CHAPTER 25 Nasal Reconstruction
Reconstruction of nasal defects is a common procedure for facial plastic surgeons. Such defects may result from trauma, tumor resection, prior aesthetic rhinoplasty, or congenital deformities. When planning for reconstruction of distorted or missing tissues of the nose, the surgeon should consider both form and function. Systematic evaluation of nasal defects involves perception of the nose as a three-layered structure: (1) a cover of skin and subcutaneous fibrofatty tissue, (2) an underlying bony and cartilaginous framework, and (3) a soft tissue lining of vestibular skin and nasal mucosa. Defects may involve any combination of these layers. The choice of tissue for reconstruction is generally determined by which elements are missing. The primary reconstructive goal is that each component be restored as closely as possible to its original anatomic state for optimal functional and aesthetic outcome.1
External Covering Layer
The nose has been described as being composed of concave and convex surfaces separated by ridges and valleys.2 These surfaces, known as aesthetic or topographic subunits, include the tip, the dorsum, the paired side walls, alar lobules, soft triangles, and the columella. When a defect involves greater than 50% of a subunit, replacement of the entire subunit is often better than replacement of only a part. Placement of scars along contour lines re-creates normal highlights and landmarks. Although this may require some healthy tissue to be discarded, the outcome is often improved.
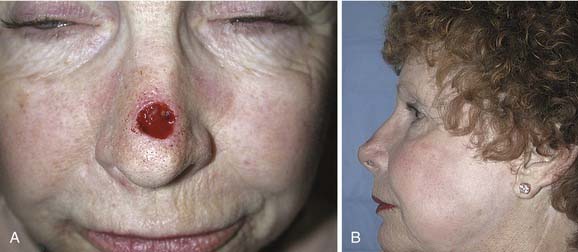
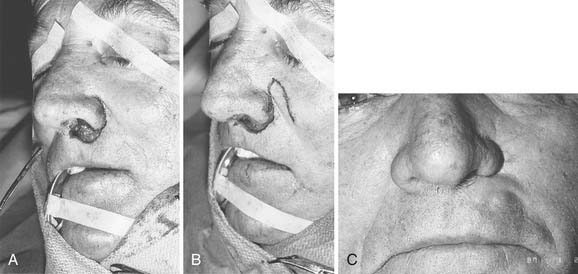
Options for repairing defects involving skin alone include healing by secondary intention, primary closure, skin grafting, and local and regional flap coverage. The choice of method is best guided by evaluation of location and depth of the wound. Healing of superficial wounds by secondary intention on concave surfaces may result in a satisfactory aesthetic outcome. For example, this method is a good choice for wounds of the medial canthal region where advancement of local tissue may distort the eyelids. However, when wounds on convex surfaces heal by secondary intention, the result is often a flattened or depressed scar. Similarly, primary closure may be used for a small defect of the mobile skin over the nasal dorsum, whereas closure of a similar defect over the tip region may result in alar retraction or asymmetry. However, in patients with tip ptosis, primary closure of a symmetrical supratip defect can improve functional as well as aesthetic results of the nose (Fig. 25-1).
Full-thickness skin grafts (FTSGs) are useful in the reconstruction of superficial defects involving convex surfaces of the nose. This is especially true in the areas of the tip and alar lobules where tissue mobility is limited for the creation of local flaps. Donor sites for FTSGs include the preauricular, postauricular, nasolabial, supraclavicular, and upper eyelid skin, each providing good color match and an easily hidden donor site. Tissue from the nasolabial crease results in the most satisfactory aesthetic reconstruction because it matches most closely the thickness, color, and texture of nasal skin.3,4 When the defect is deeper than the thickness of an FTSG, it can granulate for 7 to 14 days before the granulation tissue surface is scraped and a delayed skin graft is placed.
