growth factor is cytoprotective for intestinal epithelial cells exposed to hypoxia. J Pediatr Surg. 1998; 33:973–978.
156. Lara-Marquez, ML, Mehta, V, Michalsky, MP. Heparin-binding EGF-like growth factor down regulates proinflammatory cytokine-induced nitric oxide and inducible nitric oxide synthase production in intestinal epithelial cells. Nitric Oxide. 2002; 6:142–152.
157. Mehta, VB, Besner, GE. Inhibition of NF-kappa B activation and its target genes by heparin-binding epidermal growth factor-like growth factor. J Immunol. 2003; 171:6014–6022.
158. Halpern, M, Dominguez, JA, Dvorakova, K. Ileal cytokine dysregulation in experimental necrotizing enterocolitis is reduced by epidermal growth factor. J Pediatr Gastroenterol Nutr. 2003; 36:126–133.
159. Ricketts, RR, Jerles, ML. Neonatal necrotizing enterocolitis: Experience with 100 consecutive surgical patients. World J Surg. 1990; 14:600–605.
160. Albanese, CT, Rowe, MI. Necrotizing enterocolitis. Semin Pediatr Surg. 1995; 4:200–206.
161. Grosfeld, JL, Cheu, H, Schlatter, M, et al. Changing trends in necrotizing enterocolitis: Experience with 302 cases in two decades. Ann Surg. 1991; 214:300–307.
162. Ein, SH, Marshall, DG, Girvan, D. Peritoneal drainage under local anesthesia for perforations from necrotizing enterocolitis. J Pediatr Surg. 1977; 12:963–967.
163. Moss, RL, Dimmitt, RA, Henry, MC, et al. A meta-analysis of peritoneal drainage versus laparotomy for perforated necrotizing enterocolitis. J Pediatr Surg. 2001; 36:1210–1213.
164. Blakely, ML, Tyson, JE, Lally, KP, et al. Laparotomy versus peritoneal drainage for necrotizing enterocolitis or isolated intestinal perforation in extremely low birth weight infants: Outcomes through 18 months adjusted age. Pediatrics. 2006; 117:e680–e687.
165. Moss, RL, Dimmitt, RA, Barnhart, DC, et al. Laparotomy versus peritoneal drainage for necrotizing enterocolitis and perforation [see comment] [erratum appears in N Engl J Med. 2006 Aug 24;355(8):856]. N Engl J Med. 2006; 354:2225–2234.
166. Rees C M, Eaton S, Khoo AK. Peritoneal drainage or laparotomy in neonatal bowel perforation? A randomized controlled trial. Presented at the American Pediatric Surgical Association, Orlando, FL, 38th Annual Meeting, May 2007.
167. Laparotomy vs. Drainage for Infants With Necrotizing Enterocolitis (NEST). Available at http://www.clinicaltrial.gov/ct2/show/NCT01029353, 2012. [Accessed 06/24, 2012].
168. Nadler, EP, Upperman, JS, Ford, HR. Controversies in the management of necrotizing enterocolitis. Surg Infect. 2001; 2:113–120.
169. Cooper, A, Ross, AJ, 3rd., O’Neill, JA, Jr., et al. Resection with primary anastomosis for necrotizing enterocolitis: A contrasting view. J Pediatr Surg. 1988; 23:64–68.
170. Weber, TR, Lewis, JE. The role of second-look laparotomy in necrotizing enterocolitis. J Pediatr Surg. 1986; 21:323–325.
171. Vaughan, WG, Grosfeld, JL, West, K, et al. Avoidance of stomas and delayed anastomosis for bowel necrosis: The ‘clip and drop-back’ technique. J Pediatr Surg. 1996; 31:542–545.
172. Luzzatto, C, Previtera, C, Boscolo, R, et al. Necrotizing enterocolitis: Late surgical results after enterostomy without resection. Eur J Pediatr Surg. 1996; 6:92–94.
173. Lee, JS, Polin, RA. Treatment and prevention of necrotizing enterocolitis. Semin Neonatol. 2003; 8:449–459.
174. Stringer, MD, Brereton, RJ, Drake, DP, et al. Recurrent necrotizing enterocolitis. J Pediatr Surg. 1993; 28:979–981.
175. Frantz, ID, 3rd., L’Heureux, P, Engel, RR, et al. Necrotizing enterocolitis. J Pediatr. 1975; 86:259–263.
176. Vollman, JH, Smith, WL, Tsang, RC. Necrotizing enterocolitis with recurrent hepatic portal venous gas. J Pediatr. 1976; 88:486–487.
177. Mollitt, DL, Golladay, ES. Postoperative neonatal necrotizing enterocolitis. J Pediatr Surg. 1982; 17:757–763.
178. Oldham, KT, Coran, AG, Drongowski, RA, et al. The development of necrotizing enterocolitis following repair of gastroschisis: A surprisingly high incidence. J Pediatr Surg. 1988; 23:945–949.
179. Shanbhogue, LK, Tam, PK, Lloyd, DA. Necrotizing enterocolitis following operation in the neonatal period. Br J Surg. 1991; 78:1045–1047.
180. Ladd, AP, Rescorla, FJ, West, KW, et al. Long-term follow-up after bowel resection for necrotizing enterocolitis: Factors affecting outcome. J Pediatr Surg. 1998; 33:967–972.
181. Bisquera, JA, Cooper, TR, Berseth, CL. Impact of necrotizing enterocolitis on length of stay and hospital charges in very low birth weight infants. Pediatrics. 2002; 109:423–428.
182. Limpert, JN, Limpert, PA, Weber, TR, et al. The impact of surgery on infants born at extremely low birth weight. J Pediatr Surg. 2003; 38:924–927.
183. Patel, JC, Tepas, JJ, 3rd., Huffman, SD, et al. Neonatal necrotizing enterocolitis: The long-term perspective. Am Surg. 1998; 64:575–580.
184. Kliegman, RM, Fanaroff, AA. Necrotizing enterocolitis. N Engl J Med. 1984; 310:1093–1103.
185. Touloukian, RJ. Neonatal necrotizing enterocolitis: An update on etiology, diagnosis, and treatment. Surg Clin North Am. 1976; 56:281–298.
186. Kurscheid, T, Holschneider, AM. Necrotizing enterocolitis (NEC)—mortality and long-term results. Eur J Pediatr Surg. 1993; 3:139–143.
187. Horwitz, JR, Lally, KP, Cheu, HW, et al. Complications after surgical intervention for necrotizing enterocolitis: A multicenter review. J Pediatr Surg. 1995; 30:994–999.
188. de Souza, JC, da Motta, UI, Ketzer, CR. Prognostic factors of mortality in newborns with necrotizing enterocolitis submitted to exploratory laparotomy. J Pediatr Surg. 2001; 36:482–486.
189. Cikrit, D, Mastandrea, J, West, KW, et al. Necrotizing enterocolitis: Factors affecting mortality in 101 surgical cases. Surgery. 1984; 96:648–655.
190. Ricketts, RR. Surgical treatment of necrotizing enterocolitis and the short bowel syndrome. Clin Perinatol. 1994; 21:365–387.
191. Wilmore, DW. Factors correlating with a successful outcome following extreme intestinal resection in newborn infants. J Pediatr. 1972; 80:88–95.
192. Fasching, G, Hollwarth, ME, Schmidt, B, et al. Surgical strategies in very-low-birthweight neonates with necrotizing enterocolitis. Acta Paediatr Suppl. 1994; 396:62–64.
193. Beasley, SW, Auldist, AW, Ramanjuan, TM. The surgical management of neonatal necrotizing enterocolitis: 1975-1984. Pediatr Surg Int. 1994; 1:210–217.
194. Albanese, CT, Rowe, MI, et al. Necrotizing enterocolitis. In: O’Neill JA, Jr., Rowe MI, Grosfeld J, eds. Pediatric Surgery. St. Louis: Mosby; 1998:1297–1320.
195. Thompson, JS, Quigley, EMM, Adrian, TE. Effect of intestinal tapering and lengthening on intestinal structure and function. Am J Surg. 1995; 169:111–119.