Because of the sebaceous nature of the lower third of the nose, an FTSG may heal with a contrasting flattened, shiny appearance.5 Thus the best results are obtained when FTSGs are used to repair superficial defects in areas of the nose where the skin is naturally thin and nonsebaceous. In addition, when placing an FTSG for reconstruction, it is often best to excise sufficient normal tissue to allow replacement of an entire nasal subunit to minimize a patched appearance. Dermabrasion of the area 3 months after grafting further improves texture match between the graft and its recipient site.6
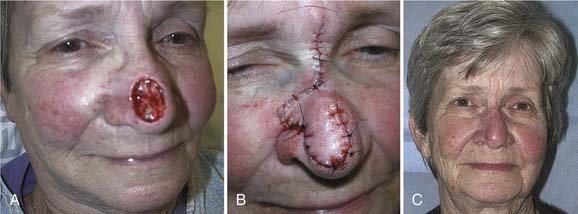
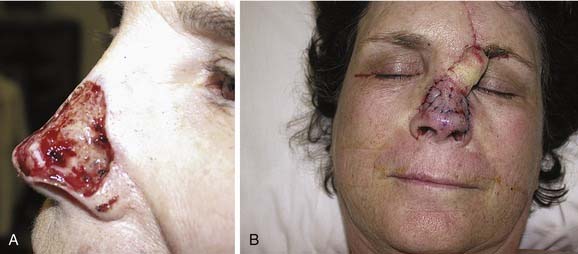
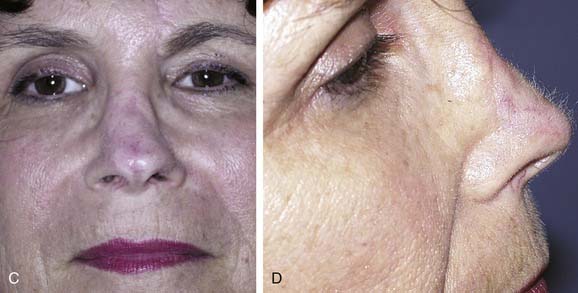
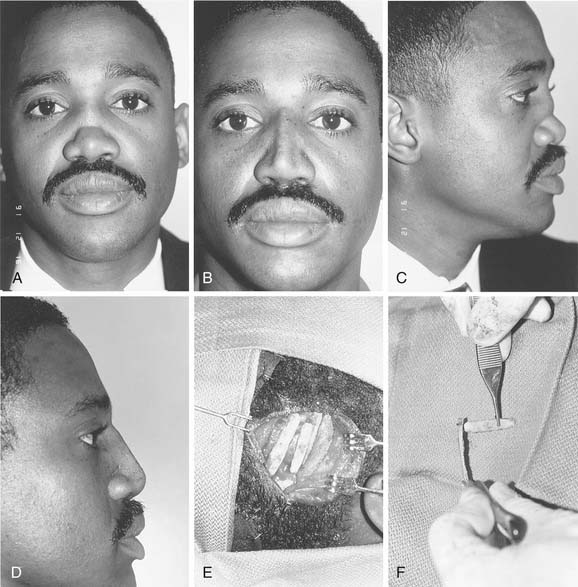
Local flaps provide several advantages over skin grafts in nasal reconstruction. Contour, color, and texture match are usually superior to FTSG, and there is usually less scar contracture, contributing to a more reliable aesthetic and functional result. Survival is greater with local flaps than with grafts because of the abundant vascular supply of the dermal and subdermal plexus. Choice among the many flaps available depends on the area of the nose to be reconstructed and the secondary deformity that will result. Simple transposition and bilobed flaps are common choices for coverage of soft-tissue defects of the dorsum or lateral walls. Using Zitelli’s modifications of the original design, the bilobed flap is the most useful of the nasal cutaneous flaps.7 This flap is most useful in the lower half of the nose where the skin from the nasal dorsum and the cheek can be recruited to close the defect of the second lobe (Fig. 25-2). Glabellar flaps are used for the more superior areas8; whereas the dorsonasal or Rieger flap is useful for single-stage repair of the more caudal regions.9 A retroauricular flap is an excellent technique for difficult nasal reconstruction in young patients. Transferring retroauricular tissue on a temporal pedicle was first described by Washio.10 Flaps should be designed so that incisions are hidden within relaxed skin tension lines and along borders of natural aesthetic units as much as possible. Wide undermining of the nasal skin and, in some cases, reduction of a dorsal hump are necessary for closure without tension. These local flaps are useful for defects of about 2 cm or less in diameter (Fig. 25-3).11