196. Goulet, OJ, Revillon, Y, Jan, D, et al. Neonatal short bowel syndrome. J Pediatr. 1991; 119:18–23.
197. Georgeson, KE, Breaux, CW, Jr. Outcome and intestinal adaptation in neonatal short-bowel syndrome. J Pediatr Surg. 1992; 27:344–350.
198. Chaet, MS, Farrell, MK, Ziegler, MM, et al. Intensive nutritional support and remedial surgical intervention for extreme short bowel syndrome. J Pediatr Gastroenterol Nutr. 1994; 19:295–298.
199. Fasoli, L, Turi, RA, Spitz, L, et al. Necrotizing enterocolitis: Extent of disease and surgical treatment. J Pediatr Surg. 1999; 34:1096–1099.
200. Andorsky, DJ, Lund, DP, Lillehei, CW, et al. Nutritional and other postoperative management of neonates with short bowel syndrome correlates with clinical outcomes. J Pediatr. 2001; 139:27–33.
201. Cooper, A, Floyd, TF, Ross, AJ, 3rd., et al. Morbidity and mortality of short-bowel syndrome acquired in infancy: An update. J Pediatr Surg. 1984; 19:711–718.
202. Weber, TR, Tracy, T, Jr., Connors, RH. Short-bowel syndrome in children: Quality of life in an era of improved survival. Arch Surg. 1991; 126:841–846.
203. Gauderer, MW, et al. Stomas of the small and large intestine. In: O’Neill JA, Jr., Rowe MI, Grosfeld J, eds. Pediatric Surgery. St. Louis, CV: Mosby; 1998:1349–1359.
204. Musemeche, CA, Kosloske, AM, Ricketts, RR. Enterostomy in necrotizing enterocolitis: An analysis of techniques and timing of closure. J Pediatr Surg. 1987; 22:479–483.
205. Alaish, SM, Krummel, TM, Bagwell, CE, et al. Loop enterostomy in newborns with necrotizing enterocolitis. J Am Coll Surg. 1996; 182:457–458.
206. O’Connor, A, Sawin, RS. High morbidity of enterostomy and its closure in premature infants with necrotizing enterocolitis. Arch Surg. 1998; 133:875–880.
207. Lemelle, JL, Schmitt, M, de Miscault, G, et al. Neonatal necrotizing enterocolitis: A retrospective and multicentric review of 331 cases. Acta Paediatr Suppl. 1994; 396:70–73.
208. Haberlik, A, Hollwarth, ME, Windhager, U, et al. Problems of ileostomy in necrotizing enterocolitis. Acta Paediatr Suppl. 1994; 396:74–76.
209. Cogbill, TH, Millikan, JS. Reconstitution of intestinal continuity after resection for neonatal necrotizing enterocolitis. Surg Gynecol Obstet. 1985; 160:330–334.
210. Gertler, JP, Seashore, JH, Touloukian, RJ. Early ileostomy closure in necrotizing enterocolitis. J Pediatr Surg. 1987; 22:140–143.
211. Sugarman, ID, Kiely, EM. Is there a role for high jejunostomy in the management of severe necrotizing enterocolitis? Pediatr Surg Int. 2001; 17:122–124.
212. Ricketts, RR. Surgical therapy for necrotizing enterocolitis. Ann Surg. 1984; 200:653–657.
213. O’Neill, JA, Jr., Holcomb, GW, Jr. Surgical experience with neonatal necrotizing enterocolitis (NNE). Ann Surg. 1979; 189:612–619.
214. Rowe, MI. Necrotizing enterocolitis. In: Welch KJ, Randolph JG, Ravitch MM, eds. Pediatric Surgery. Chicago: Year Book Medical; 1986:944–958.
215. Gobet, R, Sacher, P, Schwobel, MG. Surgical procedures in colonic strictures after necrotizing enterocolitis. Acta Paediatr Suppl. 1994; 396:77–79.
216. Schullinger, JN, Mollitt, DL, Vinocur, CD, et al. Neonatal necrotizing enterocolitis: Survival, management, and complications: A 25-year study. Am J Dis Child. 1981; 135:612–614.
217. Schimpl, G, Hollwarth, ME, Fotter, R, et al. Late intestinal strictures following successful treatment of necrotizing enterocolitis. Acta Paediatr Suppl. 1994; 396:80–83.
218. Radhakrishnan, J, Blechman, G, Shrader, C, et al. Colonic strictures following successful medical management of necrotizing enterocolitis: A prospective study evaluating early gastrointestinal contrast studies. J Pediatr Surg. 1991; 26:1043–1046.
219. Hartman, GE, Drugas, GT, Shochat, SJ. Post-necrotizing enterocolitis strictures presenting with sepsis or perforation: Risk of clinical observation. J Pediatr Surg. 1988; 23:562–566.
220. Butter, A, Flageole, H, Laberge, JM. The changing face of surgical indications for necrotizing enterocolitis. J Pediatr Surg. 2002; 37:496–499.
221. Kosloske, AM, Burstein, J, Bartow, SA. Intestinal obstruction due to colonic stricture following neonatal necrotizing enterocolitis. Ann Surg. 1980; 192:202–207.
222. Weber, TR, Tracy, TF, Jr., Silen, ML, et al. Enterostomy and its closure in newborns. Arch Surg. 1995; 130:534–537.
223. Bell, MJ, Ternberg, JL, Askin, FB, et al. Intestinal stricture in necrotizing enterocolitis. J Pediatr Surg. 1976; 11:319–327.
224. Schwartz, MZ, Richardson, CJ, Hayden, CK, et al. Intestinal stenosis following successful medical management of necrotizing enterocolitis. J Pediatr Surg. 1980; 15:890–899.
225. Kosloske, AM. Surgery of necrotizing enterocolitis. World J Surg. 1985; 9:277–284.
226. Janik, JS, Ein, SH, Mancer, K. Intestinal stricture after necrotizing enterocolitis. J Pediatr Surg. 1981; 16:438–443.
227. Robertson, JF, Azmy, AF, Young, DG. Surgery for necrotizing enterocolitis. Br J Surg. 1987; 74:387–389.
228. Born, M, Holgersen, LO, Shahrivar, F, et al. Routine contrast enemas for diagnosing and managing strictures following nonoperative treatment of necrotizing enterocolitis. J Pediatr Surg. 1985; 20:461–463.
229. Tonkin, IL, Bjelland, JC, Hunter, TB, et al. Spontaneous resolution of colonic strictures caused by necrotizing enterocolitis: Therapeutic implications. AJR Am J Roentgenol. 1978; 130:1077–1081.
230. Ball, WS, Jr., Kosloske, AM, Jewell, PF, et al. Balloon catheter dilatation of focal intestinal strictures following necrotizing enterocolitis. J Pediatr Surg. 1985; 20:637–639.
231. Stanford, A, Upperman, JS, Boyle, P, et al. Long-term follow-up of patients with necrotizing enterocolitis. J Pediatr Surg. 2002; 37:1048–1050.
232. Orellana, CB, Orellana, FA, Friesen, C. Long-term follow-up: Neurodevelopmental outcome and gastrointestinal function in infants < 801 grams diagnosed with necrotizing enterocolitis. Pediatr Res. 2003; 54:773–782.
233. Whiteman, L, Wuethrich, M, Egan, E. Infants who survive necrotizing enterocolitis. Matern Child Nurs J. 1985; 14:123–133.
234. Sonntag, J, Grimmer, I, Scholz, T, et al. Growth and neurodevelopmental outcome of very low birthweight infants with necrotizing enterocolitis. Acta Paediatr. 2000; 89:528–532.
235. Walsh, MC, Kliegman, RM, Hack, M. Severity of necrotizing enterocolitis: Influence on outcome at 2 years of age. Pediatrics. 1989; 84:808–814.
236. Stevenson, DK, Kerner, JA, Malachowski, N, et al. Late morbidity among survivors of necrotizing enterocolitis. Pediatrics. 1980; 66:925–927.
237. Cikrit, D, West, KW, Schreiner, R, et al. Long-term follow-up after surgical management of necrotizing enterocolitis: Sixty-three cases. J Pediatr Surg. 1986; 21:533–535.
238. Castro, L, Yolton, K, Haberman, B, et al. Bias in reported neurodevelopmental outcomes among extremely low birth weight survivors. Pediatrics. 2004; 114:404–410.