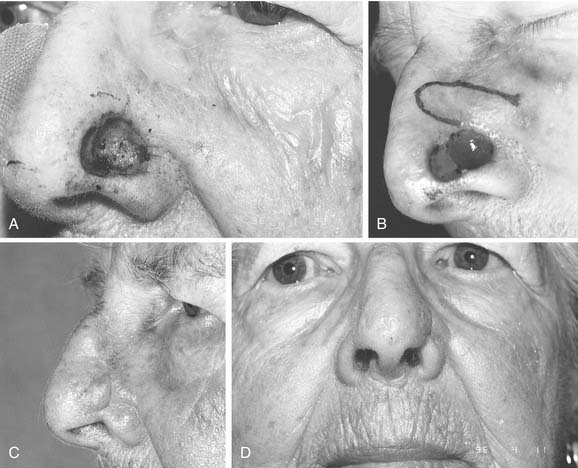
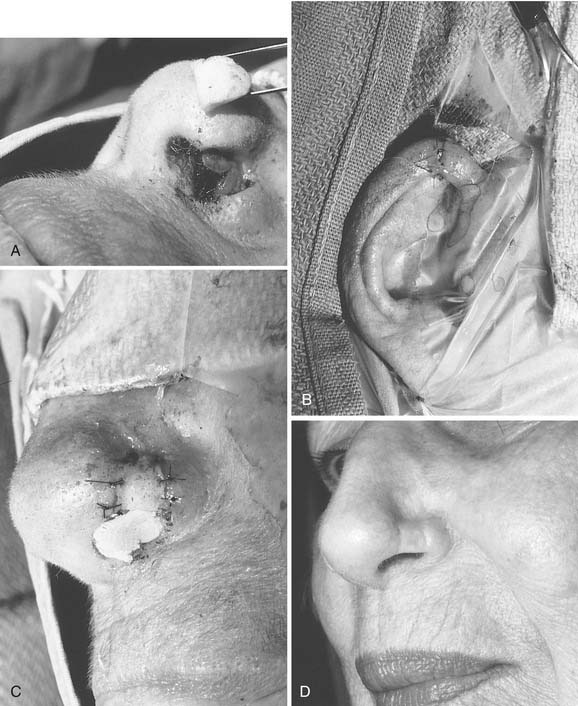
Since the earliest descriptions of the melolabial flap, many variations have been reported.12 Based superiorly, the flap may be used for defects of the lower two thirds of the nose (Fig. 25-4). With full-thickness defects, the distal portion of the flap may be turned on itself to provide internal lining coverage. When based inferiorly, the melolabial flap is appropriate for reconstruction of the upper lip, floor of nose, and columella (Fig. 25-5). Larger defects can be reconstructed using bilateral melolabial flaps or a combination of local flaps with cheek advancement.
The melolabial flap may also be used as an island flap.13 In this procedure, a circumferential skin incision is made to create a flap the size of the defect. After wide undermining of the skin, a vascular pedicle of subcutaneous tissue is made, extending from the skin island superiorly toward the infraorbital rim. The flap is then tunneled beneath the skin bridge and rotated into the defect with its subcutaneous pedicle intact. This permits one-stage reconstruction, usually without effacement of the nasofacial or alar-facial creases. The usual design of the melolabial flap requires two stages to complete, with the second procedure being a revision of the pedicle. Tacking sutures from the flap dermis to the underlying periosteum8 or temporary external mattress sutures may be used to recreate the nasofacial convexity.
The most common complications of local flaps include necrosis or “pincushioning.”14 Distortion of adjacent anatomic structures can also be a problem if flaps are not designed carefully. Necrosis with partial or complete flap loss may be caused by arterial ischemia or venous congestion. Technical problems that can contribute to complications include inadequate hemostasis, excessive tension at closure, and damage to the blood supply by excessive thinning or narrowing of the vascular pedicle.14 In addition, patients who smoke, who have received prior irradiation to the area, or who are diabetic are at increased risk for flap necrosis.