239. Hintz, SR, Kendrick, DE, Stoll, BJ, et al. Neurodevelopmental and growth outcomes of extremely low birth weight infants after necrotizing enterocolitis. Pediatrics. 2005; 115:696–703.
240. Schulzke, SM, Deshpande, GC, Patole, SK. Neurodevelopmental outcomes of very low-birth-weight infants with necrotizing enterocolitis: A systematic review of observational studies [review]. Arch Pediatr Adolesc Med. 2007; 161:583–590.
241. Rees, CM, Pierro, A, Eaton, S. Neurodevelopmental outcomes of neonates with medically and surgically treated necrotizing enterocolitis [review]. Arch Dis Child Fetal Neonatal Ed. 2007; 92:F193–F198.
242. Svenningsen, N, Lindroth, M, Lindquist, B. A comparative study of varying protein intake in low birth weight infant feeding. Acta Paediatr Scand Suppl. 1982; 296:28–31.
243. Gross, S. Growth and biochemical response of preterm infants fed human milk or modified infant formula. N Engl J Med. 1983; 308:237–241.
244. Tyson, JE, Lasky, RE, Mize, CE. Growth, metabolic response, and development in very-low-birth-weight infants fed banked human milk or enriched formula. J Pediatr. 1983; 103:95–104.
245. Cooper, PA, Rothberg, AD, Pettifor, J. Growth and biochemical response of premature infants fed pooled preterm milk or special formula. J Pediatr Gastroenterol Nutr. 1984; 3:749–754.
246. Lucas, A, Cole, T. Breast milk and neonatal necrotising enterocolitis. Lancet. 1990; 336:1519–1523.
247. Boyd, CA, Quigley, MA, Brocklehurst, P. Donor breast milk versus infant formula for preterm infants: Systematic review and meta-analysis [see comment]. Arch Dis Child Fetal Neonatal Ed. 2007; 92:F169–F175.
248. McGuire, W, Anthony, MY. Donor human milk versus formula for preventing necrotizing enterocolitis in preterm infants. Arch Dis Child Fetal Neonatal Ed. 2003; 88:F11–F15.
249. Sullivan, S, Schanler, RJ, Kim, JH, et al. An exclusively human milk-based diet is associated with a lower rate of necrotizing enterocolitis than a diet of human milk and bovine milk-based products. J Pediatr. 2010; 156:562–567.
250. Kuschel, CA, Harding, JE. Multicomponent fortified human milk for promoting growth in preterm infants. Cochrane Database Syst Rev. 2002. [CD000343].
251. Kennedy, KA, Tyson, JE. Early versus delayed initiation of progressive enteral feedings for parenterally fed low birth weight or preterm infants. Cochrane Database Syst Rev. 2000. [CD001970].
252. Kennedy, KA, Tyson, JE. Rapid versus slow rate of advancement of feedings for promoting growth and preventing necrotizing enterocolitis in parenterally fed low-birth-weight infants. Cochrane Database Syst Rev. 1998. [CD001241].
253. Berseth, CL, Bisquera, JA, Paje, VU. Prolonging small feeding volumes early in life decreases the incidence of necrotizing enterocolitis in very low birth weight infants [see comment]. Pediatrics. 2003; 111:529–534.
254. Amin, HJ, Zamora, SA, McMillan, DD, et al. Arginine supplementation prevents necrotizing enterocolitis in the premature infant [see comment]. J Pediatr. 2002; 140:425–431.
255. Shah, P, Shah, V. Arginine supplementation for prevention of necrotising enterocolitis in preterm infants. Cochrane Database Syst Rev. 2011. [CD004339].
256. Becker, RM, Wu, G, Galanko, JA, et al. Reduced serum amino acid concentrations in infants with necrotizing enterocolitis [see comment]. J Pediatr. 2000; 137:785–793.
257. Vaughn, P, Thomas, P, Clark, R. Enteral glutamine supplementation and morbidity in low birth weight infants. J Pediatr. 2003; 142:662–668.
258. Poindexter, BB, Ehrenkranz, RA, Stoll, BJ. Parenteral glutamine supplementation does not reduce the risk of mortality or late-onset sepsis in extremely low birth weight infants. Pediatrics. 2004; 113:1209–1215.