Pincushioning occurs when persistent edema of the flap results in elevation above the surrounding skin after healing. This problem occurs most frequently when C-shaped flaps are designed with the pedicle located superiorly. Causes of this inadequate lymphatic drainage include scar tissue on the dependent edges of the flap and a thick scar layer on the deep surface of the flap, which is sometimes caused by an unrecognized hematoma.14 Management options for trap-door deformities include the injection of corticosteroids into the scar tissue, Z-plasties in the parts of the scar running perpendicular to relaxed skin tension lines, and thinning of the flap with excision of the deep surface scar tissue. Although the deformity usually contributes to an unsatisfactory aesthetic result, this same process may in some instances create a favorable mounding of the flap, which helps mimic a preexisting convex contour.2
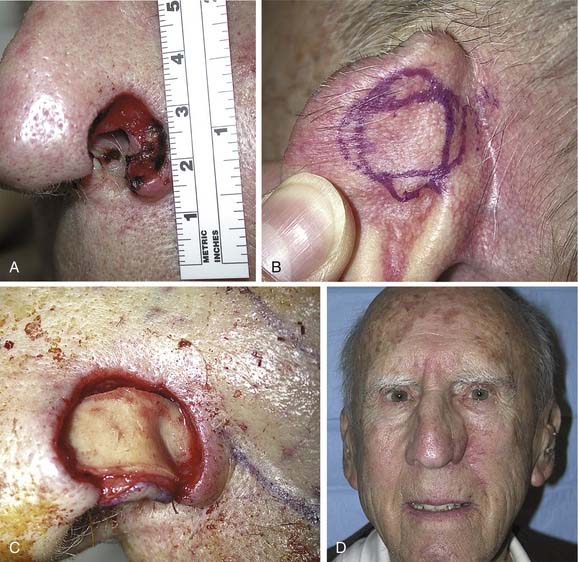
The most widely described method for reconstruction of nasal skin defects greater than 2.5 cm in diameter is the use of a forehead flap. Historically, this flap was designed to be centered in the midline of the forehead, based on one or both supratrochlear vessels. Since the description by Kazanjian in 1946,15 many variations have been proposed, including paramedian, oblique, sickle, up and down, and seagull flaps.16 The paramedian design is used most frequently.
Forehead skin provides tissue of excellent color and texture match for nasal reconstruction. A flap width of 3.5 cm or less allows primary closure of the donor site while providing enough tissue for total resurfacing of the nose. The use of tissue expanders to increase the amount of tissue available, assist in donor site closure, and increase vascularity has been reported,16–18 although it is generally not necessary.19 The paramedian flap design provides adequate tissue and allows for ease of transposition by minimizing the width at the base. In addition, the axial blood supply makes this flap extremely reliable.
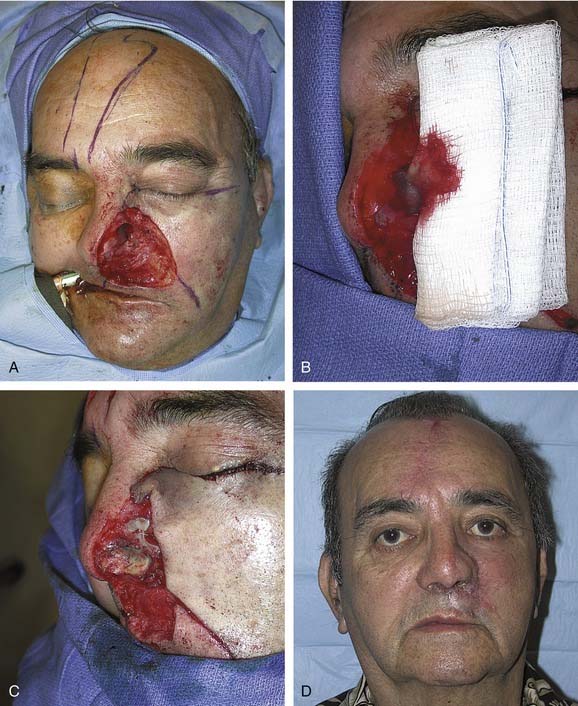
Nasal reconstruction using a forehead flap is a multistage procedure, and patients should be fully aware of the time commitment. The initial flap design is based on an exact template of the defect. Once the size is determined, the required flap length is measured and marked. Parallel skin incisions are then made with the base of the pedicle positioned over one supratrochlear artery. A Doppler device ensures that the artery is located centrally within the pedicle. The flap is elevated in a subgaleal plane. The distal half may be thinned as the vessels run superficial to the frontalis muscle superior to the eyebrows.20 Once the base has been narrowed as much as possible without jeopardizing the blood supply, the flap is rotated and set into the defect. The donor site is closed primarily after wide undermining of the forehead. The pedicle is divided 3 weeks later, and the base of the flap returned. Staged revisions involving thinning or dermabrasion of the reconstructed area are completed 3 to 6 months later.
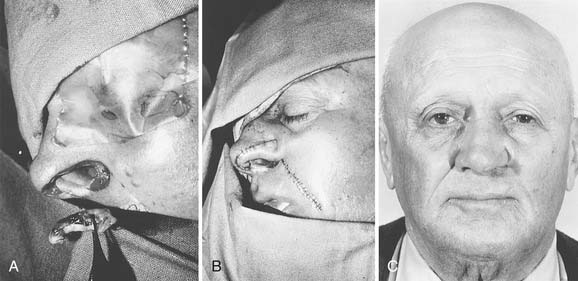
The forehead flap is versatile when used for nasal reconstruction, and it can be combined with cheek advancement, cervical rotation, or Karapandzic type flaps for larger defects that extend laterally or involve the upper lip. In cases of total or near-total nasal loss, the forehead flap is combined with cartilage or bone grafting for restoration of a stable structure (Fig. 25-6).
Framework
Solid medical grade silicone rubber, polytetrafluoroethylene carbon, and polyamide mesh are among the alloplastic (synthetic) materials that have been used for restoration of tip, alar, and septal defects and for saddle deformities.21–23 Expanded polytetrafluoroethylene (ePTFE) has been used in rhinoplasty since the mid-1970s when it attracted much attention as an alloplast.24 Calcium phosphate cement paste seems to be a suitable material for augmentation rhinoplasty. Calcium phosphate cement consisting of alpha-tricalcium phosphate, dicalcium phosphate dibasic, and tetracalcium phosphate monoxide has been used for bone replacement and augmentation because of its good biocompatibility and osteoconductivity.25 Porous high-density polyethylene implants for cleft lip nasal reconstruction are well tolerated and achieve good aesthetic results. Porous high-density polyethylene implants lend stability through fibrovascular ingrowth, with integration of the implants to the surrounding tissue.26,27
AlloDerm has been used in secondary rhinoplasty to cover the osseocartilaginous framework of the nose in an attempt to cover osseocartilaginous irregularities, to eliminate the adhesions between nasal bones and overlying skin, and to create a smooth nasal dorsum.28
Advantages of these materials include their ready availability and lack of donor site morbidity. Current alloplasts, however, lack the composition and stability necessary for permanent structural support and are better used as onlay grafts for contour deficiencies. Further disadvantages include a high extrusion rate, foreign body reaction, and susceptibility to infection.29 These disadvantages relegate alloplastics to a secondary role at this time.
Homograft tissues may also be used for nasal skeletal reconstruction. Biologic implants (e.g., irradiated cartilage,30 demineralized bone) have been used for support in total nasal reconstruction and in cases of severe saddle deformity. As with alloplasts, these materials are easily obtained and readily sculptured. They are, however, associated with a low rate of infection and extrusion. The main disadvantages of homografts are a high rate of resorption, occasional warping, and a tendency to become mobile over time.31
Autogenous cartilage and bone are the most appropriate and useful materials for reconstruction of the nasal framework. Cartilage may be used alone or in a composite graft of skin and cartilage depending on the need.32,33 Composite grafts have been used to open stenosis of the nasal airway, to repair alar retraction after rhinoplasty, and to perform columellar and alar reconstruction after tissue loss (Fig. 25-7). Revision rhinoplasty is a challenge in reconstructive rhinoplasty, both in the techniques of repair and the choice of implant material for augmentation grafting. Auricular cartilage is the best in revision rhinoplasty, because of its superior long-term survival characteristics, its ready availability in the head and neck region, its resistance to infection and resorption, and its bendability and flexibility when implanted in the nose.34
Autogenous cartilage may also be used as a free graft without attached soft tissue. The most common donor sites include the nasal septum, auricular concha, and costal cartilage. Cartilage grafts have been used for multiple purposes, including increased tip support, rebuilding of missing or weakened alar cartilages, and dorsal augmentation (Fig. 25-8).35 In addition, autogenous cartilage is especially useful in managing nasal valve collapse when used as battens or spreader grafts.36
Reconstruction of the bony nasal framework is commonly required in major facial trauma. When severe comminution or loss of bone occurs, grafting is necessary to re-create the projection and shape of the nasal dorsum and base. Although the lower turbinate bone,37 the iliac crest, and rib have been used, calvarial bone is currently the donor site of choice38 because of the easy access and low morbidity of the donor site and the decreased graft resorption noted with this membranous bone.39 Harvest is most often done from the parietal region, using outer cortical bone contoured to reconstruct the nasal dorsum or used as an L-shaped strut when dorsal and columellar support are needed (Fig. 25-9).40 Such a reconstruction is often combined with cartilaginous grafts for alar reconstruction and a forehead flap for external coverage.