259. Tubman, TRJ, Thompson, SW, McGuire, W. Glutamine supplementation to prevent morbidity and mortality in preterm infants. Cochrane Database Syst Rev. 2008. [CD001457].
260. Bury, RG, Tudehope, D. Enteral antibiotics for preventing necrotising enterocolitis in low birthweight or preterm infants. Cochrane Database Syst Rev. 2001. [2001.CD000405].
261. Martin, CR, Walker, WA. Probiotics: Role in pathophysiology and prevention in necrotizing enterocolitis. Semin Perinatol. 2008; 32:127–137.
262. Panigrahi, P, Gupta, S, Gewolb, IH, et al. Occurrence of necrotizing enterocolitis may be dependent on patterns of bacterial adherence and intestinal colonization: Studies in Caco-2 tissue culture and weanling rabbit models. Pediatr Res. 1994; 36:115–121.
263. Mack, DR, Michail, S, Wei, SH. Probiotics inhibit enteropathogenic E. coli adherence in vitro by inducing intestinal mucin gene expression. Am J Physiol. 1999; 276:G941–G950.
264. Madsen, KL, Cornish, A, Soper, P. Probiotic bacteria enhance murine and human intestinal epithelial barrier function. Gastroenterology. 2001; 121:580–591.
265. Kennedy, RJ, Kirk, SJ, Gardiner, K. Mucosal barrier function and the commensal flora. Gut. 2002; 50:441–442.
266. Orrhage, K, Nord, CE. Factors controlling the bacterial colonization of the intestine in breastfed infants. Acta Paediatr Suppl. 1999; 88:47–57.
267. Stratiki, Z, Costalos, C, Sevastiadou, S. The effect of a Bifidobacter-supplemented bovine milk on intestinal permeability of preterm infants. Early Hum Dev. 2007; 83:575–579.
268. Bernet, MF, Brassart, D, Neeser, JR. Lactobacillus acidophilus LA 1 binds to cultured human intestinal cell lines and inhibits cell attachment and cell invasion by enterovirulent bacteria. Gut. 1994; 35:483–489.
269. Collins, MD, Gibson, G. Probiotics, prebiotics and synbiotics: Approaches for modulating the microbial ecology of the gut. Am J Clin Nutr. 1999; 69:1052S–1057S.
270. Sudo, N, Sawamura, S, Tanaka, K. The requirement of intestinal bacterial flora for the development of an IgE production system fully susceptible to oral tolerance induction. J Immunol. 1997; 159:1739–1745.
271. Fukushima, Y, Kawata, Y, Hara, H. Effect of a probiotic formula on intestinal immunoglobulin A production in healthy children. Int J Food Microbiol. 1998; 42:39–44.
272. Schiffrin, EJ, Rochat, F, Link-Amster, H. Immunomodulation of human blood cells following the ingestion of lactic acid bacteria. J Dairy Sci. 1995; 78:491–497.
273. Marin, ML, Tejada-Simon, MV, Lee, JH. Stimulation of cytokine production in clonal macrophage and T-cell models by Streptococcus thermophilus: Comparison with Bifidobacterium sp. and Lactobacillus bulgaricus. J Food Prot. 1998; 61:859–864.
274. Murch, SH. Toll of allergy reduced by probiotics. Lancet. 2001; 357:1057–1059.
275. Klinman, DM, Yi, AK, Beaucage, SL. CpG motifs present in bacteria DNA rapidly induce lymphocytes to secrete interleukin 6, interleukin 12 and interferon gamma. Proc Natl Acad Sci U S A. 1996; 93:2879–2883.
276. Fujii, T, Ohtsuka, Y, Lee, T. Bifidobacterium breve enhances transforming growth factor beta1 signaling by regulating Smad7 expression in preterm infants. J Pediatr Gastroenterol Nutr. 2006; 43:83–88.
277. Kitajima, H, Sumida, Y, Tanaka, R. Early administration of Bifidobacterium breve to preterm infants: Randomized controlled trial. Arch Dis Child Fetal Neonatal Ed. 1997; 76:F101–F107.