Proper fixation of bone grafts is imperative for reestablishment of a stable skeletal structure. In addition, rigid fixation results in less resorption of the graft.41 Dorsal grafts are cantilevered to the nasal process of the frontal bone, whereas lateral grafts are fixed to the maxilla (see Fig. 25-9). Miniplates or lag screws are usually used for this purpose.13,42 Repair of nasal skeletal defects in this manner yields satisfactory functional and cosmetic results. The main disadvantage of using autologous bone grafts is their rigidity, which distorts the natural flexibility of the distal segment of the nose.
Surgical management of the nasal dorsum has traditionally been relegated to either augmentation or reduction followed by osteotomy. Reconstructive rhinoplasty demands attention to re-create a natural profile line, which may require dorsal reduction in some areas and augmentation in others. Appropriate surgical techniques are required to narrow the nasal dorsum, while maintaining dorsal height.43 Power-assisted bone removal is a useful tool in a second-stage reconstructive rhinoplasty requiring reduction of bony irregularities. Using osteotome may injure the newly reconstructed bony structure. Powered instrumentation may allow the performance of a surgical maneuver more efficiently and accurately.44,45 Endoscopic-powered rhinoplasty provides an excellent approach to the bony dorsum. It allows sculpting to be completed under direct vision, permitting precise contouring and easy visualization.42
Internal Lining
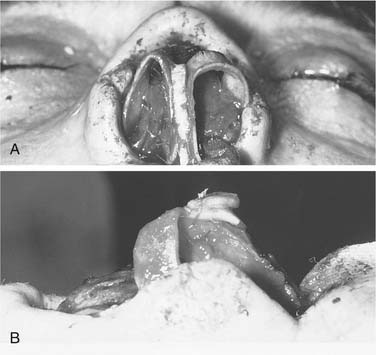
When a defect is limited to the alar margin, adjacent nasal lining may be advanced caudally as a bipedicle flap. Any remaining alar cartilage is also advanced toward the rim margin to maintain structural support. A hinge flap of septal cartilage with its contralateral mucosa attached is then used to repair the defect at the donor site.6 When more extensive tissue loss occurs or when the existing vestibular lining is severely scarred, this method of repair is usually impossible. The intranasal portion of a total nasal defect can be successfully reconstructed with a fascial forearm flap with a turbinate or mucosal graft, which allows for a thin mucosalized lining to achieve an excellent functional outcome.46 Other options include skin grafting of the undersurface of the covering flap, a turn-over flap to provide lining, and placement of a second local flap or composite ear cartilage graft that can provide good functional and aesthetic results when combined with overlying skin flaps in reconstruction of partial nasal defects (Fig. 25-10).47 Although these methods usually provide adequate internal coverage, postoperative shrinkage of a skin graft or unnatural bulkiness of a flap may cause nasal obstruction.
An ipsilateral septal mucoperichondrial flap hinged on the caudal border of the cartilaginous septum can provide adequate mucosa to reline the entire interior of the ala and nasal dome. The septal mucoperichondrial hinge flap should be designed as large as possible. Two horizontal incisions start 1 cm posterior to the caudal margin of the septal cartilage and extend 1 to 2 cm posterior to the bony cartilaginous junction where a vertical incision connects these two horizontal incisions. The dorsal horizontal incision is placed 0.5 to 1.0 cm below the roof of the middle vault. The inferior horizontal incision is placed along the junction of the nasal crest and the floor of the nose. The septal mucoperichondrial flap is dissected in the subperichondrial plane. The exposed septal cartilage and bone is left to heal by secondary intention. The septal mucoperichondrial hinge flap is supported by a framework of septal and auricular cartilage grafts. The cartilage grafts should be protected from exposure to the nasal passage. The mucoperichondrial flap will obstruct the nasal passage until it is detached from the septum in 2 to 3 weeks after the initial reconstruction (Fig. 25-11).
Baker SR. Nasal cutaneous flaps. In: Baker SR, editor. Principles of Nasal Reconstruction. St Louis: Mosby, 2002.
Burget GC, Menick FJ. The subunit principle in nasal reconstruction. Plast Reconstr Surg. 1985;76:239.
Burget GC. Aesthetic reconstruction of the tip of the nose. Oper Techniques. Plast Reconstr Surg. 1995;2:55.
Flowers RS. Nasal augmentation. Facial Plast Clin North Am. 1994;2:339.
Teichgraeber JF, Wainwright DJ. Treatment of nasal valve obstruction. Plast Reconstr Surg. 1994;93:1174.
1. Millard RD. Aesthetic reconstructive rhinoplasty. Clin Plast Surg. 1981;8:169.
2. Burget GC, Menick FJ. The subunit principle in nasal reconstruction. Plast Reconstr Surg. 1985;76:239.
3. Giebfried JW, Urken ML, Lawson W, et al. Reconstruction of nasal defects with a nasolabial island flap. Arch Otolaryngol Head Neck Surg. 1987;113:295.
4. Tardy MEJr, Boyce RG, Williams E, et al. Full-thickness skin graft reconstruction of nasal tip defects. 3rd. Facial Plast Surg. 1993;9:269.
5. Burget GC. Aesthetic reconstruction of the tip of the nose. Oper Techniques Plast Reconstr Surg. 1995;2:55.
6. Burget GC. Aesthetic restoration of the nose. Clin Plast Surg. 1985;12:463.
7. Baker SR. Nasal cutaneous flaps. In: Baker SR, editor. Principles of Nasal Reconstruction. St Louis: Mosby, 2002.
8. Larrabee WFJr, Sherris DA. Nose. In: Principles of Facial Reconstruction. Philadelphia: Lippincott-Raven; 1995.
9. Johnson TM, Swanson NA, Baker SR, et al. The Rieger flap for nasal reconstruction. Arch Otolaryngol Head Neck Surg. 1995;121:634.
10. Morrison CM, Bond JS, Leonard AG. Nasal reconstruction using the Washio retroauricular temporal flap. Br J Plast Surg. 2003;56:224.
11. Bouton FE. Aesthetic aspects of nasal reconstruction. Clin Plast Surg. 1988;15:155.
12. Becker FF, Adham RE. Melolabial flaps. In: Baker SR, Swanson NA, editors. Local Flaps in Facial Reconstruction. St Louis: Mosby, 1995.
13. Frodel JL. Primary and secondary nasal bone grafting after major facial trauma. Facial Plast Surg. 1992;8:194.
14. Cupp CL, Murakami CS. Flap necrosis and pincushioning. Oper Techniques Otolaryngol Head Neck Surg. 1993;4:82.
15. Kazanjian VH. Repair of nasal defects with median forehead flap. Surg Oncol Obstet. 1946;83:37.
16. Apesos J, Perofsky HJ. The expanded forehead flap for nasal reconstruction. Ann Plast Surg. 1993;30:411.
17. Antonyshyn O, Gruss JS, Zuker R, et al. Tissue expansion in head and neck reconstruction. Plast Reconstr Surg. 1988;82:58.
18. Kroll SS. Forehead flap nasal reconstruction with tissue expansion and delayed pedicle separation. Laryngoscope. 1989;99:448.
19. Burget GC. Personal communication (Dr. Rabinov), Rhinoplasty, Atlanta, Ga, March, 1996.
20. Shumrick KA, Smith TL. The anatomic basis of forehead flaps in nasal reconstruction. Arch Otolaryngol Head Neck Surg. 1992;118:373.