278. Mohan, R, Koebnick, C, Schildt, J. Effects of Bifidobacterium lactis B12 supplementation on intestinal microbiota of preterm infants: A double-blind, placebo-controlled, randomized study. J Clin Microbiol. 2006; 44:4025–4031.
279. Hoyos, AB. Reduced incidence of necrotizing enterocolitis associated with enteral administration of Lactobacillus acidophilus and Bifidobacterium infantis to neonates in an intensive care unit. Int J Infect Dis. 1999; 3:197–202.
280. Deshpande, G, Rao, S, Patole, S. Probiotics for prevention of necrotising enterocolitis in preterm neonates with very low birthweight: A systematic review of randomised controlled trials. Lancet. 2007; 369:1614–1620.
281. Dani, C, Biadaioli, R, Bertini, G, et al. Probiotics feeding in prevention of urinary tract infection, bacterial sepsis and necrotizing enterocolitis in preterm infants. A prospective double-blind study. Biol Neonate. 2002; 82:103–108.
282. Lin, HC, Su, BH, Chen, AC, et al. Oral probiotics reduce the incidence and severity of necrotizing enterocolitis in very low birth weight infants. Pediatrics. 2005; 115:1–4.
283. Bin-Num, A, Bromiker, R, Wilschanski, M, et al. Oral probiotics prevent necrotizing enterocolitis in very low birth weight neonates. J Pediatr. 2005; 147:192–196.
284. Kitajima, H, Sumida, Y, Tanaka, R, et al. Early administration of Bifidobacterium breve to preterm infants: Randomized conrolled trial. Arch Dis Child. 1997; 76:F101–F107.
285. Costalos, C, Skouteri, V, Gounaris, A, et al. Enteral feeding of premature infants with Saccharomyces boulardii. Early Hum Dev. 2003; 74:89–96.
286. Manzoni, P, Mostert, M, Leonessa, ML, et al. Oral supplementation with Lactobacillus casei subspecies rhamnosus prevents enteric colonization by Candida species in preterm neonates: A randomized study. Clin Infect Dis. 2006; 42:1735–1742.
287. Mohan, R, Koebnick, C, Schildt, J, et al. Effects of Bifidobacterium lactis Bb12 supplementation on intestinal microbiota of preterm neonates: A double placebo controlled, randomized study. J Clin Microbiol. 2006; 44:4025–4031.
288. Manzoni, P, Rinaldi, M, Cattani, S, et al. Bovine lactoferrin supplementation for prevention of late-onset sepsis in very low-birth-weight neonates: A randomized trial. JAMA. 2009; 302:1421–1428.
289. Braga, TD, da Silva, GA, de Lira, PI, et al. Efficacy of Bifidobacterium breve and Lactobacillus casei oral supplementation on necrotizing enterocolitis in very-low-birth-weight preterm infants: a double-blind, randomized, controlled trial. Am J Clin Nutr. 2011; 93:81–86.
290. Lin, HC, Hsu, CH, Chen, HL, et al. Oral probiotics prevent necrotizing enterocolitis in very low birth weight preterm infants: a multicenter, randomized, controlled trial. Pediatrics. 2008; 122:693–700.
291. Barclay, AR, Stenson, B, Simpson, JH, et al. Probiotics for necrotizing enterocolitis: A systematic review. J Pediatr Gastroenterol Nutr. 2007; 45:569–576.
292. Wang, Q, Dong, J, Zhu, Y. Probiotic supplement reduces risk of necrotizing enterocolitis and mortality in preterm very low-birth-weight infants: An updated meta-analysis of 20 randomized, controlled trials. J Pediatr Surg. 2012; 47:241–248.
293. Nair, RR, Warner, BB, Warner, BW. Role of epidermal growth factor and other growth factors in the prevention of necrotizing enterocolitis. Semin Perinatol. 2008; 32:107–113.
294. Grave, GD, Nelson, SA, Walker, WA, et al. New therapies and preventive approaches for necrotizing enterocolitis: Report of a research planning workshop. Pediatr Res. 2007; 62:510–514.
295. Moss, RL, Kalish, LA, Duggan, C, et al. Clinical parameters do not adequately predict outcome in necrotizing enterocolitis: Results from a prospective multi-institutional study. J Perinatol. 2008; 28:657–664.