21. Beekhuis GJ. Polyamide mesh used in facial plastic surgery. Arch Otolaryngol Head Neck Surg. 1980;106:642.
22. Flowers RS. Nasal augmentation. Facial Plast Clin North Am. 1994;2:339.
23. Mass CS, Merwin GE, Wilson J, et al. Comparison of biomaterials for facial bone augmentation. Arch Otolaryngol Head Neck Surg. 1990;116:551.
24. Ham J, Miller PJ. Expanded polytetrafluoroethylene implants in rhinoplasty: literature review, operative techniques, and outcome. Facial Plast Surg. 2003;19:331-339.
25. Okada E, Maruyama Y, Hayashi A. Nasal augmentation using calcium phosphate cement. J Craniofac Surg. 2004;15:102.
26. Romo TIII, Sclafani AP, Jacono AA. Nasal reconstruction using porous polyethylene implants. Facial Plast Surg. 2000;16:55.
27. Romo TIII, Choe KS, Sclafani AP. Cleft lip nasal reconstruction using porous high-density polyethylene. Arch Facial Plast Surg. 2003;5:175-179.
28. Jackson IT, Yavuzer R. AlloDerm for dorsal nasal irregularities. Plast Reconstr Surg. 2001;107:553.
29. Adams JS. Grafts and implants in nasal and chin augmentation: a rational approach to material selection. Otolaryngol Clin North Am. 1987;20:913.
30. Strauch B, Wallach SG. Reconstruction with irradiated homograft costal cartilage. Plast Reconstr Surg. 2003;111:2405.
31. Donald PJ. Homographic cartilage in facial implantation. Facial Plast Surg. 1992;8:157.
32. Konior RJ. Free composite grafts. Otolaryngol Clin North Am. 1994;27:81.
33. Maves MD, Yessenow RS. Use of composite auricular grafts in nasal reconstruction. Dermatol Surg Oncol. 1988;14:994.
34. Becker DG, Becker SS, Saad AA. Auricular cartilage in revision rhinoplasty. Facial Plast Surg. 2003;19:41.
35. Allcroft RA, Friedman CD, Quatella VC. Cartilage grafts for head and neck augmentation and reconstruction. Otolaryngol Clin North Am. 1994;27:69.
36. Teichgraeber JF, Wainwright DJ. Treatment of nasal valve obstruction. Plast Reconstr Surg. 1994;93:1174.
37. Gurlek A, Askar I, Bilen BT, et al. The use of lower turbinate bone grafts in the treatment of saddle nose deformities. Aesthetic Plast Surg. 2002;26:407.
38. Day TA, Vuillemin T, Laedrach K, et al. Calvarial bone grafting in craniofacial reconstruction. Facial Plast Surg Clin North Am. 1995;3:241.
39. Citardi MJ, Friedman CD. Nonvascularized autogenous bone grafts for craniofacial skeletal augmentation and replacement. Otolaryngol Clin North Am. 1994;27:891.
40. Maniglia AJ, Swin S. Parietal bone graft and titanium plate fixation in nasal reconstruction. Laryngoscope. 1993;103:1066.
41. Phillips JH, Rahn BA. Fixation effects on membranous and endochondral bone graft resorption. Plast Reconstr Surg. 1988;82:872.
42. Krouse JH. Endoscopic-powered rhinoplasty. J Otolaryngol. 1999;28:282-284.
43. Harris MO, Baker SR. Management of the wide nasal dorsum. Arch Facial Plast Surg. 2004;6:41.
44. Becker DG, Park SS, Toriumi DM. Powered instrumentation for rhinoplasty and septoplasty. Otolaryngol Clin North Am. 1999;32:683-693.
45. Davis RE, Raval J. Powered instrumentation for nasal bone reduction: advantages and indications. Arch Facial Plast Surg. 2003;5:384.
46. Winslow CP, Cook TA, Burke A, et al. Total nasal reconstruction: utility of the free radial forearm fascial flap. Arch Facial Plast Surg. 2003;5:159.
47. Keck T, Lindemann J, Kuhnemann S, et al. Healing of composite chondrocutaneous auricular grafts covered by skin flaps in nasal reconstructive surgery. Laryngoscope. 2003;113:248.